Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ni12 tetracubane cores with slow relaxation of magnetization and efficient charge utilization for photocatalytic hydrogen evolution†
Elias
Tanuhadi
 a,
Joan
Cano
a,
Joan
Cano
 b,
Samar
Batool
b,
Samar
Batool
 c,
Alexey
Cherevan
c,
Alexey
Cherevan
 *c,
Dominik
Eder
*c,
Dominik
Eder
 c and
Annette
Rompel
c and
Annette
Rompel
 *a
*a
aUniversität Wien, Fakultät für Chemie, Institut für Biophysikalische Chemie, Josef-Holaubek-Platz-2, Wien 1090, Austria. E-mail: annette.rompel@univie.ac.at; Web: https://www.bpc.univie.ac.at
bDepartment of Química Inorgànica/Instituto de Ciencia Molecular (ICMol), Facultat de Quimica, Universitat de València, C/Catedrático Jose Beltrán 2, Paterna 46980, València, Spain
cTU Wien, Institute of Materials Chemistry, Getreidemarkt 9, Vienna 1060, Austria. E-mail: alexey.cherevan@tuwien.ac.at; Web: https://www.imc.tuwien.ac.at/division_molecular_materials_chemistry/
First published on 18th October 2022
Abstract
We report two Ni12 multicubane topologies enclosed in the polyanions [Ni12(OH)9(WO4)3(PO4)(B-α-PW9O34)3]21−{Ni12W30} and [Ni12(OH)9(HPO4)3(PO4)(B-α-PW9O34)(A-α-PW9O34)2]21−{Ni12W27} that magnetically behave as Ni12 units clearly distinguishing them from typical Ni4 cubanes as shown by magnetic studies together with high field and frequency electron paramagnetic resonance (HFEPR). Beyond the unprecedented static properties, {Ni12W30} shows the unusual coexistence of slow relaxation of the magnetization and a diamagnetic ground state (S = 0), providing the unique opportunity of studying the essentially elusive magnetic relaxation behavior in excited states. The cubane-topology dependent activity of {Ni12W30} and {Ni12W27} as homogeneous HER photocatalysts unveils the structural key features significant for the design of photocatalysts with efficient charge utilization exemplified by high quantum yields (QY) of 10.42% and 8.36% for {Ni12W30} and {Ni12W27}, respectively.
The compound class of transition metal (TM) based cubane clusters with the general formulation {TM4L4} (TM = CoII, NiII, FeIII, MnII/III/IV and L = O, S, N)1 exhibits unique structural and electronic properties. Consequently, cubane-based materials have been subjected to magnetic studies.2 In solution, the structural reminiscence of enzyme active sites such as the oxygen evolving complex (OEC) {Mn4O5Ca}3 in photosystem II (PS II)4,5 and the {Fe4S4} cubane cluster in hydrogenases6 encouraged studies on cubane-based metal oxide nanoparticle catalysts.7 From a synthetic point of view, recent attention has been given to lacunary polyoxometalates (POMs).8,9 Being generated upon the removal of one or several MO6 (M = MoVI, WVI) units from their parental architectures such as the Keggin10 or Wells–Dawson types11, lacunary POMs can act as strong inorganic, diamagnetic, multidentate O-donor ligands towards electrophiles. This multidentate nature allows the construction of mono- or multinuclear transition metal substituted POMs.12 Considering the lacunary POMs’ rigidity, bulkiness, and diamagnetic nature, cubane-motif incorporating POM representatives were shown to exhibit interesting magnetic properties such as single molecule magnet (SMM) behavior as a result of large spin ground state (S) values.13a POMs generally exhibit a facile photoexcitation via near-visible or UV-light generating photoexcited species with enhanced oxidizing (higher electron affinity Eea) and reducing (lower ionization energy EI) properties.13b Their multicentered nature allows POMs to undergo multi-redox events. In addition to their versatile redox properties, the inherent water solubility of POMs and stability encouraged researchers to employ cubane-motif incorporating POMs as homogeneous photocatalysts for the water reduction catalysis (WRC)14 and oxidation catalysis (WOC)15 reactions, respectively.
