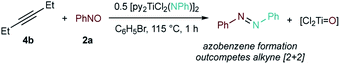Open Access Article
Open Access Articleα-Diimine synthesis via titanium-mediated multicomponent diimination of alkynes with C-nitrosos†
Connor W.
Frye
 ,
Dominic T.
Egger
,
Dominic T.
Egger
 ,
Errikos
Kounalis
,
Errikos
Kounalis
 ,
Adam J.
Pearce
,
Adam J.
Pearce
 ,
Yukun
Cheng
,
Yukun
Cheng
 and
Ian A.
Tonks
and
Ian A.
Tonks
 *
*
Department of Chemistry, University of Minnesota – Twin Cities, 207 Pleasant St SE, Minneapolis, Minnesota 55455, USA. E-mail: itonks@umn.edu
First published on 27th December 2021
Abstract
α-Diimines are commonly used as supporting ligands for a variety of transition metal-catalyzed processes, most notably in α-olefin polymerization. They are also precursors to valuable synthetic targets, such as chiral 1,2-diamines. Their synthesis is usually performed through acid-catalyzed condensation of amines with α-diketones. Despite the simplicity of this approach, accessing unsymmetrical α-diimines is challenging. Herein, we report the Ti-mediated intermolecular diimination of alkynes to afford a variety of symmetrical and unsymmetrical α-diimines through the reaction of diazatitanacyclohexadiene intermediates with C-nitrosos. These diazatitanacycles can be readily accessed in situ via the multicomponent coupling of Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR imidos with alkynes and nitriles. The formation of α-diimines is achieved through formal [4 + 2]-cycloaddition of the C-nitroso to the Ti and γ-carbon of the diazatitanacyclohexadiene followed by two subsequent cycloreversion steps to eliminate nitrile and afford the α-diimine and a Ti oxo.
NR imidos with alkynes and nitriles. The formation of α-diimines is achieved through formal [4 + 2]-cycloaddition of the C-nitroso to the Ti and γ-carbon of the diazatitanacyclohexadiene followed by two subsequent cycloreversion steps to eliminate nitrile and afford the α-diimine and a Ti oxo.
Introduction
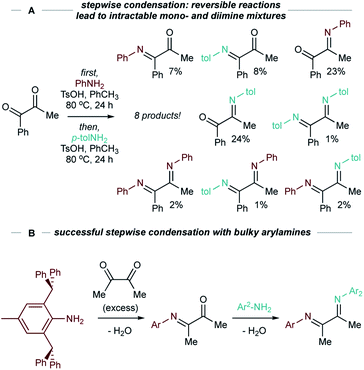
α-Diimines (1,4-diaza-1,3-dienes) are widely used as ligands for organometallic complexes,1,2 perhaps most notably in Brookhart-type catalysts for α-olefin polymerization.3–5 The α-diimine ligand scaffold has proven to be remarkably versatile due to its facile stereoelectronic tunability.6–9 For example, modifications to the backbone and N-aryl substituents of α-diimines can exert control over the molecular weight and microstructure of α-olefin polymers by attenuation of chain walking processes.1,10 As ligands, they are often redox non-innocent, which allows for richer and more complex redox processes.11–14 α-Diimines are also precursors to valuable chiral 1,2-diamines through asymmetric hydrogenation,15,16 or to N-heterocyclic carbene (NHC) ligands through cyclization with paraformaldehyde and subsequent deprotonation.17–20Typically, α-diimines are synthesized through condensation of α-diketones and amines. Despite the apparent simplicity of this approach, accessing unsymmetrical α-diimines through stepwise condensations is synthetically challenging due to poor chemoselectivity.21 Furthermore, since imine formation is reversible, attempts at sequential selective condensations can result in complex mixtures (for example, see Fig. 1A and S95–S98†). Imines are also notoriously difficult to isolate because they are prone to hydrolysis, making methods that generate product mixtures impractical. Highlighting this problem, although amine condensation with glyoxal to afford α-diimines is a common route to symmetric NHCs, examples of unsymmetric NHCs synthesized via condensation with glyoxal are rare.19,22,23
There are comparatively few examples of unsymmetrical α-diimines, and these are mostly limited to modification of N-aryl substituents, usually involving very sterically encumbered groups,3,9,24 rather than substituents on the backbone (Fig. 1B),11,25 although there are several examples from aldimine cross coupling.26,27 Selective trapping of unsymmetrical diimines via cyclization has also recently been reported.28 Given this methodology gap, developing a route to unsymmetrical α-diimines from simple feedstocks would provide a useful tool for the development of more diverse ligand scaffolds and pharmaceutically relevant building blocks.3,15,16 Our group has reported several examples of Ti-catalyzed oxidative functionalizations of alkynes for the synthesis of multisubstituted N-heterocycles that overcome limitations of classical condensation reactions.29–32 We envisioned that a complementary synthetic route to unsymmetrical α-diimines could be achieved through Ti-mediated oxidative diimination of alkynes. Examples of alkyne diamination or diimination are scarce33–36 despite many reports of alkene diamination.37–42 To the best of our knowledge, there is only a single example of a multicomponent intermolecular alkyne diamination43 and no examples of alkyne diimination. Nevertheless, there are several elegant examples of alkyne difunctionalizations44 using Ti including alkyne carboamination45–47 and iminoamination48 that provide motivation for further exploring Ti-catalyzed or -mediated diamination/diimination.
Previously, we reported that diazatitanacyclohexadienes (prepared from the multicomponent coupling of Ti imidos, alkynes, and nitriles) could undergo oxidation-induced N–N coupling to yield pyrazoles (Fig. 2, top).32 In the interest of further expanding the utility of this unique intermediate, we have begun examining its reactivity with various group transfer reagents. Herein, we report the intermolecular diimination of alkynes by Ti imidos and C-nitrosos to afford unsymmetrical α-diimines (Fig. 2, bottom). This diimination reaction proceeds through a cascading sequence of formal cycloaddition and retrocycloaddition reactions from this key diazatitanacyclohexadiene intermediate. This approach is a useful strategy for synthesizing unsymmetrical α-diimines, which are challenging to access through traditional condensation reactions.
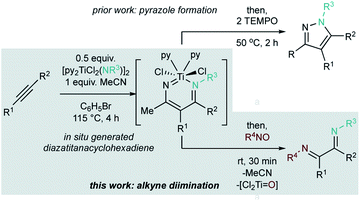 | ||
| Fig. 2 In situ generated diazatitanacyclohexadiene intermediates. (top) Previous work on oxidative N–N coupling to pyrazoles; (bottom) intermolecular diimination of alkynes (this work). | ||
Results and discussion
Addition of C-nitrosos to diazatitanacyclohexadienes
Addition of PhNO (2a) to diazatitanacycle 1 resulted in rapid, near-quantitative formation of α-diimine 3a (81% by 1H NMR) with the concomitant formation of p-tolunitrile (Fig. 3). It is proposed that this occurs through [4 + 2]-cycloaddition of PhNO to the Ti and γ-carbon of the ligand backbone, followed by [4 + 2]-retrocycloaddition to eliminate p-tolunitrile, and a second cycloreversion to afford 3a and a Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species (vide infra).
O species (vide infra).
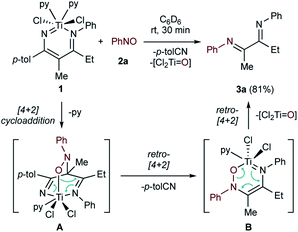 | ||
| Fig. 3 Reaction of a C-nitroso with 1 yields an α-diimine 3avia a sequence of cycloaddition/retrocycloaddition steps. | ||
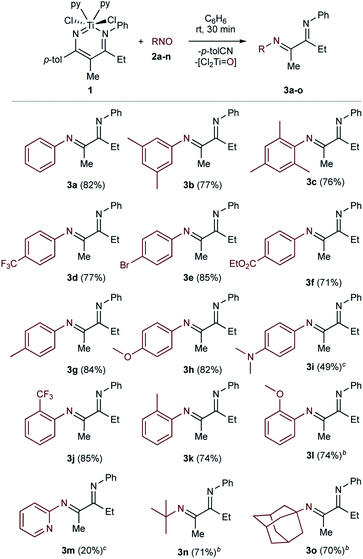
The scope of the C-nitroso reactants examined is shown in Table 1. Conveniently, nitrosoarenes can be readily prepared from the corresponding aniline via Oxone® oxidation.49 In most cases, the α-diimine products were obtained in very good isolated yields (70% to 85%).
Use of sterically demanding nitrosos 2b and 2c resulted in good yields of 3b and 3c (77% and 76%), which are commonly used as bulky α-diimine substituents.3 This method tolerates both electron-poor (3d–3f) and electron-rich (3g–3i) para-substituted (3d–3i) nitrosoarenes, as well as ortho-substituted (3j–3l) nitrosoarenes (3l is formed in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with its enamine tautomer). The reaction also proceeds cleanly with aliphatic nitrosos: 2-methyl-2-nitrosopropane 2n gives a mixture of tert-butyl substituted α-diimine 3n and its enamine tautomer in a 3.75
1 ratio with its enamine tautomer). The reaction also proceeds cleanly with aliphatic nitrosos: 2-methyl-2-nitrosopropane 2n gives a mixture of tert-butyl substituted α-diimine 3n and its enamine tautomer in a 3.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (71%). Similarly, 1-nitrosoadamantane 2o afforded α-diimine 3o and its enamine tautomer in a 3.13
1 ratio (71%). Similarly, 1-nitrosoadamantane 2o afforded α-diimine 3o and its enamine tautomer in a 3.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (70%). While the yield of the reaction was mostly consistent irrespective of nitrosoarene substituent, there were a couple of exceptions. For the reaction with electron-rich 2i, the formation of α-diimine 3i (49% yield by 1H NMR) was accompanied by the formation of unidentified side products that precluded isolation. Also, the reaction with 2-nitrosopyridine 2m yielded only a small amount of 3m by 1H NMR (20%) relative to the amount of p-tolunitrile byproduct (85%).
1 ratio (70%). While the yield of the reaction was mostly consistent irrespective of nitrosoarene substituent, there were a couple of exceptions. For the reaction with electron-rich 2i, the formation of α-diimine 3i (49% yield by 1H NMR) was accompanied by the formation of unidentified side products that precluded isolation. Also, the reaction with 2-nitrosopyridine 2m yielded only a small amount of 3m by 1H NMR (20%) relative to the amount of p-tolunitrile byproduct (85%).
In situ multicomponent diimine synthesis
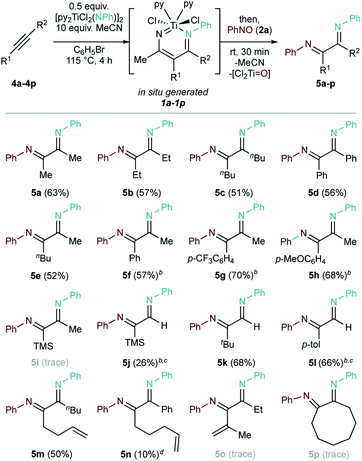
Diazatitanacycles such as 1 can be synthesized via the multicomponent coupling of Ti imidos, alkynes, and nitriles.32 With this strategy, one-pot alkyne diiminations were carried out (Fig. 4 and Table 2).| a Conditions: 0.2 mmol (0.5 equiv.) [py2TiCl2(NPh)]2, 0.4 mmol (1 equiv.) alkyne, 4.0 mmol (10 equiv.) MeCN, 4 mL PhBr, 115 °C, 4 h, N2 atmosphere (glovebox); then, 0.4 mmol (1 equiv.) PhNO, rt, 0.5 h. Isolated yields. b Mixture of stereoisomers. c Yield corrected for minor pyrrole impurities. d GC-FID yield (vs. 1,3,5-trimethoxybenzene standard). |
|---|
|
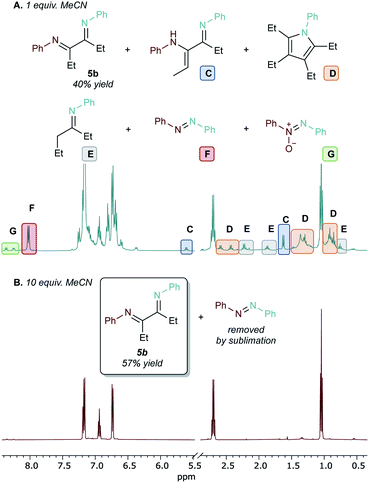
In an initial experiment, reactions with 1 equiv. MeCN led to moderate yields of 5b (40%) from reaction of 4b and PhNO (Fig. 4A). MeCN was chosen over p-tolCN (the formal nitrile component from the reactions in Table 1) because of the easier removal of MeCN in vacuo. However, several species from competing side reactions also formed that were difficult to separate from the desired product given the instability of diimines towards hydrolysis: pyrrole (D, 5%) from insertion of a second equivalent of alkyne;29 imine from the hydroamination of 3-hexyne (E, 6%); and azobenzene (F, 12%) from direct metathesis of PhNO with leftover Ti imido.50,51 Using excess MeCN (10 equiv.) suppressed these competing side reactions (Fig. 4B) and led to cleaner formation of the product, with azobenzene as the predominant side-product after removal of volatiles. Conveniently, basic aqueous extraction can remove the Ti byproducts, and azobenzene can be easily removed by sublimation—avoiding the need to perform column chromatography on the sensitive products.
Based on this result, the scope of one-pot oxidative alkyne diimination with [py2TiCl2(NPh)]2 and PhNO was examined (Table 2, 5a–5p). Here, the α-diimine yields are ultimately determined by the yield of in situ formed diazatitanacycle 1a–1p, as the subsequent oxidations are near-quantitative. Symmetrical internal alkynes with both alkyl and aryl substituents formed the respective α-diimines (5a–5d) in good isolated yields (51% to 63%).
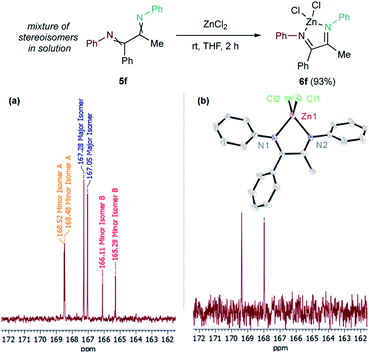
Unsymmetrical internal alkyne 4e resulted in the formation of a single α-diimine isomer (5e) in a 52% yield. In contrast, α-diimines 5f–5h give complex 1H and 13C NMR spectra, presumably due to formation of multiple imine stereoisomers. Analysis of 5f by NOESY (Fig. S53 and 54†) showed that chemical exchange occurs between each of these isomers by the presence of EXSY cross-peaks. Additionally, GC-MS revealed only one peak corresponding to the mass of 5f (Fig. S55†).
Imines are well-known to undergo rapid equilibrium between E/Z isomers through inversion (also called the lateral shift mechanism) in nonpolar solvents.52 Confirmation that the complex spectra of 5f–5h were a result of stereoisomer equilibration was obtained through further reaction of the isomer mixtures. For example, reaction of the 5f isomeric mixture with ZnCl2 resulted in 93% yield of α-diimine adduct 6f (Fig. 5). The identities of diimines 5g and 5h were similarly confirmed via ZnCl2 coordination (see ESI†).
Terminal alkynes 4j–4l were also examined. Trapping Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR + terminal alkyne [2 + 2] cycloadducts is challenging: terminal alkynes typically react faster to form pyrrole or alkyne trimerization products.53 Nevertheless, 5k was obtained cleanly in a 68% yield, while 5j and 5l were obtained as a mixture of stereoisomers with small pyrrole impurities (2.4% and 12% pyrrole, respectively).
NR + terminal alkyne [2 + 2] cycloadducts is challenging: terminal alkynes typically react faster to form pyrrole or alkyne trimerization products.53 Nevertheless, 5k was obtained cleanly in a 68% yield, while 5j and 5l were obtained as a mixture of stereoisomers with small pyrrole impurities (2.4% and 12% pyrrole, respectively).
1,5-Enyne 4m allows for clean formation of 5m (50%), while 1,6-enyne 4n results in formation of 5n (10%) as a mixture with competing carboamination47 products (6%). Here, the shorter linker of 4m compared to 4n prevents the intramolecular alkene insertion that would lead to carboamination. Conjugated enyne 4o yielded only trace 5o, while cyclooctyne 4p predominantly formed alkyne trimer and pyrrole, with only a trace amount of 5p.
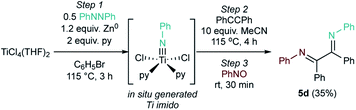
5d was also synthesized via a telescoped in situ route from TiCl4(THF)2, azobenzene, and Zn0 powder with only a moderate decrease in yield (Fig. 6). We have previously shown that this strategy is a benchtop-compatible approach to other Ti oxidative amination reactions.54 This one-pot approach facilitates the in situ formation of diazatitanacycle intermediates such as 1 without the need for specialized equipment, making the synthesis of both α-diimines and pyrazoles32 more operationally simple.
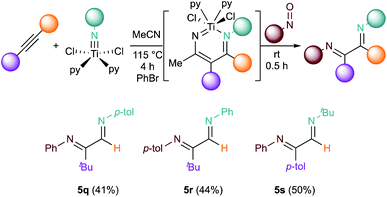
Given that diazatitanacyclohexadiene intermediate formation is regioselective,32,55–57 this method could be used to prepare regioisomeric α-diimines through different combinations of alkynes, imidos, and nitrosos (Fig. 7). For example, reaction of 4k with [py2TiCl2(Np-tol)]2 imido gives 5q (41%), while its regioisomer 5r can be synthesized by using 2g and [py2TiCl2(NPh)]2 (44%). Another regioisomer 5s can be prepared from [py2TiCl2(NtBu)]2 with a 50% yield of diimine stereoisomers, albeit with a small pyrrole impurity (4% yield).
Together, these reaction scopes demonstrate that a wide range of both symmetric and unsymmetric α-diimines can be accessed directly through a one-pot multicomponent reaction with a variety of Ti imidos, alkynes, and C-nitrosos.
Proposed mechanism for intermolecular diimination
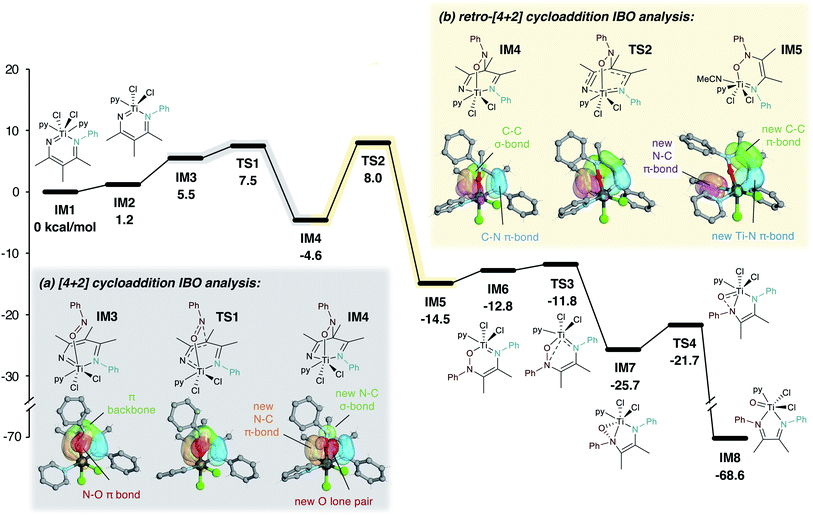
A plausible mechanism and accompanying DFT calculations for the formation of 5a from 1a (IM1) and PhNO (2a) are shown in Fig. 8. The in situ formation of IM1 from Ti imidos, alkynes, and nitriles through alkyne/imido [2 + 2]-cycloaddition and nitrile insertion has been previously established.32,58–60 The formal [4 + 2] cycloaddition of PhNO to IM1 occurs in a stepwise fashion. First, O-coordination of PhNO to Ti yields IM3. From IM3, the nucleophilic, electron-rich γ-carbon in the metallacycle backbone61 attacks the electrophilic N of coordinated PhNO, generating bicyclic IM4. This process can be further visualized using IBO analysis (Fig. 8a): coordination of PhNO results in a puckering of the γ-C orbital toward the N of coordinated PhNO. Subsequently the new N–C σ-bond is formed in IM4, with simultaneous rearrangement of the Ti–N and N–O π-bonds to a new N–C π-bond and O lone pair, respectively.The tendency of C-nitrosos to undergo Diels–Alder-type cycloaddition reactions is well known,62,63 but examples of this process with metallacycles are scarce.64,65 Titanium β-diketiminate complexes, which differ from diazatitanacyclohexadienes by a degree of unsaturation, have also been observed to undergo [4 + 2]-cycloaddition with ketenes.66,67
IM4 then undergoes rate-determining (12.6 kcal mol−1) retro-[4 + 2] cycloaddition to extrude nitrile, forming azaoxatitanacycle IM5. The retrocycloadditive nature of TS2 can also be visualized by IBO calculations (Fig. 8b), which show the elimination of nitrile by the breaking of C–C and Ti–N σ-bonds in IM4 and the formation of three new N–C, C–C, and Ti–N π-bonds in IM5. Further IBO analysis is provided in Fig. S100.† A similar tandem [4 + 2]-cycloaddition-cycloreversion process to eliminate nitriles has also been proposed in the synthesis of phosphinines.68IM5 then undergoes a haptotropic shift following loss of the coordinated nitrile (IM6) generating η2-(N,O)-bound IM7. Finally, N–O bond cleavage results in exothermic formation of a Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species with the bound α-diimine product IM8.69 Cycloreversions of group IV heterometallacycles to yield M = X (M = Ti, Zr; X= O, N) are well-precedented.70–75
O species with the bound α-diimine product IM8.69 Cycloreversions of group IV heterometallacycles to yield M = X (M = Ti, Zr; X= O, N) are well-precedented.70–75
Ultimately, nitrile serves as a promoter in the in situ reactions—first forming the key diazatitanacycle intermediate, and then being eliminated prior to product formation. Alternatively, instead of undergoing a [4 + 2]-cycloaddition, a nitroso could directly insert into the [2 + 2] alkyne/Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR cycloadduct, bypassing the need for nitrile. Indeed, C-nitrosos undergo insertions with Ti and Zr metallacycles.76,77 However, a nitrile-free control reaction resulted in exclusive formation of azobenzene through metathesis of the Ti imido with PhNO (Fig. 9), making this route unlikely.50,51
NR cycloadduct, bypassing the need for nitrile. Indeed, C-nitrosos undergo insertions with Ti and Zr metallacycles.76,77 However, a nitrile-free control reaction resulted in exclusive formation of azobenzene through metathesis of the Ti imido with PhNO (Fig. 9), making this route unlikely.50,51
Conclusions
In summary, we have demonstrated a one-pot multicomponent synthesis of α-diimines by the diimination of alkynes by Ti imidos and C-nitrosos. This reaction likely occurs by [4 + 2]-cycloaddition of a nitroso to the Ti and γ-carbon of a diazatitanacyclohexadiene intermediate, followed by two subsequent cycloreversion steps to eliminate nitrile and afford the α-diimine and Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This is an attractive route to complex, unsymmetrical α-diimines that are difficult to obtain through classical condensation reactions, providing new strategies for ligand synthesis,3,18,20 and generally adding to the library of alkyne difunctionalization reactions. Efforts are ongoing to make this cycloaddition–retrocyclization strategy generalizable to other difunctionalizations.
O. This is an attractive route to complex, unsymmetrical α-diimines that are difficult to obtain through classical condensation reactions, providing new strategies for ligand synthesis,3,18,20 and generally adding to the library of alkyne difunctionalization reactions. Efforts are ongoing to make this cycloaddition–retrocyclization strategy generalizable to other difunctionalizations.
Author contributions
CWF: experimental data collection and analysis, computational analysis; DTE: computational analysis; EK: computational analysis; AJP: experimental data collection and analysis; YC: experimental data collection and analysis; IAT: project direction and all authors contributed to the writing, editing, and revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the National Institutes of Health (R35GM119457), and the Alfred P. Sloan Foundation (I. A. T. is a 2017 Sloan Fellow). D. T. E. kindly acknowledges financial support by the ETH Zurich foundation (D. T. E. is a 2019 ESOP scholar). Y. C. acknowledges funding support from the Wayland E. Noland Fellowship (UMN). Instrumentation for the University of Minnesota Chemistry NMR facility was supported from a grant through the National Institutes of Health (S10OD011952). X-ray diffraction experiments were performed with a diffractometer purchased through a grant from NSF/MRI (1229400) and the University of Minnesota. Dr Evan Beaumier, Dr Robin Harkins, and Dr Xuelan Wen are acknowledged for helpful discussions. The Minnesota Supercomputing Institute (MSI) at the University of Minnesota for provided resources that contributed to the results reported within this paper.Notes and references
- L. K. Johnson, C. M. Killian and M. Brookhart, New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- N. G. Léonard and P. J. Chirik, Air-Stable α-Diimine Nickel Precatalysts for the Hydrogenation of Hindered, Unactivated Alkenes, ACS Catal., 2018, 8, 342–348 CrossRef.
- F. Wang and C. Chen, A continuing legend: the Brookhart-type α-diimine nickel and palladium catalysts, Polym. Chem., 2019, 10, 2354–2369 RSC.
- Z. Chen and M. Brookhart, Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts, Acc. Chem. Res., 2018, 51, 1831–1839 CrossRef CAS PubMed.
- S. D. Ittel, L. K. Johnson and M. Brookhart, Late-Metal Catalysts for Ethylene Homo- and Copolymerization, Chem. Rev., 2000, 100, 1169–1204 CrossRef CAS PubMed.
- F.-S. Liu, H.-B. Hu, Y. Xu, L.-H. Guo, S.-B. Zai, K.-M. Song, H.-Y. Gao, L. Zhang, F.-M. Zhu and Q. Wu, Thermostable α-Diimine Nickel(II) Catalyst for Ethylene Polymerization: Effects of the Substituted Backbone Structure on Catalytic Properties and Branching Structure of Polyethylene, Macromolecules, 2009, 42, 7789–7796 CrossRef CAS.
- J. L. Rhinehart, L. A. Brown and B. K. Long, A Robust Ni(II) α-Diimine Catalyst for High Temperature Ethylene Polymerization, J. Am. Chem. Soc., 2013, 135, 16316–16319 CrossRef CAS PubMed.
- S. Sa, M. Jeon and S. Y. Kim, Controlling branch distribution of polyethylenes by steric tuning of Ni α-diimine complexes based on phenanthrenequinone, J. Mol. Catal. A: Chem., 2014, 393, 263–271 CrossRef CAS.
- S. Dai, S. Zhou, W. Zhang and C. Chen, Systematic Investigations of Ligand Steric Effects on α-Diimine Palladium Catalyzed Olefin Polymerization and Copolymerization, Macromolecules, 2016, 49, 8855–8862 CrossRef CAS.
- L. Guo, S. Dai, X. Sui and C. Chen, Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization, ACS Catal., 2016, 6, 428–441 CrossRef CAS.
- N. Muresan, C. C. Lu, M. Ghosh, J. C. Peters, M. Abe, L. M. Henling, T. Weyhermöller, E. Bill and K. Wieghardt, Bis(α-diimine)iron Complexes: Electronic Structure Determination by Spectroscopy and Broken Symmetry Density Functional Theoretical Calculations, Inorg. Chem., 2008, 47, 4579–4590 CrossRef CAS PubMed.
- K. A. Kreisel, G. P. A. Yap and K. H. Theopold, Synthesis, Characterization, and Electronic Structure of Diimine Complexes of Chromium, Inorg. Chem., 2008, 47, 5293–5303 CrossRef CAS PubMed.
- H. Nishiyama, H. Ikeda, T. Saito, B. Kriegel, H. Tsurugi, J. Arnold and K. Mashima, Structural and Electronic Noninnocence of α-Diimine Ligands on Niobium for Reductive C–Cl Bond Activation and Catalytic Radical Addition Reactions, J. Am. Chem. Soc., 2017, 139, 6494–6505 CrossRef CAS PubMed.
- K. Mashima, Redox-Active α-Diimine Complexes of Early Transition Metals: From Bonding to Catalysis, Bull. Chem. Soc. Jpn., 2020, 93, 799–820 CrossRef CAS.
- M. Shimizu, M. Kamei and T. Fujisawa, Stereocontrol in the reduction of 1,2-diimine with an oxazaborolidine catalyst. Highly stereoselective preparation of (R,R)-1,2-diphenylethylenediamine, Tetrahedron Lett., 1995, 36, 8607–8610 CrossRef CAS.
- X. Zhu and H. Du, A Highly Stereoselective Metal-Free Hydrogenation of Diimines for the Synthesis of Cis-Vicinal Diamines, Org. Lett., 2015, 17, 3106–3109 CrossRef CAS PubMed.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, An overview of N-heterocyclic carbenes, Nature, 2014, 510, 485–496 CrossRef CAS PubMed.
- L. Hintermann, Expedient syntheses of the N-heterocyclic carbene precursor imidazolium salts IPr·HCl, IMes·HCl and IXy·HCl, Beilstein J. Org. Chem., 2007, 3, 22 Search PubMed.
- P. Queval, C. Jahier, M. Rouen, I. Artur, J.-C. Legeay, L. Falivene, L. Toupet, C. Crévisy, L. Cavallo, O. Baslé and M. Mauduit, Multicomponent Synthesis of Unsymmetrical Unsaturated N-Heterocyclic Carbene Precursors and Their Related Transition-Metal Complexes, Angew. Chem., Int. Ed., 2013, 52, 14103–14107 CrossRef CAS PubMed.
- S. Li, F. Yang, T. Lv, J. Lan, G. Gao and J. You, Synthesis of unsymmetrical imidazolium salts by direct quaternization of N-substituted imidazoles using arylboronic acids, Chem. Commun., 2014, 50, 3941–3943 RSC.
- M. Jeon and S. Y. Kim, Ethylene Polymerizations with Unsymmetrical (α-Diimine)nickel(II) Catalysts, Polym. J., 2008, 40, 409–413 CrossRef CAS.
- L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz and V. César, Synthetic Routes to N-Heterocyclic Carbene Precursors, Chem. Rev., 2011, 111, 2705–2733 CrossRef CAS PubMed.
- A. Dumas, R. Tarrieu, T. Vives, T. Roisnel, V. Dorcet, O. Baslé and M. Mauduit, A Versatile and Highly Z-Selective Olefin Metathesis Ruthenium Catalyst Based on a Readily Accessible N-Heterocyclic Carbene, ACS Catal., 2018, 8, 3257–3262 CrossRef CAS.
- V. Rosar, A. Meduri, T. Montini, P. Fornasiero, E. Zangrando and B. Milani, The contradictory effect of the methoxy-substituent in palladium-catalyzed ethylene/methyl acrylate cooligomerization, Dalton Trans., 2018, 47, 2778–2790 RSC.
- S. K. Ellandula, C. Opoku Amoako, J. T. Mague and P. Chandrasekaran, Crystal structure of unsymmetrical [alpha]-diimine palladium(II) complex cis-[{ArN=C(Me)-(Et)C=NAr}PdCl2] [Ar = 2,6-(iPr)2C6H3], Acta Crystallogr., Sect. E: Crystallogr. Commun., 2017, 73, 1148–1150 CrossRef CAS PubMed.
- C. Kison and T. Opatz, Synthesis of Highly Substituted Unsymmetrical 1,2-Diamines, 1,2-Diimines, Imidazolium Salts and Imidazolylidenes by Aldimine Cross-Coupling, Synthesis, 2006, 2006, 3727–3738 CrossRef.
- C. Kison, N. Meyer and T. Opatz, An Aldimine Cross-Coupling for the Diastereoselective Synthesis of Unsymmetrical 1,2-Diamines, Angew. Chem., Int. Ed., 2005, 44, 5662–5664 CrossRef CAS PubMed.
- J. Wang, X. Cheng, Y. Liu and J. Zhang, Multicomponent Synthesis of Unsymmetrical 4,5-Disubstituted Imidazolium Salts as N-Heterocyclic Carbene Precursors: Applications in Palladium-Catalyzed Cross-Coupling Reactions, J. Org. Chem., 2021, 86, 6278–6288 CrossRef CAS PubMed.
- Z. W. Gilbert, R. J. Hue and I. A. Tonks, Catalytic formal [2 + 2 + 1] synthesis of pyrroles from alkynes and diazenes via TiII/TiIV redox catalysis, Nat. Chem., 2015, 8, 63 CrossRef PubMed.
- A. N. Desnoyer, X. Y. See and I. A. Tonks, Diverse Reactivity of Diazatitanacyclohexenes: Coupling Reactions of 2H-Azirines Mediated by Titanium(II), Organometallics, 2018, 37, 4327–4331 CrossRef CAS PubMed.
- E. P. Beaumier, A. J. Pearce, X. Y. See and I. A. Tonks, Modern applications of low-valent early transition metals in synthesis and catalysis, Nat. Rev. Chem., 2019, 3, 15–34 CrossRef PubMed.
- A. J. Pearce, R. P. Harkins, B. R. Reiner, A. C. Wotal, R. J. Dunscomb and I. A. Tonks, Multicomponent Pyrazole Synthesis from Alkynes, Nitriles, and Titanium Imido Complexes via Oxidatively Induced N–N Bond Coupling, J. Am. Chem. Soc., 2020, 142, 4390–4399 CrossRef CAS PubMed.
- J. Zeng, Y. J. Tan, M. L. Leow and X.-W. Liu, Copper(II)/Iron(III) Co-catalyzed Intermolecular Diamination of Alkynes: Facile Synthesis of Imidazopyridines, Org. Lett., 2012, 14, 4386–4389 CrossRef CAS PubMed.
- V. Dwivedi, R. Kumar, K. Sharma, B. Sridhar and M. S. Reddy, Copper-Promoted Regioselective Intermolecular Diamination of Ynamides: Synthesis of Imidazo[1,2-a]pyridines, ACS Omega, 2017, 2, 2770–2777 CrossRef CAS PubMed.
- C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Heteroaromatic imidazo[1,2-a]pyridines synthesis from C–H/N–H oxidative cross-coupling/cyclization, Chem. Commun., 2012, 48, 11073–11075 RSC.
- J. Li and L. Neuville, Copper-Catalyzed Oxidative Diamination of Terminal Alkynes by Amidines: Synthesis of 1,2,4-Trisubstituted Imidazoles, Org. Lett., 2013, 15, 1752–1755 CrossRef CAS PubMed.
- K. Muñiz and C. Martínez, Development of Intramolecular Vicinal Diamination of Alkenes: From Palladium to Bromine Catalysis, J. Org. Chem., 2013, 78, 2168–2174 CrossRef PubMed.
- D. E. Olson, J. Y. Su, D. A. Roberts and J. Du Bois, Vicinal Diamination of Alkenes under Rh-Catalysis, J. Am. Chem. Soc., 2014, 136, 13506–13509 CrossRef CAS PubMed.
- Y. Zhu, R. G. Cornwall, H. Du, B. Zhao and Y. Shi, Catalytic Diamination of Olefins via N–N Bond Activation, Acc. Chem. Res., 2014, 47, 3665–3678 CrossRef CAS PubMed.
- K. Muñiz, L. Barreiro, R. M. Romero and C. Martínez, Catalytic Asymmetric Diamination of Styrenes, J. Am. Chem. Soc., 2017, 139, 4354–4357 CrossRef PubMed.
- Z. Tao, B. B. Gilbert and S. E. Denmark, Catalytic, Enantioselective syn-Diamination of Alkenes, J. Am. Chem. Soc., 2019, 141, 19161–19170 CrossRef CAS PubMed.
- S. Minakata, H. Miwa, K. Yamamoto, A. Hirayama and S. Okumura, Diastereodivergent Intermolecular 1,2-Diamination of Unactivated Alkenes Enabled by Iodine Catalysis, J. Am. Chem. Soc., 2021, 143, 4112–4118 CrossRef CAS PubMed.
- J. D. Selby, C. D. Manley, M. Feliz, A. D. Schwarz, E. Clot and P. Mountford, New ligand platforms for developing the chemistry of the Ti
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NR2 functional group and the insertion of alkynes into the N–N bond of a Ti
N–NR2 functional group and the insertion of alkynes into the N–N bond of a Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NPh2 ligand, Chem. Commun., 2007, 4937–4939 RSC.
N–NPh2 ligand, Chem. Commun., 2007, 4937–4939 RSC. - M. Manßen and L. L. Schafer, Titanium catalysis for the synthesis of fine chemicals – development and trends, Chem. Soc. Rev., 2020, 49, 6947–6994 RSC.
- F. Basuli, H. Aneetha, J. C. Huffman and D. J. Mindiola, A Fluorobenzene Adduct of Ti(IV), and Catalytic Carboamination to Prepare α,β-Unsaturated Imines and Triaryl-Substituted Quinolines, J. Am. Chem. Soc., 2005, 127, 17992–17993 CrossRef CAS PubMed.
- F. Basuli, B. Wicker, J. C. Huffman and D. J. Mindiola, Understanding the role of an easy-to-prepare aldimine–alkyne carboamination catalyst, [Ti(NMe2)3(NHMe2)][B(C6F5)4], J. Organomet. Chem., 2011, 696, 235–243 CrossRef CAS.
- Z. W. Davis-Gilbert, L. J. Yao and I. A. Tonks, Ti-Catalyzed Multicomponent Oxidative Carboamination of Alkynes with Alkenes and Diazenes, J. Am. Chem. Soc., 2016, 138, 14570–14573 CrossRef CAS PubMed.
- C. Cao, Y. Shi and A. L. Odom, A Titanium-Catalyzed Three-Component Coupling To Generate α,β-Unsaturated β-Iminoamines, J. Am. Chem. Soc., 2003, 125, 2880–2881 CrossRef CAS PubMed.
- B. Priewisch and K. Rück-Braun, Efficient Preparation of Nitrosoarenes for the Synthesis of Azobenzenes, J. Org. Chem., 2005, 70, 2350–2352 CrossRef CAS PubMed.
- S. C. Dunn, N. Hazari, A. R. Cowley, J. C. Green and P. Mountford, Synthesis and Reactions of Group 4 Imido Complexes Supported by Cyclooctatetraene Ligands, Organometallics, 2006, 25, 1755–1770 CrossRef CAS.
- S. A. Blum and R. G. Bergman, Nitro and Nitroso Metathesis Reactions with Monomeric Zirconium Imido Complexes, Organometallics, 2004, 23, 4003–4005 CrossRef CAS PubMed.
- D. Y. Curtin, E. J. Grubbs and C. G. McCarty, Uncatalyzed syn-anti Isomerization of Imines, Oxime Ethers, and Haloimines, J. Am. Chem. Soc., 1966, 88, 2775–2786 CrossRef CAS.
- X. Y. See, E. P. Beaumier, Z. W. Davis-Gilbert, P. L. Dunn, J. A. Larsen, A. J. Pearce, T. A. Wheeler and I. A. Tonks, Generation of TiII Alkyne Trimerization Catalysts in the Absence of Strong Metal Reductants, Organometallics, 2017, 36, 1383–1390 CrossRef CAS PubMed.
- Z. W. Davis-Gilbert, K. Kawakita, D. R. Blechschmidt, H. Tsurugi, K. Mashima and I. A. Tonks, In situ Catalyst Generation and Benchtop-Compatible Entry Points for TiII/TiIV Redox Catalytic Reactions, Organometallics, 2018, 37, 4439–4445 CrossRef CAS PubMed.
- J. I. Seeman, Effect of conformational change on reactivity in organic chemistry. Evaluations, applications, and extensions of Curtin–Hammett Winstein–Holness kinetics, Chem. Rev., 1983, 83, 83–134 CrossRef CAS.
- H.-C. Chiu, X. Y. See and I. A. Tonks, Dative Directing Group Effects in Ti-Catalyzed [2 + 2 + 1] Pyrrole Synthesis: Chemo- and Regioselective Alkyne Heterocoupling, ACS Catal., 2019, 9, 216–223 CrossRef CAS PubMed.
- Y. Cheng, C. K. Klein and I. A. Tonks, Synthesis of pentasubstituted 2-aryl pyrroles from boryl and stannyl alkynes via one-pot sequential Ti-catalyzed [2 + 2 + 1] pyrrole synthesis/cross coupling reactions, Chem. Sci., 2020, 11, 10236–10242 RSC.
- K. Kawakita, B. F. Parker, Y. Kakiuchi, H. Tsurugi, K. Mashima, J. Arnold and I. A. Tonks, Reactivity of terminal imido complexes of group 4–6 metals: Stoichiometric and catalytic reactions involving cycloaddition with unsaturated organic molecules, Coord. Chem. Rev., 2020, 407, 213118 CrossRef CAS PubMed.
- A. D. Schofield, A. Nova, J. D. Selby, A. D. Schwarz, E. Clot and P. Mountford, Reaction Site Diversity in the Reactions of Titanium Hydrazides with Organic Nitriles, Isonitriles and Isocyanates: Ti
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα Cycloaddition, Ti
Nα Cycloaddition, Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα Insertion and Nα–Nβ Bond Cleavage, Chem.–Eur. J., 2011, 17, 265–285 CrossRef CAS PubMed.
Nα Insertion and Nα–Nβ Bond Cleavage, Chem.–Eur. J., 2011, 17, 265–285 CrossRef CAS PubMed. - M. Manßen, S. de Graaff, M.-F. Meyer, M. Schmidtmann and R. Beckhaus, Direct Access to Titanocene Imides via Bis(η5:η1-penta-fulvene)titanium Complexes and Primary Amines, Organometallics, 2018, 37, 4506–4514 CrossRef.
- J. Barluenga, C. d. Pozo and B. Olano, Reactions of N-Unsubstituted 4-Amino-1-azadienes Towards Electrophiles, Synthesis, 1996, 1996, 133–140 CrossRef.
- S. Carosso and M. J. Miller, Nitroso Diels–Alder (NDA) reaction as an efficient tool for the functionalization of diene-containing natural products, Org. Biomol. Chem., 2014, 12, 7445–7468 RSC.
- B. Maji and H. Yamamoto, Catalytic Enantioselective Nitroso Diels–Alder Reaction, J. Am. Chem. Soc., 2015, 137, 15957–15963 CrossRef CAS PubMed.
- J. R. Bleeke, P. V. Hinkle and N. P. Rath, Synthesis, Structure, Spectroscopy, and Reactivity of a Metallathiabenzene, Organometallics, 2001, 20, 1939–1951 CrossRef CAS.
- R. L. Holland and J. M. O'Connor, Nitroso Compounds Serve as Precursors to Late-Metal η2(N,O)-Hydroxylamido Complexes, Organometallics, 2009, 28, 394–396 CrossRef CAS.
- F. Basuli, J. C. Huffman and D. J. Mindiola, Reactivity at the β-Diketiminate Ligand Nacnac- on Titanium(IV) (Nacnac- = [Ar]NC(CH3)CHC(CH3)N[Ar], Ar = 2,6-[CH(CH3)2]2C6H3). Diimine-alkoxo and Bis-anilido Ligands Stemming from the Nacnac-Skeleton, Inorg. Chem., 2003, 42, 8003–8010 CrossRef CAS PubMed.
- F. Basuli, B. C. Bailey, L. A. Watson, J. Tomaszewski, J. C. Huffman and D. J. Mindiola, Four-Coordinate Titanium Alkylidene Complexes:Synthesis ,Reactivity ,and Kinetic Studies Involving the Terminal Neopentylidene Functionality, Organometallics, 2005, 24, 1886–1906 CrossRef CAS.
- N. Avarvari, P. Le Floch, L. Ricard and F. Mathey, 1,3,2-Diazaphosphinines and -Diazaarsinines as Precursors for Polyfunctional Phosphinines and Arsinines, Organometallics, 1997, 16, 4089–4098 CrossRef CAS.
- L. Becker, F. Strehler, M. Korb, P. Arndt, A. Spannenberg, W. Baumann, H. Lang and U. Rosenthal, Unusual Nitrile–Nitrile and Nitrile–Alkyne Coupling of Fc–C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and Fc–C
N and Fc–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, Chem.–Eur. J., 2014, 20, 3061–3068 CrossRef CAS PubMed.
N, Chem.–Eur. J., 2014, 20, 3061–3068 CrossRef CAS PubMed. - G. D. Kortman, M. J. Orr and K. L. Hull, Synthesis and Reactivity of Dioxazirconacyclohexenes: Development of a Zirconium–Oxo-Mediated Alkyne–Aldehyde Coupling Reaction, Organometallics, 2015, 34, 1013–1016 CrossRef CAS.
- T. T. Nguyen, G. D. Kortman and K. L. Hull, Synthesis, Cycloaddition, and Cycloreversion Reactions of Mononuclear Titanocene–oxo Complexes, Organometallics, 2016, 35, 1713–1725 CrossRef CAS.
- K. M. Doxsee and J. K. M. Mouser, Titanium-mediated synthesis of conjugated dienes, Tetrahedron Lett., 1991, 32, 1687–1690 CrossRef CAS.
- J. L. Polse, R. A. Andersen and R. G. Bergman, Cycloaddition and Cycloreversion Reactions of a Monomeric Ti(IV) Oxo Complex with Terminal and Internal Alkynes. A Reversible Oxametallacyclobutene/Hydroxoacetylide Interconversion, J. Am. Chem. Soc., 1995, 117, 5393–5394 CrossRef CAS.
- R. T. Ruck, R. L. Zuckerman, S. W. Krska and R. G. Bergman, Carboamination: Additions of Imine C=N Bonds Across Alkynes Catalyzed by Imidozirconium Complexes, Angew. Chem., Int. Ed., 2004, 43, 5372–5374 CrossRef CAS PubMed.
- T. A. Hanna, A. M. Baranger, P. J. Walsh and R. G. Bergman, Formation of .alpha,beta.-Unsaturated Imines and Successful Trapping of Oxozirconocene in a [4 + 2] Azaoxametallacyclohexene Retrocycloaddition, J. Am. Chem. Soc., 1995, 117, 3292–3293 CrossRef CAS.
- K. M. Doxsee, J. J. J. Juliette, T. J. R. Weakley and K. Zientara, Nitrosoarene and nitrosoalkane insertion reactions of titanacyclobutenes, Inorg. Chim. Acta, 1994, 222, 305–315 CrossRef CAS.
- M. Nakamoto and T. D. Tilley, Reactions of Zirconacyclopentadienes with Nitrosobenzene. Characterization of Zirconacycle Intermediates and Formation of N-Phenylpyrroles, Organometallics, 2001, 20, 5515–5517 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectroscopic data. CCDC 2072232. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06111a |
| This journal is © The Royal Society of Chemistry 2022 |