 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environmental persistence, detection, and mitigation of endocrine disrupting contaminants in wastewater treatment plants – a review with a focus on tertiary treatment technologies
Jesús Alfredo
Rodríguez-Hernández
a,
Rafael G.
Araújo
ab,
Itzel Y.
López-Pacheco
ab,
Laura Isabel
Rodas-Zuluaga
a,
Reyna Berenice
González-González
ab,
Lizeth
Parra-Arroyo
 ab,
Juan Eduardo
Sosa-Hernández
ab,
Elda M.
Melchor-Martínez
ab,
Manuel
Martínez-Ruiz
ab,
Damià
Barceló
cde,
Lorenzo M.
Pastrana
f,
Hafiz M. N.
Iqbal
ab,
Juan Eduardo
Sosa-Hernández
ab,
Elda M.
Melchor-Martínez
ab,
Manuel
Martínez-Ruiz
ab,
Damià
Barceló
cde,
Lorenzo M.
Pastrana
f,
Hafiz M. N.
Iqbal
 *ab and
Roberto
Parra-Saldívar
*ab and
Roberto
Parra-Saldívar
 *ab
*ab
aTecnologico de Monterrey, School of Engineering and Sciences, Monterrey, 64849, Mexico. E-mail: hafiz.iqbal@tec.mx; r.parra@tec.mx
bInstitute of Advanced Materials for Sustainable Manufacturing, Tecnologico de Monterrey, Monterrey, Mexico 64849
cDepartment of Environmental Chemistry, Institute of Environmental Assessment and Water Research, IDAEA-CSIC, Jordi Girona, 18-26, 08034 Barcelona, Spain
dCatalan Institute for Water Research (ICRA-CERCA), Parc Científic i Tecnològic de la Universitat de Girona, c/Emili Grahit, 101, Edifici H2O, 17003 Girona, Spain
eSustainability Cluster, School of Engineering, University of Petroleum and Energy Studies (UPES), Dehradun, 248007, Uttarakhand, India
fInternational Iberian Nanotechnology Laboratory, Av. Mestre José Veiga s/n, 4715-330, Braga, Portugal
First published on 13th October 2022
Abstract
Endocrine disrupting chemicals are a group of contaminants that have severe effects on humans and animals when exposed, like cancer and alterations to the nervous and reproductive systems. The increasing concentrations of several endocrine disrupting chemicals in the environment are strongly related to anthropogenic activities, and as the population grows this problem becomes more relevant. Thus, wastewater is one of the main sources of endocrine disrupting chemicals, and the technologies employed during primary and secondary treatment in wastewater treatment plants cannot remove these contaminants. Due to this, researchers have tried to develop more efficient technologies for tertiary treatment of wastewater and reduce the concentration of endocrine disrupting chemicals discharged into the environment. Some of the most promising technologies include adsorption, ultrafiltration, advanced oxidation processes and biodegradation. The use of nanomaterials as adsorbents, catalysts, membranes and supports has played a key role in enhancing the efficiency of these technologies. The results showed that these technologies have great potential on the lab-scale, and even some of them have already been employed at some wastewater treatment plants. However, there are still some challenges to achieving a global implementation of these technologies, related to reducing the costs of materials and enhancing their current performance. The use of biomass/waste derived carbon materials and implementing hybrid technologies are accessible approaches for their implementation in tertiary treatment.
Environmental significanceEnvironmental pollution is a critical issue that requires proper measures for a sustainable environmental health. The current literature heavily reports on the environmental persistence, detection, and mitigation of endocrine disrupting contaminants in wastewater treatment plants. However, there is no detailed review available on tertiary treatment technologies that support the sustainable mitigation of emerging pollutants from water matrices. Herein, an effort has been made to cover this notable literature gap by stressing the efficient abatement of hazardous substances from environmental matrices. |
1. Introduction
The accentuated population growth and the consequent industrial development to comply with human needs have led to serious contamination of water.1 Currently, about 1.2 billion people consume contaminated water.2 According to the United Nations in 2025, 2.7 billion people will not have access to potable water.3 Water pollution is an issue of global importance due to the increasing awareness of innumerable pollutants, such as organic and inorganic compounds, which reduce the amount of drinking water as the demand rises.4 The discharge of millions of liters of water derived from basic human activities (flushing toilets, washing clothes and dishes, and bathing), as well as industrial and hospital activities, into sewer systems generates a very complex matrix that may include diverse emerging contaminants. Most wastewater treatment plants (WWTPs) are not designed to treat contaminants like endocrine disrupting chemicals (EDCs). Due to this, WWTP effluents that are discharged into the environment could have potentially hazardous concentrations of EDCs.5,6 EDCs are natural or artificial chemical compounds that affect the proper functioning of the endocrine system in humans as well as animals. They can include hormones (industrial chemicals, veterinary drugs, pesticides, and food additives) as well as compounds that block, interfere, or mimic endogenous hormones, such as certain pharmaceuticals. EDCs alter important physiological activities such as reproduction, growth, and development.7–9Some pharmaceuticals, like sulfamethoxazole (SMX) and carbamazepine, among others, are reported to be EDCs and can enter water systems after excretion by humans and animals. This generates large-scale contamination and consequently enters the food chain through agricultural crops later accumulating in adipose tissues of animals and humans, generating a public health problem of high concern. Foods of animal origin (meat, milk, eggs, etc.) have a greater risk of containing EDCs as they can be derived from the consumption of contaminated food and water, and farm animals sometimes are fed with illegal levels of hormones to increase their growth.8 The monitoring of EDCs is highly important as they are bioaccumulative compounds and the body's elimination of these compounds is very slow, which leads to their continuous bioaccumulation resulting in many alterations in metabolism, with the negative effects sometimes being difficult to determine, but some of them include a higher risk of cancer, alterations to the reproductive system, and interference in the immune and nervous system function.10–12
Nonylphenols, bisphenol A (BPA), as well as the degradation products of alkylphenol ethoxylate, and steroidal estrogens are some of the main EDCs that have been detected in wastewater all over the world. Effective treatments that can reduce or eliminate EDCs are important to reduce the biological risks to human health as well as the threat posed to different ecosystems.13 Different technologies for the efficient elimination of EDCs have been studied extensively (tertiary treatment). However, in most WWTPs secondary treatment, normally consisting of trickling filter treatment and activated sludge, is the last step, which is insufficient and fails at eliminating EDCs. This can cause further complications; for example EDCs derived from incomplete degradation can increase estrogenic activity and produce derivative compounds such as octylphenol and nonylphenol.13
This review focuses on the occurrence and transport of EDCs in water and consequently the long-term problems in society and the environment, focusing on the latest detection and treatment developments, conducting an extensive revision of bio-based technologies for tertiary treatment in WWTPs for the removal, degradation and inactivation of EDCs and ensuring the safe reuse of treated wastewater.
2. EDC sources, occurrence and transport in water, soils, and foods
Although the transportation and accumulation routes in the environment of EDCs are complex, studies have shown that most of them are closely related to human activities. Industrial and WWTP effluents and intensively cultivated agricultural soils are the main sources of contamination with EDCs of water systems.14,15 The cycle of contamination begins when they enter water bodies (surface and underground) and soil (Fig. 1).16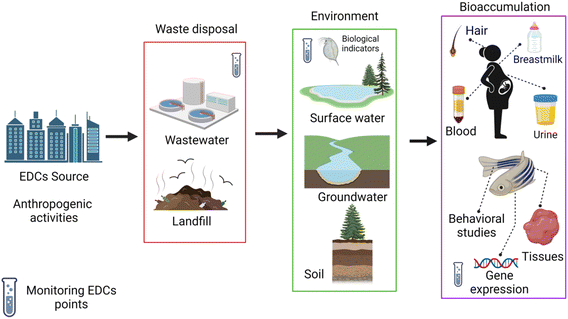 | ||
| Fig. 1 EDC migration pathway of contamination and proposed sample points for EDC monitoring. Created with https://BioRender.com. | ||
For example, natural and synthetic hormones that are not metabolized are excreted through urine or feces, and thus, sewage transports contaminated wastewater. Other anthropogenic sources are found in consumer products such as bottles and containers of plastic as well as metal, personal care products (PCPs), detergents, agricultural products (pesticides), food production and other industrial activities (Table 1).17 Humans can be exposed directly to EDCs by using containers and/or PCPs that contain these chemicals. Also, an indirect path of exposure is followed when these products end their life cycle and are discarded into landfills. Weathering causes the contained EDCs to be released into the environment and could infiltrate soil and groundwater. When animals or plants come in contact with contaminated soils or water, the bioaccumulation of EDCs may occur, and this creates another pathway of exposure through the ingestion of contaminated food.
| EDC | Source/use | IC50 (μM) | LOEC (μg L−1) | Ref. |
|---|---|---|---|---|
| a nM. b mg L−1. c μM. | ||||
| Triclosan | Personal care products | 90a | 0.66 | 147–149 |
| Triclocarban | Personal care products | 16 | 0.28 | 147, 148 and 150 |
| BPA | Plastics | 5 | 500 | 151–153 |
| BPB | Plastics | 0.93 | 0.1b | 152, 154 and 155 |
| BPC | Plastics | 46a | 15c | 152, 156 and 157 |
| Diclofenac | Pharmaceutical | 30.7a | 1 | 158–160 |
| Diethyl phthalate | Plastics | 7.9 | — | 161 and 162 |
| Caffeine | Pharmaceutical/food industry | 500 | 1 | 158, 163 and 164 |
| Chloroacetonitrile | Disinfection products | — | 1.60b | 165 and 166 |
| Tetrabromobisphenol A | Flame retardant | 0.7 | 0.3c | 167–169 |
| TBP | Flame retardant | 4 | — | 170–172 |
| Glyphosate | Pesticide | 30b | 1.7b | 173–175 |
| Fipronil | Insecticide | 1.6 | 0.32 | 176–178 |
| Fenoxycarb | Insecticide | 3 | 27.7 | 179–181 |
There are regulations for these pollutants in most countries; however, it has been determined that limits typically exceed recommended concentrations.18 Some examples of EDCs found in water samples are expressed in Table 2. Even though several EDCs, their effects and promising degradation alternatives have been studied, changes in our consumption patterns, the development of new products and technology patterns could lead to unknown EDCs being discharged into the environment.
| Sample | EDC | Concentration (ng L−1) | Country | Ref. |
|---|---|---|---|---|
| Sea water | BPS | 11.3 | Romania | 182 |
| BPF | 19.7 | |||
| BPA | 416 | |||
| Estuarine water | Methylparaben | 47 | China | 183 |
| Triclocarban | 117 | |||
| Triclosan | 122 | |||
| Ortho-phenylphenol | 141 | |||
| Surface water | 4-Tert-octylphenol | 1000 | China | 184 |
| Atrazine | 8.22 | Italy | 185 | |
| Nonylphenol | 5545 | China | 184 | |
| Nonylphenol | 36.6 | Serbia | 186 | |
| BPA | 233 | China | 184 | |
| BPA | 2180 | China | 187 | |
| BPA | 800 | Czech Republic | 188 | |
| BPA | 105.7 | Serbia | 186 | |
| BPAF | 205 | Czech Republic | 188 | |
| BPAF | 2.58 | China | 187 | |
| BPS | 133 | |||
| Caffeine | 3500 | 189 | ||
| DEHP | 1094 | 184 | ||
| Triclosan | 105 | 190 | ||
| WWTP effluent | BPA | 3 | Mexico | 191 |
| 4-Nonylphenol | 2.3 | |||
| 4-Tert-octylphenol | 5 | |||
| 17β-estradiol | 9.5 | |||
| 17α-Ethinyl estradiol | 26.8 | |||
| Swimming pool | BPA | 23.2 | China | 192 |
| EE2 | 78.8 | |||
| 4-Tert-octylphenol | 2.8 | |||
| Caffeine | 39 | |||
| Tributyl phosphate | 27 | Australia | 193 |
3. Environmental and health effects of EDCs
The presence of EDCs in the environment is a global concern because of the effects on wildlife as well as on animals for human consumption. Some EDCs are not only bioaccumulated but also biotransformed; for example, tetrabromobisphenol A exposure promoted gametogenesis and altered sex hormone levels in mussels, altering their life cycle and population dispersal.19 Also, EDC exposure alters neurodevelopment and behavior in living organisms, reducing growth in juvenile organisms, and disrupting some metabolic functions of organs like the liver (disrupts the transcript abundance of liver receptors and leptin) as in the case of Zebrafish exposed to venlafaxine.20 Also, EDCs can affect the offspring of population exposed to these pollutants, where the offspring of zebrafish exposed to flutolanil is affected in its development (gonad endocrine disruption, decreased reproduction and short body length) and its mortality rate is increased.21 Some EDCs pose a higher risk to some species over others; for example, estradiol, methyl triclosan and triclosan (TCS) showed an elevated risk for surface water aquatic species.22 Therefore, it is necessary to conduct punctual and specific EDC monitoring, to act in a timely manner and understand the consequences of their contamination in the environment.Some metals such as aluminum and manganese can act like EDCs, and the accumulation of these metals reduces relative fecundity in Astyanax altiparanae, and this accumulation in some cases is accelerated due to the availability of metals which causes the acidification of aquatic environments, making these metals more available for organisms than in alkaline environments.23 Therefore, the monitoring and removal of some metals must be performed not only at WWTPs but also in environments with high levels of these metals. Another exposure pathway to EDCs is through PCPs, as they are disposed through wastewater, sanitary landfills and other disposal ways that do not have enough treatment processes for their effective removal, leading to the discharge of EDCs in water and soil.
Furthermore, human exposure to EDCs is due to not only contact with contaminated environments but also their consumption of food and drinking water (Table 5). Fish consumption is a route of EDC exposure, di (2-ethylhexyl) phthalate bioaccumulated in Epinephelus coioides and Platycephalus indicus (widely consumed fish species from Persian Gulf), especially in liver tissue. Because of this, consuming at least two fish-based meals per week may result in a moderate health risks.24 The EDC bioaccumulation by fish occurs not only due to their presence in biological tissue but also due to the ingestion of plastics and microplastics that also act as vectors of EDCs in aquatic ecosystems mainly by sorption processes.25,26
| Nanomaterial | EDC | Degradation strategy | Degradation efficiency (%) | Time of reaction (min) | Ref. |
|---|---|---|---|---|---|
| CQDs/BiOBr nanosheets | BPA | Photocatalysis | ∼55 | 180 | 194 |
| CQDs/BiOBr | BPA | Photocatalysis | 73 | 150 | 195 |
| CQDs/polymer carbon nitride | BPA | Photocatalysis-PMS activation | 95 | 30 | 105 |
| N-CQD/SiO2 | BPF | Photocatalysis-PDS activation | 14.3 | 120 | 196 |
| N-CQD/CeZrO2 | BPF | Photocatalysis-PDS activation | 34.9 | 120 | 196 |
| N-CQD/Al2O3 | BPF | Photocatalysis-PDS activation | 81.5 | 120 | 196 |
| CQDs/Bi2WO6 | BPA | Photocatalysis | ∼55 | 120 | 197 |
| CQDs/Bi2MoO6 | BPA | Photocatalysis | 54 | 120 | 198 |
| CNQDs | BPA | Photocatalysis | ∼100 | 80 | 199 |
| EDCs | Organism | Efficiency (%) | Time | Concentration | Ref. |
|---|---|---|---|---|---|
| BPA | Pleurotus ostreatus and Pleurotus pulmonarius | 100 | 1 h | 100 mg L−1 | 200 |
| BPA | Pleurotus ostreatus and Pleurotus pulmonarius | 85 | 1 h | 200 mg L−1 | 201 |
| Nonylphenol | Ganoderma lucidum | 80 | 50 min | 5 ppm | 202 |
| Triclosan | Ganoderma lucidum | 45 | 50 min | 5 ppm | |
| BPA | Pycnoporus sanguineus (CS43) and Trametes versicolor (commercial laccase) | 100 | <24 h | 20 mg L−1 | 137 |
| Carbaryl | Pseudomonas putida KT2441 | 100 | <30 h | 50 mg L−1 | 203 |
| Carbofuran | Pseudomonas putida KT2440 | 100 | <30 h | 50 mg L−1 | 204 |
| Chloropyrifos | Pseudomonas putida KT2444 | 100 | <30 h | 50 mg L−1 | 205 |
| Di (2-ethyl hexyl) phthalate | Pleurotus ostreatus | 100 | 504 h | 206 | |
| Fanpropathrin | Pseudomonas putida KT2443 | 100 | <30 h | 50 mg L−1 | 207 |
| Parathion | Pseudomonas putida KT2442 | 100 | <30 h | 50 mg L−1 | 208 |
| Carbofuran | Cupriavidus sp. ISTL7 | 98 | 96 h | 400 ppm | 209 |
| Congo red | Oudemansiella canarii | 80 | <24 h | 50 mg L−1 | 210 |
| 17α-Ethynylestradiol | Water-sediment microcosms (bacteria from Proteobacteria, Actinobacteri, Acidobacteria, Chloroflexi, Nitrospirae, and Bacteroidetes phyla) | 44–99 | 60 days | 2 μg g−1 | 211 |
| Estrone | Haematococcus pluvialis | 97 | 40 days | 5 mg L−1 | 212 |
| Estrone | Selenastrum capricornutum | 80 | 40 days | 5 mg L−1 | |
| Estrone | Scenedesmus quadricauda | 97 | 40 days | 5 mg L−1 | |
| 17β-Estradiol | Haematococcus pluvialis | 100 | 40 days | 5 mg L−1 | |
| 17β-Estradiol | Selenastrum capricornutum | 100 | 40 days | 5 mg L−1 | |
| 17β-Estradiol | Scenedesmus quadricauda | 100 | 40 days | 5 mg L−1 | |
| 17β-Estradiol | Chlorella vulgaris | 100 | 40 days | 5 mg L−1 | |
| 17α-Ethynylestradiol | Haematococcus pluvialis | >85 | 40 days | 5 mg L−1 | |
| 17α-Ethynylestradiol | Scenedesmus quadricauda | >85 | 40 days | 5 mg L−1 | |
| 17α-Ethynylestradiol | Microbial community (Proteobacteria, Firmicutes, and Nitrospirae) | 15–90 | 70 days | 50 μg L−1 | 213 |
| 17α-Ethynylestradiol | Lentinula edodes | 100 | 21 days | 200 μg | 214 |
| Testosterone | Lentinula edodes | 100 | 21 days | 50 μg |
| Sample | EDC | Concentration (ng g−1) | Country | Ref. |
|---|---|---|---|---|
| a ng/L. | ||||
| Drinking water | DDT | 0.275a | India | 215 |
| Drinking water | γ-HCH | 55.7a | India | 215 |
| Drinking water | Nonylphenol | 7.9a | Serbia | 186 |
| Drinking water | BPA | 35.6a | Serbia | 186 |
| Drinking water | BPA | 57a | Spain | 216 |
| Drinking water | Methylparaben | 119a | Spain | 216 |
| Drinking water | Caffeine | 5.2a | Italy | 217 |
| Drinking water | Naproxen | 278a | Spain | 216 |
| Apple | DDE | 0.023 | India | 215 |
| Apple | γ-HCH | 0.44 | India | 215 |
| Tomato | DDT | 2.34 | India | 215 |
| Tomato | γ-HCH | 0.77 | India | 215 |
| Wheat | DDT | 23.2 | India | 215 |
| Wheat | γ-HCH | 3.16 | India | 215 |
| Rice | DDE | 1.46 | India | 215 |
| Rice | γ-HCH | 1.59 | India | 215 |
| Canned tuna | BPA | 69.1 | European Union | 218 |
| Plaice small | Triclosan | 142.4 | European Union | 218 |
| Mussels | BPA | 54.7 | European Union | 218 |
| Mussels | Triclosan | 42.26 | European Union | 218 |
| Mussels | BDE100 | 0.22 | European Union | 219 |
| Mussels | Methylparaben | 0.080 | European Union | 219 |
| Mullet | Carbamazepine | 1.1 | Brazil | 220 |
Therefore, EDCs are consumed by humans causing alterations in the endocrine system that can affect the life quality of populations. For example, prenatal exposure to some EDCs (organochlorines and glycol ethers), directly or indirectly, may increase the risk of hypospadias. Also, prenatal exposure to phenols is associated with an increased risk of cryptorchidism.27 A continuum mapping methodology was employed to determine the concentration of EDCs in maternal blood samples. It was found that higher levels of PBDEs in maternal blood were associated with a lower birth weight and higher levels of PCBs, and PFAS were associated with increased birth weight, demonstrating that exposure to these pollutants already has a negative effect on the health of the population.28 It is important to know that postnatal children's EDC exposure can cause neurodevelopment effects, and these effects are related to the duration of exposure as well as the type of EDC.29 With respect to the importance of the stage of development where the exposure exists, the effects of EDC exposure during puberty are increases in anxious behavior and a reduction in social interaction.30 Additionally, phthalate metabolites were found in the adult population. They include bisphenols and pesticides in urine and hair samples, demonstrating that there is a bioaccumulation of EDCs in humans. EDCs are especially found in hair samples, illustrating chronic exposure.31
EDCs from pharmaceutical sources and their metabolites affect biota as well as humans. Such compounds including levonorgestrel (contraceptive) were found in WWTP effluents and in aquatic environments. It was found that low concentrations of levonorgestrel and its fractions caused a reduction in pregnancy maintenance in some aquatic species and humans.32 Also, it was found that alkylphenol (nonylphenol and oxylphenol) and perfluoroalkyl substance exposure in women is associated with endometrial (the most common gynecological cancer in the United States) and breast cancer development.33,34 Exposure can also aggravate some diseases such as COVID-19, where some biological pathways may be dysregulated by EDCs and function as contributors to COVID-19 severity, additionally increasing the side effects of the infection.35 To take care of population health and the environment, protocols for the removal and treatment of EDCs currently found in the world should be implemented as well as promoting new methods of waste treatment, to prevent the cycle of this contamination from continuing to occur.
4. Endocrine disruptors: methods of analysis and detection by biosensors
EDC concentrations in wastewater are low, on the order of ng L−1 or μg L−1 and the most used techniques for their detection and quantification EDCs are the traditional analysis techniques such as high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS), ultra-performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS) and gas chromatography time-of-flight mass spectrometry (GC-TOF-MS).36,37 These techniques have a high sensitivity of detection and reproducibility, but require many steps of sample preparation, large sample volumes, long times of analysis, expensive consumables, and equipment. Electrochemical sensors have been reported as a new and rapid application procedure for the detection and screening of EDCs and other toxic compounds in wastewater, with very low detection limits, high sensitivity and low cost, Fig. 2.37,38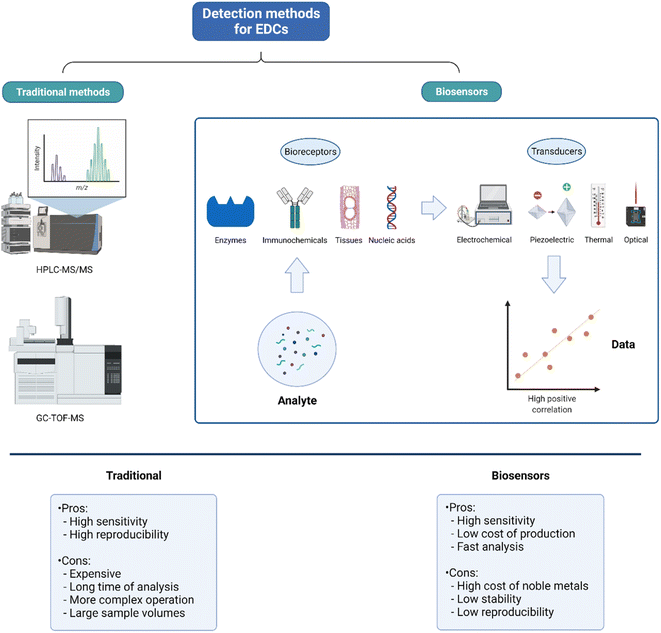 | ||
| Fig. 2 Comparative between EDC detection methods, and a diagram of how biosensors operate. Created with https://BioRender.com. | ||
Biosensors have been developed using cells, specific molecules, aptamers, antibodies, DNA, and model organisms, among others, and these sensors have been applied for the detection of different EDCs. Transducer sensors have been developed based on carbon nanomaterials such as graphene oxide (GO) and carbon nanotubes, metal oxide nanomaterials, noble metal nanomaterials, polymers and other materials.8,39,40 In a study, the authors developed a method for the electrochemical detection, quantification, and removal of BPA through low-cost carbon felt with a detection limit of 4.78 × 10−4 μM.41 The detection of BPA in real water samples demonstrated a sensitivity between 98.4 and 101%, and the sensor was reusable. An electrochemical sensor developed with covalent-organic frameworks (COFs), named CTpPa-2, using galvanostatic charge and discharge, differential, and cyclic pulse voltammetry, allowed the detection of BPA and bisphenol S (BPS) with detection limits of 0.02 and 0.09 μM, respectively.42 Most of the studies on the development of biosensors for the detection of EDCs focus on the detection of BPA and analogs, and some estrogens such as 17β-estradiol (E2).8,43
5. Fate and removal of endocrine disruptors in conventional WWTPs
The problem posed by EDCs in wastewater is the lack of specific degradation methods with high efficiencies. In developing countries most of the WWTPs are only equipped with basic treatment stages that cannot remove a wide range of contaminants such as EDCs.44,45 WWTPs typically consist of two stages: primary treatment and secondary treatment.46,47 However, some contaminants are not removed during those stages and tertiary treatment is required (Fig. 3).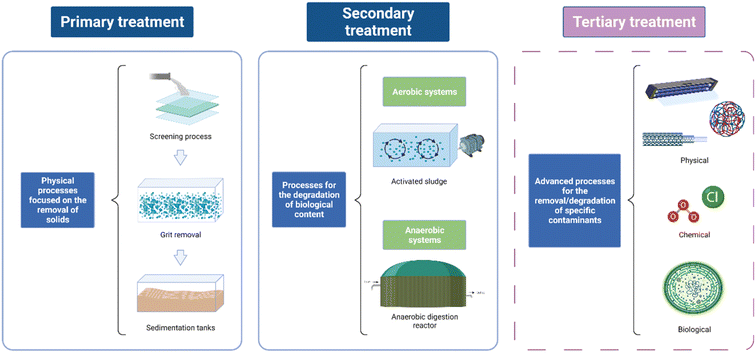 | ||
| Fig. 3 Schematic of WWTP stages and their role in water decontamination. Created with https://BioRender.com. | ||
During the first stage of treatment, physical processes are employed for the separation of solids from water. In traditional WWTPs, the primary treatment is composed of a screening process, grit removal and sedimentation. During the screening, solid waste is separated by using a device with uniform-sized openings that retain coarse materials. Grit chambers are used for the settling of sand and gravel to avoid further accumulation or damage to the mechanical instruments. Finally, during the sedimentation process, sedimentation tanks or primary settlers are employed to enable the organic suspended solids to settle as sludge.46,47
After primary treatment most pathogens and contaminants are still in the stream. Secondary treatment focuses on the degradation of the biological content, mainly by using aerobic and anaerobic treatment systems.47,48 The most common aerobic systems are trickling filters and activated sludge. In the trickling filters there is a fixed medium where microbes grow, forming biofilms that can consume the organic matter,49,50 while in the activated sludge process a high concentration of microorganisms clumped as small particles in suspension degrade the organic material.51–53 On the other hand, the anaerobic systems employed during the secondary treatment are based on anaerobic digestion. In this process, the organic matter is transformed into a mixture of biogas and sludge. The most common anaerobic treatments are an up-flow anaerobic sludge bed (UASB), expanded granular sludge bed (EGSB), and anaerobic fixed film reactor (AFFR).47
The concentration of organic pollutants in the effluents after these stages varies depending on several factors, such as the initial concentration and the susceptibility of each pollutant to be degraded by the employed techniques.54 However, in most cases the concentration of pollutants in WWTP effluents is within the range of ng L−1 to μg L−1. Nevertheless, for contaminants like EDCs even low concentrations might represent an environmental risk as most of them have potential for bioaccumulation.55 The tertiary treatment has been implemented for enhanced removal of the remaining contaminants in wastewater.
5.1 Tertiary treatment
WWTPs that include tertiary treatment are usually found in more developed countries and their aim is to ensure that wastewater has enough quality to be reused or to reduce the negative effects when it is discharged into the environment.56,57 This stage is focused on the removal of specific contaminants with advanced techniques, which are classified as chemical, physical, and biological processes.58 Ozonation, UV radiation, chlorination and reverse osmosis are among the most employed techniques in WWTPs.58 The selection of the treatment should be based on the characteristics of the target contaminant or contaminants. Although there are several technologies proven with high effectiveness on the lab scale, most of them are not yet employed in WWTPs, in most cases due to a significant reduction of their effectiveness when escalated or a high cost of operation. However, there are studies where different technologies for EDC removal were evaluated, and the results have shown that some of them could be implemented soon in the tertiary treatment for the removal of EDCs at WWTPs (Table 6).| Treatment method | Pollutant | Initial concentration | Removal efficiency (%) | Ref. |
|---|---|---|---|---|
| Adsorption with powdered activated carbon and coagulation | Bisphenol F | 1.5 ng L−1 | 95.8 | 78 |
| BPA | 1.74 ng L−1 | 99.2 | ||
| 2,4 dihydroxybenzophenone | <10 ng L−1 | >95.5 | ||
| Estrone | <2.75 ng L−1 | 96.4j | ||
| Triclosan | <50 ng L−1 | 63.7m | ||
| Ibuprofen | <0.984 ng L−1 | 99.9 | ||
| Carbamazepine | <0.0308 ng L−1 | 99.9 | ||
| Adsorption with powdered activated carbon and ultrafiltration | Carbamazepine | 169 ng L−1 | 60.2 | |
| Oxybenzone | <2.8 ng L−1 | 80.5g | ||
| Caffeine | <0.666 ng L−1 | 99.9e | ||
| Powdered activated carbon in a membrane bioreactor | Caffeine | 500 ng L−1 | >98 | 221 |
| Azithromycin | 500 ng L−1 | >99 | ||
| Ofloxacin | 500 ng L−1 | 86 | ||
| Naproxen | 500 ng L−1 | >99 | ||
| Theophylline | 500 ng L−1 | 99.9 | ||
| UV-solar/H2O2 | BPA | 25 mg L−1 | 77.40 | 222 |
| Tube-in-tube membrane microreactor for photochemical UVC/H2O2 oxidation systems | Oxytetracycline | 2 mg L−1 | 36 | 108 |
| Tube-in-tube membrane microreactor with a UVC/H2O2/TiO2 system | 17β-Estradiol | 100 μg L−1 | 48 | 109 |
| 17α-Ethinylestradiol | 101 μg L−1 | 30 | ||
| Fluidized bed reactor with stabilized laccase in porous silica | BPA | 25 mg L−1 | 90 | 134 |
| Microalgae-bacteria consortium in photobioreactors lit with a low intensity light emission diode | Sulfamethoxazole | 50 μg L−1 | 54.34 | 223 |
The selection of the technologies for EDC removal/degradation described henceforward was done based on the number of studies, where they were employed on the lab scale or pilot plants and their actual utilization in WWTPs. The studies described include evaluations in synthetic solutions, real wastewater samples, and spiked water and wastewater samples.
MOFs are usually reported as highly efficient adsorbents for a wide range of inorganic and organic contaminants, including diverse EDCs.62 For example, a study evaluated MIL-53 (Cr-based active cite MOF) for BPA removal from synthetic solutions. The authors reported a maximum adsorption capacity of 421 mg g−1 when the initial concentration of the prepared solutions was within a range of 30–80 ppm.63
MOFs based on aluminum are among the most employed for the adsorption of EDCs. For example, MIL-53 with an Al-based active site and a mesostructured MIL-53(Al) were also employed for the removal of BPA from synthetic solutions.64 The initial concentrations were between 25 and 250 mg L−1 and the maximum adsorption capacity was 325 mg g−1 for MIL-53(Al) and 465 mg g−1 for the mesostructured MIL-53(Al). Another Al-based MOF was employed for the adsorption of BPA, 17α-ethynylestradiol (EE2) and perfluorooctanoic acid (PFOA) from synthetic solutions.65 Furthermore, a competitive adsorption analysis was carried out to determine the selectivity of the adsorbent. According to the results, the maximum adsorption capacities at equilibrium were 138.4 mg g−1 for BPA, 200.4 mg g−1 for EE2, and 169.2 mg g−1 for PFOA. The adsorption mechanism was probably driven by electrostatic and hydrophobic interactions. It is demonstrated that Al-based MOFs can remove EDCs efficiently, with maximum adsorption capacities around 100 mg g−1, depending on the target contaminant.
MOFs with active sites based on other elements have also been reported. For example, a study evaluated the adsorption of BPA from synthetic solutions using two different MOFs, one Fe-based (MIL-53) and the other Ni-based (Ni-MOF).66 The authors evaluated the effect of the physicochemical characteristics of the material, pH, adsorbent dose, contaminant concentration, and contact time. Even though the specific surface area of Ni-MOF (21.71 m2 g−1) was higher than that of MIL-53 (11.39 m2 g−1), the former one had a better adsorption performance. The adsorbent dose was the parameter with a higher effect on the removal of BPA, followed by pH, contaminant concentration, and time. The experiments showed that the adsorption mechanism adjusted to a monolayer formation system, and the maximum adsorption capacity of MIL-53 was 88.6 mg g−1.
MOFs have also been employed for the adsorption of EDCs like TCS,67 ciprofloxacin,68 carbamazepine,69 or dimethyl phthalate,70 with maximum adsorption capacities above 100 mg g−1 in most cases. The reported results demonstrate that MOFs are promising materials with high potential to be implemented in the tertiary treatment of wastewater for the removal of EDCs. However, the main challenge that hinders their application in WWTPs is the scalation of the MOFs' synthesis, as their efficacy is strongly dependent on their structure; therefore cheap synthesis methods may lead to a structure with more defects. Also, the cost is a major drawback as the price per kilogram of the most efficient MOFs could be in the range of hundreds or even thousands of dollars.71
Recently, magnetic COFs have been evaluated as adsorbents for the removal of diverse EDCs. The results have shown that these materials have great potential as adsorbents of EDCs due to their high adsorption capacity. In a study by ref. 72, a magnetic COF composite was employed for the adsorption from synthetic solutions of atrazine and chlorpyrifos, pesticides classified as EDCs. The authors reported that the COF had an adsorption capacity of 54 mg g−1 for atrazine and 270 mg g−1 for chlorpyrifos, and both results were superior to those reported in the literature for other nanomaterials such as MOFs or activated carbon. Additionally, desorption studies were performed showing a recovery of >90% of the pesticides, with no major effects on the adsorption capacity of the composite up to the fifth cycle. In another study, the removal of sodium diclofenac (DCF) from water was evaluated by using two magnetic COF composites, where the effect of the material's functionalization with hydroxyl groups on its adsorption capacity was assessed by density functional theory (DFT).73 The authors evaluated the performance of a hydroxyl group-modified COF (OH–COF), and the results showed an adsorption capacity of 203.4 mg g−1 calculated by using the Langmuir model. Reusability analysis demonstrated that this material maintained its original adsorption capacity after 5 cycles.
The removal of BPA by using COFs has also been evaluated, as a study used a magnetic modified COF for BPA adsorption from synthetic solutions and DFT and molecular dynamics (MD) were employed to elucidate the followed adsorption mechanism.74 The authors reported that the COF had a BPA adsorption capacity of 114.97 mg L−1 at pH 6 and 298 K. The results of the MD and DFT showed that the aldehyde groups in the COF and the BPA were interacting by hydrogen bindings.
COFs are promising materials with desirable characteristics for an adsorbent, like high surface area, well defined porous structure, and numerous active sites. However, correspondingly to MOFs, the major challenge to full-scale application is their cost. Also, in contrast to MOFs and other adsorbents, some key aspects of COFs related to their interaction mechanisms, stability and synthesis routes are not well documented. These drawbacks have hindered their application in WWTPs in the actuality; however they remain as a promising alternative that needs to be comprehensively studied.
Another group of adsorbents are carbon-based nanomaterials, which are among the most used for adsorption. This is mainly due to their characteristics, such as high specific surface area, stability, and highly porous structure. Graphene-based materials such as GO, reduced graphene oxide (rGO), single-walled carbon nanotubes (SWCNTs), and multi-walled carbon nanotubes (MWCNTs) are a very popular group of carbon materials with diverse applications. For example, some reports have shown promising results using GO as an absorbent. A study evaluated the removal of EE2 from synthetic solutions using a rGO magnetic composite (rGO/Fe3O4).75 The authors found out that the EE2 adsorption kinetics from water by the magnetic composite fitted well with a pseudo-second-order model. The adsorption capacity was 37.33 mg g−1 at a pH of 6, and after five regeneration cycles only 20% of the initial capacity was lost. Magnetic composites, composed of carbon polyhedrons with Co crystals and rGO nanosheets as the support, were evaluated as adsorbents for the removal of BPA from synthetic solutions.76 The results showed that the Co–C/rGO composites had desirable characteristics for adsorbents, such as high surface area (284.4 m2 g−1) and highly porous structure. The adsorption capacity for BPA was 175.1 mg g−1, and the authors reported that the adsorption mechanism was probably due to π–π interactions. Regeneration analyses showed that the composite could retain 83.7% of its capacity after four cycles.
SWCNTs have also been reported as adsorbents for EDC removal from water. A study evaluated the adsorption of BPA and EE2 on SWCNTs from synthetic seawater and brackish water.77 The authors reported that SWCNTs had higher adsorption capacity for EE2 (34.6–35.71 mg g−1) than for BPA (13.39–16.05 mg g−1), probably due to hydrophobic interactions as the main adsorption mechanism. The effect of pH was also studied, and the results showed that an increase in the pH from 3.5 to 11 reduced the adsorption of BPA, but it had no effect on EE2. Furthermore, as the concentration of dissolved organic carbon (DOC) increased, the adsorption of both BPA and EE2 decreased linearly. Despite the high performance and adsorption capacity of graphene-based materials like rGO and SWCNT composites for the adsorption of diverse EDCs, the cost of industrial scale synthesis hinders their application as adsorbents in WWTPs. Nevertheless, these materials have been reported as electrocatalysts and photocatalysts when supported with different types of nanoparticles or doped with heteroatoms. The application of graphene-based materials as adsorbents might not be the best option for WWTPs, but they have great potential in advanced oxidation processes (AOPs) and as electrodes for electrochemical sensing.
Other carbon materials like activated carbon, biochar, and even raw biomass have been extensively evaluated as adsorbents, mainly due to their variety, great performance and relatively low cost compared to other nanostructured carbon materials. In a study, the authors investigated treatment technologies for removing a few new micropollutants found in real wastewater.78 Powdered activated carbon (PAC) adsorption as a regularly used consolidated water treatment technology for the removal of organic micropollutants was the target of this study. Hybrid methods that combined PAC with a coagulant (PAC/L) or ultrafiltration (PAC/U) were developed. Also, a traditional Fe(III) based coagulant (FeCl3) as well as a novel natural coagulant derived from bean seeds, Phaseolus vulgaris, were used to demonstrate PAC/L. The results showed that using PAC (20 mg L−1) and natural coagulant (37.5 L L−1) together yielded the highest removal efficiency for bisphenol F, BPA, 2,4-dihydroxybenzophenone, estrone (E1), TCS, ibuprofen, and carbamazepine, with values ranging from 60 to 95.5%. Combined PAC/UF, on the other hand, achieved removal efficiencies ranging from 60 to 99.9%. Adsorption on activated carbon has the advantage of not producing new compounds and having high removal efficiency. When used in conjunction with membranes, they also possess a disinfecting function. The authors discovered, however, that its adsorption capability varies from batch to batch and that the cost of technology implementation, such as carbon synthesis and waste management, is considerable.
The use of non-pyrolyzed biomass has also been assessed in some studies as a low-cost and simple adsorbent. The adsorption of tetracycline (TC), SMX and from synthetic solutions using sulfonated saw dust was evaluated in a study.79 The results showed that the activated biomass had higher adsorption capacity than commercial adsorbents for SMX and BPA, 295.06 and 263.75 mg g−1, respectively. Meanwhile for TC the adsorption capacity of 270.53 mg g−1 was close to that of other available adsorbents.
Some WWTPs have already employed activated carbon in tertiary treatment; however there are still challenges to overcome regarding their selectivity in real wastewater samples. Supporting different types of nanoparticles on their surface is a promising approach to enhance the adsorption performance compared to regular carbon materials. Another promising alternative for this group of materials is the generation of low-cost hybrid materials, like nanobiocatalysts by the immobilization of bacteria or enzymes. This approach could exploit the adsorption capabilities of these low-cost adsorbents, while helping to overcome some drawbacks of using free biocatalysts.
Flocculation is a widely employed technology for the removal of contaminants from water. However, most of the studies are focused on the removal of ionic contaminants, and their application in the removal of EDCs is not extensively documented. Most of the reported studies for EDC removal have shown minor efficiencies compared to other technologies like adsorption, thus making flocculation not suitable for a main removal technology. However, further research could be done focusing on coupling flocculation with other removal technologies to increase its efficiency. This could be particularly helpful for existing WWTPs with flocculation technology.
Membrane technologies have been successfully implemented in water treatment, especially RO for water purification. However, its application in WWTPs has been hindered by the costs of the membranes and the very low pressures required for their operation. The membrane technology could be evaluated for assisting other technologies where higher water quality standards are required, or even as a quaternary treatment stage.
Another study evaluated the effect of chlorination on the estrogenic/antiestrogenic activities of biologically treated water.90 The authors found out that the chlorination processes increased the antiestrogenic activity and decreased the estrogenic activity of wastewater. This is possibly related to the formation of harmful disinfection byproducts (DBPs), which are considered as EDCs.
Ozonation is an advanced oxidation technology where ozone (O3) is added to water, and due to its powerful oxidation capacity, the ozone produces reactive oxygen species that can degrade a wide range of compounds and microorganisms.95–97 This technology has also been employed in several WWTPs with promising results for the degradation of diverse contaminants, including some EDCs.
A study where the ozonation conditions were optimized for the removal of E1 and E2 from synthetic solutions was carried out by ref. 98. The experiments showed that the optimal conditions were an ozone concentration of 4 mg L−1, residence time of 5 min, and pH 6. Under these conditions, about 90% of E1 and 95% of E2 were removed. Ozone and ozone/hydrogen peroxide treatments were evaluated to remove gemfibrozil and ibuprofen from spiked treated sewage effluent by ref. 99. A complete removal of gemfibrozil and 80% removal of ibuprofen was achieved by both ozonation and AOP using O3/H2O2. Ozonation is one of the most promising approaches for degrading EDCs in WWTPs, as some plants already have the infrastructure for this technology. It is required to optimize the operation parameters to ensure higher degradation efficiency on real wastewater samples.
5.1.5.1 Photodegradation. This includes a group of processes where molecules are transformed or degraded by the absorption of photons from the UV-visible spectrum.100 Typically, three photodegradation types are distinguished: photolysis, photooxidation and photocatalysis. Heterogeneous photocatalysis is an AOP and considered a very effective strategy for wastewater treatment (Fig. 4).101 The traditional AOPs are based on the generation of hydroxyl radicals (˙OH) as the main oxidant, which can react with a wide range of pollutants at high reaction rate constants.101,102 In contrast, persulfate-based AOPs generate sulfate radicals (SO4˙−), which have a higher redox potential and a longer half-life in comparison to ˙OH, enhancing the degradation of pollutants.103,104 Ref. 105 activated peroxymonosulfate (PMS) – a commonly used persulfate – through photocatalysis of a carbon dot (CD) functionalized carbon nitride composite for the degradation of BPA from synthetic solutions under visible light. They evaluated the photocatalytic performance of the photocatalyst without CDs (termed “PCN”) and the photocatalyst with CDs (termed “PCNC-2”); the removal efficiency was 10% and 65% under visible light for 30 minutes, respectively. The authors suggest that the notably enhanced performance can be attributed to improved light absorption, inhibition of electron/hole recombination, and an enlarged surface area by the CD introduction. In the presence of PMS, the removal efficiency significantly increased to 24% and 95% with PCN and PCN-2, respectively. Similarly, other authors have reported enhanced photocatalytic systems using nanomaterials.
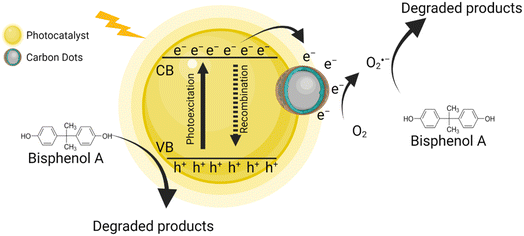 | ||
| Fig. 4 Graphical explanation of how CD assisted photocatalysis works for BPA degradation. Created with https://BioRender.com. | ||
A nanostructured poly(dimethylsiloxane) (PDMS) modified iron/Nafion/silica composite was studied as a catalyst in the catalytic UV oxidation of E1, E2, E3 and EE2 from synthetic solutions.106 The degradation of E1 achieved was greater than 90% after 60 min using 8.5 mg L−1 H2O2. The addition of the catalyst showed better conversion of E1 compared to UV or H2O2. Another study evaluated the degradation of 4NP from synthetic solutions by using a MIL-100(Fe)/ZnFe2O4/PCN (porous carbon nitride) composite as a catalyst under visible light irradiation.107 The authors prepared different composites varying the mass ratio of ZnFe2O4/PCN (ZC) to evaluate their catalytic activity. The most active ZC composite was used to produce MIL/ZC composites by adding MIL-100(Fe) in different ratios. The most active photocatalyst was 30MIL/ZC2, as it achieved 99.84% of 4NP degradation after 120 min and a dose of 30 mg of catalyst. Table 3 shows representative studies for the degradation of endocrine disruptors using different nanomaterials.
Other photooxidative methods have been advanced and improved with the coupling of membrane reactors. This is the case proposed by the study carried out by ref. 108, where a tube-in-tube membrane microreactor for photochemical UVC/H2O2 oxidation systems was developed. The main innovation of this system is the radial injection of H2O2 into the annular reaction zone through a porous membrane, resulting in a more uniform distribution of the injected chemical across the whole reactor length. The annulus of the proposed new reactor is made up of a ceramic UF membrane inner tubing and a concentric quartz outer tubing. The UF membrane is used to deliver small volumes of H2O2 into the reactor's annulus as a dosing device. UVC light is given in the annulus, where the polluted solution to be removed flows, using four mercury lamps mounted externally to the outer tube. The polluted solution's helical motion around the membrane shell side improves H2O2 radial mixing. For a synthetic solution and urban wastewater supplemented with oxytetracycline, the photochemical UVC/H2O2 process had an efficiency of 36% and 7%, respectively. The H2O2 dosage in this system was 15.8 mg L−1. Ref. 109 also designed a UVC/H2O2/TiO2 system-based tube-in-tube membrane reactor for degrading hormones from synthetic solutions. In this investigation, 48 and 30% of E2 and 17α-ethinylestradiol were found, respectively.
Ref. 110 compared direct photolysis, AOP, and photooxidation processes for the degradation of n-butylparaben (BP), 4-t-octylphenol (OP), trenbolone (TB), and boldenone (BD) from synthetic solutions. The photolysis results showed that BD had the fastest rate of decomposition as the concentration was halved after 2 s. For BP and TB, the degradation was less efficient as it took 7.5 and 26 min, respectively, to achieve 50%. Another study assessed the photolysis of BPS from synthetic solutions considering factors such as pH, source light, and the presence of other substances.111 The authors found that the process was UV-driven. With a 40 W UV-lamp, 50% of degradation was achieved after 43.1 min. The ions in water had different effects in the process, as chloride and ferric enhanced the photodegradation, while nitrate and phosphate decreased the rate of degradation. Ref. 112 studied an AOP using a solar-driven system using H2O2, for the degradation and mineralization of BPA from synthetic solutions. The optimal conditions (pH = 3, BPA concentration = 25 mg L−1, and H2O2 concentration = 350 mg L−1) allowed a BPA degradation of 77.4% and mineralization of 38.2%. Another study assessed the degradation of BPA from synthetic solutions by a photo-Fenton degradation process using persulfate (PS) and Fe2+.113 The authors found that using PS and Fe2+ in a solar-driven process was the most efficient approach compared to others. A complete degradation was achieved after 5 min using a 30 W lamp and PS/BPA ratio of 20. Ref. 114 conducted a study where a comparison between ozonation, UV-assisted ozonation (O3/UV), and photocatalytic ozonation for the degradation of parabens (methyl-, ethyl-, propyl-, butyl-, and benzyl-) from synthetic solutions was conducted. The authors evaluated the effect of parameters like pH and time. For O3/UV under optimal conditions (time: 45 min and pH 9), the removal efficiency was 65% for methylparaben, 62.5% for ethylparaben, 60% for propylparaben, 58% for butylparaben, and 53% for benzylparaben.
In general, AOPs are one of the most developed technologies for the implementation of WWTPs. Studies have shown high degradation efficiencies in most cases, and because of the variety of existing AOPs, these technologies can degrade most of the reported EDCs. Some AOPs based on photodegradation have already been successfully implemented in WWTPs and showed promising degradation efficiencies of EDCs. Yet, it is required to develop low-cost and highly efficient photocatalysts to spread their implementation in less developed countries.
Another study evaluated the role of extracellular polymeric substances (EPSs) produced by cyanobacteria Microcystis aeruginosa in the biosorption of phenanthrene (PHE).120 According to the results, EPS had a positive effect on the bioadsorption by M. aeruginosa, and with higher temperatures more PHE binding with EPS was observed. The kinetics of adsorption showed that cyanobacteria alone reached equilibrium after 24 h, while loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS) reached equilibrium in less than 2 h. The adsorption capacity at equilibrium for M. aeruginosa was 6.78 μg mg−1, while for TB-EPS and LB-TPS it was 9.47 and 12.31 μg mg−1, respectively. The adsorption mechanism was mainly attributed to electrostatic and hydrophobic interactions.
The dried biomass of three kinds of microalgae, red, brown, and green, was employed as a biosorbent for the removal of benzene and toluene, evaluating the effects of ionic strength and temperature.121 The ionic strength had a neglectable effect on the surface charge of the biomass, and the increase in temperature enhanced the adsorption and desorption processes. Brown microalgae registered the highest adsorption capacity for both benzene and toluene with 112 and 28 mg g−1, respectively. London forces and hydrophobic interactions are likely to guide the adsorption mechanism. Another study evaluated phycoremediation of water using different species of intertidal macroalgae for the removal of BPA from coastal waters.122 The authors used natural seawater and fresh Ulva pertusa, Ulva prolifera, Sargassum horneri, Gymnogongrus flabelliformis, Gracilaria lemaneiformis, and Codium fragile for the biosorption experiments. The results showed that all the studied algal species obtained above 80% of removal after 8 h, while for BPA only U. pertusa achieved approximately 100% after 36 h. A pilot-scale experiment was carried out using U. pertusa, as it was the algae with the best removal efficiency, and wastewater from a mariculture system. According to these results, BPA and nonylphenol could be efficiently removed after three cycles with an initial concentration of 100 μg L−1 for both contaminants. Furthermore, a field investigation found that in the intertidal zones with a higher presence of U. pertusa the EDC concentration was much lower. The removal mechanism was mainly driven by biosorption.
Agro-industrial wastes are another promising alternative, as there are studies that have shown their potential to be used as bio-sorbents for the removal of several pollutants, including EDCs. This alternative for the revalorization of wastes has been extensively studied in recent years due to the growing interest in the application of circular economy principles.
A corncob agro-industrial residue was employed for the biosorption of BPA from water, evaluating the effect of BPA concentration and varying pH.123 The maximum adsorption capacity of corncob ground fibers was 51.25 mg g−1 after 20 min, and the results were fitted to the monolayer adsorption model of Langmuir. The overall adsorption efficiency of the biosorbent was approximately 90%. The authors found that adsorption efficiency was higher at lower pH; however, there was no significant difference between the adsorption at pH 3, 5 and 7. At pH 9 there was significantly lower efficiency. For the BPA concentration, increasing the initial concentration resulted in a lower removal efficiency.
Another agro-industrial byproduct with potential as a biosorbent is rice husk (RH). A study evaluated the adsorption capacity of RH for removing E1, EE2 and E3 from water, evaluating the effect of pH, temperature, and competitive adsorption.124 The optimal pH and temperature conditions were neutral pH and 298 K. The equilibrium was reached after 60 min for E1 and 120 min for EE2 and E3. Kinetics experiments showed that the E1, E3, and multiple-contaminant systems fitted the pseudo-first-order model, while EE2 fitted well with both pseudo-second order and pseudo-first order models. The authors attributed this to the active sites limiting the process. Single-contaminant system adsorption data were fitted to the Langmuir model, with adsorption capacities of 2.698 mg g−1 for E1, 1.649 mg g−1 for EE2, and 0.979 mg g−1 for E3. Nevertheless, multi-contaminant system adsorption data did not fit into any isotherm model, probably due to the lack of competition between the hormones.
Biodegradation can be defined as the process where biological systems convert a substance into a simpler molecule by a simple or a sequence of biochemical reactions.125–127 There are plenty of studies where biodegradation of different pollutants from water is assessed, as shown in Table 4. Another study evaluated the biodegradation of nonylphenol, 4-tert-octylphenol (4tOP), and 2,4-dichlorophenol (2,4-DCP) by Thielavia sp. HJ22.128 The kinetics of removal showed that after 8 h 80%, 95% and 100% of degradation were achieved for 2,4-DCP, nonylphenol and 4tOP, respectively. The authors evaluated the degradation mechanisms and concluded that the laccase production increased the degradation rate.
The biosorption and biodegradation of TPT affected its coexisting ions. This mechanism was further accelerated by biodegradation using Brevibacillus.129 The pH had significant effects on the biosorption of TPT, where higher removal efficiencies were obtained in a pH range of 6.0–9.0. The removal of TPT at an initial concentration of 0.5 mg L−1 and a biomass dose of 0.3 g L−1 was 60% after 5 days. The effect of adding H2O2, glucose and rhamnolipid was evaluated. Increasing glucose concentration between 0 and 0.5 mg L−1 also increased the efficiency; however at higher concentrations the opposite effect was observed. Adding H2O2 at 6 mmol L−1 increased the TPT degradation to a maximum of 86.8%; nevertheless with higher concentrations the efficiency started to decrease. A study evaluated the degradation of DBP by Rhodovulum sp. DBP07 and the effect of biostimulation, modifying nutrient sources, pH, and temperature, and studying their effect on the degradation efficiency.130 The authors reported that a maximum degradation of 70.2% was achieved with an initial DBP concentration of 600 mg L−1. Glucose was the nutrient with the most favorable effect on the cell growth and degradation rate, removing 79.8% of a 5 g L−1 concentration, and 77.4% of 2 g L−1. Another study employed three different marine algae (Cylindrotheca Closterium, Dunaliella salina and Chaetoceros muelleri) for the biodegradation of diethyl phthalate (DEP) and DBP and to evaluate how a coexisting system would affect the degradation rates.131 The authors reported that in single contaminant systems, DBP degradation was slower than DEP degradation, while in coexisting systems the DBP degradation was faster. This was associated with the inhibition of DEP degradation by DBP. In the coexisting system, the DBP degradation efficiencies for algae were 40% for D. salina, 47.1% for C. muelleri, and 93.1% for C. Closterium.
A study evaluated the degradation of natural estrogens E1, E2, and E3, and synthetic estrogen EE2 with an aerobic reactor enriched with a bacterial community from activated sludge.132 In the system, the hormones were used as the carbon sources for the bacteria, and later sequencing analysis was performed to identify the bacteria in the system. The results showed a degradation efficiency above 98% for the natural hormones after 24 h, while EE2 degradation reached 84.5% after 34 days. The authors reported Emticicia, Nubsella, and Sphingobacterium as the bacteria responsible for the degradation of hormones. Another study assessed an enhancement of microbial degradation of E2 hormone by Shewanella oneidensis by adding biochar from reed straw as a possible promoter of the metabolization of the contaminant.133 The authors evaluated the effect of the temperature of pyrolysis of biochar on the removal efficiency, and the effect of using aerobic or anaerobic degradation. According to the results, the highest removal efficiency of E2 was 95% by anaerobic degradation using S. oneidensis with 500 °C biochar after 120 h.
Biosorption and biodegradation have shown great potential for the removal of EDCs, due to the wide variety of sorbents that can be employed, their versatility and high performance. The major challenges are related to the use of microorganisms, as it requires time-consuming processes and their production, storage and handling are far more complex than technologies where microorganisms are not required. Thus, the use of agro-industrial waste-based sorbents and immobilized instead of free microorganisms is a very promising research avenue, especially for in situ water remediation.
The removal of EE2 was assessed using Phanerochaete chrysosporium and Lentinula edodes, and the role of laccases in the transformation of the contaminant.139 The efficiency of degradation by P. chrysosporium was lower than that of L. edodes, with 43% and 50%, respectively. Also, it was observed that there was no enzymatic activity of laccase in the case of P. chrysosporium, and thus it was assumed that L. edodes had higher degradation efficiency due to the laccase activity. According to this, the authors modified the culture conditions to enhance the laccase production by L. edodes, resulting in an EE2 removal efficiency of 80%. The metabolites produced by the EE2 oxidation with purified laccase and those produced by laccase production-enhanced L. edodes cultures were the same, confirming that the EE2 degradation was carried out by laccase. The enzyme-mimicking strategy to degrade endocrine disruptors emerged as a necessity to overcome the drawbacks presented on natural enzymes (Fig. 5). Natural enzymes have been applied as an effective strategy to treat endocrine disruptors.140 For example, oxidases can catalyze estrogens to produce phenoxyl radical intermediates leading to the subsequent formation of homo-oligomers with a lower biotoxicity and bioavailability.141,142 However, their application is limited by the low stability and high-cost manufacture and storage of natural enzymes.143 Consequently, artificial nanozymes with simultaneous enzymatic and nanomaterial properties have been developed, being useful for environmental remediation purposes.141,144
 | ||
| Fig. 5 Representation of laccase-mimicking process for the degradation of BPA by nanozymes. Created with https://BioRender.com. | ||
As representative work,145 prepared a nanozyme termed “CH–Cu” through a hydrothermal synthesis method, using Cys–His dipeptide and CuCl2 as precursors. The catalytic performance on the degradation of chlorophenol and bisphenol pollutants was tested on the CH–Cu nanozyme and compared to that of natural laccases. The authors concluded that the CH–Cu nanozyme exhibited a higher capability to degrade those compounds, since CH–Cu nanozyme degraded approximately 90% of the representative compounds of chlorophenols and bisphenols, while the degradation efficiency of laccase was around 70% for the same pollutants under the same conditions. Similarly,146 evaluated six common nanozymes for the degradation of estrogen compounds, demonstrating that the dehydrogenase-like activity of gold nanoparticles (AuNPs) was useful to degrade E2. Moreover, they demonstrated the size effect on the catalytic activity evaluating the degradation performance for E2 of AuNP nanozymes of 13 nm and 5 nm. Smaller nanoparticles presented a higher catalytic performance for E2 degradation caused by a higher catalytically active site density.
6. Future prospects and challenges
The widespread use of previously discussed technologies in WWTPs or their escalation from the lab-scale to real WWTPs is strongly related to the implementation of new nanomaterials. Either as adsorbents, catalysts, membranes or supports for immobilization, there is a need to develop and implement low-cost and efficient nanomaterials that enhance the degradation/removal of EDCs reported by current technologies. Although there are novel and highly efficient nanomaterials and technologies that could significantly reduce the discharge of EDCs into the environment, in most cases the cost and complex synthesis methods hinder their implementation at WWTPs.Nevertheless, there are promising alternatives that could serve as a transition point between current methodologies and groundbreaking technologies. For example, carbon materials derived from biomass or waste are low-cost and efficient materials that can be used in several physical, chemical or biological treatment technologies. Also, there are diverse studies focused on modifications that could enhance their performance according to their application. However, an important challenge to overcome could be the reduction of the heterogeneity of these materials and providing standardized escalation routes to synthesize these materials.
In addition, an integrated approach using hybrid treatment technologies and real time monitoring of EDCs should be prioritized. As it has been proven, in most cases the conjunction of different technologies significantly enhances the degradation of EDCs. Furthermore, real time analysis of concentrations will allow us to adjust to the optimal parameters of hybrid systems.
7. Conclusions
Wastewater and industrial effluents are some of the main sources for EDC contamination. As currently most of the WWTPs are not prepared to remove EDCs from wastewater, it is required to implement more strict regulations on the monitoring, use and removal of EDCs.Due to the complex transportation pathways of EDCs, it is required to develop accessible alternatives for portable detection devices and real time monitoring of the concentration of EDCs, which will allow a faster and easier identification of the sources of contamination and determine the best strategies for the mitigation of environmental impact.
Adsorption, AOPs and enzymatic transformation are some of the technologies that are closer to being widely implemented at WWTPs for efficient EDC removal. Due to their properties, carbon-based nanomaterials have a main role in enhancing the performance of these technologies. Additionally, the use of waste or biomass derived materials could help to reduce the gap and make advanced wastewater treatment technologies more accessible all the world.