Despite the variety of existing POM-stabilized cubane motifs (Fig. S2, ESI†) that have been subjected to magnetic and/or (photo)catalytic studies (Table S1, ESI†), there are no studies exploring the correlation between a (multi)cubane-topology and its magnetic properties. Hence, advanced magnetic studies of, e.g., magnetic relaxation arising from excited states that might pave the way towards advanced spintronics remain widely elusive. While significant advances have been made in studying the cubane-dependent HER photocatalytic activity of POMs14 (Fig. S3 and Table S2, ESI†), the underlying structural features that may grant taking full advantage of photogenerated charge carriers as a key for improving the activity of photocatalysts remain essentially unexplored.16
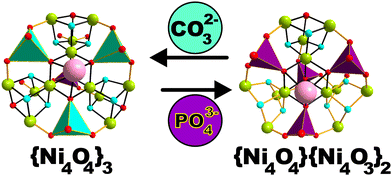
Herein, we employ phosphotungstates as rigid, all-inorganic multidentate ligands together with the simple inorganic templates PO43− or CO32− for the template-dependent stabilization of two tetracubane Ni12 topologies enclosed in the polyanions [Ni12(OH)9(WO4)3(PO4)(B-α-PW9O34)3]21−{Ni12W30} and [Ni12(OH)9(HPO4)3(PO4)(B-α-PW9O34)(A-α-PW9O34)2]21−{Ni12W27}. Given the identical number of incorporated Ni metal centers and their same net charge, {Ni12W30} and {Ni12W27} only differ in the type of POM-stabilized multicubane topology, thereby representing ideal candidates to study the topology dependent magnetic and photocatalytic behavior in the solid and solution states, respectively.
{Ni12W30} and {Ni12W27} were prepared employing a template dependent synthetic approach.17a To an aqueous reaction mixture containing the K14[P2W19O69(H2O)]·24H2O17b{P2W19} lacunary precursor, three equivalents of NiCl2 were added following the adjustment of the pH value to 9.1 via CO32− or PO43− and subsequent heating of the reaction mixture at 80 °C for 10 min. Depending on the inorganic template used for the solution's basification, {Ni12W30} (CO32−) or {Ni12W27} (PO43−) is obtained (Fig. 1, ESI†) upon slow evaporation of the solution for two weeks at 25 °C leading to yellow-plate shaped crystals of {Ni12W27} or light-green rod-shaped crystals of {Ni12W30}, respectively. When this paper was under preparation, a crystal structure with the identical anion of {Ni12W30} was reported.17c Note that for the synthesis of {Ni12W30}, different routes have been used by Lian et al. and our group (see Synthesis procedure†). The template dependent synthetic system reported in this work ultimately leads to the isolation of the novel polyanion {Ni12W27}.
Single crystal X-ray diffraction (SXRD) was performed (Tables S5–S9, ESI†) revealing that {Ni12W27} and {Ni12W30} both incorporate a Ni12 metal-oxo core that differs in the type of connectivity, and resulting in multicubane topology (Fig. 1) leading to the stabilization of three full {Ni4O4} cubane units in {Ni12W30} while {Ni12W27} comprises one full {Ni4O4} and two defect {Ni4O3} cubane motifs (Fig. 1, Fig. S14 and Tables S10, S11, ESI†). The compound's elemental composition and homogeneity were determined in the solid state by elemental analysis, IR spectroscopy (Fig. S4–S9 and Table S3, ESI†), thermogravimetric analyses (TGA) (Fig. S10–S13 and Table S4, ESI†), diffuse reflectance spectroscopy (DRS) (Fig. S17–S22, ESI†), and powder X-ray diffraction (PXRD) (Fig. S15 and S16, ESI†) as well as in solution by UV/vis spectroscopy (Fig. S31, ESI†) and cyclic voltammetry (Fig. S26–S30, ESI†).
The magnetic properties of {Ni12W30} and {Ni12W27} were studied in the solid state (Fig. 2) and solution (Fig. S42, ESI†). Plots of χMT (χM being the magnetic susceptibility per Ni12 unit) vs. T for {Ni12W30} and {Ni12W27} are displayed in Fig. 2. At 300 K, after removing the temperature-independent paramagnetism in subsequent fits (TIP = 4211 × 10−6 and 2756 × 10−6 cm3 mol−1 for {Ni12W30} and {Ni12W27}, respectively), their χMT values are ca. 15.6 ({Ni12W30}) and 13.6 cm3 mol−1 K ({Ni12W27}). These values are larger and smaller, respectively, than the spin-only value expected (ca. 14.5 cm3 mol−1 K, with g = 2.2) for twelve magnetically non-interacting high-spin NiII ions (S = 1). Hence, ferromagnetic (F) interactions are suggested to be predominant in {Ni12W30} as supported by the continuous increase of the compound's χMT upon lowering the temperature. In contrast to {Ni12W30}, a continuous decrease of χMT starting at room temperature (300 K) and reaching a minimum at 95 K accompanied by a subsequent increase is observed for {Ni12W27}. These features support the coexistence of F and antiferromagnetic (AF) interactions, with the latter being predominant. At 31 K ({Ni12W30}, Fig. 2A) and 17 K ({Ni12W27}, Fig. 2B), χMT shows maxima followed by an abrupt downturn to reach values of 5.7 and 15.0 cm3 mol−1 K at 2.0 K, respectively. Usually, zero-field splitting (zfs) could be the responsible factor for the observed decrease, as shown by the values of the local axial and rhombic zfs parameters (Table S14, see ESI,† Magnetism). The sharp downturn observed for {Ni12W30} (Fig. 2A) and {Ni12W27} (Fig. 2B) suggests additional factors apart from conventional zfs to be related to the AF inter- or intramolecular interactions. The shapes of the magnetization (M) vs. H plot at 2.0 K for both compounds (see insets of Fig. 2) show values largely below that expected for a ferromagnetic S = 12 spin ground state (26.4 Nβ, with g = 2.2). In the case of {Ni12W27}, weak or moderate H cancel the intermolecular magnetic interaction between two Ni12 units, and M tends to a saturation value of ca 12 Nβ, which is close to that expected for an S = 6 ground state. In contrast, a gentle increase of M for {Ni12W30} is observed at low fields without any trend for reaching saturation, supporting the close presence of many excited states of higher spin multiplicity that are partially populated upon increasing the magnetic field or the temperature. An estimation of the coupling constants (Ji values) obtained from DFT calculations guides the analysis of the observed magnetic behavior of both compounds suggesting a diamagnetic spin ground state S = 0 for {Ni12W30} with an energetically close excited state S = 1 (0.4 cm−1) and a ground state S = 6 with close excited states S = 5 and S = 4 at 2.0 and 10.1 cm−1 for {Ni12W27} (Fig. S32–S37 and Tables S12, S13, ESI,† see Magnetism).
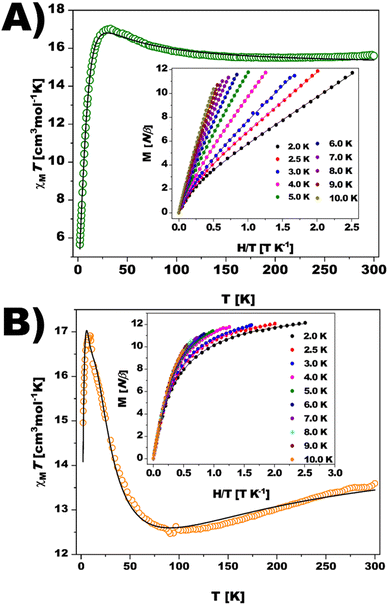 | ||
| Fig. 2 Temperature dependence of χMT and reduced magnetization curves (inset) for (A) {Ni12W30} and (B) {Ni12W27}. Solid lines are the best-fit curve and a guide to the eye for χMT and M. | ||
Alternating current (ac) magnetic susceptibility studies of {Ni12W30} and {Ni12W27} were performed to explore their magnetic relaxation properties. Out-of-phase 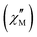 signals were observed both in the absence and under applied dc magnetic fields (Hdc) (Fig. S38 and S39, ESI†), typical for SMMs with energy barriers (Ea) risen from a D < 0 for low rhombicity (E/D ≈ 0).18,19 The occurrence of only incipient
signals were observed both in the absence and under applied dc magnetic fields (Hdc) (Fig. S38 and S39, ESI†), typical for SMMs with energy barriers (Ea) risen from a D < 0 for low rhombicity (E/D ≈ 0).18,19 The occurrence of only incipient 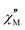 signals without maxima in the
signals without maxima in the 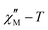 plots in both compounds precludes a correct treatment of the experimental data. Hence, a semiquantitative estimation of the Ea values was extracted from the
plots in both compounds precludes a correct treatment of the experimental data. Hence, a semiquantitative estimation of the Ea values was extracted from the 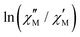 vs. 1/T plots for each frequency.18 According to the relationship
