Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultramacrocyclization in water via external templation†
Qiong
Chen
a,
Ye
Lei
a,
Guangcheng
Wu
a,
Qing
Li
*b,
Yuanjiang
Pan
 a and
Hao
Li
a and
Hao
Li
 *ac
*ac
aDepartment of Chemistry Institution, Zhejiang University, Hangzhou 310027, China. E-mail: lihao2015@zju.edu.cn
bKey Laboratory of Macrocyclic and Supramolecular Chemistry, Guizhou University, Guiyang 550025, China. E-mail: nyliqing2012@163.com
cZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou 310027, China
First published on 5th January 2022
Abstract
Condensing a dihydrazide and each of a series of cationic bisaldehyde compounds bearing polymethylene chains in weakly acidic water produces either a macrocycle in a [1 + 1] manner or its dimer namely a [2]catenane, or their mixture. The product distribution is determined by the length of the bisaldehydes. Addition of cucurbit[8]uril (CB[8]) drives the catenane/macrocycle equilibria to the side of macrocycles, by forming ring-in-ring complexes with the latter. When the polymethylene unit of the bisaldehyde is replaced with a more rigid p-xylene linker, its self-assembly with the dihydrazide leads to quantitative formation of a [2]catenane. Upon addition of CB[8], the [2]catenane is transformed into an ultra-large macrocycle condensed in a [2 + 2] manner, which is encircled by two CB[8] rings. The framework of this macrocycle contains one hundred and two atoms, whose synthesis would be a formidable task without the external template CB[8]. Removal of CB[8] with a competitive guest leads to recovery of the [2]catenane.
Introduction
Synthesis of macrocyclic compounds represents one of the major focuses in the field of supramolecular chemistry.1 When the sizes of the rings become larger, their production becomes more challenging, given that macrocyclization of rings containing more atoms implies more entropy loss. High dilution conditions2 and/or addition of feasible guest templates3 have proven to be efficient approaches to suppress or avoid oligomeric or polymeric byproducts. However, these approaches become less useful in the case that the sizes of macrocyclic products are ultra-large, especially for those rings containing more than one hundred atoms. In addition, finding a feasible internal guest template to fit within the cavity of an ultra-large macrocycle becomes more difficult, despite a few exceptions.4Our group5 and others6 discovered that hydrazone condensation represents an ideal dynamic covalent reaction that is amenable to use in water. A variety of [2]catenanes,5a,d–f rings,5c,d,6d,e and cages5b,g were self-assembled in aqueous media, thanks to the reversible nature of hydrazone that allows the systems to perform error checking. In addition, cucurbit[8]uril (CB[8])7c,e,g,i,k was observed as a host that is able to accommodate a two π-electron guest, driven by the hydrophobic effect and dipole–cation interactions in the case of cationic guests such as pyridinium derivatives. We thus envision that by taking advantage of the marriage of dynamic covalent chemistry5,6,8 and external templation based on CB[8] rings,7 we might be able to self-assemble an ultra-large macrocycle. Here, by condensing a dihydrazide and a dicationic bisaldehyde containing a poly-methylene chain in water, rings with medium sizes containing fifty to sixty atoms in their framework were self-assembled in a [1 + 1] manner as the major products. When the polymethylene chain becomes longer, the macrocycles start to undergo equilibration with their dimerized form namely [2]catenanes, driven by the hydrophobic effect. In the presence of CB[8], the macrocycle-[2]catenane equilibria shifted to the side of macrocycles, each of which was accommodated within the cavity of a CB[8] ring, forming a set of [2]pseudorotaxanes. The formation of these ring-in-ring complexes9 is favored by the CB[8]–pyridinium interactions between the host and the guest. When the poly-methylene chain in the bisaldehyde was changed to a more rigid p-xylene unit, its self-assembly with the dihydrazide linker resulted in the exclusive formation of a [2]catenane in close to a quantitative yield, thanks to the dynamic nature of hydrazone bonds that allows error checking. Addition of CB[8] led to decomposition of this [2]catenane. However, the putative [2]pseudorotaxane was not obtained as occurred in the case of the aforementioned polymethylene counterparts. This is probably on account of the p-xylene linker, which renders the [1 + 1] macrocycle too large to fit within the CB[8] cavity. Instead, the bisaldehyde and bishydrazide underwent condensation in a [2 + 2] manner, forming an ultra-large macrocycle that was encircled by two CB[8] rings. This macrocycle contains more than one hundred atoms in its framework, whose formation would be rather challenging without the external template CB[8] rings. Upon removal of the CB[8] by adding a competitive guest, the ultra-large macrocycle underwent decomposition, regaining the [2]catenane.
Results and discussion
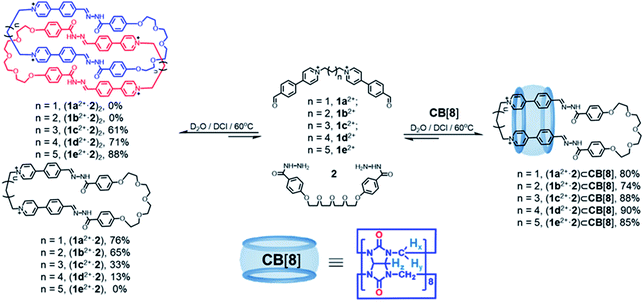
Each of the biscationic dialdehyde compounds, including 1x2+·2Br− (x = a, b, c, d and e), contains two cationic pyridinium-benzaldehydes that are bridged by a polymethylene chain containing three to seven CH2 units. A dihydrazine linker 2, contains two phenylacylhydrazide units that are connected by a glycol chain. The detailed synthetic procedures are described in the ESI.†Self-assembly was performed by combining the dihydrazine linker 2 (2.5 mM) with each of biscationic dialdehydes 1x2+·2Br− (x = a, b, c, d and e; 2.5 mM) in D2O. A catalytic amount of DCl was used to catalyze hydrazone condensation (Fig. 1 and Schemes S5–S7†). After heating the corresponding solutions at 60 °C for no less than 8 h, the corresponding 1H NMR spectra remained unchanged, showing that all the reactants were almost completely consumed. In the case of 1a2+ and 1b2+, two macrocycles (1a2+·2) and (1b2+·2), whose counteranions could be either Cl− or Br−, were self-assembled in a [1 + 1] manner as the major products, as indicated by both 1H NMR spectroscopy (Fig. S2 and S11†) and mass spectrometry (Fig. S1 and S10†). The yields of (1a2+·2) and (1b2+·2) were determined to be 76% and 65%, respectively, by using DMSO as an internal standard in the 1H NMR samples (Fig. S62B and S63B†). In the case of 1c2+ and 1d2+, the self-assembly products included both the macrocycles namely (1c2+·2) and (1d2+·2), as well as their corresponding dimerized forms namely the [2]catenanes (1c2+·2)2 and (1d2+·2)2, whose counteranions could be either Cl− or Br−. The NMR yields of the [2]catenanes (1c2+·2)2 and (1d2+·2)2, as well as the rings (1c2+·2) and (1d2+·2) were calculated to be 61%, 71%, 33% and 13%, respectively (Fig. S64B and S65B†). In the case of the biscationic dialdehyde 1e2+ containing the longest polymethylene namely a (CH2)7 chain, a [2]catenane (1e2+·2)2 (counteranions could be either Cl− or Br−) was produced as the only observable product in the 1H NMR spectrum, whose yield is 88% (Fig. S66B†). Obviously, a dialdehyde with a longer polymethylene chain favors the formation of a [2]catenane to a more significant extent compared to its shorter counterparts.
Upon addition of an equimolar amount of CB[8] to a D2O solution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
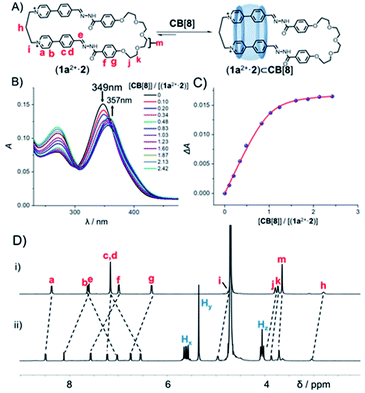
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2 (2.5 mM) and each of 1x2+·2Br− (x = a, b, c, d and e; 2.5 mM) in the presence of DCl (Fig. 1 and Scheme S8†), a set of [2]pseudorotaxanes (1x2+·2)⊂CB[8] (x = a, b, c, d and e) were observed to form as the predominant products. The yields of (1x2+·2)⊂CB[8] (x = a, b, c, d and e) (counteranions could be either Cl− or Br−) were determined to be 80%, 74%, 88%, 90% and 85% (Fig. S62A, S63A, S64A, S65A and S66A†), respectively, calculated by using an internal standard in the corresponding 1H NMR samples. The structures of these [2]pseudorotaxanes were fully characterized by both 1H NMR spectroscopy and mass spectrometry (Fig. S6, S7, S15, S16, S25, S26, S33, S34, S42 and S43†). The 1H NMR spectra of both (1a2+·2) and (1a2+·2)⊂CB[8] are shown in Fig. 2D. The resonances corresponding to the protons b and c in the guest undergo remarkable upfield shifts, indicating that the CB[8] host encircles the phenylene units in the 1a2+ residue, driven by the hydrophobic effect. As a comparison, the phenylene units in the 2 residue are located outside the pocket of CB[8], as indicated by the downfield shifts of the corresponding protons. CB[8] chooses to reside on the phenylene units in the 1a2+ residue instead of those in 2, because of the cation–dipole interactions between the CB[8] host and the pyridinium cations in the 1a2+ residue of the macrocyclic guest. The host/guest association and dissociation occurred at a relatively slow rate on the timescale of 1H NMR spectroscopy, as indicated by the observation that the two protons of CB[8] become chemically inequivalent in the 1H NMR spectrum of (1a2+·2)⊂CB[8] (Fig. 2D). Addition of an equimolar amount of CB[8] to the corresponding pre-self-assembled [2]catenanes also produced the same [2]pseudorotaxane products, indicating that the corresponding [2]pseudorotaxanes (1x2+·2)⊂CB[8] (x = a, b, c, d and e) are thermodynamic products, instead of kinetically trapped ones. Apparently, the formation of [2]pseudorotaxanes is more favored by dipole–cation interactions resulting from CB[8], compared to the homo [2]catenanes.
1 mixture of 2 (2.5 mM) and each of 1x2+·2Br− (x = a, b, c, d and e; 2.5 mM) in the presence of DCl (Fig. 1 and Scheme S8†), a set of [2]pseudorotaxanes (1x2+·2)⊂CB[8] (x = a, b, c, d and e) were observed to form as the predominant products. The yields of (1x2+·2)⊂CB[8] (x = a, b, c, d and e) (counteranions could be either Cl− or Br−) were determined to be 80%, 74%, 88%, 90% and 85% (Fig. S62A, S63A, S64A, S65A and S66A†), respectively, calculated by using an internal standard in the corresponding 1H NMR samples. The structures of these [2]pseudorotaxanes were fully characterized by both 1H NMR spectroscopy and mass spectrometry (Fig. S6, S7, S15, S16, S25, S26, S33, S34, S42 and S43†). The 1H NMR spectra of both (1a2+·2) and (1a2+·2)⊂CB[8] are shown in Fig. 2D. The resonances corresponding to the protons b and c in the guest undergo remarkable upfield shifts, indicating that the CB[8] host encircles the phenylene units in the 1a2+ residue, driven by the hydrophobic effect. As a comparison, the phenylene units in the 2 residue are located outside the pocket of CB[8], as indicated by the downfield shifts of the corresponding protons. CB[8] chooses to reside on the phenylene units in the 1a2+ residue instead of those in 2, because of the cation–dipole interactions between the CB[8] host and the pyridinium cations in the 1a2+ residue of the macrocyclic guest. The host/guest association and dissociation occurred at a relatively slow rate on the timescale of 1H NMR spectroscopy, as indicated by the observation that the two protons of CB[8] become chemically inequivalent in the 1H NMR spectrum of (1a2+·2)⊂CB[8] (Fig. 2D). Addition of an equimolar amount of CB[8] to the corresponding pre-self-assembled [2]catenanes also produced the same [2]pseudorotaxane products, indicating that the corresponding [2]pseudorotaxanes (1x2+·2)⊂CB[8] (x = a, b, c, d and e) are thermodynamic products, instead of kinetically trapped ones. Apparently, the formation of [2]pseudorotaxanes is more favored by dipole–cation interactions resulting from CB[8], compared to the homo [2]catenanes.
In order to shed more light on the formation mechanism of the ring-in-ring pseudorotaxane, we measured the binding constants (Ka) of (1a2+·2)⊂CB[8] by using UV–Vis titration spectroscopic experiments (Fig. 2B). A pure sample of (1a2+·2)·2Cl− was isolated via counterion exchange without chromatographic purification, which is water soluble. Addition of CB[8] led to a decrease of the absorption peak of (1a2+·2) centered at 349 nm, while an increase of the absorption peak centered at 357 nm that corresponds to (1a2+·2)⊂CB[8]. Ka of (1a2+·2)⊂CB[8] was determined (Fig. 2C) to be 5.6(±1.1) × 106 M−1, by fitting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 bind curve. Due to the fact that CB[8] is barely soluble in water, titration experiments were performed at ultra-low concentrations namely [(1a2+·2)] = 3.0 × 10−3 mM. Under such conditions, the concentrations of the compounds namely either (1a2+·2) or CB[8] used in titration might be pseudo-accurate, which might be responsible for the absence of a clear isosbestic point in Fig. 2B. We thus performed competitive binding experiments at higher concentrations. That is, when (1a2+·2), paraquat and CB[8], the concentration of each of which is 2.5 mM, were combined in D2O, the 1H NMR spectrum (Fig. S67†) indicated that the (1a2+·2)⊂CB[8] was formed exclusively. Such an experiment implies that (1a2+·2) has a stronger binding affinity, compared to paraquat whose Ka is 1.1 × 105 M−1.10 A similar competitive binding experiment (Fig. S68†) also clearly demonstrated that (1a2+·2)⊂CB[8] is a stronger complex compared to dodecane-1,12-diammonium⊂CB[8], whose Ka is 1.1 × 106 M−1.11 We thus propose that the value of Ka of (1a2+·2)⊂CB[8] namely 5.6(±1.1) × 106 M−1 might still be reliable, even though not accurate.
1 bind curve. Due to the fact that CB[8] is barely soluble in water, titration experiments were performed at ultra-low concentrations namely [(1a2+·2)] = 3.0 × 10−3 mM. Under such conditions, the concentrations of the compounds namely either (1a2+·2) or CB[8] used in titration might be pseudo-accurate, which might be responsible for the absence of a clear isosbestic point in Fig. 2B. We thus performed competitive binding experiments at higher concentrations. That is, when (1a2+·2), paraquat and CB[8], the concentration of each of which is 2.5 mM, were combined in D2O, the 1H NMR spectrum (Fig. S67†) indicated that the (1a2+·2)⊂CB[8] was formed exclusively. Such an experiment implies that (1a2+·2) has a stronger binding affinity, compared to paraquat whose Ka is 1.1 × 105 M−1.10 A similar competitive binding experiment (Fig. S68†) also clearly demonstrated that (1a2+·2)⊂CB[8] is a stronger complex compared to dodecane-1,12-diammonium⊂CB[8], whose Ka is 1.1 × 106 M−1.11 We thus propose that the value of Ka of (1a2+·2)⊂CB[8] namely 5.6(±1.1) × 106 M−1 might still be reliable, even though not accurate.
1f 2+·2Cl−, an analogue of 1x2+·2Br− (x = a, b, c, d and e) whose polymethylene chains were replaced by a p-xylene unit, was synthesized. 1f2+·2Cl− (2.5 mM) and 2 (2.5 mM) were combined in D2O in the presence of a catalytic amount of DCl (Fig. 4 and Scheme S9†). The solution was heated at 60 °C for 8 h. Both 1H NMR spectroscopy and mass spectrometry (Fig. 4D, S46 and S47†) demonstrated that a [2]catenane (1f2+·2)2·4Cl− was produced as the predominant product. The NMR yield was calculated to be 94% (Fig. S60†). This yield is higher than those of the catenane containing polymethylene chains including (1c2+·2)2 and (1d2+·2)2, probably because the ring (1f2+·2) contains a more rigid p-xylene unit. This linker affords the ring a more preorganized cavity that favours catenation to a more significant extent.
Upon the addition of 2 equiv. of CB[8] to a D2O solution of (1f2+·2)2 (Fig. 4 and Scheme S10†), the resonances corresponding to the catenane completely disappeared, while a new set of resonances was observed after heating the solution at 60 °C for 24 h (Fig. S57D†). Surprisingly, both the 1H NMR spectrum (Fig. 4B) and mass spectrum (Fig. S52†) indicated that a [3]pseudorotaxane namely (1f2+·2·1f2+·2)⊂2CB[8] was self-assembled, containing two CB[8] rings that encircle an ultra-large macrocycle (1f2+·2·1f2+·2), which condensed in a [2 + 2] manner. The yield of (1f2+·2·1f2+·2)⊂2CB[8] was determined to be 68%, by using an internal standard in the 1H NMR sample (Fig. S61†). It is noteworthy that a putative [2]pseudorotaxane (1f2+·2·1f2+·2)⊂CB[8] containing only one CB[8] was not observed during the self-assembly. The binding of two CB[8] rings on the (1f2+·2·1f2+·2) ring is positively cooperative. This proposition was supported by the observation that, addition of one equiv. of CB[8] to a D2O solution of (1f2+·2)2 led to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the unreacted [2]catenane and the [3]pseudorotaxane (Fig. S57C†). It is likely that upon recognition of the first CB[8] ring, the (1f2+·2·1f2+·2) guest became more preorganized to accommodate the second ring.
1 mixture of the unreacted [2]catenane and the [3]pseudorotaxane (Fig. S57C†). It is likely that upon recognition of the first CB[8] ring, the (1f2+·2·1f2+·2) guest became more preorganized to accommodate the second ring.
Adamantan-1-ol,12 a competitive guest binding with CB[8], was added into the aqueous solution of (1f2+·2·1f2+·2)⊂2CB[8] (Fig. 4 and S58†). After heating the corresponding solution at 60 °C for 48 h, the 1H NMR spectrum (Fig. S58F†) demonstrated that (1f2+·2·1f2+·2)⊂2CB[8] was almost completely transformed into adamantan-1-ol⊂CB[8], leading to recovery of the [2]catenane (1f2+·2)2. This observation indicated that (i) adamantan-1-ol represents a better guest compared to (1f2+·2·1f2+·2), with respect to the CB[8] host; (ii) without the external templation of the CB[8] ring, the ultra-large ring (1f2+·2·1f2+·2) is no longer a thermodynamically stable compound in water compared to its [2]catenane isomer namely (1f2+·2)2.
Because (1f2+·2·1f2+·2) can not be isolated, accurate determination of Ka1 and Ka2 of (1f2+·2·1f2+·2)⊂2CB[8] was difficult or impossible. However, we could qualitatively evaluate Ka2 by using competitive experiments. Upon heating a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of CB[8], the [2]catenane (1f2+·2)2 and the ring (1a2+·2) (Fig. S69†) for 24 h at 60 °C, the [2]pseudorotaxane (1a2+·2)⊂CB[8] was self-assembled selectively, leaving (1f2+·2)2 unreacted in water. As a comparison, heating a 1
2 mixture of CB[8], the [2]catenane (1f2+·2)2 and the ring (1a2+·2) (Fig. S69†) for 24 h at 60 °C, the [2]pseudorotaxane (1a2+·2)⊂CB[8] was self-assembled selectively, leaving (1f2+·2)2 unreacted in water. As a comparison, heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of the [2]catenane (1f2+·2)2, paraquat, and CB[8] yielded (1f2+·2·1f2+·2)⊂2CB[8] as the major product, accompanied by a small amount of paraquat⊂CB[8] as the minor product (Fig. S70†). Such an experiment indicated that Ka2 might be comparable to or a little larger than the Ka of paraquat⊂CB[8], namely 1.1 × 105 M−1, while smaller than the Ka of (1a2+·2)⊂CB[8].
2 mixture of the [2]catenane (1f2+·2)2, paraquat, and CB[8] yielded (1f2+·2·1f2+·2)⊂2CB[8] as the major product, accompanied by a small amount of paraquat⊂CB[8] as the minor product (Fig. S70†). Such an experiment indicated that Ka2 might be comparable to or a little larger than the Ka of paraquat⊂CB[8], namely 1.1 × 105 M−1, while smaller than the Ka of (1a2+·2)⊂CB[8].
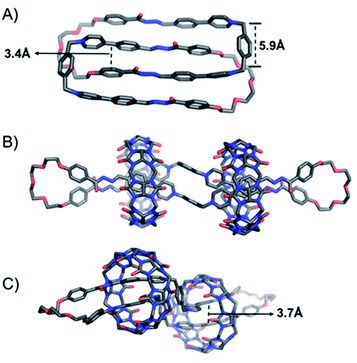
Single crystals of the [2]catenane (1f2+·2)2 and the [3]pseudorotaxane (1f2+·2·1f2+·2)⊂2CB[8] were obtained by either slow vapor diffusion of THF into the aqueous solution of (1f2+·2)2 (Fig. 3A), or slow evaporation of the aqueous solution of (1f2+·2·1f2+·2)⊂2CB[8] (Fig. 3B and C), which provided unambiguous evidence to convince their architectures. In the case of [2]catenane (1f2+·2)2, the cavity of each of the two mutually mechanically interlocked rings is almost fully occupied by another ring, leading to a variety of intercomponent close contacts. For example, each of the pyridinium building blocks in one ring undergoes π–π interactions with an adjacent phenylene unit in another ring, as inferred from their short interplane distances, i.e., around 3.4 Å (Fig. 3A). Close contacts also indicate the occurrence of CH⋯O hydrogen bonding and CH–π interactions. In the case of the ring-in-rings complex namely the [3]pseudorotaxane (1f2+·2·1f2+·2)⊂2CB[8] (Fig. 3B and C), each of the 1f2+ residues adopts a different conformation compared to that in the [2]catenane (1f2+·2)2, i.e., in the former system, the two 4-phenylpyridinium units orient in different directions with respect to the central p-xylene unit. The two methylene units in the 1f2+ residue are separated by 5.9 Å (Fig. 3A). This distance implies that the putative [1 + 1] ring, namely (1f2+·2), would be too large to fit within the cavity of CB[8]. As a consequence, the putative [2]pseudorotaxane (1f2+·2)⊂CB[8] can not form. Each of the CB[8] rings encircles two 4-phenylpyridinium units belonging to two different 1f2+ residues. Such behavior favors the formation of the ultra-large macrocycle in a [2 + 2] manner. Within the cavity of the CB[8] ring, two phenylene units undergo π–π interactions, as inferred from the close contact namely 3.7 Å (Fig. 3C). The two pyridinium units, as a comparison, exhibit a displaced parallel orientation, which helps to avoid pyridinium–pyridinium electrostatic repulsion while strengthening ion–dipole and CH–O interactions between the cationic pyridinium units and the carbonyl groups in CB[8].
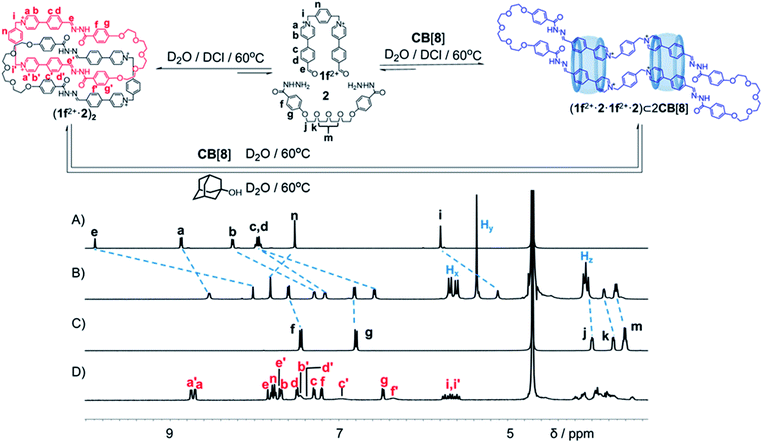 | ||
| Fig. 4 Structural formulae of a [2]catenane (1f2+·2)2 and a [3]pseudorotaxane (1f2+·2·1f2+·2)⊂2CB[8], by condensing a dicationic bisaldehyde 1f2+ and a bishydrazide 2 in water, in the absence and presence of CB[8] respectively. Partial 1H NMR spectra (500 MHz, D2O, 298 K) of (A) 1f2+, (B) (1f2+·2·1f2+·2)⊂2CB[8], (C) 2 and (D) (1f2+·2)2. The assignment of each resonance was made using the corresponding two-dimensional NMR spectra shown in the ESI.† Charges are balanced by Cl− counteranions, which are omitted here for the sake of clarity. | ||
Conclusion
In summary, by combining the corresponding bishydrazide and a set of cationic bisaldehydes bearing polymethylene chains in aqueous media, a series of [1 + 1] macrocycles were self-assembled, accompanied with the corresponding dimers namely [2]catenanes. The product distribution was determined by the length of the polymethylene chains in the bisaldehyde precursors. That is, the longer chains favor the formation of [2]catenanes, while the shorter ones favor the macrocycles more. Upon addition of CB[8], the macrocycles form a set of [2]pseudorotaxanes, driving the catenane/macrocycle equilibria to the side of macrocycles. When the polymethylene units in the bisaldehyde compounds are replaced by a p-xylene unit, the putative [1 + 1] condensed ring is too large to fit within the ring of CB[8]. Instead, an ultra-large macrocycle was self-assembled in a [2 + 2] manner, which was encircled by two CB[8] rings that act as the external templates. The framework of the ultra-large ring contains more than one hundred atoms, whose synthesis would be thermodynamically disfavored in the absence of the external template, namely CB[8]. We envision that the success in formation of a ring-in-rings complex would be taken advantage of in the synthesis of more complex architectures, such as Borromean rings. Such trials are ongoing in our laboratory.Data availability
Data for this paper, including the synthesis, structural characterization are available at ESI.†Author contributions
Conceptualization: Hao Li, Qiong Chen. Data curation: Qiong Chen, Ye Lei. Formal analysis: Qiong Chen, Hao Li. Investigation: Qiong Chen, Ye Lei. Methodology: Qiong Chen, Guangcheng Wu, Yuanjiang Pan. Project administration: Qiong Chen, Qing Li. Resources: Hao Li. Software: Qiong Chen. Supervision: Qiong Chen, Hao Li. Validation: Qiong Chen, Ye Lei. Roles/Writing – original draft: Qiong Chen. Writing – review & editing: Hao Li.Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. L. thanks the National Natural Science Foundation of China (No. 91856116, 21772173 and 21922108), the Natural Science Foundation of Zhejiang Province (No. LR18B020001), as well as the Fundamental Research Funds for the Central Universities (No. 2019FZA3007) for financial support. We thank Ms Ling He, Dr Yaqin Liu, and Prof. Mingxin Yu from the Analysis and Test Platform, Department of Chemistry, Zhejiang University for efficient NMR measurements.Notes and references
- (a) D. J. Cram and J. M. Cram, Science, 1974, 183, 803–809 CrossRef CAS PubMed; (b) D. J. Cram, Angew. Chem., Int. Ed. Engl., 1988, 27, 1009–1020 CrossRef.
- B. Odell, M. V. Reddington, A. M. Z. Slawin, N. Spencer, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1988, 27, 1547–1550 CrossRef.
- (a) M. Asakawa, W. Dehaen, G. L’abbé, S. Menzer, J. Nouwen, F. M. Raymo, J. F. Stoddart and D. J. Williams, J. Org. Chem., 1996, 61, 9591–9595 CrossRef CAS; (b) C. D. Meyer, C. S. Joiner and J. F. Stoddart, Chem. Soc. Rev., 2007, 36, 1705–1723 RSC; (c) Y. Shen, L. Zou and Q. Wang, New J. Chem., 2017, 41, 7857–7860 RSC; (d) G. Wu, C. Y. Wang, T. Jiao, H. Zhu, F. Huang and H. Li, J. Am. Chem. Soc., 2018, 140, 5955–5961 CrossRef CAS PubMed; (e) P. S. Bols, M. Rickhaus, L. Tejerina, H. Gotfredsen, K. Eriksen, M. Jirasek and H. L. Anderson, J. Am. Chem. Soc., 2020, 142, 13219–13226 CrossRef CAS.
- (a) M. C. O'Sullivan, J. K. Sprafke, D. V. Kondratuk, C. Rinfray, T. D. W. Claridge, A. Saywell, M. O. Blunt, J. N. O'Shea, P. H. Beton, M. Malfois and H. L. Anderson, Nature, 2011, 469, 72–75 CrossRef; (b) L. Favereau, A. Cnossen, J. B. Kelber, J. Q. Gong, R. M. Oetterli, J. Cremers, L. M. Herz and H. L. Anderson, J. Am. Chem. Soc., 2015, 137, 14256–14259 CrossRef CAS PubMed; (c) S. Wang, Y. Shen, B. Zhu, J. Wu and S. Li, Chem. Commun., 2016, 52, 10205–10216 RSC; (d) M. D. Peeks, T. D. W. Claridge and H. L. Anderson, Nature, 2017, 541, 200–203 CrossRef CAS PubMed; (e) J. Cremers, R. Haver, M. Rickhaus, J. Q. Gong, L. Favereau, M. D. Peeks, T. D. W. Claridge, L. M. Herz and H. L. Anderson, J. Am. Chem. Soc., 2018, 140, 5352–5355 CrossRef CAS PubMed; (f) P. S. Bols and H. L. Anderson, Acc. Chem. Res., 2018, 51, 2083–2092 CrossRef CAS PubMed.
- (a) H. Li, H. Zhang, A. D. Lammer, M. Wang, X. Li, V. M. Lynch and J. L. Sessler, Nat. Chem., 2015, 7, 1003–1008 CrossRef CAS PubMed; (b) X. Zheng, Y. Zhang, G. Wu, J. R. Liu, N. Cao, L. Wang, Y. Wang, X. Li, X. Hong, C. Yang and H. Li, Chem. Commun., 2018, 54, 3138–3141 RSC; (c) Y. Zhang, X. Zheng, N. Cao, C. Yang and H. Li, Org. Lett., 2018, 20, 2356–2359 CrossRef CAS PubMed; (d) G. Wu, C.-Y. Wang, T. Jiao, H. Zhu, F. Huang and H. Li, J. Am. Chem. Soc., 2018, 140, 5955–5961 CrossRef CAS PubMed; (e) C.-Y. Wang, G. Wu, T. Jiao, L. Shen, G. Ma, Y. Pan and H. Li, Chem. Commun., 2018, 54, 5106–5109 RSC; (f) Q. Chen, L. Chen, C.-Y. Wang, T. Jiao, Y. Pan and H. Li, Chem. Commun., 2019, 55, 13108–13111 RSC; (g) H. Wang, S. Fang, G. Wu, Y. Lei, Q. Chen, H. Wang, Y. Wu, C. Lin, X. Hong, S. K. Kim, J. L. Sessler and H. Li, J. Am. Chem. Soc., 2020, 142, 20182–20190 CrossRef CAS PubMed.
- (a) F. B. L. Cougnon, K. Caprice, M. Pupier, A. Bauza and A. Frontera, J. Am. Chem. Soc., 2018, 140, 12442–12450 CrossRef CAS PubMed; (b) K. Caprice, M. Pupier, A. Bauza, A. Frontera and F. B. L. Cougnon, Angew. Chem., Int. Ed., 2019, 58, 8053–8057 CrossRef CAS PubMed; (c) I. Neira, A. Blanco-Gomez, J. M. Quintela, C. Peinador and M. D. García, Org. Lett., 2019, 21, 8976–8980 CrossRef CAS PubMed; (d) A. Blanco-Gómez, Á. Fernández-Blanco, V. Blanco, J. Rodríguez, C. Peinador and M. D. García, J. Am. Chem. Soc., 2019, 141, 3959–3964 CrossRef PubMed; (e) A. Blanco-Gómez, I. Neira, J. L. Barriada, M. Melle-Franco, C. Peinador and M. D. García, Chem. Sci., 2019, 10, 10680–10686 RSC; (f) K. Caprice, A. Aster, F. B. L. Cougnon and T. Kumpulainen, Chem.–Eur. J., 2020, 26, 1576–1587 CrossRef CAS PubMed.
- (a) S.-Y. Kim, I.-S. Jung, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2119–2121 CrossRef CAS; (b) H.-J. Kim, J. Heo, W. S. Jeon, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 1526–1529 CrossRef CAS; (c) W. S. Jeon, A. Y. Ziganshina, J. W. Lee, Y. H. Ko, J.-K. Kang, C. Lee and K. Kim, Angew. Chem., Int. Ed., 2003, 42, 4097–4100 CrossRef CAS PubMed; (d) Y. H. Ko, H. Kim, Y. Kim and K. Kim, Angew. Chem., Int. Ed., 2008, 47, 4106–4109 CrossRef CAS; (e) L. Cera and C. A. Schalley, Chem. Sci., 2014, 5, 2560–2567 RSC; (f) S. J. Barrow, S. Kasera, M. J. Rowland, J. Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed; (g) Y. Shen, Q. Zhou, L. Zou and Q. Wang, ChemistrySelect, 2017, 2, 11977 CrossRef CAS; (h) N. A. Thompson, H. Barbero and E. Masson, Chem. Commun., 2019, 55, 12160–12163 RSC; (i) G. Wu, I. Szabó, E. Rosta and O. A. Scherman, Chem. Commun., 2019, 55, 13227–13230 RSC; (j) H. Wu, Y. Wang, L. O. Jones, W. Liu, B. Song, Y. Cui, K. Cai, L. Zhang, D. Shen, X.-Y. Chen, Y. Jiao, C. L. Stern, X. Li, G. C. Schatz and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 16849–16860 CrossRef CAS PubMed; (k) X.-K. Ma, W. Zhang, Z. Liu, H. Zhang, B. Zhang and Y. Liu, Adv. Mater., 2021, 2007476 CrossRef CAS PubMed; (l) H. Barbero and E. Masson, Chem. Sci., 2021, 12, 9962–9968 RSC.
- (a) S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef; (b) L. Wang, M. O. Vysotsky, A. Bogdan, M. Bolte and V. Böhmer, Science, 2004, 304, 1312–1314 CrossRef CAS PubMed; (c) H. Y. Au-Yeung, G. D. Pantoş and J. K. M. Sanders, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10466–10470 CrossRef CAS PubMed; (d) Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC; (e) G. Zhang, O. Presly, F. White, I. M. Oppel and M. Mastalerz, Angew. Chem., Int. Ed., 2014, 53, 1516–1520 CrossRef CAS; (f) S. Klotzbach and F. Beuerle, Angew. Chem., Int. Ed., 2015, 54, 10356–10360 CrossRef CAS PubMed; (g) Q. Wang, C. Yu, H. Long, Y. Du, Y. Jin and W. Zhang, Angew. Chem., Int. Ed., 2015, 54, 7550–7554 CrossRef CAS PubMed; (h) Q. Wang, C. Yu, C. Zhang, H. Long, S. Azarnoush, Y. Jin and W. Zhang, Chem. Sci., 2016, 7, 3370–3376 RSC; (i) S. Lee, A. Yang, T. P. Moneypenny and J. S. Moore, J. Am. Chem. Soc., 2016, 138, 2182–2185 CrossRef CAS PubMed; (j) H. Löw, E. Mena-Osteritz and M. von Delius, Chem. Commun., 2019, 55, 11434–11437 RSC; (k) J. Hu, S. K. Gupta, J. Ozdemir and M. H. Beyzavi, ACS Appl. Nano Mater., 2020, 3, 6239–6269 CrossRef CAS PubMed; (l) T. Jiao, G. Wu, Y. Zhang, L. Shen, Y. Lei, C.-Y. Wang, A. C. Fahrenbach and H. Li, Angew. Chem., Int. Ed., 2020, 59, 18350–18367 CrossRef CAS PubMed; (m) F. V. Lijsebetten, J. O. Holloway, J. M. Winne and F. E. D. Prez, Chem. Soc. Rev., 2020, 49, 8425–8438 RSC; (n) A. J. Greeniee, C. I. Wendell, M. M. Cencer, S. D. Laffoon and J. S. Moore, Trends Chem., 2020, 2, 1043–1051 CrossRef; (o) Y. Chen, G. Wu, B. Chen, H. Qu, T. Jiao, Y. Li, C. Ge, C. Zhang, L. Liang, X. Zeng, X. Cao, Q. Wang and H. Li, Angew. Chem., Int. Ed., 2021, 60, 2–8 CrossRef.
- (a) S.-Y. Kim, I.-S. Jung, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2119–2121 CrossRef CAS; (b) A. I. Day, R. J. Blanch, A. P. Arnold, S. Lorenzo, G. R. Lewis and I. Dance, Angew. Chem., Int. Ed., 2002, 41, 275–277 CrossRef CAS; (c) S.-H. Chiu, A. R. Pease, J. F. Stoddart, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2002, 41, 270–274 CrossRef CAS; (d) J. C. Loren, M. Yoshizawa, R. F. Haldimann, A. Linden and J. S. Siegel, Angew. Chem., Int. Ed., 2003, 42, 5702–5705 CrossRef CAS PubMed; (e) T. Iwanaga, R. Nakamoto, M. Yasutake, H. Takemura, K. Sako and T. Shinmyozu, Angew. Chem., Int. Ed., 2006, 45, 3643–3647 CrossRef CAS PubMed; (f) R. S. Forgan, C. Wang, D. C. Friedman, J. M. Spruell, C. L. Stern, A. A. Sarjeant, D. Cao and J. F. Stoddart, Chem.–Eur J., 2012, 18, 202–212 CrossRef CAS PubMed; (g) B. Sun, M. Wang, Z. Lou, M. Huang, C. Xu, X. Li, L. J. Chen, Y. Yu, G. L. Davis, B. Xu, H. B. Yang and X. Li, J. Am. Chem. Soc., 2015, 137, 1556–1564 CrossRef CAS PubMed; (h) J. K. Klosterman, J. Veliks, D. K. Frantz, Y. Yasui, M. Loepfe, E. Zysman-Colman, A. Linden and J. S. Siegel, Org. Chem. Front., 2016, 3, 661–666 RSC; (i) Y. Lu, H. N. Zhang and G. X. Jin, Acc. Chem. Res., 2018, 51, 2148–2158 CrossRef CAS PubMed; (j) M. C. Lipke, Y. Wu, I. Roy, Y. Wang, M. R. Wasielewski and J. F. Stoddart, ACS Cent. Sci., 2018, 4, 362–371 CrossRef CAS PubMed; (k) K. Zhu, G. Baggi and S. J. Loeb, Nat. Chem., 2018, 10, 625–630 CrossRef CAS PubMed; (l) H. Wu, Y. Wang, L. O. Jones, W. Liu, B. Song, Y. Cui, K. Cai, L. Zhang, D. Shen, X.-Y. Chen, Y. Jiao, C. L. Stern, X. Li, G. C. Schatz and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 16849–16860 CrossRef CAS PubMed.
- W. S. Jeon, H.-J. Kim, C. Lee and K. Kim, Chem. Commun., 2002, 17, 1828–1829 RSC.
- K. Baek, Y. Kim, H. Kim, M. Yoon, I. Hwang, Y. H. Ko and K. Kim, Chem. Commun., 2010, 46, 4091–4093 RSC.
- S. Moghaddam, C. Yang, M. Rekharsky, Y. H. Ko, K. Kim, Y. Inoue and M. K. Gilson, J. Am. Chem. Soc., 2011, 133, 3570–3581 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2014868, 2095808, 2096625 and 2096626. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06236k |
| This journal is © The Royal Society of Chemistry 2022 |