Open Access Article
Open Access ArticleAn expeditious FeCl3-catalyzed cascade 1,4-conjugate addition/annulation/1,5-H shift sequence for modular access of all-pyrano-moiety-substituted chromenes†
Xinwei
He
 *a,
Ruxue
Li
a,
Pui Ying
Choy
*a,
Ruxue
Li
a,
Pui Ying
Choy
 bc,
Jiahui
Duan
a,
Zhenzhen
Yin
a,
Keke
Xu
a,
Qiang
Tang
a,
Rong-Lin
Zhong
bc,
Jiahui
Duan
a,
Zhenzhen
Yin
a,
Keke
Xu
a,
Qiang
Tang
a,
Rong-Lin
Zhong
 b,
Yongjia
Shang
b,
Yongjia
Shang
 *a and
Fuk Yee
Kwong
*a and
Fuk Yee
Kwong
 *bc
*bc
aKey Laboratory of Functional Molecular Solids, Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu 241000, P. R. China. E-mail: xinweihe@mail.ahnu.edu.cn; shyj@mail.ahnu.edu.cn
bState Key Laboratory of Synthetic Chemistry and Department of Chemistry, The Chinese University of Hong Kong, New Territories, Shatin, Hong Kong SAR, P. R. China. E-mail: fykwong@cuhk.edu.hk
cShenzhen Center of Novel Functional Molecules, Shenzhen Municipal Key Laboratory of Chemical Synthesis of Medicinal Organic Molecules, CUHK Shenzhen Research Institute, No. 10. Second Yuexing Road, Shenzhen 518507, P. R. China
First published on 3rd November 2022
Abstract
ortho-Alkynyl quinone methides are well-known four-atom synthons for direct [4 + n] cycloaddition in constructing useful oxa-heterocyclic compounds owing to their high reactivity as well as the thermodynamically favored aromatization nature of this process. Herein we report an operationally simple and eco-friendly protocol for the modular and regioselective access of (E)-4-(vinyl or aryl or alkynyl)iminochromenes from propargylamines and S-methylated β-ketothioamides in the presence of FeCl3, and particularly under undried acetonitrile and air atmosphere conditions. This method exhibits a broad substrate scope and displays nice functional group compatibility, thus providing an efficient access of 3,4-disubstituted iminochromenes.
Introduction
The discovery of an advanced annulation strategy has received considerable attention in modern organic synthesis, notably in the area of polycyclic heteroarene assembly.1 Indeed, ring formation involving cyclization2 and late stage functionalization3 is a common protocol for accessing these structurally appealing frameworks, particularly in the synthesis of chromene systems.4 2H/4H-Chromenes are privileged heterocyclic compounds with versatile properties that exhibit noteworthy potency, for instance anti-cancer, anti-microbial, anti-convulsant and anti-inflammatory activities.5,6 Among these chromene systems, iminochromene has been found as one of the best potential heterocycles for new drug discovery,7 for example MEKK2 inhibitors,8 caspase activators, apoptosis inducers9 and IRAP inhibitors (Scheme 1a).10 Indeed, there is an urgent demand to develop a new modular protocol for reaching these systems which allow good structural diversity.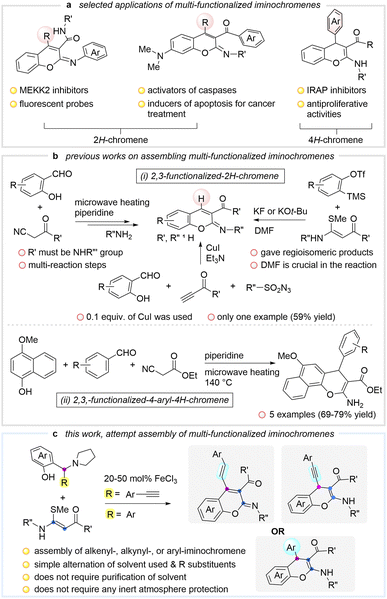 | ||
| Scheme 1 Selected applications of multi-functionalized iminochromenes, previous methods and our design for the synthesis of such compounds. | ||
Classical methods for constructing useful iminochromenes often employ salicylaldehyde as a typical synthon. Previous investigations showed the possible multicomponent reactions between terminal alkynes, sulfonyl azides and salicylaldehydes for affording 2,3-functionalized-2H-chromene using CuI as the catalyst (Scheme 1b(i)).11 Recently, Li introduced a synthetic method to obtain 2,3-disubstituted-2H-chromene using a cascade three-component coupling of arynes, dimethylformamide and N,S-keteneacetals (Scheme 1b(i)).12 In 2011, Liu and Wang reported the synthesis of 2-iminocoumarin-3-carboxamide from methyl cyanoacetate, aryl amines and salicylaldehydes via amidation and Knoevenagel condensation under microwave heating (Scheme 1b(i)).13 Remarkably, this skeleton displayed good photophysical properties for application in fluorescent intracellular imaging. Later, a modified multicomponent reaction of 4-methoxy-1-naphthol, substituted benzaldehyde and ethyl cyanoacetate allowed the formation of 2,3-functionalized-4-aryl-4H-chromenes (Scheme 1b(ii)).14 Certainly, the aforementioned methods have considerable merits in the assembly of multi-substituted iminochromenes. Nevertheless, the examples of all-pyran-functionalized iminochromenes were very limited. Only 5 relevant examples were reported. In fact, they are notable for having therapeutic potential as highlighted in Scheme 1a. Inspired by our pioneering results, in which highly reactive ortho-alkynyl quinone methide (o-AQM)15 displays the unique feature of cyclization,16 and in continuation of our interest in investigating the polyarene assembly,17 we herein report a new cascade reaction between alkylaminophenols and N,S-keteneacetals catalysed by FeCl3 (Scheme 1c). This process provides a simple, rapid, and direct approach to access alkenyl-, aryl- and alkynyl-iminochromene skeletons.
Results and discussion
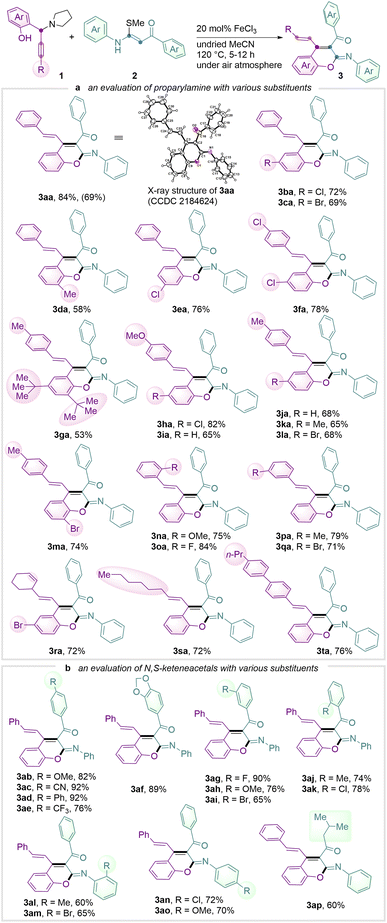
Propargylic amine 1a and N,S-keteneacetals 2a were chosen as model substrates in our prototypical investigation (see Table S1 in the ESI†). Gratifyingly, in the presence of Lewis acid FeCl3 in acetonitrile at 80 °C, the desired iminochromene 3aa was generated in 82% yield with a ratio of E/Z isomer at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Switching the Lewis acids to Sc(OTf)3, CuI, ZnI2, AgNO3 and others led to a dramatic drop in yields (entries 1–7), where no product was afforded in the absence of FeCl3 (entry 8). Utilizing 1.0 or 2.0 equivalent of 1a gave decreased yields of 72% and 76%, respectively (entries 1 vs. 9 and 10). A 20 mol% catalyst loading provided a comparable result (entries 1 vs. 11 and 12). Altering the solvent had a negative impact on the product yield (entries 11 vs. 13–17) and surprisingly the exclusion of water (i.e. anhydrous acetonitrile) proved to be unfavourable (entry 25). A survey of reaction temperature was also carried out. Decreasing the reaction temperature resulted in inferior yields and E/Z ratio (entries 11 vs. 18 and 19). Pleasingly, a 84% yield of 3aa was obtained with an improved E/Z ratio (>20
1. Switching the Lewis acids to Sc(OTf)3, CuI, ZnI2, AgNO3 and others led to a dramatic drop in yields (entries 1–7), where no product was afforded in the absence of FeCl3 (entry 8). Utilizing 1.0 or 2.0 equivalent of 1a gave decreased yields of 72% and 76%, respectively (entries 1 vs. 9 and 10). A 20 mol% catalyst loading provided a comparable result (entries 1 vs. 11 and 12). Altering the solvent had a negative impact on the product yield (entries 11 vs. 13–17) and surprisingly the exclusion of water (i.e. anhydrous acetonitrile) proved to be unfavourable (entry 25). A survey of reaction temperature was also carried out. Decreasing the reaction temperature resulted in inferior yields and E/Z ratio (entries 11 vs. 18 and 19). Pleasingly, a 84% yield of 3aa was obtained with an improved E/Z ratio (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 120 °C (entries 11 vs. 20 and 21). It is worth noting that this cascade reaction proceeds well even under a bench-top air atmosphere.
1) at 120 °C (entries 11 vs. 20 and 21). It is worth noting that this cascade reaction proceeds well even under a bench-top air atmosphere.
With the optimised reaction conditions, we next explored the substrate scope (Scheme 2). Given the unique feature of halogen-containing arene systems in halogen-bonding medicinal chemistry and chemical biology18 and the possibility of further functionalization using coupling technology,19 the substituents, e.g. –Br, –Cl and –F on the phenolic moiety and alkynyl arene moiety at the propargylic scaffold were therefore investigated. In contrast to the common Fe-catalysed coupling reaction where the halo groups would react, the present reaction system showed a halo moiety which remained intact under the stated reaction conditions (Scheme 2a, products 3ba, 3ca, 3ea, 3ha and 3qa). In addition to halo-substituents, the steric bulky tert-butyl group at the ortho-phenolic-position of propargylamine was found to be a feasible reaction partner towards the ring-forming process (product 3ga). Substrates with an ortho-hindered-substituent at the alkynyl arene moiety were also cyclised successfully to afford target iminochromenes (products 3na and 3oa). The alkenyl or alkyl unit located at propargylic amines furnished the products 3ra and 3sa in both 72% yield. Next, the reaction scope was further tested using various N,S-keteneacetal derivatives (Scheme 2b). The reactions were found to proceed smoothly and the functional groups of –Br and –Cl again were well tolerated (products 3ab–3ak). Strongly electron-withdrawing –CN and –CF3 positioned at the para-position of the arene moiety of 2 did not affect the annulation process (products 3ac and 3ae). Particularly noteworthy is that this method was found even able to accommodate ortho-substituted substrates, e.g. a chloro-substituent at the ortho-position of the arene moiety close to the carbonyl group and bromo-substituent at the ortho-position of the arene moiety near the imine group, in accessing 3ak (78%) and 3am (65%), respectively. These outcomes showed the suitability of this approach for further transformation of a π-extended system using an intramolecular annulation pathway.20
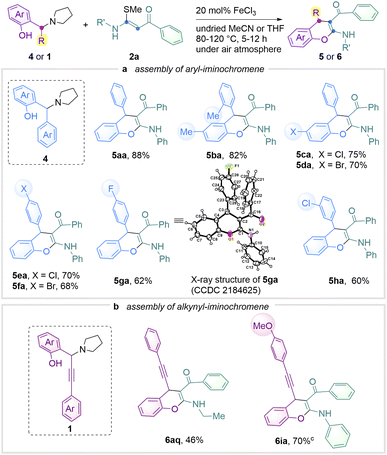
Considering the prevalence of the 4-arylchromene skeleton in drug discovery,21 we attempted to conduct the reaction using arene-containing aminoalkylphenols 4 (Scheme 3a). In fact, common modular assembly of chromene, which consists of aryl units, relies on the palladium-catalysed Suzuki–Miyaura coupling of its sulfonates/bromides with arylboronic acids in which the reaction scope is often limited by the incompatibility of –Br or –Cl groups.22Scheme 3a shows that various halogen-bearing aminophenols were tested in delivering products 5ca–5ga. Indeed, these resulting compounds exhibit rich potential for further functionalization using well-established cross-coupling technology.23 Product 5ga was unambiguously confirmed by single crystal X-ray crystallography (CCDC 2184625†). Steric bulky product 5ba was obtained in 82% yield. 4-Alkynyl-chromenes 6aq and 6ia were also afforded successfully (Scheme 3b). These products offer a high opportunity for further functionalization and thus allows new entities for material investigations.24
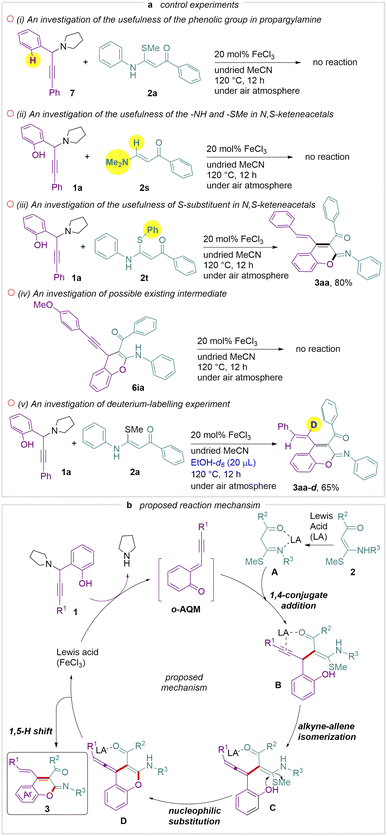
To gain more insight on this transformation, control experiments were carried out (Scheme 4a). Propargylamine 7 without a hydroxyl group did not react with 2a which indicates that the hydroxyl group plays a crucial role in the initial stage of this reaction (Scheme 4a(i)). Further attempts of 2s and 2t revealed that the –NH and –S–R groups in N,S-keteneacetals are essential for the reaction while the latter showed that the S-substituent did not affect the reaction (Scheme 4a(i) to (iii)). We were intrigued that 6ia is a probable intermediate before achieving product 3 (Scheme 4a(iv)). Yet, investigation showed that the in situ cyclisation does not occur. Furthermore, a deuterium-labelling experiment was performed, and compound 3aa–d was obtained with 85% of the deuterium atoms at the alkenyl group (Scheme 4a(v)). A mechanistic proposal is illustrated in Scheme 4b. Initially, the ortho-alkynyl quinone methide (o-AQM) intermediate is formed in the presence of Lewis acid (FeCl3), as proposed in the literature.25 Meanwhile, 2 coordinates with Lewis acid to form intermediate A with higher nucleophilicity. Intermediate B is then generated via the 1,4-conjugate addition between intermediate A and o-AQM, followed by alkyne–allene isomerization to give the intermediate C. Finally, a proton transfer followed by an intramolecular nucleophilic attack of the oxygen anion of the intermediate C yields the intermediate D,26 which further transforms to desired product 3via 1,5-H shift and releases the Lewis acid. In order to further elucidate the reaction mechanism, we also carried out theoretical investigations. Detailed information is presented in ESI Fig. S4.†26
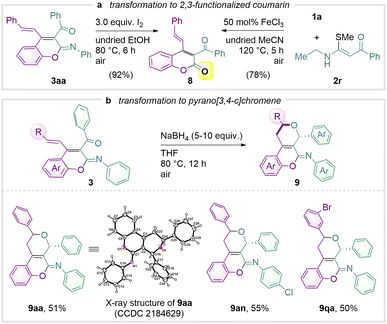
To demonstrate the synthetic versatility of 2,3,4-multi-functionalized iminochromene in resembling biological frameworks27 as well as photosensitive molecular assemblies,28 further pursuit of organic transformations was performed to show the accomplishment of multi-functionalized coumarins (Scheme 5). An iodine-promoted hydrolysis allowed the formation of coumarin 8 in 92% yield (Scheme 5a). Alternatively, the same product was able to be obtained in 78% yield when 1a was treated with 2r under standard reaction conditions. A method of accessing 1,5-dihydro-2H,4H-pyrano[3,4-c]iminochromenes is also shown in Scheme 5b (products 9aa, 9na and 9qa). The pyrano-embedded product 9aa was unambiguously confirmed by single crystal X-ray crystallography (CCDC 2184629†).
Conclusions
In conclusion, we have succeeded in showing a simple protocol for accessing all pyranyl-moiety-substituted chromenes efficiently. This FeCl3-catalyzed cascade reaction allows the formation of a variety of alkenyl-, aryl- or alkynyl-incorporated iminochromene skeletons with rich substitution patterns. Indeed, the present modular strategy meets the challenging demand of targeting diversified chromene structures for material and pharmaceutical investigations. Particularly noteworthy is that the –Br and –Cl groups which are often found impermissible in existing Pd-catalysed aromatic C–C or C–O bond ring-forming processes are well tolerated. This outcome should be beneficial for intended functionalization using versatile cross-coupling reactions.Data availability
The data supporting this study are available within the article and the ESI.† The X-ray crystallography coordinates for structures of 3aa (CCDC 2184624), 5ga (CCDC 2184625), and 9aa (CCDC 2184629) have been deposited at the Cambridge Crystallographic Data Centre.Author contributions
F. Y. K. and X. H. conceived and supervised the project. X. H., R. L., and J. D. designed and performed the experiments. R. L. and P. Y. C. carried out the data analysis. Z. Y., K. X., and Q. T. prepared the ESI.† Y. S. participated in the scientific discussions. R.-L. Z. carried out the theoretical study. X. H., P. Y. C. and F. Y. K. wrote and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Anhui Provincial Natural Science Foundation (No. 1808085MB41), the National Natural Science Foundation of China (No. 21772001), The University Synergy Innovation Program of Anhui Province (No. GXXT-2020-074), the Research Grants Council of Hong Kong (GRF: 14308121-21P), the CUHK Direct Fund (4442827), Central Funds Guiding the Local Science and Technology Development of Shenzhen Virtual University Park (2021Szvup147) and the Hong Kong Scholars Program (XJ2020-072) for financial support. We are thankful for financial support from the Innovation and Technology Commission (HKSAR, China) to the State Key Laboratory of Synthetic Chemistry.Notes and references
- (a) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301–5365 CrossRef CAS PubMed; (b) X. Y. Chen, X. Zhang and J.-P. Wan, Org. Biomol. Chem., 2022, 20, 2356–2369 RSC; (c) Q. Yang, R. Guo and J. Wang, Asian J. Org. Chem., 2019, 8, 1742–1765 CrossRef CAS.
- (a) B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937–2980 CrossRef CAS PubMed; for the most recent cycloaddition reaction in accessing chromone skeletons, see: (b) L. Meng, H. Liu, Z. Lin and J. Wang, Org. Lett., 2022, 24, 5890–5895 CrossRef CAS PubMed.
- (a) L. Guillemard, N. Kaplaneris, L. Ackermann and M. J. Johansson, Nat. Rev. Chem., 2021, 5, 522–545 CrossRef CAS; (b) L. Zhang and T. Ritter, J. Am. Chem. Soc., 2022, 144, 2399–2414 CrossRef CAS PubMed.
- (a) R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476–10526 CrossRef CAS PubMed; (b) S.-L. Zheng and L. Chen, Org. Biomol. Chem., 2021, 19, 10530–10548 RSC.
- (a) W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, Y. Wang, J. Zhao, S. Jia, J. Herich, D. Labreque, R. Storer, K. Meerovitch, D. Bouffard, R. Rej, R. Denis, C. Blais, S. Lamothe, G. Attardo, H. Gourdeau, B. Tseng, S. Kasibhatla and S. X. CaI, J. Med. Chem., 2004, 47, 6299–6310 CrossRef CAS PubMed; (b) V. Raj and J. Lee, Front. Chem., 2020, 8, 623 CrossRef CAS PubMed.
- For the most recent reference describing the synthesis of aryl-substituted 2H-chromenes and their excellent efficacy in pro-inflammation cytokine IL-1, see: (a) Q. Yang, Z. Wang, C. H. H. Hor, H. Xiao, Z. Bian and J. Wang, Sci. Adv., 2022, 8, eabm9603 CrossRef CAS PubMed; for the relevant asymmetric access of chromene and its derivatives, see: (b) Q. Yang, Y. Wang, S. Luo and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 5343–5347 CrossRef CAS PubMed.
- H. A. H. Elshemy, M. A. Zaki, A. M. Mahmoud, S. I. Khan, A. G. Chittiboyina and A. M. Kamal, Bioorg. Chem., 2022, 118, 105475 CrossRef CAS PubMed.
- S. Ahmad, V. R. S. Hilaire, S. R. Danadepally, G. L. Johnson, A. L. Williams and J. E. Scott, Biochem. Biophys. Res. Commun., 2018, 496, 205–211 CrossRef CAS PubMed.
- J. A. Drewe, S. X. Cai and Y. Wang, PCT Int. WO2001034591, 2001.
- (a) S. J. Mountford, A. L. Albiston, W. N. Charman, L. Ng, J. K. Holien, M. W. Parker, J. A. Nicolazzo, P. E. Thompson and S. Y. Chai, J. Med. Chem., 2014, 57, 1368–1377 CrossRef CAS PubMed; (b) S. Y. Chai, M. W. Parker, A. L. Albiston, F. A. O. Mendelsohn and K. G. Watson, US Pat., US8263775, 2012 Search PubMed.
- S.-L. Cui, X.-F. Lin and Y.-G. Wang, Org. Lett., 2006, 8, 4517–4520 CrossRef CAS PubMed.
- L.-R. Wen, N.-N. Man, W.-K. Yuan and M. Li, J. Org. Chem., 2016, 81, 5942–5948 CrossRef CAS PubMed.
- (a) D. Guo, T. Chen, D. Ye, J. Xu, H. Jiang, K. Chen, H. Wang and H. Liu, Org. Lett., 2011, 13, 2884–2887 CrossRef CAS PubMed; (b) D. Hu, N. Miyagi, Y. Arai, H. Oguri, T. Miura, T. Nishinaka, T. Terada, H. Gouda, O. El-Kabbani, S. Xia, N. Toyooka, A. Hara, T. Matsunaga, A. Ikari and S. Endo, Org. Biomol. Chem., 2015, 13, 7487–7499 RSC.
- (a) M. E.-A. Ahmed, M.-A.-D. Al-Anood and M. F. Ahmed, Eur. J. Chem., 2014, 5, 133–137 CrossRef; (b) F. A. Fawzia, R. M. Okasha, Z. M. Hritani, H. M. Mohamed, M. A. A. El-Nassag, A. H. Halawa, A. Mora, A. M. Fouda, M. A. Assiri, A.-A. M. Al-Dies, T. H. Afifi and A. M. El-Agrody, Bioorg. Chem., 2019, 87, 560–571 CrossRef PubMed.
- (a) B. J. Nachtsheim, Nat. Chem., 2020, 12, 326 CrossRef CAS PubMed; (b) S. Abhilash, H. Hemanta and G. Pranjal, J. Org. Chem., 2021, 86, 4883–4895 CrossRef PubMed.
- (a) J. Duan, X. He, P. Y. Choy, Q. Wang, M. Xie, R. Li, K. Xu, Y. Shang and F. Y. Kwong, Org. Lett., 2021, 23, 6455–6460 CrossRef CAS PubMed; (b) X. He, R. Li, P. Y. Choy, M. Xie, J. Duan, Q. Tang, Y. Shang and F. Y. Kwong, Commun. Chem., 2021, 4, 42 CrossRef CAS; (c) F. Y. Kwong, H. W. Lee, L. Qiu, W. H. Lam, Y. M. Li, H. L. Kwong and A. S. C. Chan, Adv. Synth. Catal., 2005, 347, 1750–1754 CrossRef CAS.
- (a) M. Li and F. Y. Kwong, Angew. Chem., Int. Ed., 2018, 57, 6512–6516 CrossRef CAS PubMed; (b) Q. Zhao, W. C. Fu and F. Y. Kwong, Angew. Chem., Int. Ed., 2018, 57, 3381–3385 CrossRef CAS PubMed.
- For a recent Perspective, see: R. Wilcken, M. O. Zimmermann, A. Lange, A. C. Joerger and F. M. Boeckler, J. Med. Chem., 2013, 56, 1363–1388 CrossRef CAS PubMed and references therein..
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed.
- L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581–590 CrossRef CAS PubMed.
- W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, C. Crogan-Grundy, D. Labreque, M. Bubenick, G. Attardo, R. Denis, S. Lamothe, H. Gourdeau, B. Tseng, S. Kasibhatla and S. X. Cai, J. Med. Chem., 2008, 51, 417–423 CrossRef CAS PubMed.
- K. R. Reddy, A. S. Reddy, D. K. Dhaked, S. K. Rasheed, A. S. Pathania, R. Shankar, F. Malik and P. Das, Org. Biomol. Chem., 2015, 13, 9285–9293 RSC.
- A. de Meijere, S. Bräse and M. Oestreich, Metal-Catalyzed Cross-Coupling Reactions and More, 1, 2, and 3, Wiley-VCH Weinheim, 2013 Search PubMed.
- G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285–2309 CrossRef CAS PubMed.
- W.-J. Bai, J. G. David, Z.-G. Feng, M. G. Weaver, K.-L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655–3664 CrossRef CAS PubMed.
- A density functional theory (DFT) calculation was further performed to elucidate the possible transformation pathways of intermediate C to D, see the ESI Computation study section for details and Fig, S4† for the DFT Gibbs energy profile of the ring-closure process.
- J. Hepworth, Pyrans and Fused Pyrans: (iii) Synthesis and Applications. in Comprehensive Heterocyclic Chemistry, ed. Katrizky, A. R. and Rees, C. W., Pergamon, Oxford, 1984, pp. 737–883 Search PubMed.
- G. P. Ellis, Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones, Wiley, New York, 1977, vol. 31 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data and copies of the NMR spectra. CCDC 2184624, 2184625 and 2184629. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc04431e |
| This journal is © The Royal Society of Chemistry 2022 |