vs. 1/T plots for each frequency.18 According to the relationship 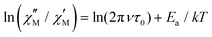 valid for a system with a single relaxation process, a collection of parallel straight lines is expected (Fig. S40 and S41, ESI†).18,19 The value of Ea would be obtained from their slope, giving Ea values of 18.1(7) and 25.0(3) cm−1 for {Ni12W30} and {Ni12W27}, respectively, with the Ea value for {Ni12W30} being close to that estimated (17.3 cm−1, the largest value between the round-trip paths) from the values of D (−15.2 cm−1) and E (0.14) proposed from the theoretical study for the first excited state S = 1. Since the negative sign of D enables a magnetic energy barrier and the good agreement between experimental and theoretical values of Ea, it can be concluded that a two-phonon Orbach mechanism governs the relaxation of the magnetization. The magnetization relaxation in {Ni12W27} occurs in the S = 6 ground state and is controlled through the energy barrier promoted by the zfs, whereas the diamagnetic singlet ground state S = 0 in {Ni12W30} suggests the observed slow relaxation of magnetization to arise from the close first excited state (S = 1, 0.4 cm−1, Fig. S36, ESI†).20a,b This excited triplet state S = 1 is only partially populated at the lowest experimental temperature being the cause of the weak
valid for a system with a single relaxation process, a collection of parallel straight lines is expected (Fig. S40 and S41, ESI†).18,19 The value of Ea would be obtained from their slope, giving Ea values of 18.1(7) and 25.0(3) cm−1 for {Ni12W30} and {Ni12W27}, respectively, with the Ea value for {Ni12W30} being close to that estimated (17.3 cm−1, the largest value between the round-trip paths) from the values of D (−15.2 cm−1) and E (0.14) proposed from the theoretical study for the first excited state S = 1. Since the negative sign of D enables a magnetic energy barrier and the good agreement between experimental and theoretical values of Ea, it can be concluded that a two-phonon Orbach mechanism governs the relaxation of the magnetization. The magnetization relaxation in {Ni12W27} occurs in the S = 6 ground state and is controlled through the energy barrier promoted by the zfs, whereas the diamagnetic singlet ground state S = 0 in {Ni12W30} suggests the observed slow relaxation of magnetization to arise from the close first excited state (S = 1, 0.4 cm−1, Fig. S36, ESI†).20a,b This excited triplet state S = 1 is only partially populated at the lowest experimental temperature being the cause of the weak 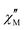 signals and precluding the data analysis in the absence of Hdc. These assumptions are additionally reinforced by high-frequency and high-field electron paramagnetic resonance (HFEPR) studies, which show an EPR-silent spectrum for {Ni12W30} in accordance with the proposed singlet ground state S = 0 (Fig. S43, ESI†), whereas HFEPR studies on {Ni12W27} reveal a weak signal at 6.6 T (Fig. S44 and S45, ESI†) further supporting the presence of a paramagnetic ground state and dipolar AF interactions arising from the {Ni12W27}2 supradimers, according to the DFT study and the crystal structure (Fig. S46, ESI,† DFT studies. Section 10.1). The observed topology-dependent magnetic behaviour and electronic properties distinguish the isolated Ni12 scaffolds from typical {Ni4O4} single-cubane motifs.
signals and precluding the data analysis in the absence of Hdc. These assumptions are additionally reinforced by high-frequency and high-field electron paramagnetic resonance (HFEPR) studies, which show an EPR-silent spectrum for {Ni12W30} in accordance with the proposed singlet ground state S = 0 (Fig. S43, ESI†), whereas HFEPR studies on {Ni12W27} reveal a weak signal at 6.6 T (Fig. S44 and S45, ESI†) further supporting the presence of a paramagnetic ground state and dipolar AF interactions arising from the {Ni12W27}2 supradimers, according to the DFT study and the crystal structure (Fig. S46, ESI,† DFT studies. Section 10.1). The observed topology-dependent magnetic behaviour and electronic properties distinguish the isolated Ni12 scaffolds from typical {Ni4O4} single-cubane motifs.
A careful inspection of the 236 GHz EPR spectrum of {Ni12W30} allows for identifying of two almost muted signals at 4.0 and 6.9 T resulting from a very close excited state (Fig. S43, ESI†). These signals do not change as the temperature increases, and their intensity decreases when using a frequency of 400 GHz. This surprising result must be attributed to several excited states very close to the ground one, as shown in Figure S36 (ESI†) from the theoretical study. A triplet spin state and a D value comparable to that proposed by CASSCF calculations (≈−15 cm−1) could reproduce the signal at 6.9 T. However, the low-field signal must appear due to a notable rhombicity in the zfs tensor (E/D ≠ 0), which agrees well with the theoretical study (0.14) and the weakness of these signals (page 59 in the ESI†).
The EPR spectrum at 236 GHz of {Ni12W27} shows five attenuated signals at 3.6, 6.2, 6.6, 7.0 and 8.0 T, which move to higher fields when increasing the frequency to 388 GHz but do not substantially modify their intensity (Fig. S45, ESI†). This pattern resembles that observed in a previous double-cubane Ni7 cluster,20c which, like {Ni12W27}, exhibits an S = 6 ground state, supporting our conclusions. The analysis of the EPR spectra of Ni7 required a nearby S = 7 excited state. However, according to our theoretical study, an analogous procedure in {Ni12W27} should consider an S = 5 rather than an S = 7 excited state. Unfortunately, the weakness of the signals on {Ni12W27} prevents further investigation.
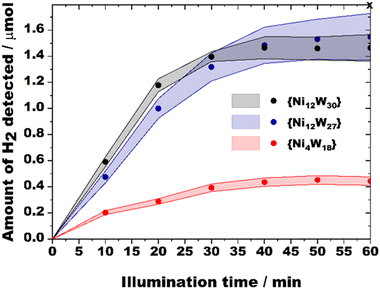
Following earlier reports on structurally relevant Ni-containing POM hydrogen evolution catalysts (HECs)21 the TBA salts of {Ni12W30} and {Ni12W27} were evaluated towards the visible-light-driven hydrogen evolution reaction (HER) employing [Ir(dtbbpy)(ppy)2]+ (dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl, ppy = 2-phenylpyridine) as the photosensitizer (PS), triethanolamine (TEOA) as an electron donor and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vol% CH3CN/DMF/H2O as a solvent mixture (see Hydrogen evolution (HER) experiments, Fig. S47 and S48, ESI†). A compositionally relevant tetra-nickel polyoxotungstate reported by Hill et al.21c referred to as {Ni4W18} was prepared, characterized (Fig. S1, ESI†), and tested under identical experimental conditions as a benchmark HEC following a comprehensive set of pre-catalytic studies employing time-dependent UV/vis studies (Fig. S31, ESI†) that demonstrated pre-catalytic stability of {Ni12W30} and {Ni12W27} as shown by the compounds’ virtually unchanged spectra over a time period of 60 min relevant to the catalytic experiment (see Section S12.1, ESI†).
4 vol% CH3CN/DMF/H2O as a solvent mixture (see Hydrogen evolution (HER) experiments, Fig. S47 and S48, ESI†). A compositionally relevant tetra-nickel polyoxotungstate reported by Hill et al.21c referred to as {Ni4W18} was prepared, characterized (Fig. S1, ESI†), and tested under identical experimental conditions as a benchmark HEC following a comprehensive set of pre-catalytic studies employing time-dependent UV/vis studies (Fig. S31, ESI†) that demonstrated pre-catalytic stability of {Ni12W30} and {Ni12W27} as shown by the compounds’ virtually unchanged spectra over a time period of 60 min relevant to the catalytic experiment (see Section S12.1, ESI†).
Table 1 contains the values of the turnover frequencies (TOFs) calculated after 10 minutes of each HER experiment. Depending on the concentrations of {Ni12W30} and {Ni12W27}, their HER TOFs lie in the range between 19.8 and 145.7 (expressed in 10−3 s−1). To allow for a valid comparison to relevant catalysts, the HER quantum yields (QYs, see General information†) were calculated (Table 1) and amount to 10.42 and 8.36% for {Ni12W30} and {Ni12W27}, respectively. These QYs greatly surpass values previously reported for Ni-containing POMs14,16 (Table S2, ESI†) and – considering that HER QY values already include absorption and recombination losses (e.g., contributions by PS-POM charge transfer) – they indicate extremely high efficiency of charge utilization for {Ni12W30} and {Ni12W27} suggesting {Ni12W30} and {Ni12W27} to be the fastest Ni-PT based HECs reported so far (Table S2, ESI†).
| Ni-POT | c/μM | H2/μmol | TON | TOF/10−3 s−1 | QY/% |
|---|---|---|---|---|---|
| {Ni12W30} | 2 | 0.56 | 140.9 | 145.7 | 6.15 |
| 5 | 0.82 | 81.6 | 84.4 | 8.90 | |
| 10 | 1.11 | 55.4 | 39.2 | 8.26 | |
| 20 | 1.47 | 36.7 | 24.7 | 10.42 | |
| {Ni12W27} | 2 | 0.52 | 130.0 | 106.3 | 4.49 |
| 5 | 0.81 | 81.0 | 56.1 | 5.92 | |
| 10 | 1.12 | 56.2 | 37.4 | 7.89 | |
| 20 | 1.55 | 38.8 | 19.8 | 8.36 | |
| {Ni4W18} | 20 | 0.45 | 11.4 | 8.8 | 3.72 |
| Ni(NO3)2 | 20 | 0.35 | 8.7 | 6.9 |
Post-catalytic studies (see the ESI† section Post-catalytic studies) featuring IR-spectroscopy demonstrated the post-catalytic bulk stability of {Ni12W30} and {Ni12W27} (Fig. S49, ESI†), which was subsequently assessed quantitatively employing X-ray fluorescence (XRF) (Table S15, ESI†). A series of reloading experiments demonstrated the recyclability of {Ni12W30} and {Ni12W27} as homogeneous HECs (Fig. S50, ESI†).
A careful analysis of the TOF values allows for structure–activity relationship (SAR) correlations between {Ni12W30} and {Ni12W27} that both feature {NiII4O4} quasi-cubanes. While higher catalyst concentrations (10 and 20 μM) yield similar TOFs, a significantly higher HER performance of {Ni12W30} over {Ni12W27} can be observed for 2 and 5 μM catalyst solutions at which the catalytic role of the elsewise isostructural Ni-PTs – that both display the same net charge of −21 – is more pronounced. One contribution to the superior activity of {Ni12W30} over {Ni12W27} can be related to the higher number of {NiII4O(OH)3} cubanes incorporated in the POT framework as compared to {Ni12W27}, which is in accordance with the findings reported by Wang and co-workers (Fig. S3, ESI†).14
To further explore the origin of the higher HER activity of {Ni12W30}, photoluminescence (PL) emission studies (Fig. S52 and S53, Section S14, ESI†) elucidating the HEC mechanism were conducted. Both steady-state and time-resolved data propose reductive quenching to dominate under the turnover conditions (Scheme S1, ESI†), which suggests that the reversible reduction of the corresponding Ni-PT (Scheme S2, ESI,† step III) – which consecutively reduces H+ upon H2 formation may represent a rate-limiting step in the HER. Based on CV studies on {Ni12W27} (Fig. S25, ESI†), {Ni12W30} (Fig. S24, ESI†), and {Ni4W18} (Fig. S23, ESI†), the onset reduction of the Ni-PTs occurs at more positive potentials in the order {Ni4W18} (−0.66 V) < {Ni12W27} (−0.25 V) < {Ni12W30} (−0.12 V). Hence, the observed activity trend can be explained by the increased tendency of {Ni12W30} to be reduced, which is additionally reflected by a substantially higher (50%) quenching rate Kq for {Ni12W30} (Kq = 13.2 × 109 M−1 s−1) compared to that of {Ni12W27} (Kq = 8.9 × 109 M−1 s−1) (Fig. S53, ESI†) illustrating its more efficient redox shuttling. A template-dependent change in the topology of the Ni12 metal-oxo core resulted in an increased number of {NiII4O(OH)3} cubanes present in {Ni12W30} and provided this Ni-PT with an enhanced charge-utilization expressed by the tuned redox-activity and increased photocatalytic HER performance as compared to {Ni12W27} and {Ni4W18}.
Conclusions
Our results highlight that a diamagnetic ground state does not necessarily preclude SMM behavior in compounds that exhibit energetically closely located paramagnetic excited spin states. Moreover, incorporating a tandem ground and nearby excited states, behaving both as SMMs or qubits, allows for envisaging advanced spintronics and quantum computing. Structure–activity studies on {Ni12W30} and {Ni12W27} probing their photocatalytic HEC activity revealed high charge utilization for {Ni12W30} and {Ni12W27}. The topology modulation of the tetracubane scaffold employing simple inorganic anionic templates represents a key to tuning their functional HEC properties upon modulating the corresponding Ni-PT's redox properties as exemplified by the high quantum yield of {Ni12W30}.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Austrian Science Fund (FWF): P33089 (to A. R.) and P32801 (to A. C.), the University of Vienna, the Spanish MINECO (Project PID2019-109735GB-I00 and Unidad de Excelencia María de Maeztu CEX2019-000919-M), and Generalitat Valenciana (AICO/2020/183 and AICO/2021/295). E. T. and A. R. acknowledge the University of Vienna for awarding a Uni:docs fellowship to E.T. The authors thank Nadiia I. Gumerova, PhD for support with ESI-MS experiments, Pablo Ayala, MSc for TXRF measurements, and Dr Heiko Geisler for assisting in CV experiments. Photoluminescence (PL) emission and lifetime measurements were performed by Sreejith P. Nandan, MSc. The authors thank Ing. Dipl.-Ing. (FH) Alexander Prado-Roller (X-ray Structure Analysis Center, Faculty of Chemistry, University of Vienna) and ao. Univ.-Prof. Mag. Dr Gerald Giester (Department of Mineralogy and Crystallography, University of Vienna) for support and valuable discussions with single crystal X-ray crystallographic measurements and data analyses. Lastly, the authors wish to thank Dipl.-Ing. Dr techn. Andreas Mautner, Privatdoz and Mag. Johannes Theiner (Mikroanalytisches Laboratorium Universität Wien) for elemental analyses. The high-field EPR spectra were recorded by Andrew Ozarowski, PhD, at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.Notes and references
- F. G. Mann, D. Purdie and A. F. Wells, J. Chem. Soc., 1936, 1503–1513 RSC.
- (a) J. E. Andrew and A. B. Blake, J. Chem. Soc. A, 1969, 1456–1461 RSC; (b) A. P. Ginsberg, J. A. Bertrand, R. I. Kaplan, C. E. Kirkwood, R. L. Martin and R. C. Sherwood, Inorg. Chem., 1971, 10, 240–246 CrossRef CAS.
- R. M. Cinco, J. H. Robblee, A. Rompel, C. Fernandez, V. K. Yachandra, K. Sauer and M. P. Klein, J. Phys. Chem. B, 1998, 102, 8248–8256 CrossRef CAS PubMed.
- J. Yano and V. K. Yachandra, Chem. Rev., 2014, 114, 4175–4205 CrossRef CAS PubMed.
- M. L. Helm, M. P. Stewart, R. M. Bullock, M. R. DuBois and D. L. DuBois, Science, 2011, 333, 863–866 CrossRef CAS PubMed.
- J. Liu, S. Chakraborty, P. Hosseinzadeh, Y. Yu, S. Tian, I. Petrik, A. Bhagi and Y. Lu, Chem. Rev., 2014, 114, 4366–4469 CrossRef CAS PubMed.
- (a) S. Berardi, G. La Ganga, M. Natali, I. Bazzan, F. Puntoriero, A. Sartorel, F. Scandola, S. Campagna and M. Bonchio, J. Am. Chem. Soc., 2012, 134, 11104–11107 CrossRef CAS PubMed; (b) B. Zhang, F. Li, F. Yu, X. Wang, X. Zhou, H. Li, Y. Jiang and L. Sun, ACS Catal., 2014, 4, 804–809 CrossRef CAS.
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer Verlag, Berlin, 1983, vol. 2, pp. 10–26 Search PubMed.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 RSC.
- G. L. Miessler and D. A. Tarr, Inorganic Chemistry, Pearson Prentice Hall, Upper Saddle River, N.J., 3rd edn, Internat. edn, 2004 Search PubMed.
- L. Briand and H. J. Thomas, Appl. Catal., A, 2003, 256, 37–50 CrossRef CAS.
- Z.-J. Liu, X.-L. Wang, C. Qin, Z.-M. Zhang, Y.-G. Li, W.-L. Chen and E.-B. Wang, Coord. Chem. Rev., 2016, 313, 94–110 CrossRef CAS.
- (a) M. Ibrahim, Y. Lan, B. S. Bassil, Y. Xiang, A. Suchopar, A. K. Powell and U. Kortz, Angew. Chem., Int. Ed., 2011, 50, 4708–4711 CrossRef CAS PubMed; (b) C. Streb, K. Kastner and J. Tucher, Phys. Sci. Rev., 2019, 4, 368–378 Search PubMed.
- X.-B. Han, C. Qin, X.-L. Wang, Y.-Z. Tan, X.-J. Zhao and E.-B. Wang, Appl. Catal., B, 2017, 211, 349–356 CrossRef CAS.
- X.-B. Han, Y.-G. Li, Z.-M. Zhang, H.-Q. Tan, Y. Lu and E.-B. Wang, J. Am. Chem. Soc., 2015, 137, 5486–5493 CrossRef CAS PubMed.
- P. Zhang, T. Wang, X. Chang and J. Gong, Acc. Chem. Res., 2016, 49, 911–921 CrossRef CAS PubMed.
- (a) S. Li, H. Wang, H. Su, H. Chen, M. Du, L. Long, X. Kong and L. Zheng, Small Methods, 2021, 5, 2000777 CrossRef CAS PubMed; (b) C. M. Tourné and G. F. Tourné, J. Chem. Soc., Dalton Trans., 1988, 9, 2411–2420 RSC; (c) C. Lian, H.-L. Li and G.-Y. Yang, Inorg. Chem., 2022, 61, 11335–11341 CrossRef CAS PubMed.
- F. Luis, J. Bartolomé, J. F. Fernández, J. Tejada, J. M. Hernández, X. X. Zhang and R. Ziolo, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 11448–11456 CrossRef CAS.
- A. Pascual-Álvarez, J. Vallejo, E. Pardo, M. Julve, F. Lloret, J. Krzystek, D. Armentano, W. Wernsdorfer and J. Cano, Chem. – Eur. J., 2015, 21, 17299–17307 CrossRef PubMed.
- (a) D. Cabrosi, C. Cruz, V. Paredes-García and P. Alborés, Dalton Trans., 2021, 50, 1402–1412 RSC; (b) J. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729–1733 CrossRef CAS PubMed; (c) S. Petit, P. Neugebauer, G. Pilet, G. Chastanet, A.-L. Barra, A. B. Antunes, W. Wernsdorfer and D. Luneau, Inorg. Chem., 2012, 51, 6645–6654 CrossRef CAS PubMed.
- (a) H. Lv, Y. Chi, J. van Leusen, P. Kögerler, Z. Chen, J. Bacsa, Y. V. Geletii, W. Guo, T. Lian and C. L. Hill, Chem. – Eur. J., 2015, 21, 17363–17370 CrossRef CAS PubMed; (b) W. Guo, H. Lv, J. Bacsa, Y. Gao, J. S. Lee and C. L. Hill, Inorg. Chem., 2016, 55, 461–466 CrossRef CAS PubMed; (c) H. Lv, W. Guo, K. Wu, Z. Chen, J. Bacsa, D. G. Musaev, Y. V. Geletii, S. M. Lauinger, T. Lian and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 14015–14018 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2102166 and 2102167. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2tc03508a |
| This journal is © The Royal Society of Chemistry 2022 |