Open Access Article
Open Access ArticlePreparation and electrical properties of inorganic electride [Y2Ti2O6]2+(2e−)
Yong Liuabc,
Hengwei Weib,
Xiaoming Wang b,
Huan Jiao
b,
Huan Jiao *b and
Xiping Jing*c
*b and
Xiping Jing*c
aThe Guyuan Museum of Ningxia, Guyuan 756000, P. R. China
bKey Laboratory of Macromolecular Science of Shaanxi Province, College of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, P. R. China. E-mail: jiaohuan@snnu.edu.cn
cThe State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P. R. China. E-mail: xpjing@pku.edu.cn
First published on 5th October 2022
Abstract
Oxygen-depleted samples [Y2Ti2O7−x]2x+(2xe−) (0 ≤ x ≤ 1.0) were prepared by reducing Y2TiO7 powders at 500 °C to 650 °C using CaH2 as a reductive agent, where x represents the content of 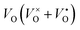 , which was determined by thermogravimetric analysis. Powder X-ray diffraction patterns illustrate that the pure pyrochlore phase is kept for the samples with x ≤ 1.0, whereas the apparent x values surpass 1.0, and the impurity phase Y2O3 appears. The electride [Y2Ti2O7−x]2x+(2xe−) (x ≈ 1.0) can be obtained under a reductive condition, in which the concentration of VO is 7.75 × 1021 cm−3. The electron paramagnetic resonance measurements gave the concentration of unpaired electrons in the electride as 1.30 × 1021 cm−3, indicating that the degree of the ionization of
, which was determined by thermogravimetric analysis. Powder X-ray diffraction patterns illustrate that the pure pyrochlore phase is kept for the samples with x ≤ 1.0, whereas the apparent x values surpass 1.0, and the impurity phase Y2O3 appears. The electride [Y2Ti2O7−x]2x+(2xe−) (x ≈ 1.0) can be obtained under a reductive condition, in which the concentration of VO is 7.75 × 1021 cm−3. The electron paramagnetic resonance measurements gave the concentration of unpaired electrons in the electride as 1.30 × 1021 cm−3, indicating that the degree of the ionization of 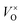 is less than 10%. Conductivity measurements for a sintered pellet sample (relative density ∼ 70%) indicate that the electride has quite high conductivity (∼1.09 S cm−1 at 300 K). The conduction was interpreted by using the variable range hopping mechanisms.
is less than 10%. Conductivity measurements for a sintered pellet sample (relative density ∼ 70%) indicate that the electride has quite high conductivity (∼1.09 S cm−1 at 300 K). The conduction was interpreted by using the variable range hopping mechanisms.
Introduction
Electrides are a kind of material in which electrons serve as anions.1 It was found that electrides were formed when the alkali metal and alkali earth metals were dissolved into liquid ammonia at low temperatures. As an example, when the metal Na is dissolved into liquid ammonia, the Na atoms are ionized and form solvated Na+ [Na(am)n+, am = ammonia] as cations. At the same time, the generated electrons are also solvated [e(am)m−], which play the role of anions. The reactions between alkali metals and crown ethers also lead to the formation of electrides. In the crystal of Cs(18crown-6)2 complex, Cs+ is coordinated by two 18crown-6, forming [Cs(18crown-6)2]+ as cations, while the “electron-anions” generated from the ionization of the Cs atoms take the interstitial positions constructed by the [Cs(18crown-6)2]+ cations. Due to the existence of the solvated electrons, the electrides are very strongly reductive agents.1 However, these electrides are only stable at low temperatures and very sensitive to air and moisture. Thus, their applications are impeded.Recently, Matsushi et al. reported an inorganic solid electride–fully reduced mayenite [Ca12Al14O32]2+(2e−),2 which is stable at ambient temperature and atmosphere. The original composition of mayenite can be represented as Ca12Al14O33, which is a neutral material. This material has a tetragonal unit cell, containing two molecules (Ca12Al14O33 × 2). The lattice framework is constructed by [Ca12Al14O33 × 2]4+ with the positive charge composing 12 cages, 2 relatively loosely bonded O2− take the positions in 2 out of 12 cages.2,3 When the material is thermally treated in a reductive atmosphere, part of the loosely bonded O2− ions may escape as O2 from the lattice and leave electrons in the cages, considered as the electron captured oxygen vacancy 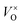 , or F-center. With the proper reductive condition, all of the loosely bonded O2− ions can be removed from the lattice. In this case, the relatively free electrons captured at
, or F-center. With the proper reductive condition, all of the loosely bonded O2− ions can be removed from the lattice. In this case, the relatively free electrons captured at 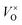 play the role of anions. Thus the material is considered as the electride. The concentration of the “free electrons” is high in the lattice. Therefore, the electride has high conductivity. Since some of the “free electrons” are unpaired, the magnetic properties are also attractive. The inorganic electrides would be potentially used as reductive agents, electron-emitters, etc2,4. Additionally, it was also reported5,6 that these materials were applied as catalysts for the NH3 synthesis and CO oxidation. The finding of the inorganic solid electride [Ca12Al14O32]2+(2e−) stimulates the research interest in exploring more inorganic solid electrides.
play the role of anions. Thus the material is considered as the electride. The concentration of the “free electrons” is high in the lattice. Therefore, the electride has high conductivity. Since some of the “free electrons” are unpaired, the magnetic properties are also attractive. The inorganic electrides would be potentially used as reductive agents, electron-emitters, etc2,4. Additionally, it was also reported5,6 that these materials were applied as catalysts for the NH3 synthesis and CO oxidation. The finding of the inorganic solid electride [Ca12Al14O32]2+(2e−) stimulates the research interest in exploring more inorganic solid electrides.
