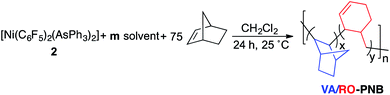Open Access Article
Open Access ArticleA different polynorbornene backbone by combination of two polymer growth pathways: vinylic addition and ring opening via β-C elimination†
Ignacio
Pérez-Ortega
 and
Ana C.
Albéniz
and
Ana C.
Albéniz
 *
*
IU CINQUIMA/Química Inorgánica, Universidad de Valladolid, 47071 Valladolid, Spain. E-mail: albeniz@qi.uva.es
First published on 20th January 2022
Abstract
A new polynorbornene skeleton has been found that contains bicyclic norbornane units and cyclohexenyl methyl linkages. The polymers have been synthesized using a nickel catalyst in the presence of a controlled amount of ligands with low or moderate coordination ability. The backbone structure is the result of a vinylic addition polymerization, via sequential insertions of norbornene into a Ni–C bond (bicyclic units) combined with an unusual ring opening of the norbornene structure by a β-C elimination (cyclohexenyl methyl units) to give a new Ni–C(alkyl) bond that continues the polymerization. The ring opening events are favored when the rate of propagation of the vinylic addition polymerization decreases, and this can be modulated by making the coordination of norbornene to the metal center less favorable using additional ligands.
Introduction
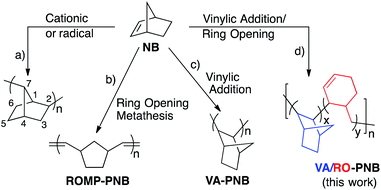
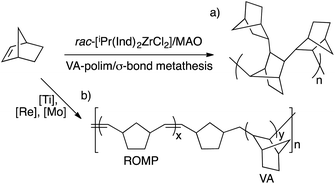
Bicyclo[2.2.1]hept-2-ene or norbornene (NB) can be polymerized in several ways to give different polymer structures (Scheme 1).1 Radical or cationic routes lead to low molecular weight polymers that show a 2,7-linkage of the bicyclic moieties (Scheme 1a). Norbornene and many norbornene derivatives are amenable to ring opening metathesis polymerization, which produces unsaturated polymeric structures (ROMP-PNB, Scheme 1b).2 The vinylic addition polynorbornenes (VA-PNBs) result from a metal-catalyzed double bond insertion polymerization leading to an aliphatic backbone where the bicyclic units are preserved and show an exo-2,3-enchainment (Scheme 1c).3 Both ROMP-PNBs and VA-PNBs can be obtained as high molecular weight polymers with a wide range of applications and commercial availability.4,5 It has been shown that using suitable catalysts the ROMP route can be controlled to synthesize cyclic unsaturated polynorbornenes via what is called ring expansion metathesis polymerization or REMP.6Few exceptions to these polymeric structures have been found and the reported examples combine two types of the known polymeric arrangements in Scheme 1a–c. For example, a few metal catalysts are capable of bringing about the ROMP and VA-polymerization of norbornene by switching during the process between a metal carbene species and a metal alkyl through an α-elimination–readdition process. The outcome is a polymer structure that mixes 2,3-bicyclic moieties and ring opened olefinic units as shown in Scheme 2b.7 A norbornene oligomer was reported by Fink et al. that shows 2,3- and 2,7- enchained bicyclic units. This structure is the result of a σ-bond metathesis between a Zr–C and a C(7)–H bond of the growing polymer chain in a VA-polymerization with a Zr metallocene (Scheme 2a).8
We report here a new type of polynorbornene structure (Scheme 1d) that includes in the polymer backbone both 2, 3-bicyclic and cyclohexenylmethyl fragments via vinylic addition polymerization and a ring opening of norbornene (NB) by β-C elimination, and therefore can be labeled as VA/RO-PNB.
Results and discussion
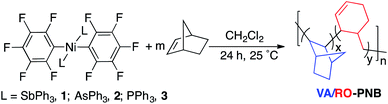
Complexes [Ni(RF)2L2] (RF = C6F5, C6Cl2F3), where L is a labile ligand, are very efficient catalysts in the VA-polymerization of norbornene.9 We have been using for some time [Ni(C6F5)2(SbPh3)2] (1) which is very active in the VA-polymerization of norbornene and some norbornene derivatives such as haloalkylnorbornenes,10b generally much more reluctant to this type of polymerization, as well as in the copolymerization of functionalized norbornenes such as haloalkyl-,10b alkenyl-,10c and stannylated norbornenes,10a with norbornene.4b In the course of these studies, we observed that when the activity of the catalyst was low as, for example, in the copolymerization of substituted norbornenes with carbonyl groups, the polymers obtained in low yields showed an olefinic signal at about 5.7 ppm in the 1H NMR.11 We decided to look into the origin of this unsaturation and we started by carrying out the polymerization of norbornene with complexes [Ni(C6F5)2L2] with ligands of different coordination ability and low NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni mol ratios. Some of the polymers obtained showed the abovementioned unsaturation (Fig. 1a) and, as it is shown in Fig. 1, this signal is not consistent with the presence of ROMP units in the polymer (cf. spectra, Fig. 1a and c).
Ni mol ratios. Some of the polymers obtained showed the abovementioned unsaturation (Fig. 1a) and, as it is shown in Fig. 1, this signal is not consistent with the presence of ROMP units in the polymer (cf. spectra, Fig. 1a and c).
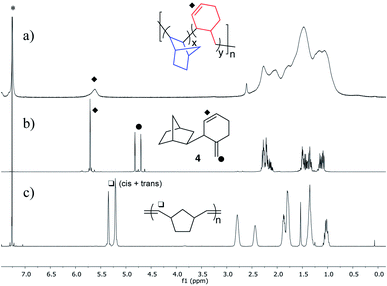 | ||
| Fig. 1 1H NMR (CDCl3) of: (a) VA/RO-PNB (NBVA/NBRO = 7.6/1). (b) dimer 4; (c) ROMP-PNB. *Signal corresponding to the residual solvent. | ||
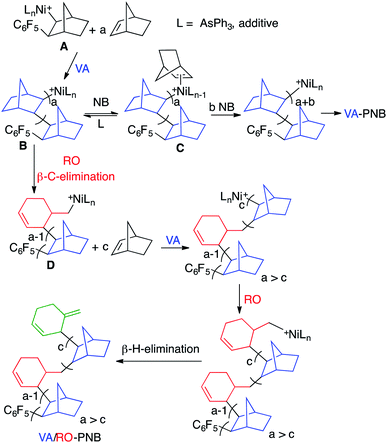
The chemical shifts in the 1H and 13C NMR are in agreement with an endocyclic cyclohexenyl double bond that could result from β-C elimination in a Ni–norbornyl complex during the polymerization as shown in eqn (1). β-C (or β-alkyl) elimination is not as common for group 10 metals as it is for early transition metals.12 However, examples of C–C cleavage by group 10 metal complexes can be found,13 and the process is gaining great importance in the context of norbornene-mediated palladium catalyzed regioselective reactions, that rely on a reversible norbornene insertion into a Pd–C bond.14 In the latter processes the norbornene cyclic structure remains intact and the type of ring opening of norbornene shown in eqn (1) is very scarce in the literature. Catellani et al. reported in 1983 the ring opening by β-C elimination of norbornene after two sequential insertions of norbornene into a Pd–Ar bond.15 Milstein also reported a similar process when studying the Heck reaction using norbornene.16 In the context of the Pd-catalyzed VA-polymerization of an ester derivative of norbornene, Rhodes et al. proposed the occurrence of the ring opening process in eqn (1) followed by β-H elimination to explain the chain termination observed.17 For Ni complexes, only the dimerization of norbornene mediated by an in situ generated nickel hydride has been reported and it leads to 4, shown in Fig. 1b.18 As can be seen when comparing the spectra in Fig. 1a and b, the polymer unsaturation is consistent with the endocyclic double bond of a cyclohexenyl fragment similar to that in dimer 4 synthesized independently. In the polymerization, the cyclohexenyl methyl nickel complex (eqn (1)) undergoes a new monomer insertion instead of a β-H elimination and the exocyclic double bond is not formed.
 | (1) |
Table 1 shows the polymerization experiments with complexes 1–3. The polymerization of norbornene requires a weakly coordinating ligand, i.e. SbPh3 or AsPh3. The more donating PPh3 renders the complex inactive (cf. entries 1, 3 and 6, Table 1). The presence of ring-opened norbornene units (NBRO) was observed when the reactions were carried out at low initial concentration of monomer (entries 2 and 4, Table 1). This effect is more important for the more coordinating AsPh3vs. SbPh3 (cf. entries 2 and 4, Table 1). The amount of cyclohexenylmethyl units (or ring-opened norbornene, NBRO) can be calculated by comparison of the intensity of the olefinic signal at about 5.7 ppm with the aliphatic region in the 1H NMR spectra of the polymers (for details see page S4, ESI†). The composition of the polymers is given in the tables as the ratio of bicyclic units (NBVA) to ring-opened units (NBRO) as well as the percentage of NBRO in the polymers. Table 1 (entry 5) also shows that a decrease in the catalyst amount, using the same initial NB concentration, disfavors the ring opening process. The use of a lower concentration of the nickel complex implies that the concentration of free ligand L, dissociated during the polymerization, is also lower. Therefore, all these results show that the formation of NBRO units is favored by a low initial monomer (NB) concentration and the presence of free AsPh3, dissociated from the nickel complex.
| Entry | [Ni] | m | [NB]0a | NBVA/NBROb | % NBROc | Yield | M w | Đ |
|---|---|---|---|---|---|---|---|---|
| a Initial molar concentration of norbornene (NB). b The mol ratio NBVA/NBRO was calculated by comparison of the integral of the 1H NMR signals (olefinic vs. aliphatic) of the polymer (see ESI). c The mol% of NBRO was calculated from the mol ratio NBVA/NBRO. d M w (Da) and Đ (Mw/Mn) determined by GPC in CHCl3 using polystyrene standards. | ||||||||
| 1 | 1 | 75 | 0.34 | No NBRO | 0% | 90% | 162090 | 4.4 |
| 2 | 1 | 75 | 0.061 | 67/1 | 1.5% | 75% | 49065 | 1.9 |
| 3 | 2 | 75 | 0.34 | No NBRO | 0% | 95% | 138411 | 4.2 |
| 4 | 2 | 75 | 0.061 | 14.3/1 | 6.5% | 67% | 14071 | 2.3 |
| 5 | 2 | 225 | 0.061 | No NBRO | 0% | 74% | 87947 | 1.9 |
| 6 | 3 | 75 | 0.34 | — | — | — | — | — |
The polymers were characterized by NMR (see below and ESI for details†) and they show unimodal distributions in GPC, indicating that they are not mixtures of two types of polymers. A higher amount of NBRO units is associated with a lower yield of the polymerization as well as a lower Mw for the polymer (Table 1), therefore a less efficient polymer growth. Thus, we tested the polymerization in the presence of small amounts of compounds that have shown to hamper the VA-polymerization of norbornene when used as solvents.9b,19 These are coordinating solvents, such as ketones, acetonitrile or amides. Table 2 shows that polymers with up to 12% of NBRO units can be obtained in good or moderate yields using a controlled amount of ketones (acetone or acetophenone) as additives in a polymerization of norbornene with complex 2 (entries 1–5, Table 2). The more coordinating DMA can also be used but a smaller amount is required (entries 8–11, Table 2). The presence of MeCN halts the polymerization. The experiments with the ketones show that there is a direct correlation between the amount of additive and the percentage of NBRO units (entries 2–4, Table 2). Also, a larger amount of NBRO is observed when the coordinating ability of the additive increases (cf. entries 2 and 5, Table 2).20 The effect of the temperature in the polymerization was tested in the small range allowed by the significant catalyst decomposition (T > 45 °C) and the very low conversion at 0 °C. The relative amount of ring opened units (% NBRO) decreases when lowering the reaction temperature (entries 6 and 7, Table 2). The data in Table 2 show that the VA/RO-PNBs have molecular weights in the range 1–3 x 104 Da and Tgs between 150–230 °C. As the percentage of NBRO units increases, a trend toward lower Mws (entries 2–4, Table 2) and lower Tgs is observed (cf. entries 1 and 5, Table 2, for polymers with similar size). The thermogravimetric analysis shows the decomposition of the polymers at about 430 °C with no significant differences between VA/RO-PNBs with different percentage of NBRO units (Fig. S32, ESI†).
| Entry | [NB]0a | m solvent | NBVA/NBROb | % NBROc | Yield | M w | Đ | T g (°C) |
|---|---|---|---|---|---|---|---|---|
| a Initial molar concentration. b The mol ratio NBVA/NBRO was calculated by comparison of the integral of the 1H NMR signals (olefinic vs. aliphatic) of the polymer (see ESI). c The molar% was calculated from the mol ratio NBVA/NBRO. d M w (Da) and Đ (Mw/Mn) determined by GPC in CHCl3 using polystyrene standards. e Determined by DSC. f T = 45 °C; some catalyst decomposition was observed. g T = 12.5 °C. | ||||||||
| 1 | 0.34 | 160 Me2CO | 15.7/1 | 6.0% | 70% | 18769 | 2.4 | 227 |
| 2 | 0.061 | 160 Me2CO | 12.1/1 | 7.6% | 65% | 14302 | 2.2 | |
| 3 | 0.061 | 320 Me2CO | 9.4/1 | 9.6% | 63% | 11770 | 2.6 | 169 |
| 4 | 0.061 | 640 Me2CO | 8.5/1 | 10.5% | 50% | 10900 | 2.3 | 164 |
| 5 | 0.061 | 160 PhMeCO | 7.6/1 | 11.6% | 64% | 17391 | 1.5 | 159 |
| 6f | 0.061 | 160 PhMeCO | 8.1/1 | 10.9% | 50% | 12141 | 2.0 | |
| 7g | 0.061 | 160 PhMeCO | 16.1/1 | 5.8% | 43% | 19297 | 1.8 | |
| 8 | 0.061 | 20 DMA | 7.0/1 | 12.5% | 34% | 12570 | 1.4 | |
| 9 | 0.34 | 20 DMA | 13.9/1 | 6.7% | 74% | 33605 | 1.6 | |
| 10 | 0.34 | 40 DMA | 8.4/1 | 10.6% | 55% | 22366 | 1.7 | |
| 11 | 0.061 | 160 DMA | — | — | 0% | — | — | |
| 12 | 0.34 | 20 MeCN | — | — | 0% | — | — | |
In order to learn about the distribution of NBRO units in the polymer, experiments were carried out in the conditions of entry 5, Table 2, quenching the polymerization at different reaction times, i.e. at different conversions (Table S1, ESI†). Fig. 2a, shows that at short reaction times, the % of NBRO units is low but it increases with time, as the conversion increases and norbornene is consumed. This is consistent with the results in Table 1 (entries 3 and 4) and the experiments shown in Fig. 2b, where low initial NB concentrations favor the ring opening for polymerizations quenched at the same reaction time (Table S2, ESI†). As the reaction progresses the NB concentration also decreases leading to a higher relative ratio of NBRO.
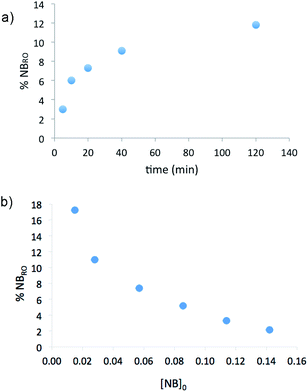 | ||
| Fig. 2 Plot of the amount or ring opened units (% of NBRO) vs. the polymerization reaction time (a) or the initial norbornene concentration (b). | ||
A short polymer was synthesized in order to gather some information about the end-groups of the materials. Using a lower NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni mol ratio in the presence of acetophenone (NB
Ni mol ratio in the presence of acetophenone (NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhCOMe = 5
PhCOMe = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
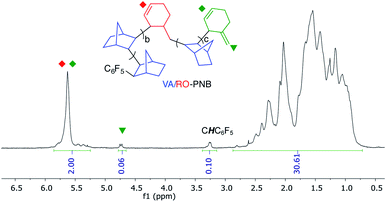
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) a polymer with Mw = 2270 Da was prepared, which contains pentafluorophenyl groups as clearly shown in its 19F NMR spectrum (Fig. S8, ESI†). This indicates that the polymerization initiates by insertion of a norbornene into a Ni–C6F5 bond, as it has been observed for this type of complexes before.9 The 1H NMR spectrum of this polymer also shows a signal corresponding to the CH–C6F5 group at 3.25 ppm, as well as two singlets at about 4.7 ppm characteristic of a terminal exocyclic methylenecyclohexenyl double bond (Fig. 3, S23 and S24, ESI†). This fragment is the result of a β-H elimination in a cyclohexenylmethyl nickel growing polymer chain and it is a termination pathway for the polymerization. However, when comparing the integral value of the CH–C6F5 (chain initiation) in Fig. 3 and the C
15) a polymer with Mw = 2270 Da was prepared, which contains pentafluorophenyl groups as clearly shown in its 19F NMR spectrum (Fig. S8, ESI†). This indicates that the polymerization initiates by insertion of a norbornene into a Ni–C6F5 bond, as it has been observed for this type of complexes before.9 The 1H NMR spectrum of this polymer also shows a signal corresponding to the CH–C6F5 group at 3.25 ppm, as well as two singlets at about 4.7 ppm characteristic of a terminal exocyclic methylenecyclohexenyl double bond (Fig. 3, S23 and S24, ESI†). This fragment is the result of a β-H elimination in a cyclohexenylmethyl nickel growing polymer chain and it is a termination pathway for the polymerization. However, when comparing the integral value of the CH–C6F5 (chain initiation) in Fig. 3 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 resonances (chain termination) it is clear that the terminal olefinic signal is less intense than expected, and therefore the β-H elimination route is important but not the only termination pathway. We could not identify any other end-group that could shed light into it.‡ In these polymerizations the metal stays attached to the growing chain long enough to be trapped by a carbonylation process, indicating that the termination step is slow.21 We quenched a polymerization under the same conditions used for the synthesis of the polymer mentioned above, by bubbling CO through the solution and adding NaOMe in MeOH. The obtained polymer showed characteristic -COOMe resonance in the 1H NMR (3.68 ppm, Fig. S11, ESI†) and a carbonyl resonance at 174 ppm as a cross peak in a long range 1H–13C HMBC NMR experiment. This ester group was also found when a VA-polymerization (conditions of Table 1, entry 1, 15 min) was quenched in the same way (Fig. S16, ESI†).
CH2 resonances (chain termination) it is clear that the terminal olefinic signal is less intense than expected, and therefore the β-H elimination route is important but not the only termination pathway. We could not identify any other end-group that could shed light into it.‡ In these polymerizations the metal stays attached to the growing chain long enough to be trapped by a carbonylation process, indicating that the termination step is slow.21 We quenched a polymerization under the same conditions used for the synthesis of the polymer mentioned above, by bubbling CO through the solution and adding NaOMe in MeOH. The obtained polymer showed characteristic -COOMe resonance in the 1H NMR (3.68 ppm, Fig. S11, ESI†) and a carbonyl resonance at 174 ppm as a cross peak in a long range 1H–13C HMBC NMR experiment. This ester group was also found when a VA-polymerization (conditions of Table 1, entry 1, 15 min) was quenched in the same way (Fig. S16, ESI†).
According to all these data a plausible polymerization scheme can be drawn as depicted in Scheme 3. The polymerization initiates by insertion of norbornene into a Ni–C6F5 bond, as supported by the presence of pentafluorophenyl groups in the polymer. The initiation is slower than the propagation of the polymerization as shown by the high molecular weight of the polymers obtained when compared to the amount of catalyst used. For example, most of the polymerizations were carried out with a mol ratio Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NB = 1
NB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75; if every Ni atom starts a polymer chain the maximum Mw of the polymer should be 7062 Da (i.e. 75 × MWNB, MWNB = 94.16). In all cases the Mw found is higher than expected, so only a fraction of the Ni catalyst, which undergoes the slower initiation, is responsible for the polymer growth.
75; if every Ni atom starts a polymer chain the maximum Mw of the polymer should be 7062 Da (i.e. 75 × MWNB, MWNB = 94.16). In all cases the Mw found is higher than expected, so only a fraction of the Ni catalyst, which undergoes the slower initiation, is responsible for the polymer growth.
The equilibrium between intermediates B and C determines the pathway for the polymer growth. 2,3-Insertion of NB (NBVA units) is favored by a high concentration of intermediate C and this occurs when L is labile and the amount of free ligand is low (i.e. no additive added) as well as when there is a high concentration of NB. If the equilibrium is shifted to intermediate B the insertion is disfavored and the β-C elimination with concomitant ring opening occurs (NBRO units).
This scenario is favored for more coordinating L, higher L concentration and low NB concentration. The latter inevitably occurs as the polymerization progresses and the relative number of NBRO events increases accordingly as we have observed (Fig. 2a). At the end of the polymerization the probability of β-C elimination vs. a new NB insertion is highest so this pathway is a viable termination in the VA-polymerization of norbornene with group 10 metal complexes.17 We have carried out the monitorization of a polymerization reaction in the conditions described above for the synthesis of a low molecular weight VA/RO-PNB (mol ratio NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhCOMe = 5
PhCOMe = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). The kinetic experimental data conforms to the scenario described above as shown by microkinetic modeling using the COPASI software.22 All the details can be found in the ESI (Section 1.10†).
15). The kinetic experimental data conforms to the scenario described above as shown by microkinetic modeling using the COPASI software.22 All the details can be found in the ESI (Section 1.10†).
An increase in the percentage of NBRO units was observed upon increasing the polymerization temperature (entries 6 and 7, Table 2) and this could be rationalized considering the entropic effects affecting the VA and RO routes. The ring opening (β-C elimination) is a unimolecular process and it is reasonable to assume that it will have a small entropy of activation. In contrast the formation of C is a ligand substitution reaction, which in an associative scenario would imply a more ordered transition state (negative entropic term). Thus, an increase in temperature would disfavor the VA route while little affecting the ring opening and therefore increasing the relative amount of NBRO units.
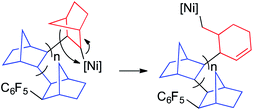
The β-H elimination in the Ni–cyclohexenylmethyl complex D is not very fast and the insertion of a new norbornene molecule in the primary Ni–alkyl bond competes efficiently leading to the incorporation of the ring-opened moiety in the polymer. Because of the sluggish β-H elimination and comparably faster olefin insertion,23 nickel complexes are more convenient to obtain these type of VA/RO-PNBs than palladium complexes. Eventually, at the end of the polymerization the formation of an exocyclic double bond by β-H elimination to give a methylene cyclohexene end group can occur, as we have observed.
Conclusions
A new type of polynorbornene backbone has been found that results from a typical vinylic addition polymerization, leading to bicyclic norbornyl units (NBVA) combined with an unusual ring opening via a β-C elimination, which forms cyclohexenylmethyl units (NBRO). This mixed VA/RO-PNB skeleton appears when the coordination of the monomer to the Ni center used as catalyst is disfavored by lowering the norbornene concentration or by using competing ligands of moderated coordination ability. These results show that the β–γ–C–C cleavage of a nickel bound norbornyl group is facile whereas the β-H elimination in the primary Ni-cyclohexenylmethyl moiety formed is not too fast and further olefin insertions into the Ni–alkyl bond occur. This leads to the incorporation of the ring-opened norbornene fragments into the polymer, and to a new VA/RO-polynorbornene structure.The C–C cleavage in the norbornyl-M fragment is more prevalent at the end of the polymerization and therefore this is a probable termination pathway in the conventional vinylic addition polymerization of norbornene or norbornene derivatives where some of the common termination pathways, such as a direct β-H elimination, are not possible.21
Data availability
Data supporting this article have been uploaded as ESI.†Author contributions
I. P. O. conducted the investigation under A. C. A. supervision. A. C. A. wrote the manuscript and I. P. O prepared the ESI.† All authors contributed to the conceptualization of the project and the review and editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support of the Spanish MICINN (PID2019-111406GB-I00) and the Junta de Castilla y León-FEDER (VA224P20).Notes and references
- R. Madan, A. Srivastava, R. C. Anand and I. K. Varma, Prog. Polym. Sci., 1998, 23, 621–663 CrossRef CAS.
- C. W. Bielawski and R. G. Grubbs, Prog. Polym. Sci., 2007, 32, 1–29 CrossRef CAS.
- (a) F. Blank and C. Janiak, Coord. Chem. Rev., 2009, 253, 827–861 CrossRef CAS; (b) M. V. Bermeshev and P. P. Chapala, Prog. Polym. Sci., 2018, 84, 1–46 CrossRef CAS.
- (a) M. Eo, D. Han, M. H. Park, M. Hong, Y. Do, S. Yoo and M. H. Lee, Eur. Polym. J., 2014, 51, 37–44 CrossRef CAS; (b) R. García-Loma and A. C. Albéniz, Asian J. Org. Chem., 2019, 8, 304–315 CrossRef; (c) X. Wang, T. J. Wilson, D. Alentiev, M. Gringolts, E. Finkelstein, M. Bermeshev and B. K. Long, Polym. Chem., 2021, 12, 2947–2977 RSC.
- ROMP-PNB is commercialized as Norsorex® (oil absorption properties) and VA-PNB as AvatrelTM (dielectric material).
- (a) Y. Xia, A. J. Boydston and R. H. Grubbs, Angew. Chem., Int. Ed., 2011, 50, 5882–5885 CrossRef CAS PubMed; (b) S. A. Gonsales, T. Kubo, M. K. Flint, K. A. Abboud, B. S. Sumerlin and A. S. Veige, J. Am. Chem. Soc., 2016, 138, 4996–4999 CrossRef CAS PubMed; (c) T.-W. Wang, P.-R. Huang, J. L. Chow, W. Kaminsky and M. R. Golder, J. Am. Chem. Soc., 2021, 143, 7314–7319 CrossRef CAS PubMed.
- (a) M. A. Alonso, K. E. Bower, J. A. Johnston and M. F. Farona, Polym. Bull., 1988, 19, 211–216 CrossRef CAS; (b) J. A. Johnston, M. Tokles, G. S. Hatvany, P. L. Rinaldi and M. F. Farona, Macromolecules, 1991, 24, 5532–5534 CrossRef CAS; (c) R. Manivannan, G. Sundararajan and W. Kaminsky, Macromol. Rapid Commun., 2000, 21, 968–972 CrossRef CAS; (d) R. Manivannan, G. Sundararajan and W. Kaminsky, J. Mol. Catal. A: Chem., 2000, 160, 85–95 CrossRef CAS; (e) M. R. Buchmeiser, S. Camadanli, D. Wang, Y. Zou, U. Decker, C. Kühnel and I. Reinhardt, Angew. Chem., Int. Ed., 2011, 50, 3566–3571 CrossRef CAS PubMed; (f) Y. Zou, D. Wang, K. Wurst, C. Kühnel, I. Reinhardt, U. Decker, V. Gurram, S. Camadanli and M. R. Buchmeiser, Chem. –Eur. J., 2011, 17, 13832–13846 CrossRef CAS PubMed.
- C. Karafilidis, H. Hermann, A. Rufiñska, B. Gabor, R. J. Mynott, G. Breitenbruch, C. Weidenthaler, J. Rust, W. Joppek, M. S. Brookhart, W. Thiel and G. Fink, Angew. Chem., Int. Ed., 2004, 43, 2444–2446 CrossRef CAS PubMed.
- (a) D. A. Barnes, G. M. Benedikt, B. L. Goodall, S. S. Huang, H. A. Kalamarides, S. Lenhard, L. H. McIntosh, K. T. Selvy, R. A. Shick and L. F. Rhodes, Macromolecules, 2003, 36, 2623–2632 CrossRef CAS; (b) J. A. Casares, P. Espinet, J. M. Martín-Alvarez, J. M. Martínez-Ilarduya and G. Salas, Eur. J. Inorg. Chem., 2005, 3825–3831 CrossRef CAS.
- (a) N. Carrera, E. Gutiérrez, R. Benavente, M. M. Villavieja, A. C. Albéniz and P. Espinet, Chem. –Eur. J., 2008, 14, 10141–10148 CrossRef CAS PubMed; (b) S. Martínez-Arranz, A. C. Albéniz and P. Espinet, Macromolecules, 2010, 43, 7482–7487 CrossRef; (c) J. A. Molina de la Torre, I. Pérez-Ortega, Á. Beltrán, M. R. Rodríguez, M. M. Díaz-Requejo, P. J. Pérez and A. C. Albéniz, Chem. –Eur. J., 2019, 25, 556–563 CrossRef CAS PubMed.
- J. A. Molina de la Torre and A. C. Albéniz, Eur. J. Inorg. Chem., 2017, 2911–2919 CrossRef CAS.
- M. E. O'Reilly, S. Dutta and A. S. Veige, Chem. Rev., 2016, 116, 8105–8145 CrossRef PubMed.
- (a) R. Noyori and H. Takaya, J. Chem. Soc. D, 1969, 525 RSC; (b) T. Hosokawa and P. M. Maitlis, J. Am. Chem. Soc., 1972, 94, 3238–3240 CrossRef CAS; (c) A. C. Albéniz, P. Espinet and Y. S. Lin, J. Am. Chem. Soc., 1996, 118, 7145–7152 CrossRef; (d) J. Campora, E. Gutierrez-Puebla, J. A. Lopez, A. Monge, P. Palma, D. del Rio and E. Carmona, Angew. Chem., Int. Ed., 2001, 40, 3641–3644 CrossRef CAS; (e) G. Fumagalli, S. Stanton and J. F. Bower, Chem. Rev., 2017, 117, 9404–9432 CrossRef CAS PubMed.
- (a) M. Catellani, F. Frignani and A. Rangoni, Angew. Chem., Int. Ed. Engl., 1997, 36, 119–122 CrossRef CAS; (b) N. Della Ca, M. Fontana, E. Motti and M. Catellani, Acc. Chem. Res., 2016, 49, 1389–1400 CrossRef CAS PubMed; (c) J. Wang and G. Dong, Chem. Rev., 2019, 119, 7478–7528 CrossRef CAS PubMed; (d) H. –G. Cheng, S. Chen, R. Chen and Q. Zhou, Angew. Chem., Int. Ed. Engl., 2019, 58, 5832–5844 CrossRef CAS PubMed.
- M. Catellani and G. P. Chiusoli, J. Organomet. Chem., 1983, 247, C59–C62 CrossRef CAS.
- M. Portnoy, Y. Ben-David, I. Rousso and D. Milstein, Organometallics, 1994, 13, 3465–3479 CrossRef CAS.
- J. McDermott, C. Chun, L. F. Martín, L. F. Rhodes, G. M. Benedikt and R. P. Lattimer, Macromolecules, 2008, 41, 2984–2986 CrossRef CAS.
- A. Tenaglia, A. Terranova and B. Waegell, J. Mol. Catal., 1987, 40, 281–287 CrossRef CAS.
- (a) A. D. Hennis, J. D. Polley, G. S. Long, A. Sen, D. Yandulov, J. Lipian, G. M. Benedikt, L. F. Rhodes and J. Huffman, Organometallics, 2001, 20, 2802–2812 CrossRef CAS; (b) J. K. Funk, C. E. Andes and A. Sen, Organometallics, 2004, 23, 1680–1683 CrossRef CAS; (c) M. Kang and A. Sen, Organometallics, 2004, 23, 5396–5398 CrossRef CAS.
- (a) M. Munakata and S. Kitagawa, Inorg. Chim. Acta, 1990, 169, 225–234 CrossRef CAS; (b) S. Álvarez, Chem. –Eur. J., 2020, 26, 4350–4377 CrossRef PubMed.
- C. Mehler and W. Risse, Macromolecules, 1992, 25, 4226–4228 CrossRef CAS.
- COmplex PAthway Simulator (COPASI) is an easily available free software: S. Hoops, S. Sahle, R. Gauges, C. Lee, J. Pahle, N. Simus, M. Singhal, L. Xu, P. Mendes and U. Kummer, Bioinformatics, 2006, 22, 3067–3074 CrossRef CAS PubMed.
- (a) M. D. Leatherman, S. A. Svejda, L. K. Johnson and M. Brookhart, J. Am. Chem. Soc., 2003, 125, 3068–3081 CrossRef CAS PubMed; (b) H. Xu, P. B. White, C. Hu and T. Diao, Angew. Chem., Int. Ed., 2017, 56, 1535–1538 CrossRef CAS PubMed; (c) H. Xu, C. T. Hu, X. Wang and T. Diao, Organometallics, 2017, 36, 4099–4102 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/d1sc07028b |
| ‡ A termination by involvement of the MeOH used to quench the polymerization could be plausible, via methoxy coordination, β-H elimination to give a Ni–H, and reductive elimination. We carried out several experiment using CD3OD but could not detect the incorporation of deuterium in the polymer. A chain transfer to the monomer via sigma bond metathesis has also been suggested and this aliphatic termination would not be detected in the polymer. |
| This journal is © The Royal Society of Chemistry 2022 |