Open Access Article
Open Access ArticleBioimaging agents based on redox-active transition metal complexes
Shan-Shan
Xue†
 ,
Yingbo
Pan†
,
Wei
Pan
,
Yingbo
Pan†
,
Wei
Pan
 ,
Shujie
Liu
,
Na
Li
,
Shujie
Liu
,
Na
Li
 * and
Bo
Tang
* and
Bo
Tang
 *
*
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Centre of Functionalized Probes for Chemical Imaging in Universities of Shandong, Institute of Molecular and Nano Science, Shandong Normal University, Jinan, 250014, P. R. China. E-mail: lina@sdnu.edu.cn; tangb@sdnu.edu.cn
First published on 27th July 2022
Abstract
Detecting the fluctuation and distribution of various bioactive species in biological systems is of great importance in determining diseases at their early stages. Metal complex-based probes have attracted considerable attention in bioimaging applications owing to their unique advantages, such as high luminescence, good photostability, large Stokes shifts, low toxicity, and good biocompatibility. In this review, we summarized the development of redox-active transition metal complex-based probes in recent five years with the metal ions of iron, manganese, and copper, which play essential roles in life and can avoid the introduction of exogenous metals into biological systems. The designing principles that afford these complexes with optical or magnetic resonance (MR) imaging properties are elucidated. The applications of the complexes for bioimaging applications of different bioactive species are demonstrated. The current challenges and potential future directions of these probes for applications in biological systems are also discussed.
1. Introduction
The homeostasis of bioactive species plays a vital role in determining the physiological and pathological states of living systems.1,2 The fluctuation and distribution of bioactive species are various for different cells at different states, while many diseases, such as cancer and neurological diseases, are closely related to the abnormal fluctuation and distribution of these bioactive species.3–6 Therefore, selective and accurate detection of these species holds tremendous promise for accurate diagnosis and treatment of diseases. A lot of probes have been developed in the past few decades using different imaging modalities including optical imaging (OI),7,8 magnetic resonance imaging (MRI),9–11 X-ray computed tomography (CT),12,13 positron emission tomography (PET),14etc. Metal complex-based bioimaging agents have attracted extensive attention due to their properties of low toxicity and good biocompatibility, as well as rich and stable photophysical and electromagnetic properties.9,15–18Transition metal complexes usually consist of one or more metal ions and some organic molecules as ligands. By changing the metals and ligands, the geometric structures and properties of complexes will be various.19–21 Therefore, different functions of complexes could be realized by modifying their metal ions or ligand structures. Some metal complexes essential for life are commonly used as catalytic centers in metalloenzymes. The biomimetic studies using metal complexes as biologically relevant catalysts have been inspiring.22–27 These biologically relevant transition metals, including iron, manganese, zinc, and copper, can bind with different ligands to form complexes with the properties of catalytic reactivities and good fluorescence.7,20,28–30 The application of the metals essential for life can avoid the introduction of exogenous metals and the problems of biotoxicity and metabolism.31,32 In recent years, a series of metal complex-based probes have been developed with OI and MRI modalities, which can overcome the shortages of organic molecular probes and avoid the metabolic difficulty and biotoxicity of nano-sized probes.9,16,19 More importantly, metal complex-based probes have the potential to be used as bioimaging agents in clinics. Developing more efficient probes for bioimaging applications based on these biologically relevant metal complexes is very desirable.
Recently, some excellent reviews on metal complex-based molecular probes have been published from different perspectives, such as various optical properties determined by electronic configurations,19 targeting design for biomolecules,15,33 imaging and therapeutic bifunctionality for specific diseases,18 responsive lanthanide luminescence,34 and functionalization with dendrimers.17 This review will focus on summarizing the research progress of redox-active metal (iron, manganese, and copper) complexes, which play essential roles in life, as bioimaging agents for both optical and MR images in recent five years. The review will be divided into four parts: the introduction about this topic, the designing principles of metal complex-based probes, the metal complexes used for bioimaging, and conclusion and perspectives for further development.
2. Designing principles of transition metal complex-based probes
Metal complexes are mainly constructed from two functional units, metal ions and ligands. As a result, the molecular probes based on metal complexes could be achieved by recognizing and reacting the targets using metal ions or ligands. The redox-active transition metal complexes discussed in this review are based on Fe, Mn, and Cu, which are redox-active transition metal ions with rich valent states. The ligands of these complexes are usually poly- or macrocyclic pyridine and porphyrin derivatives, using 4 to 8 nitrogen (N) and oxygen (O) atoms as coordination sites to bind with the metal ions.35,36 The configurations and properties of complexes could be varied by changing the ligands, even using the same metal ion as the coordination center. Through reacting with the targets, the metal valence or ligand will change, and the fluorescent or magnetic signals will show up.16 The OI or MRI or dual modalities of these probes in detecting the bioactive species in biological systems could be selected according to their property changes.OI is one of the most widely used imaging techniques to monitor the physiological and pathological processes in biological systems owing to the advantages of high resolution, good sensitivity, and low cost.19,37–39 Roughly speaking, the complexes will form new energy level structures, such as highest occupied molecular orbitals (HOMOs) or lowest unoccupied molecular orbitals (LUMOs), which makes the electron transfer between the energy levels much easier and quantum yield higher for complexes than that of the ligands.40,41 The luminescence mechanisms of complexes mainly include intramolecular ligand charge transfer (ILCT), ligand-to-metal charge transfer (LMCT), metal-to-ligand charge transfer (MLCT), and ligand-to-ligand charge transfer (LLCT).40,42,43 The complexes could be utilized as OI agents when the excited states of the target-reacted species could be generated through each one of these mechanisms.44 For the Fe and Mn complex-based probes, OI usually could be achieved from the outstanding optical properties of inherent excited states using appropriate polypyridine and porphyrin derivatives as ligands, or by connecting an organic fluorophore (not participating in coordination) to the ligand.45 Cu complexes with tetrahedral configuration are commonly used as OI agents, while some examples are also reported using a fluorophore as ligand, through oxidizing or reducing the Cu valence, and de-metallization of the fluorophore and the optical properties will obviously change.46,47
MRI is another widely used noninvasive diagnostic imaging technique, which can generate high resolution images through excitation of nucleus using long wavelength radio frequency.9 For the paramagnetic metal ions, the complexes could be utilized as MRI contrast agents (CAs). The redox-active transition metal ions discussed in this review can change their valence to result in the paramagnetic to antimagnetic property changes through reacting with the active species. Some Fe and Mn complexes have been reported as MRI CAs through the metal magnetic property changes, to detect oxidative bioactive species (e.g., H2O2) efficiently.48–50 Besides, several MRI-based techniques have been developed, including proton (1H) MRI, 19F MRI, and chemical exchange saturation transfer (CEST).51 1H MRI is the most commonly used, which could provide clear images of tissues due to the abundance of protons in the body. 19F MRI and CEST have attracted growing interest, and some examples have been published using metal complexes. 19F MRI uses F for quantitative analysis,52 and the 19F signal could be enhanced by the paramagnetic metals through reducing longitudinal relaxation times (T1), resulting in more efficient images.53 Therefore, complexes with proper F containing ligands and paramagnetic metal ions could be used as efficient 19F MRI agents. The MRI modality using the CEST mechanism works through irradiation of protons that can exchange with bulk H2O.54 The magnetization transfer results in the signal decrease of the bulk H2O.55,56 The CEST signal has inherent sensitivity towards pH, which makes the modality suitable for accurately detecting pH values in vivo.56 For the paramagnetic metals, the oxidation potentials of the complexes are usually lower than the reduction potentials of the bioactive targets, which makes the redox reactions happen and the magnetic properties of complexes change. For the other three magnetic techniques, Fe and Mn complexes usually use macrocyclic ligands, while Cu complexes are four-coordinated with aliphatic ligands. With the increasing research interest, the OI and MRI using metal complexes can potentially offer an attractive solution to the unmet clinical applications in detecting diseases.
3. Metal complexes in bioimaging application
3.1 Iron complexes
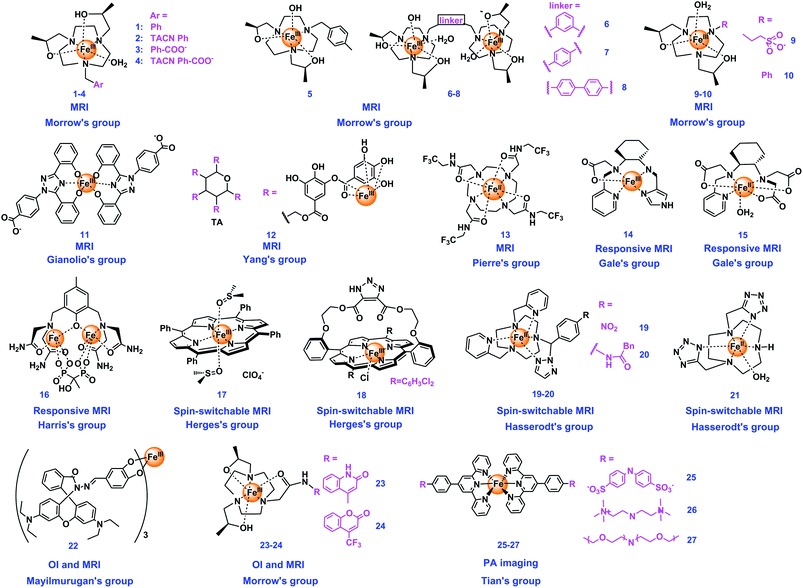
Iron is one of the most important essential trace metals for life.57 Biological systems utilize iron in numerous vital biochemical processes, including oxygen transport, DNA replication, and various redox reactions, owing to its capacity of electron transfer and cycling between different oxidation states.58,59 Iron complexes are required as a catalytic center for many redox enzymes, such as cytochrome P450, to mediate many redox processes that are crucial for energy production and intermediate metabolism.60 In recent years, the applications of iron complexes as sensors have been widely studied due to their rich valence state changes and excellent photomagnetic properties, especially for optical and MR imaging. The structures of Fe complex-based probes summarized in this review are shown in Fig. 1. The properties of these agents have been summarized in Table 1.| Imaging agents | Type of agents | Contrast/fluorescence enhancement | Magnetic field | Relaxivity (mM−1 s−1) | Detection mechanisms | Targets | Ref |
|---|---|---|---|---|---|---|---|
| 1–4 | MRI | Δr1 of 1 was 6-fold higher in the kidney than for Gd(DTPA) at 30 minutes | 4.7 T, 37 °C | 1: r1 = 3.8, r2 = 6.2, 3: r1 = 1.8, r2 = 3.0 | Proton relaxivity of water from second-sphere interactions with Fe | Kidney image | 66 |
| 5–8 | The most effective 7 increased 3-fold in r1 relaxivity compared to 5 | 4.7 T, 37 °C | 5: r1 = 1.83, 6: r1 = 4.06, 7: r1 = 5.26, 8: r1 = 4.36 | Proton relaxivity of inner-sphere water exchange | Blood pool and kidney | 64 | |
| 9, 10 | Enhanced bladder and kidney contrast | 4.7 T, 37 °C | 9: r1 = 2.0, 10: r1 = 0.97 | Proton relaxivity of water from second-sphere interactions | Kidney image | 71 | |
| 11 | MRI | Δr1 = 1.4 mM−1 s−1 with HSA | 0.47 T, 37 °C | 11: r1 = 1.7, 11/HSA: r1 = 3.1 | Proton relaxivity, second sphere water molecules; binding BSA | Human serum albumin (BSA); cancer | 65 |
| 12 | MRI | Significant increase | 1 T | 12/HSA: r1 = 3.47 | Inner-sphere water proton relaxivity; binding BSA | In vivo imaging and PTT | 72 |
| 13 | 19F and CEST dual modal MRI | CEST signal increase between pH 6.9 and 7.4; 19F independent of pH | — | Detection limitation: 2 mM | 19F and CEST MRI signals at different pH | In vivo pH mapping | 73 |
| 14 | pH-responsive MRI | Δr1 = 1.56 mM−1 s−1, over 7-fold increase | 4.7 T, 37 °C | Dimer: r1 = 0.14 monomer: r1 = 1.7 | Proton relaxivity, deprotonation of the dimer forms the high-relaxivity monomeric complex | pH changes | 74 |
| 15 | L-Cys, H2O2 responsive MRI | 10- to 15-fold increase | 4.7 T, 37 °C | Fe(II): r1 = 0.18, Fe(III): r1 = 2.4 | H2O2 rapidly oxidizes 14 from Fe(II) to Fe(III), resulting in higher relaxivity | L-Cys, H2O2 pathological change in vivo | 48 |
| 16 | Redox-dependent PARACEST MRI | — | 9.4 T | — | Redox-dependent PARACEST | Ratiometric quantitation of the redox environment | 49 |
| 17 | Spin switchable MRI | Δr1 = 10.0 mM−1 s−1, over 15-fold increase | — | High spin: r1 = 10.6, low spin: r1 = 0.6 | Light-controlled spin switching; inner sphere water proton relaxation | High-spin catalytic species generation of artificial enzyme systems | 75 |
| 18 | — | — | — | Light switching of deprotonated and photodissociable ligand bound high-spin Fe(III) | pH responsive CA | 76 | |
| 19, 20 | T 1 decrease of 80–90% | 11.4 T, 37 °C | — | Analyte switched high-spin Fe(II), inner sphere water proton relaxation | Catalytic hydrogenation and penicillin amidase | 78 | |
| 21 | — | 7 T | r 1 = 0.57 | Analyte switched high-spin Fe(II), inner sphere water proton relaxation | A specific targeted enzyme | 79 | |
| 22 | OI–MRI dual modal | r 1 relaxivity significantly enhanced | — | — | Rhodamine catecholate and inner sphere water proton relaxation | NO and acidic pH | 80 |
| 23, 24 | — | 4.7 T, 37 °C | +HSA: 23: r1 = 1.10, 24: r1 = 1.06 | Second-sphere water proton relaxation | Yeast cells | 67 | |
| 25–27 | PA imaging | — | — | — | Photoacoustic signal | Multi-modal imaging | 81 |
Gianolio's group also designed an Fe(III)-based MRI T1 CA (11, Fig. 1). 11 possesses an excellent thermodynamic stability, a high binding affinity with human serum albumin that three complex molecules can bind to protein at the same time, and a good relaxivity that increases in the range of 20–80 MHz. The reason for relaxation enhancement was revealed that the second sphere water molecules probably formed hydrogen bonds with the coordinating phenol-oxygen, while further enhancement was observed by binding albumin to form the supramolecular adduct. The contrast ability of 11 was comparable to that of the commercial agent GdIII (DTPA). Furthermore, the complex could be completely removed within 24 h from blood samples of healthy mice, which can avoid the issues brought by exogenous Gd. In this work, the Fe(III) center of 11 is highly robust to any redox, and the reduction of 11 to Fe(II) species is not anticipated under physiological conditions.65 Yang's group developed a strategy to develop a nontoxic Fe(III) complex, constructed by using tannic acid (TA), a large natural polyphenol, and bovine serum albumin (BSA), 12@BSA, which slows the molecular spin and increase the relaxivity of protons (Fig. 1). 12@BSA shows not only good stability and biocompatibility, but also exhibits a good T1 MRI enhancement effect. In addition, 12@BSA can be used as a photothermal agent for effective tumor elimination.72
Pierre's group presented an eight-coordinated stable Fe(II) complex (13, Fig. 1) as a dual CA of 19F and chemical exchange saturation transfer (CEST) for ratiometric pH imaging. The intensity of the CEST response depends on the concentration of 13 and pH values, with a significant increase in saturation transfer at pH = 6.9–7.4, while the 19F imaging signal is not pH-dependent. And the rare 8-coordinate geometry Fe(II) complex is stable in air, making the ratiometric pH-mapping and accurate differentiation at pH = 6.9–7.4 being achieved.73
Besides, switchable spin-crossover Fe complexes have significant potential to be used as OFF/ON mode MRI agents. Some examples using the spin-switching Fe complexes as stimuli-responsive MRI CAs have been reported. Herges's group developed a series of switchable spin-crossover iron complexes. For example, they designed an Fe(III) porphyrin (17, Fig. 1) as light-controlled switching MRI with a photochromic axial ligand. The spin state of 17 can be changed reversibly between low-spin (S = 1/2) and high-spin (S = 5/2) under irradiation of two wavelengths (365 and 435 nm), with the switching efficiency reaching up to 76% at room temperature and no fatigue observed after over 1000 switching cycles. This complex can not only be a simple artificial mimic for the first step of the cytochrome P450 catalytic cycle, but also can be used as an MRI agent with the relaxation time T1 of water protons switching 15 times as the spin switch. This was the first example of a light-controlled molecular spin switch using an Fe(III) complex.75 The same group constructed a novel two-component system based on Fe(III) porphyrins bridged with 1,2,3-triazole ligands and one separate ligand (18, Fig. 1) for spin switching. The mechanism illustrated that spin state changes happened when the axial ligands changed. During deprotonation, the triazolate anion as the axial ligand coordinating to the Fe(III) makes the complex a high-spin neutral complex, while pyridine coordinating to the second axial coordination site makes 18 change from high-spin to low-spin. Besides, the spin state of the complex can be changed reversibly in the presence of a photoswitchable ligand. 18 can be a potential photoswitchable responsive CA.76
Hasserodt's group also published some interesting studies using the spin switchable iron complexes.77 They designed two binary Fe(II) complexes (19–20, Fig. 1) of a large ring hexadentate N6 ligand with two picolyl pendent arms with high stability and low-spin diamagnetic state. The probes can detect chemically transforming analytes through reacting with the respective stimulus, the reacted third pendent arm of the complexes will disappear entirely and the new complex will be converted to a high-spin paramagnetic state (from 0 to 2). Therefore, 19 and 20 can successfully detect the analyte and accomplish the spin state changes under simulated physiological conditions.78 They also prepared a neutral Fe(II) complex (21, Fig. 1) with a novel macrocyclic chelator as a CA for MRI, which is the first example of using an iron(II) complex to examine the enhanced MRI contrast in vitro and in vivo of mice. The results constitute an important step to discover an iron(II)-based CA that passes from the low-spin (S = 0, off) to the high-spin (S = 2, on) state, upon the presence of and transformation by a specific target enzyme in live animals.79
3.2 Manganese complexes
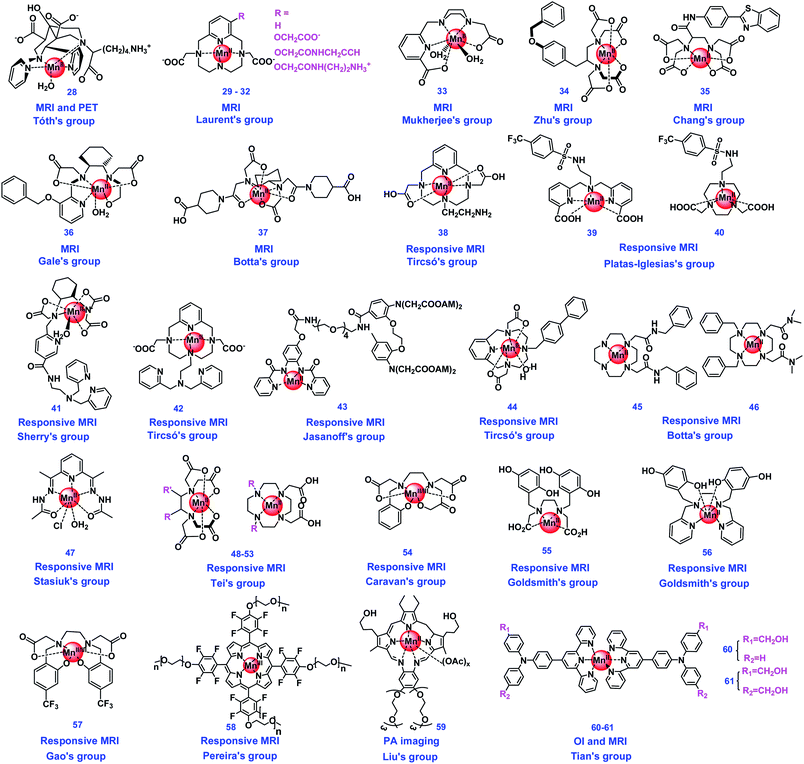
Manganese is another essential metal ion of life.82 The water splitting complex of Photosystem II in plants and cyanobacteria, CaMn4O4, was constituted using manganese as the key element.83 Besides, manganese is one of the main metals for metalloenzymes,23 for example, manganese binding superoxide dismutase (Mn-SOD) is one of the main SODs.32 The oxidation states of manganese are usually +2, +4, and +7.32 In addition to numerous biomimetic studies utilizing manganese being reported, manganese complex-based bioimaging agents are also constructed in recent years. The structures of Mn complex-based probes summarized in this review are shown in Fig. 2. The properties of these manganese complex-based bioimaging agents have been summarized in Table 2.| Imaging agents | Type of agent | Contrast/fluorescence enhancement | Magnetic field | Relaxivity (mM−1 s−1) | Detection mechanisms | Targets | Ref |
|---|---|---|---|---|---|---|---|
| 28 | MRI and PET | Δr1 = 1.75 mM−1 s−1 increased in human serum | 4.7 T, 37 °C | r 1 = 3.37 | Proton relaxation of inner- and second-sphere water interacted with Mn | In vivo imaging | 84 |
| 29–32 | MRI | — | 9.4 T, 37 °C | r 1 = 29: 2.8; 30: 2.7; 31: 3.1; 32: 2.95 | Proton relaxation of inner sphere water interacted with Mn | Potential to be clinical MRI CAs | 85 |
| 33 | Increase with the increase of concentration | 1.41 T, 25 °C | r 1 = 4.28 | 86 | |||
| 34 | Δr1 = 11.47 mM−1 s−1 | 0.47 T, 32 °C | 34: r1 = 4.34, 34/BSA: r1 = 15.81 | Proton relaxation; binding with BSA | Liver specific imaging | 87 | |
| 35 | Δr1 = 11.6 mM−1 s−1 | 1.5 T, 24 °C | 35: r1 = 3.5, 35/HSA: r1 = 15.1 | Liver targeting, tumor imaging | 88 | ||
| 36 | Δr1 = 6.6 mM−1 s−1 | 1.4 T, 37 °C | In Tris: r1 = 2.4, in plasma: r1 = 9.0 | Proton relaxation | Liver tumor | 89 | |
| 37 | MRI | Δr1 = 15.0 mM−1 s−1 | 0.5 T, 25 °C | 37: r1 = 3.5, 37/SiNPs: r1 = 18.5 | Proton relaxation, inner sphere water interaction with Mn(II) | Low field (up to 1.5 T) MRI applications in vitro and in vivo | 90 |
| 38 | pH responsive MRI | Δr1 = 1.4 mM−1 s−1 | 0.49 T, 25 °C | pH 8.4: r1 = 2.1, pH 6.0: r1 = 3.5 | pH in vitro | 91 | |
| 39, 40 | Δr1 = 6.0 mM−1 s−1 | 0.5 T, 25 °C | pH 9.0: r1 = 3.8, pH 4.0: r1 = 9.8 | pH in vitro | 92 | ||
| 41 | Metal-sensitive MRI | Δr1 = 13.7 mM−1 s−1 | 1.4 T, 37 °C | 41: r1 = 3.7, 41 + Zn2+ + HSA: r1 = 17.4 | Proton relaxation; binding with Zn2+ and BSA | Glucose-stimulated zinc secretion from the mouse pancreas and prostate | 93 |
| 42 | Δr1 = 5.92 mM−1 s−1 | 1.41 T, 37 °C | 42: r1 = 3.11, 42 + Zn2+ + HSA: r1 = 9.03 | Glucose-stimulated zinc | 31 | ||
| 43 | Δr1 = 1.5 mM−1 s−1 | — | 43: r1 = 3.6, 43 + Ca2+: r1 = 5.1 | Proton relaxation; binding with Ca2+ | Intracellular Ca2+ sensing | 94 | |
| 44 | HSA-sensitive MRI | Δr1 = 31.9 mM−1 s−1 | 0.49 T, 37 °C | 44: r1 = 3.8, 44 + HSA: r1 = 35.7 | Proton relaxation; inner sphere water interaction with Mn(II) and HSA | HSA of vascular | 95 |
| 45, 46 | Δr1 = 15.7 and 24.6 mM−1 s−1 for 51 and 52, respectively | 0.5 T, 37 °C | r 1 = 2.8 for both r1 = 18.5, 27.4 with HSA | HSA | 96 | ||
| 47 | BSA-sensitive MRI | Δr1 = 15.4 mM−1 s−1 | 0.5 T, 25 °C | r 1 = 5.7, r1 = 21.1 with HSA | BSA | 97 | |
| 48–53 | MRI | Increased 1.8–13.3 fold | 0.75 T, 25 °C | r 1 values for 48–53: 4.6, 5.9, 18.4, 2.2, 12.6, 15.3 | HSA binding enhanced MRI | 98 | |
| 54 | Redox-active MRI | 3-fold increase in relaxivity | 4.7 T, 37 °C | — | Exchange between Mn(II) and Mn(III) | GSH and H2O2 | 99 |
| 55 | — | — | — | Proton relaxation; inner sphere water interactions | H2O2 | 100 | |
| 56 | Δr1 = 1.71 mM−1 s−1 | 3 T, 25 °C | r 1 = 5.46, r1 = 7.17 with H2O2 | H2O2 | 101 | ||
| 57 | Δr1 = 2.0 mM−1 s−1 | 0.5 T, 37 °C | Mn(II): r1 = 0.7, Mn(III): r1 = 2.7 | 1H/19F MRI | GSH and H2O2 | 102 | |
| 58 | Obviously enhanced for Mn(II) | 0.5 T, 25 °C | Mn(II): r1 = 24.3 | Exchange between Mn(II) and Mn(III) | Ascorbic acid or β-mercaptoethanol, O2 | 103 | |
| 59 | PA | Intensity increased 3.1-fold 24 h after injection | — | — | Nonradiative conversion of light energy | RAW 264.7 cells and in vivo | 104 |
| 60, 61 | OI and MRI | — | — | — | Two-photon OI and MRI | In cellulo and ex vivo | 105 |
To improve the detection contrast, some organ-specific CAs have been developed. Zhu's group constructed a novel Mn(II) complex (34, Fig. 2) binding with a lipophilic group-modified ethylenediaminetetraacetic acid (EDTA), as a new liver-specific MRI CA. In vivo in mice biodistribution confirmed that 34 can be hepatic specifically ingested with a combination of hepatobiliary and renal clearance pathways. Bromosulfophthalein (BSP) inhibition imaging, biodistribution, and cellular uptake studies confirmed that the amphiphilic anion of 34 promotes the hepatic targeting mediated by organic anion transporting polypeptides (OATPs) expressed by functional hepatocytes. The new CA 34 may contribute to the MRI diagnosis of cancer with altered OATP expression.87 Chang's group designed and synthesized a novel manganese(II) complex (35, Fig. 2) based on an EDTA coordination cage bearing a benzothiazole aniline (BTA) moiety, which was used as a potential liver-specific MRI CA. The liver-specificity was demonstrated because the new Mn chelate could be rapidly taken up by liver hepatocytes and excreted by the kidneys and biliary system. 35 showed much higher kinetic inertness and R1 relaxivity than MnDPDP, a clinically approved liver-specific MRI CA.88 Gale's group developed a series of manganese-based CAs containing the ligands of PyC3A derivatives, which were used as liver-specific MRI CAs, and the structure–activity relationships were also studied. Among these complexes, the PyC3A-3-OBn ligand containing 36 (Fig. 2) emerged as the lead candidate due to the properties of high relaxivity, rapid blood clearance, and avid hepatocellular uptake. 36 can render liver tumors conspicuously hypo-intense in a murine model and be wholly eliminated within 24 h of injection. The SAR generated in this study suggested that liver specific properties could be affected by the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values.89
P values.89
Owing to the development of nanotechnology, many medical studies used nanoparticles as carriers. As for the MRI agents, some complexes were loaded onto nanomaterials to increase the relaxivity of complexes. Botta's group obtained novel Mn(II)-based nanoprobes as high contrast enhancing agents MRI by anchoring a Mn(II)-CDTA derivative (37, Fig. 2) to the surface of organo-modified silica nanoparticles (SiNPs). High image contrast was found for the 37-based nanoprobes in T1w-MRI images collected on phantoms containing relatively small amounts of CA, and low cellular toxicity was observed. Preliminary in vivo studies demonstrated the efficiency of the 37-based nanoparticle as T1w-MRI probes, resulting in significant contrast enhancement in the liver.90
Some metal ion-sensitive MRI studies have also been reported. Sherry's group prepared a Mn(II)-based zinc-sensitive MRI CA (41, Fig. 2) to detect glucose-stimulated zinc secretion (GSZS) from the mouse pancreas and prostate in vivo. 41 was revealed to have superior kinetic inertness from the thermodynamic and kinetic stability experiments, compared with GdDTPA. When compared with other gadolinium-based zinc sensors, 41 appeared to be an alternative for β-cell function images in the pancreas, and GSZS from the prostate. The two BPEN moieties each bound with a single Zn2+ ion resulted in a new complex forming a ternary complex with albumin, which resulted in an increase of r1 relaxivity.93 Tircsó's group presented another zinc-responsive Mn(II)-based MRI CA candidate (42, Fig. 2) derived from pyclen-3,9-diacetate (3,9-PC2A) possessing a di(2-picolyl)amine (DPA) moiety as an active arm. 42 has a relatively high relaxation rate, which cannot be affected by the zinc concentration directly, however, in the co-presence of 0.7 mM human serum albumin (HSA), the relaxation rate values increased significantly owing to the efficient binding of 42 to the protein. The increase can be visualized by MRI experiments in vitro, and in vivo tests confirmed that GSZS could be observed in the prostate of a healthy mouse.31 Jasanoff's group developed a Mn-based MRI CA (43, Fig. 2) as a sensor for intracellular calcium ions (Ca2+). This cell-permeable complex will undergo esterase cleavage, and allow intracellular Ca2+ levels to be monitored by MRI. Their results indicated that 43 is a Ca2+ sensor that can achieve spatiotemporal mapping of calcium signaling processes, and possess the properties of being compatible with the extensive expanded depth and field of view afforded by MRI.94
Some studies reported enhanced relaxivity while the complexes bind with proteins. Tircsó’s group prepared a macrocyclic chelate Mn(II) complex (44, Fig. 2) which possesses high thermodynamic stability and kinetic inertness as well as remarkable relaxivity in the presence of HSA. These properties make 44 have a significant MRI signal intensity increase in the vasculature even at low dose of it.95 Botta's group synthesized two novel macrocyclic ligands Mn(II) based on the 1,4-DO2AM platform and containing two benzyl groups (45 and 46, Fig. 2), which could form relatively strong adducts with HSA. The interaction with HSA slows down the rotational tumbling of 45 and 46 in solution, resulting in adducts endowed with remarkably high proton relaxivities.96 Stasiuk's group developed a Mn(II)-based T1 CA (47, Fig. 2) with a Schiff-base type diacetylpyridylcarbo-hydrazide ligand using a simple single-pot template reaction. 47 displays optimized r1 relaxivities at both medium (20 and 64 MHz) and high (300 and 400 MHz) magnetic fields. Upon binding to BSA (Ka = 4.2 × 103 M−1), a much enhanced r1 value was achieved. The studies in vivo showed that 47 could be cleared intact into the bladder through renal excretion, and has a prolonged blood half-life compared to commercially avaliable Gd CA Magnevist.97 Tei's group reported that the Mn(II) complexes of six original amphiphilic ligands (three EDTA-like ligands and three 1,4-DO2A derivatives) embodying one or two aliphatic chains (48–53, Fig. 2) were evaluated as potential MRI CAs. The relaxivity (r1) enhancement was observed for the Mn(II) micelles, resulting from the increased molecular tumbling rate of the supramolecular aggregate. 48–53 could bind to HSA tightly using their amphiphilic chelates with large association constants, and achieved remarkable relaxivity values.98
Some studies reported the detection of bioactive species using Mn complexes, with the relaxivity changing along with the change of the Mn oxidation state. Caravan's group has reported a simple Mn coordination complex (54, Fig. 2) as a redox-activated MR CA in 2013; the Mn(II)/Mn(III) redox couple could convert reversibly in the presence of glutathione (GSH) and H2O2 followed by relaxivity changes.99 Goldsmith's group designed a Mn(II) complex (55, Fig. 2) with quinol-containing ligands as MRI CA sensors to H2O2. The metal center could be oxidized by H2O2 to the less paramagnetic Mn(III), which can eliminate the r1 response of this complex.100 Upon modifying the ligand, H2O2 may oxidize the ligand and result in the opposite relaxivity changes. The same group reported another redox-responsive mononuclear Mn(II) complex (56, Fig. 2) as a MRI CA by using the ligand with two quinols. The introduction of the second quinol improves the relaxivity response of 56 to H2O2 and reduces its cytotoxicity. The authors found that H2O2 can partially oxidize the quinol subunits to para-quinones, and the oxidation of the ligand enables more H2O to coordinate with the metal ion due to para-quinones being unable to deprotonate to an anionic form, resulting in the enhancement of relaxivity.101
Gao's group prepared a redox-responsive manganese complex (57, Fig. 2), which can convert the Mn(III)/Mn(II) couple reversibly by GSH and H2O2, respectively. The complexes in both states are water soluble and biologically interconvertible. Therefore, 57 can be used as a probe in 1H/19F MRI for detecting and imaging biological redox species. Mn(III)/Mn(II) in this system have been demonstrated to be a redox reversible couple. This work provides a pathway to access redox information associated with various diseases.102 Pereira's group constructed a biocompatible redox MRI probe based on a Mn porphyrin complex (58, Fig. 2). The complex can reversibly switch between Mn(II)/Mn(III) oxidation states in aqueous solutions. The Mn(III) ion can be reduced to the Mn(II) form in the presence of ascorbic acid or β-mercaptoethanol, and the Mn(II) would be slowly but fully reversed back to Mn(III) in the presence of O2. This redox imaging probe showed good water solubility, biocompatibility, non-toxicity, and a strong “turn on” relaxivity response upon reduction.103
3.3 Copper complexes
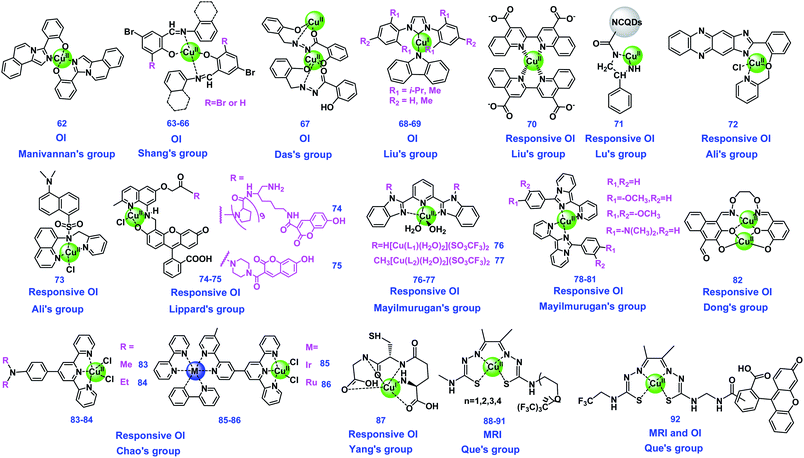
Copper is the third most abundant essential trace metal for life, which usually exists through binding with proteins to form metalloproteins.20 Similar to the two metal ions discussed above, copper complexes can be the catalytic center of some metalloenzymes, such as copper, zinc-superoxide dismutase (Cu, Zn-SOD).106 Copper also plays a vital role in other living possesses, including gene expression, electron transfer and redox processes, to maintain the metabolism of life.107 The imaging studies using copper complexes have attracted much attention in recent decades through both optical and MR imaging. The structures of Cu complex-based probes summarized in this review are shown in Fig. 3, and their properties have been summarized in Table 3.| Imaging agents | Type of agents | Contrast/fluorescence enhancement | Magnetic field | Relaxivity (mM−1 s−1) | Detection mechanisms | Targets | Ref |
|---|---|---|---|---|---|---|---|
| 62 | OI | Obvious color change | — | — | Colorless to yellowish green | CN− | 108 |
| 63–66 | Over 80-fold increase of fluorescence intensity | — | — | Color change and fluorescence enhancement | HS−; H2S level in vivo | 109 | |
| 67 | 156-fold fluorescence enhancement after incubation with Cyt C | — | — | Interaction with Cyt C, fluorescence enhancement | Cytochrome C (Cyt C) | 110 | |
| 68–69 | RTP is achieved with a lifetime of 140 ms | — | — | Fluorescence and phosphorescence dual-emission | — | 111 | |
| 70 | Stimuli-responsive OI | The LODs of rabbit lgG and PSA are 0.05 ng mL−1 and 0.38 ng mL−1, respectively | — | — | Reduction of Cu(II) to Cu(I), colorimetric and fluorescence methods | Rabbit IgG and prostate specific antigen (PSA) | 112 |
| 71 | The LODs of H2O2 by colorimetric and fluorescent methods are 80 nM and 2.5 μM | — | — | Cu(I) may be formed Cu(II)-LPQDs; colorimetric and fluorescence changes | H2O2 | 113 | |
| 72 | 5-fold and 6-fold fluorescence enhancement upon treatment with NO and HNO, respectively | — | — | Reduction of Cu(II) to Cu(I) resulted in a remarkable fluorescence recovery | NO and HNO | 46 | |
| 73 | Fluorescence intensity restored up to six-fold (90%) | — | — | Fluorescence enhancement through PET | HNO | 114 | |
| 74, 75 | 11-fold ratiometric turn-on | — | — | Cu(II) reduced to Cu(I), de-metallization, and N-nitrosated ligand products recovered fluorescence | Ratiometric detection of NO | 115 | |
| 76, 77 | The fluorescence intensity of 76 and 77 increased 28 and 143 times, respectively | — | — | Reduction of Cu(II) to Cu(I) and the displacement of copper recovered fluorescence | Cys | 116 | |
| 78–81 | The fluorescence intensity increased 103-fold in the presence of Cys | — | — | Reduction of Cu(II) to Cu(I) resulted in fluorescence recovery | Cys | 117 | |
| 82 | The LODs of S2O32− and GSH are 149 and 131 nM, respectively | — | — | Cu(II) was reduced to Cu(I), released free ligand, and fluorescence recovered by the ICT process | S2O32− and GSH | 118 | |
| 83–86 | 1500-fold luminescence intensity enhancement of 84 in the detection of Hcy | — | — | Cu(II) was reduced by Hcy to form four-coordinated terpyridine-Cu(I) and recovered luminescence | Hcy | 119 | |
| 87 | OI (and PET) | — | — | — | Luminescent Cu(I) oxidized to non-luminescent Cu(II) | Oxidation states in vivo | 120 |
| 88–91 | 19F MRI | T 2-increase of 129-fold between one of the Cu2+ complexes and its reduced system | 7.0 T, 22 °C | — | Hypoxia-targeting 19F MRI | Hypoxia cells | 124 |
| 92 | 19F MRI and OI | The SNR of 19F increased 7-fold and the fluorescence intensity increased 3.5-fold in hypoxia | — | — | 19F MR and fluorescence signals being turned on when reduced to Cu(I) | Hypoxic cells | 122 |
The photophysical properties and fluorescence mechanisms of copper complexes may change as the metal valence changed. Liu's group developed a colorimetric alkaline phosphatase (ALP)-linked immunoassay method for highly sensitive determination of rabbit IgG and prostate specific antigen (PSA) using a Cu(II)-BCA (70, Fig. 3) based color generation system. In the presence of IgG or PSA, ALP was introduced to catalyze L-ascorbic acid 2-phosphate (AAO) to L-ascorbic acid (AA), which results in Cu(II) of 70 being reduced to Cu(I). The reduced Cu(I) species of 70 showed a purple color, and was utilized as a promising chromogenic reporter for the visual immunoassay. This method holds many advantages, and provides a promising colorimetric immunoassay platform for bio-chemical analysis in different fields.112 Lu's group constructed a Cu(II)-functionalized nanoplatform by coupling a Cu(II) complex with L-phenylalaninamide (LPN) (71, Fig. 3) with N-doped carbon quantum dots (LPQDs), which was used as mimetic-peroxidase with catalytic properties for sensitive monitoring of H2O2. The detection of H2O2 in biological samples could be realized with the detection limits of 80 nM and 2.5 μM by colorimetric and fluorescent methods, respectively, through capturing the appearance of colorimetric and fluorescence changes. During the H2O2 detection process, Cu(I)-LPQDs may form through discharging electrons by aromatic species in LPQDs into Cu(II)-LPQDs. These findings provide clues for the construction of intelligent biomimetic nanoplatforms using copper for sensing applications and exhibit high peroxidase mimetic activity.113 Ali's group synthesized a phenazine-based Cu(II) complex (72, Fig. 3) for the selective detection of NO and HNO in biological systems. 72 was nonfluorescent, however, fluorescence can remarkably regenerate upon treatment with NO and HNO under physiological conditions, where 72 was reduced to Cu(I) species. The photophysical properties of the ligand and complexes have been studied using DFT. 72 was found to be almost nontoxic and cell permeable, making it available to image the exogenous and endogenous NO and HNO in A549 and Raw 264.7 cells.46 The same group also synthesized a novel Cu(II) complex-based fluorescent probe (73, Fig. 3) which exhibits a high selectively significant fluorescence turn-on response towards HNO. HNO can induce the reduction of paramagnetic 73 to diamagnetic Cu(I) species with fluorescence obviously enhanced through the photoinduced electron transfer (PET) mechanism. Additionally, 73 could detect HNO effectively in the biological pH range, and the properties of water friendliness, low cytotoxicity and cell permeability, make 73 useful for the detection of HNO in living cells.114
The reduction of Cu(II) to Cu(I) can result in displacement of the complexes, and the fluorescence will be restored along with the free ligands, which probably further reacted with the bioactive molecules. Lippard's group presented the first two Cu(II)-based fluorescent sensors (74 and 75, Fig. 3) for direct ratiometric detection of nitric oxide (NO), which is one of their series of studies of directly imaging NO production in living cells by turn-on Cu(II)-based fluorescence. The probes work by energy transfer between hydroxycoumarin and fluorescein chromophores by a FRET mechanism, containing polyproline or piperazine as rigid spacers. In the presence of NO, the Cu(II) of probes was reduced to Cu(I), followed by amine deprotonation and nitrosation, and the fluorescence was restored for the N-nitrosated products. Notably, they elicited a rapid and selective ratiometric response upon direct reaction with NO at physiological pH values.115
Mayilmurugan's group constructed two Cu(II) complexes (76 and 77, Fig. 3) using 2,6-bis(benzimidazolyl)pyridine derivatives as the ligands, for highly L-cysteine (Cys) selective “turn-on” optical probes. The properties of 76 and 77 were compared, and both of them showed selective and efficient “turn-on” fluorescence behavior in the presence of Cys over other amino acids. The detection was realized by the reduction of the Cu(II) center to Cu(I) followed by the displacement of copper.116 They also synthesized four imidazopyridine-based Cu(II) complexes (78–81, Fig. 3) as turn-on OI probes for Cys in cancer cells. The molecular structures of these complexes were studied using different techniques. The detection studies indicated that 79 showed selective and efficient turn-on fluorescence behavior towards Cys among the probes. The selective detection may originate from a nearly perfect trigonal plane of 79 adopted around a Cu(II) center, which required minimum structural change during the reduction of Cu(II) to Cu(I) when imaging Cys, while other complexes need more reorganizational energy. 79 was used for OI of Cys in HeLa cells and macrophages.117 Dong's group designed and exploited a novel turn-on fluorogenic aldehyde-appended salamo-like Cu(II) complex (82, Fig. 3) for the simultaneous detection of thiosulfate (S2O32−) ions and GSH through an intramolecular charge transfer (ICT) process. 82 showed highly selective and sensitive fluorescence recovery towards S2O32− and GSH in aqueous DMF medium with the detection limits of 149 and 131 nM, respectively. During detection of S2O32− and GSH, Cu(II) was reduced to Cu(I) ions and displaced from the coordination sphere, resulting in the release of the free ligand.118
Some luminescent Cu(I) complexes as probes have been reported owing to the changeable valence states. Chao's group designed four terpyridine-based Cu(II) complexes (83–86, Fig. 3) for the detection of homocysteine (Hcy) in water and cells through aggregation enhanced luminescence. 84 and 86 were found to have better performance than 83 and 85. The enhanced luminescence was because these Cu(II) complexes were reduced by Hcy to form four-coordinated terpyridine-Cu(I) species, while the aggregation of reduced 83 and 85 enhanced the inhibition of oxygen-induced luminescence quenching. The cellular imaging experiments revealed that 84 could localized in the whole, while 86 only stained cytoplasm.119 Some redox-active ligands can be oxidized together with the metal ions to generate new complexes. For example, Yang's group demonstrated a luminescent glutathione-mediated Cu(I) complex (Cu(I)-GSH, 87, Fig. 3) for in vivo imaging studies. 87 was revealed to have much more efficient renal clearance and obviously lower liver accumulation than that of its oxidation states, which may be due to the strong protein binding capability of the partial Cu(II)-GSSG complex formed. The radioactive 87 have the potential to be a PET imaging agent in clinical translation.120
There are also some reports using copper complexes to make biosensors, such as nano copper complexes as a potentiometric membrane biosensor for the early detection of the prostate-specific antigen (PSA).48 And the studies using the copper complexes as theranostic agents are also reported.47,125–127
4. Conclusion and perspectives
In summary, redox-active transition metal complexes have shown great potential as bioimaging agents through optical or MR imaging modalities owing to their diverse structures and functions. We overviewed the development and bioimaging applications of molecular probes based on iron, manganese, and copper complexes in recent five years. Some of these probes have been approved as clinical MRI CAs (e.g., MnDPDP). These probes can be activated in biological systems by different bioactive species, including H2O2, H2S, pH, hypoxia, proteins etc., through the changes of responsive metal ions or ligands. However, the research in this field is still in its infancy, and many unresolved problems and challenges remain to be solved.We propose that the potential future development of this field could include the following directions: (1) the development of more dual/multi modal bioimaging agents. Each detection modality has its shortcomings, while the agents combining imaging modalities of optical and MR were expected to avoid the shortages and maintain their advantages. Besides, combined with the two imaging technologies, the rationally designed molecular probes could image the subcellular organelles and biomolecules more accurately. Although a few complex-based dual modal probes have been reported, developing more efficient agents with the combination of different advantages is still challenging. (2) Design of “double/multi-locked” bioimaging agents. The “single-locked” imaging agents that only respond to single stimulus may suffer from false positive signals of the normal cells/tissues. The agents that can respond to double even multiple stimuli can efficiently increase the detection accuracy. (3) The imaging application of these bioimaging agents can be extended. The transition metal complexes possess many specific advantages compared with organic molecular probes, therefore many other applications, such as the lifetime imaging, CT, ultrasound imaging etc., could be achieved. And the real-time dynamic imaging and imaging for deeper tissues, which are challengeable for current imaging studies, are expected to be overcome. (4) Finally, it is necessary to investigate the structure–activity relationship (SAR) of metal complex-based bioimaging agents systematically, and further explore the possibility of their clinical translation. Some manganese probes have been proved for clinical usage, however, more transition metal complex-based probes for clinical translation are needed. We believe that more novel efficient probes based on transition metal complexes will be developed for bio-applications in the following years.
Author contributions
B. Tang and N. Li proposed the conception, and led the project; S.-S. Xue and Y. Pan drafted the manuscript; all the authors wrote and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Shandong Provincial Key Research and Development Program (Major Scientific and Technological Innovation Project) (2021CXGC010515) and Youth Innovation Science and Technology Program of Higher Education Institution of Shandong Province (2019KJC022).Notes and references
- W. G. Kaelin Jr and C. B. Thompson, Nature, 2010, 465, 562–564 CrossRef PubMed.
- L. Satriano, M. Lewinska, P. M. Rodrigues, J. M. Banales and J. B. Andersen, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 748–766 CrossRef CAS PubMed.
- H. Sies and D. P. Jones, Nat. Rev. Mol. Cell Biol., 2020, 21, 363–383 CrossRef CAS PubMed.
- J. Muri and M. Kopf, Nat. Rev. Immunol., 2021, 21, 363–381 CrossRef CAS PubMed.
- C. Gorrini, I. S. Harris and T. W. Mak, Nat. Rev. Drug Discovery, 2013, 12, 931–947 CrossRef CAS.
- T. C. Jorgenson, W. Zhong and T. D. Oberley, Cancer Res., 2013, 73, 6118–6123 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed.
- L. Wu, J. Liu, P. Li, B. Tang and T. D. James, Chem. Soc. Rev., 2021, 50, 702–734 RSC.
- A. Gupta, P. Caravan, W. S. Price, C. Platas-Iglesias and E. M. Gale, Inorg. Chem., 2020, 59, 6648–6678 CrossRef CAS PubMed.
- L. Wu, F. Liu, S. Liu, X. Xu, Z. Liu and X. Sun, Int. J. Nanomed., 2020, 15, 7377–7395 CrossRef CAS PubMed.
- G. Katti, S. A. Ara and A. Shireen, Int. J. Dent. Clin., 2011, 3, 65–70 Search PubMed.
- H. Lusic and M. W. Grinstaff, Chem. Rev., 2013, 113, 1641–1666 CrossRef CAS PubMed.
- S. D. Rawson, J. Maksimcuka, P. J. Withers and S. H. Cartmell, BMC Biol., 2020, 18, 21 CrossRef PubMed.
- A. Sun, X. Liu and G. Tang, Front. Chem., 2017, 5, 124 CrossRef PubMed.
- W. Liu, J. Chen and Z. Xu, Coord. Chem. Rev., 2021, 429, 213638 CrossRef CAS.
- N. Kwon, D. Kim, K. M. K. Swamy and J. Yoon, Coord. Chem. Rev., 2021, 427, 213581 CrossRef CAS.
- A.-M. Caminade, A. Hameau, C.-O. Turrin, R. Laurent and J.-P. Majoral, Coord. Chem. Rev., 2021, 430, 213739 CrossRef CAS.
- D. L. Ma, C. Wu, G. Li, T. L. Yung and C. H. Leung, J. Mater. Chem. B, 2020, 8, 4715–4725 RSC.
- Y. Ning, G. Q. Jin, M. X. Wang, S. Gao and J. L. Zhang, Curr. Opin. Chem. Biol., 2022, 66, 102097 CrossRef CAS PubMed.
- S. Nasiri Sovari and F. Zobi, Chemistry, 2020, 2, 418–452 CrossRef.
- D. A. Iovan, S. Jia and C. J. Chang, Inorg. Chem., 2019, 58, 13546–13560 CrossRef CAS PubMed.
- Y. Lu, N. Yeung, N. Sieracki and N. M. Marshall, Nature, 2009, 460, 855–862 CrossRef CAS.
- C. Glorieux and P. B. Calderon, Biol. Chem., 2017, 398, 1095–1108 CAS.
- S. Yoshikawa and A. Shimada, Chem. Rev., 2015, 115, 1936–1989 CrossRef CAS PubMed.
- G. R. Warner, Y. Somasundar, K. C. Jansen, E. Z. Kaaret, C. Weng, A. E. Burton, M. R. Mills, L. Q. Shen, A. D. Ryabov, G. Pros, T. Pintauer, S. Biswas, M. P. Hendrich, J. A. Taylor, F. S. Vom Saal and T. J. Collins, ACS Catal., 2019, 9, 7023–7037 CrossRef CAS.
- E. Kuah, S. Toh, J. Yee, Q. Ma and Z. Gao, Chem.–Eur. J., 2016, 22, 8404–8430 CrossRef CAS PubMed.
- C. Wang, N. Zhang, C.-Y. Hou, X.-X. Han, C.-H. Liu, Y.-H. Xing, F.-Y. Bai and L.-X. Sun, Transition Met. Chem., 2020, 45, 423–433 CrossRef CAS.
- K. J. Bruemmer, S. W. M. Crossley and C. J. Chang, Angew. Chem., Int. Ed., 2020, 59, 13734–13762 CrossRef CAS PubMed.
- H. Singh, K. Tiwari, R. Tiwari, S. K. Pramanik and A. Das, Chem. Rev., 2019, 119, 11718–11760 CrossRef CAS PubMed.
- N. Kumar, Roopa, V. Bhalla and M. Kumar, Coord. Chem. Rev., 2021, 427, 213550 CrossRef CAS.
- R. Botár, E. Molnár, Z. Garda, E. Madarasi, G. Trencsényi, J. Kiss, F. K. Kálmán and G. Tircsó, Inorg. Chem. Front., 2022, 9, 577–583 RSC.
- A. Sinopoli, N. T. La Porte, J. F. Martinez, M. R. Wasielewski and M. Sohail, Coord. Chem. Rev., 2018, 365, 60–74 CrossRef CAS.
- J. Berrones Reyes, M. K. Kuimova and R. Vilar, Curr. Opin. Chem. Biol., 2021, 61, 179–190 CrossRef CAS PubMed.
- D. Parker, J. D. Fradgley and K. L. Wong, Chem. Soc. Rev., 2021, 50, 8193–8213 RSC.
- V. A. Larson, B. Battistella, K. Ray, N. Lehnert and W. Nam, Nat. Rev. Chem., 2020, 4, 404–419 CrossRef CAS.
- J. A. Drewry and P. T. Gunning, Coord. Chem. Rev., 2011, 255, 459–472 CrossRef CAS.
- H. Li, D. Kim, Q. Yao, H. Ge, J. Chung, J. Fan, J. Wang, X. Peng and J. Yoon, Angew. Chem., Int. Ed., 2021, 60, 17268–17289 CrossRef CAS PubMed.
- H. Xiao, W. Zhang, P. Li, W. Zhang, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2019, 59, 4216–4230 CrossRef PubMed.
- A. C. Sedgwick, L. Wu, H. H. Han, S. D. Bull, X. P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- A. Vogler and H. Kunkely, Luminescent Metal Complexes: Diversity of Excited States, Springer, Berlin, 2001 Search PubMed.
- Y. Y. Chia and M. G. Tay, Dalton Trans., 2014, 43, 13159–13168 RSC.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- M. L. Aulsebrook, B. Graham, M. R. Grace and K. L. Tuck, Coord. Chem. Rev., 2018, 375, 191–220 CrossRef CAS.
- X. Zhen, R. Qu, W. Chen, W. Wu and X. Jiang, Biomater. Sci., 2021, 9, 285–300 RSC.
- D. Wu, L. Chen, Q. Xu, X. Chen and J. Yoon, Acc. Chem. Res., 2019, 52, 2158–2168 CrossRef CAS PubMed.
- A. S. M. Islam, M. Sasmal, D. Maiti, A. Dutta, S. Ganguly, A. Katarkar, S. Gangopadhyay and M. Ali, ACS Appl. Bio Mater., 2019, 2, 1944–1955 CrossRef CAS PubMed.
- Y. Gou, M. Chen, S. Li, J. Deng, J. Li, G. Fang, F. Yang and G. Huang, J. Med. Chem., 2021, 64, 5485–5499 CrossRef CAS PubMed.
- H. Wang, V. C. Jordan, I. A. Ramsay, M. Sojoodi, B. C. Fuchs, K. K. Tanabe, P. Caravan and E. M. Gale, J. Am. Chem. Soc., 2019, 141, 5916–5925 CrossRef CAS PubMed.
- K. Du, E. A. Waters and T. D. Harris, Chem. Sci., 2017, 8, 4424–4430 RSC.
- G. S. Loving, S. Mukherjee and P. Caravan, J. Am. Chem. Soc., 2013, 135, 4620–4623 CrossRef CAS PubMed.
- M. Yu, B. S. Bouley, D. Xie and E. L. Que, Dalton Trans., 2019, 48, 9337–9341 RSC.
- J. Ruiz-Cabello, B. P. Barnett, P. A. Bottomley and J. W. Bulte, NMR Biomed., 2011, 24, 114–129 CrossRef CAS PubMed.
- K. L. Peterson, K. Srivastava and V. C. Pierre, Front. Chem., 2018, 6, 160 CrossRef PubMed.
- P. C. M. van Zijl and N. N. Yadav, Magn. Reson. Med., 2011, 65, 927–948 CrossRef CAS PubMed.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS PubMed.
- S. Zhang, M. Merritt, D. E. Woessner, R. E. Lenkinski and A. D. Sherry, Acc. Chem. Res., 2003, 36, 783–790 CrossRef CAS PubMed.
- J. H. McCullough, Evol. Med. Public Health, 2015, 2015, 149 Search PubMed.
- J. Wade, D. J. Byrne, C. J. Ballentine and H. Drakesmith, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2109865118 CrossRef CAS PubMed.
- T. Ganz and E. Nemeth, Nat. Rev. Immunol., 2015, 15, 500–510 CrossRef CAS PubMed.
- K. D. Dubey and S. Shaik, Acc. Chem. Res., 2019, 52, 389–399 CrossRef PubMed.
- B. C. Bales, B. Grimmond, B. F. Johnson, M. T. Luttrell, D. E. Meyer, T. Polyanskaya, M. J. Rishel and J. Roberts, Contrast Media Mol. Imaging, 2019, 2019, 8356931 Search PubMed.
- P. Papan, J. Kantapan, P. Sangthong, P. Meepowpan and N. Dechsupa, Contrast Media Mol. Imaging, 2020, 2020, 8877862 Search PubMed.
- M. Y. Lee, D. Choi, M. S. Jang and J. H. Lee, Bioconjugate Chem., 2018, 29, 2426–2435 CrossRef CAS PubMed.
- D. Asik, S. M. Abozeid, S. G. Turowski, J. A. Spernyak and J. R. Morrow, Inorg. Chem., 2021, 60, 8651–8664 CrossRef CAS PubMed.
- L. Palagi, E. Di Gregorio, D. Costanzo, R. Stefania, C. Cavallotti, M. Capozza, S. Aime and E. Gianolio, J. Am. Chem. Soc., 2021, 143, 14178–14188 CrossRef CAS PubMed.
- E. M. Snyder, D. Asik, S. M. Abozeid, A. Burgio, G. Bateman, S. G. Turowski, J. A. Spernyak and J. R. Morrow, Angew. Chem., Int. Ed., 2020, 59, 2414–2419 CrossRef CAS PubMed.
- A. Patel, D. Asik, J. A. Spernyak, P. J. Cullen and J. R. Morrow, J. Inorg. Biochem., 2019, 201, 110832 CrossRef CAS PubMed.
- C. Das, A. Mondal, S. Sengupta, C. Cardin and S. K. Chattopadhyay, Spectrochim. Acta, Part A, 2022, 273, 120943 CrossRef CAS PubMed.
- P. B. Tsitovich, F. Gendron, A. Y. Nazarenko, B. N. Livesay, A. P. Lopez, M. P. Shores, J. Autschbach and J. R. Morrow, Inorg. Chem., 2018, 57, 8364–8374 CrossRef CAS PubMed.
- S. M. Abozeid, M. S. I. Chowdhury, D. Asik, J. A. Spernyak and J. R. Morrow, ACS Appl. Bio Mater., 2021, 4, 7951–7960 CrossRef CAS PubMed.
- D. Asik, R. Smolinski, S. M. Abozeid, T. B. Mitchell, S. G. Turowski, J. A. Spernyak and J. R. Morrow, Molecules, 2020, 25, 2291 CrossRef CAS PubMed.
- L. An, Y. Cai, Q. Tian, J. Lin and S. Yang, Sci. China Mater., 2020, 64, 498–509 CrossRef.
- K. Srivastava, G. Ferrauto, V. G. Young, Jr, S. Aime and V. C. Pierre, Inorg. Chem., 2017, 56, 12206–12213 CrossRef CAS PubMed.
- H. Wang, A. Wong, L. C. Lewis, G. R. Nemeth, V. C. Jordan, J. W. Bacon, P. Caravan, H. S. Shafaat and E. M. Gale, Inorg. Chem., 2020, 59, 17712–17721 CrossRef CAS PubMed.
- S. Shankar, M. Peters, K. Steinborn, B. Krahwinkel, F. D. Sonnichsen, D. Grote, W. Sander, T. Lohmiller, O. Rudiger and R. Herges, Nat. Commun., 2018, 9, 4750 CrossRef PubMed.
- M. K. Peters, S. Hamer, T. Jakel, F. Rohricht, F. D. Sonnichsen, C. von Essen, M. Lahtinen, C. Naether, K. Rissanen and R. Herges, Inorg. Chem., 2019, 58, 5265–5272 CrossRef CAS PubMed.
- J. Salaam, M. Rivat, T. Fogeron and J. Hasserodt, Analysis & Sensing, 2020, 1, 11–29 Search PubMed.
- F. Touti, P. Maurin and J. Hasserodt, Angew. Chem., Int. Ed., 2013, 52, 4654–4658 CrossRef CAS PubMed.
- F. Touti, A. K. Singh, P. Maurin, L. Canaple, O. Beuf, J. Samarut and J. Hasserodt, J. Med. Chem., 2011, 54, 4274–4278 CrossRef CAS PubMed.
- D. Maheshwaran, T. Nagendraraj, T. Sekar Balaji, G. Kumaresan, S. Senthil Kumaran and R. Mayilmurugan, Dalton Trans., 2020, 49, 14680–14689 RSC.
- P. Xiang, Y. Shen, J. Shen, Z. Feng, M. Sun, Q. Zhang, S. Li, D. Li, G. Zhang, Z. Wu, Y. Tian, Z. Zhang and X. Tian, Inorg. Chem. Front., 2020, 7, 2753–2758 RSC.
- V. C. Culotta and M. J. Daly, Antioxid. Redox Signaling, 2013, 19, 933–944 CrossRef CAS PubMed.
- D. K. Dogutan and D. G. Nocera, Acc. Chem. Res., 2019, 52, 3143–3148 CrossRef CAS PubMed.
- D. Ndiaye, M. Sy, A. Pallier, S. Meme, I. de Silva, S. Lacerda, A. M. Nonat, L. J. Charbonniere and É. Tóth, Angew. Chem., Int. Ed., 2020, 59, 11958–11963 CrossRef CAS PubMed.
- M. Devreux, C. Henoumont, F. Dioury, S. Boutry, O. Vacher, L. V. Elst, M. Port, R. N. Muller, O. Sandre and S. Laurent, Inorg. Chem., 2021, 60, 3604–3619 CrossRef CAS PubMed.
- B. Phukan, C. Mukherjee, U. Goswami, A. Sarmah, S. Mukherjee, S. K. Sahoo and S. C. Moi, Inorg. Chem., 2018, 57, 2631–2638 CrossRef CAS PubMed.
- K. Chen, P. Li, C. Zhu, Z. Xia, Q. Xia, L. Zhong, B. Xiao, T. Cheng, C. Wu, C. Shen, X. Zhang and J. Zhu, J. Med. Chem., 2021, 64, 9182–9192 CrossRef CAS PubMed.
- M. K. Islam, S. Kim, H. K. Kim, S. Park, G. H. Lee, H. J. Kang, J. C. Jung, J. S. Park, T. J. Kim and Y. Chang, J. Med. Chem., 2017, 60, 2993–3001 CrossRef CAS PubMed.
- J. Wang, H. Wang, I. A. Ramsay, D. J. Erstad, B. C. Fuchs, K. K. Tanabe, P. Caravan and E. M. Gale, J. Med. Chem., 2018, 61, 8811–8824 CrossRef CAS PubMed.
- D. Lalli, G. Ferrauto, E. Terreno, F. Carniato and M. Botta, J. Mater. Chem. B, 2021, 9, 8994–9004 RSC.
- R. Botar, E. Molnar, G. Trencsenyi, J. Kiss, F. K. Kalman and G. Tircsó, J. Am. Chem. Soc., 2020, 142, 1662–1666 CrossRef CAS PubMed.
- R. Uzal-Varela, A. Rodriguez-Rodriguez, M. Martinez-Calvo, F. Carniato, D. Lalli, D. Esteban-Gomez, I. Brandariz, P. Perez-Lourido, M. Botta and C. Platas-Iglesias, Inorg. Chem., 2020, 59, 14306–14317 CrossRef CAS PubMed.
- S. Chirayil, V. C. Jordan, A. F. Martins, N. Paranawithana, S. J. Ratnakar and A. D. Sherry, Inorg. Chem., 2021, 60, 2168–2177 CrossRef CAS PubMed.
- A. Barandov, B. B. Bartelle, C. G. Williamson, E. S. Loucks, S. J. Lippard and A. Jasanoff, Nat. Commun., 2019, 10, 897 CrossRef PubMed.
- F. K. Kalman, V. Nagy, B. Varadi, Z. Garda, E. Molnar, G. Trencsenyi, J. Kiss, S. Meme, W. Meme, É. Tóth and G. Tircsó, J. Med. Chem., 2020, 63, 6057–6065 CrossRef CAS PubMed.
- A. Forgacs, L. Tei, Z. Baranyai, D. Esteban-Gomez, C. Platas-Iglesias and M. Botta, Dalton Trans., 2017, 46, 8494–8504 RSC.
- S. Anbu, S. H. L. Hoffmann, F. Carniato, L. Kenning, T. W. Price, T. J. Prior, M. Botta, A. F. Martins and G. J. Stasiuk, Angew. Chem., Int. Ed., 2021, 60, 10736–10744 CrossRef CAS PubMed.
- G. Rolla, V. De Biasio, G. B. Giovenzana, M. Botta and L. Tei, Dalton Trans., 2018, 47, 10660–10670 RSC.
- G. S. Loving, S. Mukherjee and P. Caravan, J. Am. Chem. Soc., 2013, 135, 4620–4623 CrossRef CAS PubMed.
- T. E. Hutchinson, A. Bashir, M. Yu, R. J. Beyers and C. R. Goldsmith, Inorg. Chim. Acta, 2019, 496, 119045 CrossRef CAS.
- M. Yu, M. B. Ward, A. Franke, S. L. Ambrose, Z. L. Whaley, T. M. Bradford, J. D. Gorden, R. J. Beyers, R. C. Cattley, I. Ivanovic-Burmazovic, D. D. Schwartz and C. R. Goldsmith, Inorg. Chem., 2017, 56, 2812–2826 CrossRef CAS PubMed.
- H. Chen, X. Tang, X. Gong, D. Chen, A. Li, C. Sun, H. Lin and J. Gao, Chem. Commun., 2020, 56, 4106–4109 RSC.
- S. M. A. Pinto, M. J. F. Calvete, M. E. Ghica, S. Soler, I. Gallardo, A. Pallier, M. B. Laranjo, A. M. S. Cardoso, M. Castro, C. M. A. Brett, M. M. Pereira, É. Tóth and C. Geraldes, Dalton Trans., 2019, 48, 3249–3262 RSC.
- Y. Ren, A. C. Sedgwick, J. Chen, G. Thiabaud, C. V. Chau, J. An, J. F. Arambula, X. P. He, J. S. Kim, J. L. Sessler and C. Liu, J. Am. Chem. Soc., 2020, 142, 16156–16160 CrossRef CAS PubMed.
- X. Tian, L. Xiao, Y. Shen, L. Luo, G. Zhang, Q. Zhang, D. Li, J. Wu, Z. Wu, Z. Zhang and Y. Tian, Inorg. Chem. Front., 2019, 6, 2914–2920 RSC.
- M. K. Tiwari, P. M. Hagglund, I. M. Moller, M. J. Davies and M. J. Bjerrum, Redox Biol., 2019, 26, 101262 CrossRef CAS PubMed.
- R. Memary, D. Giurco, G. Mudd and L. Mason, J. Cleaner Prod., 2012, 33, 97–108 CrossRef CAS.
- S. Mahata, S. Dey, B. B. Mandal and V. Manivannan, J. Photochem. Photobiol., A, 2022, 427, 113795 CrossRef CAS.
- X. Shang, X. Yue, Y. Chen, C. Li, H. Chen and T. Wang, Inorg. Chem. Commun., 2019, 99, 1–10 CrossRef CAS.
- S. Khanra, S. Ta, A. Paladhi, M. Ghosh, S. Ghosh, S. K. Hira, P. P. Manna, P. Brandao, V. Felix and D. Das, Chem. Commun., 2020, 56, 6563–6566 RSC.
- J. Li, L. Wang, Z. Zhao, X. Li, X. Yu, P. Huo, Q. Jin, Z. Liu, Z. Bian and C. Huang, Angew. Chem., Int. Ed., 2020, 59, 8210–8217 CrossRef CAS PubMed.
- L. Lei, W. Xie, Z. Chen, Y. Jiang and Y. Liu, Sens. Actuators, B, 2018, 273, 35–40 CrossRef CAS.
- D. Zhang, X. Mao, Z. Zhang, S. Zhang, J. Chen, D. Shan and X. Lu, Sens. Actuators, B, 2019, 284, 684–694 CrossRef CAS.
- D. Maiti, A. S. M. Islam, A. Dutta, M. Sasmal, C. Prodhan and M. Ali, Dalton Trans., 2019, 48, 2760–2771 RSC.
- A. Loas and S. J. Lippard, J. Mater. Chem. B, 2017, 5, 8929–8933 RSC.
- D. Maheshwaran, S. Priyanga and R. Mayilmurugan, Dalton Trans., 2017, 46, 11408–11417 RSC.
- S. Priyanga, T. Khamrang, M. Velusamy, S. Karthi, B. Ashokkumar and R. Mayilmurugan, Dalton Trans., 2019, 48, 1489–1503 RSC.
- Y.-H. Deng, R.-Y. Li, J.-Q. Zhang, Y.-F. Wang, J.-T. Li, W.-T. Guo and W.-K. Dong, New J. Chem., 2021, 45, 8597–8607 RSC.
- D. Chao and Y. Zhang, Sens. Actuators, B, 2017, 245, 146–155 CrossRef CAS.
- S. N. Yin, Y. Liu, C. Zhou and S. Yang, Nanomater, 2017, 7, 132 CrossRef PubMed.
- J. S. Enriquez, M. Yu, B. S. Bouley, D. Xie and E. L. Que, Dalton Trans., 2018, 47, 15024–15030 RSC.
- R. T. Kadakia, D. Xie, D. Martinez, M. Yu and E. L. Que, Chem. Commun., 2019, 55, 8860–8863 RSC.
- K. E. Prosser, D. Xie, A. Chu, G. A. MacNeil, B. R. Varju, R. T. Kadakia, E. L. Que and C. J. Walsby, Chem.–Eur. J., 2021, 27, 9839–9849 CrossRef CAS PubMed.
- D. Xie, S. Kim, V. Kohli, A. Banerjee, M. Yu, J. S. Enriquez, J. J. Luci and E. L. Que, Inorg. Chem., 2017, 56, 6429–6437 CrossRef CAS PubMed.
- M. A. Akl, E. R. El-Gharkawy, N. A. El-Mahdy, S. M. El-Sheikh and S. M. Sheta, Dalton Trans., 2020, 49, 15769–15778 RSC.
- A. Bhattacharyya, A. Jameei, A. Garai, R. Saha, A. A. Karande and A. R. Chakravarty, Dalton Trans., 2018, 47, 5019–5030 RSC.
- C. Vidya Rani, M. P. Kesavan, S. Haseena, R. Varatharaj, J. Rajesh and G. Rajagopal, Appl. Biochem. Biotechnol., 2020, 191, 1515–1532 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |