 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Activation energy of magnesite (MgCO3) precipitation: recent insights from olivine carbonation studies
Quin R. S.
Miller
 * and
H. Todd
Schaef
* and
H. Todd
Schaef
 *
*
Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA, USA. E-mail: quin.miller@pnnl.gov; todd.schaef@pnnl.gov
First published on 18th July 2022
Abstract
We present two new activation energies for magnesite precipitation during forsteritic olivine (Mg2−xFexSiO4; 0.18 ≤ x ≤ 0.26) carbonation in high-pressure carbon dioxide. These new activation energies of 89 ± 6 and 85 ± 1 kJ mol−1 are consistent with the literature for magnesite precipitation in aqueous media and extend the temperature range to encompass 90 °C to 50 °C. These insights will help improve understanding of mineral transformation kinetics in the subsurface, including carbon storage in mafic-ultramafic environments, and aid in the development of carbon dioxide removal (CDR) and net negative-emissions technologies.
Environmental significanceOlivine is a key constituent of reactive geologic formations and industrial wastes that are targets for permanent carbon storage via mineralization. The relative paucity of kinetic parameters for olivine transformation to magnesite via coupled dissolution and carbonate precipitation hinders efforts to predict rate and design efficient mineralization strategies. Our calculations of two new olivine carbonation activation energies help address these knowledge gaps relevant to natural and engineered environmental carbon-management processes. |
The concept of carbon dioxide removal (CDR) through carbon capture and sequestration is an integral component of current climate mitigation strategies and pursuit of net-negative emissions technologies. A promising CDR approach involves injection of carbon dioxide (CO2) into reactive mafic and ultramafic rocks to form stable carbonate minerals, enabling rapid permanent carbon storage.1–8 In this context, understanding rates of mineral carbonation is crucial for predicting fate and transport of subsurface CO2.
Olivine (Mg2−xFexSiO4) is a key reactive component of mafic and ultramafic rocks, and its dissolution, hydration, and carbonation rates have received considerable scrutiny (c.f., ref. 9–13). The recent quantitative kinetics analyses and compilations of Miller et al.11 and Sendula et al.12 fit the Avrami model14 and shrinking particle model (SPM),12,15–17 respectively, to the broad olivine carbonation literature. The more recent and comprehensive study of Sendula et al.12 provided 35 new experiments, nearly doubling the amount of available datasets, and the SPM proved most flexible and adaptable for the diverse olivine carbonation literature. The goal of the present Communication is to extract carbonation activation energy parameters from recently compiled olivine carbonation studies.11,12 To do so we critically reviewed the datasets to identify two12,18 suitable internally-consistent collections of reaction rate vs. temperature data for magnesite precipitation during olivine carbonation. These datasets were suitable as they included reaction kinetics for at least three distinct temperatures.
The San Carlos olivine used in Sendula et al.12 has ∼88–91% of the divalent metal sites occupied with Mg2+ (Fo88–Fo91; Mg1.76Fe0.24SiO4 to Mg1.82Fe0.18SiO4),19–23 and the composition of the Gadikota et al.18 olivine is Fo87. The most rapid olivine carbonation occurs at ∼185–200 °C. (c.f., ref. 11 and 12) Indeed, the high-temperature datapoints of Sendula et al.12 (200 °C) and Gadikota et al.18 (185 °C) are lower than expected based on the calculated activation energies, consistent with this 185–200 °C temperature range being an inflection point for rate vs. temperature.
