Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of TiVO4 photoelectrode for photoelectrochemical application†
Manal Alruwaili *ab,
Anurag Roy
*ab,
Anurag Roy a,
Srijita Nundya and
Asif Ali Tahir
a,
Srijita Nundya and
Asif Ali Tahir *a
*a
aEnvironment and Sustainability Institute, Faculty of Environment, Science and Economy, University of Exeter, Penryn TR10 9FE, UK. E-mail: ma942@exeter.ac.uk; a.tahir@exeter.ac.uk
bPhysics Department, Faculty of Science, Jouf University, PO Box 2014, Sakaka 42421, Saudi Arabia
First published on 2nd December 2022
Abstract
Photoelectrochemical (PEC) water splitting is one of the promising, environmentally friendly, carbon emission-free strategies for the cost-effective production of hydrogen. The interest in developing effective approaches for solar-to-hydrogen production with stable and visible light active semiconductors directed many researchers to develop stable and efficient materials. For the first time, a nanostructured TiVO4 photoanode was fabricated at a substrate temperature of 250 °C and further annealed at 600 °C using the spray pyrolysis technique and it obtained an optical band gap of ∼2.18 eV. The photoanode underwent photoelectrochemical testing, where it exhibited a high photocurrent density of 0.080 mA cm−2 at 1.23 V (vs. reversible hydrogen electrode), which can be stable up to 110 min. Further, various physicochemical characterizations were employed to understand the phase purity and thin film growth mechanism. A systematic substrate and annealed temperatures were monitored during the fabrication process. The transmission electron microscopy (TEM) studies revealed agglomeration of TiVO4 nanoparticles with an average size of ∼100 nm accompanying dendritic orientation at the outer edge. This study envisages the design and development of a novel photocatalyst for water splitting under visible light irradiation, an ideal route to a cost-effective, large-scale, sustainable route for hydrogen production.
1. Introduction
The most incredible mission of the current scientific generation is to address the considerable energy demands.1 Researchers are devoting their efforts to searching for secure, safe, and sustainable energy options to cope with expected nonrenewable energy shortages and eliminate environmental pollutants. Solar energy is expected to be the leading and safe option that can solve the energy crisis, targeted explicitly through improvements to ensure access to reliable, sustainable, affordable and modern energy. An understanding of renewable-energy solutions for water splitting, which is the process of hydrogen production by direct water decomposition, heavily depends on further developments in cost-effectiveness and environmental technologies. The photoelectrochemical (PEC) water splitting is considered one of the most promising H2 generation methods with required potential (ΔG° = 237 kJ mol−1), due to its use of the unlimited energy source of solar light without producing any CO2.2–5 Since 1972, after the pioneering work by Fujishima and Honda using TiO2 as a photocatalyst in a water splitting system.6 To achieve high performance of PEC materials, suitable band gap, diffusion length, bulk defects and surface recombination, i.e. accessible surface area, porosity, pore volume and high crystallinity, are critical parameters to affect the overall PEC performance.7 Particularly, porosity and pore fall into the requirements for low diffusion length materials, such as some metal oxides.8–10Fe2O3,11 Co3O4,12 TiO2,13 MoOx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and VO2
14 and VO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 are materials that have been used widely in PEC applications, but some drawbacks suppress their work, such as fast charge carriers recombination and low charge transport. In addition, these semiconductors' band edge potentials are incompatible with the redox potentials for some specific photocatalytic redox reactions. Hence, if these bimetallic semiconductors can be combined with other novel photoactive materials such as NiO,16 ZnO,17 CdO (ref. 18) etc., to construct heterojunctions such as Co3O4/TiO2,19 Fe2O3/Au/TiO2,20 ZnO/Fe2O3,21 Fe2O3/CdO,22 MoOx/GaP
15 are materials that have been used widely in PEC applications, but some drawbacks suppress their work, such as fast charge carriers recombination and low charge transport. In addition, these semiconductors' band edge potentials are incompatible with the redox potentials for some specific photocatalytic redox reactions. Hence, if these bimetallic semiconductors can be combined with other novel photoactive materials such as NiO,16 ZnO,17 CdO (ref. 18) etc., to construct heterojunctions such as Co3O4/TiO2,19 Fe2O3/Au/TiO2,20 ZnO/Fe2O3,21 Fe2O3/CdO,22 MoOx/GaP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and ZnO/NiO
23 and ZnO/NiO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 etc. with proper band alignment, they could serve as efficient PEC material as compared to single-phase metal oxide. It can be combined with other dopants to extend those materials' spectral response towards visible light.25–27
24 etc. with proper band alignment, they could serve as efficient PEC material as compared to single-phase metal oxide. It can be combined with other dopants to extend those materials' spectral response towards visible light.25–27
Recently, bimetallic oxide materials (BiVO4,28 FexVxO4,29 CuxVxOx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 have become promising photoelectrode due to their tuned bandgap, suitable for PEC water splitting. They have shown an effective usage of solar irradiation owing to their synergic absorption in the visible and ultraviolet (UV) regions.23,31 Numerous pioneering reports highlighted the superior performance of TiV systems as composites VOx/TiO2 or as doping compared to TiVO4 – bimetal oxide on their plausible photocatalysis application.32,33 Samdarshi et al. (2010) evaluated the effect of silver on the titanium vanadium mixed metal (Ag/TiV) oxides.34 They observed that the system could absorb a large portion of the visible spectrum, where favourable electron transfer could be experienced anatase-rutile mixed phase where silver dominated through its scavenging action to reduce hole recombination. On the other hand, the Ag-doped TiV films were developed by the sol–gel technique, increasing antibacterial activity when exposed to visible light.35 The visible transmittance can be substantially improved, resulting in a higher photocatalytic property under visible light irradiation for the Ti–V system.36 Alternatively, an efficient way to use the majority of sunlight could be achieved using nanocomposite semiconductors such as TiO2–V2O5.37 Li et al. (2016) investigated V4+ ion doping to decorate TiO2 nanocrystals, which tailored energy level alingment of TiO2 nanostructure by shortening the electronic transfer lifetime to 34.7% compared to only TiO2.38 By contrast, no reports have gone on to utilize TiV systems in PEC applications. Extending these advanced characteristics to PEC systems will undoubtedly bring breakthroughs in PEC-driven photo-to-energy conversions. The main challenge now is to design optical paths to achieve high light absorption with high activity, durability, and selectivity during the PEC analysis.
30 have become promising photoelectrode due to their tuned bandgap, suitable for PEC water splitting. They have shown an effective usage of solar irradiation owing to their synergic absorption in the visible and ultraviolet (UV) regions.23,31 Numerous pioneering reports highlighted the superior performance of TiV systems as composites VOx/TiO2 or as doping compared to TiVO4 – bimetal oxide on their plausible photocatalysis application.32,33 Samdarshi et al. (2010) evaluated the effect of silver on the titanium vanadium mixed metal (Ag/TiV) oxides.34 They observed that the system could absorb a large portion of the visible spectrum, where favourable electron transfer could be experienced anatase-rutile mixed phase where silver dominated through its scavenging action to reduce hole recombination. On the other hand, the Ag-doped TiV films were developed by the sol–gel technique, increasing antibacterial activity when exposed to visible light.35 The visible transmittance can be substantially improved, resulting in a higher photocatalytic property under visible light irradiation for the Ti–V system.36 Alternatively, an efficient way to use the majority of sunlight could be achieved using nanocomposite semiconductors such as TiO2–V2O5.37 Li et al. (2016) investigated V4+ ion doping to decorate TiO2 nanocrystals, which tailored energy level alingment of TiO2 nanostructure by shortening the electronic transfer lifetime to 34.7% compared to only TiO2.38 By contrast, no reports have gone on to utilize TiV systems in PEC applications. Extending these advanced characteristics to PEC systems will undoubtedly bring breakthroughs in PEC-driven photo-to-energy conversions. The main challenge now is to design optical paths to achieve high light absorption with high activity, durability, and selectivity during the PEC analysis.
Single-phase TiVO4 is still not explored much and received little attention, which can promise effective PEC characteristics reported by various TiV systems. Comparing TiVO4 to TiO2, its interfacial structure is superior for higher electron diffusion during high-temperature fabrication, creating a low impedance contact possessing high optical transparency and providing long-term stability. By comparing the requirements of novel PEC materials, TiVO4 can be achieved with similar small-radius cations, which are directed to narrow band gap and have transition metals as the cation component, which is earth-abundant, cost-effective, and low-toxicity, benefiting wide usage in the future.
This work synthesized a phase pure TiVO4 as a photoanode using the scalable spray pyrolysis technique. The photoanode produced by spray pyrolysis has a large surface area of substrate coverage potential, a cost-effective method and homogeneity of mass synthesis. The influence of the thin films' substrate and annealing temperatures is further studied and exhibited remarkable hierarchical structures. Furthermore, the spray pyrolyzed TiVO4 photoanode significantly resulted in a narrower visible band gap with a high photocurrent, further employed for the PEC water splitting application. The following sections thoroughly investigated and discussed an underlying phenomenon for this superior performance.
2. Materials and methods
2.1. Fabrication of TiVO4 thin film via spray pyrolysis
All chemicals used for the fabrication of TiVO4 were purchased from Merck Life Science Products (UK) and used without further purification.TiVO4 photoanodes were prepared via spray pyrolysis. The solution was prepared by dissolving vanadium acetylacetonate, and titanium isopropoxide in 15 mL of ethanol with a total concentration of 0.05 M. Next, 0.05 mL of trifluoroacetate acid (99%) was added to the mixture. It was kept under stirring for 2 h to get a clear homogenous solution. The solution was employed in the spray pyrolysis system, which comprises a syringe pump (New Era Pump system NE-1000), an attached vortex, and an ultrasonic atomizer (Sonozap) assisted with a nozzle of 1 mm (diameter). In this process, the prepared spray droplets are transported to the substrate through an atomizing nozzle. The evaporation of the solvent within the spray droplets leads to the formation of a uniform coating on the substrate. The parameters of the spray pyrolysis were maintained as follows, 10 mL of solution was sprayed on the 1 cm × 1 cm cleaned fluorine-doped tin oxide (FTO) glasses kept at a distance of 12 cm from the nozzle head at a flow rate of 1 mL min−1, which is assisted with the compressed air (4 L min−1). The deposition on the FTO glass was performed under various temperatures from 150 °C to 300 °C at an interval of 50 °C, referred to as substrate temperatures. Once the spray FTO finished, substrates were then taken for annealing at different temperatures from 500 °C to 650 °C, at an interval of 50 °C, for 2 h in a muffle furnace. The TiVO4-coated FTO substrates were allowed to cool to room temperature before being taken for further analysis. Fig. 1 represents a schematic illustration of a TiVO4 photoanode fabrication process using spray pyrolysis technique under optimised conditions.
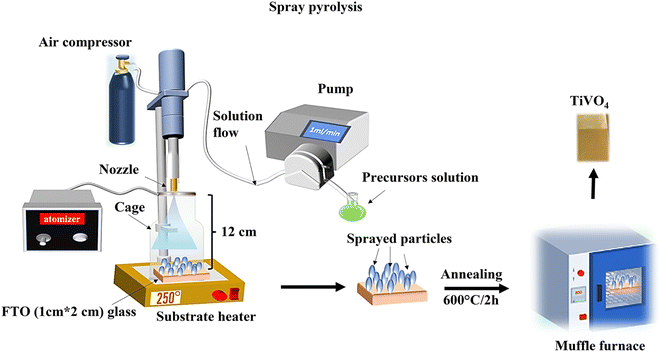 | ||
| Fig. 1 Schematic representation of a TiVO4 photoanode fabrication process using spray pyrolysis technique under optimised conditions. | ||
2.2 Materials characterisations
Thin film microstructure analysis was performed using the TESCAN VEGA3 scanning electron microscopy (SEM) equipped with the energy dispersive spectroscopy (EDS) from Oxford Instruments. The structure and phases of the TiVO4 thin films were characterised using a Bruker D8 X-ray diffractometer (XRD) assisted with a monochromatic CuKα (λ = 0.154 nm) radiation. XPS Analysis was performed using a Thermo NEXSA XPS fitted with a monochromated Al Kα X-ray source (1486.7 eV). All sample data was recorded at a pressure below 10−8 Torr, and a room temperature of 294 K. Data was analysed using CasaXPS v2.3.20PR1.0 and calibrated with C 1s peak at 284.8 eV. Corresponding transmission electron microscopy (HR-TEM) analysis was carried out using JEOL JEM-2100F TEM (200 kV). The absorption and transmission spectra of the thin film were executed by PerkinElmer's UV-VIS-NIR UV-3600 Plus spectrophotometer.TiVO4 photoanode was employed for PEC studies, which were conducted utilising the Metrohm Autolab (PGSTAT302N) workstation consisting of three-electrode compartments. Where a platinum wire was used as the counter electrode, a 3 M aqueous solution of Ag/AgCl in KCl was considered the reference electrode, and 1 M aqueous solution of NaOH (pH of 13.6) was employed as the electrolyte for the electrochemical testing. The light intensity was simulated to achieve 1 SUN condition (100 mW cm−2) from Newport, consisting of a 300 W xenon lamp with AM 1.5 filter and 420 nm cut-off filter to remove the UV part of the sunlight. The photoanode's voltages (potential vs. Ag/AgCl) were recorded at a scan rate of 0.01 V s−1 from negative to a positive potential direction (−0.3 V and +0.8) under light, dark and chopping conditions. All potentials were then converted to reversible hydrogen electrode (RHE) potential according to the Nernst eqn (1) as,
| ERHE = EAg/AgCl + 0.0591(pH) + 0.1976 V | (1) |
3. Results and discussions
3.1. Structural and optical analysis
The XRD pattern of synthesized TiVO4 photoanode exhibits single-phase material at 600 °C, as shown in Fig. 2a. The diffraction peaks correspond to 27.5°, 41.3° and 54.4°, representing the tetragonal lattice planes of (110), (111) and (211) for TiVO4 structure. These results also corroborate with the JCPDS file 01-770332.34 Besides, with almost similar intensity, the distinct peaks indicate FTO-glass substrate, and XRD confirms TiVO4 thin film formation. No additional peaks of either TiO2 or VO2 hence admittedly manifested pure phase of TiVO4 tetragonal structure. However, the XRD analysis was also performed at different substrate temperatures during the spray pyrolysis and further different annealing temperatures (Fig. S1, ESI†) to optimize the phase pure TiVO4 thin film development. The optimized substrate and annealed temperatures were found to be 250 °C and 600 °C, respectively. Below these temperatures, either the tetragonal TiVO4 phase does not entirely form, or the film deposition was not homogeneously covering the FTO surface; as a result, the XRD patterns revealed dominated peaks attributed to the FTO glass.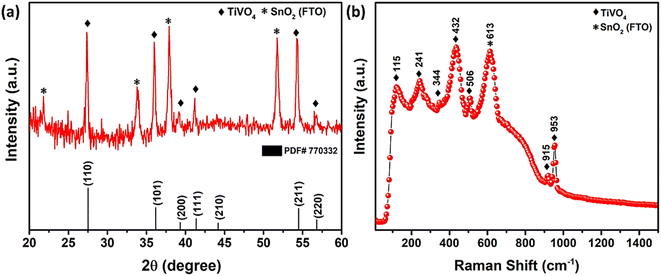 | ||
| Fig. 2 (a) XRD pattern, (b) Raman spectrum of spray pyrolyzed TiVO4 photoanode deposited on FTO glass. | ||
Fig. 2b revealed the Raman spectrum of the optimized TiVO4 thin film, confirming the successful formation of tetragonal TiVO4 on the FTO glass. The stretching modes of V–O combining Ti–O and V–O occurred at higher wavenumber bands at ∼915 and 953 cm−1.39 The bending and stretching modes appeared in the 400–600 cm−1 region. Other <400 cm−1 bands were external modes from the lattice, translational, and vibrational motions. Noticeably, Raman analysis of TiVO4 (tetragonal) is rare; however, the concerning result has been compared with the CrVO4 (orthorhombic) and FeVO4 (triclinic) Raman bands relevant to TiVO4.40 It is anticipated that due to the difference in electronegativity of these metal, all the strongest peak of TiVO4 exhibits a slightly lower intensity compared to CrVO4 and FeVO4. Also, because of its tetragonal and nondegenerate vibrations, the Raman bands of TiVO4 were between CrVO4 and FeVO4. A peak at ∼613 cm−1 corresponds to the A1g vibrational mode of F-doped SnO2 originating from the FTO glass.41
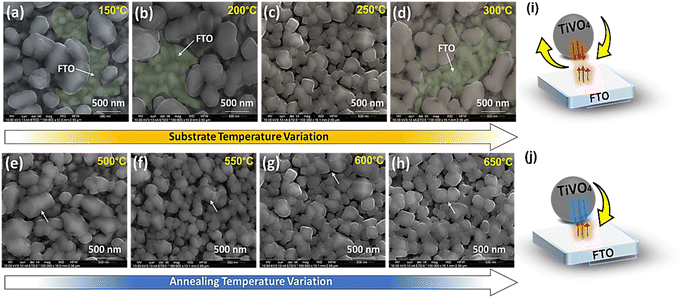
Fig. 3a–d reveals SEM microstructural images of TiVO4 photoanode developed at different substrate temperatures of 150 °C, 200 °C, 250 °C and 300 °C, where the annealing temperature was maintained at 600 °C. The substrate temperature profoundly impacts the morphology and performance.42–45 At lower substrate temperatures of 150 °C to 200 °C, the spray droplets splash directly onto the substrate (FTO) and decompose without evaporating the solvent in (Fig. 3a and b).46 As a result, the particles agglomerated with an average size of ∼700 nm, leaving bare FTO as shown in SEM (green coloured). This is due to the rapid droplet flux reaching the substrate without a complete nucleation growth process, leading to rough films and an ununiform distribution of the material on FTO glass. Whereas at the substrate temperature of 250 °C, the solvent will leave before reaching the substrate, and a solid precipitate of precursor will fall on the substrate providing a homogenous coating. As a result, growth is uniform, and the bead-like structure with reduced particle size narrowed down to 300 nm, securing better coverage and homogeneous deposition across the FTO glass, as shown in Fig. 3c. However, at 300 °C substrate temperature, the decomposition of precursor starts before reaching the substrate, resulting in less adhesion to the substrate and giving powder particles on the surface, which peel off during the annealing process leaving bare FTO as shown in (Fig. 3d) (green coloured).47,48 Based on the morphology observed by SEM; it is clear that the optimum substrate spray temperature for uniform and smooth films was found to be 250 °C.
Fig. 3e–h displays the impact of the various annealing temperatures from 500 °C to 650 °C on the films deposited at a substrate temperature of 250 °C. The images from Fig. 3e–h show that the only significant difference in morphology of the films is observed on those annealed at 500 °C. The morphology at this annealing temperature shows the wide distribution of the particle's size ranging from 600 to 150 nm, whereas the morphology of all those films annealed at 550 to 650 °C shows almost similar size distribution and more uniform growth. As well as Fig. 3e and f indicate that particle boundaries are agglomerated. While once the annealed temperature rises to ≥600 °C, the particles are comparatively distinct. The increase in porosity found in the films can be attributed to increased temperature, and the film becomes denser without cracks at higher temperatures. The thermophoretic effect during the high and optimized substrate temperatures of the TiVO4 particle deposition was explained through a schematic, as shown in Fig. 3i and j, respectively. The substrate temperatures determine the rate of particle formation and their homogenous distribution. The growth of particles and concerning coverage maximized at 250 °C, and calcination temperature governs the phase purity and morphology of the fabricated particles. At higher substrate temperatures, particles' supersaturation rate and mobility were expected to be high and therefore yielded higher nucleation rates. In contrast, higher mobility facilitates nucleation collision and different growth rates of individual particles. Hence, by understanding the substrate and annealing temperature effect on morphology and homogeneity of the TiVO4 photoanode, the optimised spray annealed temperatures are 250 °C, and 600 °C were taken for further analyses.
Furthermore, the high-resolution TEM was conducted to get detailed information and understand the nanostructured morphology and crystallinity of the TiVO4. The TEM images shown in Fig. 4a display scattered nanoparticle assemblies of TiVO4, leading to an average diameter of ∼100 nm. The TiVO4 particles exhibit porous features, with some dendrites on their outer edge. Nanoparticles have appeared to form some extent of designed agglomeration. Fig. 4b represents corresponding high-resolution TEM images and selected area diffraction patterns (HR-TEM) images in the inset, indicating the tetragonal lattice fringes match well with the (111), (200) and (110) crystal planes of TiVO4, indicating the presence of Ti, V in the composite form. T EDS spectrum was measured to further confirm the doping of vanadium ions into the TiO2 lattice, as shown in Fig. 4c and S2, ESI.† The spectrum demonstrates the distinct peaks corresponding to Ti (Kα1) and V (Kα1) with a ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V as 1
V as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (16.4% and 16.0%), providing the composite to be formed as TiVO4. Furthermore, elemental color mapping was also carried out, aimed to be a suitable technique to validate the presence of TiVO4 onto the FTO glass as shown for the TiVO4 thin film as shown in Fig. 4d. It shows the coexistence of Ti, V, Sn and O belonging to TiVO4. This result further confirms the deposition of TiVO4 onto the FTO glass.
1 (16.4% and 16.0%), providing the composite to be formed as TiVO4. Furthermore, elemental color mapping was also carried out, aimed to be a suitable technique to validate the presence of TiVO4 onto the FTO glass as shown for the TiVO4 thin film as shown in Fig. 4d. It shows the coexistence of Ti, V, Sn and O belonging to TiVO4. This result further confirms the deposition of TiVO4 onto the FTO glass.
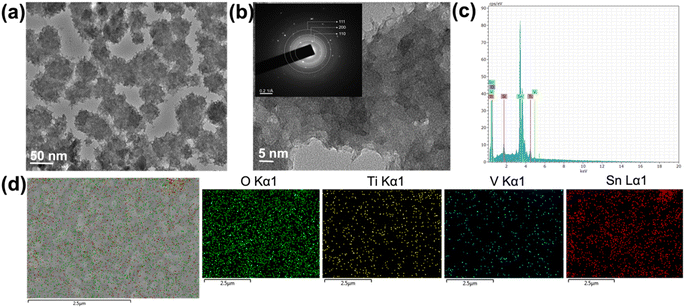 | ||
| Fig. 4 (a) TEM bright field image, (b) corresponding HRTEM and SAED images (inset), (c) EDS spectrum and (d) corresponding element colour mapping of the TiVO4 photoanode. | ||
As photoelectrochemical activities are surface-driven phenomena, to understand the oxidation state of the elements present on the outermost surface, the surface composition of the sample was analysed by XPS integrated peak area analysis as shown in Fig. 5. The XPS survey spectra of TiVO4 photoanode indicates the presence of Ti 2p, V 2p and O 1s, without any impurity and successfully deposited on the FTO glass, indicated by Sn 3d3/2 peak as displayed in Fig. 5a. The XPS peaks at 458.7 eV and 464.5 eV corresponding to the binding energies of the Ti 2p3/2 and 2p1/2 (Fig. 5b). The spin–orbit splitting energy of 5.8 eV is the characteristic of Ti4+ oxidation state present in the TiVO4 structure.49 In addition, the Ti 2p core level spectra can be fitted into Ti3+ peaks at ∼457.8 eV and ∼461.6 eV corresponding to Ti3+ 2p3/2 and Ti3+ 2p1/2, originating due to the partial reduction of Ti4+ state by the generation of oxygen vacancies in the reducing atmosphere. It is reported that Ti3+ species and/or oxygen vacancies may significantly improve the observed electronic conductivity of the material.50 However, compared to the Ti4+ oxidation state, the Ti3+ oxidation state is negligible.
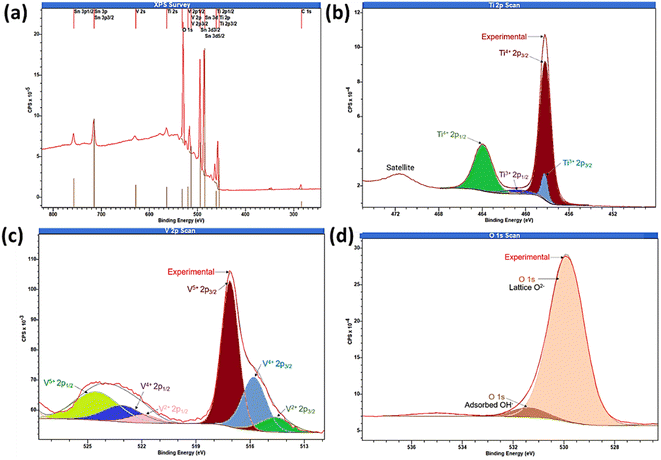 | ||
| Fig. 5 X-ray photoelectron spectroscopy (a) survey spectrum and core level spectrum of (b) Ti 2p, (c) V 2p and (d) O 1s regions of TiVO4 thin film deposited on a FTO glass. | ||
There are strong hybridization between V 2p and O 1s states and thus exposed various oxidation states of V 2p during TiVO4 formation. The peaks at 516.3 and 517.2 eV correspond to the binding energies of V 2p3/2 for V4+ and V5+, respectively, as shown in Fig. 5c. Besides, the V 2p1/2 peaks are positioned at 524.4, and 523.1 eV corresponding to V5+ and V4+.51 V5+ might result from the surface oxidation of the samples in the air, which is common in vanadium-based samples.52 The observed broadening of the V 2p3/2, the linewidth is due to the decrease of the oxidation state. As a result, V2+ peaks were also observed.52 However, the formation of VO has the oxidation state V2+, which is not reported in any previous study. Therefore we cannot unambiguously assign the third component to a vanadium oxidation state. The overlapped mixed-valence oxidation states of V5+ and V4+ are usually observed in ternary vanadium-based oxides.53 Such overlapping peaks contain mixed valences of V5+ and V4+. Also, the V2p binding energies of TiVO4 were lower than the bare V2O5 and VO3 due to the ternary oxide composition. Eventually, the pristine of TiVO4 show a ratio V/O ∼0.27, in agreement with the ratio found in the TiVO4 (0.25); thus, it is expected that only V4+ is present in the sample. The existence of V5+ species in the TiVO4 thin film suggests that during the chemical synthesis, possible interactions of between the Ti–V sol produce a partial shift to higher binding energy in concordance with the V5+ species.
Further, two peaks for oxygen (O 1s) were observed, one for O2− in the metal oxide as interaction strength of the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, and O
O bonds, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) V–O–V4+(V5+)–O–Ti4+ to form TiVO4. While the other one at 532.2 eV characteristics of chemisorbed oxygen on transition-metal as shown in Fig. 5d.54 The surface atomic percentages calculated from the XPS data were Ti 2p ∼9%, V 2p ∼8.2%, O 1s ∼36% and Sn 3d ∼4%. The elemental composition and distribution are corroborated with the XRD, EDS and XPS measurements. These results further proved that the nanostructures of TiVO4 had been successfully grown on the surface of FTO glass.
V–O–V4+(V5+)–O–Ti4+ to form TiVO4. While the other one at 532.2 eV characteristics of chemisorbed oxygen on transition-metal as shown in Fig. 5d.54 The surface atomic percentages calculated from the XPS data were Ti 2p ∼9%, V 2p ∼8.2%, O 1s ∼36% and Sn 3d ∼4%. The elemental composition and distribution are corroborated with the XRD, EDS and XPS measurements. These results further proved that the nanostructures of TiVO4 had been successfully grown on the surface of FTO glass.
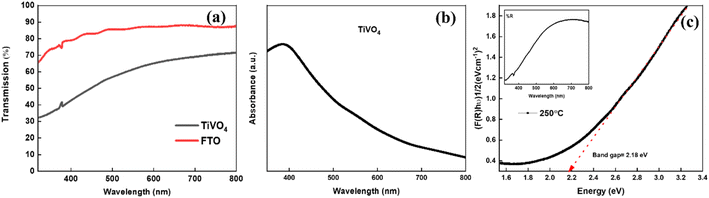
Fig. 6a exhibits the transmittance spectra of the TiVO4 photoanode and compares it with the FTO glass. The FTO glass shows an average transmittance of ∼80%, whereas the TiVO4 thin film exhibits slightly less steady transmittance to ∼50. Reduction in transmittance indicates the formation of a thin film on FTO glass. During the spray pyrolysis process, the thin film retained semi-transparent behaviour for all the samples. This is also evident from the thin film's colour, which visually appears light yellow. During the spray pyrolysis process, the thin film retained semi-transparent behaviour for all the samples. This is also evident from the thin film's colour, which visually appears light yellow. Fig. 6b shows the maximum absorption edge of TiVO4 ∼ 400 nm, which shows an exponential decrease towards the visible wavelength region.
Moreover, the absorption edge of the composite has been extended to the visible region up to 600 nm due to incorporating V4+ in the TiO2 structure, which could help harvest the UV and visible components of the solar radiation. The optical bandgap estimation was calculated using reflectance measurement from the Kubelka Munk equation, as shown in Fig. 6c. The band gap of TiVO4 is estimated to be ∼2.18 eV. TiVO4 bandgap was narrower than TiO2, where V4+ incorporation plays a crucial role. This may be because the ionic radii of V4+ are 0.72 Å, which is close to the Ti4+ ionic radii (0.74 Å), which signifies interstitial doping of V4+ within TiO2 lattice TiO2 enables tuning its light-absorbing capacity. Narrowing the wide bandgap indicates TiVO4 could be used as an anodic electrode for water-splitting applications.
3.2. Photoelectrochemical analysis
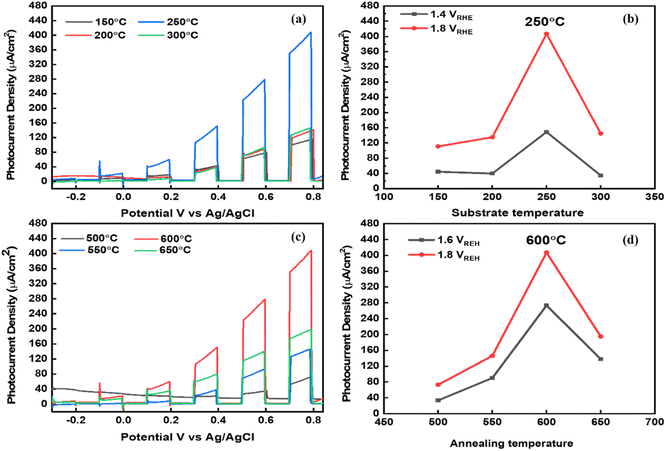
Considering the optical analysis, nanostructure, and phase pure properties, the fabricated TiVO4 photoanodes were employed for PEC investigation with varied substrate and annealing temperatures. The linear sweep voltage (LSV) plot (Fig. 7a), i.e., photocurrent (μA) vs. potential (vs. Ag/AgCl), was performed at a scan rate of 1 mV s−1 of the TiVO4 as a working electrode was recorded under chopped conditions (light and dark with chopping at an interval of 0.2 V vs. Ag/AgCl) for various substrate temperatures fabricated films. Among the various substrate temperatures, the photoanode developed at 250 °C recorded the highest photocurrent of 400 μA at 0.8 V vs. Ag/AgCl (1.8 V vs. RHE), which is much higher than the other samples sprayed at 150 °C (108 μA), 200 °C (135 μA) and 300 °C (145 μA). Fig. 7b indicates the trend of photocurrent obtained by the TiVO4 photoanode developed at various substrate temperatures at 1.4 and 1.8 V vs. RHE. The photocurrent slightly increases with an increase in temperature from 150 to 200 °C, i.e., from 108 to 135 μA. Intermediate adhesion and incomplete phase development with low coverage of the particles on FTO glass could have resulted in low photocurrent.55 At 250 °C, it drastically enhanced to 400 μA. This phenomenon is mainly attributed to the sufficient temperature required to evaporate the organic materials, forming the pure phase material. At an optimised substrate temperature, maximum nucleation density and proper atomic diffusion can be achieved, resulting in the material's complete growth.56,57 However, at 300 °C, the photocurrent decreases significantly beyond this temperature to 140 μA. This is ascribed to the agglomeration and decomposition of the materials at higher temperatures and corroborates with the SEM data. The overall trend of photocurrent generation for different substrate temperatures was followed as 150 °C < 200 °C ∼250 °C > 300 °C.Next, to evaluate the effect of different annealing temperatures on the TiVO4 photoanodes, the LSV photocurrent transients were obtained, as shown in Fig. 7c. Here, the substrate temperature was maintained at the optimized temperature, i.e., 250 °C. A significant enhancement in the photocurrent is noticed for the thin film annealed at 600 °C. The photocurrent seemed to increase monotonically from 73 to 400 μA with an increase in annealing temperature from 500 to 600 °C. This was due to better coverage on the substrate, uniformity of the thin film as observed by SEM, and improved crystallinity improvement as confirmed by XRD. The overall trend of photocurrent generation for different substrate temperatures was followed as 500 °C < 550 °C < 600 °C > 650 °C. The trend for photocurrent achievement at different annealing temperatures at 1.4 and 1.8 V vs. RHE was further described in Fig. 7d. Nevertheless, higher annealing temperature (650 °C) displays a drop in the photocurrent to 196 μA, primarily due to the increasing its overall resistivity of films which may be due to the sintering of particles to the increased size and reduce the surface area.58
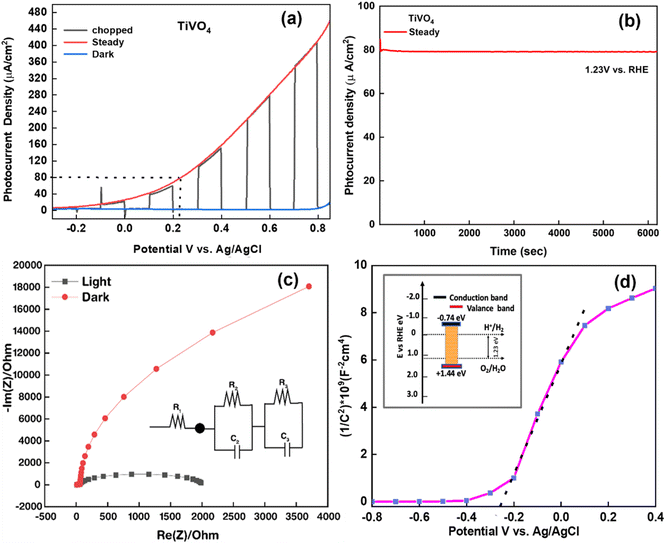
The linear sweep voltammogram (LSV) of the optimised TiVO4 photoanode was plotted in Fig. 8a and measured under dark and illumination conditions with an onset potential of ∼0.8 V (vs. RHE). Herein, the photocurrent was significantly increased over the positive potential scan range, which indicates that TiVO4 is working as an effective photoanode material. At the bias potential of 1.8 V vs. RHE, photocurrent density was measured to be the highest value of 400 μA cm−2. Also, the stability of optimised TiVO4 was investigated using the chronoamperometry technique by keeping the film under illumination at a maintained applied bias potential of 0.23 V vs. Ag/AgCl (1.23 V vs. RHE), representing the required PEC water potential for 6000 s Fig. 8b. Steady-state of photocurrent density was extrapolated over tested time without degradation of the total of photocurrent density. Furthermore, to investigate the charge transport kinetics of the TiVO4, electrochemical impedance measurements were carried out under both dark and 1 SUN illumination (100 mW cm−2). The Nyquist plots of electrochemical impedance spectra of obtained data are shown in Fig. 8c, with an equivalent circuit (R1 + R2/C2 + R3/C3) in the inset used to evaluate resistance values. R1 represents the total solution resistances of the circuit among FTO, titanium vanadate film and connecting wires, R2 is a resistance that arises from charge transfer at the electrode/electrolyte interface, and R3 represents the resistance of charge transport of the bulk TiVO4 film.59 Simultaneously, C2 and C3 are ascribed to the bulk material's capacitance and the associated surface states. Under light conditions, resistance values of R1, R2 and R3 were found to be 12.41 Ω, 1983 Ω and 113 Ω, respectively, indicating the facile charge transfer of the obtained film and thus promoting PEC activity.
The Mott–Schottky calculation was carried out to determine the space-charge capacitance over potential scan ranges and the flat band potential of the optimized photoanode. The Mott–Schottky plot elucidated the n-type performance of TiVO4 film, as shown in Fig. 8d. Flat band potential was estimated to be −0.26 V vs. Ag/AgCl and used in the Mott–Schottky eqn (2) to calculate the concentration of the dopants (ND)
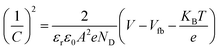 | (2) |
In order to explain the overall water splitting in a PEC with an n-type semiconductor TiVO4 photoanode, hydrogen is generated at the cathode while oxygen is evolved at the photoanode surface. Thus, the reaction products can be collected in separate chambers. In PEC water splitting, similar to the basic principle of photoanode, i.e., on light irradiation, photoexcitation of the charge carriers followed by their migration to the photoanode surface take place. These photo-generated electrons (e−) and holes (h+) that migrate to the surface of the photocatalyst without recombination can produce gaseous hydrogen and oxygen, respectively, by reducing and/or oxidizing the water molecules, respectively adsorbed on the photoanode surface. The photo-oxidation of H2O by the holes in the valence band causes a surface back reaction which demerits the H2 evolution rate.
In this work, the narrow band gap of the obtained film at ∼2.18 eV is suitable to absorb a wide range of incident visible light and thus form electron–hole pairs. The generated charge carries participation into the electrolyte with the species of O2 and OH˙ to trigger redox reactions. In brief, photo-generated electrons on the photoanode travel towards FTO, drifting to the cathode surface to carry out the reduction process (H2 evolution).60 Most importantly, some energy losses may occur due to recombining charge carriers, resulting in low photocurrent density. Next, photo-generated holes remain on the photoanode and usually experience slow kinetics at the interface between photoanode/electrolyte, resulting in hole accumulation, causing charge recombination, thus initiating oxygen evolution. The photoanode of the titanium vanadate film has shown enhanced PEC activity with lower charge transfer resistance observed from the impedance data than in the binary oxide of TiO2 and VO2 films.6,15
In addition, the higher photocurrent density of TiVO4 lead to a promising alternative for the PEC application in future, and the observed data has been compared with the other reported materials in Table 1. Impressive photocurrent density and stability tested time were observed for the TiVO4 photoanode compared with the other reported thin films developed via spray pyrolysis.
| Serial no. | Sample | Fabrication/deposition technique | Highest photocurrent (mA cm−2) (at 1.23 V vs. RHE) | Stability tested time (h) | Reference |
|---|---|---|---|---|---|
| 1 | LaFeO3 | Spray pyrolysis | 0.0180 | 21 | 61 |
| 2 | Bi2WO6 | Spray pyrolysis | 0.042 | — | 62 |
| 3 | BiFeO3 | Spray pyrolysis | 0.012 | — | 63 |
| 4 | ZnFe2O4 | Spray pyrolysis | 0.130 | 1.37 | 64 |
| 5 | TiVO4 | Spray pyrolysis | 0.080 | 1.66 | This study |
Although significant improvements have been achieved in the construction of highly efficient ternary TiVO4 PEC materials, the recombination rate of photo-generated charge carriers for TiVO4-based photocatalysts is still considerably high, accounting for poor reduction ability in the photoexcited electrons at a low potential of the conduction band edge, which is efficiently quenched by defects and holes. Morphological engineering could improve the PEC activity of TiVO4, regulating the crystal structure, particle size, and surface area leading to a large-scale preparation, which would enormously improve the separation efficiency of the photo-generated charge carriers. Further, TiVO4 could pave the way as a promising not only for PEC but also for smart coating and energy conversion.
4. Conclusion
This work describes the development of single-phase tetragonal nanostructure TiVO4, which is not explored as a PEC material. TiVO4 photoanode on FTO glass was fabricated using the scalable spray pyrolysis technique, a simple and efficient synthesis route showing a semi-transparent characteristic with a band gap of ∼2.18 eV. XRD, Raman and XPS spectroscopic analysis confirms the formation of tetragonal phase pure TiVO4 on an FTO glass. The development of TiVO4 photoanode was studied for various substrate and annealing temperatures. The SEM microstructure analyses revealed the temperature effect of the TiVO4 photoanode development. The optimized substrate temperature was found at 250 °C; the annealed temperature was 600 °C to achieve a single-phase TiVO4 photoanode. The TEM analyses signify that the homogeneous distribution of TiVO4 nanoparticles' agglomeration formed a dendritic outer edge fashion. The highest photocurrent density at 1.8 V vs. RHE of TiVO4 was recorded at 400 μA cm−2 for the substrate temperature at 250 °C. Additionally, a narrower band gap (2.18 eV) of n-type semiconductor TiVO4 makes it a beneficial light absorber, thus driving PEC water splitting for hydrogen generation. These results further inspire TiVO4 employment as a photoanode material with a particular emphasis on their applications in PEC solar water splitting under 1 SUN.Furthermore, sprayed TiVO4 photoanode showed excellent stability up to 6000 s. Besides, the photocurrent density monotonically improved from 73 to 400 μA cm−2 at 1.8 V vs. RHE with an increase in annealing temperature from 500 to 600 °C. Thus, the underlying photo-absorbing semiconductors could be fulfilled the lack of efficient photoanodes for the water-splitting reactions while not compromising either material's performance and achieving long-term passivation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Engineering and Physical Sciences Research Council (EPSRC) under the research grant no. EP/V049046/1. MA would like to acknowledge the financial support from the Saudi Arabia Culture Bureau in the United Kingdom. The X-ray photoelectron (XPS) data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”), operated by Cardiff University and UCL, under Contract No. PR16195. The authors acknowledge the help rendered for the SEM and TEM characterizations by Dr Hong Chang, Imaging Suite Manager, Harrison Building, University of Exeter, Streatham Campus, U.K.References
- R. Van de Krol and M. Grätzel, Photoelectrochemical hydrogen production, Springer, 2012 Search PubMed.
- Y. Wang, J. Zhang, M.-S. Balogun, Y. Tong and Y. Huang, Oxygen vacancy-based metal oxides photoanodes in photoelectrochemical water splitting, Mater. Today Sustain., 2022, 18, 100118 CrossRef.
- H. Shi, H. Guo, S. Wang, G. Zhang, Y. Hu, W. Jiang and G. Liu, Visible Light Photoanode Material for Photoelectrochemical Water Splitting: A Review of Bismuth Vanadate, Energy Fuels, 2022, 36(19), 11404–11427 CrossRef CAS.
- S. Li, W. Xu, L. Meng, W. Tian and L. Li, Recent Progress on Semiconductor Heterojunction-Based Photoanodes for Photoelectrochemical Water Splitting, Small Sci., 2022, 2100112 CrossRef CAS.
- A. R. Fareza, F. A. A. Nugroho, F. Abdi and V. Fauzia, Nanoscale metal oxides-2D materials heterostructures for photoelectrochemical water splitting—a review, J. Mater. Chem. A, 2022, 10, 8656–8686 RSC.
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- D. Bae, B. Seger, P. C. Vesborg, O. Hansen and I. Chorkendorff, Strategies for stable water splitting via protected photoelectrodes, Chem. Soc. Rev., 2017, 46, 1933–1954 RSC.
- R. Abe, Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation, J. Photochem. Photobiol., C, 2010, 11, 179–209 CrossRef CAS.
- Q. Huang, Z. Ye and X. Xiao, Recent progress in photocathodes for hydrogen evolution, J. Mater. Chem. A, 2015, 3, 15824–15837 RSC.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Artificial photosynthesis for solar water-splitting, Nat. Photonics, 2012, 6, 511–518 CrossRef CAS.
- A. A. Tahir, K. U. Wijayantha, S. Saremi-Yarahmadi, M. Mazhar and V. McKee, Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation, Chem. Mater., 2009, 21, 3763–3772 CrossRef CAS.
- T. Hong, Z. Liu, X. Zheng, J. Zhang and L. Yan, Efficient photoelectrochemical water splitting over Co3O4 and Co3O4/Ag composite structure, Appl. Catal., B, 2017, 202, 454–459 CrossRef CAS.
- S. Hoang, S. P. Berglund, N. T. Hahn, A. J. Bard and C. B. Mullins, Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: synergistic effects between Ti3+ and N, J. Am. Chem. Soc., 2012, 134, 3659–3662 CrossRef CAS PubMed.
- J. Bullock, A. Cuevas, T. Allen and C. Battaglia, Molybdenum oxide MoOx: a versatile hole contact for silicon solar cells, Appl. Phys. Lett., 2014, 105, 232109 CrossRef.
- I. N. Reddy, A. Sreedhar, J. Shim and J. S. Gwag, Multifunctional monoclinic VO2 nanorod thin films for enhanced energy applications: photoelectrochemical water splitting and supercapacitor, J. Electroanal. Chem., 2019, 835, 40–47 CrossRef CAS.
- E. A. Gibson, L. Le Pleux, J. Fortage, Y. Pellegrin, E. Blart, F. Odobel, A. Hagfeldt and G. Boschloo, Role of the triiodide/iodide redox couple in dye regeneration in p-type dye-sensitized solar cells, Langmuir, 2012, 28, 6485–6493 CrossRef CAS PubMed.
- H. M. Chen, C. K. Chen, Y. C. Chang, C. W. Tsai, R. S. Liu, S. F. Hu, W. S. Chang and K. H. Chen, Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: true efficiency for water splitting, Angew. Chem., 2010, 122, 6102–6105 CrossRef.
- D. Carballeda-Galicia, R. Castanedo-Perez, O. Jimenez-Sandoval, S. Jimenez-Sandoval, G. Torres-Delgado and C. Zuniga-Romero, High transmittance CdO thin films obtained by the sol–gel method, Thin Solid Films, 2000, 371, 105–108 CrossRef CAS.
- Q. Ding, L. Gou, D. Wei, D. Xu, W. Fan and W. Shi, Metal–organic framework derived Co3O4/TiO2 heterostructure nanoarrays for promote photoelectrochemical water splitting, Int. J. Hydrogen Energy, 2021, 46, 24965–24976 CrossRef CAS.
- Y. Fu, et al., A ternary nanostructured α-Fe2O3/Au/TiO2 photoanode with reconstructed interfaces for efficient photoelectrocatalytic water splitting, Appl. Catal., B, 2020, 260, 118206 CrossRef CAS.
- Y.-K. Hsu, Y.-C. Chen and Y.-G. Lin, Novel ZnO/Fe2O3 core–shell nanowires for photoelectrochemical water splitting, ACS Appl. Mater. Interfaces, 2015, 7, 14157–14162 CrossRef CAS PubMed.
- M. Alhabradi, S. Nundy, A. Ghosh and A. A. Tahir, Vertically Aligned CdO-Decked α-Fe2O3 Nanorod Arrays by a Radio Frequency Sputtering Method for Enhanced Photocatalytic Applications, ACS Omega, 2022, 7(32), 28396–28407 CrossRef CAS PubMed.
- D. Bae, G. Kanellos, K. Wedege, E. Dražević, A. Bentien and W. A. Smith, Tailored energy level alignment at MoOX/GaP interface for solar-driven redox flow battery application, J. Chem. Phys., 2020, 152, 124710 CrossRef CAS PubMed.
- P. Sahoo, A. Sharma, S. Padhan and R. Thangavel, Construction of ZnO@NiO heterostructure photoelectrodes for improved photoelectrochemical performance, Int. J. Hydrogen Energy, 2021, 46, 36176–36188 CrossRef CAS.
- Z. Dong, D. Ding, T. Li and C. Ning, Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting, Appl. Surf. Sci., 2018, 443, 321–328 CrossRef CAS.
- M. Altomare, K. Lee, M. S. Killian, E. Selli and P. Schmuki, Ta-doped TiO2 nanotubes for enhanced solar-light photoelectrochemical water splitting, Chem.–Eur. J., 2013, 19, 5841–5844 CrossRef CAS PubMed.
- C. Cheng and Y. Sun, Carbon doped TiO2 nanowire arrays with improved photoelectrochemical water splitting performance, Appl. Surf. Sci., 2012, 263, 273–276 CrossRef CAS.
- S. Hernández, S. M. Thalluri, A. Sacco, S. Bensaid, G. Saracco and N. Russo, Photo-catalytic activity of BiVO4 thin-film electrodes for solar-driven water splitting, Appl. Catal., A, 2015, 504, 266–271 CrossRef.
- H. Mandal, S. Shyamal, P. Hajra, A. Bera, D. Sariket, S. Kundu and C. Bhattacharya, Development of ternary iron vanadium oxide semiconductors for applications in photoelectrochemical water oxidation, RSC Adv., 2016, 6, 4992–4999 RSC.
- S. S. Kalanur and H. Seo, Facile growth of compositionally tuned copper vanadate nanostructured thin films for efficient photoelectrochemical water splitting, Appl. Catal., B, 2019, 249, 235–245 CrossRef CAS.
- Q. Jia, K. Iwashina and A. Kudo, Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11564–11569 CrossRef CAS PubMed.
- D. Mei, L. Li, C. Zhu, X. Zhao and Y. Yuan, Phy-chemical Attributes of Nano-scale V2O5/TiO2 Catalyst and Its' Effect on Soot Oxidation, Bull. Chem. React. Eng. Catal., 2016, 11, 161–169 CrossRef CAS.
- K. Y. Jung, Y. R. Jung, J.-K. Jeon, J. H. Kim and Y.-K. Park, Catalytic conversion of 1,2-dichlorobenzene over mesoporous V2O5/TiO2 prepared from spray pyrolysis, J. Nanosci. Nanotechnol., 2011, 11, 1710–1713 CrossRef CAS PubMed.
- A. Tripathi, R. G. Nair and S. Samdarshi, Visible active silver sensitized vanadium titanium mixed metal oxide photocatalyst nanoparticles through sol–gel technique, Sol. Energy Mater. Sol. Cells, 2010, 94, 2379–2385 CrossRef CAS.
- A. Wren, B. Adams, D. Pradhan, M. Towler and N. Mellott, Titanium–vanadium oxide nanocomposite thin films: synthesis, characterization and antibacterial activity, Mater. Chem. Phys., 2014, 144, 538–546 CrossRef CAS.
- P.-Y. Zhou, C.-C. Cheng, C.-H. Huang and J.-K. Chen, Hexagonal pillar structure of heteroepitaxial titania–vanadia nanocrystal films for high performance in thermochromic and photocatalytic properties, Phys. Chem. Chem. Phys., 2016, 18, 9088–9101 RSC.
- H. Liu, X. Wang, Z. Lan and H. Xu, Titanium Vanadium Oxide Thin Films Prepared by Thermal Oxidation of Titanium–Vanadium Alloy as Photocatalyst for Methylene Blue Degradation under Visible Light, J. Mater. Eng. Perform., 2021, 30, 1711–1722 CrossRef CAS.
- X. Jin, W. Sun, C. Chen, T. Wei, Y. Cheng, P. Li and Q. Li, Efficiency enhancement via tailoring energy level alignment induced by vanadium ion doping in organic/inorganic hybrid solar cells, RSC Adv., 2014, 4, 46008–46015 RSC.
- X. He, C. Zhang and D. Tian, The structure, vibrational spectra, and thermal expansion study of AVO4 (A = Bi, Fe, Cr) and Co2V2O7, Materials, 2020, 13, 1628 CrossRef CAS PubMed.
- G. Bera, V. Reddy, P. Rambabu, P. Mal, P. Das, N. Mohapatra, G. Padmaja and G. Turpu, Triclinic–monoclinic–orthorhombic (T–M–O) structural transitions in phase diagram of FeVO4–CrVO4 solid solutions, J. Appl. Phys., 2017, 122, 115101 CrossRef.
- X. Wang, X. Wang, Q. Di, H. Zhao, B. Liang and J. Yang, Mutual effects of fluorine dopant and oxygen vacancies on structural and luminescence characteristics of F doped SnO2 nanoparticles, Materials, 2017, 10, 1398 CrossRef PubMed.
- F. Zahedi, R. Dariani and S. Rozati, Effect of substrate temperature on the properties of ZnO thin films prepared by spray pyrolysis, Mater. Sci. Semicond. Process., 2013, 16, 245–249 CrossRef CAS.
- A. Bouzidi, N. Benramdane, H. Tabet-Derraz, C. Mathieu, B. Khelifa and R. Desfeux, Effect of substrate temperature on the structural and optical properties of MoO3 thin films prepared by spray pyrolysis technique, Mater. Sci. Eng., B, 2003, 97, 5–8 CrossRef.
- R. Irani, S. Rozati and S. Beke, Structural and optical properties of nanostructural V2O5 thin films deposited by spray pyrolysis technique: effect of the substrate temperature, Mater. Chem. Phys., 2013, 139, 489–493 CrossRef CAS.
- A. A. Tahir, M. A. Ehsan, M. Mazhar, K. U. Wijayantha, M. Zeller and A. Hunter, Photoelectrochemical and photoresponsive properties of Bi2S3 nanotube and nanoparticle thin films, Chem. Mater., 2010, 22, 5084–5092 CrossRef CAS.
- D. Perednis and L. J. Gauckler, Thin film deposition using spray pyrolysis, J. Electroceram., 2005, 14, 103–111 CrossRef CAS.
- A. Tricoli and T. D. Elmøe, Flame spray pyrolysis synthesis and aerosol deposition of nanoparticle films, AIChE J., 2012, 58, 3578–3588 CrossRef CAS.
- A. B. Khatibani and S. Rozati, Synthesis and characterization of amorphous aluminum oxide thin films prepared by spray pyrolysis: effects of substrate temperature, J. Non-Cryst. Solids, 2013, 363, 121–133 CrossRef.
- A. Roy, S. Mukhopadhyay, P. S. Devi and S. Sundaram, Polyaniline-layered rutile TiO2 nanorods as alternative photoanode in dye-sensitized solar cells, ACS Omega, 2019, 4, 1130–1138 CrossRef CAS PubMed.
- L. Wang, et al., Structural and electrochemical characteristics of Ca-doped “flower-like” Li4Ti5O12 motifs as high-rate anode materials for lithium-ion batteries, Chem. Mater., 2018, 30, 671–684 CrossRef CAS.
- J. Wang, X. Zhang, Y. Zhang, A. Abas, X. Zhao, Z. Yang, Q. Su, W. Lan and E. Xie, Lightweight, interconnected VO2 nanoflowers hydrothermally grown on 3D graphene networks for wide-voltage-window supercapacitors, RSC Adv., 2017, 7, 35558–35564 RSC.
- G. Silversmit, D. Depla, H. Poelman, G. B. Marin and R. De Gryse, Determination of the V 2p XPS binding energies for different vanadium oxidation states (V5+ to V0+), J. Electron Spectrosc. Relat. Phenom., 2004, 135, 167–175 CrossRef CAS.
- X. Zhao, Y. Yan, L. Mao, M. Fu, H. Zhao, L. Sun, Y. Xiao and G. Dong, A relationship between the V4+/V5+ ratio and the surface dispersion, surface acidity, and redox performance of V2O5–WO3/TiO2 SCR catalysts, RSC Adv., 2018, 8, 31081–31093 RSC.
- A. Roy, S. Bhandari, A. Ghosh, S. Sundaram and T. K. Mallick, Incorporating solution-processed mesoporous WO3 as an interfacial cathode buffer layer for photovoltaic applications, J. Phys. Chem. A, 2020, 124, 5709–5719 CrossRef CAS PubMed.
- R. Swapna and M. S. Kumar, The role of substrate temperature on the properties of nanocrystalline Mo doped ZnO thin films by spray pyrolysis, Ceram. Int., 2012, 38, 3875–3883 CrossRef CAS.
- T. S. Rao, C. Halpin, J. Webb, J. Noad and J. McCaffrey, Effect of substrate temperature on the growth rate and surface morphology of heteroepitaxial indium antimonide layers grown on (100) GaAs by metalorganic magnetron sputtering, J. Appl. Phys., 1989, 65, 585–590 CrossRef CAS.
- M. Mousavi, A. Kompany, N. Shahtahmasebi and M. Bagheri-Mohagheghi, Study of structural, electrical and optical properties of vanadium oxide condensed films deposited by spray pyrolysis technique, Adv. Manuf., 2013, 1, 320–328 CrossRef CAS.
- G. Rahman and O.-S. Joo, Photoelectrochemical water splitting at nanostructured α-Fe2O3 electrodes, Int. J. Hydrogen Energy, 2012, 37, 13989–13997 CrossRef CAS.
- S. S. Kalanur, Y. J. Lee and H. Seo, Exploring the synthesis, band edge insights, and photoelectrochemical water splitting properties of lead vanadates, ACS Appl. Mater. Interfaces, 2021, 13, 25906–25917 CrossRef CAS PubMed.
- Z. Chen, H. N. Dinh and E. Miller, Photoelectrochemical water splitting, Springer, 2013 Search PubMed.
- G. S. Pawar and A. A. Tahir, Unbiased spontaneous solar fuel production using stable LaFeO3 photoelectrode, Sci. Rep., 2018, 8, 1–9 CAS.
- B. Y. Alfaifi, A. A. Tahir and K. U. Wijayantha, Fabrication of Bi2WO6 photoelectrodes with enhanced photoelectrochemical and photocatalytic performance, Sol. Energy Mater. Sol. Cells, 2019, 195, 134–141 CrossRef CAS.
- G. Liu, S. K. Karuturi, H. Chen, D. Wang, J. W. Ager, A. N. Simonov and A. Tricoli, Enhancement of the photoelectrochemical water splitting by perovskite BiFeO3 via interfacial engineering, Sol. Energy, 2020, 202, 198–203 CrossRef CAS.
- J. W. Park, et al., Activation of a highly oriented columnar structure of ZnFe2O4 for photoelectrochemical water splitting: orchestrated effects of two-step quenching and Sn4+ diffusion, Sol. Energy Mater. Sol. Cells, 2018, 187, 207–218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05894d |
| This journal is © The Royal Society of Chemistry 2022 |