Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A redox-mediator pathway for enhanced multi-colour electrochemiluminescence in aqueous solution†
Emily
Kerr
 *a,
David J.
Hayne
*a,
David J.
Hayne
 a,
Lachlan C.
Soulsby
b,
Joseph C.
Bawden
b,
Steven J.
Blom
a,
Lachlan C.
Soulsby
b,
Joseph C.
Bawden
b,
Steven J.
Blom
 b,
Egan H.
Doeven
b,
Luke C.
Henderson
b,
Egan H.
Doeven
b,
Luke C.
Henderson
 a,
Conor F.
Hogan
a,
Conor F.
Hogan
 c and
Paul S.
Francis
c and
Paul S.
Francis
 *b
*b
aInstitute for Frontier Materials, Deakin University, Geelong, Victoria 3220, Australia. E-mail: emily.kerr@deakin.edu.au
bSchool of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, Geelong, Victoria 3220, Australia. E-mail: paul.francis@deakin.edu.au
cDepartment of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia
First published on 15th December 2021
Abstract
The classic and most widely used co-reactant electrochemiluminescence (ECL) reaction of tris(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+) and tri-n-propylamine is enhanced by an order of magnitude by fac-[Ir(sppy)3]3− (where sppy = 5′-sulfo-2-phenylpyridinato-C2,N), through a novel ‘redox mediator’ pathway. Moreover, the concomitant green emission of [Ir(sppy)3]3−* enables internal standardisation of the co-reactant ECL of [Ru(bpy)3]2+. This can be applied using a digital camera as the photodetector by exploiting the ratio of R and B values of the RGB colour data, providing superior sensitivity and precision for the development of low-cost, portable ECL-based analytical devices.
Introduction
Electrochemiluminescence (ECL) is a highly sensitive mode of detection1,2 frequently employed in clinical diagnostic assays such as those of the Roche cobas e automated immunoassay system.3 Conventional ECL bioassays incorporate an electrochemiluminophore (label) and a ‘co-reactant’ that enables the light-producing reaction to be initiated at a single applied potential in aqueous solution.4 The majority of ECL applications, and all commercial ECL technologies, use the tris(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+) luminophore (or a closely related derivative) and tri-n-propylamine (TPrA) co-reactant.5 Efforts to enhance the sensitivity of this fundamental ECL detection system have included the development of ‘poly-Ru2+’ labels, exploiting dendrimers6 or nanoparticles,7 but the diminishing increase in ECL intensity with the number of metal centres, and greater background signals due to higher non-specific binding has limited their use in clinical diagnostics.3 Alternatively, numerous Ir(III) complexes have shown great promise as high quantum yield luminophores for ECL in organic media,8 and their diverse electrochemical potentials and emission wavelengths have enabled the preliminary development of potential-resolved and multi-colour ECL systems,2,9 towards the long-held goals of multiplexed9–11 and internally standardised, ratiometric12 ECL detection.‡13–16 The poor solubility and/or reduced ECL performance of many Ir(III) complexes in aqueous solution, however, has restricted their analytical application.To enhance the water solubility, ECL intensity, and/or binding specificity of Ru(II) and Ir(III) complex electrochemiluminophores, researchers have incorporated 4,7-diphenyl-1,10-phenanthroline-disulfonate ligands17,18 or synthesised new ligands with polar functional groups such as methanesulfonate, tetraethylene-glycol and saccharides.3,19,20 Comparisons with [Ru(bpy)3]2+ in the ‘ProCell’ buffer solution (containing TPrA co-reactant and a surfactant) used in commercial ECL immunoassay system, however, have revealed that only a few outperform the conventional luminophore.18,20,21 One of the most promising candidates, an Ir(III) complex containing two sulfonate-bearing phenylphenanthridine derivatives and a phenylisoquinoline-based ligand for bioconjugation, exhibits 3–4 fold greater ECL intensity than the Ru(II) complex.3 Multi-colour ECL systems incorporating Ir(III) complexes have to date been predominantly limited to organic solvents11,22–24 and polymer-ionic-liquid gels,25 but have been coupled with assays in aqueous solution through closed bipolar electrochemistry,24,26 or by loading the luminophores into polystyrene beads that support the assay, followed by their release in the organic solvent for detection.27
The most commonly used Ir(III) complex in multi-colour ECL is fac-tris(2-phenylpyridinato)iridium(III) (fac-Ir(ppy)3),10,11,26–33 due to the large differences in its electrochemical potentials and emission wavelengths compared to [Ru(bpy)3]2+, and the effective quenching of its ECL at high overpotentials under certain conditions when TPrA is used as the co-reactant,28,29 which enables the complete resolution of its emission from other luminophores through the applied potential. Moreover, the addition of Ir(ppy)3 to the annihilation ECL reaction of [Ru(bpy)3]2+ in acetonitrile was shown to enhance (∼25-fold) the emission from the Ru(II) complex, through the ‘mixed annihilation ECL’ reaction of the reduced [Ru(bpy)3]+ with oxidised [Ir(ppy)3]+ (reaction (1)).31
| [Ru(bpy)3]+ + [Ir(ppy)3]+ → [Ru(bpy)3]2+* + Ir(ppy)3 | (1) |
Considering that the multiple reaction pathways of the co-reactant ECL of [Ru(bpy)3]2+ and TPrA involve oxidation and/or reduction of the Ru(II) complex,5 we sought to use Ir(ppy)3 as an alternative means to enhance this most widely applied system. The Ir(ppy)3 complex is insoluble in aqueous solution, but Wenger et al.34,35 recently outlined a convenient synthetic strategy to add a sulfonate group to each ligand of the intact complex, which they used for photoredox catalysis in aqueous solution. Herein, we adopt this synthetic approach to explore [Ir(sppy)3]3− (Fig. 1) as a novel luminophore for aqueous ECL, which enables the first multi-colour ECL from a mixture of metal complexes in aqueous solution. We then demonstrate that the [Ir(sppy)3]3− complex can enhance the ECL of [Ru(bpy)3]2+ with TPrA co-reactant by over an order of magnitude, while providing an unprecedented means for internal standardisation of this important ECL system. Finally, we show that the enhancement and internal standardisation can be exploited when using a digital camera as the photodetector (via the ratio of R and B values of RGB colour data), providing a new approach to overcome the reproducibility and sensitivity limitations of low-cost, portable analytical devices with ECL detection.
Results and discussion
Electrochemistry and photoluminescence
The photophysical and electrochemical properties of [Ir(sppy)3]3− are compared to those of Ir(ppy)3, [Ru(bpy)3]2+, and a previously reported20 water-soluble Ir(III) complex electrochemiluminophore, [Ir(ppy)2(pt-TEG)]+, in Table 1. [Ir(sppy)3]3− readily dissolved at 5 mM in phosphate buffer solution (PBS) and exhibited a reversible oxidation (Fig. S1†) at 0.79 V vs. SCE, marginally higher than the oxidation potential of Ir(ppy)3 in acetonitrile (0.71 V vs. SCE36). The sulfonated derivative was insoluble in acetonitrile (at 0.1 mM) and we therefore determined the potential of the [Ir(sppy)3]3−/4− couple (E0′ = −2.48 V vs. SCE) in dimethylformamide. The [Ir(sppy)3]3− complex exhibited green photoluminescence in aqueous solution (λmax = 515 nm; Fig. S2†) at slightly higher energy than Ir(ppy)3 in acetonitrile (λmax = 520 nm (ref. 36)). A small difference in emission energy was also observed in the low temperature (85 K) emission spectra in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol
1 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (Table 1). Wenger and co-workers reported an excited state oxidation potential for [Ir(sppy)3]3− at −1.89 V vs. SCE,37 based on the ground-state oxidation potential (E0′(M+/M)) and the energy of the emissive triplet state (E0–0) estimated from the short-wavelength edge of the emission (at 10% of the maximum intensity) at room temperature. Our measurement of both ground state potentials and the E0–0 estimated from the λmax of the low-temperature emission spectrum enabled calculation of the excited state oxidation and reduction potentials as −1.82 V and 0.57 V vs. SCE.
methanol (Table 1). Wenger and co-workers reported an excited state oxidation potential for [Ir(sppy)3]3− at −1.89 V vs. SCE,37 based on the ground-state oxidation potential (E0′(M+/M)) and the energy of the emissive triplet state (E0–0) estimated from the short-wavelength edge of the emission (at 10% of the maximum intensity) at room temperature. Our measurement of both ground state potentials and the E0–0 estimated from the λmax of the low-temperature emission spectrum enabled calculation of the excited state oxidation and reduction potentials as −1.82 V and 0.57 V vs. SCE.
| [Ru(bpy)3]2+ | [Ir(ppy)2(pt-TEG)]+ | [Ir(sppy)3]3− | Ir(ppy)3 | |
|---|---|---|---|---|
a Metal complexes at 10 μM in water (or acetonitrile for Ir(ppy)3) at ambient temperature.
b Corrected for the change in instrument sensitivity over the wavelength range.
c Metal complexes at 5 μM in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (4 methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 85 K.
d Energy gap between the zeroth vibrational levels of the ground and excited states, estimated from the λmax of the low-temperature emission spectrum.
e Aqueous solution; deaerated.
f Aqueous (ProCell) solution; aerated.
g Acetonitrile; deaerated.
h Dichloromethane, deaerated.
i 2-Methyltetrahydrofuran (2-MeTHF), deaerated.
j Converted to SCE from Ag/AgCl (3.5 M KCl) by subtracting 40 mV.45
k Converted to SCE from Ag/AgCl (sat. KCl) by subtracting 46 mV.45
l Converted to SCE from Fc+/Fc0 (in ACN with 0.1 M TBAPF6 electrolyte) by adding 0.38 V.46
m Converted to SCE from Fc+/Fc0 (in DMF with 0.1 M TBAPF6 electrolyte) by adding 0.47 V.46
n Estimated using E0′(M+/M) − E0–0(M–M*).
o Estimated using E0′(M/M−) + E0–0.
p Integrated ECL intensity upon application of 1.2 V (vs. Ag/AgCl) for 10 s, measured using CCD spectrometer.
q Metal complex at 100 μM.
r Metal complex at 10 μM.
s By definition.
t Error represents standard deviation of five replicates. 1) at 85 K.
d Energy gap between the zeroth vibrational levels of the ground and excited states, estimated from the λmax of the low-temperature emission spectrum.
e Aqueous solution; deaerated.
f Aqueous (ProCell) solution; aerated.
g Acetonitrile; deaerated.
h Dichloromethane, deaerated.
i 2-Methyltetrahydrofuran (2-MeTHF), deaerated.
j Converted to SCE from Ag/AgCl (3.5 M KCl) by subtracting 40 mV.45
k Converted to SCE from Ag/AgCl (sat. KCl) by subtracting 46 mV.45
l Converted to SCE from Fc+/Fc0 (in ACN with 0.1 M TBAPF6 electrolyte) by adding 0.38 V.46
m Converted to SCE from Fc+/Fc0 (in DMF with 0.1 M TBAPF6 electrolyte) by adding 0.47 V.46
n Estimated using E0′(M+/M) − E0–0(M–M*).
o Estimated using E0′(M/M−) + E0–0.
p Integrated ECL intensity upon application of 1.2 V (vs. Ag/AgCl) for 10 s, measured using CCD spectrometer.
q Metal complex at 100 μM.
r Metal complex at 10 μM.
s By definition.
t Error represents standard deviation of five replicates.
|
||||
| Abs. (λmax)/nma | 243, 285, 452 | 251, 376 | 245, 271, 360 | 242, 280, 380 (ref. 38) |
| PL (λmax)/nma,b | 622 | 476, 505 | 515 | 520 |
| PL (λmax; 85 K)/nmb,c | 581, 629 (ref. 20) | 471, 506, 536 (ref. 20) | 481, 516 | 494, 532 (ref. 36) |
| E 0–0/eVd | 2.13 | 2.63 | 2.58 | 2.51 |
| QY (ΦPL)/% | 6.3e,41 | 14f,20 | 72.9e,34 | 70g,42, 90h,43, 97i,44, 99g,38 |
| E 0′(M+/M)/V vs. SCE | 1.06 (Aq)j, 1.27 (ACN)l,20 | 1.08 (Aq)j, 1.24 (ACN)l,20 | 0.79 (Aq)j, 0.77k,37, 0.76 (DMF)m | 0.71 (ACN)l,36 |
| E 0′(M/M−)/V vs. SCE | −1.35 (ACN)l,20 | −1.82 (ACN)l,20 | −2.01 (DMF)m | −2.29 (ACN)l,36 |
| E 0(M+/M*)/V vs. SCEn | −0.86 | −1.39 | −1.82 (−1.89)37 | −1.78 |
| E 0(M*/M−)/V vs. SCEo | 0.78 | 0.81 | 0.57 | 0.24 |
| ECL λmax/nm | 623 | 504 | 516 | 520 |
| ECL Irel (10 mM TPrA)p,q | 100s | 29.2 ± 1.8t | 0.27 ± 0.01t | 0.40g,38 |
| ECL Irel (100 mM TPrA)p,r | 100s | 24.6 ± 2.2t | 0.18 ± 0.01t | — |
| ECL Irel (ProCell)p,r | 100s | 36.5 ± 1.5t | 1.09 ± 0.03t | — |
Electrochemiluminescence
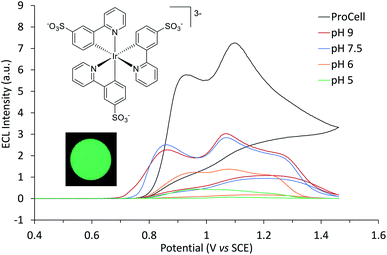
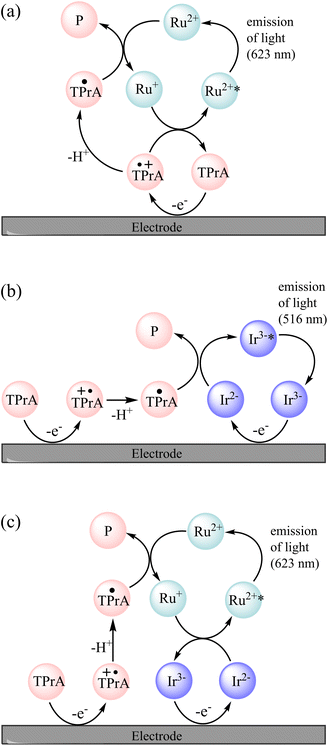
[Ir(sppy)3]3− exhibited green ECL (λmax = 516 nm, Fig. S3†) with TPrA as a co-reactant, which increased in intensity from pH 5 to 7.5 in 0.1 M PBS (Fig. 1). The ECL intensity with 10 mM TPrA was 0.27% that of [Ru(bpy)3]2+ with the same co-reactant, which is similar to the relative intensity of Ir(ppy)3 (0.40%) to [Ru(bpy)3]2+ with 10 mM TPrA in acetonitrile.38 Greater ECL from [Ir(sppy)3]3− (1.09% that of [Ru(bpy)3]2+) was obtained in ‘ProCell’ solution, a commercially available ECL reaction matrix composed of 180 mM TPrA, surfactant (0.1%) and preservative in 0.3 M PBS at pH 6.8, developed for commercial ECL analysers.21Possible pathways to attain the [Ir(sppy)3]3−* excited state can be drawn from those of the well-known co-reactant ECL5 of [Ru(bpy)3]2+ and TPrA (reactions (2)–(10), where TPrA˙+ is Pr3N˙+ and TPrA˙ is Pr2NC˙HCH2CH3) with consideration of the corresponding redox potentials and excited state energy of [Ir(sppy)3]3− (Table 1).39 The TPrA˙ radical (E0ox ≈ −1.7 V vs. SCE) can reduce [Ru(bpy)3]2+ (E0′ = −1.35 V vs. SCE) but not [Ir(sppy)3]3− (E0′ = −2.01 V vs. SCE), which means that reactions (7)–(9) are not feasible for the [Ir(sppy)3]3− complex. Moreover, unlike [Ru(bpy)3]3+, [Ir(sppy)3]2− cannot oxidise TPrA (E0′ ≈ 0.79–0.91 V vs. SCE5), which removes the ‘catalytic route’5 shown as reaction (4). The light-producing pathway for [Ir(sppy)3]3− with TPrA as a co-reactant is therefore limited to reactions (2), (3), (5), (6) and (10) (Fig. 2b).40
| TPrA → TPrA˙+ + e− | (2) |
| M → M+ + e− | (3) |
| M+ + TPrA → M + TPrA˙+ | (4) |
| TPrA˙+ → TPrA˙ + H+ | (5) |
| M+ + TPrA˙ → M* + P | (6) |
| M + TPrA˙ → M− + P | (7) |
| M+ + M− → M* + M | (8) |
| M− + TPrA˙+ → M* + TPrA | (9) |
| M* → M + hv | (10) |
The two ECL intensity maxima observed when increasing the applied potential (Fig. 1) are reminiscent of the ‘two waves’ of ECL of the [Ru(bpy)3]2+ or certain Ir(III) complexes such as [Ir(bt)2(pt-TEG)]+, with the TPrA co-reactant.5,20,47 This arises from the two distinct pathways to the excited state5,20 involving electrooxidation of (i) TPrA only (reactions (2), (5), (7), (9) and (10); as depicted in Fig. 2a), which is referred to as the ‘remote’ mechanism,§40,48 or (ii) both TPrA and the metal complex (reactions (2)–(6), (8) and (10)), referred to as the ‘direct’ mechanism.48 However, as the first pathway is not feasible for [Ir(sppy)3]3−, this effect is not involved here. In acetonitrile, the co-reactant ECL of Ir(ppy)3 with TPrA has been found to ‘switch-off’ at high overpotentials, ascribed to oxidative quenching by the TPrA˙+ intermediate (reaction (11)).28,29 The ECL of an analogue containing an electron withdrawing fluorine group (Ir(F-ppy)(ppy)2) was considerably less quenched.30 Partial quenching could also be anticipated in the co-reactant ECL of [Ir(sppy)3]3− (as its excited state is a less powerful reductant than Ir(ppy)3*; Table 1), which may explain the local intensity minima at ∼1 V in Fig. 1.
| M* + TPrA˙+ → M+ + TPrA | (11) |
Multi-colour and ratiometric ECL
We previously demonstrated switching between the green and red ECL of Ir(ppy)3 and a [Ru(bpy)3]2+ derivative within the same acetonitrile solution (with TPrA co-reactant) simply by changing the applied potential.28 This potential-resolved ECL2 exploited the distinct E0′(M+/M) of the metal complexes and the quenching of Ir(ppy)3* at high overpotentials. The [Ir(sppy)3]3− complex exhibits similar photophysical and electrochemical characteristics to Ir(ppy)3 (Table 1), but as noted above, its co-reactant ECL with TPrA is not strongly quenched at high overpotentials in aqueous phosphate buffer or ProCell solution (Fig. 1). For a mixture of [Ru(bpy)3]2+ and [Ir(sppy)3]3− in ProCell solution, the ECL of both luminophores ‘switches on’ at approximately 0.85 V (Fig. 3 and S4†), through the mechanisms shown in Fig. 2a and b, and was observed over the remainder of the potential range (to 1.46 V vs. SCE).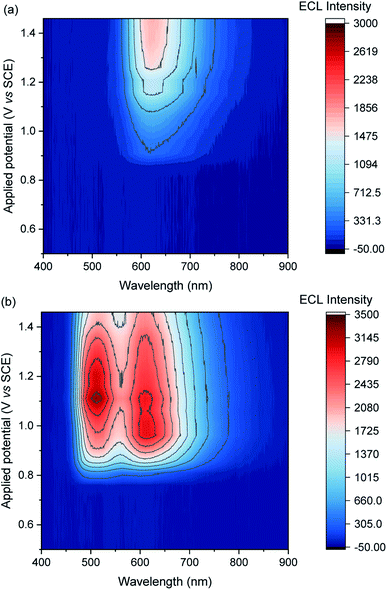 | ||
| Fig. 3 Contour plots of ECL vs. wavelength and applied potential for (a) [Ru(bpy)3]2+ (0.75 μM) or (b) a mixture of [Ru(bpy)3]2+ (0.75 μM) and [Ir(sppy)3]3− (150 μM), in ProCell solution, prepared by applying the potentials (10 s chronoamperometric pulses) in 50 mV intervals. The contour plot for [Ir(sppy)3]3− (150 μM) is shown in Fig. S5.† A plot of the ECL intensity at 620 nm vs. applied potential is shown in Fig. S7.† | ||
To obtain similar ECL intensities from the two luminophores in the mixture (Fig. 3 and S5†), a much greater concentration of [Ir(sppy)3]3− than [Ru(bpy)3]2+ was required, due to their relative co-reactant ECL efficiencies, and a considerable enhancement of the [Ru(bpy)3]2+ ECL when combined with [Ir(sppy)3]3−. Energy transfer from [Ir(sppy)3]3−* to [Ru(bpy)3]2+ was ruled out as a major source of this enhancement because: (i) there is little overlap between the emission of [Ir(sppy)3]3− and the MLCT absorption band of [Ru(bpy)3]2+ (Fig. S6†); and (ii) the [Ru(bpy)3]2+ complex has a lower luminescence quantum yield, but the integrated ECL intensity of the mixture is higher than that of the individual complexes. Moreover, energy transfer was not observed from (Ir(ppy)3)* to [Ru(bpy)2(L)]2+ (where L is a 4,4′-dicarboxamide derivative of 2,2′-bipyridine) in the potential-resolved co-reactant ECL system in acetonitrile.28 The enhancement of [Ru(bpy)3]2+ ECL can therefore be ascribed to electron transfer between the intermediates of the two metal-complexes.
The onset of ECL from both luminophores occurs in the potential region at which both [Ir(sppy)3]3− and TPrA are oxidised, and as noted above, the TPrA˙ radical can reduce [Ru(bpy)3]2+, but not [Ir(sppy)3]3−. Of the available species under these conditions, the only possible electron transfer between the ground oxidation states of the Ir and Ru complexes is from [Ru(bpy)3]+ to [Ir(sppy)3]2−, which is sufficiently energetic to attain [Ru(bpy)3]2+* (reaction (12); ΔE0′ ≈ E0–0), but not [Ir(sppy)3]3−* (ΔE0′ < E0–0).
A similar reaction (1) occurs in the mixed annihilation ECL of [Ru(bpy)3]2+ and Ir(ppy)3 in acetonitrile, which was isolated from other light-producing pathways by alternating between potentials sufficient to oxidise only Ir(ppy)3 and reduce only [Ru(bpy)3]2+.23,31,33 The feasibility of generating [Ru(bpy)3]2+* via reaction (12) was verified by selectively reducing [Ru(bpy)3]2+ and oxidising [Ir(sppy)3]3− under annihilation ECL conditions in 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 acetonitrile
20 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution (Fig. S8†). The ECL mechanisms for [Ru(bpy)3]2+ and [Ir(sppy)3]3− with TPrA as co-reactant in aqueous solution described above are summarised in Fig. 2.
water solution (Fig. S8†). The ECL mechanisms for [Ru(bpy)3]2+ and [Ir(sppy)3]3− with TPrA as co-reactant in aqueous solution described above are summarised in Fig. 2.
| [Ru(bpy)3]+ + [Ir(sppy)3]2− → [Ru(bpy)3]2+* + [Ir(sppy)3]3− | (12) |
In the mixed co-reactant ECL system, the electrochemically oxidised [Ir(sppy)3]2− will not react with the excess TPrA co-reactant, providing a longer lived alternative to TPrA˙+ for the generation of [Ru(bpy)3]2+* via reaction (9). This mechanism of enhancement (reaction (12)) would therefore be expected to have a greater effect on the co-reactant ECL of [Ru(bpy)3]2+ at applied potentials between 0.81 V and 1.06 V (vs. SCE), which are sufficient to oxidise TPrA and [Ir(sppy)3]3−, and generate [Ru(bpy)3]2+* via reaction (9), but not oxidise [Ru(bpy)3]2+ (which would enable the generation of [Ru(bpy)3]2+* via reactions (6) and (8)). As shown in Fig. 3 and S7,† this is indeed the case. Deconvolution of the contributions from the two luminophores (e.g., Fig. S9c†) shows that by adding 100 μM [Ir(sppy)3]3−, the co-reactant ECL intensity of [Ru(bpy)3]2+ increased by 10.8-fold when applying a potential of 0.86 V, but only 1.5-fold at 1.16 V (Fig. S10†). Importantly, the enhanced pathway is the predominant route for ECL detection in magnetic-bead supported assays,3 where only a small fraction of the [Ru(bpy)3]2+ electrochemiluminophore is close enough to the electrode surface to undergo direct oxidation.5,49
To summarise the available ECL pathways described above: at potentials between 0.81 V and 1.06 V vs. SCE, and at higher potentials if the [Ru(bpy)3]2+ luminophore is not close enough to the electrode for its direct oxidation, the ECL of [Ru(bpy)3]2+ can only proceed via the remote5,48 pathway (Fig. 2a), and regardless of the applied potential, the ECL of [Ir(sppy)3]3− can only proceed via the direct5,48 pathway (Fig. 2b). But when both metal complexes are present under these conditions, the reduced ruthenium complex can also react with the oxidised iridium complex to form [Ru(bpy)3]2+* (reaction (12)), thereby enhancing the ECL intensity of [Ru(bpy)3]2+ (Fig. 2c).
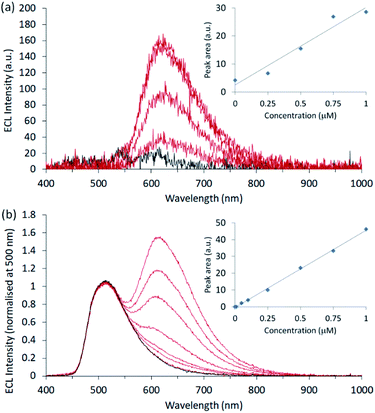
The [Ir(sppy)3]3− complex is an excellent candidate for ratiometric ECL detection of [Ru(bpy)3]2+ under aqueous conditions. It is highly soluble and the spectral distribution of its emission is sufficiently different from the Ru(II) complex to enable deconvolution of their contributions. Moreover, due to its lower efficiency and strong enhancement of the co-reactant ECL of [Ru(bpy)3]2+, relatively high concentrations of [Ir(sppy)3]3− are required to attain similar intensities. Comparison of the ECL response over the range 1–100 μM [Ir(sppy)3]3− in the presence and absence of 0.75 μM [Ru(bpy)3]2+ (Fig. S10†) shows not only the enhancement of the [Ru(bpy)3]2+* emission, but also the absence of significant quenching of the Ir(III) complex ECL, revealing that [Ir(sppy)3]3− could serve simultaneously as an enhancer and internal standard. As a preliminary demonstration, we prepared calibrations using seven [Ru(bpy)3]2+ standards from 10 nM to 1 μM, with and without 100 μM [Ir(sppy)3]3−. Between each experiment, the ECL cell was disassembled and the working electrode was polished. The slight variation in the re-alignment of electrode and the collimating lens had a significant influence on signal intensity. Under these conditions, the ECL signals without the enhancer exhibited relatively poor precision (RSD of ∼20% at 0.5 μM) and signal-to-noise (Fig. 4a), resulting in a limit of detection of approximately 0.25 μM.
In the presence of the enhancer, the intensity of the [Ru(bpy)3]2+ emission was markedly increased, but the deleterious variation in signal intensity was compounded by the overlap with the long-wavelength edge of [Ir(sppy)3]3−. Deconvolution of the emissions could minimise this effect but would not remove the inherent instrumental variability of the [Ru(bpy)3]2+ signal. However, using [Ir(sppy)3]3− as an internal standard (by simply normalising the spectra at 500 nm and subtracting the blank signal; Fig. 4b), the precision (RSD of 2% at 0.25 μM) and linearity (R2 = 0.9987) were vastly improved, resulting in a limit of detection of 40 nM, without the need for signal deconvolution.
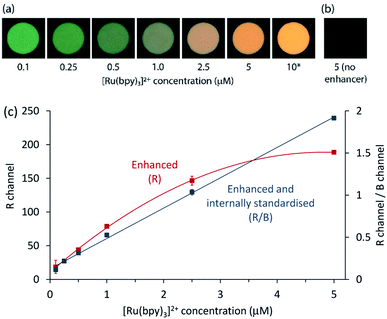
Finally, we sought to apply the enhancement and internal standardisation to co-reactant [Ru(bpy)3]2+ ECL captured as a digital image. ECL is a promising mode of detection for portable analytical systems integrating consumer devices such as cameras or smartphones,50 which can serve as both a power source to initiate the electrochemical reaction and a photodetector to measure the emission.51–53 As shown in Fig. 5a, digital photographs of the ECL at the working electrode surface for a solution of 0.1–10 μM [Ru(bpy)3]2+ in ProCell with 100 μM [Ir(sppy)3]3− showed a clear change in colour from that dominated by the green emission of the enhancer to the orange emission of the Ru(II) complex. The corresponding images of the extracted RGB data are shown in Fig. S11.† Under these conditions, no ECL could be detected from [Ru(bpy)3]2+ in ProCell solution without the enhancer (e.g., Fig. 5b).
The RGB colour data extracted from the digital images showed a quadratic relationship between the R value and [Ru(bpy)3]2+ concentration (red plot in Fig. 5c). Using the R/G ratio improved the precision (from 10% to 4% RSD at 0.25 μM), but the relationship was also quadratic (Fig. S12†), due to the significant contribution of both luminophores to the R and G channels. As the [Ru(bpy)3]2+ emission had a relatively minor influence on the B value, the R/B ratio provided effective internal standardisation from [Ir(sppy)3]3−, generating a linear calibration (R2 = 0.9982), as depicted by the blue plot in Fig. 5c. The greater precision of the R/B calibration (3% RSD at 0.25 μM) resulted in a lower limit of detection (80 nM; 3σ) than that prepared using R values only (0.4 μM).
Experimental section
Chemicals and general details
Reagents and solvents were purchased from various commercial sources and used without further purification. Tris(2,2′-bipyridine)ruthenium(II) dichloride hexahydrate ([Ru(bpy)]3Cl2·6H2O) was purchased from Strem (USA). [Ir(df-ppy)2(pt-TEG)]Cl was prepared as previously described.20,52 Potassium phosphate monobasic and dibasic salt, sodium chloride, TPrA and fac-Ir(ppy)3 were purchased from Sigma-Aldrich Australia. ProCell buffer solution containing the TPrA co-reactant was purchased from Roche Australia. NMR spectra were acquired on a Bruker Biospin AV400 spectrometer. 1H NMR spectra were acquired at 400 MHz, 13C{1H} NMR spectra were acquired at 101 MHz. All NMR spectra were recorded at 298 K. Chemical shifts were referenced to residual solvent peaks and are quoted in terms of parts per million (ppm), relative to tetramethylsilane (Si(CH3)4).Synthesis
The [Ir(sppy)3]3− complex was synthesised according to the previously reported procedure37 with some modification. A mixture of concentrated sulfuric acid (99 mg, 1.0 mmol) and trifluoroacetic anhydride (10 mL) was set stirring at ambient temperature in an N2 atmosphere. After 1 h, a solution of fac-Ir(ppy)3 (195 mg, 0.3 mmol) in dichloromethane (40 mL) was added dropwise and the reaction mixture was stirred for 24 h. The solvent was removed by evaporation under a stream of N2(g) followed by addition of a few drops of saturated aqueous sodium carbonate to the residue. The mixture was lyophilised and the solid was washed several times with methanol. The volume of methanol was reduced under a stream of N2(g) and acetonitrile was added then the mixture was stored at −20 °C and a precipitate was observed. The precipitate was isolated by filtration and washed with acetonitrile then dried at the pump, and purified by column chromatography (SiO2, 30% NH4OH/methanol/acetonitrile, 1/5/50) to afford a yellow powder (75 mg, 0.08 mmol, 27%). NMR spectra matched those previously reported for this compound.37Luminescence measurements
Photoluminescence (PL) spectra were obtained using a Cary Eclipse fluorescence spectrophotometer and UV-visible absorbance spectra were obtained using a Cary 300 spectrophotometer from 10 μM metal complex in deionised water using a quartz cuvette with a cell path length of 1 cm. For the PL spectra, the PMT voltage was 600 V and the excitation wavelength was 271 nm for [Ir(sppy)3]3−, 620 V and 258 nm for [Ir(ppy)2(pt-TEG)]+, and 700 V and 285 nm for [Ru(bpy)3]2+. The data interval for all collected spectra was 1 nm with an excitation bandpass filter of 250–395 nm for all analytes. The emission filter was 430–1100 nm for [Ru(bpy)3]2+ and [Ir(sppy)3]3−, and 360–1100 nm for [Ir(ppy)2(pt-TEG)]+. Low temperature PL spectra were obtained using 5 μM metal complex in an ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (4
methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture that was cooled to 85 K using an OptistatDN Variable Temperature Liquid Nitrogen Cryostat equipped with custom-made quartz sample holder.54 The temperature of 85 K was used to avoid damage to the spectroscopic cuvettes which occurred at 77 K.55 We previously found no significant difference in λmax in the spectra of [Ru(bpy)3]2+ and Ir(ppy)3 measured at these two temperatures under these instrumental conditions.54 All PL emission spectra were corrected for the change in instrumental sensitivity over the wavelength range by multiplication with correction curves established using a quartz halogen tungsten lamp.
1) mixture that was cooled to 85 K using an OptistatDN Variable Temperature Liquid Nitrogen Cryostat equipped with custom-made quartz sample holder.54 The temperature of 85 K was used to avoid damage to the spectroscopic cuvettes which occurred at 77 K.55 We previously found no significant difference in λmax in the spectra of [Ru(bpy)3]2+ and Ir(ppy)3 measured at these two temperatures under these instrumental conditions.54 All PL emission spectra were corrected for the change in instrumental sensitivity over the wavelength range by multiplication with correction curves established using a quartz halogen tungsten lamp.
Electrochemistry and ECL
We used a previously described custom cell design31 with glassy carbon working, platinum wire counter (CH Instruments) and leakless Ag/AgCl reference (model ET069; eDAQ Australia) electrodes to collect all electrochemical and ECL data. We polished the glassy carbon electrode using 1 μm and 0.05 μm alumina polishing powder (CH Instruments) followed by sonicating in ethanol prior to each experiment. An Autolab PGSTAT204 or Autolab PGSTAT128N was used for all electrochemical measurements. Potentials were referenced to SCE from Ag/AgCl (3.5 M KCl) by subtracting 40 mV.45 The cell was interfaced with a photomultiplier tube (PMT, extended-range trialkali S20 PMT, ET Enterprises model 9828B) to measure ECL intensity, a charge coupled device (CCD, QEPro, Ocean Optics) to obtain ECL spectra, or a digital camera (Canon EOS 6D DSLR camera, fitted with a Tonika AT-X PRO MACRO 100 mm f/2.8 D lens) to collect images of the ECL at the working electrode surface, as previously described.29,31,53 ECL experiments were conducted in ProCell or 0.1 M phosphate buffer solution (PBS) with 100 mM TPrA with pH adjusted using NaOH or HCl to obtain the desired pH. When required, the ECL spectra were deconvoluted31 into the two characteristic emission bands (defined by the ECL spectrum of each metal complex) using the Solver function of Excel, where the concentrations of [Ru(bpy)3]2+ (cRu) and [Ir(sppy)3]3− (cIr) were solved by minimising the sum of the squared differences between the model (Itotal = cRuIRu + cIrIIr) and the measured intensity (Imeas) at each wavelength. RBG data were extracted from the digital images of ECL at the working electrode surface using ImageJ (https://imagej.nih.gov/ij/).56Fig. 3 and S5† were prepared using the ‘Contour’ plot type in OriginPro, Version 2021b (OriginLab Corporation, Northampton, MA, USA).Conclusions
The water-soluble [Ir(sppy)3]3− electrochemiluminophore not only enhanced the co-reactant ECL of [Ru(bpy)3]2+ at 0.86 V (vs. SCE) by over an order of magnitude, but also served as an effective internal standard, providing superior precision. The ability to apply these advances in sensitivity and precision to ECL measured by digital photography through the ratio of R to B colour data is particularly promising for the development of low-cost, portable analytical devices. Furthermore, considering the importance of Ir(ppy)3 in the fundamental development of multi-colour ECL in organic solvents, these findings are an important step in its translation to analytical applications in aqueous solution. More generally, this synthetic approach is a convenient alternative to the synthesis of Ir(III) complexes from novel ligands containing polar functional groups that could enable a wide range of promising electrochemiluminophores to be utilised under aqueous conditions.Data availability
The data supporting the findings of this study are included in the main text and in the ESI file.†Author contributions
Emily Kerr: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. David Hayne: Methodology, Investigation, Supervision, Writing – original draft, Writing – review & editing. Lachlan Soulsby: Methodology, Investigation, Writing – review & editing. Joseph Bawden: Investigation, Writing – original draft, Writing – review & editing. Steven Blom: Investigation, Writing – review & editing. Egan Doeven: Methodology, Supervision, Writing – review & editing. Luke Henderson: Methodology, Supervision, Funding acquisition, Writing – review & editing. Conor Hogan: Methodology, Writing – review & editing. Paul Francis: Conceptualization, Methodology, Validation, Resources, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Australian Research Council (DP200102947) for funding this work. EK gratefully acknowledges the National Health and Medical Research Council of Australia (GNT1161573).Notes and references
- H. Qi and C. Zhang, Anal. Chem., 2020, 92, 524–534 CrossRef CAS PubMed; K. Muzyka, M. Saqib, Z. Liu, W. Zhang and G. Xu, Biosens. Bioelectron., 2017, 92, 241–258 CrossRef PubMed; L. Li, Y. Chen and J.-J. Zhu, Anal. Chem., 2017, 89, 358–371 CrossRef PubMed; Z. Liu, W. Qi and G. Xu, Chem. Soc. Rev., 2015, 44, 3117–3142 RSC.
- E. H. Doeven, G. J. Barbante, C. F. Hogan and P. S. Francis, ChemPlusChem, 2015, 80, 456–470 CrossRef CAS PubMed.
- E. Faatz, A. Finke, H.-P. Josel, G. Prencipe, S. Quint and M. Windfuhr, in Analytical Electrogenerated Chemiluminescence: From Fundamentals to Bioassays, ed. N. Sojic, The Royal Society of Chemistry, 2020, pp. 443–470 Search PubMed.
- I. Rubinstein and A. J. Bard, J. Am. Chem. Soc., 1981, 103, 512–516 CrossRef CAS.
- W. Miao, J.-P. Choi and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 14478–14485 CrossRef CAS PubMed.
- M. Staffilani, E. Höss, U. Giesen, E. Schneider, F. Hartl, H.-P. Josel and L. De Cola, Inorg. Chem., 2003, 42, 7789–7798 CrossRef CAS PubMed; D. N. Lee, H. S. Park, E. H. Kim, Y. M. Jun, J.-Y. Lee, W.-Y. Lee and B. H. Kim, Bull. Korean Chem. Soc., 2006, 27, 99–105 CrossRef; P. Chikhaliwala and S. Chandra, J. Org. Chem, 2016, 821, 78–90 CrossRef; H. Wang, Y. Yuan, Y. Zhuo, Y. Chai and R. Yuan, Anal. Chem., 2016, 88, 5797–5803 CrossRef PubMed.
- Y. Feng, F. Sun, N. Wang, J. Lei and H. Ju, Anal. Chem., 2017, 89, 7659–7666 CrossRef CAS PubMed; G. Valenti, E. Rampazzo, S. Kesarkar, D. Genovese, A. Fiorani, A. Zanut, F. Palomba, M. Marcaccio, F. Paolucci and L. Prodi, Coord. Chem. Rev., 2018, 367, 65–81 CrossRef; A. Zanut, F. Palomba, M. R. Scota, S. Rebeccani, M. Marcaccio, D. Genovese, E. Rampazzo, G. Valenti, F. Paolucci and L. Prodi, Angew. Chem., Int. Ed., 2020, 59, 21858–21863 CrossRef PubMed.
- A. Kapturkiewicz, Anal. Bioanal. Chem., 2016, 408, 7013–7033 CrossRef CAS PubMed; S. Laird and C. F. Hogan, in Iridium(III) in Optoelectronic and Photonics Applications, ed. E. Zysman-Colman, John Wiley & Sons, Inc., Chichester, UK, 2017, pp. 359–414 Search PubMed.
- C. F. Hogan, P. S. Francis and E. H. Doeven, in Analytical Electrogenerated Chemiluminescence: From Fundamentals to Bioassays, ed. N. Sojic, The Royal Society of Chemistry, 2020, pp. 200–246 Search PubMed.
- D. Bruce and M. M. Richter, Anal. Chem., 2002, 74, 1340–1342 CrossRef CAS PubMed.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, C. F. Hogan, N. W. Barnett and P. S. Francis, Angew. Chem., Int. Ed., 2012, 51, 4354–4357 CrossRef CAS PubMed.
- X.-L. Huo, H.-J. Lu, J.-J. Xu, H. Zhou and H.-Y. Chen, J. Mater. Chem. B, 2019, 7, 6469–6475 RSC; F. Du, Y. Chen, C. Meng, B. Lou, W. Zhang and G. Xu, Curr. Opin. Electrochem., 2021, 28, 100725 CrossRef CAS.
- K. Shao, B. Wang, A. Nie, S. Ye, J. Ma, Z. Li, Z. Lv and H. Han, Biosens. Bioelectron., 2018, 118, 160–166 CrossRef CAS PubMed; H. Chen, H. Zhang, R. Yuan and S. Chen, Anal. Biochem., 2017, 89, 2823–2829 Search PubMed.
- H.-R. Zhang, J.-J. Xu and H.-Y. Chen, Anal. Chem., 2013, 85, 5321–5325 CrossRef CAS PubMed; X.-L. Huo, N. Zhang, H. Yang, J.-J. Xu and H.-Y. Chen, Anal. Chem., 2018, 90, 13723–13728 CrossRef PubMed.
- Y.-L. Wang, F.-R. Liu, J.-T. Cao, S.-W. Ren and Y.-M. Liu, Biosens. Bioelectron., 2018, 102, 525–530 CrossRef CAS PubMed; Y. Lv, S. Chen, Y. Shen, J. Ji, Q. Zhou, S. Liu and Y. Zhang, J. Am. Chem. Soc., 2018, 140, 2801–2804 CrossRef PubMed.
- Y. Lin, J. Jia, R. Yang, D. Chen, J. Wang, F. Luo, L. Guo, B. Qiu and Z. Lin, Anal. Chem., 2019, 91, 3717–3724 CrossRef CAS PubMed.
- R. V. Kiran, E. M. Zammit, C. F. Hogan, B. D. James, N. W. Barnett and P. S. Francis, Analyst, 2009, 134, 1297–1298 RSC; L. Yu, Y. Liu and M. Zhou, Anal. Bioanal. Chem., 2016, 408, 7095–7103 CrossRef CAS PubMed.
- L. Yu, Z. Huang, Y. Liu and M. Zhou, J. Organomet. Chem., 2012, 718, 14–21 CrossRef CAS.
- M.-J. Li, P. Jiao, W. He, C. Yi, C.-W. Li, X. Chen, G.-N. Chen and M. Yang, Eur. J. Inorg. Chem., 2011, 197–200 CrossRef CAS; S. Zanarini, M. Felici, G. Valenti, M. Marcaccio, L. Prodi, S. Bonacchi, P. Contreras-Carballada, R. M. Williams, M. C. Feiters, R. J. M. Nolte, L. De Cola and F. Paolucci, Chem.–Eur. J., 2011, 17, 4640–4647 CrossRef PubMed; E. Kerr, E. H. Doeven, G. J. Barbante, T. U. Connell, P. S. Donnelly, D. J. D. Wilson, T. D. Ashton, F. M. Pfeffer and P. S. Francis, Chem.–Eur. J., 2015, 21, 14987–14995 CrossRef PubMed; B. Newman, L. Chen, L. C. Henderson, E. H. Doeven, P. S. Francis and D. J. Hayne, Front. Chem., 2020, 8, 583631 CrossRef PubMed.
- L. Chen, D. J. Hayne, E. H. Doeven, J. Agugiaro, D. J. D. Wilson, L. C. Henderson, T. U. Connell, Y. H. Nai, R. Alexander, S. Carrara, C. F. Hogan, P. S. Donnelly and P. S. Francis, Chem. Sci., 2019, 10, 8654–8667 RSC.
- J. M. Fernandez-Hernandez, E. Longhi, R. Cysewski, F. Polo, H.-P. Josel and L. De Cola, Anal. Chem., 2016, 88, 4174–4178 CrossRef CAS PubMed.
- B. D. Muegge and M. M. Richter, Anal. Chem., 2004, 76, 73–77 CrossRef CAS; K. N. Swanick, S. Ladouceur, E. Zysman-Colman and Z. Ding, Angew. Chem., Int. Ed., 2012, 51, 11079–11082 CrossRef PubMed; M. A. Haghighatbin, S.-C. Lo, P. L. Burn and C. F. Hogan, Chem. Sci., 2016, 7, 6974–6980 RSC; W. Sun, S. Sun, N. Jiang, L. Gao and G. Zheng, J. Organomet. Chem., 2017, 846, 367–371 CrossRef; A. S. Danis, J. B. Gordon, K. P. Potts, L. I. Stephens, S. C. Perry and J. Mauzeroll, Anal. Chem., 2019, 91, 2312–2318 CrossRef PubMed.
- E. Kerr, E. H. Doeven, G. J. Barbante, C. F. Hogan, D. Bower, P. S. Donnelly, T. U. Connell and P. S. Francis, Chem. Sci., 2015, 6, 472–479 RSC.
- Y.-Z. Wang, S.-Y. Ji, H.-Y. Xu, W. Zhao, J.-J. Xu and H.-Y. Chen, Anal. Chem., 2018, 90, 3570–3575 CrossRef CAS PubMed.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, J. Am. Chem. Soc., 2014, 136, 3705–3712 CrossRef CAS PubMed; L. C. Soulsby, E. H. Doeven, T. T. Pham, D. J. Eyckens, L. C. Henderson, B. M. Long, R. M. Guijt and P. S. Francis, Chem. Commun., 2019, 55, 11474–11477 RSC; D.-K. Kwon and J.-M. Myoung, Chem. Eng. J., 2019, 379, 122347 CrossRef.
- Y.-Z. Wang, C.-H. Xu, W. Zhao, Q.-Y. Guan, H.-Y. Chen and J.-J. Xu, Anal. Chem., 2017, 89, 8050–8056 CrossRef CAS PubMed; M. R. Moghaddam, S. Carrara and C. F. Hogan, Chem. Commun., 2019, 55, 1024–1027 RSC.
- W. Guo, H. Ding, C. Gu, Y. Liu, X. Jiang, B. Su and Y. Shao, J. Am. Chem. Soc., 2018, 140, 15904–15915 CrossRef CAS PubMed.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, P. S. Francis, N. W. Barnett and C. F. Hogan, Chem. Sci., 2013, 4, 977–982 RSC.
- E. H. Doeven, G. J. Barbante, E. Kerr, C. F. Hogan, J. A. Endler and P. S. Francis, Anal. Chem., 2014, 86, 2727–2732 CrossRef CAS PubMed.
- G. J. Barbante, N. Kebede, C. M. Hindson, E. H. Doeven, E. M. Zammit, G. R. Hanson, C. F. Hogan and P. S. Francis, Chem.–Eur. J., 2014, 20, 14026–14031 CrossRef CAS PubMed.
- E. Kerr, E. H. Doeven, G. J. Barbante, C. F. Hogan, D. J. Hayne, P. S. Donnelly and P. S. Francis, Chem. Sci., 2016, 7, 5271–5279 RSC.
- H. Li, L. Bouffier, S. Arbault, A. Kuhn, C. F. Hogan and N. Sojic, Electrochem. Commun., 2017, 77, 10–13 CrossRef CAS; L. C. Soulsby, D. J. Hayne, E. H. Doeven, L. Chen, C. F. Hogan, E. Kerr, J. L. Adcock and P. S. Francis, ChemElectroChem, 2018, 5, 1543–1547 CrossRef.
- N. S. Adamson, A. G. Theakstone, L. C. Soulsby, E. H. Doeven, E. Kerr, C. F. Hogan, P. S. Francis and L. Dennany, Chem. Sci., 2021, 12, 9770–9777 RSC.
- C. Kerzig, X. Guo and O. S. Wenger, J. Am. Chem. Soc., 2019, 141, 2122–2127 CrossRef CAS PubMed.
- C. Kerzig and O. S. Wenger, Chem. Sci., 2019, 10, 11023–11029 RSC.
- L. Chen, E. H. Doeven, D. J. D. Wilson, E. Kerr, D. J. Hayne, C. F. Hogan, W. Yang, T. T. Pham and P. S. Francis, ChemElectroChem, 2017, 4, 1797–1808 CrossRef CAS.
- X. Guo, Y. Okamoto, M. R. Schreier, T. R. Ward and O. S. Wenger, Chem. Sci., 2018, 9, 5052–5056 RSC.
- G. J. Barbante, E. H. Doeven, E. Kerr, T. U. Connell, P. S. Donnelly, J. M. White, T. Lópes, S. Laird, C. F. Hogan, D. J. D. Wilson, P. J. Barnard and P. S. Francis, Chem.–Eur. J., 2014, 20, 3322–3332 CrossRef CAS PubMed.
- E. Kerr, E. H. Doeven, D. J. D. Wilson, C. F. Hogan and P. S. Francis, Analyst, 2016, 141, 62–69 RSC.
- A. Zanut, A. Fiorani, S. Canola, T. Saito, N. Ziebart, S. Rapino, S. Rebeccani, A. Barbon, T. Irie, H.-P. Josel, F. Negri, M. Marcaccio, M. Windfuhr, K. Imai, G. Valenti and F. Paolucci, Nat. Commun., 2020, 11, 2668 CrossRef CAS PubMed.
- K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi and S. Tobita, Phys. Chem. Chem. Phys., 2009, 11, 9850–9860 RSC.
- A. Kapturkiewicz and G. Angulo, Dalton Trans., 2003, 3907–3913 RSC.
- T. Hofbeck and H. Yersin, Inorg. Chem., 2010, 49, 9290–9299 CrossRef CAS PubMed.
- T. Sajoto, P. I. Djurovich, A. B. Tamayo, J. Oxgaard, W. A. Goddard and M. E. Thompson, J. Am. Chem. Soc., 2009, 131, 9813–9822 CrossRef CAS PubMed.
- H. Kahlert, in Electroanalytical Methods, ed. F. Scholz, Springer-Verlag, Heidelberg, 2nd edn, 2010, pp. 291–308 Search PubMed.
- J. R. Aranzaes, M.-C. Daniel and D. Astruc, Can. J. Chem., 2006, 84, 288–299 CrossRef.
- S. Zanarini, E. Rampazzo, L. Della Ciana, M. Marcaccio, E. Marzocchi, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 2260–2267 CrossRef CAS PubMed; K. Imai, G. Valenti, E. Villani, S. Rapino, E. Rampazzo, M. Marcaccio, L. Prodi and F. Paolucci, J. Phys. Chem. C, 2015, 119, 26111–26118 CrossRef.
- A. Zanut, M. Rossetti, M. Marcaccio, F. Ricci, F. Paolucci, A. Porchetta and G. Valenti, Anal. Chem., 2021, 93, 10397–10402 CrossRef CAS PubMed.
- M. Sentic, M. Milutinovic, F. Kanoufi, D. Manojlovic, S. Arbault and N. Sojic, Chem. Sci., 2014, 5, 2568–2572 RSC.
- M. Rezazadeh, S. Seidi, M. Lid, S. Pedersen-Bjergaard and Y. Yamini, Trends Anal. Chem., 2019, 118, 548–555 CrossRef CAS; A. Ainla, M. P. S. Mousavi, M.-N. Tsaloglou, J. Redston, J. G. Bell, M. T. Fernández-Abedul and G. M. Whitesides, Anal. Chem., 2018, 90, 6240–6246 CrossRef PubMed.
- J. L. Delaney and C. F. Hogan, in Mobile Health Technologies: Methods and Protocols, ed. A. Rasooly and K. E. Herold, Springer, New York, 2015, p. 277 CrossRef CAS PubMed; L. Qi, Y. Xia, W. Qi, W. Gao, F. Wu and G. Xu, Anal. Chem., 2016, 88, 1123–1127 CrossRef CAS PubMed; L. Zhu, S. Li, W. Liu, J. Chen, Q. Yu, Z. Zhang, Y. Li, J. Liu and X. Chen, Biosens. Bioelectron., 2021, 187, 113284 CrossRef PubMed; W. Chen, Y. Yao, T. Chen, W. Shen, S. Tang and H. K. Lee, Biosens. Bioelectron., 2021, 172, 112788 CrossRef PubMed.
- E. H. Doeven, G. J. Barbante, A. J. Harsant, P. S. Donnelly, T. U. Connell, C. F. Hogan and P. S. Francis, Sens. Actuators, B, 2015, 216, 608–613 CrossRef CAS.
- E. Kerr, R. Farr, E. H. Doeven, Y. H. Nai, R. Alexander, R. M. Guijt, B. Prieto-Simon, P. S. Francis, M. Dearnley, D. J. Hayne, L. C. Henderson and N. H. Voelcker, Sens. Actuators, B, 2021, 330, 129261 CrossRef CAS.
- L. C. Soulsby, D. J. Hayne, E. H. Doeven, D. J. D. Wilson, J. Agugiaro, T. U. Connell, L. Chen, C. F. Hogan, E. Kerr, J. L. Adcock, P. S. Donnelly, J. M. White and P. S. Francis, Phys. Chem. Chem. Phys., 2018, 20, 18995–19006 RSC.
- C. Mallet, A. Bolduc, S. Bishop, Y. Gautier and W. G. Skene, Phys. Chem. Chem. Phys., 2014, 16, 24382–24390 RSC.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc05609c |
| ‡ Other reported ratiometric ECL approaches (predominantly applied with nanoparticle-based luminophores) exploit phenomena such as competition for co-reactants13 or resonance energy transfer between luminophores,14 or involve internal standardisation with distinct ECL systems (e.g., [Ru(bpy)3]2+ or luminol).15 Internal standardisation was also demonstrated for the co-reactant ECL of a [Ru(bpy)3]2+ derivative and 2-(dibutylamino)ethanol (DBAE) using the internal electrochemical reference signal of methylene blue.16 |
| § Zanut et al.40 recently revealed an additional pathway involving the oxidative C–N bond cleavage of TPrA to form dipropylamine radicals, but for the purposes of this discussion, we group those short-lived intermediates with TPrA˙+ and TPrA˙. |
| This journal is © The Royal Society of Chemistry 2022 |