Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Palladium nanoparticles immobilized on polyethylenimine-derivatized gold surfaces for catalysis of Suzuki reactions: development and application in a lab-on-a-chip context
Prasad Anaspure,
Subramanian Suriyanarayanan* and
Ian A. Nicholls
Linnaeus University Centre for Biomaterials Chemistry, Bioorganic and Biophysical Chemistry Laboratory, Department of Chemistry and Biomedical Sciences, Linnaeus University, SE-39182 Kalmar, Sweden. E-mail: subramanian.suriyanarayanan@lnu.se
First published on 2nd December 2022
Abstract
Correction for ‘Palladium nanoparticles immobilized on polyethylenimine-derivatized gold surfaces for catalysis of Suzuki reactions: development and application in a lab-on-a-chip context’ by Prasad Anaspure et al., RSC Adv., 2021, 11, 35161–35164. https://doi.org/10.1039/D1RA06851B.
The authors regret that the turnover numbers (TONs) were not correctly given in the original article.
In the abstract on page 35161, the corrected number should read 3.4 × 104.
The corrected versions of Table 1 and 2 are shown below.
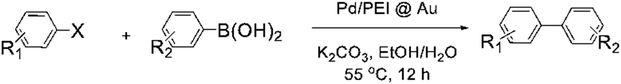
| Entry | R1 | X | R2 | Amount of Pd, μg | Yield | TON |
|---|---|---|---|---|---|---|
| a General procedure: 1.0 mmol of aryl halide, 1.2 mmol of arylboric acid, 2.0 mmol of k2CO3 in H2O/EtOH. Turnover number TON = mol product/mol Pd. n. r. = no reaction. | ||||||
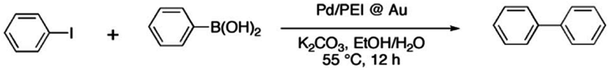
| 1 | H | I | H | 3.2 | 93% | 3.1 × 104 |
| 2 | H | Br | H | 2.8 | 95% | 3.4 × 104 |
| 3 | H | I | 2-CH3 | 3.9 | 82% | 2.2 × 104 |
| 4 | H | I | 3-OCH3 | 4.0 | 57% | 1.5 × 104 |
| 5 | H | I | 4-OCH3 | 3.7 | 84% | 2.4 × 104 |
| 6 | H | I | 2-CN | 3.99 | 15% | 0.4 × 104 |
| 7 | H | I | 4-CN | 3.6 | 95% | 2.8 × 104 |
| 8 | 4-CH3 | Br | H | 6.2 | 88% | 1.5 × 104 |
| 9 | 4-OCH3 | Br | H | 8.4 | 95% | 1.2 × 104 |
| 10 | H | I | H3–NH2 | 3.5 | n. r. | — |
| 11 | H | Cl | H | 1.0 | 94% | 10.0 × 104 |
| 12 | 4-OCH3 | Cl | H | 1.62 | 80% | 5.3 × 104 |
| 13 | 4-CoCH3 | Cl | H | 1.5 | n. r. | — |
| Entry | Run | Conc. of Pd, αg | Yield | TON |
|---|---|---|---|---|
| a General procedure: 1.0 mmol of aryl halide, 1.2 mmol of arylboronic acid, 2.0 mmol of K2CO3 in H2O/EtOH. TON = mol product/mol. | ||||
| 1 | 1st | ±2.3 | 93% | 4.4 × 104 |
| 2 | 2nd | ±2.22 | 89% | 4.3 × 104 |
| 3 | 3rd | ±2.22 | 85% | 4.0 × 104 |
| 4 | 4th | ±2.15 | 80% | 3.9 × 104 |
Accordingly, Table 1-SI, Table 2-SI, Table 3-SI, and Table 4-SI in the original ESI have been revised; the ESI has been updated online.
An independent expert has viewed the corrected tables and has concluded that they are consistent with the discussions and conclusions presented.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2022 |