Open Access Article
Open Access ArticleMetal-free hydrosulfonylation of α,β-unsaturated ketones: synthesis and application of γ-keto sulfones†
Xiufang Cheng‡
a,
Shuo Wang‡a,
Yibo Wei‡a,
Huamin Wang *a and
Ying-Wu Lin
*a and
Ying-Wu Lin *ab
*ab
aSchool of Chemistry and Chemical Engineering, University of South China, Hengyang, P. R. China. E-mail: huaminwang@usc.edu.cn; linlinying@hotmail.com
bLaboratory of Protein Structure and Function, University of South China Medical School, Hengyang, P. R. China
First published on 13th December 2022
Abstract
γ-Keto sulfones are versatile building blocks and valuable intermediates in organic synthesis and pharmaceutical chemistry. Motivated by their excellent properties, we herein report a green, convenient, metal-free hydrosulfonylation method for a variety of ynones, vinyl ketones, and sodium sulfinates in the absence of stoichiometric oxidants. This operationally simple protocol provides straightforward and practical access to a wide range of γ-keto sulfones with broad functional group tolerance from easily available starting materials. Moreover, the β,γ-unsaturated keto sulfones could further react with 2,3-butadienoate to generate cyclopentenes in phosphine-mediated [3 + 2] cycloaddition.
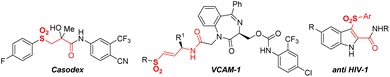
As a useful common structural fragment in a broad number of pharmaceuticals1 and functional materials,2 keto sulfones are usually present in promising biologically active molecules such as Casodex,3 VCAM-1 (ref. 4) and anti-HIV-1 (ref. 5) (Fig. 1). Furthermore, a valuable synthetic impression is associated with the role of reactive intermediates in various high-demand synthetic transformations,6 including total synthesis.7 Owing to their excellent properties, and efficient and practical synthesis methods keto sulfones are in high demand.
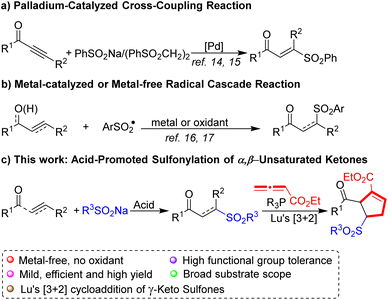
In the past decades, a variety of protocols have been developed to construct β-keto sulfones.8 Whereas succinct synthetic routes toward structurally related γ-keto sulfones are scarce,9 traditionally, γ-keto sulfones were synthesized via the nucleophilic substitution of sodium sulfinates by 2-chlorovinyl ketones,10 the elimination of the bromo derivatives of saturated keto sulfones11 and the oxidation of the corresponding sulfides or sulfoxides.12 However, the principal drawback is that these procedures were strongly limited by multiple steps, narrow substrate scope, or poor stereoselectivity.
Indeed, several streamlined strategies for the preparation of γ-keto sulfones involves addition reaction of alkenes or alkynes have been developed.13 Li's group14 reported the synthesis of (E)-vinyl sulfones through Pd-catalyzed conjugate additions of alkynes with 1,2-bis(phenylsulfonyl)ethane. In 2013 Jiang and co-workers15 showed that a Pd-catalyzed sulfonylation of alkynoates with sodium sulfinates affords γ-keto sulfones (Scheme 1a). Li and coworkers16 reported that BPO triggered the hydrosulfonylation of chalcones with arylsulfonyl hydrazides producing γ-keto sulfones. Subsequently, Bi's group17 developed a Ag2CO3-promoted sulfonylation of allyl/propargyl alcohols with sodium sulfinates for the preparation of γ-keto sulfones (Scheme 1b). Nevertheless, most cases still have to use large excess oxidants, noble metal catalysts, or require high temperatures. Accordingly, an efficient, mild and practical method to furnish γ-keto sulfones is worthwhile studying.
With growing demand for sustainable chemistry, an “ideal” reaction system for such transformations would be “metal-free” due to cost efficiency and possible advantages regarding toxicity, as well as selectivity. With this intent, we herein describe a simple and efficient acid-mediated sulfonylation of sodium sulfinates and α,β-unsaturated ketones for the selective synthesis of γ-keto sulfones (Scheme 1c). The significant advantages of this method are high efficiency, metal-free and mild reaction conditions, thus providing a potential application in natural product synthesis and medicinal chemistry.
