 Open Access Article
Open Access ArticleSecond-phase-induced fluorescence quenching in non-equivalent substituted red phosphors†
Jun Chena,
Xianfeng Yangc,
Chunyan Jianga,
Yunfeng Wang*ab,
Lei Zhou *a and
Mingmei Wu
*a and
Mingmei Wu *a
*a
aSchool of Chemistry/School of Marine Science/School of Chemical Engineering and Technology, Sun Yat-Sen University, Guangzhou 510275/Zhuhai 519082, P. R. China. E-mail: wangyunfeng@nyist.edu.cn; zhoul8@mail.sysu.edu.cn; ceswmm@mail.sysu.edu.cn
bSchool of Information Engineering, Nanyang Institute of Technology, Nanyang 473004, P. R. China
cAnalytical and Testing Center, South China University of Technology, Guangzhou, 510640, P. R. China
First published on 21st October 2022
Abstract
Concentration quenching, which generally originates from serious energy migrations among the uniformly distributed luminescent centers in the host matrix, is a key factor to influence the luminescence properties of materials. Different from previous reports, we demonstrate a novel fluorescence-quenching mechanism attributable to the second-phase Eu2W2O9 in non-equivalent substituted SrWO4:xEu3+ phosphors. The crystal structure, elemental distribution, and luminescence properties of the as-prepared SrWO4:xEu3+ phosphors are systematically investigated. A second-phase Eu2W2O9 is confirmed when the Eu3+-doping concentration exceeds 20%, which produces the new structure defects and energy-transfer paths, resulting in fluorescence quenching in this material. This finding gives a new perspective to analyze the concentration-quenching mechanism of the non-equivalent substituted phosphors and can help in the design of new, efficient luminescence materials. In addition, the as-prepared SrWO4:xEu3+ phosphors exhibit a strong intrinsic excitation in the range of 355–425 nm, which is accompanied by the Commission Internationale de I'Eclairage (CIE) coordinates at (0.653, 0.347) and stable color purity of up to 94.52%. A packaged white light-emitting diode with CIE chromaticity coordinates of (0.398, 0.335), correlated color temperature of 3132 K, and color rendering index of 84.3 is fabricated by SrWO4:20%Eu3+ phosphors with blue BAM:Eu2+ and green YAGB:Tb3+ phosphors in a near-ultraviolet chip.
Introduction
In recent years, solid-state lighting based on inorganic luminescent materials has attracted considerable attention due to its wide range of application fields, such as lighting, display, plant growth, solar-cell utilization, optical communication, photocatalysis, and biosensors.1–9 It is well known that concentration quenching is a common phenomenon that influences the luminescence property of doping ions. Investigations on fluorescence concentration quenching have mostly focused on equivalent and matching size substitution, such as K2SiF6:Mn4+, Y3Al5O12:Ce3+, Y2O3:Eu3+, YVO4:Dy3+, NaYF4:Yb3+, Er3+, Y2SiO5:Eu3+, Bi3+, BaAl12O19:Eu2+, and CaZnOS:Mn2+.10–17 In these reports, fluorescence activators can homogeneously disperse into the interior host lattice by replacing the equivalent ions that have similar ionic radii. According to the theoretical perspectives of Blasse, non-radiative energy can migrate among the excited emitters and reach the quenching center, which is unfavorable to luminescence.18 Generally, a higher doping concentration can generate a much closer distance between the dopants and accelerate energy migrations. Therefore, the traditional concentration-quenching phenomenon is often observed in inorganic luminescent materials when the doping concentration exceeds a certain value.In addition, various studies related to non-equivalent substitution have been proposed with excellent luminous performance in rare-earth- and transition-metal-ion-doped inorganic optical materials such as K3AlF6:Mn4+, K3ScF6:Mn4+, ZrO2:Eu3+, TiO2:Sm3+, Zn2SiO4:Eu3+, MMoO4:Eu3+ (M = Ca, Sr, Ba), (CaMg)x(NaSc)1−xSi2O6:Eu2+, and NaYF4:Yb3+, Er3+, Mn2+.19–27 Based on these reports, the luminescence centers in the non-equivalent substituted inorganic materials can produce a higher luminous efficiency owing to the perfect size matching to the replaced ions, accomplishing similar electronic densities and coordination numbers.28 Nevertheless, the concomitant charge imbalance from non-equivalent substitution can induce some intrinsic microstructure defects such as traps, which may considerably deteriorate the luminescence performance of the as-prepared phosphor materials.29 Some research groups have attempted to use the charge compensation method to cover the shortage and enhance the photoluminescence.30–32 Further, many published articles on non-equivalent substituted fluorescent materials often do not represent structure defects owing to the relatively low emitter doping concentrations.33–35 Moreover, many studies on phosphors have pointed out the fact that non-equivalent foreign-ion doping can alter the local structure symmetry of the primary host lattice and result in an obvious phase-structure change.36–39 Consequently, whether such a possibility exists for the typical luminescent property of the non-equivalent substituted samples mainly depends on the subsistent phase separation. To the best of our knowledge, this kind of work has not been thoroughly studied in previous literature yet.
Here, we chose the familiar SrWO4:Eu3+ phosphors as our research object. Many experiments have confirmed that SrWO4 is an excellent luminescence host due to the perfect matching size of Sr2+ (1.26 Å) and Eu3+ (1.07 Å),40 high thermal and mechanical stability, simple route of synthesis, etc.30 Therefore, scheelite-based tungsten structure hosts have attracted widespread attention and interest for use in white light-emitting diodes (WLEDs). Based on an extensive study of the concentration-quenching mechanism, the non-equivalent substituted Eu3+-doped SrWO4 red phosphors were prepared by a facile sol–gel method. The crystal structure, elemental distribution, and photoluminescence properties with different Eu3+-doping concentrations are comprehensively studied. Accordingly, a novel concentration-quenching mechanism using phase separation has been proposed in non-equivalent substituted luminescence materials. This may provide a new perspective to investigate the inorganic luminescent materials with emitters of inconsistent charges.
Experimental section
Sample preparation
All the chemicals are of the analytical grade and used as received without further purification. Europium nitrate [Eu(NO3)3·6H2O] is purchased from the National Engineering Research Centre of Rare Earth Metallurgy and Function Materials. Strontium nitrate, ammonium metatungstate, and citric acid are received from Aladdin.SrWO4:xEu3+ phosphors were synthesized by a simple, mild sol–gel method. First, strontium nitrate, ammonium metatungstate, and europium nitrate with the corresponding stoichiometric ratio (the total number of Sr2+ and Eu3+ ions were 3 mmol) were dissolved in a mixture solution of deionized water and ethanol at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 ml); 100 mg citric acid was added as the complexant. After stirring for several minutes, the homogeneous transparent solution was transferred into an oven at 70 °C to remove the deionized water and ethanol. Finally, annealing was carried out at an elevated temperature rate (180 °C h−1) up to a certain high temperature within 500–1200 °C and maintained for 3 h. As a comparison, Eu2W2O9 was prepared by the same method as described above.
1 (5 ml); 100 mg citric acid was added as the complexant. After stirring for several minutes, the homogeneous transparent solution was transferred into an oven at 70 °C to remove the deionized water and ethanol. Finally, annealing was carried out at an elevated temperature rate (180 °C h−1) up to a certain high temperature within 500–1200 °C and maintained for 3 h. As a comparison, Eu2W2O9 was prepared by the same method as described above.
Sample characterization
The phase structures of the samples were characterized by an X-ray diffractometer (XRD) using a Rigaku D-Max 2200 XRD system with Cu Kα radiation at 40 kV and 26 mA (λ = 1.5405 Å). The high-resolution transmission electron microscopy (HR-TEM) images, elemental mapping, and energy-dispersive X-ray spectroscopy (EDX) data of the samples were recorded on a JEOL JEM 2100F TEM instrument with an acceleration voltage of 200 kV. The morphology and rough elemental composition were analyzed on an FEI Quanta scanning electron microscopy (SEM) instrument equipped with an energy-dispersive spectrometer (EDS). The Raman spectra were characterized by a Horiba Jobin Yvon HR800 Raman spectrometer. The excitation source was a 488 nm laser and the diameter of the laser spot was 1.0 μm. The photoluminescence (PL) emission spectra, photoluminescence excitation (PLE) spectra, and decay curves were measured by an FLS 980-combined time-resolved and steady-state fluorescence spectrometer (Edinburgh Instruments) equipped with Xe/nF/μF lamps. Temperature-dependent PL spectra and dynamics were measured on the same instrument within a temperature range of 77–500 K. The quantum yield (QY) was obtained with a Hamamatsu C9920-03G absolute QY measurement system.Results and discussion
Crystal structure and elemental distribution of SrWO4:Eu3+
The lattice structure, morphology, and elemental distribution of the SrWO4:xEu3+ samples prepared by the simple sol–gel technique are analyzed by XRD, TEM, and SEM. The XRD patterns of the as-prepared SrWO4:xEu3+ phosphors at an annealing temperature of 900 °C with different Eu3+-doping concentrations are shown in Fig. 1a. Evidently, the samples exhibit a pure phase structure and the detected diffraction peaks match well with the International Centre for Diffraction Data (ICDD) card no. 08-0490 when the Eu3+ concentration varies from 2% to 20%, which seemingly indicates that the Eu3+ ions can homogeneously incorporate into the SrWO4 host. However, when the increasing Eu3+ concentration is above 20%, the XRD patterns appear to have obvious impure diffraction peaks, which are ascribed to a second-phase Eu2W2O9 distributing in the SrWO4:Eu3+ main part with ICDD card no. 23-1073.The comparison of the diffraction peaks of SrWO4:40%Eu3+ and SrWO4:6%Eu3+ (Fig. 1b) and pure Eu2W2O9 (Fig. S1†) can definitively confirm the second phase in the as-prepared non-equivalent substituted materials. It can be deduced that some Eu3+ ions are not integrated into the SrWO4 host lattice and combine with W6+ and O2− groups to generate a new Eu2W2O9 phase structure, which is derived from the existing charge balance and radius difference between Eu3+ and Sr2+ ions with a gradually increasing Eu3+ concentration. Different from traditional uniform Eu3+-ion doping into the lattice matrix, the Eu2W2O9 second-phase separation could be a major role to influence the photoluminescence properties in a non-equivalent substituted optical material. In addition, it can be easily observed (Fig. S2†) that the diffraction peaks gradually shift to a larger angle from 2% to 40%, which is due to the ionic radius of the replaced Sr2+ (r = 1.26 Å, CN = 8), larger than Eu3+ (r = 1.07 Å, CN = 8) emitters; this also implies that some of the Eu3+ ions can be evenly doped into the SrWO4 host lattice. In order to better understand the crystal structure and coordination environment, a schematic of tetragonal SrWO4:xEu3+ with the I41/a space group is modelled (Fig. 1c). Regarding the crystallographic structure, the SrWO4 host has a common tetragonal structure with the space group of I41/a and the point-group symmetry of C64h. The lattice parameters and unit-cell volumes of SrWO4 have been calculated as a = b = 0.542 nm, c = 1.196 nm, and V = 0.351 nm3, with four SrWO4 formula units per tetragonal cell. In this type of structure, Sr2+ and W6+ ions are coordinated by eight and four oxygen atoms in the form of dodecahedra and tetrahedra, respectively, and the site symmetry of Sr2+, W6+, and O2− ions is S4, Td, and C1, respectively. In the unit cell of SrWO4:xEu3+, Eu3+ and Sr2+ ions are coordinated to eight oxygen ions and they form a distorted dodecahedron; W6+ ions are surrounded by four oxygen ions and constitute a slightly distorted tetrahedron.41
To better understand the Eu2W2O9 second-phase separation, elemental mapping with EDX and HR-TEM images of SrWO4:20%Eu3+ were specifically checked. In Fig. 2a, one microparticle with areas of different shapes was selected, and its elemental composition of Sr, W, Eu, and O was preliminarily detected by using the EDX spectrum (Fig. S3†). From Fig. 2b–d, it is evident that W, O, and Sr are uniformly distributed throughout the as-prepared material. However, in Fig. 2e and f, some parts are apparently rich with Eu3+ ions, which suggests the possible second-phase Eu2W2O9 existing in SrWO4:20%Eu3+ phosphors beyond the range of the XRD detection limit. The phase structure of Eu2W2O9 can be confirmed by the HR-TEM images (Fig. 2g and S4†). The measurement result (Fig. 2g) reveals the presence of monoclinic crystalline Eu2W2O9 possessing lattice fringes of 0.3123 and 0.2613 nm, which correspond to the (112) and (032) (Fig. S4†) crystallographic planes, respectively. Furthermore, the HR-TEM images shown in Fig. 2h and S5† exhibit the typically tetragonal crystalline SrWO4 host lattice with a fringe of 0.4958 nm, which corresponds to the crystallographic plane of (101). In addition, the elementary composition and EDS by SEM images reveal that the atomic ratios of Eu, Sr, W, and O are 3.08, 13.81, 16.96, and 66.14, respectively (Fig. S6†), which is consistent with the predetermined SrWO4:20%Eu3+. According to the above analysis, the second-phase Eu2W2O9 is confirmed in the non-equivalent substituted SrWO4:xEu3+ phosphors as the Eu3+ concentration increases, which may have a great impact on the luminescence behavior and properties of this material.
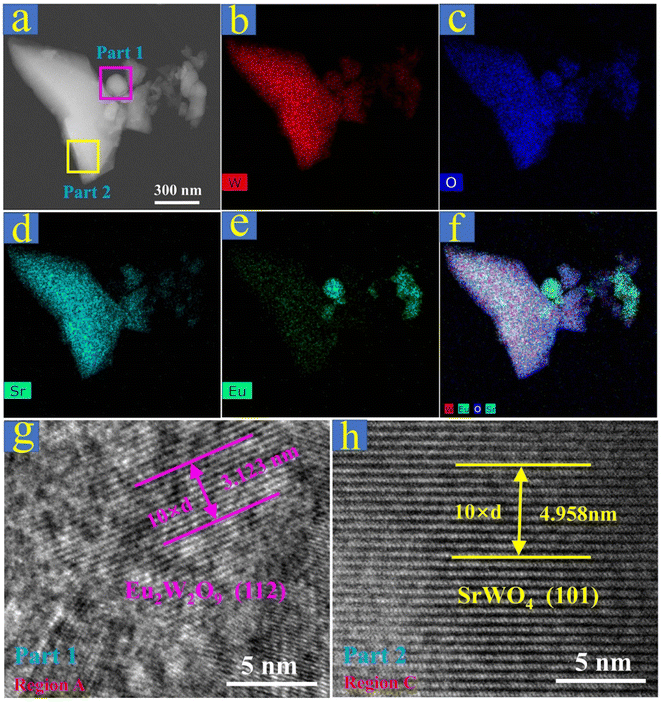 | ||
| Fig. 2 (a)–(f) Scanning TEM and elemental mapping images of the as-prepared SrWO4:20%Eu3+ sample. (g) Magnified TEM image of region A in part 1. (h) Magnified TEM image of region C in part 2. | ||
Concentration-dependent luminescence and dynamics properties
It is well known that Eu3+ has the most popular red emission property because of its representative 5DJ–7FJ transitions.42 The typical PLE and PL spectra of SrWO4:xEu3+ phosphors are measured and shown (Fig. 3a) and the main population of Eu3+ ions at the lower-energy levels is shown in Fig. 3c for a better understanding of the PLE and PL processes of SrWO4:xEu3+. In the left-hand side of Fig. 3a, the PLE spectra of SrWO4:xEu3+ are collected by monitoring the intense emission intensities at 612 nm (5D0–7F2) with different Eu3+-doping concentrations from 2% to 40%. Some strong PLE peaks are located at 360, 380, 394, 413, and 463 nm, which are assigned to the transitions of 7F0–5D4, 7F0–5GJ/5L7, 7F0–5L6, 7F0–5D3, and 7F0–5D2, respectively.33 This can provide a worthwhile advantage for producing efficient luminescence under near-UV LED chip excitation. Moreover, the diffuse reflection spectra of pure SrWO4 host and SrWO4:20%Eu3+ are recorded (Fig. S7†). An obvious absorption band of 200–350 nm is attributed to the typical energy transfer band (CTB), and some sharp absorption peaks in the longer-wavelength region of 350–650 nm show the intrinsic absorption sites of Eu3+ ions from the 7F0 ground state to the rich excited states.43 These characteristics are consistent with the PLE spectra shown in Fig. 3a.In general, the luminescent properties of materials strongly depend on the doping concentration of the activators. The right-hand side of Fig. 3a shows the typical emission lines of SrWO4:xEu3+ (x = 2%, 6%, 10%, 15%, 20%, 30%, and 40%) under the optimal 394 nm excitation. Evidently, all the emission peaks are almost entirely covered by 5D0–7FJ transitions, which is due to the serious non-radiative relaxations of 5D1–5D0 in tungstate host lattices with a large phonon threshold of 921 cm−1 (Fig. S8†).
Because of the very low intensity of 5D1–7FJ transitions, the color purity on red emissions of SrWO4:xEu3+ is expected to be further optimized and marginally changed. Regarding the calculated results shown in Fig. 4, the color purity of SrWO4:xEu3+ reaches up to 94.52% and the float does not exceed 1.1% with different Eu3+ concentrations (Fig. 4a).43 The Commission Internationale de I'Eclairage (CIE) coordinates of SrWO4:20%Eu3+ are (0.653, 0.357), which are close to the standard red emission of (0.670, 0.330), as shown in Fig. 4b. To systematically investigate the concentration-dependent PL emission properties further, Fig. 3b shows the variation tendency of the SrWO4:xEu3+ phosphors on integral PL intensities. According to the experimental results, the integral intensities show an upward tendency before the Eu3+-doping concentration reaches its optimal ratio of 20% and then gradually decreases with an increasing Eu3+ concentration. This common change in regularity has been reported in many rare-earth-ion-doped inorganic luminescent materials.42 With the traditional views, Eu3+ ions can uniformly distribute into the SrWO4 host lattices; the higher Eu3+-doping concentration produces a much closer distance between the Eu3+ emitters and accelerates the energy migration among them. Therefore, an increasing amount of excitation energy migrates to the quenching sites and results in luminescence quenching.
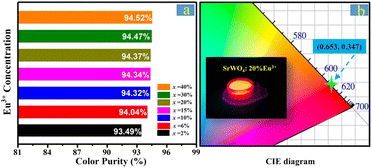 | ||
| Fig. 4 (a) Color purity of SrWO4:xEu3+ phosphors. (b) CIE chromaticity coordinates of SrWO4:20%Eu3+, where the inset shows a digital photograph of SrWO4:20%-Eu3+ under near-UV excitation. | ||
However, another fluorescence-quenching path could be highlighted in the non-equivalent substituted SrWO4:xEu3+ materials. Based on the XRD patterns and elemental distribution analyses, not all Eu3+ dopants can be coordinated into the SrWO4 host with the increasing doping concentration as well as an increasingly serious charge imbalance. The excessive Eu3+ ions can combine with W6+ and O2− groups to generate the second-phase Eu2W2O9, which produces the new local structure defects and energy-transfer paths. Further, the excitation energy can migrate to Eu2W2O9 or transmit inside it easily with an increasing Eu3+-doping concentration. This establishes a novel concentration-quenching mechanism in rare-earth-ion-doped non-equivalent substituted materials, and the schematic of the energy-transfer relationship in SrWO4:xEu3+ phosphors is shown in Fig. 5. Regarding the traditional concentration-quenching mechanism, the distance between the luminescence emitters is closer as the doping concentration increases, which accelerates the energy migration to make an increasing amount of energy transform into the quenching sites. Developed from the traditional concentration-quenching mechanism, a novel mechanism is proposed in which a higher doping concentration not only reduces the distance between the luminescent ions but also produces the second phase that acts as the quenching site to deteriorate the luminescence performance. Therefore, as the x values of SrWO4:xEu3+ increases, the high Eu3+-doping concentration decreases the distance of Eu3+ ions, on one hand, to accelerate the energy transfer between them; on the other hand, second-phase Eu2W2O9 is generated as a special quenching site, which causes significant fluorescence quenching in this non-equivalent substituted phosphor. Further, it should be noted that under the same excitation wavelength of 394 nm, the integral emission intensity of SrWO4:20%Eu3+ is 51% higher than that of Eu2W2O9 (Fig. S9†), showing excellent luminescence performance. To better understand the quenching mechanism, the relation between the doping concentration and emission intensity is calculated (Fig. S10†). The experimental results can be linearly fitted with a slope of −1.58, demonstrating the dominant dipole–dipole interaction in the concentration-quenching mechanism of SrWO4:xEu3+ phosphors.43 We consider the internal QY η, which is defined as the ratio of the emitted to the absorbed photon number, as another reliable parameter to reflect the applicability of luminescence centers for lighting application. The QY values of SrWO4:xEu3+ are measured under 394 nm excitation (Fig. 6). The measurement results show that the QY of the optimal sample is up to 61% with 20% Eu3+-doping concentration, which is consistent with the best emission intensity.
Fluorescence dynamics is a forceful means to investigate the microscopic population process of rare-earth activators. The comprehensive decay times of SrWO4:xEu3+ have been measured and calculated through the following first-order exponential equation:33
I(t) = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) exp(−t/τ)
| (1) |
| τ−1 ∝ Rc + RET[Eu3+] | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 cm−1, about 18 times that of the phonon energy of the SrWO4 host), it can be inferred that the longer fluorescence lifetime in Eu2W2O9 on 5D0–7F2 transition is mainly due to its larger non-radiative transition rate, which derives from the inherent smaller Eu3+–Eu3+ ion spacing.
268 cm−1, about 18 times that of the phonon energy of the SrWO4 host), it can be inferred that the longer fluorescence lifetime in Eu2W2O9 on 5D0–7F2 transition is mainly due to its larger non-radiative transition rate, which derives from the inherent smaller Eu3+–Eu3+ ion spacing.
Temperature-dependent emission spectra and dynamics
It is well known that the emission intensities of phosphors strongly depend on the environmental temperature, which is called the thermal-quenching effect. To reveal the thermal-quenching property, the emission spectra of SrWO4:20%Eu3+ with a varying temperature range of 300–500 K are displayed in Fig. 8. Evidently, the locations of the emission peaks did not show any change, which is the merit for achieving stable emitting color in the high-temperature range (Fig. S13†). Nevertheless, the emission intensities gradually decrease due to the harmful thermal-quenching effect in the material. Meanwhile, the emission intensity of SrWO4:20%Eu3+ can be maintained at 96%, 84%, 68%, and 56% when the temperature is 325, 350, 375, and 400 K, respectively. This indicates that the phosphor has relatively better thermal stability for potential applications in warm WLEDs.45–48 In order to further reveal the temperature-quenching mechanism of the emission intensity, the temperature-dependent dynamics of 5D1–7F1 (535 nm) and 5D0–7F2 (612 nm) transitions in SrWO4:20%Eu3+ phosphor are also measured (Fig. S14†). The fluorescence lifetimes of 535 and 612 nm are gradually shorter with an increasing temperature, illustrating the important impact on the spontaneous radiation process. Generally, the environmental temperature is an important factor to activate the non-radiative path by raising the phonon vibrational modes and creating an unfavorable condition for photoluminescence. | ||
| Fig. 8 Temperature-dependent PL intensity of SrWO4:20%Eu3+ ranging from 300 to 500 K; the inset shows a fitting function on the related intensity. | ||
Applications in red and warm WLEDs
The concentration control of doped Eu3+ emitting ions and the increase in efficiency is of critical importance for practical applications. A red-emitting LED device is encapsulated by combining an InGaN near-UV chip (∼377 nm) and the SrWO4:20%Eu3+ phosphor (Fig. 9a). Clearly, the device emits a bright and pure red emission that can be seen with the naked eye (Fig. 9a, inset), which certifies that the as-prepared SrWO4:Eu3+ sample has a satisfactory absorption efficiency and a potential application for warm WLEDs. To further verify the application of quality indoor illumination, a WLED is fabricated by coating the commercial BAM:Eu2+ blue-emitting phosphors, YGAB:Tb3+ green-emitting phosphors, and the as-prepared SrWO4:20%Eu3+ onto a near-UV chip (Fig. 9b). As expected, the combined WLED shows a bright white light with a marginal amount of yellow (Fig. 9b, inset). The as-fabricated WLED displays an effective lighting performance with the CIE chromaticity coordinates of (0.398, 0.335) (Fig. 9c), correlated color temperature (CCT) of 3132 K, and color rendering index (CRI) of 84.3. These experimental results indicate that the facilely prepared non-equivalent substituted SrWO4:xEu3+ phosphors have potential application prospects for warm WLEDs.Conclusions
In summary, a series of highly efficient non-equivalent substituted SrWO4:xEu3+ phosphors are successfully synthesized by a simple and facile sol–gel method. The crystal structure, elemental distribution, and PL properties of the as-prepared samples are investigated in detail via XRD, EDS, HR-TEM, PL spectra, and fluorescence decay curves. It should be first highlighted that a distinct second-phase Eu2W2O9 can be observed in the SrWO4:xEu3+ phosphors as the Eu3+ concentration is over 20%, accompanied with a decrease in the emission intensity, which represents a potential fluorescence-quenching mechanism by an additional path of phase separation in non-equivalent substituted luminescence materials. It is also discovered that the as-prepared SrWO4:xEu3+ phosphors exhibit excellent absorption characteristics in the near-UV spectrum range of 355–425 nm and emit bright red light with the CIE coordinates of (0.653, 0.347) and a highly stable color purity of 94.52%. Finally, the fabricated device displays an effective warm white-light performance with the appropriate CIE coordinates of (0.398, 0.335), low CCT of 3132 K, and favorable CRI of 84.3. In brief, the experimental results show a fresh perspective to view the fluorescence-quenching mechanism of non-equivalent substituted inorganic luminescent materials in warm WLED applications and will provide more opportunities for developing novel efficient luminescent materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (No. 52102194 and 51902355), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515010449), Key Research Projects of Colleges and Universities in Henan Province (No. 21A430027).Notes and references
- Y. J. Zhang, Z. L. Zhang, X. D. Liu, G. Z. Shao, L. L. Shen, J. M. Liu, W. D. Xiang and X. J. Liang, Chem. Eng. J., 2020, 401, 125983 CrossRef CAS.
- Y. Y. Zhou, H. Ming, S. Zhang, T. T. Deng, E. H. Song and Q. Y. Zhang, Chem. Eng. J., 2021, 415, 128974 CrossRef CAS.
- D. Zhang, B. F. Zheng, Z. B. Zheng, L. Li, Q. Yang, Y. H. Song, B. Zou and H. F. Zou, Chem. Eng. J., 2022, 431, 133805 CrossRef CAS.
- Y. Wei, Z. Y. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 RSC.
- X. Y. Huang, J. Liang, B. Li, L. L. Sun and J. Lin, Opt. Lett., 2018, 43, 3305–3308 CrossRef CAS PubMed.
- C. Chen, Y. J. Wu, L. Liu, Y. B. Gao, X. F. Chen, W. B. Bi, X. Liu, D. L. Chen, Q. L. Dai and H. W. Song, Adv. Sci., 2019, 6, 1802046 CrossRef PubMed.
- A. Forbes, A. Dudley and M. McLaren, Adv. Opt. Photonics, 2016, 8, 200–227 CrossRef.
- N. Jallouli, L. M. Pastrana-Martinez, A. R. Ribeiro, N. F. F. Moreira, J. L. Faria, O. Hentati, A. M. T. Silva and M. Ksibi, Chem. Eng. J., 2018, 334, 976–984 CrossRef CAS.
- H. Rabie, Y. Zhang, N. Pasquale, M. J. Lagos, P. E. Batson and K. B. Lee, Adv. Mater., 2019, 31, 1970104 CrossRef.
- H. M. Zhu, C. C. Lin, W. Q. Luo, S. T. Shu, Z. G. Liu, Y. S. Liu, J. T. Kong, E. Ma, Y. G. Cao, R. S. Liu and X. Y. Chen, Nat. Commun., 2014, 5, 4312 CrossRef CAS PubMed.
- R. Zhang, H. Lin, Y. L. Yu, D. Q. Chen, J. Xu and Y. S. Wang, Laser Photonics Rev., 2014, 8, 158–164 CrossRef CAS.
- R. S. Meltzer, S. P. Feofilov, B. Tissue and H. B. Yuan, Phys. Rev. B, 1999, 60, 14012–14015 CrossRef.
- M. Yu, J. Lin, Z. Wang, J. Fu, S. Wang, H. J. Zhang and Y. C. Han, Chem. Mater., 2002, 14, 2224–2231 CrossRef CAS.
- C. D. S. Brites, X. J. Xie, M. L. Debasu, X. Qin, R. F. Chen, W. Huang, J. Rocha, X. G. Liu and L. D. Carlos, Nat. Nanotechnol., 2016, 11, 851–856 CrossRef CAS PubMed.
- F. W. Kang, Y. Zhang and M. Y. Peng, Inorg. Chem., 2015, 54, 1462–1473 CrossRef CAS PubMed.
- K. S. Sohn, E. S. Park, C. H. Kim and H. D. Park, J. Electrochem. Soc., 2000, 147, 4368–4373 CrossRef CAS.
- C. J. Duan, A. C. A. Delsing and H. T. Hintzen, Chem. Mater., 2009, 21, 1010–1016 CrossRef CAS.
- G. Blasse, Recl. Trav. Chim. Pays-Bas, 1986, 105, 143–149 CrossRef CAS.
- E. Song, J. Wang, J. Shi, T. Deng and Q. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 8805–8812 CrossRef CAS PubMed.
- H. Ming, S. Liu, L. Liu, J. Peng, J. Fu, F. Du and X. Ye, ACS Appl. Mater. Interfaces, 2018, 10, 19783–19795 CrossRef CAS PubMed.
- L. M. Chen, Y. N. Liu and Y. D. Li, J. Alloys Compd., 2004, 381, 266–271 CrossRef CAS.
- M. G. Brik, I. Sildos and V. Kiisk, Phys. B, 2010, 405, 2450–2456 CrossRef CAS.
- Z. M. El-Bahy, A. A. Ismail and R. M. Mohamed, J. Hazard. Mater., 2009, 166, 138–143 CrossRef CAS PubMed.
- L. Dacanin, S. R. Lukic, D. M. Petrovic, M. Nikolic and M. D. Dramicanin, Phys. B, 2011, 406, 2319–2322 CrossRef CAS.
- Y. Jin, J. H. Zhang, S. Z. Lu, H. F. Zhao, X. Zhang and X. J. Wang, J. Phys. Chem. C, 2008, 112, 5860–5864 CrossRef CAS.
- Z. Xia, G. Liu, J. Wen, Z. Mei, M. Balasubramanian, M. S. Molokeev, L. Peng, L. Gu, D. J. Miller, Q. Liu and K. R. Poeppelmeier, J. Am. Chem. Soc., 2016, 138, 1158–1161 CrossRef CAS PubMed.
- G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- S. K. Gupta, P. S. Ghosh, A. K. Yadav, N. Pathak, A. Arya, S. N. Jha, D. Bhattacharyya and R. M. Kadam, Inorg. Chem., 2016, 55, 1728–1740 CrossRef CAS PubMed.
- K. Li, M. Shang, H. Lian and J. Lin, Inorg. Chem., 2015, 54, 7992–8002 CrossRef CAS PubMed.
- S. K. Gupta, K. Sudarshan, A. K. Yadav, R. Gupta, D. Bhattacharyya, S. N. Jha and R. M. Kadam, Inorg. Chem., 2018, 57, 821–832 CrossRef CAS PubMed.
- Y. Wei, J. S. Gao, G. C. Xing, G. G. Li, P. P. Dang, S. S. Liang, Y. S. Huang, C. C. Lin, T. S. Chan and J. Lin, Inorg. Chem., 2019, 58, 6376–6387 CrossRef CAS PubMed.
- L. Cheng, W. T. Zhang, Y. H. Li, S. Y. Dai, X. F. Chen and K. H. Qiu, Ceram. Int., 2017, 43, 11244–11249 CrossRef CAS.
- Maheshwary, B. P. Singh, J. Singh and R. A. Singh, RSC Adv., 2014, 4, 32605–32621 CAS.
- F. A. Rabuffetti, S. P. Culver, J. S. Lee and R. L. Brutchey, Nanoscale, 2014, 6, 2909–2914 RSC.
- Y. G. Su, L. P. Li and G. S. Li, Chem. Mater., 2008, 20, 6060–6067 CrossRef CAS.
- Y. A. Zulueta, T. C. Lim and J. A. Dawson, J. Phys. Chem. C, 2017, 121, 23642–23648 CrossRef CAS.
- J. A. Dawson, H. Chen and I. Tanaka, J. Phys. Chem. C, 2014, 118, 14485–14494 CrossRef CAS.
- L. W. W. Fang, R. Zhao, M. Li, K. G. Lim, L. Shi, T. C. Chong and Y. C. Yeo, J. Appl. Phys., 2010, 107, 104506 CrossRef.
- S. R. Bathe and P. S. Patil, Solid State Ionics, 2008, 179, 314–323 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- P. F. S. Pereira, A. P. de Moura, I. C. Nogueira, M. V. S. Lima, E. Longo, P. C. de Sousa Filho, O. A. Serra, E. J. Nassar and I. L. V. Rosa, J. Alloys Compd., 2012, 526, 11–21 CrossRef CAS.
- J. Li, J. Yan, D. Wen, W. U. Khan, J. Shi, M. Wu and Q. Su, J. Mater. Chem. C, 2016, 4, 8611–8623 RSC.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef CAS.
- Y. Wang, W. Xu, S. Cui, S. Xu, Z. Yin, H. Song, P. Zhou, X. Liu, L. Xu and H. Cui, Nanoscale, 2015, 7, 1363–1373 RSC.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef CAS.
- X. Huang, B. Li and H. Guo, Ceram. Int., 2017, 43, 10566–10571 CrossRef CAS.
- H. Deng, Z. Gao, N. Xue, J. H. Jeong and R. Yu, J. Lumin., 2017, 192, 684–689 CrossRef CAS.
- J. Li, Q. Liang, Y. Cao, J. Yan, J. Zhou, Y. Xu, L. Dolgov, Y. Meng, J. Shi and M. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 41479–41486 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05647j |
| This journal is © The Royal Society of Chemistry 2022 |






