Open Access Article
Open Access ArticleAccess to chiral β-sulfonyl carbonyl compounds via photoinduced organocatalytic asymmetric radical sulfonylation with sulfur dioxide†
Fu-Sheng
He‡
a,
Chun
Zhang‡
a,
Minghui
Jiang
a,
Lujun
Lou
a,
Jie
Wu
 *abc and
Shengqing
Ye
*abc and
Shengqing
Ye
 *a
*a
aSchool of Pharmaceutical and Materials Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou 318000, China. E-mail: shengqing.ye@tzc.edu.cn; jie_wu@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 8th July 2022
Abstract
An organocatalytic enantioselective radical reaction of potassium alkyltrifluoroborates, DABCO·(SO2)2 and α,β-unsaturated carbonyl compounds under photoinduced conditions is developed, which provides an efficient pathway for the synthesis of chiral β-sulfonyl carbonyl compounds in good yields with excellent enantioselectivity (up to 96% ee). Aside from α,β-unsaturated carbonyl compounds with auxiliary groups, common chalcone substrates are also well compatible with this organocatalytic system. This method proceeds through an organocatalytic enantioselective radical sulfonylation under photoinduced conditions, and represents a rare example of asymmetric transformation involving sulfur dioxide insertion.
Introduction
The catalytic asymmetric conjugate addition of α,β-unsaturated carbonyl compounds is one of the most powerful synthesis strategies for the synthesis of chiral β-substituted carbonyl compounds.1 Compared with the well-developed classic Michael addition, the photoinduced radical addition process provides more promising opportunities since the high activity of radical species can overcome the inherent deficiencies of the traditional pathway in terms of substrate activity and steric hindrance. Since the pioneering work of Bach in photoinduced catalytic enantioselective radical addition,2 research in this field has developed rapidly. Various carbon-centered radicals or N-centered radicals are compatible in this transformation, leading to diverse chiral β-substituted carbonyl compounds.3,4Chiral sulfur-containing molecules are ubiquitous in market drugs and bioactive molecules.5 Great attention has been devoted to the catalytic asymmetric synthesis of enantioenriched sulfones and considerable progress has been made in recent years.6 However, construction of chiral sulfones through a catalytic enantioselective radical process remains challenging. There are few reports on catalytic asymmetric sulfonyl radical conjugate addition to α,β-unsaturated carbonyl compounds, which would afford biologically interesting enantioenriched β-sulfonyl carbonyl compounds (Scheme 1a). In 2017, Meggers and co-workers described a photoinduced, chiral Rh catalyzed enantioselective reaction of allyl sulfones with α,β-unsaturated N-acylpyrazoles, affording a sulfonyl radical asymmetric addition product.7 In 2019, Wu and co-workers reported one example to access a chiral β-sulfonyl carbonyl compound using sulfinic acid as a sulfonyl radical source.8 Recently, Gong and co-workers demonstrated a photoinduced asymmetric sulfonyl radical addition to α,β-unsaturated carbonyl compounds under chiral nickel catalysis.9 The sulfonyl radical was generated in situ from the reaction of the C(sp3)–H precursor and DABCO·(SO2)2 in this transformation. Despite significant advances, the aforementioned metal-catalyzed radical additions still suffer from substrate limitations, where the coordination of an auxiliary group to the metal center is essential for substrate activation and stereochemical control. The development of a novel catalytic system for the asymmetric synthesis of chiral β-sulfonyl carbonyl compounds with excellent enantioselectivity and broader substrate scope is still highly desirable.
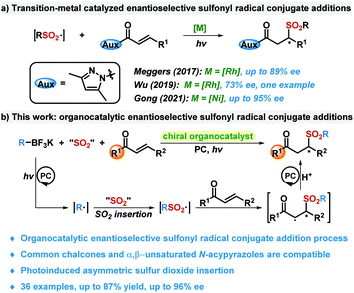 | ||
| Scheme 1 Catalytic asymmetric sulfonyl radical conjugate addition to α,β-unsaturated carbonyl compounds. | ||
On the other hand, construction of a sulfonyl nucleus through the radical-based sulfur dioxide insertion strategy has been developed rapidly.10,11 Based on our continuous interest in radical-based sulfur dioxide insertion and recent success in squaramide catalyzed asymmetric addition of sulfonyl radicals to VQMs (vinylidene ortho-quinone methides),12 we develop an organocatalytic enantioselective radical reaction of α,β-unsaturated carbonyl compounds, potassium alkyltrifluoroborates, and DABCO·(SO2)2 under visible light irradiation, affording chiral β-sulfonyl carbonyl compounds in good yields with excellent enantioselectivity (up to 96% ee). This method proceeds through a photoinduced organocatalytic enantioselective sulfonyl radical conjugate addition process, and represents a rare example of asymmetric transformation involving sulfur dioxide insertion (Scheme 1b).
