Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-catalysed C–H arylation of benzophospholes with aryl halides†
Shibo
Xu
a,
Kazutoshi
Nishimura
b,
Kosuke
Saito
b,
Koji
Hirano
 *ab and
Masahiro
Miura
*ab and
Masahiro
Miura
 *a
*a
aInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University, Suita, Osaka 565-0871, Japan. E-mail: k_hirano@chem.eng.osaka-u.ac.jp; miura@chem.eng.osaka-u.ac.jp
bDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
First published on 30th August 2022
Abstract
A palladium-catalysed C–H arylation of benzophospholes with aryl halides has been developed. The reaction with aryl iodides and bromides proceeds well even under phosphine ligand-free Pd(OAc)2 catalysis whereas the Pd(PCy3)2 is effective for the coupling with less reactive aryl chlorides. The optimal conditions are also applicable to the double arylations with organic dihalides and annulation reaction with ortho-dihalogenated benzenes, making the corresponding benzophosphole-based acceptor–donor–acceptor-type molecules and highly condensed heteroacene-type molecules of potent interest in materials chemistry. Although there are many reports of catalytic C–H functionalisations of related benzoheteroles such as indoles, benzothiophenes, and benzofurans, this is the first successful example of the catalytic direct C–H transformation of benzophospholes, to the best of our knowledge. The preliminary optoelectronic properties of some newly synthesized benzophosphole derivatives are also investigated.
Introduction
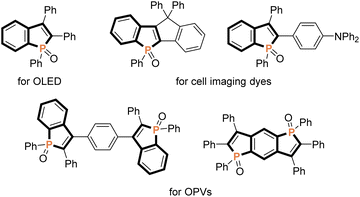
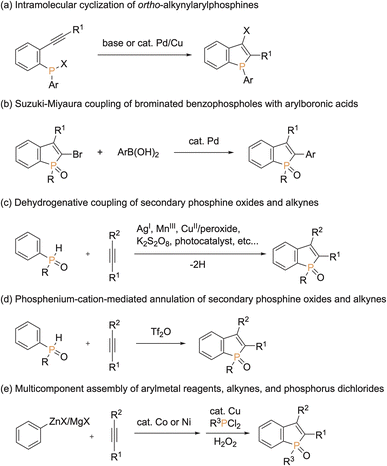
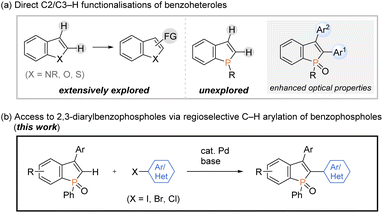
Because of unique optical, electronical, and physical properties, benzophosphole derivatives have attracted attention in the field of organic functional materials. Among well-known examples are organic light-emitting diodes (OLEDs),1 photovoltaics,2 and cell imaging dyes3 (Fig. 1). Accordingly, the development of synthetic strategies for the preparation of benzophospholes, particularly, multiply substituted ones, has been one of the long-standing research subjects in the synthetic community.4 The most reliable protocols are the cyclization–functionalisation sequence from the ortho-alkynylarylphosphines (Scheme 1a) and the related Suzuki–Miyaura cross-coupling of brominated benzophospholes with arylboronic acids (Scheme 1b), which can control the substituent position on the phosphole nucleus.5 However, the starting substrates are prepared in multiple steps, often from unstable and sensitive substrates/reagents, and thus the functional group compatibility is still problematic. Other protocols are the radical- and cation-mediated annulation reactions of secondary phosphine oxides with alkynes (Schemes 1c and d).6,7 The direct use of stable and readily available starting materials is the significant advantage, but the regioselectivity is difficult to control when unsymmetrical internal diaryl alkynes are employed. Recently, Yoshikai reported the modular approach from internal alkynes, chlorophosphines, and arylzincs or -magnesiums through the cobalt- or nickel-catalysed carbometalation reaction (Scheme 1e).8 However, also in this case, the reaction with unsymmetrical diaryl alkynes generally encounters difficulty in controlling of the regioselectivity. Thus, the rapid, concise, and selective synthesis of substituted benzophospholes, particularly, that bear two different aryl groups at the C2 and C3 positions is strongly appealing.On the other hand, transition-metal-promoted C–H functionalisation has been proven to be one of the most powerful strategies in the conversion of simple starting materials to the diverse and value-added molecules.9 Among them, the direct C–H arylation of benzoheteroles such as indoles, benzothiophenes, and benzofurans, to construct functional aryl-heteroaryl linkages has received tremendous attention and has made remarkable progress (Scheme 2a, left).10 However, the direct catalytic C–H transformation of phosphorus analogues has not been successful so far, and only a formal C–H arylation of P-aryl phospholes was recently reported under Cu catalysis.11 Given the significant optical performance of C2- and C3-diarylated benzophospholes,1–3 the development of C–H arylation strategy can provide a potentially more practical alternative for the rapid construction of benzophosphole-based π-electron materials (Scheme 2a, right).
Meanwhile, our research group has been interested in the development of efficient methodologies for the synthesis of benzophosphole derivatives7,12 and recently disclosed profitable access to the highly π-conjugated dibenzophospholes from simple biaryls via phosphenium dication strategy in one operation.13 Notably, this protocol was also productive in the reaction with 1,1-diphenylethylene to give the corresponding benzophosphole bearing a free C2–H bond, which provides accessible space for the further transformation based on C–H activation. During our continuing interest in this chemistry, we herein report a concise and general process for the synthesis of structurally useful C2,C3-diarylated benzophospholes via the Pd-catalysed regioselective C2–H arylation with aryl halides (Scheme 2b). Owing to the broad scope of aryl halides, the C–H arylation reaction flexibly introduces various aryl groups at the C2 position, which is complementary to reported strategies in Scheme 1 for the synthesis of C2,C3-diarylated benzophosphole derivatives. It is important to note that the identity of C2-aryl-substituent is known to largely affect the optical properties.3 More attractively, the double arylation with aromatic dihalides and annulation reaction with ortho-dihalogenated benzenes are also applicable to afford the corresponding benzophosphole-based acceptor–donor–acceptor-type molecules and highly condensed heteroacene-type molecules of potent interest in material chemistry. Additionally, we evaluated the cardinal optoelectronic properties of several new compounds. Our preliminary mechanistic studies revealed that the C–H cleavage occurred by the base-promoted deprotonation.
Results and discussion
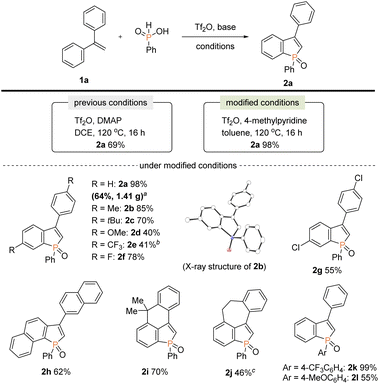
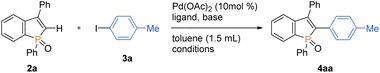
Our attention was initially focused on preparation of the starting C2–H benzophospholes 2 from 1,1-diarylethylenes 1via the phosphenium dication-mediated cyclization reaction (Scheme 3). Although the 1,1-diphenylethylene 1a directly furnished the corresponding benzophosphole 2a, previous N,N-dimethyl-4-aminopyridine (DMAP)-involved conditions still suffer from a moderate yield, poor scalability, and electronic sensitivity to substrates, which hamper applications in the scalable synthesis.13 To address this problem, 1a was selected as model substrate, and we started re-investigation of reaction parameters including base, solvent, and reaction temperature (see the ESI for more details†). To our delight, the reaction efficiency was dramatically improved when 4-methylpyridine was used instead of DMAP in toluene at 120 °C to deliver the desired product 2a in 98% yield. The gram-scale reaction also proceeded well affording 2a in 64% yield. Under the modified conditions, several 1,1-diarylethylenes bearing electron-donating and -withdrawing groups successfully furnished the corresponding C2–H benzophospholes 2b–g in moderate to good yields. The structure of 2b was unambiguously confirmed by X-ray crystallographic analysis (CCDC 2174057†). The C–P bond formation exclusively occurred at the more electron-rich α position in the case of naphthyl substituent, and the desired 2h was obtained in 62% yield. Additionally, the structurally novel benzophosphole skeletons 2i and 2j that contain the six- and seven-membered systems, respectively, were readily accessible. Unfortunately, the heterocyclic system such as thiophene gave a complicated mixture or inseparable regiomixtures (see the ESI for details†). The substitution effect on phosphinic acid was also investigated. A reactivity trend similar to our previous work13,14 was observed: the electron-deficient CF3 group furnished 2k in a quantitative yield, whereas the electron-rich MeO-substituted one gave the corresponding 2l with decreased efficiency.We next selected 3-phenylbenzophosphole 2a and 4-iodotoluene 3a as model substrates and commenced optimization studies on C–H arylation under Pd(OAc)2 catalysis (Table 1). Pleasingly, the C–H arylation occurred to form the coupling product 4aa in 66% yield even in the absence of any supporting ligands when NaOtBu was used as base (entry 1). The addition of phosphine ligands gave negative or negligible results (entries 2–5, see the ESI for more details†). The choice of base is critical to the reaction: KOtBu showed a comparable reactivity, while LiOtBu resulted in a low conversion, and even no reaction was observed in the case of Cs2CO3 (entries 6–8). The amount of base is also important, and the conversion significantly dropped when NaOtBu was reduced to 1.5 equiv. from 2 equiv. (entry 9). The additive TBAB has been demonstrated to improve the reaction efficiency in the Pd-catalysed C–H arylation under ligand-free conditions,15 but in our case, the conversion largely dropped (entry 10). The reaction period was greatly shortened with the assistance of microwave irradiation, and the yield was increased up to 72% (entry 11). The temperature effect was obvious, and the reaction showed a dramatically reduced efficiency at 80 °C (entry 12). The yield could be furtherly improved under more concentrated conditions (entry 13). Additionally notable is the high C2–H regioselectivity in spite of the possibility of phosphole P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-directed C–H functionalisation of the phenyl group on phosphorus.16 The P
O-directed C–H functionalisation of the phenyl group on phosphorus.16 The P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-directed second arylation of the C2–H arylation product was actually detected in ca. 10% yield in the prolonged reaction periods with the conventional oil bath heating (data not shown), but the formation of such a diarylation byproduct could be avoided under the microwave irradiation. The palladium loading could be reduced to 5 mol% on a 0.20 mmol scale, and the arylation product was isolated in 79% yield (entry 14).
O-directed second arylation of the C2–H arylation product was actually detected in ca. 10% yield in the prolonged reaction periods with the conventional oil bath heating (data not shown), but the formation of such a diarylation byproduct could be avoided under the microwave irradiation. The palladium loading could be reduced to 5 mol% on a 0.20 mmol scale, and the arylation product was isolated in 79% yield (entry 14).
| Entry | Ligand | Base (equiv.) | Conditions | Yield of 4aab (%) |
|---|---|---|---|---|
| a Conditions: 2a (0.10 mmol), 3a (0.15 mmol), Pd(OAc)2 (0.010 mmol), ligand (0.020 mmol), base (0.20 mmol), toluene (1.5 mL), under the indicated conditions. b Determined by 1H NMR using triethylphosphate as internal standard. Isolated yield is in parentheses. c In 1.0 mL of toluene. d On a 0.20 mmol scale with Pd(OAc)2 (0.010 mmol, 5 mol%) in 2.0 mL of toluene. TBAB = tetrabutylammonium bromide. | ||||
| 1 | NaOtBu (2.0) | 90 °C, 16 h | 66 | |
| 2 | SPhos | NaOtBu (2.0) | 90 °C, 16 h | 21 |
| 3 | XPhos | NaOtBu (2.0) | 90 °C, 16 h | 19 |
| 4 | PPh2Cy | NaOtBu (2.0) | 90 °C, 16 h | 56 |
| 5 | PPh3 | NaOtBu (2.0) | 90 °C, 16 h | 39 |
| 6 | KOtBu (2.0) | 90 °C, 16 h | 68 | |
| 7 | LiOtBu (2.0) | 110 °C, 16 h | 21 | |
| 8 | Cs2CO3 (2.0) | 110 °C, 16 h | 0 | |
| 9 | NaOtBu (1.5) | 90 °C, 16 h | 31 | |
| 10 | NaOtBu (2.0)/TBAB (1.0) | 90 °C, 16 h | 22 | |
| 11 | NaOtBu (2.0) | μW, 90 °C, 1 h | 72 | |
| 12 | NaOtBu (2.0) | μW, 80 °C, 2 h | 35 | |
| 13c | NaOtBu (2.0) | μW, 90 °C, 1 h | 83 | |
| 14d | NaOtBu (2.0) | μW, 90 °C, 1 h | (79) | |
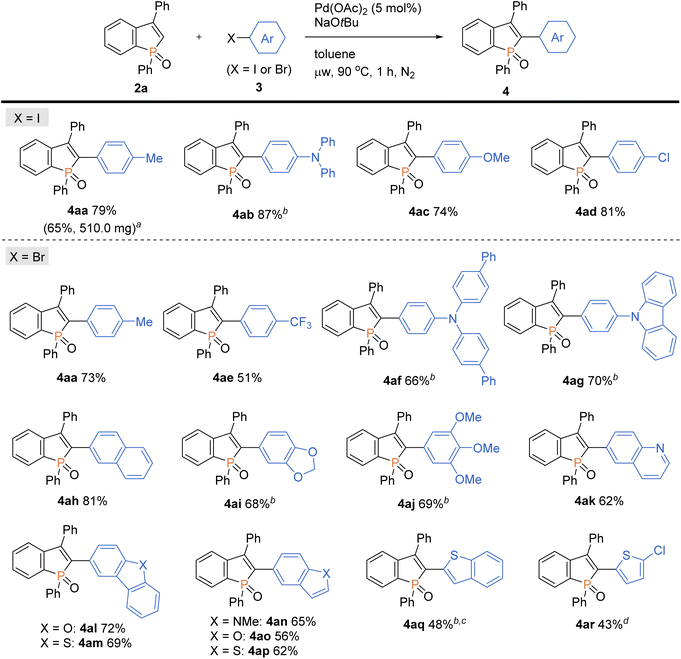
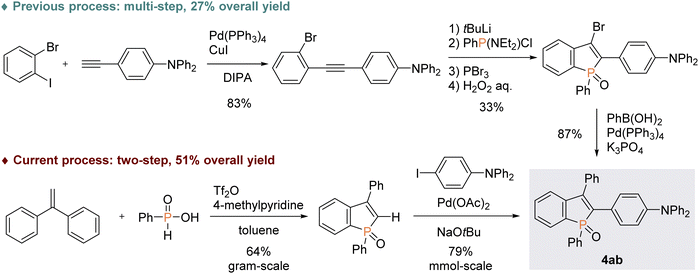
With the optimal conditions in hand, we investigated the practicality and generality of the palladium-catalysed C–H arylation reaction (Scheme 4). The model reaction could be easily performed on a 10-fold larger scale to afford 4aa in 65% yield. The 4-iodoarenes 3 bearing both electron-donating and -withdrawing groups were good coupling partners. 4-Iodotriphenylamine 3b participated in the C–H transformation to produce the arylation product 4ab in a high yield; this molecule is particularly useful and has been used for fluorescent probe.3a As illustrated in Scheme 5, the synthesis of such a valuable molecule 4ab was available through the Suzuki–Miyaura coupling reaction, however, the C3-brominated benzophosphole intermediate should be prepared in several steps including the Sonogashira coupling and intramolecular cyclization with sensitive reagents. In contrast, our current strategy features the short step synthesis, operational simplicity, scalability, and synthetically useful yield. As shown in Scheme 4, the MeO- and Cl-substituted iodobenzenes were also tolerated to give the coupling products 4ac and 4ad. Of note, under the standard ligand-free conditions, the reaction of 2a with 4-bromotoluene also occurred smoothly to provide the desired 4aa in a good yield. Considering better availability of aryl bromides than aryl iodides, our attempts were then moved to investigate the scope of aryl bromides in detail. The electron-withdrawing CF3 substituent was tolerated to deliver the arylating benzophosphole 4ae in a synthetically useful yield. Notably, at 100 °C, the aryl bromides bearing strongly electron-donating diarylamino and carbazolyl groups successfully furnished the corresponding products 4af and 4ag, which are of great potential for applications in functional materials.17 Furthermore, 2-bromonaphthalene 3h, 5-bromobenzodioxole 3i, and 5-bromotrimethoxybenzene 3j were also viable coupling partners to produce the donor–acceptor-type molecules 4ah–aj in good yields. Moreover, a variety of heterocyclic bromides could also be employed in the C–H arylation. For example, 6-bromoquinoline, 4-bromodibenzofuran, and 4-bromodibenzothiophene underwent the C–C coupling to give the highly π-extended frameworks (4ak–am) without any difficulties. Particularly notable is the successful application of 5-bromoindole, 5-bromobenzofuran, and 5-bromobenzothiophene that bear potentially reactive C2/C3–H bonds under the C–H activation conditions.10 The phosphole C2–H showed higher reactivity, and the corresponding arylated benzophospholes (4an–ap) were dominantly generated under the standard conditions. Additionally, our direct C–H arylation protocol was applicable in the reaction with 2-bromothiophenes to form the phosphole–thiophene linkages (4aq and 4ar), albeit in moderate yields.
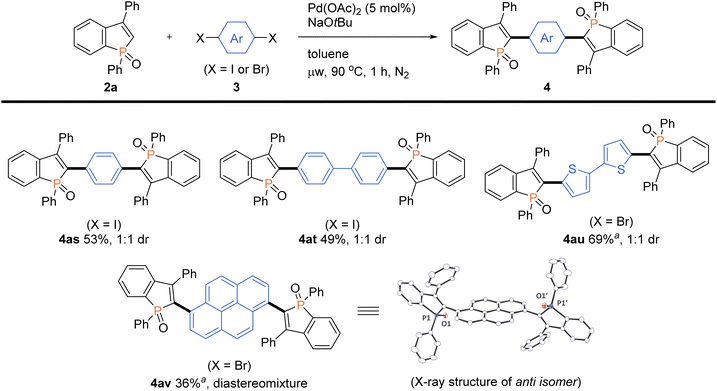
More intriguingly, the optimal conditions are also applicable to the double arylations with aromatic dihalides, enabling the rapid construction of benzophosphole-based acceptor–donor–acceptor-type molecules. As shown in Scheme 6, 1,4-diiodobenzene and 4,4′-diiodobiphenyl underwent the double C–C bond formation to give the expected molecules 4as and 4at, respectively. This type of structure is of great interest for applications in OLEDs and thin-film photovoltaics.2a As a promising electron-donor, 5,5′-dibromo-2,2′-bithiophene was also effective in the double C–C coupling to provide the highly conjugated molecules 4au in a good yield. Furthermore, as an outstanding chromophore, 1,6-dibromopyrene was coupled with two benzophosphole molecules to furnish the largely π-extended 4av, and the structure of its anti isomer was unambiguously confirmed by X-ray analysis (CCDC 2166424†).
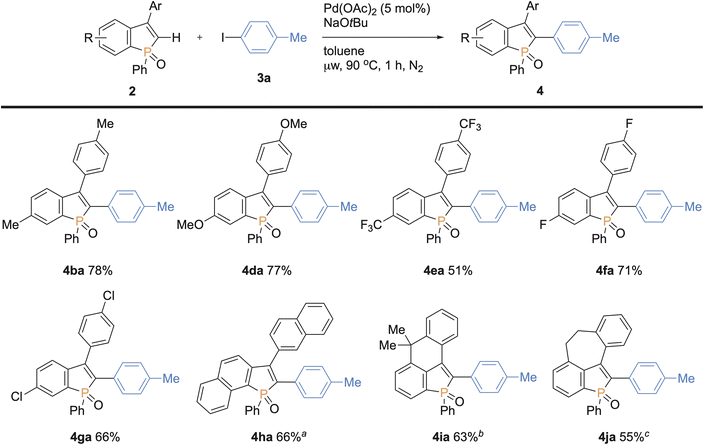
We next examined the scope of benzophospholes with 4-iodotoluene 3a as the coupling partner (Scheme 7). Various C2–H free benzophospholes were smoothly arylated under our standard conditions via the C–H bond cleavage, thus easily accessing the structurally useful C2,C3-diarylated benzophospholes. The electron-donating (Me and OMe) and electron-withdrawing (CF3 and F) substituents were well tolerated to deliver the functionalized benzophospholes 4ba–fa in acceptable to good yields. The chloro-substituted benzophosphole 2g also furnished the coupling product with the Ar–Cl moiety left intact. Additionally, the more condensed naphthophosphole 2h could also be functionalized under the modified conditions using KOtBu in place of NaOtBu. It should be noted that the C2-arylated naphthophospholes also show unique optical properties, and our synthetic method provides a rapid access to such interesting skeletons.5f,18 Moreover, the benzophospholes bearing six- and seven-membered cyclic systems underwent the C–C coupling to deliver the multi-ring fused products 4ia–ja in acceptable yields. Thus, additional salient feature of our synthetic platform is the controllable introduction of two different aryl substituents at the C2 and C3 positions of benzophospholes.
To further expand the generality of our protocol, we then focused on the less reactive aryl chlorides for the C–H arylation reaction (Scheme 8a). However, under standard ligand-free conditions, no target product was detected. After extensive screening of palladium catalysts and ligands (see the ESI for details†), we were pleased to find that the Pd(Cy3P)2 complex was optimal, and the arylating product 4aa was formed in a good yield. The Pd(Cy3P)2 catalyst was effective for both the electron-rich and -deficient aryl chlorides to afford the corresponding products in synthetically useful yields (4ac and 4ae).
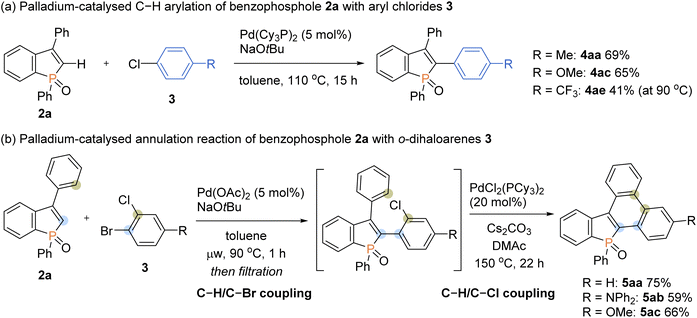 | ||
| Scheme 8 Palladium-catalysed regioselective C–H arylation of benzophosphole 2a with aryl chlorides 3 and application to annulation reactions with o-dihaloarenes 3. | ||
Taking advantage of distinct reactivity of Ar–Br and Ar–Cl moieties, we attempted the annulation reaction with bromochloroarenes via sequential C–H/C–X coupling (Scheme 8b). The 2-bromochloroarene 3 and benzophosphole 2a were subjected to the ligand-free Pd(OAc)2-catalysed C–H arylation conditions, which was followed by PdCl2(PCy3)2-catalysed intramolecular C–H arylation of phenyl ring with the C–Cl moiety to furnish the highly condensed framework 5aa in a good overall yield. The donor–acceptor systems 5ab and 5ac were also readily prepared with acceptable efficiencies.
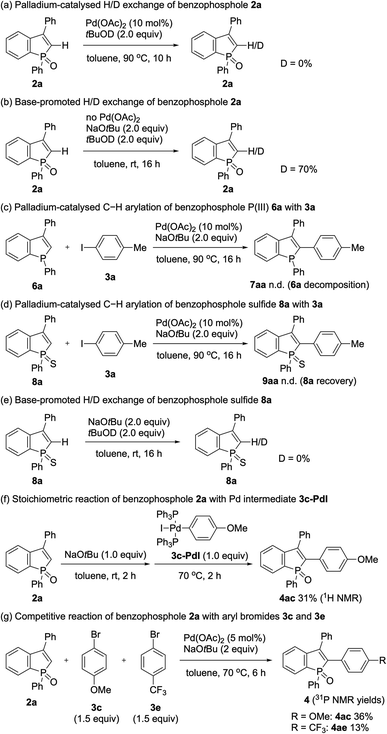
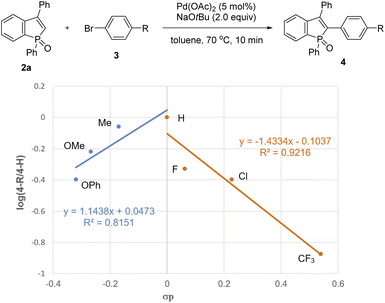
To gain insight into the reaction pathway, we performed several control experiments (Scheme 9). The C–H bond cleavage step was first investigated by H/D scrambling experiments with benzophosphole 2a and tBuOD in the absence of the aryl halide coupling partner. The H/D exchange of 2a was not observed at all in the presence of Pd(OAc)2 alone (Scheme 9a). In contrast, 70% deuterium incorporation at the C2 position was detected when 2a was treated with 2 equiv. of NaOtBu at room temperature even in the absence of Pd(OAc)2 (Scheme 9b). These results apparently indicate that the C–H bond cleavage of benzophosphole can occur by deprotonation with basic NaOtBu.19 To investigate the effect of phosphorus moiety in the reaction, we tested the corresponding P(III) benzophosphole 6a and benzophosphole sulfide 8a with 4-iodotoluene (3a) under the standard conditions (Schemes 9c and d, respectively). There was no detectable arylation product in both cases; 6a underwent decomposition because of its stability issue, while no reaction occurred with 8a probably due to the lower acidity of the C2–H bond.20 We actually did not observe any deuterium incorporation when 8a was treated with NaOtBu and tBuOD (Scheme 9e). Although we could not completely preclude the directing effect of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, the acidity of C2–H bond seems to be critical in the regioselective arylation. Additionally, when we independently prepared the palladium complex 3c-PdI21 and subjected it to a mixture of 2a and NaOtBu, the arylation product 4ac was indeed formed in 31% 1H NMR yield, indicating that the reaction proceeds via a Pd(0)/Pd(II) catalytic cycle (Scheme 9f). We also observed an inverted V-shaped Hammett plot by the reaction of 1a with several para-substituted aryl bromides 3 (Fig. 2): a positive slope of ρ = 1.14 was obtained from the electron-donating groups, whereas the electron-withdrawing groups resulted in a negatively sharper slope of ρ = −1.43 (see the ESI for more details†). Thus, the rate-limiting step would change, dependent on the electronic nature of the substituent on the aryl bromide. Further competitive experiment of substrate 2a with aryl bromides 3c and 3e revealed that the more electron-rich 3c showed higher reactivity than the electron-deficient 3e (Scheme 9g).
O group, the acidity of C2–H bond seems to be critical in the regioselective arylation. Additionally, when we independently prepared the palladium complex 3c-PdI21 and subjected it to a mixture of 2a and NaOtBu, the arylation product 4ac was indeed formed in 31% 1H NMR yield, indicating that the reaction proceeds via a Pd(0)/Pd(II) catalytic cycle (Scheme 9f). We also observed an inverted V-shaped Hammett plot by the reaction of 1a with several para-substituted aryl bromides 3 (Fig. 2): a positive slope of ρ = 1.14 was obtained from the electron-donating groups, whereas the electron-withdrawing groups resulted in a negatively sharper slope of ρ = −1.43 (see the ESI for more details†). Thus, the rate-limiting step would change, dependent on the electronic nature of the substituent on the aryl bromide. Further competitive experiment of substrate 2a with aryl bromides 3c and 3e revealed that the more electron-rich 3c showed higher reactivity than the electron-deficient 3e (Scheme 9g).
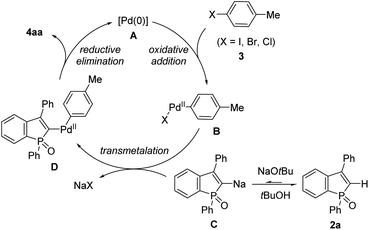
On the basis of the aforementioned outcomes, our proposed reaction mechanism of 2a with 3 is illustrated in Scheme 10. Oxidative addition of Pd(0) A to the aryl electrophile 3 results in the formation of Ar–Pd(II)–X complex B. A dynamic deprotonation/metalation of benzophosphole with NaOtBu (2a ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) C) is followed by transmetalation to Pd, giving the Ar–Pd(II)–phosphole intermediate D.22 Subsequent reductive elimination forms the arylated benzophosphole 4aa with regeneration of the starting Pd(0) species A to complete the catalytic cycle. The result in Scheme 9g suggests that the oxidative addition step is somewhat influential, but the reductive elimination is a more predominant step in the product formation.
C) is followed by transmetalation to Pd, giving the Ar–Pd(II)–phosphole intermediate D.22 Subsequent reductive elimination forms the arylated benzophosphole 4aa with regeneration of the starting Pd(0) species A to complete the catalytic cycle. The result in Scheme 9g suggests that the oxidative addition step is somewhat influential, but the reductive elimination is a more predominant step in the product formation.
We finally examined the optical properties of several newly synthesized compounds in CH2Cl2 solution. UV/Vis absorption and fluorescence spectra of selected compounds 4 and 5 are shown in Fig. 3, and the absorption/emission properties (λabs/λem) and fluorescence quantum yields (ΦF) are summarized in Table 2. Compared with the starting compound 2a, all arylated benzophosphole derivatives were fluorescent in solution (Fig. 4, 2avs. selected compounds). Most compounds gave a relatively narrow range of their longest wavelength absorption maxima (370–395 nm), whereas the electron-donating diarylamino-substituted 4af and 5ab, and bithiophenyl-derived 4au showed large bathochromic shifts of their λabs values (533–572 nm) with higher molar extinction coefficients (ε) and fluorescence in the green to yellow colour regions. Additionally, 4ag and 4av also exhibited intense fluorescence with broadened emission bands in the visible region. Although the quantum yield was moderate, 4af showed absorption and emission maxima at the even longer wavelength regions with a larger molar extinction coefficient relative to a similar framework 4ab. It is also noteworthy that, in comparison with a series of biaryl-type compounds 4, the more condensed 5 exhibited distinctly smaller Stokes shifts with good to excellent quantum yields, probably because of their highly rigid structures.
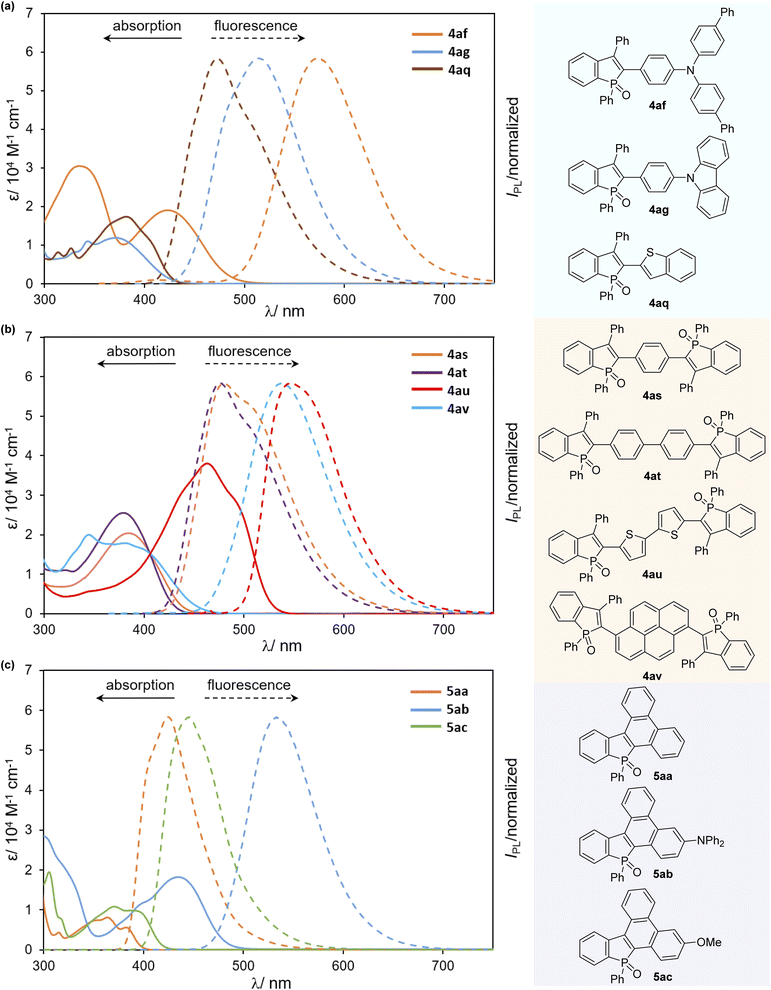 | ||
| Fig. 3 UV-Vis absorption (solid line) and fluorescence spectra (dashed line) of (a) 4af–ag, and 4aq, (b) 4as–av, and (c) 5aa–ac in CH2Cl2 (c = 1.0 × 10−5 M). | ||
| 4/5 | λ abs (nm) (ε (104 M−1 cm−1)) | λ em (nm) | Φ F (%) | Δνc (cm−1) |
|---|---|---|---|---|
| a Excited at λabs. b Absolute fluorescence quantum yields. c Stokes shifts. d The optical data of 4ab in CH2Cl2 was reported by Yamaguchi et al. in ref. 3a. e Absorption maxima at the longest wavelength. | ||||
| 4ab | 415 (1.73) | 565 | 90 | 6397 |
| 4af | 334 (3.05), 422 (1.90)e | 572 | 48 | 6214 |
| 4ag | 294 (1.87), 348 (1.04), 370 (1.19)e | 512 | 83 | 7496 |
| 4aq | 380 (1.73) | 471 | 20 | 5085 |
| 4as | 388 (2.03) | 481, 505 | 23 | 4983 |
| 4at | 380 (2.55) | 479, 499 | 39 | 5439 |
| 4au | 438 (3.08), 465 (3.78), 490 (2.89)e | 548 | 25 | 2160 |
| 4av | 344 (2.0), 385 (1.77)e | 540 | 32 | 7455 |
| 5aa | 297 (1.97), 364 (0.81), 382 (0.56)e | 425 | 56 | 2649 |
| 5ab | 278 (3.19), 304 (2.78), 438 (1.81)e | 533 | 87 | 4069 |
| 5ac | 306 (0.19), 370 (0.11), 395 (0.10)e | 446 | 95 | 2894 |
 | ||
| Fig. 4 Fluorescence images of some compounds in CH2Cl2 (c = 1.0 × 10−5 M) under UV irradiation (365 nm). | ||
We also investigated the electrochemical properties of the aforementioned compounds 4 and 5 by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in MeCN with tetrabutylammonium hexafluorophosphate (Bu4NPF6) as an electrolyte versus ferrocene/ferrocenium ion (Fc/Fc+) (Fig. S2–S12†), and their HOMO and LUMO levels were estimated according to the first oxidation potentials and the optical band gaps (Eoptg). The data is summarized in Table 3. The CV of 4ab, 4af, 4au, and 5ab showed reversible first oxidation waves, and their E1/2ox values are significantly shifted in a negative direction. Notably, compared with 4ab, identical HOMO levels and even lower LUMO levels were estimated for the 4af and 5ab, which may suggest their larger intramolecular charge transfer abilities. Density functional theory (DFT) calculations at the PBE0/6-31+G(d) level of theory were performed for 4ab,3a4at, and 4av, and their HOMO levels were estimated as −5.35 eV, −5.91 eV, and −5.48 eV, respectively (Fig. S14 and S15†). These values are in good agreement with those obtained from the CV and DPV experiments.
| 4/5 | λ absonset (nm) | E optg (eV) | E 1/2ox (V) | E HOMO (eV) | E LUMO (eV) |
|---|---|---|---|---|---|
| a Measured in CH2Cl2. b Determined from the onset of the absorption spectra. c Performed in MeCN in the presence of Bu4NPF6. ν = 0.1 V s−1 (4ag), 0.05 V s−1 (4af, 4aq, 4as–av, and 5aa), 0.03 V s−1 (4at, 5ab–ac). Values determined by CV (4ab, 4af, 4au, and 5ab) or DPV (4ag, 4aq, 4as, 4at, 4av, 5aa, and 5ac), versus Fc/Fc+. d The approximation for Fc/Fc+ level is −4.8 eV versus vacuum: EHOMO = −4.8 − E1/2ox. e Estimated from EHOMO and Eoptg: ELUMO = EHOMO + Eoptg. f The value was cited from ref. 3a. | |||||
| 4ab | 441f | 2.81 | 0.535 | −5.34 | −2.53 |
| 4af | 485 | 2.56 | 0.509 | −5.31 | −2.75 |
| 4ag | 424 | 2.92 | 0.87 | −5.67 | −2.75 |
| 4aq | 428 | 2.90 | 1.06 | −5.86 | −2.96 |
| 4as | 440 | 2.82 | 1.18 | −5.98 | −3.16 |
| 4at | 429 | 2.89 | 1.16 | −5.96 | −3.07 |
| 4au | 525 | 2.36 | 0.714 | −5.51 | −3.15 |
| 4av | 455 | 2.73 | 0.89 | −5.69 | −2.96 |
| 5aa | 397 | 3.12 | 1.32 | −6.12 | −3.0 |
| 5ab | 481 | 2.58 | 0.553 | −5.35 | −2.77 |
| 5ac | 418 | 2.96 | 1.03 | −5.83 | −2.87 |
Conclusions
In conclusion, we have developed the first example of palladium-catalysed regioselective C2–H arylation of benzophospholes with aryl halides. The reaction proceeds well with various aryl iodides, bromides, and even more challenging aryl chlorides, thus providing a modular synthetic platform for the construction of C2,C3-diarylated benzophospholes. Moreover, the double arylations with aromatic dihalides and annulation with ortho-dihalogenated arenes are also achieved to give the corresponding π-extended molecules of potent interest in material chemistry. Additionally, preliminary investigations of cardinal optoelectronic properties of some newly synthesized benzophospholes are also conducted. We anticipate that this strategy will find wide applications in the design and synthesis of benzophosphole-based materials and provide an entry point to other C–H functionalisations of benzophosphole derivatives.Data availability
All experimental procedures and spectroscopic data can be found in the ESI.†Author contributions
S. X. and K. N. performed optimization studies and scope investigations of C–H arylation. K. S. investigated the synthesis of starting benzophospholes. S. X. and K. H. prepared the manuscript. The project was supervised by K. H. and M. M. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant No. JP 22H02077 (Grant-in-Aid for Scientific Research(B), to K. H.), JP 17H06092 (Grant-in-Aid for Specially Promoted Research, to M. M.), and JP 21J10947 (Grant-in-Aid for JSPS Research Fellow, to K. N.) as well as by JST FOREST Program, Grant Number JPMJFR 211X to K. H. We thank Dr Yuji Nishii (Osaka University) for his assistance with the X-ray analysis.Notes and references
- Reviews: (a) T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681–4727 CrossRef CAS PubMed; (b) Y. Matano and H. Imahori, Org. Biomol. Chem., 2009, 7, 1258–1271 RSC . Selected examples; (c) C. Fave, T.-Y. Cho, M. Hissler, C.-W. Chen, T.-Y. Luh, C.-C. Wu and R. Réau, J. Am. Chem. Soc., 2003, 125, 9254–9255 CrossRef CAS PubMed; (d) H.-C. Su, O. Fadhel, C.-J. Yang, T.-Y. Cho, C. Fave, M. Hissler, C.-C. Wu and R. Réau, J. Am. Chem. Soc., 2006, 128, 983–995 CrossRef CAS PubMed; (e) H. Tsuji, K. Sato, Y. Sato and E. Nakamura, J. Mater. Chem., 2009, 19, 3364–3366 RSC; (f) O. Fadhel, M. Gras, N. Lemaitre, V. Deborde, M. Hissler, B. Geffroy and R. Réau, Adv. Mater., 2009, 21, 1261–1265 CrossRef CAS; (g) Y. Zhou, S. Yang, J. Li, G. He, Z. Duan and F. Mathey, Dalton Trans., 2016, 45, 18308–18312 RSC.
- (a) H. Tsuji, K. Sato, Y. Sato and E. Nakamura, Chem.–Asian J., 2010, 5, 1294–1297 CAS; (b) A. Kira, Y. Shibano, S. Kang, H. Hayashi, T. Umeyama, Y. Matano and H. Imahori, Chem. Lett., 2010, 39, 448–450 CrossRef CAS; (c) Y. Matano, A. Saito, Y. Suzuki, T. Miyajima, S. Akiyama, S. Otsubo, E. Nakamoto, S. Aramaki and H. Imahori, Chem.–Asian J., 2012, 7, 2305–2312 CrossRef CAS PubMed; (d) Y. Matano, H. Ohkubo, T. Miyata, Y. Watanabe, Y. Hayashi, T. Umeyama and H. Imahori, Eur. J. Inorg. Chem., 2014, 2014, 1620–1624 CrossRef CAS; (e) K. H. Park, Y. J. Kim, G. B. Lee, T. K. An, C. E. Park, S. K. Kwon and Y. H. Kim, Adv. Funct. Mater., 2015, 25, 3991–3997 CrossRef CAS.
- (a) E. Yamaguchi, C. Wang, A. Fukazawa, M. Taki, Y. Sato, T. Sasaki, M. Ueda, N. Sasaki, T. Higashiyama and S. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 4539–4543 CrossRef CAS; (b) C. Wang, A. Fukazawa, M. Taki, Y. Sato, T. Higashiyama and S. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 15213–15217 CrossRef CAS; (c) C. Wang, M. Taki, Y. Sato, A. Fukazawa, T. Higashiyama and S. Yamaguchi, J. Am. Chem. Soc., 2017, 139, 10374–10381 CrossRef CAS.
- For a recent review: (a) B. Wu and N. Yoshikai, Org. Biomol. Chem., 2016, 14, 5402–5416 RSC . Selected examples; (b) T. M. Balthazor, J. Org. Chem., 1980, 45, 2519–2522 CrossRef CAS; (c) J. G. Cordaro, D. Stein and H. Grützmacher, J. Am. Chem. Soc., 2006, 128, 14962–14971 CrossRef CAS PubMed.
- (a) G. Märkl, G. Y. Jin and K. P. Berr, Tetrahedron Lett., 1993, 34, 3103–3106 CrossRef; (b) H. Tsuji, K. Sato, L. Ilies, Y. Itoh, Y. Sato and E. Nakamura, Org. Lett., 2008, 10, 2263–2265 CrossRef CAS PubMed; (c) T. Sanji, K. Shiraishi, T. Kashiwabara and M. Tanaka, Org. Lett., 2008, 10, 2689–2692 CrossRef CAS PubMed; (d) A. Fukazawa, Y. Ichihashi, Y. Kosaka and S. Yamaguchi, Chem.–Asian J., 2009, 4, 1729–1740 CrossRef CAS; (e) Y. Zhou, Z. Gan, B. Su, J. Li, Z. Duan and F. Mathey, Org. Lett., 2015, 17, 5722–5724 CrossRef CAS PubMed . For the related Suzuki-Miyaura cross-coupling reactions, see:; (f) Y. Matano, Y. Motegi, S. Kawatsu and Y. Kimura, J. Org. Chem., 2015, 80, 5944–5950 CrossRef CAS; (g) M. Taki, H. Ogasawara, H. Osaki, A. Fuazawa, Y. Sato, K. Ogasawara, T. Higashiyama and S. Yamaguchi, Chem. Commun., 2015, 51, 11880–11883 RSC; (h) P. Aillard, M. Gicquel, K. Yavari, P. Retailleau, A. Voituriez and A. Marinetti, Eur. J. Org. Chem., 2018, 2018, 5853–5860 CrossRef CAS.
- (a) Y. Unoh, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2013, 52, 12975–12979 CrossRef CAS PubMed; (b) Y.-R. Chen and W.-L. Duan, J. Am. Chem. Soc., 2013, 135, 16754–16757 CrossRef CAS PubMed; (c) W. Ma and L. Ackermann, Synthesis, 2014, 2014, 2297–2304 Search PubMed; (d) P. Zhang, Y. Gao, L. Zhang, Z. Li, Y. Liu, G. Tang and Y. Zhao, Adv. Synth. Catal., 2016, 358, 138–142 CrossRef CAS; (e) D. M. Ma, W. Z. Chen, G. B. Hu, Y. Zhang, Y. X. Gao, Y. W. Yin and Y. F. Zhao, Green Chem., 2016, 18, 3522–3526 RSC; (f) V. Quint, F. Morlet-Savary, J. F. Lohier, J. Lalevee, A. C. Gaumont and S. Lakhdar, J. Am. Chem. Soc., 2016, 138, 7436–7441 CrossRef CAS PubMed.
- K. Nishimura, Y. Unoh, K. Hirano and M. Miura, Chem.–Eur. J., 2018, 24, 13089–13092 CrossRef CAS.
- (a) B. Wu, M. Santra and N. Yoshikai, Angew. Chem., Int. Ed., 2014, 53, 7543–7546 CrossRef CAS PubMed; (b) B. Wu, R. Chopra and N. Yoshikai, Org. Lett., 2015, 17, 5666–5669 CrossRef CAS PubMed.
- Selected reviews on metal-mediated C–H functionalisations: (a) F. Kakiuchi and T. Kochi, Synthesis, 2008, 2008, 3013–3039 CrossRef; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed; (c) T. Satoh and M. Miura, Chem.–Eur. J., 2010, 16, 11212–11222 CrossRef CAS PubMed; (d) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780–1824 CrossRef CAS PubMed; (e) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS; (f) V. P. Boyarskiy, D. S. Ryabukhin, N. A. Bokach and A. V. Vasilyev, Chem. Rev., 2016, 116, 5894–5986 CrossRef CAS; (g) R.-Y. Zhu, M. E. Farmer, Y.-Q. Chen and J.-Q. Yu, Angew. Chem., Int. Ed., 2016, 55, 10578–10599 CrossRef CAS PubMed; (h) M. Gulías and J. L. Mascareñas, Angew. Chem., Int. Ed., 2016, 55, 11000–11019 CrossRef PubMed; (i) L. Ping, D. S. Chung, J. Bouffard and S. Li, Chem. Soc. Rev., 2017, 46, 4299–4328 RSC; (j) M. T. Mihai, G. R. Genov and R. J. Phipps, Chem. Soc. Rev., 2018, 47, 149–171 RSC; (k) J. C. K. Chu and T. Rovis, Angew. Chem., Int. Ed., 2018, 57, 62–101 CrossRef CAS PubMed; (l) C. Sambiagio, D. Schonbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnurch, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC; (m) S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788–1887 CrossRef CAS PubMed.
- Selected reviews: (a) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200–205 CrossRef CAS; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (d) K. Hirano and M. Miura, Chem. Lett., 2015, 44, 868–873 CrossRef CAS; (e) I. A. Stepek and K. Itami, ACS Mater. Lett., 2020, 2, 951–974 CrossRef CAS; (f) B. Li, A. I. M. Ali and H. Ge, Chem, 2020, 6, 2591–2657 CrossRef CAS; (g) H.-Y. Huang, A. Benzai, X. Shi and H. Doucet, Chem. Rec., 2021, 21, 343–356 CrossRef CAS.
- P. Polák, J. Čejka and T. Tobrman, Org. Lett., 2020, 22, 2187–2190 CrossRef.
- K. Nishimura, K. Hirano and M. Miura, Org. Lett., 2019, 21, 1467–1470 CrossRef CAS.
- K. Nishimura, K. Hirano and M. Miura, Org. Lett., 2020, 22, 3185–3189 CrossRef CAS.
- A similar trend was observed in the previous electrophilic phosphination of alkynes: Y. Unoh, K. Hirano and M. Miura, J. Am. Chem. Soc., 2017, 139, 6106–6109 CrossRef CAS PubMed.
- (a) K. C. Majumdar, A. K. Pal, A. Taher and P. Debnath, Synthesis, 2007, 2007, 1707–1711 CrossRef; (b) M.-T. Nolan, J. T. W. Bray, K. Eccles, M. S. Cheung, Z. Lin, S. E. Lawrence, A. C. Whitwood, I. J. S. Fairlamb and G. P. McGlacken, Tetrahedron, 2014, 70, 7120–7127 CrossRef CAS.
- (a) Y. Unoh, T. Satoh, K. Hirano and M. Miura, ACS Catal., 2015, 5, 6634–6639 CrossRef CAS; (b) X. Luo, J. Yuan, C.-D. Yue, Z.-Y. Zhang, J. Chen, G.-A. Yu and C.-M. Che, Org. Lett., 2018, 20, 1810–1814 CrossRef CAS; (c) C. Sire, H. Cattey, A. Tsivery, J.-C. Hierso and J. Roger, Adv. Synth. Catal., 2022, 364, 440–452 CrossRef CAS.
- Y.-H. Cheng, E. Y.-H. Hong, M.-Y. Leung, S.-L. Lai and V. W.-W. Yam, SmartMat, 2021, 2, 406–418 CrossRef.
- N. Yoshikai, M. Santra and B. Wu, Organometallics, 2017, 36, 2637–2645 CrossRef CAS.
- For relatively low pKa values of phosphole derivatives, see: (a) D. Delaere, N. Pham-Tran and M. T. Nguyen, J. Phys. Chem. A, 2003, 107, 7514–7523 CrossRef CAS; (b) L. D. Quin, J. G. Bryson and C. G. Moreland, J. Am. Chem. Soc., 1969, 91, 3308–3316 CrossRef CAS.
- Ph2P
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shows a larger Hammett substituent constant than Ph2P
O shows a larger Hammett substituent constant than Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and Ph2P (σm = 0.38, 0.29, and 0.11, respectively), see: C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
S and Ph2P (σm = 0.38, 0.29, and 0.11, respectively), see: C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS. - D. Whitaker, J. Burés and I. Larrosa, J. Am. Chem. Soc., 2016, 138, 8384–8387 CrossRef CAS PubMed.
- Another possibility is the formation of Ar–Pd–phosphole intermediate by the deprotonation with Ar–Pd–OtBu species, which can be generated through a ligand exchange with NaOtBu.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2166424 and 2174057. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc04311d |
| This journal is © The Royal Society of Chemistry 2022 |