In this work, we choose Y2Ti2O7 with the pyrochlore structure as a starting material to prepare electrides. As we know, Ti4+ ions in titanates have the potential to be reduced into Ti3+ when they are treated at high temperatures or in reductive atmospheres. At the same time, some O2+ ions may escape from the lattice as O2, leaving the oxygen vacancies VO (including 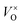 and
and 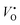 ) in the lattice.7,8 The materials containing VO with relatively high concentrations are named oxygen-depleted (simplified as O-depleted) materials. The composition of the O-depleted Y2Ti2O7 can be represented as Y2Ti2O7−x. Additionally, the pyrochlore structure, Y2Ti2O7 has one oxygen not bonded to Ti atoms. Thus it might be possible to prepare the O-depleted Y2Ti2O7 with a high concentration of VO, or even let the x value approach 1.0, with which the material would be considered as an electride.
) in the lattice.7,8 The materials containing VO with relatively high concentrations are named oxygen-depleted (simplified as O-depleted) materials. The composition of the O-depleted Y2Ti2O7 can be represented as Y2Ti2O7−x. Additionally, the pyrochlore structure, Y2Ti2O7 has one oxygen not bonded to Ti atoms. Thus it might be possible to prepare the O-depleted Y2Ti2O7 with a high concentration of VO, or even let the x value approach 1.0, with which the material would be considered as an electride.
Actually, the research to prepare the O-depleted pyrochlore could be found in the 1960s. In Goodenough's work,9 the Pb-noble metal pyrochlores (Pb2M2O7−x, M = Ru, Ir, Re) with O-depletion were prepared, and the materials with x = 1.0 were obtained. However, in this work, the concept of electride was not mentioned, and the functional properties (i.e. conductive or magnetic properties) were not studied. The research for the O-depleted Y2Ti2O7 (Y2Ti2O7−x) appeared in this century,10,11 in which the materials with x ≈ 1.0 were prepared, and the conductivities and magnetic susceptibilities were measured. It is a pity that the concept of the electride was not applied to interpret the work either. Therefore, further research on the O-depleted Y2Ti2O7 is imperative.
In this work, the reductive conditions were explored for preparing the O-depleted Y2Ti2O7, and the electride with the composition Y2Ti2O6 (x ≈ 1.0) was obtained, which would be represented as [Y2Ti2O6]2+(2e−). The contents of VO and the concentration of unpaired electrons in the lattice were measured. The conductive properties of the electride Y2Ti2O6 were characterized.
2 Experimental
Powder samples of the starting material Y2Ti2O7 were synthesized by solid-state reactions from stoichiometric mixtures of the raw materials TiO2 (A. R.) and Y2O3 (99.99%) at 1400 °C for 13 h in air. Prior to heating, the raw materials were thoroughly mixed by grindings in an agate mortar with a pestle. As-prepared powder samples of Y2Ti2O7 have a white (or pale) body color.The powder samples of the O-depleted Y2Ti2O7 were prepared from the powder of Y2Ti2O7 by using the solid reducing approach, in which the solid reductive agent CaH2 was applied. The powder mixtures of Y2Ti2O7 (∼0.5 g) and CaH2 in various mass ratios were ground in a glovebox (Mikrouns, Ar atmosphere, O2 < 1 ppm, H2O < 1 ppm), and then sealed in vacuumed quartz ampoules (∼5 ml). The sealed samples were heated at various temperatures ranging from 500–650 °C with various time 5–40 h. After heating, crash the quartz ampoules and take the samples out. The samples were washed using deionized water for several times until the supernatant approached to neutral in order to remove Ca2+ and OH− ions. Finally, the washed samples were dried at 140 °C for 2 h in an oven. The reduced treatments lead to the samples becoming dark.
For conductivity measurements, the pellets of the O-depleted samples were sintered. The powder samples were mixed with a few drops of 5% polyvinyl alcohol solution as a binder and then were pressed into pellets with 10 MPa pressure in stainless steel die. The pellets were sealed in the vacuumed quartz ampoules and sintered at 950 °C for a short time ∼2 h, in order to avoid a large change in the sample status. The obtained pellets had the dimension ∼1.0 cm in diameter and ∼0.15 cm in thickness with a relative density of ∼70%.
The phase purity of samples was characterized by X-ray diffraction (XRD) using a Rigaku Dmax2000 X-ray powder diffractometer (Japan) with Cu Kα radiation (λ = 0.15418 nm) at 40 kV and 100 mA. The XRD patterns were collected in the 2θ range 10° ∼ 80° with the scanning rate of 8° min−1. Electron paramagnetic resonance (EPR) measurements were performed at ∼9.4 GHz (X-band) using a Bruker A300 10-12 EPR spectrometer (Germany). The concentrations of unpaired electrons in the samples were determined by using the method supplied in ref. 12, in which the areas of the microwave absorption bands were calculated by twice integrations of the original EPR spectra (“dA/dH–H′′ plots, A is the microwave absorption of the samples and H is the strength of the magnetic field), and CuSO4·5H2O, diluted by Al2O3 powders with the mass ratio CuSO4·5H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3 = 1
Al2O3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, was applied as a standard. Both the XRD and EPR measurements were carried out at room temperature. Thermal analyses were conducted on a simultaneous differential scanning calorimeter–thermogravimetric analyzer (DSC-TGA, Q600STD, Thermal Analysis, USA). The data were collected from room temperature to 1200 °C with the temperature increasing rate of 20 °C min−1 and air as flowing gas.
9, was applied as a standard. Both the XRD and EPR measurements were carried out at room temperature. Thermal analyses were conducted on a simultaneous differential scanning calorimeter–thermogravimetric analyzer (DSC-TGA, Q600STD, Thermal Analysis, USA). The data were collected from room temperature to 1200 °C with the temperature increasing rate of 20 °C min−1 and air as flowing gas.
The conductivities of the sintered pellets were measured in the temperature range 80–450 K using a four-wire setup, and Pt wires were cemented by Ag paste on the two opposite surfaces of the pellet samples. The temperature environment was provided by a closed cycle refrigerator (Janis VPF-100, UAS) using the liquid N2 as the refrigerant. The temperature was regulated by a M331 temperature controller (LakeShore, USA). A SB118 current source and a PZ158A voltmeter (Shanghai Qianfeng Electronic Instruments, China) were used for data collection.
3 Results and discussion
3.1 Description of the Y2Ti2O7 structure
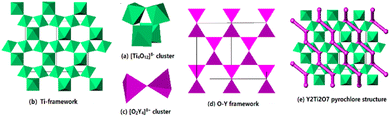
Y2Ti2O7 takes a pyrochlore structure with the face-center cubic unit (a = 1.00979 nm) and the space group Fd3m (no. 227), which contains 8 Y2Ti2O7.13 The structure is constructed by Ti–O framework and O–Y framework. In the Ti–O framework, the [Ti4O18]20− cluster is the framework unit, in which each TiO6 octahedron shares its three corners with other 3 TiO6 octahedrons [Fig. 1(a)]. Each [Ti4O18]20− cluster shares its 12 outer corners with the other 8 [Ti4O18]20− clusters, extending to 3-dimensional framework. The average composition of the Ti–O framework is [Ti4O12]. The [Ti4O12] framework has 3-member rings, denoted as [T], and 6-member rings, denoted as [H] viewing along [110] direction. The number ratio [T]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [H] is 2
[H] is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Fig. 1(b)]. In the O–Y framework, 2 OY4 tetrahedrons connecting each other by sharing one corner construct the [O2Y7]17+ cluster as the framework unit [Fig. 1(c)]. Each [O2Y7]17+ cluster contributes its six outer corners to link other 6 [O2Y4]8+ clusters, forming 3D framework [Fig. 1(d)]. The average composition of the O–Y network is [O2Y4]8+. The Ti–O framework and the O–Y framework interpenetrate each other, building the 3-dimensional pyrochlore structure, in which the O–Y framework extends through the [H] rings of the Ti–O framework and the [T] rings are left empty [Fig. 1(e)]. The structure data indicated in the formula Y2Ti2O7, 6 O link to Ti, constructing the TiO6 octahedron and 1 O links to Y, taking the site at the center of the OY4 tetrahedron. In the 1970s, some authors14–16 calculated the electrostatic energies of the [Ti4O12] and [O2Y4] frameworks, indicating that the former is much higher than the later. Therefore, it might be deduced that the oxygen atoms in the OY4 tetrahedrons are easier to be removed out from the lattice than the oxygen atoms in the TiO6 octahedrons. If Y2Ti2O7 is thermally treated in a reductive atmosphere, and the oxygen vacancies VO tend to take the sites at the centers in the OY4 tetrahedrons.
1 [Fig. 1(b)]. In the O–Y framework, 2 OY4 tetrahedrons connecting each other by sharing one corner construct the [O2Y7]17+ cluster as the framework unit [Fig. 1(c)]. Each [O2Y7]17+ cluster contributes its six outer corners to link other 6 [O2Y4]8+ clusters, forming 3D framework [Fig. 1(d)]. The average composition of the O–Y network is [O2Y4]8+. The Ti–O framework and the O–Y framework interpenetrate each other, building the 3-dimensional pyrochlore structure, in which the O–Y framework extends through the [H] rings of the Ti–O framework and the [T] rings are left empty [Fig. 1(e)]. The structure data indicated in the formula Y2Ti2O7, 6 O link to Ti, constructing the TiO6 octahedron and 1 O links to Y, taking the site at the center of the OY4 tetrahedron. In the 1970s, some authors14–16 calculated the electrostatic energies of the [Ti4O12] and [O2Y4] frameworks, indicating that the former is much higher than the later. Therefore, it might be deduced that the oxygen atoms in the OY4 tetrahedrons are easier to be removed out from the lattice than the oxygen atoms in the TiO6 octahedrons. If Y2Ti2O7 is thermally treated in a reductive atmosphere, and the oxygen vacancies VO tend to take the sites at the centers in the OY4 tetrahedrons.
3.2 Investigation of the reductive conditions
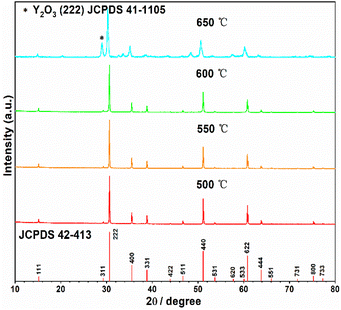
The starting material Y2Ti2O7 was prepared by the routine solid state reactions, which was described in the “experimental” section. The XRD pattern of the as-prepared sample shown in Fig. 2 illustrates that the pattern matches well to the related JCPDS file 42-413. Thus, the as-prepared Y2Ti2O7 is a pure phase sample. In order to change the starting material to the O-depleted material or the electride, thermally reducing treatments are necessary for Y2Ti2O7. The reducing process was also described in the “experimental” section. For obtaining high content of VO, the reductive conditions should be chosen as strong as possible, but the phase purity should be kept.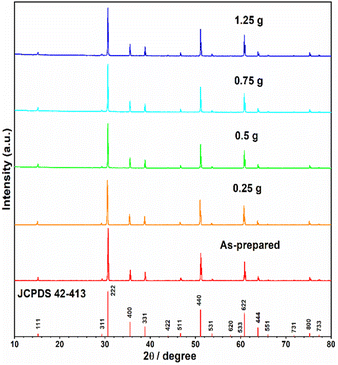 | ||
| Fig. 2 XRD patterns of JCPDS file (42-413), the as-prepared samples as well as the samples (0.5 g) mixed with various weights of CaH2 annealed at 500 °C for 16 h in vacuumed quartz ampoules. | ||
Firstly, the influence of the mass of CaH2 to the phase purity was investigated. The Y2Ti2O7 powder samples (0.5 g for each) were mixed with various amounts of CaH2 (0.25 g–1.25 g), and annealed at 500 °C for 16 h. After the reducing treatment, the white powders of Y2Ti2O7 became dark, which can be explained by the defect reactions and chemical reactions. When Y2Ti2O7 is annealed at a high temperature (e.g. above 500 °C), the defect reaction (1) occurs, and the oxygen vacancies 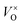 are produced:
are produced:
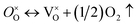 | (1) |
In the ambient atmosphere, eqn (1) only shifts to right slightly, and the 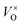 content is not very high. In this research, the solid reductive agent CaH2 was added, thus the below chemical reaction was involved in the process and O2 produced in eqn (1) was reduced by CaH2:
content is not very high. In this research, the solid reductive agent CaH2 was added, thus the below chemical reaction was involved in the process and O2 produced in eqn (1) was reduced by CaH2:
| O2 + CaH2 → Ca(OH)2 | (2) |
Thus the addition of CaH2 lead to right-shift of eqn (1) in a large extent and the 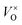 content increased significantly. In this research, it is respected that 1 mole O atoms should be removed, and 1 mole
content increased significantly. In this research, it is respected that 1 mole O atoms should be removed, and 1 mole 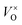 should be produced. In addition,
should be produced. In addition, 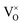 may be ionized and transfer an electron to
may be ionized and transfer an electron to 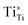 :
:
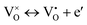 | (3) |
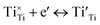 | (4) |
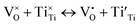 | (5) |
For brief discussion, the second step of the ionization 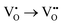 is omitted. Since all three kinds of defects
is omitted. Since all three kinds of defects 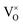 ,
, 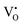 and
and 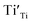 have loosely bound electrons, the levels of which may be located in the band gap below the conduction band. These loosely bound electrons may be excited to the conduction band by the visible light, resulting in the dark body color of the reduced samples.
have loosely bound electrons, the levels of which may be located in the band gap below the conduction band. These loosely bound electrons may be excited to the conduction band by the visible light, resulting in the dark body color of the reduced samples.
The XRD patterns of the samples thermally treated with various amounts of CaH2 are shown in Fig. 2. The patterns of all the samples are in good agreement with that of JCPDS file 42-413 and no clear peaks related to any impurity phases were observed, thus, all the reduced samples are phase pure, which indicated that the mass of CaH2 was not very sensitive to the phase purity of the reduced samples and the CaH2 weights used in this work were all suitable. However, in order to keep a relatively strong reductive conditions, the CaH2 weight of 0.75 g was chosen for the further work.
Then optimized reducing temperature was investigated. Several powder samples of Y2Ti2O7 (0.5 g for each) mixed with 0.75 g CaH2 were annealed at various temperatures (500 °C–650 °C). The XRD patterns represented in Fig. 3 indicate that if the reducing temperature is below 600 °C, the reduced samples are phase pure; whereas if it is above 650 °C, impurity phase of Y2O3 appears. The suitable temperature was decided as 600 °C for the reducing annealing.
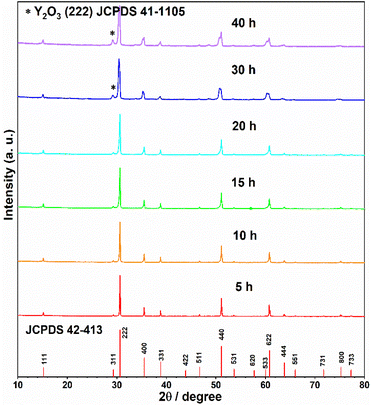
Furthermore, the influence of annealing time to the phase purity was explored. The powder samples of Y2Ti2O7 (0.5 g for each) mixed with 0.75 g CaH2 were annealed at 600 °C for various time (5–40 h). The XRD patterns (in Fig. 4) clearly shows that the reducing time 20 h may be considered as an optimized time. If the reducing time surpasses 20 h, the peaks of the impurity phase Y2O3 appear.
In summary, to prepare electrides or O-depleted samples, the suitable reductive conditions are decided: the starting material Y2Ti2O7 0.5 g mixed with the reductive agent CaH2 0.75 g is annealed at 600 °C for 20 h.
3.3 Measurements of the content of the oxygen vacancies VO
In 3.2 section, it was deduced that after reducing treatment, the oxygen vacancies VO (including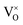 and
and 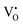 ) may be formed. In this section, the existence of VO would be proved by TG measurements.
) may be formed. In this section, the existence of VO would be proved by TG measurements.
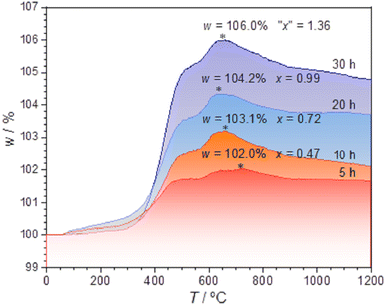
The TG measurements (Fig. 5) were conducted for the samples previously annealed with 0.75 g CaH2 at 600 °C for various time, 5–40 h. The TG data indicate that with the increase of the temperature from ∼320–∼620 °C, the weights of the samples enhance clearly and the weight increments are about several percent. The samples previously annealed longer time in the reductive conditions have larger increments.
In 3.2 section, it was deduced that after reduction by CaH2, the samples have quit high contents of VO. In this case, the composition of the material can be represented by the formula Y2Ti2O7−x. The x represents the content of VO in the lattice. The reaction that occurred during TG measurements can be represented as:
| Y2Ti2O7−x + (x/2) O2 → Y2Ti2O7 | (6) |
Eqn (6) means that during TG measurements, the VO−contained samples are oxidized by O2 and the O atoms enter the lattice filling the oxygen vacancies VO, leading to the weight increments, i.e. Eqn (1) shifts to left. The larger the x value, the larger the weight increment is. According to the values of the weight increments, the x values (the VO contents) can be calculated, showed in Fig. 5. For the sample previously annealed with CaH2 for 5 h, the VO content x is 0.47. With an increase of the annealing time, the x value increases. When the annealing time increases to 20 h, the x value approaches 1.0, which illustrates that one mole of oxygen atoms are removed out from the lattice, and one mole VO is formed. As we know, 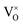 has two loosely bound electrons (maybe, some of them are ionized and transferred to the
has two loosely bound electrons (maybe, some of them are ionized and transferred to the 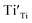 sites, but the total number of the loosely bound electrons are not changed), which may play the role of the anions. Thus this sample is considered as an electride, the composition of which can be represented as [Y2Ti2O6]2+(2e−). For the sample with annealing time 30 h, the apparent “x” value is 1.36 (over 1.0). However, the XRD data (Fig. 4) indicate that this sample is over reduced and is not phase pure, thus this “x” value is insignificance. The TG curves also show that the samples have slight weight loss above 620 °C. It is mostly possible that eqn (1) slightly shifts to right at higher temperatures since it is an entropy increasing reaction (solid O becomes gaseous O).
sites, but the total number of the loosely bound electrons are not changed), which may play the role of the anions. Thus this sample is considered as an electride, the composition of which can be represented as [Y2Ti2O6]2+(2e−). For the sample with annealing time 30 h, the apparent “x” value is 1.36 (over 1.0). However, the XRD data (Fig. 4) indicate that this sample is over reduced and is not phase pure, thus this “x” value is insignificance. The TG curves also show that the samples have slight weight loss above 620 °C. It is mostly possible that eqn (1) slightly shifts to right at higher temperatures since it is an entropy increasing reaction (solid O becomes gaseous O).
There is another possibility that the reducing treatments does not produce VO, but the loss of oxygen leads to the reduction of Ti4+ to Ti3+. If this is the case, for the sample x = 1, 1 O was lost, which would result in 2 Ti4+ were reduced to Ti3+. In other words, in this sample, all Ti were reduced from Ti4+ to Ti3+. XRD pattern (Fig. 4) revealed this sample has pure phase of pyrochlore. It could not be imaged that Ti3+ compound could have the same structure as Ti4+ compound. Therefore, it is believed that the reducing treatments produce 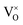 in the lattice, rather than reduce Ti4+ to Ti3+. It is known that the ionization of
in the lattice, rather than reduce Ti4+ to Ti3+. It is known that the ionization of 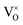 may produce Ti3+, described in eqn (4) or (5), but only small amount of Ti4+ can be reduced (see 3.4 section), thus the pyrochlore structure can be kept. Actually, ref. 10 and 11 support the formation of VO in the lattice after reducing treatments.
may produce Ti3+, described in eqn (4) or (5), but only small amount of Ti4+ can be reduced (see 3.4 section), thus the pyrochlore structure can be kept. Actually, ref. 10 and 11 support the formation of VO in the lattice after reducing treatments.
In 3.1 section, it was described that the Y2Ti2O7 structure was constructed by two frameworks of [Ti4O12] and [O2Y4] interpenetrated each other. Theoretic calculations14–16 indicated that the electrostatic energy of the [Ti4O12]8− cluster was much larger than that of [O2Y4]8+. Thus, it is deduced that the oxygen vacancies VO tend to be formed by removing the oxygen atoms out from the center of the OY4 tetrahedrons. Goff, et al.10 conducted the structure refinement of the O-depleted Y2Ti2O6.79 based on the neutron diffraction data. The site occupancies also indicated that the oxygen vacancies VO are prefer to stay at the centers of the OY4 tetrahedrons. As we know, in Y2Ti2O7, 6 O link Ti and only 1 O links Y. For the sample with the value x ≈ 1.0, all the oxygens at the centers of the OY4 tetrahedrons were removed out.
3.4 Measurements of the concentration of the unpaired electrons
In 3.2 section, it was presumed that once the oxygen vacancies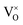 were formed in the lattice, they would be ionized and transfer electrons to Ti, represented by eqn (3) and (4). In this case, two kinds of defects
were formed in the lattice, they would be ionized and transfer electrons to Ti, represented by eqn (3) and (4). In this case, two kinds of defects 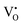 and
and 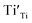 would exist, each of which has one unpaired electron. These defects can be characterized by using EPR.
would exist, each of which has one unpaired electron. These defects can be characterized by using EPR.
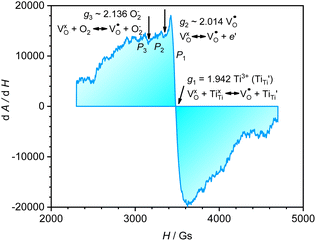
A selected EPR spectrum for the sample annealed with 0.75 g CaH2 at 600 °C for 20 h is illustrated Fig. 6. The composition of the sample in Fig. 6 can be represented as Y2Ti2O7−x (x ≈ 1.0). This formula indicates that the sample has 1 mole of VO in the lattice, which is a quite high concentration of the defect. The high concentration of VO leads to lattice distortion, further it causes the broadening, deformation and overlapping of the EPR peaks, thus it is not easy to give clear assignments for this spectrum. Here, we would like to consider the peaks P1 to P3. Refer to the assignments given in ref. 17–20 for some titania and titanates, the peak with g < 2.0 (P2) is assigned to 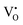 ; the peak with g < 2.0 (P1) is assigned to Ti3+
; the peak with g < 2.0 (P1) is assigned to Ti3+ 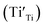 . In fact, besides the electron transferring processes described in eqn (3)–(5), in the lattice, there are other electron transferring processes, e.g. the electrons transfer to the O2 absorbed on the surface:
. In fact, besides the electron transferring processes described in eqn (3)–(5), in the lattice, there are other electron transferring processes, e.g. the electrons transfer to the O2 absorbed on the surface:
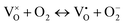 | (7) |
P3 has a g value larger than 2.0, which may be assigned to O2−. Actually, there are some peaks with poor resolution at lower magnetic fields, which might be related to some unknown defects containing unpair electrons, but they could not be assigned at this stage. The assignments for the above EPR peaks support the discussions in 3.2 and 3.2 sections about the formation of VO as well as the electron transfer processes, thus they also support that the sample related to Fig. 6 (Y2Ti2O7−x, x ≈ 1.0) is an electride.
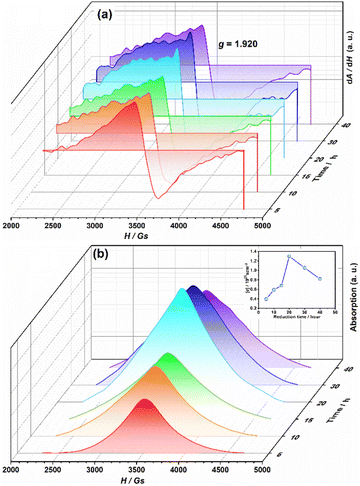
A series EPR spectra were collected for the samples annealed with 0.75 g CaH2 at 600 °C for various times, i. e. for the samples Y2Ti2O7−x with various values of x, represented in Fig. 7(a). The spectra in Fig. 7(a) have a similar profile to that in Fig. 6, which means these samples have the defects 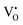 ,
, 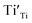 and O2−. By using these EPR data, the concentrations of the unpaired electrons varied with annealing time (or x) can be calculated. The calculation equation was recommended by Weil, et al.12 In these calculations, CuSO4·5H2O powder (mixed with Al2O3 in the mass ratio WCuSO4·H2O
and O2−. By using these EPR data, the concentrations of the unpaired electrons varied with annealing time (or x) can be calculated. The calculation equation was recommended by Weil, et al.12 In these calculations, CuSO4·5H2O powder (mixed with Al2O3 in the mass ratio WCuSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WAl2O3 = 1
WAl2O3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was used as the standard. The EPR spectra for both the samples and the standard were collected in the same measuring conditions so as the parameters related to the instrument set-up, the signal amplification, and the scanning scale in the equation can be reduced. On the other hand, all of the defects
9) was used as the standard. The EPR spectra for both the samples and the standard were collected in the same measuring conditions so as the parameters related to the instrument set-up, the signal amplification, and the scanning scale in the equation can be reduced. On the other hand, all of the defects 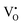 and
and 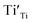 , as well as Cu2+ in the standard are the one-unpaired-electron centers and they all have the total spin quantum number S = 1/2, thus the quantum number S also can be eliminated from the equation. The simplified equation for calculating the concentration of the unpaired electrons [e] can be represented as:
, as well as Cu2+ in the standard are the one-unpaired-electron centers and they all have the total spin quantum number S = 1/2, thus the quantum number S also can be eliminated from the equation. The simplified equation for calculating the concentration of the unpaired electrons [e] can be represented as:
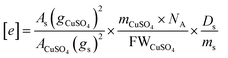 | (8) |
The calculation results are illustrated in the inset in Fig. 7(b). For the sample annealed with CaH2 for five h (x = 0.47), the concentration of the unpaired electrons [e] is about 3.93 × 1020 cm−3. When the annealing time increases, the [e] value increases and it approaches a maximum of ∼1.30 × 1021 cm−3 for the sample with an annealing time of 20 h (x ≈ 1.0). Further increasing the annealing time, [e] decreases gradually since they were over-reduced and impurity phase appeared.
For the sample with an annealing time of 20 h, the VO content x is about 1.0 (refer to the formula Y2Ti2O7−x), which corresponds to the concentration of VO (it includes both 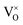 and
and 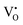 ) as 7.75 × 1021 cm−3. However, [e] is about 1.30 × 1021 cm−3, much less than [VO], probably due to incomplete ionization. According to eqn (3)–(5) and (7), [e] is the sum of the concentrations of
) as 7.75 × 1021 cm−3. However, [e] is about 1.30 × 1021 cm−3, much less than [VO], probably due to incomplete ionization. According to eqn (3)–(5) and (7), [e] is the sum of the concentrations of 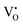 ,
, 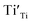 , O2− as well as some unknown defects containing unpaired electrons. Except for
, O2− as well as some unknown defects containing unpaired electrons. Except for 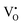 , all the unpaired electrons on the various defects come from the ionization of
, all the unpaired electrons on the various defects come from the ionization of 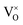 , thus
, thus 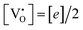 . Further, the degree of ionization (α) of
. Further, the degree of ionization (α) of 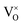 can be calculated as
can be calculated as
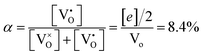 | (9) |
Only less than 10% of 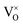 is ionized. Although the concentration of VO is quite high, the concentrations of
is ionized. Although the concentration of VO is quite high, the concentrations of 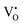 and
and 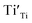 are not high. Most VO takes the form of
are not high. Most VO takes the form of 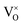 , i.e., most loosely bound electrons are trapped at
, i.e., most loosely bound electrons are trapped at 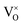 . Therefore, it is reasonable to consider the sample Y2Ti2O7−x (x ≈ 1.0) as an electride, and it is better to represent it as [Y2Ti2O6]2+(2e−).
. Therefore, it is reasonable to consider the sample Y2Ti2O7−x (x ≈ 1.0) as an electride, and it is better to represent it as [Y2Ti2O6]2+(2e−).
Ref. 2 reported the properties of the electride [Ca12Al14O32]2+(2e−): [e] ≈ 5 × 1019 cm−3, [VO] ≈ 2 × 1021 cm−3. The degree of ionization is calculated as α ≈ 1.3%. The α value for [Ca12Al14O32]2+(2e−) is smaller than that of [Y2Ti2O6]2+(2e−), possibly because [Ca12Al14O32]2+(2e−) does not have valence variable ions, such as Ti4+.
3.5 DC conductivity measurements
Based on the above discussions, it is known that after annealing with CaH2 at 600 °C for 20 h, Y2Ti2O7 can be reduced to an electride, and its composition can be represented as [Y2Ti2O6]2+(2e−). Since the electride has loosely bound electrons (2e−), it should have good conductivity. The DC conductivity of the electride was measured in the temperature range 100 K–500 K. Prior to the measurements. The pellet samples were sintered in vacuumed quartz ampoules at 900 °C for 2 h (2.1 section). The data are illustrated in Fig. 8. The electride [Y2Ti2O6]2+(2e−) has relatively high conductivity, e.g. at 300 K, the conductivity σ = 1.09 S cm−1. In this case, Arrhenius equation (σ = σA0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−Ea/kT, Ea is activation energy, k is Boltzmann constant and σA0 is a constant) is not suitable to describe the relationship between the conductivity (σ) and temperature (T). Nevertheless, the variable range hopping molds may be applied to this material. Following Chaudhuri's idea,21 for the electride [Y2Ti2O6]2+(2e−), above 230 K, Mott equation is applied to analyze the “σ ∼ T” data, while below this temperature, Efros–Shklovskii equation is used.
e−Ea/kT, Ea is activation energy, k is Boltzmann constant and σA0 is a constant) is not suitable to describe the relationship between the conductivity (σ) and temperature (T). Nevertheless, the variable range hopping molds may be applied to this material. Following Chaudhuri's idea,21 for the electride [Y2Ti2O6]2+(2e−), above 230 K, Mott equation is applied to analyze the “σ ∼ T” data, while below this temperature, Efros–Shklovskii equation is used.
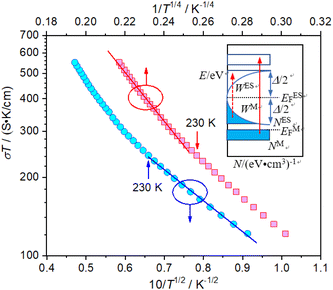 | ||
Fig. 8 Variations of conductivity with temperature. Upper: Mott plot (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tσ–T1/4 plot); down: Efros–Shklovskii plot (log Tσ–T1/4 plot); down: Efros–Shklovskii plot (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tσ–T1/2 plot); inset: schematic diagram of DOS. Tσ–T1/2 plot); inset: schematic diagram of DOS. | ||
Since [Y2Ti2O6]2+(2e−) has quite high conductivity and the conductivity does not change very much with temperature, the lattice scattering to the electrons cannot be omitted. The pre-exponential factor in Mott equation should be modified from “σM0” to “σM0/T”:
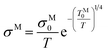 | (10) |
The density of state (DOS) at Fermi energy NM is represented as:
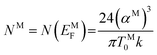 | (11) |
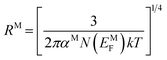 | (12) |
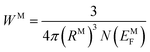 | (13) |
The calculated results (T = 400 K) are listed in Table 1.
| E–S eqn | T0ES/K | αES/nm−1 | NES/(eV cm3)−1 | RES/nm | WES/meV | Δ/meV | T/K | kT meV−1 |
| 7.16 × 102 | 0.33 | 4.10 × 1021 | 1.35 | 16 | 18 | 200 | 17 |
As the same reason, the pre-exponential factor of Efros–Shklovskii eqution is also modified as “σES0/T”:
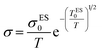 | (14) |
The Coulomb gap Δ is calculated as:
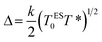 | (15) |
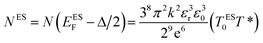 | (16) |
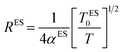 | (17) |
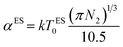 | (18) |
The value of N2 is given as
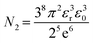 | (19) |
The hopping energy is given as:
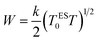 | (20) |
The calculated results are listed in Table 1 (T = 200 K).
Mott's theory and Efros–Shklovskii's theory are based on different DOS (density of state) features, which are schematically illustrated in the inset in Fig. 8. In Efros–Shklovskii's theory, the DOS varies as parabolic function near Fermi level, which is applied in low temperature range. At higher temperature range, DOS has flat or constant feature by Mott's theory. The parameters listed in Table 1 are basically reasonable. Hopping energy WM is larger than WES; both WM and WES are larger than the related kT. The hopping distance RM is shorter than RES, but both of them are about 1.2 nm. According to the structure of Y2TiO7, the electrons in the electride [Y2Ti2O6]2+(2e−) have three possible transporting channels (Fig. 9): (i) 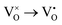 through [O2Y4] framework; (ii) Ti3+→Ti4+ through [Ti4O12] framework; (iii)
through [O2Y4] framework; (ii) Ti3+→Ti4+ through [Ti4O12] framework; (iii) 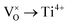 cross two frameworks. In all three channels, the distances (d) for the electron transportation are all about 0.4 nm, which are the minimum distances. RM and RES are ∼3 times d. Thus, the values of RM and RES are in a reasonable scale.
cross two frameworks. In all three channels, the distances (d) for the electron transportation are all about 0.4 nm, which are the minimum distances. RM and RES are ∼3 times d. Thus, the values of RM and RES are in a reasonable scale.
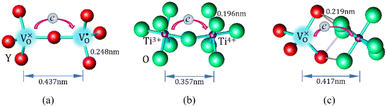 | ||
Fig. 9 Schematic diagrams for three possible conduction channels for electrons: (a) 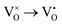 through O–Y framework; (b) Ti3+ → Ti4+ through Ti–O framework; (c) through O–Y framework; (b) Ti3+ → Ti4+ through Ti–O framework; (c) 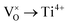 cross two frameworks. cross two frameworks. | ||
The conductivity of [Y2Ti2O6]2+(2e−) was also reported by Hayward,11 σ = 10−4 S cm−1 (295 K). In Hayward's work, conductivity measurements were carried out on pressed powders. Due to poor connection among the grains, the conductivity was low. In this work, sintered pellets were used. Since the connections among the grains were improved, the conductivity was increased significantly (σ = 1.09 S cm−1). However, the relative densities of the sintered pellets in this work were about 75%. If denser pellets could be obtained, the conductivity may be further improved.
