Open Access Article
Open Access ArticleA (μ-hydrido)diborane(4) anion and its coordination chemistry with coinage metals†
Xiaofeng
Mao
,
Jie
Zhang
 ,
Zhenpin
Lu‡
,
Zhenpin
Lu‡
 * and
Zuowei
Xie
* and
Zuowei
Xie
 *
*
Department of Chemistry, State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, N. T., Hong Kong, China. E-mail: zhenpilu@cityu.edu.hk; zxie@cuhk.edu.hk
First published on 11th February 2022
Abstract
A tetra(o-tolyl) (μ-hydrido)diborane(4) anion 1, an analogue of [B2H5]− species, was facilely prepared through the reaction of tetra(o-tolyl)diborane(4) with sodium hydride. Unlike common sp2–sp3 diborane species, 1 exhibited a σ-B–B bond nucleophilicity towards NHC-coordinated transition-metal (Cu, Ag, and Au) halides, resulting in the formation of η2-B–B bonded complexes 2 as confirmed by single-crystal X-ray analyses. Compared with 1, the structural data of 2 imply significant elongations of B–B bonds, following the order Au > Cu > Ag. DFT studies show that the diboron ligand interacts with the coinage metal through a three-center-two-electron B–M–B bonding mode. The fact that the B–B bond of the gold complex is much prolonged than the related Cu and Ag compounds might be ascribed to the superior electrophilicity of the gold atom.
Introduction
Diboron compounds are fascinating molecules,1 and have demonstrated their potential as useful diboration reagents in organic synthesis2 and unique ligands for the construction of σ/π-B–B bonded metal complexes.3 Most of these studies focused on the development of neutral diboron species.2b,3a Until recently, anionic diboron compounds also received considerable research interest.4 On the one hand, anionic diboron compounds can be used as a type of nucleophilic boron reagents. Because of the high value in the preparation of new boron-element bonds in organic synthesis, since the first synthesis of nucleophilic boryllithium reagent by Yamashita, Nozaki, and co-workers in 2006,5 the studies on nucleophilic boron compounds have been an ongoing research interest of many groups.6 Anionic diboron compounds, such as [(RO)2B–B(OR)2(OR′)]−, can react as an equivalent of [:B(OR)2]− with electrophiles for further transformations, providing an alternative approach to nucleophilic boron compounds.7 However, for non-heteroatom-substituted diboron compounds, the example is scarce.8On the other hand, diboron compounds are considered as B–B bonded ligands for transition metal complexes.9 [B2H5]−, probably the simplest anionic diboron species, is not stable as a free molecule. A computational study of [B2H5]− reveals that the non-classical bonding structure, where the two boron atoms are bridged by a hydrogen atom, is more stable in energy than the classical structure featured with a B–B σ bond (Fig. 1a).10,11 Most of the reported [B2H5]− complexes with transition metals endowed the structures with a non-classical [B2H5]− core (A, B, and C; Fig. 1b).12 The example of diboron compounds with the classical [B2H5]− core is extremely rare. Until 2019, Ghosh et al. reported a tantalum complex with the coordination of a classical [B2H5]−, which is stabilized as a result of the electron donation from the tantalum template to the sp2-boron atom (D; Fig. 1b).11 For the study on organic derivatives [B2R4H]− of [B2H5]−, Braunschweig et al. demonstrated that neutral, base-stabilized diborane(5) compounds can be utilized as neutral analogues of [B2H5]− ligands to form related transition metal complexes with a characteristic σ B–B bond (E; Fig. 1b).13 Wagner et al. synthesized a derivative of [B2R4H]−, F, from dimeric 9-H-9-borafluorene, which is stabilized through the π conjugated rings (F; Fig. 1b).8 Despite these endeavours, the reported examples of anionic non-heteroatom-stabilized diboron(5) compounds remain rare species probably due to the challenges in preparation. We envision that the reaction of diborane(4) species with metal hydride might provide a facile synthesis to new [B2R4H]− derivatives for further investigation. Herein, we report the synthesis of a novel diboron(5) anion 1, which shows nucleophilic reactivity towards transition metal halides to generate σ-B–B bonded metal complexes 2 (Fig. 1c), and the results are presented in this article.
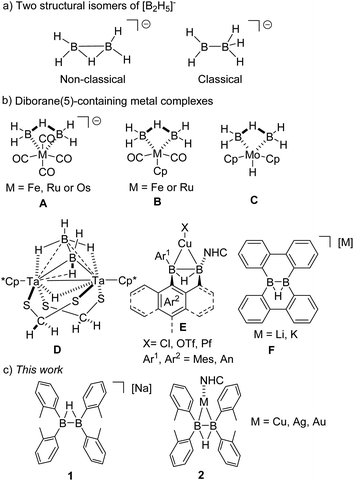 | ||
| Fig. 1 (a) Structure of non-classical and classical [B2H5]−; (b) reported diborane(5)-containing metal complexes; (c) new anionic diboron compound reported in this study. | ||
Results and discussion
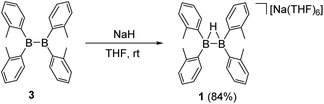
Synthesis of tetra(o-tolyl)(μ-hydrido)diborane(4) anion 1
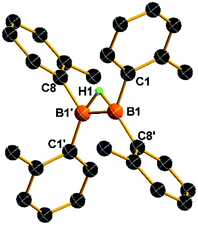
The reaction of tetra(o-tolyl)diborane(4)143 with NaH in THF afforded the desired anionic diboron product 1 in 84% isolated yield. The 11B NMR of compound 1 in THF-d8 displayed only a broad singlet at 30.2 ppm, indicating a symmetrical structure of this diboron compound in solution. This measured value is significantly upfield shifted concerning the 88.6 ppm observed in the 11B NMR spectrum of 3,14 but is close to those (31–38 ppm) found in B2(o-tolyl)42− dianion.15 Colorless crystals of 1 were obtained from recrystallization in a THF solution at −30 °C. Single-crystal X-ray analyses reveal that 1 is an ionic salt, consisting of a discrete cation [Na(THF)6]+ and a hydrogen-bridged anion [(o-tolyl)2B(μ-H)B(tolyl)2]−. The molecular structure of the anion in 1 is shown in Fig. 2. The C(8)–B(1′)–B(1)–C(8′)/C(1′)–B(1′)–B(1)–C(1) torsion angles in 1 are almost zero, which is similar to that observed in the B2(o-tolyl)42− dianion15 but in sharp contrast to those in 3 (75.7(7)–93.2(7)°).14 The B–B bond distance (1.628(5) Å) in 1 is very close to that of B2(o-tolyl)42− dianion (1.633(3), 1.639(6) Å), slightly shorter than that observed in compound F (1.651(6) Å) (Fig. 1b),8 but is considerably shorter than the one in 3 (1.686(9), 1.694(9) Å) and other neutral diborane species (1.67–1.68 Å).13 The aforementioned data suggest that the B–B bond in 1 has a double bond character (Scheme 1).Synthesis of η2-B–B bonded complexes 2
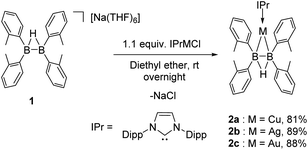
In our attempt to explore the nucleophilic reactivity of this new anionic diboron species, compound 1 was treated with different NHC-supported transition-metal halides (Scheme 2). Although compound 1 did not work as an equivalent of BR2− to afford metal boryl species,16 the nucleophilic B–B σ bond of 1 can interact with the transition metals to generate new η2-bonded complexes 2. They were fully characterized by NMR spectra, HRMS, and single-crystal X-ray analyses. The 11B NMR spectra of 2 exhibited a broad singlet at 24–27 ppm, which was upfield-shifted compared with 1 (30 ppm), suggesting a back-donation from the metal to the boron atoms.The electrochemical properties of compounds 1 and 2 were investigated by cyclic voltammetry (CV). Both compounds 2a and 2b exhibited a quasi-reversible reduction potential at around −1.6 V (vs. Cp2Fe/Cp2Fe+), whereas compound 2c showed a similar one at −1.9 V (Fig. S10, S15, and S20†). In comparison, compound 1 was not stable under electrochemical conditions, and no clear reduction potential was observed from the CV spectra (Fig. S5†), which indicated that the formation of B–B bonded transition metal complexes renders this anionic diboron species more robust upon the electrical reduction process.
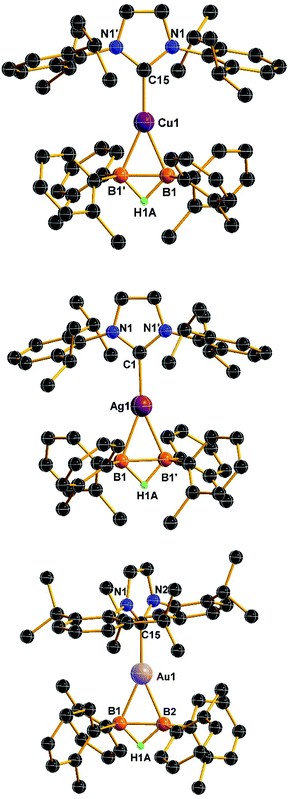
The molecular structures of compounds 2 confirm the formation of two new metal-boron bonds that the B–B bond is bonded to the metal in an η2 fashion (Fig. 3). Similar to that of 1, the BHB unit is observed in compound 2. Compounds 2a and 2b have C2 symmetries, whereas 2c is packing in an orthorhombic crystal system. For compound 2a, the Cu–B bond distance (2.177(3) Å) is close to that of reported copper-based dianionic diboron complexes (2.178–2.186 Å)9 and copper(I) diborane complexes (2.146(3) Å and 2.149(3) Å).17 For the silver complex, the Ag–B length in 2b (2.328(5) Å) is similar to the reported symmetric diborane Ag(I) species (2.318(4) Å and 2.3664 Å).18 The Au–B bond length in 2c (2.235(5) Å and 2.238(5) Å) are considerably longer than those of reported sp2 boryl gold complexes (2.07–2.09 Å)19 and boride gold (2.11–2.19 Å),20 but comparable to those of sp3 boryl gold complexes (2.21–2.30 Å)21 and borylene gold species (2.17–2.22 Å).22
Notably, the B–B bond lengths of compounds 2 (2a: 1.763(7) Å, 2b: 1.761(10) Å, and 2c: 1.822(8) Å) were significantly elongated compared with that of compound 1 (1.628(5) Å). These values are slightly longer than those of reported copper(I) diboron dianionic species (1.68–1.72 Å),9 but comparable to the reported based-stabilized diborane(5) metal complexes (1.76–1.79 Å)13 as well as those of metal-stabilized non-classical [B2H5]− complexes (A, B and C; Fig. 1b) (1.77–1.79 Å).12 Particularly for 2c, the B–B bond distance (1.822(8) Å) is considerably longer than common B(sp3)–B(sp3) single bond (1.76 Å).23
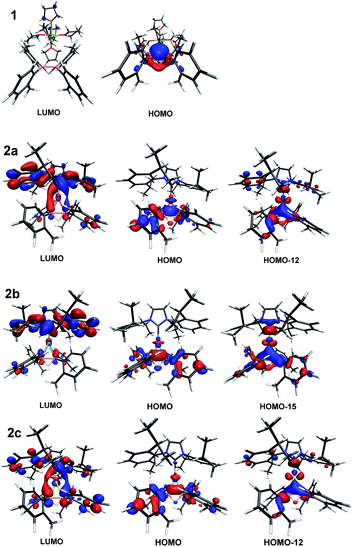
DFT computational studies were conducted to elucidate the electronic structures of compounds 1 and 2 (Fig. 4). The HOMO of 1 consists of both σ- and π-orbitals, suggesting the presence of B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond characteristics as evidenced by the Wiberg bond index (WBI) of 1.13 in 1. In contrast, only σ-orbitals of B–B bond can be clearly seen from the HOMOs of compounds 2, indicating the presence of metal to B–B bond back donation. Accordingly, the WBIs of the B–B bond in compounds 2 are all reduced (2a: 1.02, 2b: 0.99, and 2c: 0.83). These results are in good agreement with the measured B–B bond distances. In addition, the HOMO−12 of 2a, HOMO−15 of 2b, and HOMO−12 of 2c are partially polarized towards the metal atoms, resulting in the generation of metallacycle-like structures.24 It is also noted that gold atom exhibits a superior electrophilicity than Cu and Ag, which might explain that the B–B bond distance of 2c is the longest one among these three derivatives.25 The LUMOs of compounds 2 remain mainly on the NHC group, although for 2a and 2c the existence of π*-orbitals of B–B bond could also be observed.
B double bond characteristics as evidenced by the Wiberg bond index (WBI) of 1.13 in 1. In contrast, only σ-orbitals of B–B bond can be clearly seen from the HOMOs of compounds 2, indicating the presence of metal to B–B bond back donation. Accordingly, the WBIs of the B–B bond in compounds 2 are all reduced (2a: 1.02, 2b: 0.99, and 2c: 0.83). These results are in good agreement with the measured B–B bond distances. In addition, the HOMO−12 of 2a, HOMO−15 of 2b, and HOMO−12 of 2c are partially polarized towards the metal atoms, resulting in the generation of metallacycle-like structures.24 It is also noted that gold atom exhibits a superior electrophilicity than Cu and Ag, which might explain that the B–B bond distance of 2c is the longest one among these three derivatives.25 The LUMOs of compounds 2 remain mainly on the NHC group, although for 2a and 2c the existence of π*-orbitals of B–B bond could also be observed.
Natural bond orbital (NBO) analyses were carried out to investigate the bonding situation between the anionic B–B bond and the metal atom in compounds 2. The second-order perturbation analyses indicate that donor–acceptor interaction from the σ-orbitals of the B–B bond to the metal atom is dominant (2a: 54.28 kcal mol−1, 2b: 60.24 kcal mol−1 and 2c: 174.24 kcal mol−1), in which the stabilization energy of gold complex 2c is much higher than the other two compounds. Moreover, localized molecular orbital (LMO) analyses suggested the presence of three-center-two-electron B–M–B bonding interaction between the two boron atoms and metal in compounds 2, in addition to B–H–B 3c–2e bonding interaction (Fig. S21 in ESI†). These data imply that the σ-B–B bond is highly polarized to the metal center, particularly in 2c, leading to a significant activation of the B–B bond. In addition, a back donation from the occupied p/d-orbital of the metal atom to the B–B bond can also be observed: however, such interactions are far less significant (1.18 to 9.62 kcal mol−1, see the ESI†).
Conclusions
A new anionic diboron 1, which can be viewed as an analogue of [B2H5]−, was conveniently synthesized in 84% isolated yield by the treatment of tetra(o-tolyl)diborane(4) with sodium hydride. Compound 1 demonstrated the nucleophilicity towards NHC-supported transition-metal halides to afford corresponding η2-bonded complexes. Both structural data and DFT computational results showed that the B–B bonds were dramatically activated with the introduction of transition metals. The reactivity of 1 with other electrophiles is currently under investigation in our laboratory.Data availability
Experimental procedure, NMR spectra, computational details are available in the ESI.† Details of the crystal structures were deposited in the Cambridge Crystallographic Data Centre with CCDC 2133420–2133423 for 1, 2a, 2b, and 2c, respectively.Author contributions
Z. X. and Z. L. conceived and directed the project. X. M. did the experiments. J. Z. performed the computational studies. All authors discussed the results and wrote the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by grants from the Research Grants Council of The Hong Kong Special Administration Region (Project No. 14303621 and 14307421).Notes and references
- V. M. Dembitsky, H. A. Ali and M. Srebnik, Adv. Organomet. Chem., 2004, 51, 193–250 CrossRef CAS.
- (a) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890–931 CrossRef CAS PubMed; (b) R. D. Dewhurst, E. C. Neeve, H. Braunschweig and T. B. Marder, Chem. Commun., 2015, 51, 9594–9607 RSC; (c) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed.
- (a) R. Borthakur, K. Saha, S. Kar and S. Ghosh, Coord. Chem. Rev., 2019, 399, 213021 CrossRef CAS; (b) H. Braunschweig, A. Damme, R. D. Dewhurst and A. Vargas, Nat. Chem., 2013, 5, 115–121 CrossRef CAS PubMed.
- (a) A. B. Cuenca, R. Shishido, H. Ito and E. Fernández, Chem. Soc. Rev., 2017, 46, 415–430 RSC; (b) C. Kleeberg, L. Dang, Z. Lin and T. B. Marder, Angew. Chem., Int. Ed., 2009, 48, 5350–5354 CrossRef CAS PubMed; (c) A. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás and E. Fernández, Angew. Chem., Int. Ed., 2011, 50, 7158–7161 CrossRef CAS PubMed; (d) A.-F. Pécharman, M. S. Hill, G. McMullon, C. L. McMullin and M. F. Mahon, Chem. Sci., 2019, 10, 6672–6682 RSC; (e) A.-F. Pécharman, N. A. Rajabi, M. S. Hill, C. L. McMullin and M. F. Mahon, Chem. Commun., 2019, 55, 9035–9038 RSC; (f) H. Budy, J. Gilmer, T. Trageser and M. Wagner, Eur. J. Inorg. Chem., 2020, 4148–4162 CrossRef CAS.
- Y. Segawa, M. Yamashita and K. Nozaki, Science, 2006, 314, 113–115 CrossRef CAS PubMed.
- (a) A. F. Pécharman, A. L. Colebatch, M. S. Hill, C. L. McMullin, M. F. Mahon and C. Weetman, Nat. Commun., 2017, 8, 15022 CrossRef PubMed; (b) H. Braunschweig, C. W. Chiu, K. Radacki and T. Kupfer, Angew. Chem., Int. Ed., 2010, 49, 2041–2044 CrossRef CAS PubMed; (c) J. Gilmer, H. Budy, T. Kaese, M. Bolte, H. W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2020, 59, 5621–5625 CrossRef CAS PubMed; (d) J. Landmann, J. A. P. Sprenger, R. Bertermann, N. Ignat'ev, V. Bernhardt-Pitchougina, E. Bernhardt, H. Willner and M. Finze, Chem. Commun., 2015, 51, 4989–4992 RSC; (e) K. E. Wentz, A. Molino, S. L. Weisflog, A. Kaur, D. A. Dickie, D. J. D. Wilson and R. J. Gilliard Jr, Angew. Chem., Int. Ed., 2021, 60, 13065–13072 CrossRef CAS PubMed.
- R. D. Dewhurst, E. C. Neeve, H. Braunschweig and T. B. Marder, Chem. Commun., 2015, 51, 9594–9607 RSC.
- (a) T. Trageser, M. Bolte, H. W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2020, 59, 7726–7731 CrossRef CAS PubMed; (b) T. Kaese, A. Hübner, M. Bolte, H. W. Lerner and M. Wagner, J. Am. Chem. Soc., 2016, 138, 6224–6233 CrossRef CAS PubMed.
- (a) S. Akiyama, S. Ikemoto, S. Muratsugu, M. Tada and M. Yamashita, Organometallics, 2020, 39, 500–504 CrossRef CAS; (b) S. Akiyama and M. Yamashita, Chem. Lett., 2020, 49, 721–723 CrossRef CAS.
- K. Lammertsma and T. Ohwada, J. Am. Chem. Soc., 1996, 118, 7247–7254 CrossRef CAS.
- K. Saha, S. Ghorai, S. Kar, S. Saha, R. Halder, B. Raghavendra, E. D. Jemmis and S. Ghosh, Angew. Chem., Int. Ed., 2019, 58, 17684–17689 CrossRef CAS PubMed.
- (a) J. S. Plotkin and S. G. Shore, J. Organomet. Chem., 1979, 182, C15–C19 CrossRef CAS; (b) T. J. Coffy, G. Medford, J. Plotkin, G. J. Long, J. C. Huffman and S. G. Shore, Organometallics, 1989, 8, 2404–2409 CrossRef CAS; (c) P. D. Grebenik, M. L. H. Green, M. A. Kelland, J. B. Leach, P. Mountford, G. Stringer, N. M. Walker and L. L. Wong, J. Chem. Soc., Chem. Commun., 1988, 799–801 RSC; (d) G. Medford and S. G. Shore, J. Am. Chem. Soc., 1978, 100, 3953–3954 CrossRef CAS.
- S. R. Wang, D. Prieschl, J. D. Mattock, M. Arrowsmith, C. Pranckevicius, T. E. Stennett, R. D. Dewhurst, A. Vargas and H. Braunschweig, Angew. Chem., Int. Ed., 2018, 57, 6347–6351 CrossRef CAS PubMed.
- N. Tsukahara, H. Asakawa, K. H. Lee, Z. Lin and M. Yamashita, J. Am. Chem. Soc., 2017, 139, 2593–2596 CrossRef CAS PubMed.
- S. Akiyama, K. Yamada and M. Yamashita, Angew. Chem., Int. Ed., 2019, 58, 11806–11810 CrossRef CAS PubMed.
- R. B. Bedford, P. B. Brenner, E. Carter, T. Gallagher, D. M. Murphy and D. R. Pye, Organometallics, 2014, 33, 5940–5943 CrossRef CAS.
- P. Bissinger, A. Steffen, A. Vargas, R. D. Dewhurst, A. Damme and H. Braunschweig, Angew. Chem., Int. Ed., 2015, 54, 4362–4366 CrossRef CAS PubMed.
- P. Bissinger, H. Braunschweig, A. Damme, T. Kupfer and A. Vargas, Angew. Chem., Int. Ed., 2012, 51, 9931–9934 CrossRef CAS PubMed.
- (a) W. Lu, H. Hu, Y. Li, R. Ganguly and R. Kinjo, J. Am. Chem. Soc., 2016, 138, 6650–6661 CrossRef CAS PubMed; (b) Y. Segawa, M. Yamashita and K. Nozaki, Angew. Chem., Int. Ed., 2007, 46, 6710–6713 CrossRef CAS PubMed.
- H. Braunschweig, P. Brenner, R. D. Dewhurst, M. Kaupp, R. Müller and S. Östreicher, Angew. Chem., Int. Ed., 2009, 48, 9735–9738 CrossRef CAS PubMed.
- H. Braunschweig, K. Radacki and R. Shang, Chem. Commun., 2013, 49, 9905–9907 RSC.
- (a) W. Lu and R. Kinjo, Chem.–Eur. J., 2018, 24, 15656–15662 CrossRef CAS PubMed; (b) H. Braunschweig, K. Radacki and R. Shang, Chem. Sci., 2015, 6, 2989–2996 RSC.
- (a) W. Clegg, C. Dai, F. J. Lawlor, T. B. Marder, P. Nguyen, N. C. Norman, N. L. Pickett, W. P. Power and A. J. Scott, J. Chem. Soc., Dalton Trans., 1997, 839–846 RSC; (b) C. P. Brock, M. K. Das, R. P. Minton and K. Niedenzu, J. Am. Chem. Soc., 1988, 110, 817–822 CrossRef CAS; (c) W. VanDoorne, A. W. Cordes and G. W. Hunt, Inorg. Chem., 1973, 12, 1686–1689 CrossRef CAS; (d) Y. Shoji, S. Kaneda, H. Fueno, K. Tanaka, K. Tamao, D. Hashizume and T. Matsuo, Chem. Lett., 2014, 43, 1587–1589 CrossRef CAS.
- P. Bissinger, A. Steffen, A. Vargas, R. D. Dewhurst, A. Damme and H. Braunschweig, Angew. Chem., Int. Ed., 2015, 54, 4362–4366 CrossRef CAS PubMed.
- (a) P. Motloch, J. Jašík and J. Roithová, Organometallics, 2021, 40, 1492–1502 CrossRef CAS PubMed; (b) A. Fedorov, E. P. A. Couzijn, N. S. Nagornova, O. V. Boyarkin, T. R. Rizzo and P. Chen, J. Am. Chem. Soc., 2010, 132, 13789–13798 CrossRef CAS PubMed; (c) H. V. R. Dias, J. A. Flores, J. Wu and P. Kroll, J. Am. Chem. Soc., 2009, 131, 11249–11255 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, complete characterization data, NMR spectra. CCDC 2133420–2133423 for 1, 2a, 2b and 2c. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2sc00318j |
| ‡ Present address: Department of Chemistry, City University of Hong Kong, 83 Tat Chee Ave, Hong Kong, China. |
| This journal is © The Royal Society of Chemistry 2022 |