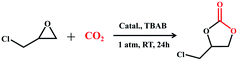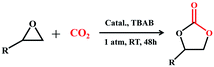Open Access Article
Open Access ArticleStepwise assembly and reversible structural transformation of ligated titanium coated bismuth-oxo cores: shell morphology engineering for enhanced chemical fixation of CO2†
Qing-Rong
Ding
ab,
Yinghua
Yu
a,
Changsheng
Cao
 a,
Jian
Zhang
a,
Jian
Zhang
 a and
Lei
Zhang
a and
Lei
Zhang
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: Lzhang@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 1st February 2022
Abstract
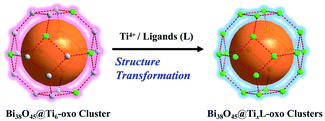
Herein, we report the stepwise assembly and reversible transformation of atomically precise ligated titanium coated bismuth-oxide core nanostructures. The soluble and stable Bi38O45@Ti6-oxo clusters with weakly coordinated surface salicylate ligands were first prepared as precursors. Owing to the high surface reactivity of the Bi38O45 inner core, its shell composition and morphology could be systemically modified by assembly with various Ti ions and auxiliary ligands (L), especially those with different flexibility, bridging ability and steric hindrance. As a result, a series of new core–shell Bi38O44/45@TixL-oxo (x = 14, 16, 18 or 20) clusters containing gradually increasing shell Ti atoms were successfully synthesized. Among them, the Bi38Ti20-oxo cluster is the largest one in the family of heterometallic Bi/Ti-oxo clusters to date. In addition, the sensitized titanium outer shell can effectively improve the photocurrent response under visible light irradiation. More remarkably, the obtained core–shell Bi38O44/45@TixL-oxo clusters can serve as stable and efficient catalysts for CO2 cycloaddition with epoxides under ambient conditions, whose activity was significantly influenced by the outer ligated titanium shell structure. This work provides a new insight into the construction of atomically precise heterometallic core–shell nanostructures and also an interesting shell engineering strategy for tuning their physicochemical properties.
Introduction
Core–shell nanostructures have attracted a lot of research interest in chemical and materials science due to their improved physicochemical performances in comparison with individual components.1,2 In particular, the active interfaces between the inner core and outer shell layers can facilitate outstanding synergistic functions to provide a variety of novel applications in catalysis, batteries, supercapacitors and photonics.3–6 In the past few decades, there have been numerous core–shell structured materials reported, including metal nanoparticles as typical cores and metal oxides or porous carbon as major shells.7,8 However, the majority of known core–shell structures belong to atomically heterogenous nanosized materials. It is rather difficult to determine and control their precise structures and compositions at the atomic level, which are crucial to the understanding of the structure–activity relationship.9 Therefore, the construction of atomically precise core–shell nanostructures is quite appealing to better achieve the future rational design and performance optimization.10–16As the molecular models of functional metal oxides, atomically precise metal-oxo clusters recently became a prominent research topic.17,18 A growing number of interesting oxo clusters have been prepared, including representative complexes of Mo,19 W,20,21 V,22 Nb,23 Ti,24–26 Sn,27 Pd28,29 and so on. The construction of metal-oxo clusters not only provides opportunities for mechanism research, but also sometimes brings unprecedented functionalities.30,31 Moreover, the precise structural information of metal-oxo clusters also makes it possible to utilize them to construct atomically accurate core–shell structures. Indeed, we have just successfully used hollow titanium-oxo clusters to encapsulate and stabilize silver nanoclusters, resulting in several core–shell Agn@Tim-oxo structures.32,33 Therefore, by changing the core/shell compositions, there will be a very broad space to build metal-oxo cluster-based core–shell nanostructures.
On the other hand, it has been well confirmed that the construction of metal oxide heterojunctions, e.g. Bi2O3–TiO2, can dramatically improve their catalytic activities.34 Accordingly, no matter from the perspective of new structures or new functions, molecular BinOx@TimOy core–shell nanostructures would be of great research value. Unfortunately, although both Bi- and Ti-oxo clusters are widely reported and bismuth was ever applied to stabilize Ti-oxo sulfate clusters by Nyman and co-workers,17,24,35–38 the core–shell assembly between them is still quite challenging.
Recently, we have found that the Ti(SAC)3 (H2SAC = salicylic acid) moieties can act as versatile metalloligands to stabilize Ag clusters.39 Similarly, these Ti(SAC)3 species might also be applied to coat multinuclear Bi-oxo cores to form the desired core–shell nanostructures. Following this consideration, herein we successfully prepared the first core–shell Bi–Ti oxo cluster, [Ti6Bi38O45(SAC)18(HSAC)12]·2(H2O)·4(NBA)·11(DMF) (NBA = 1-butanol) (PTC-281). Single crystal X-ray diffraction analysis indicates that PTC-281 contains a Bi38O45 core which is surrounded by 12 weakly coordinated HSAC ligands and six Ti(SAC)3 moieties. More interestingly, the shell of PTC-281 has high surface reactivity, making it an ideal candidate for the stepwise assembly of core–shell Bi38O45@TixL-oxo structures with different numbers of shell titanium atoms (Scheme 1). Thereupon, another six Bi38O44/45@TixL-oxo clusters with 14, 16, 18 and 20 titanium atoms and various auxiliary ligands (L) surrounding the Bi38-oxo core were successfully synthesized (Table 1). In addition, interesting reversible structural transformation has been discovered between some Bi38O44@TixL-oxo clusters, further confirming the shell modifiability of these core–shell structures. Finally, the sensitized Ti shells result in different visible-light driven photocurrent responses and distinct catalytic activities towards the coupling reaction of epoxides with CO2.
| Complex | Formula | Core | Shell | |
|---|---|---|---|---|
| Ti(SAC)3 | Others components | |||
| a Abbreviations: HNBA = 1-butanol; PhOH = phenol; DMF = dimethylformamide; H2DEA = diethanolamine; H2DPC = cis-4-cyclohexene-1,2-dicarboxylic acid; H4DTPP = di(trimethylolpropane); H2DMT = 2,2′-biphenol. | ||||
| PTC-281 | [Ti6Bi38O45(SAC)18(HSAC)12]·2(H2O)·4(NBA)·11(DMF) | Bi38O45 | 6 | 12× HSAC |
| PTC-282 | H2[Ti14Bi38O50(SAC)30(EtO)12]·12(DMF) | Bi38O44 | 2 | 6× [Ti2O(SAC)4(EtO)2] |
| PTC-283 | [Ti16Bi38O51(SAC)28(HSAC)2(DEA)4(PhO)10]·2(CH3CN) | Bi38O45 | 2 | 2× [Ti3O(SAC)5(DEA)(PhO)2]; 2× [Ti4O(SAC)6(DEA)(PhO)3] |
| PTC-284 | [Ti16Bi38O44(SAC)34(DPC)6(PhO)8]·4(CH3CN) | Bi38O44 | 2 | 2× [Ti3(SAC)6(DPC)2(PhO)]; 2× [Ti4(SAC)8(DPC)(PhO)3] |
| PTC-285 | [Ti18Bi38O50(SAC)24(DTPP)6(DMT)6][OiPr]2·12(DMF) | Bi38O44 | 6 | 6× [Ti2O(SAC)(DTPP)(DMT)] |
| PTC-286 | H2[Ti20Bi38O50(SAC)30(DTPP)6(CH3O)12]·4(DMF) | Bi38O44 | 8 | 6× [Ti2O(SAC)(DTPP)(CH3O)2] |
Results and discussion
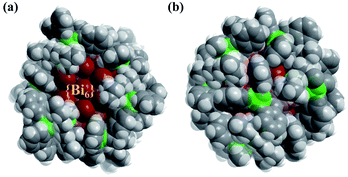
Yellow crystals of PTC-281 were synthesized by the reaction of bismuth subsalicylate, salicylic acid and Ti(OiPr)4 in a mixed DMF and NBA solution at 80 °C for three days. Single-crystal X-ray diffraction (SXRD) analysis revealed that PTC-281 crystallized in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The core structure of PTC-281 is similar to the previously reported bismuth-oxo clusters, [Bi38O45(hfac)24] (Hhfac = hexafluoroacetylacetone)40 and [Bi38O44(HSAC)26(Me2CO)16(H2O)2]·(Me2CO)4.41 As shown in Fig. 1, the 38 bismuth atoms from the Bi38O45 inner core of PTC-281 form a triple nested structure, Bi6@Bi8@Bi24. Thus, the outermost Bi24 is a twisted truncated octahedron consisting of eight hexagonal {Bi6} planes and six quadrilateral {Bi4} planes. Among them, six hexagonal {Bi6} planes are each coordinated by a Ti(SAC)3 metalloligand with calixarene-like arrangement filled by coordination-active oxygen sites (Fig. S2†), and the remaining two {Bi6} planes are each coordinated by three HSAC ligands. Meanwhile, all six {Bi4} planes are each capped by one HSAC ligand. In order to clarify the position relationship between the outer shell Ti atoms and Bi38O45 inner core, an unfolded diagram of the Bi24 truncated octahedron is also given in Fig. 1. Clearly, six Ti atoms are each distributed on the hexagonal planes of the expanded truncated octahedron. The Ti and Bi components were further confirmed by inductively coupled plasma (ICP) analysis (Table S5†).
space group. The core structure of PTC-281 is similar to the previously reported bismuth-oxo clusters, [Bi38O45(hfac)24] (Hhfac = hexafluoroacetylacetone)40 and [Bi38O44(HSAC)26(Me2CO)16(H2O)2]·(Me2CO)4.41 As shown in Fig. 1, the 38 bismuth atoms from the Bi38O45 inner core of PTC-281 form a triple nested structure, Bi6@Bi8@Bi24. Thus, the outermost Bi24 is a twisted truncated octahedron consisting of eight hexagonal {Bi6} planes and six quadrilateral {Bi4} planes. Among them, six hexagonal {Bi6} planes are each coordinated by a Ti(SAC)3 metalloligand with calixarene-like arrangement filled by coordination-active oxygen sites (Fig. S2†), and the remaining two {Bi6} planes are each coordinated by three HSAC ligands. Meanwhile, all six {Bi4} planes are each capped by one HSAC ligand. In order to clarify the position relationship between the outer shell Ti atoms and Bi38O45 inner core, an unfolded diagram of the Bi24 truncated octahedron is also given in Fig. 1. Clearly, six Ti atoms are each distributed on the hexagonal planes of the expanded truncated octahedron. The Ti and Bi components were further confirmed by inductively coupled plasma (ICP) analysis (Table S5†).
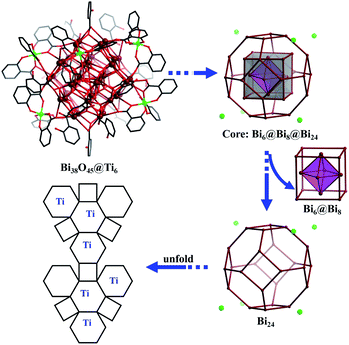 | ||
| Fig. 1 Molecular structure and structural evolution with unfolded diagrams of PTC-281. Color codes: dark red Bi; green Ti; black C; red O. H atoms are omitted for clarity. | ||
The crystals of PTC-281 exhibit good solubility in DMF solvent with a saturated solubility of 12.5 g L−1 at 80 °C. Interestingly, a yellow rod-like crystal could be obtained in one day, named PTC-281R (Fig. S4†), after cooling to room temperature. X-ray diffraction analysis indicated that PTC-281R presented the same Bi38O45@Ti6 structure as PTC-281, confirming the high solution stability of the clusters in PTC-281. In addition, scale up synthesis of PTC-281 was realized by increasing the amounts of reactants (>25 g, Fig. S5†), which could provide sufficient samples for the subsequent stepwise assembly studies.
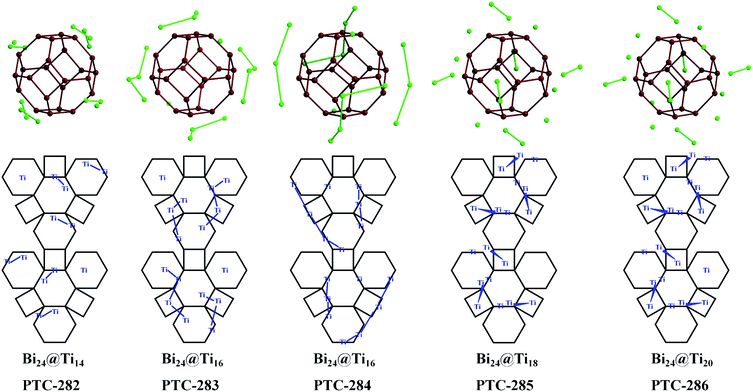
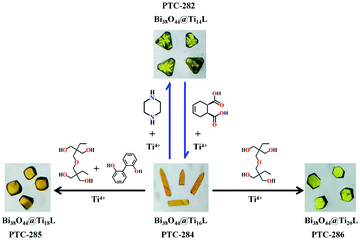
Based on the excellent solubility and stability of the Bi38O45@Ti6 clusters in organic solvents, their stepwise assembly with additional titanium ions and different auxiliary ligands was then investigated. At the beginning, salicylic acid, piperazine and Ti(OiPr)4 were added to the DMF/EAC solution of PTC-281, which was then heated at 80 °C for one night, forming yellow block crystals of PTC-282 (Fig. 2). Structural analysis revealed that PTC-282 also contains a similar Bi38O44 cluster inner core, but 8 more Ti atoms are present in the outer shell. Different from PTC-281, in the structure of PTC-282, 24 SAC ligands are completely deprotonated to coordinate to form 12 Ti(SAC)2 moieties, and then further connected together by a μ3-O atom and one μ2-EtO molecule to form a binuclear [Ti2O(SAC)4(EtO)2] unit (Fig. S6a†). As shown in Fig. 3, six such [Ti2O(SAC)4(EtO)2] units decorate six {Bi6} hexagons, and the remaining two {Bi6} planes are occupied by two Ti(SAC)3 metalloligands, giving rise to the total 6 × 2 + 2 = 14 Ti atoms.
 | ||
| Fig. 2 Reaction scheme of the stepwise assembly from PTC-281 to the Bi38O44/45@TixL-oxo cluster family, highlighting the outer shell molecular compositions of PTC-282 to PTC-286. | ||
To capture more Ti atoms in the outer shell, the steric hindrance and bridging ability of the surface ligands should be considered. Therefore, more Bi38O45@TixL-oxo assembly procedures were studied by selecting small flexible ligands with polyhydroxy groups. Following this idea, the diethanolamine (DEA) ligand was introduced into the reaction of salicylic acid and Ti(OiPr)4 in a CH3CN/phenol solution of PTC-281, giving rise to PTC-283 (Fig. 2). Compared with PTC-282, two more Ti atoms were incorporated, increasing the shell composition of PTC-283 to 16 titanium atoms. The shell structure of PTC-283 is composed of three kinds of subunits: two Ti(SAC)3 metalloligands, two trinuclear [Ti3O(SAC)5(DEA)(PhO)2] moieties and two tetranuclear [Ti4O(SAC)6(DEA)(PhO)3] moieties, all of which are symmetrically distributed (Table 1 and Fig. S7†). It's worth noting that the {Ti3} subunit appears in a triangular arrangement across three adjacent planes (two {Bi6} and one {Bi4}), while the {Ti4} subunit is arranged in a Z shape across four adjacent planes (three {Bi6} and one {Bi4}) (Fig. 3).
In addition, when cis-4-cyclohexene-1,2-dicarboxylic acid (H2DPC) was applied instead of DEA, red crystals of PTC-284 were synthesized, whose shell also contains 16 Ti atoms (Table 1 and Fig. S9†). Interestingly, a Ti3+ ion was found in the structure of PTC-284, as confirmed by bond valence sum calculation studies and a strong signal at g = 1.98 in the ESR spectrum (Table S4 and Fig. S10†).42 There are 48 surface ligands in the shell of PTC-284, which are more than the 30 surface ligands for PTC-280, 32 surface ligands for PTC-281, 42 surface ligands for PTC-282 and 44 surface ligands for PTC-283 (Table 1). Therefore, in order to accommodate these ligands, as shown in Fig. 3, all the titanium atoms in PTC-284 were shifted to the edges of the hexagonal {Bi6} planes and quadrilateral {Bi4} planes.
It is interesting that when larger DTPP and DMT ligands were simultaneously used, PTC-285 was obtained whose outer shell holds 18 Ti atoms and 42 surface ligands (Fig. 2 and Table 1). Due to the steric hindrance of the larger DTPP and DMT ligands, the arrangement of Ti atoms in PTC-285 is quite unique, with six binuclear [Ti2O(SAC)(DTPP)(DMT)] units anchored to the six quadrilateral {Bi4} planes of the Bi38O44 core in a head-to-head fashion (Fig. S12a†), while, the Ti atoms in PTC-280 to PTC-284 all adopt a side-by-side fashion. Herein, the head-to-head anchored fashion in PTC-285 makes one Ti atom from the binuclear unit protrude out of the {Bi4} plane to overcome the steric hindrance effect (Fig. 3). In addition, there are six more Ti(SAC)3 metalloligands to coordinate six hexagonal {Bi6} planes, forming the total 6 × 2 + 6 = 18 Ti atoms in the outer shell of PTC-285. It is worth noting that there are still two {Bi6} planes unoccupied by any ligands, showing the potential to accommodate more Ti atoms.
Fortunately, yellow block crystals of PTC-286 with two more shell Ti atoms were obtained by using less methanol instead of DMT (Fig. 2). As expected, the shell of PTC-286 is similar to that of PTC-285, with six quadrilateral {Bi4} planes also anchored by six binuclear [Ti2O(SAC)(DTPP)(CH3O)2] units in a head-to-head fashion (Fig. S13†). Attributed to the weakening of the steric hindrance effect in the outer shell, all eight hexagonal {Bi6} planes of PTC-286 are coordinated by Ti(SAC)3 metalloligands, giving rise to the total 6 × 2 + 8 = 20 Ti atoms in the shell.
From the above results, the robustness of the Bi38-oxo inner core and the variability of its outer shell composition have been clearly confirmed. Such unique characteristics might also produce post-synthetic structural transformation between the obtained Bi38O44@TixL-oxo core–shell clusters. To further verify this point, the assembly behavior of PTC-284 was studied (Fig. 4). A series of stepwise assembly reactions of PTC-284 were carried out, giving rise to crystals with structures of PTC-282, PTC-285 and PTC-286 (Fig. S15c, S16b and c†). More interestingly, some of these structural transformations are reversible, like those between PTC-284 and PTC-282 (Fig. S16a†), while, the transformations from PTC-284 to PTC-285 and PTC-286 only happen in one direction. Such differences might be attributed to the different coordination abilities of the corresponding auxiliary ligands. These results fully confirm that the obtained Bi38O44@TixL-oxo core–shell clusters have very rich assembly chemistry, which could be used as versatile platforms for new structures and functionalities.
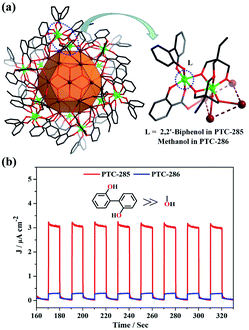
The solid-state UV-vis absorption spectra of these complexes display intense absorption in the visible region, and the calculated optical bandgaps are in the range from 2.00 eV to 2.34 eV (Fig. S20 to S22†). Based on these results, the visible-light driven photocurrent responses of the Bi38O44/45@TixL-oxo core–shell clusters were measured, and the measurements were carried out in a 0.20 mol L−1 Na2SO4 electrolyte solution under on-off cycling irradiation by UV-vis light with a 420 nm cutoff filter (intervals of 10 s). As shown in Fig. 5b and S23,† upon repetitive irradiation, steady photocurrent responses were observed for all the complexes used. Notably, for PTC-285 and PTC-286 with the same DTPP ligand, when the surface methanol molecules were replaced by the DMT ligand (Fig. 5a), the photocurrent density greatly increased (Fig. 5b). Thus, PTC-285 (DMT) exhibited the optimal photocurrent response among all the Bi38O44/45@TixL-oxo clusters, and its current density value reached 3.23 μA cm−2 which was about 11.54 times that of PTC-286 (methanol, 0.28 μA cm−2). Therefore, the DMT ligand can be considered as more photosensitive to better harvest visible light.24
It is worth mentioning that some Bi atoms in the above Bi38O44/45@TixL core–shell clusters are exposed to the surface, e.g. the hexagonal {Bi6} planes in PTC-285, making them possible Lewis acid sites for catalytic reactions (Fig. 6a).43 To explore this hypothesis, the transformation of CO2 with epoxides was chosen as a model reaction. The experiments were carried out at room temperature and 1 atm pressure in the presence of a 0.5 mol‰ catalyst combined with tetrabutylammonium bromide, and the yields of these compounds range from 63% to 86%, indicating that they exhibit different catalytic activities. Among them, PTC-285 with exposed {Bi6} sites indeed showed the best yield of 86% (Table 2, entry 1). As for PTC-286 with a deeply embedded Bi38O44 inner core and all occupied {Bi6} planes (Fig. 6b), it should be more difficult to expose surface Bi sites, producing a much lower yield than PTC-285 (Table 2, entry 6). Therefore, the outer shell structures of the Bi38O44/45@TixL clusters have significant influence on their catalytic activities towards chemical CO2 fixation.
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reaction conditions: epichlorohydrin (10 mmol), catalysts (0.005 mmol), nBu4NBr (1 mmol), room temperature, 24 h, CO2 (1 atm gauge pressure). b Total yield was determined by 1H NMR spectroscopy using dibromomethane as an internal standard (Fig. S31 to S36). | ||
| 1 | PTC-285 | 86 |
| 2 | PTC-282 | 84 |
| 3 | PTC-281 | 76 |
| 4 | PTC-284 | 75 |
| 5 | PTC-283 | 70 |
| 6 | PTC-286 | 63 |
| 7 | Blank | 44 |
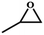
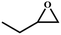
To further explore optimal catalytic performance and acquire some mechanistic information, PTC-285 and PTC-286 were selected as representatives for detailed studies. It was reported that the conversion of epoxides into cyclic carbonates could be effectively improved by increasing the reaction temperature and pressure or prolonging the reaction time.44–47 Indeed, although carried out under room temperature and 1 atm pressure, the yields of propylene carbonate and 1,2-butylene carbonate by PTC-285 could still be increased to 99% and 95% by prolonging the reaction time to 48 hours (Table 3, entry 1, 3). In addition, the size effect of the substrate was investigated. When the larger styrene oxide was applied, PTC-285 showed lower catalytic activity to give only 60% yield of phenylethylene carbonate (Table 3, entry 5); while with the largest substrate of cyclohexene oxide, the lowest product yield of 30% was obtained (Table 3, entry 7). Such an obvious steric effect further indicates that the epoxide substrates used need to go through the TixL outer shell to approach the Bi active sites on the inner Bi38-oxo core. Correspondingly, lower yields were observed for larger substrates due to steric hindrance. Moreover, as shown in Table 3, when PTC-285 was replaced by PTC-286, the yields of all four carbonates were greatly reduced, further indicating that the catalytic activity of PTC-285 is much better than that of PTC-286. From the above structural analysis, the most significant difference between PTC-285 and PTC-286 is that PTC-285 holds surface exposed {Bi6} sites. Therefore, these results further confirm the effect of shell morphology on the catalytic activities.
| Entry | Catalyst | Epoxides | Products | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: epoxides (10 mmol), catalysts (0.005 mmol), nBu4NBr (1 mmol), room temperature, 48 h, CO2 (1 atm gauge pressure). b Total yield was determined by 1H NMR spectroscopy using dibromomethane as an internal standard (Fig. S37 to S44). | ||||
| 1 | PTC-285 |
|
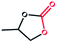
|
99 |
| 2 | PTC-286 | 62 | ||
| 3 | PTC-285 |
|

|
95 |
| 4 | PTC-286 | 60 | ||
| 5 | PTC-285 |
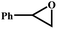
|

|
60 |
| 6 | PTC-286 | 32 | ||
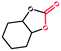
| 7 | PTC-285 |
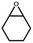
|
|
30 |
| 8 | PTC-286 | 10 | ||
The catalytic stability of the Bi38O44/45@TixL-oxo core–shell clusters used was also studied. The solution-state UV-vis spectra and solid-state IR spectra of these catalysts before and after catalytic studies were basically unchanged (Fig. S25 to S29†). Furthermore, the sample of PTC-285 could be reused three successive times which maintained its catalytic performance to a large extent (Fig. S30†). These results demonstrated the high stability of the applied Bi38O44/45@TixL-oxo cluster catalysts.
According to the reported mechanism of the cycloaddition of epoxides with CO2,43,48 we also proposed a tentative reaction mechanism for our studies (Fig. S30†). First, epoxides are activated by the exposed Lewis acidic Bi sites in the Bi38-oxo inner core. Subsequently, Br− from TBAB attacks the coordinated epoxide to promote its ring opening effectively. Finally, CO2 around the active sites attacks the activated C atom and Br− is removed to complete the catalytic reaction.
Conclusions
In summary, a core–shell Bi38O45@Ti6-oxo cluster with high solubility and solution stability in organic solvents was prepared and applied as a versatile precursor for the stepwise assembly of a series of Bi38O44/45@TixL-oxo core–shell nanostructures. Attributed to its high surface reactivity, the outer ligated titanium shell composition has been successfully increased to 14, 16, 18 and 20 Ti atoms with the assistance of auxiliary ligands with different flexibility, coordination behavior and geometric effects. Furthermore, interesting reversible structural transformation has been realized between some Bi38O44@TixL-oxo clusters, further confirming the shell modifiability of these core–shell structures. In addition, the sensitized Ti outer shell showed a great effect on their physicochemical properties, including excellent visible-light driven photocurrent responses and enhanced catalytic activities for the coupling reaction between epoxides and CO2 under normal temperature and pressure. Especially, the outer ligated titanium shell structures determined the exposure degree of Bi active sites and shuttle behavior of epoxide substrates, resulting in steric hindrance dependent product yields. Therefore, an effective stepwise strategy has been successfully established for the assembly of atomically precise BinOx@TimOy core–shell nanostructures, which could be extended to the construction of more core–shell materials with tunable composition and optimizable applications.Data availability
All experimental supporting data and procedures are available in the ESI.†Author contributions
L. Z. designed the study and supervised the project. Q. D., Y. Y., and C. C. performed the experiments. All the authors discussed the results and co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21922111 and 91961108).Notes and references
- R. G. Chaudhuri and S. Paria, Chem. Rev., 2012, 112, 2373–2433 CrossRef PubMed.
- S. Das, J. Pérez-Ramírez, J. Gong, N. Dewangan, K. Hidajat, B. C. Gates and S. Kawi, Chem. Soc. Rev., 2020, 49, 2937–3004 RSC.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Chem. Rev., 2020, 120, 1184–1249 CrossRef CAS PubMed.
- Z. Yi, Q. Han, P. Zan, Y. Cheng, Y. Wu and L. Wang, J. Mater. Chem. A, 2016, 4, 12850–12857 RSC.
- M. Wang, Z. Li, C. Wang, R. Zhao, C. Li, D. Guo, L. Zhang and L. Yin, Adv. Funct. Mater., 2017, 27, 1701014–1701023 CrossRef.
- D. Huo, M. J. Kim, Z. Lyu, Y. Shi, B. J. Wiley and Y. Xia, Chem. Rev., 2019, 119, 8972–9073 CrossRef CAS PubMed.
- X. Liu, J. Iocozzia, Y. Wang, X. Cui, Y. Chen, S. Zhao, Z. Li and Z. Lin, Energy Environ. Sci., 2017, 10, 402–434 RSC.
- X. Xia, Y. Zhang, Z. Fan, D. Chao, Q. Xiong, J. Tu, H. Zhang and H. J. Fan, Adv. Energy Mater., 2014, 1401709–1401717 Search PubMed.
- C. Hu, R. Chen and N. Zheng, Adv. Mater., 2021, 2006159–2006168 CrossRef CAS PubMed.
- X.-K. Wan, X.-L. Cheng, Q. Tang, Y.-Z. Han, G. Hu, D. Jiang and Q.-M. Wang, J. Am. Chem. Soc., 2017, 139, 9451–9454 CrossRef CAS PubMed.
- Z. Wang, H.-F. Su, C.-H. Tung, D. Sun and L.-S. Zheng, Nat. Commun., 2018, 9, 4407–4417 CrossRef PubMed.
- H. Yang, J. Zhang, M. Luo, W. Wang, H. Lin, Y. Li, D. Li, P. Feng and T. Wu, J. Am. Chem. Soc., 2018, 140, 11189–11192 CrossRef CAS PubMed.
- K. Yonesato, H. Ito, H. Itakura, D. Yokogawa, T. Kikuchi, N. Mizuno, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2019, 141, 19550–19554 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- S.-Z. Zhan, G.-H. Zhang, J.-H. Li, J.-L. Liu, S.-H. Zhu, W. Lu, J. Zheng, S. W. Ng and D. Li, J. Am. Chem. Soc., 2020, 142, 5943–5947 CrossRef CAS PubMed.
- Y. Jin, C. Zhang, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Soc. Rev., 2021, 50, 2297–2319 RSC.
- W.-H. Fang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2018, 47, 404–421 RSC.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 RSC.
- H. N. Miras, G. J. T. Cooper, D.-L. Long, H. Bogge, A. Muller, C. Streb and L. Cronin, Science, 2010, 327, 72–74 CrossRef CAS PubMed.
- C.-H. Zhan, R. S. Winter, Q. Zheng, J. Yan, J. M. Cameron, D.-L. Long and L. Cronin, Angew. Chem., Int. Ed., 2015, 127, 14516–14520 CrossRef.
- S. An, J. C. Liu, H. Zhang, L. Wu and Y. F. Song, Sci. China: Chem., 2019, 62, 159–161 CrossRef CAS.
- L. K. Mahnke, A. Kondinski, U. Warzok, C. Näther, J. Leusen, C. A. Schalley, K. Yu. Monakhov, P. Kçgerler and W. Bensch, Angew. Chem., Int. Ed., 2018, 57, 2972–2975 CrossRef CAS PubMed.
- Y.-L. Wu, X.-X. Li, Y.-J. Qi, H. Yu, L. Jin and S.-T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572–8576 CrossRef CAS PubMed.
- Q.-Y. Zhu and J. Dai, Coord. Chem. Rev., 2021, 430, 213664–213676 CrossRef CAS.
- N. Li, J. Liu, J.-J. Liu, L.-Z. Dong, S.-L. Li, B.-X. Dong, Y.-H. Kan and Y.-Q. Lan, Angew. Chem., Int. Ed., 2019, 58, 17260–17264 CrossRef CAS PubMed.
- C. Zhao, Y.-Z. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16252–16256 CrossRef CAS PubMed.
- S. Saha, D.-H. Park, D. C. Hutchison, M. R. Olsen, L. N. Zakharov, D. Marsh, S. Goberna-Ferrón, R. T. Frederick, J. T. Diulus, N. Kenane, G. S. Herman, D. W. Johnson, D. A. Keszler and M. Nyman, Angew. Chem., Int. Ed., 2017, 56, 10140–10144 CrossRef CAS PubMed.
- S. Bhattacharya, U. Basu, M. Haouas, P. Su, M. F. Espenship, F. Wang, A. Solé-Daura, D. H. Taffa, M. Wark, J. M. Poblet, J. Laskin, E. Cadot and U. Kortz, Angew. Chem., Int. Ed., 2021, 60, 3632–3639 CrossRef CAS PubMed.
- P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed.
- X. Fan, J. Wang, K. Wu, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2019, 58, 1320–1323 CrossRef CAS PubMed.
- G. Zhang, C. Liu, D.-L. Long, L. Cronin, C.-H. Tung, G. Zhang, W. Li, C. Liu, J. Jia, C.-H. Tung and Y. Wang, J. Am. Chem. Soc., 2018, 140, 66–69 CrossRef CAS PubMed.
- S. Chen, W.-H. Fang, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2018, 57, 11252–11256 CrossRef CAS PubMed.
- X. Fan, F. Yuan, D. Li, S. Chen, Z. Cheng, Z. Zhang, S. Xiang, S.-Q. Zang, J. Zhang and L. Zhang, Angew. Chem., Int. Ed., 2021, 60, 12949–12954 CrossRef CAS PubMed.
- M. Ge, C. Cao, S. Li, S. Zhang, S. Deng, J. Huang, Q. Li, K. Zhang, S. S. Al-Deyab and Y. Lai, Nanoscale, 2015, 7, 11552–11560 RSC.
- M. Mehring, Coord. Chem. Rev., 2007, 251, 974–1006 CrossRef CAS.
- K. Chintakrinda, N. Narayanam, Y.-Z. Li, F. Wang, C. Kashi, Q.-H. Li, G. Xu, L. Zhang and J. Zhang, CCS Chem., 2020, 2, 209–215 CrossRef CAS.
- J. C. Liu, Q. Han, L. J. Chen, J. W. Zhao, C. Streb and Y. F. Song, Angew. Chem., Int. Ed., 2018, 57, 8416–8420 CrossRef CAS PubMed.
- P. I. Molina, K. Kozma, M. Santala, C. Falaise and M. Nyman, Angew. Chem., Int. Ed., 2017, 56, 16277–16281 CrossRef CAS PubMed.
- M.-Y. Gao, K. Wang, Y. Sun, D. Li, B.-Q. Song, Y. H. Andaloussi, M. J. Zaworotko, J. Zhang and L. Zhang, J. Am. Chem. Soc., 2020, 142, 12784–12790 CrossRef CAS PubMed.
- E. V. Dikarev, H. Zhang and B. Li, Angew. Chem., Int. Ed., 2006, 45, 5448–5451 CrossRef CAS PubMed.
- P. C. Andrews, G. B. Deacon, C. M. Forsyth, P. C. Junk, I. Kumar and M. Maguire, Angew. Chem., Int. Ed., 2006, 45, 5638–5642 CrossRef CAS PubMed.
- J. Wang, Y. Wang, W. Wang, T. Peng, J. Liang, P. Li, D. Pan, Q. Fan and W. Wu, Environ. Pollut., 2020, 262, 114373–114383 CrossRef CAS PubMed.
- G. Zhai, Y. Liu, L. Lei, J. Wang, Z. Wang, Z. Zheng, P. Wang, H. Cheng, Y. Dai and B. Huang, ACS Catal., 2021, 11, 1988–1994 CrossRef CAS.
- E. López-Maya, N. M. Padial, J. Castells-Gil, C. R. Ganivet, A. Rubio-Gaspar, F. G. Cirujano, N. Almora-Barrios, S. Tatay, S. Navalón and C. Martí-Gastaldo, Angew. Chem., Int. Ed., 2021, 60, 11868–11873 CrossRef PubMed.
- H. Xu, C.-S. Cao, H.-S. Hu, S.-B. Wang, J.-C. Liu, P. Cheng, N. Kaltsoyannis, J. Li and B. Zhao, Angew. Chem., Int. Ed., 2019, 58, 6022–6027 CrossRef CAS PubMed.
- G. Li, X. Sui, X. Cai, W. Hu, X. Liu, M. Chen and Y. Zhu, Angew. Chem., Int. Ed., 2021, 60, 10573–10576 CrossRef CAS PubMed.
- X. M. Kang, Y. Shi, C. S. Cao and B. Zhao, Sci. China: Chem., 2019, 62, 622–628 CrossRef CAS.
- J. Dong, P. Cui, P.-F. Shi, P. Cheng and B. Zhao, J. Am. Chem. Soc., 2015, 137, 15988–15991 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2104507, 2104508, and 2104511–2104515. For ESI and crystallographic data in CIF or other electronic formats see DOI: 10.1039/d1sc06847d |
| This journal is © The Royal Society of Chemistry 2022 |