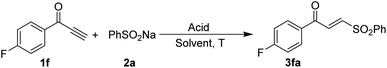
Further studies were commenced with the optimization of the conditions for the hydrosulfonylation of the ynone 1f with sodium benzosulfonate 2a (Table 1). Acetate buffer solution (pH = 3.5)13e and acetyl chloride/H2O,19 as used in the previous study, were completely ineffective due to several unknown complex products being formed (entry 1). Gratifyingly, the desired γ-keto sulfone 3fa was isolated in a 49% yield (E/Z = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as the major product for the reaction mediated by AcOH (entry 3). Encouraged by this initial result, we screened an array of acids. The results showed that 4-chlorobenzoic acid (PCBA) gave the best result, leading to the isolation of γ-keto sulfone 3fa in a yield of 80% (E/Z = 98
10) as the major product for the reaction mediated by AcOH (entry 3). Encouraged by this initial result, we screened an array of acids. The results showed that 4-chlorobenzoic acid (PCBA) gave the best result, leading to the isolation of γ-keto sulfone 3fa in a yield of 80% (E/Z = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02) (entries 4–15). Solvent screening indicated that mesitylene could improve the yield to 85% (E/Z = 95
02) (entries 4–15). Solvent screening indicated that mesitylene could improve the yield to 85% (E/Z = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05) (entry 21). Further investigations on the reduced usage of PCBA to 2.0 equivalents, the yield of 3fa was slightly reduced (entry 22, 83% yield, E/Z = 95
05) (entry 21). Further investigations on the reduced usage of PCBA to 2.0 equivalents, the yield of 3fa was slightly reduced (entry 22, 83% yield, E/Z = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05). The amounts of sodium benzosulfonate 2a and the reaction temperature have deleterious effects on the reaction yields (entries 23–27). Thus, the optimized reaction conditions were successfully established as 1f (1.0 equiv.), 2a (2.5 equiv.), PCBA (2.0 equiv.), and mesitylene (2.0 mL) at 30 °C in this process.
05). The amounts of sodium benzosulfonate 2a and the reaction temperature have deleterious effects on the reaction yields (entries 23–27). Thus, the optimized reaction conditions were successfully established as 1f (1.0 equiv.), 2a (2.5 equiv.), PCBA (2.0 equiv.), and mesitylene (2.0 mL) at 30 °C in this process.
| Entry | Acid (x equiv.) | Solvent | Yieldb (%) | E/Zc |
|---|---|---|---|---|
| a Reaction conditions: 1f (0.1 mmol), 2a (0.25 mmol), acid (x equiv.), solvent (1.0 mL), 30 °C, 48 h.b Isolated yields.c Determined by RP-HPLC.d With 2.0 equiv. 2a.e 50 °C.f 80 °C. PCBA = 4-chlorobenzoic acid. PNBA = p-nitrobenzoic acid. | ||||
| 1 | Buffer (pH = 3.5) | DMF | NR | — |
| 2 | Acetyl chloride/H2O | CHCl3 | NR | — |
| 3 | AcOH (3.0) | Toluene | 49 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 4 | HCO2H (3.0) | Toluene | 23 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 5 | HCl (3.0) | Toluene | 36 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 6 | HNO3 (3.0) | Toluene | 36 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 7 | Benzoic acid (3.0) | Toluene | 66 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 04 04 |
| 8 | p-Toluic acid (3.0) | Toluene | 52 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 04 04 |
| 9 | 4-Acetylbenzoic acid (3.0) | Toluene | 57 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 04 04 |
| 10 | 4-Fluorobenzoic acid (3.0) | Toluene | 73 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02 02 |
| 11 | PCBA (3.0) | Toluene | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02 02 |
| 12 | 4-Bromobenzoic acid (3.0) | Toluene | 72 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 05 |
| 13 | PNBA (3.0) | Toluene | 57 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 14 | 2-Naphthoic acid (3.0) | Toluene | 48 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 06 06 |
| 15 | 2-Nitrobenzoic acid (3.0) | Toluene | 29 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 06 06 |
| 16 | PCBA (3.0) | o-Xylene | 73 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
| 17 | PCBA (3.0) | p-Xylene | 70 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 18 | PCBA (3.0) | m-Xylene | 72 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 04 04 |
| 19 | PCBA (3.0) | DMF | NR | — |
