Open Access Article
Open Access ArticleRecent progress in ZnCo2O4 and its composites for energy storage and conversion: a review
Josué M.
Gonçalves
 *a,
Matheus I.
da Silva
a,
Murillo N. T.
Silva
b,
Paulo R.
Martins
*a,
Matheus I.
da Silva
a,
Murillo N. T.
Silva
b,
Paulo R.
Martins
 c,
Edson
Nossol
b,
Henrique E.
Toma
c,
Edson
Nossol
b,
Henrique E.
Toma
 a and
Lucio
Angnes
a and
Lucio
Angnes
 *a
*a
aInstituto de Química, Universidade de São Paulo, Av. Prof. Lineu Prestes 748, 05508-000, São Paulo-SP, Brazil. E-mail: josuemartins@usp.br; luangnes@iq.usp.br
bInstitute of Chemistry, Federal University of Uberlândia, Av. João Naves de Ávila 2121, 38400-902, Uberlândia-MG, Brazil
cInstituto de Química, Universidade Federal de Goiás, Av. Esperança s/n, 74690-900, Goiânia-GO, Brazil
First published on 3rd October 2022
Abstract
Transition metal oxides have attracted growing attention for application in energy storage and conversion technologies. In particular, spinel-based materials, such as ZnCo2O4, exhibit structures suitable for performing as multifunctional electrodes in energy devices. In fact, great efforts have been dedicated to the design of micro- and nanomaterials based on ZnCo2O4, using different synthesis approaches and controlled conditions. Consequently, interesting morphologies and structures have been recently obtained, exhibiting outstanding electrochemical performance. Hence, in this review we report a comprehensive survey of the progress of multifunctional ZnCo2O4-based materials, focusing on the development of supercapacitor devices and batteries. The top 10 electrode materials for each application are highlighted, including key findings in the development of slurry-cast or binder-free electrodes. In addition, the main strategies in the design of ZnCo2O4-based electrocatalysts for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) are reviewed, including electrocatalysts capable of performing tetra-electron oxygen reduction reactions (ORRs).
1. Introduction
Clean, sustainable and efficient technologies for energy production, conversion, and storage are becoming crucial for the energy crisis which is confronting the world.1 In this regard, the development of new electrode materials may play a primary role, impacting the performance of these energy systems.1 Therefore, materials chemistry is becoming the key to the design of systems that can overcome the current challenges of our modern society. Among the emerging challenges, one can highlight the development of electrode materials capable of using solar energy and/or electricity to promote the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) by electrochemical and/or photochemical water-splitting processes, respectively. This corresponds to the conversion of renewable energy into a high-energy-content chemical species, approaching the ultimate clean energy resource due to the zero emission of carbonaceous species.2 Another challenging step is how to store energy more efficiently, especially in a faster way, e.g., by assembling devices with high energy and power density. This is the case of hybrid supercapacitors (HSCs) combining the outstanding power density of supercapacitive materials with the high-energy density of battery-type materials.3Among the emerging materials recently studied, transition metal oxides (TMOs) deserve special consideration because of their rich redox chemistry and abundant density of active sites, in addition to their low cost, environmental friendliness, and excellent electrochemical performance.4,5 In fact, special attention has been given to spinel materials with a bimetallic oxide structure of the typical chemical formula AB2O4. Spinels consist of cation A, typically charged as 2+, in tetrahedral sites (Td), and cation B charged as 3+ occupying octahedral sites (Oh).6 The interest in this type of material is justified by its higher electrochemical activity, electrical conductivity, and more abundant redox reactions, compared with monometallic oxides of the types A3O4 and B3O4.7,8
It is also important to mention that among various spinel-type oxides, structures based on bimetallic cobaltite (MCo2O4, where M = Mg, Ni, Zn, Cu, Fe, and Mn) have been most widely reported,9 as recently summarized in several review articles. In particular, one can highlight the use of nickel cobaltite spinel (NiCo2O4) in different applications such as in supercapacitors,9,10 batteries11 and sensors.12 Similarly, Gonçalves et al.8 summarized the main advances in MnCo2O4-based materials for energy applications and the main strategies used for the design of these materials, including HSCs, LIBs and MABs, as well as the advancements achieved as electrocatalysts for water-splitting, more specifically for the HER and OER. Similarly, Wu and colleagues13 highlighted the current research progress regarding synthetic strategies for MgCo2O4-based electrode materials and their applications in supercapacitors, Li-ion batteries, Mg-ion batteries, and some other rechargeable ion batteries. J. Sun, C. Xu & H. Chen14 reviewed the synthesis of CuCo2O4-based electrode materials and their applications in supercapacitors, while Gao et al.15 briefly summarized the recent applications of FeCo2O4 (and CoFe2O4) in energy storage and conversion, as well as the current understanding of the mechanisms and especially the relevance of morphologies and structures and composites to the electrochemical performance.
As shown above, several review articles show the progress made for Mg, Mn, Fe, Cu and especially Ni cobaltite. However, to our knowledge, more than 800 papers report the syntheses and/or use of ZnCo2O4 spinel for various applications, including sensor, and energy conversion and storage applications. Its multifunctionality and excellent electrochemical properties are closely related to its structure which presents a regular spinel structure where Zn2+ only replaces Co2+ in the Td sites in Co3O4, leaving the Co3+ content in the Oh sites unchanged, while Ni and Mn mainly occupy Oh sites in NiCo2O4 and MnCo2O4.16 In fact, the effect of the oxidation state and cation distribution in the spinel on the electrocatalytic activity for the OER in an alkaline solution has been studied, and a comparison of the electrochemical and physicochemical behavior of MCo2O4 (where M = Mn, Fe, Co, Ni, and Zn) was made by M. Harada, F. Kotegawa, & M. Kuwa.16 Interestingly, their catalytic activity for the OER follows the order: ZnCo2O4 > NiCo2O4 > FeCo2O4 > Co3O4 > MnCo2O4. According to the authors, the active sites for the OER are M3+ species in the octahedral site, and their activities are significantly dependent on the Co3+/Co2+ and M3+/M2+ content ratios in the octahedral site as demonstrated according to XPS and in situ X-ray absorption fine structure (XAFS) measurements, demonstrating the importance of the presence of Zn2+ ions in ZnCo2O4.16 Complementarily, ZnCo2O4 is a promising energy storage material which shows advantageous properties, including low cost, low-toxicity, different morphologies, high electrical conductivity,17,18 and high theoretical capacity in comparison with unitary ZnO and CoO and binary Co3O4.17
Inspired by the above considerations, and despite being the second most reported cobaltite, as far as we know, there is no review work summarizing recent progress in ZnCo2O4 in energy applications. Therefore, in this review article we focus on ZnCo2O4 and its composites as electrode materials for energy technologies, including the main strategies used for the design (Scheme 1) of HSCs, LIBs and MABs, as well as the advancements as electrocatalysts for water-splitting (HER and OER) and the ORR. The pros and cons of using this spinel in the different devices are critically discussed, encompassing the perspectives and possible future directions.
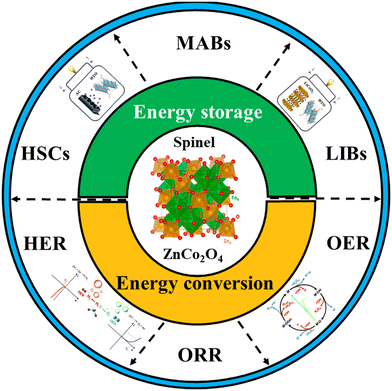 | ||
| Scheme 1 Applications of ZnCo2O4-based materials. Reproduced with permission.19 Copyright © 2018, The Author(s). Creative Commons CC BY license. Reproduced with permission.20 Copyright Royal Society of Chemistry, 2017. Reproduced with permission.21 Copyright: © 2021 by the authors. Licensee MDPI, Basel, Switzerland (CC BY). | ||
2. Water-splitting and electrochemical energy storage systems
2.1. Water-splitting
Electrocatalytic water-splitting is an effective way to produce hydrogen with high purity.22 The overall reaction includes two half reactions, e.g., HER and OER, taking place, respectively, at the cathode and the anode,23 as shown in Fig. 1A. In addition, the water splitting reactions are dependent on the pH, as expected for reactions involving protons,23 as demonstrated by the equations presented in Fig. 1B. For instance, for the HER, there are two main steps on the electrode surface, described by the Volmer–Heyrovsky and Volmer–Tafel mechanisms proposed for acidic and basic solutions24 (Fig. 1C). On the other hand, the OER is a more complex, requiring a high energy to overcome the sluggish kinetic barrier associated with the four-electron transfer process, and involves a larger overpotential25 (Fig. 1C).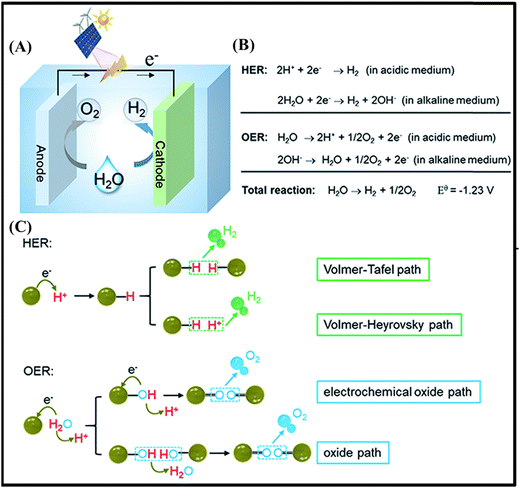 | ||
| Fig. 1 (A) Scheme of a conventional water electrolyzer. (B) Water splitting reactions under acidic and alkaline conditions. (C) Proposed mechanisms of the HER and OER in an acidic aqueous solution. Reproduced with permission.26 Copyright Marketplace™, Royal Society of Chemistry. | ||
The electrocatalytic performance is usually measured by linear sweep voltammetry (LSV), cyclic voltammetry (CV)25 and electrochemical chrono-methods where several parameters are used to classify catalysts according to their performance, and even to unravel the reaction mechanisms. Among the electrochemical activity criteria, the overpotential (η), Tafel slope and stability are the most used ones to study the performance of electrocatalysts based on metal oxides/hydroxides.
The overpotential (η) is one of the essential criteria to evaluate the activity of electrocatalysts. It represents the difference between the potentials for achieving a specific current density and the onset potential to start the reaction (HER = 0 V and OER = 1.23 V).25 Generally, the overpotentials at a current density of 10 mA cm−2 (η10) are used to compare the electrocatalytic activity between different catalysts. This corresponds to the equivalent efficiency of 12.3% for photoelectrochemical water splitting.25 In practice, a catalyst providing an overpotential in the range of 300–400 mV is considered to be an excellent catalyst for the OER.27,28 However, η10 has a great influence on the loading mass even considering the same geometrical area; thus it cannot be the only criterion to evaluate the activity.25 In this regard, Tafel analysis provides additional information to understand the reaction kinetics and mechanism, such as the magnitude of the slope, which helps in establishing the rate-determining step and the response sensitivity.27 For instance, Tafel slopes of 120, 40 and 30 mV dec−1 were observed, respectively, for the Volmer, Heyrovsky and Tafel determining rate steps. The smaller value of the Tafel slope means a faster electron-transfer kinetics of the electrocatalyst.22,24,25
In addition to low overpotential and Tafel slope values, a good electrocatalyst should also be stable for long periods of time, under operating conditions. This evaluation can be performed by different techniques, including continuous CV cycling and LSV. The measurements allow comparing the overpotentials before and after cycling.25 Another way to obtain information about the stability of electrocatalysts is by galvanostatic or potentiostatic electrolysis, registering the variation of potential or current density.25
In summary, the overpotential (η), Tafel slope and stability are the main criteria to categorize electrocatalysts based on ZnCo2O4, for the OER and HER.
2.2. Electrochemical energy storage systems
In an effort to overcome past limitations, recent years have seen intense research efforts in energy storage areas, such as fuel cells, capacitors, supercapacitors, and batteries. Electrochemical energy storage systems (EESSs) play a critical role in renewable energy integration applications. They serve as energy sources to provide power supply and/or energy buffers to improve efficiency and the overall economy. This has triggered intensive research efforts in the past three decades, which have resulted in the advent of modern EESSs such as batteries and supercapacitors.29–31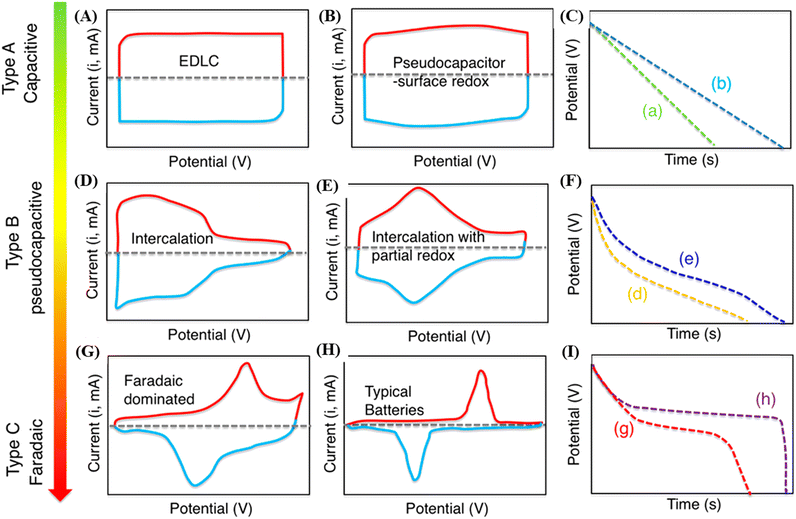 | ||
| Fig. 2 Illustrative cyclic voltammograms (A, B, D, E, G and H) and the corresponding GCD curves (C, F and I) for different types of energy-storage materials. Electrochemical double-layer capacitors: CV profile (A) and the corresponding discharge curve (C). A pseudocapacitive electrode could present an electrochemical response of one, or a mix, of the following categories: (B and C) surface redox materials and (D–F) intercalation-type materials. Electrochemical profiles in (G–I) correspond to battery-like materials. Reproduced with permission.32 Copyright © 2018 American Chemical Society. | ||
Distinct from conventional capacitors, supercapacitors store charges electrochemically but show high-energy density compared to the former, with high rate capability and excellent cycling stability. According to their charge storage mechanisms, supercapacitors are divided into two categories, namely, electrochemical double-layer capacitors (EDLCs) and pseudocapacitors.33 In EDLCs, the electrochemical behavior is due to the storage of charges at the electrode/electrolyte interface by establishing electrochemical double layers through a non-faradaic process (Fig. 2A and C.a). In pseudocapacitors, the electrochemical behavior in terms of current is neither totally capacitive nor entirely faradaic (like batteries). These electrodes present fast and reversible oxidation/reduction reactions through either intercalation or surface ion processes and quasi-rectangular CVs and quasi-linear GCD curves.34 In surface-redox pseudocapacitors, the charge storage is mostly assigned to the charge transfers occurring at the surface of the material. As can be seen in Fig. 2B and C.b, the CV and the GCD characteristics for surface-redox pseudocapacitors present the linear dependency of the charge storage over the entire potential window, storing charges through surface faradaic and double layer mechanisms. Intercalation-type materials involve the core of the electrode materials and are expressed by the intercalation of charges between layers or in channels originating from the faradaic reaction and lack of phase changes during cycling (Fig. 2D–F).35
EDLCs can reach fast charging/discharging rates and high cycling stability. However, the energy density of this type of material is relatively low, due to the deficient contact at the electrode/electrolyte interface. On the other hand, the capacitance of pseudocapacitors is attributed to the fast and reversible redox process of materials, such as some transition metal oxides/hydroxides and conducting polymers. Hence, pseudocapacitors can provide higher specific capacitance but present lower power density,2 due to the low conductivity of pseudocapacitive materials. In this way, one strategy to increase the performance of electrodes is the preparation of nanocomposites containing carbon structures.36
The configuration of conventional supercapacitors is based on button cells or spiral-wound designs, which are composed of two collectors, two electrodes, and a separator, all soaked in electrolyte.37 Distinct from the case of conventional supercapacitors, the development of materials in smart configurations (films, fibers, and micro-scale supercapacitors) has increased, aiming for the construction of thin, flexible, and even foldable devices. Thin-film electrodes are prepared with a layer of active material with its thickness varying from nanometers to micrometers, resulting in short charge and ion transport distances, and thus promoting fast physical or chemical processes during charge storage.38 Fiber supercapacitors are commonly designed like 1D wires with diameters varying from micrometers to millimeters and constructed based on parallel, twisted, coaxial, or woven structures.39 Micro-supercapacitors generally consist of a vertical structure composed of two electrodes and electrolyte sandwiched in the middle of both or, in the case of the in-plane interdigital device architecture, electrodes are separated by an insulated gap, with no need for separators in the construction of the device. The electrolyte is subsequently deposited on the top of devices to guarantee ion transport between electrodes. The total size of micro-supercapacitors could be in the order of millimeters.36,40
Among a number of different energy storage technologies, metal-ion batteries, in particular lithium-ion batteries (LIBs), have recently been accepted as the leading candidate for commercial EESSs. LIBs, as the main power source, dominate the portable device market due to their high energy density, high output voltage, long life and environmentally friendly operation.31,44 It is important to mention that many review works already published highlighting recent progress,45–47 issues and challenges facing rechargeable LIBs,48 as well as rechargeable sodium-ion batteries (SIBs) as potential alternatives to current LIBs,49 which can be used to obtain more detailed information about these EESSs.
On the other hand, metal–air batteries (MABs) are a family of electrochemical cells powered by metal oxidation and oxygen reduction; in this system oxygen is used as the active cathode material. This oxygen is obtained from air, which diffuses into the electrolyte from the atmosphere and undergoes reduction at the cathode, exhibiting a great advantage regarding theoretical energy density, which is about 3–30 times higher than those of commercial LIBs.50–52
In typical continuum-based models, the cathode material is considered as a porous medium and the structure is represented by several parameters, such as porosity, permeability, and tortuosity.52 In addition, it is necessary to design oxygen electrode catalysts with special structures for use in MABs to overcome the sluggish kinetics of the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER).53–55
3. ZnCo2O4-based materials for energy storage applications
3.1. ZnCo2O4-based electrode materials for supercapacitive applications
One of the best electrochemical performances is observed for RuO2 as a supercapacitor electrode material, exhibiting a high specific capacitance of 1580 F g−1. However, because of its high cost and element scarcity, it becomes necessary to seek environmentally friendly and low-cost alternative electrode materials. Recently, transition metal oxides (TMOs) such as MnO2, NiO, and Co3O4 have been studied as promising electrode materials for supercapacitor applications,56 especially those based on doped-Co3O4. The bimetallic oxide ZnCo2O4 has recently attracted much attention because of its excellent electrochemical properties, with lower activation energy, higher conductivity and electroactivity in comparison with pristine Co3O4. It also exhibits high theoretical capacitance (2604 F g−1), and is environmentally compatible and a cost-effective and abundant material. In addition, ZnCo2O4 presents a p-type semiconducting nature, which influences the electrical conductivity of the material, and shares the same Co3O4 spinel crystal structure. The replacement of Co2+ ions with Zn2+ ions, with Zn occupying the tetrahedral sites and Co occupying the octahedral sites, results in much richer redox reactions. However, ZnCo2O4 has the disadvantage of exhibiting an intrinsically poor electrical conductivity, involving large volume changes through the charge/discharge processes. This leads to some intrinsic electrical insulation, showing rapidly fading capacitance at higher current densities and during charge/discharge cycles, thus usually presenting low rate-capability and cycling stability.57,58Therefore, to overcome these limitations, rational design of suitable electrode materials is imperative, since the electrochemical performance strongly depends on their mechanical properties. To surpass the limitations of ZnCo2O4-based electrodes for supercapacitor applications, it is important to seek for an optimized morphology that can provide high active surface area, short lengths and high rates of ion and electron diffusion. Plenty of redox sites should be available. For this reason, pristine ZnCo2O4 has been synthesized as microparticles,59 microsheets,60 microspheres,61–64 microflowers,65,66 nanoparticles (NPs),67,68 nanocubes,69 nanosheets,70–73 nanoplates,56 nanoflowers,74 nanorods,75 nanospheres,76 and nanotubes77 to produce slurry-cast supercapacitive electrodes (Table 1).
| Electrode material | Specific capacitance or specific capacity | Potential window (V)/ref. electrode | Rate capability/current density range | Stability retention/cycle numbers | Highest energy density (W h kg−1) | Highest power density (W kg−1) | Negative electrode material in SCs | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnCo2O4 nanocubes | 434 F g−1 at 5 mV s−1 | −0.5 to 0.5/Ag/AgCl | 9.9%/5–50 (mV s−1) | — | — | — | — | 69 |
| Peony-like ZnCo2O4 nanoparticles | 440 F g−1 at 1 A g−1 | 0.0–0.55/SCE | 67.7%/1–10 | 155.6%/3000 | 29.76 at 398.53 W kg−1 | — | AC | 68 |
| Mesoporous ZnCo2O4 nanosheets | 3.3 F cm−2 at 1.01 mA cm−2 | 0.0–0.35/SCE | 59.8%/1–10 | 96.5%/5000 | 33.98 at 800 W kg−1 | 4800 at 16.67 W h kg−1 | AC | 70 |
| Hollow ZnCo2O4 microspheres | 78.89 mA h g−1 at 1 A g−1 | 0.0–0.5/SCE | 56%/1–10 | 145%/2000 | 27.78 at 158.5 W kg−1 | 920.8 at 12.62 W h kg−1 | AC | 62 |
| Porous Zn1.36Co1.64O4 nanoplates | 805.3 F g−1 at 1 A g−1 | 0.0–0.5/Ag/AgCl | 56%/1–25 | 88%/5100 | — | — | Symmetric | 56 |
| ZnCo2O4 nanosheets | 290.5 F g−1 at 0.5 A g−1 | 0.0–0.45/Ag/AgCl | 64.3%/0.5–10 | — | 0.46 at 22.44 W kg−1 | 107.53 at 0.21 W h kg−1 | Symmetric | 71 |
| ZnCo2O4 microparticles | 158 F g−1 at 5 mV s−1 | −0.4 to 0.6/SCE | 7.6%/5–200 (mV s−1) | 75%/1000 | — | — | — | 59 |
| Urchin-like ZnCo2O4 microspheres | 677 F g−1 at 1 A g−1 | 0.0–0.45/SCE | 77.5%/1–15 | 107.3%/5000 | — | — | — | 64 |
| Porous ZnCo2O4 microflowers | 689 F g−1 at 1 A g−1 | 0.0–0.44/SCE | 81.3%/1–15 | 98.7%/5000 | — | — | — | 65 |
| Porous ZnCo2O4 microspheres | 126 F g−1 at 1 A g−1 | 0.0–0.6/SCE | 77.7%/1–7 | — | — | — | — | 61 |
| Sphere-like ZnCo2O4 nanoparticles | 843 F g−1 at 1 A g−1 | 0.0–0.45/Ag/AgCl | 72.7%/1–3 | 97%/5000 | 26.28 at 716 W kg−1 | 3850 at 3.85 W h kg−1 | AC | 67 |
| Porous ZnCo2O4 nanosheets | 3.07 F cm−2 at 1.04 mA cm−2 | 0.0–0.35/SCE | 61.2%/1.04–10.4 | 96.3%/5000 | 42.83 at 425 W kg−1 | 8500 at 12.99 W h kg−1 | AC | 72 |
| Sheet-like ZnCo2O4 microstructures | 16.13 mF cm−2 at 10 μA cm−2 | 0.0–0.4/Ag/AgCl | 19.9%/10–1000 | 170%/1000 | — | — | — | 60 |
| Mesoporous ZnCo2O4 microflowers | 680 F g−1 at 1 A g−1 | 0.0–0.4/Ag/AgCl | 89.4%/0.35–1 | 90%/2000 | — | — | — | 66 |
| Porous Zn1.5Co1.5O4−δ nanoflowers | 763.32 F g−1 at 1 A g−1 | 0.0–0.5/Hg/HgO | 55.31%/1–30 | 89.42%/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
40.49 at 397.37 W kg−1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 at 20.87 W h kg−1 080 at 20.87 W h kg−1 |
AC | 74 |
| Rod-like ZnCo2O4 nanoparticles | 135 F g−1 at 1 A g−1 | 0.0–0.45/Ag/AgCl | — | — | — | — | — | 75 |
| ZnCo2O4 microspheres | 460 F g−1 at 1 A g−1 | 0.0–0.45/Ag/AgCl | 54.8%/1–5 | 165%/1000 | — | — | — | 63 |
| Mesoporous ZnCo2O4 nanosheets | 835.26 F g−1 at 1 A g−1 | 0–0.38/SCE | 35.6%/1–10 | 73.28%/1000 | — | — | — | 73 |
| Porous sphere-like ZnCo2O4 nanoparticles | 420 F g−1 at 0.5 A g−1 | −0.1 to 0.45/Hg/HgO | ∼71.4%/0.5–10 | — | 28.6 at 100 W kg−1 | 2500 at 18 W h kg−1 | NPC | 76 |
| Hollow ZnCo2O4 nanotubes | 362 F g−1 (181 C g−1) at 0.5 A g−1 | 0.0–0.5/SCE | 75.1%/0.5–10 | 97.4%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10.42 at 375.12 W kg−1 | 7503.75 at 6.67 W h kg−1 | AC | 77 |
Other strategies rely on the production of hybrid materials, such as composites and core@shell structures, which will be discussed later, and/or combining the morphology design and hybrid materials engineering with binder-free and self-supporting architectures. The use of binders to produce slurry-cast electrodes for supercapacitor applications significantly reduces the electronic conductivity, limits the active material availability, hinders the ion-diffusion, and increases the mass density as “dead-mass”. Additionally, after repetitive redox reactions, the material can lose the integrity and/or peel-off from the substrate, reducing the capacitance retention through several charge/discharge cycles. Therefore, the above-mentioned downsides can be resolved by the growth of ZnCo2O4 electroactive materials directly on the surfaces of electrode substrates, such as nickel foam (NF), carbon foam (CF) and carbon cloth (CC). These strategies not only avoid “dead-mass” but also greatly improve the electroactive surface area, and offer fast electron transportation and short ion diffusion paths. In addition, these strategies will decrease the resistance between the electroactive material and current collector, provide efficient ion-diffusion channels, ensure excellent mechanical strength, enhance the electrical conductivity and accommodate the volume changes through cycling. Therefore, the challenge to fabricating highly efficient binder-free electrode materials capable of storing rapidly larger amounts of energy, at low cost, can be solved by using ZnCo2O4-modified electrodes. Hence, binder-free electrodes based on pristine ZnCo2O4 nanorods,78,79 nanobelts,80 nanoribbons,81 nanoflowers,82 nanoflakes,83,84 nanosheets,85–89 nanomuscles,90 nanowires,65,91 nanoleaves,92 nanocubes,93 micro-urchins,94,95 and nanoneedles89 were also reviewed for supercapacitor applications (Table 2) and will be discussed along with slurry-cast electrodes according to their morphology.
| Electrode material | Specific capacitance or specific capacity | Potential window (V)/ref. electrode | Rate capability/current density range | Stability retention/cycle numbers | Highest energy density (W h kg−1) | Highest power density (W kg−1) | Negative electrode material in SCs | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnCo2O4 nano-rods on CC | 5.18 F cm−2 at 5 mA cm−2 | 0–0.6/Ag/AgCl | 59.8%/5–100 | 92.8%/3000 | 2.3 mW h cm−2 at 7.82 mW cm−2 | — | PPy/CC | 78 |
| ZnCo2O4 nano-belt-decorated CC | 1197.14 F g−1 at 2 A g−1 | 0–0.7/Ag/AgCl | 75.2%/2–10 | 95.01%/5000 | 79.48 at 894.24 W kg−1 | 8900 at 62.1 W h kg−1 | AC/CC | 80 |
| Porous ZnCo2O4 nanoribbons on NF | 1957.7 F g−1 at 3 mA cm−2 | 0–0.5/SCE | 61.7%/3–60 | 84%/3000 | — | — | — | 81 |
| ZnCo2O4 nano-flowers on NF | 1657 F g−1 at 1 A g−1 | 0–0.5/Hg/HgO | ∼45%/1–16 | 89%/2000 | 40 at 1016 W kg−1 | ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at ∼22 W h kg−1 000 at ∼22 W h kg−1 |
Symmetric | 82 |
| Flake-like ZnCo2O4 nano-structures on CC | ∼41 mA h g−1 at 2 A cm−2 | 0–0.35/SCE | ∼36%/2–20 | 94.8%/2000 | — | — | — | 83 |
| Ultra-thin ZnCo2O4 curved sheets on NF | 1848.9 F g−1 (832 C g−1) at 5 A g−1 | 0–0.45/Ag/AgCl | 88.6%/5–15 | 85.5%/5000 | 20.31 at 855 W kg−1 | 4250 at 10.2 W h kg−1 | AC/NF | 85 |
| ZnCo2O4 nano-muscle networks on NF | 1156.3 F g−1 462.5 C g−1 at 1 A g−1 | 0–0.4/Ag/AgCl | 71.5%/1–8 | 97.4%/5000 | — | — | — | 90 |
| ZnCo2O4 nano-wires on NF | 2049 F g−1 at 2 A cm−2 | 0–0.5/SCE | 83.6%/2–30 | 88.8%/3000 | 37.5 at 358.2 W kg−1 | 4776.1 at 19.9 W h kg−1 | AC/NF | 96 |
| ZnCo2O4 nano-flake-decorated porous 3D-Ni | 1170 F g−1 at 2 A g−1 | 0–0.6/Ag/AgCl | 51.3%/2–30 | 95%/3000 | 28.8 mW h cm−2 at 3 W cm−2 | — | Fe2O3/3D-Ni | 84 |
| Porous ZnCo2O4 nanosheet networks on NF | 3.19 F cm−2 at 2 mA cm−2 | 0–0.5/Hg/HgO | 83.9%/2–30 | 72.5%/2500 | 50.7 at 187.6 W kg−1 | 2950.4 at 37.7 W h kg−1 | AC/NF | 86 |
| Leaf-like ZnCo2O4 nano-structures on NF | 1700 F g−1 at 1 A g−1 | 0–0.4/SCE | ∼36.8%/1–10 | 110%/8000 | 63 at 795.5 W kg−1 | — | AC/NF | 92 |
| ZnCo2O4 intertwined heterostructured nanocubes on NF | 2040 F g−1 at 20 A g−1 | 0–0.5/SCE | 47.7%/50–200 (mV s−1) | 92%/1000 | — | — | — | 93 |
| Urchin-like ZnCo2O4 microspheres on FSSM | 127.8 F g−1 at 1 mA cm−2 | 0–0.5/Ag/AgCl | 64%/1–10 | 80.7%/3000 | 94 | |||
| Porous ZnCo2O4 micro-urchins on NF | 1527.2 F g−1 at 1 A g−1 | 0–0.5/Hg/HgO | 78.8%/1–10 | 86%/2000 | 69.2 at 774.6 W kg−1 | 7742.2 at 35.7 W h kg−1 | AC/NF | 91 |
| ZnCo2O4 intertwined nanosheets on CC | 1750 F g−1 at 1.5 A g−1 | 0–0.8/Ag/AgCl | 72%/1.5–10 | 96.8%/3000 | 117.92 at 1490.4 W kg−1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 at 76.69 W h kg−1 520 at 76.69 W h kg−1 |
NPC/CC | 87 |
| ZnCo2O4 nano-rods on FSSM | 315 F g−1 at 2 A g−1 | 0–0.35/Ag/AgCl | 92.4%/2–10 | 87.09%/6000 | 25.45 at 3620 W kg−1 | ∼6050 at ∼5 W h kg−1 | FeCo2O4/FSSM | 79 |
| ZnCo2O4 nano-sheets on NF | 400 F g−1 at 1 A g−1 | 0–0.45/SCE | 81.8%/1–10 | 93%/5000 | 14.1 at 375 W kg−1 | 6000 at 4.4 W h kg−1 | N-doped AC/NF | 88 |
| ZnCo2O4 micro-urchins on NF | 390 F g−1 at 1 A g−1 | 0.1–0.5/Hg/HgO | 69%/1–16 | 82.5%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.27 mW h cm−2 at 14.18 W cm−2 | 62.27 at 0.66 mW h cm−2 | AC/NF | 95 |
| Porous ZnCo2O4 micro-urchins on NF | 1000 F g−1 at 20 A g−1 | 0–0.5/SCE | 52.6%/10–50 | 93%/5000 | — | — | — | 89 |
| Porous Al0.5Zn0.5Co2O4 nanosheets on NF | ∼1200 F g−1 at 20 A g−1 | 0–0.5/SCE | 56.7%/10–50 | 95%/5000 | — | — | — |
In the case of Co3O4-based materials, the direct comparison between different MCo2O4 materials can only be understood by further analyzing their morphologies instead of just their composition, as reported by Merabet et al.59 (M = Zn, Ni, Mn, and Cu) and Alqahtani et al.61 (M = Zn, Ni, Mn, Cu, and Fe). Both author groups synthesized sphere-like ZnCo2O4 microparticles for slurry-cast electrodes, but different morphologies were obtained for other MCo2O4 species, impacting their performance. Since their sphere-like ZnCo2O4 microparticles presented bulkier morphologies and lower electroactive surface, they exhibited the lowest electrochemical performance, delivering 158 F g−1 at 5 mV s−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 and 126 F g−1 at 1 A g−1.61
59 and 126 F g−1 at 1 A g−1.61
Notwithstanding, there are some structural strategies that can be applied to optimize 3D ZnCo2O4 morphologies for slurry-cast electrodes, e.g., nanocubes (434 F g−1 at 5 mV s−1),69 sphere-like NPs (843 F g−1 at 1 A g−1)67 and rod-like NPs (135 F g−1 at 1 A g−1).75 However, even though nanocubes69 and sphere-like NPs67 presented cycling stability (97% after 5000 cycles) and relatively good specific capacitance in comparison to bulk microspheres59 and nanorods,75 they showed poor rate capability. Nonetheless, the overall stability can be further enhanced by producing hollow (78.89 mA h g−1 at 1 A g−1)62 and porous microspheres (460 F g−1 at 1 A g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 and 420 F g−1 at 0.5 A g−1
63 and 420 F g−1 at 0.5 A g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76). Differently from bulk and smooth microparticles59 (Fig. 3A), which presented 75% of their initial specific capacitance after 1000 cycles, the initial specific capacity of hollow ZnCo2O4 microspheres62 (Fig. 3B) increased to 145% after 2000 cycles, while porous microspheres63 (Fig. 3C) delivered 165% of their initial specific capacitance after 1000 cycles. These results suggest that porous and hollow particles show superior cycling performance due to the facile mass transfer from the interconnected structure of NPs and the void/space in between the particles, alleviating the strain effects of the volume changes during charge/discharge processes. As for binder-free electrodes, there are ZnCo2O4 connected nanomuscle network microstructures uniformly grown onto NF (1156.3 F g−1 at 1 A g−1),90 which originate from agglomerated nanosheets and present a highly porous 3D structure. This can partially buffer the strain effect through the charge–discharge processes, improve the specific surface area and active site availability, and lower the interior resistance, facilitating electron transfer and resulting in such high specific capacitance.
76). Differently from bulk and smooth microparticles59 (Fig. 3A), which presented 75% of their initial specific capacitance after 1000 cycles, the initial specific capacity of hollow ZnCo2O4 microspheres62 (Fig. 3B) increased to 145% after 2000 cycles, while porous microspheres63 (Fig. 3C) delivered 165% of their initial specific capacitance after 1000 cycles. These results suggest that porous and hollow particles show superior cycling performance due to the facile mass transfer from the interconnected structure of NPs and the void/space in between the particles, alleviating the strain effects of the volume changes during charge/discharge processes. As for binder-free electrodes, there are ZnCo2O4 connected nanomuscle network microstructures uniformly grown onto NF (1156.3 F g−1 at 1 A g−1),90 which originate from agglomerated nanosheets and present a highly porous 3D structure. This can partially buffer the strain effect through the charge–discharge processes, improve the specific surface area and active site availability, and lower the interior resistance, facilitating electron transfer and resulting in such high specific capacitance.
 | ||
| Fig. 3 (A) SEM image of bulk ZnCo2O4 microparticles. Reproduced with permission.59 Copyright © 2018 Elsevier Ltd and Techna Group S.r.l. All rights reserved. (B) SEM image of hollow ZnCo2O4 microspheres. Reproduced with permission.62 Copyright © 2018 Elsevier Ltd. All rights reserved. (C) SEM image of porous ZnCo2O4 microspheres. Reproduced with permission.63 Copyright © 2018, Springer-Verlag GmbH Germany, part of Springer Nature. | ||
Aside from 3D ZnCo2O4 NPs, the literature has reported a series of 2D-structured ZnCo2O4, such as micro-60 and nanosheets,70–73,85–89 nanoplates,56 nanoflakes,83,84 nanoleaves,92 nanobelts,80 nanoribbons,81 and those based on radial growth of nanosheets, i.e., micro-65,66 and nanoflowers.74,82 In addition, there are 1D-structured ZnCo2O4, such as urchin-like microspheres,64 nanorods75,78,79 and nanotubes.77 Two-dimensional sheet-like morphologies are known to be generally more suitable than traditional bulk (3D) materials for supercapacitor applications, once they present high specific surface area, higher surface area-to-volume ratios, and shorter ion transportation channels due to their greatly reduced thickness in one dimension, thus improving the availability of electroactive sites for redox reactions, electrical conductivity, cycling stability and ion-diffusion rates.56,60,70–73
All sheet-like ZnCo2O4 materials for slurry-cast electrodes encountered in this review had superior capacitance retention through cycling in comparison to bulk spherical and cubic ZnCo2O4 nanoparticles due to their more stable morphology. However, they also presented some limitations in rate capability, with a significant decrease in specific capacitance at increasing current density. Presumably, the electrolyte ions have insufficient time to diffuse into the electrode material and to access the active sites at higher scan rates. Even though the highest specific capacitance between slurry-cast electrodes of 835.26 F g−1 at 1 A g−1 was achieved by mesoporous ZnCo2O4 nanosheets produced by Xiao et al.73 (Fig. 4A), similar results were achieved with porous Zn1.36Co1.64O4 nanoplates (805.3 F g−1 at 1 A g−1),56 as well as with mesoporous ZnCo2O4 nanosheets (3.3 F cm−2 at 1.01 mA cm−2) and porous ZnCo2O4 nanosheets (3.07 F cm−2 at 1.04 mA cm−2).72 In fact, these results are attributed to their porosity and nanosized morphology. Smooth ZnCo2O4 nanosheets71 delivered only 290.5 F g−1 at 0.5 A g−1 and ZnCo2O4 microsheets60 delivered the poorest areal capacitance (16.13 mF cm−2 at 10 μA cm−2) among all materials, along with rather low rate-capability, owing to their inferior specific surface area and the lower availability of electroactive sites, especially at higher current densities.
 | ||
| Fig. 4 (A) SEM image of mesoporous ZnCo2O4 nanosheets. Reproduced with permission.73 Copyright © 2017, Springer-Verlag GmbH Germany, part of Springer Nature. (B) FESEM image of ultra-thin ZnCo2O4 curved nanosheets/NF. Reproduced with permission.85 Copyright © 2019 Elsevier Ltd. All rights reserved. (C) SEM image of porous ZnCo2O4 nanoribbons/NF. Reproduced with permission.81 Copyright © 2017 Elsevier Ltd. All rights reserved. (D) SEM image of porous ZnCo2O4 microflowers. Reproduced with permission.65 Copyright © 2019 Elsevier Ltd and Techna Group S.r.l. All rights reserved. (E and F) SEM images of ZnCo2O4 nanoflowers/NF. Reproduced with permission.82 Copyright © 2017 Elsevier Ltd. All rights reserved. | ||
When assembled in binder-free electrodes, on the other hand, sheet-like ZnCo2O4 materials present superb specific capacitance, rate capability and cycling stability and are quite competitive.80,81,83–89,92 Nanosheets are the most studied 2D morphology of ZnCo2O4,85–88 featuring porous nanosheet networks on NF (3.19 F cm−2 at 2 mA cm−2),86 intertwined nanosheet arrays on CC (1750 F g−1 at 1.5 A g−1)87 and NF (400 F g−1 at 1 A g−1),88 ultra-thin curved nanosheet arrays on NF (1848.9 F g−1 at 5 A g−1)85 (Fig. 4B). These materials presented high rate-capabilities and cycling stabilities, besides porous nanosheet networks on NF,86 with a capacitance retention of 72.5% after 2500 cycles. Such good rate capabilities are achieved owing to the nanosheet array arrangements with adequate space between individual nanosheets, composed of many NPs and pores85,86,88 or intertwined nanosheets,87 which facilitates the transport path for ion-diffusion in charge/discharge processes.
Other binder-free electrodes based on 2D ZnCo2O4 materials were also produced in recent years, i.e. nanobelts (1197.14 F g−1 at 2 A g−1),80 nanoribbons (1957.7 F g−1 at 3 mA cm−2),81 flake-like nanostructures (∼41 mA h g−1 at 2 A cm−2),83 nanoflakes (1170 F g−1 at 2 A g−1),84 nanoleaves (1700 F g−1 at 1 A g−1),92 and porous Al0.5Zn0.5Co2O4 nanosheet arrays on NF (∼1200 F g−1 at 20 A g−1).89
ZnCo2O4 nanobelt-decorated CC80 had a similar structure to interconnected nanosheets and thus provided similar performance to ZnCo2O4 nanosheet materials,85–88 while flake-like ZnCo2O4 nanostructures on CC,83 leaf-like ZnCo2O4 nanostructures on NF92 and Al0.5Zn0.5Co2O4 nanosheet arrays on NF89 presented poor rate capabilities despite their high cycling stabilities. The trimetallic oxide-based electrode delivered slightly better performance than pristine ZnCo2O4 micro-urchin arrays on NF produced in the same work (approximately 1000 F g−1 at 20 A g−1) due to the incorporation of a third metallic center that can enhance even further the ZnCo2O4 electrochemical behavior. The leaf-like ZnCo2O4 nanostructures on NF92 delivered high initial specific capacitance, due to their high specific surface area and electroactive site availability, but limited morphology for fast ion-diffusion. The poor specific capacity of flake-like ZnCo2O4 nanostructures on CC83 seems to be caused by their smooth surface and low specific surface area.
On the other hand, porous ZnCo2O4 nanoribbon arrays on NF81 (Fig. 4C) not only delivered the highest specific capacitance among binder-free 2D ZnCo2O4-modified electrodes, but also demonstrated a good rate capability of 61.7% upon a 20-fold current density increase. In this case, good cycling stability was noticed, maintaining 84% of the initial specific capacitance after 3000 cycles, attributed to the appropriately spaced and highly porous nanoribbon arrays, which provided multiple and facile channels for fast ion-diffusion.
Compared with 2D nanomaterials based on radial growth of nanosheets, i.e., micro-65,66 and nanoflowers,74 slurry-cast electrodes presented even better rate capability, high specific capacitance and cycling stability. Mesoporous ZnCo2O4 microflowers66 (680 F g−1 at 1 A g−1), porous ZnCo2O4 microflowers65 (689 F g−1 at 1 A g−1) and porous Zn1.5Co1.5O4−δ nanoflowers74 (763.32 F g−1 at 1 A g−1) (Fig. 4D) presented 89.4% (0.35 to 1 A g−1), 81.3% (1 to 15 A g−1) and 55.31% (1 to 30 A g−1) capacitance retention, respectively.
Micro- and nanoflower NPs combine the benefits of strongly interconnected sheet-like structures with a highly porous and hierarchical structure. They also present high specific surface area, promoting reduced mechanical stress. This arises from the huge volumetric expansion during the charge/discharge processes, facilitating electrolyte penetration and ion diffusion into the electroactive material. There is a high availability of electroactive sites even at high current densities and numerous charge/discharge cycles. The binder-free electrode with radial growth of ZnCo2O4 nanoflowers on an NF electrode82 (Fig. 4E and F) delivered 1657 F g−1 at 1 A g−1, and was designed along with a flake-like ZnCo2O4-modified NF electrode, which delivered 1803 F g−1 at 1 A g−1. They presented, respectively, ∼45% and ∼33.3% rate capability at 16 A g−1, which makes ZnCo2O4 nanoflowers on the NF electrode a better candidate for supercapacitive applications even though they still present low specific capacitance retention at higher current densities. Presumably, nanoflower structures are more stable under high current conditions and repeated charge/discharge cycles. The abundance of ion-diffusion channels can improve the electrolyte and electron transport. However, it is still very limited, and the parallelly grown structures can be more suitable for binder-free electrodes in comparison to those radially grown.
There are also some recent works about pristine 1D structured ZnCo2O4. These structures can have some advantages, exhibiting optimal specific surface area and material mass ratios which are only surpassed by typical 0D materials, such as quantum-dots. In this case, it is important to mention their extremely reduced length in two dimensions, shorter ion diffusion lengths and facile electrical transport exclusively in the axial direction. Also relevant are the quantum confinement effects, altering the material properties in such a way that photons can be absorbed at one wavelength and transmitted at another.97 These advantages can be further enhanced in slurry-cast electrodes by producing hollow nanotubes (362 F g−1 at 0.5 A g−1)77 (Fig. 5A), with low density, superior specific surface area and shorter ion transport path. Alternatively, urchin-like microspheres (677 F g−1 at 1 A g−1)64 (Fig. 5B) with radially grown porous nanorods have almost the same benefits of porous microflowers, both with high rate capability and capacitance retention through cycling for slurry-cast electrodes.
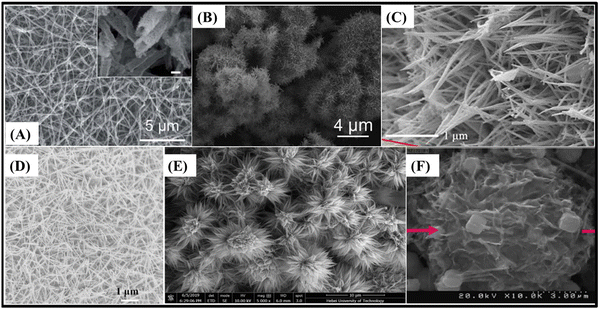 | ||
| Fig. 5 SEM images of (A) hollow ZnCo2O4 nanotubes,77 (B) urchin-like ZnCo2O4 microspheres,64 (C) ZnCo2O4 nanorods/CC,78 (D) ZnCo2O4 nanowire arrays/NF,96 (E) porous ZnCo2O4 micro-urchins/NF91 and (F) ZnCo2O4 intertwined heterostructured nanocubes/NF.93 Panel A: Reproduced with permission.77 Attribution 3.0 Unported (CC BY 3.0), Royal Society of Chemistry. Panel B: Reproduced with permission.64 Copyright © 2018 Elsevier Ltd and Techna Group S.r.l. All rights reserved. Panel C: Reproduced with permission.78 Copyright © 2018 Elsevier B.V. All rights reserved. Panel D: Reproduced with permission.96 Panel E: Reproduced with permission.91 Copyright © 2019, Springer-Verlag GmbH Germany, part of Springer Nature. Panel F: Reproduced with permission.93 Copyright © 2019 Korean Physical Society. Published by Elsevier B.V. All rights reserved. | ||
Binder-free electrodes, ZnCo2O4 nanorods/CC (5.18 F cm−2 at 5 mA cm−2),78 nanorods/flexible stainless-steel mesh (FSSM) (315 F g−1 at 2 A g−1),79 nanowires (2049 F g−1 at 2 A cm−2),65 micro-urchins/FSSM (127.8 F g−1 at 1 mA cm−2),94 micro-urchins/NF (390 F g−1 at 1 A g−1),95 and two porous ZnCo2O4 micro-urchins on NF electrodes (1000 F g−1 at 20 A g−1;89 1527.2 F g−1 at 1 A g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91) were also assembled. The ZnCo2O4 nano-rod arrays on CC78 (Fig. 5C) presented one of the highest rate capabilities based on 1D-morphology, with 59.8% capacitance retention and a 20-fold current density increase, along with a good areal capacitance. The other nanorod-modified electrode, ZnCo2O4 nano-rod arrays on FSSM,79 delivered not only low specific capacitance but also good rate-capability. This is attributed to the uniform thickness, length, and parallel oriented distribution of ZnCo2O4 nanorods with suitable spaces between them, allowing rapid ion-diffusion and active site availability. Nanowire arrays of ZnCo2O4 on NF96 (Fig. 5D) share almost the same properties of nanorod-modified electrodes, but instead they deliver one of the highest specific capacitances and rate-capabilities among all pristine 1D ZnCo2O4-modified binder-free electrodes, with 83.6% capacitance retention after a 15-fold increase in current density. They also exhibit the unusually high cycling stability observed for binder-free electrodes (88.8%, 3000 cycles). This superior performance is inferred to be caused by the higher length of the nanowires in comparison to nanorods. As a result, an interconnective mesoporous structure with very high specific area, abundant available electroactive sites, and shortened distances of electron transportation is obtained. There are also suitable spaces between nanowires for allowing fast and effective ion-diffusion.
91) were also assembled. The ZnCo2O4 nano-rod arrays on CC78 (Fig. 5C) presented one of the highest rate capabilities based on 1D-morphology, with 59.8% capacitance retention and a 20-fold current density increase, along with a good areal capacitance. The other nanorod-modified electrode, ZnCo2O4 nano-rod arrays on FSSM,79 delivered not only low specific capacitance but also good rate-capability. This is attributed to the uniform thickness, length, and parallel oriented distribution of ZnCo2O4 nanorods with suitable spaces between them, allowing rapid ion-diffusion and active site availability. Nanowire arrays of ZnCo2O4 on NF96 (Fig. 5D) share almost the same properties of nanorod-modified electrodes, but instead they deliver one of the highest specific capacitances and rate-capabilities among all pristine 1D ZnCo2O4-modified binder-free electrodes, with 83.6% capacitance retention after a 15-fold increase in current density. They also exhibit the unusually high cycling stability observed for binder-free electrodes (88.8%, 3000 cycles). This superior performance is inferred to be caused by the higher length of the nanowires in comparison to nanorods. As a result, an interconnective mesoporous structure with very high specific area, abundant available electroactive sites, and shortened distances of electron transportation is obtained. There are also suitable spaces between nanowires for allowing fast and effective ion-diffusion.
Interestingly, two of the reported micro-urchin architectures deliver relatively low specific capacitances for a binder-free ZnCo2O4-based electrode,94,95 although much higher than those of slurry-cast micro-urchin electrodes produced by a similar synthesis route.95 Porous ZnCo2O4 micro-urchins on NF electrodes89,91 deliver good specific capacitances, due to their larger spatial and porous structure (Fig. 5E), which greatly improves electroactive site availability and promotes better charge transport and ion-diffusion. However, all these mentioned materials presented limited rate-capability as 2D-morphology-based microflowers, due to the lack of parallel orientation. There is no adequate space between the nanostructures for optimizing the electrolyte penetration. Additionally, by combining 1D and 2D features in cubic structures, ZnCo2O4 intertwined heterostructured nanocubes on an NF electrode93 (Fig. 5F) were produced. They encompass mixed nanowires and nanosheets directly grown onto NF, with connective channels for electron transfer and suitable pores facilitating rapid ion-diffusion. This results in a high specific capacitance, e.g., 2040 F g−1, at a high current density of 20 A g−1.
| Electrode material | Specific capacitance or specific capacity | Potential window (V)/ref. electrode | Rate capability/current density range | Stability retention/cycle numbers | Highest energy density (W h kg−1) | Highest power density (W kg−1) | Negative electrode material in SCs | Ref. |
|---|---|---|---|---|---|---|---|---|
| NiCo2O4/ZnCo2O4 heterostructure | 1870.9 F g−1 (1029 C g−1) at 1 A g−1 | 0–0.55/Hg/HgO | 58.4%/1–20 | 91%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
101.6 at 1600 W kg−1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 at 11 W h kg−1 500 at 11 W h kg−1 |
NACC | 104 |
| ZnCo2O4/CNT nanoflowers | 1203.8 F g−1 at 1 A g−1 | 0–0.625/Ag/AgCl | 56.6%/1–20 | 87%/3000 | 24.46 at 750 W kg−1 | — | AC | 129 |
| ZnCo2O4/C nanofibers | 327.5 F g−1 at 0.5 A g−1 | 0–0.4/SCE | 27.5%/0.5–8 | 125%/1000 | — | — | — | 97 |
| Snowflake-like ZnCo2O4/ZnO microstructures | 826.7 F g−1 (372 C g−1) at 1 A g−1 | 0–0.45/SCE | 69.6%/1–15 | 68.7%/5000 | — | — | — | 99 |
| Hydrangea-like ZnCo2O4/Ni3V2O8 nanostructures | 1734 F g−1 at 1 A g−1 | 0–0.5/SCE | 90%/1–10 | 96%/8000 | 90 at 812 W kg−1 | 7909 at 75 W h kg−1 | AC | 107 |
| CNP/ZnO/ZnCo2O4 nanosheets | 593.6 F g−1 at 0.25 A g−1 | 0–0.4/Ag/AgCl | 18.3%/0.25–15 | 89%/1500 | — | — | — | 100 |
| ZnCo2O4/rGO ultrathin nanosheets | — | — | — | — | 31.8 mW h cm−3 at 280 mW cm−3 | 3880 mW cm−3 at 8.3 mW h cm−3 | Symmetric | 130 |
| Nanosheet-like ZnCo2O4/N-GO/PANI | 720 F g−1 at 1.5 A g−1 | 0–0.5/SCE | — | ∼96.4%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — | — | 131 |
| N-doped C supported P-ZnCo2O4 nanosheets | 1581.5 F g−1 at 1 A g−1 | 0–0.5/Hg/HgO | 90.6%/1–10 | — | 47.8 at 800 W kg−1 | — | AC | 132 |
| Porous NiCo2O4/ZnCo2O4/Co3O4 hollow nanocages | 1892.5 F g−1 at 1 A g−1 | 0–0.4/SCE | 64.1%/1–10 | 66%/2000 | 83.11 at 800 W kg−1 | — | AC | 105 |
| Cauliflower-like AuNP/rGO–ZnCo2O4 | 54.1 mA h g−1 at 25 mA cm−2 | 0–0.5/SCE | — | 97%/2000 | — | 2121 at 31 W h kg−1 | AC | 133 |
| Marigold-like ZnO/ZnCo2O4 | 705.1 F g−1 at 0.3 A g−1 | 0–0.5/Ag/AgCl | 89.4%/0.3–1 | ∼90%/2500 | — | — | — | 101 |
| Heterostructured NiCo2O4–ZnCo2O4/rGO nanosheets | 2176.4 F g−1 (1197 C g−1) at 1 A g−1 | 0–0.55/Hg/HgO | 58.2%/1–20 | 93.8%/5000 | 62 at 720 W kg−1 | 4540 at 7 W h kg−1 | Symmetric | 106 |
| 71 at 980 W kg−1 | 6040 at 17 W h kg−1 | rGO | ||||||
| MWCNT/ZnCo2O4 hexagonal nanoplates | 64 mA h g−1 at 1 A g−1 | 0–0.45/Ag/AgCl | 76.6%/1–3 | 88.1%/2000 | — | — | AC | 134 |
| Nanosheet-based hollow ZnO/ZnCo2O4/NiO microspheres | 1136.4 F g−1 at 1 A g−1 | 0–0.5/SCE | ∼31.2%/1–30 | 86.5%/5000 | 46.04 at 799.99 W kg−1 | 7987.5 at ∼21 W h kg−1 | AC | 102 |
| Ultrathin ZnCo2O4/MnO2 nanosheets | 286 F g−1 at 1 A g−1 | 0–0.5/Hg/HgO | 61.5%/1–10 | — | 16.94 at 750 W kg−1 | 7500 at 11.3 W h kg−1 | AC | 98 |
| Nanosheet-like g-C3N4/ZnCo2O4 | 1386 F g−1 (154 mA h g−1) at 4 A g−1 | 0–0.4/Ag/AgCl | ∼66%/4–8 | 90%/2500 | 39 at 1478 W kg−1 | — | Symmetric | 135 |
| PANI/ZnCo2O4 nanoparticles | 867 F g−1 at 0.5 A g−1 | 0–0.4/Ag/AgCl | 64%/0.5–4 | 98.9%/1000 | — | — | Symmetric | 136 |
| ZnCo2O4/MnCo2O4 heterojunction nanosheets | 254 F g−1 at 1 A g−1 | Hg/HgO | 73%/1–10 | — | 19.5 at 750 W kg−1 | 7494 at 15.4 W h kg−1 | AC | 103 |
| Electrode material | Specific capacitance or specific capacity | Potential window (V)/ref. electrode | Rate capability/current density range | Stability retention/cycle numbers | Highest energy density (W h kg−1) | Highest power density (W kg−1) | Negative electrode material in SCs | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mesoporous ZnCo2O4/NiO flower-like clusters on NF | 2797 F g−1 at 1 A g−1 | 0–0.5/SCE | 81.8%/1–10 | ∼100%/3000 | — | — | — | 111 |
| Porous ZnCo2O4/MnO2 heterostructures on NF | 2057 F g−1 at 1 A g−1 | 0–0.4/SCE | 65%/1–15 | 96.5%/5000 | 69 at 400 W kg−1 | 4900 at 21.7 W h kg−1 | AC/NF | 108 |
| ZnCo2O4/rGO intertwined sheets on NF | 3222 F g−1 at 1 A g−1 | 0–0.5/HgO/Hg | 26.7%/1–20 | 65%/5000 | 49.1 at ∼600 W kg−1 | 7625 at 18.8 W h kg−1 | AC | 137 |
| ZnCo2O4/NC hollow nanowall arrays on CT | ∼2003.8 F g−1 at ∼1.79 A g−1 | 0.05–0.45/Ag/AgCl | 74.7%/1.79–57.14 | ∼99.4%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.32 mW h cm−3 at 33.3 mW cm−3 | 166.7 mW cm−3 at 1.70 mW h cm−3 | Fe3O4/r-GO//CT | 138 |
| Sandwich-like ZnCo2O4 hollow spheres/rGO lamellar films | 1075.4 F g−1 at 1 A g−1 | 0–0.4 | — | 89.3%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — | — | 139 |
| 3D flower-like ZnCo2O4/PVP | 761 F g−1 at 0.35 A g−1 | 0–0.4/Ag/AgCl | ∼89.4%/0.35–1 | 90%/2000 | — | — | — | 66 |
| NC ZnCo2O4 honey nest nanostructures | 1289 F g−1 at 3.5 A g−1 | 0.2–0.45/Ag/AgCl | 70%/3.5–20 | 86%/2000 | 41.9 at 1065.1 W kg−1 | ∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 at ∼29 W h kg−1 900 at ∼29 W h kg−1 |
AC/CC | 140 |
| Heterostructured ZnCo2O4/N-rGO on NF | 1600 F g−1 at 1 A g−1 | −0.2 to 0.4/SCE | 78.1%/1–30 | — | 66.1 at 701 W kg−1 | 7016 at 43.66 W h kg−1 | AC | 141 |
| MnO2-decorated ZnCo2O4 nanosheets on rGO-doped NF | 3405.2 F g−1 at 2 A g−1 | 0–0.5/SCE | 64.9%/2–20 | 91.2%/5000 | 46.85 at 166.67 W kg−1 | 1666.67 at 17.13 W h kg−1 | rGO/NF | 109 |
| ZnCo2O4/Co3S4 nanowires on NF | 2.02 C g−1 at 0.8 A g−1 | — | — | 95.3%/6000 | 0.0798 at 1795 W kg−1 | 9760 at 0.0732 W h kg−1 | — | 112 |
| Porous ZnCo2O4 nanosheets on rGO-doped NF | 680 F g−1 at 1 A g−1 | 0–0.45/Hg/HgO | 88%/1–5 | 95.6%/3000 | 31.25 at 375 W kg−1 | 3750 at 11.46 W h kg−1 | AC | 142 |
| ZnCo2O4/ZnO heterostructured nanorods on ITO | 150 μF cm−2 at 1.2 μA cm−2 (UV-radiation) | 0–0.6/symmetric SC | 174%/off–on (UV) | — | 11.8 10−3 μW h cm−2 at 1.2 μA cm−2 (UV) | — | Symmetric | 110 |
| Electrode material | Specific capacitance or specific capacity | Potential window (V)/ref. electrode | Rate capability/current density range | Stability retention/cycle numbers | Highest energy density (W h kg−1) | Highest power density (W kg−1) | Negative electrode material in SCs | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnCo2O4@MnO2 nanowires on NF | 4.98 F cm−2 at 2 mA cm−2 | 0–0.45/SCE | ∼78.9%/2–16 | 106.2%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.058 mW h cm−3 at 7150 W cm−3 | — | AC | 115 |
| ZnCo2O4@Ni3S2 heterostructured nanowires on NF | 2200 F g−1 at 2 A g−1 | 0–0.4/Ag/AgCl | 55.7%/2–10 | 88.9%/1000 | — | — | — | 117 |
| ZnCo2O4@NiMoO4 heterostructured nanowires on NF | 2316 F g−1 (1158 C g−1) at 10 mA cm−2 | 0–0.5/SCE | 75.3%/3–40 | 103.4%/5000 | 25.3 at 787.9 W kg−1 | 9467.5 at 18.4 W h kg−1 | AC | 119 |
| ZnCo2O4@NiMoO4 heterostructured nanowires on NF | 1912 F g−1 at 1 A g−1 | 0–0.5/Ag/AgCl | 55%/1–20 | — | 57.5 at 900 W kg−1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at 30 W h kg−1 000 at 30 W h kg−1 |
CNT/NF | 120 |
| ZnCo2O4@ZnWO4 heterostructured nanowires on NF | 13.4 F cm−2 at 4 mA cm−2 | 0–0.4/Ag/AgCl | 28.1%/4–64 | 98.5%/5000 | 24 at 400 W kg−1 | 2001.07 at 16.68 W h kg−1 | AC | 123 |
| ZnCo2O4@CoMoO4 nanosheets on NF | 2192.2 F g−1 (1096.1 C g−1) at 10 mA cm−2 | 0–0.5/SCE | 73.8%/3–40 | 104.1%/5000 | 29.24 at 884.57 W kg−1 | 10526.32 at 20.76 W h kg−1 | AC | 122 |
| ZnO–ZnCo2O4@Ni(OH)2 heterostructured nanowires on NF | 1901.6 F g−1 (237.7 mA h g−1) at 2 A g−1 | 0–0.45/Ag/AgCl | 85.7%/2–20 | 98.7%/5000 | 80.10 at 662.06 W kg−1 | 9200 at 64.75 W h kg−1 | Fe2O3/NF | 125 |
| ZnCo2O4@Ni–Co–S nanosheet-based microspheres on NF | 1762.6 F g−1 at 1 A g−1 | 0–0.5/Hg/HgO | 81.3%/1–50 | 81.4%/5000 | 37.1 at 433.1 W kg−1 | 5124.3 at 28.3 W h kg−1 | AC | 118 |
| ZnCo2O4@Co–Al LDH nanowires on NF | 2041 F g−1 at 1 A g−1 | 0–0.5/SCE | 70%/1–10 | — | 50.1 at 400 W kg−1 | 6200 at 16.53 W h kg−1 | AC | 127 |
| r-ZnCo2O4@NiMoO4·H2O heterostructured nanowires on NF | 3.53 F cm−2 at 1 mA cm−2 | 0–0.5/Ag/AgCl | — | 95.4%/5000 | 2.55 mW h cm−3 at 0.033 W cm−3 | 0.169 W cm−3 at 0.39 mW h cm−3 | CNT | 121 |
| Porous ZnCo2O4/C nanowires on NF | 2340 F g−1 (7.02 F cm−2) at 1 mA cm−2 | 0–0.5/Hg/HgO | ∼57%/1–20 | 92.6%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35.75 at 73.17 W kg−1 | ∼1900 at ∼4.5 W h kg−1 | AC | 143 |
| Porous ZnCo2O4@Ni(OH)2 nanosheets on NF | 1021.1 F g−1 (3.06 F cm−2) at 1 mA cm−2 | 0–0.5/SCE | 55.3%/1–10 | 50.1%/5000 | 40.0 at 802.7 W kg−1 | 8020 at 17.6 W h kg−1 | AC | 126 |
| ZnCo2O4@PPy nanostructures on NF | 1210 F g−1 (605 C g−1) at 1 A g−1 | 0–0.5/Ag/AgCl | 56%/1–10 | 93.5%/9000 | 141.3 at 2700.5 W kg−1 | ∼27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at ∼90 W h kg−1 000 at ∼90 W h kg−1 |
AC | 57 |
| ZnCo2O4@NiWO4 heterostructures on NF | 1782 F g−1 (2.14 F cm−2) at 1 mA cm−2 | 0–0.5/Hg/HgO | 35.5%/1–10 | 95.4%/5000 | 42.2 at 716 W kg−1 | 3087 at 34.3 W h kg−1 | AC | 124 |
| ZnCo2O4@MnO2 hierarchical nanosheets on NF | 2170 F g−1 (2.6 F cm−2) at 3 mA cm−2 | 0–0.5/SCE | 50.4%/3–40 | 117.5% 2500 | 29.41 at 628.42 W kg−1 | 8378.38 at 6.98 W h kg−1 | AC | 58 |
| Leaf-like ZnCo2O4@NiCo2S4@PPy on NF | 2507.0 F g−1 (3.75 F cm−2) at 0.5 A g−1 | 0–0.5/Hg/HgO | ∼69%/0.5–20 | 83.2%/5000 | 44.15 at 850 W kg−1 | 4250 at 33.06 W h kg−1 | AC | 144 |
| ZnCo2O4@CdS nanoflowers on NF | 5.91 F cm−2 (2.66 C cm−2) at 25 mA | 0–0.45/SCE | 62.2%/25–40 | — | — | — | — | 116 |
| Mesoporous Co3O4@ZnCo2O4 nanowires on NF | 2255.5 F g−1 (1240.5 C g−1) at 2 mA cm−2 | 0–0.55/Hg/HgO | 59.0%/2–30 | 90.9%/3000 | 37.3 at 800 W kg−1 | 8000 at 21.3 W h kg−1 | AC | 128 |
| Flower-like ZnCo2O4@ZnCo2S4 nanostructures on NF | 1057.78 F g−1 at 1 A g−1 | SCE | 54.6%/1–10 | — | 127.4 at 2520 W kg−1 | 36497.16 at 40.55 W h kg−1 | CNTs | 113 |
| ZnCo2O4@Zn–Co–S hybrid nanowires on CNTFs | ∼1.35 F cm−2 at 0.5 mA cm−2 | −0.1 to 0.6/SCE | — | — | 32.01 μW h cm−2 at 698.42 μW cm−2 | 6999.99 μW cm−2 at 12.38 μW h cm−2 | H-Co3O4/CoNC/CNTFs | 113 |
MnO2 has been considered to be an ideal electrode active material owing to its superior electrochemical activity and high theoretical capacitance (about 1370 F g−1); however, its poor conductivity still precludes practical application in high-performance energy storage devices. Nevertheless, the addition of MnO2 NPs and nanostructures onto more conductive materials, such as ZnCo2O4, can further enhance the electrochemical performance of nanocomposite-based electrodes. As a result, a MnO2 NP-decorated ultrathin ZnCo2O4 nanosheet slurry-cast electrode (286 F g−1 at 1 A g−1)98 presents more electroactive sites and specific surface area in comparison to pristine ZnCo2O4. Consequently, it provides better transmission channels for electrons due to the superior electrical conduction and suitable morphology of ZnCo2O4 support, while the appropriate content of MnO2 NPs further improves its electrochemical properties, acting as a highly active co-catalyst. Similarly, concerning binder-free electrodes, porous ZnCo2O4 nanoflakes of interconnected NPs, with sufficient space to serve as the backbone for the growth of MnO2 nanosheets, were used to produce a ZnCo2O4/MnO2 heterostructure on NF108 (Fig. 6A). This drastically increased the availability of electroactive sites and specific surface area, but maintained the space needed for electrolyte diffusion at higher current densities. This new structure was able to deliver 2057 F g−1 at 1 A g−1 with a cycling stability of 96.5% after 5000 cycles and a rate capability of 65% after a 15-fold current density increase.
