Open Access Article
Open Access ArticleWaste acrylonitrile butadiene styrene (ABS) incorporated with polyvinylpyrrolidone (PVP) for potential water filtration membrane
Syarifa Nur'ainia,
Akmal Zulfi*b,
Bagas Haqi Arrosyida,
Ande Fudja Rafryantoa,
Alfian Noviyanto *ac,
Dian Ahmad Hapidind,
Dafit Feriyanto
*ac,
Dian Ahmad Hapidind,
Dafit Feriyanto c,
Kurniawan Eko Saputro
c,
Kurniawan Eko Saputro a,
Khairurrijal Khairurrijal
a,
Khairurrijal Khairurrijal d and
Nurul Taufiqu Rochmane
d and
Nurul Taufiqu Rochmane
aNano Center Indonesia, Jalan Raya PUSPIPTEK, South Tangerang, Banten 15314, Indonesia. E-mail: a.noviyanto@nano.or.id
bResearch Center for Environmental and Clean Technology, National Research and Innovation Agency, Bandung Advanced Science and Creative Engineering Space (BASICS), Jl. Cisitu, Bandung 40135, Indonesia. E-mail: akmal.zulfi.m@brin.go.id
cDepartment of Mechanical Engineering, Mercu Buana University, Jl. Meruya Selatan, Kebun Jeruk, Jakarta 11650, Indonesia. E-mail: alfian.noviyanto@mercubuana.ac.id
dDepartment of Physics, Institut Teknologi Bandung, Jalan Ganesa 10, Bandung 40132, Indonesia
eResearch Center for Metallurgy and Materials, National Research and Innovation Agency, South Tangerang, Banten 15314, Indonesia
First published on 24th November 2022
Abstract
Acrylonitrile butadiene styrene (ABS) is one of the most common fused-filament feedstocks for 3D printing. The rapid growth of the 3D printing industry has resulted in huge demand for ABS filaments; however, it generates a large amount of waste. This study developed a novel method using waste ABS to fabricate electrospun nanofiber membranes (ENMs) for water filtration. Polyvinylpyrrolidone (PVP) was employed to modify the properties of waste ABS, and the effect of PVP addition in the range of 0–5 wt% was investigated. The results showed that adding PVP increased the viscosity and surface tension but decreased the conductivity of the precursor solution. After electrospinning, PVP could reduce the number of beads, increase the porosity and fiber diameter, and improve the wettability of the fabricated fibers. Moreover, the bilayer of ABS ENMs achieved a high flux value between 2951 and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 L m−2 h−1 and a high rejection rate of 99%. Our study demonstrates a sustainable strategy to convert waste plastics to inexpensive materials for wastewater treatment membranes.
041 L m−2 h−1 and a high rejection rate of 99%. Our study demonstrates a sustainable strategy to convert waste plastics to inexpensive materials for wastewater treatment membranes.
Introduction
Acrylonitrile butadiene styrene (ABS) is a thermoplastic polymer consisting of 70–80% styrene–acrylonitrile copolymer (SAN) and 20–30% polybutadiene rubber.1,2 Owing to its excellent heat resistance, glossy surfaces, high hardness, and easy processability,3 ABS has been widely used as housing materials for automotive systems, air conditioners, computers, and electronic appliances.3–7 Rapid technology development requires design freedom for customized parts; therefore, 3D printing has become popular for polymer fabrication in many fields.8 This remarkable technology creates a high demand for filaments as feedstock, and ABS is one of the most common polymer feedstock.9,10 The high demand for ABS filament also increases its waste. ABS waste is the major component of waste electrical and electronic equipment (WEEE).11,12 The WEEE streams contributed to 20% of all plastic waste types and reached 53.6 million tons in 2019.13 The common methods of plastic waste treatment include landfilling and incineration, which are not environmentally safe.14 The landfill process may pollute groundwater, and the incineration of WEEE causes toxic dust containing heavy metals, dioxins, and other harmful substances.15–17 Therefore, it is urgently required to develop a sustainable solution to recycle ABS waste.Several studies have attempted to utilize ABS waste in higher-value products such as additive bitumen binders,18 green catalysts for oil and metal recovery,19 recycled filaments for reuse in 3D printing,20 and epoxy materials.4 Recently, ABS waste has been recycled into nanofiber membranes for air filtration.21,22 Nanofiber membranes made from recycled ABS waste exhibited filtration efficiencies of approximately 95% for PM 2.5 because the interconnected pore structures and the large surface area were suitable for particle capture during gas filtration. Nanofiber membranes also have a unique pore structure, narrow pore size, and high selectivity,23 which are ideal for water treatment and filtration.24–28 Excellent performance has been achieved with an efficiency of 99.99% and a flux of 5000–35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1.29–32 However, to the best of the authors knowledge, there are no reports on recycling ABS waste into water filtration membranes.
000 L m−2 h−1.29–32 However, to the best of the authors knowledge, there are no reports on recycling ABS waste into water filtration membranes.
Electrospinning is a well-known technique for fabricating nanofiber membranes from various polymers. This technique is versatile and has the ability to control nanofiber morphology by adjusting the solution and process parameters.33 ABS nanofiber membranes have hydrophobic surfaces,21,34 which are unfavorable for water filtration because they can increase energy consumption.35 Therefore, water-soluble materials are often added to enhance the membrane flux. Polyvinylpyrrolidone (PVP) and polydopamine (PDA) increased the surface hydrophilicity and flux rate of the membranes.36 Another study by Park et al. reported that the modification of a hydrophobic membrane with polyvinyl alcohol (PVA) could enhance membrane performance.37
The present work aimed to recycle ABS to electrospun nanofiber membranes (ENMs) for microfiltration in a 2500 ppm antacid solution. This study also investigated the surface modification of ENMs by blending waste ABS with PVP. Adding PVP is expected to improve the hydrophilicity of ENMs and enhance the permeate flux as well as filtration efficiency. The ENMs prepared with waste ABS in this study have comparable filtration performance to the membranes prepared using expensive polymers. In addition, recycling ABS waste has a positive impact on the environment.
Experimental materials
ABS was obtained as 3D-printing filament waste from Shenzhen eSun Industrial Co. Ltd PVP (Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich. N,N-dimethylformamide (DMF) was used as the solvent (Sigma-Aldrich). In addition, commercial nonwoven polyethylene terephthalate (PET) was used as the substrate to deposit the nanofiber, and a commercial antacid was used as a particle model in the membrane performance test.
000) was purchased from Sigma-Aldrich. N,N-dimethylformamide (DMF) was used as the solvent (Sigma-Aldrich). In addition, commercial nonwoven polyethylene terephthalate (PET) was used as the substrate to deposit the nanofiber, and a commercial antacid was used as a particle model in the membrane performance test.
Synthesis of ENMs
ABS filament waste was washed and cut into pieces to facilitate the subsequent dissolution process. ABS and PVP with various weight percentages were dissolved in DMF at 40–50 °C under magnetic stirring until the solution became homogeneous. A conductometer (Mettler Toledo, Seveneasy Conductivity, Switzerland), Fenske–Oswald viscometer (Fisher Scientific, 50 A643), and du Noüy ring tensiometer (Fisher) were used to measure the conductivity, viscosity, and surface tension of the precursor solutions, respectively. The measurements were performed at room temperature (25 ± 5 °C). Table 1 lists the weight percentages (wt%) of ABS and PVP used in each precursor solution and its measured conductivity, viscosity, and surface tension.| Parameter/code | ABSP0 | ABSP1 | ABSP3 | ABSP5 |
|---|---|---|---|---|
| ABS (wt%) | 28 | 27 | 25 | 23 |
| PVP (wt%) | 0 | 1 | 3 | 5 |
| Viscosity (cP) | 153.91 | 384.82 | 858.12 | 1259.81 |
| Conductivity (μS) | 9.07 | 4.05 | 3.71 | 2.87 |
| Surface tension (dyne cm−1) | 62.72 | 64.95 | 65.37 | 66.43 |
The precursor solution was loaded into a commercial syringe-fitted stainless-steel needle with an inner diameter of 0.7 mm. The filled syringe was controlled by a syringe pump at a constant rate of 0.3 mL hour−1. The tip of the needle was connected to a high-voltage source operated at 12 kV. The distance between the needle tip and the drum collector was 12–15 cm. The drum collector was grounded and rotated to deposit the spun fibers. To compile the nanofibers easily, the drum collector was also wrapped with nonwovens. The apparatus was set up in a horizontal-spinning configuration at room temperature (25 ± 5 °C) under a relative humidity of 60%. ENMs were continuously synthesized for 7 h to obtain nanofibers on an electrospinning apparatus (ILMI-N101, Integrated Laboratory of Materials and Instrumentation, ITB, Indonesia).
Characterizations
X-ray diffraction (XRD, Rigaku MiniFlex 600) was used to measure the crystallinity of the ENMs at room temperature in the 2θ range of 5°–90°. The chemical bonds in the fiber were analyzed by Fourier transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet iS10). The size and morphology of the ENMs were observed by scanning electron microscopy (SEM, Hitachi SU3500). Before SEM observation, the ENMs were coated with Au to enhance conductivity and minimize charging. The fiber size distribution was calculated based on 250 random fibers, while the diameter of the beads was estimated by measuring the length and width of the beads in the SEM images using the Image J software. The surface porosity was obtained by two-dimensional calculation on the SEM image, as described in a previous study.38–40Because the ENMs are projected for water filtration, the water contact angle (WCA) is an important parameter to ensure hydrophilicity. Therefore, WCA was investigated using a contact angle apparatus (Nachriebe 320, Nachriebe). Water (5 μL) was used to create a droplet and the shape of the droplet on the membrane surface was captured using a camera, and the image was digitally processed using Image J software to obtain the WCA.
Performance and filtration test
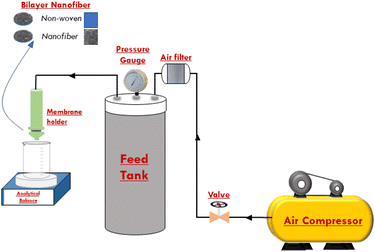
The performance of the ENMs was assessed using the flux value of pure water and the efficiency of particle rejection. Dead-end flux was evaluated using a system containing a compressors, valves, prefilters, low-pressure regulators, membrane holders, and an analytical balance (Fig. 1).36,40 The pressure for the pure water flux (PWF) test was controlled between 0.025 and 0.1 bar, while the pressure for filtration evaluation was 0.025 bar. Low pressure was applied in the microfiltration process.28 Pressurized clean air flowed through the ENMs to obtain the permeate volume. The bilayer nanofiber is an assembly of two pieces of ENMs and substrates arranged alternately. The PWF value indicated the maximum amount of fluid that was streamed through the ENMs. The higher the flux value, the less energy consumed.41–43 The flux value (J) can be expressed by eqn (1).
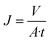 | (1) |
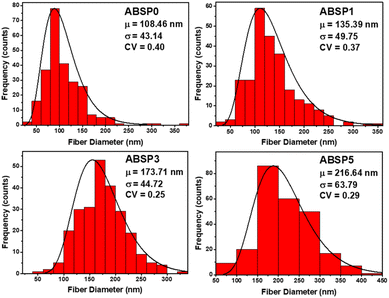
The feed and permeate concentrations were measured using a UV/Vis spectrophotometer (Labtron LUS-B13). The concentration was determined based on a calibration curve.45 The concentrations used for calibration were 2500, 1875, 1250, 625, 317, and 0 ppm. The permeate concentration was derived from the calibration curve. The retention ratio (%) was calculated using eqn (2):46,47
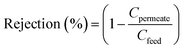 | (2) |
Results and discussion
Nanofiber characterization
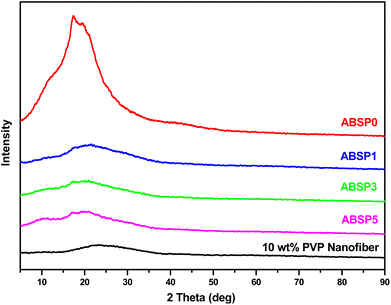
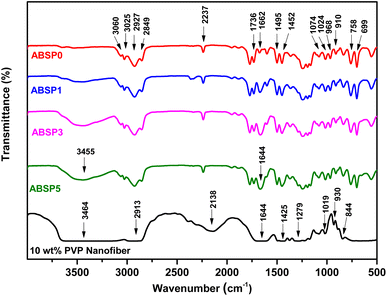
Nanofibers were successfully prepared using the solution of 23–28 wt% ABS waste and 1–5 wt% PVP. The XRD patterns of ABSPs (Fig. 3) agreed with the characteristics of amorphous materials.48 ABSP0 had a broad peak between 10° and 30° similar to the pure ABS reported in a previous study.49 However, the peak intensity of the ABSP0 was higher. Some reports attributed the high intensity to the molding process and the intermolecular hydrogen bonding.50,51 Nevertheless, the XRD pattern of ABSP0 was in agreement with the study using ABS as an amorphous feedstock polymer.9,52 For comparison, the ENM with 10 wt% PVP was prepared to show the typical peak of pure PVP. Therefore, it can be understood the difference in the XRD patterns of ABSP1, ABSP3, and ABSP5 are due to the addition of PVP. In line with the previous study, the addition of other polymer with high molecular weight will decrease the intensity of XRD pattern.53Fig. 4 shows the FTIR spectra of the ENMs. ABS was formed by polymerizing three monomers, i.e., acrylonitrile, polybutadiene, and styrene. The spectra of ABS waste were similar to the previous report.54,55 The peak at 3000–3100 cm−1 was the absorption of asymmetric C–H aromatic bonds in styrene compounds, while the peak at 2800–3000 cm−1 corresponded to the aliphatic C–H bond, and that at 2237 cm−1 was the deformation of nitrile C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds. The C
N bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic double bond stretching vibration was found at 1602 cm−1. The peaks between 1495 and 1452 cm−1 corresponded to the stretching of C–C in the aromatic ring, and the stretching of unsaturated C
C aromatic double bond stretching vibration was found at 1602 cm−1. The peaks between 1495 and 1452 cm−1 corresponded to the stretching of C–C in the aromatic ring, and the stretching of unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in polybutadiene. The bending C–H in-plane and (mono)-substituted out-of-plane vibrations were at 1074 and 1024 cm−1 and 758 and 699 cm−1, respectively. In our ABS waste, a carbonyl (C