Author contributions
Jesús Alfredo Rodríguez-Hernández: writing – original draft preparation, visualization, and conceptualization. Rafael G. Araújo: writing – original draft preparation, visualization, supervision, and conceptualization. Itzel Y. López-Pacheco: writing – original draft preparation. Laura Isabel Rodas-Zuluaga: writing – original draft preparation. Reyna Berenice González-González: writing – original draft preparation and visualization. Lizeth Parra-Arroyo: writing – original draft preparation. Juan Eduardo Sosa-Hernández: writing – review & editing. Elda M. Melchor-Martínez: writing – review & editing. Manuel Martínez-Ruiz: writing – review & editing. Damià Barceló: writing – review & editing. Lorenzo M. Pastrana: writing – review & editing. Hafiz M. N. Iqbal: writing – review & editing. Roberto Parra-Saldívar: writing – review & editing and funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is part of the project entitled “Contaminantes emergentes y prioritarios en las aguas reutilizadas en agricultura: riesgos y efectos en suelos, producción agrícola y entorno ambiental” funded by CSIC-Tecnológico de Monterrey under the i-Link + program (LINKB20030). The author “Jesús Alfredo Rodríguez-Hernández” acknowledges Consejo Nacional de Ciencia y Tecnología (CONACyT) for awarding a scholarship for a PhD in nanotechnology (CVU: 924193). CONACyT is thankfully acknowledged for partially supporting this work under the Sistema Nacional de Investigadores (SNI) program awarded to Rafael G. Araújo (CVU: 714118), Juan Eduardo Sosa-Hernández (CVU: 375202), Elda M. Melchor-Martínez (CVU: 230784), Manuel Martinez-Ruiz (CVU: 418151), Hafiz M. N. Iqbal (CVU: 735340) and Roberto Parra-Saldívar (CVU: 35753). The authors are also thankful to “Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo” in the Latin American development network “Lacasas Inmovilizadas para la Degradación de Compuestos Aromáticos en Aguas Residuales” (LIDA, project 318RT0552). All listed authors are also grateful to their representative universities/institutes for providing literature facilities and the Biorender online program for the elaboration of the graphical abstract and Fig. 1–5.References
- T. K. Kasonga, M. A. A. Coetzee, I. Kamika, V. M. Ngole-Jeme and M. N. Benteke Momba, Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review, J. Environ. Manage., 2021, 277, 111485 CrossRef CAS PubMed.
- United Nations Children’s Fund (UNICEF), World Health Organization. Progress on Household Drinking Water, Sanitation and Hygiene I 2000–2017. New York; 2019 Search PubMed.
- United Nations World Water Assessment Programme (WWAP). The United Nations World Water Development Report 2015: Water for a Sustainable World. Paris; 2015 Search PubMed.
- S. Ahuja. Water quality worldwide. Handbook of Water Purity and Quality. 2021; pp. 19–33 Search PubMed.
- R. M. Blair, S. Waldron and C. Gauchotte-Lindsay, Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period, Water Res., 2019, 163, 114909 CrossRef CAS.
- M. S. Recsetar, K. M. Fitzsimmons, J. L. Cuello, C. Hoppe-Jones and S. A. Snyder, Evaluation of a recirculating hydroponic bed bioreactor for removal of contaminants of emerging concern from tertiary-treated wastewater effluent, Chemosphere, 2021, 262, 128121 CrossRef CAS.
- A. C. Gore, V. A. Chappell, S. E. Fenton, J. A. Flaws, A. Nadal and G. S. Prins, et al., EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals, Endocr. Rev., 2015, 36(6), E1–E150 CrossRef CAS . Available from: https://academic.oup.com/edrv/article/36/6/E1/2354691.
- X. Lu, J. Sun and X. Sun, Recent advances in biosensors for the detection of estrogens in the environment and food, TrAC, Trends Anal. Chem., 2020, 127, 115882 CrossRef CAS.
- W. T. Vieira, M. B. de Farias, M. P. Spaolonzi, M. G. C. da Silva and M. G. A. Vieira, Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review, Environ. Chem. Lett., 2020, 18(4), 1113–1143 CrossRef CAS . Available from: https://link.springer.com/article/10.1007/s10311-020-01000-1.
- N. A. H. Ismail, S. Y. Wee, N. H. Kamarulzaman and A. Z. Aris, Quantification of multi-classes of endocrine-disrupting compounds in estuarine water, Environ. Pollut., 2019, 249, 1019–1028 CrossRef CAS.
- M. A. la Merrill, L. N. Vandenberg, M. T. Smith, W. Goodson, P. Browne and H. B. Patisaul, et al., Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification, Nat. Rev. Endocrinol., 2020, 16(1), 45–57, DOI:10.1038/s41574-019-0273-8.
- X. Lu, J. Sun and X. Sun, Recent advances in biosensors for the detection of estrogens in the environment and food, TrAC, Trends Anal. Chem., 2020, 127, 115882 CrossRef CAS.
- L. A. Hamilton, L. A. Tremblay, G. L. Northcott, M. Boake and R. P. Lim, The impact of variations of influent loading on the efficacy of an advanced tertiary sewage treatment plant to remove endocrine disrupting chemicals, Sci. Total Environ., 2016, 560–561, 101–109 CrossRef CAS.
- N. Zwart, W. Jonker, R. t. Broek, J. de Boer, G. Somsen and J. Kool, et al., Identification of mutagenic and endocrine disrupting compounds in surface water and wastewater treatment plant effluents using high-resolution effect-directed analysis, Water Res., 2020, 168, 115204 CrossRef CAS.
- W. T. Vieira, M. B. de Farias, M. P. Spaolonzi, M. G. C. da Silva and M. G. A. Vieira, Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways—A critical review, J. Environ. Chem. Eng., 2021, 9(1), 104558 CrossRef CAS.
- I. Y. López-Pacheco, A. Silva-Núñez, C. Salinas-Salazar, A. Arévalo-Gallegos, L. A. Lizarazo-Holguin and D. Barceló, et al., Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects, Sci. Total Environ., 2019, 690, 1068–1088 CrossRef.
- NIEHS. Endocrine Disruptors. 2021. Available from: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm Search PubMed.
- O. Alofe, E. Kisanga, S. H. Inayat-Hussain, M. Fukumura, R. Garcia-Milian and L. Perera, et al., Determining the endocrine disruption potential of industrial chemicals using an integrative approach: Public databases, in vitro exposure, and modeling receptor interactions, Environ. Int., 2019, 131, 104969 CrossRef CAS.
- S. Wang, C. Ji, F. Li, J. Zhan, T. Sun and J. Tang, et al., Tetrabromobisphenol A induced reproductive endocrine-disrupting effects in mussel Mytilus galloprovincialis, J. Hazard. Mater., 2021, 416, 126228 CrossRef CAS.
- W. A. Thompson and M. M. Vijayan, Environmental levels of venlafaxine impact larval behavioural performance in fathead minnows, Chemosphere, 2020, 259, 127437 CrossRef CAS.
- M. Teng, C. Wang, M. Song, X. Chen, J. Zhang and C. Wang, Chronic exposure of zebrafish (Danio rerio) to flutolanil leads to endocrine disruption and reproductive disorders, Environ. Res., 2020, 184, 109310 CrossRef CAS.
- M. Ashfaq, Q. Sun, C. Ma, A. Rashid, Y. Li and S. I. Mulla, et al., Occurrence, seasonal variation and risk evaluation of selected endocrine disrupting compounds and their transformation products in Jiulong river and estuary, China, Mar. Pollut. Bull., 2019, 145, 370–376 CrossRef CAS.
- T. G. Correia, V. A. R. O. Vieira, A. de Moraes Narcizo, R. A. Zampieri, L. M. Floeter-Winter and R. G. Moreira, Endocrine disruption caused by the aquatic exposure to aluminum and manganese in Astyanax altiparanae (Teleostei: Characidae) females during the final ovarian maturation, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2021, 249, 109132 CAS.
- R. Akhbarizadeh, G. Russo, S. Rossi, K. Golianova, F. Moore and M. Guida, et al., Emerging endocrine disruptors in two edible fish from the Persian Gulf: Occurrence, congener profile, and human health risk assessment, Mar. Pollut. Bull., 2021, 166, 112241 CrossRef CAS PubMed.
- T. Chenet, A. Mancia, G. Bono, F. Falsone, D. Scannella and C. Vaccaro, et al., Plastic ingestion by Atlantic horse mackerel (Trachurus trachurus) from central Mediterranean Sea: A potential cause for endocrine disruption, Environ. Pollut., 2021, 284, 117449 CrossRef CAS.
- L. Z. Lara, C. Bertoldi, N. M. Alves and A. N. Fernandes, Sorption of endocrine disrupting compounds onto polyamide microplastics under different environmental conditions: Behaviour and mechanism, Sci. Total Environ., 2021, 796, 148983 CrossRef CAS PubMed.
- Y. Wu, J. Wang, Y. Wei, J. Chen, L. Kang and C. Long, et al., Contribution of prenatal endocrine-disrupting chemical exposure to genital anomalies in males: The pooled results from current evidence, Chemosphere, 2022, 286, 131844 CrossRef CAS.
- J. L. Pearce, B. Neelon, M. S. Bloom, J. P. Buckley, C. v. Ananth and F. Perera, et al., Exploring associations between prenatal exposure to multiple endocrine disruptors and birth weight with exposure continuum mapping, Environ. Res., 2021, 200, 111386 CrossRef CAS.
- V. Ramírez, Y. Gálvez-Ontiveros, P. J. González-Domenech, M. Á. Baca, L. Rodrigo and A. Rivas, Role of endocrine disrupting chemicals in children's neurodevelopment, Environ. Res., 2022, 203, 111890 CrossRef PubMed.
- S. Zhang, Z. Jiao, X. Zhao, M. Sun and X. Feng, Environmental exposure to 17β-trenbolone during adolescence inhibits social interaction in male mice, Environ. Pollut., 2021, 289, 117710 CrossRef CAS PubMed.
- F. Fäys, E. M. Hardy, P. Palazzi, S. Haan, C. Beausoleil and B. M. R. Appenzeller, Biomonitoring of fast-elimination endocrine disruptors – Results from a 6-month follow up on human volunteers with repeated urine and hair collection, Sci. Total Environ., 2021, 778, 146330 CrossRef.
- J. F. Narváez, H. Grant, V. C. Gil, J. Porras, J. C. Bueno Sanchez and L. F. Ocampo Duque, et al., Assessment of endocrine disruptor effects of levonorgestrel and its photoproducts: Environmental implications of released fractions after their photocatalytic removal, J. Hazard. Mater., 2019, 371, 273–279 CrossRef.
- M. C. Velarde, A. F. O. Chan, v. Sajo MEJ, I. Zakharevich, J. Melamed and G. L. B. Uy, et al., Elevated levels of perfluoroalkyl substances in breast cancer patients within the Greater Manila Area, Chemosphere, 2022, 286, 131545 CrossRef CAS PubMed.
- H. J. Wen, T. C. Chang, W. H. Ding, S. F. Tsai, C. A. Hsiung and S. L. Wang, Exposure to endocrine disruptor alkylphenols and the occurrence of endometrial cancer, Environ. Pollut., 2020, 267, 115475 CrossRef CAS PubMed.
- Q. Wu, X. Coumoul, P. Grandjean, R. Barouki and K. Audouze, Endocrine disrupting chemicals and COVID-19 relationships: A computational systems biology approach, Environ. Int., 2021, 157, 106232 CrossRef CAS.
- C. Castillo-Zacarías, M. E. Barocio, E. Hidalgo-Vázquez, J. E. Sosa-Hernández, L. Parra-Arroyo and I. Y. López-Pacheco, et al., Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection, Sci. Total Environ., 2021, 757, 143722 CrossRef.
- I. ben Chabchoubi, N. Belkhamssa, M. Ksibi and O. Hentati, Trends in the detection of pharmaceuticals and endocrine-disrupting compounds by Field-Effect Transistors (FETs), Trends Environ. Anal. Chem., 2021, 30, e00127 CrossRef CAS.
- J. Ali, Biosensors: Their Fundamentals, Designs, Types and Most Recent Impactful Applications: A Review. Biosensors: Their Fundamentals, Designs, Types and Most Recent Impactful Applications: A Review, J. Biosens. Bioelectron., 2017, 8(1), 235 Search PubMed.
- N. Jaffrezic-Renault, J. Kou, D. Tan and Z. Guo, New trends in the electrochemical detection of endocrine disruptors in complex media, Anal. Bioanal. Chem., 2020, 412(24), 5913–5923 CrossRef CAS PubMed . Available from: https://link.springer.com/article/10.1007/s00216-020-02516-9.
- S. I. Kaya, A. Cetinkaya, N. K. Bakirhan and S. A. Ozkan, Trends in sensitive electrochemical sensors for endocrine disruptive compounds, Trends Environ. Anal. Chem., 2020, 28, e00106 CrossRef CAS.
- M. Kim, Y. E. Song, J. Q. Xiong, K. Y. Kim, M. Jang and B. H. Jeon, et al., Electrochemical detection and simultaneous removal of endocrine disruptor, bisphenol A using a carbon felt electrode, J. Electroanal. Chem., 2021, 880, 114907 CrossRef CAS.
- Y. H. Pang, Y. Y. Huang, L. Wang, X. F. Shen and Y. Y. Wang, Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor, Environ. Pollut., 2020, 263, 114616 CrossRef CAS.
- X. Zhu, G. Wu, Y. Xing, C. Wang, X. Yuan and B. Li, Evaluation of single and combined toxicity of bisphenol A and its analogues using a highly-sensitive micro-biosensor, J. Hazard. Mater., 2020, 381, 120908 CrossRef CAS PubMed.
- H. Awad, M. Gar Alalm and H. K. El-Etriby, Environmental and cost life cycle assessment of different alternatives for improvement of wastewater treatment plants in developing countries, Sci. Total Environ., 2019, 660, 57–68 CrossRef CAS.
- M. F. M. A. Zamri, R. Bahru, F. Suja’, A. H. Shamsuddin, S. K. Pramanik and I. M. R. Fattah, Treatment strategies for enhancing the removal of endocrine-disrupting chemicals in water and wastewater systems, J. Water Proc. Eng., 2021, 41, 102017 CrossRef.
- C. P. Gerba and I. L. Pepper. Municipal Wastewater Treatment. Environmental and Pollution Science. 2019; pp. 393–418. https://linkinghub.elsevier.com/retrieve/pii/B9780128147191000227 Search PubMed.
- B. Mandal, A. Purkayastha, A. A. Prabhu and V. V. Dasu. Development in wastewater treatment plant design. Emerging Technologies in Environmental Bioremediation. 2020; pp. 311–321 Search PubMed.
- A. Sonune and R. Ghate, Developments in wastewater treatment methods, Desalination, 2004, 167(1–3), 55–63 CrossRef CAS.
- R. A. Hamza, O. T. Iorhemen and J. H. Tay, Advances in biological systems for the treatment of high-strength wastewater, J. Water Proc. Eng., 2016, 10, 128–142 CrossRef.
- J. R. Buchanan, Decentralized Wastewater Treatment. Comprehensive Water Quality and Purification. 2014; vol. 3: pp. 244–267 Search PubMed.
- R. Stott, Fate and behaviour of parasites in wastewater treatment systems. Handbook of Water and Wastewater Microbiology. 2003; pp. 491–521 Search PubMed.
- M. Scholz. Activated Sludge Processes. Wetlands for Water Pollution Control. 2016; pp. 91–105 Search PubMed.
- P. Rajasulochana and V. Preethy, Comparison on efficiency of various techniques in treatment of waste and sewage water – A comprehensive review, Resour.-Effic. Technol., 2016, 2(4), 175–184 Search PubMed.
- J. Karpińska and U. Kotowska. Removal of Organic Pollution in the Water Environment. 2019; vol. 11(10): p. 2017 Search PubMed.
- H. J. Geyer, G. G. Rimkus, I. Scheunert, A. Kaune, K. W. Schramm, A. Kettrup, et al., Bioaccumulation and Occurrence of Endocrine-Disrupting Chemicals (EDCs), Persistent Organic Pollutants (POPs), and Other Organic Compounds in Fish and Other Organisms Including Humans. Bioaccumulation – New Aspects and Developments. 2000; pp. 1–166 Search PubMed.
- S. C. Ameta. Introduction. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology. 2018; pp. 1–12 Search PubMed.
- I. v. Muralikrishna and V. Manickam. in: Chapter Twelve – Wastewater Treatment Technologies. ed. Muralikrishna I. v. and Manickam V. B. T. E. M., Butterworth-Heinemann; 2017. pp. 249–293. Available from: https://www.sciencedirect.com/science/article/pii/B9780128119891000129 Search PubMed.
- R. S. Dhodapkar and K. N. Gandhi. Pharmaceuticals and personal care products in aquatic environment: chemicals of emerging concern?, Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology Emerging Contaminants and Micro Pollutants. 2019; pp. 63–85 Search PubMed.
- S. Ray and G. Das. Adsorption. Process Equipment and Plant Design. 2020; pp. 351–384. https://linkinghub.elsevier.com/retrieve/pii/B9780128148853000129 Search PubMed.
- A. Bakhtyari, M. Mofarahi and C. H. Lee. CO2 adsorption by conventional and nanosized zeolites. Advances in Carbon Capture. 2020; pp. 193–228 Search PubMed.
- S. Singh, K. L. Wasewar and S. K. Kansal. Low-cost adsorbents for removal of inorganic impurities from wastewater. Inorganic Pollutants in Water. 2020; pp. 173–203 Search PubMed.
- S. Dhaka, R. Kumar, A. Deep, M. B. Kurade, S. W. Ji and B. H. Jeon, Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments, Coord. Chem. Rev., 2019, 380, 330–352 CrossRef CAS.
- E. Y. Park, Z. Hasan, N. A. Khan and S. H. Jhung, Adsorptive removal of bisphenol-a from water with a metal–organic framework, a porous chromium-benzenedicarboxylate, J. Nanosci. Nanotechnol., 2013, 13(4), 2789–2794 CrossRef CAS.
- M. Zhou, Y. Wu, J. Qiao, J. Zhang, A. McDonald and G. Li, et al., The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al), J. Colloid Interface Sci., 2013, 405, 157–163 CrossRef CAS.
- B. M. Jun, H. S. Hwang, J. Heo, J. Han, M. Jang and J. Sohn, et al., Removal of selected endocrine-disrupting compounds using Al-based metal organic framework: Performance and mechanism of competitive adsorption, J. Ind. Eng. Chem., 2019, 79, 345–352 CrossRef CAS.
- S. Rezania, J. Cho, Z. Derakhshan Nejad, A. Barghi, K. K. Yadav and E. M. Ahmed, et al., Microporous metal–organic frameworks against endocrine-disruptor bisphenol A: parametric evaluation and optimization, Colloids Surf., A, 2021, 626, 127039 CrossRef CAS.
- J. Y. Song and S. H. Jhung, Adsorption of pharmaceuticals and personal care products over metal–organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption, Chem. Eng. J., 2017, 322, 366–374 CrossRef CAS.
- S. Li, X. Zhang and Y. Huang, Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water, J. Hazard. Mater., 2017, 321, 711–719 CrossRef CAS PubMed.
- C. Chen, D. Chen, S. Xie, H. Quan, X. Luo and L. Guo, Adsorption Behaviors of Organic Micropollutants on Zirconium Metal–Organic Framework UiO-66: Analysis of Surface Interactions, ACS Appl. Mater. Interfaces, 2017, 9(46), 41043–41054 CrossRef CAS.
- Z. Li, Y. N. Wu, J. Li, Y. Zhang, X. Zou and F. Li, The Metal–Organic Framework MIL-53(Al) Constructed from Multiple Metal Sources: Alumina, Aluminum Hydroxide, and Boehmite, Chem.–Eur. J., 2015, 21(18), 6913–6920 CrossRef CAS.
- K. E. Diab, E. Salama, H. S. Hassan, A. Abd El-moneim and M. F. Elkady, Biocompatible MIP-202 Zr-MOF tunable sorbent for cost-effective decontamination of anionic and cationic pollutants from waste solutions, Sci. Rep., 2021, 11(1), 1–13 CrossRef PubMed.
- V. Romero, S. P. S. Fernandes, P. Kovář, M. Pšenička, Y. v. Kolen’ko and L. M. Salonen, et al., Efficient adsorption of endocrine-disrupting pesticides from water with a reusable magnetic covalent organic framework, Microporous Mesoporous Mater., 2020, 307, 110523 CrossRef CAS.
- X. Mi, S. Zhou, Z. Zhou, M. Vakili, Y. Qi and Y. Jia, et al., Adsorptive removal of diclofenac sodium from aqueous solution by magnetic COF: Role of hydroxyl group on COF, Colloids Surf., A, 2020, 603, 125238 CrossRef CAS.
- D. Wei, J. Li, Z. Chen, L. Liang, J. Ma and M. Wei, et al., Understanding bisphenol-A adsorption in magnetic modified covalent organic frameworks: Experiments coupled with DFT calculations, J. Mol. Liq., 2020, 301, 112431 CrossRef CAS.
- Z. Luo, H. Li, Y. Yang, H. Lin and Z. Yang, Adsorption of 17α-ethinylestradiol from aqueous solution onto a reduced graphene oxide-magnetic composite, J. Taiwan Inst. Chem. Eng., 2017, 80, 797–804 CrossRef CAS.
- Y. Wang, D. Chen, Y. Yu, Y. Ding, X. Cao and M. Fu, et al., Magnetic porous carbon nanopolyhedron modified rGO composites as recyclable sorbent for effective removal of bisphenol A from water, J. Environ. Chem. Eng., 2021, 9(5), 105911 CrossRef CAS.
- L. Joseph, J. Heo, Y. G. Park, J. R. V. Flora and Y. Yoon, Adsorption of bisphenol A and 17α-ethinyl estradiol on single walled carbon nanotubes from seawater and brackish water, Desalination, 2011, 281(1), 68–74 CrossRef CAS.
- M. Bogunović, I. Ivančev-Tumbas, M. Česen, T. D. Sekulić, J. Prodanović and A. Tubić, et al., Removal of selected emerging micropollutants from wastewater treatment plant effluent by advanced non-oxidative treatment – A lab-scale case study from Serbia, Sci. Total Environ., 2021, 765, 142764 CrossRef PubMed.
- M. A. Ahsan, M. T. Islam, C. Hernandez, E. Castro, S. K. Katla and H. Kim, et al., Biomass conversion of saw dust to a functionalized carbonaceous materials for the removal of Tetracycline, Sulfamethoxazole and Bisphenol A from water, J. Environ. Chem. Eng., 2018, 6(4), 4329–4338 CrossRef CAS.
- B. M. Baraniak and E. Waleriańczyk. Flocculation. Encyclopedia of Food Sciences and Nutrition. 2003; pp. 2531–2535. https://linkinghub.elsevier.com/retrieve/pii/B012227055X004855 Search PubMed.
- H. Bridle, K. Jacobsson and A. C. Schultz. Sample Processing. Waterborne Pathogens: Detection Methods and Applications. 2014; pp. 67–114 Search PubMed.
- Z. Yang, K. Ren, E. Guibal, S. Jia, J. Shen and X. Zhang, et al., Removal of trace nonylphenol from water in the coexistence of suspended inorganic particles and NOMs by using a cellulose-based flocculant, Chemosphere, 2016, 161, 482–490 CrossRef CAS PubMed.
- T. H. Shah and A. Rawal. Textiles in filtration. Handbook of Technical Textiles. 2016; pp. 57–110 Search PubMed.
- X. Si, Z. Hu, D. Ding and X. Fu, Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent, J. Environ. Sci., 2019, 76, 57–64 CrossRef CAS.
- M. N. Nguyen, P. B. Trinh, C. J. Burkhardt and A. I. Schäfer, Incorporation of single-walled carbon nanotubes in ultrafiltration support structure for the removal of steroid hormone micropollutants, Sep. Purif. Technol., 2021, 264, 118405 CrossRef CAS.
- K. O. Agenson, J. I. Oh and T. Urase, Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: controlling parameters of process, J. Membr. Sci., 2003, 225(1–2), 91–103 CrossRef CAS.
- S. S. Anand, B. K. Philip and H. M. Mehendale. Chlorination Byproducts. Encyclopedia of Toxicology, 3rd edn, 2014; pp. 855–859 Search PubMed.
- C. Prasse, D. Stalter, U. Schulte-Oehlmann, J. Oehlmann and T. A. Ternes, Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies, Water Res., 2015, 87, 237–270 CrossRef CAS PubMed.
- C. Noutsopoulos, D. Mamais, V. Samaras, T. Bouras, M. Marneri and K. Antoniou, Effect of wastewater chlorination on endocrine disruptor removal, Water Sci. Technol., 2013, 67(7), 1551–1556, DOI:10.2166/wst.2013.025.
- Q. Y. Wu, H. Y. Hu, X. Zhao and Y. X. Sun, Effect of chlorination on the estrogenic/antiestrogenic activities of biologically treated wastewater, Environ. Sci. Technol., 2009, 43(13), 4940–4945 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/abs/10.1021/es8034329.
- S. Dave and J. Das. Technological model on advanced stages of oxidation of wastewater effluent from food industry. Advanced Oxidation Processes for Effluent Treatment Plants. 2021; pp. 33–49 Search PubMed.
- M. Moreno-Benito, E. Yamal-Turbay, A. Espuña, M. Pérez-Moya and M. Graells, Optimal recipe design for Paracetamol degradation by advanced oxidation processes (AOPs) in a pilot plant, Comput.-Aided Chem. Eng., 2013, 32, 943–948 CAS.
- W. Zong, Z. Guo, M. Wu, X. Yi, H. Zhou and S. Jing, et al., Synergistic multiple active species driven fast estrone oxidation by δ-MnO2 in the existence of methanol, Sci. Total Environ., 2021, 761, 143201 CrossRef CAS PubMed.
- S. Ziembowicz, M. Kida, P. Koszelnik, J. Czarnota and M. Miąsik, Fenton-like degradation of di-n-butyl phthalate in landfill leachate by endogenous catalysts or iron, copper and manganese loaded bottom sediments, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100551 CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2215153221001264.
- I. S. Arvanitoyannis and A. Kassaveti. Olive Oil Waste Management: Treatment Methods and Potential Uses of Treated Waste. Waste Management for the Food Industries. 2008; pp. 453–568 Search PubMed.
- M. R. Kiani and M. R. Rahimpour. Aquatic/water environment contamination, treatment, and use. Current Trends and Future Developments on (Bio-) Membranes: Membranes in Environmental Applications. 2020; pp. 213–238 Search PubMed.
- K. Poddar and A. Sarkar. Emerging treatment strategies of pharmaceutical pollutants: Reactive physiochemical and innocuous biological approaches. Removal of Toxic Pollutants Through Microbiological and Tertiary Treatment. 2020; pp. 431–451 Search PubMed.
- k. h. Abbaszadeh Maroufan, H. Mirzaei, A. A. Matin, A. Javadi and A. Amani-gadim, Optimizing water ozonation conditions with the aim of removing estrone and 17β-estradiol hormones, Food Hygiene, 2020, 10(3), 57–66 Search PubMed . Available from: https://jfh.iaut.ac.ir/article_677374.html.
- H. Farzaneh, K. Loganathan, J. Saththasivam and G. McKay, Ozone and ozone/hydrogen peroxide treatment to remove gemfibrozil and ibuprofen from treated sewage effluent: Factors influencing bromate formation, Emerging Contam., 2020, 6, 225–234 CrossRef.
- M. P. Callao and M. S. Larrechi. Simultaneous Determination of Organic Dyes Using Second-Order Data. Data Handling in Science and Technology. 2015; vol. 29: pp. 399–426 Search PubMed.
- J. Rivera-Utrilla, M. Sánchez-Polo, M. Á. Ferro-García, G. Prados-Joya and R. Ocampo-Pérez, Pharmaceuticals as emerging contaminants and their removal from water. A review, Chemosphere, 2013, 93(7), 1268–1287 CrossRef CAS.
- K. Kümmerer. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks. Springer Science & Business Media; 2008 Search PubMed.
- S. Xiao, M. Cheng, H. Zhong, Z. Liu, Y. Liu and X. Yang, et al., Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review, Chem. Eng. J., 2020, 384, 123265 CrossRef CAS.
- J. Yang, M. Zhu and D. D. Dionysiou, What is the role of light in persulfate-based advanced oxidation for water treatment?, Water Res., 2021, 189, 116627 CrossRef CAS.
- H. Ming, D. Wei, Y. Yang, B. Chen, C. Yang and J. Zhang, et al., Photocatalytic activation of peroxymonosulfate by carbon quantum dots functionalized carbon nitride for efficient degradation of bisphenol A under visible-light irradiation, Chem. Eng. J., 2021, 424, 130296 CrossRef CAS.
- N. Baycan and G. Li Puma, Nanostructured catalysts for photo-oxidation of endocrine disrupting chemicals, J. Photochem. Photobiol., A, 2018, 364, 274–281 CrossRef CAS.
- K. Xu, L. Jiao, C. Wang, Y. Bu, Y. Tang and L. Qiu, et al., Nonylphenol photodegradation by novel ternary MIL-100(Fe)/ZnFe2O4/PCN composite under visible light irradiation via double charge transfer process, J. Environ. Sci., 2022, 111, 93–103 CrossRef CAS.
- V. J. P. Vilar, P. Alfonso-Muniozguren, J. P. Monteiro, J. Lee, S. M. Miranda and R. A. R. Boaventura, Tube-in-tube membrane microreactor for photochemical UVC/H2O2 processes: A proof of concept, Chem. Eng. J., 2020, 379, 122341 CrossRef CAS.
- R. M. Castellanos, J. Paulo Bassin, M. Dezotti, R. A. R. Boaventura and V. J. P. Vilar, Tube-in-tube membrane reactor for heterogeneous TiO2 photocatalysis with radial addition of H2O2, Chem. Eng. J., 2020, 395, 124998 CrossRef CAS.
- D. Błedzka, M. Gmurek, M. Gryglik, M. Olak, J. S. Miller and S. Ledakowicz, Photodegradation and advanced oxidation of endocrine disruptors in aqueous solutions, Catal. Today, 2010, 151(1–2), 125–130 CrossRef.
- G. Cao, J. Lu and G. Wang, Photolysis kinetics and influencing factors of bisphenol S in aqueous solutions, J. Environ. Sci., 2012, 24(5), 846–851 CrossRef CAS.
- C. F. Zorzo, J. J. Inticher, F. H. Borba, L. C. Cabrera, J. S. Dugatto and S. Baroni, et al., Oxidative degradation and mineralization of the endocrine disrupting chemical bisphenol-A by an eco-friendly system based on UV-solar/H2O2 with reduction of genotoxicity and cytotoxicity levels, Sci. Total Environ., 2021, 770, 145296 CrossRef CAS.
- M. Khandarkhaeva, A. Batoeva, M. Sizykh, D. Aseev and N. Garkusheva, Photo-Fenton-like degradation of bisphenol A by persulfate and solar irradiation, J. Environ. Manage., 2019, 249, 109348 CrossRef CAS PubMed.
- E. Asgari, A. Esrafili, R. Rostami and M. Farzadkia, O3, O3/UV and O3/UV/ZnO for abatement of parabens in aqueous solutions: Effect of operational parameters and mineralization/biodegradability improvement, Process Saf. Environ. Prot., 2019, 125, 238–250 CrossRef CAS.
- K. Vijayaraghavan and R. Balasubramanian, Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions, J. Environ. Manage., 2015, 160, 283–296 CrossRef CAS.
- S. Zainith, G. Saxena, R. Kishor and R. N. Bharagava. Application of microalgae in industrial effluent treatment, contaminants removal, and biodiesel production: Opportunities, challenges, and future prospects. Bioremediation for Environmental Sustainability. 2021; pp. 481–517 Search PubMed.
- S. Singh, V. Kumar, D. Singh Dhanjal, S. Datta, S. Singh and J. Singh. Biosorbents for heavy metal removal from industrial effluents. Bioremediation for Environmental Sustainability. 2021; pp. 219–233 Search PubMed.
- M. A. Usmani, I. Khan, U. Gazal, M. K. Mohamad Haafiz and A. H. Bhatk. Interplay of polymer bionanocomposites and significance of ionic liquids for heavy metal removal. Polymer-based Nanocomposites for Energy and Environmental Applications: A Volume in Woodhead Publishing Series in Composites Science and Engineering. 2018; pp. 441–463 Search PubMed.
- J. Gao, J. Ye, J. Ma, L. Tang and J. Huang, Biosorption and biodegradation of triphenyltin by Stenotrophomonas maltophilia and their influence on cellular metabolism, J. Hazard. Mater., 2014, 276, 112–119 CrossRef CAS PubMed.
- L. Bai, H. Xu, C. Wang, J. Deng and H. Jiang, Extracellular polymeric substances facilitate the biosorption of phenanthrene on cyanobacteria Microcystis aeruginosa, Chemosphere, 2016, 162, 172–180 CrossRef CAS PubMed.
- C. E. Flores-Chaparro, L. F. Chazaro Ruiz, M. C. Alfaro de la Torre, M. A. Huerta-Diaz and J. R. Rangel-Mendez, Biosorption removal of benzene and toluene by three dried macroalgae at different ionic strength and temperatures: Algae biochemical composition and kinetics, J. Environ. Manage., 2017, 193, 126–135 CrossRef CAS PubMed.
- C. Zhang, J. Lu and J. Wu, Enhanced removal of phenolic endocrine disrupting chemicals from coastal waters by intertidal macroalgae, J. Hazard. Mater., 2021, 411, 125105 CrossRef CAS PubMed.
- J. C. S. Golveia, M. F. Santiago, L. B. Silva, L. C. Campos and F. Schimidt, Utilization of the corncob agro-industrial residue as a potential adsorbent in the biosorption of bisphenol-A, J. Braz. Chem. Soc., 2021, 32(7), 1396–1404 CAS.
- J. F. Honorio, M. T. Veit, P. Y. R. Suzaki, P. F. Coldebella, E. Sloboda Rigobello and C. R. G. Tavares, Adsorption of naturals hormones estrone, 17β-estradiol, and estriol by rice husk: monocomponent and multicomponent kinetics and equilibrium, Environ. Technol., 2020, 41(9), 1075–1092, DOI:10.1080/09593330.2018.1521472.
- R. L. Crawford. Biodegradation: Principles, Scope, and Technologies. Comprehensive Biotechnology, 2nd edn. 2011; vol. 6: pp. 3–13 Search PubMed.
- S. E. Jørgensen. Biodegradation. Encyclopedia of Ecology, Five-Volume Set. 2008; pp. 366–367 Search PubMed.
- C. D. Shackelford. Geoenvironmental Engineering. Reference Module in Earth Systems and Environmental Sciences. 2013, https://linkinghub.elsevier.com/retrieve/pii/B9780124095489054245 Search PubMed.
- R. Mtibaà, A. Ezzanad, E. Aranda, C. Pozo, B. Ghariani and J. Moraga, et al., Biodegradation and toxicity reduction of nonylphenol, 4-tert-octylphenol and 2,4-dichlorophenol by the ascomycetous fungus Thielavia sp HJ22: Identification of fungal metabolites and proposal of a putative pathway, Sci. Total Environ., 2020, 708, 135129 CrossRef.
- J. Ye, H. Yin, H. Peng, J. Bai, D. Xie and L. Wang, Biosorption and biodegradation of triphenyltin by Brevibacillus brevis, Bioresour. Technol., 2013, 129, 236–241 CrossRef CAS PubMed.
- A. Baker, B. Ahmad, K. M. Alarjani, A. N. saleem and M. S. Khan, Biostimulation of Rhodovulum sp., for enhanced degradation of di-n-butyl phthalate under optimum conditions, Chemosphere, 2021, 266, 128998 CrossRef CAS.
- J. Gao and J. Chi, Biodegradation of phthalate acid esters by different marine microalgal species, Mar. Pollut. Bull., 2015, 99(1–2), 70–75 CrossRef CAS.
- B. J. K. Brasil, J. B. Tiago, E. da. C. Rayra, A. L. R. Maria, D. S. Daniele and D. de. A. Rafael, et al., Bacterial Community Structure Applied to Hormone Degradation, J. Environ. Eng., 2019, 145(12), 4019086, DOI:10.1061/(ASCE)EE.1943-7870.0001607.
- H. Dai, S. Gao, C. Lai, H. He, F. Han and X. Pan, Biochar enhanced microbial degradation of 17β-estradiol, Environ. Sci.: Processes Impacts, 2019, 21(10), 1736–1744, 10.1039/C9EM00168A.
- M. Piao, D. Zou, X. Ren, S. Gao, C. Qin and Y. Piao, High efficiency biotransformation of bisphenol A in a fluidized bed reactor using stabilized laccase in porous silica, Enzyme Microb. Technol., 2019, 126, 1–8 CrossRef CAS PubMed.
- L. Alvarado-Ramírez, M. Rostro-Alanis, J. Rodríguez-Rodríguez, C. Castillo-Zacarías, J. E. Sosa-Hernández and D. Barceló, et al., Exploring current tendencies in techniques and materials for immobilization of laccases – A review, Int. J. Biol. Macromol., 2021, 181, 683–696 CrossRef PubMed.
- G. Bayramoglu, B. Karagoz and M. Y. Arica, Cyclic-carbonate functionalized polymer brushes on polymeric microspheres: Immobilized laccase for degradation of endocrine disturbing compounds, J. Ind. Eng. Chem., 2018, 60, 407–417 CrossRef CAS.
- C. Barrios-Estrada, M. de Jesús Rostro-Alanis, B. D. Muñoz-Gutiérrez, H. M. N. Iqbal, S. Kannan and R. Parra-Saldívar, Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation – A review, Sci. Total Environ., 2018, 612, 1516–1531 CrossRef CAS.
- M. Fu, J. Xing and Z. Ge, Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A, Sci. Total Environ., 2019, 651, 2857–2865 CrossRef CAS PubMed.
- H. C. Eldridge, A. Milliken, C. Farmer, A. S. Hampton, N. Wendland and L. Coward, et al., Efficient remediation of 17α-ethinylestradiol by Lentinula edodes (shiitake) laccase, Biocatal. Agric. Biotechnol., 2017, 10, 64–68 CrossRef.
- S. Beck, E. Berry, S. Duke, A. Milliken, H. Patterson and D. L. Prewett, et al., Characterization of Trametes versicolor laccase-catalyzed degradation of estrogenic pollutants: Substrate limitation and product identification, Int. Biodeterior. Biodegrad., 2018, 127, 146–159 CrossRef CAS.
- K. Sun, Q. Liu, S. Li, Y. Qi and Y. Si, MnO2 nanozyme-driven polymerization and decomposition mechanisms of 17β-estradiol: Influence of humic acid, J. Hazard. Mater., 2020, 393, 122393 CrossRef CAS PubMed.
- L. Mao, Q. Huang, J. Lu and S. Gao, Ligninase-mediated removal of natural and synthetic estrogens from water: I. Reaction behaviors, Environ. Sci. Technol., 2009, 43(2), 374–379 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/abs/10.1021/es801791v.
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Nanozyme: new horizons for responsive biomedical applications, Chem. Soc. Rev., 2019, 48(14), 3683–3704 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2019/cs/c8cs00718g.
- X. Wu, Y. Zhang, T. Han, H. Wu, S. Guo and J. Zhang, Composite of graphene quantum dots and Fe3O4 nanoparticles: peroxidase activity and application in phenolic compound removal, RSC Adv., 2013, 4(7), 3299–3305 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2014/ra/c3ra44709j.
- J. Wang, R. Huang, W. Qi, R. Su, B. P. Binks and Z. He, Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants, Appl. Catal., B, 2019, 254, 452–462 CrossRef CAS.
- Z. Zhang, L. M. Bragg, M. R. Servos and J. Liu, Gold nanoparticles as dehydrogenase mimicking nanozymes for estradiol degradation, Chin. Chem. Lett., 2019, 30(9), 1655–1658 CrossRef CAS.
- C. Geiß, K. Ruppert, T. Heidelbach and J. Oehlmann, The antimicrobial agents triclocarban and triclosan as potent modulators of reproduction in Potamopyrgus antipodarum (Mollusca: Hydrobiidae), J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2016, 51(13), 1173–1179 CrossRef PubMed.
- M. Han, Y. Wang, C. Tang, H. Fang, D. Yang and J. Wu, et al., Association of triclosan and triclocarban in urine with obesity risk in Chinese school children, Environ. Int., 2021, 157, 106846 CrossRef CAS.
- J. Stec, C. Vilchèze, S. Lun, A. L. Perryman, X. Wang and J. S. Freundlich, et al., Biological Evaluation of Potent Triclosan-Derived Inhibitors of the Enoyl–Acyl Carrier Protein Reductase InhA in Drug-Sensitive and Drug-Resistant Strains of Mycobacterium tuberculosis, ChemMedChem, 2014, 9(11), 2528–2537 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cmdc.201402255.
- H. Li, Y. Zhao, L. Chen, Y. Su, X. Li and L. Jin, et al., Triclocarban and Triclosan Inhibit Human Aromatase via Different Mechanisms, BioMed Res. Int., 2017, 2017, 8284097 Search PubMed.
- H. S. Kim, S. Y. Han, S. D. Yoo, B. M. Lee and K. L. Park, Potential Estrogenic Effects of Bisphenol-A Estimated by in vitro and in vivo combination assays, J. Toxicol. Sci., 2001, 26(3), 111–118 CrossRef CAS.
- J. Lin, L. Deng, M. Sun, Y. Wang, S. Lee and K. Choi, et al., An in vitro investigation of endocrine disrupting potentials of ten bisphenol analogues, Steroids, 2021, 169, 108826 CrossRef CAS.
- A. Tabata, N. Watanabe, I. Yamamoto, Y. Ohnishi, M. Itoh and T. Kamel, et al., The effect of bisphenol A and chlorinated derivatives of bisphenol A on the level of serum vitellogenin in Japanese medaka (Oryzias latipes), Water Sci. Technol., 2004, 50(5), 125–132 CrossRef CAS PubMed.
- H. Serra, C. Beausoleil, R. Habert, C. Minier, N. Picard-Hagen and C. Michel, Evidence for Bisphenol B Endocrine Properties: Scientific and Regulatory Perspectives, Environ. Health Perspect., 127(10), 106001 CrossRef PubMed . Available from: https://ehp.niehs.nih.gov/doi/10.1289/EHP5200.
- Q. Yang, X. Yang, J. Liu, W. Ren, Y. Chen and S. Shen, Exposure to Bisphenol B Disrupts Steroid Hormone Homeostasis and Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis of Zebrafish, Water, Air, Soil Pollut., 2017, 228(3), 1–12 CrossRef CAS . Available from: https://link.springer.com/article/10.1007/s11270-017-3282-z.
- K. Owczarek, B. Kudłak, V. Simeonov, Z. Mazerska and J. Namieśnik, Binary Mixtures of Selected Bisphenols in the Environment: Their Toxicity in Relationship to Individual Constituents, Molecules, 2018, 23(12), 3226 CrossRef . Available from: https://www.mdpi.com/1420-3049/23/12/3226/htm.
- C. Pinto, R. Hao, M. Grimaldi, S. Thrikawala, A. Boulahtouf and S. Aït-Aïssa, et al., Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo, Toxicol. Appl. Pharmacol., 2019, 380, 114709 CrossRef CAS.
- F. G. A. Godoi, M. Muñoz-Peñuela, A. D. O. Gomes, C. E. Tolussi, G. Brambila-Souza and G. S. Branco, et al., Endocrine disruptive action of diclofenac and caffeine on Astyanax altiparanae males (Teleostei: Characiformes: Characidae), Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2020, 231, 108720 CAS.
- T. Kida, S. Kozai, H. Takahashi, M. Isaka, H. Tokushige and T. Sakamoto, Pharmacokinetics and Efficacy of Topically Applied Nonsteroidal Anti-Inflammatory Drugs in Retinochoroidal Tissues in Rabbits, PLoS One, 2014, 9(5), e96481 CrossRef PubMed . Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0096481.
- R. Triebskorn, H. Casper, A. Heyd, R. Eikemper, H. R. Köhler and J. Schwaiger, Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss), Aquat. Toxicol., 2004, 68(2), 151–166 CrossRef CAS PubMed.
- D. Balabanič, M. Filipič, A. Krivograd Klemenčič and B. Žegura, Genotoxic activity of endocrine disrupting compounds commonly present in paper mill effluents, Sci. Total Environ., 2021, 794, 148489 CrossRef.
- L. Kaun-Yu, T. Fu-Wei, W. Chia-Jung and L. Pei-Shan, Suppression by phthalates of the calcium signaling of human nicotinic acetylcholine receptors in human neuroblastoma SH-SY5Y cells, Toxicology, 2004, 200(2–3), 113–121 CrossRef.
- S. Zhou, Q. Chen, C. di Paolo, Y. Shao, H. Hollert and T. B. Seiler, Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals, Sci. Total Environ., 2019, 664, 89–98 CrossRef CAS.
- C. Willson, The clinical toxicology of caffeine: A review and case study, Toxicol. Rep., 2018, 5, 1140–1152 CrossRef CAS.
- C. Y. Chen, Y. J. Wang and C. F. Yang, Estimating low-toxic-effect concentrations in closed-system algal toxicity tests, Ecotoxicol. Environ. Saf., 2009, 72(5), 1514–1522 CrossRef CAS PubMed.
- C. G. Park, K. C. Jung, D. H. Kim and Y. J. Kim, Monohaloacetonitriles induce cytotoxicity and exhibit different mode of action in endocrine disruption, Sci. Total Environ., 2021, 761, 143316 CrossRef CAS.
- O. A. Ogunbayo, P. F. Lai, T. J. Connolly and F. Michelangeli, Tetrabromobisphenol A (TBBPA), induces cell death in TM4 Sertoli cells by modulating Ca2+ transport proteins and causing dysregulation of Ca2+ homeostasis, Toxicol. in Vitro, 2008, 22(4), 943–952 CrossRef CAS PubMed.
- X. M. Ren, L. Yao, Q. Xue, J. Shi, Q. Zhang and P. Wang, et al., Binding and Activity of Tetrabromobisphenol A Mono-Ether Structural Analogs to Thyroid Hormone Transport Proteins and Receptors, Environ. Health Perspect., 2020, 128(10), 1–14 CrossRef PubMed . Available from: https://ehp.niehs.nih.gov/doi/10.1289/EHP6498.
- S. Wang, C. Ji, F. Li, J. Zhan, T. Sun and J. Tang, et al., Tetrabromobisphenol A induced reproductive endocrine-disrupting effects in mussel Mytilus galloprovincialis, J. Hazard. Mater., 2021, 416, 126228 CrossRef CAS PubMed.
- M. Ezechiáš, K. Svobodová and T. Cajthaml, Hormonal activities of new brominated flame retardants, Chemosphere, 2012, 87(7), 820–824 CrossRef.
- J. Fu, Y. Guo, M. Wang, L. Yang, J. Han and J. S. Lee, et al., Bioconcentration of 2,4,6-tribromophenol (TBP) and thyroid endocrine disruption in zebrafish larvae, Ecotoxicol. Environ. Saf., 2020, 206, 111207 CrossRef CAS.
- G. A. Knudsen, A. W. Trexler, A. C. Richards, S. M. Hall, M. F. Hughes and L. S. Birnbaum, 2,4,6-Tribromophenol Disposition and Kinetics in Rodents: Effects of Dose, Route, Sex, and Species, Toxicol. Sci., 2019, 169(1), 167–179 CrossRef CAS PubMed . Available from: https://academic.oup.com/toxsci/article/169/1/167/5320422.
- K. Gandhi, S. Khan, M. Patrikar, A. Markad, N. Kumar and A. Choudhari, et al., Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants, Environ. Challenges, 2021, 4, 100149 CrossRef CAS.
- K. K. Jaiswal, V. Kumar, M. S. Vlaskin and M. Nanda, Impact of glyphosate herbicide stress on metabolic growth and lipid inducement in Chlorella sorokiniana UUIND6 for biodiesel production, Algal Res., 2020, 51, 102071 CrossRef.
- P. Kalofiri, G. Balias and F. Tekos, The EU endocrine disruptors' regulation and the glyphosate controversy, Toxicol. Rep., 2021, 8, 1193–1199 CrossRef CAS PubMed.
- K. Hu, L. Zhou, Y. Gao, Q. Lai, H. Shi and M. Wang, Enantioselective endocrine-disrupting effects of the phenylpyrazole chiral insecticides in vitro and in silico, Chemosphere, 2020, 252, 126572 CrossRef CAS.
- T. Narahashi. Neurophysiological Effects of Insecticides. Hayes' Handbook of Pesticide Toxicology. 2010; pp. 799–817 Search PubMed.
- C. Paredes, J. Iannacone and L. Alvariño, Efecto subletal del fipronil en el desenvolvimiento de embriones de oncorhynchus mykiss “trucha arco iris”, Biotempo, 2010, 10, 9–14 CrossRef . Available from: https://revistas.urp.edu.pe/index.php/Biotempo/article/view/848.
- T. Y. Jeong and M. J. Simpson, Reproduction stage specific dysregulation of Daphnia magna metabolites as an early indicator of reproductive endocrine disruption, Water Res., 2020, 184, 116107 CrossRef CAS.
- M. Sha, S. Li, Y. Lu and G. Cheng, Direct and indirect effects of fenoxycarb on freshwater systems dominated by Neocaridina palmata (Decapoda: Atyidae) and macrophyte Ceratophyllum demersum, Ecotoxicol. Environ. Saf., 2021, 219, 112304 CrossRef CAS.
- C. J. G. M. Smulders, T. J. H. Bueters, R. G. D. M. van Kleef and H. P. M. Vijverberg, Selective effects of carbamate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase, Toxicol. Appl. Pharmacol., 2003, 193(2), 139–146 CrossRef CAS.
- F. L. Chiriac, F. Pirvu and I. Paun, Investigation of endocrine disruptor pollutants and their metabolites along the Romanian Black Sea Coast: Occurrence, distribution and risk assessment, Environ. Toxicol. Pharmacol., 2021, 86, 103673 CrossRef CAS PubMed.
- S. Lu, C. Lin, K. Lei, M. Xin, B. Wang and W. Ouyang, et al., Endocrine-disrupting chemicals in a typical urbanized bay of Yellow Sea, China: Distribution, risk assessment, and identification of priority pollutants, Environ. Pollut., 2021, 287, 117588 CrossRef CAS PubMed.
- D. Fan, W. Yin, W. Gu, M. Liu, J. Liu and Z. Wang, et al., Occurrence, spatial distribution and risk assessment of high concern endocrine-disrupting chemicals in Jiangsu Province, China, Chemosphere, 2021, 285, 131396 CrossRef CAS.
- M. Triassi, P. Montuori, D. P. Provvisiero, E. de Rosa, F. di Duca and P. Sarnacchiaro, et al., Occurrence and spatial-temporal distribution of atrazine and its metabolites in the aquatic environment of the Volturno River estuary, southern Italy, Sci. Total Environ., 2022, 803, 149972 CrossRef CAS PubMed.
- M. Čelić, B. D. Škrbić, S. Insa, J. Živančev, M. Gros and M. Petrović, Occurrence and assessment of environmental risks of endocrine disrupting compounds in drinking, surface and wastewaters in Serbia, Environ. Pollut., 2020, 262, 114344 CrossRef.
- Z. Huang, J. L. Zhao, Y. Y. Yang, Y. W. Jia, Q. Q. Zhang and C. E. Chen, et al., Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: Urban rainfall runoff as a potential source for receiving rivers, Environ. Pollut., 2020, 263, 114361 CrossRef CAS.
- P. Šauer, H. Švecová, K. Grabicová, F. Gönül Aydın, T. Mackuľak and V. Kodeš, et al., Bisphenols emerging in Norwegian and Czech aquatic environments show transthyretin binding potency and other less-studied endocrine-disrupting activities, Sci. Total Environ., 2021, 751, 141801 CrossRef.
- B. Yao, S. Yan, L. Lian, X. Yang, C. Wan and H. Dong, et al., Occurrence and indicators of pharmaceuticals in Chinese streams: A nationwide study, Environ. Pollut., 2018, 236, 889–898 CrossRef CAS PubMed.
- Y. Y. Yang, J. L. Zhao, Y. S. Liu, W. R. Liu, Q. Q. Zhang and L. Yao, et al., Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination, Sci. Total Environ., 2018, 616–617, 816–823 CrossRef CAS.
- K. López-Velázquez, J. L. Guzmán-Mar, T. J. Montalvo-Herrera, S. Y. Mendiola-Alvarez and M. Villanueva-Rodríguez, Efficient photocatalytic removal of four endocrine-disrupting compounds using N-doped BiOBr catalyst under UV-Vis radiation, J. Environ. Chem. Eng., 2021, 9(5), 106185 CrossRef.
- X. Zhou, F. Peng, Z. Luo, Y. Li, H. Li and Z. Yang, Assessment of water contamination and health risk of endocrine disrupting chemicals in outdoor and indoor swimming pools, Sci. Total Environ., 2020, 704, 135277 CrossRef CAS.
- T. L. L. Teo, H. M. Coleman and S. J. Khan, Presence and select determinants of organophosphate flame retardants in public swimming pools, Sci. Total Environ., 2016, 569–570, 469–475 CrossRef CAS.
- J. Xia, J. Di, H. Li, H. Xu, H. Li and S. Guo, Ionic liquid-induced strategy for carbon quantum dots/BiOX (X = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis, Appl. Catal., B, 2016, 181, 260–269 CrossRef CAS.
- M. Ji, Z. Zhang, J. Xia, J. Di, Y. Liu and R. Chen, et al., Enhanced photocatalytic performance of carbon quantum dots/BiOBr composite and mechanism investigation, Chin. Chem. Lett., 2018, 29(6), 805–810 CrossRef CAS.
- J. Hou, H. Li, Y. Tang, J. Sun, H. Fu and X. Qu, et al., Supported N-doped carbon quantum dots as the highly effective peroxydisulfate catalysts for bisphenol F degradation, Appl. Catal., B, 2018, 238, 225–235 CrossRef CAS.
- J. Di, J. Xia, Y. Ge, H. Li, H. Ji and H. Xu, et al., Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight, Appl. Catal., B, 2015, 168–169, 51–61 CrossRef CAS.
- J. Di, J. Xia, M. Ji, H. Li, H. Xu and H. Li, et al., The synergistic role of carbon quantum dots for the improved photocatalytic performance of Bi2MoO6, Nanoscale, 2015, 7(26), 11433–11443 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2015/nr/c5nr01350j.
- R. Guo, D. Zeng, Y. Xie, Y. Ling, D. Zhou and L. Jiang, et al., Carbon nitride quantum dots (CNQDs)/TiO2 nanoparticle heterojunction photocatalysts for enhanced ultraviolet-visible-light-driven bisphenol a degradation and H2 production, Int. J. Hydrogen Energy, 2020, 45(43), 22534–22544 CrossRef CAS.
- J. Mamo, M. J. García-Galán, M. Stefani, S. Rodríguez-Mozaz, D. Barceló and H. Monclús, et al., Fate of pharmaceuticals and their transformation products in integrated membrane systems for wastewater reclamation, Chem. Eng. J., 2018, 331, 450–461 CrossRef CAS.
- E. N. de Freitas, G. A. Bubna, T. Brugnari, C. G. Kato, M. Nolli and T. G. Rauen, et al., Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products, Chem. Eng. J., 2017, 330, 1361–1369 CrossRef.
- M. Bilal, M. Asgher, H. M. N. Iqbal, H. Hu and X. Zhang, Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates, Environ. Sci. Pollut. Res., 2017, 24(8), 7035–7041 CrossRef CAS . Available from: https://link.springer.com/article/10.1007/s11356-017-8369-y.
- J. M. Marín-Benito, L. Alletto, E. Barriuso, C. Bedos, P. Benoit and V. Pot, et al., Pesticide fate modelling in conservation tillage: Simulating the effect of mulch and cover crop on S-metolachlor leaching, Sci. Total Environ., 2018, 628–629, 1508–1517 CrossRef.
- T. Gong, X. Xu, Y. Dang, A. Kong, Y. Wu and P. Liang, et al., An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates, Sci. Total Environ., 2018, 628–629, 1258–1265 CrossRef CAS PubMed.
- Y. Jia, T. Xiao, J. Sun, F. Yang and P. C. Baveye, Microcolumn-based speciation analysis of thallium in soil and green cabbage, Sci. Total Environ., 2018, 630, 146–153 CrossRef CAS.
- M. Ahuactzin-Pérez, S. Tlecuitl-Beristain, J. García-Dávila, E. Santacruz-Juárez, M. González-Pérez and M. C. Gutiérrez-Ruíz, et al., A novel biodegradation pathway of the endocrine-disruptor di(2-ethyl hexyl) phthalate by Pleurotus ostreatus based on quantum chemical investigation, Ecotoxicol. Environ. Saf., 2018, 147, 494–499 CrossRef PubMed.
- T. Gong, X. Xu, Y. Dang, A. Kong, Y. Wu and P. Liang, et al., An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates, Sci. Total Environ., 2018, 628–629, 1258–1265 CrossRef CAS.
- M. Cellura, M. A. Cusenza and S. Longo, Energy-related GHG emissions balances: IPCC versus LCA, Sci. Total Environ., 2018, 628–629, 1328–1339 CrossRef CAS PubMed.
- J. Gupta, R. Rathour, R. Singh and I. S. Thakur, Production and characterization of extracellular polymeric substances (EPS) generated by a carbofuran degrading strain Cupriavidus sp. ISTL7, Bioresour. Technol., 2019, 282, 417–424 CrossRef CAS PubMed.
- D. Iark, A. J. d. R. Buzzo, J. A. A. Garcia, V. G. Côrrea, C. V. Helm and R. C. G. Corrêa, et al., Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii, Bioresour. Technol., 2019, 289, 121655 CrossRef CAS.
- L. Bai, Q. Zhang, Q. Ju, C. Wang, H. Zhang and H. Jiang, Priming effect of autochthonous organic matter on enhanced degradation of 17α-ethynylestradiol in water-sediment system of one eutrophic lake, Water Res., 2020, 184, 116153 CrossRef CAS PubMed.
- Y. Wang, Q. Sun, Y. Li, H. Wang, K. Wu and C. P. Yu, Biotransformation of estrone, 17β-estradiol and 17α-ethynylestradiol by four species of microalgae, Ecotoxicol. Environ. Saf., 2019, 180, 723–732 CrossRef CAS.
- W. Ma, J. Sun, Y. Li, X. Lun, D. Shan and C. Nie, et al., 17α-Ethynylestradiol biodegradation in different river-based groundwater recharge modes with reclaimed water and degradation-associated community structure of bacteria and archaea, J. Environ. Sci., 2018, 64, 51–61 CrossRef CAS.
- B. Muszyńska, P. Żmudzki, J. Lazur, K. Kała, K. Sułkowska-Ziaja and W. Opoka, Analysis of the biodegradation of synthetic testosterone and 17α-ethynylestradiol using the edible mushroom Lentinula edodes. 3, Biotech, 2018, 8(10), 1–15 Search PubMed . Available from: https://link.springer.com/article/10.1007/s13205-018-1458-x.
- R. Sharma, N. Verma, Y. Lugani, S. Kumar and M. Asadnia. Conventional and advanced techniques of wastewater monitoring and treatment. Green Sustainable Process for Chemical and Environmental Engineering and Science. 2021; pp. 1–48 Search PubMed.
- E. Carmona, V. Andreu and Y. Picó, Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water, Sci. Total Environ., 2014, 484(1), 53–63 CrossRef CAS.
- F. Riva, S. Castiglioni, E. Fattore, A. Manenti, E. Davoli and E. Zuccato, Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk, Int. J. Hyg. Environ. Health, 2018, 221(3), 451–457 CrossRef CAS PubMed.
- D. Álvarez-Muñoz, S. Rodríguez-Mozaz, S. Jacobs, A. Serra-Compte, N. Cáceres and I. Sioen, et al., Pharmaceuticals and endocrine disruptors in raw and cooked seafood from European market: Concentrations and human exposure levels, Environ. Int., 2018, 119, 570–581 CrossRef.
- R. N. Alves, A. L. Maulvault, V. L. Barbosa, S. Cunha, C. J. A. F. Kwadijk and D. Álvarez-Muñoz, et al., Preliminary assessment on the bioaccessibility of contaminants of emerging concern in raw and cooked seafood, Food Chem. Toxicol., 2017, 104, 69–78 CrossRef CAS.
- F. v. Mello, S. C. Cunha, F. H. S. Fogaça, M. B. Alonso, J. P. M. Torres and J. O. Fernandes, Occurrence of pharmaceuticals in seafood from two Brazilian coastal areas: Implication for human risk assessment, Sci. Total Environ., 2022, 803, 149744 CrossRef CAS PubMed.
- M. B. Asif, B. Ren, C. Li, T. Maqbool, X. Zhang and Z. Zhang, Powdered activated carbon – Membrane bioreactor (PAC-MBR): Impacts of high PAC concentration on micropollutant removal and microbial communities, Sci. Total Environ., 2020, 745, 141090 CrossRef CAS PubMed.
- C. F. Zorzo, J. J. Inticher, F. H. Borba, L. C. Cabrera, J. S. Dugatto and S. Baroni, et al., Oxidative degradation and mineralization of the endocrine disrupting chemical bisphenol-A by an eco-friendly system based on UV-solar/H2O2 with reduction of genotoxicity and cytotoxicity levels, Sci. Total Environ., 2021, 770, 145296 CrossRef CAS PubMed.
- D. A. da Silva Rodrigues, C. C. R. F. da Cunha, M. G. Freitas, A. L. C. de Barros, P. B. N. e Castro and A. R. Pereira, et al., Biodegradation of sulfamethoxazole by microalgae-bacteria consortium in wastewater treatment plant effluents, Sci. Total Environ., 2020, 749, 141441 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |
