Open Access Article
Open Access ArticleDesign and synthesis of stable four-coordinated benzotriazole-borane with tunable fluorescence emission†
Qi
Tang
a,
Shi-Jun
Li
 b,
Xiaohan
Ye
a,
Teng
Yuan
a,
Kai
Zhao
a,
Ying
He
a,
Chuan
Shan
b,
Xiaohan
Ye
a,
Teng
Yuan
a,
Kai
Zhao
a,
Ying
He
a,
Chuan
Shan
 a,
Lukasz
Wojtas
a,
David
Richardson
c,
Yu
Lan
a,
Lukasz
Wojtas
a,
David
Richardson
c,
Yu
Lan
 *b and
Xiaodong
Shi
*b and
Xiaodong
Shi
 *a
*a
aDepartment of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, Florida 33620, USA. E-mail: xmshi@usf.edu
bCollege of Chemistry, Institute of Green Catalysis, Zhengzhou University, Zhengzhou, Henan 450001, China
cDepartment of Chemistry, University of Central Florida, Orlando, Florida 32816, USA
First published on 18th April 2022
Abstract
A new class of stable four-coordinated benzotriazole-borane compounds was developed via gold-catalyzed alkyne hydroboration. The application of polymeric (BH2CN)n reagent gave the formation of cyano-amine-boranes (CAB) complexes with less basic N-heterocyclic amines and anilines. Various new CABs were investigated in catalytic hydroboration to synthesize N–B cycles. The 1,2,3-benzotriazoles were identified as the only feasible N-source, giving the four coordinated borane N–B cycles (BTAB) in excellent yields (up to 90%) with good functional group tolerability. This new class of polycyclic N–B compounds showed excellent stability toward acid, base, high temperature, and photo-irradiation. The facile synthesis, excellent stability, strong and tunable fluorescence emission make BTAB interesting new fluorescent probes for future chemical and biological applications.
Introduction
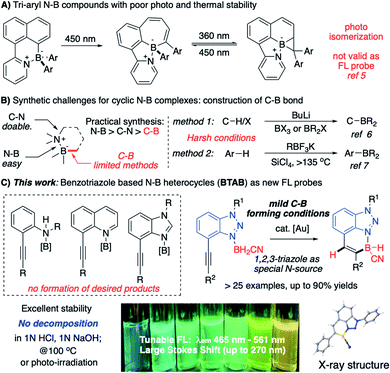
With the good binding affinity between nitrogen lone-pair electrons and boron's empty orbital, the amine–borane complexes have been widely applied as versatile synthetic reagents.1 Recently, discovering interesting photo/electronic properties associated with the cyclic N–B compounds makes them an important class of heterocycles in chemical, material, and biomedical research.2 With the discovery of BODIPY and its derivatives, the four-coordinate boron “ate” complexes have received great attention due to their interesting fluorescence (FL) properties.3 Recently, the discovery of aryl-modified boron complexes (containing C–B–N moiety) led to a new direction for developing molecular probes with novel structural skeletons.4 However, with labile B–Ar bonds, these compounds undergo rapid photo-initiated isomerization (Scheme 1A), impeding them from being valid FL probes.5The synthesis of N–B heterocycles could be challenging. As shown in Scheme 1B, N–B heterocycle formation involves the selected construction of N–B, C–N, and C–B bonds. Compared with N–B and C–N bonds, the C–B bond construction is the most challenging due to limited available methods. Currently, strategies for cyclic N–B complex synthesis include (A) carbon anion (from n-BuLi C–H deprotonation) nucleophilic addition6 and (B) LA promoted aryl C–H activation.7 The lack of effective practical approaches for large-scale synthesis and the strong need to access new N–B modified polycyclic molecular skeletons make developing a new synthetic strategy toward the N–B modified polycyclic system highly desirable.8 In this work, we report the preparation of benzotriazole borane (BTAB) as a new class of stable four-coordinate N–B heterocycles through gold-catalyzed alkyne hydroboration. These new N–B heterocycles give strong and tunable FL emission (λmax = 465 nm to 561 nm) with excellent stability toward acid (1 N HCl), base (1 N NaOH), thermal (100 °C, 24 h), and photo radiation (254 nm and 365 nm, 24 h), making them promising new molecular probes for chemical research (Scheme 1C).
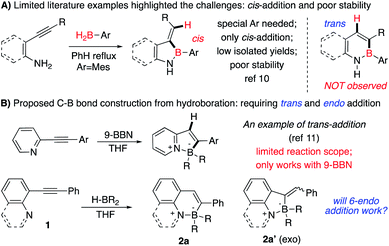
One effective strategy for vinyl-C–B bond synthesis is alkyne hydroboration.9e Though this method has been applied for many B-containing compound preparation, it could not be feasible in the N–B heterocycles synthesis. As shown in Fig. 1A, cis-addition of alkyne in most catalytic system leads to a “wrong” regioisomer against the sequential endo-type cyclization.10 Although Wang and coworkers reported an interesting route to N–B heterocycles with the assistance of directing nitrogen (Fig. 1B),11 it only works with 9-BBN as borane source, and the resulting 5-membered N–B heterocycles have poor stability, even decomposing upon the separation. To test whether this method would be valid for broader N–B heterocycles synthesis, we prepared alkynes 1a–1e (Fig. 2C) to react with 9-BBN using Wang's condition. No cyclic N–B compounds were observed in any cases. This result, again, highlighted the challenges associated with the synthesis of polycyclic four-coordinated N–B compounds.
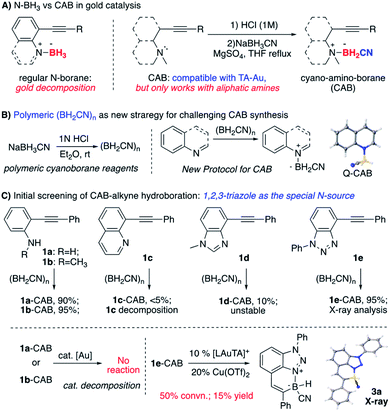
Our group recently reported that the triazole-gold (TA-Au) catalyzed alkyne hydroboration to synthesize cyclic N–B compounds (N as aliphatic amine).12 As we reported previously, triazole gold [L-Au-TA]+ showed significantly improved stability over [L-Au]+. However, treating TA-Au with [N]–BH3 still led to rapid gold decomposition, highlighting the challenge of applying gold catalysis under a reductive environment. Fortunately, the cyano-amino-borane (CAB, N–BH2CN) was identified as the valid hydride source, giving hydroboration with TA-Au as the catalyst ([L-Au]+ gave rapid decomposition). The initial preparation of CABs could be tricky by treating the aliphatic amine HCl salts with NaBH3CN in THF. As a result, this protocol could not be used for the polycyclic N–B compound synthesis since both aniline and N-heterocycles failed to produce CABs using the initial conditions (Fig. 2A).
Over the past several years, we have devoted efforts to exploring new strategies for plausible CAB synthesis with aniline or N-heterocycles as the N-source. After many attempts, we identified polymeric cyano-borane (BH2CN)n as a new reagent in CAB synthesis.13 As shown in Fig. 2B, this polymeric (BH2CN)n reagent could be readily prepared by treating NaBH3CN with 1 N HCl (see ESI†). The resulting THF solution can be used directly to react with quinoline, giving the desired Q-CAB in 90% yields. With this new reagent, the desired CABs of aniline 1a or 1b were prepared in excellent yields. Other N-heterocycles were also tested. Both quinoline alkyne 1c and benzimidazole alkyne 1d gave low yields of CAB due to poor stability (substrate decomposition). Interestingly, the 1,2,3-benzotriazole alkyne 1e gave the desired CAB with excellent yield (95%).
Results and discussion
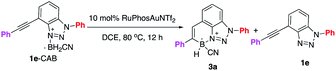
Encouraged by the synthesis of aniline and triazole CABs, the alkyne-CAB complexes were charged with gold-catalyzed hydroboration under various conditions. After exploring various gold-catalyzed conditions, the aniline CABs (1a and 1b) failed to give any hydroboration products with rapid gold decomposition, likely due to the more reactive B–H. Interestingly, under previously optimal conditions, 10 mol% [(ArO)3PAu(TA-Ph)]OTf and 20 mol% Cu(OTf)2 at 60 °C, benzotriazole modified 1e-CAB successfully generated the desired cyclic N–B product 3a, though in low yields (Fig. 2C, 50% conversion, 15% yields). To our surprise, this new N–B heterocycle 3a was very stable toward air, moisture, and even elevated temperature (vide infra). Therefore, the low yield results from gold catalyst decomposition (low conversation) and 1e-CAB decomposition (formation of 1e). This result was exciting since it confirmed that 1,2,3-benzotriazole, as a special N-heterocycle, could be successfully applied to construct the N–B heterocycle as a new molecular skeleton. Comprehensive condition screening was performed, and RuPhosAuNTf2 (10 mol%) in DCE at 80 °C was identified as the optimal one, giving the BTAB 3a in 87% isolated yields. Comparison with selected alternative conditions is summarized in Table 1 (see detailed conditions screening in ESI†).| Entry | Variation from “standard conditions” | Convn. (%) | 3a (%) | 1e (%) |
|---|---|---|---|---|
| a Conditions: 1e-CAB (0.1 mmol), Au cat. (0.01 mmol), DCE (2 mL), 80 °C, 12 h. b 1H NMR yields using 1,3,5-tribromobenzene as an internal standard (isolated yields). | ||||
| 1 | None | 100 | 90 (87) | <5 |
| 2 | [Au] = PPh3AuNTf2 | <5 | n.d. | n.d. |
| 3 | IPrAuNTf2 | 70 | 20 | 38 |
| 4 | JohnPhosAuNTf2 | 80 | 45 | 24 |
| 5 | (ArO)3PAuNTf2 | 52 | 10 | 35 |
| 6 | SPhosAuNTf2 | 95 | 78 | 10 |
| 7 | TA-Au: RuPhosAu(TA-H)OTf | 43 | 8 | 30 |
| 8 | [Au] + [Cu]: RuPhosAu(TA-H)OTf + Cu(OTf)2 | 20 | <5 | n.d. |
| 9 | Other solvents (CH3CN, THF, tol, MeOH. See details in ESI) | <89 | <71 | — |
| 10 | 5% [Au] | 65 | 54 | <5 |
| 11 | 60 °C | 65 | 30 | — |
| 12 | Other metal cat. (Ag, Cu, Zr, Zn etc. See ESI) | <10% | n.d. | — |
As shown in Table 1, among various tested metal complexes (including Ag, Cu, Zr, Zn etc. entry 12), the cationic gold is the only one that gives desired hydroboration products, which highlighted the critical role of gold catalyst for this transformation. The primary ligand on gold plays a crucial role by providing balanced reactivity and catalyst stability. The Buchwald type bis-aryl ligands increased conversion with the improved gold cation stability (entries 5–6). Notably, while triazole helps to improve gold stability, TA-Au gave low conversion (entry 7), likely due to the competing TA-borane coordination. Reducing reaction temperature to 60 °C caused a slow reaction rate with gold decomposition over time. With the optimal condition in hand, the substrate scope was evaluated.
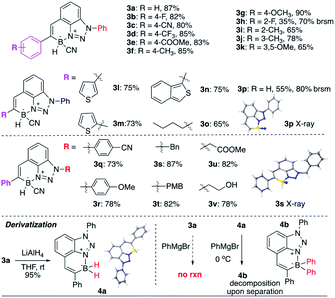
As shown in Table 2, this new transformation works well with aromatic and aliphatic substituted alkynes, giving the desired BTAB in good yields. Both EDG and EWG modified aryl alkynes work well (3a–3g), except ortho-substituted benzene (3h, slow conversion, likely due to the combination of electronic effect and steric hindrance). Impressively, thiophene substituted alkynes (3l–3n) are suitable substrates for this reaction, albeit the potential Au–S binding. Terminal alkyne (3p) and aliphatic alkyne (3o) give slightly reduced yields. The substituted group on the N-1 position of benzotriazole was also tested. Both N-aryl (3q) and N-alkyl (3s) substituted benzotriazole are suitable substrates. In addition, both EDG and EWG modified N-aryl triazoles gave the desired N–B heterocycles in good yields (3q, 3r). Various N-alkyl substituted triazoles (3s–3u) were applied, giving the BTABs in good yields, suggesting the excellent functional group tolerability and easy modification for future applications (with N-1 position as the synthetic handle).
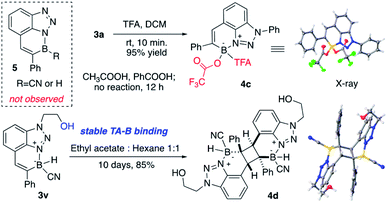
Treating BTAB 3a with LiAlH4 gave the dihydro borane 4a (R2BH2) in excellent yield, confirming the feasibility of sequential modification on boron after cyclization. However, the reaction of 3a with Grignard reagents gave no CN substitution. Instead, the reaction between 4a (R2BH2) and excess PhMgBr gave the formation of diphenyl substituted borane 4b (decomposition upon separation, based on crude NMR).14 The polyaromatic hydrocarbon (PAH) compounds have received tremendous attention over the past decades due to their interesting photo and electronic properties.15 With the incorporation of “electron” and “hole” pair, the NB modified PAH is undoubtedly one interesting modification of typical PAH. As a six-membered N–B heterocycle, one question is whether this new class BTAB would transform into three-coordinated azaborine as polyaromatic hydrocarbons, especially with active B–H bond (losing hydride).
To explore this idea, BTAB 3a was treated by various RCOOH. As shown in Fig. 3, while acetic acid and PhCOOH gave no reaction, treating 3a with TFA gave the rapid formation of TFA substituted BTAB 4c in excellent yield (structure confirmed by singles crystal X-ray structure determination). Interestingly, the hydroxy modified BTAB 3v could give the [2 + 2] cycloaddition product 4d (X-ray) over a long period (10 days) without N–B bond dissociation in the hexane and ethyl acetate solvent mixture. These results suggested the excellent N–B bond stability of this four-coordinated BTAB polycycles.
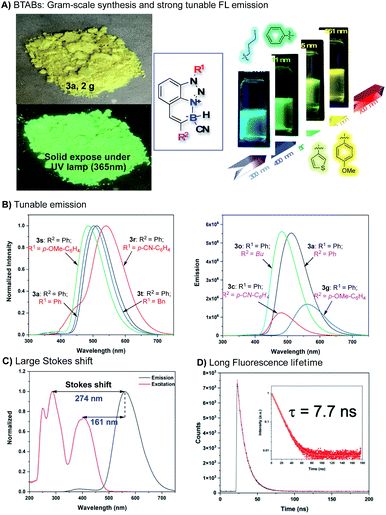
Comprehensive photophysical properties evaluations of these new stable N–B polycyclic molecular skeletons were performed. As expected, strong photoluminescence activity was observed. The detailed excitation (λex), emission (λem), adsorption (ε), Stokes shift, quantum yield (φF), and fluorescence lifetime (FLT) date are summarized in SI. Some representative data are highlighted in Fig. 4. First, all BTABs gave strong fluorescence emission in the solid-state and in the solution with emission λmax between 465 nm and 561 nm (Fig. 4A). In addition, this N–B polycyclic skeleton showed impressive stability with no decomposition in acid (1 N HCl, 24 h), base (1 N NaOH, 24 h), thermal (100 °C, 24 h), and photo-irradiation (254 nm, 24 h). Both N-substituted group R1 and alkyne C-substitute group R2 would influence the overall fluorescence performance, giving tunable emissions. As shown in Fig. 4B, extended conjugation on both C and N positions gave the expected red shift (3avs.3t; 3rvs.3t). Similarly, since TA is electron deficient, the electron-withdrawing group (EWG, R1) modified 3r gave further redshift compared to the electron-donating group (EDG) modified 3s. In contrast, EDG modified R2 substrates cause the red shift compared to the EWG modified R2 substrates due to this extended conjugation effect (3gvs.3c). Impressively, a large Stokes shift (193 nm to 274 nm, Fig. 4C) and long fluorescence lifetime (7.7 ns, Fig. 4D) were obtained with these BTABs, suggesting promising application scope as this new FL probe.16
Finally, density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were performed to explore the emission mechanism. The calculation of 3a at PBE0 level shows the first two peaks of absorption are 404 nm and 297 nm, which are very close to the experimental results (375 nm and 287 nm). The calculated energy gap of HOMO and LUMO was 3.8 eV, corresponding to the first absorption peak. The analysis of those orbitals shows that HOMO is majorly located on azaborine moiety while LUMO is majorly located on benzotriazole moiety, suggesting a possible charge transfer process from the azaborine to benzotriazole in the exited process. Based on this computational model, the emission was determined as 522 nm, which was in very good agreement with the experimental data (511 nm), suggesting the good correlation of the computational model in explaining the fluorescence emission mechanism for these new BTAB compounds.
Conclusions
In summary, with the application of polymeric (BH2CN)n reagent to form cyanoborane complexes of less reactive N-heterocycles, we identified 1,2,3-benzotriazole as the critical building block and successfully developed a highly efficient synthesis to achieve a new class of N–B heterocycles BTABs. These new compounds showed strong, tunable fluorescence emission with excellent stability toward acid, base, and photoirradiation. The combination of easy gram-scale synthesis, long lifetime, and large Stokes shift makes BTABs a promising new fluorophore for future chemical and material applications.Data availability
All experimental and characterization data, including experiment procedure, NMR spectra, crystallographic data, photophysical spectra, and calculations are available in the ESI.†Author contributions
X. S. designed and directed the investigations. Q. T. performed the experiments. Q. T., X. Y., T. Y., K. Z., and Y. H. analyzed and discussed the experiment results. S. L. and Y. L. carried out the calculations. C. S. and L. W. carried out the X-ray crystallographic measurements. D. R. collected the 19B NMR spectroscopic data. All authors were involved in the preparation of the manuscript. The final manuscript was approved by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the NSF (CHE-2054180) and NIH (1R01GM120240-01) for financial support. This work has been supported in part by University of South Florida Interdisciplinary NMR Facility and the Chemical Purification, Analysis, and Screening (CPAS) Core Facility.Notes and references
- (a) D. H. A. Boom, A. R. Jupp and J. C. Slootweg, Chem.–Eur. J., 2019, 25, 9133–9152 CrossRef CAS PubMed; (b) S. Lau, D. Gasperini and R. L. Webster, Angew. Chem., Int. Ed., 2021, 60, 14272–14294 CrossRef CAS PubMed; (c) A. Rossin and M. Peruzzini, Chem. Rev., 2016, 116, 8848–8872 CrossRef CAS PubMed; (d) A. Staubitz, A. P. M. Robertson, M. E. Sloan and I. Manners, Chem. Rev., 2010, 110, 4023–4078 CrossRef CAS PubMed; (e) Y. Liu, R. Puig de la Bellacasa, B. Li, A. B. Cuenca and S.-Y. Liu, J. Am. Chem. Soc., 2021, 143, 14059–14064 CrossRef CAS PubMed.
- (a) F. Yang, M. Zhu, J. Zhang and H. Zhou, MedChemComm, 2018, 9, 201–211 RSC; (b) Z. X. Giustra and S. Y. Liu, J. Am. Chem. Soc., 2018, 140, 1184–1194 CrossRef CAS PubMed; (c) C. R. McConnell and S. Y. Liu, Chem. Soc. Rev., 2019, 48, 3436–3453 RSC; (d) A. Bafana, S. S. Devi and T. Chakrabarti, Environ. Rev., 2011, 19, 350–370 CrossRef CAS; (e) P. G. Campbell, A. J. V. Marwitz and S. Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074–6092 CrossRef CAS PubMed; (f) X. Gao, Y. Yao and X. Meng, Mater. Sci. Semicond. Process., 2020, 120, 105256 CrossRef CAS.
- (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (b) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC; (c) Y. Ni and J. S. Wu, Org. Biomol. Chem., 2014, 12, 3774–3791 RSC; (d) N. Boens, B. Verbelen and W. Dehaen, Eur. J. Org. Chem., 2015, 2015, 6577–6595 CrossRef CAS; (e) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC; (f) J. Zhang, N. N. Wang, X. Ji, Y. F. Tao, J. M. Wang and W. L. Zhao, Chem.–Eur. J., 2020, 26, 4172–4192 CrossRef CAS PubMed.
- (a) S. K. Mellerup and S. Wang, Chem. Soc. Rev., 2019, 48, 3537–3549 RSC; (b) S. K. Mellerup and S. N. Wang, Trends Chem., 2019, 1, 77–89 CrossRef CAS; (c) D.-T. Yang, S. K. Mellerup, J.-B. Peng, X. Wang, Q.-S. Li and S. Wang, J. Am. Chem. Soc., 2016, 138, 11513–11516 CrossRef CAS PubMed.
- Z.-C. He, S. K. Mellerup, L. Liu, X. Wang, C. Dao and S. Wang, Angew. Chem., Int. Ed., 2019, 58, 6683–6687 CrossRef CAS PubMed.
- (a) P.-F. Zhang, J.-C. Zeng, F.-D. Zhuang, K.-X. Zhao, Z.-H. Sun, Z.-F. Yao, Y. Lu, X.-Y. Wang, J.-Y. Wang and J. Pei, Angew. Chem., Int. Ed., 2021, 60, 23313–23319 CrossRef CAS PubMed; (b) S. K. Mellerup, L. Häfele, A. Lorbach, X. Wang and S. Wang, Org. Lett., 2017, 19, 3851–3854 CrossRef CAS PubMed; (c) S. Wang, K. Yuan, M.-F. Hu, X. Wang, T. Peng, N. Wang and Q.-S. Li, Angew. Chem., Int. Ed., 2018, 57, 1073–1077 CrossRef CAS PubMed.
- (a) S. A. Iqbal, J. Pahl, K. Yuan and M. J. Ingleson, Chem. Soc. Rev., 2020, 49, 4564–4591 RSC; (b) K. Yang and Q. L. Song, Acc. Chem. Res., 2021, 54, 2298–2312 CrossRef CAS PubMed.
- A. M. Priegert, B. W. Rawe, S. C. Serin and D. P. Gates, Chem. Soc. Rev., 2016, 45, 922–953 RSC.
- (a) D. M. Tellers, S. J. Skoog, R. G. Bergman, T. B. Gunnoe and W. D. Harman, Organometallics, 2000, 19, 2428–2432 CrossRef CAS; (b) C. M. Crudden and D. Edwards, Eur. J. Org. Chem., 2003, 14, 4695–4712 CrossRef; (c) Z. H. Huang and E. Negishi, J. Am. Chem. Soc., 2007, 129, 14788–14792 CrossRef CAS PubMed; (d) S. Q. Xu, C. T. Lee, H. H. Rao and E. Negishi, Adv. Synth. Catal., 2011, 353, 2981–2987 CrossRef CAS PubMed; (e) L. Weber, D. Eickhoff, J. Halama, S. Werner, J. Kahlert, H. G. Stammler and B. Neumann, Eur. J. Inorg. Chem., 2013, 14, 2608–2614 CrossRef; (f) Z. Q. Zuo, J. Yang and Z. Huang, Angew. Chem., Int. Ed., 2016, 55, 10839–10843 CrossRef CAS PubMed; (g) J. V. Obligacion and P. J. Chirik, Nat. Rev. Chem., 2018, 2, 15–34 CrossRef CAS PubMed; (h) R. J. Procter, M. Uzelac, J. Cid, P. J. Rushworth and M. J. Ingleson, ACS Catal., 2019, 9, 5760–5771 CrossRef CAS; (i) K. Skoch, C. G. Daniliuc, G. Kehr and G. Erker, Angew. Chem., Int. Ed., 2021, 60, 6757–6763 CrossRef CAS PubMed; (j) L. Weber, D. Eickhoff, J. Halama, S. Werner, J. Kahlert, H.-G. Stammler and B. Neumann, Eur. J. Inorg. Chem., 2013, 2013, 2608–2614 CrossRef CAS.
- K. Yuan, X. Wang, S. K. Mellerup, I. Kozin and S. N. Wang, J. Org. Chem., 2017, 82, 13481–13487 CrossRef CAS PubMed.
- K. Yuan, N. Suzuki, S. K. Mellerup, X. Wang, S. Yamaguchi and S. N. Wang, Org. Lett., 2016, 18, 720–723 CrossRef CAS PubMed.
- (a) Q. Y. Wang, S. E. Motika, N. G. Akhmedov, J. L. Petersen and X. D. Shi, Angew. Chem., Int. Ed., 2014, 53, 5418–5422 CrossRef CAS PubMed; (b) S. E. Motika, Q. Y. Wang, N. G. Akhmedov, L. Wojtas and X. D. Shi, Angew. Chem., Int. Ed., 2016, 55, 11582–11586 CrossRef CAS PubMed.
- B. F. Spielvogel, R. F. Bratton and C. G. Moreland, J. Am. Chem. Soc., 1972, 94, 8597–8598 CrossRef CAS.
- (a) Y. G. Shi, D. T. Yang, S. K. Mellerup, N. Wang, T. Peng and S. N. Wang, Org. Lett., 2016, 18, 1626–1629 CrossRef CAS PubMed; (b) M. J. D. Bosdet and W. E. Piers, Can. J. Chem., 2009, 87, 8–29 CrossRef.
- (a) P. Kissel, R. Erni, W. B. Schweizer, M. D. Rossell, B. T. King, T. Bauer, S. Götzinger, A. D. Schlüter and J. Sakamoto, Nat. Chem., 2012, 4, 287–291 CrossRef CAS PubMed; (b) S. Kim, C. M. Park, M. Jang, A. Son, N. Her, M. Yu, S. Snyder, D.-H. Kim and Y. Yoon, Chemosphere, 2018, 212, 1104–1124 CrossRef CAS PubMed.
- Y. Zhang, J. Lee and S. R. Forrest, Nat. Commun., 2014, 5, 5008 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, NMR spectra, ESI-MS spectra and crystallographic data. CCDC 2113561, 2113563, 2113565–2113567, 2113568–2113571, 2114086, 2114265, 2114505 and 2113568. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01103d |
| This journal is © The Royal Society of Chemistry 2022 |