4 Conclusions
The O-depleched pyrochlore samples Y2Ti2O7−x were prepared by the solid reducing method. The x value representing the content of VO was determined by TG analyses. In a proper reductive condition, the sample with x = 1.0 was obtained, which is considered as an electride. The composition of the electride is represented as [Y2Ti2O6]2+(2e−). Here (2e−) are two loosely trapped electrons in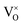 , which may be ionized, resulting in the production of unpaired electrons. Quantitative analysis of EPR data gave the concentration of unpaired electrons as 1.3 × 1021 cm−3. Since the concentration of loosely bound electrons in electride is quite high, its performance satisfied conductivity (1.09 S cm−1 at 300 K). In the preparation process, the samples underwent water-washing and oven-drying. Thus, the electride [Y2Ti2O6]2+(2e−) obtained in this work is stable in moisture and oven temperatures (i.e. below 150 °C).
, which may be ionized, resulting in the production of unpaired electrons. Quantitative analysis of EPR data gave the concentration of unpaired electrons as 1.3 × 1021 cm−3. Since the concentration of loosely bound electrons in electride is quite high, its performance satisfied conductivity (1.09 S cm−1 at 300 K). In the preparation process, the samples underwent water-washing and oven-drying. Thus, the electride [Y2Ti2O6]2+(2e−) obtained in this work is stable in moisture and oven temperatures (i.e. below 150 °C).
Although electrides were found quite early, inorganic solid electrides were found quite recently (2003). Due to their special crystal structure and electronic structure, these kinds of materials may have unique properties on electronics, magnetics, and catalysis. However, only a few inorganic solid electrides were reported. It is imperative to search for more novel electrides.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for financial support from National Natural Science Foundations of China (21371015 and 22271180). Thanks are also given to Ms Yufeng Guo and Mr Jinlong Wang at School of Environment in Tsinghua University for EPR measurements.Notes and references
- P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, Inorganic Chemistry, 5th edn, W. H. Freeman and Company, New York, 2010, vol. 130, p. 306 Search PubMed.
- S. Matsuishi, Y. Toda, M. Miyakawa, K. Hayashi, T. Kamiya, M. Hirano, I. Tanaka and H. Hosono, Science, 2003, 301, 626 CrossRef CAS PubMed.
- J. Huang, L. Valenzano and G. Sant, Chem. Mater., 2015, 27, 4731 CrossRef CAS.
- Y. Toda, S. Matuishi, K. Hayashi, K. Ueda, T. Kamiya, M. Hirano and H. Hosono, Adv. Mater., 2004, 16, 685 CrossRef CAS.
- Y. Inoue, M. Kitano, S.-W. Kim, T. Yokoyama, M. Hara and H. Hosono, ACS Catal., 2014, 4, 674 CrossRef CAS.
- J. Sharif, M. Kitano, Y. Inoue, Y. Niwa, H. Abe, T. Yokoyama, M. Hara and H. Hosono, J. Phys. Chem. C, 2015, 119, 11725 CrossRef.
- N.-H. Chan, R. K. Sharma and D. M. Smyth, J. Am. Ceram. Soc., 1981, 64, 556 CrossRef CAS.
- X. Kuang, X. Jing and Z. Tang, J. Am. Ceram. Soc., 2006, 89, 241 CrossRef CAS.
- J. M. Longo, P. M. Raccah and J. B. Goodenough, Mater. Res. Bull., 1969, 4, 191 CrossRef CAS.
- G. Sala, M. J. Gutmann, D. Prabhakaran, D. Pomaranski, C. Mitchelitis, J. B. Kycia, D. G. Porter, C. Castelnovo and J. P. Goff, Nat. Mater., 2014, 13, 488 CrossRef CAS.
- M. A. Hayward, Chem. Mater., 2005, 17, 670 CrossRef CAS.
- J. A. Weil, J. R. Bolton and J. E. Wertz, Electron Paramagnetic Resonance. John Wiley & Sons, Inc. New York, 1994, p. 496 Search PubMed.
- F. Matteucci, G. Cruciani, M. Dondi, G. Baldi and A. Barzanti, Acta Mater., 2007, 55, 2229 CrossRef CAS.
- M. A. Subramanian, G. Aravamudan and G. V. Subba Rao, Prog. Solid State Chem., 1983, 15, 55 CrossRef CAS.
- W. W. Barker, P. S. White and O. Knop, Can. J. Chem., 1976, 54, 2316 CrossRef CAS.
- A. W. Sleight, Mater. Res. Bull., 1971, 6, 775 CrossRef CAS.
- S. K. Misra, S. I. Andronenko, D. Tipikin, J. H. Freed, V. Somani and O. Prakash, J. Magn. Magn. Mater., 2016, 401, 495 CrossRef CAS PubMed.
- M. Ivanovskaya, K. Chernyakova, E. Ovodok, S. Poznyak, D. Kotsikau and I. Azarko, Mater. Chem. Phys., 2022, 278, 125703 CrossRef CAS.
- D. Zhang, X. Ma, H. Zhang, Y. Liao and Q. Xiang, Mater. Today Energy, 2018, 10, 132 CrossRef.
- K. Xie, N. Umezawa, N. Zhang, P. Reunchan, Y. Zhang and J. Ye, Energy Environ. Sci., 2011, 4, 4211 RSC.
- A. Ganguly, S. K. Mandal, S. Chaudhuri and A. K. Pal, J. Appl. Phys., 2001, 90, 5652 CrossRef CAS.
- M. H. Sage, G. R. Blake and T. T. M. Palstra, Phys. Rev., 2008, B77, 155121 CrossRef.
- T.-T. Tao, L.-X. Wang and Q.-T. Zhang, J. Alloy. Compd, 2009, 486, 606 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |