Open Access Article
Open Access ArticleThe chemistry behind the body art: unveiling the elemental profile and heavy metal content of natural tattoos and dyes by ICP-MS†
Laura Rubio *ab,
Pedro Barrulas
*ab,
Pedro Barrulas *c,
Mafalda Costa
*c,
Mafalda Costa c,
Carmen Garcia-Jaresab,
Marta Lores
c,
Carmen Garcia-Jaresab,
Marta Lores b and
Cristina Barrocas Dias
b and
Cristina Barrocas Dias cd
cd
aCRETUS, Department of Analytical Chemistry, Nutrition and Food Science, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain. E-mail: laura.rubio.lareu@usc.es; Tel: +34-881-814-379carmen.garcia.jares@usc.es
bLaboratory of Research and Development of Analytical Solutions (LIDSA), Department of Analytical Chemistry, Nutrition and Food Science, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain. E-mail: marta.lores@usc.es
cHERCULES Laboratory, University of Évora, Palácio do Vimioso, Largo Marquês de Marialva 8, 7000-809, Évora, Portugal. E-mail: pbarrulas@uevora.pt; mcosta@uevora.pt; cmbd@uevora.pt; Tel: +351-266740800
dChemistry and Biochemistry Department, School of Sciences and Technology, Rua Romão Ramalho, 59, 7000-671, Évora, Portugal
First published on 30th November 2022
Abstract
Temporary tattoos and dyes are increasing popularity as an alternative to permanent ones. However, knowledge on its elementary composition is scarce and, this scientific gap can potentially make them a source of heavy metal exposure on humans. The present research aimed to explore the possibility of using the inorganic chemical signature to characterize natural pigments-based products and to evaluate the heavy metal content in jagua temporary tattoos and henna temporary hair dyes and tattoos. Thirty-four different samples of commercial products were analyzed for the presence and quantity of eleven trace elements (Al, Cr, Mn, Co, Ni, Cu, Zn, As, Cd, Ba, and Pb) by inductively coupled plasma mass spectrometry (ICP-MS). The overall mean concentrations varied between 0.02 and 973 μg g−1 in solid samples or 0.01–1878 μg g−1 for paste ones, wherein potential fake products were uncovered. None of the 34 samples considered comply with the current European Cosmetics Regulation. Samples were differentiated according to their Cr, Zn, Ba, and Pb content. The overall results revealed highly variable levels in the investigated samples, which leads us to suggest detailed quality controls of these materials, due the fact that their continued use can origin potential threat to human health.
1. Introduction
Personal care products and facial cosmetics are one of the main pillars of daily consumption, involving a high degree of exposure among the population. Direct application of cosmetics on human hair or skin makes the society vulnerable to a wide variety of chemical compounds. Despite the protecting function of the skin, these substances may produce local effects such as irritation, sensitization, or allergy. Among the hazardous substances contained in cosmetics, such as allergenic fragrances, aromatic amines, or phthalates, heavy metals are widespread in color makeup products.1,2Henna is a typical example of a traditional cosmetic used in different parts of the world since ancient times. In the recent decades, its use as temporary tattoos or hair dyes has become popular as well as other natural dyes that have emerged such as jagua. Henna is a natural pigment derived from dried and crushed leaves of the Lawsonia inermis plant (from Lythraceae family), that contains lawsone (2-hydroxy-1,4-naphthoquinone) as the bioactive compound, which is responsible for the typical red-brown coloration.3 Jagua is a natural colorant extracted from Genipa americana L. plant (from Rubiaceae family) and contains geniposide and genipin as active ingredients.4
The safety of henna and its main active ingredient has been evaluated in specific hair dye formulations5 but no similar assessment has been performed for henna temporary tattoos. Simultaneously, although their allergenic potential has been demonstrated,3,6 the regulatory consideration of jagua temporary tattoos has not yet been approached. Both types of temporary tattoos can be considered as new-format cosmetics or borderline products from the point of view of European regulations.7 According to the guidelines of the Borderline Products Manual, they should simultaneously comply with the Cosmetics Regulation8 and the Toys Directive.9 However, the reality is quite different: the chemical composition of these body-decorating products is diverse, most of them are not correctly or fully labelled, do not have proper regulations, strict specific legislation, implemented procedures, or any legal market control.10,11
The final chemical composition of theses natural pigments can be severely impacted by natural and anthropogenic factors but also through plants post-processing. Plants used to produce henna and jagua pigments may be grown in contaminated areas, and the derived products can be mixed with other plant extracts or materials. Additional chemicals such as synthetic dyes, solvents, or metallic salts may also be added to alter the color and make it more intense. Consequently, marketed products can contain relatively significant amounts of heavy metals12 present in the ingredients or unintentionally introduced during the different steps of preparation. In this sense, the European cosmetics legislation8 lists the metals allowed or prohibited as ingredients throughout its annexes, as well as the technically unavoidable metal impurity levels that come primarily from colorants. To accurately evaluate the heavy metal content in henna products, electrothermal or flame atomic absorption spectrometry (ETAAS or FAAS12–18) and inductively coupled plasma optical or atomic emission spectroscopy (ICP-OES or ICP-AES19–22) have been used in previous studies. It is important to mention that most of these works are exclusively focused on Pb or trace amounts of Cd, Ni, or Co in order to evaluate possible contaminations of these metals without the intention to characterize the cosmetic products.
However, among the various techniques suggested for heavy metal analysis in cosmetic products, inductively coupled plasma mass spectrometry (ICP-MS) is strongly recommended23–26 because it is a highly accurate, precise, and sensitive analytical technique, allowing the measurement of multi-elemental composition at trace levels in a single analysis.27,28 There are some studies using ICP-MS approach to analyze trace metal contaminations in cosmetics, including henna samples.29–34 Their results will be compared with those obtained in our study. However, given the lack of information on these decorative cosmetic products, the purpose is to go further than an analysis of certain metals, trying to go as far as characterization. In addition, innovative jagua products have not yet been included in any published work, therefore here we present and describe the first paper where ICP-MS have been applied to this type of samples.
Keeping in mind the increasing use of natural pigments and their possible side effects and health impacts, the present research aims to investigate the metal composition of hair dye and temporary tattoo samples, estimate the levels of some trace metals, and explore the possibility of using the elemental composition to characterize this kind of samples. The present study will contribute to the existing knowledge by providing an informative overview of the heavy metal contents in this type of products, as well as a discussion based on the literature of the potential risks associated with their exposure and potential health impacts to ensure consumer safety. Acid digestion followed by ICP-MS was the methodology approach used for the analysis of 11 trace elements in 34 henna and jagua commercial samples included in this study.
2. Materials and methods
2.1. Chemical and reagents
All chemicals and reagents were used as received. Nitric acid (HNO3, Suprapur® grade 65% (w/v), Merck®) was used both for digestion and for standard solution preparation. Hydrogen peroxide (H2O2, 30% (w/v) OPTIMA grade, Fluka Chemicals) and hydrochloric acid (HCl, 37% (w/v) OPTIMA grade, Fluka Chemicals) were also used on the digestion procedures. For sample dilution, ultrapure water (18.2 MΩ cm quality) obtained from a Milli-Q Integral 3 system (Millipore, Merck, Darmstadt, Germany) was used. ICP-MS tuning solution was obtained by Agilent Technologies (Palo Alto, CA, USA) and it was employed for ICP-MS instrument optimization. Three calibration standards supplied by High-Purity Standards® (Charleston, SC, USA) were used for standard addition calibration: two standard solutions (solution A and B, ICP-MS-68B-A and ICP-MS-68B-B, respectively) were employed. A third solution (ICP-MS-68B-C) containing Ru, Rh, and Ir, was used as internal standard solution for drift monitoring during analysis.To avoid sample contamination by any traces of metal, the digestion Teflon beakers used were first soaked overnight in 5% (v/v) HNO3 and then soaked in distilled water for another 24 hours. They were then rinsed thoroughly with ultrapure water, completely dried in the oven at 50 °C and stored until use.
2.2. Samples
A total of 34 samples were acquired comprising henna tattoos (17, HT), jagua tattoos (6, JT), henna hair dyeing samples (7, HD) and henna samples for both purposes (3, HTD). One plant-based tattoo (HPT) sample of unknown origin and composition was also included and analyzed as another jagua. Henna samples were purchased at through a well-known site available to anyone on the Internet (Amazon, September 2020 to February 2021) from different sellers and at a very inexpensive price, with the reception of two that were obtained from a local store in Morocco (HT-13 and HT-14). Jagua samples, all those that were conveniently available at the time of acquisition were also purchased online. Table S1† details the characteristics of the samples included in this study.Samples were divided into two groups according to their physical state: solid (11) and paste (23) and were correlatively named. Paste samples included 17 hennas, five jagua and HPT; solid samples included 10 hennas and one jagua. Regarding the color of the samples, the paste hennas presented a greater range of tonalities from more common colors to more vivid hues. However, all other hennas were greenish powders. Finally, all the jaguas and the HPT sample were black. Most samples were not labelled, as happened with paste hennas or HPT, being marketed without information labels indicating their composition. Some samples report on their labels the presence of natural extracts as well as other ingredients allowed in cosmetics as preservatives, such as potassium sorbate, or state that they do not contain metallic salts or heavy metals.
All samples were stored at room temperature under non-moisture conditions and kept in their original containers protected from light until the analysis.
2.3. Sample preparation
Prior to the complete sample digestion procedure, samples were submitted to a pre-digestion step due to their high organic content. Two independent sample preparation and digestion procedures (one for solid samples and other for paste ones, detailed in Fig. 1b) were established in this work, in order to determine the concentrations of 11 trace elements (Ba, Pb, Al, Cr, Mn, Co, Ni, Cu, Zn, Cd, and As). Fig. 1a provides a general picture of the complete digestion process while Fig. 1b shows the different digestion procedures according to the sample type.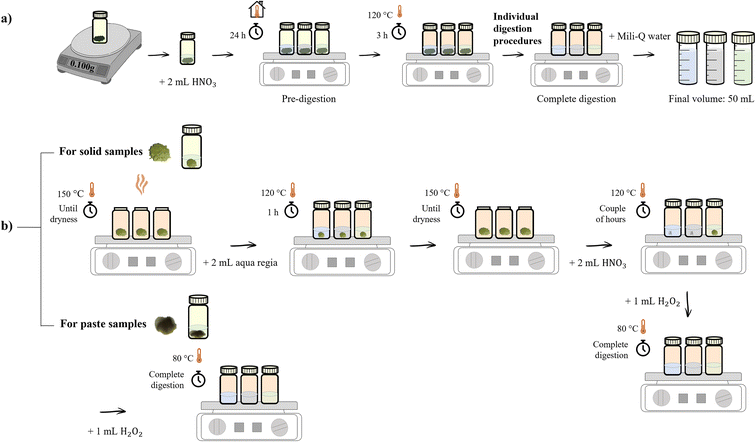 | ||
| Fig. 1 (a) General digestion process; (b) schematic digestion procedures according to the type of sample: solid or paste. | ||
Briefly, in both procedures, approximately 0.1 g of each sample were accurately weighted in dry and clean PFA (perfluoroalkoxy) Savillex® beakers and exposed to a pre-digestion step with 2 mL of concentrated HNO3 at room temperature for 24 h. After the pre-digestion period, samples were digested in closed beakers over a hotplate at 120 °C for 3 h (during this time the digestion could be monitored through the formation of an orange atmosphere). Subsequently, in the digestion method for solid products, all samples were evaporated until dryness over a hotplate at 150 °C, followed by cooling and addition of 2 mL of aqua regia freshly prepared (HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl, 1
HCl, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The second digestion step took place over a hotplate at 120 °C for 1 h. Samples were evaporated again and 2 mL of concentrated HNO3 were added. The third digestion step was then started by heating up the samples at 120 °C for a couple of hours. This step was completed through addition, at room temperature, of 1 mL of H2O2. However, in the method for paste samples only 1 mL of H2O2 was added to all samples at room temperature. In both cases, they are then placed on the hotplate at 80 °C. According to safety rules in chemical laboratory, this procedure was carried out in a fume hood. When digestion was complete (colorless samples), samples were cooled, transferred to PFA volumetric flasks, and fulfilled with Milli-Q water up to 50 mL, yielding a final matrix of 2% HNO3 for analysis. Two blank control solutions were prepared and analyzed following the same digestions procedures for solid and paste samples, respectively. Digested samples and blanks were stored and kept at 4 °C until the analysis.
3 v/v). The second digestion step took place over a hotplate at 120 °C for 1 h. Samples were evaporated again and 2 mL of concentrated HNO3 were added. The third digestion step was then started by heating up the samples at 120 °C for a couple of hours. This step was completed through addition, at room temperature, of 1 mL of H2O2. However, in the method for paste samples only 1 mL of H2O2 was added to all samples at room temperature. In both cases, they are then placed on the hotplate at 80 °C. According to safety rules in chemical laboratory, this procedure was carried out in a fume hood. When digestion was complete (colorless samples), samples were cooled, transferred to PFA volumetric flasks, and fulfilled with Milli-Q water up to 50 mL, yielding a final matrix of 2% HNO3 for analysis. Two blank control solutions were prepared and analyzed following the same digestions procedures for solid and paste samples, respectively. Digested samples and blanks were stored and kept at 4 °C until the analysis.
2.4. ICP-MS analysis
Multi-elemental quantification was performed using an Agilent 8800 Triple Quadrupole ICP-MS instrument (Agilent Technologies, Japan), equipped with a MicroMist nebulizer and a quartz spray chamber. Parameters and operational conditions are provided in Table 1.| ICP-MS 8800 QQQ Agilent Technologies | ||
|---|---|---|
| Scan type | MS/MS | |
| Plasma parameters | RF power | 1550 W |
| RF matching | 1.70 V | |
| Carrier gas | 1.20 L min−1 | |
| Nebulizer pump | 0.10 rps | |
| Collision reaction cell (CRC) gases | He | 4.0 mL min−1 |
| O2 | 0.50 mL min−1 | |
| Acq parameters | Spectrum mode | |
| Spectrum mode option | Q2 peak pattern | 1 point |
| Replicates | 3 | |
| Sweeps/replicate | 10 | |
| Isotope/CRC gas mode | He | 27Al, 52Cr, 55Mn, 59Co, 60Ni, 63Cu, 66Zn, 95Mo |
| O2 | 75As | |
| No gas | 55Mn, 107Ag, 111Cd, 137Ba, 208Pb, 209Bi | |
In accordance with the analytes of interest, the collision/reaction cell was in “He mode”, “O2 mode” and “no-gas mode”. Prior to the analysis, the equipment was calibrated with a tuning solution containing 10 μg L−1 each of Ce, Co, Li, Tl, and Y in a matrix of 2% HNO3, the sensitivity and resolution were optimized, and the doubly charged ions and oxides species (<1.04%) were minimized. Along ICP-MS analysis, a solution containing 400 μg L−1 of the three internal standards (101Ru, 103Rh, 193Ir) was added online to correct the data for possible instrumental drifts and matrix effects.
Standard addition calibration method was carried out for the quantification. One sample of each type was used for the calibration, HTD-2 and HT-4 for powders and pastes, respectively, fortified at 0, 5, 10, 20, 50, 100, 200, 400, 800, and 1600 μg L−1 for each element. Quality controls were done analyzing a standard solution of known concentration (400 μg L−1) every 10 samples. Method precision was evaluated running each sample (HD-1 for powders and HT-1 for pastes) ten times and reporting the relative standard deviation (RSD). Due to the unavailability of certified reference material for metal analysis in jagua and henna, the method was validated in terms of accuracy by performing analyte recovery studies at six concentrations (2, 5, 10, 20, 50, and 100 μg L−1), using sample HD-2 as representative for powder group and sample HT-15 as representative for pastes.
The instrumental detection limits (LOD) were experimentally obtained before the quantification of the samples. Quantification limits (LOQ) were calculated as three times the detection limit values.
2.5. Statistical analysis
Thirty-four henna and jagua samples were quantitatively analyzed by ICP-MS and characterized by eleven descriptors (elemental concentrations) measured. The final matrix data obtained contained 34 rows and 11 columns. For subsequent chemometric processing, all samples belonging to the different types were assigned numerical codes: 1 (HD), 2 (HTD), 3 (HT), 4 (JT) and 5 (HPT) and solid samples were assigned to code 0 and pastes, 6. The basic chemometric characterization of the investigated samples was performed by cluster analysis (CLU) which is used as a feature to group the key variables that explain the principal data dimensionality.STATGRAPHICS Centurion software ver. 18 (2017) was used for statistical treatment of the ICP-MS measurement results.
3. Results and discussion
3.1. Analytical method performance
Linearity coefficients of determination (R2), ranged from 0.9941–0.9998 to 0.9986–1.0000 for solid and paste samples, respectively, and the overall mean value was higher than 0.9986 (see ESI Table S2†). Results of the method accuracy and precision evaluation are summarized in Table 2, showing excellent to good recoveries (87–118% for paste-type samples and from 97% to 116% for solid-type samples) and RSD values (6% for pastes and 5% for solid samples). The LODs and LOQs for the elements analyzed are depicted in Tables S2† and 2, respectively.LODs ranged between 0.004–16 μg g−1 for solids and 0.002–1.8 μg g−1 for paste samples. LOQs for the solid samples ranged from 0.012 to 5.8 μg g−1, although they slightly increase for Mn (20 μg g−1) and especially for Al (48 μg g−1). For paste samples, LOQs ranged from 0.0058 to 1.5 μg g−1, being higher for Al (5.4 μg g−1).
| Element | Solid samples | Paste samples | ||||
|---|---|---|---|---|---|---|
| Precision (RSD, %) | Recovery ± RSD (%) | LOQ (μg g−1) | Precision (RSD, %) | Recovery ± RSD (%) | LOQ (μg g−1) | |
| Al | 6.8 | 102 ± 9 | 48 | 3.9 | 118 ± 6 | 5.4 |
| Cr | 4.7 | 101 ± 6 | 5.8 | 6.3 | 87 ± 2 | 0.15 |
| Mn | 6.3 | 99 ± 4 | 20 | 5.8 | 108 ± 3 | 0.14 |
| Co | 6.3 | 112 ± 10 | 0.29 | 9.1 | 95 ± 2 | 0.04 |
| Ni | 5.7 | 111 ± 12 | 1.8 | 8.8 | 110 ± 2 | 0.26 |
| Cu | 6.2 | 102 ± 9 | 1.1 | 3.5 | 97 ± 2 | 0.27 |
| Zn | 6.2 | 116 ± 8 | 1.9 | 2.1 | 103 ± 14 | 1.5 |
| As | 1.2 | 99 ± 3 | 0.06 | 9.9 | 91 ± 2 | 0.025 |
| Cd | 5.9 | 113 ± 7 | 0.012 | 7.7 | 90 ± 8 | 0.0058 |
| Ba | 4.5 | 97 ± 5 | 0.88 | 2.5 | 103 ± 11 | 0.13 |
| Pb | 3.2 | 101 ± 11 | 0.15 | 4.1 | 95 ± 11 | 0.028 |
3.2. Heavy metal content in henna and jagua samples
The division of the purchased samples into two main groups according to their physical state: solids and pastes, has been kept for the results evaluation. It is mandatory to carefully chose the elements according to their importance and their relationship with toxicity and health. Therefore, Al, Cr, Mn, Co, Ni, Cu, Zn, As, Cd, Ba, and Pb were selected to be analyzed by ICP-MS. Affinities and differences between the samples were explored according to the typology considered.A first look at the obtained data (see Table 3), revealed that the physical state of the natural tattoos and dyes studied can be easily discriminated according to their chemical composition. Both groups contained considerable amounts of heavy metals and showed a wide variation among the samples. Since solid samples revealed more information than the pastes and generally with higher concentrations, this approach may be used to chemically distinguish between pastes and solids, providing an analytical tool with the evident application on potential fraud control on the global market. A closer look shows that the elements Ba and Pb can be used to discriminate among the pastes group samples. In the case of the solid samples, a similar situation occurs, but the two discriminating elements are now Al and Zn. It is relevant to mention at this point that other three elements that we initially considered (Mo, Ag, and Bi) were discarded since their presence was not detected using the methodology adopted in this work.
| Al | Cr | Mn | Co | Ni | Cu | Zn | As | Cd | Ba | Pb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid samples | HD-1 | 718 | 37 | 80 | 0.68 | 5.4 | 11 | 16 | 0.22 | 0.04 | 20 | 0.90 |
| HD-2 | 218 | 20 | 117 | 0.50 | 6.3 | 9.0 | 29 | 0.09 | 0.04 | 78 | 0.28 | |
| HD-3 | 193 | 50 | 113 | 0.80 | 13 | 11 | 30 | <0.06 | 0.018 | 88 | 0.35 | |
| HD-4 | 414 | 44 | 101 | 0.60 | 6.6 | 8.9 | 14 | 0.14 | 0.06 | 15 | 0.72 | |
| HD-5 | 420 | 23 | 118 | 0.59 | 4.9 | 8.7 | 12 | 0.14 | 0.08 | 15 | 0.40 | |
| HD-6 | 457 | 40 | 91 | 0.56 | 5.7 | 9.7 | 20 | 0.13 | 0.04 | 16 | 0.56 | |
| HD-7 | 711 | 57 | 99 | 0.98 | 8.9 | 8.4 | 30 | 0.21 | 0.05 | 12 | 0.49 | |
| HTD-1 | 974 | <5.8 | 75 | 0.90 | <1.8 | 7.8 | 17 | 0.53 | 0.05 | 23 | 0.39 | |
| HTD-2 | 850 | 57 | 141 | 1.0 | 8.1 | 12 | 13 | 0.37 | 0.09 | 16 | 0.62 | |
| HTD-3 | 318 | 23 | 131 | 0.55 | 4.7 | 8.5 | 23 | 0.13 | 0.07 | 66 | 0.42 | |
| JT-4 | 51 | <5.8 | <20 | <0.29 | <1.8 | 3.8 | 699 | 0.074 | <0.012 | <0.88 | 0.33 | |
| Paste samples | HT-1 | 25 | 3.3 | 0.38 | <0.04 | <0.26 | 0.56 | 6.7 | 0.05 | 0.009 | 0.32 | 0.08 |
| HT-2 | 8.4 | 1.4 | 0.28 | <0.04 | <0.26 | <0.27 | <1.5 | 0.033 | 0.011 | 1.7 | 0.09 | |
| HT-3 | 185 | 0.17 | 0.53 | <0.04 | <0.26 | <0.27 | 4.4 | 0.11 | 0.008 | 1878 | 0.06 | |
| HT-4 | 7.8 | 130 | 1.1 | <0.04 | <0.26 | 0.51 | 3.7 | <0.025 | 0.010 | 0.56 | 0.11 | |
| HT-5 | <5.4 | <0.15 | 0.15 | 0.04 | <0.26 | <0.27 | 2.0 | <0.025 | 0.010 | 0.21 | 0.038 | |
| HT-6 | <5.4 | 0.29 | 1.1 | <0.04 | <0.26 | <0.27 | 3.1 | <0.025 | 0.008 | 0.55 | 0.33 | |
| HT-7 | 7.1 | 0.25 | 1.3 | <0.04 | <0.26 | <0.27 | 5.7 | 0.026 | 0.009 | 0.30 | 0.06 | |
| HT-8 | 8.9 | 0.16 | 0.36 | <0.04 | <0.26 | <0.27 | 5.5 | 0.026 | 0.010 | 0.19 | 3.1 | |
| HT-9 | <5.4 | 0.63 | 1.4 | <0.04 | <0.26 | <0.27 | 2.6 | <0.025 | 0.009 | 0.24 | 0.41 | |
| HT-10 | 9.7 | 0.87 | 0.35 | 0.05 | <0.26 | 0.40 | 3.1 | 0.026 | 0.009 | 1.8 | 0.05 | |
| HT-11 | 260 | 14 | 39 | 0.37 | 5.2 | 2.4 | 3.6 | 0.09 | 0.024 | 4.0 | 0.27 | |
| HT-12 | 8.8 | 0.95 | 0.28 | <0.04 | <0.26 | <0.27 | <1.5 | 0.029 | 0.010 | 0.20 | 0.06 | |
| HT-13 | <5.4 | 0.23 | 0.16 | <0.04 | <0.26 | <0.27 | 6.4 | <0.025 | 0.008 | <0.13 | 0.05 | |
| HT-14 | <5.4 | 0.16 | <0.14 | <0.04 | <0.26 | <0.27 | 1.6 | <0.025 | 0.006 | <0.13 | 0.044 | |
| HT-15 | <5.4 | <0.15 | 0.22 | <0.04 | <0.26 | <0.27 | 2.8 | <0.025 | 0.006 | 0.23 | 0.035 | |
| HT-16 | 235 | 11 | 60 | 0.24 | 1.7 | 3.3 | 7.6 | 0.10 | 0.034 | 7.2 | 0.27 | |
| HT-17 | 9.6 | 0.16 | 0.53 | <0.04 | <0.26 | <0.27 | 4.2 | <0.025 | 0.008 | <0.13 | 4.5 | |
| JT-1 | <5.4 | 0.15 | 1.8 | <0.04 | <0.26 | 0.75 | 2.3 | 0.026 | 0.011 | 0.48 | 0.039 | |
| JT-2 | <5.4 | <0.15 | 0.24 | <0.04 | <0.26 | <0.27 | 2.3 | <0.025 | 0.006 | <0.13 | 0.032 | |
| JT-3 | 8.0 | <0.15 | 0.21 | <0.04 | <0.26 | <0.27 | 3.0 | <0.025 | <0.0058 | <0.13 | 0.039 | |
| JT-5 | <5.4 | 0.15 | <0.14 | <0.04 | <0.26 | <0.27 | <1.5 | <0.025 | <0.0058 | <0.13 | 0.032 | |
| JT-6 | <5.4 | 0.51 | <0.14 | <0.04 | 3.3 | 7.1 | 12 | <0.025 | 0.008 | 0.17 | 0.41 | |
| HPT | 6.0 | 274 | <0.14 | <0.04 | <0.26 | <0.27 | 17 | <0.025 | <0.0058 | <0.13 | 0.029 |
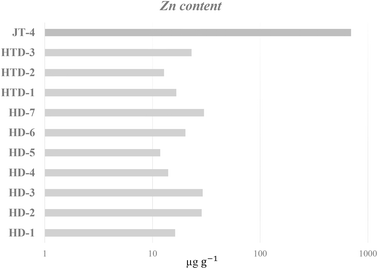
Based on the showed overall mean concentrations, the heavy metal levels in the solid-type products were in the following decreasing order: Al > Mn > Cr > Ba > Zn > Cu > Ni > Co > Pb > As > Cd. However, it is important to note that the solid JT-4 jagua data was not considered to calculate the Zn mean, because it distorted the value upwards. In fact, the Zn concentration of the JT-4 is almost 700 μg g−1, enabling this element to be used to discriminate henna from jaguas within the solid sample group as seen in Fig. 2.
A careful inspection of the data also revealed that Al differentiates samples HD-1, HD-7, HTD-1, and HTD-2 from the remaining solid samples displaying concentrations higher than 500 μg g−1. All other elements were present in similar concentrations in all solid samples, with Cd being present in the lowest concentration.
For the paste-type samples, the decreasing order of concentrations was as follows: Al > Cr > Mn > Zn > Ni > Cu > Ba > Pb > Co > As > Cd. As in the case of Zn in the solid samples group, sample HT-3 was not considered here for the calculation of the mean Ba concentration. For the HPT sample, Cr and Zn were detected in the highest concentrations among the group, with Cr reaching 300 μg g−1. The high Ba concentration in the white sample HT-3 stands out, so Ba differentiates this sample from the other ones. Certain samples are notable for their high content of a particular element such as the orange HT-4 with Cr, JT-6 with Cu or Zn higher than 10 μg g−1 for JT-6 and HPT. Two brown hennas, HT-11 and HT-16, showed higher concentrations of Mn, Ni, or Co.
A comparison within the two sample types, hennas and jaguas, is discussed below. Despite the commercial differences of the selected henna powder samples, they do not chemically differ from each other regarding the proportion of the elements, as shown in Fig. 3. Manganese, Zn, and Pb were the elements that appeared in the most henna samples in general, while Ni and Co were the least frequent. The metal content values do not differ if we compare the two henna claims: hair dyes (HTD) or temporary tattoos (HT) beyond the previously commented differences between solid and paste samples. Some observations can be made by looking at the color of the henna pastes. The red HT-14 is the sample, according with the chosen elements, with lower elemental content, which can indicate that its color maybe due to organic nature. Since Pb discriminated blue samples HT-8 and HT-17 and considering its organic components, it is likely that Pb present in the mixture is part of the chromophore responsible for the sample color. HT-3 is widely noted for its high Ba concentration and has a white color. This can be explained by the use of Ba-based white pigments, such as barium sulfate, which is widely used in cosmetic products35 and probably used in the preparation of this henna paste. Finally, samples HT-11 and HT-16 are very different when compared to the other pastes, as their concentrations were relatively higher for all the 11 elements detected. Therefore, these two products are more similar to the solid hennas in terms of their metal content proportion (Fig. 3), which probably indicates the same type of recipe in the preparation of these dyes. A possible reason for this is that these two samples have been reported as real hennas, while others studied may be frauds considering their active components.36,37 Regarding all jaguas, Co was not detected in any of them. Some trace metals appeared in groups of two samples such as Cr in JT-6 and HPT, or high amount of Pb for JT-4 and JT-6, or Al for JT-3 and HPT, but there were also other elements that were detected in only one sample such as Ni for JT-6 and As for JT-1. Only two elements were found for sample JT-5 and the concentration of Cr in HPT was remarkable. Zinc was detected in most of the samples, with the high concentration in the unique solid jagua distinguishing it from the others.
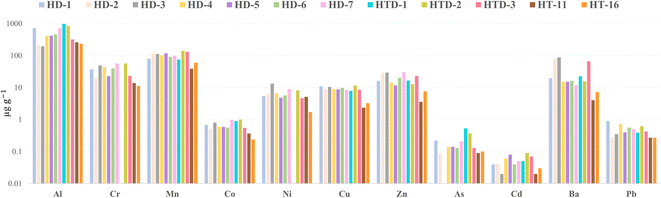 | ||
| Fig. 3 Similar element distribution in the different henna samples: solid ones and two brown paste samples (in logarithmic scale). | ||
The results obtained in this work were compared with those available in the few works published to date. In fact, there are very few references that study the metallic composition of this type of samples, and in the case of jagua products there is no previous literature on their analysis by ICP-MS. Therefore, in the case of henna products such as those considered in this work, most of the articles focused on a limited number of elements, with the inclusion of Pb among them being common. In henna powder, concentrations of Pb > Cd > As were found,29 while in the solid samples we found Pb > As > Cd, coinciding in that Pb appeared in all the samples studied and in the highest concentration. In this work, 0.2 g of sample was used and prior drying of the sample was necessary. The presence of elements common to those found in HTD samples was reported in the analysis of 7 samples of henna powder for tattooing.30 Between 0.1 and 0.2 g of sample is involved but microwave-assisted digestion is used.29,30 Ba, Pb, Al, Ni, Cu, and Zn were also found in a sample of hair dye henna.31 In this case, the amount used by the authors was higher at 1 g of sample and they employed a dangerous acid such as hydrofluoric acid (HF). In black henna-based temporary tattoo products, Pb levels were similar.32,33 Two different methods for sample digestion: using a heating block or a water bath were compared in one work.33 The analysis of two henna analyzed among different cosmetics showed a frequency distribution of Cr > Ni > Cu > Pb34 identical to that found in the henna paste samples in the present work. A possible explanation for the disparity of data in the literature on heavy metal content between henna samples is the difference in the way the product is manufactured (different chemical additives such as dyes or other natural extracts) and the origin of the product, which varies depending on climatic conditions and soil.
The comparison of the obtained results with regulated values and the EU legislation is of utmost importance.8,38 The metals analyzed in this study are not listed as ingredients on any of the products, and some labels even declare the absence of heavy metals. Chromium, Ni, As, Cd, Co, and Pb are the six out of the 11 studied elements in this work that are banned in cosmetic products. As shown in Table 3, Ni, Co, and As were present in 12, 14, or 20 of the 34 samples, respectively; Cr were absent in six of them while Cd in four, with the lowest concentrations of As and Cd. In other words, approximately 35 to 60% of the samples contain Ni, Co, or As while more than 80% contain Cr and Cd while Pb was detected in 100% of the samples. In contrast, the regulation lists other substances allowed with specific uses, such as coloring agents as Al (Color Index number, CI, 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and Cu (CI 77
000) and Cu (CI 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) as white and brown dyes, respectively.
400) as white and brown dyes, respectively.
Finally, from the perspective of public health, positive reactions to Co and Ni have already been observed in a patient with allergic contact dermatitis caused by henna dye used for tattooing.14 So, analyzing and controlling heavy metals in commercial henna or similar mixtures is important because these substances may still be present in cosmetics as metallic contaminants.
3.3. Sample study by cluster analysis
The application of untargeted analysis to the studied samples can provide an overview of the differences in the metallic composition of the analyzed samples. Cluster analysis is a multivariate technique that shows the similarity of objects by classifying them into clusters, such that they are very similar within a class, but significantly different from those in other classes with respect to the predetermined selection criterion.39,40 Agglomerative hierarchical clustering is the most common approach,41 which is usually illustrated with a dendrogram. The individual samples were classified into clusters based on the standardized squared Euclidian distance applying the centroid method as the agglomerative hierarchical clustering algorithm to gather in a cluster those variables in which the distance between two clusters is the distance between the centroids of each cluster.The results of elements comprised in the studied samples clustering are shown in Fig. 4.
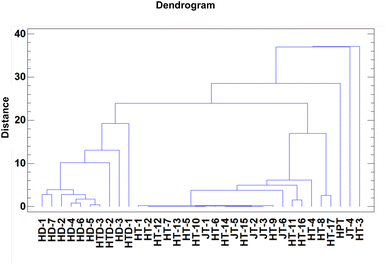 | ||
| Fig. 4 Dendrogram of 11 variables (element concentrations) including 34 samples and clustering technique (squared Euclidean) for cluster analysis. | ||
The dendrogram shows that two main groups of the samples can be differentiated: cluster I is formed by the hair dye samples (HD) or those of the two claims (HTD), and cluster II groups tattoo samples, both hennas (HT) and jaguas (JT), being all of them of paste type. This group includes jaguas with robustly low metallic content and the fake hennas. The two real hennas pasta (HT-11 and HT-16), brown in color and similar metal content to the solid hennas are perfectly separated. Clustering of blue hennas pasta with higher Pb content (samples HT-8 and HT-17) can also be shown. Three outliers were isolated due to their high content of certain elements: HPT for Cr, JT-4 for Zn and HT-3 for Ba. In summary, the cluster analysis demonstrated that the elemental composition of the samples can serve to differentiate them.
3.4. Heavy metals in cosmetics: associated risks and impact on human health
Heavy metal toxicity to human is well documented.42 Metal contents have been reported in different cosmetic products but the association with potential health risks is challenging to interpret. When tattoos or hair dyes are used, they come into direct contact with human skin, scalp, or hair. There are many complications associated with the use of metals and metal salts in these cosmetics, with local inflammation, infection, and allergic reactions43 being the most common adverse effects and the main safety concern. Since the pigments used in the studied products are complex mixtures with a variable metallic composition, allergic reactions to these substances are potentially frequent. Local exposure and accumulation44 can induce various long term health disorders that may include headaches, loss of hair, respiratory diseases, cardiovascular problems, organ dysfunction and a serious impact on mental health.45Chromium, Ni, As, Cd, Co, and Pb are banned as intentional ingredients in cosmetics as mentioned above. However, due to the lack of worldwide uniform legislation on the presence of toxic metals in cosmetics and the technically unavoidable levels that come mainly from permitted pigments, they are in some cases present as accidental contaminants or impurities in trace amounts. Lead, Cd, and As are extremely toxic with a wide variety of chronic human health long-term effects,46,47 while Cr, Ni, and Co are well known skin sensitizers.48 Some metals are used as dyes, such as Cr, but chromium is also corrosive, and its toxicity can cause skin reactions, ulcerations, kidney, and liver damage or respiratory diseases. Nickel allergy is quite common and can cause severe contact dermatitis and also result in skin irritation and hypersensitivity. Despite the generally low As concentrations in the studied samples, As has a high affinity for skin and keratin, present in hair and nails, and its harmful effects include skin lesions, nail striation, and alopecia.49 Cadmium is used in cosmetic products due to its color property and has been used as a pigment in many industries.50 Although Cd concentrations in natural tattoo and dye samples were minimal, even at very low levels of exposure and taking into account its bioaccumulation, Cd can cause kidney and bone damages. Cobalt and its salts are widely used as coloring agents in make-up and light brown hair dyes.51 However, different researchers point to the capacity of Co to cause skin sensitization.14 Finally, Pb is highly toxic for the fetuses, babies, and children whose nervous systems are still developing.52
While Al and Cu can be present in dyes according to the European Cosmetic Regulations, Al can induce granulomatous reactions and although the application of Cu to the skin may initially provide beneficial effects, its high potential to induce skin irritation reactions is often underestimated.53,54 In contrast, some metals such as Mn or Zn are essential trace minerals with various functions in the human body.
Regarding the samples intended only as hair dyes, the safety assessment guideline of L. inermis (henna) as a hair dye by the SCCS5 established impurity levels of heavy metals such as Pb, Cr, and Ni at 1.04, 9.4 and 8.06 ppm, respectively. As such, given the results obtained in this study for these three elements (Table 3), only Pb fulfils the SCCS requirements for all HD samples. In the case of Ni, one of the samples (HD-3) shows a higher concentration than specified and nine of the ten henna dye samples exceed the mentioned Cr concentration.
Although some concentrations of the elements analyzed were relatively low, it is important to consider that they remain on the skin for a certain period of time because they are products for temporary use, and it is difficult to completely exclude the possibility that they induce adverse effects. Moreover, some of them are also toxic even at low concentrations. Thus, the prolonged use of these plant-based cosmetics can increase the absorption of heavy metals into the human body and act as health hazards. On the basis of this background, the metallic ingredients of temporary tattoos and dyes should be analyzed, and a systematic risk assessment should be carried out to ensure their safety and to reduce unnecessary exposure to toxic metals.
4. Conclusions
Natural pigment-based temporary tattoo and dye products are highly prevalent and available to anyone, even through Internet sources. Characterization of henna and jagua temporary tattoos and hair dyes according to their elemental content have been achieved by ICP-MS. Differences between the two groups of samples (solid and pastes) as well as between the henna and the jagua samples have been studied, together with hypothetic impact on public and human health.Both groups contained considerable amounts of the selected heavy metals showing a wide variability among the samples, with solids containing higher concentrations than the pastes. The low method LOQs allowed characterization and differentiation between both groups, finding all the studied elements in ten samples.
The heavy metal variation in the results points to the fact that these products lack any quality control during the material sourcing and manufacturing and may be produced from impure and low quality substances. It is significant that, according to the measured concentrations of Cr, Ni, As, Cd, Co, or Pb, none of the 34 samples analyzed comply with the current European Cosmetics Regulation. Some of the products containing the highest levels of these elements do not provide label information about their presence, with some labels indicating the absence of heavy metals.
A declaration of additives, synthetic dyes and metallic contaminants with appropriate labelling is needed. The cumulative effects of prolonged exposure to metals and their potential toxicity may be of concern. The widespread use of these products, especially when children are involved, may constitute a public health risk. Therefore, extensive quality measures would be recommended for products designed to come into direct contact with the skin or hair when regulations are unclear. Thus, the proposed ICP-MS analytical methodology could contribute to develop a useful tool in monitoring this type of beauty products.
Author contributions
L. Rubio: methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation. P. Barrulas: conceptualization, methodology, software, validation, formal analysis, resources, data curation, writing—review and editing, visualization, supervision. M. Costa: conceptualization, resources, writing—review and editing. C. Garcia-Jares: writing—review and editing, project administration, funding acquisition. M. Lores: writing—review and editing, project administration, funding acquisition. C. Barrocas Dias: conceptualization, resources, writing—review and editing, project administration.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This research was supported by project ED431B 2020/06 (Galician Competitive Research Groups, Xunta de Galicia, Spain). The authors L. Rubio and C. Garcia-Jares belong to the CRETUS Strategic Partnership (ED431E 2018/01). All these programs are co-funded by FEDER (EU). L. Rubio acknowledges Xunta de Galicia for her predoctoral contract (ED481A-2018/227) and Erasmus + program for her mobility grant. This work has also been financially supported by the UIDB/04449/2020 and UIDP/04449/2020 projects, funded by Fundação para a Ciência e a Tecnologia (FCT) and by the European Regional Development Fund.References
- O. Al-Dayel, J. Hefne and T. Al-Ajyan, Human exposure to heavy metals from cosmetics, Orient. J. Chem., 2011, 27(1), 1 CAS.
- S. Safavi, R. Najarian, M. Rasouli-Azad, S. Masoumzadeh, A. Ghaderi and R. Eghtesadi, A narrative review of heavy metals in cosmetics; health risks, Int. J. Pharm. Res., 2019, 11(4), 182–190, DOI:10.31838/ijpr/2019.11.04.031.
- J. Kazandjieva, L. Grozdev and N. Tsankov, Temporary henna tattoos, Clin. Dermatol., 2007, 25, 383–387, DOI:10.1016/j.clindermatol.2007.05.013.
- M. Maarouf, C. Saberian, R. J. Segal and V. Y. Shi, A new era for tattoos, with new potential complications, J. Clin. Aesthet. Dermatol., 2019, 12, 37–38 Search PubMed , PMID: 30881582, PMCID: PMC6415705..
- Scientific Committee on Consumer Safety (SCCS), Opinion on Lawsonia inermis (Henna) COLIPA no. C169, 19 September 2013 SCCS/1511/13, https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_140.pdf, accessed 19 September 2022.
- J. Waton, F. Brault and E. Laveine, A putative case of allergic contact dermatitis caused by a jagua tattoo, Contact Dermatitis, 2021, 76, 296–321, DOI:10.1111/cod.12767.
- Manual of the Working Group on Cosmetic Products (Sub-Group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No. 1223/2009 (Art. 2(1)(A)) Version 3.1, 2017, https://ec.europa.eu/docsroom/documents/29002, accessed 19 September 2022.
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast), Off. J. Eur. Union L 342, 2009, 59, https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223, accessed 19 September 2022 Search PubMed.
- Directive 2009/48/EC of the European Parliament and of the Council of 18 June 2009 on the Safety of Toys, Off. J. Eur. Union L 170, 2009, 1–37, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020SC0287%26rid=6, accessed 19 September 2022 Search PubMed.
- M. Lores, M. Celeiro, L. Rubio, M. Llompart and C. Garcia-Jares, Extreme cosmetics and borderline products: An analytical-based survey of European regulation compliance, Anal. Bioanal. Chem., 2018, 410, 7085–7102, DOI:10.1007/s00216-018-1312-3.
- L. Rubio, E. Guerra, C. Garcia-Jares and M. Lores, Body-decorating products: Ingredients of permanent and temporary tattoos from analytical and European regulatory perspectives, Anal. Chim. Acta, 2019, 1079, 59–72, DOI:10.1016/j.aca.2019.06.052.
- N. Lekouch, A. Sedki, A. Nejmeddine and S. Gamon, Lead and traditional Moroccan pharmacopeia, Sci. Total Environ., 2001, 280, 39–43, DOI:10.1016/S0048-9697(01)00801-4.
- M. A. Nouioui, S. Mahjoubi, A. Ghorbe, M. B. Y. Yahia, D. Amira, H. Ghorbel and A. Hedhili, Health Risk Assessment of Heavy Metals in Traditional Cosmetics Sold in Tunisian Local Markets, Int. Scholarly Res. Not., 2016, 2016, 1–12, DOI:10.1155/2016/6296458.
- I.-K. Kang and M.-H. Lee, Quantification of para-phenylenediamine and heavy metals in henna dye, Contact Dermatitis, 2006, 55, 26–29, DOI:10.1111/j.0105-1873.2006.00845.x.
- I. A. Al-Saleh and L. Coate, Lead exposure in Saudi Arabia from the use of traditional cosmetics and medical remedies, Environ. Geochem. Health, 1995, 17, 29–31, DOI:10.1007/BF00188629.
- A. Y. Ahmed, A. Asada and I. A. A. Hamza, Cobalt and lead concentrations in cosmetic products sold at local markets in Saudi Arabia, Toxicol. Rep., 2021, 8, 1693–1698, DOI:10.1016/j.toxrep.2021.09.004.
- M. Yahya, S. Kesekler, I. Durukan and C. Arpa, Determination of prohibited lead and cadmium traces in hair dyes and henna samples using ultrasound assisted-deep eutectic solvent-based liquid phase microextraction followed by microsampling-flame atomic absorption spectrometry, Anal. Methods, 2021, 13, 1058–1068, 10.1039/D0AY02235G.
- N. Ozbek and S. Akman, Determination of lead, cadmium and nickel in hennas and other hair dyes sold in Turkey, Regul. Toxicol. Pharmacol., 2016, 79, 49–53, DOI:10.1016/j.yrtph.2016.05.013.
- K. N. Jallad and C. Espada-Jallad, Lead exposure from the use of Lawsonia inermis (Henna) in temporary paint-on-tattooing and hair dying, Sci. Total Environ., 20018, 397, 244–250, DOI:10.1016/j.scitotenv.2008.02.055.
- A. Ait Sidi Mou, M. Daoudi, M. E. A. Ghanjaoui, B. Elgamany and R. Slimani, Principal component analysis applied to compare the quality of trace elements in Moroccan and Indian henna, J. Anal. Sci. Appl. Biotechnol., 2020, 2, 32–37, DOI:10.48402/IMIST.PRSM/jasab-v2i1.19558.
- M. E. Ghanjaoui, M. Cervera, M. El Rhazi and M. de la Guardia, Assessment of trace elements in traditional Moroccan cosmetics by inductively coupled plasma atomic emission spectroscopy, Int. J. Sci. Res., 2014, 3(10), 104–112 Search PubMed.
- N. Ozbek, Elemental analysis of henna samples by MP AES, J. Turk. Chem. Soc., Sect. A, 2018, 5, 857–868, DOI:10.18596/jotcsa.423820.
- A. K. Salama, Assessment of metals in cosmetics commonly used in Saudi Arabia, Environ. Monit. Assess., 2016, 188, 553, DOI:10.1007/s10661-016-5550-6.
- M. G. Volpe, M. Nazzaro, R. Coppola, F. Rapuano and R. P. Aquino, Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA, Microchem. J., 2012, 101, 65–69, DOI:10.1016/j.microc.2011.10.008.
- M. F. Mesko, D. L. R. Novo, V. C. Costa, A. S. Henn and E. M. M. Flores, Toxic and potentially toxic elements determination in cosmetics used for make-up: A critical review, Anal. Chim. Acta, 2020, 1098, 1–26, DOI:10.1016/j.aca.2019.11.046.
- N. M. Hepp, W. R. Mindak and J. Cheng, Determination of total lead in lipstick: development and validation of a microwave-assisted digestion, inductively coupled plasma–mass spectrometric method, J. Cosmet. Sci., 2009, 60, 405–414 CAS , PMID: 19691936..
- R. Thomas, Practical Guide to ICP-MS, CRC Press, Boca Raton, 2013 Search PubMed.
- L. Balcaen, E. Bolea-Fernandez, M. Resano and F. Vanhaecke, Inductively coupled plasma – Tandem mass spectrometry (ICP-MS/MS): a powerful and universal tool for the interference-free determination of (ultra)trace elements – a tutorial review, Anal. Chim. Acta, 2015, 894, 7–19, DOI:10.1016/j.aca.2015.08.053.
- A. M. Bobaker, I. Alakili, S. B. Sarmani, N. Al-Ansari and Z. M. Yaseen, Determination and Assessment of the Toxic Heavy Metal Elements Abstracted from the Traditional Plant Cosmetics and Medical Remedies: Case Study of Libya, Int. J. Environ. Res. Public Health, 2019, 16, 1957, DOI:10.3390/ijerph16111957.
- S. Y. Ibrahim and R. A. Maguid, Evaluation of Metals Content among Different Cosmetic Products in the Arabian Market, J. Pharmacol. Clin. Toxicol., 2016, 4, 1062, DOI:10.14419/ijpt.v4i1.5942.
- M. A. Al-Qutob, H. M. Alatrash and S. Abol-Ola, Determination of different heavy metals concentrations in cosmetics purchased from the Palestinian markets by ICP/MS, AES Bioflux, 2013, 5, 287–293 Search PubMed.
- A. Aktas Sukuroglu, D. Battal and S. Burgaz, Monitoring of lawsone, p-phenylenediamine and heavy metals in commercial temporary black henna tattoos sold in Turkey, Contact Dermatitis, 2021, 76, 89–95, DOI:10.1111/cod.12702.
- S. Y. Ibrahim, M. M. Fawzi, M. G. Saad and S. M. A. Rahman, Determination of Heavy Metals and Other Toxic Ingredients in Henna (Lawsonia inermis), J. Environ. Anal. Toxicol., 2016, 6, 364, DOI:10.4172/2161-0525.1000364.
- S. Kilic, M. Kilic and M. Soylak, The Determination of Toxic Metals in some Traditional Cosmetic Products and Health Risk Assessment, Biol. Trace Elem. Res., 2021, 199, 2272–2277, DOI:10.1007/s12011-020-02357-8.
- W. Johnson Jr, W. F. Bergfeld, D. V. Belsito, R. A. Hill, C. D. Klaassen, D. C. Liebler, J. G. Marks Jr, R. C. Shank, T. J. Slaga, P. W. Snyder, L. J. Gill and B. Heldreth, Safety Assessment of Barium Sulfate as Used in Cosmetics, Int. J. Toxicol., 2018, 37, 5S–11S, DOI:10.1177/1091581818799346.
- L. Rubio, M. Lores and C. Garcia-Jares, Monitoring of natural pigments in henna and jagua tattoos for fake detection, Cosmetics, 2020, 7, 74, DOI:10.3390/cosmetics7040074.
- L. Rubio, C. Garcia-Jares and M. Lores, High-resolution mass spectrometry for the comprehensive characterization of plant-pigment-based tattoos and dyes formulations, Cosmetics, 2021, 8, 55, DOI:10.3390/cosmetics8020055.
- Commission Regulation (EU) 2021/850 of 26 May 2021 amending and correcting Annex II and amending Annexes III, IV and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R0850, accessed 19 September 2022.
- R. A. Johnson and D. W. Wichern, Applied Multivariate Statistical Analysis, Prentice Hall, Englewood Cliffs, NJ, 1992 Search PubMed.
- M. J. Adams, The principles of multivariate data analysis, in Analytical Methods of Food Authentication, ed. P. R. Ashurst and M. J. Dennis, Blackie Academic & Professional, London, UK, 1998, p. 350 Search PubMed.
- J. E. McKenna Jr, An enhanced cluster analysis program with bootstrap significance testing for ecological community analysis, Environ. Model. Software, 2003, 18, 205–220, DOI:10.1016/S1364-8152(02)00094-4.
- G. F. Nordberg, B. A. Fowler, M. Nordberg and L. Friberg, Handbook of the Toxicology of Metals, Academic Press, London, UK, 2007 Search PubMed.
- G. J. Nohynek, E. Antignac, T. Re and H. Toutain, Safety assessment of personal care products/cosmetics and their ingredients, Toxicol. Appl. Pharmacol., 2010, 243, 239–259, DOI:10.1016/j.taap.2009.12.001.
- S. Borowska and M. M. Brzóska, Metals in cosmetics: implications for human health, J. Appl. Toxicol., 2015, 35, 551–572, DOI:10.1002/jat.3129.
- B. Bocca, A. Pino, A. Alimonti and G. Forte, Toxic metals contained in cosmetics: a status report, Regul. Toxicol. Pharmacol., 2014, 68, 447–467, DOI:10.1016/j.yrtph.2014.02.003.
- G. Forte, F. Petrucci and B. Bocca, Metal allergens of growing significance: epidemiology, immunotoxicology, strategies for testing and prevention, Inflammation Allergy: Drug Targets, 2008, 7, 145–162, DOI:10.2174/187152808785748146.
- J. P. Thyssen and T. Menné, Metal allergy – a review on exposures, penetration, genetics, prevalence, and clinical implications, Chem. Res. Toxicol., 2010, 23, 309–318, DOI:10.1021/tx9002726.
- L. Kanerva, R. Jolanki, T. Estlander, K. Alanko and A. Savela, Incidence rates of occupational allergic contact dermatitis caused by metals, Am. J. Contact Dermatitis, 2000, 3, 155–160, DOI:10.1053/ajcd.2000.7186.
- R. Guy, J. J. Hostynek, R. S. Hinz and C. R. Lorence, Metals and the Skin: Topical Effects and Systemic Absorption, CRC Press, New York, 1999 Search PubMed.
- J. Godt, F. Scheidig, C. Grosse-Siestrup, V. Esche, P. Brandenburg, A. Reich and D. A. Gronenberg, The toxicity of cadmium and resulting hazards for human health, J. Occup. Med. Toxicol., 2006, 1, 1–6, DOI:10.1186/1745-6673-1-22.
- A. Fischer, B. Brodziak-Dopierała, K. Loska and J. Stojko, The Assessment of Toxic Metals in Plants Used in Cosmetics and Cosmetology, Int. J. Environ. Res. Public Health, 2017, 14(10), 1280, DOI:10.3390/ijerph14101280.
- T. Sanders, Y. Liu, V. Buchner and P. B. Tchounwou, Neurotoxic effects and biomarkers of lead exposure: a review, Rev. Environ. Health, 2009, 24, 15–45, DOI:10.1515/REVEH.2009.24.1.15.
- J. G. Ayenimo, A. M. Yusuf, A. S. Adekunle and O. W. Makinde, Heavy metal exposure from personal care products, Bull. Environ. Contam. Toxicol., 2010, 84, 8–14, DOI:10.1007/s00128-009-9867-5.
- H. Li, P. Z. Toh, J. Y. Tan, M. T. Zin, C. Y. Lee, B. Li, M. Leolukman, H. Bao and L. Kang, Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds, Sci. Rep., 2016, 6, 37664, DOI:10.1038/srep37664.
Footnote |
| † Electronic supplementary information (ESI) available: Coefficient of determination and instrumental detection limits obtained for the different elements. See DOI: https://doi.org/10.1039/d2ra06126k |
| This journal is © The Royal Society of Chemistry 2022 |