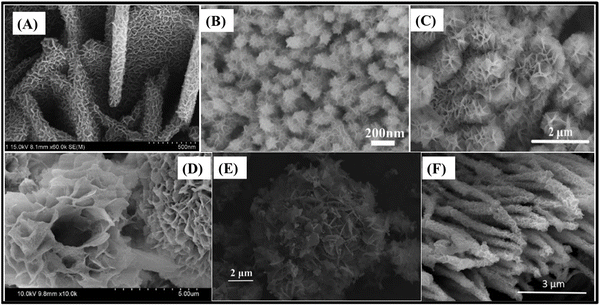 | ||
| Fig. 6 SEM images of (A) porous ZnCo2O4/MnO2 heterostructures/NF,108 (B) ZnCo2O4@MnO2 nanowires/NF,115 (C) mesoporous ZnCo2O4/NiO flower-like clusters/NF,111 (D) ZnCo2O4@CdS nanoflowers/NF,116 (E) hydrangea-like ZnCo2O4/Ni3V2O8 nanostructures/NF107 and (F) ZnCo2O4@Ni3S2 heterostructured nanowires/NF.117 Panel A: Reproduced with permission.108 Copyright © 2018 Elsevier Ltd. All rights reserved. Panel B: Reproduced with permission.115 Copyright MarketplaceTM, Royal Society of Chemistry. Panel C: Reproduced with permission.111 Copyright © 2018 Elsevier Ltd. All rights reserved. Panel D: Reproduced with permission.116 Copyright © 2020 Korean Physical Society. Published by Elsevier B.V. All rights reserved. Panel E: Reproduced with permission.107 Copyright © 2019 Elsevier Ltd and Techna Group S.r.l. All rights reserved. Panel F: Reproduced with permission.117 Copyright © 2017 Elsevier B.V. All rights reserved. | ||
In fact, heterostructured nanosheet architectures are among the best for ZnCo2O4 composite-based binder-free electrodes due to their electrochemical stability, large specific surface area and optimal space between the nanosheets, which maximizes the availability of electroactive sites even at high current density. Therefore, ZnCo2O4@MnO2 hierarchical nanosheet arrays on NF (2170 F g−1 at 3 mA cm−2),58 based on the growth of MnO2 nanosheets onto porous ultrathin ZnCo2O4 nanosheets, delivered a high specific capacitance similarly to the ZnCo2O4/MnO2 heterostructure on NF.108 The capacitance retention was 117.5% after 2500 charge/discharge cycles, and the rate capability was 50.4% at 40 mA cm−2, due to the slow diffusion of electrolyte between the spaces of abundant MnO2 nanosheets. Moreover, such core@shell materials were also studied in 1D morphology as ZnCo2O4@MnO2 nanowire arrays on NF (4.98 F cm−2 at 2 mA cm−2)115 (Fig. 6B), encompassing smooth ZnCo2O4 nanowires uniformly covered with a porous MnO2 thin film. This largely increased the specific surface area of the electrode, delivering 5 times more specific capacitance than that of pristine ZnCo2O4 nanowires, in addition to exhibiting much better cycling stability (106.2% vs. 87.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and rate capability (∼78.9% vs. 57.4% at 16 mA cm−2).
000 cycles) and rate capability (∼78.9% vs. 57.4% at 16 mA cm−2).
Binder-free electrodes were studied in recent years, using the same strategy of designing decorated nanocomposites, ZnCo2O4/Co3S4 nanowire arrays on NF (2.02 C g−1 at 0.8 A g−1)112 and mesoporous ZnCo2O4/NiO flower-like clusters on NF (2797 F g−1 at 1 A g−1),111 as well as slurry-cast electrodes and ZnCo2O4@CdS nanoflowers on NF (5.91 F cm−2 at 25 mA).116 Mesoporous ZnCo2O4/NiO flower-like clusters on NF111 (Fig. 6C) make use of highly electroactive NiO nanosheets, with a theoretical specific capacitance of 3750 F g−1, assembled onto ZnCo2O4 microspheres. In this case, the NiO nanosheets form flower-like clusters, and relieve the internal stress and restrain the capacitance decay through the charge–discharge processes. In addition, they help in reducing the ion-diffusion path length and increasing the specific surface area. As a result, very high specific capacitance and overall stability are achieved, with a retention of 81.8% upon a 10-fold current density increase and ∼100% after 3000 cycles. CdS is another highly electroactive semiconductor candidate for supercapacitive applications due to its excellent conductivity and high theoretical capacity of 1675 F g−1. Therefore, it is assembled with CdS nanoparticles as the coating shell and ZnCo2O4 nanoflowers as the core (Fig. 6D),116 delivering more than 10 times the specific capacitance of pristine ZnCo2O4 nanoflowers. There is a low rate capability, mainly due to the intrinsically low electrolyte diffusion at higher current densities for the flower-like structured binder-free electrode, and also some limitation of ion-diffusion through the CdS nanoparticle shell.
In addition, Ni3V2O8 and Ni3S2 have also been used for supercapacitive applications owing to their high performance and capacity. In fact, slurry-cast hydrangea-like ZnCo2O4/Ni3V2O8 nanostructures composed of ZnCo2O4 nanospheres and Ni3V2O8 nanosheets107 (Fig. 6E) present nanoflower-like heterostructures that can provide open space for ion-diffusion pathways, exposing various redox active sites for electrochemical reactions and electron transport, delivering 1734 F g−1 at 1 A g−1. It also presents superior cycling stability and rate capability, retaining 96% of its initial specific capacitance after 8000 cycles and 90% from 1 to 10 A g−1. The binder-free core@shell ZnCo2O4@Ni3S2//NF electrode (2200 F g−1 at 2 A g−1)117 (Fig. 6F) exhibits interconnected Ni3S2 nanosheets coated on the surfaces of the highly ordered and dense ZnCo2O4 nanowire arrays. It delivered high specific capacitance because of the two-dimensional (2D) nanosheet coating, which largely increases the specific surface area. However, 55.7% rate capability at 5-fold current density is due to the reduced space between nanowires that limits the electrolyte diffusion at higher current densities.
Zinc oxide (ZnO), an n-type semiconductor with a wide band gap (∼3.37 eV), can synergize well with ZnCo2O4, a p-type semiconductor, to produce ZnCo2O4/ZnO heterostructures with p–n junctions. The n-type region has a high electron concentration and the p-type, a high hole concentration; so electrons diffuse from the n-type side to the p-type side. Therefore, the electrons generated at ZnO sites in charge/discharge cycles can rapidly diffuse into the ZnCo2O4 matrix, potentially enhancing the overall electronic conduction of the composite. All ZnCo2O4/ZnO composites used in slurry-cast electrodes found in the literature presented very high specific surface area, due to radially grown structures such as snowflake-like ZnCo2O4/ZnO (826.7 F g−1 at 1 A g−1)99 and marigold-like ZnO/ZnCo2O4 (705.1 F g−1 at 0.3 A g−1),101 or hollow structures, such as nanosheet-based hollow ZnO/ZnCo2O4/NiO microspheres (1136.4 F g−1 at 1 A g−1).102 Snowflake-like ZnCo2O4/ZnO microstructures99 (Fig. 7A) presented superior performance not only in specific capacitance but also in rate capability. They delivered 69.6% of their initial capacitance after a 15-fold increase in current density, due to the more suitable open space for the ion-diffusion pathway in comparison to tight nanosheets in marigold-like ZnO/ZnCo2O4 (89.4%, 0.3–1 A g−1).101
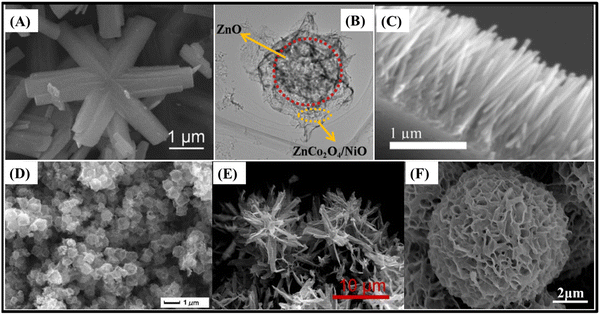 | ||
| Fig. 7 (A) SEM image of snowflake-like ZnCo2O4/ZnO microstructures.99 (B) TEM image of nanosheet-based hollow ZnO/ZnCo2O4/NiO microspheres,102 and SEM images of (C) ZnCo2O4/ZnO heterostructured nanorods/ITO,110 (D) porous NiCo2O4/ZnCo2O4/Co3O4 hollow nanocages,105 (E) flower-like ZnCo2O4@ZnCo2S4 nanostructures/NF,113 and (F) ZnCo2O4@Ni–Co–S nanosheet-based microspheres/NF.118 Panel A: Reproduced with permission.99 © 2019 Elsevier Ltd and Techna Group S.r.l. All rights reserved. Panel B: Reproduced with permission.102 Copyright MarketplaceTM, Royal Society of Chemistry. Panel C: Reproduced with permission.110 Copyright © 2018 American Chemical Society. Panel D: Reproduced with permission.105 Copyright © 2018 Elsevier B.V. All rights reserved. Panel E: Reproduced with permission.113 Copyright © 2020, Springer Science Business Media, LLC, part of Springer Nature. Panel F: Reproduced with permission.118 Copyright © 2020 Elsevier B.V. All rights reserved. | ||
Hollow ZnO/ZnCo2O4/NiO microspheres102 are covered with numerous ultrathin nanosheets and decorated with tiny pores (Fig. 7B), which provide optimized specific surface area and access to plenty of electrolyte. Such characteristics are beneficial for the exposure of electroactive sites, buffering the effect of volume changes and promoting suitable channels to facilitate rapid ion/electron diffusion during the charge/discharge processes. The result encompasses 86.5% capacitance retention after 5000 cycles and, due to the tiny pore sizes and spaces within the ultrathin nanosheets, rate capabilities of ∼31.2% and 54.9% with 30- and 10-fold current density increases, respectively. As for binder-free electrodes, there are ZnCo2O4/ZnO heterostructured nanorods on an ITO electrode (150 μF cm−2 at 1.2 μA cm−2 with UV-radiation)110 (Fig. 7C), which feature both the photoelectric effect and direct electron transportation pathway. Photoinduced electrons and holes, under UV radiation, participate directly in the electrolyte ion separation process to boost the overall capacitive response, thus delivering 174% (2.7 times) more specific capacitance under UV illumination as compared to the absence of UV.
Differently from the mentioned core@shell materials, the mesoporous Co3O4@ZnCo2O4/NF electrode (2255.5 F g−1 at 2 mA cm−2)128 features ZnCo2O4 as the shell material, due to its excellent rate capability and cycling stability. In this way it could improve the practical application of the electrode; even so, it has better electrical conductivity than its core. The directly grown needle-like Co3O4 nanowire arrays are composed of numerous polycrystalline interconnected nanoparticles, which provides good roughness, increasing the specific surface area and facilitating the uniform coating with the ZnCo2O4 thin film composed of multiple nanoparticles. As a result, the electrode delivers high specific capacitance, about 3-times more than that of Co3O4/NF, with a capacitance retention of 59.0% and 90.9% after a 15-fold current density increase and 3000 cycles, respectively.
Composites based on heterojunctions of ZnCo2O4 and other MCo2O4 usually present: (i) richer and more abundant redox reaction sites and, thus, higher specific capacitances; (ii) more stability, since both have high contents of Co2O42−; and (iii) similar lattice parameters, in which the internal resistance of the adjacent interfaces is greatly reduced during the charge/discharge processes and facilitates the electron transport. In this context, ZnCo2O4/MnCo2O4 heterojunction nanosheets (254 F g−1 at 1 A g−1)103 and NiCo2O4/ZnCo2O4 heterostructures (1870.9 F g−1 at 1 A g−1)104 composed of ZnCo2O4 nanosheets and urchin-like NiCo2O4, and porous NiCo2O4/ZnCo2O4/Co3O4 hollow nanocages (1892.5 F g−1 at 1 A g−1)105 (Fig. 7D) formed by interconnecting ultra-small nanoparticles with many voids that results in porous multiple shells have been reported. Despite delivering relatively low specific capacitance, slurry-cast ZnCo2O4/MnCo2O4 heterojunction nanosheet electrodes103 presented higher specific capacitance and overall stability than pristine ZnCo2O4 and MnCo2O4. In fact, the other two composites also presented much better performance than their counterparts in slurry-cast electrodes NiCo2O4/Co3O4 and ZnCo2O4/Co3O4, with NiCo2O4/ZnCo2O4/Co3O4 hollow nanocages,105 and urchin-like NiCo2O4 and sheet-like ZnCo2O4 for NiCo2O4/ZnCo2O4 heterostructures. Notwithstanding, these NiCo2O4/ZnCo2O4-based composites exhibit the second and third highest specific capacitances among all reported slurry-cast electrodes. They also show superior electrical conductivity, rich and abundant electrochemically active sites, high specific surface area, and good rate capability and cycling stability,104,105 with a capacitance retention of 58.4% and 91% after a 20-fold current density increase and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively.
000 cycles, respectively.
Transition metal sulfides display higher electrical conductivity than their oxide counterparts because the replacement of oxygen with sulfur allows easier electron transport, lower electronegativity and smaller band-gaps, making them good candidates for supercapacitive applications and thus improving the energy storage properties of ZnCo2O4 in composite architectures. From this perspective, there are binder-free electrodes based on core@shell ZnCo2O4@ZnxCo3−xS4 materials, such as those based on flower-like ZnCo2O4@ZnCo2S4 arrays//NF (1057.78 F g−1 at 1 A g−1)113 and ZnCo2O4@Zn–Co–S hybrid arrays//CNTFs (∼1.35 F cm−2 at 0.5 mA cm−2)114 and microsphere-structured ZnCo2O4@Ni–Co–S nanosheets (1762.6 F g−1 at 1 A g−1).118 The flower-like ZnCo2O4@ZnCo2S4//NF113 electrode (Fig. 7E) delivered good specific capacitance and, even so the hierarchical micro-nanostructured features could further improve the electrochemical properties of the electrode by offering larger spacing for the penetration of electrolyte into the structure. Thus, it could increase the availability of electroactive sites at higher current densities and electron transfer. A capacitance retention of 54.6% was achieved by a 10-fold increase in current density, as expected for a flower-like structure-based binder-free electrode.
Among bimetallic sulfides, nickel–cobalt sulfides have attracted a lot of attention due to their excellent conductivity, superior to nickel and cobalt sulfide counterparts and about 100 times higher than those of the corresponding oxides, and better capacitance performance compared with other metallic sulfides, such as NiS, Ni3S2, and CoS. In this context, slurry-cast core@shell ZnCo2O4@Ni–Co–S microspheres composed of radially grown ZnCo2O4 nanosheets, with a rough surface of electrodeposited Ni–Co–S118 (Fig. 7F), delivered higher specific capacitance, rate capability and cycling stability than their pristine ZnCo2O4 and Ni–Co–S counterparts. The increase of capacitance occurs mainly due to their hierarchical micro-nanostructure that has an open network of individual nanosheets. They facilitate ion-diffusion and help in maintaining the structural integrity. The highly conductive Ni–Co–S shell can efficiently decrease the charge transfer resistance, leading to a fast reversible redox reaction, ample redox active site availability and short ion diffusion pathways, thus resulting in a capacitance retention of 81.3% at a 50-fold increase in current density and 81.4% after 5000 cycles.
Notwithstanding, other materials have also attracted great attention as promising electrodes for energy storage devices, such as molybdenum- and tungsten-based metal oxides, nickel hydroxides and layered double hydroxides (LDHs). Core@shell structures along with ZnCo2O4 have been studied as shell materials, to achieve competitive supercapacitive performance. Molybdenum-based metal oxides such as NiMoO4119–121 and CoMoO4122 in core@shell architectures with ZnCo2O4 were studied, due to their high theoretical specific capacity attributed to Ni and Co ions, and excellent electrical conductivity, attributed to the multiple redox reactions of Mo ions.119–122 All three reviewed core@shell ZnCo2O4@NiMoO4 materials had hierarchical nanowire and nanosheet architectures grown on NF, although with some differences that were relevant to their electrochemical performance, respectively: intercrossed ZnCo2O4 nanowires covered with NiMoO4 nanosheets (2316 F g−1 at 10 mA cm−2)119 (Fig. 8A); ZnCo2O4 nanowires covered with an ultrathin porous NiMoO4 nanosheet network (1912 F g−1 at 1 A g−1)120 (Fig. 8B); and smooth reduced-ZnCo2O4 nanowires covered with NiMoO4 nanosheets (3.53 F cm−2 at 1 mA cm−2)121 (Fig. 8C). Comparing all three electrodes, the first one119 not only had the best cycling stability and specific capacitance, but also presented hierarchical heterostructures for the nanowires with the smallest diameter, which facilitated ion-diffusion. The rate-capability was the best one, although it was still relatively low as a nanowire-based binder-free electrode. On the other hand, the core@shell ZnCo2O4@CoMoO4/NF electrode (2192.2 F g−1 at 10 mA cm−2) presented smooth honeycomb-like ZnCo2O4 nanosheets covered with interconnected rough CoMoO4 nanosheets. In this way, it could effectively shorten the ion transport distance and increase the availability of electroactive sites, thus delivering high specific capacitance and excellent cycling stability, along with good rate-capability.
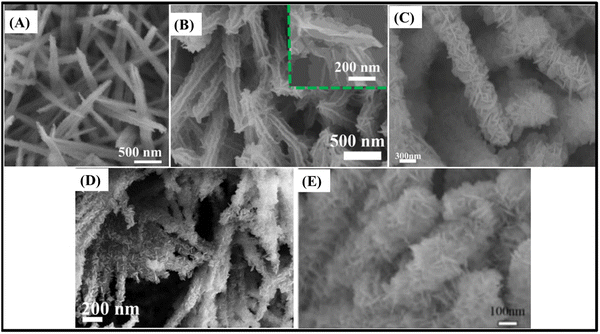 | ||
| Fig. 8 SEM images of (A) ZnCo2O4@NiMoO4 heterostructured nanowires/NF,119 (B) ZnCo2O4@NiMoO4 heterostructured nanowires/NF,120 (C) r-ZnCo2O4@NiMoO4·H2O heterostructured nanowires/NF,121 (D) ZnCo2O4@ZnWO4 heterostructured nanowires/NF123 and (E) ZnCo2O4@NiWO4 heterostructures/NF.124 Panel A: Reproduced with permission.119 Copyright © 2020 Elsevier Ltd. All rights reserved. Panel B: Reproduced with permission.120 Copyright © 2020 Elsevier Ltd. All rights reserved. Panel C: Reproduced with permission.121 © 2018 Elsevier B.V. All rights reserved. Panel D: Reproduced with permission.123 Copyright © 2018 Published by Elsevier Inc. Panel E: Reproduced with permission.124 Copyright © 2020, Springer-Verlag GmbH Germany, part of Springer Nature. | ||
Tungsten-based metal oxides with wolframite structure, such as ZnWO4123 and NiWO4,124 are promising materials for sensor, photocatalyst and energy storage systems. They allow supercapacitive applications, with high theoretical specific capacitance, where both Zn/Ni and W elements participate in the faradaic redox reactions and have high electrical conductivity. Core@shell ZnCo2O4@ZnWO4 (13.4 F cm−2 at 4 mA cm−2)123 (Fig. 8D) and ZnCo2O4@NiWO4 (1782 F g−1 and 2.14 F cm−2 at 1 mA cm−2)124 (Fig. 8E) present heterostructured ultrathin and interconnected nanosheet-covered nanowires on NF architecture. They can deliver high specific and areal capacitance, especially in the case of ZnCo2O4@ZnWO4 where the highly conductive ZnCo2O4 nanowire arrays rationally overcome the poor conductivity of ZnWO4 nanosheets which could shorten the ion-diffusion and electron transport pathways. Additionally, both electrodes present relatively poor rate-capability as nanowire-based binder-free electrodes, with a capacitance retention of 28.1% at 64 mA cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 and 35.5%124 at 10 mA cm−2, respectively.
123 and 35.5%124 at 10 mA cm−2, respectively.
Considering hydroxide-based shell materials, recent reports can be found in the literature for Ni(OH)2125,126 and Co–Al LDH.127 The ZnCo2O4@Ni(OH)2/NF electrode (1021.1 F g−1 and 3.06 F cm−2 at 1 mA cm−2)126 (Fig. 9A) based on crosslinked ultrathin nanoflakes, covering porous nanosheets with a thick triangular shape, delivered good specific capacitance but low rate-capability and cycling stability. They have been ascribed to the bulkiness of the ZnCo2O4 nanosheets and reduced space between them, which hinders the ion-diffusion and electron transfer. This also reduces the control of the strain effects due to volume changes through cycling. Conversely, ZnO–ZnCo2O4@Ni(OH)2/NF (1901.6 F g−1 at 2 A g−1)125 (Fig. 9B) delivered not only higher specific capacitance, but also high rate-capability for a heterostructured nanowire-based binder-free electrode, with 85.7% capacitance retention at 20 A g−1, along with high cycling stability, retaining 98.7% of its initial capacitance after 5000 cycles. Additionally, the ZnO–ZnCo2O4@ZnO/NF, ZnO–ZnCo2O4@CoO/NF and ZnO–ZnCo2O4/NF electrodes have been studied for comparison purposes, delivering approximately 54%, 40% and 31%, respectively, of the specific capacitance of ZnO–ZnCo2O4@Ni(OH)2/NF. The superior performance of ZnO–ZnCo2O4@Ni(OH)2/NF is attributed to the nanoflake-covered interconnected nanowires forming a hierarchical porous 2D network on top of the NF. This provides a high surface area, with plenty of space for electrolyte diffusion, which in conjunction with the available electroactive sites facilitates the electron transport through the ZnO–ZnCo2O4 nanowires.
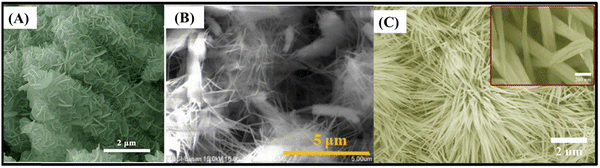 | ||
| Fig. 9 SEM images of (A) porous ZnCo2O4@Ni(OH)2 nanosheets/NF,126 (B) ZnO–ZnCo2O4@Ni(OH)2 heterostructured nanowires/NF,125 and (C) ZnCo2O4@Co–Al LDH nanowires on NF.127 Panel A: Reproduced with permission.126 Copyright © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Panel B: Reproduced with permission.125 Copyright © 2020 Elsevier Ltd. All rights reserved. Panel C: Reproduced with permission.127 Copyright © 2019 Elsevier Ltd and Techna Group S.r.l. All rights reserved. | ||
Layered double hydroxides (LDHs) have high theoretical capacity, low cost and environmental compatibility. However, their inherent low conductivity and aggregation effects hinder charge transportation, leading to low electrochemical performance. However, when an LDH is assembled as a shell material combined with a highly conductive core, such as ZnCo2O4, superior performance is expected. In fact, ZnCo2O4@Co–Al LDH nanowires on NF (2041 F g−1 at 1 A g−1)127 (Fig. 9C), composed of urchin-like porous ZnCo2O4 nanowires, which were uniformly covered with Co–Al LDH nanosheets, delivered higher specific capacitance and rate-capability than pristine ZnCo2O4, Ni–Al LDH and Co–Al LDH, and core@shell ZnCo2O4@Ni–Al LDH electrodes, retaining 70% of the initial capacitance at 10 A g−1 due to the increase in specific surface area, the high electroactivity of the Co–Al LDH shell, and band alignments between ZnCo2O4 and Co–Al LDH, thus facilitating the charge transfer.
Polymers, e.g., PANI,131,136 g-C3N4135 and PVP,66 can act as support materials for ZnCo2O4, while PPy57,144 has been explored as a shell material in core@shell architectures. Embedding ZnCo2O4 in g-C3N4, a mesoporous sheet-like soft polymer, can produce g-C3N4@ZnCo2O4 (1386 F g−1 at 4 A g−1)135 (Fig. 10A) with the benefit of the highly active nitrogen sites, large specific surface area and good overall stability, in addition to low-cost. However, in comparison to pristine ZnCo2O4, only 66% of the initial specific capacity was maintained for a 2-fold density current increase. PVP is a bulky, non-toxic, non-ionic polymer containing carbonyl, amine, and alkyl functional groups that can be used as a surfactant, reducing agent, shape controlling agent, and dispersant in nanoparticle synthesis. The self-assembly of PVP was used to produce binder-free hierarchical microflowers of ZnCo2O4/PVP composites (761 F g−1 at 0.35 A g−1)66 (Fig. 10B) via an assisted hydrothermal method. These materials presented relatively poor rate capability, as expected for a flower-like structured material-based binder-free electrode. Notwithstanding, PANI, a semi-flexible rod-like polymer, exhibits a good electrical conductivity with multi-redox activity involving protonation, and can modify ZnCo2O4 particles’ sizes and shapes thanks to its strong interactions, shortening electron/ion pathways and increasing surface area due to interconnective rod-like structures. As a result, nanosheet-like ZnCo2O4/N-GO/PANI (720 F g−1 at 1.5 A g−1)131 (Fig. 10C), based on ZnCo2O4/N-GO coverage with multifaceted PANI, and PANI/ZnCo2O4 nanoparticle (867 F g−1 at 0.5 A g−1)136 (Fig. 10D) slurry-cast electrodes exhibited significant changes in size, shape, specific surface area, bond length, electron density, and other parameters. Both delivered excellent cyclability and specific capacitance in comparison to the ZnCo2O4/N-GO nanocomposite131 and pristine ZnCo2O4 NPs.136
 | ||
| Fig. 10 SEM images of (A) nanosheet-like g-C3N4/ZnCo2O4,135 (B) 3D flower-like ZnCo2O4/PVP,66 (C) nanosheet-like ZnCo2O4/N-GO/PANI,131 (D) PANI/ZnCo2O4 nanoparticles,136 (E) ZnCo2O4@PPy nanostructures/NF57 and (F) core@shell ZnCo2O4@NiCo2S4@PPy.144 Panel A: Reproduced with permission.135 Copyright © 2020, The Author(s). Panel B: Reproduced with permission.66 Copyright © 2020 John Wiley & Sons Ltd. Panel C: Reproduced with permission.131 © 2020 Elsevier B.V. All rights reserved. Panel D: Reproduced with permission.136 Copyright © 2019, Springer-Verlag GmbH Germany, part of Springer Nature. Panel E: Reproduced with permission.57 CC BY 3.0. Royal Society of Chemistry. Panel F: Reproduced with permission.144 Copyright MarketplaceTM. IOP Publishing. | ||
PPy is considered to be a promising electrode material owing to its high electrical conductivity, greatly improving the specific capacitance and cycle performance as well as decreasing the overpotential attributed to the promotion of electron transport and reduction of internal resistance.57,144 ZnCo2O4@PPy/NF (1210 F g−1 at 1 A g−1)57 (Fig. 10E), architectured as ultrathin PPy film-coated ZnCo2O4 nanowires, delivered about 9 times more specific capacitance than pristine spinel species. On the other hand, the core@shell ZnCo2O4@NiCo2S4@PPy/NF electrode (2507.0 F g−1 and 3.75 F cm−2 at 0.5 A g−1)144 (Fig. 10F) presented much better rate-capability, with 69% capacitance retention after a 40-fold increase in current density. This result is associated with its composition, since NiCo2S4 exhibits abundant valence states and high theoretical specific capacitance in addition to the more suitable architecture. It resembles porous leaf-like ZnCo2O4 nanosheets covered hierarchically with thin and abundant NiCo2S4 nanosheets and a thin PPy film. This core@shell structure formed by three materials created a bi-interface that can promote the contact with the electrolyte and facilitate ion-diffusion, accelerate the electron transfer, and increase the availability of electroactive sites. However, PPy can contribute to the pseudocapacitance through doping and de-doping redox reactions, increasing the volume changes along the cycling and thus reducing the mechanical stability of the material. Slightly poorer cycling stability than that of ZnCo2O4@NiCo2S4/NF was observed, without PPy coating, but, in contrast, the specific capacitance almost doubled after the coating.
Carbon (C) is also considered to be a promising candidate to form a composite material for ZnCo2O4-based electrodes in supercapacitive applications, due to its good volume expansion tolerance and excellent electron transport. The use of C can effectively improve the overall electrical conductivity of the material, decrease the volume expansion, and inhibit the agglomeration of ZnCo2O4 in the redox reaction process, thus improving the specific capacitance and cycling stability. This is the case of the core@shell ZnCo2O4@C/NF electrode (2340 F g−1 and 7.02 F cm−2 at 1 mA cm−2),143 composed of agglomerated ZnCo2O4 nanoparticles as porous nanowire arrays, covered with a thin amorphous carbon layer, leading to high specific capacitance and good cycling stability (capacitance retention of 92.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).
000 cycles).
Notwithstanding, N-doped carbon (NC) supported P-ZnCo2O4 nanosheets (1581.5 F g−1 at 1 A g−1)132 (Fig. 11A), in which the NC acted as a 3D continuous network, provided a highly electrically conductive support with large surface area for the growth of P-doped ZnCo2O4 nanosheets. They showed much better results, with 90.6% rate capability after a 10-fold current density increase. The triangular-shaped P-doped ZnCo2O4 nanosheets are rich in oxygen vacancies, due to their substitution for phosphorus ions. In this way, ion-diffusion and the absorption of OH− are facilitated. There are a large interface contact area and shortened electron/ion diffusion paths, which is an interesting strategy to improve ZnCo2O4 electrochemical performances in slurry-cast electrodes.
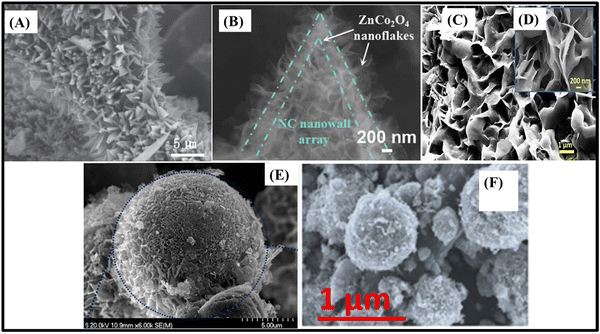 | ||
| Fig. 11 (A) SEM image of N-doped C supported P-ZnCo2O4 nanosheets.132 (B) TEM image of ZnCo2O4/N-doped carbon hollow nanowall arrays/CT.138 SEM images of (C and D) N-doped carbon/ZnCo2O4 honey nest nanostructures,140 (E) cauliflower-like AuNP/rGO–ZnCo2O4133 and (F) NiCo2O4–ZnCo2O4/rGO nanosheets.106 Panel A: Reproduced with permission.132 © 2020 Elsevier B.V. All rights reserved. Panel B: Reproduced with permission.138 © 2019 Published by Elsevier B.V. Panels C and D: Reproduced with permission.140 Copyright © 2020, Springer Science Business Media, LLC, part of Springer Nature. Panel E: Reproduced with permission.133 Copyright © 2019 Elsevier B.V. All rights reserved. Panel F: Reproduced with permission.106 Copyright © 2020 American Chemical Society. | ||
As for binder-free electrodes, in recent years the relevant systems studied were ZnCo2O4/NC hollow nanowall arrays/flexible carbon textiles (CT) (∼2003.8 F g−1 at ∼1.79 A g−1)138 and NC/ZnCo2O4 honeycomb-like nanostructures (1289 F g−1 at 3.5 A g−1).140 The first one138 (Fig. 11B) is based on NC hollow nanowall arrays that serve as the backbone and conductive connection for porous ultrathin ZnCo2O4 nanoflakes. They increase the contact area with the electrolyte and enable fast redox reaction, featuring high specific surface area and short ion diffusion paths. This leads to high rate-capability and cycling stability, with 74.7% and ∼99.4% capacitance retention, when increasing the current density to 57.14 A g−1 and after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively. The second one140 (Fig. 11C) shows less competitive performance, but it involves an interesting strategy for the production of NC using high fructose corn syrup as a green, abundant, and inexpensive carbon source for producing 3D porous ultrathin nanoflakes in a honeycomb-like morphology. The arrangement facilitates the penetration of the electrolyte, providing small contact impedance, and improved ion and electron transportation, yielding relatively good rate capability and cycling stability, with 70% and 86% capacitance retention at 20 A g−1 and after 2000 cycles.
000 cycles, respectively. The second one140 (Fig. 11C) shows less competitive performance, but it involves an interesting strategy for the production of NC using high fructose corn syrup as a green, abundant, and inexpensive carbon source for producing 3D porous ultrathin nanoflakes in a honeycomb-like morphology. The arrangement facilitates the penetration of the electrolyte, providing small contact impedance, and improved ion and electron transportation, yielding relatively good rate capability and cycling stability, with 70% and 86% capacitance retention at 20 A g−1 and after 2000 cycles.
As for carbon nanoparticles (CNPs), there are examples in which they were dispersed onto ZnO/ZnCo2O4 nanosheets to produce CNP/ZnO/ZnCo2O4 derivatives (593.6 F g−1 at 0.25 A g−1).100 Electrospun 1D ZnCo2O4/C nanofibers, consisting of a ZnCo2O4 and carbon nanoparticle mixture (327.5 F g−1 at 0.5 A g−1), have also been reported.97 Both materials don’t use carbon as a conductive support, but in the form of dispersed nanoparticles. Therefore, the ions should diffuse through them to reach the electroactive material. As a result, they present low specific capacitances and very poor rate capabilities, despite the high cycling stability due to their optimized morphologies and CNP incorporation.97,100
Other highly conductive carbon materials, such as CNTs129 and rGO,106,133 have also been studied as supports for ZnCo2O4 in slurry cast electrodes, and both presented remarkable results. It should be noted that rGO has a large specific surface area, high electrical conductivity, good thermal stability, and excellent mechanical flexibility, displaying all benefits of 2D morphologies and superb possibilities as a support material. Nonetheless, π–π interactions and van der Waals forces between graphene sheets cause a restacking effect of rGO at higher current densities. This can limit its electrochemical performance, due to reduction in the specific surface area and creation of difficult channels for electrolyte ion transportation. The cauliflower-like AuNP/rGO–ZnCo2O4 (54.1 mA h g−1 at 25 mA cm−2)133 (Fig. 11D) was based on the incorporation of AuNPs within rGO nanosheets to prevent the restacking effect. However, rGO nanosheets were coated with flower-like ZnCo2O4 in order to increase their specific surface area. Therefore, this material did not work as a support material. The electrode delivered low specific capacity although it presented high cycling stability. In contrast, heterostructured NiCo2O4–ZnCo2O4/rGO nanosheets (2176.4 F g−1 at 1 A g−1)106 (Fig. 11E), composed of spherical NiCo2O4@ZnCo2O4 heterostructures (urchin-like NiCo2O4 and sheetlike ZnCo2O4) that were supported on rGO nanosheets, delivered the highest specific capacitance among all reviewed slurry-cast electrodes. This material afforded 58.2% rate capability after a 20-fold current density increase and 93.8% capacitance retention after 5000 charge/discharge cycles. Not coincidentally, the three best slurry-cast electrodes were those based on NiCo2O4–ZnCo2O4 composites supported onto rGO.
Binder-free electrode materials based on ZnCo2O4 and rGO composites have also been studied in recent years.109,137,139,141,142 ZnCo2O4/rGO intertwined sheets on NF (3222 F g−1 at 1 A g−1)137 (Fig. 12A) presented specific capacitance superior to other ZiCo-based composite materials containing rGO such as ZnCo-layered double hydroxide@rGO/NF (2142.0 F g−1),145 ZnCo-sulfide–rGO 3D hollow microsphere flowers (1225.1 F g−1)146 and CoO–ZnO/rGO/NF (1951.8 F g−1),147 but with poor rate capability and cycling stability, retaining only 26.7% and 65% after a 20-fold current density increase and 5000 cycles. This is due to the slow ion-diffusion rates induced by the fused porous ultrathin ZnCo2O4 curl nanosheets coated onto the vertically interconnected rGO nanosheets, limiting the penetration of electrolyte. Porous ZnCo2O4 nanosheets directly grown on rGO-coated NF (680 F g−1 at 1 A g−1)142 presented the poorest specific capacitance among all reviewed electrodes, but it is inferred that the rGO can effectively buffer ZnCo2O4 nanosheets’ volume changes through cycling and enhance the electrical conductivity. It can act as bridges for electron transfer, but the rGO-coated NF seems to not actively promote the ion-diffusion rates, exhibiting 88% capacitance retention after just a 5-fold current density increase. Lamellar films of ZnCo2O4/rGO hollow spheres (1075.4 F g−1 at 1 A g−1)139 (Fig. 12B) present a sandwich-like structure. The sandwiched hollow nanospheres can expand the inner-space and minimize the aggregation of rGO, facilitating and accelerating the electrolyte diffusion and increasing the cycling stability. On the other hand, heterostructured ZnCo2O4/N-rGO on NF (1600 F g−1 at 1 A g−1)141 (Fig. 12C) features ultrathin and porous honeycomb-like nanosheets and nanofeathers, with a hierarchical double-morphology. These characteristics, respectively, increase the active surface area and hinder the volume change through cycling. The N-doped rGO seems to parallelly orient the growth of ZnCo2O4 nanosheets, thus delivering 78.1% of the initial capacitance even after a 30-fold current density increase. Finally, MnO2-decorated ZnCo2O4 nanosheets on rGO-doped NF (3405.2 F g−1 at 2 A g−1)109 (Fig. 12D and E) feature the combined benefits of composites based on MnO2 and rGO. They were electrodeposited onto porous ZnCo2O4 nanosheets and on rGO-coated NF, thus delivering very high specific capacitance and good cycling stability (91.2%, 5000 cycles) but relatively poor rate capability (64.9%, 10-fold increase). In this way, they behave as porous ZnCo2O4 nanosheets on rGO-doped NF,142 because the rGO coating limits the ion-diffusion at higher current densities.
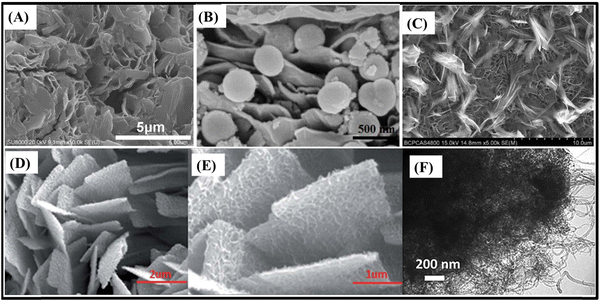 | ||
| Fig. 12 SEM images of (A) ZnCo2O4/rGO intertwined sheets/NF,137 (B) sandwich-like ZnCo2O4 hollow spheres/rGO lamellar film,139 (C) heterostructured ZnCo2O4/N-rGO/NF,141 and (D and E) MnO2-decorated ZnCo2O4 nanosheets on rGO-doped NF.109 (F) TEM image of ZnCo2O4/CNT nanoflowers.129 Panel A: Reproduced with permission.137 Copyright © 2018 Elsevier B.V. All rights reserved. Panel B: Reproduced with permission.139 Copyright © 2020 Published by Elsevier B.V. Panel C: Reproduced with permission.141 CC BY-NC 3.0. Royal Society of Chemistry. Panels D and E: Reproduced with permission.109 CC BY-NC 3.0. Royal Society of Chemistry. Panel F: Reproduced with permission.129 Copyright © 2019 Elsevier B.V. All rights reserved. | ||
CNTs present all advantages of 1D materials along with the increased conductivity of a carbon material. Therefore, when used as a support and connective material, they provide improved charge and electron transfer pathways.129,134 A MWCNT/ZnCo2O4 slurry-cast electrode (64 mA h g−1 at 1 A g−1)134 presented nearly double the specific capacity of pristine ZnCo2O4 due to its hexagonal nanoplates connected by multiwalled carbon nanotubes, even though it delivered very low specific capacity and rate capability. On the other hand, ZnCo2O4/CNT nanoflowers129 (Fig. 12F) delivered a high specific capacitance of 1203.8 F g−1 at 1 A g−1, in which CNTs interpenetrate the ZnCo2O4 flowers acting as both a conductive additive and a buffer material. This facilitates ion diffusion rates and rapid electron transfer and reduces interior stress and volume expansion during electrochemical reactions, increasing the cycling stability and electrochemical performances of the electrode.
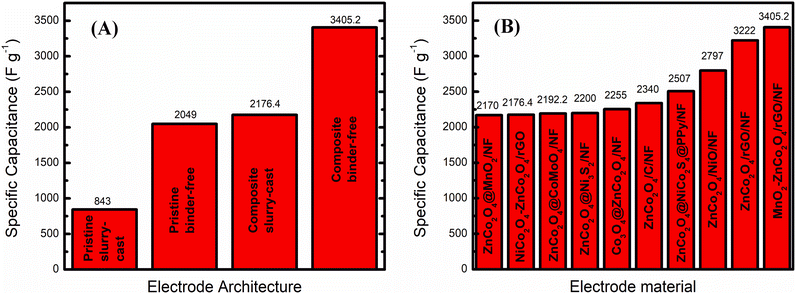 | ||
| Fig. 13 (A) Best specific capacitance for each ZnCo2O4-based electrode type: pristine slurry-cast; pristine binder-free; composite slurry-cast and composite binder-free (ref. 11, 26, 58 and 62, respectively). (B) Top 10 specific capacitances delivered by ZnCo2O4-based electrodes (ref. 62, 43, 38, 90, 84, 67, 75, 79, 58 and 89, respectively). | ||
Notwithstanding, there is still a possible limitation to be taken into consideration: in such architectures, the space between the nanostructures plays an important role in the ion-diffusion rates and in the availability of electroactive sites at higher current densities. This is pretty evident in binder-free electrodes based on ZnCo2O4 nanowires that can present very high specific capacitance, but low rate-capability. As a result, the best rate-capabilities are achieved by these binder-free electrodes with suitably spaced nanostructures and high availability of ion-diffusion channels.
3.2. Batteries
To increase mass transfer and contact between electrodes and electrolyte, Zhang et al.152 reported nickel foam supported hierarchical ZnCo2O4 nanosheets prepared by the solution-based method. A reversible specific capacity of 773 mA h g−1 at 0.25 A g−1 over 500 cycles was found for the porous ZnCo2O4 nanosheets. Song et al.157 also reported the synthesis of ZnCo2O4 nanosheets; when evaluated as an anode material for LIBs, the electrode showed an initial specific capacity of 1979 mA h g−1 and a stable discharge capacity of 688 mA g−1 at 0.5 A g−1 after 1000 cycles. Another ZnCo2O4 nanosheet material reported in the literature delivered a reversible capacity of 1640.8 mA h g−1 at a current density of 100 mA g−1 after 50 cycles.156
The morphology of the material plays a crucial role in the overall electrochemical performance, and thus, various morphologies have been intensively pursued and well designed. For example, Chen et al.160 synthesized ZnCo2O4 nanospheres with the desired shape via a one-step solution method. The ZnCo2O4 nanospheres showed an initial discharge capacity of 1320 mA h g−1 at a current density of 100 mA g−1 and a capacity retention rate of 76.22% after 50 charge and discharge cycles. Cheng et al.167 synthesized 1D porous ZnCo2O4 tailored cuboids with green natural soybean oil by a micro-emulsion strategy. This material exhibited an initial coulombic efficiency of 80.6% and a specific capacity of 1029.3 mA h g−1 at 1000 mA g−1 over 400 cycles. Lately, Li et al.153 synthesized 3D mesoporous ZnCo2O4 architectures by the ethylene glycol combustion strategy. The average specific capacity of the ZnCo2O4 electrode can return to about 778.7 mA h g−1 at a current density of 200 mA g−1 over 50 cycles. 3D hierarchical ZnCo2O4 nanocubes prepared by a hydrothermal method delivered a reversible specific capacity of 775 mA h g−1 after 100 cycles at 500 mA g−1.159
Hollow nanostructures have attracted considerable attention; their unique structure enables a high specific surface area, tunable chemical composition, and short charge transport pathway. Xue et al.164 developed a universal self-template approach to synthesize multishelled hollow ZnCo2O4 spheres (Fig. 14A and B), which displayed a specific capacity of 1020 mA h g−1 at 100 mA g−1 (Fig. 14C), a cycling durability of 1200 mA h g−1 after 200 cycles at 0.1 A g−1 and a rate capability of 730 mA h g−1 at 5.0 A g−1. Similarly, Deng et al.168 proposed a citrate-assisted hydrothermal synthesis to generate hollow ZnCo2O4 octahedral particles (Fig. 14D and E). Battery tests demonstrated a specific capacity of 1110 mA h g−1 at 0.2 A g−1 (Fig. 14F) and a capacity retention of 60% at 5 A g−1 over 60 cycles.
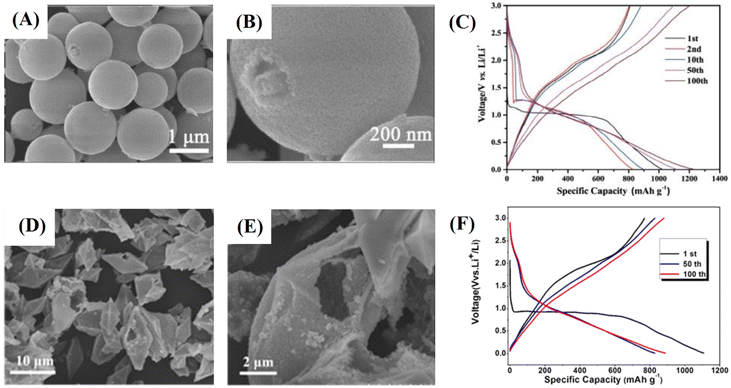 | ||
| Fig. 14 (A and B) SEM images of ZnCo2O4 multishelled hollow spheres at different magnifications. (C) Galvanostatic charge/discharge curves of a ZnCo2O4 multi-shelled hollow sphere anode at a current density of 100 mA g−1. Reproduced with permission.164 Copyright MarketplaceTM. Royal Society of Chemistry. (D and E) SEM images of ZnCo2O4 hollow structures at different magnifications. (F) Galvanostatic charge/discharge curves of a ZnCo2O4 hollow anode at a current density of 0.2 A g−1. Reproduced with permission.168 Copyright © 2017 Published by Elsevier B.V. | ||
The main electrochemical performances for ZnCo2O4 with different morphologies are summarized and listed in Table 6. Hollow porous structures composed of 2D structures of ZnCo2O4, such as nanosheets, showed superior electrochemical performance to other nanostructures or microstructures in LIBs, due to the interior hollow structure which can accommodate the huge volume expansion and provide more active lithiation sites; thus, ZnCo2O4 structures exhibit higher capacity and cycling stability than the other materials, and second, the porous structures ensure sufficient contact between active materials and electrolyte. Therefore, it can be concluded that 2D nanostructures of ZnCo2O4 would be considered as an optimum architecture for high-performance ZnCo2O4.
| Type | Strategy | Material | Initial discharge (mA h g−1) | Potential window (V vs.) | Reversible capacity (Nth) mA h g−1 | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| CC: carbon cloth, CF: carbon fiber, CNTs: carbon nanotubes, CSF: carbonized silk fabric, NC: N-doped carbon, NF: nickel foam, PAN: polyaniline, rGO: reduced graphene oxide. | |||||||
| Li-ion | Pristine | Hollow polyhedral ZnCo2O4 | 1097.3 | 0.01–3 Li/Li+ | 891.7 (200)/100 mA g−1 | — | 169 |
| ZnCo2O4 nanoribbons | 2161 | 0.01–3 Li/Li+ | 1422 (80)/200 mA g−1 | 71%/80 cycles | 81 | ||
| Hierarchical micro-sized ZnCo2O4 assembled with nanosheets | 1005.8 (180)/500 mA g−1 | — | 170 | ||||
| ZnxCo3−xO4 hollow nanoboxes | 1141.7 | 0.01–3 Li/Li+ | 625 (800)/500 mA g−1 | 85%/800 cycles | 155 | ||
| ZnCo2O4 nanosheets | 1979 | 0.01–3 Li/Li+ | 688 (1000)/5 A g−1 | — | 157 | ||
| ZnCo2O4 nanosheets/NF | 1297 | 0.01–3 Li/Li+ | 773 (500)/0.25 A g−1 | 87%/500 cycles | 152 | ||
| ZnCo2O4 nanosheets | 1710.2 | 0.01–3 Li/Li+ | 1640.8 (50)/100 mA g−1 | — | 156 | ||
| 1D porous ZnCo2O4 cuboids | 1376 | 0.01–2.5 Li/Li+ | 1029.3 (400)/1000 mA g−1 | — | 167 | ||
| 3D ZnCo2O4 nanocubes | 1049 | 0.01–3 Li/Li+ | 775 (100)/500 mA g−1 | — | 159 | ||
| ZnCo2O4 micro-cubes | 1087 | 0.01–3 Li/Li+ | 588 (1000)/1 A g−1 | 76%/1000 cycles | 158 | ||
| Microcube-like ZnCo2O4 | 1179 mA h cm−3 | 0.01–3 Li/Li+ | 412 (600)/1200 mA g−1 | — | 171 | ||
| ZnCo2O4 nanocages | 1328 | 0.01–3 Li/Li+ | 1025 (200)/500 mA g−1 | — | 166 | ||
| Micro–nanoporous ZnCo2O4 spheres | 1307.8 | 0.02–3 Li/Li+ | 950 (90)/0.1C | 99.7%/90 cycles | 163 | ||
| Yolk–shell ZnCo2O4 microspheres | 1466 | 0.01–3 Li/Li+ | 1063 (50)/200 mA g−1 | — | 162 | ||
| Yolk–shell ZnCo2O4 spheres | 1586 | 0.01–3 Li/Li+ | 910 (300)/1 A g−1 | 92.3%/300 cycles | 161 | ||
| Multi-shelled hollow ZnCo2O4 spheres | 1020 | 0.01–3 Li/Li+ | 1200 (200)/0.1 mA g−1 | — | 164 | ||
| 3D ZnCo2O4 microspheres | 2094 | 0.01–3 Li/Li+ | 1296.91 (200)/100 mA g−1 | — | 172 | ||
| 3D Zn0.2Ni0.8Co2O4 microspheres | 1482 | 0.01–3 Li/Li+ | 681 (40)/C/20 | — | 173 | ||
| ZnCo2O4 nanospheres | 1320 | 0.01–3 Li/Li+ | 625 (50)/100 mA g−1 | 76.22%/50 cycles | 160 | ||
| Nanosheathed ZnCo2O4 spheroids | 1477 | 0.01–3 Li/Li+ | 815 (500)/500 mA g−1 | — | 174 | ||
| Needle-like ZnCo2O4 | 1413 | 0.005–3 Li/Li+ | 516 (50)/60 mA g−1 | — | 175 | ||
| 3D mesoporous ZnCo2O4 nanoparticles | 1128.0 | 0.01–3 Li/Li+ | 779.6 (50)/200 mA g−1 | 94%/50 cycles | 153 | ||
| Hollow ZnCo2O4 octahedrons | 1110 | 0.01–3 Li/Li+ | 880 (160)/0.2 A g−1 | 60%/60 cycles | 161 | ||
| Zn defective ZnCO2O4 nanorods | 1398.8 | 0.01–3 Li/Li+ | 1140 (200)/0.4 A g−1 | — | 176 | ||
| ZnCo2O4 nanotubes | 1353 | 0.01–3 Li/Li+ | 1180 (275)/200 mA g−1 | — | 165 | ||
| Composites with oxides | 3D porous ZnCo2O4/Co3O4 | 1350.0 | 0.01–3 Li/Li+ | 481.9 (105)/0.3 A g−1 | 64.2%/105 cycles | 177 | |
| Co3O4/ZnCo2O4 microspheres | 1567 | 0.01–3 Li/Li+ | 754 (800)/2 A g−1 | — | 178 | ||
| Co3O4/ZnCo2O4 | 1051 | 0.01–3 Li/Li+ | 890 (120)/0.1 A g−1 | — | 179 | ||
| 3D ZnO/ZnCo2O4/Co3O4/Cu | 1480 | 0.01–3 Li/Li+ | 1428 (100)/200 mA g−1 | — | 180 | ||
| Ni–NiCo2O4@ZnCo2O4 yolk–shell nanotetrahedrons | 1541 | 0.01–3 Li/Li+ | 1097.5 (600)/1 A g−1 | — | 181 | ||
| ZnCo2O4@Fe2O3–C | 1501 | 0.01–3 Li/Li+ | 952 (100)/100 mA g−1 | — | 182 | ||
| N-doped ZnCo2O4/CoO | 1303.9 | 0.01–3 Li/Li+ | 978 (500)/1 A g−1 | — | 183 | ||
| ZnCo2O4/Co–B | 1385 | 0.01–3 Li/Li+ | 946 (1000)/1 A g−1 | — | 184 | ||
| 3D porous ZnCo2O4@NiO/NF | 1595.8 | 0.01–3 Li/Li+ | 730.5 (200)/800 mA g−1 | — | 185 | ||
| Zn1−xCoxO/ZnCo2O4 | 1265 | 0.01–3 Li/Li+ | 741 (800)/1000 mA g−1 | — | 186 | ||
| Composites with carbon materials | Yolk–shell ZnCo2O4 spheres/rGO | 1587 | 0.01–3 Li/Li+ | 997 (500)/1.0 A g−1 | — | 187 | |
| ZnCo2O4/rGO | 1093 | 0.01–3 Li/Li+ | 1613 (400)/500 mA g−1 | — | 188 | ||
| rGO@ZnCo2O4 nanosheets | 801.5 | 0.01–3 Li/Li+ | 1107.2 (100)/100 mA g−1 | — | 189 | ||
| ZnCo2O4 microspheres/rGO | 963.9 | 0.01–3 Li/Li+ | 908.7 (500)/500 mA g−1 | — | 190 | ||
| ZnCo2O4@3D graphene film@Ni foams | 2024 | 0.01–3 Li/Li+ | 1223 (240)/500 mA g−1 | — | 191 | ||
| Nano-ZnCo2O4@rGO | ∼1230 | 0.01–3 Li/Li+ | ∼746 (250)/1 A g−1 | — | 192 | ||
| Graphene/porous ZnCo2O4 | 1146 | 0.01–3 Li/Li+ | 791 (1000)/1 A g−1 | 97.3%/1000 cycles | 193 | ||
| ZnCo2O4–graphene | 1937 | 0.01–3 Li/Li+ | 1100 (2000)/4000 mA g−1 | 66%/2000 cycles | 194 | ||
| ZnCo2O4/CC | 1087 | 0.01–3 Li/Li+ | 701 (60)/0.25 A g−1 | — | 195 | ||
| ZnCo2O4@CC | 1886.2 | 0.01–3 Li/Li+ | 1375 (200)/1 A g−1 | — | 196 | ||
| ZnCo2O4 nanoplates on CC | 2.78 mA h cm−2 | 0.01–3 Li/Li+ | 3.01 mA h cm−2 (100)/0.24 mA cm−2 | — | 197 | ||
| ZnCo2O4/ZnO/carbon nanotubes | 1893 | 0.005–3 Li/Li+ | 1440 (200)/100 mA g−1 | — | 198 | ||
| C/ZnCo2O4/CNTs | 1947.1 | 0.01–3 Li/Li+ | 430.4 (1000)/2 A g−1 | — | 199 | ||
| ZnCo2O4@CNTs | 2553 | 0.005–3 Li/Li+ | 1507 (200)/100 mA g−1 | — | 200 | ||
| ZnCo2O4/CNT microflowers | 1300 | 0.01–3 Li/Li+ | 1200 (120)/200 mA g−1 | — | 129 | ||
| ZnCo2O4/ZnO/C | 1589 | 0.005–3 Li/Li+ | 800 (400)/1 A g−1 | — | 201 | ||
| ZnCo2O4–C | 1521.9 | 0.01–3 Li/Li+ | 622.5 (1000)/4 A g−1 | — | 202 | ||
| ZnCo2O4/C | 1703.7 | 0.01–3 Li/Li+ | ∼760.3 (100)/0.1C | — | 203 | ||
| ZnCo2O4/C microhydrangea | 1418.1 | 0.01–3 Li/Li+ | 704.4 (1000)/4 A g−1 | — | 204 | ||
| ZnCo2O4/C@carbon fibers | 733 | 0.0–3 Li/Li+ | 463 (100)/50 mA g−1 | — | 205 | ||
| Porous ZnCo2O4/C nanofibers | 1707 | 0.01–3 Li/Li+ | 1145 (100)/0.1 A g−1 | — | 206 | ||
| ZnCo2O4@NC polyhedrons | 1495 | 0.01–3 Li/Li+ | 1082 (300)/0.1 A g−1 | — | 207 | ||
| ZnCo2O4@NC | 1592.1 | 0.01–3 Li/Li+ | 1146.6 (100)/0.5 A g−1 | — | 208 | ||
| Carbon-coated ZnCo2O4 nanowires | 1951.4 | 0.01–3 Li/Li+ | 886.4 (100)/200 mA g−1 | — | 209 | ||
| PAN-CF/ZnCo2O4 | 927.2 | 0.01–3 Li/Li+ | 787.2 (150)/100 mA g−1 | — | 210 | ||
| ZnCo2O4@C3N4-B | 1636.34 | 0.01–3 Li/Li+ | 919.76 (500)/0.2 A g−1 | 97.8%/1000 cycles | 211 | ||
| ZnCo2O4/CSF | 3164 | 0.01–3 Li/Li+ | 778 (100)/100 mA g−1 | — | 212 | ||
| Hybrid carbon/ZnCo2O4 nanotubes | 2247 | 0.01–3 Li/Li+ | 494 (600)/5 A g−1 | 75%/600 cycles | 213 | ||
| Other strategies | ZnCo2O4/NiCl2−xFx hydrate | 1312 | 0.01–3 Li/Li+ | 700 (1000)/1 A g−1 | — | 214 | |
| Ni-substituted ZnCo2O4 nanograins | 1067 | 0.01–3 Li/Li+ | 386 (100)/1 A g−1 | 68%/100 cycles | 215 | ||
| N-doped ZnCo2O4 nanoparticles | 1025 | 0.01–3 Li/Li+ | 650 (100)/1C | 63%/100 cycles | 216 | ||
| ZnCo2O4@Ag hollow spheres | 830 | 0.01–3 Li/Li+ | 616 (900)/1 A g−1 | — | 217 | ||
| Na-ion | Pristine | ZnCo2O4 nanowires | ∼1180 | 0.01–2.5 Na/Na+ | 70.8 (100)/100 mA g−1 | — | 218 |
| ZnCo2O4 nanosheets | ∼1150 | 0.01–2.5 Na/Na+ | 191.9 (100)/100 mA g−1 | — | 218 | ||
| ZnxCo3−xO4 hollow nanoboxes | 350 | 0.01–3 Na/Na+ | 310 (100)/200 mA g−1 | 90.4%/100 cycles | 155 | ||
| ZnCo2O4 nanosheets | 415.1 | 0.01–3 Na/Na+ | 330 (100)/100 mA g−1 | — | 219 | ||
| ZnCo2O4 nanosheets | 800 | 0.01–3 Na/Na+ | 463 (60)/0.1 A g−1 | — | 219 | ||
| Composites with carbon materials | Yolk–shell ZnCo2O4 spheres/rGO | 827.7 | 0.01–3 Na/Na+ | 280 (1000)/1.0 A g−1 | — | 187 | |
| ZnCo2O4@rGO | 407 | 0.01–3 Na/Na+ | 134 (300)/100 mA g−1 | — | 220 | ||
| ZnCo2O4/rGO | 569.3 | 0.01–3 Na/Na+ | 101.7 (500)/1000 mA g−1 | — | 221 | ||
Although the theoretical capacity of ZnCo2O4 as an anode material is high (900 mA h g−1),154 tremendous efforts have been paid, in recent years, to increasing the conductivity and overcoming the volume expansion of ZnCo2O4 caused by lithium-ion insertion/extraction, which results in its fast fading of capacity. One strategy is the combination with transition metal oxides such as ZnO/ZnCo2O4/Co3O4,180 ZnCo2O4/Co3O4,177–179 N-ZnCo2O4/CoO,183 ZnCo2O4@NiO,185 Ni–NiCo2O4@ZnCo2O4,181 and ZnCo2O4@Fe2O3–C,182 which can alleviate the problem through the synergy effect of bimetallic oxides.
The construction of hollow and 3D porous structures is another effective strategy, promoting the generation of voids, which can alleviate the structural stress and buffer the volume variation. For example, a novel route to prepare hollow Co3O4 nanospheres doped with ZnCo2O4 was demonstrated by Song et al.179 (Fig. 15A). This nanocomposite shows a specific capacity of 890 mA h g−1 at a current density of 0.1 A g−1 and displays a similar specific capacity at 1 A g−1 after 120 cycles (Fig. 15B). Guo et al.177 reported the synthesis of a 3D porous ZnCo2O4/Co3O4 composite on carbon cloth (Fig. 15C). The as-prepared composite exhibits an enhanced lithium storage property of 1350.0 mA h g−1 at 0.3 A g−1 and a cycling performance of 64% over 105 cycles at 0.3 A g−1 (Fig. 15D). Li et al.178 also prepared ZnCo2O4/Co3O4 hierarchical hollow ZnCo2O4/Co3O4 microspheres via solvothermal synthesis followed by thermal annealing. When used as an anode material for LIBs, this material exhibits a rate capability of 842 mA h g−1 at a current density of 4 A g−1 and a cycle life of 754 mA h g−1 after 800 cycles at a current density of 2 A g−1. The development of hollow structures based on ZnCo2O4/Co3O4 composites demonstrated that the hierarchical hollow structure with high porosity relieves the volume expansion and increases the contact area between the electrode and electrolyte, increasing discharge capacity and cycling performance.
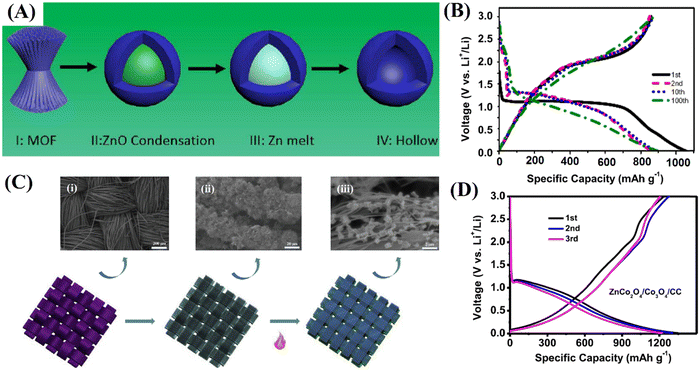 | ||
| Fig. 15 (A) Schematic representation of the formation process of the hollow structure. (I) ZnCo MOF growth; (II) ZnO condensation to the middle of the pyrolyzed particle; (III) Zn reduction and melt from the middle; and (IV) hollow Co3O4 doped with ZnCo2O4 after losing Zn. (B) Charge/discharge curves of hollow Co3O4/ZnCo2O4 spheres at 0.1 A g−1. Reproduced with permission.179 Copyright © 2018 Published by Elsevier B.V. (C) Schematic illustration of a ZnCo2O4/Co3O4 composite grown on carbon cloth; SEM images of (i) carbon cloth; (ii) Zn/Co precursor/CC; and (iii) ZnCo2O4/Co3O4/CC. (D) Charge–discharge curves at a current density of 0.1 A g−1 Reproduced with permission.177 Copyright © 2020 Elsevier B.V. All rights reserved. | ||
Another strategy to solve concerns in terms of lithium diffusion kinetics, electronic transport, volume change, and particle agglomeration is to anchor ZnCo2O4 structures onto electrically conductive nanostructured carbon materials. Hence, some carbonaceous materials including carbon nanotubes (CNTs),129,198,199 reduced graphene oxide (rGO),187–192 polyaniline (PAN),210 n-doped carbon layers,208,222 carbon cloth (CC),194,195,223 and carbon porous structures202–204 were used as inert and conductive matrices in ZnCo2O4 based anode materials. For instance, binder-free and self-supporting anode materials were prepared based on carbon-coated ZnCo2O4 composites. The lithium storage properties were as follows: a high initial discharge (1951.4 mA h g−1) and good capacity after cycling (88.6.4 mA h g−1 over 100 cycles at 200 mA g−1).209 In addition, Huang et al.223 prepared a ZnCo2O4@CC nanocomposite with a reversible capacity of 1376 mA h g−1 even after 200 cycles at a current density of 1 A g−1.
Graphene has attracted widespread attention due to its unique properties such as mechanical flexibility, excellent conductivity (1600 S m−1), large specific surface area (2630 m2 g−1), and chemical stability.224–227 The introduction of graphene into ZnCo2O4 structures can accommodate serious volume expansion, prevent agglomeration of ZnCo2O4 material over continuous lithiation/delithiation cycles, and, meanwhile, improve the electrical conductivity of the hybrids.187,228,229 For example, Wang et al.191 prepared interconnected mesoporous ZnCo2O4 nanosheets on 3D graphene foam (Fig. 16A), which had a discharge capacity of 1233 mA h g−1 at 500 mA g−1 after 240 cycles (Fig. 16B). Ren et al.188 fabricated a ZnCo2O4@rGO nanocomposite to be used as a LIB anode. The ZnCo2O4@rGO electrode exhibited cycling stability (1589 mA h g−1 at 100 mA g−1 after 140 cycles) (Fig. 16C). Xie et al. developed a rapid laser-irradiation methodology for the synthesis of oxygen-vacancy abundant nano-ZnCo2O4/porous rGO hybrids as anodes for LIBs (Fig. 16E). The results showed that the nano-ZnCo2O4/porous rGO has a reversible capacity of ∼1053 mA h g−1 at 0.05 A g−1 and a cycling stability of ∼746 mA h g−1 at 1.0 A g−1 after 250 cycles (Fig. 16D).192 In these cases, the rGO acts as a conductive substrate for anchoring the ZnCo2O4 structure, which increases the electrical conductivity and avoid the structure collapse upon cycling (Fig. 16F).
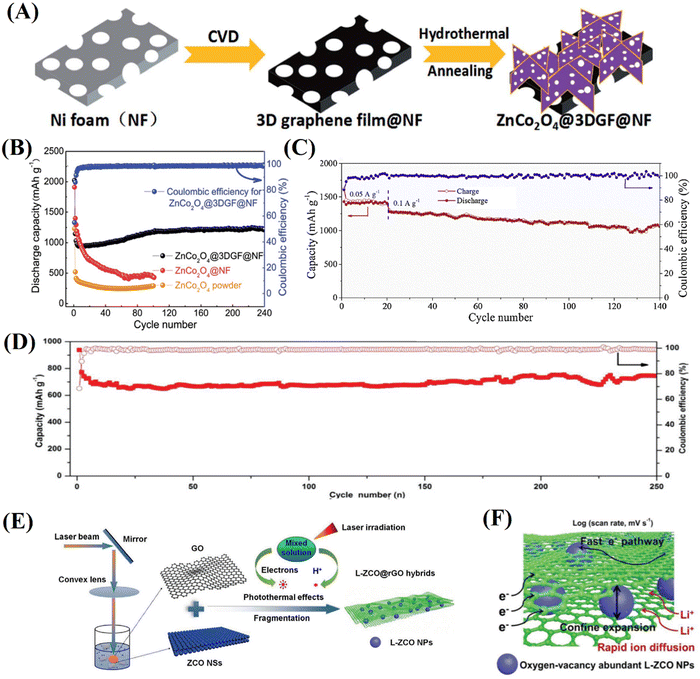 | ||
| Fig. 16 (A) Schematic illustration of the synthesis of ZnCo2O4@3D graphene foam@nickel foam composites. (B) Comparison of cycle performance of the ZnCo2O4@3DGF@NF, ZnCo2O4@NF, and ZnCo2O4 powder electrodes at a current density of 500 mA g−1. Reproduced with permission.191 CC BY-NC 3.0. Royal Society of Chemistry. (C) Cycling capacity of the ZCO@rGO//LiCoO2 full cell at a current density of 100 mA g−1. Reproduced with permission.188 Copyright © 2018 Elsevier Ltd. All rights reserved. (D) Cycling performance and coulombic efficiency data at 1.0 A g−1 of L-ZCO@rGO-30. (E) The schematic diagram for the formation of L-ZCO@rGO hybrids. (F) Schematic illustration of the fast electron/ion transfer and rapid electrochemical kinetics of the L-ZCO@rGO-30 electrode. Reproduced with permission.192 Copyright © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The combination in the composites, taking full use of the good conductivity and high surface area of carbon materials, efficaciously heightens the undesirable conductivity of ZnCo2O4, thereby affording enhanced electrochemical behaviors in LIBs. The carbon coated ZnCo2O4 nanocomposites have large surface areas, resulting in better electrolyte wettability and high conductivity, which contribute to cycling stability. This effective approach to fabricating material composites not only has the advantages of all the constituents, but also overcomes the disadvantages of the individual components.
From the perspective of material application, an energy storage device balances the power supply and demand of large-grid energy storage. Several factors can be addressed to evaluate the performance of an electrode material in a battery cell, such as first discharge, stability, reversible capacity and the potential window. Among the many types of ZnCo2O4 materials previously shown, we summarize in Fig. 17 the electrodes with the 10 biggest first discharge. The best one is the ZnCo2O4/carbonized silk fabric (CSF) (3164 mA h g−1); the high initial discharge of this material is endowed by the hydrothermal method that improves the bonding between active materials and the flexible substrate, and avoids capacity reduction from the active substance detaching from the substrate during the charge and discharge cycle; the unique weave structure of the CSF gives it good mechanical flexibility and the 3D network structure of the CSF provides a fast electron transport path to enhance the composite material's energy storage performance.212
Nine out of the top ten anode materials demonstrate the benefits of a nanocomposite based on ZnCo2O4/carbon nanomaterials. Use of these nanocomposites was shown to be a remarkable strategy to improve the electrochemical performance of anode electrodes, as the carbon nanomaterials have many great electrochemical abilities, including enhancing the electrical conductivity of the electrode and preventing the volume change and aggregation found with ZnCo2O4 electrodes. The second (ZnCo2O4@CNTs, 2553 mA h g−1),200 seventh (C/ZnCo2O4/CNT, 1947.1 mA h g−1)199 and ninth (ZnCo2O4/ZnO/CNT, 1893 mA h g−1)198 materials with the best performances demonstrate the advantages due to the presence of carbon nanotubes; this can be attributed to the efficient electron transport and CNT network, which could shorten the diffusion pathway of lithium-ions and buffer the volume expansion/constriction, as well as enlarge the surface area for more electrochemically active species.209 Likewise, the tenth (ZnCo2O4@CC, 1886.2 mA h g−1),196 eighth (ZnCo2O4–graphene, 1937 mA h g−1),194 sixth (carbon-coated ZnCo2O4 nanowires, 1951.4 mA h g−1),209 fifth (ZnCo2O4@3D graphene film@Ni foam, 2024 mA h g−1)191 and third (hybrid carbon/ZnCo2O4 nanotubes, 2247 mA h g−1)213 best materials demonstrate improved electrochemical performance, which may be assigned to the carbon nanomaterial structure, which can enlarge the electrode–electrolyte contact area, greatly strengthen the electroconductivity and structural stability and improve the energy density.
It's worth highlighting that the second, seventh and tenth best materials mentioned above are based on MOF-derived materials. This strategy of preparation of materials has many advantages; for example, it endows the materials with large specific area, regular porosity, shearing capability and topological diversity, which can demonstrate that the best electrochemical performance is associated with the effects of the preparation method and the electrode architecture.230 The fourth (ZnCo2O4 nanoribbons, 2161 mA h g−1)81 best material had its highlighted role due to its unique morphology, as well as the tenth best materials. In fact, the size of nanostructures of ZnCo2O4 provided more active sites, large surface area and shorter diffusion paths for ions and electrons, bringing remarkable enhancement in their electrochemical performance.81,196
In summary, ZnCo2O4 with excellent electrochemical performance should have nanostructures or a unique morphology or be associated with a carbon nanomaterial as a nanocomposite. These improved electrochemical performance can be attribute to the greater number of electrochemically active sites, the high surface area, a good diffusion length of ions and electrons, and a satisfactory volume expansion from the insertion/extraction of Li ions.
In addition to SIBs, Zn-ion and Mg-ion batteries afford some attributes required of an alternative energy storage technology, such as nondendritic formations, Zn and Mg metal anode material delivers a high capacity of 820 mA g−1 and 2205 mA h g−1, respectively, and abundant and non-toxic raw materials.233,234 Recently, ZnCo2O4 structures have been developed as potential cathode materials for these types of batteries. Baby et al.235 reported the synthesis of a ZnMnCoO4 cathode material with the first discharge of 109.4 mA h g−1 in Zn-ion batteries, whereas Shimokawa et al.236 reported the synthesis of ZnCo2O4 used as a cathode material for rechargeable magnesium batteries with a discharge capacity in the first cycle of ∼100 mA h g−1.
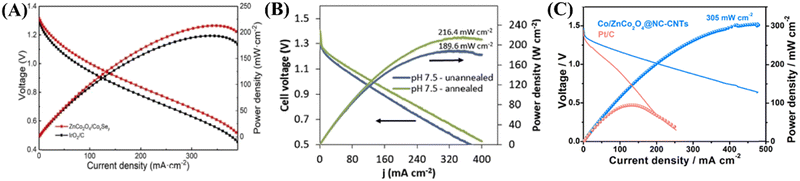 | ||
| Fig. 18 Discharge polarization curves and the corresponding power density plots of a battery based on (A) ZnCo2O4/CoxSey and IrO2/C (Reproduced with permission.253 Copyright MarketplaceTM. Royal Society of Chemistry), (B) annealed and unannealed W–Co oxide electrodes (Reproduced with permission.252 Copyright © 2020 Hydrogen Energy Publications LLC. Published by Elsevier Ltd. All rights reserved) and (C) Pt/C and Co/ZnCo2O4@NC-CNTs (Reproduced with permission.255 Copyright © 2020 Elsevier Ltd. All rights reserved). | ||
To improve catalyst performance, composite materials have been synthesized and used as catalysts in both the ORR and OER. Generally, metal oxides combined with carbon materials such as graphene and carbon nanotubes (CNTs) can not only improve the conductivity of the catalyst but also increase the specific surface area and improve electrochemical stability.254–257 Combining Co/ZnCo2O4 with N-doped carbon microplates interwoven with CNTs, Yan et al.255 developed a Co/ZnCo2O4@NC-CNT-based flexible solid-state Zn–air battery with a competitive power density of 151 mW cm−2 at 50 mA cm−2 (Fig. 18C), robust flexibility and integrality. Xu et al.254 prepared ZnCo2O4/CNTs by inserting zinc ions. When used as a cathode material in a rechargeable Zn–air battery, this material exhibits a power density of 249.4 mW cm−2, and a charge–discharge durability of 240 cycles.
4. ZnCo2O4-based electrocatalysts for energy conversion and storage applications
4.1. ORR electrocatalysts in energy storage
As mentioned in the previous topic, both the oxygen reduction and oxygen evolution reactions (ORR/OER) play an important role in the electrochemical energy conversion process, not only in metal–air rechargeable batteries but also in fuel cells.258 In fact, because of their high associated activation energies, such reactions are usually sluggish and require catalysts to enhance the kinetics.235 In this sense, great efforts have been directed towards the development of inexpensive, efficient, noble metal-free and stable electrocatalysts for next-generation sustainable energy technologies.258 Thus, ZnCo2O4 and its composites show great potential as electrocatalysts due to their high intrinsic activity,259 and in many cases exhibit both ORR and OER activity simultaneously.As shown in Table 7, despite being considered as very promising electrocatalysts, less than two dozen ZnCo2O4-based materials have been reported in the past 5 years for application in the ORR, indicating that these materials are still to be explored, especially in the design of bifunctional electrocatalysts. However, some improvement strategies for these materials can be highlighted, as a guide for future research. For instance, many catalysts with different morphologies such as nanosheets,130,260 flower like structures130 and near-spherical particles235 have been recently reported.
| ORR catalysts | Incorporated material or atom | Substrate | E ORR onset potential (V vs. RHE) | Half wave potential (V vs. RHE) | Overpotential EORR at −3 mA cm−1 (V) | Overpotential EOER at 10 mA cm−1 (V) | ΔE, EOER −EORR (V vs. RHE) | Current density (mA cm−1) | Tafel slope (mV dec−1) | Average electron transfer number (n) | Retention%–stability (h) | pH conditions for the ORR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-doped MWCNTs = nitrogen-doped multi-walled carbon nanotubes, CNTs = carbon nanotubes, 3D-G = three-dimensional graphene, LFs = lilac flowers, N–C = N-doped carbon, pNGr = N-doped porous graphene, D-AC = AC-based defective carbon, and MC = graphene and porous carbon.a V vs. Ag/AgCl.b V vs. SHE.c ΔE = |Ej10| − |E1/2|. | |||||||||||||
| ZnCo2O4 ultrathin nanosheets | — | GCE | — | — | — | 0.34 | — | — | — | 4.1 | 12 | 0.1 M KOH | 130 |
| Flower like ZnCo2O4 | — | GCE | 0.81 | 0.75 | 0.696 | 0.41 | 0.944 | — | — | 3.05–3.4 | — | 1 M KOH | 261 |
| Near-spherical particles of ZnCo2O4 | — | GCE | 0.83b | 0.62b | — | — | — | 2.97 | — | 3.99 | — | 0.1 M KOH | 235 |
| ZnCo2O4 nanosheets | — | GCE | 0.8 | — | — | — | — | 5.6 | — | 3.77–3.95 | 95% | 0.1 M KOH | 260 |
| 2.77 | |||||||||||||
| ZnCo2O4 LFs | — | GCE | 0.77 | 0.68 | — | — | — | — | — | ∼3.5 | ∼96% | 0.1 M KOH | 262 |
| 1.94 h | |||||||||||||
| Near-spherical particles of ZnMnCoO4 | Mn | GCE | 0.94b | 0.74b | — | — | — | 5.22 | — | 3.99 | — | 0.1 M KOH | 235 |
| ZnCo2O4-CNTs | CNTs | GCE | 0.97 | 0.76 | — | 0.49 | — | 5.72 | — | 3.89 | 2000 cycles CVs | 0.1 M KOH | 254 |
| Co/ZnCo2O4@NC-CNTs | NC-CNTs | GCE | 1.01 | 0.90 | — | 0.37 | 0.70c | 4.6 | 91 | 4.0 | 87% | 0.1 M KOH | 255 |
| >10 | |||||||||||||
| ZnCo2O4-MC | MC | GCE | — | — | — | — | — | 4.24 | — | — | — | 0.1 M KOH | 254 |
| ZnCo2O4–graphene | Graphene | GCE | — | — | — | — | — | 4.49 | — | — | — | 0.1 M KOH | 254 |
| rGO–ZnCo2O4 | RGO | GCE | 0.95 | 0.87 | 0.851 | 0.30 | 0.679 | 6.11 | — | 3.7–3.95 | 12 | 1 M KOH | 261 |
| ZnO/ZnCo2O4/C | ZnO + C | GCE | −0.14a | −0.25a | — | — | — | — | 87.39 | 3.41 | 95.6% | 0.1 M KOH | 263 |
| 3.33 | |||||||||||||
| ZnO/ZnCo2O4/C@rGO | ZnO + C + rGO | GCE | −0.05a | −0.15a | — | — | — | — | 46.70 | 3.95 | 99.7% | 0.1 M KOH | 263 |
| 3.33 | |||||||||||||
In one of these studies, Chakrabarty et al.261 synthesized a flower-like porous ZnCo2O4 microstructure by the one-step solvothermal method, as confirmed by SEM and HRTEM images (Fig. 19A and B). The ZnCo2O4 microstructure achieves a nearly 4-electron assisted oxygen reduction (n ≈ 3.4) with onset and half wave potentials observed at 0.81 V and 0.75 V vs. RHE (Table 7). It is important to highlight that despite the interesting results obtained by designing the morphology of ZnCo2O4 nanostructures, better results are clearly improved by the formation of composites, especially by combining them with conductive carbonaceous materials. For example, Chakrabarty et al.261 also showed the activity of electrocatalysts predated by the simultaneous growth of ZnCo2O4 and reduction of GO (Fig. 19D), achieving a more positive ORR onset potential (0.95 V vs. RHE) with higher cathodic peak current density compared to ZnCo2O4 and n ≈ 3.95, demonstrating that the presence of a conductive matrix is essential in the design of high-performance electrocatalysts. Furthermore, the bifunctional electroactivity of the rGO–ZnCo2O4 and ZnCo2O4 was determined from the potential difference (ΔE) between the OER (EOER, at 10 mA cm−2) and ORR (EORR, at −3 mA cm−2). The ΔE for rGO–ZnCo2O4 was 0.679 V vs. RHE (Fig. 19C), which is less than that obtained using ZnCo2O4 (0.944 V vs. RHE), demonstrating the synergistic effect achieved by increasing the catalytic surface area and efficient electron transfer through the RGO sheet in the composite catalyst.261
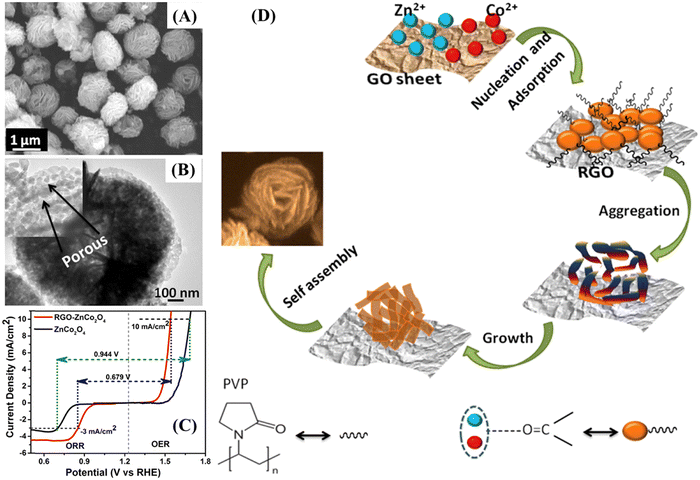 | ||
| Fig. 19 (A) SEM image of ZnCo2O4 microspheres and (B) TEM images of a ZnCo2O4 microsphere. The inset of (B) shows the magnified portion of the image that shows the porous structure. (C) Oxygen electrode activities of both the catalysts within the range of potential for the ORR and OER in O2-saturated 1 M KOH electrolyte at 1200 rpm. (D) Growth mechanism of rGO–ZnCo2O4 flower-like microstructures. Reproduced with permission.261 Copyright © 2018 Hydrogen Energy Publications LLC. Published by Elsevier Ltd. All rights reserved. | ||
Employing a similar strategy, Yan and coworkers255 reported the preparation of a 3D bifunctional oxygen electrocatalyst based on Co/ZnCo2O4 nanoparticles derived from CoZn-ZIF-L sandwiched in leaf-like nitrogen-doped carbon microplates interwoven with carbon nanotubes (Co/ZnCo2O4@NC-CNTs, Fig. 20A), as confirmed by the SEM images in Fig. 20B and C and TEM images in Fig. 20D and E. As shown in Table 7, the Co/ZnCo2O4@NC-CNT material is among the best bifunctional electrocatalysts as revealed by its excellent onset potential of 1.01 V, E1/2 of 0.90 V, Tafel slope of 91 mV dec−1, limiting current density of 4.6 mA cm−2 for the ORR and small ΔE of 0.70 V for ORR/OER activities. The excellent activity of this composite is due to the large amount of metal–Nx and Co3+ active sites as well as the interwoven CNTs on the surfaces of the carbon microplates which are beneficial to the charge transfer in the ORR/OER processes.255
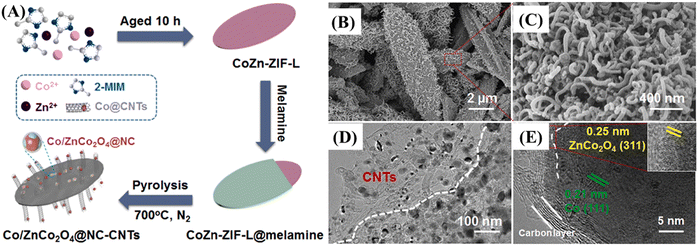 | ||
| Fig. 20 (A) Schematic synthesis process of the Co/ZnCo2O4@NC-CNT electrocatalyst. (B and C) SEM images, (D) TEM image, and (E) HRTEM image of the as-prepared Co/ZnCo2O4@NC-CNTs. Reproduced with permission.255 Copyright © 2020 Elsevier Ltd. All rights reserved. | ||
4.2. Water-splitting electrocatalysts for energy conversion (OER and HER)
Electrochemical water-splitting has been considered as a promising method to obtain H2 and O2 through the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), respectively. However, the production of H2 is limited by the sluggish OER kinetics at the anode due to the multi-electron transfer coupled with protons, which leads to high overpotentials.264 Benchmark catalysts such as RuO2 and IrO2 have been used in the water-splitting process to overcome this issue. Nevertheless, due to the scarcity and high cost of these noble metals, their commercial implementation has been unfeasible.265 In this sense, it is necessary to search for new electrode materials with low cost, which are not scarce, besides they have a superior electrochemical behavior.In recent years, cobaltite spinel oxides MxCo3−xO4 (where M = Ni, Mn, Zn, and Fe) have been used as electrode materials for efficient water oxidation.61,266,267 Among these electrode materials, ZnCo2O4 has drawn attention due to its rich redox chemistry, which has led to enhanced electrochemical performance. Indeed, ZnCo2O4 presents a better catalytic activity for the OER when compared to other cobaltite spinel oxides,268 and the reason for this lies in how Zn2+ ions replace Co ions in the Co3O4 spinel structure.
In the Co3O4 spinel structure, Co2+ and Co3+ ions are found, respectively, in the tetrahedral and octahedral sites. Kim and colleagues269 demonstrated that Zn2+, when inserted into the Co3O4 spinel structure to form ZnCo2O4, only replaces Co2+ found in the tetrahedral interstices, leaving Co3+ (highly active species for the OER) unchanged in the octahedral sites. Nevertheless, other metal ions like Ni and Mn, when inserted into Co3O4 to form NiCo2O4 and MnCo2O4, respectively, can suppress the catalytic activity for the OER,261 due to occupation of tetrahedral and octahedral sites in the Co3O4 spinel structure. In addition, M. Harada, F. Kotegawa, & M. Kuwa16 demonstrated that the active sites are controlled by the balance of M3+/M2+ cation distribution in Oh and Td sites and by the bond strength between M and oxygen atoms at the electrocatalyst surface before and after the exposure to OER conditions, where the catalytic activity of the OER decreases in the order of ZnCo2O4 > NiCo2O4 > FeCo2O4 > Co3O4 > MnCo2O4.
In this sense, ZnCo2O4 has been used as an electrode material for the OER and has shown good results. For instance, Bao et al.130 prepared ZnCo2O4 ultrathin nanosheets by thermal treatment of ZnCo-LDH (where LDH = layered double hydroxide). The electrode material was deposited on a GCE (glassy carbon electrode) and tested for OER performance in KOH 1.0 mol L−1. The as-prepared ZnCo2O4 ultrathin nanosheet presented an overpotential of 340 mV at 10 mA cm−2, and a Tafel slope of 38 mV dec−1, compared to RuO2 (33 mV dec−1). The authors attributed these results to the large surface area of ZnCo2O4 ultrathin nanosheets that provides more exposed active sites on the surface, easing the catalytic reaction. Moreover, Xiang and colleagues270 synthesized ZnCo2O4 nanosheets with abundant oxygen vacancies (OV), named OV-ZnCo2O4, through the hydrothermal method and NaBH4 reduction process. The results showed that the presence of oxygen vacancies in ZnCo2O4 was beneficial for the OER. In fact, OV-ZnCo2O4 achieved an overpotential of 324 mV at 10 mA cm−2, while pristine ZnCo2O4 showed an overpotential of 427 mV at the same current density. The catalytic kinetics for the OER also was evaluated and OV-ZnCo2O4 presented a Tafel slope of 56.9 mV dec−1, which is lower than that of pristine ZnCo2O4 (74.4 mV dec−1).
Although the studies aforementioned seem to be encouraging, the electrochemical performance of ZnCo2O4 is still restricted by its poor electronic conductivity, which leads to suppression of electrocatalytic activity towards the OER. Thus, most works reported in the literature presented ZnCo2O4 combined with other compounds, especially with conductive polymers and conductive carbon-based materials to enhance its electronic conductivity, resulting in a better catalytic activity for the OER, as can be seen in Table 8.
| Catalyst | Preparation method | Overpotential at 10 mA cm−2 (E η10) (mV vs. RHE) | Tafel slope (mV dec−1) | Stability (h) | pH conditions (mol L−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| HPs = hollow polyhedrons; ZIFs = zeolitic imidazolate frameworks.a At 50 mA cm−2. | |||||||
| OER | ZnCo2O4 | Sol–gel method | 650 | 51 | — | KOH 0.1 | 271 |
| ZnCo2O4 nanosheets | Thermal treatment of Zn–Co LDH | 340 | 38 | — | KOH 1.0 | 130 | |
| MOF-derived ZnCo2O4 | Calcination process | 389 | 61.8 | 2 | KOH 1.0 | 272 | |
| OV-ZnCo2O4 | Hydrothermal method | 324 | 56.9 | 30 | KOH 0.1 | 270 | |
| m-ZnCo2O4 | Calcination process | 300 | 54 | — | KOH 1.0 | 273 | |
| PVP-ZnCo2O4 NPs | Hydrothermal method | 282 | 79.9 | 24 | KOH 1.0 | 274 | |
| ZnCo2O4@PPy-200 | Hydrothermal and electrochemical deposition | 254 | 60.77 | 42 | KOH 1.0 | 275 | |
| ZnCo2O4-CNTs | Hydrothermal method | 490 | — | — | KOH 0.1 | 254 | |
| ZnCo2O4@C-MWCNTs | Calcination process | 327 | 65 | 25 | KOH 1.0 | 276 | |
| ZnCo2O4@NC/CT | Carbonization–oxidation process | 196.4 | 61.3 | 45 | KOH 1.0 | 138 | |
| rGO–ZnCo2O4 | Solvothermal method | 300 | 59.2 | 12 | KOH 1.0 | 261 | |
| Co/ZnCo2O4@NC-CNTs | Pyrolysis treatment | 370 | 64 | 30 | KOH 1.0 | 255 | |
| ZnCo2O4@Ni(OH)2 – 2.0 | Hydrothermal method | 280.2a | 64.62 | 17 | KOH 1.0 | 277 | |
| ZnCo2O4@ZnCo-LDHs | Hydrolysis | 375 | 73 | — | KOH 1.0 | 278 | |
| ZnCo2O4@NiFe-LDH | Hydrothermal method | 249 | 96.7 | 20 | KOH 1.0 | 279 | |
| ZnCo2O4/FeOOH HPs | Thermal treatment of ZnCo/ZIFs | 299 | 69 | 15 | KOH 1.0 | 280 | |
| ZnCo2O4/Au/CNTs | Hydrothermal method | 440 | 46.2 | — | KOH 1.0 | 281 | |
| ZnCo2O4/CoxSey | Solvothermal method | 324 | 79.3 | 50 | KOH 1.0 | 253 | |
| C/ZnCo2O4/ZnO | Annealing | 279 | 72 | 24 | KOH 1.0 | 282 | |
| HER | Co2P/CoO/ZnCo2O4 | Hydrothermal followed by phosphorization process | 112 | 62 | 24 | KOH 1.0 | 283 |
| ZnCo2O4@PPy-50 | Hydrothermal and electrochemical deposition | 133 | 62.4 | — | KOH 1.0 | 57 | |
| ZnCo2O4@PPy-200 | Hydrothermal and electrochemical deposition | 183.52 | 60.77 | 22 | KOH 1.0 | 275 | |
For instance, Tomboc et al.274 prepared ZnCo2O4 nanoparticles with a nanocactus morphology in the presence of polyvinylpyrrolidone (PVP) (here denoted as PVP-ZnCo2O4) using a one-step hydrothermal method followed by calcination treatment. The authors demonstrated that in the presence of PVP the electrocatalytic activity of ZnCo2O4 was enhanced when compared to ZnCo2O4 without PVP. Indeed, PVP-ZnCo2O4 exhibited an overpotential of 282 mV at 10 mA cm−2, while ZnCo2O4 without PVP showed an overpotential of 343 mV. PVP-ZnCo2O4 also presented an overpotential lower than PVP-NiCo2O4 (298 mV), synthesized under the same conditions.
Recently, Zhao and colleagues275 electropolymerized polypyrrole (PPy) on ZnCo2O4 nanowires under a constant potential of 0.9 V for 60, 100, 200 and 300 s, and the electrodes were denoted as ZnCo2O4@PPy-60, ZnCo2O4@PPy-100, ZnCo2O4@PPy-200, and ZnCo2O4@PPy-300, respectively. The SEM image of ZnCo2O4@PPy-200 in Fig. 21B reveals that nanowires were coated by a thin layer of PPy, in comparison to ZnCo2O4 (Fig. 21A), and from the TEM images in Fig. 21C and D it is possible to observe that nanowires are composed of many nanoparticles. In addition, ZnCo2O4@PPy-200 presented a surface area of 56 m2 g−1 higher than pristine ZnCo2O4 (39 m2 g−1). Among these samples, ZnCo2O4@PPy-200 exhibited a lower overpotential (250 mV) at 10 mV cm−2 (Fig. 21E) and a lower Tafel slope (60.77 mV dec−1). Chronoamperometric studies were performed to evaluate the durability and stability of the ZnCo2O4@PPy-200 electrode, and even after 42 hours the catalyst remained steady, revealing its excellent stability.
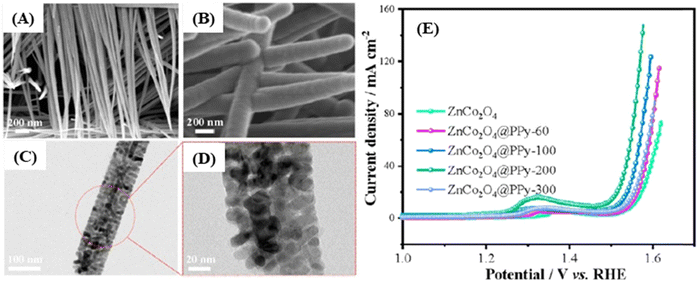 | ||
| Fig. 21 SEM images of ZnCo2O4 (A) and ZnCo2O4@PPy-200 (B). TEM images of ZnCo2O4@PPy-200 (C and D). Linear sweep voltammetry at 2 mV s−1 for ZnCo2O4 and ZnCo2O4@PPy samples (E). Reproduced with permission.275 Copyright © 2021 Elsevier Ltd. All rights reserved. | ||
In addition to conductive polymers, carbon-based materials (carbon nanotubes and graphene) have been widely used with cobaltite spinel oxides to improve their electronic conductivity,284,285 thus providing a conducting platform. Furthermore, these materials, when combined, present a synergistic effect in the OER owing to their high surface area, providing more electrocatalytically active sites for charge transport between the electrode/electrolyte interface. For instance, Yan and co-authors255 reported the synthesis of Co/ZnCo2O4 from a MOF (CoZn-ZIF-L) sandwiched in N-doped carbon interconnected with carbon nanotubes (denoted Co/ZnCo2O4@NC-CNTs) as an electrode material for OER activity. The composite presented an overpotential of 370 mV at a current density of 10 mA cm−2 and a low Tafel slope of 64 mV dec−1. Similarly, Liu et al.276 embedded two different MOFs (metal–organic frameworks) ZIF-8 and ZIF-67 into MWCNTs (multi-walled carbon nanotubes) and obtained ZnCo2O4@C-MWCNTs by the calcination process. The electrode material exhibited a low overpotential of 327 mV at 10 mA cm−2 and a Tafel slope of 65 mV dec−1. In addition, the electrocatalytic activity of ZnCo2O4@C-MWCNTs remained unchanged, even after 25 hours of tests, demonstrating the reliability of the material.
In the same way, Kong et al.,138 using a ZnCo MOF, prepared an electrode material based on zinc-cobalt oxide nanoflakes@N-doped carbon hollow nanowall arrays anchored onto carbon textile (ZnCo2O4@NC/CT). The SEM images of ZnCo2O4@NC/CT show that the compound grown vertically on a carbon textile electrode (Fig. 22A) and holes can be observed in its structure (Fig. 22B), caused by cation exchange between Co2+ and Zn2+. Furthermore, the hollow structure is confirmed through the contrast between the shell and core (hollow), as can be seen in Fig. 22C. The electrode exhibited an outstanding low overpotential of 196.4 mV at 10 mV cm−2, a low Tafel slope of 61.3 mV dec−1, and a long-term durability of 45 hours (Fig. 22D). The authors attributed the excellent results to (i) the decreased resistance at the interface between the substrate and the electrode material due to the direct growth of N-doped carbon nanowalls on the substrate surface, leading to improvement of the ion/electron transfer rates and (ii) the easy penetration of electrolyte, leading to faster faradaic reactions and ion diffusion rates, thanks to the high surface area of the porous structure of the ZnCo2O4 nanoflake shell, as shown in Fig. 22E and F.
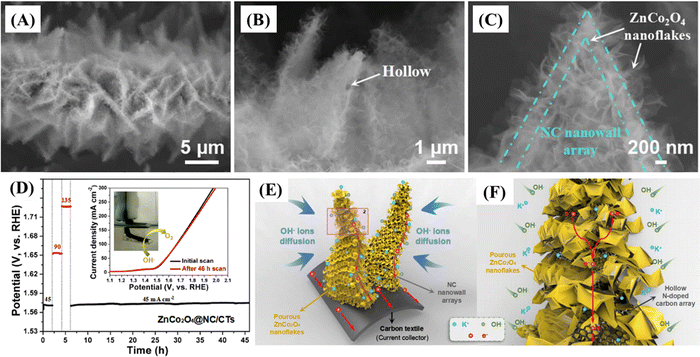 | ||
| Fig. 22 (A–C) SEM images of ZnCo2O4@NC/CT. (D) Stability measurements of ZnCo2O4@NC/CT at different current densities and (E and F) schematic illustration of the ZnCo2O4@NC/CT electrode. Reproduced with permission.138 Copyright © 2019 Published by Elsevier B.V. | ||
Graphene has also been combined with spinel oxides to improve the electrocatalytic activity for the OER.284,286,287 To enhance the catalytic activity of ZnCo2O4 towards the OER, Chakabarty et al.261 prepared a ZnCo2O4 grafted onto reduced graphene oxide (rGO) sheet through the solvothermal method. The SEM and TEM images in Fig. 19A and B revealed that the structure of the ZnCo2O4 microsphere is highly porous, as well as composed of several nanoparticles with an average size of 10 nm. The highly porous structure of ZnCo2O4 was maintained in the rGO–ZnCo2O4, as shown in Fig. 19B. The rGO–ZnCo2O4 composite presented the lowest overpotential at 10 mA cm−2 for the OER (300 mV) when compared to rGO (510 mV), ZnCo2O4 (410 mV), and benchmark IrO2 (340 mV), or a rGO/ZnCo-layered double hydroxide composite (onset overpotential ∼330 mV).288 Moreover, rGO–ZnCo2O4 presented high stability and the current density remained stable from the beginning to the end of the measurement (12 h), differently from ZnCo2O4 that presented a decrease of current density, caused by gas bubble formation. In addition, the electrocatalytic activity of rGO–ZnCo2O4 towards the OER was evaluated by SECM measurement. It is possible to observe that a small current density is detected from 1.4 V, indicating the beginning of the OER process. As the potential increases to 1.45 V and 1.5 V the current density also increases.
In addition to carbon-based materials, other compounds such as LDH and oxides have been associated with ZnCo2O4, as can be seen in Table 8. For instance, Pan et al.278 reported the synthesis of ZnCo2O4@ZnCo-LDH yolk–shell nanospheres. The electrode material exhibited an overpotential of 375 mV at 10 mA cm−2 and a Tafel slope of 73 mV dec−1. Its electrochemical performance was attributed to the large surface area, the synergistic effect between ZnCo2O4 and ZnCo-LDH, and the interconnection among the nanosheets which consisted of the nanospheres, causing the reduction of the transportation path of electrolyte ions. Que et al.279 obtained a core–shell structure of ZnCo2O4@NiFe-LHD that presented an overpotential of 249 mV at 10 mA cm−2. The authors explained that the low overpotential achieved by the electrode material was due to the synergistic effect between core@shell structure components. Xiong et al.282 prepared a C/ZnCo2O4/ZnO material, combining two strategies (preparation of MOF-derived ZnCo2O4 and the formation of a hierarchical core@shell structure). As a consequence, the electrode material required 279 mV overpotential to reach 10 mA cm−2 current density. Besides, the electrocatalyst did not present significant degradation after a 24 h stability test.
Possible strategies and tendencies in the preparation of electrode materials based on ZnCo2O4 for OER catalysis can be seen in Fig. 23, where the electrocatalysts are summarized according to their low overpotential (η10 ≤ 300 mV). Analyzing the electrode materials displayed in Fig. 23 we figured out that three of the nine electrocatalysts based on ZnCo2O4 are MOF derivatives, and one of them presented the best electrochemical performance for OER catalysis among the electrocatalysts reported. In fact, ZnCo2O4@NC/CT, C/ZnCo2O4/ZnO and ZnCo2O4/FeOOH HPs exhibited an overpotential of 196.4, 279 and 299 mV, respectively. The best electrocatalyst ZnCo2O4@NC/CT presented an overpotential of ∼102 mV lower than the seventh electrocatalyst also based on MOF-derivative ZnCo2O4/FeOOH HPs (299 mV). Although both of them were designed from the MOF, the former was combined with a carbon material that enhanced the electronic conductivity of the electrode. However, the rGO–ZnCo2O4 electrocatalyst occupied the eighth position along with m-ZnCo2O4 and both of them presented an overpotential of 300 mV.
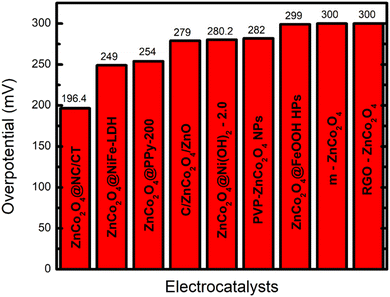 | ||
| Fig. 23 The top 9 electrocatalysts based on ZnCo2O4 for the OER that presented an overpotential ≤300 mV (η10 ≤ 300 mV). | ||
Among the electrode materials displayed in Fig. 23, it can be noticed that the combination of ZnCo2O4 with conductivity polymers can also be a good strategy to improve the electrochemical performance of the electrocatalyst. Indeed, intermediate overpotential values were reached for ZnCo2O4@PPy-200 (254 mV) and PVP-ZnCo2O4 NPs (282 mV) electrodes, occupying, respectively, the third and sixth positions.
The design of hierarchical structures as core@shell providing a shortened ion/electron transport pathways and a large surface area with a large number of electrocatalytic sites exposed, favoring faradaic reactions, seems to be another interesting strategy to improve the electrochemical performance for OER catalysis. Thus, it can be highlighted that the electrocatalyst based on ZnCo2O4@NiFe-LDH presented the second-best electrochemical performance with an overpotential of 249 mV, and the synergistic effect between the core and shell materials in the structure contributed to the excellent result. It is noteworthy that the chosen shell material also was fundamental to achieving the results. In fact, recent studies have shown that NiFe-LDH and ternary NiFe-LDH derivatives are promising electrode materials for the OER catalysis.289 The fifth position was occupied by the electrocatalyst ZnCo2O4@Ni(OH)2 – 2.0, presenting an overpotential of 280.2 mV, where the presence of Ni(OH)2 as a shell material improved the electrochemical performance of the electrode materials, since the Ni(OH)2 nanosheets made transporting electrons/ions easier. In fact, the overpotential value for the ZnCo2O4@Ni(OH)2 is much smaller than those of many other pristine materials such as NiO (310 mV),290 ZnO (340 mV)291 and Ni(OH)2 (340 mV).292
Although many works using ZnCo2O4 as an electrode material for the catalysis of OER processes have been reported, few articles using the same material were found in the literature for HER electrocatalysis, as can be seen in Table 8. The main reason for this is that the production of high-purity hydrogen from the water-splitting method293 still is restricted by the sluggish kinetics of the OER.264
Among these works, we can highlight that reported by Zhang and colleagues283 where zinc cobalt oxide/phosphide (Co2P/CoO/ZnCo2O4) hollow submicron boxes were obtained and used as an electrode material for the HER. The electrocatalyst showed an overpotential of 112 mV at −10 mA cm−2 current density; for comparison purposes the commercial Pt/C electrode also was tested and presented an overpotential of 19 mV at the same current density. Furthermore, the electrode materials exhibited a Tafel slope of 62 mV dec−1, indicating that the reaction pathway obeys the Volmer–Heyrovsky mechanism with a fast Volmer step for the HER.
5. Conclusion and outlook
The morphology of ZnCo2O4 has a huge impact on its electrochemical performance, and can be improved by means of a rational design. The specific capacitance, electrocatalytic activity, rate-capability and cycling stability of ZnCo2O4-based electrodes heavily depend on ZnCo2O4 mechanical properties. Bulkier micro- and nanoparticles usually have low specific surface area (even lower if they are not porous or having at least rough surfaces), heavily suffering from the strain effects throughout the charge–discharge cycling, and high internal electrical resistance due to low surface area-to-volume ratios.The design of 1D and 2D morphologies, along with hollow and/or porous structures, can partially overcome these limitations, specially aligned with suitable spaces between these 1D and 2D structures. 1D and 2D structures present increased specific surface areas, promoting electrolyte diffusion and electroactive site availability; greatly reduced one or more dimensions, providing shortened electron transfer pathways and alleviating the strain effects caused by volume changes; and in the case of electrocatalysts the high porosity and pore sizes, enhancing specific surface area and facilitating electrolyte adsorption and product release (e.g., O2 in the OER and H2 in the HER).
In fact, for supercapacitive applications, 1D structures can deliver high specific capacitances at lower current densities, owing to their unidimensional electron pathways and high specific surface area, which enhances the electroactive site availability. However, usually at higher current densities the electrolyte diffusion is hindered due to the entanglement of such 1D structures, which reduces the area for electrolyte penetration within the structure, limiting the electroactive site availability and cyclability. This effect can also be observed in 2D structures, which, even being the most commonly synthesized and being known for their high specific capacitance, can present strain effects caused by volume changes if the space between the structures is not suitable for fast electrolyte diffusion at higher current densities. Thus, it is extremely beneficial to engineering electrodes based on ZnCo2O4 with wide-open 1D or 2D nanostructures, which, along with all the benefits of such structures, facilitates the electrolyte diffusion even at higher current densities and further alleviates the strain effects of continuous charge–discharge cycling processes.
As for electrocatalytic applications, similarly to supercapacitive applications, it is interesting to synthesize wide-open and porous nanostructures. 1D nanostructures usually present unsuitable specific surface area, pore sizes and porosity for efficient electrolyte adsorption and product desorption, hindering the electrocatalyst performance of such structures, especially in comparison to 2D structured nanoparticles. 2D nanostructures commonly present the most optimal mesoporous and microporous sizes and volumes for the promotion of electrocatalytic activity, which can be even further enhanced according to the spaces between such structures by the facilitation of electrolyte penetration and enhancement of electroactive site availability.
Additionally, the electrochemical performance of ZnCo2O4-based electrodes can be even more improved by the incorporation of composites and/or binder-free electrode production, along with the morphology control. The use of slurry-cast electrodes with binders that can significantly reduce the electronic conductivity, limiting the availability of active materials, and hindering the ion-diffusion. It can also increase the mass density as “dead-mass” and reduce the material integrity through cycling. So, binder-free electrodes should be preferred to circumvent all the above-mentioned downsides. ZnCo2O4 composites can be produced with highly electrically conductive and/or electrochemically active carbonaceous and other transition metal materials, such as oxides, hydroxides and sulfides, as both support and coating components. They can provide bigger specific surface area, faster electron transfer, and short and more efficient ion-diffusion paths. In addition to the more active sites and richer redox reactions, the overall stability is greatly improved. One can also morphologically orient the growth of ZnCo2O4 when it is used as a support material.
The strategies for synthesis and application of pristine ZnCo2O4 and its composites in LIBs, SIBs, Li–S batteries and metal–air batteries are summed up. Although ZnCo2O4 has been applied in energy storage and has proved to be a promising electrode material, there are a few challenges to mention, e.g., its poor electrical conductivity, slow lithium diffusion and short cycling life. This is associated with the volume expansion during the lithium insertion and extraction process. Many prospective strategies should be developed for the application of ZnCo2O4 electrode materials, and we hope that this review article will facilitate further studies and advancements in this area.
Improving the conductivity is always a key issue in the development of electrode materials based on ZnCo2O4. Generally synthesis of electrode materials with nanoscale dimension ZnCo2O4 has already been proven to be effective for obtaining high-power density, high-energy density, better stability and other admirable electrochemical performances. Nanostructured ZnCo2O4 composites with conductive materials such as polymers and carbon were also demonstrated to improve their electrochemical performance. These strategies can effectively enhance the conductivity and alleviate the volume change of ZnCo2O4 electrode materials. Therefore, ZnCo2O4 has been gaining more and more attention in the field of energy storage in recent years.
For application as electrocatalysts in energy technologies, ZnCo2O4 and its composites show great potential due to their high intrinsic activity. In many cases they can exhibit bifunctionalities, encompassing both ORR and OER activity. In fact, it is important to highlight that the main strategies employed in electrode materials for the ORR consisted of the design of new catalysts with different morphologies, and the formation of composites with conducting nanocarbons, such as carbon nanotubes and graphene.
Similarly, although ZnCo2O4 exhibits an overpotential close to 300 mV as an electrode material for OER catalysis, it is limited by its poor conductivity. For this reason, ZnCo2O4-based OER electrocatalysts have been combined with conducting carbon materials and polymers, as well as with compounds such as metal oxides/hydroxides. Curiously, among the top 9 (η10 ≤ 300 mV) electrode materials for OER catalysis, three electrocatalysts are based on MOF-derivatives. Deriving ZnCo2O4 electrodes using the MOF strategies can be interesting, since the main features will be preserved, such as a high porous structure and large surface area.294 They will improve the electrochemical performance of the electrocatalyst. The design of the electrode material is fundamental for obtaining a good electrochemical performance. For instance, hierarchical core@shell structures can yield excellent results because of their large surface area, while the exposed electrocatalytic sites can improve the faradaic reaction, and shorten the ion/electron transport pathway. In fact, the regulation strategies for improving the electrocatalytic performance of ZnCo2O4-based electrodes follow trends also reported in other works6,289,295 and can be summarized mainly as: (a) reducing electrical resistance using conductive supports and (b) increasing active sites by nanostructuration, morphology engineering and porous structure construction.27
Despite the important advances in the design of new materials based on ZnCo2O4 aforementioned, many challenges still need to be overcome regarding a full exploration and implementation in practical/real application in electrochemical energy storage and conversion. For instance, currently it is mandatory the development of devices that are able to withstand high current density with long-term cycling stability, aiming to reduce the charge time, e.g., devices that can provide high energy density at a high-power density during the long-term charge/discharge cycling process. However, the excellent performances generally reported in the literature, especially in studies using three-electrode systems, and even in two-electrode devices, may not fully represent a real application, since on a laboratory scale it usually takes 2–3 mg cm−2 of the electrode material, but commercially always demands high mass loading (>10 mg cm−2). In this sense, the design and manufacture of more robust devices with greater thickness and mass loading should be further studied.
In fact, we are convinced that much research needs to be done to further improve electrochemical and electrocatalytic materials based on ZnCo2O4, where site engineering and a conductivity optimization approach should be used in the quest for ideal electrode materials.27 For instance, the incorporation (or metal-ion doping) of third and fourth metal ions3 or the development of high entropy materials296 can be decisive for improving energy storage and for electrocatalytic activity.289 In fact, these strategies can also help in the challenge of minimizing the use of Co, which are pushing a new trend of emerging low-Co (and Co-free) materials as next-generation electrode materials for energy applications.297 In addition, the research should seek to increase the conductivity and porosity of ZnCo2O4/carbon composites as strategies for manufacturing electrodes with high mass loading for real application. From this perspective, the preparation of ZnCo2O4/carbon derived from MOFs should be studied more deeply, especially those derived from the zeolitic imidazolate framework (ZIF-67, ZIF-8, ZIF-67 + ZIF-8, etc.). In fact, MOF-derived composites have been regarded as excellent new functional electrode materials for many applications, exhibiting exceptional conductivity, stability, porous/hollow structures with tunable shapes, and tailored compositions and electrochemical activity, overcoming the relatively low conductivity and missing chemical and/or structural robustness of precursor MOFs.13,294,298 Therefore, these are some future directions for the development of ZnCo2O4 based materials for their commercial/real applications towards a more sustainable society.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the São Paulo Research Foundation (FAPESP 2018/21489-1, 2017/13137-5, 2014/50867-3 and 2013/24725-4) and the National Council for Scientific and Technological Development (CNPq 401581/2016-0, 408222/2016-6, 442599/2019-6, 311847/2018-8), in addition to the fellowships granted to J. M. G. (FAPESP 2018/16896-7), M. I. S. (CAPES 141853/2015-8) and M. N. T. S. (CAPES 88882.429165/2019.01). The Grupo de Materiais Inorgânicos do Triângulo (GMIT) research group was supported by FAPEMIG (APQ-00330-14), Brazilian Institute of Science and Technology (INCT) in Carbon Nanomaterials.References
- S. Liu, L. Hu, X. Xu, A. A. Al-Ghamdi and X. Fang, Nickel Cobaltite Nanostructures for Photoelectric and Catalytic Applications, Small, 2015, 11, 4267–4283 CrossRef CAS PubMed.
- J. M. Gonçalves, A. Kumar, M. I. da Silva, H. E. Toma, P. R. Martins, K. Araki, M. Bertotti and L. Angnes, Nanoporous Gold-Based Materials for Electrochemical Energy Storage and Conversion, Energy Technol., 2021, 9, 2000927 CrossRef.
- J. M. Gonçalves, M. I. da Silva, H. E. Toma, L. Angnes, P. R. Martins and K. Araki, Trimetallic oxides/hydroxides as hybrid supercapacitor electrode materials: a review, J. Mater. Chem. A, 2020, 8, 10534–10570 RSC.
- S. J. Uke, V. P. Akhare, D. R. Bambole, A. B. Bodade and G. N. Chaudhari, Recent Advancements in the Cobalt Oxides, Manganese Oxides, and Their Composite As an Electrode Material for Supercapacitor: A Review, Front. Mater., 2017, 4, 21 CrossRef.
- J. G. Ruiz-Montoya, V. L. Quispe-Garrido, J. C. Calderón Gómez, A. M. Baena Moncada and J. M. Gonçalves, Recent Progress and Prospects on Supercapacitor Materials based on Metal Oxide or Hydroxide/Biomass-Derived Carbon Composites, Sustainable Energy Fuels, 2021, 5, 5332–5365 RSC.
- J. M. Gonçalves, D. P. Rocha, M. N. T. Silva, P. Roberto Martins, E. Nossol, L. Angnes, C. S. Rout and R. A. Abarza Munoz, Feasible strategies to promote the sensing performances of spinel MCo2O4 (M = Ni, Fe, Mn, Cu and Zn) based electrochemical sensors: A review, J. Mater. Chem. C, 2021, 9, 7852–7887 RSC.
- Y. Li, X. Han, T. Yi, Y. He and X. Li, Review and prospect of NiCo2O4-based composite materials for supercapacitor electrodes, J. Energy Chem., 2019, 31, 54–78 CrossRef.
- J. M. Gonçalves, M. N. T. Silva, K. K. Naik, P. R. Martins, D. P. Rocha, E. Nossol, R. A. A. Munoz, L. Angnes and C. S. Rout, Multifunctional spinel MnCo2O4 based materials for energy storage and conversion: a review on emerging trends, recent developments and future perspectives, J. Mater. Chem. A, 2021, 9, 3095–3124 RSC.
- X. Zhao, L. Mao, Q. Cheng, J. Li, F. Liao, G. Yang, L. Xie, C. Zhao and L. Chen, Two-dimensional Spinel Structured Co-based Materials for High Performance Supercapacitors: A Critical Review, Chem. Eng. J., 2020, 387, 124081 CrossRef CAS.
- J. P. Cheng, W. D. Wang, X. C. Wang and F. Liu, Recent research of core–shell structured composites with NiCo2O4 as scaffolds for electrochemical capacitors, Chem. Eng. J., 2020, 393, 124747 CrossRef CAS.
- X. Han, X. Gui, T.-F. Yi, Y. Li and C. Yue, Recent progress of NiCo2O4-based anodes for high-performance lithium-ion batteries, Curr. Opin. Solid State Mater. Sci., 2018, 22, 109–126 CrossRef CAS.
- R. Kumar, NiCo2O4 Nano-/Microstructures as High-Performance Biosensors: A Review, Nano–Micro Lett., 2020, 12, 122 CrossRef CAS PubMed.
- R. Wu, J. Sun, C. Xu and H. Chen, MgCo2O4-based electrode materials for electrochemical energy storage and conversion: a comprehensive review, Sustainable Energy Fuels, 2021, 5, 4807–4829 RSC.
- J. Sun, C. Xu and H. Chen, A review on the synthesis of CuCo2O4-based electrode materials and their applications in supercapacitors, J. Materiomics, 2021, 7, 98–126 CrossRef.
- H. Gao, S. Liu, Y. Li, E. Conte and Y. Cao, A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion, Energies, 2017, 10(11), 1787, DOI:10.3390/en10111787.
- M. Harada, F. Kotegawa and M. Kuwa, Structural Changes of Spinel MCo2O4 (M = Mn, Fe, Co, Ni, and Zn) Electrocatalysts during the Oxygen Evolution Reaction Investigated by In Situ X-ray Absorption Spectroscopy, ACS Appl. Energy Mater., 2022, 5, 278–294 CrossRef CAS.
- S. Gedi, R. Manne, G. Manjula, L. V. Reddy, C. P. Reddy, N. Marraiki, W. K. Kim, K. Mallikarjuna and M. S. Pratap, Reddy, Tunability of the self-assemblies of porous polygon-like zinc cobaltite architectures using mixed solvents for high-performance supercapacitors, J. Phys. Chem. Solids, 2022, 163, 110587 CrossRef CAS.
- J. A. Rajesh and K.-S. Ahn, Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires, Catalysts, 2021, 11(12), 1516, DOI:10.3390/catal11121516.
- S.-H. Lee, J. H. Kim and J.-R. Yoon, Laser Scribed Graphene Cathode for Next Generation of High Performance Hybrid Supercapacitors, Sci. Rep., 2018, 8, 8179 CrossRef PubMed.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- M. Bohra, V. Alman and R. Arras, Nanostructured ZnFe2O4: An Exotic Energy Material, Nanomaterials, 2021, 11(5), 1286, DOI:10.3390/nano11051286.
- L. Sun, Q. Luo, Z. Dai and F. Ma, Material libraries for electrocatalytic overall water splitting, Coord. Chem. Rev., 2021, 444, 214049 CrossRef CAS.
- J. M. Gonçalves, P. R. Martins, K. Araki and L. Angnes, Recent progress in water splitting and hybrid supercapacitors based on nickel-vanadium layered double hydroxides, J. Energy Chem., 2021, 57, 496–515 CrossRef.
- Y. Wang, X. Huang and Z. Wei, Recent developments in the use of single-atom catalysts for water splitting, Chin. J. Catal., 2021, 42, 1269–1286 CrossRef CAS.
- P. Chen, J. Ye, H. Wang, L. Ouyang and M. Zhu, Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting, J. Alloys Compd., 2021, 883, 160833 CrossRef CAS.
- Y. Yan, T. He, B. Zhao, K. Qi, H. Liu and B. Y. Xia, Metal/covalent–organic frameworks-based electrocatalysts for water splitting, J. Mater. Chem. A, 2018, 6, 15905–15926 RSC.
- J. M. Gonçalves, T. A. Matias, K. C. F. Toledo and K. Araki, in Advances in Inorganic Chemistry, ed. R. V. Eldik and C. Hubbard, Elsevier, 2019, vol. 74, p. 63 Search PubMed.
- M. Tahir, L. Pan, F. Idrees, X. Zhang, L. Wang, J.-J. Zou and Z. L. Wang, Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review, Nano Energy, 2017, 37, 136–157 CrossRef CAS.
- A. Chakraborty, S. Kunnikuruvan, S. Kumar, B. Markovsky, D. Aurbach, M. Dixit and D. T. Major, Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1–x–yCoxMnyO2 and LiNi1–x–yCoxAlyO2, Chem. Mater., 2020, 32, 915–952 CrossRef CAS.
- M. Winter and R. J. Brodd, What Are Batteries, Fuel Cells, and Supercapacitors?, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- C. Zou, L. Zhang, X. Hu, Z. Wang, T. Wik and M. Pecht, A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors, J. Power Sources, 2018, 390, 286–296 CrossRef CAS.
- Y. Gogotsi and R. M. Penner, Energy Storage in Nanomaterials – Capacitive, Pseudocapacitive, or Battery-like?, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- J. Cherusseri, D. Pandey and J. Thomas, Symmetric, Asymmetric, and Battery-Type Supercapacitors Using Two-Dimensional Nanomaterials and Composites, Batteries Supercaps, 2020, 3, 860–875 CrossRef CAS.
- N. R. Chodankar, H. D. Pham, A. K. Nanjundan, J. F. S. Fernando, K. Jayaramulu, D. Golberg, Y.-K. Han and D. P. Dubal, True Meaning of Pseudocapacitors and Their Performance Metrics: Asymmetric versus Hybrid Supercapacitors, Small, 2020, 16, 2002806 CrossRef CAS PubMed.
- J. M. Gonçalves, M. I. da Silva, M. Hasheminejad, H. E. Toma, K. Araki, P. R. Martins and L. Angnes, Recent Progress in Core@Shell Sulfide Electrode Materials for Advanced Supercapacitor Devices, Batteries Supercaps, 2021, 4, 1397 CrossRef.
- R. Wang, M. Yao and Z. Niu, Smart supercapacitors from materials to devices, InfoMat, 2020, 2, 113–125 CrossRef CAS.
- M. D. Stoller, S. A. Stoller, N. Quarles, J. W. Suk, S. Murali, Y. Zhu, X. Zhu and R. S. Ruoff, Using coin cells for ultracapacitor electrode material testing, J. Appl. Electrochem., 2011, 41, 681 CrossRef CAS.
- M. Yu and X. Feng, Thin-Film Electrode-Based Supercapacitors, Joule, 2019, 3, 338–360 CrossRef CAS.
- D. Chen, K. Jiang, T. Huang and G. Shen, Recent Advances in Fiber Supercapacitors: Materials, Device Configurations, and Applications, Adv. Mater., 2020, 32, 1901806 CrossRef CAS.
- F. Bu, W. Zhou, Y. Xu, Y. Du, C. Guan and W. Huang, Recent developments of advanced micro-supercapacitors: design, fabrication and applications, npj Flexible Electron., 2020, 4, 31 CrossRef.
- V. Augustyn, P. Simon and B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- D. P. Chatterjee and A. K. Nandi, A review on the recent advances in hybrid supercapacitors, J. Mater. Chem. A, 2021, 9, 15880–15918 RSC.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gómez-Romero, Hybrid energy storage: the merging of battery and supercapacitor chemistries, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- D. Qian, C. Ma, K. L. More, Y. S. Meng and M. Chi, Advanced analytical electron microscopy for lithium-ion batteries, NPG Asia Mater., 2015, 7, e193 CrossRef CAS.
- H. Cheng, J. G. Shapter, Y. Li and G. Gao, Recent progress of advanced anode materials of lithium-ion batteries, J. Energy Chem., 2021, 57, 451–468 CrossRef CAS.
- Y. Han, Y. Lei, J. Ni, Y. Zhang, Z. Geng, P. Ming, C. Zhang, X. Tian, J.-L. Shi, Y.-G. Guo and Q. Xiao, Single-Crystalline Cathodes for Advanced Li-Ion Batteries: Progress and Challenges, Small, 2022, 2107048 CrossRef PubMed.
- Y. Lu, Q. Zhang and J. Chen, Recent progress on lithium-ion batteries with high electrochemical performance, Sci. China: Chem., 2019, 62, 533–548 CrossRef CAS.
- J. M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- Y. Li and J. Lu, Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice?, ACS Energy Lett., 2017, 2, 1370–1377 CrossRef CAS.
- H.-F. Wang and Q. Xu, Materials Design for Rechargeable Metal-Air Batteries, Matter, 2019, 1, 565–595 CrossRef CAS.
- K. Yoo, S. Banerjee, J. Kim and P. Dutta, A Review of Lithium-Air Battery Modeling Studies, Energies, 2017, 10, 1748 CrossRef.
- Y. Huang, Y. Wang, C. Tang, J. Wang, Q. Zhang, Y. Wang and J. Zhang, Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal-Air Batteries, Adv. Mater., 2019, 31, 1803800 CrossRef.
- Z. Ma, X. Yuan, L. Li, Z.-F. Ma, D. P. Wilkinson, L. Zhang and J. Zhang, A review of cathode materials and structures for rechargeable lithium–air batteries, Energy Environ. Sci., 2015, 8, 2144–2198 RSC.
- K. Song, B. Yang, Z. Li, Y. Lv, Y. Yu, L. Yuan, X. Shen and X. Hu, Direct synthesis of ACo2O4 (A = Ni, Cu, Fe, Zn) nanowires on carbon cloth as an oxygen electrode catalyst for rechargeable lithium-oxygen batteries, Appl. Surf. Sci., 2020, 529, 147064 CrossRef CAS.
- T. Ramachandran and F. Hamed, Electrochemical performance of plate-like zinc cobaltite electrode material for supercapacitor applications, J. Phys. Chem. Solids, 2018, 121, 93–101 CrossRef CAS.
- X. Liu, Q. Li, Y. Qin and Y. Jiang, Constructing high-performance electrode materials using core–shell ZnCo2O4@PPy nanowires for hybrid batteries and water splitting, RSC Adv., 2020, 10, 28324–28331 RSC.
- D. Yu, Z. Zhang, Y. n Meng, Y. Teng, Y. Wu, X. Zhang, Q. Sun, W. Tong, X. Zhao and X. Liu, The synthesis of hierarchical ZnCo2O4@MnO2 core–shell nanosheet arrays on Ni foam for high-performance all-solid-state asymmetric supercapacitors, Inorg. Chem. Front., 2018, 5, 597–604 RSC.
- L. Merabet, K. Rida and N. Boukmouche, Sol-gel synthesis, characterization, and supercapacitor applications of MCo2O4 (M = Ni, Mn, Cu, Zn) cobaltite spinels, Ceram. Int., 2018, 44, 11265–11273 CrossRef CAS.
- K. Prasad, G. Rajasekhara Reddy, M. Rajesh, P. R. Babu, G. Shanmugam, N. J. Sushma, M. S. Pratap Reddy and B. Deva, Electrochemical Performance of 2D-Hierarchical Sheet-Like ZnCo2O4 Microstructures for Supercapacitor Applications, Crystals, 2020, 10, 566, DOI:10.3390/cryst10070566.
- D. M. Alqahtani, C. Zequine, C. K. Ranaweera, K. Siam, P. K. Kahol, T. P. Poudel, S. R. Mishra and R. K. Gupta, Effect of metal ion substitution on electrochemical properties of cobalt oxide, J. Alloys Compd., 2019, 771, 951–959 CrossRef CAS.
- Y. Shang, T. Xie, C. Ma, L. Su, Y. Gai, J. Liu and L. Gong, Synthesis of hollow ZnCo2O4 microspheres with enhanced electrochemical performance for asymmetric supercapacitor, Electrochim. Acta, 2018, 286, 103–113 CrossRef CAS.
- B. Saravanakumar, G. Ravi, R. Yuvakkumar, V. Ganesh, S. Ravichandran, M. Thambidurai and A. Sakunthala, Hydrothermal synthesis and electrochemical properties of ZnCo2O4 microspheres, Ionics, 2019, 25, 353–360 CrossRef CAS.
- H. Chen, J. Wang, X. Han, F. Liao, Y. Zhang, X. Han and C. Xu, Simple growth of mesoporous zinc cobaltite urchin-like microstructures towards high-performance electrochemical capacitors, Ceram. Int., 2019, 45, 4059–4066 CrossRef CAS.
- H. Chen, J. Wang, X. Han, F. Liao, Y. Zhang, L. Gao and C. Xu, Facile synthesis of mesoporous ZnCo2O4 hierarchical microspheres and their excellent supercapacitor performance, Ceram. Int., 2019, 45, 8577–8584 CrossRef CAS.
- R. R. Gutturu, T. V. M. Sreekanth, R. Rajavaram, D. P. R. Borelli, G. R. Dillip, P. C. Nagajyothi and J. Shim, Effect of reaction time and PVP contents on morphologies of hierarchical 3D flower-like ZnCo2O4 microstructures for energy storage devices, Int. J. Energy Res., 2020, 44, 11233–11247 CrossRef CAS.
- J. Bhagwan, Sk. Khaja Hussain and J. S. Yu, Aqueous asymmetric supercapacitors based on ZnCo2O4 nanoparticles via facile combustion method, J. Alloys Compd., 2020, 815, 152456 CrossRef CAS.
- Y. Shang, T. Xie, Y. Gai, L. Su, L. Gong, H. Lv and F. Dong, Self-assembled hierarchical peony-like ZnCo2O4 for high-performance asymmetric supercapacitors, Electrochim. Acta, 2017, 253, 281–290 CrossRef CAS.
- M. Priya, V. K. Premkumar, P. Vasantharani and G. Sivakumar, Structural and electrochemical properties of ZnCo2O4 nanoparticles synthesized by hydrothermal method, Vacuum, 2019, 167, 307–312 CrossRef CAS.
- D. Song, J. Zhu, J. Li, T. Pu, B. Huang, C. Zhao, L. Xie and L. Chen, Free-standing Two-dimensional Mesoporous ZnCo2O4 Thin Sheets Consisting of 3D Ultrathin Nanoflake Array Frameworks for High Performance Asymmetric Supercapacitor, Electrochim. Acta, 2017, 257, 455–464 CrossRef CAS.
- A. J. C. Mary and A. C. Bose, Surfactant assisted ZnCo2O4 nanomaterial for supercapacitor application, Appl. Surf. Sci., 2018, 449, 105–112 CrossRef CAS.
- J. Zhu, D. Song, T. Pu, J. Li, B. Huang, W. Wang, C. Zhao, L. Xie and L. Chen, Two-dimensional porous ZnCo2O4 thin sheets assembled by 3D nanoflake array with enhanced performance for aqueous asymmetric supercapacitor, Chem. Eng. J., 2018, 336, 679–689 CrossRef CAS.
- X. Xiao, G. Wang, M. Zhang, Z. Wang, R. Zhao and Y. Wang, Electrochemical performance of mesoporous ZnCo2O4 nanosheets as an electrode material for supercapacitor, Ionics, 2018, 24, 2435–2443 CrossRef CAS.
- P. Sivakumar, P. Nakhanivej, C. J. Raj and H. S. Park, 3D flower-like oxygen-deficient non-stoichiometry zinc cobaltite for high performance hybrid supercapacitors, Int. J. Energy Res., 2021, 45, 10832–10842 CrossRef CAS.
- A. J. C. Mary, S. Thilagavathi and A. C. Bose, Influence of different synthesis approach on ZnCo2O4 nanomaterial and its supercapacitor behavior, AIP Conf. Proc., 2018, 1942, 140042 CrossRef.
- D. He, Y. Gao, Y. Yao, L. Wu, J. Zhang, Z.-H. Huang and M.-X. Wang, Asymmetric Supercapacitors Based on Hierarchically Nanoporous Carbon and ZnCo2O4 From a Single Biometallic Metal-Organic Frameworks (Zn/Co-MOF), Front. Chem., 2020, 8, 719 CrossRef CAS PubMed.
- S. Zhao, X. Yu, H. Chen, K. Tao, Y. Hu and L. Han, Zeolitic imidazolate framework derived ZnCo2O4 hollow tubular nanofibers for long-life supercapacitors, RSC Adv., 2020, 10, 13922–13928 RSC.
- X. Wu, L. Meng, Q. Wang, W. Zhang and Y. Wang, Highly flexible and large areal/volumetric capacitances for asymmetric supercapacitor based on ZnCo2O4 nanorods arrays and polypyrrole on carbon cloth as binder-free electrodes, Mater Lett., 2019, 234, 1–4 CrossRef CAS.
- G. P. Kamble, A. A. Kashale, S. S. Kolekar, I. W. P. Chen, B. R. Sathe and A. V. Ghule, Reflux temperature-dependent zinc cobaltite nanostructures for asymmetric supercapacitors, J. Mater. Sci.: Mater. Electron., 2021, 32, 5859–5869 CrossRef CAS.
- M. S. Javed, A. J. Khan, S. Asim, S. S. A. Shah, T. Najam, S. H. Siyalg, M. F. Tahir, Z. Zhao and W. Mai, Insights to pseudocapacitive charge storage of binary metal-oxide nanobelts decorated activated carbon cloth for highly-flexible hybrid-supercapacitors, J. Energy Storage, 2020, 31, 101602 CrossRef.
- Z. Zhang, X. Zhang, Y. Feng, X. Wang, Q. Sun, D. Yu, W. Tong, X. Zhao and X. Liu, Fabrication of porous ZnCo2O4 nanoribbon arrays on nickel foam for high-performance supercapacitors and lithium-ion batteries, Electrochim. Acta, 2018, 260, 823–829 CrossRef CAS.
- G. Rajeshkhanna and G. Ranga, Rao, High energy density symmetric capacitor using zinc cobaltate flowers grown in situ on Ni foam, Electrochim. Acta, 2018, 261, 265–274 CrossRef CAS.
- G. J. H. Lim, X. Liu, C. Guan and J. Wang, Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage, Electrochim. Acta, 2018, 291, 177–187 CrossRef CAS.
- B. Saravanakumar, T. H. Ko and B.-S. Kim, Rational design of binder-free ZnCo2O4 and Fe2O3 decorated porous 3D Ni as high-performance electrodes for asymmetric supercapacitor, Ceram. Int., 2018, 44, 10635–10645 CrossRef CAS.
- Y. zhou, L. Chen, Y. Jiao, Z. Li and Y. Gao, Controllable fabrication of ZnCo2O4 ultra-thin curved sheets on Ni foam for high-performance asymmetric supercapacitors, Electrochim. Acta, 2019, 299, 388–394 CrossRef CAS.
- X. Li, M. Zhang, L. Wu, Q. Fu and H. Gao, Annealing temperature dependent ZnCo2O4 nanosheet arrays supported on Ni foam for high-performance asymmetric supercapacitor, J. Alloys Compd., 2019, 773, 367–375 CrossRef CAS.
- M. S. Javed, N. Shaheen, S. Hussain, J. Li, S. S. A. Shah, Y. Abbas, M. A. Ahmad, R. Raza and W. Mai, An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra, J. Mater. Chem. A, 2019, 7, 946–957 RSC.
- W. Wang, L. Chen, J. Qi, Y. Sui, Y. He, Q. Meng, F. Wei and Z. Sun, All-solid-state asymmetric supercapacitor based on N-doped activated carbon derived from polyvinylidene fluoride and ZnCo2O4 nanosheet arrays, J. Mater. Sci.: Mater. Electron., 2018, 29, 2120–2130 CrossRef CAS.
- M. Saghafi, S. A. Hosseini, S. Zangeneh, A. H. Moghanian and S. Mohajerzadeh, Ternary nanostructured MZnCo oxides (M = Al, Mg, Cu, Fe, Ni) prepared by hydrothermal method as excellent charge storage devices, Ionics, 2020, 26, 1491–1505 CrossRef CAS.
- Y. A. Kumar, K. D. Kumar and H.-J. Kim, Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application, Electrochim. Acta, 2020, 330, 135261 CrossRef CAS.
- C. Du, E. Han, L. Sun, S. Qiao and L. Li, Template agent for assisting in the synthesis of ZnCo2O4 on Ni foam for high-performance supercapacitors, Ionics, 2020, 26, 383–391 CrossRef CAS.
- L. Cheng, M. Xu, Q. Zhang, G. Li, J. Chen and Y. Lou, NH4F assisted and morphology-controlled fabrication of ZnCo2O4 nanostructures on Ni-foam for enhanced energy storage devices, J. Alloys Compd., 2019, 781, 245–254 CrossRef CAS.
- M. Saghafi and S. Zangeneh, Zn-Co oxide electrodes with excellent capacitive behavior for using supercapacitor application, Curr. Appl. Phys., 2019, 19, 745–755 CrossRef.
- G. P. Kamble, A. A. Kashale, S. S. Dhanayat, S. S. Kolekar and A. V. Ghule, Binder-free synthesis of high-quality nanocrystalline ZnCo2O4 thin film electrodes for supercapacitor application, Bull. Mater. Sci., 2019, 42, 272 CrossRef.
- D. Xie, R.-j Zhong, D. Ren, L.-y Tuo, G.-h Song and F. Hu, Self-assembled ZnCo2O4 micro-urchins as high-performance electrode materials for energy storage device, J. Mater. Sci.: Mater. Electron., 2019, 30, 6439–6447 CrossRef CAS.
- S. Wang, Y. Teng, X. Liu, D. Yu, Y. n Meng, Y. Wu, S. Sun, X. Zhao and X. Liu, Facile synthesis of mesoporous ZnCo2O4 nanowire arrays and nanosheet arrays directly grown on nickel foam for high-performance supercapacitors, Inorg. Chem. Commun., 2019, 101, 16–22 CrossRef CAS.
- H. Yu, H. Zhao, Y. Wu, B. Chen and J. Sun, Electrospun ZnCo2O4/C composite nanofibers with superior electrochemical performance for supercapacitor, J. Phys. Chem. Solids, 2020, 140, 109385 CrossRef CAS.
- H. Li, L. Wang, Y. Guan, Y. Su, J. Mu, H. Che, A. Liu and Z. Guo, Facile solvothermal synthesis of ZnCo2O4/MnO2 nanosheets composite with enhanced electrochemical properties as supercapacitor electrodes, Appl. Phys. A: Mater. Sci. Process., 2018, 124, 485 CrossRef.
- J. Sun, S. Li, X. Han, F. Liao, Y. Zhang, L. Gao, H. Chen and C. Xu, Rapid hydrothermal synthesis of snowflake-like ZnCo2O4/ZnO mesoporous microstructures with excellent electrochemical performances, Ceram. Int., 2019, 45, 12243–12250 CrossRef CAS.
- H. M. Shaikh, N. Siva Kumar and A. Mahmood, Synthesis of carbon nanoparticles/zinc oxide/zinc cobalt oxide composite and its electrochemical properties, Ceram. Int., 2020, 46, 18096–18100 CrossRef CAS.
- N. S. Kumar, V. R. Minnam Reddy, M. Asif, M. Boumaza and K. Mallikarjuna, Reaction time dependent in situ synthesized and morphology altered chrysanthemum to marigold -flower like zinc cobaltite and zinc oxide composite for energy storage devices, J. Taiwan Inst. Chem. Eng., 2020, 116, 92–100 CrossRef CAS.
- C. Huang, C. Hao, Z. Ye, S. Zhou, X. Wang, L. Zhu and J. Wu, In situ growth of ZIF-8-derived ternary ZnO/ZnCo2O4/NiO for high performance asymmetric supercapacitors, Nanoscale, 2019, 11, 10114–10128 RSC.
- L. Wang, Y. Guan, X. Zhao, J. Mu, H. Che, H. Li and Z. Guo, ZnCo2O4@MnCo2O4 heterojunction structured nanosheets for high-performance supercapacitor, J. Mater. Sci.: Mater. Electron., 2018, 29, 5782–5790 CrossRef CAS.
- A. J. Christina Mary, C. I. Sathish, P. S. Murphin Kumar, A. Vinu and A. C. Bose, Fabrication of hybrid supercapacitor device based on NiCo2O4@ZnCo2O4 and the biomass-derived N-doped activated carbon with a honeycomb structure, Electrochim. Acta, 2020, 342, 136062 CrossRef CAS.
- S. Zhou, C. Hao, J. Wang, X. Wang and H. Gao, Metal-organic framework templated synthesis of porous NiCo2O4/ZnCo2O4/Co3O4 hollow polyhedral nanocages and their enhanced pseudocapacitive properties, Chem. Eng. J., 2018, 351, 74–84 CrossRef CAS.
- A. J. C. Mary, C. I. Sathish, A. Vinu and A. C. Bose, Electrochemical Performance of rGO/NiCo2O4@ZnCo2O4 Ternary Composite Material and the Fabrication of an all-Solid-State Supercapacitor Device, Energy Fuels, 2020, 34, 10131–10141 CrossRef CAS.
- Y. Huang, X. Feng, C. Li, Y. Li, X. Chen, X. Gao, C. Chen, Z. Guang and P. Liu, Construction of hydrangea-like ZnCo2O4/Ni3V2O8 hierarchical nanostructures for asymmetric all-solid-state supercapacitors, Ceram. Int., 2019, 45, 15451–15457 CrossRef CAS.
- V. S. Kumbhar and D.-H. Kim, Hierarchical coating of MnO2 nanosheets on ZnCo2O4 nanoflakes for enhanced electrochemical performance of asymmetric supercapacitors, Electrochim. Acta, 2018, 271, 284–296 CrossRef CAS.
- W. Cong, R. Miao, B. Tao and F. Miao, MnO2/ZnCo2O4 with binder-free arrays on nickel foam loaded with graphene as a high performance electrode for advanced asymmetric supercapacitors, RSC Adv., 2019, 9, 32889–32897 RSC.
- B. D. Boruah and A. Misra, Voltage Generation in Optically Sensitive Supercapacitor for Enhanced Performance, ACS Appl. Energy Mater., 2019, 2, 278–286 CrossRef CAS.
- T.-F. Yi, Y.-M. Li, J.-Z. Wu, Y. Xie and S. Luo, Hierarchical mesoporous flower-like ZnCo2O4@NiO nanoflakes grown on nickel foam as high-performance electrodes for supercapacitors, Electrochim. Acta, 2018, 284, 128–141 CrossRef CAS.
- Y. Tong, X. Cheng, D. Qi, B. Chi and W. Zhang, Hybrid ZnCo2O4@Co3S4 Nanowires for High-Performance Asymmetric Supercapacitors, J. Nanoelectron. Optoelectron., 2020, 15, 1552–1558 CrossRef CAS.
- X. Wang, P. Wu, Z. Zhao, L. Sun, Q. Deng, Z. Yin and X. Chen, Construction of flower-like ZnCo2S4/ZnCo2O4 arrays on Ni foam for high-performance asymmetric supercapacitors, J. Mater. Sci.: Mater. Electron., 2020, 31, 4895–4904 CrossRef CAS.
- J. Zhao, H. Li, C. Li, Q. Zhang, J. Sun, X. Wang, J. Guo, L. Xie, J. Xie, B. He, Z. Zhou, C. Lu, W. Lu, G. Zhu and Y. Yao, MOF for template-directed growth of well-oriented nanowire hybrid arrays on carbon nanotube fibers for wearable electronics integrated with triboelectric nanogenerators, Nano Energy, 2018, 45, 420–431 CrossRef CAS.
- X. Jia, X. Wu and B. Liu, Formation of ZnCo2O4@MnO2 core–shell electrode materials for hybrid supercapacitor, Dalton Trans., 2018, 47, 15506–15511 RSC.
- D. S. Patil, A. M. Teli, W. J. Choi, S. A. Pawar, J. C. Shin and H. J. Kim, An all chemical route to design a hybrid battery-type supercapacitor based on ZnCo2O4/CdS composite nanostructures, Curr. Appl. Phys., 2020, 20, 1416–1423 CrossRef.
- D. Yang, Y. Wang, Q. Wang, W. Wang, T. Wei and Y. Sun, Preparation and supercapacitive properties of hierarchical ZnCo2O4@Ni3S2 core/shell nanowire arrays on Ni foam, Mater. Lett., 2018, 213, 222–226 CrossRef CAS.
- H. Xuan, H. Li, J. Gao, Y. Guan, Z. Xie, X. Liang, H. Li, P. Han and Y. Wu, Construction of hierarchical core-shell ZnCo2O4@Ni–Co–S nanosheets with a microsphere structure on nickel foam for high-performance asymmetric supercapacitors, Appl. Surf. Sci., 2020, 513, 145893 CrossRef CAS.
- Y. n Meng, D. Yu, Y. Teng, H. Qi, X. Liu, Y. Wu, X. Zhao and X. Liu, Coating of the NiMoO4 nanosheets on different-morphology ZnCo2O4 nanoarrays on Ni foam and their application in battery-supercapacitor hybrid devices, J. Energy Storage, 2020, 29, 101195 CrossRef.
- J. Wang, S. Wang, Y. Tian, X. Jin and J. Dong, 3D heterogeneous ZnCo2O4@NiMoO4 nanoarrays grown on Ni foam as a binder-free electrode for high-performance energy storage, J. Energy Storage, 2020, 32, 101899 CrossRef.
- C. Chen, S. Wang, X. Luo, W. Gao, G. Huang, Y. Zeng and Z. Zhu, Reduced ZnCo2O4@NiMoO4·H2O heterostructure electrodes with modulating oxygen vacancies for enhanced aqueous asymmetric supercapacitors, J. Power Sources, 2019, 409, 112–122 CrossRef CAS.
- Y. n Meng, D. Yu, Y. Teng, X. Liu and X. Liu, A high-performance electrode based on the ZnCo2O4@CoMoO4 core-shell nanosheet arrays on nickel foam and their application in battery-supercapacitor hybrid device, Electrochim. Acta, 2020, 347, 136278 CrossRef CAS.
- L. Xie, Y. Liu, H. Bai, C. Li, B. Mao, L. Sun and W. Shi, Core-shell structured ZnCo2O4@ZnWO4 nanowire arrays on nickel foam for advanced asymmetric supercapacitors, J. Colloid Interface Sci., 2018, 531, 64–73 CrossRef CAS PubMed.
- L. Ma, Z. Chang, L. Guo, T. Li, G. Li and K. Wang, String-like core-shell ZnCo2O4@NiWO4 nanowire/nanosheet arrays on Ni foam for binder-free supercapacitor electrodes, Ionics, 2020, 26, 2537–2547 CrossRef CAS.
- Y. Anil Kumar, S. Sambasivam, S. Ahmed Hira, K. Zeb, W. Uddin, T. N. V. Krishna, K. Dasha Kumar, I. M. Obaidat and H.-J. Kim, Boosting the energy density of highly efficient flexible hybrid supercapacitors via selective integration of hierarchical nanostructured energy materials, Electrochim. Acta, 2020, 364, 137318 CrossRef.
- X. Han, Y. Yang, J.-J. Zhou, Q. Ma, K. Tao and L. Han, Metal–Organic Framework Templated 3D Hierarchical ZnCo2O4@Ni(OH)2 Core–Shell Nanosheet Arrays for High-Performance Supercapacitors, Chem. – Eur. J., 2018, 24, 18106–18114 CrossRef CAS PubMed.
- X. Bai, D. Cao and H. Zhang, Constructing ZnCo2O4@LDH Core–Shell hierarchical structure for high performance supercapacitor electrodes, Ceram. Int., 2019, 45, 14943–14952 CrossRef CAS.
- M. Li, W. Yang, Y. Huang and Y. Yu, Hierarchical mesoporous Co3O4@ZnCo2O4 hybrid nanowire arrays supported on Ni foam for high-performance asymmetric supercapacitors, Sci. China Mater., 2018, 61, 1167–1176 CrossRef CAS.
- L. Wu, L. Sun, X. Li, Q. Zhang, H. Si, Y. Zhang, K. Wang and Y. Zhang, Mesoporous ZnCo2O4-CNT microflowers as bifunctional material for supercapacitive and lithium energy storage, Appl. Surf. Sci., 2020, 506, 144964 CrossRef CAS.
- J. Bao, Z. Wang, W. Liu, L. Xu, F. Lei, J. Xie, Y. Zhao, Y. Huang, M. Guan and H. Li, ZnCo2O4 ultrathin nanosheets towards the high performance of flexible supercapacitors and bifunctional electrocatalysis, J. Alloys Compd., 2018, 764, 565–573 CrossRef CAS.
- A. Kathalingam, S. Ramesh, H. M. Yadav, J.-H. Choi, H. S. Kim and H.-S. Kim, Nanosheet-like ZnCo2O4@nitrogen doped graphene oxide/polyaniline composite for supercapacitor application: Effect of polyaniline incorporation, J. Alloys Compd., 2020, 830, 154734 CrossRef CAS.
- X. Wei, H. Wu and L. Li, 3D N-doped carbon continuous network supported P-doped ZnCo2O4 nanosheets with rich oxygen vacancies for high-performance asymmetric pseudocapacitor, J. Alloys Compd., 2021, 861, 158544 CrossRef CAS.
- S. J. Patil, D. P. Dubal and D.-W. Lee, Gold nanoparticles decorated rGO-ZnCo2O4 nanocomposite: A promising positive electrode for high performance hybrid supercapacitors, Chem. Eng. J., 2020, 379, 122211 CrossRef CAS.
- J. Bhagwan, G. Nagaraju, B. Ramulu and J. S. Yu, Promotive Effect of MWCNT on ZnCo2O4 Hexagonal Plates and Their Application in Aqueous Asymmetric Supercapacitor, J. Electrochem. Soc., 2019, 166, A217–A224 CrossRef CAS.
- M. Sharma and A. Gaur, Designing of Carbon Nitride Supported ZnCo2O4 Hybrid Electrode for High-Performance Energy Storage Applications, Sci. Rep., 2020, 10, 2035 CrossRef CAS PubMed.
- V. Shanmugavalli, O. V. Saravanan, K. Vishista and R. Saravanan, A study of charge density distribution and enhanced electrochemical properties of zinc cobaltite/polyaniline nanocomposite for supercapacitor application, Ionics, 2019, 25, 4393–4408 CrossRef CAS.
- Z. Gao, L. Zhang, J. Chang, Z. Wang, D. Wu, F. Xu, Y. Guo and K. Jiang, ZnCo2O4-reduced graphene oxide composite with balanced capacitive performance in asymmetric supercapacitors, Appl. Surf. Sci., 2018, 442, 138–147 CrossRef CAS.
- D. Kong, Y. Wang, S. Huang, J. Hu, Y. V. Lim, B. Liu, S. Fan, Y. Shi and H. Y. Yang, 3D self-branched zinc-cobalt Oxide@N-doped carbon hollow nanowall arrays for high-performance asymmetric supercapacitors and oxygen electrocatalysis, Energy Storage Mater., 2019, 23, 653–663 CrossRef.
- M. Yan, F. Jiang, Y. Liu, L. Sun, H. Bai, F. Zhu and W. Shi, Flexible mixed metal oxide hollow spheres/RGO hybrid lamellar films for high performance supercapacitors, Colloids Surf., A, 2021, 612, 125902 CrossRef CAS.
- A. Asghari, S. H. Kazemi and M. Khanmohammadi, Facile and binder-free synthesis of N-doped carbon/ZnCo2O4 hybrid nanostructures on nickel foam for high-performance solid-state asymmetric supercapacitor, J. Mater. Sci.: Mater. Electron., 2020, 31, 4354–4363 CrossRef CAS.
- Z. Wang, S. Lu, G. He, A. Lv, Y. Shen and W. Xu, In situ construction of dual-morphology ZnCo2O4 for high-performance asymmetric supercapacitors, Nanoscale Adv., 2019, 1, 3086–3094 RSC.
- J. Qi, J. Mao, A. Zhang, L. Jiang, Y. Sui, Y. He, Q. Meng, F. Wei and X. Zhang, Facile synthesis of mesoporous ZnCo2O4 nanosheet arrays grown on rGO as binder-free electrode for high-performance asymmetric supercapacitor, J. Mater. Sci., 2018, 53, 16074–16085 CrossRef CAS.
- Y. Lu, L. Wang, M. Chen, Y. Wu, G. Liu, P. Qi, M. Fu, H. Wu and Y. Tang, Rationally designed hierarchical ZnCo2O4/C core-shell nanowire arrays for high performance and stable supercapacitors, J. Alloys Compd., 2021, 876, 160037 CrossRef CAS.
- J. Zhu, Y. Wang, X. Zhang and W. Cai, MOF-derived ZnCo2O4@NiCo2S4@PPy core–shell nanosheets on Ni foam for high-performance supercapacitors, Nanotechnology, 2021, 32, 145404 CrossRef CAS PubMed.
- J. Gao, H. Xuan, Y. Xu, T. Liang, X. Han, J. Yang, P. Han, D. Wang and Y. Du, Interconnected network of zinc-cobalt layered double hydroxide stick onto rGO/nickel foam for high performance asymmetric supercapacitors, Electrochim. Acta, 2018, 286, 92–102 CrossRef CAS.
- P. Chen, C. Yang, P. Gao, X. Chen, Y.-J. Cheng, J. Liu and K. Guo, Distinctive Formation of Bifunctional ZnCoS-rGO 3D Hollow Microsphere Flowers with Excellent Energy Storage Performances, Chem. Mater., 2022, 34, 5896–5911 CrossRef CAS.
- M. Xu, M. Sun, S. u Rehman, K. Ge, X. Hu, H. Ding, J. Liu and H. Bi, One-pot synthesis of CoO–ZnO/rGO supported on Ni foam for high-performance hybrid supercapacitor with greatly enhanced cycling stability, Chin. Chem. Lett., 2021, 32, 2027–2032 CrossRef CAS.
- J. B. Goodenough and K.-S. Park, The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- X. Liu, J.-Q. Huang, Q. Zhang and L. Mai, Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries, Adv. Mater., 2017, 29, 1601759 CrossRef PubMed.
- B. Xu, D. Qian, Z. Wang and Y. S. Meng, Recent progress in cathode materials research for advanced lithium ion batteries, Mater. Sci. Eng., R, 2012, 73, 51–65 CrossRef CAS.
- S. Goriparti, E. Miele, F. De Angelis, E. Di Fabrizio, R. Proietti Zaccaria and C. Capiglia, Review on recent progress of nanostructured anode materials for Li-ion batteries, J. Power Sources, 2014, 257, 421–443 CrossRef CAS.
- J. Zhang, R. Chu, Y. Chen, H. Jiang, Y. Zhang, N. M. Huang and H. Guo, In-situ grown hierarchical ZnCo2O4 nanosheets on nickel foam as binder-free anode for lithium ion batteries, Ceram. Int., 2018, 44, 16219–16226 CrossRef CAS.
- Z. Li, D. Wang, A. Gu, W. Wei, H. Lv, Z. Lou and Q. Zhou, Ethylene glycol combustion strategy towards 3D mesoporous ZnCo2O4 as anodes for Li-ion batteries, Solid State Ionics, 2020, 356, 115461 CrossRef CAS.
- R. A. Adams, V. G. Pol and A. Varma, Tailored Solution Combustion Synthesis of High Performance ZnCo2O4 Anode Materials for Lithium-Ion Batteries, Ind. Eng. Chem. Res., 2017, 56, 7173–7183 CrossRef CAS.
- M. Wang, Y. Huang, Y. Zhu, N. Zhang, J. Zhang, X. Qin and H. Zhang, Synthesis of porous ZnxCo3−xO4 hollow nanoboxes derived from metal-organic frameworks for lithium and sodium storage, Electrochim. Acta, 2020, 335, 135694 CrossRef CAS.
- J. Du, Y. Tang, Y. Wang, P. Shi, J. Fan, Q. Xu and Y. Min, A MOF-derived method to construct well-arranged porous nanosheets for lithium ion batteries, Dalton Trans., 2018, 47, 7571–7577 RSC.
- Y. Song, M. Zhao, Z. Pan, L. Jiang, Y. Jiang, B. Fu, J. Xu and L. Hu, Thermal transformation of ZnCo1.5(OH)4.5Cl0.5·0.45H2O into hexagonal ZnCo2O4 nanosheets for high-performance secondary ion batteries, J. Alloys Compd., 2019, 783, 455–459 CrossRef CAS.
- H. Xu, Y. Zhang, X. Song, X. Kong, T. Ma and H. Wang, Synthesis of porous ZnCo2O4 micro-cube with large tap density and its application in anode for lithium-ion battery, J. Alloys Compd., 2020, 821, 153289 CrossRef CAS.
- L. Wang, M. Zhen, F. Hu, H. Wang, H. Jia and M. Wei, Construction of three-dimensional ZnCo2O4 hierarchical nanocubes for enhanced lithium storage performances, Mater. Lett., 2021, 286, 129231 CrossRef CAS.
- Z. Chen, F. Chen, Y. Wang and Y. Jiang, Facile template-free one-pot fabrication of ZnCo2O4 nanospheres for advanced lithium storage capability, Ionics, 2017, 23, 3323–3328 CrossRef CAS.
- J. Deng, X. Yu, X. Qin, B. Li and F. Kang, Carbon sphere-templated synthesis of porous yolk-shell ZnCo2O4 spheres for high-performance lithium storage, J. Alloys Compd., 2019, 780, 65–71 CrossRef CAS.
- Y. Gu, Y. Xuan, H. Zhang, X. Deng, Y. Sun and L. Wang, A facile route to prepare mixed transition metal oxide yolk–shell microspheres for enhanced lithium storage, Dalton Trans., 2019, 48, 10604–10609 RSC.
- H. Liu and Q. Hu, Novel secondary assembled micro/nano porous spheres ZnCo2O4 with superior electrochemical performances as lithium ion anode material, Nanotechnology, 2018, 29, 325603 CrossRef PubMed.
- D. Xue, F. Xue, X. Lin, F. Zong, J. Zhang and Q. Li, Coordination polymer derived general synthesis of multi-shelled hollow metal oxides for lithium-ion batteries, Nanoscale, 2019, 11, 17478–17484 RSC.
- H. Liu, X. Wang, H. Xu, J. Wang, Q. Ma, W. Yu, Y. Yang, X. Dong, G. Liu and Y. Zhao, Controllable synthesis of nanostructured ZnCo2O4 as high-performance anode materials for lithium-ion batteries, RSC Adv., 2018, 8, 39377–39383 RSC.
- B. Liu, H. Liu, M. Liang, L. Liu, Z. Lv, H. Zhou and H. Guo, Controlled synthesis of hollow octahedral ZnCo2O4 nanocages assembled from ultrathin 2D nanosheets for enhanced lithium storage, Nanoscale, 2017, 9, 17174–17180 RSC.
- S. Cheng, Q. Ru, P. Liu, H. Yan, Z. Shi, X. Hou, S. Su, L. Zhao and F. Chi-Chung, Ling, Micro-emulsion strategy used to prepare soybean oil-tailored 1D porous ZnCo2O4 cuboid morphology providing a durable performance of the anodes of lithium ion batteries, J. Alloys Compd., 2019, 809, 151703 CrossRef CAS.
- J. Deng, X. Yu, X. Qin, B. Liu, Y.-B. He, B. Li and F. Kang, Controlled synthesis of anisotropic hollow ZnCo2O4 octahedrons for high-performance lithium storage, Energy Storage Mater., 2018, 11, 184–190 CrossRef.
- L. Zhang and S. Zhu, Hollow polyhedral ZnCo2O4 anode materials for lithium-ion batteries, Mater. Lett., 2019, 236, 337–341 CrossRef CAS.
- H. Du, K. Huang, W. Dong and B. Geng, A general gelatin-assisted strategy to hierarchical porous transition metal oxides with excellent lithium-ion storage, Electrochim. Acta, 2018, 279, 66–73 CrossRef CAS.
- J. Liu, Y. Xuan, D. G. D. Galpaya, Y. Gu, Z. Lin, S. Zhang, C. Yan, S. Feng and L. Wang, A high-volumetric-capacity and high-areal-capacity ZnCo2O4 anode for Li-ion batteries enabled by a robust biopolymer binder, J. Mater. Chem. A, 2018, 6, 19455–19462 RSC.
- M.-H. Jung, The two-dimensional to three-dimensional transition structures of ZnCo2O4 for the application of lithium-ion batteries, Appl. Surf. Sci., 2018, 427, 293–301 CrossRef CAS.
- C. R. Mariappan, V. Kumar, R. Azmi, L. Esmezjan, S. Indris, M. Bruns and H. Ehrenberg, High electrochemical performance of 3D highly porous Zn0.2Ni0.8Co2O4 microspheres as an electrode material for electrochemical energy storage, CrystEngComm, 2018, 20, 2159–2168 RSC.
- Y. Mo, J. Liu, S. Wang, M. Xiao, S. Ren, D. Han and Y. Meng, Low-Carbon and Nanosheathed ZnCo2O4 Spheroids with Porous Architecture for Boosted Lithium Storage Properties, Research, 2019, 2019, 1354829 CrossRef CAS PubMed.
- D. Darbar, M. R. Anilkumar, V. Rajagopalan, I. Bhattacharya, H. I. Elim, T. Ramakrishnappa, F. I. Ezema, R. Jose and M. V. Reddy, Studies on spinel cobaltites, MCo2O4 (M = Mn, Zn, Fe, Ni and Co) and their functional properties, Ceram. Int., 2018, 44, 4630–4639 CrossRef CAS.
- M. Carbone, Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries, J. Electroanal. Chem., 2018, 815, 151–157 CrossRef CAS.
- S. Guo, J. Liu, Q. Zhang and H. Wang, 3D porous ZnCo2O4/Co3O4 composite grown on carbon cloth as high-performance anode material for lithium-ion battery, Mater. Lett., 2020, 267, 127549 CrossRef CAS.
- F. Li, M. Zheng, Y. You, D. Jiang, H. Yuan, Z. Zhai, W. Zhang, L. Ma and W. Shen, Hierarchical Hollow Bimetal Oxide Microspheres Synthesized through a Recrystallization Mechanism for High-Performance Lithium-Ion Batteries, ChemElectroChem, 2020, 7, 3468–3477 CrossRef CAS.
- W. Song, K. Ji, A. Aguadero, P. R. Shearing, D. J. L. Brett, F. Xie and D. J. Riley, Co3O4 hollow nanospheres doped with ZnCo2O4via thermal vapor mechanism for fast lithium storage, Energy Storage Mater., 2018, 14, 324–334 CrossRef.
- H. Mao, P. Shen, G. Yang, L. Zhao, X. Qiu, H. Wang and Q. Jiang, 3D highly oriented metal foam: a competitive self-supporting anode for high-performance lithium-ion batteries, J. Mater. Sci., 2020, 55, 11462–11476 CrossRef CAS.
- H. Xin, D. Li, L. Shi, M. Ji, Y. Lin, J. Yu, B. Yang, C. Li and C. Zhu, A simple approach to fabricate of Ni-NiCo2O4@ZnCo2O4 yolk-shell nano-tetrahedron composite as high-performance anode material for lithium-ion batteries, Chem. Eng. J., 2018, 341, 601–609 CrossRef CAS.
- L. Wang and Q. Yang, ZnCo2O4 nanoflakes loaded on a Cu-supported Fe2O3-C network as an integrated lithium-ion battery anode, J. Alloys Compd., 2019, 792, 750–758 CrossRef CAS.
- X. Deng, S. Li, J. Wang, D. Nan, J. Dong and J. Liu, Nitrogen-doped zinc/cobalt mixed oxide micro-/nanospheres for high-rate lithium-ion battery anode, J. Mater. Res., 2019, 34, 3204–3211 CrossRef CAS.
- J. Deng, X. Yu, X. Qin, D. Zhou, L. Zhang, H. Duan, F. Kang, B. Li and G. Wang, Co–B Nanoflakes as Multifunctional Bridges in ZnCo2O4 Micro-/Nanospheres for Superior Lithium Storage with Boosted Kinetics and Stability, Adv. Energy Mater., 2019, 9, 1803612 CrossRef.
- L. Zhang, S. Zhu, X. Li, H. Fang, L. Wang, Y. Song and X. Jia, 3D porous ZnCo2O4@NiO on nickel foam as advanced electrodes for lithium storage, Ionics, 2020, 26, 2157–2164 CrossRef CAS.
- J. Yu, Y. Wang, L. Mou, D. Fang, S. Chen and S. Zhang, Nature-Inspired 2D-Mosaic 3D-Gradient Mesoporous Framework: Bimetal Oxide Dual-Composite Strategy toward Ultrastable and High-Capacity Lithium Storage, ACS Nano, 2018, 12, 2035–2047 CrossRef CAS PubMed.
- Z. Zhang, Y. Huang, X. Liu, X. Wang and P. Liu, Yolk-shell structured ZnCo2O4 spheres anchored on reduced graphene oxide with enhance lithium/sodium storage performance, Electrochim. Acta, 2020, 342, 136104 CrossRef CAS.
- H. Ren, W. Wang, S. Woo Joo, Y. Sun and C. Gu, Preparation of ZnCo2O4@reduced graphene oxide nanocomposite for high-capacity Li-ion battery anodes, Mater. Res. Bull., 2019, 111, 34–42 CrossRef CAS.
- Q. Feng, Y. Du, S. Liang and H. Li, Reduced graphene oxide supported quasi-two-dimensional ZnCo2O4 nanosheets for lithium ion batteries with high electrochemical stability, Nanotechnology, 2019, 31, 045402 CrossRef PubMed.
- Q. Ru, Z. Wang, S. Cheng, P. Liu, X. Hou, S. Su and F. C.-C. Ling, Self Assembled Rice Ball-Like ZnCo2O4 Inlaid on rGO as Flexible Anodes with High Lithium Storage Capability and Superior Cycling Stability, Energy Technol., 2018, 6, 1899–1903 CrossRef CAS.
- X. Wang, Q. Chen, P. Zhao and M. Wang, Synthesis of interconnected mesoporous ZnCo2O4 nanosheets on a 3D graphene foam as a binder-free anode for high-performance Li-ion batteries, RSC Adv., 2018, 8, 33717–33727 RSC.
- L. Li, Z. Xie, G. Jiang, Y. Wang, B. Cao and C. Yuan, Efficient Laser-Induced Construction of Oxygen-Vacancy Abundant Nano-ZnCo2O4/Porous Reduced Graphene Oxide Hybrids toward Exceptional Capacitive Lithium Storage, Small, 2020, 16, 2001526 CrossRef CAS PubMed.
- H. Cao, X. Zhou, W. Deng, Z. Ma, Y. Liu and Z. Liu, Layer structured graphene/porous ZnCo2O4 composite film for high performance flexible lithium-ion batteries, Chem. Eng. J., 2018, 343, 654–661 CrossRef CAS.
- Y. Yang, Z. Li, S. Xu, Y. Tang, C.-S. Lee and W. Zhang, Hierarchically nanostructured ZnCo2O4 particles in 3D graphene networks for high-rate and long-life lithium ion batteries, Mater. Today Energy, 2019, 12, 46–52 CrossRef.
- Z. Zhao, G. Tian, V. Trouillet, L. Zhu, J. Zhu, A. Missiul, E. Welter and S. Dsoke, In Operando analysis of the charge storage mechanism in a conversion ZnCo2O4 anode and the application in flexible Li-ion batteries, Inorg. Chem. Front., 2019, 6, 1861–1872 RSC.
- T. Huang, Z. Lou, Y. Lu, R. Li, Y. Jiang, G. Shen and D. Chen, Metal-Organic-Framework-Derived MCo2O4 (M = Mn and Zn) Nanosheet Arrays on Carbon Cloth as Integrated Anodes for Energy Storage Applications, ChemElectroChem, 2019, 6, 5836–5843 CrossRef CAS.
- T. Liu, W. Wang, M. Yi, Q. Chen, C. Xu, D. Cai and H. Zhan, Metal-organic framework derived porous ternary ZnCo2O4 nanoplate arrays grown on carbon cloth as binder-free electrodes for lithium-ion batteries, Chem. Eng. J., 2018, 354, 454–462 CrossRef CAS.
- P. Huang, M. Zhang, J. Kang, H. Feng, Q. Su, G. Du, Y. Yu and B. Xu, Rapid microwave-irradiation synthesis of ZnCo2O4/ZnO nanocrystals/carbon nanotubes composite as anodes for high-performance lithium-ion battery, J. Mater. Sci., 2018, 54, 4154–4167 CrossRef.
- Y. Wang, X. Zhu, D. Liu, H. Tang, G. Luo, K. Tu, Z. Xie, J. Lei, J. Li, X. Li and D. Qu, Synthesis of MOF-74-derived carbon/ZnCo2O4 nanoparticles@CNT-nest hybrid material and its application in lithium ion batteries, J. Appl. Electrochem., 2019, 49, 1103–1112 CrossRef CAS.
- X. Tang, M. Liang, Y. Zhang, W. Sun and Y. Wang, Ultrafine ternary metal oxide particles with carbon nanotubes: a metal–organic-framework-based approach and superior lithium-storage performance, Dalton Trans., 2019, 48, 4413–4419 RSC.
- H. Liang, J. Wu, M. Wang, H. Fan and Y. Zhang, Pseudocapacitance-dominated high-performance and stable lithium-ion batteries from MOF-derived spinel ZnCo2O4/ZnO/C heterostructure anode, Dalton Trans., 2020, 49, 13311–13316 RSC.
- C. Ding, W. Xu, X. Zeng, M. Wang, W. Wang and Z. Qing, Citrate-directed hydrothermal synthesis of ZnCo2O4-in-carbon porous microspheres for highly reliable lithium-ion batteries, Ionics, 2019, 26, 703–710 CrossRef.
- L. Wang, D. Li, J. Zhang, C. Song, H. Xin and X. Qin, Porous flower-like ZnCo2O4 and ZnCo2O4@C composite: a facile controllable synthesis and enhanced electrochemical performance, Ionics, 2020, 26, 4479–4487 CrossRef CAS.
- C. Ding, W. Xu, M. Wang, X. Zeng and W. Wang, Microstructure controlled ZnCo2O4/C microhydrangea nanocomposites as highly reliable anodes for lithium-ion batteries, Int. J. Energy Res., 2019, 44, 977–987 CrossRef.
- H. Li, S. Wang, M. Feng, J. Yang and B. Zhang, MOF-derived ZnCo2O4/C wrapped on carbon fiber as anode materials for structural lithium-ion batteries, Chin. Chem. Lett., 2019, 30, 529–532 CrossRef CAS.
- Z. Dai, Z. Long, R. Li, C. Shi, H. Qiao, K. Wang and K. Liu, Metal–Organic Framework-Structured Porous ZnCo2O4/C Composite Nanofibers for High-Rate Lithium-Ion Batteries, ACS Appl. Energy Mater., 2020, 3, 12378–12384 CrossRef CAS.
- R. Sun, Z. Qin, Z. Li, H. Fan and S. Lu, Binary zinc-cobalt metal-organic framework derived mesoporous ZnCo2O4@NC polyhedron as a high-performance lithium-ion battery anode, Dalton Trans., 2020, 49, 14237–14242 RSC.
- X. Wu, M. Zeng, L. Wang and J. Li, CTAB-assisted synthesis of ZnCo2O4 nanoparticles embedded in N-doped carbon as superior anode materials for lithium-ion battery, J. Alloys Compd., 2019, 780, 897–906 CrossRef CAS.
- W. Zhao, Z. Shi, Y. Qi and J. Cheng, The Carbon-Coated ZnCo2O4 Nanowire Arrays Pyrolyzed from PVA for Enhancing Lithium Storage Capacity, Processes, 2020, 8, 1501 CrossRef CAS.
- Q. Han, X. Zhang, W. Zhang, Y. Li and Z. Zhang, Preparation of multifunctional structural P-CF@ZnCo2O4 composites used as structural anode materials, J. Alloys Compd., 2020, 842, 155743 CrossRef CAS.
- H. Xiao, G. Ma, J. Tan, S. Ru, Z. Ai and C. Wang, Three-dimensional hierarchical ZnCo2O4@C3N4-B nanoflowers as high-performance anode materials for lithium-ion batteries, RSC Adv., 2020, 10, 32609–32615 RSC.
- P. Pan, Y. Hu, K. Wu, Z. Cheng, Z. Shen, L. Jiang, J. Mao, C. Ni, Y. Ge and Z. Wang, Growth of ZnCo2O4 nanocubes on flexible biochar substrate derived from natural silk waste fabric for lithium-ion battery anode, J. Alloys Compd., 2020, 814, 152306 CrossRef CAS.
- H. Li, L. Lv, W. Wang, X. Huang and D. Chen, A network of porous carbon/ZnCo2O4 nanotubes derived from shell-hybridized worm-like micelles for lithium storage, J. Mater. Chem. A, 2019, 7, 22642–22649 RSC.
- J. Deng, X. Yu, J. Tang, L. Zhang, K. Zhang, S. Lin and B. Li, Highly reversible lithium storage in a conversion-type ZnCo2O4 anode promoted by NiCl2−xFx hydrate, J. Mater. Chem. A, 2020, 8, 2356–2363 RSC.
- A. Ghaffar, G. Ali, S. Zawar, M. Hasan, G. M. Mustafa, S. Atiq and S. M. Ramay, Electrochemical performance of Li+ insertion/extraction in Ni-substituted ZnCo2O4 as an emerging highly efficient anode material, RSC Adv., 2020, 10, 28550–28559 RSC.
- C. Liu, B.-H. Chen, W.-R. Liu and J.-G. Duh, Synthesis and theoretical calculations of N-doped ZnCo2O4 anode for lithium-ion anode via gradient pressure-induced processes and theoretical calculations, J. Alloys Compd., 2019, 797, 978–985 CrossRef CAS.
- W.-T. Koo, H.-Y. Jang, C. Kim, J.-W. Jung, J. Y. Cheong and I.-D. Kim, MOF derived ZnCo2O4 porous hollow spheres functionalized with Ag nanoparticles for a long-cycle and high-capacity lithium ion battery anode, J. Mater. Chem. A, 2017, 5, 22717–22725 RSC.
- W. Zhao, Y. Qi, J. Dong, J. Xu, P. Wu and C. Zhang, New insights into the structure-property relation in ZnCo2O4 nanowire and nanosheet arrays, J. Alloys Compd., 2020, 817, 152692 CrossRef CAS.
- X. Cao, Y. Yang and A. Li, Facile Synthesis of Porous ZnCo(2)O(4) Nanosheets and the Superior Electrochemical Properties for Sodium Ion Batteries, Nanomaterials, 2018, 8(6), 377, DOI:10.3390/nano8060377.
- X. Yang, P. Wang, Y. Tang, C. Peng, Y. Lai, J. Li and Z. Zhang, Bimetallic ZIF–derived polyhedron ZnCo2O4 anchored on the reduced graphene oxide as an anode for sodium-ion battery, Ionics, 2019, 25, 2945–2950 CrossRef CAS.
- J. Xu, B. Yan, H. Maleki Kheimeh Sari, Y. Hao, D. Xiong, S. Dou, W. Liu, H. Kou, D. Li and X. Li, Mesoporous ZnCo2O4/rGO nanocomposites enhancing sodium storage, Nanotechnology, 2019, 30, 234005 CrossRef CAS PubMed.
- J. Liu, J. Wu, C. Zhou, P. Zhang, S. Guo, S. Li, Y. Yang, K. Li, L. Chen and M. Wang, Single-phase ZnCo2O4 derived ZnO–CoO mesoporous microspheres encapsulated by nitrogen-doped carbon shell as anode for high-performance lithium-ion batteries, J. Alloys Compd., 2020, 825, 153951 CrossRef CAS.
- T. Huang, Z. Lou, Y. Lu, R. Li, Y. Jiang, G. Shen and D. Chen, Metal-Organic-Framework-Derived MCo2O4 (M = Mn and Zn) Nanosheet Arrays on Carbon Cloth as Integrated Anodes for Energy Storage Applications, ChemElectroChem, 2019, 6, 5836–5843 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- C. N. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Graphene: the new two-dimensional nanomaterial, Angew. Chem., Int. Ed., 2009, 48, 7752–7777 CrossRef CAS PubMed.
- C. N. Rao, K. S. Subrahmanyam, H. S. Ramakrishna Matte, B. Abdulhakeem, A. Govindaraj, B. Das, P. Kumar, A. Ghosh and D. J. Late, A study of the synthetic methods and properties of graphenes, Sci. Technol. Adv. Mater., 2010, 11, 054502 CrossRef CAS PubMed.
- C.-H. Park, F. Giustino, C. D. Spataru, M. L. Cohen and S. G. Louie, Angle-Resolved Photoemission Spectra of Graphene from First-Principles Calculations, Nano Lett., 2009, 9, 4234–4239 CrossRef CAS PubMed.
- S. Sahoo, S.-H. Bae, Y.-S. Lee, J.-M. Lee, J.-M. Ahn, C.-G. Kim and I.-K. Oh, Defect-engineered mesoporous ternary nanoarchitecture of zinc-cobalt-oxide/nitrogen-doped graphene as anode material in lithium ion batteries, Carbon, 2015, 94, 455–463 CrossRef CAS.
- X. Song, X. Li, Z. Bai, B. Yan, D. Xiong, L. Lin, H. Zhao, D. Li and Y. Shao, Rationally-designed configuration of directly-coated Ni3S2/Ni electrode by RGO providing superior sodium storage, Carbon, 2018, 133, 14–22 CrossRef CAS.
- J. M. Gonçalves, P. R. Martins, M. I. da Silva, J. G. Ruiz-Montoya, L. V. Quispe-Garrido and A. Lucio, in Handbook of Energy Materials, ed. R. Gupta, Springer Nature Singapore, Singapore, 2022, pp. 1–46 DOI:10.1007/978-981-16-4480-1_19-1.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Research Development on Sodium-Ion Batteries, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- J. Muldoon, C. B. Bucur and T. Gregory, Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond, Chem. Rev., 2014, 114, 11683–11720 CrossRef CAS PubMed.
- C. Pan, R. Zhang, R. G. Nuzzo and A. A. Gewirth, ZnNixMnxCo2–2xO4 Spinel as a High-Voltage and High-Capacity Cathode Material for Nonaqueous Zn-Ion Batteries, Adv. Energy Mater., 2018, 8, 1800589 CrossRef.
- A. Baby, B. Senthilkumar and P. Barpanda, Low-Cost Rapid Template-Free Synthesis of Nanoscale Zinc Spinels for Energy Storage and Electrocatalytic Applications, ACS Appl. Energy Mater., 2019, 2, 3211–3219 CrossRef CAS.
- K. Shimokawa, T. Atsumi, M. Harada, R. E. Ward, M. Nakayama, Y. Kumagai, F. Oba, N. L. Okamoto, K. Kanamura and T. Ichitsubo, Zinc-based spinel cathode materials for magnesium rechargeable batteries: toward the reversible spinel–rocksalt transition, J. Mater. Chem. A, 2019, 7, 12225–12235 RSC.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Li–O2 and Li–S batteries with high energy storage, Nat. Mater., 2011, 11, 19–29 CrossRef PubMed.
- M. Du, Q. Li, Y. Zhao, C.-S. Liu and H. Pang, A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites, Coord. Chem. Rev., 2020, 416, 213341 CrossRef CAS.
- X. Ji, K. T. Lee and L. F. Nazar, A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- M. Yan, W.-P. Wang, Y.-X. Yin, L.-J. Wan and Y.-G. Guo, Interfacial design for lithium–sulfur batteries: From liquid to solid, EnergyChem, 2019, 1, 100002 CrossRef.
- G. Zhou, Y. Zhao and A. Manthiram, Dual-Confined Flexible Sulfur Cathodes Encapsulated in Nitrogen-Doped Double-Shelled Hollow Carbon Spheres and Wrapped with Graphene for Li–S Batteries, Adv. Energy Mater., 2015, 5, 1402263 CrossRef.
- Q. Sun, B. Xi, J.-Y. Li, H. Mao, X. Ma, J. Liang, J. Feng and S. Xiong, Nitrogen-Doped Graphene-Supported Mixed Transition-Metal Oxide Porous Particles to Confine Polysulfides for Lithium-Sulfur Batteries, Adv. Energy Mater., 2018, 8, 1800595 CrossRef.
- J. S. Yeon, T. H. Park, Y. H. Ko, P. Sivakumar, J. S. Kim, Y. Kim and H. S. Park, 2D spinel ZnCo2O4 microsheet-coated functional separator for promoted redox kinetics and inhibited polysulfide dissolution, J. Energy Chem., 2021, 55, 468–475 CrossRef CAS.
- F. Wu, J. Bai, J. Feng and S. Xiong, Porous mixed metal oxides: design, formation mechanism, and application in lithium-ion batteries, Nanoscale, 2015, 7, 17211–17230 RSC.
- H. Zhang, J. Liu, X. Lin, Y. Zhong, J. Ren, Z. Wang, T. Han and J. Li, A metal organic foam-derived zinc cobalt sulfide with improved binding energies towards polysulfides for lithium–sulfur batteries, Ceram. Int., 2020, 46, 14056–14063 CrossRef CAS.
- M. A. Rahman, X. Wang and C. Wen, High Energy Density Metal-Air Batteries: A Review, J. Electrochem. Soc., 2013, 160, A1759–A1771 CrossRef CAS.
- Q. Wang, L. Shang, R. Shi, X. Zhang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc–air batteries’ cathodes, Nano Energy, 2017, 40, 382–389 CrossRef CAS.
- M. Dong, X. Liu, L. Jiang, Z. Zhu, Y. Shu, S. Chen, Y. Dou, P. Liu, H. Yin and H. Zhao, Cobalt-doped Mn3O4 nanocrystals embedded in graphene nanosheets as a high-performance bifunctional oxygen electrocatalyst for rechargeable Zn–Air batteries, Green Energy Environ., 2020, 5, 499–505 CrossRef.
- Q. Wang, L. Shang, R. Shi, X. Zhang, Y. Zhao, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, NiFe Layered Double Hydroxide Nanoparticles on Co,N-Codoped Carbon Nanoframes as Efficient Bifunctional Catalysts for Rechargeable Zinc–Air Batteries, Adv. Energy Mater., 2017, 7, 1700467 CrossRef.
- Y. Huang, Y. Wang, C. Tang, J. Wang, Q. Zhang, Y. Wang and J. Zhang, Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal–Air Batteries, Adv. Mater., 2019, 31, 1803800 CrossRef PubMed.
- J.-C. Kim, G.-H. Lee, S. Lee, S.-I. Oh, Y. Kang and D.-W. Kim, Tailored Porous ZnCo2O4 Nanofibrous Electrocatalysts for Lithium–Oxygen Batteries, Adv. Mater. Interfaces, 2018, 5, 1701234 CrossRef.
- J. M. Costa, M. P. Clark, A. F. de Almeida Neto and D. G. Ivey, In-situ transformation of electrodeposited W–Co oxide to ZnCo2O4 nanoparticles as an effective bifunctional catalysts in Zn–air batteries, Int. J. Hydrogen Energy, 2020, 45, 16122–16132 CrossRef CAS.
- Z. Mai, W. Duan, K. Wang, Z. Tang and S. Chen, Integrating ZnCo2O4 submicro/nanospheres with CoxSey nanosheets for the oxygen evolution reaction and zinc–air batteries, Sustainable Energy Fuels, 2020, 4, 2184–2191 RSC.
- N. Xu, Q. Nie, J. Liu, H. Huang, J. Qiao and X.-D. Zhou, Insert Zn2+ in Tetrahedral Sites of Bi-metal Zn-Co Spinel Oxides with High Oxygen Catalytic Performance for Liquid and Flexible Zinc-Air Batteries, J. Electrochem. Soc., 2020, 167, 050512 CrossRef CAS.
- L. Yan, Z. Xu, W. Hu, J. Ning, Y. Zhong and Y. Hu, Formation of sandwiched leaf-like CNTs-Co/ZnCo2O4@NC-CNTs nanohybrids for high-power-density rechargeable Zn–air batteries, Nano Energy, 2021, 82, 105710 CrossRef CAS.
- Y.-P. Deng, Y. Jiang, D. Luo, J. Fu, R. Liang, S. Cheng, Z. Bai, Y. Liu, W. Lei, L. Yang, J. Zhu and Z. Chen, Hierarchical Porous Double-Shelled Electrocatalyst with Tailored Lattice Alkalinity toward Bifunctional Oxygen Reactions for Metal–Air Batteries, ACS Energy Lett., 2017, 2, 2706–2712 CrossRef CAS.
- W. Yan, X. Cao, J. Tian, C. Jin, K. Ke and R. Yang, Nitrogen/sulfur dual-doped 3D reduced graphene oxide networks-supported CoFe2O4 with enhanced electrocatalytic activities for oxygen reduction and evolution reactions, Carbon, 2016, 99, 195–202 CrossRef CAS.
- T.-W. Chen, P. Kalimuthu, G. Anushya, S.-M. Chen, R. Ramachandran, V. Mariyappan and D. C. Muthumala, High-Efficiency of Bi-Functional-Based Perovskite Nanocomposite for Oxygen Evolution and Oxygen Reduction Reaction: An Overview, Materials, 2021, 14, 2976, DOI:10.3390/ma14112976.
- J. H. Li, M. Y. Liu, Y. Li, L. Yuan, P. Zhang, Z. Cai, H. Chen and J. L. Zou, ZIF-8@ZIF-67-derived ZnCo2O4@nitrogen–doped carbon/carbon nanotubes wrapped by a carbon layer: a stable oxygen reduction catalyst with a competitive strength in acid media, Mater. Today Energy, 2021, 19, 100574 CrossRef CAS.
- N. Tao, J. Liu, B. Wang, C. Liang, S. Lin, Y. Du, D. Fan, H. Yang, Y. Wang and K. Huang, Hydrothermal two-dimensionalisation to porous ZnCo2O4 nanosheets non-platinum ORR catalyst, Micro Nano Lett., 2019, 14, 665–668 CrossRef CAS.
- S. Chakrabarty, A. Mukherjee, W.-N. Su and S. Basu, Improved bi-functional ORR and OER catalytic activity of reduced graphene oxide supported ZnCo2O4 microsphere, Int. J. Hydrogen Energy, 2019, 44, 1565–1578 CrossRef CAS.
- T. V. M. Sreekanth, P. C. Nagajyothi, K. C. Devarayapalli, J. Shim and K. Yoo, Lilac flower-shaped ZnCo2O4 electrocatalyst for efficient methanol oxidation and oxygen reduction reactions in an alkaline medium, CrystEngComm, 2020, 22, 2849–2858 RSC.
- Y. Liu, H. Jiang, J. Hao, Y. Liu, H. Shen, W. Li and J. Li, Metal–Organic Framework-Derived Reduced Graphene Oxide-Supported ZnO/ZnCo2O4/C Hollow Nanocages as Cathode Catalysts for Aluminum–O2 Batteries, ACS Appl. Mater. Interfaces, 2017, 9, 31841–31852 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S.-Z. Qiao, Molecule-Level g-C3N4 Coordinated Transition Metals as a New Class of Electrocatalysts for Oxygen Electrode Reactions, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef CAS PubMed.
- Y. P. Zhu, T. Y. Ma, M. Jaroniec and S. Z. Qiao, Self-Templating Synthesis of Hollow Co3O4 Microtube Arrays for Highly Efficient Water Electrolysis, Angew. Chem., Int. Ed., 2017, 56, 1324–1328 CrossRef CAS PubMed.
- J. Béjar, L. Álvarez-Contreras, J. Ledesma-García, N. Arjona and L. G. Arriaga, Electrocatalytic evaluation of Co3O4 and NiCo2O4 rosettes-like hierarchical spinel as bifunctional materials for oxygen evolution (OER) and reduction (ORR) reactions in alkaline media, J. Electroanal. Chem., 2019, 847, 113190 CrossRef.
- J. Ge, W. Zhang, J. Tu, T. Xia, S. Chen and G. Xie, Suppressed Jahn–Teller Distortion in MnCo2O4@Ni2P Heterostructures to Promote the Overall Water Splitting, Small, 2020, 16, 2001856 CrossRef CAS PubMed.
- Y. Tan, C. Wu, H. Lin, J. Li, B. Chi, J. Pu and L. Jian, Insight the effect of surface Co cations on the electrocatalytic oxygen evolution properties of cobaltite spinels, Electrochim. Acta, 2014, 121, 183–187 CrossRef CAS.
- T. W. Kim, M. A. Woo, M. Regis and K.-S. Choi, Electrochemical Synthesis of Spinel Type ZnCo2O4 Electrodes for Use as Oxygen Evolution Reaction Catalysts, J. Phys. Chem. Lett., 2014, 5, 2370–2374 CrossRef CAS PubMed.
- K. Xiang, D. Wu, Y. Fan, W. You, D. Zhang, J.-L. Luo and X.-Z. Fu, Enhancing bifunctional electrodes of oxygen vacancy abundant ZnCo2O4 nanosheets for supercapacitor and oxygen evolution, Chem. Eng. J., 2021, 425, 130583 CrossRef CAS.
- S. Sun, Y. Sun, Y. Zhou, J. Shen, D. Mandler, R. Neumann and Z. J. Xu, Switch of the Rate-Determining Step of Water Oxidation by Spin-Selected Electron Transfer in Spinel Oxides, Chem. Mater., 2019, 31, 8106–8111 CrossRef CAS.
- D. Zhang, Z. Wang, J. Li, C. Hu, X. Zhang, B. Jiang, Z. Cao, J. Zhang and R. Zhang, MOF-derived ZnCo2O4 porous micro-rice with enhanced electro-catalytic activity for the oxygen evolution reaction and glucose oxidation, RSC Adv., 2020, 10, 9063–9069 RSC.
- A. Amirzhanova, N. Akmanşen, I. Karakaya and Ö. Dag, Mesoporous MnCo2O4, NiCo2O4, and ZnCo2O4 Thin-Film Electrodes as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions, ACS Appl. Energy Mater., 2021, 4, 2769–2785 CrossRef CAS.
- G. M. Tomboc, F. O. Agyemang and H. Kim, Improved electrocatalytic oxygen evolution reaction properties using PVP modified direct growth Co-based metal oxides electrocatalysts on nickel foam, Electrochim. Acta, 2018, 263, 362–372 CrossRef CAS.
- D. Zhao, M. Dai, H. Liu, X. Zhu and X. Wu, PPy film anchored on ZnCo2O4 nanowires facilitating efficient bifunctional electrocatalysis, Mater. Today Energy, 2021, 20, 100637 CrossRef CAS.
- J. Liu, Y. Xie, Y. Nan, G. Gou, X. Li, Y. Fang, X. Wang, Y. Tang, H. Yang and J. Ma, ZnCo2O4 nanoparticles derived from dual-metal-organic-frameworks embedded in Multiwalled Carbon Nanotubes: a favorable electrocatalyst for the water splitting, Electrochim. Acta, 2017, 257, 233–242 CrossRef CAS.
- M. Dai, H. Liu, D. Zhao, X. Zhu, A. Umar, H. Algarni and X. Wu, Ni Foam Substrates Modified with a ZnCo2O4 Nanowire-Coated Ni(OH)2 Nanosheet Electrode for Hybrid Capacitors and Electrocatalysts, ACS Appl. Nano Mater., 2021, 4, 5461–5468 CrossRef CAS.
- J. Pan, F. Wang, L. Zhang, S. Song and H. Zhang, Clean synthesis of ZnCo2O4@ZnCo-LDHs yolk–shell nanospheres composed of ultra-thin nanosheets with enhanced electrocatalytic properties, Inorg. Chem. Front., 2019, 6, 220–225 RSC.
- R. Que, S. Liu, Y. Yang and Y. Pan, High catalytic performance of core-shell structure ZnCo2O4@NiFe LDH for oxygen evolution reaction, Mater. Lett., 2021, 298, 129982 CrossRef CAS.
- Z. Yu, Y. Bai, N. Zhang, W. Yang, J. Ma, Z. Wang, W. Sun, J. Qiao and K. Sun, Metal-organic framework-derived heterostructured ZnCo2O4@FeOOH hollow polyhedrons for oxygen evolution reaction, J. Alloys Compd., 2020, 832, 155067 CrossRef CAS.
- H. Cheng, C.-Y. Su, Z.-Y. Tan, S.-Z. Tai and Z.-Q. Liu, Interacting ZnCo2O4 and Au nanodots on carbon nanotubes as highly efficient water oxidation electrocatalyst, J. Power Sources, 2017, 357, 1–10 CrossRef CAS.
- T. Xiong, Z. Tan, Y. Mi, Q. Huang, Y. Tan, X. Yin and F. Hu, On-site generated metal organic framework-deriving core/shell ZnCo2O4/ZnO nanoarray for better water oxidation, Nanotechnology, 2019, 30, 495405 CrossRef CAS PubMed.
- C. Zhang, Y. Pan, C. Ouyang, X. Li, X. Quan, Z. Hong and M. Zhi, Template-Free Synthesis of Zinc Cobalt Oxides/Phosphides (Co2P/CoO/ZnCo2O4) Hollow Sub-Micron Boxes as Hydrogen Evolution Reaction Catalysts, ChemistrySelect, 2021, 6, 1685–1691 CrossRef CAS.
- S. Debata, S. Patra, S. Banerjee, R. Madhuri and P. K. Sharma, Controlled hydrothermal synthesis of graphene supported NiCo2O4 coral-like nanostructures: An efficient electrocatalyst for overall water splitting, Appl. Surf. Sci., 2018, 449, 203–212 CrossRef CAS.
- C. Shenghai, S. Liping, K. Fanhao, H. Lihua and Z. Hui, Carbon-coated MnCo2O4 nanowire as bifunctional oxygen catalysts for rechargeable Zn–air batteries, J. Power Sources, 2019, 430, 25–31 CrossRef CAS.
- X. Jia, S. Gao, T. Liu, D. Li, P. Tang and Y. Feng, Controllable Synthesis and Bi-functional Electrocatalytic Performance towards Oxygen Electrode Reactions of Co3O4/N-RGO Composites, Electrochim. Acta, 2017, 226, 104–112 CrossRef CAS.
- A. Rebekah, S. Anantharaj, C. Viswanthan and N. Ponpandian, Zn-substituted MnCo2O4 nanostructure anchored over rGO for boosting the electrocatalytic performance towards methanol oxidation and oxygen evolution reaction (OER), Int. J. Hydrogen Energy, 2020, 45, 14713–14727 CrossRef CAS.
- D. Tang, Y. Han, W. Ji, S. Qiao, X. Zhou, R. Liu, X. Han, H. Huang, Y. Liu and Z. Kang, A high-performance reduced graphene oxide/ZnCo layered double hydroxide electrocatalyst for efficient water oxidation, Dalton Trans., 2014, 43, 15119–15125 RSC.
- J. M. Gonçalves, P. R. Martins, L. Angnes and K. Araki, Recent advances in ternary layered double hydroxide electrocatalysts for the oxygen evolution reaction, New J. Chem., 2020, 44, 9981–9997 RSC.
- J. P. Kumar, S. D. Giri and A. Sarkar, Mesoporous NiO with different morphology: Synthesis, characterization and their evaluation for oxygen evolution reaction, Int. J. Hydrogen Energy, 2018, 43, 15639–15649 CrossRef CAS.
- M. Kashif, M. Fiaz and M. Athar, One-step hydrothermal synthesis of ZnO nanorods as efficient oxygen evolution reaction catalyst, Inorg. Nano-Met. Chem., 2022, 52, 101–107 CAS.
- N. Kim, D. Lim, Y. Choi, S. E. Shim and S.-H. Baeck, Hexagonal β-Ni(OH)2 nanoplates with oxygen vacancies as efficient catalysts for the oxygen evolution reaction, Electrochim. Acta, 2019, 324, 134868 CrossRef CAS.
- X. Du, Z. Yang, Y. Li, Y. Gong and M. Zhao, Controlled synthesis of Ni(OH)2/Ni3S2 hybrid nanosheet arrays as highly active and stable electrocatalysts for water splitting, J. Mater. Chem. A, 2018, 6, 6938–6946 RSC.
- M. I. da Silva, Í. R. Machado, H. E. Toma, K. Araki, L. Angnes and J. M. Gonçalves, Recent progress in water-splitting and supercapacitor electrode materials based on MOF-derived sulfides, J. Mater. Chem. A, 2022, 10, 430–474 RSC.
- L. Zhang, Q. Fan, K. Li, S. Zhang and X. Ma, First-row transition metal oxide oxygen evolution electrocatalysts: regulation strategies and mechanistic understandings, Sustainable Energy Fuels, 2020, 4, 5417–5432 RSC.
- T. G. Ritter, A. H. Phakatkar, M. G. Rasul, M. T. Saray, L. V. Sorokina, T. Shokuhfar, J. M. Gonçalves and R. Shahbazian-Yassar, Electrochemical synthesis of high entropy hydroxides and oxides boosted by hydrogen evolution reaction, Cell Rep. Phys. Sci., 2022, 3, 100847 CrossRef CAS.
- L. Yu, T. Liu, R. Amine, J. Wen, J. Lu and K. Amine, High Nickel and No Cobalt—The Pursuit of Next-Generation Layered Oxide Cathodes, ACS Appl. Mater. Interfaces, 2022, 14(20), 23056–23065, DOI:10.1021/acsami.1c22091.
- J. M. Gonçalves, P. Roberto Martins, D. P. Rocha, T. A. Matias, M. S. Julião, R. A. Abarza Munoz and L. Angnes, Recent trends and perspectives in electrochemical sensors based on MOF-derived materials, J. Mater. Chem. C, 2021, 9(28), 8718–8745, 10.1039/d1tc02025k.
| This journal is © The Royal Society of Chemistry 2022 |