Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible-light-driven SAQS-catalyzed aerobic oxidative dehydrogenation of alkyl 2-phenylhydrazinecarboxylates†
Van Hieu Tranab and
Hee-Kwon Kim *ab
*ab
aDepartment of Nuclear Medicine, Molecular Imaging & Therapeutic Medicine Research Center, Jeonbuk National University Medical School and Hospital, Jeonju, 54907, Republic of Korea. E-mail: hkkim717@jbnu.ac.kr
bResearch Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, 54907, Republic of Korea
First published on 24th October 2022
Abstract
Azo compounds are useful molecules with a wide range of applications in organic chemistry. Here, a novel visible-light-driven oxidative dehydrogenation of alkyl 2-phenylhydrazinecarboxylates is used for the synthesis of azo compounds. This synthetic method was conducted under an aerobic environment with mild reaction conditions. Sodium anthraquinone sulfonate (SAQS) was employed as the crucial organic photocatalyst in a visible-light-driven reaction to generate various azo compounds in high yields. In addition, aerobic transformation of hydrazobenzenes to azobenzenes using visible light was successfully carried out under SAQS-mediated reaction conditions. This procedure is a practical and promising synthetic approach to produce useful azo compounds.
Introduction
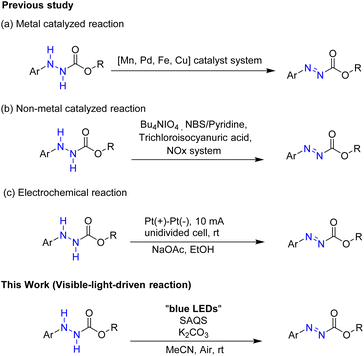
Azo compounds are widely used in materials science, medicinal chemistry, and organic chemistry. Azo compounds have been employed as pigments,1 dyes,2 metallochromic indicators,3 and medicinal agents.4Alkyl 2-phenylazocarboxylates, well known azo compounds with an alkoxy carbonyl group, have received significant attention for potential uses in organic chemistry. They have been used as a catalyst for the Mitsunobu reaction5,6 or as building blocks for synthetic chemistry.7 And several phenylazocarboxylate salts have participated in cycloaddition reactions. Additionally, alkyl 2-phenylazocarboxylates were also used for Mizoroki–Heck reactions.8,9
Various methods for synthesis of alkyl 2-phenylazocarboxylates have been reported (Scheme 1). They were produced from hydrazine derivatives via reactions utilizing various metal oxidants such as MnO2,10 Pb(OAc)4,11 Pd/C,12 and iron phthalocyanine13 or using an HZIF@TCPP-Fe/Fe system.14 Several reaction systems using copper have been developed to prepare alkyl 2-phenylazocarboxylate compounds: CuCl and DMAP,15 Cu(OAc)2·H2O and 1,10-Phen,16 and PdCl2/CuI.17
Besides, several non-metal catalytic systems using n-Bu4NIO4,18 NBS and pyridine,19 trichloroisocyanuric acid,20 and NOx 21 to afford alkyl 2-phenylazocarboxylates have been reported. In another method, electrochemical dehydrogenation of hydrazines was developed to give azo compounds.22
Visible-light-driven reaction systems have received significant attention as a promising strategy in organic synthesis due to high synthetic yields and wide tolerance of functional groups.23–29 Moreover, many visible-light-driven reactions are achieved under mild reaction conditions to produce desired products.30–32 Photocatalysts play an important role in visible-light-driven reaction systems to increase reaction efficiency.33 Besides, several photo-dehydrogenation reactions were reported.34–36
Anthraquinone (AQ) derivatives, which are useful components in medicines, were employed as organic catalysts in photo-redox or photooxygenation reactions to produce reactive oxygen species (ROS).37 In particular, sodium anthraquinone sulfonate (SAQS) has been effectively used as a beneficial catalyst for numerous chemical transformations such as oxidation of alcohols, asymmetric aldol reactions, and bromination.38–44
We are interested in development of highly efficient and practical synthetic procedures to prepare azo compounds such as alkyl 2-phenylazocarboxylates and reasoned that a reaction using visible light could be promising to achieve oxidation and dehydrogenation.
In this paper, we describe a novel visible-light-driven reaction using SAQS as an organo-photocatalyst to produce azo compounds under mild reaction conditions (Scheme 1).
Results and discussion
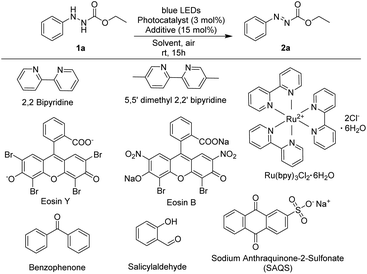
As shown in Table 1, ethyl 2-phenylhydrazine-1-carboxylate was used as a model substrate and the reaction was carried out in the presence of a photocatalyst (0.03 equiv.) and additive in MeCN (2 mL) in an aerobic environment under irradiation with blue LEDs.| Entry | Photocatalyst | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: compound 1a (1.0 mmol), photocatalyst (0.03 mmol), additive (0.15 mmol), solvent (2 mL), room temperature, irradiation with 5 W blue LEDs for 15 h.b Isolated yield after purification by flash column chromatography.c No reaction. | ||||
| 1 | — | K2CO3 | MeCN | NRc |
| 2 | 2,2-Bipyridine | K2CO3 | MeCN | 5 |
| 3 | 5.5′-Dimethyl | |||
| 2,2′-Bipyridine | K2CO3 | MeCN | 7 | |
| 4 | Ru(bpy)3Cl26H2O | K2CO3 | MeCN | 82 |
| 5 | Eosin Y | K2CO3 | MeCN | 78 |
| 6 | Eosin B | K2CO3 | MeCN | 83 |
| 7 | Benzophenone | K2CO3 | MeCN | 71 |
| 8 | Salicyladehyde | K2CO3 | MeCN | NRc |
| 9 | SAQS | K2CO3 | MeCN | 97 |
Several commercially available photocatalysts were screened to investigate their efficiency in the oxidation reaction (Table 1, entries 2–9). When 2,2-bipyridine or 5,5-dimethyl-2,2-bipyridine was utilized as a photocatalyst, the target product was produced in less than 10% yield (5–7%) (Table 1, entries 2 and 3). Reactions using photocatalysts such as Eosin Y, Ru(bpy)3Cl2·6H2O, benzophenone, and Eosin B produced the desired products in yields ranging from 71% to 85% (Table 1, entries 4–7) whereas utilization of salicylaldehyde did not produce the target product. However, in reaction using sodium anthraquinone sulfonate (SAQS), ethyl (E)-2-phenyldiazene-1-carboxylate, the desired product, was produced in 97% yield in 15 hours (Table 1, entry 9).
To optimize the reaction conditions, a variety of bases was utilized as additives. In the presence of DIPEA, Et3N, DMAP, and NaHCO3, reactions proceeded in low yields (4–34%) (Table S1,† entries 1–5). Cs2CO3 and DBU increased the reaction performance to yields of 65% and 76%, respectively (Table S1,† entries 6 and 7). Interestingly, the target product was generated in good yield in the absence of base. K2CO3 was a useful additive as the target product was obtained in 97% yield, suggesting that the efficiency of the reaction was significantly affected by K2CO3.
Second, solvent was a crucial factor influencing the efficiency of the reaction. Performing the reaction in toluene, DCE, CH2Cl2, or 1,4-dioxane led to ethyl (E)-2-phenyldiazene-1-carboxylate in less than 20% yield (Table S1,† entries 9–12). The reaction in DMF and THF yielded the target product in 61% and 65%, respectively (Table S1,† entries 13–14). Remarkably, MeCN was the optimal solvent for this oxidation process, with 97% reaction yield. Thus, MeCN was selected as the solvent for further experiments.
Next, the influence of light on the reaction was studied (Table S1,† entries 15–19). When the reaction was carried out in the dark, the target product was not formed. Green LEDs, white LEDs, and compact fluorescent lights (CFLs) accelerated the visible-light-driven process and showed yields of 65%, 72%, and 70%, respectively, which were less effective oxidation reactions than using blue LEDs (97%). This finding implies that blue LEDs are more suitable for such oxidation. Additionally, reaction under ambient conditions using sunlight was also conducted, and the target product was obtained in 88% yield (Table S1,† entry 19).
A number of reactions with varied amounts of photocatalyst and additive was carried out to optimize reaction conditions (Table S2†). Better reaction efficiency was observed when using 3 mol% SAQS (Table S2,† entries 1–5). Varying the amount of base revealed that the most efficient oxidation resulted when 15% K2CO3 was employed (Table S2,† entries 3, 6–11).
The substrate scope of oxidative dehydrogenation of arylhydrazides was investigated after optimal conditions were determined (Table 2). First, ethyl 2-phenylhydrazine-1-carboxylate was converted to ethyl (E)-2-phenyldiazene-1-carboxylate (2a) in high yield. Several ethyl (E)-2-aryldiazene-1-carboxylates with electron-donating groups including methyl, ethyl, and methoxy groups at the para position were successfully synthesized in high yields using the visible-light-driven procedure (2b–2d). The reaction method was also effective with substrates containing electron-withdrawing groups such as chloro, bromo, and nitro groups to yield the products (2e–2g). Furthermore, visible-light-driven oxidation of substrates with a substituent at the ortho or meta position occurred readily to produce the target products (2h and 2i). Using this procedure, di-substituted substrates with dimethyl or dichloro groups were successfully converted to the corresponding products in high yields (95% and 93%, respectively) (2j and 2k), and reactions of substrates containing hetero rings and naphthalene successfully generated the desired products (2l and 2m).
| a Reaction conditions: compound 1 (1.0 mmol), SAQS (0.03 mmol), K2CO3 (0.15 mmol), MeCN (2 mL), room temperature, irradiation with 5 W blue LEDs for 15 h. |
|---|
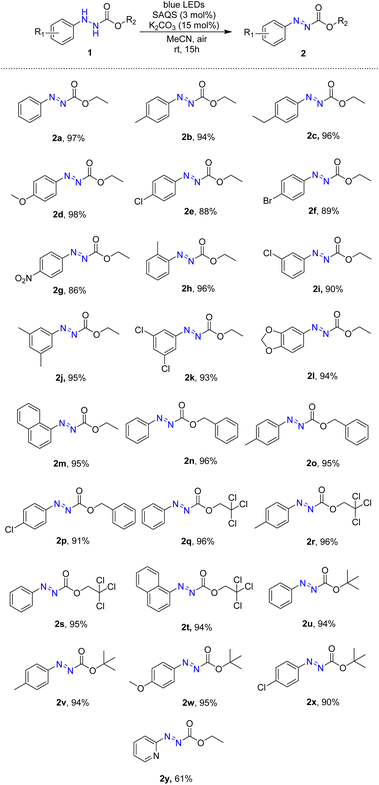 |
Various alkyl 2-phenylhydrazine-1-carboxylates containing benzyl, trichloroethyl, and tert-butyl groups were tested for this method. Under visible light, benzyl carboxylates of arylhydrazine were converted to the corresponding azo compounds in high yields (91–96%) (2n–2q). Oxidation with trichloroethyl carboxylate substrates resulted in generation of the corresponding products in 94–96% yields (2r–2t). Additionally, several tert-butyl azocarboxylates were synthesized in high yields via the visible-light-driven reaction (2u–2x). Heteroarene hydrazine carboxylates was also employed for the reaction, and the desired product was obtained in 61% yield.
In an extended application, this visible-light-driven reaction was employed in the preparation of azobenzene compounds. Reactions of hydrazobenzenes for preparation of azobenzene compounds were conducted based on the optimal reaction conditions using SAQS and K2CO3 under blue LED light. A series of substituted symmetrical and unsymmetrical hydrazobenzenes was tested for photocatalytic oxidative dehydrogenation, and these reactions provided efficient photocatalytic oxidation (Table 3).
| a Reaction conditions: compound 3 (1.0 mmol), SAQS (0.03 mmol), K2CO3 (0.15 mmol), MeCN (2 mL), room temperature, irradiation with 5 W blue LEDs for 15 h. |
|---|
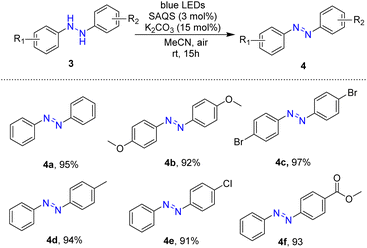 |
Symmetrical hydrazobenzenes containing various different electron-donating and electron-withdrawing substituents (methoxy and bromo groups) at the para-position of the phenyl rings were well tolerated in reactions, and the target products were obtained in high yields (4b and 4c). The reaction was also effective with unsymmetrical hydrazobenzenes with an electron-donating substituent such as a methyl group, affording the desired products in high yields (94%) (4d). Reactions of a substrate with halogen on the phenyl ring occurred smoothly, giving the desired product in 91% yield (4e). Synthesis of methyl (E)-4-(phenyldiazenyl)benzoate was achieved in 93% yield via the visible-light-driven reaction (4f).
In additions, reaction of benzyl protected phenylhydrazine, a alkyl-protected phenylhydrazine, was performed (Scheme 2), and the target product was successfully prepared in 87% yields (6a).
Next, a large-scale oxidative dehydrogenation of alkyl 2-phenylazocarboxylates was performed to demonstrate the utility of the procedure (Scheme 3). In reaction of ethyl 2-phenylhydrazine-1-carboxylate 1a (10.0 mmol, 1.81 g), the target product was obtained in 82% yield via the same reaction condition, suggesting that this reaction method can be scalable and practical.
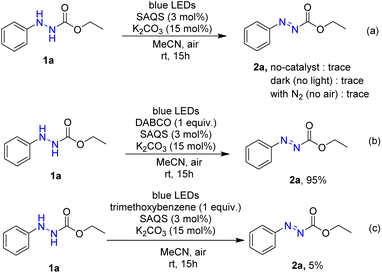
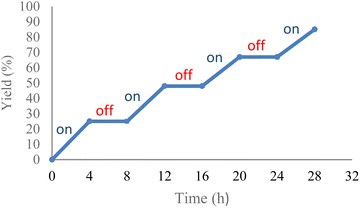
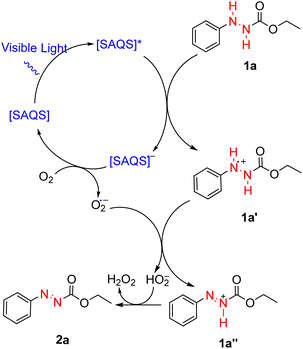
Several control experiments were carried out to better ascertain the reaction mechanism (Scheme 4). In the absence of photocatalyst, the product 2a was not synthesized. Under dark conditions, no conversion of 1a to product 2a was observed. Furthermore, a light turn on/turn off reaction was performed (Fig. 1) and indicated that light irradiation was a crucial factor for a successful transformation. The reaction was performed under N2, and product 2a was not produced, suggesting that oxygen affects the efficiency of the reaction. A singlet oxygen (1O2) quenching experiment was then conducted to determine whether this novel reaction followed an oxidative quenching mechanism. The singlet oxygen (1O2) quenching experiment was carried out under standard conditions with addition of 4-diazabicyclo[2.2.2]-octane (DABCO) as quencher to afford the target product in 95% yield. This suggests that 1O2 was not involved in the reaction pathway. The role of superoxide anion (O2˙−) was investigated by performing the reaction with trimethoxybenzene. In that reaction, only 5% product 2a was formed, which indicated that the reaction was inhibited by (O2˙−).
Based on the control experiments and literature findings,45,46 a possible reaction mechanism is proposed in Scheme 5. Under visible light, SAQS is converted into [SAQS]*, an excited state species. [SAQS]* undergoes single electron transfer (SET) with phenylhydrazine carboxylate (1a) to produce the anion radical [SAQS]˙− with concomitant generation of the radical of phenylhydrazine carboxylate (1a′). Further electron transfer between the anion radical [SAQS]˙− and oxygen leads to the formation of superoxide anion (O2˙−), which allows [SAQS]˙− to recover to its ground state. On the other hand, the superoxide anion abstracts one proton from (1a′) to generate the radical cation (1a′′) and peroxide radical. Finally, transfer of a proton results in conversion of the radical cation (1a′′) to the target product (2a) together with formation of H2O2.
Conclusions
In conclusion, the visible-light-driven, photocatalyzed oxidative dehydrogenation of phenylhydrazinecarboxylates for the synthesis of azo compounds was described. In this procedure, sodium anthraquinone sulfonate, an organic molecule, was employed as an efficient photocatalyst to produce the desired products. This procedure utilizes visible light and air, suggesting that it is environmentally friendly and practical. A variety of substrates with different functional groups was tolerated in the reaction, and target phenylazocarboxylates were successfully synthesized in high yields (up to 98%) under mild reaction conditions. The novel visible-light-driven, photocatalyzed oxidative dehydrogenation of phenylazocarboxylate was an efficient approach to the synthesis of valuable azo compounds.Data availability
The data supporting this study are available within the article and the ESI†.Author contributions
H.-K. K. conceived the study. V. H. T. and H.-K. K. conducted the experiments and analysed the data. V. H. T. and H.-K. K. prepared the manuscript. V. H. T. prepared the ESI.† All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF/2021R1A2C1011204).References
- E. Merino, Chem. Soc. Rev., 2011, 40, 3835–3853 RSC.
- N. DiCesare and J. R. Lakowicz, Org. Lett., 2001, 3, 3891–3893 CrossRef CAS PubMed.
- P. N. D. Ashutosh and J. K. Mehrotra, Colourage, 1979, 26, 25 Search PubMed.
- S. H. Lee, E. Moroz, B. Castagner and J. C. Leroux, J. Am. Chem. Soc., 2014, 136, 12868–12871 CrossRef CAS PubMed.
- D. Hirose, M. Gazvoda, J. Košmrlj and T. Taniguchi, Chem. Sci., 2016, 7, 5148–5159 RSC.
- J. A. Buonomo and C. C. Aldrich, Angew. Chem., 2015, 127, 13233–13236 CrossRef.
- H. Jasch, S. B. Höfling and M. R. Heinrich, J. Org. Chem., 2012, 77, 1520–1532 CrossRef CAS PubMed.
- S. K. Fehler, G. Pratsch and M. R. Heinrich, Angew. Chem., Int. Ed., 2014, 53, 11361–11365 CrossRef CAS PubMed.
- R. Lasch, S. K. Fehler and M. R. Heinrich, Org. Lett., 2016, 18, 1586–1589 CrossRef CAS PubMed.
- O. Tšubrik, R. Sillard and U. Mäeorg, Synthesis, 2006, 5, 843–846 Search PubMed.
- V. Srinivasan, D. J. Jebaratnam and D. E. Budil, J. Org. Chem., 1999, 64, 5644–5649 CrossRef CAS PubMed.
- M. A. Dekeyser, P. T. McDonald and G. W. Angle Jr, J. Agric. Food Chem., 1995, 43, 1705–1707 CrossRef CAS.
- T. Hashimoto, D. Hirose and T. Taniguchi, Adv. Synth. Catal., 2015, 357, 3346–3352 CrossRef CAS.
- D. Zhang, J. Liu, P. Du, Z. Zhang, X. Ning, Y. Deng, D. Yin, J. Chen, Z. Han and X. Lu, Small, 2020, 16, 1905889 CrossRef CAS PubMed.
- M. H. Kim and J. Kim, J. Org. Chem., 2018, 83, 1673–1679 CrossRef CAS PubMed.
- J. Q. Zhang, G. B. Huang, J. Weng, G. Lu and A. S. Chan, Org. Biomol. Chem., 2015, 13, 2055–2063 RSC.
- J. Q. Zhang, J. Cao, W. Li, S. M. Li, Y. K. Li, J. T. Wang and L. Tang, New J. Chem., 2017, 41, 437–441 RSC.
- C. L. Molina, C. P. Chow and K. J. Shea, J. Org. Chem., 2007, 72, 6816–6823 CrossRef CAS PubMed.
- M. Zhu and N. Zheng, Synthesis, 2011, 14, 2223–2236 Search PubMed.
- Y. Su, X. Liu, J. Yu, G. Cao, R. Zhang, Y. Zhao, D. Huang, K. H. Wang, C. Huo and Y. Hu, Synthesis, 2020, 52, 1103–1112 CrossRef CAS.
- G. Jo, M. H. Kim and J. Kim, Org. Chem. Front., 2020, 7, 834–839 RSC.
- K. S. Du and J. M. Huang, Green Chem., 2019, 21, 1680–1685 RSC.
- D. A. Nicewicz and D. W. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- C. K. Prier, D. A. Rankic and D. W. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- T. T. Bui, V. H. Tran and H.-K. Kim, Adv. Synth. Catal., 2022, 364, 341–347 CrossRef CAS.
- K. C. Aganda and A. Lee, Adv. Synth. Catal., 2021, 363, 5149–5154 CrossRef CAS.
- T. T. Bui and H.-K. Kim, Chem.–Asian J., 2021, 16, 2155–2167 CrossRef CAS PubMed.
- L. T. Giang, T. T. Bui and H.-K. Kim, RSC Adv., 2022, 12, 17499–17504 RSC.
- J. Park and D. Y. Kim, Bull. Korean Chem. Soc., 2022, 43, 941–945 CrossRef CAS.
- T. T. Bui, W. P. Hong and H.-K. Kim, J. Fluorine Chem., 2021, 247, 109794 CrossRef CAS.
- L. T. Giang, Y. Jung and H.-K. Kim, Molecules, 2021, 26, 7380 CrossRef PubMed.
- C. Mejuto, L. Ibáñez-Ibáñez, G. Guisado-Barrios and J. A. Mat, ACS Catal., 2022, 12, 6238–6245 CrossRef CAS PubMed.
- J.-J. Zhong, W.-P. To, Y. Liu, W. Lu and C.-M. Che, Chem. Sci., 2019, 10, 4883–4889 RSC.
- K. Yu, H. Zhang, C. Su and Y. Zhu, Eur. J. Org. Chem., 2020, 2020, 1956–1960 CrossRef CAS.
- J. Cervantes-González, D. A. Vosburg, S. E. Mora-Rodriguez, M. A. Vázquez, L. G. Zepeda, C. Villegas Gomez and S. Lagunas-Rivera, ChemCatChem, 2020, 12, 3811–3827 CrossRef.
- C. F. Wells, J. Chem. Soc., 1961, 57, 1703–1718 Search PubMed.
- C. F. Wells, J. Chem. Soc., 1961, 57, 1719–1731 CAS.
- K. P. Clark and H. I. Stonehill, J. Chem. Soc., 1972, 68, 577–590 CAS.
- K. P. Clark and H. I. Stonehill, J. Chem. Soc., 1972, 68, 1676–1686 CAS.
- W. Zhang, J. Gacs, I. W. Arends and F. Hollmann, ChemCatChem, 2017, 9, 3821–3826 CrossRef CAS PubMed.
- A. Fujiya, T. Nobuta, E. Yamaguchi, N. Tada, T. Miura and A. Itoh, RSC Adv., 2015, 5, 39539–39543 RSC.
- D. Petzold and B. König, Adv. Synth. Catal., 2018, 360, 626–630 CrossRef CAS.
- X. Wang, X. Wang, C. Xia and L. Wu, Green Chem., 2019, 21, 4189–4193 RSC.
- H. Lv, R. D. Laishram, J. Li, Y. Zhou, D. Xu, S. More, Y. Dai and B. Fan, Green Chem., 2019, 21, 4055–4061 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05842a |
| This journal is © The Royal Society of Chemistry 2022 |