Open Access Article
Open Access ArticleSodium silylsilanolate as a precursor of silylcopper species†
Hiroki
Yamagishi
 ,
Kenshiro
Hitoshio
,
Kenshiro
Hitoshio
 ,
Jun
Shimokawa
,
Jun
Shimokawa
 * and
Hideki
Yorimitsu
* and
Hideki
Yorimitsu
 *
*
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan. E-mail: shimokawa@kuchem.kyoto-u.ac.jp; yori@kuchem.kyoto-u.ac.jp
First published on 21st March 2022
Abstract
Silylcoppers function as convenient and effective sources of silicon functional groups. Commonly used precursors for those species have been limited to certain symmetric disilanes and silylboranes. This fact renders the development of silylcopper precursors desirable so that more diverse silyl groups could be efficiently delivered. Here we extend the utility of sodium silylsilanolates as competent precursors of silylcoppers. A silanolate unit operates as an auxiliary to transfer a variety of silyl groups to the copper centre, which was demonstrated in the copper-catalysed hydrosilylation of internal alkynes, α,β-unsaturated ketones, and allenes. Our mechanistic studies through DFT calculation suggested that a copper silylsilanolate undergoes intramolecular oxidative addition of the Si–Si bond to the copper centre to generate a silylcopper, in contrast to the typical formal σ-bond metathesis mechanism for conventional disilanes or silylboranes and copper alkoxides. Accordingly, sodium silylsilanolate has been established as an expeditious precursor of a variety of silylcopper species.
Introduction
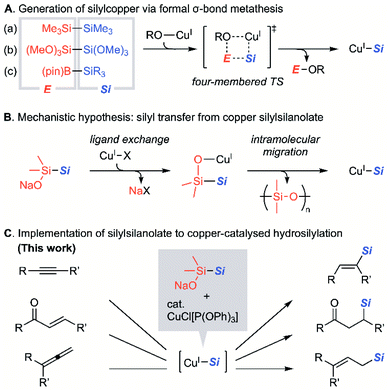
Organosilicon compounds are gathering increased attention in the areas of materials1 and pharmaceutical sciences.2 One of the most versatile reactions for the synthesis of organosilicon compounds is copper-catalysed silylation that is favoured for its high functional group tolerance and the availability of inexpensive copper salts.3 The efficiency of the reaction is critically affected by the choice of the source of the silicon functional group. Early literature on copper-mediated or -catalysed silylation reactions employed silyllithium or silylzinc as silicon sources that normally need to be prepared in situ.3b,4 Hexaorganodisilanes could also act as the precursor of silylcoppers. These species are used in a limited number of studies, since the reactions require harsh reaction conditions due to the low reactivity for activation of the Si–Si bond through a formal σ-bond metathesis (Fig. 1A(a)).3b,5 When disilanes are substituted with multiple heteroatoms in such form as hexaalkoxydisilanes, it is known that silylcoppers are smoothly generated on treatment with copper alkoxide via the efficient formation of the silicate intermediate.6 This phenomenon originates in the higher Lewis acidity of the silicon centre (Fig. 1A(b)). Meanwhile, such active disilanes and the silylated products are moisture-sensitive and the efforts to utilise these alkoxydisilanes for copper-catalysed silylation remain rare. Silylboranes have thus been used as popular precursors to silylcopper reagents because of the high efficiency of the formation of active silylcopper species.7 It was demonstrated by means of stoichiometric experiments8 that the silylcopper species was generated through the reaction between a silylborane and a copper alkoxide with concomitant formation of an alkoxyborane via formal σ-bond metathesis (Fig. 1A(c)). Despite the increasing utility of silylboranes by the refined preparative methods,9 sensitivity to air/moisture remains to inflict certain limitations.10 From the viewpoint of the structural diversity of silyl groups and the disadvantageous susceptibility to air and moisture, silicon sources such as hexaalkoxydisilanes or silylboranes leave room for more development. Thus, a new silylating reagent that could be complementarily used with disilanes or silylboranes has been desired as key reagents for versatile silylcupration.Recently, we developed a new silylating reagent, sodium silylsilanolate, which has a nucleophilic silanolate and one Si–Si bond in the molecular structure. These reagents are chemically stable to air and moisture,11 and could be used in combination with palladium or nickel catalysts for the silylation reactions of aryl halides.12 The reaction conditions are generally mild and allow the introduction of silyl groups, including tert-butyldimethylsilyl and allyldimethylsilyl groups that were not previously applied for coupling reactions using conventional silylating reagents. Remarkably, the reports on transferring the simplest triorganosilyl group, trimethylsilyl group, copper species such as Me3SiCu or (Me3Si)2CuLi has relied upon using Me3SiLi as a precursor,13 except for the one report that employs hexamethyldisilane.5a This fact inevitably indicates the absence of a conventional method for delivering a trimethylsilyl group in copper chemistry. These results inspired us to study silylsilanolates as precursors of silylcoppers via an unconventional activation mechanism that is distinct from the ones for disilanes or silylboranes (Fig. 1B). We hypothesised that silylsilanolate-coordinated copper(I) formed in situ would undergo an activation scheme via four-membered transition state, which affords silylcopper(I) with concomitant formation of polysiloxane, a polymer form of the detached silanone species.
Herein, we report our studies on the copper-catalysed hydrosilylation across the unsaturated bonds of internal alkynes, α,β-unsaturated ketones, and allenes. With the aid of DFT calculation, our study demonstrates the utility of silylsilanolates as precursors of silylcopper species that function distinctively to the conventional silicon sources (Fig. 1C).
Results and discussion
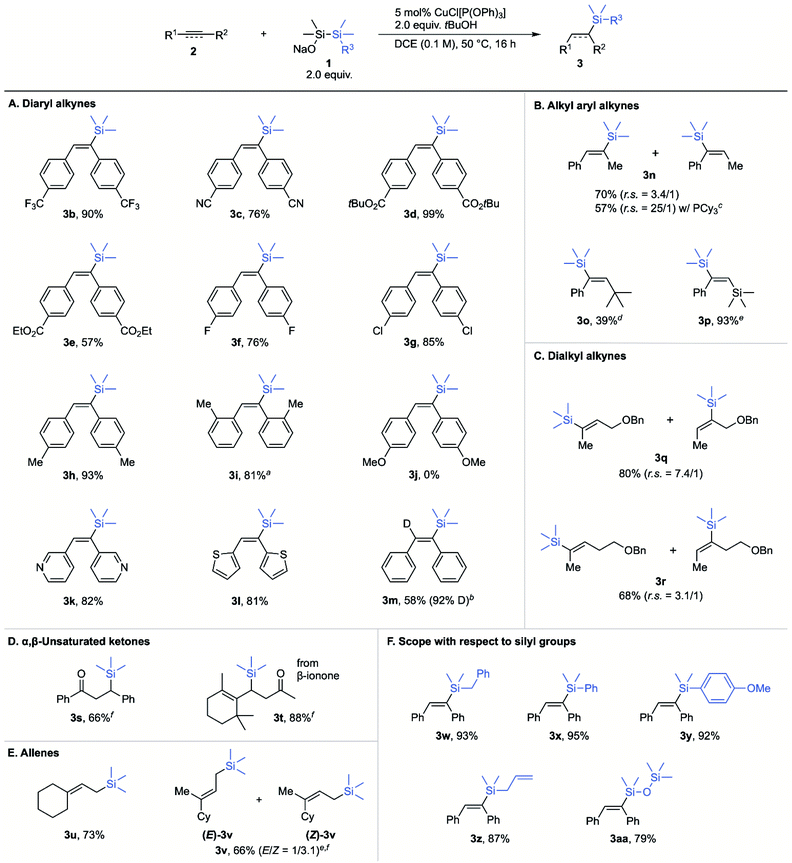
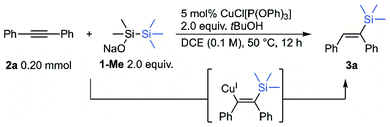
Initially, we investigated the use of sodium trimethylsilyldimethylsilanolate (1-Me) to evaluate silylsilanolate as a precursor of silylcopper through the hydrosilylation of internal triple bond of diphenylacetylene (2a) as a benchmark reaction.14 Extensive screening of reaction conditions revealed that the conditions using 5 mol% of CuCl[P(OPh)3] in DCE (1,2-dichloroethane), 2.0 equiv. tBuOH, 50 °C, 12 h were chosen as the standard reaction conditions. Protonation of the intermediary alkenylcopper would generate alkenylsilane 3a that was obtained in 89% NMR yield (entry 1). In the absence of a copper catalyst or in the presence of a catalytic amount of CuCl with no ligand, no or low conversion of 2a was observed (entries 2 and 3). Other disilanes (tBuOMe2SiSiMe3, Me3SiSiMe3) in combination with NaOSiMe3 as a base resulted in no formation of the product, showing the importance of the silanolate unit for the reactivity (entries 4 and 5). Regarding the ligands of copper catalysts, slightly lower yields (68% and 84%) were observed with monodentate phosphine ligand, PPh3, or 2,2′-bipyridyl (entries 6 and 7). Much lower yields (27% and 5%) were observed with a bidentate phosphine ligand, dppe, or an N-heterocyclic carbene ligand, IPr (entries 8 and 9). The reaction in toluene or THF was similarly efficient (60% and 84%) though almost no product was obtained in CH3CN due to the low conversion (8%) of the substrate (entries 10–12). MeOH showed almost the identical utility with tBuOH as a proton source and the desired product was obtained in a slightly lower yield of 86% (entry 13). The use of lithium or potassium trimethylsilyldimethylsilanolate showed much lower efficiency (entries 14 and 15). Finally, extending the reaction time to 16 h forced the reaction to the completion to give the desired product in 95% NMR yield and 86% isolated yield (entry 16). The conditions for entry 16 were set to be optimal for this reaction.Next, the reaction scope with respect to symmetric diaryl alkynes was surveyed under the optimised reaction conditions (Scheme 1A). The reaction could tolerate various electron-withdrawing groups at para-positions on aromatic rings. The substrate with trifluoromethyl or cyano group was transformed to 3b or 3c in excellent yield. Tert-Butoxycarbonyl group was compatible under the reaction conditions to give 3d while ethoxycarbonyl group seemed to be partially hydrolysed and 3e was afforded in 57% yield. Substrates with fluoro and chloro substituents gave the hydrosilylated products (3f, 3g) in 76% and 85% respective yields. In the case of methyl groups as substituents, both p-tolyl and o-tolyl substrates gave the products 3h and 3i in good yields. For the sterically hindered o-tolyl product 3i, increased amounts of silylsilanolate and tBuOH were needed. In the case of p-methoxy substrate, no hydrosilylated product 3j was observed while recovering the substrate in 85% yield. Heteroaryl-substituted acetylenes were also successfully transformed into the corresponding alkenylsilanes in good yields (3k, 3l). When CH3OD was used in place of tBuOH for the hydrosilylation of 2a, the corresponding deuterated alkenylsilane 3m was obtained in 58% yield with 92% deuterium incorporation. This result indicates that alkenylcopper generated in situ is protonated by alcohols. The scope of alkyl aryl acetylenes is shown in Scheme 1B.
1-Phenyl-1-propyne (2n) was converted to the desired alkenylsilane 3n in good yield, but with low regioselectivity (r.s. = 3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this case, PCy3 instead of P(OPh)3 improved the regioselectivity up to r.s. = 25
1). In this case, PCy3 instead of P(OPh)3 improved the regioselectivity up to r.s. = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1-Phenyl-2-tert-butylacetylene (2o) showed low conversion and provided 3o only in 39% yield even at higher temperature. 3o was obtained selectively as a single isomer, probably due to the steric hindrance of the tert-butyl group that drives the incoming silyl group away to the distal position. The substrate bearing bulky trimethylsilyl group 2p also gave a sole regioisomer, and 3p was obtained in excellent yield (93%). The difference of the yield between 3o and 3p could be ascribed to the fact that the intermediate alkenylcopper for 3p is thermodynamically stabilised by the presence of the adjacent silicon–carbon bond.15 Dialkyl alkynes are also applicable and hydrosilylated products 3q, 3r were obtained in good yields with moderate to low regioselectivity (Scheme 1C). The regioselectivity is seemingly affected by the intramolecular coordination of the ether moiety to the silylcopper intermediate, which would direct the copper atom to the proximal position of the triple bond. Ethynylbenzene was found unreactive and no hydrosilylated product was obtained. This result indicates that terminal alkyne substrate is not compatible. It is noteworthy that the current copper-catalysed hydrosilylation could also be applied to α,β-unsaturated ketones4c,5a,16 and allenes17 which are known to be good substrates for these reactions (Scheme 1D and 1E).3b Chalcone (2s) and β-ionone (2t) were amenable to the optimised reaction conditions, affording the products of conjugate silylation 3s and 3t in 66% and 88% respective yields.18 Symmetric terminal allene 2u provided the corresponding allylsilane 3u in good yield. Terminal allene 2v was also converted to allylsilane 3v as a mixture of E and Z isomers (E/Z = 1/3.1). We believe that this stereoselectivity is due to the steric hindrance around the bulkier cyclohexyl group that propels the silylcopper to the opposite face. Aldehydes19 and imines20 are known as good substrates in copper-catalysed silylation reactions in the presence of silylborane. However, in the current reaction system, benzaldehyde and N-tosylbenzenemethanimine afforded no desired products since these substrates are not compatible with silylsilanolate reagents.
1. 1-Phenyl-2-tert-butylacetylene (2o) showed low conversion and provided 3o only in 39% yield even at higher temperature. 3o was obtained selectively as a single isomer, probably due to the steric hindrance of the tert-butyl group that drives the incoming silyl group away to the distal position. The substrate bearing bulky trimethylsilyl group 2p also gave a sole regioisomer, and 3p was obtained in excellent yield (93%). The difference of the yield between 3o and 3p could be ascribed to the fact that the intermediate alkenylcopper for 3p is thermodynamically stabilised by the presence of the adjacent silicon–carbon bond.15 Dialkyl alkynes are also applicable and hydrosilylated products 3q, 3r were obtained in good yields with moderate to low regioselectivity (Scheme 1C). The regioselectivity is seemingly affected by the intramolecular coordination of the ether moiety to the silylcopper intermediate, which would direct the copper atom to the proximal position of the triple bond. Ethynylbenzene was found unreactive and no hydrosilylated product was obtained. This result indicates that terminal alkyne substrate is not compatible. It is noteworthy that the current copper-catalysed hydrosilylation could also be applied to α,β-unsaturated ketones4c,5a,16 and allenes17 which are known to be good substrates for these reactions (Scheme 1D and 1E).3b Chalcone (2s) and β-ionone (2t) were amenable to the optimised reaction conditions, affording the products of conjugate silylation 3s and 3t in 66% and 88% respective yields.18 Symmetric terminal allene 2u provided the corresponding allylsilane 3u in good yield. Terminal allene 2v was also converted to allylsilane 3v as a mixture of E and Z isomers (E/Z = 1/3.1). We believe that this stereoselectivity is due to the steric hindrance around the bulkier cyclohexyl group that propels the silylcopper to the opposite face. Aldehydes19 and imines20 are known as good substrates in copper-catalysed silylation reactions in the presence of silylborane. However, in the current reaction system, benzaldehyde and N-tosylbenzenemethanimine afforded no desired products since these substrates are not compatible with silylsilanolate reagents.
Sodium silylsilanolates were also confirmed to be viable for the introduction of other silyl groups (Scheme 1F). Alkenylsilane 3w substituted with benzyldimethylsilyl group was obtained from the corresponding silylsilanolate 1-Bn in 93% yield. For aryldimethylsilyl groups, both phenyl- and anisyl-substituted alkenylsilanes 3x, 3y were obtained in excellent 95% and 92% yields regardless of the electronic property of the aryl groups. Allyl-substituted silylsilanolate 1-allyl enabled the transfer of the allyldimethylsilyl group and gave 3z in 87% yield. Delivery of the siloxysilyl group with silylsilanolate 1-OSiMe3 afforded the corresponding adduct 3aa in 79% yield. Among these silyl groups, this study represents the first case of introducing anisyldimethylsilyl and dimethyl(trimethylsiloxy)silyl groups through copper-catalysed silylation reactions. These results demonstrate that silylsilanolates represent important substitutes for the conventional silylating reagents that mediate copper-catalysed silylation reactions.
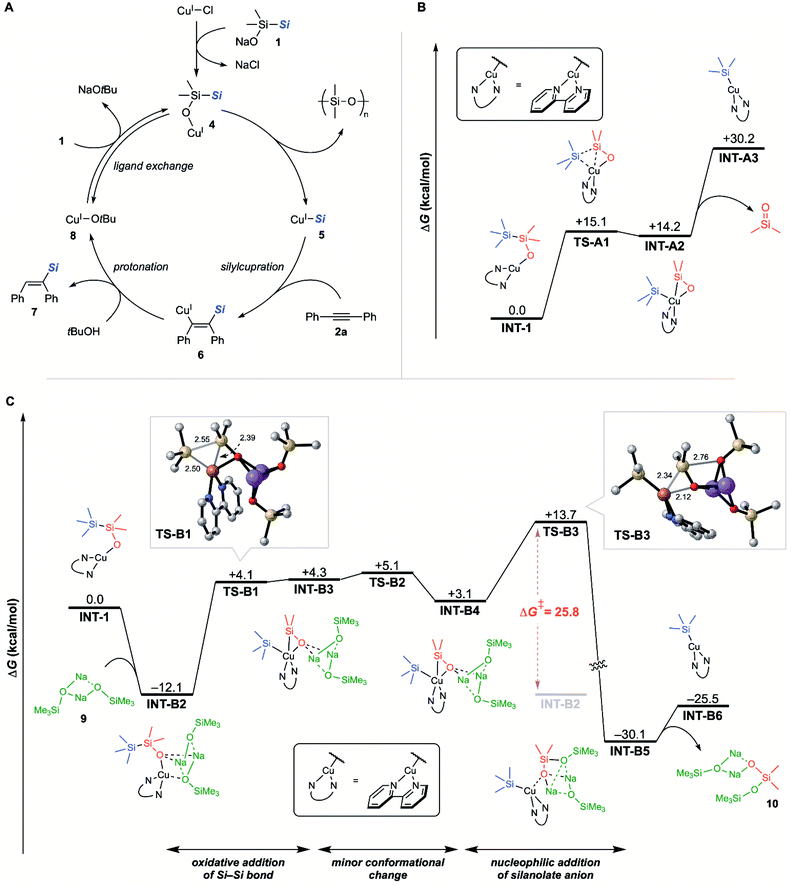
A possible overall reaction mechanism for the hydrosilylation of diphenylacetylene (2a) is shown in Fig. 2A. Initially, copper(I) silylsilanolate 4 would be generated by the reaction between copper(I) chloride and sodium silylsilanolate 1. Copper(I) silylsilanolate 4 would provide silylcopper 5 accompanied by the generation of polysiloxane. Silylcopper 5 would undergo silylcupration across 2a to afford alkenylcopper 6. Keeping in mind that the pKa of silanol is generally smaller than that of alcohol,21tBuOH would be the only protic species in the reaction mixture. Thus, the protonation of 6 with tBuOH would give CuOtBu (8) with the formation of alkenylsilane 7. Finally, the ligand exchange of 8 with sodium silylsilanolate 1 would furnish NaOtBu with the regeneration of 4. DFT calculations revealed that the sum of free energies of copper(I) silylsilanolate 4 and NaOtBu is almost the same as that of CuOtBu and sodium silylsilanolate 1 (ΔG = 0.57 kcal mol−1, see ESI†). These data support the potential equilibrium of ligand exchange between the silanolate and the alkoxide on the copper atom.
To figure out the mechanism of the migration of the silyl groups of silylsilanolates to the copper atom, DFT calculations were carried out. Given that the optimised copper catalyst CuCl[P(OPh)3] is known to form a cluster that could complicate the calculation,22 2,2′-bipyridyl, the second-best ligand in the hydrosilylation reaction, was employed to model the calculation. Since disilanes were believed to undergo σ-bond metathesis via four-membered transition state as in Fig. 1A,5e one possible pathway to form silylcopper could be the reaction between CuOtBu generated in situ and sodium silylsilanolate, which would furnish silylcopper through the σ-bond metathesis between the Si–Si bond of silylsilanolate and the Cu–O bond of CuOtBu, without going through silylsilanolate-coordinated copper(I) like 4 in Fig. 2A. From the fact that hexamethyldisilane and monoalkoxydisilane without anionic silanolate unit did not give any product (Table 1, entries 4,5), the formal σ-bond metathesis mechanism seems unlikely. Our extensive effort on DFT calculation toward locating the transition state for such a pathway led only to a futile result. Next, copper complex INT-1 coordinated with silylsilanolate like 4 was chosen as a starting point of the calculated pathway. The pathway for the migration of the silyl group directly from INT-1 to afford silylcopper was initially examined (Fig. 2B). The pathway from INT-1 to INT-A3 was found to be endergonic because of the thermodynamically unfavourable generation of a dimethylsilanone. Thus, we calculated the migration pathway in the presence of activators.
| Entry | Deviations from the standard conditions | Yielda (%) |
|---|---|---|
| a Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. b Isolated yield (0.50 mmol scale). | ||
| 1 | None | 89 |
| 2 | No copper catalyst | 0 |
| 3 | No ligand | 11 |
| 4 | tBuOMe2SiSiMe3/NaOSiMe3 instead of 1-Me | 0 |
| 5 | Me3SiSiMe3/NaOSiMe3 instead of 1-Me | 0 |
| 6 | 5 mol% CuCl + 5 mol% PPh3 | 68 |
| 7 | CuCl(bpy) | 84 |
| 8 | CuCl(dppe) | 27 |
| 9 | CuCl(IPr) | 5 |
| 10 | Toluene | 60 |
| 11 | THF | 84 |
| 12 | CH3CN | 1 |
| 13 | MeOH instead of tBuOH | 86 |
| 14 | Countercation: Li | 41 |
| 15 | Countercation: K | 7 |
| 16 | Reaction time: 16 h | 95 (86)b |
|
||
In our previous report, the pathway for the migration of the silyl group to the palladium atom was calculated through the activation by sodium trimethylsilanolate dimer 9 that was modelled as a simplified form of sodium silylsilanolate dimer.12a Based on this result, the energy profile of the migration pathway with the aid of 9 was calculated. The result is summarised in Fig. 2C. The complexation of INT-1 with 9 was found to be exergonic to provide INT-B2. Oxidative addition of the Si–Si bond to the copper atom in INT-B2 affords INT-B3viaTS-B1 that bears the elongated Si–Si bond (2.55 Å).23 This is followed by a minor conformational change to provide INT-B4viaTS-B2. The intramolecular attack of trimethylsilanolate (green) to the silicon atom (red) viaTS-B3 (Si–O = 2.76 Å) provides INT-B5 through sufficiently low activation barrier (ΔG‡ = 25.8 kcal mol−1). Finally, dissociation of a silanolate bearing disiloxane moiety 10 from INT-B5 affords INT-B6. This calculation results show that the formation of the Cu–Si bond proceeds via oxidative addition of the Si–Si bond to the copper atom. The presence of the Si–Si bond in the vicinity of the copper atom in INT-B2 may kinetically promote oxidative addition of the Si–Si bond. To the best of our knowledge, this is the first example of an indication that silylcopper could be generated through oxidative addition.24 Compared with the pathway to silylcopper without any activation shown in Fig. 2B, the pathway with the assistance of the sodium trimethylsilanolate dimer 9 is found to be overall exergonic probably due to the formation of a thermodynamically stable disiloxane 10 instead of the formation of a silanone. Mass spectroscopic analysis of the finished reaction mixture shows molecular weight distribution that fitted with the Gaussian distribution with interval of 74 mass units corresponding to the repeat unit of polydimethylsiloxane, dimethylsilanone (see ESI†). This result indicates the formation of dimethylsilanone surrogates such as 10 that is assumed in our DFT calculations. It was also found that the migration process of the silyl group could proceed using NaOtBu dimer as an activator instead of 9 with a little higher activation barrier (ΔG‡ = 28.4 kcal mol−1, see ESI†). This result indicates that the NaOtBu generated in situ could also be involved in the migration pathway of silyl groups as an activator.
Conclusions
We revealed that sodium silylsilanolates function as precursors to silylcopper species in the presence of an appropriate copper catalyst. Through the demonstrations of hydrosilylation that was applied for alkynes, α,β-unsaturated ketones, and allenes, facile in situ generation of reactive silylcopper species was corroborated. The reaction allows the delivery of a series of silyl groups by the choice of the silylsilanolate reagent. DFT calculations unveiled the possible reaction mechanism for the formation of the silylcopper from copper(I) silylsilanolates, which proceeds through an unprecedented pathway via oxidative addition of the Si–Si bond to the copper(I) centre. We believe the present study would propose a new versatile approach for the generation of silylcopper species.Data availability
Data for all compounds in this manuscript are available in the ESI,† which includes experimental details, characterisation data and copies of 1H, 13C and 29Si NMR spectra.Author contributions
J. S. and H. Ya. conceived the project. J. S. and H. Yo. directed the research. H. Ya. and K. H. performed the experiments. H. Ya. conducted the DFT calculations. H. Ya. and J. S. composed the manuscript and the ESI† section. All authors contributed to the editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP21H01934, JP20J23393, JP19H00895, and partly by JST CREST Grant Number JPMJCR19R4, Japan. H. Ya. acknowledges a JSPS Predoctoral Fellowship.Notes and references
- (a) Silicon Polymers, ed. A. M. Muzafarov, Springer, Heidelberg, 2011 Search PubMed; (b) Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications, ed. R. G. Jones, W. Ando and J. Chojnowski, Kluwer Academic: Dordrecht, 2000 Search PubMed; (c) S. Yamaguchi and K. Tamao, Chem. Lett., 2005, 34, 2–7 CrossRef CAS; (d) D. Sun, Z. Ren, M. R. Bryce and S. Yan, J. Mater. Chem. C, 2015, 3, 9496–9508 RSC; (e) M. Shimizu, Chem. Rec., 2015, 15, 73–85 CrossRef CAS PubMed.
- For reviews about silicon-containing drugs, see: (a) R. Tacke and S. Dörrich, Drug Design Based on the Carbon/Silicon Switch Strategy. in Atypical Elements in Drug Design, ed. J. Schwarz, Topics in Medicinal Chemistry, vol. 17, 2016, pp. 29–59 Search PubMed; (b) G. A. Showell and J. S. Mills, Drug Discovery, 2003, 8, 551–556 CAS; (c) S. McN. Sieburth and C.-A. Chen, Eur. J. Org. Chem., 2006, 311–322 CrossRef CAS; (d) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS PubMed; (e) A. K. Franz and S. O. Wilson, J. Med. Chem., 2013, 56, 388–405 CrossRef CAS PubMed; (f) S. Fujii and Y. Hashimoto, Future Med. Chem., 2017, 9, 485–505 CrossRef CAS PubMed; (g) R. Ramesh and D. S. Reddy, J. Med. Chem., 2018, 61, 3779–3798 CrossRef CAS PubMed.
- (a) Y. Tsuji and T. Fujihara, Chem. Rec., 2016, 16, 2294–2313 CrossRef CAS PubMed; (b) J. R. Wilkinson, C. E. Nuyen, T. S. Carpenter, S. R. Harruff and R. Van Hoveln, ACS Catal., 2019, 9, 8961–8979 CrossRef CAS; (c) W. Xue and M. Oestreich, ACS Cent. Sci., 2020, 6, 1070–1081 CrossRef CAS PubMed.
- (a) For preparation of silyllithium and silylzinc, see: Science of Synthesis, ed. I. Fleming, Thieme, Stuttgart, 2002. vol 4 Search PubMed; (b) D. J. Ager and I. Fleming, J. Chem. Soc., Chem. Commun., 1978, 177–178 RSC; (c) D. J. Ager, I. Fleming and S. K. Patel, J. Chem. Soc., 1981, 2520–2526 CAS; (d) D. J. Vyas and M. Oestreich, Chem. Commun., 2010, 46, 568–570 RSC; (e) A. Weickgennant and M. Oestreich, Chem.–Eur. J., 2010, 16, 402–412 CrossRef PubMed; (f) C. K. Hazra and M. Oestreich, Org. Lett., 2012, 14, 4010–4013 CrossRef CAS PubMed; (g) Preparation of a storable solid silylzinc has been reported: R. Chandrasekaran, F. T. Pulikkottil, K. S. Elamaa and R. Rasappan, Chem. Sci., 2021, 12, 15719–15726 RSC.
- (a) H. Ito, T. Ishizuka, J. Tateiwa, M. Sonoda and A. Hosomi, J. Am. Chem. Soc., 1998, 120, 11196–11197 CrossRef CAS; (b) C. T. Clark, J. F. Lake and K. A. Scheidt, J. Am. Chem. Soc., 2004, 126, 84–85 CrossRef CAS PubMed; (c) L. Iannazzo and G. A. Molander, Eur. J. Org. Chem., 2012, 4923–4926 CrossRef CAS PubMed; (d) Y. Minami, K. Shimizu, C. Tsuruoka, T. Komiyama and T. Hiyama, Chem. Lett., 2014, 43, 201–203 CrossRef CAS; (e) H. Ito, Y. Horita and M. Sawamura, Adv. Synth. Catal., 2012, 354, 813–817 CrossRef CAS.
- B. J. McCarty, B. M. Thomas, M. Zeller and R. Van Hoveln, Organometallics, 2018, 37, 2937–2940 CrossRef CAS.
- (a) K.-S. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2010, 132, 2898–2900 CrossRef CAS PubMed; (b) D. J. Vyas and M. Oestreich, Angew. Chem., Int. Ed., 2010, 47, 8513–8515 CrossRef PubMed; (c) D. J. Vyas, R. Fröhlich and M. Oestreich, Org. Lett., 2011, 13, 2094–2097 CrossRef CAS PubMed; (d) A. Welle, J. Petrignet, B. Tinant, J. Wouters and O. Riant, Chem.–Eur. J., 2010, 16, 10980–10983 CrossRef CAS PubMed; (e) I. Ibrahem, S. Santoro, F. Himo and A. Córdova, Adv. Synth. Catal., 2011, 353, 245–252 CrossRef CAS; (f) M. Tobisu, H. Fujihara, K. Koh and N. Chatani, J. Org. Chem., 2010, 75, 4841–4847 CrossRef CAS PubMed; (g) P. Wang, X.-L. Yeo and T. P. Loh, J. Am. Chem. Soc., 2011, 133, 1254–1256 CrossRef CAS PubMed; (h) K. Kubota and H. Ito, Catalytic Generation of Silicon Nucleophiles. in Organosilicon Chemistry, ed. T. Hiyama and M. Oestreich, Wiley-VCH, Weinheim, 2019, pp. 1–32 Search PubMed.
- (a) C. Kleeberg, M. S. Cheung, Z. Lin and T. B. Marder, J. Am. Chem. Soc., 2011, 133, 19060–19063 CrossRef CAS PubMed; (b) L.-J. Cheng and N. P. Mankad, J. Am. Chem. Soc., 2020, 142, 80–84 CrossRef CAS PubMed.
- (a) M. Suginome, T. Matsuda and Y. Ito, Organometallics, 2000, 19, 4647–4649 CrossRef CAS; (b) T. A. Boebel and J. F. Hartwig, Organometallics, 2008, 27, 6013–6019 CrossRef CAS; (c) R. Shishido, M. Uesugi, R. Takahashi, T. Mita, T. Ishiyama, K. Kubota and H. Ito, J. Am. Chem. Soc., 2020, 142, 14125–14133 CrossRef CAS PubMed.
- E. Yamamoto, R. Shishido, T. Seki and H. Ito, Organometallics, 2017, 36, 3019–3022 CrossRef CAS.
- The stability of sodium trimethylsilyldimethylsilanolate under aerial conditions was verified according to the method reported by Ito et al.10 See ESI† for details..
- (a) H. Yamagishi, H. Saito, J. Shimokawa and H. Yorimitsu, ACS Catal., 2021, 11, 10095–10103 CrossRef CAS; (b) K. Hitoshio, H. Yamagishi, J. Shimokawa and H. Yorimitsu, Chem. Commun., 2021, 57, 6867–6870 RSC.
- (a) B. M. Trost and D. M. T. Chan, J. Am. Chem. Soc., 1982, 104, 3733–3735 CrossRef CAS; (b) J. G. Smith, N. R. Quinn and M. Viswanathan, Synth. Commun., 1983, 13, 1–5 CrossRef CAS; (c) J. E. Audia and J. A. Marshall, Synth. Commun., 1983, 13, 531–535 CrossRef CAS; (d) I. Fleming and T. W. Newton, J. Chem. Soc., Perkin Trans., 1984, 1, 1805–1808 RSC; (e) S. Kamio, T. Imagawa, M. Nakamoto, M. Oestreich and H. Yoshida, Synthesis, 2021, 53, 4678–4681 CrossRef CAS.
- (a) G. Auer and M. Oestreich, Chem. Commun., 2006, 311–313 RSC; (b) Q.-Q. Xuan, C.-L. Ren, L. Liu, D. Wang and C.-J. Li, Org. Biomol. Chem., 2015, 13, 5871–5874 RSC.
- (a) E. A. Brinkman, S. Berger and J. I. Brauman, J. Am. Chem. Soc., 1994, 116, 8304–8310 CrossRef CAS; (b) B. Römer, G. G. Gatev, M. Zhong and J. I. Brauman, J. Am. Chem. Soc., 1998, 120, 2919–2924 CrossRef.
- (a) B. H. Lipshutz, J. A. Sclafani and T. Takanami, J. Am. Chem. Soc., 1998, 120, 4021–4022 CrossRef CAS; (b) M. Oestreich and B. Weiner, Synlett, 2004, 2139–2142 CrossRef CAS.
- (a) I. Fleming and F. J. Pulido, J. Chem. Soc., Chem. Commun., 1986, 1010–1011 RSC; (b) J. Rae, Y. C. Hu and D. J. Procter, Chem.–Eur. J., 2014, 20, 13143–13145 CrossRef CAS PubMed.
- Attempted enantioselective hydrosilylation of 2s with several chiral phosphine ligands afforded the hydrosilylated products in moderate to good yields, albeit without any noticeable enantioselectivity in all cases. See ESI† for details.
- C. Kleeberg, E. Feldmann, E. Hartmann, D. J. Vyas and M. Oestreich, Chem.–Eur. J., 2011, 17, 13538–13543 CrossRef CAS PubMed.
- D. J. Vyas, R. Fröhlich and M. Oestreich, Org. Lett., 2011, 13, 2094–2097 CrossRef CAS PubMed.
- (a) R. West and R. H. Baney, J. Am. Chem. Soc., 1959, 81, 6145–6148 CrossRef CAS; (b) R. West, R. H. Baney and D. L. Powell, J. Am. Chem. Soc., 1960, 82, 6269–6272 CrossRef CAS; (c) T. Kagiya, Y. Sumida and T. Tachi, Bull. Chem. Soc. Jpn., 1970, 43, 3716–3722 CrossRef CAS.
- (a) R. D. Pike, W. H. Starnes, Jr. and G. B. Carpenter, Acta Crystallogr., 1999, 55, 162–165 Search PubMed; (b) F.-F. Jian, K.-F. Wang, H.-L. Xiao and Y.-B. Qiao, Acta Crystallogr., 2005, 61, m1324–m1325 CAS.
- The Gibbs free energy of INT-B3 is higher than that of TS-B1 as shown in Fig. 2C. As this reversal may seem contradictory, electronic energy of INT-B3 is lower than that of TS-B1 on potential energy surface. IRC calculations also confirmed that TS-B1 connects to INT-B3.
- DFT calculations by Maiti et al. have suggested that the transfer of silyl groups onto palladium atoms proceeds via oxidative addition of Si−Si bond. A. Maji, S. Guin, S. Feng, A. Dahiya, V. Singh, P. Liu and D. Maiti, Angew. Chem., Int. Ed., 2017, 56, 14903–14907 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and full characterisation data, details of the computational study and NMR spectra. See DOI: 10.1039/d2sc00227b |
| This journal is © The Royal Society of Chemistry 2022 |