Open Access Article
Open Access ArticleOne-pot chlorocarboxylation of toluenes with chlorine dioxide under photoirradiation†
Shohei Ohno a,
Haruyasu Asahara
a,
Haruyasu Asahara ab,
Tsuyoshi Inoueab and
Kei Ohkubo
ab,
Tsuyoshi Inoueab and
Kei Ohkubo *bc
*bc
aGraduate School of Pharmaceutical Sciences, Osaka University, Yamada-oka 1-6, Suita, Osaka, 565-0871, Japan
bInstitute for Open and Transdisciplinary Research Initiatives, Osaka University, Yamada-oka 1-6, Suita, Osaka, 565-0871, Japan
cInstitute for Advanced Co-Creation Studies, Osaka University, Yamada-oka 1-6, Suita, Osaka, 565-0871, Japan. E-mail: ohkubo@irdd.osaka-u.ac.jp
First published on 2nd November 2022
Abstract
Chlorocarboxylation of toluene was achieved by photoirradiation of a chlorine dioxide radical (ClO2˙) under ambient conditions (298 K and 1 atm). 2- and 4-Chlorobenzoic acids were obtained in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yield ratio. This is a single step synthesis under metal-free and mild conditions.
1 yield ratio. This is a single step synthesis under metal-free and mild conditions.
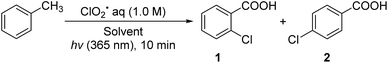
The chlorocarboxylation reaction is of practical importance in organic synthesis as chloro and carboxy groups are introduced into the substrate in a single step. In the 1980s, chlorocarboxylation of polymer materials such as polyethylene was performed using toxic dry chlorine gas and/or maleic anhydride at high temperatures (90–110 °C).1 To the best of our knowledge, there have been no reports of chlorocarboxylation of small molecules thus far.2,3 However, reactions such as chloroesterification and chlorocarbamoylation in the presence of transition metal catalysts have been reported.4 The substrates used in these reactions were limited to only alkenes and alkynes; chlorocarboxylation of aromatic compounds have not been reported to date. In this study, we developed a photoinduced chlorocarboxylation of toluene initiated by a chlorine dioxide radical (ClO2˙) at room temperature without the use of a metal catalyst [eqn. (1)]. We hypothesized that ClO2˙ is first photochemically activated to generate Cl˙ and O2, which would react with toluene to produce 2- and 4-chlorobenzoic acids in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yield ratio.
1 yield ratio.
 | (1) |
Previous reports have demonstrated the synthesis of 2- and 4-chlorobenzoic acids via a two-step process, where chlorination occurs first followed by the oxidation of toluene in the presence of toxic metals.5 In contrast, our method is green and sustainable as the products are obtained in a single step under metal-free conditions.
ClO2˙ (50 mM), which is a water-soluble gas and a stable radical at room temperature, was prepared by mixing sodium chlorite (NaClO2, 200 mg, 1.75 mmol) and 35% aq. HCl (100 μL) in aqueous solution (28 mL), as described in a previously reported method.6–9 The generation of ClO2˙ gas is shown in eqn (2).
| 5NaClO2 + 4HCl → 4ClO2˙ + 5NaCl + 2H2O | (2) |
UV-vis absorption (λmax = 358 nm) and electron spin resonance (ESR) spectroscopy (g = 2.015, a(Cl) = 16 G) of the aqueous solution confirmed the generation of ClO2˙ gas.7 Chlorocarboxylation of toluene did not occur in the dark because ClO2˙ cannot be activated without photoirradiation. In contrast, when an LED (λ = 365 nm, 20 mW cm−1) was used, ClO2˙ was photochemically activated to yield Cl˙ radical and singlet oxygen (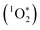 ) [eqn (3)].7
) [eqn (3)].7
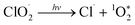 | (3) |
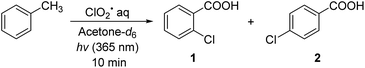
First, we studied the effects of different solvents on the chlorocarboxylation of toluene (Table 1). This reaction does not proceed in solvents with C–H bonds because ClO2˙ reacts with a hydrogen atom from the solvent. When acetone-d6 (entry 1) was used, 2-chlorobenzoic acid 1 and 4-chlorobenzoic acid 2 were produced in a total yield of 68% (35% and 33%, respectively). This implies that chlorination proceeded selectively at the electron-rich ortho and para positions. When acetonitrile-d3 and dimethylformamide-d6 were used under identical conditions (entries 2 and 3), the product yields (33% and 31%) were comparable to that of entry 1.10 When methanol-d4, N,N-dimethylformamide-d7, tetrahydrofuran-d8, benzene-d6, perfluorocarbons, and carbon tetrachloride were used (entries 4–9), the reaction did not proceed at all.