C in polybutadiene. The bending C–H in-plane and (mono)-substituted out-of-plane vibrations were at 1074 and 1024 cm−1 and 758 and 699 cm−1, respectively. In our ABS waste, a carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching peak was observed at 1736 cm−1 because of the oxidation during its usage and application.4 The spectral peak of the pure PVP was in line with previous studies. The peak at 3464 cm−1 was related to the stretching of a hydroxyl group (hydrogen bonding). The peaks at 2913 cm−1, 1644 cm−1, 1425 cm−1, and 1279 cm−1 were assigned to the asymmetric CH2 stretching, carbonyl group C
O) stretching peak was observed at 1736 cm−1 because of the oxidation during its usage and application.4 The spectral peak of the pure PVP was in line with previous studies. The peak at 3464 cm−1 was related to the stretching of a hydroxyl group (hydrogen bonding). The peaks at 2913 cm−1, 1644 cm−1, 1425 cm−1, and 1279 cm−1 were assigned to the asymmetric CH2 stretching, carbonyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, bending of C–H bond, C–N bond stretching, respectively. The peaks at 1019 cm−1, 930 cm−1, and 844 cm−1 were attributed to the C–C bending. In the spectra of ABSPs, sharp peaks at 3455 cm−1 and 1644 cm−1 were observed due to the stretching of the hydrogen bonding (O–H) and carbonyl group (C
O, bending of C–H bond, C–N bond stretching, respectively. The peaks at 1019 cm−1, 930 cm−1, and 844 cm−1 were attributed to the C–C bending. In the spectra of ABSPs, sharp peaks at 3455 cm−1 and 1644 cm−1 were observed due to the stretching of the hydrogen bonding (O–H) and carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. The reaction might occur between the C
O), respectively. The reaction might occur between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the pyrrolidone ring and the hydrocarbons from polybutadiene or benzene compound36 to form O–H and C
O groups in the pyrrolidone ring and the hydrocarbons from polybutadiene or benzene compound36 to form O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. DMF as a solution was not detected in the spectra of all samples, confirming that the solvent was completely evaporated.56
O bonds. DMF as a solution was not detected in the spectra of all samples, confirming that the solvent was completely evaporated.56
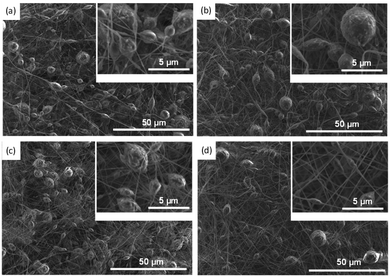
The SEM morphology of the ENMs (Fig. 5) shows the formation of bead nanofibers. A similar bead nanofiber was reported by Jiang et al. when 15 wt% of ABS in DMSO and CHCl3 was used for electrospinning. The same method generated bead-free nanofiber when the ABS content was increased to 20–25 wt%.34 Bead-free ABS nanofibers were obtained by mixing the solvent (DMF and THF) at a ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as reported by Moradi et al.57 Zulfi et al. prepared bead-free nanofibers from 30 wt% of ABS waste.21 Therefore, the formation of bead nanofibers in this study was more likely due to the low concentration of ABS waste. Moreover, the source of ABS, solvent, and other additives might influence the formation of bead nanofiber.
1, as reported by Moradi et al.57 Zulfi et al. prepared bead-free nanofibers from 30 wt% of ABS waste.21 Therefore, the formation of bead nanofibers in this study was more likely due to the low concentration of ABS waste. Moreover, the source of ABS, solvent, and other additives might influence the formation of bead nanofiber.
Interestingly, the total number of beads was closely related to the weight percentage of PVP. For instance, the total number of beads for ABSP0, ABSP1, ABSP3, and ABSP5 were ∼643, ∼550, ∼454, and ∼262, respectively. PVP increased the viscosity of the precursor solution and improved jet stability, resulting in a decrease in bead formation. Moreover, the average bead diameter reduced with increasing PVP content. The average bead diameters of the ABSP0, ABSP1, ABSP3, and ABSP5 ENMs were 5.832, 5.382, 3.485, and 3.137 μm, respectively (Fig. 6). In addition to decreasing the number and size of the beads, PVP changed the beads from spherical to elongated shapes.58 In this study, the shape change was not evident, and the coefficient of variance (CV) of the size was >0.3, suggesting the uniformity of beads or fibers.59 The small number of beads in ABSP5 indicated that the size of the beads changed from large to small, eventually forming bead-free fibers. As shown in Table 1, a higher PVP content in the solution increased the viscosity and surface tension, and reduced the conductivity. This result is in agreement with published reports that showed bead-free fibers with higher PVP content.60–62 However, the dependence of surface tension on the formation of beads was inconsistent in the literature. Almetwally et al. found that higher surface tension produced more beads on fiber.63 However, Liu et al. reported that a high surface tension could yield a bead-free fiber.64
 | ||
| Fig. 6 Distribution of beads diameter based on the SEM images in Fig. 5. | ||
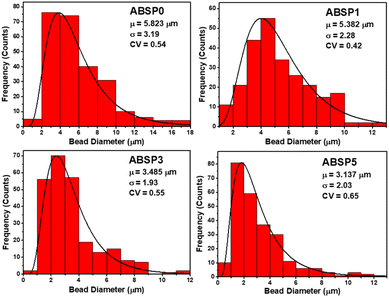
Fig. 7 shows the distribution of fiber diameters when different contents of PVP were added. The mean fiber diameters of the ABSP0, ABSP1, ABSP3, and ABSP5 ENMs were 108.46, 136.39, 173.71, and 216.64 nm, respectively. The fiber diameter increased with PVP, as reported previously.65 The fiber diameters of ABSP3 and ABSP5 were considered a homogeneous distribution, characterized by CV < 0.3. In contrast, the CV values of ABSP0 and ABSP1 were 0.37 and 0.4, respectively, implying an inhomogeneous fiber size. As shown in Table 1, the conductivity of the solution decreased with PVP; therefore, the thin fiber of ABSP0 was due to the high conductivity of the solution, consistent with a previous report that high conductivity generated thin fibers.27
 | ||
| Fig. 7 Distribution of fiber diameter based on the SEM images in Fig. 5. | ||
The morphology of the ENMs also influenced the surface porosity. A high PVP concentration was reported to increase the porosity, which agreed with the present study that the porosities of ABSP1, ABSP3, and ABSP5 increased from 64.18, 70.33, to 71.78%, respectively. Porosity was more likely to be affected by the size of the fiber rather than the size of the bead. ABSP5 had a small bead diameter; however, the fiber diameter increased by a factor of two or more, resulting in its high porosity (71.78%). Comparatively, despite the large bead diameter of ABSP0, its porosity was only 65.23% owing to the small fiber diameter.
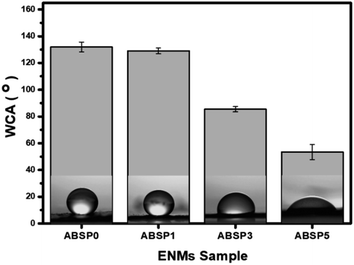
Fig. 8 shows the WCA of the ENMs that represent the hydrophilicity of membrane.66 The two mechanisms of water passing through the membrane are (1) water penetrates the porous membranes and (2) water seeps into the membranes. As mentioned above, PVP is a water-soluble material;67,68 thus, a high PVP content will increase the permeability of the membrane. Therefore, it was evident that the lowest WCA was found in ABSP5. Moreover, ABSP5 had more pores than the others, making it easier for water to pass through.48 The average WCAs of ABSP0, ABSP1, ABSP3, and ABSP5 were 131.97°, 129.09°, 85.48°, and 53.40°, respectively, as shown in Fig. 8. It should be noted that ABSP0 was hydrophobic, consistent with previous studies.21,34 Although PVP was water-soluble, adding 1 wt% PVP into ABS did not change the WCA. The WCA of ABS was reduced by adding at least 3 wt% of PVP. The ABSP3 had a WCA of 85.48° and could be categorized as hydrophilic, as well as ABSP5 which had WCA of 53.40°. Hydrophilic behavior is characterized by WCA < 90°.69
ENMs performance
Before the microfiltration test, the PWF values of the ENMs were examined to define the volume of permeate per unit time and the transmembrane pressure. The surface area directly in contact with the pure water and feed solution was 17.14 cm2. The energy consumption was estimated for the permeate to pass through the membranes (permeability). Fig. 9(a) presents the PWF values of the ENMs at a fixed pressure of 0.025 bar. Indeed, the PWF was in accordance with the wettability, as explained in the previous subsection of nanofiber characterization. ABSP0 and ABSP1 had similarly low PWF values because of the hydrophobic properties of the ENMs. ABSP3 had a higher PWF due to increased hydrophilicity, and the PWF was the highest for ABSP5 owing to its hydrophilic properties. As shown in Fig. 9(a), average PWF values for ABSP0, ABSP1, ABSP3, and ABSP5 were 2820.2 ± 159, 3538.3 ± 56, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 512.5 ± 152, and 17
512.5 ± 152, and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 395 ± 163 L m−2 h−1, respectively. Fig. 9(b) shows that the PWF increased with increasing applied pressure. The fluid flowing through a porous medium in a low-pressure condition follows Darcy's law,70 which is derived from the Navier–Stokes equation:71
395 ± 163 L m−2 h−1, respectively. Fig. 9(b) shows that the PWF increased with increasing applied pressure. The fluid flowing through a porous medium in a low-pressure condition follows Darcy's law,70 which is derived from the Navier–Stokes equation:71
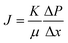 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 L m−2 h−1, as shown in Fig. 9(b). High permeability is one of the essential requirements for high-performance membranes because it can reduce energy consumption and manufacturing costs.72 In contrast, the appearance of beads increased the pressure drop, which was another reason for the decreased permeability of ENMs with a large number of beads.73
041 L m−2 h−1, as shown in Fig. 9(b). High permeability is one of the essential requirements for high-performance membranes because it can reduce energy consumption and manufacturing costs.72 In contrast, the appearance of beads increased the pressure drop, which was another reason for the decreased permeability of ENMs with a large number of beads.73
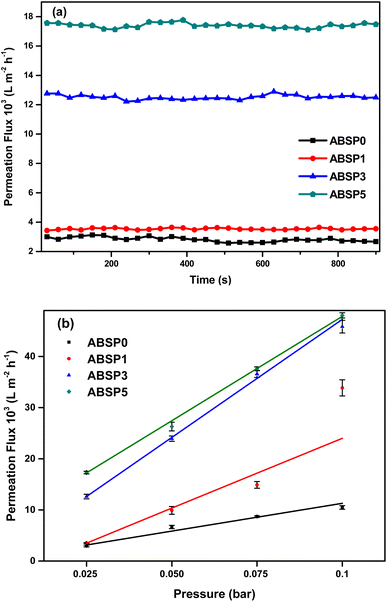 | ||
| Fig. 9 Performance evaluation of ENMs (a) permeation flux at a fixed pressure (0.025 bar), and (b) permeation flux as a function of pressures. | ||
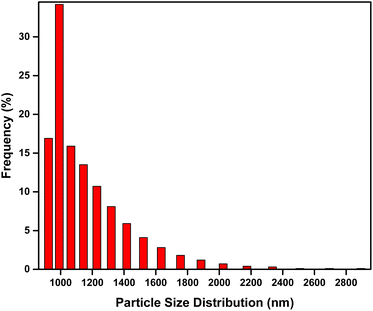
This study used microfiltration because the particle model size was between 900 and 2600 nm. Therefore, besides the suspended solid larger than 0.1 μm, the other contaminants, such as oil emulsion, bacteria, cells, and colloidal haze, will be suspended in the membrane.74 Fig. 10(a) shows the permeability of the ENMs using the particle model of antacids (Fig. 2). The rejection rates of ABSP1, ABSP3, and ABSP5 were 99%. Hence, the permeate solutions of ABSP1, ABSP3, and ABSP5 were clear, as shown in Fig. 10(b). In contrast, the permeate solution of ABSP0 was turbid in Fig. 10(b), corresponding to a rejection rate of 83%. These results indicated the importance of adding PVP to enhance the permeability of ABS ENMs. To the best of our knowledge, adding PVP to ABS waste has rarely been reported, particularly for water filtration applications. Moreover, this study successfully modified the surface characteristics of ABS waste, changing it from hydrophobicity suitable for air filtration21 to hydrophilicity with the addition of PVP. Although the porosity of ABSP0 was smaller than that of the other ENMs, the filtration ability was not solely dependent on porosity. For instance, ABSP0 had a porosity of 65.23% and a rejection rate of 83%. Meanwhile, the rejection rate of ABSP1 was 99%, despite its similar porosity to ABSP0 (64.18%). Therefore, it can be concluded that PVP enhances the applicability of ABS waste for water filtration.
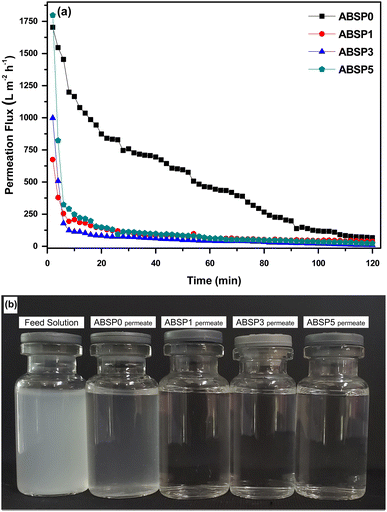 | ||
| Fig. 10 (a) Permeation flux while filtration at 0.05 bar; (b) photographs of feed and permeate solutions after the filtration process. | ||
Three mechanisms are involved in the filtration process, i.e., sieving, adsorption, and cake formation. Sieving occurs first as larger particles are retained on the surface of the membranes. Smaller particles flow through the inner membrane and are adsorbed on the fiber surface. Lastly, the particles in the membrane pores are retained on the surface and form a cake layer.75 In this study, the cake layer was formed in two-hour filtration with 2500 ppm feed solution and remove manually. From the SEM images of ABSP1 after the filtration process (Fig. 11), depth filtration was confirmed in ABSP1. This membrane first accumulated particles on the surface, then small particles remained in the inner layer, resulting in a clear permeate. It seemed the cake formation mainly occurred on the surface for ABSP1. A previous study reported that depth filtration in bead fibers would extend the lifetime of the membrane, whereas filtration onto the membranes had a short lifetime and high-pressure drop.76 Recently, clean-up processes for depth clogging have been developed to enhance the reusability of the membranes. Latest studies to achieve surface filtration, remove a cake layer, and improve the mechanical and morphological properties are important contributions to minimizing the disadvantages.77
 | ||
| Fig. 11 SEM images of the first layer of ABSP1 ENMs after the filtration process and heating at 40 °C (a) filtered particles from surface view, and (b) cross-section view. | ||
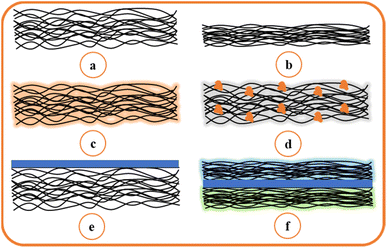
Based on this significant result, nanofiber membranes can be used in more advanced applications. However, challenges remain to make the membranes sufficiently strong, antifouling, and applicable to all types of waste treatment. The cake layer of pollutants in the nanofiber membranes is easy to remove and expected to render the membranes reusability. To restrict the pollutant on the surface, the nanofiber membranes should have a sufficiently strong surface to separate the pollutant. Fig. 12 depicts six types of surface modification methods in recent studies: (a) normal nanofiber synthesis, (b) heat treatment with annealing or direct warming at a specific temperature, (c) blending polymers with different properties or chemical crosslinking, (d) incorporating functional materials to obtain specific properties, (e) layering the nanofiber, and (f) combining two types of modified nanofiber.83 The heat-annealed ENMs shown in Fig. 12(b) have several advantages, such as small pore size and excellent mechanical strength.31,77 Blending with other copolymers makes the fiber more hydrophilic, and crosslinking with other materials can enhance the mechanical strength,36,84 as shown in Fig. 12(c). Incorporating various functional material can achieve antibacterial, hydrophobic, and antifouling properties,85–87 as shown in Fig. 12(d). The coating layer in Fig. 12(e) is usually made from water-soluble materials, enhancing the water flux and retaining the pollutant on the surface.37 Furthermore, Fig. 12(f) is usually applied in membrane distillation applications, wherein two types of different polymers with modifications in each layer are incorporated; each layer can be heated to decrease the surface, crosslinked with other materials, or developed with a support layer.88
Table 2 compares the precursors, electrospinning parameters, ENMs characteristics, and efficiencies reported in the literature. Indeed, most of the published reports in Table 2 used non waste precursors to fabricate effective and high-performance nanofiber membranes. This study employed ABS waste to reduce the cost of the precursor and alleviate the environmental burden. As shown in Table 2, the fiber diameter in this study was comparable to that in other published reports. Moreover, compared with polylactic acid (PLA), polyether sulfones (PES), cellulose acetate (CA), polyimides (PI), and polysulfone (PSU) precursors with fiber diameters of 500–1700 nm, our study showed a reduced fiber diameter (108.46–216.64 nm). The rejection rate in this study was comparable to other ENMs, as shown in Table 2. Based on the comparison, nanofibers prepared by ABS waste with a simple PVP modification are suitable for the microfiltration process. This study provides a new strategy to reduce the amounts of WEEE in the environment, enhance the added value of recycled products and help to manage water resources. This result provides a promising way to utilize waste for more advanced membranes in the future.
| Precursor solution (polymer/solvent) | Electrospinning parameters concentration (wt%), voltage (kV), flow rate (mL h−1), diameter needle tip (mm), collector distance (cm), humidity (%) | ENMs characteristics diameter (nm), porosity (%), water flux | Efficiency (%) |
|---|---|---|---|
| a PAN, polyacrylonitrile; PLA, polylactic acid; PES, polyether sulfones; PVA, polyvinyl alcohol; CA, cellulose acetate; PI, polyimides; PSU, polysulfone; NMP, N-methyl-2-pyrrolidone, THF tetrahydrofuran. | |||
| PAN/DMF78 | 7–12, 18, 0.5, 0.4, NA, NA | 150–250, 34 to 86, 500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 000 L m−2 h−1 |
99.3 |
| PLA/acetone77 | 11–13, 20, 0.5, 0.4, 10, 30 | 500–1200, 5–70, 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65 000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 000 L m−2 h−1 |
10–85 |
| PAN-PET/DMF32 | 4–18, 35, NA, 0.6, 7–19, 40 | 100–500, 61–74, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–35 500–35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 000 L m−2 h−1 |
93–99 |
| PES/NMP31 | 25–40 w/v%, 10–15, 0.5, 1, 10–20, 50–60 | 550–1300, NA, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006 L m−2 h−1 bar−1 006 L m−2 h−1 bar−1 |
100 |
| PVA/distilled water79 | 10, 32, 0.6, 1, 10, 50–60 | 100–182, NA, ∼5417 L m−2 h−1 bar−1 | ∼95 |
| CA/acetic acid–acetone80 | 10, 10, 10 mL h−1, 0.4, 15, NA | 500–1700, NA, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 L m−2 h−1 bar−1 900 L m−2 h−1 bar−1 |
87.7 |
| PAN/DMF29 | 8–12, 20, 1.5–3, NA, 15, NA | 151–535, NA, 5000–7000 L m−2 h−1 | 81.4–99 |
PI/NMP–xylene (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)81 1)81 |
12, 16, 0.4, 0.4, NA, NA | <1000, NA, 514–17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607 L m−2 h−1 607 L m−2 h−1 |
96.7 |
PVA/citric acid–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)30 8)30 |
10, 22, 2 μL min−1, NA, 12/5, NA | 510–535, NA, 7500–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 L m−2 h−1 500 L m−2 h−1 |
99 |
PSU/DMF–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)82 1)82 |
20, 16, 2.5, 1.825, 10, 38–41 | 690–830, NA, NA | 75.3 |
| R-ABS-PVP/DMF (this study) | 28, 12, 0.3, 0.7, 12–15, 60 | 108.46–216.64, 64.18–71.78, 2951–48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 L m−2 h−1 041 L m−2 h−1 |
83–99 |
Conclusions
ABS waste was successfully recycled as nanofiber membranes for microfiltration using an electrospinning technique. All the samples had similar characteristics, showing the presence of beads in the fiber. The average fiber diameter was 108.46–216.64 nm using a total 28 wt% ABS waste in DMF with a PVP concentration between 0 and 5 wt%. A high concentration of PVP (5 wt%) could decrease the number of beads, increase fiber diameter and surface porosity, also reduce WCA to achieve hydrophilic behavior. These properties influenced the performance of membrane filtration. The PWF was 2951–48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 L m−2 h−1. A small amount of PVP (1 wt%) could enhance the rejection rate significantly from 83% (ABSP0) to 99% (ABSP1). This study emphasizes that polymeric waste could be a future material for inexpensive ENMs for advanced wastewater applications.
041 L m−2 h−1. A small amount of PVP (1 wt%) could enhance the rejection rate significantly from 83% (ABSP0) to 99% (ABSP1). This study emphasizes that polymeric waste could be a future material for inexpensive ENMs for advanced wastewater applications.
Author contributions
Syarifa Nur'aini: formal analysis, methodology, investigation, writing – original draft. Akmal Zulfi: conceptualization, methodology, investigation, supervision, writing – review & editing. Bagas Haqi Arrosyid: formal analysis, investigation. Ande Fudja Rafriyanto: formal analysis, investigation. Alfian Noviyanto: conceptualization, formal analysis, supervision, funding acquisition, writing – review & editing. Dian Ahmad Hapidin: resources, supervision, writing – review & editing. Dafit Feriyanto: resources. Kurniawan Eko Saputro: formal analysis, resources. Khairurrijal: resources, writing – review & editing. Nurul T. Rochman: resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Kerjasama Dalam Negeri Scheme (KDN) [grant number 02-5/1082/B-SPK/III/2022], Mercu Buana University. The authors would like to express their gratitude to the Nano Center Indonesia for their facilities and characterization.References
- D. M. Kulich, S. K. Gaggar, V. Lowry and R. Stepien, Acrylonitrile–Butadiene–Styrene (ABS) Polymers, John Wiley & Sons, Ltd, Hoboken, NJ, USA, 2003, vol. 1 Search PubMed.
- R. Balart, J. López, D. García and M. D. Salvador, Eur. Polym. J., 2005, 41, 2150–2160 CrossRef CAS.
- A. C. Ferreira, M. F. Diniz and E. da C. Mattos, Polimeros, 2018, 28, 6–14 CrossRef.
- J. Wang, Y. Li, J. Song, M. He, J. Song and K. Xia, Polym. Degrad. Stab., 2015, 112, 167–174 CrossRef CAS.
- R. Scaffaro, L. Botta and G. Di Benedetto, Eur. Polym. J., 2012, 48, 637–648 CrossRef CAS.
- P. Żur, A. Kołodziej, A. Baier and G. Kokot, Eur. J. Eng. Sci. Technol., 1970, 3, 44–51 CrossRef.
- L. Gripon, I. Belyamani, B. Legros, K. Seaudeau-Pirouley, E. Lafranche and L. Cauret, J. Waste Manage., 2021, 131, 313–322 CrossRef CAS PubMed.
- L. Han, C. Chen, L. Shen, H. Lin, B. Li, Z. Huang, Y. Xu, R. Li and H. Hong, J. Mater. Chem. A, 2022, 10, 12055–12061 RSC.
- D. Vaes and P. Van Puyvelde, Prog. Polym. Sci., 2021, 118, 101411 CrossRef CAS.
- Z. Liu, Y. Wang, B. Wu, C. Cui, Y. Guo and C. Yan, Int. J. Adv. Manuf. Technol., 2019, 102, 2877–2889 CrossRef.
- Y. Cui, Y. Li, W. Wang, X. Wang, J. Lin, X. Mai, G. Song, N. Naik and Z. Guo, Sep. Purif. Technol., 2021, 269, 118767 CrossRef CAS.
- T. D. Nguyen, T. M. Al Tahtamouni, P. T. Huong and P. Q. Thang, J. Environ. Chem. Eng., 2019, 7, 103354 CrossRef.
- G. F. Cardamone, F. Ardolino and U. Arena, J. Waste Manage., 2021, 126, 119–132 CrossRef CAS PubMed.
- R. Al-Jarallah and E. Aleisa, J. Waste Manage., 2014, 34, 952–960 CrossRef CAS PubMed.
- H. Wang, X. L. Chen, Y. Bai, C. Guo and L. Zhang, J. Waste Manage., 2012, 32, 1297–1305 CrossRef CAS PubMed.
- C. Ma, J. Yu, B. Wang, Z. Song, J. Xiang, S. Hu, S. Su and L. Sun, Renewable Sustainable Energy Rev., 2016, 61, 433–450 CrossRef CAS.
- W. C. Qing, H. Wang, J. G. Fu and Y. N. Liu, J. Waste Manage., 2015, 41, 28–38 CrossRef PubMed.
- P. K. Singh, S. K. Suman and M. Kumar, Transp. Res. Procedia, 2020, 48, 3668–3677 CrossRef.
- C. Areeprasert and C. Khaobang, Fuel Process. Technol., 2018, 182, 26–36 CrossRef CAS.
- M. I. Mohammed, A. Das, E. Gomez-Kervin, D. Wilson and I. Gibson, in Solid Freeform Fabrication: Proceedings of the 28th annual international solid freeform fabrication symposium - an Additive Manufacturing Conference, 2017, pp. 532–542 Search PubMed.
- A. Zulfi, D. A. Hapidin, M. M. Munir, F. Iskandar and K. Khairurrijal, RSC Adv., 2019, 9, 30741–30751 RSC.
- M. M. Munir, A. I. Kamil and M. Burhanuddin, J. Mater. Res. Technol., 2022, 18, 4564–4577 CrossRef CAS.
- R. Sarbatly, J. Sariau and M. F. I. Alam, Mater. Today Proc., 2021, 46, 2118–2121 CrossRef CAS.
- S. Agrawal, R. Ranjan, B. Lal, A. Rahman, S. P. Singh, T. Selvaratnam and T. Nawaz, Processes, 2021, 9, 1779 CrossRef CAS.
- M. Khraisheh, S. Elhenawy, F. Almomani, M. Al-Ghouti, M. K. Hassan and B. H. Hameed, Membranes, 2021, 11, 995 CrossRef CAS PubMed.
- Z. Uddin, F. Ahmad, T. Ullan, Y. Nawab, S. Ahmad, F. Azam, A. Rasheed and M. S. Zafar, Int. J. Environ. Sci. Technol., 2022, 19, 9149–9176 CrossRef CAS.
- S. S. Ray, S. S. Chen, C. W. Li, N. C. Nguyen and H. T. Nguyen, RSC Adv., 2016, 6, 85495–85514 RSC.
- N. S. Abd Halim, M. D. H. Wirzal, S. M. Hizam, M. R. Bilad, N. A. H. M. Nordin, N. S. Sambudi, Z. A. Putra and A. R. M. Yusoff, J. Environ. Chem. Eng., 2021, 9, 104613 CrossRef CAS.
- T. Aslan, S. Arslan, M. Eyvaz, S. Güçlü, E. Yüksel and İ. Koyuncu, J. Memb. Sci., 2016, 520, 616–629 CrossRef CAS.
- E. Karimi, A. Raisi and A. Aroujalian, Polymer, 2016, 99, 642–653 CrossRef CAS.
- J. Bae, I. Baek and H. Choi, Water Res., 2016, 105, 406–412 CrossRef CAS PubMed.
- S. M. Seyed Shahabadi, S. A. Mousavi and D. Bastani, J. Taiwan Inst. Chem. Eng., 2016, 76, 474–483 CrossRef.
- A. Mamun, T. Blachowicz and L. Sabantina, Polymers, 2021, 13, 1–14 CrossRef PubMed.
- S. Jiang, H. Schmalz, S. Agarwal and A. Greiner, Adv. Fiber Mater., 2020, 2, 34–43 CrossRef CAS.
- S. Kumarage, I. Munaweera and N. Kottegoda, Beilstein J. Nanotechnol., 2022, 13, 137–159 CrossRef CAS PubMed.
- Y. Wu, J. Zeng, Y. Zeng, H. Zhou, G. Liu, J. Jian and J. Ding, Chin. J. Chem. Eng., 2021, 38, 84–97 CrossRef.
- M. J. Park, R. R. Gonzales, A. Abdel-Wahab, S. Phuntsho and H. K. Shon, Desalination, 2018, 426, 50–59 CrossRef CAS.
- M. Abdullah and K. Khairurrijal, Indones. J. Phys., 2016, 20, 37–40 CrossRef.
- C. D. Tsakiroglou, M. A. Ioannidis, E. Amirtharaj and O. Vizika, Chem. Eng. Sci., 2009, 64, 847–859 CrossRef CAS.
- A. Sawitri, M. M. Munir, D. Edikresnha, A. Sandi, A. Fauzi, A. Rajak, D. Natalia and K. Khairurrijal, Mater. Res. Express, 2018, 5, 54003 CrossRef.
- R. Wang, Y. Liu, B. Li, B. S. Hsiao and B. Chu, J. Memb. Sci., 2012, 392–393, 167–174 CrossRef CAS.
- K. Oshvandi, R. Kavyannejad, R. S. Borzuo and M. Gholyaf, Nurs. Midwifery Stud., 2014, 3, e21764 Search PubMed.
- S. R. Selvi and R. Baskaran, in APCBEE Procedia, Elsevier BV, 2014, vol. 9, pp. 97–101 Search PubMed.
- T. Mudalige, H. Qu, D. Van Haute, S. M. Ansar, A. Paredes and T. Ingle, Nanomater. Food Appl., 2019, 313–353 Search PubMed.
- R. Gopal, S. Kaur, Z. Ma, C. Chan, S. Ramakrishna and T. Matsuura, J. Membr. Sci., 2006, 281, 581–586 CrossRef CAS.
- K. M. Dobosz, C. A. Kuo-Leblanc, T. J. Martin and J. D. Schiffman, Ind. Eng. Chem. Res., 2017, 56, 5724–5733 CrossRef CAS PubMed.
- Y. Liu, L. Shen, Z. Huang, J. Liu, Y. Xu, R. Li, M. Zhang, H. Hong and H. Lin, J. Membr. Sci., 2022, 641, 119925 CrossRef CAS.
- A. Rajak, D. A. Hapidin, F. Iskandar, M. M. Munir and K. Khairurrijal, J. Waste Manage., 2020, 103, 76–86 CrossRef CAS PubMed.
- H. Sanaeepur, A. Ebadi Amooghin, A. Moghadassi, A. Kargari, S. Moradi and D. Ghanbari, Polym. Adv. Technol., 2012, 23, 1207–1218 CrossRef CAS.
- I. N. Strain, Q. Wu, A. M. Pourrahimi, M. S. Hedenqvist, R. T. Olsson and R. L. Andersson, J. Mater. Chem. A, 2015, 3, 1632–1640 RSC.
- M. Gu, K. Wang, W. Li, C. Qin, J. J. Wang and L. Dai, Fibers Polym., 2011, 12, 65–72 CrossRef CAS.
- R. Kumar, R. Singh and I. Farina, PSU Res. Rev., 2018, 2, 115–137 CrossRef.
- J. Lv, W. Gu, X. Cui, S. Dai, B. Zhang and G. Ji, Sci. Rep., 2019, 9, 1–11 CrossRef CAS PubMed.
- I. Turku, S. Kasala and T. Kärki, Recycling, 2018, 3, 57 CrossRef.
- G. Liu, Y. Liao and X. Ma, J. Waste Manage., 2017, 61, 315–326 CrossRef CAS PubMed.
- A. Fauzi, D. A. Hapidin, M. M. Munir, F. Iskandar and K. Khairurrijal, RSC Adv., 2020, 10, 17205–17216 RSC.
- E. Moradi, H. Ebrahimzadeh and Z. Mehrani, Talanta, 2019, 205, 120080 CrossRef CAS PubMed.
- B. Ozbey-Unal, E. Gezmis-Yavuz, B. Eryildiz, D. Y. Koseoglu-Imer, B. Keskinler and I. Koyuncu, J. Environ. Chem. Eng., 2020, 8, 104113 CrossRef CAS.
- J. Matulevicius, L. Kliucininkas, T. Prasauskas, D. Buivydiene and D. Martuzevicius, J. Aerosol Sci., 2016, 92, 27–37 CrossRef CAS.
- M. Borrego, J. E. Martín-Alfonso, M. C. Sánchez, C. Valencia and J. M. Franco, Int. J. Biol. Macromol., 2021, 180, 212–221 CrossRef CAS PubMed.
- I. Sriyanti, D. Edikresnha, A. Rahma, M. M. Munir, H. Rachmawati and K. Khairurrijal, J. Nanomater., 2017, 2017, 9687896 Search PubMed.
- W. T. Kim, D. C. Park, W. H. Yang, C. H. Cho and W. Y. Choi, Nanomaterials, 2021, 11, 26–28 Search PubMed.
- A. A. Almetwally, M. El-Sakhawy, M. H. Elshakankery and M. H. Kasem, J. Text. Assoc., 2017, 78, 5–14 Search PubMed.
- Y. Liu, J. H. He, J. Y. Yu and H. M. Zeng, Polym. Int., 2008, 57, 632–636 CrossRef CAS.
- S. Higashi, T. Hirai, M. Matsubara, H. Yoshida and A. Beniya, Sci. Rep., 2020, 10, 13427 CrossRef CAS PubMed.
- Z. Huang, L. Shen, H. Lin, B. Li, C. Chen, Y. Xu, R. Li, M. Zhang and D. Zhao, J. Membr. Sci., 2022, 661, 120949 CrossRef CAS.
- I. M. Alarifi, A. R. Alharbi, M. Khan, W. S. Khan, A. Usta and R. Asmatulu, Int. J. Mater. Sci. Res., 2018, 2, 43–49 CrossRef.
- A. Gençtürk, E. Kahraman, S. Güngör, Y. Özsoy and A. S. Saraç, Turkish J. Pharm. Sci., 2020, 17, 638–644 CrossRef PubMed.
- I. Tlili and T. A. Alkanhal, J. Water Reuse Desalin., 2019, 9, 232–247 CrossRef CAS.
- A. Atangana, in Fractional Operators with Constant and Variable Order with Application to Geo-Hydrology, Academic Press, 2018, pp. 15–47 Search PubMed.
- K. Damak, A. Ayadi, B. Zeghmati and P. Schmitz, Desalination, 2004, 161, 67–77 CrossRef CAS.
- Z. Zhou and X. F. Wu, Mater. Lett., 2015, 160, 423–427 CrossRef CAS.
- A. Zulfi, M. M. Munir, D. A. Hapidin, A. Rajak, D. Edikresnha, F. Iskandar and K. Khairurrijal, Mater. Res. Express, 2018, 5, 035049 CrossRef.
- O. Abdalla, M. A. Wahab and A. Abdala, Curr. Pharm. Biotechnol., 2020, 22, 1686–1704 Search PubMed.
- N. Zhou, R. Liang and A. Hu, Nanotechnology for water treatment and purification, Springer International Publishing, Switzerland, 2014, vol. 22 Search PubMed.
- L. Bao, K. Seki, H. Niinuma, Y. Otani, R. Balgis, T. Ogi, L. Gradon and K. Okuyama, Sep. Purif. Technol., 2016, 159, 100–107 CrossRef CAS.
- L. Li, R. Hashaikeh and H. A. Arafat, J. Membr. Sci., 2013, 436, 57–67 CrossRef CAS.
- Z. Wang, C. Crandall, R. Sahadevan, T. J. Menkhaus and H. Fong, Polymer, 2017, 114, 64–72 CrossRef CAS.
- Y. Liu, R. Wang, H. Ma, B. S. Hsiao and B. Chu, Polymer, 2013, 54, 548–556 CrossRef CAS.
- L. A. Goetz, B. Jalvo, R. Rosal and A. P. Mathew, J. Membr. Sci., 2016, 510, 238–248 CrossRef CAS.
- A. K. Gautam, C. Lai, H. Fong and T. J. Menkhaus, J. Membr. Sci., 2014, 466, 142–150 CrossRef CAS.
- P. Arribas, M. C. García-Payo, M. Khayet and L. Gil, Sep. Purif. Technol., 2019, 226, 323–336 CrossRef CAS.
- H. Saleem, L. Trabzon, A. Kilic and S. J. Zaidi, Desalination, 2020, 478, 114178 CrossRef CAS.
- E. F. C. Chaúque, L. N. Dlamini, A. A. Adelodun, C. J. Greyling and J. C. Ngila, Phys. Chem. Earth, 2017, 100, 201–211 CrossRef.
- X. Yin, Y. He, Y. Wang, H. Yu, J. Chen and Y. Gao, Korean J. Chem. Eng., 2020, 37, 1751–1760 CrossRef CAS.
- S. Hartati, A. Zulfi, P. Y. D. Maulida, A. Yudhowijoyo, M. Dioktyanto, K. E. Saputro, A. Noviyanto and N. T. Rochman, ACS Omega, 2022, 7, 10516–10525 CrossRef CAS PubMed.
- X. Wang, L. Zhai, Y. Wang, R. Li, X. Gu, Y. Di Yuan, Y. Qian, Z. Hu and D. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 37848–37855 CrossRef CAS PubMed.
- T. Zhou, J. Li, X. Guo, Y. Yao, P. Zhu and R. Xiang, Desalin. Water Treat., 2019, 165, 63–72 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |