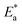DOI:
10.1039/D2RA05803K
(Paper)
RSC Adv., 2022,
12, 29350-29374
Corrosion mitigation for steel in acid environment using novel p-phenylenediamine and benzidine coumarin derivatives: synthesis, electrochemical, computational and SRB biological resistivity
Received
15th September 2022
, Accepted 3rd October 2022
First published on 13th October 2022
Abstract
Three novel p-phenylenediamine and benzidine coumarin derivatives were synthetized, namely: 4,4′-((((1,4-phenylenebis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-diyl))bis(diazene-2,1-diyl))dibenzenesulfonic acid (PhODB), 4,4′-(((-([1,1′-biphenyl]-4,4′-diylbis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-diyl))bis(diazene-2,1-diyl))dibenzenesulfonic acid (BODB) and 4,4′-(((-((3,3′-dimethoxy-[1,1′-biphenyl]-4,4′-diyl)bis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-iyl))bis(diazene-2,1-diyl))dibenzenesulfonic acid (DODB). Their chemical structures were proved by performing Fourier-transform infrared spectroscopy, proton nuclear magnetic resonance and mass spectrometry analysis. The synthesized p-phenylenediamine and benzidine coumarin derivatives were tested as corrosion inhibitors for mild steel (MS) in 1 M HCl solution using weight loss, electrochemical, morphological, and theoretical studies. The compound 3,3′-dimethoxy benzidine coumarin derivative (DODB) was proved to give the highest efficiency with 94.98% obtained from weight loss measurements. These compounds are mixed inhibitors, as seen by the polarization curves. Impedance diagrams showed that when the concentration of these derivatives rose, the double-layer capacitance fell and the charge transfer resistance increased. Calculated thermodynamic parameters were computed and the mechanism of adsorption was also studied for the synthesized p-phenylenediamine and benzidine coumarin derivatives. The ability of the synthesized derivatives to protect the surface against corrosion was investigated by scanning electron microscope (SEM), UV-visible spectroscopy and energy dispersive X-ray spectroscopy (EDX). Theoretical chemical calculations (DFT) and biological resistivity (SRB) were investigated.
1. Introduction
The corrosion process is considered an electrochemical process performed naturally by the tendency of the metal to become more stable by conversion to its corresponding oxide. The use of metals is very widespread in the petroleum industry, pipelines, petrochemicals, power stations, turbines, and boilers. Using acids to remove the scales and rust formed on the metal surface during different operations is a very destructive process. The corrosion process can be mitigated by using inhibitors.1 Organic compounds with heteroatoms are preferred as corrosion inhibitors because they have good ability for electron donation.2 The ability of organic inhibitors to be more effective in the inhibition process increases when they contain the heteroatoms sulfur (S) and nitrogen (N), and sulfur (S) gives them the highest ability for electron donation.3 Electron donation to the d orbital of a metal is easier for organic compounds with available electrons to share located on their double bonds or via hereto atoms and this donation makes organic compounds potential corrosion inhibitors.4–8 Diethyl(phenyl(phenylamino)methyl)phosphonate (DEPAMP) and diethyl((2-methoxyphenyl)(phenylamino)methyl)phosphonate (o-DEPAMP), when utilized as corrosion inhibitors for XC48 steel in a 1 M HCl solution, yield 89.27% and 90.72% inhibition efficiencies at 10−3 M, respectively.9 2,6-Bis(hydroxymethyl)-4-methoxyphenol (1) or 4-chloro-2,6-bis(hydroxymethyl)phenol (2) showed 93% and 84% inhibition efficiency at 5 × 10−2 M, respectively.10 When utilized as a corrosion inhibitor for XC48 carbon steel in 0.5 M H2SO4 solutions, (E)-1-(3-nitrobenzylidene)-2-(p-tolyl) hydrazine (E-NBPTH) demonstrated an inhibitory efficiency of 86.52% at 10−3 M.11 When used as corrosion inhibitors for XC48 carbon steel in 0.5 M H2SO4 solutions, (E)-N,N-dimethyl-4-((phenylimino)methyl)aniline (E-NDPIMA) and diethyl((4-(dimethylamino)phenyl)(phenylamino)methyl)phosphonate (α-APD) yielded respective results of 85.83% and 92.81% at 10−3 M.12 When used as a corrosion inhibitor for carbon steel in 0.5 M H2SO4 solution, 4-(2-[ethoxy(hydroxy)phosphonyl](3-nitrophenyl)methylhydrazinyl) benzoic acid achieved 88.63% inhibition at 10−3 M.13 Derivatives of quinoxaline, indole, benzimidazole and asphenyl-benzothiazole are various types of inhibitors used previously as potential corrosion inhibitors.8,14–17 Organic inhibitors are considered the best choice due to their easy synthesis, low cost, low toxicity, high purity and environmental friendliness among other advantages.18,19 Coumarins and their derivatives are organic compounds containing heterocycles, heteroatoms, double bonds and aryl rings with high ability for electron donation and use as potential corrosion inhibitors. Compounds containing an azo double bond can donate electrons to the metal surface and form a complex with it.20,21 The use of coumarin and its derivatives is widespread now because of their stability, availability and ability to donate electrons.22,23 Coumarin derivatives are widely used antibacterial, antifungal and antimicrobial, anti-inflammatory, anti-coagulant and antitumor agents. Furthermore, due to the green property of coumarin derivatives, they are also used as fixative and flavouring agents.24–29 In oil and gas industries, a common reason for pitting corrosion is microbial-influenced corrosion (MIC).30 The capacity for MIC increases as the deposits and accumulations in tubes and pipelines increase due to cathodic depolarization and galvanic cell formation.31 Twenty percent of corrosion costs are caused by microbial corrosion.32,33 Sulfate-reducing bacteria are the main microorganism responsible for sulfide generation.34,35 Desulfotomaculum and Desulfovibrio strains are the most familiar SRB strains. These strains have a great ability to survive even in aggressive conditions like high pressure (507 bar), high temperature (40 °C) and also different pH values (4–8).36 Hydrogen sulfide gas (H2S), sulfate and metal sulfides are the most commonly generated products for SRB via an oxidation–reduction mechanism. Hydrogen sulfide gas (H2S) is liberated with sufficient concentration through this oxidation–reduction mechanism and drives the electrochemical process, leading to a localized fatigue corrosion mechanism.37,38 Eco-friendly organic compounds with biocidal properties are used in the petroleum industry and are added to decrease the bio corrosion process.39 The presence of biofilm causes resistance to the transfer of heat in heat exchangers and cooling towers.40
In the current study, novel p-phenylenediamine and benzidine coumarin derivatives were synthesized. The corrosion mitigation aptitude for the new organic coumarin derivatives to prevent steel corrosion in 1 M hydrochloric acid was examined by electrochemical methods and weight loss. Furthermore, morphological examination, DFT theoretical computational studies, UV-visible studies and action against SRB bacteria were also carried out.
2. Experimental techniques
2.1. Electrolytes and electrodes
Concentrated hydrochloric acid 37% (Merck) was diluted using demineralized pure water to prepare the required solution from (1.0 M) hydrochloric acid. Then, the diluted hydrochloric acid (1.0 M) was used for the preparation of multiple molar concentrations according to the molecular weight of each coumarin derivative. Due to the spontaneous corrosion process for the selected corrosive electrolyte, no stimulus or shaking was needed to proceed.
The dimensions of the mild steel specimen used in the weight loss measurements were 2.5 × 0.3 × 6 cm and the total area was 35.1 cm2. The wt% composition was Fe = 99.10, Mn = 0.45, Si = 0.25, C = 0.11, S = 0.05 and P = 0.04. In order to remove the undesired layer on the steel specimen surface, various emery paper grades (80–2000) were used for cleaning and polishing. Then, demineralized pure water and acetone were used for washing, and the specimens were dehydrated using a desiccator before performing the experimental procedures.
2.2. Synthesis of p-phenylenediamine and benzidine coumarin derivative inhibitors
Using the previously synthesized acetyl nucleus from our previous study,41 three p-phenylenediamine and benzidine coumarin derivatives were prepared, as shown in Fig. 1. One mole each of p-phenylenediamine, benzidine and 3,3′-dimethoxy benzidine were reacted with two moles of 4-((3-acetyl-2-oxo-2H-chromen-6-yl)diazenyl)benzenesulfonic acid (Start), respectively. All reactions were performed in ethanol as solvent with a few drops of piperidine and glacial acetic acid as catalysts under refluxing for 2 h. The resulting products are 4,4′-((((1,4-phenylenebis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-diyl)) bis(diazene-2,1-diyl))dibenzenesulfonic acid (PhODB), 4,4′-(((-([1,1′-biphenyl]-4,4′-diylbis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-diyl))bis(diazene-2,1-diyl))dibenzenesulfonic acid (BODB) and 4,4′-(((-((3,3′-dimethoxy-[1,1′-biphenyl]-4,4′-diyl)bis(azaneylylidene))bis(ethan-1-yl-1-ylidene))bis(2-oxo-2H-chromene-3,6-iyl))bis(diazene-2,1-diyl))dibenzenesulfonic acid (DODB), respectively. Washing, drying and recrystallization were performed for all products. The products were solid powders with an orange reddish color, orange to brown color and rose to brown color for (PhODB), (BODB) and (DODB), respectively. The melting point was >300 °C for all the synthesized derivatives and sufficient yields were achieved, with 82.71%, 82.57% and 65.16% for (PhODB), (BODB) and (DODB), respectively.
 |
| | Fig. 1 Preparation scheme for synthesized coumarin derivatives: (A) PhODB, (B) BODB and (C) DODB. | |
2.3. Electrochemical measurements
In an electrochemical cell, a mild steel electrode with a (1 cm2 area) flat surface restrained using an epoxy holder was used as the working electrode (WE). A saturated calomel electrode (SCE) was used as the reference electrode and the counter electrode (CE) was made from graphite. The working electrode was exposed to 100 ml of acid electrolyte with different concentrations. The electrochemical analysis was carried out using a potentiostat/galvanostat/ZRA (Gamry-3000) analyzer with Gamry framework software for data acquisition (version 7.8.2). Fitting, plotting and graphing of the output data were determined with Gamry Echem Analyst software (version 7.8.2). Before performing every electrochemical test, the WE was first immersed in acid electrolyte for 3600 s to reach a stable steady state for the open circuit potential (OCP). The adjusted values for measuring the electrochemical impedance spectroscopy (EIS) were very low voltage (10 mV) with frequency range 100 kHz to 0.01 mHz and 10 points per decade at 25 °C. The adjusted potential values for potentiodynamic polarization measurements (PDP) ranged from −500 mV to 500 mV with a 1 mV s−1 scan rate at 25 °C. The adjusted frequency values for electrochemical frequency modulation (EFM) were 2 Hz and 5 Hz at 25 °C. The inhibition efficiency was calculated for EFM, EIS and PDP from the following equations:42–44| |
 | (1) |
| |
 | (2) |
where,  and icorr are current density for corrosion electrolytes without and with inhibitors, respectively.
and icorr are current density for corrosion electrolytes without and with inhibitors, respectively.| |
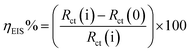 | (3) |
where, Rct(i) and Rct(0) are the charge transfer resistance with and without inhibitor, respectively, using 1.0 M HCl electrolyte medium.
2.4. Gravimetric weight loss measurements
After carefully cleaning the mild steel (MS) coupons, weight loss (WL) measurements were performed in 100 ml of corrosive electrolyte with and without inhibitors. WL was investigated at various temperatures (298, 303, 308, 313 and 318 K) using a water bath. Emery papers of various grades (80–2000) were used for cleaning and abrading the MS coupons. Demineralized pure water followed by acetone were used for the cleaning and washing steps and then the MS coupons were dried before starting the measurements. The exposure period for an MS coupon was 24 h of immersion in 100 ml of 1.0 M HCl corrosive electrolyte. The process of exposing the MS to 1.0 M HCl corrosive electrolyte was repeated using different concentrations of inhibitors dissolved in the same corrosive electrolyte (1.0 M HCl).
The corrosion rate was obtained using the following formula:
where, Δ
W = (
W1 (at initial time) −
W2 (after 24 h)) in mg,
A is the (MS) coupon surface area in cm
2,
t is the exposure time (h) and the overall units for the resulting value are mg cm
−2 h
−1.
45
The calculation of (WL) ηWL% inhibition efficiency can be performed using the following equations:
| | |
ηWL% = (W0 − Wi/W0) × 100
| (6) |
where,
θ = surface coverage,
W0 = WL value without an inhibitor and
Wi = WL value with an inhibitor.
2.5. Spectral surface analysis: UV-visible, SEM and EDX
After 24 h of exposure to a corrosive electrolyte at room temperature, a UV-vis spectrophotometer (Thermo Fisher Scientific) was used to prove complex formation between the synthesized coumarin derivatives and MS cations by measuring the changes in the wavelength values. Surface morphological examination was carried out with SEM-EDX (JEOL JSM-IT200 SEM). MS coupons with dimensions of 2.5 cm × 2.5 cm × 0.3 cm were abraded, scratched and cleaned using emery papers of various grades (1000–2000) before exposure to the corrosive electrolyte for 24 h before examination. After 24 h and before the examination, the MS coupons were washed with demineralized water and dehydrated. The MS coupon was fixed in the sample holder and SEM analysis was performed with (1000×) magnification to obtain a good detailed image of the examined (MS) coupon. EDX was used to measure the organic elements deposited from the synthesized derivatives on the MS surface which can prove surface protection via complexation between the synthesized derivatives and MS cations.
2.6. Bio-corrosion mitigation
Hydrogen sulfide (H2S) can be easily generated by sulfate-reducing (SRB) bacteria. Liberation of H2S increases the fatigue damage caused by corrosion. SRB (SRB-BART™ – DBI) vials were selected to monitor the bacterial growth of SRB, due to the low test period (11 days max) and high approximate population results. Once the vial has turned black, the test is complete and the SRB population can be recognized. The results can be achieved within eleven days, which is the maximum period for the test time. Each single day represents a specified quantity of SRB present and the test is complete when the first black sign appears on the test vial.41
2.7. Quantum chemical computation
Optimization of PhODB, BODB and DODB molecules was performed with semi-empirical (PM6), Hartree–Fock (631G) and DFT (6311G) basis set methods. DFT was used with 3 exchange function parameters for Beck's (B3LYP – Lee–Yang–Parr) correlation. Recently, DFT has been preferred due to its accuracy.46 The calculations were performed using the Gaussian 09 and Gauss View 06 packages.47 EHOMO and ELUMO are known as the energies of the frontier molecular orbital (FMO), where EHOMO and ELUMO refer to the highest occupied and the lowest unoccupied molecular orbitals, respectively. According to Koopman's theory, the energy values for EHOMO and ELUMO can be expressed with other values like the energy gap (ΔE), ionization potential (IP), electron affinity (EA), electronegativity (χ), electrophilicity (ω), transferred electrons (ΔN), softness (σ), hardness (η), dipole moment (μ), total energy E(RB3LYP), molecular volume (MV) and total negative charge (TNC).48 The values of the abovementioned parameters can be calculated from the following equations:49–54| |
 | (10) |
| |
 | (12) |
| |
 | (13) |
where the theoretical values are χFe = 7.0 eV and ηFe = zero,| | |
χ = 0.5(LUMO + HOMO)
| (14) |
3. Results and discussion
3.1. Confirmation of the synthesis of p-phenylenediamine and benzidine coumarin derivatives
The chemical structures for p-phenylenediamine and benzidine coumarin (PhODB, BODB and DODB) derivatives were confirmed by performing Fourier-transform infrared spectroscopy, proton nuclear magnetic resonance and mass spectrometry analysis. The FTIR of PhODB showed peaks at 3062.46 (aromatic C–H), 1754.93 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1511.39, 1565.82 (N
O), 1511.39, 1565.82 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1204.67, 1039.57 (δ lactone, O–C
N, azo groups, bis azo compound), 1204.67, 1039.57 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1620.23 (C
O), 1620.23 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), and 3442.83 (SO3H group). The FTIR of BODB showed peaks at 3062.84 (aromatic C–H), 1754.90 (C
N), and 3442.83 (SO3H group). The FTIR of BODB showed peaks at 3062.84 (aromatic C–H), 1754.90 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O, coumarin), 1492.43, 1565.71 (N
O, coumarin), 1492.43, 1565.71 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1620.96 (C
N, azo groups, bis azo compound), 1620.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 1205.39, 1038.55 (δ lactone, O–C
N), 1205.39, 1038.55 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), and 3443.43 (SO3H group). The DODB compound showed FTIR peaks at 3063.04 (aromatic C–H), 2855.46, 2938.14, 2962.67 (aliphatic C–H), 1755.21 (C
O), and 3443.43 (SO3H group). The DODB compound showed FTIR peaks at 3063.04 (aromatic C–H), 2855.46, 2938.14, 2962.67 (aliphatic C–H), 1755.21 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1459.38, 1566.51 (N
O), 1459.38, 1566.51 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1205.10, 1039.25 (δ lactone, O–C
N, azo groups, bis azo compound), 1205.10, 1039.25 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1621.31 (C
O), 1621.31 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 3437.10 (SO3H group). All detailed FTIR values are given in Table 1 and Fig. 2. From the 1H NMR analysis (DMSO-d6), 400 MHz; PhODB showed bands at δ = 2.62 (6H, s, N
N), 3437.10 (SO3H group). All detailed FTIR values are given in Table 1 and Fig. 2. From the 1H NMR analysis (DMSO-d6), 400 MHz; PhODB showed bands at δ = 2.62 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 10.36 ppm (SO3H), δ = 7.07–8.83 ppm (18H, m, Ar-H), δ = 9.06, 9.25 ppm (2H, s, coumarin-4-H). But BODB showed bands at δ = 2.61 (6H, s, N
C–CH3), δ = 10.36 ppm (SO3H), δ = 7.07–8.83 ppm (18H, m, Ar-H), δ = 9.06, 9.25 ppm (2H, s, coumarin-4-H). But BODB showed bands at δ = 2.61 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 10.37 ppm (SO3H), δ = 7.11–8.82 ppm (22H, m, Ar-H), δ = 9.22, 9.26 ppm (2H, s, coumarin-4-H). The 1H NMR of DODB showed bands at δ = 2.61 (6H, s, N
C–CH3), δ = 10.37 ppm (SO3H), δ = 7.11–8.82 ppm (22H, m, Ar-H), δ = 9.22, 9.26 ppm (2H, s, coumarin-4-H). The 1H NMR of DODB showed bands at δ = 2.61 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 3.03 (6H, s, CH3–O-Ar), δ = 10.32 ppm (SO3H), δ = 6.64–8.82 ppm (20H, m, Ar-H), δ = 9.25, 9.29 ppm (2H, s, coumarin-4-H). All detailed 1H NMR values are given in Table 1 and Fig. 3. From mass analysis at m/z (%); (M+.) = 816 (66.03%) for PhODB, (M+˙) = 892 (43.42%) for BODB and (M+˙) = 952 (66.15%) for DODB. The molecular ion peaks (base peak) are at m/z = 621 (100%) for PhODB, m/z = 724 (100%) for (BODB) and m/z = 950 (100%) for DODB. The other additional peaks are listed in Table 1 and Fig. 4.
C–CH3), δ = 3.03 (6H, s, CH3–O-Ar), δ = 10.32 ppm (SO3H), δ = 6.64–8.82 ppm (20H, m, Ar-H), δ = 9.25, 9.29 ppm (2H, s, coumarin-4-H). All detailed 1H NMR values are given in Table 1 and Fig. 3. From mass analysis at m/z (%); (M+.) = 816 (66.03%) for PhODB, (M+˙) = 892 (43.42%) for BODB and (M+˙) = 952 (66.15%) for DODB. The molecular ion peaks (base peak) are at m/z = 621 (100%) for PhODB, m/z = 724 (100%) for (BODB) and m/z = 950 (100%) for DODB. The other additional peaks are listed in Table 1 and Fig. 4.
Table 1 FTIR, 1H NMR and mass spectroscopy values for synthesized coumarin derivatives (PhODB, BODB and DODB)
| Assignment |
PhODB |
BODB |
DODB |
| FTIR (wave number, cm−1) |
| Aromatic C–H |
3062 |
3062.84 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643.04 643.04 |
| Aliphatic C–H |
— |
— |
2855.46, 2938.14, 2962.67 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (coumarin) O (coumarin) |
1754.93 |
1754.90 |
1755.21 |
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (azo groups) N (azo groups) |
1511.39, 1565.82 |
1492.43, 1565.71 |
1459.38, 1566.51 |
δ lactone (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
1204.67, 1039.57 |
1205.39, 1038.55 |
1205.10, 1039.25 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1620.23 |
1620.96 |
1620.23 |
| SO3H |
3442.83 |
3443.43 |
3437.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| 1H NMR (DMSO-d6), 400 MHz, δ (ppm) |
 |
2.62 (6H, s) |
2.61 (6H, s) |
2.61 (6H, s) |
| Aromatic-H |
7.07–8.83 (18H, m) |
7.11–8.82 (22H, m) |
7.07–8.83 (18H, m) |
| Coumarin-4-H |
9.06, 9.25 (2H, s) |
9.22, 9.26 (2H, s) |
9.25, 9.29 (2H, s) |
| SO3H |
10.36 |
10.37 |
10.32 |
| CH3–O-Ar |
— |
— |
3.03 (6H, s) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Mass spectrum m/z (%) |
| (M+.) |
816 (66.03%) |
892 (43.42%) |
952 (66.15%) |
| Molecular ion peak (base peak) |
621 (100%) |
724 (100%) |
950 (100%) |
| Other peaks |
734 (18.15%) |
778 (93.95%) |
404 (63.44%) |
| 341 (74.47%) |
404 (15.70%) |
| 369 (26.45%) |
363 (17.32%) |
| |
257 (18.62%) |
| |
177 (30.18%) |
 |
| | Fig. 2 FTIR spectra for synthesized coumarin derivatives: (a) PhODB, (b) BODB and (c) DODB. | |
 |
| | Fig. 3 1H NMR spectra for synthesized coumarin derivatives: (a) PhODB, (b) BODB and (c) DODB. | |
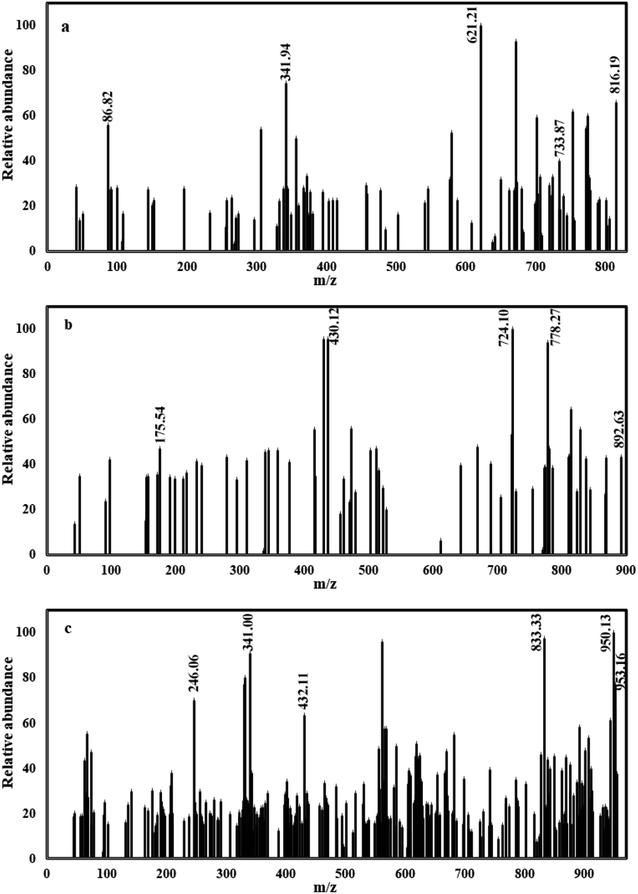 |
| | Fig. 4 Mass spectra for synthesized coumarin derivatives: (a) PhODB, (b) BODB and (c) DODB. | |
3.2. Electrochemical analysis
3.2.1. Electrochemical frequency modulation measurements (EFM). Before performing every electrochemical measurement for each concentration of the inhibitor, the working electrode (WE) was first immersed in acid electrolyte (1.0 M HCl) with and without inhibitors for 3600 s (1 h) to reach a stable steady state for the open circuit potential (OCP) (Fig. 5). EFM is a nondestructive measurement technology that uses a tiny electrical signal from an amphoteric current (AC) with varying frequencies to detect the rate of corrosion while producing two separate sine waves simultaneously. Intermodulation spectra at various concentrations for the DODB synthesized coumarin derivative are plotted in Fig. 6. Not only are the inputted frequencies included in the response of the current, but also their sums, multiplicities and differences.55 The frequency selection must be selective and tiny. The resulting data from EFM at higher peak can be used to determine the icorr value without using the Tafel constants (βa and βc). Causality factor values (CF-2 and CF-3) can be utilized to self-validate experimental results at various doses or concentrations. The recorded CF values are similar to the theoretical numbers (2 and 3) according to Table 2, and increasing the inhibitor dosages leads to a reduction in the current density values. By increasing the dosage of the coumarin derivative inhibitor, the severity of corrosion was reduced according to the estimated EFM values. According to the EFM results, the inhibition order is DODB > BODB > PhODB.
 |
| | Fig. 5 OCP curves for the corrosion of MS in 1.0 M HCl with and without different concentrations of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C. | |
 |
| | Fig. 6 EFM curves for the corrosion of MS in 1.0 M HCl with and without different concentrations of synthesized inhibitor (BODB, for example) at 25 °C. | |
Table 2 Electrochemical kinetic parametersa obtained by the EFM technique for MS in the absence and presence of various concentrations of PhODB, BODB and DODB inhibitors in 1.0 M HCl at 30 °C
| Inhibitor name |
Conc. (M) |
Icorr (μA cm−2) |
βa (mV dec−1) |
−βc (mV dec−1) |
CF-2 |
CF-3 |
k (mpy) |
θ |
ηEFM% |
| Ecorr is the corrosion potential; Icorr is the corrosion current density: βa and βc are the Tafel constants for both anode and cathode; k is the corrosion rate; θ is the surface coverage; ηEFM is the inhibition efficiency. |
| Blank |
— |
2791 |
100.4 |
113.1 |
1.763 |
3.155 |
1275.00 |
— |
— |
| PhODB |
0.50 × 10−4 |
720.7 |
107.4 |
129.7 |
1.973 |
3.124 |
329.3 |
0.7418 |
74.18 |
| 0.75 × 10−4 |
539.3 |
116.0 |
158.8 |
1.957 |
2.476 |
246.4 |
0.8068 |
80.68 |
| 2.50 × 10−4 |
395.3 |
119.1 |
131.7 |
2.050 |
2.947 |
180.6 |
0.8584 |
85.84 |
| 5.00 × 10−4 |
304.2 |
105.0 |
115.5 |
1.976 |
3.098 |
139.0 |
0.8910 |
89.10 |
| 7.50 × 10−4 |
151.9 |
95.56 |
111.5 |
1.695 |
2.930 |
69.39 |
0.9456 |
94.56 |
| BODB |
0.50 × 10−4 |
653.7 |
100.6 |
124.5 |
1.994 |
3.256 |
298.70 |
0.7658 |
76.58 |
| 0.75 × 10−4 |
485 |
125.2 |
132.3 |
2.040 |
3.121 |
221.60 |
0.8262 |
82.62 |
| 2.50 × 10−4 |
347.2 |
105.0 |
112.8 |
2.308 |
3.023 |
158.7 |
0.8756 |
87.56 |
| 5.00 × 10−4 |
215.9 |
97.59 |
101.8 |
2.574 |
3.387 |
98.66 |
0.9226 |
92.26 |
| 7.50 × 10−4 |
114.9 |
100.8 |
120.0 |
1.994 |
3.100 |
52.52 |
0.9588 |
95.88 |
| DODB |
0.50 × 10−4 |
608.3 |
87.65 |
117.7 |
1.974 |
2.910 |
278.00 |
0.7820 |
78.20 |
| 0.75 × 10−4 |
431.9 |
97.7 |
120.2 |
1.997 |
3.120 |
197.4 |
0.8453 |
84.53 |
| 2.50 × 10−4 |
320 |
121.3 |
177.8 |
2.026 |
3.001 |
146.20 |
0.8853 |
88.53 |
| 5.00 × 10−4 |
167.7 |
103.6 |
121.1 |
2.139 |
3.437 |
76.64 |
0.9399 |
93.99 |
| 7.50 × 10−4 |
94.32 |
84.1 |
90.7 |
1.395 |
3.106 |
43.10 |
0.9662 |
96.62 |
3.2.2. Electrochemical impedance spectroscopy (EIS) measurements. To study the interface characteristics and adsorption performance of the inhibitors, EIS is a very helpful measurement tool. EIS gives details of the kinetics and properties of electrochemical processes for a thorough knowledge of the corrosion inhibition process. Furthermore, EIS is another non-destructive method for analyzing a metal's corrosion inhibition performance in an acidic electrolyte. Using the EIS procedure, the inhibition characteristics of the three synthetic p-phenylenediamine and benzidine coumarin derivatives (PhODB, BODB and DODB) at different concentrations were identified at 25 °C. The comparable Nyquist plots and corresponding circuit model derived from EIS are displayed in Fig. 7. As can be seen from Fig. 7, a boost in inhibitor concentration forces the Nyquist plots to enlarge in diameter, which also leads their semi-circular shape to expand. As a result, the primary cause of the diameter increase is the formation of an inhibitor molecule film on the metal surface.56 For PhODB, BODB, and DODB at various concentrations, Fig. 7 illustrates the existence of a single capacitive loop, illuminating the activity of the examined inhibitors as major interface inhibitors and their adsorption onto the metal specimen surface. The Bode and phase angle plots for the investigated p-phenylenediamine and benzidine coumarin inhibitors (PhODB, BODB and DODB) are shown in Fig. 8. In order to analyze the electrode/electrolyte correlation by modeling the experimental graphs to plot the resulting data from EIS, a suitable circuit is necessary. To fit the Nyquist curve plots, many compartments are used to build up the equivalent circuit, electrolyte resistance (Rs) with constant phase element (CPE) in a parallel combination together, and charge transfer resistance (Rct). Typically, CPE gives a suitable representation for the electrochemical process in place of capacitance.57 The CPE values can be represented by the following equation:where, Y0 is CPE (constant), n is an exponent, j is the imaginary value and ω is the angular frequency. The measured Cdl values can be represented with the next equation:58where, Y0 is CPE (constant) and n is the CPE exponent. Table 3 presents the measured values from EIS and it can be realized from these values that the Rct values increase with a rise in inhibitor concentration, while Cdl demonstrates the opposite dependency. Nyquist and Bode graphs vary for the same inhibitor with different doses and also for the various investigated compounds. Even when using the same inhibitor, the strength of the highest peak increases with increasing concentration. Resistance may be indicated by the peak diameter, and resistance increases with increasing inhibitor concentration. Higher resistance (low Cdl values) might be amazing evidence for the presence of a layer from the evaluated inhibitors above the metal, providing excellent metal preservation and slowing down the corrosion rate.59 When inhibitors are present compared to when they are absent, the curve and loop diameter of the Nyquist plot are larger. With reference to the values in Table 3, the difference between Rct and Rs values becomes greater with increasing inhibitor dosage and also with increasing Rct values, and the Cdl values are lower due to inhibitor adsorption on the electrode surface being affected by increasing the thickness because of occupation of the electrode surface by inhibitors instead of water or acid electrolytes. The approximate values of n (close to unity) revealed that the electric double layer in the current investigation performed like a pseudo-capacitor type.60,61 As the concentration of PhODB, BODB, or DODB increases, the phase angle becomes wider due to the high frequency, as plotted in Fig. 8. By plotting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (Ω cm2) against log frequency (Hz), the capacitance properties of the adsorption behavior of the investigated compounds on the MS surface were confirmed.62 The observed values from EIS indicate that, at higher inhibitor dosage, the highest mitigation results can be achieved. According to the EIS results, the inhibition order is DODB > BODB > PhODB.
Z (Ω cm2) against log frequency (Hz), the capacitance properties of the adsorption behavior of the investigated compounds on the MS surface were confirmed.62 The observed values from EIS indicate that, at higher inhibitor dosage, the highest mitigation results can be achieved. According to the EIS results, the inhibition order is DODB > BODB > PhODB.
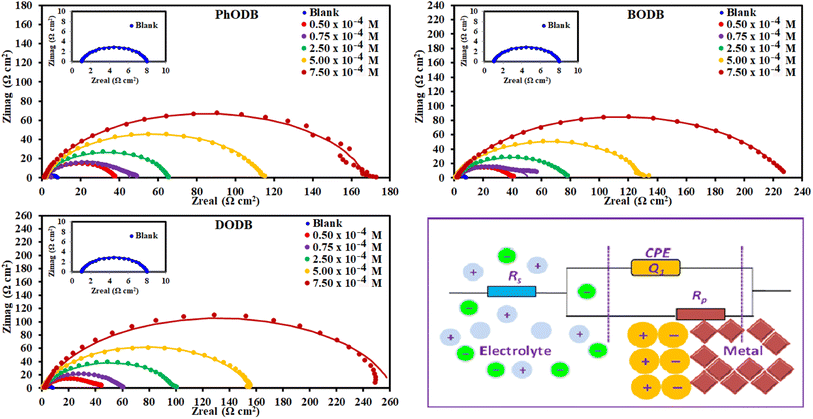 |
| | Fig. 7 Nyquist plots for MS in 1.0 M HCl with and without different concentrations of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C and the equivalent circuit model for fitting the EIS data. | |
 |
| | Fig. 8 Bode and phase angle plots for steel in 1.0 M HCl with and without different concentrations of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C. | |
Table 3 EIS parameters for corrosion of MS in 1.0 M HCl in the absence and presence of different concentrations of PhODB, BODB and DODB inhibitors at 25 °Ca
| Inhibitor |
Conc. (M) |
Rs (Ru) (Ω cm2) |
Rct (Rp) (Ω cm2) |
Y0 (μΩ−1 sn cm−2) |
n |
Cdl (μF cm−2) |
χ2 (chi squared) |
S |
α° |
τ (mS) |
θ |
ηz% |
| Rs = solution resistance, Rct = charge transfer resistance, Y0, n = constant phase elements, Cdl = double layer capacitance, S = the slopes of the Bode impedance magnitude at intermediate frequencies, α° = maximum phase angle, □ = the relaxation time, θ = surface coverage, ηz = inhibition efficiency. |
| Blank |
— |
1.082 |
6.884 |
478.50 |
0.8836 |
225.363 |
2.25 × 10−2 |
−0.365 |
−42.07 |
3.29 |
— |
— |
| PhODB |
0.50 × 10−4 |
2.265 |
35.55 |
1063.00 |
0.8760 |
668.585 |
3.33 × 10−3 |
−0.481 |
−53.43 |
37.79 |
0.8064 |
80.64 |
| 0.75 × 10−4 |
2.368 |
43.20 |
395.60 |
0.8434 |
185.830 |
3.83 × 10−2 |
−0.588 |
−52.62 |
17.09 |
0.8406 |
84.06 |
| 2.50 × 10−4 |
1.785 |
63.88 |
532.10 |
0.8833 |
340.376 |
5.06 × 10−3 |
−0.661 |
−61.75 |
33.99 |
0.8922 |
89.22 |
| 5.00 × 10−4 |
2.029 |
112.1 |
317.40 |
0.8708 |
193.487 |
3.34 × 10−3 |
−0.646 |
−64.00 |
35.58 |
0.9386 |
93.86 |
| 7.50 × 10−4 |
1.458 |
166.4 |
68.48 |
0.8637 |
33.798 |
9.11 × 10−4 |
−0.678 |
−67.71 |
11.40 |
0.9586 |
95.86 |
| BODB |
0.50 × 10−4 |
2.067 |
37.56 |
886.40 |
0.8273 |
435.682 |
5.12 × 10−3 |
−0.545 |
−51.52 |
33.29 |
0.8167 |
81.67 |
| 0.75 × 10−4 |
2.440 |
49.30 |
1176.00 |
0.738 |
427.903 |
7.34 × 10−3 |
−0.513 |
−46.87 |
57.98 |
0.8604 |
86.04 |
| 2.50 × 10−4 |
1.729 |
77.18 |
738.60 |
0.8004 |
361.547 |
4.73 × 10−3 |
−0.620 |
−56.11 |
57.01 |
0.9108 |
91.08 |
| 5.00 × 10−4 |
1.086 |
129.6 |
121.00 |
0.8390 |
54.512 |
1.59 × 10−3 |
−0.741 |
−65.86 |
15.68 |
0.9469 |
94.69 |
| 7.50 × 10−4 |
2.206 |
225.9 |
704.70 |
0.8201 |
470.907 |
5.07 × 10−4 |
−0.721 |
−62.86 |
159.19 |
0.9695 |
96.95 |
| DODB |
0.50 × 10−4 |
2.360 |
40.69 |
1354.00 |
0.766 |
558.5319 |
4.83 × 10−3 |
−0.482 |
−47.66 |
55.09 |
0.8308 |
83.08 |
| 0.75 × 10−4 |
2.252 |
57.06 |
587.60 |
0.818 |
276.055 |
7.74 × 10−2 |
−0.598 |
−54.43 |
33.53 |
0.8794 |
87.94 |
| 2.50 × 10−4 |
1.762 |
98.15 |
494.40 |
0.8373 |
274.626 |
5.42 × 10−3 |
−0.676 |
−60.41 |
48.53 |
0.9299 |
92.99 |
| 5.00 × 10−4 |
1.093 |
154.0 |
116.10 |
0.8618 |
60.894 |
2.11 × 10−3 |
−0.775 |
−68.15 |
17.88 |
0.9553 |
95.53 |
| 7.50 × 10−4 |
2.106 |
260.0 |
1733.00 |
0.8667 |
1533.021 |
1.96 × 10−3 |
−0.771 |
−68.92 |
450.58 |
0.9735 |
97.35 |
3.2.3. Potentiodynamic polarization measurements (PDP). In the current study, PDP measurements were used to evaluate the adsorptive capability of synthesized coumarin derivatives i.e., PhODB, BODB and DODB. The PDP method is applied to explain the interaction between the electrical charge and the electrode potential. PDP analysis was performed using various dosages of inhibitors (PhODB, BODB and DODB) at 25 °C (Fig. 9). Cathodic (βc), and anodic (βa) Tafel slopes, current density (icorr), corrosion potential (Ecorr), degree of surface coverage (θ) and inhibition efficiency (ηPDP%) values were obtained and are listed in Table 4. Using inhibitors and as a result of blocking the active points on the electrode surface due to formation of a protective layer, inhibition efficiency (ηPDP%) increased, and corrosion rate and current density values were reduced. No significant or valuable shift were realized in the Ecorr results, just a tiny shift to the positive side direction. As recorded in other studies,63–65 if Ecorr values are higher than 85 mV, the inhibitor can be anodic or cathodic according to the Ecorr values recorded for the acidic electrolyte. Furthermore, inhibitors with values of Ecorr lower than 85 mV are confirmed to be mixed type (anodic and cathodic types together). From the Ecorr results in Table 4, the PhODB, BODB, and DODB inhibitors are also considered to be mixed-type inhibitors, due to the slight changes in βc and βa. The Tafel lines are parallel, indicating that there was no change in the mechanism of the process in the presence and absence of inhibitors. As the θ values increased, icorr values reduced as a result of an anodic protective film formed on the electrode due to the presence of coumarin inhibitors. According to the PDP results, the inhibition order is DODB > BODB > PhODB.
 |
| | Fig. 9 PDP curves for the corrosion of steel in 1.0 M HCl with and without different concentrations of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C. | |
Table 4 Electrochemical parametersa for steel dissolution in 1.0 M HCl solution containing different concentrations of the PhODB, BODB and DODB inhibitors obtained from polarization measurements at 25 °C
| Inhibitor name |
Conc. (M) |
Ecorr vs.. SCE (mV) |
Icorr (μA cm−2) |
βa (mV dec−1) |
−βc (mV dec−1) |
k (mpy) |
ΔEcorr (mV) |
θ |
ηPDP% |
| Ecorr is the corrosion potential; Icorr is the corrosion current density: βa and βc are Tafel constants for both anode and cathode; k is the corrosion rate; θ is the surface coverage; ηPDP is the inhibition efficiency. |
| Blank |
— |
−523 |
8624 |
305.0 |
333.0 |
4137 |
— |
— |
— |
| PhODB |
0.50 × 10−4 |
−571 |
2340 |
246.9 |
212.6 |
1234 |
−48 |
0.729 |
72.85 |
| 0.75 × 10−4 |
−471 |
1350 |
233.7 |
240.8 |
617.9 |
52 |
0.843 |
84.34 |
| 2.50 × 10−4 |
−459 |
778 |
211.8 |
230.7 |
355.5 |
64 |
0.910 |
90.97 |
| 5.00 × 10−4 |
−460 |
580 |
199.9 |
210.3 |
265.2 |
63 |
0.933 |
93.27 |
| 7.50 × 10−4 |
−452 |
406 |
175.7 |
192.6 |
185.5 |
71 |
0.953 |
95.29 |
| BODB |
0.50 × 10−4 |
−465 |
1440 |
220.7 |
232.0 |
659.0 |
58 |
0.833 |
83.29 |
| 0.75 × 10−4 |
−472 |
1050 |
226.8 |
233.0 |
480.1 |
51 |
0.878 |
87.82 |
| 2.50 × 10−4 |
−447 |
652 |
191.8 |
207.7 |
297.8 |
76 |
0.924 |
92.44 |
| 5.00 × 10−4 |
−441 |
478 |
175.0 |
213.0 |
218.5 |
82 |
0.945 |
94.45 |
| 7.50 × 10−4 |
−444 |
302 |
166.7 |
187.1 |
138.0 |
79 |
0.965 |
96.50 |
| DODB |
0.50 × 10−4 |
−465 |
1370 |
234.4 |
246.1 |
624.9 |
58 |
0.841 |
84.11 |
| 0.75 × 10−4 |
−468 |
937 |
215.3 |
224.3 |
427.9 |
55 |
0.891 |
89.13 |
| 2.50 × 10−4 |
−443 |
569 |
182.0 |
203.8 |
260.1 |
80 |
0.934 |
93.40 |
| 5.00 × 10−4 |
−440 |
419 |
169.8 |
197.7 |
191.2 |
83 |
0.951 |
95.14 |
| 7.50 × 10−4 |
−455 |
207 |
163.1 |
174 |
94.73 |
68 |
0.976 |
97.60 |
3.3. Gravimetric measurements (weight loss)
The WL gravimetric measurement technique is a successful and frequently utilized procedure that does not need a well-established research facility to execute in order to investigate the actual character of organic inhibitors. The effect of different dosages of PhODB, BODB and DODB inhibitors on MS in corrosive electrolyte was investigated and the same behavior was also investigated at various temperatures.
3.3.1. Effect of different concentrations. Using various dosages of PhODB, BODB, and DODB inhibitors, the corrosion rate for MS in (1.0 M HCl) corrosive electrolyte was investigated. The MS electrode was submerged in the corrosive electrolyte for 24 h and the weight was determined before and after submersion. The effect of adding five different concentrations of PhODB, BODB, and DODB inhibitors on the corrosion rate was investigated. Furthermore, the same effect was studied at various temperatures (298, 303, 308, 313 and 318 K). Several variables like corrosion rate (CR(k) = mg cm−2 h−1), surface coverage (θ) and inhibition efficiency (ηWL%) were measured and are listed in Table 5. From the values presented in Table 5, as the dosage of the inhibitors is raised, the ηWL% values improve and the CR values reduce. At 318 K, the ηWL% values are 95.85%, 93.19% and 94.98% at the highest concentration (7.5 × 10−4 M) for the studied inhibitors PhODB, BODB, and DODB, respectively. At the same mentioned temperature and without adding inhibitors, the CR value was 03.1736 mg cm−2 h−1 and after adding inhibitors (7.5 × 10−4 molar concentration) the CR values are reduced to become 0.2269 mg cm−2 h−1, 0.2162 mg cm−2 h−1 and 0.1592 mg cm−2 h−1 for PhODB, BODB, and DODB, respectively. From the indicated values in Table 5 and Fig. 10, the ηWL% results increase with increases in both temperatures and concentrations of PhODB, BODB and DODB inhibitors, indicating chemical adsorption. According to the WL results, the inhibition order is DODB > BODB > PhODB.
Table 5 Corrosion rate, surface coverage and percentage of inhibition efficiency of steel in 1.0 HCl of the PhODB, BODB and DODB inhibitors at different temperatures
| Inhibitor |
Inhibitor conc. (M) |
25 °C |
30 °C |
35 °C |
40 °C |
45 °C |
| CR (k) (mg cm−2 h−1) |
θ |
ηw (%) |
CR (k) (mg cm−2 h−1) |
θ |
ηw (%) |
CR (k) (mg cm−2 h−1) |
θ |
ηw (%) |
CR (k) (mg cm−2 h−1) |
θ |
ηw (%) |
CR (k) (mg cm−2 h−1) |
θ |
ηw (%) |
| Blank |
0.00 × 10−4 |
0.3365 |
— |
— |
0.5765 |
— |
— |
1.3534 |
— |
— |
1.9141 |
— |
— |
3.1736 |
— |
— |
| PhODB |
0.50 × 10−4 |
0.1309 |
0.611 |
61.11 |
0.1913 |
0.668 |
66.82 |
0.3541 |
0.738 |
73.84 |
0.4537 |
0.763 |
76.30 |
0.5290 |
0.833 |
83.33 |
| 0.75 × 10−4 |
0.1084 |
0.678 |
67.78 |
0.1547 |
0.732 |
73.17 |
0.2472 |
0.817 |
81.74 |
0.3264 |
0.830 |
82.95 |
0.4680 |
0.853 |
85.25 |
| 2.50 × 10−4 |
0.0846 |
0.749 |
74.87 |
0.1075 |
0.814 |
81.35 |
0.1870 |
0.862 |
86.18 |
0.2407 |
0.874 |
87.42 |
0.3366 |
0.894 |
89.39 |
| 5.00 × 10−4 |
0.0788 |
0.766 |
76.58 |
0.0947 |
0.836 |
83.57 |
0.1717 |
0.873 |
87.32 |
0.2041 |
0.893 |
89.33 |
0.3074 |
0.903 |
90.31 |
| 7.50 × 10−4 |
0.0505 |
0.850 |
85.00 |
0.0824 |
0.857 |
85.71 |
0.1514 |
0.888 |
88.81 |
0.1780 |
0.907 |
90.70 |
0.2269 |
0.929 |
92.85 |
| BODB |
0.50 × 10−4 |
0.1233 |
0.634 |
63.38 |
0.1801 |
0.688 |
68.75 |
0.3346 |
0.753 |
75.28 |
0.3706 |
0.806 |
80.64 |
0.4815 |
0.848 |
84.83 |
| 0.75 × 10−4 |
0.1063 |
0.684 |
68.40 |
0.1396 |
0.758 |
75.79 |
0.2399 |
0.823 |
82.27 |
0.3192 |
0.833 |
83.32 |
0.3986 |
0.874 |
87.44 |
| 2.50 × 10−4 |
0.0835 |
0.752 |
75.17 |
0.0990 |
0.828 |
82.83 |
0.1762 |
0.870 |
86.98 |
0.2170 |
0.887 |
88.66 |
0.2773 |
0.913 |
91.26 |
| 5.00 × 10−4 |
0.0766 |
0.772 |
77.23 |
0.0772 |
0.866 |
86.60 |
0.1424 |
0.895 |
89.48 |
0.1804 |
0.906 |
90.58 |
0.2600 |
0.918 |
91.81 |
| 7.50 × 10−4 |
0.0485 |
0.856 |
85.58 |
0.0723 |
0.875 |
87.46 |
0.1415 |
0.895 |
89.55 |
0.1542 |
0.919 |
91.94 |
0.2162 |
0.932 |
93.19 |
| DODB |
0.50 × 10−4 |
0.1184 |
0.648 |
64.82 |
0.1685 |
0.708 |
70.77 |
0.3240 |
0.761 |
76.06 |
0.3263 |
0.830 |
82.96 |
0.4566 |
0.856 |
85.61 |
| 0.75 × 10−4 |
0.1007 |
0.701 |
70.08 |
0.1335 |
0.768 |
76.85 |
0.2247 |
0.834 |
83.40 |
0.2895 |
0.849 |
84.87 |
0.3780 |
0.881 |
88.09 |
| 2.50 × 10−4 |
0.0789 |
0.766 |
76.56 |
0.0930 |
0.839 |
83.87 |
0.1563 |
0.884 |
88.45 |
0.2013 |
0.895 |
89.48 |
0.2471 |
0.922 |
92.21 |
| 5.00 × 10−4 |
0.0725 |
0.784 |
78.44 |
0.0734 |
0.873 |
87.27 |
0.1330 |
0.902 |
90.17 |
0.1588 |
0.917 |
91.71 |
0.2004 |
0.937 |
93.69 |
| 7.50 × 10−4 |
0.0407 |
0.879 |
87.90 |
0.0671 |
0.884 |
88.36 |
0.1212 |
0.910 |
91.04 |
0.1445 |
0.925 |
92.45 |
0.1592 |
0.950 |
94.98 |
 |
| | Fig. 10 Effect of various temperatures and concentrations of synthesized coumarin derivatives: (a) PhODB, (b) BODB and (c) DODB on the corrosion rate of steel in 1.0 M HCl using the weight loss method. | |
3.3.2. Adsorption isotherm. Understanding how the inhibitors and active points on the metal electrode surface interact is the main goal of the adsorption isotherm. In the current investigation, a variety of isotherms were utilized for fitting, with the Langmuir model providing the best match since the linear regression coefficients (R2) are nearly all equal to one.66 The R2 values for Freundlich, Langmuir, Frumkin, Temkin, Flory–Huggins and kinetic–thermodynamic adsorption isotherms models are listed and plotted in Table 6 and Fig. 11, respectively. The next formula is used to describe the Langmuir adsorption isotherm:67where, C = inhibitor concentration, θ = surface coverage and K = binding constant. By plotting C vs. (C/θ), straight lines were achieved for the Langmuir model (Fig. 11). Considering the intercept, the Gibb's standard free energy  can be calculated from the following formula:68
can be calculated from the following formula:68| |
 | (19) |
where, R is the universal gas constant (8.314 J mol−1 K−1), T is the temperature (kelvin) and the numerical value (55.5 mole per liter) is the water concentration. The calculated  and Kads values for all adsorption isotherm models are listed in Table 6 at 298 K. Spontaneous nature of adsorption and stability of the adsorbed layer are expected for the studied organic derivatives towards the electrode metal surface due to the negative calculated values for
and Kads values for all adsorption isotherm models are listed in Table 6 at 298 K. Spontaneous nature of adsorption and stability of the adsorbed layer are expected for the studied organic derivatives towards the electrode metal surface due to the negative calculated values for 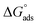 . Based on previously accepted research,69,70 the spontaneous adsorption behavior is a steady process that cannot be reversed. The adsorption process behavior depends on the
. Based on previously accepted research,69,70 the spontaneous adsorption behavior is a steady process that cannot be reversed. The adsorption process behavior depends on the  values: physisorption if
values: physisorption if  ≥ −20 kJ mol−1, chemisorption if
≥ −20 kJ mol−1, chemisorption if  ≥ −40 kJ mol−1 and mixed type if
≥ −40 kJ mol−1 and mixed type if  values are between −20 and −40 kJ mol−1.71 The
values are between −20 and −40 kJ mol−1.71 The  results are −36.10, −36.18 and 36.15 kJ mol−1 for PhODB, BODB and DODB, respectively, according to the Langmuir adsorption model, so the adsorption of these compounds on MS surfaces are of mixed type (physisorption and chemisorption, but mainly chemical).
results are −36.10, −36.18 and 36.15 kJ mol−1 for PhODB, BODB and DODB, respectively, according to the Langmuir adsorption model, so the adsorption of these compounds on MS surfaces are of mixed type (physisorption and chemisorption, but mainly chemical).
Table 6 Adsorption isotherm models of the inhibitors with values of R2, slopes, intercepts, Kads and  obtained by using data from WL measurementsa
obtained by using data from WL measurementsa
 |
| | Fig. 11 Different adsorption isotherms for synthesized coumarin derivatives (PhODB, BODB and DODB) using the weight loss method. | |
According to the Van't Hoff equation, the adsorption thermodynamic parameters for the synthesized inhibitors (PhODB, BODB and DODB) on the MS electrode surface are essential for understanding the adsorption process and this equation can be represented as follows:72,73
| |
 | (20) |
By fitting (1/T) vs. (Kads), the adsorption heat value  can be retrieved as a result of the slope
can be retrieved as a result of the slope  . The
. The 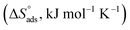 standard adsorption entropy can retrieved from the following basic thermodynamic equation:74
standard adsorption entropy can retrieved from the following basic thermodynamic equation:74
| |
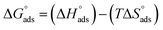 | (21) |
The values of the adsorption parameters are depicted in Table 7. The adsorption mechanism can be identified according to the resulting  values: if the values are negative, it is an exothermic physisorption or chemisorption mechanism; and if the values are positive, it is an endothermic or chemisorption mechanism.75 The values were +ve and between 48.77 and 56.00 kJ mol−1. These values are more than 41.8 kJ mol−1, indicating that the adsorption is chemical and the +ve sign indicates that the adsorption process is endothermic, i.e. chemisorption. The values of
values: if the values are negative, it is an exothermic physisorption or chemisorption mechanism; and if the values are positive, it is an endothermic or chemisorption mechanism.75 The values were +ve and between 48.77 and 56.00 kJ mol−1. These values are more than 41.8 kJ mol−1, indicating that the adsorption is chemical and the +ve sign indicates that the adsorption process is endothermic, i.e. chemisorption. The values of  are positive, indicating that the increase in disorderly due to the replacement of water molecules from the MS surface.
are positive, indicating that the increase in disorderly due to the replacement of water molecules from the MS surface.
Table 7 Adsorption parameters obtained from the Langmuir isotherm for steel dissolution in 1.0 M HCl in the presence of PhODB, BODB and DODB inhibitors at different temperatures
| Inhibitor |
Temp. (K) |
Kads (kJ mol−1) |
ΔGads (kJ mol−1) |
ΔSads (J mol−1 K−1) |
ΔHads (kJ mol−1) |
| PhODB |
298 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 105 |
−36.10 |
282.78 |
48.77 |
| 303 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
−37.55 |
284.87 |
| 308 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061 061 |
−39.53 |
286.68 |
| 313 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 |
−40.13 |
284.04 |
| 318 |
107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920 |
−41.26 |
283.11 |
| BODB |
298 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183 183 |
−36.18 |
317.20 |
58.94 |
| 303 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072 072 |
−37.75 |
319.10 |
| 308 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126 |
−39.72 |
320.31 |
| 313 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071 071 |
−40.39 |
317.33 |
| 318 |
153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 898 |
−42.20 |
318.03 |
| DODB |
298 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
36.15 |
307.23 |
56.00 |
| 303 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 915 |
−37.91 |
309.95 |
| 308 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196 |
−39.64 |
310.52 |
| 313 |
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 823 |
−40.84 |
309.39 |
| 318 |
130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 415 415 |
−41.76 |
307.42 |
3.3.3. Thermodynamic and activation parameters. Monitoring the corrosion behavior at various temperatures is very important to produce various related activation (energy  , entropy (ΔS*) and enthalpy (ΔH*)) parameters, as listed in Table 8. The correlation between temperature (T) and corrosion rate (k) is typically represented by the Arrhenius equation76 as follows:
, entropy (ΔS*) and enthalpy (ΔH*)) parameters, as listed in Table 8. The correlation between temperature (T) and corrosion rate (k) is typically represented by the Arrhenius equation76 as follows:| |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CR(k) = −log CR(k) = −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − (Ea/2.303RT) A − (Ea/2.303RT)
| (22) |
where, A is a frequency factor, Ea is the activation energy, R is the molar gas constant (8.314 J mol−1 K−1) and T is the absolute temperature in K. Fig. 12(a) shows the plots for the Arrhenius relation between (1/T) and log corrosion rate (k = mg cm−2 h−1) for PhODB, BODB and DODB inhibitors. From Table 8,  = 89.76 kJ mol−1 for an uninhibited corrosive electrolyte and the values changed to 59.72, 59.21 and 55.37 kJ mol−1 for PhODB, BODB and DODB, respectively, at 7.50 × 10−4 molar concentration for all organic inhibitors. This change in
= 89.76 kJ mol−1 for an uninhibited corrosive electrolyte and the values changed to 59.72, 59.21 and 55.37 kJ mol−1 for PhODB, BODB and DODB, respectively, at 7.50 × 10−4 molar concentration for all organic inhibitors. This change in  numbers is a result of the chemisorption adsorption behavior of these inhibitors. The transition state equation can be represented as in the next equation:77
numbers is a result of the chemisorption adsorption behavior of these inhibitors. The transition state equation can be represented as in the next equation:77| |
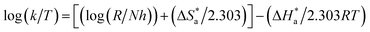 | (23) |
where, 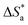 and
and  are the activation entropy and enthalpy, respectively. N is Avogadro's number (6.022 × 1023 mol−1), h is Planck's constant (6.626176 × 10−34 J s) and T is the temperature in kelvin (K). Fig. 12(b) shows plots of the transition state relation between (1/T) and log(k/T). The DODB compound has the lowest activation value among the investigated synthesized organic derivatives and because of this, it is suggested that it would be a more effective inhibitor for MS against an acidic electrolyte. According to thermodynamic and activation parameter results, the inhibition order is DODB > BODB > PhODB.
are the activation entropy and enthalpy, respectively. N is Avogadro's number (6.022 × 1023 mol−1), h is Planck's constant (6.626176 × 10−34 J s) and T is the temperature in kelvin (K). Fig. 12(b) shows plots of the transition state relation between (1/T) and log(k/T). The DODB compound has the lowest activation value among the investigated synthesized organic derivatives and because of this, it is suggested that it would be a more effective inhibitor for MS against an acidic electrolyte. According to thermodynamic and activation parameter results, the inhibition order is DODB > BODB > PhODB.
Table 8 Activation parameters values for steel in 1.0 M HCl in the absence and presence of different concentrations of the PhODB, BODB and DODB compounds
| Inhibitor |
Conc. of inhibitor (M) |
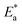
(kJ mol−1) |
ΔH* (kJ mol−1) |
ΔS* (J mol−1 K−1) |
| Blank |
0.00 × 10−4 |
89.76 |
87.20 |
38.86 |
| PhODB |
0.50 × 10−4 |
57.82 |
55.26 |
−75.91 |
| 0.75 × 10−4 |
57.89 |
55.34 |
−77.67 |
| 2.50 × 10−4 |
56.24 |
53.68 |
−85.59 |
| 5.00 × 10−4 |
54.98 |
52.42 |
−90.57 |
| 7.50 × 10−4 |
59.72 |
57.16 |
−77.09 |
| BODB |
0.50 × 10−4 |
54.48 |
51.92 |
−87.52 |
| 0.75 × 10−4 |
54.76 |
52.20 |
−88.40 |
| 2.50 × 10−4 |
50.24 |
47.68 |
−105.84 |
| 5.00 × 10−4 |
51.79 |
49.23 |
−102.12 |
| 7.50 × 10−4 |
59.21 |
56.65 |
−79.46 |
| DODB |
0.50 × 10−4 |
53.11 |
50.55 |
−92.50 |
| 0.75 × 10−4 |
53.95 |
51.39 |
−91.57 |
| 2.50 × 10−4 |
48.18 |
45.62 |
−113.22 |
| 5.00 × 10−4 |
44.18 |
41.63 |
−127.75 |
| 7.50 × 10−4 |
55.37 |
52.81 |
−93.21 |
 |
| | Fig. 12 Arrhenius plots (a) and transition state plots (b) for steel dissolution with and without various dosages from synthesized coumarin derivatives (PhODB, BODB and DODB) in 1.0 M HCl solution. | |
3.4. Spectral UV-visible analysis
Applying UV-visible spectroscopic analysis, one can ascertain how the coumarin derivatives and metallic cations form a complex. The MS electrode was exposed to (1.0 M HCl) corrosive electrolyte for 24 h at 25 °C without using any inhibitor (blank = MS + 1.0 M HCl). Also, a significant molar concentration (1.25 × 10−4) of each coumarin derivative inhibitor was dissolved in the same corrosive electrolyte to be used for the blank sample (solution A = inhibitor + 1.0 M HCl). Furthermore, another solution contained an MS electrode and defined significant molar dosages of the inhibitors (1.25 × 10−4) dissolved and immersed again in the same corrosive electrolyte (1.0 M HCl) for 24 h at 25 °C (solution B = inhibitor + MS + 1.0 M HCl). The UV was measured for the three different solutions for each coumarin inhibitor and the absorption wavelengths were recorded, as plotted in Fig. 13. The measured absorption wavelength for the blank (MS + 1.0 M HCl) solution was 205 nm. For solution A (inhibitor + 1.0 M HCl), the absorption values were 220 nm, 230 nm and 234 nm for PhODB, BODB and DODB, respectively, as a result of π–π* transitions. Also, other values were obtained for the same solutions at 334 nm, 316 nm and 342 nm for PhODB, BODB and DODB, respectively, as a result of n–π* transitions (hypsochromic shift). Furthermore, for solution B (inhibitor + MS + 1.0 M HCl), the measured absorption values were 340 nm, 328 nm and 336 nm for PhODB, BODB and DODB, respectively, as a result of π–π* transitions (bathochromic shift). This variation in absorption data might be interpreted as a sign that the PhODB, BODB and DODB coumarin inhibitors and the metallic electrode surface are forming a complex.78
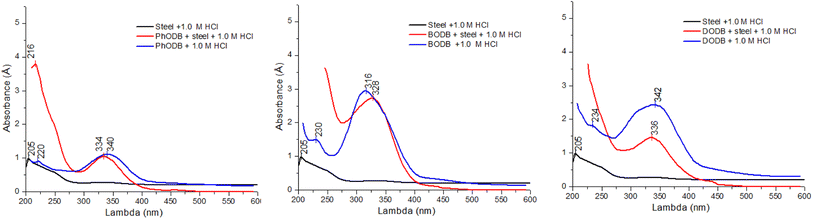 |
| | Fig. 13 UV-visible spectra for various solutions for steel, 1.0 M HCl and PhODB, BODB and DODB inhibitors at 25 °C after immersion for 24 h. | |
3.5. SEM and EDX
In order to validate the electrochemical measurements, quantitative EDX studies and qualitative microscopic SEM analyses were carried out. The surface states of the MS samples are shown in Fig. 14 before and after 24 hours of immersion in 1 M HCl solution and in the presence of (7.5 × 10−4) PhODB, BODB and DODB inhibitors. Without inhibitors, the sample has been substantially degraded by the medium and has become heterogeneous as a result of the acid's aggressive attack (Fig. 15(a)). The MS surface is noticeably enhanced, has fewer holes, is smoother, and is more heterogeneous in the presence of the PhODB, BODB and DODB inhibitors at 7.5 × 10−4 M, as seen in Fig. 14, which confirms the inhibitory effect. The inhibitors acted as an isolation layer deposited on and protecting the MS surface from Cl− ion attack. To identify the composition of the elements deposited on the MS surface, EDX measurements were applied. The obtained EDX results are plotted in Fig. 15 and the percentages of detected ions are listed in Table 9. For the blank sample (no inhibitor added), the major detected ions are mainly Fe (99.94 wt%) and Cl (0.06 wt%). In the case of adding PhODB, BODB and DODB inhibitors, new sufficient concentrations were detected for new elements (C, S, N and O) and corrosive chloride ions were absent. This behavior may suggest that the synthesized organic compounds have high adsorption characteristics for deposition on MS and a preventative film from the inhibitors has formed on the metal surface.79,80
 |
| | Fig. 14 SEM images for steel surface after immersion in 1.0 M HCl for 24 h in the absence and presence of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C. | |
 |
| | Fig. 15 EDX spectra for steel surface after immersion in 1.0 M HCl for 24 h in the absence and presence of synthesized coumarin derivatives (PhODB, BODB and DODB) at 25 °C. | |
Table 9 EDX analysis for steel surface after 24 h immersion in 1.0 M HCl in the presence and absence of the synthesized inhibitors PhODB, BODB and DODB
| Element |
Blank (HCl–Fe) |
PhODB-Fe |
BODB-Fe |
DODB-Fe |
| Mass% |
Atom% |
Mass% |
Atom% |
Mass% |
Atom% |
Mass% |
Atom% |
| Cl |
0.06 |
0.10 |
— |
— |
— |
— |
— |
— |
| C |
— |
— |
3.48 |
13.24 |
0.20 |
0.56 |
31.40 |
56.99 |
| N |
— |
— |
0.34 |
1.12 |
0.04 |
0.05 |
3.02 |
4.68 |
| O |
— |
— |
3.39 |
9.69 |
27.91 |
57.11 |
12.66 |
17.25 |
| S |
— |
— |
0.06 |
0.10 |
0.36 |
0.37 |
1.46 |
1 |
| Fe |
99.94 |
99.90 |
92.73 |
75.85 |
71.49 |
41.91 |
51.46 |
20.08 |
| Total |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
3.6. SRB biological resistivity
The SRB (sulfate-reducing bacteria) source was a water sample coming from an Egyptian gas field. We have previously discussed the water analysis, SRB population and monitoring procedures carried out using SRB (BART) vials (capacity = 15 ml).41 A small concentration (1 ppm mol−1) from each inhibitor was prepared in ultra-pure water and only 1 ml was added to the SRB test vial in addition to the water sample (15 ml) containing the SRB source. Another vial with only 15 ml of SRB water source without any added inhibitors was prepared as a blank. All vials were incubated at 35 °C inside an incubator. According to the test procedures, the maximum SRB test period is only 11 days but could be less according to realizing the first black sign had appeared on the test vials. After only 4 days, the test was completed for the blank with an aggressive population value of approximately 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (cfu ml−1). For BODB, the test was completed after 7 days giving 325 (cfu ml−1) as the population value, with conversion of the severity of SRB to moderate instead of aggressive in the case of the blank sample. For both PhODB and DODB, the observed values were obtained after 8 days with a high effectiveness against SRB bacteria, giving 75 cfu ml−1 population (not aggressive), as listed in Table 10. The results clearly provide a valuable indication of a reduction in SRB reactivity. Furthermore, due to the biological activity of PhODB, BODB and DODB inhibitors, the corrosion resulting from the presence of SRB can be mitigated.
000 (cfu ml−1). For BODB, the test was completed after 7 days giving 325 (cfu ml−1) as the population value, with conversion of the severity of SRB to moderate instead of aggressive in the case of the blank sample. For both PhODB and DODB, the observed values were obtained after 8 days with a high effectiveness against SRB bacteria, giving 75 cfu ml−1 population (not aggressive), as listed in Table 10. The results clearly provide a valuable indication of a reduction in SRB reactivity. Furthermore, due to the biological activity of PhODB, BODB and DODB inhibitors, the corrosion resulting from the presence of SRB can be mitigated.
Table 10 Approximate SRB population for tested inhibitors
3.7. The correlation between quantum chemical calculations and the corrosion parameters
Using different basis sets (Semi-empirical PM6, HF-631G and DFT/B3LYP/6-311+G), quantum chemical calculations were carried out to investigate the reactivity, adsorption and interaction behavior between the inhibitors (PhODB, BODB and DODB) and MS.81,82 Many parameters were calculated using the basis sets (Semi-empirical PM6, HF-631G and DFT/B3LYP/6-311+G): highest occupied and lowest unoccupied molecular orbitals (EHOMO and ELUMO, respectively), energy gap (ΔE), ionization potential (IP), electron affinity (EA), electronegativity (χ), electrophilicity (ω), transferred electrons (ΔN), softness (σ), hardness (η), dipole moment (μ), total energy E (RB3LYP), molecular volume (MV) and total negative charge (TNC), as listed in Table 11. Furthermore, the optimized molecular structures, HOMO, LUMO and ESP (electrostatic potential) resulting from (DFT/B3LYP/6-311+G) calculations for PhODB, BODB and DODB inhibitors are displayed in Fig. 16. An organic molecule's ability to donate electrons is generally demonstrated via its HOMO. In general, a molecule's ability to donate electrons is stronger with a higher EHOMO value. Due to the lone pair of electrons as a result of heteroatoms and π electron as a result of the aryl ring there is greater capability for electron donation, and Fig. 16 shows that the HOMO is much more localized toward them.83–87 From the values listed in Table 11, the EHOMO values are equal to −8.8862, −8.8451 and −8.6592 eV using Semi-empirical PM6, −8.1895, −7.8587 and −7.7710 eV using HF-631G, and −5.9715, −5.8948 and −5.6592 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. From the abovementioned EHOMO results, DODB has the highest EHOMO values and so it is has the greatest ability to donate electrons to the MS surface, leading to the formation a protective layer on its surface which is stronger than for BODB or PhODB. The energy gap (ΔE) is the difference between ELUMO and EHOMO values, and molecules with lower energy gaps deposit on metal surfaces more successfully because of the lower ionization energy that results from the lower energy gap, which makes it easier to remove the electron from the final orbital of the molecule.88 ΔE values equal 7.0829, 7.0608 and 6.9960 eV using semi-empirical PM6, 8.4483, 8.1188 and 8.0837 eV using HF-631G, and 2.4107, 2.3328 and 2.1211 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. Furthermore, the ionization potential (IP) values equal 8.8862, 8.8451 and 8.6592 eV using semi-empirical PM6, 8.1895, 7.8587 and 7.7710 eV using HF-631G, and 5.9715, 5.8948 and 5.6592 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. From the abovementioned ΔE and IP results, DODB has the lowest values, then BODB and PhODB have the highest values for the two parameters and, according to these values, DODB will have the highest reactivity to adsorb on the MS surface as a more effective corrosion inhibitor than BODB which will be more active than the PhODB inhibitor. Similarly, the compound with the lowest electronegativity (χ) values is the compound that most easily donates electrons to the MS surface and the same concept is also applicable to the total negative charge (TNC). According to the results in Table 11, the electronegativity (χ) values are equal to 5.3447, 5.3147 and 5.1612 eV using semi-empirical PM6, 3.9654, 3.7993 and 3.7292 eV using HF-631G, and 4.7662, 4.7284 and 4.5986 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. In addition, the total negative charge (TNC) results equal −14.5915, −15.3029 and −16.2161 eV using semi-empirical PM6, −14.9896, −15.8206 and −17.2966 eV using HF-631G, and −10.4903, −11.1590 and −12.6524 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. Therefore, DODB is considered to be the compound with higher protection ability than BODB or PhODB. Also, softness (σ) and hardness (η) are two chemical parameters which are related to each other. Chemical hardness prevents chemical molecules from deforming, and global hardness is negatively correlated with softness, so the highest softness compounds have the lowest hardness values.89 From the values in Table 11, the softness (σ) values are equal to 0.2824, 0.2823 and 2859 eV−1 using semi-empirical PM6, 0.2367, 0.2463 and 0.274 eV−1 using HF-631G, and 0.8296, 0.8573 and 0.9424 eV−1 using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. In addition, the hardness (η) results are equal to 3.541, 3.530 and 3.498 eV using semi-empirical PM6, 4.224, 4.059 and 4.041 eV using HF-631G, and 1.205, 1.166 and 1.060 eV using DFT/B3LYP/6-311+G for PhODB, BODB and DODB, respectively. It is clear that the DODB compound has the highest softness values, and also the lowest hardness values and the highest ability to protect the MS surface, but PhODB has the lowest softness values, the highest hardness values and the lowest ability to protect the MS surface and finally BODB lies in between DODB and PhODB. Referring to ESP in Fig. 16, the electrophilic and nucleophilic reactivity can be predicted through the change in the color of the region: the blue color refers to nucleophilic reactivity and a positive region, but red and yellow colors refer to electrophilic reactivity and negative regions.90 Furthermore, the ability of the inhibitor to protect the metal surface increases with the increasing molecular volume (MV) of the inhibitor. From the values in Table 11, DODB has a higher MV than BODB or PhODB by using different calculation methods: semi-empirical PM6, HF-631G and DFT/B3LYP/6-311+G. Also, the number of electrons transferred (ΔN) provided good proof of the inhibitor's ability to donate electrons to the metal surface, and from the obtained results DODB has the highest ability for electron donation, but PhODB has the lowest ability. The unshared electrons can act as a Lewis base and be easily donated to the metal ion (acting as a Lewis acid) via the vacant d orbital. By sharing the electrons from the inhibitor to the vacant d orbitals on the metal ion, a coordination bond is easily formed, resulting in complex formation between the inhibitor and the metal surface. The result is protection of the metal from attack by the corrosive electrolyte.91–93 The regression values (R2) for the calculated quantum chemical parameters and the ηEFM% are plotted in Fig. 17 and listed in Table 12. The values resulting from the theoretical quantum chemical calculation are in a good agreement with the values from the
experimental results and suggest the inhibition order is DODB > BODB > PhODB.
Table 11 The calculated quantum chemical parameters using 3 different optimization basis sets: semi-empirical PM6, HF-631G and DFT/B3LYP/6-311G
| OPT |
Molecule |
EHOMO (eV) |
ELUMO (eV) |
ΔE (eV) |
IP (eV) |
μ (D) |
MV (cm3 mol−1) |
TNC (e) |
σ (eV−1) |
ω (eV) |
χ (eV) |
η (eV) |
ΔN (e) |
ηPDP% |
| Semi-empirical PM6 |
PhODB |
−8.8862 |
−1.8033 |
7.0829 |
8.8862 |
0.9283 |
631.388 |
−14.5915 |
0.2824 |
4.0331 |
5.3447 |
3.541 |
0.2337 |
95.29 |
| BODB |
−8.8451 |
−1.7843 |
7.0608 |
8.8451 |
4.5997 |
814.211 |
−15.3029 |
0.2833 |
4.0003 |
5.3147 |
3.530 |
0.2387 |
96.50 |
| DODB |
−8.6592 |
−1.6632 |
6.9960 |
8.6592 |
5.4661 |
846.2280 |
−16.2161 |
0.2859 |
3.8076 |
5.1612 |
3.498 |
0.2628 |
97.60 |
| HF-631G |
PhODB |
−8.1895 |
0.2588 |
8.4483 |
8.1895 |
2.8506 |
545.8450 |
−14.9896 |
0.2367 |
1.8612 |
3.9654 |
4.224 |
0.3592 |
95.29 |
| BODB |
−7.8587 |
0.2601 |
8.1188 |
7.8587 |
2.7910 |
559.3780 |
−15.8206 |
0.2463 |
1.7779 |
3.7993 |
4.059 |
0.3942 |
96.50 |
| DODB |
−7.7710 |
0.3127 |
8.0837 |
7.7710 |
2.4156 |
631.3660 |
−17.2966 |
0.2474 |
1.7204 |
3.7292 |
4.041 |
0.4046 |
97.60 |
| DFT/B3LYP/6-311G |
PhODB |
−5.9715 |
−3.5609 |
2.4107 |
5.9715 |
0.0024 |
537.5310 |
−10.4903 |
0.8296 |
9.4235 |
4.7662 |
1.205 |
0.9266 |
95.29 |
| BODB |
−5.8948 |
−3.5620 |
2.3328 |
5.8948 |
0.7567 |
571.0750 |
−11.1590 |
0.8573 |
9.5839 |
4.7284 |
1.166 |
0.9738 |
96.50 |
| DODB |
−5.6592 |
−3.5380 |
2.1211 |
5.6592 |
3.5620 |
719.8810 |
−12.6524 |
0.9429 |
9.9697 |
4.5986 |
1.060 |
1.1321 |
97.60 |
 |
| | Fig. 16 Optimized structures, HOMO, LUMO and ESP for synthesized coumarin derivatives (PhODB, BODB and DODB) using DFT/B3LYP/6-311+G. | |
 |
| | Fig. 17 The regression value (R2) obtained from correlation between the calculated quantum chemical parameters and ηEFM% for the synthesized coumarin derivatives (PhODB, BODB and DODB). | |
Table 12 The calculated R2 values using different optimization basis sets: semi-empirical PM6, HF-631G and DFT/B3LYP/6-311G
| Optimized basis sets |
EHOMO (eV) |
ELUMO (eV) |
ΔE (eV) |
IP (eV) |
μ (D) |
MV (cm3 mol−1) |
TNC (e) |
σ (eV−1) |
ω (eV) |
χ (eV) |
η (eV) |
ΔN (e) |
| Semi-empirical PM6 |
0.7583 |
0.7188 |
0.8204 |
0.7583 |
0.9674 |
0.9507 |
0.9468 |
0.8228 |
0.727 |
0.7429 |
0.8217 |
0.7502 |
| HF-631G |
0.9738 |
0.9904 |
0.9003 |
0.9738 |
0.7196 |
0.7383 |
0.9003 |
0.9294 |
0.9969 |
0.9952 |
0.9273 |
0.9728 |
| DFT/B3LYP/6-311G |
0.8133 |
0.6803 |
0.8639 |
0.8133 |
0.7849 |
0.7608 |
0.8639 |
0.8123 |
0.8519 |
0.6052 |
0.8324 |
0.8000 |
4. Conclusions
Three novel coumarin derivatives were synthesized and characterized by different analyses. The inhibition efficiency increases when the inhibitor concentration and temperature of the environment are raised, which indicates that the adsorption is mainly chemical. Adsorption of these derivatives onto the MS surface in 1 M HCl solution obeys the Langmuir adsorption model. Potentiodynamic polarization studies reveal that these derivatives are mixed-type inhibitors. Electrochemical impedance measurements indicate the formation of a protective film on the MS surface in HCl solution. FTIR spectroscopic data suggest that the protective film consists of an Fe–additive molecule complex. SEM and XRD analyses clearly indicate the presence of a protective surface layer on the MS surface. The results showed that corrosion related to SRB can be controlled by these novel coumarin derivatives. Theoretical calculation show an amazing match with the experimental results. The suggested inhibition order according to the resulting values from theoretical and experimental techniques is as follows: DODB > BODB > PhODB.
Author contributions
Hani M. Elaryian: methodology and taking experiment part of inhibitors synthesized and tested, writing – original draft preparation. Mahmoud A. Bedair: supervision, software, resources, conceptualization, experimental, validation, formal analysis, FMO computations, review and editing article. Ahmed H. Bedair: supervision, resources. Rabab M. Aboushahba: supervision. Abd El-Aziz S. Fouda: supervision, conceptualization, investigation, software, validation, review and editing article.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- R. Hsissou, F. Benhiba, O. Dagdag, M. El Bouchti, K. Nouneh, M. Assouag, S. Briche, A. Zarrouk and A. Elharfi, J. Colloid Interface Sci., 2020, 574, 43–60 CrossRef CAS PubMed
 .
. - A. M. Abuelela, M. A. Bedair, W. M. Zoghaib, L. D. Wilson and T. A. Mohamed, J. Mol. Struct., 2021, 1230, 129647 CrossRef CAS
 .
. - A. M. Nagiub, M. H. Mahross, H. F. Y. Khalil, B. N. A. Mahran, M. M. Yehia and M. M. B. El-Sabbah, Port. Electrochim. Acta, 2013, 31, 119–139 CrossRef CAS
 .
. - M. A. Bedair, A. M. Abuelela, W. M. Zoghaib and T. A. Mohamed, J. Mol. Struct., 2021, 1244, 130927 CrossRef CAS
 .
. - J. Zhang, Int. J. Electrochem. Sci., 2020, 1437–1449 CrossRef CAS
 .
. - A. Singh, K. R. Ansari, J. Haque, P. Dohare, H. Lgaz, R. Salghi and M. A. Quraishi, J. Taiwan Inst. Chem. Eng., 2018, 82, 233–251 CrossRef CAS
 .
. - J. Liu, Int. J. Electrochem. Sci., 2020, 2499–2510 CrossRef CAS
 .
. - Z. Salarvand, M. Amirnasr, M. Talebian, K. Raeissi and S. Meghdadi, Corros. Sci., 2017, 114, 133–145 CrossRef CAS
 .
. - O. Moumeni, S. Chafaa, R. Kerkour, K. Benbouguerra and N. Chafai, J. Mol. Struct., 2020, 1206, 127693 CrossRef CAS
 .
. - L. Ouksel, R. Bourzami, S. Chafaa and N. Chafai, J. Mol. Struct., 2020, 1222, 128813 CrossRef CAS
 .
. - N. Chafai, S. Chafaa, K. Benbouguerra, A. Hellal and M. Mehri, J. Mol. Struct., 2019, 1181, 83–92 CrossRef CAS
 .
. - M. Djenane, S. Chafaa, N. Chafai, R. Kerkour and A. Hellal, J. Mol. Struct., 2019, 1175, 398–413 CrossRef CAS
 .
. - N. Chafai, S. Chafaa, K. Benbouguerra, D. Daoud, A. Hellal and M. Mehri, J. Taiwan Inst. Chem. Eng., 2017, 70, 331–344 CrossRef CAS
 .
. - Y. El Aoufir, H. Lgaz, H. Bourazmi, Y. Kerroum, Y. Ramli, A. Guenbour, R. Salghi, F. El-Hajjaji, B. Hammouti and H. Oudda, J. Mater. Environ. Sci., 2016, 7, 4330–4347 CAS
 .
. - F. Z. Qachchachi, Y. Kandri Rodi, H. Elmsellem, H. Steli, A. Haoudi, A. Mazzah, Y. Ouzidan, N. K. Sebbar and E. M. Essassi, J. Mater. Environ. Sci., 2016, 7, 2897–2907 CAS
 .
. - C. Verma, M. A. Quraishi, E. E. Ebenso, I. B. Obot and A. El Assyry, J. Mol. Liq., 2016, 219, 647–660 CrossRef CAS
 .
. - I. B. Obot, A. Madhankumar, S. A. Umoren and Z. M. Gasem, J. Adhes. Sci. Technol., 2015, 29, 2130–2152 CrossRef CAS
 .
. - A. Singh, K. R. Ansari, D. S. Chauhan, M. A. Quraishi, H. Lgaz and I.-M. Chung, J. Colloid Interface Sci., 2020, 560, 225–236 CrossRef CAS PubMed
 .
. - E. Kamali Ardakani, E. Kowsari and A. Ehsani, Colloids Surf., A, 2020, 586, 124195 CrossRef CAS
 .
. - S. A. Soliman, M. S. Metwally, S. R. Selim, M. A. Bedair and M. A. Abbas, J. Ind. Eng. Chem., 2014, 20, 4311–4320 CrossRef CAS
 .
. - M. A. Bedair, M. M. B. El-Sabbah, A. S. Fouda and H. M. Elaryian, Corros. Sci., 2017, 128, 54–72 CrossRef CAS
 .
. - A. Asan, S. Soylu, T. Kıyak, F. Yıldırım, S. G. Öztaş, N. Ancın and M. Kabasakaloğlu, Corros. Sci., 2006, 48, 3933–3944 CrossRef CAS
 .
. - F. Touhami, A. Aouniti, Y. Abed, B. Hammouti, S. Kertit, A. Ramdani and K. Elkacemi, Corros. Sci., 2000, 42, 929–940 CrossRef CAS
 .
. - J. R. Casley-Smith and J. R. Casley-Smith, Med. J. Aust., 1995, 162, 391 CrossRef CAS PubMed
 .
. - I. Kostova, S. Raleva, P. Genova and R. Argirova, Bioinorg. Chem. Appl., 2006, 2006, 1–9 Search PubMed
 .
. - J.-C. Jung, Y.-J. Jung and O.-S. Park, Synth. Commun., 2001, 31, 1195–1200 CrossRef CAS
 .
. - K. Fylaktakidou, D. Hadjipavlou-Litina, K. Litinas and D. Nicolaides, Curr. Pharm. Des., 2004, 10, 3813–3833 CrossRef CAS PubMed
 .
. - G. Smitha and C. Sanjeeva Reddy, Synth. Commun., 2004, 34, 3997–4003 CrossRef CAS
 .
. - Z. Nofal, M. El-Zahar and S. Abd El-Karim, Molecules, 2000, 5, 99–113 CrossRef CAS
 .
. - P. A. Rasheed, K. A. Jabbar, K. Rasool, R. P. Pandey, M. H. Sliem, M. Helal, A. Samara, A. M. Abdullah and K. A. Mahmoud, Corros. Sci., 2019, 148, 397–406 CrossRef
 .
. - D. Enning and J. Garrelfs, Appl. Environ. Microbiol., 2014, 80, 1226–1236 CrossRef PubMed
 .
. - H. Wang, Z. Wang, H. Hong and Y. Yin, Mater. Chem. Phys., 2010, 124, 791–794 CrossRef CAS
 .
. - J. Duan, B. Hou and Z. Yu, Mater. Sci. Eng., C, 2006, 26, 624–629 CrossRef CAS
 .
. - K. Rasool, A. Shahzad and D. S. Lee, J. Hazard. Mater., 2016, 318, 641–649 CrossRef CAS PubMed
 .
. - K. Rasool and D. S. Lee, J. Nanosci. Nanotechnol., 2016, 16, 4456–4463 CrossRef CAS PubMed
 .
. - S. P. Tambe, S. D. Jagtap, A. K. Chaurasiya and K. K. Joshi, Prog. Org. Coat., 2016, 94, 49–55 CrossRef CAS
 .
. - P. J. Antony, R. K. S. Raman, R. Raman and P. Kumar, Corros. Sci., 2010, 52, 1404–1412 CrossRef CAS
 .
. - C. Sun, J. Xu and F. Wang, Ind. Eng. Chem. Res., 2011, 50, 12797–12806 CrossRef CAS
 .
. - Y. Xue and G. Voordouw, Front. Microbiol., 2015, 6, 1387–1398 Search PubMed
 .
. - K. P. Meesters, J. Van Groenestijn and J. Gerritse, Water Res., 2003, 37, 525–532 CrossRef CAS PubMed
 .
. - H. M. Elaryian, M. A. Bedair, A. H. Bedair, R. M. Aboushahba and A. E.-A. S. Fouda, J. Mol. Liq., 2022, 346, 118310 CrossRef CAS
 .
. - M. N. Majeed, Q. A. Yousif and M. A. Bedair, ACS Omega, 2022, 7, 29850–29857 CrossRef CAS PubMed
 .
. - R. Haldhar, S.-C. Kim, D. Prasad, M. A. Bedair, I. Bahadur, S. Kaya, O. Dagdag and L. Guo, J. Mol. Struct., 2021, 1242, 130822 CrossRef CAS
 .
. - A. Gangan, M. ElSabbagh, M. A. Bedair, H. M. Ahmed, M. El-Sabbah, S. M. El-Bahy and A. Fahmy, Arabian J. Chem., 2021, 14, 103391 CrossRef CAS
 .
. - A. Hassan, B. Heakal, A. Younis, M. Bedair, Z. El - Billy and M. Mohamed, Egypt. J. Chem., 2019, 62(9), 1603–1624 Search PubMed
 .
. - M. M. Elsenety, B. A. Elsayed, I. A. Ibrahem and M. A. Bedair, Inorg. Chem. Commun., 2020, 121, 108213 CrossRef CAS
 .
. - M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed
 .
. - A. Singh, K. R. Ansari, A. Kumar, W. Liu, C. Songsong and Y. Lin, J. Alloys Compd., 2017, 712, 121–133 CrossRef CAS
 .
. - J. Foresman, Lancet, 1973, 2, 139–141 Search PubMed
 .
. - L. Pauling, The Nature of the Chemical Bond: An Introduction to Modern Structural Chemistry, Cornell University Press, 3rd edn, 1960 Search PubMed
 .
. - P. Senet, Chem. Phys. Lett., 1997, 275, 527–532 CrossRef CAS
 .
. - R. G. Pearson, Inorg. Chem., 1988, 27, 734–740 CrossRef CAS
 .
. - M. A. Bedair, S. A. Soliman, M. F. Bakr, E. S. Gad, H. Lgaz, I.-M. Chung, M. Salama and F. Z. Alqahtany, J. Mol. Liq., 2020, 317, 114015 CrossRef CAS
 .
. - R. G. Parr, L. v. Szentpály and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS
 .
. - M. A. Bedair, A. S. Fouda, M. A. Ismail and A. Mostafa, Ionics, 2019, 25, 2913–2933 CrossRef CAS
 .
. - M. Yadav, T. K. Sarkar and I. B. Obot, RSC Adv., 2016, 6, 110053–110069 RSC
 .
. - M. A. Bedair, E. H. Alosaimi and S. Melhi, J. Adhes. Sci. Technol., 2021, 1–31 CrossRef
 .
. - E. A. Badr, M. A. Bedair and S. M. Shaban, Mater. Chem. Phys., 2018, 219, 444–460 CrossRef CAS
 .
. - K. Zakaria, M. A. Abbas and M. A. Bedair, J. Mol. Liq., 2022, 352, 118689 CrossRef CAS
 .
. - M. Ouakki, M. Galai, Z. Benzekri, C. Verma, E. Ech-chihbi, S. Kaya, S. Boukhris, E. E. Ebenso, M. E. Touhami and M. Cherkaoui, Colloids Surf., A, 2021, 611, 125810 CrossRef CAS
 .
. - M. Ouakki, M. Rbaa, M. Galai, B. Lakhrissi, E. H. Rifi and M. Cherkaoui, J. Bio- Tribo-Corros., 2018, 4, 35 CrossRef
 .
. - Y. Qiang, S. Zhang, S. Yan, X. Zou and S. Chen, Corros. Sci., 2017, 126, 295–304 CrossRef CAS
 .
. - A. Bousskri, A. Anejjar, M. Messali, R. Salghi, O. Benali, Y. Karzazi, S. Jodeh, M. Zougagh, E. E. Ebenso and B. Hammouti, J. Mol. Liq., 2015, 211, 1000–1008 CrossRef CAS
 .
. - W. Al Zoubi, D. K. Yoon, Y. G. Kim and Y. G. Ko, J. Colloid Interface Sci., 2020, 573, 31–44 CrossRef CAS PubMed
 .
. - K. Hu, J. Zhuang, J. Ding, Z. Ma, F. Wang and X. Zeng, Corros. Sci., 2017, 125, 68–76 CrossRef CAS
 .
. - M. A. Mostafa, A. M. Ashmawy, M. A. M. A. Reheim, M. A. Bedair and A. M. Abuelela, J. Mol. Struct., 2021, 1236, 130292 CrossRef CAS
 .
. - M. Bedair, M. Metwally, S. Soliman, A. Al-Sabagh, A. Salem and T. Mohamed, Al-Azhar Bull. Sci., 2015, 26, 1–14 CrossRef
 .
. - M. A. Khaled, M. A. Ismail, A. A. El-Hossiany and A. E.-A. S. Fouda, RSC Adv., 2021, 11, 25314–25333 RSC
 .
. - S. S. Alarfaji, I. H. Ali, M. Z. Bani-Fwaz and M. A. Bedair, Molecules, 2021, 26, 3183 CrossRef CAS PubMed
 .
. - M. A. Abbas, M. A. Bedair, O. E. El-Azabawy and E. S. Gad, ACS Omega, 2021, 6, 15089–15102 CrossRef CAS PubMed
 .
. - M. A. Abbas and M. A. Bedair, Z. Phys. Chem., 2019, 233, 225–254 CrossRef CAS
 .
. - M. M. B. El-Sabbah, M. A. Bedair, M. A. Abbas, A. Fahmy, S. Hassaballa and A. A. Moustafa, Z. Phys. Chem., 2019, 233, 627–649 CrossRef CAS
 .
. - M. A. Hegazy, A. S. El-Tabei, A. H. Bedair and M. A. Sadeq, Corros. Sci., 2012, 54, 219–230 CrossRef CAS
 .
. - M. A. Bedair, S. A. Soliman, M. A. Hegazy, I. B. Obot and A. S. Ahmed, J. Adhes. Sci. Technol., 2019, 33, 1139–1168 CrossRef CAS
 .
. - E. A. Noor and A. H. Al-Moubaraki, Mater. Chem. Phys., 2008, 110, 145–154 CrossRef CAS
 .
. - X. Li, S. Deng, H. Fu and G. Mu, Corros. Sci., 2009, 51, 620–634 CrossRef CAS
 .
. - M. A. Gebril, M. A. Bedair, S. A. Soliman, M. F. Bakr and M. B. I. Mohamed, J. Mol. Liq., 2022, 349, 118445 CrossRef CAS
 .
. - H. Mehta, G. Kaur, G. R. Chaudhary and N. Prabhakar, Corros. Sci., 2021, 179, 109101 CrossRef CAS
 .
. - A. M. Ashmawy, R. Said, I. A. Naguib, B. Yao and M. A. Bedair, ACS Omega, 2022, 7, 17849–17860 CrossRef CAS PubMed
 .
. - M. Goyal, S. Kumar, C. Verma, I. Bahadur, E. E. Ebenso, H. Lgaz and I.-M. Chung, J. Mol. Liq., 2020, 298, 111995 CrossRef CAS
 .
. - M. A. Abbas, E. I. Arafa, E. S. Gad, M. A. Bedair, O. E. El-Azabawy and H. I. Al-Shafey, Inorg. Chem. Commun., 2022, 143, 109758 CrossRef CAS
 .
. - M. M. Khalaf, A. H. Tantawy, K. A. Soliman and H. M. Abd El-Lateef, J. Mol. Struct., 2020, 1203, 127442 CrossRef CAS
 .
. - M. M. Abdelsalam, M. A. Bedair, A. M. Hassan, B. H. Heakal, A. Younis, Z. I. Elbialy, M. A. Badawy, H. E.-D. Fawzy and S. A. Fareed, Arabian J. Chem., 2022, 15, 103491 CrossRef CAS
 .
. - M. A. Bedair, S. A. Soliman and M. S. Metwally, J. Ind. Eng. Chem., 2016, 41, 10–22 CrossRef CAS
 .
. - M. K. Awad, M. S. Metwally, S. A. Soliman, A. A. El-Zomrawy and M. A. Bedair, J. Ind. Eng. Chem., 2014, 20, 796–808 CrossRef CAS
 .
. - W. N. Ahmaeed, A. N. Abd and A. A. Khadom, Int. J. Corros. Scale Inhib., 2019, 8(4), 1097–1111 CAS
 .
. - H. M. Abd El-Lateef and A. O. Alnajjar, J. Mol. Liq., 2020, 303, 112641 CrossRef CAS
 .
. - E. E. Ebenso, T. Arslan, F. Kandemirli, N. Caner and I. Love, Int. J. Quantum Chem., 2010, 110, 1003–1018 CrossRef CAS
 .
. - S. Kaya, C. Kaya and N. Islam, Comput. Theor. Chem., 2016, 1080, 72–78 CrossRef CAS
 .
. - M. A. Bedair, J. Mol. Liq., 2016, 219, 128–141 CrossRef CAS
 .
. - C. Lai, X. Guo, J. Wei, B. Xie, L. Zou, X. Li, Z. Chen and C. Wang, Open Chem., 2018, 16, 904 CrossRef
 .
. - P. Preethi Kumari, P. Shetty and S. A. Rao, Arabian J. Chem., 2017, 10, 653–663 CrossRef CAS
 .
. - W. Zhang, H.-J. Li, M. Wang, L.-J. Wang, F. Shang and Y.-C. Wu, J. Phys. Chem. C, 2018, 122, 25349–25364 CrossRef CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ab,
Mahmoud A. Bedair
ab,
Mahmoud A. Bedair *ac,
Ahmed H. Bedaira,
Rabab M. Aboushahbad and
Abd El-Aziz S. Fouda*e
*ac,
Ahmed H. Bedaira,
Rabab M. Aboushahbad and
Abd El-Aziz S. Fouda*e


 and icorr are current density for corrosion electrolytes without and with inhibitors, respectively.
and icorr are current density for corrosion electrolytes without and with inhibitors, respectively.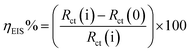



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1511.39, 1565.82 (N
O), 1511.39, 1565.82 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1204.67, 1039.57 (δ lactone, O–C
N, azo groups, bis azo compound), 1204.67, 1039.57 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1620.23 (C
O), 1620.23 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), and 3442.83 (SO3H group). The FTIR of BODB showed peaks at 3062.84 (aromatic C–H), 1754.90 (C
N), and 3442.83 (SO3H group). The FTIR of BODB showed peaks at 3062.84 (aromatic C–H), 1754.90 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O, coumarin), 1492.43, 1565.71 (N
O, coumarin), 1492.43, 1565.71 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1620.96 (C
N, azo groups, bis azo compound), 1620.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 1205.39, 1038.55 (δ lactone, O–C
N), 1205.39, 1038.55 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), and 3443.43 (SO3H group). The DODB compound showed FTIR peaks at 3063.04 (aromatic C–H), 2855.46, 2938.14, 2962.67 (aliphatic C–H), 1755.21 (C
O), and 3443.43 (SO3H group). The DODB compound showed FTIR peaks at 3063.04 (aromatic C–H), 2855.46, 2938.14, 2962.67 (aliphatic C–H), 1755.21 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1459.38, 1566.51 (N
O), 1459.38, 1566.51 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N, azo groups, bis azo compound), 1205.10, 1039.25 (δ lactone, O–C
N, azo groups, bis azo compound), 1205.10, 1039.25 (δ lactone, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O), 1621.31 (C
O), 1621.31 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 3437.10 (SO3H group). All detailed FTIR values are given in Table 1 and Fig. 2. From the 1H NMR analysis (DMSO-d6), 400 MHz; PhODB showed bands at δ = 2.62 (6H, s, N
N), 3437.10 (SO3H group). All detailed FTIR values are given in Table 1 and Fig. 2. From the 1H NMR analysis (DMSO-d6), 400 MHz; PhODB showed bands at δ = 2.62 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 10.36 ppm (SO3H), δ = 7.07–8.83 ppm (18H, m, Ar-H), δ = 9.06, 9.25 ppm (2H, s, coumarin-4-H). But BODB showed bands at δ = 2.61 (6H, s, N
C–CH3), δ = 10.36 ppm (SO3H), δ = 7.07–8.83 ppm (18H, m, Ar-H), δ = 9.06, 9.25 ppm (2H, s, coumarin-4-H). But BODB showed bands at δ = 2.61 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 10.37 ppm (SO3H), δ = 7.11–8.82 ppm (22H, m, Ar-H), δ = 9.22, 9.26 ppm (2H, s, coumarin-4-H). The 1H NMR of DODB showed bands at δ = 2.61 (6H, s, N
C–CH3), δ = 10.37 ppm (SO3H), δ = 7.11–8.82 ppm (22H, m, Ar-H), δ = 9.22, 9.26 ppm (2H, s, coumarin-4-H). The 1H NMR of DODB showed bands at δ = 2.61 (6H, s, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), δ = 3.03 (6H, s, CH3–O-Ar), δ = 10.32 ppm (SO3H), δ = 6.64–8.82 ppm (20H, m, Ar-H), δ = 9.25, 9.29 ppm (2H, s, coumarin-4-H). All detailed 1H NMR values are given in Table 1 and Fig. 3. From mass analysis at m/z (%); (M+.) = 816 (66.03%) for PhODB, (M+˙) = 892 (43.42%) for BODB and (M+˙) = 952 (66.15%) for DODB. The molecular ion peaks (base peak) are at m/z = 621 (100%) for PhODB, m/z = 724 (100%) for (BODB) and m/z = 950 (100%) for DODB. The other additional peaks are listed in Table 1 and Fig. 4.
C–CH3), δ = 3.03 (6H, s, CH3–O-Ar), δ = 10.32 ppm (SO3H), δ = 6.64–8.82 ppm (20H, m, Ar-H), δ = 9.25, 9.29 ppm (2H, s, coumarin-4-H). All detailed 1H NMR values are given in Table 1 and Fig. 3. From mass analysis at m/z (%); (M+.) = 816 (66.03%) for PhODB, (M+˙) = 892 (43.42%) for BODB and (M+˙) = 952 (66.15%) for DODB. The molecular ion peaks (base peak) are at m/z = 621 (100%) for PhODB, m/z = 724 (100%) for (BODB) and m/z = 950 (100%) for DODB. The other additional peaks are listed in Table 1 and Fig. 4.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (Ω cm2) against log frequency (Hz), the capacitance properties of the adsorption behavior of the investigated compounds on the MS surface were confirmed.62 The observed values from EIS indicate that, at higher inhibitor dosage, the highest mitigation results can be achieved. According to the EIS results, the inhibition order is DODB > BODB > PhODB.
Z (Ω cm2) against log frequency (Hz), the capacitance properties of the adsorption behavior of the investigated compounds on the MS surface were confirmed.62 The observed values from EIS indicate that, at higher inhibitor dosage, the highest mitigation results can be achieved. According to the EIS results, the inhibition order is DODB > BODB > PhODB.


 can be calculated from the following formula:68
can be calculated from the following formula:68
 and Kads values for all adsorption isotherm models are listed in Table 6 at 298 K. Spontaneous nature of adsorption and stability of the adsorbed layer are expected for the studied organic derivatives towards the electrode metal surface due to the negative calculated values for
and Kads values for all adsorption isotherm models are listed in Table 6 at 298 K. Spontaneous nature of adsorption and stability of the adsorbed layer are expected for the studied organic derivatives towards the electrode metal surface due to the negative calculated values for 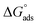 . Based on previously accepted research,69,70 the spontaneous adsorption behavior is a steady process that cannot be reversed. The adsorption process behavior depends on the
. Based on previously accepted research,69,70 the spontaneous adsorption behavior is a steady process that cannot be reversed. The adsorption process behavior depends on the  values: physisorption if
values: physisorption if  ≥ −20 kJ mol−1, chemisorption if
≥ −20 kJ mol−1, chemisorption if  ≥ −40 kJ mol−1 and mixed type if
≥ −40 kJ mol−1 and mixed type if  values are between −20 and −40 kJ mol−1.71 The
values are between −20 and −40 kJ mol−1.71 The  results are −36.10, −36.18 and 36.15 kJ mol−1 for PhODB, BODB and DODB, respectively, according to the Langmuir adsorption model, so the adsorption of these compounds on MS surfaces are of mixed type (physisorption and chemisorption, but mainly chemical).
results are −36.10, −36.18 and 36.15 kJ mol−1 for PhODB, BODB and DODB, respectively, according to the Langmuir adsorption model, so the adsorption of these compounds on MS surfaces are of mixed type (physisorption and chemisorption, but mainly chemical).
 obtained by using data from WL measurementsa
obtained by using data from WL measurementsa
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = log
θ = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K + 1/n
K + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C
C
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105
105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800





 can be retrieved as a result of the slope
can be retrieved as a result of the slope  . The
. The 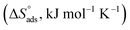 standard adsorption entropy can retrieved from the following basic thermodynamic equation:74
standard adsorption entropy can retrieved from the following basic thermodynamic equation:74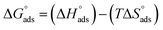
 values: if the values are negative, it is an exothermic physisorption or chemisorption mechanism; and if the values are positive, it is an endothermic or chemisorption mechanism.75 The values were +ve and between 48.77 and 56.00 kJ mol−1. These values are more than 41.8 kJ mol−1, indicating that the adsorption is chemical and the +ve sign indicates that the adsorption process is endothermic, i.e. chemisorption. The values of
values: if the values are negative, it is an exothermic physisorption or chemisorption mechanism; and if the values are positive, it is an endothermic or chemisorption mechanism.75 The values were +ve and between 48.77 and 56.00 kJ mol−1. These values are more than 41.8 kJ mol−1, indicating that the adsorption is chemical and the +ve sign indicates that the adsorption process is endothermic, i.e. chemisorption. The values of  are positive, indicating that the increase in disorderly due to the replacement of water molecules from the MS surface.
are positive, indicating that the increase in disorderly due to the replacement of water molecules from the MS surface.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105
105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526
526![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061
061![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836
836![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920
920![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072
072![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071
071![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898
898![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915
915![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196
196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823
823![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 415
415 , entropy (ΔS*) and enthalpy (ΔH*)) parameters, as listed in Table 8. The correlation between temperature (T) and corrosion rate (k) is typically represented by the Arrhenius equation76 as follows:
, entropy (ΔS*) and enthalpy (ΔH*)) parameters, as listed in Table 8. The correlation between temperature (T) and corrosion rate (k) is typically represented by the Arrhenius equation76 as follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CR(k) = −log
CR(k) = −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − (Ea/2.303RT)
A − (Ea/2.303RT)
 = 89.76 kJ mol−1 for an uninhibited corrosive electrolyte and the values changed to 59.72, 59.21 and 55.37 kJ mol−1 for PhODB, BODB and DODB, respectively, at 7.50 × 10−4 molar concentration for all organic inhibitors. This change in
= 89.76 kJ mol−1 for an uninhibited corrosive electrolyte and the values changed to 59.72, 59.21 and 55.37 kJ mol−1 for PhODB, BODB and DODB, respectively, at 7.50 × 10−4 molar concentration for all organic inhibitors. This change in  numbers is a result of the chemisorption adsorption behavior of these inhibitors. The transition state equation can be represented as in the next equation:77
numbers is a result of the chemisorption adsorption behavior of these inhibitors. The transition state equation can be represented as in the next equation:77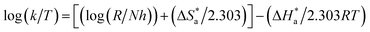
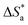 and
and  are the activation entropy and enthalpy, respectively. N is Avogadro's number (6.022 × 1023 mol−1), h is Planck's constant (6.626176 × 10−34 J s) and T is the temperature in kelvin (K). Fig. 12(b) shows plots of the transition state relation between (1/T) and log(k/T). The DODB compound has the lowest activation value among the investigated synthesized organic derivatives and because of this, it is suggested that it would be a more effective inhibitor for MS against an acidic electrolyte. According to thermodynamic and activation parameter results, the inhibition order is DODB > BODB > PhODB.
are the activation entropy and enthalpy, respectively. N is Avogadro's number (6.022 × 1023 mol−1), h is Planck's constant (6.626176 × 10−34 J s) and T is the temperature in kelvin (K). Fig. 12(b) shows plots of the transition state relation between (1/T) and log(k/T). The DODB compound has the lowest activation value among the investigated synthesized organic derivatives and because of this, it is suggested that it would be a more effective inhibitor for MS against an acidic electrolyte. According to thermodynamic and activation parameter results, the inhibition order is DODB > BODB > PhODB.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (cfu ml−1). For BODB, the test was completed after 7 days giving 325 (cfu ml−1) as the population value, with conversion of the severity of SRB to moderate instead of aggressive in the case of the blank sample. For both PhODB and DODB, the observed values were obtained after 8 days with a high effectiveness against SRB bacteria, giving 75 cfu ml−1 population (not aggressive), as listed in Table 10. The results clearly provide a valuable indication of a reduction in SRB reactivity. Furthermore, due to the biological activity of PhODB, BODB and DODB inhibitors, the corrosion resulting from the presence of SRB can be mitigated.
000 (cfu ml−1). For BODB, the test was completed after 7 days giving 325 (cfu ml−1) as the population value, with conversion of the severity of SRB to moderate instead of aggressive in the case of the blank sample. For both PhODB and DODB, the observed values were obtained after 8 days with a high effectiveness against SRB bacteria, giving 75 cfu ml−1 population (not aggressive), as listed in Table 10. The results clearly provide a valuable indication of a reduction in SRB reactivity. Furthermore, due to the biological activity of PhODB, BODB and DODB inhibitors, the corrosion resulting from the presence of SRB can be mitigated.


.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.