| 20 | PCBA (3.0) | MeOH | 68 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 21 | PCBA (3.0) | Mesitylene | 85 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 05 |
| 22 | PCBA (2.0) | Mesitylene | 83 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 05 |
| 23 | PCBA (1.2) | Mesitylene | 76 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
| 24 | PCBA (0.5) | Mesitylene | 44 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 25d | PCBA (2.0) | Mesitylene | 79 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 26e | PCBA (2.0) | Mesitylene | 80 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 05 |
| 27f | PCBA (2.0) | Mesitylene | 53 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 03 03 |
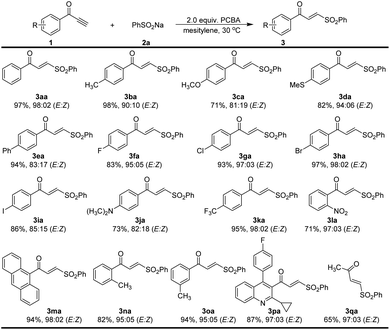
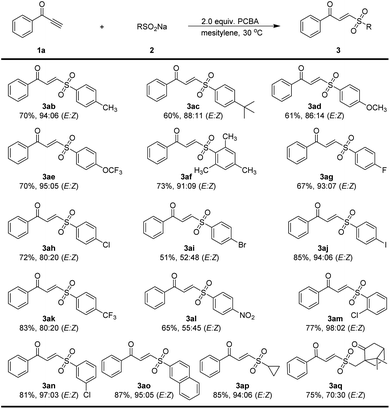
We then sought to explore the generality of the method for the synthesis of α,β-unsaturated γ-keto sulfones, using various ynones in reactions with 2a under the optimized conditions (Scheme 2). The reaction of the 1-phenylprop-2-yn-1-one 1a with 2a proceeded reasonably to provide an excellent yield of the corresponding γ-keto sulfone 3aa (97% yield, E/Z = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02). To our delight, the reaction worked successfully with a range of ynones 1 bearing various substituents on the aromatic ring. Substituents such as methyl, thiomethylmethoxy, phenyl, halogen and dimethylamino atoms could be tolerated and gave the corresponding products 3ba–3ja with high to excellent yields (71–98% yield) and stereoselectivity (E/Z = 82
02). To our delight, the reaction worked successfully with a range of ynones 1 bearing various substituents on the aromatic ring. Substituents such as methyl, thiomethylmethoxy, phenyl, halogen and dimethylamino atoms could be tolerated and gave the corresponding products 3ba–3ja with high to excellent yields (71–98% yield) and stereoselectivity (E/Z = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 to 98
18 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02). Trifluoromethyl and nitro substituents on the aromatic ring were also compatible and products 3ka and 3la were afforded 95% and 71% yields, respectively. 9-Anthracenee-derived ynone successfully afforded 3ma in a 94% yield (E/Z = 98
02). Trifluoromethyl and nitro substituents on the aromatic ring were also compatible and products 3ka and 3la were afforded 95% and 71% yields, respectively. 9-Anthracenee-derived ynone successfully afforded 3ma in a 94% yield (E/Z = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02). The methyl group in the ortho or meta positions of the aromatic ring gave the desired γ-keto sulfones in 82% and 94% yield, respectively. The desired product 3pa bearing a pitavastatin unit could be readily prepared in a yield of 87%. When alkyl terminal alkynone 1q was subjected to the reaction, affording the desired product 3qa in 65% yield (E/Z = 97
02). The methyl group in the ortho or meta positions of the aromatic ring gave the desired γ-keto sulfones in 82% and 94% yield, respectively. The desired product 3pa bearing a pitavastatin unit could be readily prepared in a yield of 87%. When alkyl terminal alkynone 1q was subjected to the reaction, affording the desired product 3qa in 65% yield (E/Z = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 03).
03).
 | (1) |
Inspired by the above results, the nonterminal alkyne was used as the substrate to react with PhSO2Na at 30 °C for 36 h. The reaction provided E and Z-β-sulfonyl-α,β-unsaturated carbonyl mixed compounds 3qa13e (86% yield, E/Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The results of ynone 1a reacting with a number of sodium sulfinates under the optimized condition are depicted in Scheme 3. Gratifyingly, no matter whether the phenyl ring of sodium sulfinate was substituted with either a sterically hindered, electron-donating, or electron-withdrawing group, all of them smoothly furnished the corresponding products in moderate to excellent yields with a high range of E/Z ratios from 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 to 97
48 to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 03 (3ab–3an). Likewise, 2-napthyl and cyclopropyl substituted sodium sulfinates were both effective in this reaction with a yield of 87% and 85%, respectively (3ao and 3ap). Additionally, L-10-camphorsulfonyl sulfinate 2q was also suitable for this reaction.
03 (3ab–3an). Likewise, 2-napthyl and cyclopropyl substituted sodium sulfinates were both effective in this reaction with a yield of 87% and 85%, respectively (3ao and 3ap). Additionally, L-10-camphorsulfonyl sulfinate 2q was also suitable for this reaction.
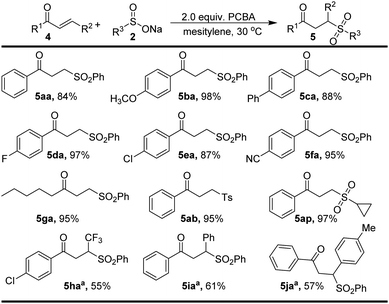
Interestingly, the treatment of the vinyl ketone 4a with PhSO2Na (2a) under the standard conditions furnished sulfone 5aa (Scheme 4). The substrate scope was also explored in Scheme 4. Delightfully, it was perfectly tolerable to introduce both electron-donating (OCH3 and Ph) and electron-withdrawing (F, Cl, and CN) groups at the para position of the phenyl ring, affording the corresponding products (5ba–5fa) in excellent yields. 4-Toluene sulfonate and cyclopropane sulfonate also reacted well with substrate 2a to form γ-keto sulfone in excellent yields. We were pleased to find that the β-trifluoromethylated enone 4h and trans-chalcone (4i–4j) could be successfully employed to give desired products (5ha–5ja, 55–61% yields). Unfortunately, no reaction occurred for 2-cyclopentenone.
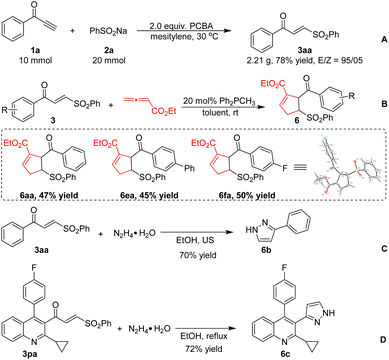
Additionally, the synthetic utility of the γ-keto sulfones obtained by the present method was explored (Scheme 5). Gram-scale ynone 1a was reacted with sodium benzosulfonate 2a to form product 3aa with an excellent E/Z ratio (A). Lu's [3 + 2] cycloaddition of 2,3-butadienoate with α,β-unsaturated γ-keto sulfones 3 mediated by phosphine produced cycloadducts 6 (ref. 18) in good yields (B). Moreover, pyrazole derivative 6b could be efficiently obtained from 3aa under ultrasound (US) irradiation conditions (C). Next, γ-keto sulfone 3pa derived from the biologically active pitavastatin could also react with hydrazine to give a high yield of 6c (D).
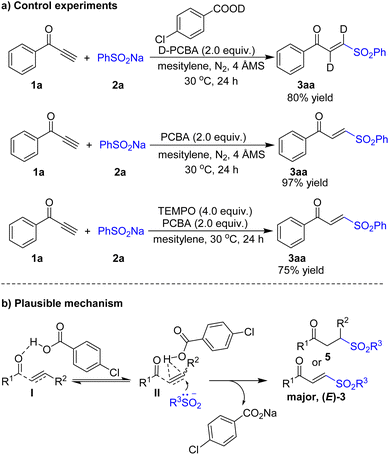
To understand the reaction mechanism, control reactions of 1a with 2a were examined (Scheme 6a). When 1a and 2a was subjected to the standard reaction conditions except using deuterated 4-chlorobenzoic acid system, the 3a were detected with 80% yield. An attempt to run the reaction of 1a and 2a in a anhydrous solvent system under an N2 atmosphere also successfully delivered 3a in 97% yield.20 The results unambiguously disclosed that the incorporated hydrogen atoms in 3a originated from acid rather than water. The reaction using 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and 2,4-di-tert-butyl-4-methylphenol (BHT) as the radical scavengers showed no observable radical intermediates and unaffected desired products formation, which suggests that the radical process could be ruled out.20 On the basis of the results presented above and previous reports, we propose the following mechanism in Scheme 6b. The 4-chlorobenzoic acid activates the carbonyl group in α,β-unsaturated ketones 1 (4) to afford intermediate I or tautomerize to intermediate II. Finally, sulfonyl anion can add to the unsaturated bond of intermediate II to afford the products 3 (5).
Conclusions
In summary, we developed a simple and efficient acid-mediated approach for the formation of γ-keto sulfones from sodium sulfinates and α,β-unsaturated ketones. This environmentally friendly methodology features a convenient, mild, efficient, C–S sulfonylation approach without the use of any metal catalysts and stoichiometric oxidants. The procedure results in good to excellent yields with various substituted ynones or vinyl ketones, as well as good functional group tolerance. The sulfonylation was easily scaled up and successfully integrated into Lu's [3 + 2] cycloaddition based on transformations of α,β-unsaturated γ-ketosulfones (3). All these advantages make the new method highly attractive to the organic chemist in both academia and industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support for this work from the National Natural Science Foundation of China (No. 21977042), the Hunan Provincial Natural Science Foundation of China (No. 2022JJ40362). We thank Professor Wei-Min He (USC) for helpful discussions on this manuscript.Notes and references
- (a) R. Ahmadi and S. Emami, Recent applications of vinyl sulfone motif in drug design and discovery, Eur. J. Med. Chem., 2022, 114255 CrossRef CAS PubMed; (b) P. Pakavathkumar, A. Noël, C. Lecrux, A. Tubeleviciute-Aydin, E. Hamel, J. E. Ahlfors and A. C. LeBlanc, Caspase vinyl sulfone small molecule inhibitors prevent axonal degeneration in human neurons and reverse cognitive impairment in Caspase-6-overexpressing mice, Mol. Neurodegener., 2017, 12, 22 CrossRef PubMed; (c) J. Xiang, M. Ipek, V. Suri, M. Tam, Y. Xing, N. Huang, Y. Zhang, J. Tobin, T. S. Mansour and J. McKew, β-Keto sulfones as inhibitors of 11β-hydroxysteroid dehydrogenase type I and the mechanism of action, Bioorg. Med. Chem., 2007, 15, 4396 CrossRef CAS PubMed; (d) C. Curti, M. Laget, A. O. Carle, A. Gellis and P. Vanelle, Rapid synthesis of sulfone derivatives as potential anti-infectious agents, Eur. J. Med. Chem., 2007, 42, 880 CrossRef CAS PubMed; (e) W. M. Wolf, The fungicidal activity of β-keto sulfones. Molecular conformation of α-phenylhydrazono-β-ketosulfones as determined by an X-ray analysis, J. Mol. Struct., 1999, 474, 113 CrossRef CAS.
- (a) S. Patai, C. Z. Rappoport and J. M. Stirling, The Chemistry of Sulfones and Sulfoxides, Wiley, New York, 1988 Search PubMed; (b) N. S. Simpkins, Radical cyclisation of dienes and enynes using TolSO2SePh, Pergamon Press, Oxford, 1993 Search PubMed.
- P. Iversen, C. J. Tyrrell, A. V. Kaisary, J. B. Anderson, H. E. I. N. Van Poppel, T. L. Tammela and I. Melezinek, Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup, J. Urol., 2000, 164, 1579 CrossRef CAS PubMed.
- R. Ettari, E. Nizi, M. E. D. Francesco, M. A. Dude, G. Pradel, R. Vicik, T. Schirmeister, N. Micale, S. Grasso and M. Zappala, Development of Peptidomimetics with a Vinyl Sulfone Warhead as Irreversible Falcipain-2 Inhibitors, J. Med. Chem., 2008, 51, 988 CrossRef CAS PubMed.
- (a) G. La Regina, A. Coluccia, A. Brancale, F. Piscitelli, V. Gatti, G. Maga, A. Samuele, C. Pannecouque, D. Schols, J. Balzarini, E. Novellino and R. Silvestri, New Nitrogen Containing Substituents at the Indole-2-carboxamide Yield High Potent and Broad Spectrum Indolylarylsulfone HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, J. Med. Chem., 2012, 55, 6634 CrossRef CAS PubMed; (b) V. Famiglini and R. Silvestri, Indolylarylsulfones, a fascinating story of highly potent human immunodeficiency virus type 1 non-nucleoside reverse transcriptase inhibitors, Antiviral Chem. Chemother., 2018, 26, 2040206617753443 CAS.
- (a) A.-N. R. Alna, X. Companyó and R. Rios, Sulfones: new reagents in organocatalysis, Chem. Soc. Rev., 2010, 39, 2018 RSC; (b) K. Inanaga, T. Fukuyama, M. Kubota, Y. Komatsu, H. Chiba, A. Kayano and K. Tagami, Novel and Efficient Chromium(II)-Mediated Desulfonylation of α-Sulfonyl Ketone, Org. Lett., 2015, 17, 3158 CrossRef CAS PubMed; (c) M.-Y. Chang, H.-Y. Chen and Y.-L. Tsai, Temperature-Controlled Desulfonylative Condensation of α-Sulfonyl o-Hydroxyacetophenones and 2-Formyl Azaarenes: Synthesis of Azaaryl Aurones and Flavones, J. Org. Chem., 2018, 84, 326 CrossRef PubMed; (d) Y.-D. Shao and D.-J. Cheng, Catalytic Asymmetric 1,2-Difunctionalization of Indolenines with α-(Benzothiazol-2-ylsulfonyl) Carbonyl Compounds, Adv. Synth. Catal., 2017, 359, 2549 CrossRef CAS; (e) R. E. Swenson, T. J. Sowin and H. Q. Zhang, Synthesis of Substituted Quinolines Using the Dianion Addition of N-Boc-anilines and α-Tolylsulfonyl-α,β-unsaturated Ketones, J. Org. Chem., 2002, 67, 9182 CrossRef CAS PubMed; (f) W.-M. He, Y.-W. Lin and D.-H. Yu, Uranyl photocatalysis:precisely controlled oxidation of sulfides with ground-state oxygen, Sci. China: Chem., 2020, 63, 291 CrossRef CAS.
- H. Yang, R. G. Carter and L. N. Zakharov, Enantioselective Total Synthesis of Lycopodine, J. Am. Chem. Soc., 2008, 130, 9238 CrossRef CAS PubMed.
- For examples, see: (a) Q. Tian, P. He and C. Kuang, Copper-catalyzed arylsulfonylation of N-arylsulfonyl-acrylamides with arylsulfonohydrazides: synthesis of sulfonated oxindoles, Org. Biomol. Chem., 2014, 12, 6349 RSC; (b) S. Handa, J. Fennewald and B. Lipshutz, Aerobic Oxidation in Nanomicelles of Aryl Alkynes, inWater at Room Temperature, Angew. Chem., Int. Ed., 2014, 53, 3432 CrossRef CAS PubMed; (c) Y.-L. Zhu, B. Jiang, W.-J. Hao, A.-F. Wang, J.-K. Qiu, P. Wei, D.-C. Wang, G. Li and S.-J. Tu, A new cascade halosulfonylation of 1,7-enynes toward 3,4-dihydroquinolin-2(1H)-ones via sulfonyl radical-triggered addition/6-exo-dig cyclization, Chem. Commun., 2016, 52, 1907 RSC; (d) W. Yu, P. Hu, Y. Fan, C. Yu, X. Yan, X. Li and X. Xu, Metal-free TBAI-catalyzed arylsulfonylation of activated alkenes with sulfonylhydrazides, Org. Biomol. Chem., 2015, 13, 3308 RSC; (e) X. Li, X. Xu, P. Hu, X. Xiao and C. Zhou, Synthesis of Sulfonated Oxindoles by Potassium Iodide Catalyzed Arylsulfonylation of Activated Alkenes with Sulfonylhydrazides in Water, J. Org. Chem., 2013, 78, 7343 CrossRef CAS PubMed; (f) M.-Z. Zhang, P.-Y. Ji, Y.-F. Liu, J.-W. Xu and C.-C. Guo, Disulfides as Sulfonylating Precursors for the Synthesis of Sulfone-Containing Oxindoles, Adv. Synth. Catal., 2016, 358, 2976 CrossRef CAS; (g) W. Wei, J. Wen, D. Yang, J. Du, J. You and H. Wang, Catalyst-free direct arylsulfonylation of N-arylacrylamides with sulfinic acids: a convenient and efficient route to sulfonated oxindoles, Green Chem., 2014, 16, 2988 RSC; (h) L. Liu, H. Xiao, F. H. Xiao, Y. J. Xie, H. W. Huang and G.-J. Deng, Synthesis of β-Ketosulfone from Sodium Sulfinate and Aryl Ethyl Ketone/Indanone, Chin. J. Org. Chem., 2021, 41, 4749 CrossRef; (i) I. Chikunova, Y. Kukushkin and Y. Dubovtsev, Atom-economic synthesis of β-ketosulfones based on gold-catalyzed highly regioselective hydration of alkynylsulfones, Green Chem., 2022, 24, 3314 RSC; (j) F. Xiao, C. Liu, D. Wang, H. Huang and G.-J. Deng, Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly, Green Chem., 2018, 20, 973–977 RSC; (k) S. Zhong, Z. Zhou, F. Zhao, G. Mao, G.-J. Deng and H. Huang, Deoxygenative C-S Bond Coupling with Sulfinates via Nickel/Photoredox Dual Catalysis, Org. Lett., 2022, 24, 1865–1870 CrossRef CAS PubMed.
- (a) S. Liang, K. Hofman, M. Friedrich, J. Keller and G. Manolikakes, Recent Progress and Emerging Technologies towards a Sustainable Synthesis of Sulfones, ChemSusChem, 2021, 14, 4878 CrossRef CAS PubMed; (b) P. Li, L. Wang and X. Wang, Recent advances on the pesticidal activity evaluations of sulfone derivatives: A 2010 to 2020 decade in mini-review, J. Heterocycl. Chem., 2021, 58, 28 CrossRef CAS; (c) D. Yadav and R. S. Menon, Recent developments in the chemistry of allenyl sulfones, Org. Biomol. Chem., 2020, 18, 365 RSC; (d) W. Guo, K. Tao, W. Tan, M. Zhao, L. Zheng and X. Fan, Recent advances in photocatalytic C–S/P–S bond formation via the generation of sulfur centered radicals and functionalization, Org. Chem. Front., 2019, 6, 2048 RSC; (e) G. Qiu, K. Zhou, L. Gao and J. Wu, Insertion of sulfur dioxide via a radical process: an efficient route to sulfonyl compounds, Org. Chem. Front., 2018, 5, 691 RSC; (f) B. Qin, S. Huang, J.-Q. Chen, W. Xiao and J. Wu, Metal-free synthesis of sulfonylated indolo[2,1-a]isoquinolines from sulfur dioxide, Org. Chem. Front., 2022, 9, 3521 RSC; (g) X.-L. Chen, C.-Y. Wu, J.-T. Ma, S.-Y. Zhuang, Z.-C. Yu, Y.-D. Wu and A.-X. Wu, Rongalite as C1 Synthon and Sulfone Source: A Practical Sulfonylmethylation Based on the Separate-Embedding Strategy, Org. Lett., 2022, 24, 223 CrossRef CAS PubMed.
- (a) D. B. Reddy, N. C. Babu, V. Padmavathi and R. P. Sumathi, A Novel Route for the Synthesis of Unsaturated Oxo Sulfones and Bissulfones, Synthesis, 1999, 3, 491 CrossRef; (b) B. Cavalchi, D. Landini and F. Montanari, Stereospecific synthesis of cis- and trans-2-halogenovinyl ketones. Stereochemistry of nucleophilic substitutions at vinylic carbon, J. Chem. Soc. C, 1969, 1204 RSC.
- E. P. Kohler and G. R. Larsen, The Properties of Unsaturated Sulfur Compounds. II. Alpha, Beta-Unsaturated Ketosulfones, J. Am. Chem. Soc., 1935, 57, 1448 CrossRef CAS.
- (a) B. K. Peters, T. Zhou, J. Rujirawanich, A. Cadu, T. Singh, W. Rabten, S. Kerdphon and P. G. Andersson, An Enantioselective Approach to the Preparation of Chiral Sulfones by Ir-Catalyzed Asymmetric Hydrogenation, J. Am. Chem. Soc., 2014, 136, 16557 CrossRef CAS PubMed; (b) V. Padmavathi, B. J. M. Reddy and A. Padmaja, A novel and simple route for the synthesis of 3,4-disubstituted pyrroles, J. Heterocycl. Chem., 2005, 42, 333 CrossRef CAS; (c) S. Hinterbergera, O. Hofer and H. Greger, Synthesis of amides from Glycosmis species: Methylthiopropenoic acid, methylsulfonylpropenoic acid, thiocarbamic acid S-methyl ester, and senecioic acid amides, Tetrahedron, 1998, 54, 487 CrossRef; (d) E. P. ohler and G. R. Larsen, The Properties of Unsaturated Sulfur Compounds. I. Alpha Beta Unsaturated Sulfones, J. Am. Chem. Soc., 1935, 57, 1316 CrossRef.
- (a) E. P. Kohler and G. R. Barrett, Some addition reactions of phenyl benzoyl acetylene, J. Am. Chem. Soc., 1924, 46, 747 CrossRef CAS; (b) W. Shi, B. Zhang, B. Liu, F. Xu, F. Xiao, J. Zhang, S. Zhang and J. Wang, Unusual reaction of β-hydroxy α-diazo carbonyl compounds with TsNHN CHCOCl/Et3N, Tetrahedron Lett., 2004, 45, 4563 CrossRef CAS; (c) Y. L. Zhu, B. Jiang, W. J. Hao, A. F. Wang, J. K. Qiu, P. Wei, D. C. Wang, G. Li and S. J. Tu, A new cascade halosulfonylation of 1,7-enynes toward 3,4-dihydroquinolin-2(1H)-ones via sulfonyl radical-triggered addition/6-exo-dig cyclization, Chem. Commun., 2016, 52, 1907 RSC; (d) J.-K. Qiu, C. Shan, D.-C. Wang, P. Wei, B. Jiang, S.-J. Tu, G. Li and K. Guo, Metal-Free Radical-Triggered Selenosulfonation of 1,7-Enynes for the Rapid Synthesis of 3,4-Dihydroquinolin-2(1H)-ones in Batch and Flow, Adv. Synth. Catal., 2017, 359, 4332 CrossRef CAS; (e) W. Zhang, G. Johnsonb, Z. Guan and Y.-H. He, Regio- and Stereoselective Hydrosulfonylation of Electron-Deficient Alkynes: Access to Both E- and Z-β-Sulfonyl-α,β-Unsaturated Carbonyl Compounds, Adv. Synth. Catal., 2018, 360, 4562 CrossRef CAS; (f) J. Jiang, Z. Wang and W.-M. He, Electrosynthesis of 1-indanones, Chin. Chem. Lett., 2021, 32, 1591 CrossRef CAS; (g) X.-X. Meng, Q.-Q. Kang, J.-Y. Zhang, Q. Li, W.-T. Wei and W.-M. He, Visible-light-initiated regioselective sulfonylation/cyclization of 1,6-enynes under photocatalyst- and additive-free conditions, Green Chem., 2020, 22, 1388 RSC; (h) Y. Gu, L. Dai, J. Zhang, X. Lu, X. Liu, C. Wang, J. Zhang and L. Rong, Silver-Catalyzed Radical Cascade Sulfonation/Cycloaddition for the Construction of Multifunctional Succimides Containing Separable Z/E-Isomers, J. Org. Chem., 2021, 86, 2173 CrossRef CAS PubMed.
- R.-J. Song, Y. Liu, Y.-Y. Liu and J.-H. Li, Palladium-Catalyzed Conjugate Addition to Electron-Deficient Alkynes with Benzenesulfinic Acid Derived from 1,2-Bis(phenylsulfonyl)ethane: Selective Synthesis of (E)-Vinyl Sulfones, J. Org. Chem., 2011, 76, 1001 CrossRef CAS PubMed.
- Y. Xu, J. Zhao, X. Tang, W. Wu and H. Jiang, Chemoselective Synthesis of Unsymmetrical Internal Alkynes or Vinyl Sulfones via Palladium-Catalyzed Cross-Coupling Reaction of Sodium Sulfinates with Alkynes, Adv. Synth. Catal., 2014, 356, 2029 CrossRef CAS.
- Z.-Z. Chen, S. Liu, W.-J. Hao, G. Xu, S. Wu, J.-N. Miao, B. Jiang, S.-L. Wang, S.-J. Tu and G. Li, Catalytic arylsulfonyl radical-triggered 1, 5-enyne-bicyclizations and hydrosulfonylation of α, β-conjugates, Chem. Sci., 2015, 6, 6654 RSC.
- G. Fang, J. Liu, W. Shang, Q. Liu and X. Bi, Silver(I)-Promoted Radical Sulfonylation of Allyl/Propargyl Alcohols: Efficient Synthesis of g-Keto Sulfones, Chem.–Asian J., 2016, 11, 3334 CrossRef CAS PubMed.
- The structure of 6fa (CCDC 2181007†) was clearly determined by X-ray crystallographic analysis.
- Z. Fang, Y. Zhang, Z. Zhang, Q. Song, Y. Wu, Z. Liu and Y. Ning, Synthesis of gem-Disulfonyl Enamines via an Iminyl-Radical-Mediated Formal 1,3-HAT/Radical Coupling Cascade, Org. Lett., 2022, 24, 6374 CrossRef CAS PubMed.
- More detail information for control experiments in ESI.†.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2181007. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra06784f |
| ‡ X. F. C., S. W. and Y. B. W. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |