Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium/GF-Phos-catalyzed asymmetric carbenylative amination to access chiral pyrrolidines and piperidines†
Yue
Sun
a,
Chun
Ma
a,
Zhiming
Li
 *a and
Junliang
Zhang
*a and
Junliang
Zhang
 *ab
*ab
aDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai 200438, China. E-mail: zmli@fudan.edu.cn; junliangzhang@fudan.edu.cn
bZhuhai Fudan Innovation Institute, Hengqing District, Zhuhai 519000, China
First published on 1st September 2022
Abstract
The cross-coupling of N-tosylhydrazones has emerged as a powerful method for the construction of structurally diverse molecules, but the development of catalytic enantioselective versions still poses considerable challenges and only very limited examples have been reported. We herein report an asymmetric palladium/GF-Phos-catalyzed carbenylative amination reaction of N-tosylhydrazones and (E)-vinyl iodides pendent with amine, which allows facile access to a range of chiral pyrrolidines and piperidines in good yields (45–93%) with up to 96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 er. Moreover, mild conditions, general substrate scope, scaled-up preparation, as well as the efficient synthesis of natural product (−)-norruspoline are practical features of this method.
3.5 er. Moreover, mild conditions, general substrate scope, scaled-up preparation, as well as the efficient synthesis of natural product (−)-norruspoline are practical features of this method.
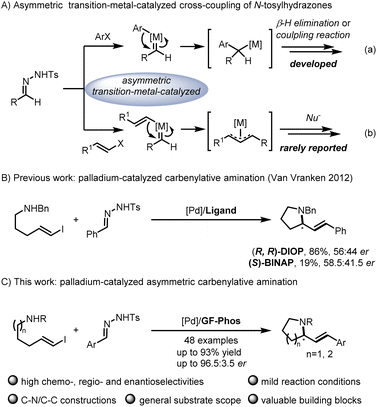
N-tosylhydrazones, readily prepared from aldehydes or ketones, served as a safe source of carbene precursors and have attracted much attention of chemists.1N-tosylhydrazone-mediated applications have been continuously developed, such as cyclopropanation or cyclopropenation, X–H insertion, ylide formation, cycloaddition, aza-Wacker-type cyclization, asymmetric allylic substitution, etc.2 Among them, transition-metal-catalyzed cross-coupling is one of the powerful protocols for C–X or C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation in organic synthesis involving versatile intermediates, of which in situ generation of diazo compounds and carbene migratory insertion are considered key steps.3–5 Over the past decades, considerable progress has been made in the asymmetric cross-coupling reactions of N-tosylhydrazones with various coupling partners, including cyclobutanols, terminal alkynes, silacyclobutanes and so on.4 Relatively, only a few examples focus on the cross-coupling reactions of aryl halides with N-tosylhydrazones involving benzyl metal intermediates [Scheme 1A, eqn. (a)].6 For example, Gu,6a Wu,6b Lassaletta6c and coworkers have developed a palladium-catalyzed asymmetric synthesis of axial chiral compounds from aryl bromides and N-tosylhydrazones, ending with β-H elimination. Very recently, we realized palladium/GF-Phos catalyzed asymmetric three component cross-coupling reactions of aryl halides, N-tosylhydrazones, with terminal alkynes.6f In contrast, much less progress has been made in N-tosylhydrazone-based carbenylative insertions from vinyl halides, which would generate a π-allylic metal intermediate followed by nucleophile attack, providing a unique approach for building C–X bonds, especially for N-heterocyclic compounds [Scheme 1A, eqn. (b)].7N-heterocycles are important structural motifs for the development of various types of valuable chemicals and materials.8 Importantly, optically active 2-substituted pyrrolidine and piperidine derivatives are privileged scaffolds in many natural products and pharmaceuticals with a wide range of biological activities,9 as well as the backbone of organocatalysts in asymmetric catalysis (Fig. 1).10
C bond formation in organic synthesis involving versatile intermediates, of which in situ generation of diazo compounds and carbene migratory insertion are considered key steps.3–5 Over the past decades, considerable progress has been made in the asymmetric cross-coupling reactions of N-tosylhydrazones with various coupling partners, including cyclobutanols, terminal alkynes, silacyclobutanes and so on.4 Relatively, only a few examples focus on the cross-coupling reactions of aryl halides with N-tosylhydrazones involving benzyl metal intermediates [Scheme 1A, eqn. (a)].6 For example, Gu,6a Wu,6b Lassaletta6c and coworkers have developed a palladium-catalyzed asymmetric synthesis of axial chiral compounds from aryl bromides and N-tosylhydrazones, ending with β-H elimination. Very recently, we realized palladium/GF-Phos catalyzed asymmetric three component cross-coupling reactions of aryl halides, N-tosylhydrazones, with terminal alkynes.6f In contrast, much less progress has been made in N-tosylhydrazone-based carbenylative insertions from vinyl halides, which would generate a π-allylic metal intermediate followed by nucleophile attack, providing a unique approach for building C–X bonds, especially for N-heterocyclic compounds [Scheme 1A, eqn. (b)].7N-heterocycles are important structural motifs for the development of various types of valuable chemicals and materials.8 Importantly, optically active 2-substituted pyrrolidine and piperidine derivatives are privileged scaffolds in many natural products and pharmaceuticals with a wide range of biological activities,9 as well as the backbone of organocatalysts in asymmetric catalysis (Fig. 1).10
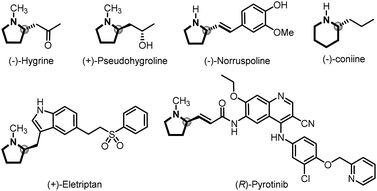 | ||
| Fig. 1 Selected natural products and pharmaceuticals containing chiral 2-substituted pyrrolidine and piperidine units. | ||
Notably, Van Vranken and coworkers reported an elegant palladium-catalyzed carbenylative amination reaction of N-tosylhydrazones and (E)-vinyl iodides pendent with amine, providing facile access to pyrrolidine and piperidine ring systems that are common to alkaloid natural products (Scheme 1B).11 Unfortunately, only up to 58.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.5 er was obtained after they made a lot of efforts to screen a series of chiral phosphine ligands, indicating that this asymmetric reaction indeed poses considerable challenges in addition to competitive side reactions such as the dimerization of vinyl iodides,12 the formation of diene via the palladatropic rearrangement/β-H elimination or allene via β-H elimination from Csp2,13 and the π-allylpalladium intermediate trapped by the byproduct sulfinic acid salt.14 Given the significance of chiral pyrrolidines and piperidines as core structures in alkaloid natural products, the development of an asymmetric version of this elegant carbenylative amination reaction is highly desirable. In recent years, our group has developed a series of chiral sulfinamide phosphine ligands (so-called Sadphos), which showed unique potential in asymmetric transition-metal catalysis,6f,15 so we wondered whether Sadphos could address this challenging asymmetric carbenylative amination reaction (Scheme 1C).
41.5 er was obtained after they made a lot of efforts to screen a series of chiral phosphine ligands, indicating that this asymmetric reaction indeed poses considerable challenges in addition to competitive side reactions such as the dimerization of vinyl iodides,12 the formation of diene via the palladatropic rearrangement/β-H elimination or allene via β-H elimination from Csp2,13 and the π-allylpalladium intermediate trapped by the byproduct sulfinic acid salt.14 Given the significance of chiral pyrrolidines and piperidines as core structures in alkaloid natural products, the development of an asymmetric version of this elegant carbenylative amination reaction is highly desirable. In recent years, our group has developed a series of chiral sulfinamide phosphine ligands (so-called Sadphos), which showed unique potential in asymmetric transition-metal catalysis,6f,15 so we wondered whether Sadphos could address this challenging asymmetric carbenylative amination reaction (Scheme 1C).
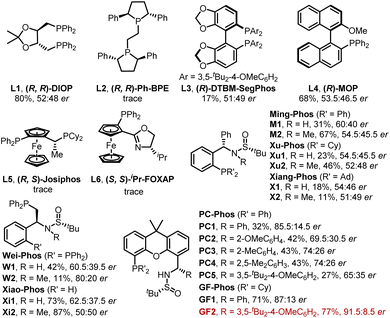
Initially, our study began with (E)-vinyl iodide 1a and N-tosylhydrazone 2a in the presence of Pd2(dba)3, t-BuOLi, Et3N, and triethylbenzylammonium chloride (TEBAC) in THF at 30 °C. A series of commercially available chiral ligands were first screened (Fig. 2). Only (R, R)-DIOP (L1), (R)-DTBM-SegPhos (L3) and (R)-MOP (L4) provided the desired product 3aa with poor enantioselectivity and other ligands such as (R, R)-Ph-BPE (L2), (R, S)-Josiphos (L5) and (S, S)-iPr-FOXAP (L6) showed low reactivity. We next turned to systematically investigate Sadphos, such as Wei-Phos,16 Xiao-Phos,15d,17 Ming-Phos,15a,18 Xu-Phos,15b,19 Xiang-Phos20 and PC-Phos15c,21 (Fig. 2). To our delight, PC1 delivered 3aa in 32% yield and 85.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.5 er. Inspired by this result, we further screened PC2–PC5 which vary in the substituent of phenyl, but unfortunately none of them showed better results. Surprisingly, the reactivity of this reaction could be greatly improved with our recently developed GF-Phos GF1, delivering 71% yield. When steric hindered tert-butyl groups were introduced on the phenyl group (GF2), the product 3aa was obtained in 77% yield with 91.5
14.5 er. Inspired by this result, we further screened PC2–PC5 which vary in the substituent of phenyl, but unfortunately none of them showed better results. Surprisingly, the reactivity of this reaction could be greatly improved with our recently developed GF-Phos GF1, delivering 71% yield. When steric hindered tert-butyl groups were introduced on the phenyl group (GF2), the product 3aa was obtained in 77% yield with 91.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 er. After screening different palladium catalysts and solvents (Table 1, entries 1–10), the er value has been slightly increased. Additionally, lowering reaction temperature led to an increase in enantioselectivity but a decrease in yield (Table 1, entry 11).
8.5 er. After screening different palladium catalysts and solvents (Table 1, entries 1–10), the er value has been slightly increased. Additionally, lowering reaction temperature led to an increase in enantioselectivity but a decrease in yield (Table 1, entry 11).
| Entry | [Pd] | Base | Solvent | Yieldb (%) | er |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.16 mmol), [Pd] (5 mol%), GF2 (15 mol%), t-BuOLi (2.2 equiv.), TEBAC (1.0 equiv.), base (2.0 equiv.) in 0.1 M solvent at 30 °C for 12 h. b Determined by GC analysis with n-tetradecane as an internal standard. c The er value was determined by chiral HPLC. d 15 °C for 12 h. e Without TEBAC. f 15 mol% Ag2CO3. THF = tetrahydrofuran. MTBE = tert-butyl methyl ether. DMF = N,N-dimethylformamide. DCE = 1,2-dichloroethane. DMSO = dimethyl sulfoxide. | |||||
| 1 | Pd2(dba)3 | Et3N | THF | 77 | 91.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 8.5 |
| 2 | Pd(acac)2 | Et3N | THF | 89 | 86.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.5 13.5 |
| 3 | Pd(OAc)2 | Et3N | THF | 82 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 4 | PdBr2 | Et3N | THF | 78 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 5 | Pd2(dba)3·CHCl3 | Et3N | THF | 75 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 6 | Pd2(dba)3·CHCl3 | Et3N | Toluene | 23 | 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
| 7 | Pd2(dba)3·CHCl3 | Et3N | DMF | 90 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 8 | Pd2(dba)3·CHCl3 | Et3N | MTBE | 28 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 9 | Pd2(dba)3·CHCl3 | Et3N | 1,4-Dioxane | 38 | 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5 11.5 |
| 10 | Pd2(dba)3·CHCl3 | Et3N | 2-Me-THF | 89 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 11d | Pd2(dba)3·CHCl3 | Et3N | 2-Me-THF | 26 | 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
| 12 | Pd2(dba)3·CHCl3 | DABCO | 2-Me-THF | 76 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 13 | Pd2(dba)3·CHCl3 | Cs2CO3 | 2-Me-THF | 93 | 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
| 14 | Pd2(dba)3·CHCl3 | KOH | 2-Me-THF | 89 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 15 | Pd2(dba)3·CHCl3 | None | 2-Me-THF | 83 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 16e | Pd2(dba)3·CHCl3 | None | 2-Me-THF | 69 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 17f | Pd2(dba)3·CHCl3 | None | 2-Me-THF | 81 | 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
We also found that, besides t-BuOLi, there was little effect on the yield or enantioselectivity by changing another base. The study was therefore continued without it (Table 1, entries 12–15). Moreover, in the absence of TEBAC, 3aa was produced in only 69% yield and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er. TEBAC probably helps to increase the solubility of the anion of N-tosylhydrazones (Table 1, entry 16). Interestingly, we investigated a series of additives, and the results indicated that the addition of Ag2CO3 could further provide slightly higher enantioselectivity (94.5
12 er. TEBAC probably helps to increase the solubility of the anion of N-tosylhydrazones (Table 1, entry 16). Interestingly, we investigated a series of additives, and the results indicated that the addition of Ag2CO3 could further provide slightly higher enantioselectivity (94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 er) (Table 1, entry 17, see the ESI for more details†).
5.5 er) (Table 1, entry 17, see the ESI for more details†).
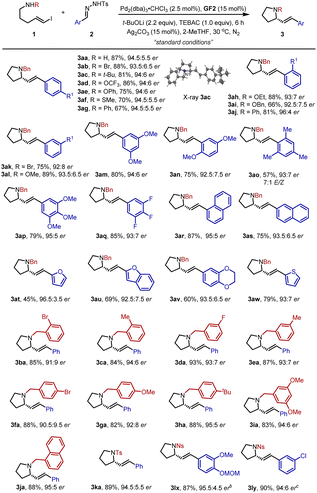
The scope of the carbenylative amination reaction was then studied using the optimized reaction conditions (Table 2). A wide range of N-tosylhydrazones 2 bearing electron-withdrawing or donating groups at the ortho-, meta- or para-position of the phenyl ring were tested, giving the corresponding products 3aa–3aj in moderate to good yields with 92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5–96
7.5–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. The absolute configuration of 3ac was confirmed as S by single crystal X-ray diffraction analysis.22 Multisubstituted phenyl and naphthyl groups were also well-tolerated (3am, 3an, 3ap–3as). It is note-worthy that the 2,4,6-trimethylphenyl-substituted substrate delivered 3ao in 57% yield with 7/1 E/Z selectivity, probably due in part to the steric hindrance. Moreover, N-tosylhydrazones containing heterocycles reacted smoothly to furnish the expected products 3at–3aw. Besides diverse substituted N-tosylhydrazones 2, various kinds of vinyl iodide derivatives 1 with functional groups such as halides, methyl, tert-butyl, methoxy and 1-naphthyl at different positions on the phenyl ring also worked well and afforded 3ba–3ja in good yields. Surprisingly, when the protective group on the nitrogen atom was replaced by a p-toluenesulfonyl or p-nitrophenylsulfonyl group, the corresponding cyclic products 3ka, 3lx, and 3ly were successfully produced in high yields and enantioselectivities.
4 er. The absolute configuration of 3ac was confirmed as S by single crystal X-ray diffraction analysis.22 Multisubstituted phenyl and naphthyl groups were also well-tolerated (3am, 3an, 3ap–3as). It is note-worthy that the 2,4,6-trimethylphenyl-substituted substrate delivered 3ao in 57% yield with 7/1 E/Z selectivity, probably due in part to the steric hindrance. Moreover, N-tosylhydrazones containing heterocycles reacted smoothly to furnish the expected products 3at–3aw. Besides diverse substituted N-tosylhydrazones 2, various kinds of vinyl iodide derivatives 1 with functional groups such as halides, methyl, tert-butyl, methoxy and 1-naphthyl at different positions on the phenyl ring also worked well and afforded 3ba–3ja in good yields. Surprisingly, when the protective group on the nitrogen atom was replaced by a p-toluenesulfonyl or p-nitrophenylsulfonyl group, the corresponding cyclic products 3ka, 3lx, and 3ly were successfully produced in high yields and enantioselectivities.
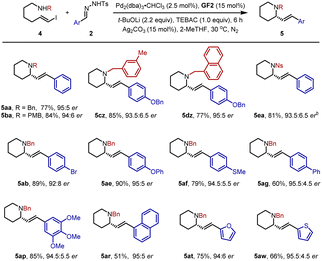
Subsequently, we further turned our efforts to the synthesis of piperidine derivatives. As shown in Table 3, the desired six-membered heterocycles 5aa–5dz could be obtained efficiently in 77–85% yields with 93.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5–95
6.5–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er under standard conditions. Similarly, the p-nitrophenylsulfonyl group was also a compatible partner to give 5ea in 81% yield with 93.5
5 er under standard conditions. Similarly, the p-nitrophenylsulfonyl group was also a compatible partner to give 5ea in 81% yield with 93.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 er. In parallel, a variety of N-tosylhydrazones 2 mentioned above were studied, affording structurally diverse piperidines 5ab–5ar smoothly. In addition, 2-furan- and thienyl-substituted N-tosylhydrazones were transformed into 5at and 5aw in good yields with high er values.
6.5 er. In parallel, a variety of N-tosylhydrazones 2 mentioned above were studied, affording structurally diverse piperidines 5ab–5ar smoothly. In addition, 2-furan- and thienyl-substituted N-tosylhydrazones were transformed into 5at and 5aw in good yields with high er values.
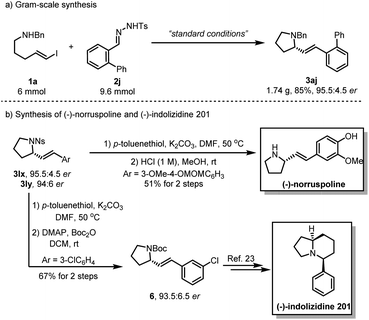
To evaluate the synthetic utility of this asymmetric carbenylative amination reaction, we carried out a gram–scale reaction under standard conditions, providing the product 3aj in 85% yield with 95.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 er (Scheme 2a). Of note, a 2-step deprotection of 3lx with p-toluenethiol/K2CO3 and HCl (1 M) enabled the synthesis of natural product (−)-norruspoline in 51% overall yield. Additionally, replacing the protecting group of 3ly with the Boc group afforded 6 in 67% yield without the loss of enantioselectivity and it has been previously shown that 6 is a synthetic intermediate for the preparation of natural product (−)-indolizidine 201 (Scheme 2b).23 A linear relationship was demonstrated by a nonlinear effect study on the ee value of GF2 and product 3aa, which implied that the catalytically active structure contains only a single chiral ligand. (please find more details in the ESI†).
4.5 er (Scheme 2a). Of note, a 2-step deprotection of 3lx with p-toluenethiol/K2CO3 and HCl (1 M) enabled the synthesis of natural product (−)-norruspoline in 51% overall yield. Additionally, replacing the protecting group of 3ly with the Boc group afforded 6 in 67% yield without the loss of enantioselectivity and it has been previously shown that 6 is a synthetic intermediate for the preparation of natural product (−)-indolizidine 201 (Scheme 2b).23 A linear relationship was demonstrated by a nonlinear effect study on the ee value of GF2 and product 3aa, which implied that the catalytically active structure contains only a single chiral ligand. (please find more details in the ESI†).
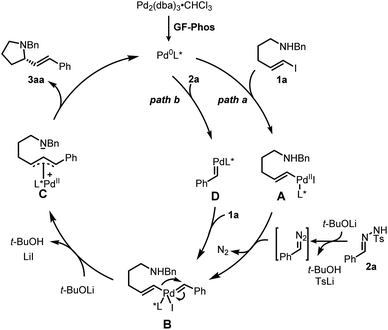
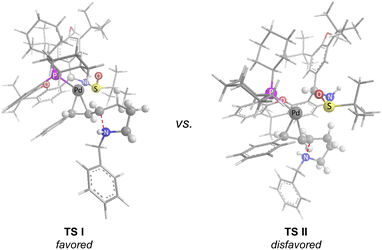
Based on our study and previous work,24 a catalytic cycle pathway to rationalize the synthesis of chiral pyrrolidines is illustrated in Scheme 3. First, the oxidative addition of vinyl iodide 1a to a Pd0/GF-Phos complex would generate vinyl PdII species A. In the presence of a base, N-tosylhydrazone 2ain situ generated a diazo intermediate and formed palladium carbene B with vinyl PdII species A, followed by migratory insertion to generate the π-allylpalladium intermediate C, as displayed in path a. Alternatively, the reaction proceeds in a palladium carbene/oxidative addition sequence as in path b. Next, the nucleophilic attack of the nitrogen atom on π-allylpalladium delivered product 3aa and regenerated the Pd0 complex, thus completing the entire catalytic cycle. In light of the structure of the chiral ligand GF2 and the absolute configuration of product (S)-3, a chirality induction model for stereochemical induction was proposed (Fig. 3).
In conclusion, we have developed a palladium/GF-Phos catalyzed asymmetric carbenylative amination of (E)-vinyl iodides with N-tosylhydrazones via a carbene migratory insertion/Tsuji-Trost sequence to build C–N/C–C more efficiently. This catalytic system exhibits general functional group tolerance and enables rapid access to a variety of chiral 2-substituted pyrrolidines and piperidines in moderate to good yields with high chemo-, regio-, enantioselectivities under mild conditions. Our approach can be applied to the direct synthesis of significant natural product (−)-norruspoline and provides an alternative route for the formal synthesis of (−)-indolizidine 201.
Data availability
All experimental data and detailed experimental procedures are available in the ESI.†Author contributions
Y. S. conducted the experiments and analysed the data. C. M. conducted the preparation of the starting materials. Z. L. and J. Z. directed the project. Y. S., Z. L. and J. Z. prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the funding support of the National Key R&D Program of China (2021YFF0701601), NSFC (22031004 and 21921003), and Shanghai Municipal Education Commission (20212308).Notes and references
-
(a) D. G. Farnum, J. Org. Chem., 1963, 28, 870–872 CrossRef CAS
; (b) J. R. Fulton, V. K. Aggarwal and J. de Vicente, Eur. J. Org. Chem., 2005, 8, 1479–1492 CrossRef
.
- For books, see:
(a)
J. Wang, C.-M. Che and M. P. Doyle, Transition Metal-Catalyzed Carbene Transformations, Wiley-VCH, Weinheim, Germany, 2022. For reviews, see CrossRef
; (b) M. Jia and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 9134–9166 CrossRef CAS PubMed
; (c) D. Arunprasath, B. D. Bala and G. Sekar, Adv. Synth. Catal., 2019, 361, 1172–1207 CrossRef CAS
; (d) R. Singhal, S. P. Choudhary, B. Malik and M. Pilania, ChemistrySelect, 2022, 7, e20220013 CrossRef
; For recent examples, see:; (e) W. C. Lee, D.-S. Wang, C. Zhang, J. Xie, B. Li and X. P. Zhang, Chem, 2021, 7, 1588–1601 CrossRef CAS
; (f) J. Ke, W. C. Lee, X. Wang, Y. Wang, X. Wen and X. P. Zhang, J. Am. Chem. Soc., 2022, 144, 2368–2378 CrossRef CAS PubMed
; (g) L.-L. Yang, J. Ouyang, H.-N. Zou, S.-F. Zhu and Q.-L. Zhou, J. Am. Chem. Soc., 2021, 143, 6401–6406 CrossRef CAS PubMed
; (h) L. Ma, F. Jin, X. Cheng, S. Tao, G. Jiang, X. Li, J. Yang, X. Bao and X. Wan, Chem. Sci., 2021, 12, 9823–9830 RSC
; (i) K. Xu, Y. Zheng, Y. Ye, D. Liu and W. Zhang, Org. Lett., 2020, 22, 8836–8841 CrossRef CAS PubMed
; (j) X. Kou, Q. Shao, C. Ye, G. Yang and W. Zhang, J. Am. Chem. Soc., 2018, 140, 7587–7597 CrossRef CAS PubMed
.
- For selected reviews and recent examples, see:
(a) Z. Shao and H. Zhang, Chem. Soc. Rev., 2012, 41, 560–572 RSC
; (b) Y. Xia and J. Wang, Chem. Soc. Rev., 2017, 46, 2306–2362 RSC
; (c) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS
; (d) J. Barluenga and C. Valdés, Angew. Chem., Int. Ed., 2011, 50, 7486–7500 CrossRef CAS
; (e) Y. Xia and J. Wang, J. Am. Chem. Soc., 2020, 142, 10592–10605 CrossRef CAS PubMed
; (f) J. Radolko, P. Ehlers and P. Langer, Adv. Synth. Catal., 2021, 363, 3616–3654 CrossRef CAS
; (g) Y. Ping, R. Wang, Q. Wang, T. Chang, J. Huo, M. Lei and J. Wang, J. Am. Chem. Soc., 2021, 143, 9769–9780 CrossRef CAS PubMed
; (h) A. Yanagimoto, Y. Uwabe, Q. Wu, K. Muto and J. Yamaguchi, ACS Catal., 2021, 11, 10429–10435 CrossRef CAS
; (i) N. F. C. Ritchie, A. J. Zahara and S. M. Wilkerson-Hill, J. Am. Chem. Soc., 2022, 144, 2101–2106 CrossRef CAS PubMed
.
-
(a) A. Yada, S. Fujita and M. Murakami, J. Am. Chem. Soc., 2014, 136, 7217–7220 CrossRef CAS PubMed
; (b) W.-D. Chu, F. Guo, L. Yu, J. Hong, Q. Liu, F. Mo, Y. Zhang and J. Wang, Chin. J. Chem., 2018, 36, 217–222 CrossRef CAS
; (c) T. Osako, M. Nagaosa, G. Hamasaka and Y. Uozumi, Synlett, 2018, 29, 2251–2256 CrossRef CAS
; (d) J. Huo, K. Zhong, Y. Xue, M. Lyu, Y. Ping, Z. Liu, Y. Lan and J. Wang, J. Am. Chem. Soc., 2021, 143, 12968–12973 CrossRef CAS
.
-
(a) W. R. Bamford and T. S. Stevens, J. Chem. Soc., 1952, 4735–4740 RSC
; (b) V. K. Aggarwal, E. Alonso, I. Bae, G. Hynd, K. M. Lydon, M. J. Palmer, M. Patel, M. Porcelloni, J. Richardson, R. A. Stenson, J. R. Studley, J. L. Vasse and C. L. Winn, J. Am. Chem. Soc., 2003, 125, 10926–10940 CrossRef CAS PubMed
.
-
(a) J. Feng, B. Li, Y. He and Z. Gu, Angew. Chem., Int. Ed., 2016, 55, 2186–2190 CrossRef CAS PubMed
; (b) H. Wu, Z. S. Han, B. Qu, D. Wang, Y. Zhang, Y. Xu, N. Grinberg, H. Lee, J. J. Song, F. Roschangar, G. Wang and C. H. Senanayake, Adv. Synth. Catal., 2017, 359, 3927–3933 CrossRef CAS
; (c) S. Kattela, C. R. D. Correia, A. Ros, V. Hornillos, J. Iglesias-Sigüenza, R. Fernández and J. M. Lassaletta, Org. Lett., 2022, 24, 3812–3816 CrossRef CAS PubMed
; (d) X. Ning, Y. Chen, F. Hu and Y. Xia, Org. Lett., 2021, 23, 8348–8352 CrossRef CAS PubMed
; (e) R.-X. Liang, K. Wang, Q. Wu, W.-J. Sheng and Y.-X. Jia, Organometallics, 2019, 38, 3927–3930 CrossRef CAS
; (f) G. Zhao, Y. Wu, H.-H. Wu, J. Yang and J. Zhang, J. Am. Chem. Soc., 2021, 143, 17983–17988 CrossRef CAS PubMed
.
-
(a) P.-X. Zhou, Y.-Y. Ye and Y.-M. Liang, Org. Lett., 2013, 15, 5080–5083 CrossRef CAS PubMed
; (b) I. D. U. A. Premachandra, T. A. Nguyen, C. Shen, E. S. Gutman and D. L. Van Vranken, Org. Lett., 2015, 17, 5464–5467 CrossRef CAS PubMed
; (c) Y. Xia, Y. Xia, Y. Zhang and J. Wang, Org. Biomol. Chem., 2014, 12, 9333–9336 RSC
; (d) X. S. Shang, N. T. Li, H. X. Siyang and P. N. Liu, J. Org. Chem., 2015, 80, 4808–4815 CrossRef CAS PubMed
; (e) M. Kitamura, R. Yuasa and D. L. Van Vranken, Tetrahedron Lett., 2015, 56, 3027–3031 CrossRef CAS
; (f) M. Paraja, R. Barroso, M. P. Cabal and C. Valdés, Adv. Synth. Catal., 2017, 359, 1058–1062 CrossRef CAS
; (g) P.-X. Zhou, Y.-Y. Ye, L.-B. Zhao, J.-Y. Hou, X. Kang, D.-Q. Chen, Q. Tang, J.-Y. Zhang, Q.-X. Huang, L. Zheng, J.-W. Ma, P.-F. Xu and Y.-M. Liang, Chem. - Eur. J., 2014, 20, 16093–16096 CrossRef CAS PubMed
.
-
(a) A. Mochizuki, Y. Nakamoto, H. Naito, K. Uoto and T. Ohta, Bioorg. Med. Chem. Lett., 2008, 18, 782–787 CrossRef CAS PubMed
; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed
; (c) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed
; (d) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347–361 CrossRef CAS PubMed
.
-
(a) T. K. Beng and R. E. Gawley, J. Am. Chem. Soc., 2010, 132, 12216–12217 CrossRef CAS PubMed
; (b) K. T. Wanner and G. Hoefner, Arch. Pharm. (Weinheim, Ger.), 1990, 323, 977–986 CrossRef CAS
; (c) C. Bhat and S. G. Tilve, Tetrahedron Lett., 2011, 52, 6566–6568 CrossRef CAS
; (d) M. Liniger, K. Estermann and K.-H. Altmann, J. Org. Chem., 2013, 78, 11066–11070 CrossRef CAS
; (e) K. Beaumont, I. Gardner, K. Chapman, M. Hall and M. Rowland, J. Pharm. Sci., 2011, 100, 4518–4535 CrossRef CAS PubMed
; (f) B. Su, T. Huang, Y. Jin, H. Yin, H. Qiu and X. Yuan, Gastric Cancer, 2021, 24, 352–367 CrossRef CAS PubMed
.
- For selected reviews and recent examples, see:
(a) S. Sulzer-Mossé and A. Alexakis, Chem. Commun., 2007, 30, 3123–3135 RSC
; (b) M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC
; (c) Y. Hayashi and N. Umekubo, Angew. Chem., Int. Ed., 2018, 57, 1958–1962 CrossRef CAS PubMed
; (d) A. Vega-Peñaloza, S. Paria, M. Bonchio, L. Dell'Amico and X. Companyó, ACS Catal., 2019, 9, 6058–6072 CrossRef
; (e) Y. Oka, S. Tsuzukib and K. Moriyama, Chem. Commun., 2021, 57, 11457–11460 RSC
.
- A. Khanna, C. Maung, K. R. Johnson, T. T. Luong and D. L. Van Vranken, Org. Lett., 2012, 14, 3233–3235 CrossRef CAS
.
-
(a) A. Khanna, I. D. U. A. Premachandra, P. D. Sung and D. L. Van Vranken, Org. Lett., 2013, 15, 3694–3697 CrossRef CAS PubMed
; (b) D. P. Ojha and K. R. Prabhu, J. Org. Chem., 2013, 78, 12136–12143 CrossRef PubMed
.
-
(a) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Adv. Synth. Catal., 2010, 352, 3235–3240 CrossRef CAS
; (b) G. Zhang, Y.-K. Song, F. Zhang, Z.-J. Xue, M.-Y. Li, G.-S. Zhang, B.-B. Zhu, J. Wei, C. Li, C.-G. Feng and G.-Q. Lin, Nat. Commun., 2021, 12, 728–737 CrossRef CAS PubMed
.
- P.-X. Zhou, Y. Zhang, C. Ge, Y.-M. Liang and C. Li, J. Org. Chem., 2018, 83, 4762–4768 CrossRef CAS PubMed
.
-
(a) Z.-M. Zhang, P. Chen, W. Li, Y. Niu, X.-L. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4350–4354 CrossRef CAS PubMed
; (b) Z.-M. Zhang, B. Xu, L. Wu, Y. Wu, Y. Qian, L. Zhou, Y. Liu and J. Zhang, Angew. Chem., Int. Ed., 2019, 58, 14653–14659 CrossRef CAS
; (c) H. Chu, J. Cheng, J. Yang, Y.-L. Guo and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 21991–21996 CrossRef CAS PubMed
; (d) Q. Dai, L. Liu and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 27247–27252 CrossRef CAS PubMed
.
- For Wei-phos ligands, see: W. Zhou, X. Su, M. Tao, C. Zhu, Q. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14853–14857 CrossRef CAS PubMed
.
- For Xiao-phos ligands, see:
(a) X. Su, W. Zhou, Y. Li and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6874–6877 CrossRef CAS PubMed
; (b) Q. Dai, L. Liu, Y. Qian, W. Li and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 20645–20650 CrossRef CAS PubMed
.
- For Ming-phos ligands, see:
(a) Z.-M. Zhang, B. Xu, S. Xu, H.-H. Wu and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 6324–6328 CrossRef CAS
; (b) S. Li, Q. Chen, Z.-M. Zhang and J. Zhang, Green Synth. Catal., 2021, 2, 374–376 CrossRef
.
- For Xu-phos ligands, see:
(a) Y.-L. Li, P.-C. Zhang, H.-H. Wu and J. Zhang, J. Am. Chem. Soc., 2021, 143, 13010–13015 CrossRef CAS
; (b) B. Xu, D. Ji, L. Wu, L. Zhou, Y. Liu, Z.-M. Zhang and J. Zhang, Chem, 2022, 8, 836–849 CrossRef CAS
.
- For Xiang-phos ligands, see: L. Wang, K. Zhang, Y. Wang, W. Li, M. Chen and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 4421–4427 CrossRef CAS PubMed
.
- For PC-phos ligands, see:
(a) L. Wang, M. Chen, P.-C. Zhang, W. Li and J. Zhang, J. Am. Chem. Soc., 2018, 140, 3467–3473 CrossRef CAS PubMed
; (b) P.-C. Zhang, J. Han and J. Zhang, Angew. Chem., Int. Ed., 2019, 58, 11444–11448 CrossRef CAS PubMed
.
- The absolute configuration of 3ac was determined by X-ray crystallographic analysis. CCDC number: 2129469.
- H. Zhang, C. Huang, X.-A. Yuan and S. Yu, J. Am. Chem. Soc., 2022, 144, 10958–10967 CrossRef CAS
.
-
(a) N. A. Butta and W. Zhang, Chem. Soc. Rev., 2015, 44, 7929–7967 RSC
; (b) L. M. Lutete, I. Kadota and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 1622–1623 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2129469 (3ac). For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc03999k |
| This journal is © The Royal Society of Chemistry 2022 |