| Entry | Solvent | Yield (%)a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|---|---|---|
| a The products were identified and quantified using GC-MS by comparing them with the characterization data of authentic samples; ND = not detected. | ||
| 1 | Acetone-d6 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| 2 | Acetonitrile-d3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 3 | Dimethyl sulfoxide-d6 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 4 | Methanol-d6 | ND |
| 5 | DMF-d7 | ND |
| 6 | THF-d8 | ND |
| 7 | Benzene-d6 | ND |
| 8 | Perfluorohexane | ND |
| 9 | Tetrachloromethane | ND |
Next, we optimized the reaction conditions—concentration of ClO2˙ and excitation wavelength (Table 2). We observed that the reaction proceeded most efficiently when a 0.05 M ClO2˙ solution was used (entry 2). Other concentrations of ClO2˙ solutions produced the products in lower yields (entries 3, 4 and 5) than those obtained for entry 2. However, the reaction proceeded well even with visible light (λ = 405 nm) (entry 6). Therefore, the optimal conditions were 0.05 M ClO2˙ and an excitation wavelength of 365 nm.
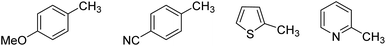
Subsequently, we attempted the chlorocarboxylation of other substrates (Fig. 1). When an electron-donating group-bearing toluene (p-methoxy toluene) was used di- or trichlorination occurred instead of the desired reaction. In contrast, when an electron-withdrawing group-bearing toluene (p-cyano toluene) was used, the reaction did not proceed and the starting material was recovered. Furthermore, the desired reaction did not proceed when electron-rich or electron-deficient heterocycles (2-methyl thiophene and 2-methyl pyridine, respectively) were used as substrates.
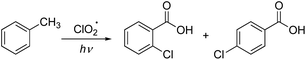
Finally, control experiments were performed to investigate the reaction mechanism (Fig. 2). First, the reaction was examined using 18O-labeled water (H2O18) under nitrogen atmosphere; however, no labeled product was observed [Fig. 2(a)]. This result suggests that the carboxylate oxygen atoms in the product were not derived from water. In contrast, when the reaction was conducted in toluene-d8, only monochlorinated toluene was obtained without any carboxylated products [Fig. 2(b)]. This result suggests that the carboxylation step in chlorocarboxylation begins with hydrogen abstraction at the benzyl position of toluene. Moreover, when we studied the chlorination of benzoic acid under ClO2˙ photoreaction conditions, chlorination did not occur [Fig. 2(c)]. The reactions of 2- and 4-chlorotoluene yielded the corresponding chlorocarboxylic acids in good yields (66% and 75%, respectively) [Fig. 2(d)]. These results indicate that the chlorocarboxylation of toluene begins with chlorination, in which a chlorine radical attacks the aromatic ring.
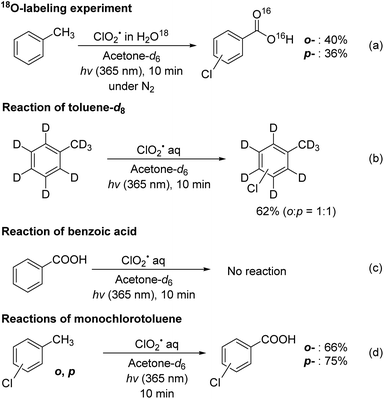 | ||
| Fig. 2 Control experiments to elucidate the reaction mechanism of the chlorocarboxylation of toluene. | ||
Based on these experimental results, we propose the following mechanism (Scheme 1).:11 First, the photoirradiation of ClO2˙ produces a Cl˙ radical and singlet O2. Then the Cl˙ radical attacks toluene to generate an aryl radical. Subsequently, another Cl˙ radical abstracts a hydrogen from the aryl radical to produce chlorotoluene. Next, another hydrogen abstraction from chlorotoluene generates a benzyl radical.
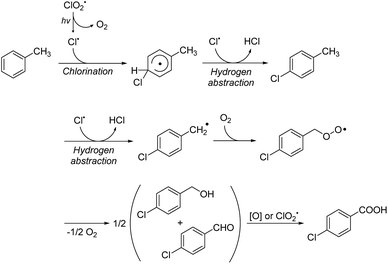 | ||
| Scheme 1 Plausible reaction mechanism for the chlorocarboxylation of toluene using ClO2˙. hν represents photo-irradiation and [O] represents autoxidation. | ||
This benzyl radical reacts with a singlet oxygen in the solution to form a peroxy radical intermediate. This peroxy radical releases one oxygen atom to form chlorobenzyl alcohol and chlorobenzaldehyde, which are then oxidized through Pinnick oxidation or autoxidation to yield the final product, chlorobenzoic acid.
In conclusion, for the first time in the literature, we demonstrated that ClO2˙ is an efficient reagent for the chlorocarboxylation of toluene in a single step. A plausible mechanism was proposed based on control experiments. This single-step synthesis of chlorobenzoic acids under metal-free conditions is a green and sustainable method that has potential applications in organic synthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (JP20K05606 to H. A.; JP20H02779 and JP20H04819 to K. O.), New Energy and Industrial Technology Development Organization (NEDO) grant (17101509-0 to H. A.), and JST Open Innovation with Enterprises, Research Institute, and Academia (OPERA) grant (JPMJOP1861 to T. I).Notes and references
- (a) S. G. Joshi and A. A. Natu, Angew. Makromol. Chem., 1986, 140, 99–112 CrossRef CAS; (b) S. G. Joshi and A. A. Natu, Angew. Makromol. Chem., 1986, 143, 115–130 CrossRef CAS; (c) S. G. Joshi and A. A. Natu, Angew. Makromol. Chem., 1986, 144, 113–127 CrossRef CAS; (d) S. G. Joshi and A. A. Natu, Angew. Makromol. Chem., 1986, 161, 135–143 CrossRef.
- (a) M. S. Chen and M. C. White, Science, 2010, 327, 566–571 CrossRef CAS PubMed; (b) M. A. Bigi, S. A. Reed and M. C. White, Nat. Chem., 2011, 3, 216–222 CrossRef CAS PubMed; (c) P. E. Gormisky and M. C. White, J. Am. Chem. Soc., 2011, 133, 12584–12589 CrossRef CAS PubMed; (d) M. C. White, Science, 2012, 335, 807–809 CrossRef CAS PubMed.
- (a) K. Ohkubo, K. Mizushima, R. Iwata and S. Fukuzumi, Chem. Sci., 2011, 2, 715–722 RSC; (b) B. Huang, Y. Zhao, C. Yang, Y. Gao and W. Xia, Org. Lett., 2017, 19, 3799–3802 CrossRef CAS PubMed; (c) Y. Li, T. Mou, L. Lu and X. Jiang, Chem. Commun., 2019, 55, 14299–14302 RSC; (d) L. Lu, Y. Li and X. Jiang, Green Chem., 2020, 22, 5989–5994 RSC.
- (a) R. Hua, S. Shimada and M. Tanaka, J. Am. Chem. Soc., 1998, 120, 12365–12366 CrossRef CAS; (b) R. Hua, S. Y. Onozawa and M. Tanaka, Chem.– Eur. J., 2005, 11, 3621–3630 CrossRef CAS PubMed; (c) B. Wang, S. Wang, P. Li and L. Wang, Chem. Commun., 2010, 46, 5891–5893 RSC; (d) T. Iwai, T. Fujihara, J. Terao and Y. Tsuji, J. Am. Chem. Soc., 2012, 134, 1268–1274 CrossRef CAS PubMed; (e) C. M. Le, X. Hou, T. Sperger, F. Schoenebeck and M. Lautens, Angew. Chem., Int. Ed., 2015, 54, 15897–15900 CrossRef CAS PubMed; (f) D. Bag, S. Mahajan and S. D. Sawant, Adv. Synth. Catal., 2020, 362, 3948–3970 CrossRef CAS.
- (a) H. T. Clarke and E. R. Taylor, Org. Synth., 1930, 10, 20 CrossRef CAS; (b) T. Maki and K. Takeda, Ullmann's Encycl. Ind. Chem., 2012, 5, 330–342 Search PubMed.
- Caution! ClO2˙ gas is explosive. Do not use in the high concentration (>10%) in air or at the high temperature (T > 100 °C).
- (a) K. Ohkubo and K. Hirose, Angew. Chem., Int. Ed., 2018, 57, 2126–2129 CrossRef CAS PubMed; (b) K. Ohkubo, H. Asahara and T. Inoue, Chem. Commun., 2019, 55, 4723–4726 RSC; (c) H. Asahara, N. Takao, M. Moriguchi, T. Inoue and K. Ohkubo, Chem. Commun., 2022, 58, 6174–6179 RSC.
- K. Ohkubo, K. Hirose, T. Shibata, K. Takamori and S. Fukuzumi, J. Phys. Org. Chem., 2017, 30, e3619 CrossRef.
- B. R. Deshwal, H.-D. Jo and H.-K. Lee, Can. J. Chem. Eng., 2004, 82, 619–632 CrossRef CAS.
- There is a linear proportion between reaction time and substrate concentration for photochemical reactions.
- (a) K. Ohkubo, K. Suga, K. Morikawa and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 12850–12859 CrossRef CAS PubMed; (b) K. Ohkubo, K. Hirose and S. Fukuzumi, RSC Adv., 2016, 6, 41011–41014 RSC; (c) A. Pokutsa, K. Ohkubo, A. Zaborovski and P. Bloniarz, Asia-Pac. J. Chem. Eng., 2021, 16, 2714 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06591f |
| This journal is © The Royal Society of Chemistry 2022 |