Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydroxy-directed fluorination of remote unactivated C(sp3)–H bonds: a new age of diastereoselective radical fluorination†
Stefan Andrew
Harry
 ,
Michael Richard
Xiang
,
Eric
Holt
,
Andrea
Zhu
,
Fereshte
Ghorbani
,
Dhaval
Patel
and
Thomas
Lectka
*
,
Michael Richard
Xiang
,
Eric
Holt
,
Andrea
Zhu
,
Fereshte
Ghorbani
,
Dhaval
Patel
and
Thomas
Lectka
*
Department of Chemistry, Johns Hopkins University, 3400 N. Charles St., Baltimore, MD 21218, USA. E-mail: lectka@jhu.edu
First published on 30th May 2022
Abstract
We report a photochemically induced, hydroxy-directed fluorination that addresses the prevailing challenge of high diastereoselectivity in this burgeoning field. Numerous simple and complex motifs showcase a spectrum of regio- and stereochemical outcomes based on the configuration of the hydroxy group. Notable examples include a long-sought switch in the selectivity of the refractory sclareolide core, an override of benzylic fluorination, and a rare case of 3,3′-difluorination. Furthermore, calculations illuminate a low barrier transition state for fluorination, supporting our notion that alcohols are engaged in coordinated reagent direction. A hydrogen bonding interaction between the innate hydroxy directing group and fluorine is also highlighted for several substrates with 19F–1H HOESY experiments, calculations, and more.
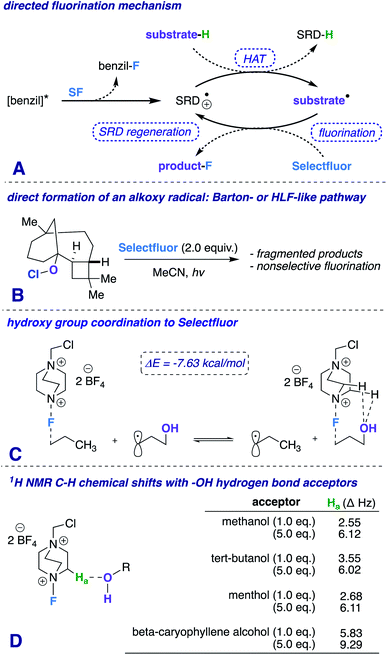
The hydroxy (OH) group is treasured and versatile in chemistry and biology.1 Its ubiquity in nature and broad spectrum of chemical properties make it an attractive source as a potential directing group.2 The exploitation of the mild Lewis basicity exhibited by alcohols has afforded several elegant pathways for selective functionalization (e.g., Sharpless epoxidation,3 homogeneous hydrogenation,4 cross-coupling reactions,5 among others6). Recently, we reported a photochemically promoted carbonyl-directed aliphatic fluorination, and most notably, established the key role that C–H⋯O hydrogen bonds play in the success of the reaction.7 Our detailed mechanistic investigations prompt us to postulate that other Lewis basic functional groups (such as –OH) can direct fluorination in highly complementary ways.8 In this communication, we report a hydroxy-directed aliphatic fluorination method that exhibits unique directing properties and greatly expands the domain of radical fluorination into the less established realm governing high diastereoselectivity.9
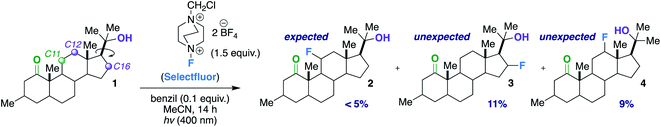
Our first inclination that functional groups other than carbonyls may influence fluorination regiochemical outcomes was obtained while screening substrates for our published ketone-directed radical-based method (Scheme 1).8a In this example, we surmised that oxidation of the tertiary hydroxy group on substrate 1 cannot occur and would demonstrate functional group tolerance (directing to C11, compound 2). Surprisingly, the two major regioisomers (products 3 and 4) are derivatized by Selectfluor (SF) on C12 and C16 – indicative of the freely rotating hydroxyl directing fluorination. Without an obvious explanation of how these groups could be involved in dictating regiochemistry, we continued the mechanistic study of carbonyl-directed fluorination (Scheme 2A). We established that the regioselective coordinated hydrogen atom abstraction occurs by hydrogen bonding between a strategically placed carbonyl and Selectfluor radical dication (SRD).7 However, we noted that the subsequent radical fluorination is not diastereoselective due to the locally planar nature of carbonyl groups. Thus, we posed the question: are there other directing groups that can provide both regio- and diastereoselectivity? Such a group would optimally be attached to a sp3 hybridized carbon; thus the “three dimensional” hydroxy carbon logically comes to mind as an attractive choice, and Scheme 1 illustrates the first positive hint.
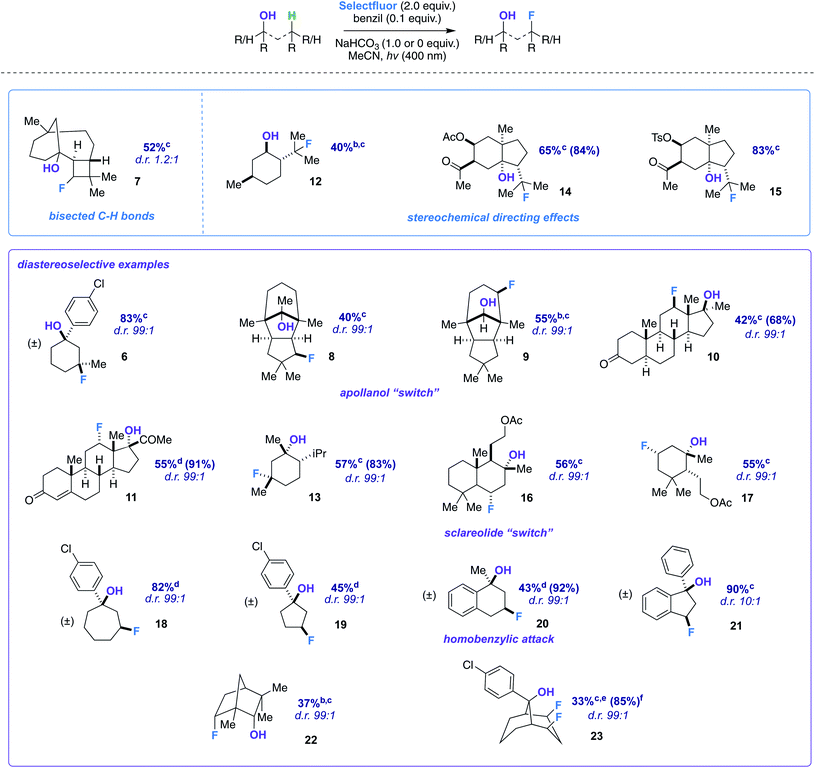
We began our detailed study with a simple substrate that contains a tertiary hydroxyl group. Alcohol 5 was synthesized stereoselectively by the reaction of 3-methylcyclohexanone, FeCl3, and 4-chlorophenylmagnesium bromide;10 the 4-chlorophenyl substituent allows for an uncomplicated product identification and isolation (aromatic chromophore). We sought to determine optimal reaction conditions by examination of numerous photosensitizers, bases, solvents, and light sources (Table 1). To our satisfaction, fluorination not only provides the intended regioisomer but only a single diastereomer is formed (compound 6). A photosensitizer screen shows that benzil (in MeCN solvent) generally affords the highest yield (83%).7 Although we utilize cool blue LEDs (sharp cutoff ca. 400 nm), CFLs (small amount of UVB (280–315 nm) and UVA (315–400 nm)) are useable as well.11 A mild base additive was also found to neutralize adventitious HF and improve yields in the substrates indicated (Table 2). Control substrates, such as methylcyclohexane, afford low or no yields of product mixtures under identical conditions.
| Entry | Sensitizer | 19F yield |
|---|---|---|
| a Unless stated otherwise: substrate (0.25 mmol, 1.0 equiv.), Selectfluor (0.50 mmol, 2.0 equiv.), NaHCO3 (0.25 mmol, 1.0 equiv.), and sensitizer (0.025 mmol, 10 mol%) were dissolved in MeCN (4.0 mL) and irradiated with cool white LEDs for 14 h. | ||
| 1 | None | 0% |
| 2 | Benzil | 83% |
| 3 | Benzil, no base | 63% |
| 4 | Benzil, K2CO3 | 68% |
| 5 | Benzil, CFL light source | 75% |
| 6 | 5-Dibenzosuberenone | 15% |
| 7 | 4,4′-Difluorobenzil | 63% |
| 8 | 9,10-Phenantherenequinone | 71% |
| 9 | Perylene | 8% |
| 10 | Methyl benzoylformate | 42% |
| a Unless otherwise specified, the substrate (0.25 mmol, 1.0 equiv.), Selectfluor (0.50 mmol, 2.0 equiv.), NaHCO3 (0.25 mmol, 1.0 equiv. or 0.0 equiv.), and benzil (0.025 mmol 10 mol%) were stirred in MeCN (4.0 mL) and irradiated with cool white LEDs for 14 h. Yields were determined by integration of 19F NMR signals relative to an internal standard and confirmed by isolation of products through column chromatography on silica gel. Yields based on recovered starting material in parentheses. Major diastereomer (with respect to C–F bond) depicted where known. b 1.2 equiv. of Selectfluor used. c 1.0 equiv. of NaHCO3. d 0.0 equiv. of NaHCO3. e 3.0 equiv. of Selectfluor used. f Including the monofluoride (approx. 11%) with starting material. |
|---|
|
The screening concurrently buttresses our claim that hydroxy-directed fluorination is proceeding through a mechanism involving a network of C–H⋯OH hydrogen bonds.12 Other N–F reagents (for example, N-fluorobenzenesulfonimide and N-fluoropyridinium tetrafluoroborate) do not provide the desired fluorinated product 6. The 1,3-diaxial relationship shown in Fig. 1 presents an intramolecular competition: tertiary vs. secondary C–H abstraction (O⋯H–C calculated distances: 2.62 and 2.70 Å at B3LYP 6-311++G**, respectively). The tertiary fluoride is the major product in this case.
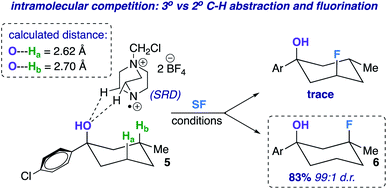 | ||
| Fig. 1 Example of an intramolecular competition (secondary vs. tertiary C–H abstraction/fluorination) and calculated C–H⋯O distances of compound 5 (B3LYP/6-311++G**). | ||
With optimized conditions established, we assessed the site-selectivity of the method with a molecule derived from the acid catalyzed cyclization of α-caryophyllene, β-caryophyllene alcohol (commonly used as a fragrance ingredient in cosmetics, soaps, and detergents).13 When subjected to fluorination conditions, it targets the strained cyclobutane ring (substrate 7) in 52% yield (Table 2). Based on computational modeling (B3LYP/6-311++G**), the hydroxy group bisects the cyclobutane ring; thus, the diastereomeric ratio is only 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, this observation suggests that diastereoselectivity is incumbent upon the relative position of the hydroxy group in space to the carbon radical, and that substrates should be assessed geometrically for suitability. Products 12–15, on the other hand, illustrate that directionality may in turn influence site-selectivity.
1. However, this observation suggests that diastereoselectivity is incumbent upon the relative position of the hydroxy group in space to the carbon radical, and that substrates should be assessed geometrically for suitability. Products 12–15, on the other hand, illustrate that directionality may in turn influence site-selectivity.
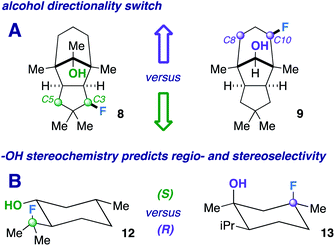
Next, we pursued a substrate that supports our notion that hydroxy group orientation can influence both site selectivity and diastereoselectivity favorably. Another unique, rare natural product derived from α-caryophyllene came to mind; 11-apollanol (α-caryophyllene alcohol 9).14 The hydroxy group stereochemistry is poised to direct fluorination to either the C8 or C10 positions (compound 9) due to the plane of symmetry (Fig. 3A). Moreover, we synthesized a complementary derivative through PCC oxidation followed by a Grignard reaction, thereby switching directionality of the hydroxy group (Fig. 3A) to target the C3 or C5 positions instead (compound 8). We found the resultant fluorinated products to be what one expects if engaged in coordinated hydrogen atom transfer (HAT) (55% and 40% for molecules 9 and 8) – a change in regiochemistry based on the stereochemistry of the alcohol. Additionally, only a single stereoisomer is produced for both (d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and reinforce this study as a salient example of diastereoselective radical fluorination.
1) and reinforce this study as a salient example of diastereoselective radical fluorination.
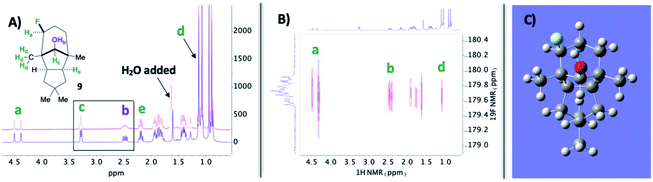
In the midst of characterizing compound 9, we uncovered a noteworthy hydrogen bonding interaction. Firstly, our plan was to identify the –OH peak within the 1H NMR spectrum and determine if there is a through-space interaction with fluorine in the 19F–1H HOESY NMR spectrum (ultimately aiding in assigning the stereochemistry of the fluorine).15 At first glance, no peaks were immediately discernible as the –OH; however, when a stoichiometric amount of H2O is added, it becomes apparent that the –OH group and geminal proton to the hydroxy peaks broaden by rapid proton exchange (Fig. 2A). Upon closer examination of the dry 1H NMR spectrum, the –OH peak appears to be a sharp doublet of doublets: one bond coupling to the geminal C–H proton of 9 Hz and one of the largest reported through-space couplings to fluorine of 20 Hz. The 19F–1H HOESY spectrum also supports our regio- and stereochemical assignment – a strong interaction between fluorine and Ha, Hb, and Hd, as well as no apparent interaction with Hc and He (Fig. 2B). Consequently, we postulate that intramolecular hydrogen bonding is responsible for the considerable coupling constant. This conclusion is also supported by calculations at B3LYP/6-311++G** (Fig. 2C): the O–H–F angle is given as 140° and F⋯H–O bond distance is 1.97 Å.
Appreciating the complexity and biological significance of steroids,16 we derivatized dehydroepiandrosterone to afford fluorinated substrate 10 (42%; d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Computational modeling assisted in verifying that the β-hydroxy group targets the C12 position (B3LYP/6-311++G**); furthermore, the β-fluoro isomer is the major product (validated by NOESY, 1H, and 19F NMR). Additionally, we subjected 17α-hydroxyprogesterone (endogenous progestogen steroid hormone17) to fluorination conditions and found the α-fluoro product (11) as the major diastereomer in 55% yield (99
1). Computational modeling assisted in verifying that the β-hydroxy group targets the C12 position (B3LYP/6-311++G**); furthermore, the β-fluoro isomer is the major product (validated by NOESY, 1H, and 19F NMR). Additionally, we subjected 17α-hydroxyprogesterone (endogenous progestogen steroid hormone17) to fluorination conditions and found the α-fluoro product (11) as the major diastereomer in 55% yield (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.). To investigate further the notion of coordinated fluorination and explanation of the observed stereoisomers (e.g., β-hydroxy/β-fluoro and α-hydroxy/α-fluoro), we calculated a simplified system comparing the fluorination of 1-propyl radical and γ-propanol radical (Scheme 2C). The reaction can be distilled into two key steps: a site-selective HAT, followed by a diastereoselective fluorination reaction. The following isodesmic relation (ωB97xd/6-31+G*, −7.63 kcal mol−1) illustrates the stabilizing energetic role that the hydroxy group plays in commanding diastereoselectivity. The transition states represent low barrier processes; a solvent dielectric was necessary to find saddle points.
1 d.r.). To investigate further the notion of coordinated fluorination and explanation of the observed stereoisomers (e.g., β-hydroxy/β-fluoro and α-hydroxy/α-fluoro), we calculated a simplified system comparing the fluorination of 1-propyl radical and γ-propanol radical (Scheme 2C). The reaction can be distilled into two key steps: a site-selective HAT, followed by a diastereoselective fluorination reaction. The following isodesmic relation (ωB97xd/6-31+G*, −7.63 kcal mol−1) illustrates the stabilizing energetic role that the hydroxy group plays in commanding diastereoselectivity. The transition states represent low barrier processes; a solvent dielectric was necessary to find saddle points.
Additionally, a simple Protein Data Bank (PDB) survey showed numerous intermolecular close contacts between hydroxy groups and H–C–+NR3 moieties.18 What is more, solutions of Selectfluor with various alcohols at different concentrations reveal characteristic H–C–+NR3 downfield chemical shifts in the 1H NMR spectra (Scheme 2D).19 Both of these observations buttress the claim of a putative hydrogen bonding interaction between Selectfluor and the hydroxy group.
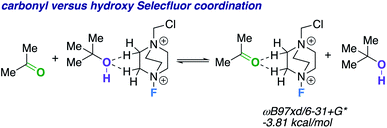
We theorize that the regioselective HAT step proceeds similarly to the reported carbonyl-directed pathway (Scheme 2A) involving Selectfluor radical cation coordination (considering the likenesses in conditions and aforementioned Lewis basicity logic). Alternatively, one can imagine the reaction proceeding through a Barton20 or Hofmann–Löffler–Freytag21 style mechanism. To probe this possibility, we employed a β-caryophyllene alcohol hypochlorite derivative to form the alkoxy radical directly, and found that under standard conditions there is complex fragmentation and nonselective fluorination (Scheme 2B). Lastly, we compared the hydroxy versus carbonyl group SF coordination computationally. The carbonyl group is preferred to bind to SF through nonclassical C–H⋯O hydrogen bonds preferentially over the hydroxy group, as the following isodesmic relation shows (acetone and t-BuOH as models; ωB97xd/6-31+G*, −3.81 kcal mol−1), but, once again, rigidity and propinquity are ultimately more important factors in determining directing effects (Scheme 3).
The tetrahedral nature of hydroxy groups provides unique access to previously unobtainable sites. For example, we compared menthol and an alkylated congener to form products 12 and 13 (Fig. 3B). The hydroxy group in the precursor to 12 is in the equatorial position, mandating the exocyclic isopropyl group as the reactive site (40% yield).22 In the precursor to 13, the methyl and isopropyl substituent lock the hydroxy group into the axial position, targeting its endocyclic tertiary site through a 1,3-diaxial relationship to afford fluorinated product in 57% yield (d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In all, the comparison showcases the versatility in directing ability, offering a choice of regio- and stereoselectivity based on the stereochemistry of the hydroxy group. The directing system only necessitates two features based on our results: (1) the hydroxy group must be either secondary or tertiary (primary tends to favor oxidation) and (2) the oxygen atom must be within the range of 2.4–3.2 Å of the targeted secondary or tertiary hydrogen.
1). In all, the comparison showcases the versatility in directing ability, offering a choice of regio- and stereoselectivity based on the stereochemistry of the hydroxy group. The directing system only necessitates two features based on our results: (1) the hydroxy group must be either secondary or tertiary (primary tends to favor oxidation) and (2) the oxygen atom must be within the range of 2.4–3.2 Å of the targeted secondary or tertiary hydrogen.
Among the several biologically active compounds we screened, caratol derivatives 14 and 15 were found to be attractive candidates that reveal directed fluorination to an exocyclic isopropyl group (Table 2). Comprising ca. 40% of carrot seed oil, caratol is its major constituent and has shown allelopathic interactions (e.g., as insecticidal, antifungal, and herbicidal agents23).24 After extraction, isolation, and derivatization, molecules 14 and 15 are afforded in 65% and 83% yield (Table 2). Acetates and oxidized sulfur-containing functional groups were well tolerated.
Another natural product we modified to a tertiary alcohol was sclareolide, which has a rich history of fluorinating on its C2 and C3 positions due to polar effects, as reported by Tang,25 Groves,9f Britton,26 and others.27 The derived alcohol finally overrode this natural tendency and directed to the predicted position in 56% (d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (product 16). Smaller amounts of competitive polar effect fluorination were observed at the C2 and C3 positions, highlighting how challenging a problem the functionalization of the sclareolide core presents.28,29
1) (product 16). Smaller amounts of competitive polar effect fluorination were observed at the C2 and C3 positions, highlighting how challenging a problem the functionalization of the sclareolide core presents.28,29
An altered dihydroactinidiolide was found to participate in the fluorination through a 1,3-diaxial guided HAT and fluorination in 55% yield (product 17, d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). We next modeled several more substrates that participated in similar 1,3 relationships; however, each exhibited a variation from one another (e.g., ring size or fused aromatic ring). Products 19 and 18 displayed the reaction's capability to direct to the desired positions with an expanded (65%; d.r. 99
1). We next modeled several more substrates that participated in similar 1,3 relationships; however, each exhibited a variation from one another (e.g., ring size or fused aromatic ring). Products 19 and 18 displayed the reaction's capability to direct to the desired positions with an expanded (65%; d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and reduced (45%; d.r. 99
1) and reduced (45%; d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
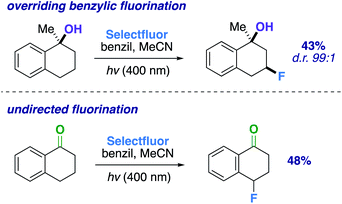
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ring system when compared to the previous 6-membered ring examples. Additionally, we examined a methylated α-tetralone derivative. The desired 3-fluoro product 20 forms in 43% yield (d.r. 99
1) ring system when compared to the previous 6-membered ring examples. Additionally, we examined a methylated α-tetralone derivative. The desired 3-fluoro product 20 forms in 43% yield (d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), overriding benzylic fluorination (Scheme 4).30 Under identical conditions α-tetralone provides 4-fluorotetralone in 48% yield. In similar motif, 1-phenylindanol, we intentionally targeted the benzylic position in a 90% and 10
1), overriding benzylic fluorination (Scheme 4).30 Under identical conditions α-tetralone provides 4-fluorotetralone in 48% yield. In similar motif, 1-phenylindanol, we intentionally targeted the benzylic position in a 90% and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. (product 21). Unlike the methylated α-tetralone derivative, the geometry of the starting material calculated at B3LYP/6-311++G** shows the hydroxy group is not truly axial and is 4.30 Å from the targeted C–H bond, explaining the dip in diastereoselectivity.
1 d.r. (product 21). Unlike the methylated α-tetralone derivative, the geometry of the starting material calculated at B3LYP/6-311++G** shows the hydroxy group is not truly axial and is 4.30 Å from the targeted C–H bond, explaining the dip in diastereoselectivity.
Next, we examined an isomer of borneol that is widely used in perfumery, fenchol.31 The secondary alcohol displays a diastereoselective fluorination in 38% (d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (product 22). Our last designed motif was ideally constructed to have a doubly-directing effect. Our observations show that a well-positioned hydroxy group not only provides sequential regioselective hydrogen atom abstraction but also displays a powerful demonstration of Selectfluor guidance to afford the cis-difluoro product (23) in 33% yield (85% brsm, d.r. 99
1) (product 22). Our last designed motif was ideally constructed to have a doubly-directing effect. Our observations show that a well-positioned hydroxy group not only provides sequential regioselective hydrogen atom abstraction but also displays a powerful demonstration of Selectfluor guidance to afford the cis-difluoro product (23) in 33% yield (85% brsm, d.r. 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Spectroscopically (1H, 13C, and 19F NMR), the product possesses apparent Cs symmetry and showcases close interactions (e.g., diagnostic couplings and chemical shifts). cis-Polyfluorocycloalkanes are of intense current interest in materials chemistry, wherein faces of differing polarity can complement one another.32
1). Spectroscopically (1H, 13C, and 19F NMR), the product possesses apparent Cs symmetry and showcases close interactions (e.g., diagnostic couplings and chemical shifts). cis-Polyfluorocycloalkanes are of intense current interest in materials chemistry, wherein faces of differing polarity can complement one another.32
All in all, this photochemical hydroxy-directed fluorination report represents one of the first steps in commanding diastereoselectivity within the field of radical fluorination. An ability to dictate regio- and stereoselectivity is demonstrated in a variety of substrates by simply switching the stereochemistry of the hydroxy group. Computations support the key role of Selectfluor coordination to the key hydroxy group in the fluorination step. Future studies will seek to uncover other compatible Lewis basic functional groups, expanding further the versatility of radical fluorination.
Data availability
Experimental and computational data is located in the ESI.†Author contributions
S. Harry designed probes, synthesized compounds, and provided drafts of the manuscript. M. Xiang, E. Holt, A. Zhu, F. Ghorbani, and D. Patel synthesized compounds for isolation/characterization and assisted in compiling data. T. Lectka supervised the project and reviewed/edited the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
T.L. thanks the National Science Foundation (NSF) (CHE grants 1800510 and 2102116) for financial support. Mass spectral data were obtained at the University of Delaware's mass spectrometry center.References
- (a) P. Chauhan and S. S. Chimni, RSC Adv., 2012, 2, 737–758 RSC; (b) G. D. Rose and R. Wolfenden, Annu. Rev. Biophys. Biomol. Struct., 1993, 22, 381–415 CrossRef CAS PubMed; (c) X. Wu and C. Zhu, Chem. Commun., 2019, 55, 9747–9756 RSC; (d) T. Steiner, Crystallogr. Rev., 2003, 9, 177–228 CrossRef CAS; (e) S. Zhang, F. Zhang and Y. Tu, Chem. Soc. Rev., 2011, 4, 1937–1949 RSC; (f) A. S. Kertes and C. J. King, Chem. Rev., 1987, 87, 687–710 CrossRef CAS; (g) W. Kroutil, H. Mang, K. Edegger and K. Faber, Adv. Synth. Catal., 2004, 2, 125–142 CrossRef.
- (a) C. Zhang, J. Ji and P. Sun, J. Org. Chem., 2014, 79, 3200–3205 CrossRef CAS PubMed; (b) G. T. Hoang, Z. Pan, J. T. Brethorst and C. J. Douglas, J. Org. Chem., 2014, 79, 11383–11394 CrossRef CAS PubMed; (c) A. M. Camelio, T. Barton, F. Guo, T. Shaw and D. Siegel, Org. Lett., 2011, 13, 1517–1519 CrossRef CAS PubMed; (d) J. Sun, G. Zheng, T. Xiong, Q. Zhang, J. Zhao, Y. Li and Q. Zhang, ACS Catal., 2016, 6, 3674–3678 CrossRef CAS; (e) X. Wu, H. Zhang, N. Tang, Z. Wu, D. Wang, M. Ji, Y. Xu, M. Wang and C. Zhu, Nat. Commun., 2018, 9, 3343 CrossRef PubMed.
- (a) T. Katsuki and K. B. Sharpless, J. Am. Chem. Soc., 1980, 102, 5976–5978l CrossRef; (b) H. Brunner and A. Sicheneder, Angew. Chem., Int. Ed. Engl., 1988, 27, 718–719 CrossRef; (c) V. Jäger, D. Schröter and B. Koppenhoefer, Tetrahedron, 1991, 47, 2195–2210 CrossRef.
- (a) J. Brown, Angew. Chem., Int. Ed. Engl., 1987, 26, 190–203 CrossRef; (b) R. H. Crabtree and M. W. Davis, Organometallics, 1983, 2, 682–684 CrossRef; (c) R. H. Crabtree and M. W. Davis, J. Org. Chem., 1986, 51, 2655–2661 CrossRef CAS; (d) G. Stork and D. E. Kahne, J. Am. Chem. Soc., 1983, 105, 1072–2073 CrossRef CAS.
- (a) T. P. Blaisdell and J. P. Morken, J. Am. Chem. Soc., 2015, 137, 8712–8715 CrossRef CAS PubMed; (b) K. Manabe and S. Ishikawa, Synthesis, 2008, 16, 2645–2649 CrossRef; (c) E. M. Simmons and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 17092–17095 CrossRef CAS PubMed.
- (a) E. M. Simmons and J. F. Hartwig, Nature, 2012, 483, 70–73 CrossRef CAS PubMed; (b) X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Angew. Chem., Int. Ed. Engl., 2018, 57, 1640–1644 CrossRef CAS PubMed; (c) T. P. Blaisdell, T. C. Caya, L. Zhang, A. Sanz-Marco and J. P. Morken, J. Am. Chem. Soc., 2014, 136, 9264–9267 CrossRef CAS PubMed; (d) X. Wang, Y. Lu, H. Dai and J. Yu, J. Am. Chem. Soc., 2010, 132, 12203–12205 CrossRef CAS PubMed; (e) Y. Lu, D. Wang, K. M. Engle and J. Yu, J. Am. Chem. Soc., 2010, 132, 5916–5921 CrossRef CAS PubMed; (f) S. Saito, T. Ishikawa, A. Kuroda, K. Koga and T. Moriwake, Tetrahedron, 1992, 48, 4067–4086 CrossRef CAS.
- F. Ghorbani, S. A. Harry, J. N. Capilato, C. R. Pitts, J. Joram, G. Peters, J. Tovar, I. Smajlagic, M. Siegler, T. Dudding and T. Lectka, J. Am. Chem. Soc., 2020, 142, 14710–14724 CrossRef CAS PubMed.
- (a) D. D. Bume, C. R. Pitts, F. Ghorbani, S. A. Harry, J. N. Capilato, M. A. Siegler and T. Lectka, Chem. Sci., 2017, 8, 6918–6923 RSC; (b) D. D. Bume, S. A. Harry, C. R. Pitts and T. Lectka, J. Org. Chem., 2018, 83, 1565–1575 CrossRef CAS PubMed; (c) C. R. Pitts, D. D. Bume, S. A. Harry, M. A. Sielger and T. Lectka, J. Am. Chem. Soc., 2017, 139, 2208–2211 CrossRef CAS PubMed.
- (a) Z. Yuan, H. Wang, X. Mu, P. Chen, Y. Guo and G. Liu, J. Am. Chem. Soc., 2015, 137, 2468–2471 CrossRef CAS PubMed; (b) W. Zhang, Y. Gu, J. Lin and J. Xiao, Org. Lett., 2020, 22, 6642–6646 CrossRef CAS PubMed; (c) Z. Li, Z. Wang, L. Zhu, X. Tan and C. Li, J. Am. Chem. Soc., 2014, 136, 16439–16443 CrossRef CAS PubMed; (d) H. Shigehisa, E. Nishi, M. Fujisawa and K. Hiroya, Org. Lett., 2013, 15, 5158–5161 CrossRef CAS PubMed; (e) W. Liu, X. Huang and J. T. Groves, Nat. Protoc., 2013, 8, 2348–2354 CrossRef CAS PubMed; (f) W. Liu, X. Huang, M. J. Cheng, R. J. Nielsen, W. A. Goddard III and J. T. Groves, Science, 2012, 337, 1322–1325 CrossRef CAS PubMed.
- M. T. Reetz and S. Stanchev, J. Chem. Soc., Chem. Commun., 1993, 3, 328–330 RSC.
- G. A. Olah, Angew. Chem., 1973, 85, 183–225 CrossRef CAS.
- (a) Y. Gu, T. Kar and S. Scheiner, J. Am. Chem. Soc., 1999, 121, 9411–9422 CrossRef CAS; (b) S. G. Olesen and S. Hammerum, J. Phys. Chem. A, 2009, 113, 7940–7944 CrossRef CAS PubMed; (c) X. Wang, H. Woo, B. Kiran and L. Wang, Angew. Chem., 2005, 117, 5048–5052 CrossRef.
- (a) S. P. Bhatia, C. S. Letizia and A. M. Api, Food Chem. Toxicol., 2008, 46, 95–96 CrossRef PubMed; (b) M. Skold, A. Karlberg, M. Matura and A. Börje, Food Chem. Toxicol., 2006, 44, 538–545 CrossRef PubMed.
- G. G. Henderson, R. Osborne, O. McCrone and J. M. Robertson, J. Am. Chem. Soc., 1929, 1368–1372 RSC.
- L. E. Combettes, P. Clausen-Thue, M. A. King, B. Odell, A. L. Thompson, V. Gouverneur and T. D. W. Claridge, Chem.–Eur. J., 2012, 12, 13133–13141 CrossRef PubMed.
- (a) S. L. Greenspan, D. S. Oppenheim and A. Klibanski, Ann. Intern. Med., 1989, 110, 526–531 CrossRef CAS PubMed; (b) J. M. Tanner, R. H. Whitehouse, P. C. R. Hughes and B. S. Carter, J. Pediatr., 1976, 89, 1000–1008 CrossRef CAS PubMed; (c) A. Y. Bagrov, J. I. Shapiro and O. V. Fedorova, Pharmacol. Rev., 2009, 61, 9–38 CrossRef CAS PubMed.
- D. Tulchinsky and C. J. Hobel, Am. J. Obstet. Gynecol., 1973, 117, 884–893 CrossRef CAS PubMed.
- Y. Itoh, Y. Nakashima, S. Tsukamoto, T. Kurohara, M. Suzuki, Y. Sakae, M. Oda, Y. Okamoto and T. Suzuki, Sci. Rep., 2019, 9, 1–12 CAS.
- J. Capilato, S. A. Harry, M. Seigler and T. Lectka, Chem.–Eur. J., 2021, 28, e202103922 Search PubMed.
- D. H. Barton, R. Hesse, M. Pechet and L. Smith, J. Chem. Soc., Perkin Trans. 1, 1979, 115, 1159–1165 RSC.
- G. Majetich and K. Wheless, Tetrahedron, 1995, 55, 7095–7129 CrossRef.
- E. Hayashi, S. Hara, H. Shirato, T. Hatekeyama, T. Fukuhara and N. Yoneda, Chem. Lett., 1995, 3, 205–206 CrossRef.
- A. Cakir, H. Özer, T. Aydin, S. Kordali, A. T. Cavusoglu, T. Akcin, E. Mete and A. Akcin, Rec. Nat. Prod., 2016, 10, 154–167 CAS.
- S. Sridhar, R. V. Rajagopal, R. Rajavel, S. Masilamani and S. Narasimhan, J. Agric. Food Chem., 2003, 51, 7596–7599 CrossRef CAS PubMed.
- X. Zhang, S. Guo and P. Tang, Org. Chem. Front., 2015, 2, 806–810 RSC.
- S. D. Halperin, H. Fan, S. Chang, R. E. Martin and R. Britton, Angew. Chem., 2014, 126, 4778–4781 CrossRef.
- (a) Y. Takahira, M. Chen, Y. Kawamata, P. Mykhailiuk, H. Nakamura, M. K. Peters, S. H. Reisberg, C. Li, L. Chen, T. Hoshikawa, T. Shibuguchi and P. S. Baran, Synlett, 2019, 30, 1178–1182 CrossRef CAS PubMed; (b) S. Bloom, J. L. Knippel and T. Lectka, Chem. Sci., 2014, 5, 1175–1178 RSC.
- (a) C. Walling, Free Radicals in Solution, Wiley, New York, NY, 1957 Search PubMed; (b) R. Bernardi, R. Galli and F. Minisci, J. Chem. Soc. B, 1968, 324–325 RSC; (c) F. Minisci, R. Galli and R. Bernardi, Chem. Commun., 1967, 17, 903–904 RSC; (d) A. A. Zavitsas and J. A. Pinto, J. Am. Chem. Soc., 1972, 94, 7390–7396 CrossRef CAS; (e) T. Newhouse and P. S. Baran, Angew. Chem., Int. Ed., 2011, 50, 3362–3374 CrossRef CAS PubMed.
- (a) Y. Amaoka, M. Nagamoto and M. Inoue, Org. Lett., 2013, 15, 2160–2163 CrossRef CAS PubMed; (b) C. R. Pitts, B. Ling, R. Woltornist, R. Liu and T. Lectka, J. Org. Chem., 2014, 79, 8895–8899 CrossRef CAS PubMed; (c) S. Bloom, C. R. Pitts, D. Miller, N. Haselton, M. G. Holl, E. Urheim and T. Lectka, Angew. Chem., Int. Ed., 2012, 51, 10580–10583 CrossRef CAS PubMed; (d) J.-B. Xia, C. Zhu and C. Chen, Chem. Commun., 2014, 50, 11701–11704 RSC; (e) J. G. West, T. A. Bedell and E. J. Sorensen, Angew. Chem., Int. Ed., 2016, 55, 8923–8927 CrossRef CAS PubMed; (f) J.-B. Xia, Y. Ma and C. Chen, Org. Chem. Front., 2014, 1, 468–472 RSC; (g) C. W. Kee, K. F. Chin, M. W. Wong and C.-H. Tan, Chem. Commun., 2014, 50, 8211–8214 RSC; (h) S. D. Halperin, H. Fan, S. Chang, R. E. Martin and R. Britton, Angew. Chem., Int. Ed., 2014, 53, 4690–4693 CrossRef CAS PubMed; (i) P. Champagne, J. Desroches, J. Hamel, M. Vandamme and J.-F. Paquin, Chem. Rev., 2015, 115, 9073–9174 CrossRef CAS PubMed.
- (a) A. M. Hua, D. N. Mai, R. Martinez and R. D. Baxter, Org. Lett., 2017, 19, 2949–2952 CrossRef CAS PubMed; (b) D. Cantillo, O. de Frutos, J. A. Rincon, C. Mateos and O. C. Kappe, J. Org. Chem., 2014, 79, 8486–8490 CrossRef CAS PubMed; (c) S. Bloom, C. R. Pitts, R. Woltornist, A. Griswold, M. G. Holl and T. Lectka, Org. Lett., 2013, 15, 1722–1724 CrossRef CAS PubMed; (d) W. Liu and J. T. Groves, Angew. Chem., Int. Ed., 2013, 52, 6024–6027 CrossRef CAS PubMed; (e) S. Bloom, S. A. Sharber, M. G. Holl, J. L. Knippel and T. Lectka, J. Org. Chem., 2013, 78, 11082–11086 CrossRef CAS PubMed; (f) J.-B. Xia, C. Zhu and C. Chen, J. Am. Chem. Soc., 2013, 135, 17494–17500 CrossRef CAS PubMed; (g) S. Bloom, M. McCann and T. Lectka, Org. Lett., 2014, 16, 6338–6341 CrossRef CAS PubMed; (h) M. B. Nodwell, A. Bagai, S. D. Halperin, R. E. Martin, H. Knust and R. Britton, Chem. Commun., 2015, 51, 11783–11786 RSC; (i) D. D. Bume, C. R. Pitts, R. T. Jokhai and T. Lectka, Tetrahedron, 2016, 72, 6031–6036 CrossRef CAS; (j) A. Koperniku, H. Liu and P. B. Hurley, Eur. J. Org. Chem., 2016, 871–886 CrossRef CAS.
- G. K. Oloyede, Arabian J. Chem., 2016, 9, 840–845 CrossRef.
- (a) M. Zupan, A. Gregorcic and A. Pollak, J. Org. Chem., 1976, 42, 1562–1565 CrossRef; (b) M. Kaj, S. Banik and E. Jacobsen, Org. Lett., 2019, 21, 4919–4923 CrossRef PubMed; (c) Q. Huchet, B. Kuhn, B. Wagner, N. Kratochwil, H. Fischer, M. Kansy, D. Zimmerli, E. Carreira and K. Müller, J. Med. Chem., 2015, 58, 9041–9060 CrossRef CAS PubMed; (d) C. Thiehoff, Y. Rey and R. Gilmour, Isr. J. Chem., 2017, 57, 92–100 CrossRef CAS; (e) I. Yamamoto, M. Jordan, N. Gavande, M. Doddareddy, M. Chebib and L. Hunter, Chem. Commun., 2012, 48, 829–831 RSC; (f) X. Hu, D. Thomas, R. Griffith and L. Hunter, Angew. Chem., Int. Ed., 2014, 53, 6176–6179 CrossRef CAS PubMed; (g) M. Schüler, D. O'Hagan and A. Slawin, Chem. Commun., 2005, 4324–4326 RSC; (h) I. G. Molnμr, C. Thiehoff, M. C. Holland and R. Gilmour, ACS Catal., 2016, 6, 7167–7173 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc01907h |
| This journal is © The Royal Society of Chemistry 2022 |