Plots of the Sendula et al.12 (Se21, 50–150 °C) and Gadikota et al.18 (Ga14, 90–150 °C) carbonation rates on Arrhenius plots (Fig. 1a and b) illustrate the linear relationships needed to calculate apparent activation energies. The linearity of the Arrhenius plots indicates that temperature is the dominant control, and other possible variations in chemical affinity and pressure12 (Fig. 1c) are negligible, at least for these far-from-equilibrium high-pressure carbonation studies. The olivine to magnesite activation energy values are “apparent” as they encompass contributions from all elementary reactions involved in the complex dissolution–precipitation processes. The calculations revealed the apparent activation energies of 89 ± 6 (Se21) and 85 ± 1 (Ga14) kJ mol−1. These newly-determined activation energies are consistent with the literature for magnesite precipitation in aqueous media (Table 1). This present analysis extended the temperature range of the Table 1 dataset down from 90 °C to 50 °C. Although the studies compiled in Table 1 span a range of aqueous-mediated processes, including olivine carbonation, hydromagnesite transformation, and step advancement on magnesite, all values are presented given the paucity of literature data. Our group at Pacific Northwest National Laboratory has also studied the influence of adsorbed water nanofilm thickness on the activation energy of forsterite to magnesite carbonation, demonstrating a linear relationship between reported monolayer H2O thickness and activation energy, from ∼34 to ∼130 kJ mol−1.24–26 Given the occurrence of multiphase CO2–H2O fluids, it is vital to understand the barriers to magnesite precipitation in aqueous media to predict and interpret experiments conducted in non-aqueous regimes (e.g., water films).
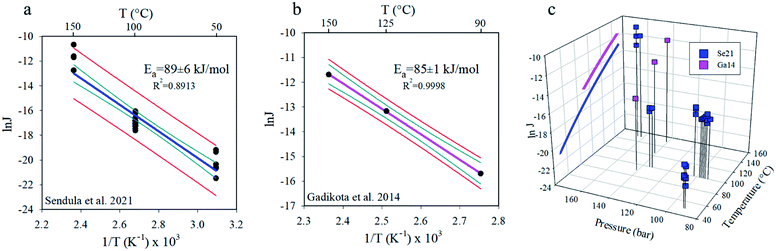 | ||
Fig. 1 Arrhenius plots using the carbonation rate results of (a) Sendula et al.12 (Se21) and (b) Gadikota et al.18 (Ga14), showing the variation of the natural logarithm of the olivine to magnesite transformation rates (J, mol m−2 s−1) as a function of 1000 times the reciprocal absolute temperature (T) of the experiments. Temperature (°C) is labelled on the upper x-axis for reference. The calculated apparent activation energies, coefficient of determination, and uncertainties are given next to the linear best fits. Red and dark cyan curves denote 95% prediction band and 95% confidence bands, respectively. In panel (c), the Arrhenius trends have both been plotted on the °C vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J plane, while the Sendula et al.12 and Gadikota et al.18 rates used to construct the Arrhenius plots are shown in the context of pressure and temperature conditions. The reference drop lines from the points to the P–T plane help clarify the 3D perspective. J plane, while the Sendula et al.12 and Gadikota et al.18 rates used to construct the Arrhenius plots are shown in the context of pressure and temperature conditions. The reference drop lines from the points to the P–T plane help clarify the 3D perspective. | ||
| Magnesite (MgCO3) precipitation apparent activation energies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activation energy (kJ mol−1) | Temperature (°C) | Ref. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Based on the solution-mediated transformation reaction of hydromagnesite [(Mg5(CO3)4(OH)2·4H2O)] to magnesite. Multiple Zhang et al.43 values are due to different fluid compositions, and multiple values for Di Lorenzo et al.42 were due to their use of two different kinetic models. b Arvidson and Mackenzie46 used the approach of Lippmann47 in conjunction with the 39.3 kJ mol−1 calcite (CaCO3) activation energy of Kazmierczak et al.48 to calculate their magnesite precipitation activation energy. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Present communication | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 89 ± 6 | 50–150 | This study, based on olivine carbonation kinetics reported by Sendula et al.12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 85 ± 1 | 90–150 | This study, based on Sendula et al.12 calculation of Gadikota et al.18 olivine to magnesite carbonation rates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Literature values | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 159 ± 17 | 90–100 | Saldi et al. 2009 (ref. 41) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 122.6 ± 20a | 120–180 | Di Lorenzo et al. 2014 (ref. 42) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 100a | 110–200 | Zhang et al. 2000 (ref. 43) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 93.3 ± 3.3a | 120–180 | Di Lorenzo et al. 2014 (ref. 42) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 85.1 ± 7.7 | 100–146 | Gautier et al. 2016 (ref. 44) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 81a | 110–200 | Zhang et al. 2000 (ref. 43) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 80.2 | 100–200 | Saldi et al. 2012 (ref. 45) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 92.9 ± 3.8b | 15–35b | Arvidson and Mackenzie 2000 (ref. 46) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In summary, this Communication presents two new robust activation energies for the olivine to magnesite carbonation reaction. These types of monomineralic studies are important for delineating controlling reaction mechanisms and kinetic interpretation of mafic-ultramafic rock carbonation studies (e.g. ref. 22, 27–35). Further insights from dynamic kinetic model36 and reactive force-field37,49 development, along with additional carbonation kinetics studies,12,16,38–40 are vital for clarifying the multiscale mechanisms and rates of silicate carbonation transformations. Our analysis provides a basis for focusing future work on key mechanistic and kinetic unknowns that could improve understanding of mineral transformation kinetics in the subsurface, including carbon storage in mafic–ultramafic rocks, and aid in the development of carbon dioxide removal and net negative-emissions technologies.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
QRSM was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences program at Pacific Northwest National Laboratory (PNNL). HTS was supported by the U.S. Department of Energy’s Carbon Storage Program and thanks Darin Damiani from DOE-HQ. HTS also acknowledges partial support from the Carbon Utilization and Storage Partnership (CUSP). We also thank the three anonymous reviewers for their close attention and helpful comments.References
- B. P. McGrail, F. A. Spane, E. C. Sullivan, D. H. Bacon and G. Hund, The Wallula Basalt Sequestration Pilot Project, Energy Procedia, 2011, 4, 5653–5660 CrossRef.
- B. P. McGrail, H. T. Schaef, A. M. Ho, Y.-J. Chien, J. J. Dooley and C. L. Davidson, Potential for carbon dioxide sequestration in flood basalts, J. Geophys. Res.: Solid Earth, 2006, 111, B12 CrossRef.
- H. T. Schaef, B. P. McGrail and A. T. Owen, Carbonate mineralization of volcanic province basalts, Int. J. Greenhouse Gas Control, 2010, 4, 249–261 CrossRef CAS.
- J. M. Matter and P. B. Kelemen, Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation, Nat. Geosci., 2009, 2, 837–841 CrossRef CAS.
- B. P. McGrail, H. T. Schaef, F. A. Spane, J. B. Cliff, O. Qafoku, J. A. Horner, C. J. Thompson, A. T. Owen and C. E. Sullivan, Field validation of supercritical CO2 reactivity with basalts, Environ. Sci. Technol. Lett., 2017, 4, 6–10 CrossRef CAS.
- J. M. Matter, M. Stute, S. Ó. Snæbjörnsdottir, E. H. Oelkers, S. R. Gislason, E. S. Aradottir, B. Sigfusson, I. Gunnarsson, H. Sigurdardottir, E. Gunnlaugsson, G. Axelsson, H. A. Alfredsson, D. Wolff-Boenisch, K. Mesfin, D. F. d. l. R. Taya, J. Hall, K. Dideriksen and W. S. Broecker, Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions, Science, 2016, 352, 1312–1314 CrossRef CAS PubMed.
- B. M. Tutolo, A. Awolayo and C. Brown, Alkalinity Generation Constraints on Basalt Carbonation for Carbon Dioxide Removal at the Gigaton-per-Year Scale, Environ. Sci. Technol., 2021, 55, 11906–11915 CrossRef CAS PubMed.
- S. K. White, F. A. Spane, H. T. Schaef, Q. R. S. Miller, M. D. White, J. A. Horner and B. P. McGrail, Quantification of CO2 Mineralization at the Wallula Basalt Pilot Project, Environ. Sci. Technol., 2020, 54, 14609–14616 CrossRef CAS PubMed.
- E. H. Oelkers, J. Declercq, G. D. Saldi, S. R. Gislason and J. Schott, Olivine dissolution rates: A critical review, Chem. Geol., 2018, 500, 1–19 CrossRef CAS.
- J. D. Rimstidt, S. L. Brantley and A. A. Olsen, Systematic review of forsterite dissolution rate data, Geochim. Cosmochim. Acta, 2012, 99, 159–178 CrossRef CAS.
- Q. R. S. Miller, H. T. Schaef, J. P. Kaszuba, G. Gadikota, B. P. McGrail and K. M. Rosso, Quantitative Review of Olivine Carbonation Kinetics: Reactivity Trends, Mechanistic Insights, and Research Frontiers, Environ. Sci. Technol. Lett., 2019, 6, 431–442 CrossRef CAS.
- E. Sendula, H. M. Lamadrid, J. D. Rimstidt, M. Steele-MacInnis, D. M. Sublett, L. E. Aradi, C. Szabó, M. J. Caddick, Z. Zajacz and R. J. Bodnar, Synthetic Fluid Inclusions XXIV. In situ Monitoring of the Carbonation of Olivine Under Conditions Relevant to Carbon Capture and Storage Using Synthetic Fluid Inclusion Micro-Reactors: Determination of Reaction Rates, Front. Clim., 2021, 3, 722447 CrossRef.
- H. M. Lamadrid, Z. Zajacz, F. Klein and R. J. Bodnar, Synthetic fluid inclusions XXIII. Effect of temperature and fluid composition on rates of serpentinization of olivine, Geochim. Cosmochim. Acta, 2021, 292, 285–308 CrossRef CAS.
- M. Avrami, Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei, J. Chem. Phys., 1940, 8, 212–224 CrossRef CAS.
- H. M. Lamadrid, J. D. Rimstidt, E. M. Schwarzenbach, F. Klein, S. Ulrich, A. Dolocan and R. J. Bodnar, Effect of water activity on rates of serpentinization of olivine, Nat. Commun., 2017, 8, 16107 CrossRef CAS PubMed.
- E. Sendula, H. M. Lamadrid and R. J. Bodnar, Reaction Rates Of Olivine Carbonation-An Experimental Study Using Synthetic Fluid Inclusions As Micro-Reactors, AGU Fall Meeting Abstracts, 2017, 2017AGUFMOS2053D1243S, https://ui.adsabs.harvard.edu/abs/2017AGUFMOS2053D1243S/abstract Search PubMed.
- J. D. Rimstidt, Geochemical Rate Models: An Introduction to Geochemical Kinetics, Cambridge University Press, 2013 Search PubMed.
- G. Gadikota, J. Matter, P. Kelemen and A.-h. A. Park, Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3, Phys. Chem. Chem. Phys., 2014, 16, 4679–4693 RSC.
- H. Ueda, Y. Sawaki and S. Maruyama, Reactions between olivine and CO2-rich seawater at 300 °C: Implications for H2 generation and CO2 sequestration on the early Earth, Geosci. Front., 2017, 8, 387–396 CrossRef CAS.
- F. Klein and T. M. McCollom, From serpentinization to carbonation: New insights from a CO2 injection experiment, Earth Planet. Sci. Lett., 2013, 379, 137–145 CrossRef CAS.
- Y. Sekine, T. Shibuya, F. Postberg, H. W. Hsu, K. Suzuki, Y. Masaki, T. Kuwatani, M. Mori, P. K. Hong, M. Yoshizaki, S. Tachibana and S. Sirono, High-temperature water-rock interactions and hydrothermal environments in the chondrite-like core of Enceladus, Nat. Commun., 2015, 6, 8604 CrossRef CAS PubMed.
- O. Sissmann, F. Brunet, I. Martinez, F. Guyot, A. Verlaguet, Y. Pinquier and D. Daval, Enhanced Olivine Carbonation within a Basalt as Compared to Single-Phase Experiments: Reevaluating the Potential of CO2 Mineral Sequestration, Environ. Sci. Technol., 2014, 48, 5512–5519 CrossRef CAS PubMed.
- R. Lafay, G. Montes-Hernandez, E. Janots, R. Chiriac, N. Findling and F. Toche, Simultaneous precipitation of magnesite and lizardite from hydrothermal alteration of olivine under high-carbonate alkalinity, Chem. Geol., 2014, 368, 63–75 CrossRef CAS.
- Q. R. S. Miller, J. P. Kaszuba, H. T. Schaef, M. E. Bowden, B. P. McGrail and K. M. Rosso, Anomalously low activation energy of nanoconfined MgCO3 precipitation, Chem. Commun., 2019, 55, 6835–6837 RSC.
- Q. R. S. Miller, J. P. Kaszuba, S. N. Kerisit, H. T. Schaef, M. E. Bowden, B. P. McGrail and K. M. Rosso, Emerging investigator series: ion diffusivities in nanoconfined interfacial water films contribute to mineral carbonation thresholds, Environ. Sci.: Nano, 2020, 7, 1068–1081 RSC.
- S. N. Kerisit, S. T. Mergelsberg, C. J. Thompson, S. K. White and J. S. Loring, Thin Water Films Enable Low-Temperature Magnesite Growth Under Conditions Relevant to Geologic Carbon Sequestration, Environ. Sci. Technol., 2021, 55, 12539–12548 CrossRef CAS PubMed.
- J. Hovelmann, H. Austrheim and B. Jamtveit, Microstructure and porosity evolution during experimental carbonation of a natural peridotite, Chem. Geol., 2012, 334, 254–265 CrossRef CAS.
- A. P. Gysi and A. Stefánsson, CO2-water–basalt interaction. Low temperature experiments and implications for CO2 sequestration into basalts, Geochim. Cosmochim. Acta, 2012, 81, 129–152 CrossRef CAS.
- R. J. Rosenbauer, B. Thomas, J. L. Bischoff and J. Palandri, Carbon sequestration via reaction with basaltic rocks: Geochemical modeling and experimental results, Geochim. Cosmochim. Acta, 2012, 89, 116–133 CrossRef CAS.
- H. Ueda, T. Shibuya, Y. Sawaki, M. Saitoh, K. Takai and S. Maruyama, Reactions between komatiite and CO2-rich seawater at 250 and 350 °C, 500 bars: implications for hydrogen generation in the Hadean seafloor hydrothermal system, Prog. Earth Planet. Sci., 2016, 3, 35 CrossRef.
- T. Shibuya, M. Yoshizaki, Y. Masaki, K. Suzuki, K. Takai and M. J. Russell, Reactions between basalt and CO2-rich seawater at 250 and 350 °C, 500 bars: Implications for the CO2 sequestration into the modern oceanic crust and the composition of hydrothermal vent fluid in the CO2-rich early ocean, Chem. Geol., 2013, 359, 1–9 CrossRef CAS.
- N. G. Grozeva, F. Klein, J. S. Seewald and S. P. Sylva, Experimental study of carbonate formation in oceanic peridotite, Geochim. Cosmochim. Acta, 2017, 199, 264–286 CrossRef CAS.
- A. J. Luhmann, B. M. Tutolo, B. C. Bagley, D. F. Mildner, P. P. Scheuermann, J. M. Feinberg, K. Ignatyev and W. E. Seyfried Jr, Chemical and physical changes during seawater flow through intact dunite cores: An experimental study at 150–200 °C, Geochim. Cosmochim. Acta, 2017, 214, 86–114 CrossRef CAS.
- A. J. Luhmann, B. M. Tutolo, B. C. Bagley, D. F. Mildner, W. E. Seyfried and M. O. Saar, Permeability, porosity, and mineral surface area changes in basalt cores induced by reactive transport of CO2-rich brine, Water Resour. Res., 2017, 53, 1908–1927 CrossRef.
- A. J. Luhmann, B. M. Tutolo, C. Y. Tan, B. M. Moskowitz, M. O. Saar and W. E. Seyfried, Whole rock basalt alteration from CO2-rich brine during flow-through experiments at 150 °C and 150 bar, Chem. Geol., 2017, 453, 92–110 CrossRef CAS.
- A. M. Bremen, T. Ploch, A. Mhamdi and A. Mitsos, A mechanistic model of direct forsterite carbonation, Chem. Eng. J., 2021, 404, 126480 CrossRef CAS.
- S. Zare and M. J. A. Qomi, Reactive force fields for aqueous and interfacial magnesium carbonate formation, Phys. Chem. Chem. Phys., 2021, 23, 23106–23123 RSC.
- F. Wang, D. Dreisinger, M. Jarvis and T. Hitchins, Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration, Miner. Eng., 2019, 131, 185–197 CrossRef CAS.
- F. Wang, D. Dreisinger, M. Jarvis and T. Hitchins, Kinetic evaluation of mineral carbonation of natural silicate samples, Chem. Eng. J., 2021, 404, 126522 CrossRef CAS.
- F. Wang, D. Dreisinger, M. Jarvis, T. Hitchins and D. Dyson, Quantifying kinetics of mineralization of carbon dioxide by olivine under moderate conditions, Chem. Eng. J., 2019, 360, 452–463 CrossRef CAS.
- G. D. Saldi, G. Jordan, J. Schott and E. H. Oelkers, Magnesite growth rates as a function of temperature and saturation state, Geochim. Cosmochim. Acta, 2009, 73, 5646–5657 CrossRef CAS.
- F. Di Lorenzo, R. M. Rodriguez-Galan and M. Prieto, Kinetics of the solvent-mediated transformation of hydromagnesite into magnesite at different temperatures, Mines Mag., 2014, 78, 1363–1372 CrossRef.
- P. Zhang, H. L. Anderson, J. W. Kelly, J. L. Krumhansl and H. W. Papenguth, Kinetics and mechanisms of formation of magnesite from hydromagnesite in brine, Technical Report SAN099-19465, Sandia National Labs., Albuquerque, NM (US), 2000 Search PubMed.
- Q. Gautier, P. Benezeth and J. Schott, Magnesite growth inhibition by organic ligands: An experimental study at 100, 120 and 146 °C, Geochim. Cosmochim. Acta, 2016, 181, 101–125 CrossRef CAS.
- G. D. Saldi, J. Schott, O. S. Pokrovsky, Q. Gautier and E. H. Oelkers, An experimental study of magnesite precipitation rates at neutral to alkaline conditions and 100-200 °C as a function of pH, aqueous solution composition and chemical affinity, Geochim. Cosmochim. Acta, 2012, 83, 93–109 CrossRef CAS.
- R. S. Arvidson and F. T. Mackenzie, Temperature dependence of mineral precipitation rates along the CaCO3-MgCO3 join, Aquat. Geochem., 2000, 6, 249–256 CrossRef CAS.
- F. Lippmann, Sedimentary Carbonate Minerals, Springer Berlin Heidelberg, Berlin, Heidelberg, 1973 Search PubMed.
- T. F. Kazmierczak, M. B. Tomson and G. H. Nancollas, Crystal growth of calcium carbonate. A controlled composition kinetic study, J. Phys. Chem., 1982, 86, 103–107 CrossRef CAS.
- S. Zare, Formation and Dissolution of Surface Metal Carbonate Complexes: Implications for Interfacial Carbon Mineralization in Metal Silicates, J. Phys. Chem. C, 2022 DOI:10.1021/acs.jpcc.2c02981.
| This journal is © The Royal Society of Chemistry 2022 |