Results and discussion
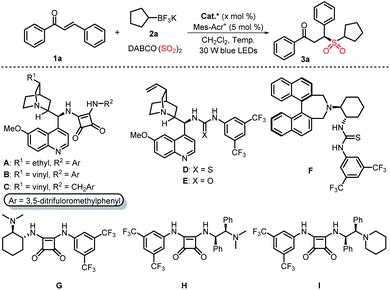
We commenced this study by using commercially available chalcone 1a and potassium cyclopentyltrifluoroborate 2a as model substrates with DABCO·(SO2)2 as the sulfur dioxide surrogate to explore suitable reaction conditions (Table 1). Pleasingly, the reaction proceeded smoothly with A as the organocatalyst, and Mes-Acr+ClO4− as the photocatalyst in CH2Cl2 at −5 °C, providing the desired product 3a in 80% NMR yield with −68% ee (Table 1, entry 1). Encouraged by this result, we then examined the reaction with other bifunctional organocatalysts B–I (Table 1, entries 2–9). It was found that H was the optimal oraganocatalyst in terms of yield and enantioselectivity (Table 1, entry 8). No reaction occurred when other Ir or Ru photocatalysts were used in this reaction (Table 1, entries 10–12). Lowering the reaction temperature to −20 °C resulted in an improvement of enantioselectivity, albeit with a slightly decreased yield (Table 1, entry 13). Furthermore, the screening of solvents including CHCl3, EtOAc, iPrOH, MTBE, and MeCN showed that MeCN was the ideal solvent for this transformation (Table 1, entries 14–18). Remarkably, when the loading of catalyst H was decreased to 1 mol%, an improved yield and ee value of 3a was obtained (Table 1, entry 19). Control experiments without an organocatalyst, light irradiation, or a photocatalyst suggested that these components were all essential to achieve a product with excellent enantioselectivity (Table 1, entries 20 and 21).| Entry | PC (mol%) | Cat* (mol%) | T (°C) | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: chalcone 1a (0.1 mmol), potassium cyclopentyltrifluoroborate 2a (0.2 mmol), DABCO·(SO2)2 (0.1 mmol), Mes-Acr+ClO4− (5 mol%), solvent (2.0 mL), 30 W blue LED, 72 h. b Determined by 1H NMR analysis (isolated yield in parentheses). c Determined by HPLC analysis on a chiral stationary phase. d In the dark. e In the absence of Mes-Acr+. | ||||||
| 1 | Mes-Acr+ClO4− | A (5) | −5 | CH2Cl2 | 80 | −68 |
| 2 | Mes-Acr+ClO4− | B (5) | −5 | CH2Cl2 | 78 | −73 |
| 3 | Mes-Acr+ClO4− | C (5) | −5 | CH2Cl2 | 78 | −60 |
| 4 | Mes-Acr+ClO4− | D (5) | −5 | CH2Cl2 | 78 | −60 |
| 5 | Mes-Acr+ClO4− | E (5) | −5 | CH2Cl2 | 88 | −68 |
| 6 | Mes-Acr+ClO4− | F (5) | −5 | CH2Cl2 | 84 | −46 |
| 7 | Mes-Acr+ClO4− | G (5) | −5 | CH2Cl2 | 72 | 60 |
| 8 | Mes-Acr+ClO4− | H (5) | −5 | CH2Cl2 | 84 | 80 |
| 9 | Mes-Acr+ClO4− | I (5) | −5 | CH2Cl2 | 78 | 80 |
| 10 | fac-Ir(ppy)3 | H (5) | −5 | CH2Cl2 | n.r. | — |
| 11 | [Ir(dFCF3ppy)2bpy]PF6 | H (5) | −5 | CH2Cl2 | n.r. | — |
| 12 | Ru(bpy)3Cl2 | H (5) | −5 | CH2Cl2 | n.r. | — |
| 13 | Mes-Acr+ClO4− | H (5) | −20 | CH2Cl2 | 54 | 89 |
| 14 | Mes-Acr+ClO4− | H (5) | −20 | CHCl3 | 70 | 87 |
| 15 | Mes-Acr+ClO4− | H (5) | −20 | EtOAc | 30 | 93 |
| 16 | Mes-Acr+ClO4− | H (5) | −20 | iPrOH | 20 | 86 |
| 17 | Mes-Acr+ClO4− | H (5) | −20 | MTBE | 32 | 73 |
| 18 | Mes-Acr+ClO4− | H (5) | −20 | MeCN | 56 | 93 |
| 19 | Mes-Acr+ClO4− | H (1) | −20 | MeCN | 64 (60) | 95 |
| 20 | Mes-Acr+ClO4− | — | −20 | MeCN | Trace | — |
| 21d | Mes-Acr+ClO4− | H (1) | −20 | MeCN | n.r. | — |
| 22e | Mes-Acr+ClO4− | H (1) | −20 | MeCN | n.r. | — |
Under optimal reaction conditions, the substrate scope of this organocatalytic asymmetric three-component reaction with respect to α,β-unsaturated ketones 1 and potassium alkyltrifluoroborates 2 was investigated (Table 2). In general, the reaction proceeded smoothly with a wide range of substrates to give the desired products in moderate to good yields with universally high enantioselectivities. For example, α,β-unsaturated ketones bearing both electron-donating or -withdrawing substituents on the phenyl group participated in the reaction well to furnish the corresponding products 3b–3g. Changing the aryl group of R2 to an alkyl group had little effect on the enantioselectivities (3i–3m). Moreover, the reaction was also compatible with primary potassium alkyltrifluoroborates, leading to the chiral sulfones 3n–3p in 47–87% yields with 86–90% ee. The absolute configuration of 3d was determined to be S by single-crystal X-ray diffraction analysis.13 No product was obtained when phenyltrifluoroborate was utilized in this reaction under the standard reaction conditions.
| a Isolated yield based on α,β-unsaturated ketone 1. |
|---|
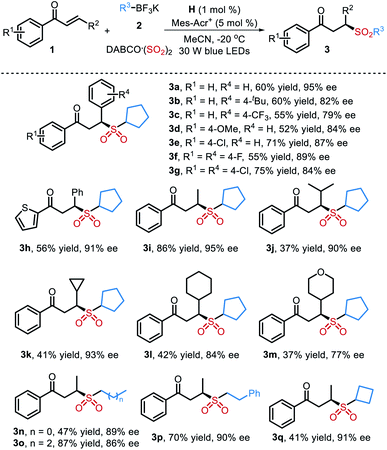
|
Subsequently, the generality of this transformation utilizing α,β-unsaturated N-acylpyrazoles as radical acceptors was also explored. As summarized in Table 3, a variety of structurally diverse chiral sulfones were afforded under slightly modified reaction conditions (see the ESI for details). It was found that α,β-unsaturated N-acylpyrazoles with different substituents (R1) were all suitable for this reaction, providing the chiral products 5a–5i in moderate to good yields with high ee values. The absolute configuration of 5a was assigned as S by comparison with Gong's work.9 Additionally, both primary and secondary potassium alkyltrifluoroborates worked well and delivered the target products 5j–5q in 31–80% yields with 80–95% ee.
| a Isolated yield based on α,β-unsaturated N-acylpyrazole 4. |
|---|
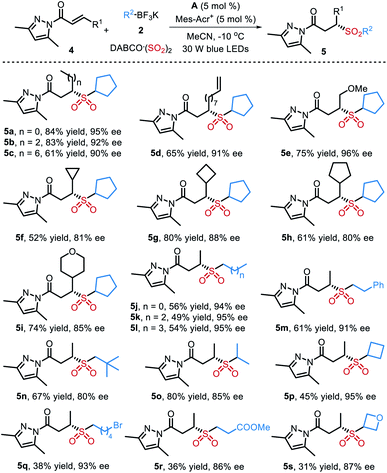
|
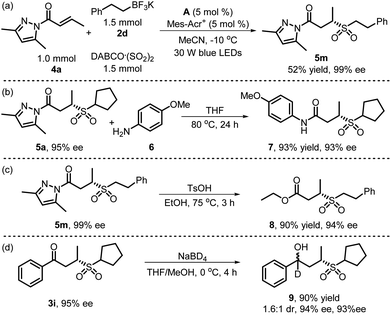
To further evaluate the practicality of this method, a 1 mmol scale reaction of α,β-unsaturated N-acylpyrazole 4a, potassium phenethyltrifluoroborate 2d and DABCO·(SO2)2 was carried out under standard conditions, affording the desired product 5m in 52% yield and with 99% ee (Scheme 2a). In addition, the pyrazole moiety of chiral sulfone product 5a could be substituted by 4-methoxyaniline 6 and delivered the amide derivative 7 in 91% yield with 93% ee (Scheme 2b). Furthermore, esterification product 8 could be generated in 90% yield with 94% ee from the reaction of chiral sulfone product 5m with ethanol (Scheme 2c). Next, a γ-hydroxy sulfone product could be constructed by the hydrogenation of the carbonyl group of chiral sulfone product 3i, and γ-hydroxy sulfone product 9 could be obtained in 90% yield with 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (94% ee, 93% ee) (Scheme 2d).
1 dr (94% ee, 93% ee) (Scheme 2d).
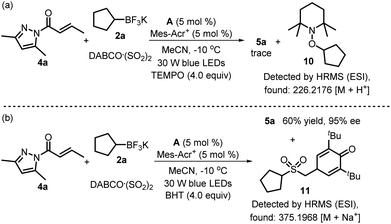
Next, two control experiments were performed to elucidate the reaction mechanism. As shown in Scheme 3a, the reaction of α,β-unsaturated N-acylpyrazole 4a, potassium cyclopentyltrifluoroborate 2a and DABCO·(SO2)2 was completely suppressed in the presence of 4.0 equiv. of radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) under standard conditions, and radical trapping adduct 10 was detected by HRMS (high-resolution mass spectrometry). Moreover, the addition of 4.0 equiv. of another radical scavenger BHT (butylated hydroxytoluene) led to a decreased yield of product 5a with retention of enantioselectivity, and confirmed the existence of a sulfonyl radical upon the detection of compound 11 (Scheme 3b). Taken together, these results suggested that the organocatalytic asymmetric reaction involved a radical process.
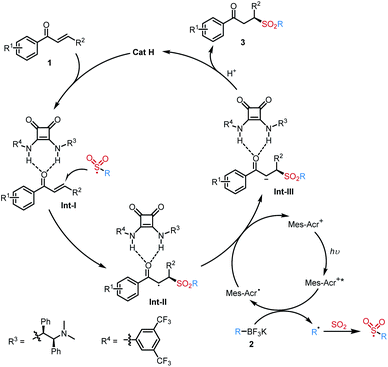
Based on the above experiment results and previous reports on the organocatalytic asymmetric radical transformations,14 a plausible reaction pathway for this photoinduced enantioselective radical sulfonylation with sulfur dioxide is shown in Scheme 4. Initially, potassium alkyltrifluoroborate 2 could be oxidized by a photocatalyst under visible light irradiation to afford the alkyl radical, which would react with sulfur dioxide to generate the alkylsulfonyl radical. The asymmetric addition of a sulfonyl radical to substrate 1 was achieved by the hydrogen-bond interaction in the presence of chiral squaramide catalyst H, giving rise to the chiral radical intermediate Int II in the S configuration. Subsequently, Int II would undergo single electron transfer (SET) reduction to produce the anion intermediate Int-III. The desired product 3 was obtained by the protonation of Int-III and regeneration of the organocatalyst H.
Conclusions
In conclusion, we have developed a photoinduced enantioselective organocatalytic radical conjugate addition to access enantioenriched β-sulfonyl carbonyl compounds through a three-component reaction of potassium alkyltrifluoroborates, DABCO·(SO2)2 and α,β-unsaturated carbonyl compounds. Chiral β-sulfonyl carbonyl compounds were achieved with excellent enantioselectivity and good yields. This process features mild reaction conditions and broad substrate scope. Not only α,β-unsaturated carbonyl compounds with auxiliary groups, but also common chalcone substrates were workable in this reaction. This method represents a rare example of asymmetric transformation involving sulfur dioxide insertion as well.Data availability
The data supporting this study are available within the article and the ESI.† The X-ray crystallographic coordinates for the structure of 3d have been deposited at the Cambridge Crystallographic Data Center (CCDC: 2165683).Author contributions
F.-S. H. and C. Z. contributed equally to this work. S. Y. conceived the study. F.-S. H. and C. Z. conducted the experiments and analysed the data. M. J. and L. L. conducted the preparation of the starting materials. S. Y. and J. W. directed the project. S. Y. and F.-S. H. prepared the manuscript. C. Z. prepared the ESI.† All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21901178), the Natural Science Foundation of Zhejiang Province (LY21B020002), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD04) is gratefully acknowledged.Notes and references
- (a) A. Córdova, Catalytic Asymmetric Conjugate Reactions, Wiley-VCH, Weinheim, 2010 CrossRef; (b) J. L. Vicario, E. Reyes, L. Carrillo and U. Uria, Organocatalytic Asymmetric Nucleophilic Addition to Electron−Deficient Alkenes, Comprehensive Organic Synthesis (Second Edition), 4.03 Organocatalytic Asymmetric Nucleophilic Addition to Electron-Deficient Alkenes, WileyVCH, Weinheim, 2014, pp. 119–188 Search PubMed; (c) M. Mauduit, O. Basle, H. Clavier, C. Crévisy and A. Denicourt-Nowicki, Metal-Catalyzed Asymmetric Nucleophilic Addition to Electron−Deficient Alkenes, Comprehensive Organic Synthesis (Second Edition), 4.04 Organocatalytic Asymmetric Nucleophilic Addition to Electron-Deficient Alkenes, Wiley-VCH, Weinheim, 2014, pp. 189–341 Search PubMed; (d) C. Hui, F. Pu and J. Xu, Chem.–Eur. J., 2017, 23, 4023–4036 CrossRef CAS PubMed; (e) K. Zheng, X. Liu and X. Ming, Chem. Rev., 2018, 118, 7586–7656 CrossRef CAS PubMed.
- A. Bauer, F. Westkämper, S. Grimme and T. Bach, Nature, 2005, 436, 1139–1140 CrossRef CAS PubMed.
- For selected examples, see: (a) L. Ruiz Espelt, I. S. McPherson, E. M. Wiensch and T. P. Yoon, J. Am. Chem. Soc., 2015, 137, 2452–2455 CrossRef CAS PubMed; (b) J. J. Murphy, D. Bastida, S. Paria, M. Fagnoni and P. Melchiorre, Nature, 2016, 532, 218–222 CrossRef CAS PubMed; (c) C. Wang, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 13495–13498 CrossRef CAS PubMed; (d) H. Huo, K. Harms and E. Meggers, J. Am. Chem. Soc., 2016, 138, 6936–6939 CrossRef CAS PubMed; (e) M. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti and P. Melchiorre, Nat. Chem., 2017, 9, 868–873 CrossRef CAS PubMed; (f) Z. Zhou, Y. Li, B. Han, L. Gong and E. Meggers, Chem. Sci., 2017, 8, 5757–5763 RSC; (g) S.-X. Lin, G.-J. Sun and Q. Kang, Chem. Commun., 2017, 53, 7665–7668 RSC; (h) P. Bonilla, Y. P. Rey, C. M. Holden and P. Melchiorre, Angew. Chem., Int. Ed., 2018, 57, 12819–12823 CrossRef CAS PubMed; (i) X. Shen, Y. Li, Z. Wen, S. Cao, X. Hou and L. Gong, Chem. Sci., 2018, 9, 4562–4568 RSC; (j) Z.-Y. Cao, T. Ghosh and P. Melchiorre, Nat. Commun., 2018, 9, 3274 CrossRef PubMed; (k) J. Ma, J. Lin, L. Zhao, K. Harms, M. Marsch, X. Xie and E. Meggers, Angew. Chem., Int. Ed., 2018, 57, 11193–11197 CrossRef CAS PubMed; (l) Y. Yin, Y. Dai, H. Jia, J. Li, L. Bu, B. Qiao, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2018, 140, 6083–6087 CrossRef CAS PubMed; (m) K. Zhang, L.-Q. Lu, Y. Jia, Y. Wang, F.-D. Lu, F. Pan and W.-J. Xiao, Angew. Chem., Int. Ed., 2019, 58, 13375–13379 CrossRef CAS PubMed; (n) E. L. Saux, D. Ma, P. Bonilla, C. M. Holden, D. Lustosa and P. Melchiorre, Angew. Chem., Int. Ed., 2021, 60, 5357–5362 CrossRef PubMed.
- For selected reviews, see: (a) K. Nakajima, Y. Miyake and Y. Nishibayashi, Acc. Chem. Res., 2016, 49, 1946–1956 CrossRef CAS PubMed; (b) T. P. Yoon, Acc. Chem. Res., 2016, 49, 2307–2315 CrossRef CAS PubMed; (c) M. Silvi and P. Melchiorre, Nature, 2018, 554, 41–49 CrossRef CAS PubMed; (d) B.-C. Hong, Org. Biomol. Chem., 2020, 18, 4298–4353 RSC; (e) A. L. Gant Kanegusuku and J. L. Roizen, Angew. Chem., Int. Ed., 2021, 60, 21116–21149 CrossRef CAS PubMed; (f) T. Xiong and Q. Zhang, Chem. Soc. Rev., 2021, 50, 8857–8873 RSC; (g) M. J. Genzink, J. B. Kidd, W. B. Swords and T. P. Yoon, Chem. Rev., 2022, 122, 1654–1716 CrossRef CAS PubMed; (h) S. Ye and J. Wu, Acta Chim. Sin., 2019, 77, 814–831 CrossRef CAS.
- (a) N. A. McGrath, M. Brichacek and J. T. Niardarson, J. Chem. Educ., 2010, 87, 1348–1349 CrossRef CAS; (b) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed; (c) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed.
- For selected examples, see: (a) X.-S. Wu, Y. Chen, M.-B. Li, M.-G. Zhou and S.-K. Tian, J. Am. Chem. Soc., 2012, 134, 14694–14697 CrossRef CAS PubMed; (b) Z. Jin, J. Xu, S. Yang, B.-A. Song and Y. R. Chi, Angew. Chem., Int. Ed., 2013, 52, 12354–12358 CrossRef CAS PubMed; (c) J. Choi, P. Martín-Gago and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 12161–12165 CrossRef CAS PubMed; (d) K. M.-H. Lim and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 3201–3204 CrossRef CAS PubMed; (e) S. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng and H. Yan, J. Am. Chem. Soc., 2018, 140, 7056–7060 CrossRef CAS PubMed; (f) J. Long, L. Shi, X. Li, H. Lv and X. Zhang, Angew. Chem., Int. Ed., 2018, 57, 13248–13251 CrossRef CAS PubMed; (g) Q. Yan, G. Xiao, Y. Wang, G. Zi, Z. Zhang and G. Hou, J. Am. Chem. Soc., 2019, 141, 1749–1756 CrossRef CAS PubMed; (h) A. Cai and A. W. Kleij, Angew. Chem., Int. Ed., 2019, 58, 14944–14949 CrossRef CAS PubMed; (i) Q. Zhang, D. Dong and W. Zi, J. Am. Chem. Soc., 2020, 142, 15860–15869 CrossRef CAS PubMed.
- X. Huang, S. Luo, O. Burghaus, R. D. Webster, K. Harms and E. Meggers, Chem. Sci., 2017, 8, 7126–7131 RSC.
- Y. Kuang, K. Wang, X. Shi, X. Huang, E. Meggers and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 16859–16863 CrossRef CAS PubMed.
- S. Cao, W. Hong, Z. Ye and L. Gong, Nat. Commun., 2021, 12, 2377 CrossRef CAS PubMed.
- For selected reviews, see: (a) P. Vogel, M. Turks, L. Bouchez, D. Marković, A. Varela-Álvarez and J. Á. Sordo, Acc. Chem. Res., 2007, 40, 931–942 CrossRef CAS PubMed; (b) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592–1599 RSC; (c) E. J. Emmett and M. C. Willis, Asian J. Org. Chem., 2015, 4, 602–611 CrossRef CAS; (d) G. Qiu, K. Zhou, L. Gao and J. Wu, Org. Chem. Front., 2018, 5, 691–705 RSC; (e) D. Zheng and J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017 CrossRef; (f) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561–12569 RSC; (g) K. Suta and M. Turks, Chem. Heterocycl. Compd., 2018, 54, 584–586 CrossRef CAS; (h) S. Ye, G. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013–1019 RSC; (i) S. Ye, M. Yang and J. Wu, Chem. Commun., 2020, 56, 4145–4155 RSC; (j) S. P. Blum, K. Hofman, G. Manolikakes and S. R. Waldvogel, Chem. Commun., 2021, 57, 8236–8249 RSC.
- For selected examples, see: (a) D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451–2454 CrossRef CAS PubMed; (b) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55, 11925–11929 CrossRef CAS PubMed; (c) F. Liu, J.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Angew. Chem., Int. Ed., 2017, 56, 15570–15574 CrossRef CAS PubMed; (d) H. Wang, S. Sun and J. Cheng, Org. Lett., 2017, 19, 5844–5847 CrossRef CAS PubMed; (e) Y. Wang, L. Deng, J. Zhou, X. Wang, H. Mei, J. Han and Y. Pan, Adv. Synth. Catal., 2018, 360, 1060–1065 CrossRef CAS; (f) Y. Meng, M. Wang and X. Jiang, Angew. Chem., Int. Ed., 2020, 59, 1346–1353 CrossRef CAS PubMed; (g) K. Chen, W. Chen, B. Han, W. Chen, M. Liu and H. Wu, Org. Lett., 2020, 22, 1841–1845 CrossRef CAS PubMed; (h) Y. Liu, Q.-L. Wang, Z. Chen, H. Li, B.-Q. Xiong, P.-L. Zhang and K.-W. Tang, Chem. Commun., 2020, 56, 3011–3014 RSC; (i) Y. Liu, D. Yu, Y. Guo, J.-C. Xiao, Q.-Y. Chen and C. Liu, Org. Lett., 2020, 22, 2281–2286 CrossRef CAS PubMed; (j) Z. Chen, N.-W. Liu, M. Bolte, H. Ren and G. Manolikakes, Green Chem., 2018, 20, 3059–3070 RSC; (k) N. Von Wolff, J. Char, X. Frogneux and T. Cantat, Angew. Chem., Int. Ed., 2017, 56, 5616–5619 CrossRef CAS; (l) K. Zhou, J. Chen and J. Wu, Chin. Chem. Lett., 2020, 31, 2996–2998 CrossRef CAS; (m) F.-S. He, M. Yang, S. Ye and J. Wu, Chin. Chem. Lett., 2021, 32, 461–464 CrossRef CAS; (n) Y. Liu, L. Wang, L.-H. Zeng, Y. Zhao, T. Zhu and J. Wu, Chin. Chem. Lett., 2022, 33, 2383–2386 CrossRef CAS; (o) Y. Liu, X. Zhang, J. Lv, C. Zhang, X. Chang, S. Ye and J. Wu, Org. Chem. Front., 2022, 9, 450–455 RSC.
- C. Zhang, Z. Tang, Y. Qiu, J. Tang, S. Ye, Z. Li and J. Wu, Chem Catal., 2022, 2, 164–177 CrossRef.
- CCDC 2165683 contains the supplementary crystallographic data for this paper.†.
- (a) S. Mondal, F. Dumur, D. Gigmes, M. P. Sibi, M. P. Bertrand and M. Nechab, Chem. Rev., 2022, 122, 5842–5976 CrossRef CAS; (b) D. Liang, J.-R. Chen, L.-P. Tan, Z.-W. He and W.-J. Xiao, J. Am. Chem. Soc., 2022, 144, 6040–6049 CrossRef CAS PubMed; (c) Y. Yin, Y. Dai, H. Jia, J. Li, L. Bu, B. Qiao, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2018, 140, 6083–6087 CrossRef CAS PubMed; (d) Y. Zhu, L. Zhang and S. Luo, J. Am. Chem. Soc., 2014, 136, 14642–14645 CrossRef CAS PubMed; (e) L. J. Rono, H. G. Yayla, D. Y. Wang, M. F. Armstrong and R. R. Knowles, J. Am. Chem. Soc., 2013, 135, 17735–17738 CrossRef CAS; (f) M.-C. Fu, R. Shang, B. Zhao, B. Wang and Y. Fu, Science, 2019, 363, 1429–1434 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data and copies of 1H and 13C NMR spectra. CCDC 2165683. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc02497g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |