Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair
Hui
Lin
ab,
Liyun
Zhang
ab,
Qiyue
Zhang
ab,
Qiang
Wang
ab,
Xue
Wang
*a and
Guangqi
Yan
 *a
*a
aSchool and Hospital of Stomatology, China Medical University, Shenyang, China. E-mail: yanguangqi_1982@163.com
bLiaoning Provincial Key Laboratory of Oral Diseases, China Medical University, Shenyang, China
First published on 29th September 2023
Abstract
Bioceramics have attracted considerable attention in the field of bone repair because of their excellent osteogenic properties, degradability, and biocompatibility. To resolve issues regarding limited formability, recent studies have introduced 3D printing technology for the fabrication of bioceramic bone repair scaffolds. Nevertheless, the mechanisms by which bioceramics promote bone repair and clinical applications of 3D-printed bioceramic scaffolds remain elusive. This review provides an account of the fabrication methods of 3D-printed degradable bioceramic scaffolds. In addition, the types and characteristics of degradable bioceramics used in clinical and preclinical applications are summarized. We have also highlighted the osteogenic molecular mechanisms in biomaterials with the aim of providing a basis and support for future research on the clinical applications of degradable bioceramic scaffolds. Finally, new developments and potential applications of 3D-printed degradable bioceramic scaffolds are discussed with reference to experimental and theoretical studies.
1 Introduction
Bone tissues exhibit a certain degree of self-healing. For a critical bone defect with an extent of less than 2.5 cm,1 the differentiation of bone progenitor cells into osteoblasts can result in complete bone regeneration.2 However, extreme clinical circumstances, including high-energy injury, degenerative disease, or removal of bone tumors, can change the bone healing capacity, resulting in severe dimensional defects and bone regeneration failure.3 Various bone repair materials have been developed to meet the growing demand for the treatment of critically sized bone defects.Although autografts are considered the gold standard for bone grafts, they have several limitations, including donor site morbidity and limited availability.4,5 Allografts provide a different treatment option for critically sized bone defects, but their applications are restricted owing to significant drawbacks, such as immune response complications, risk of infection, and ethical constraints.5–7 Hence, successful scaffold designs that mimic the structure and composition of natural bone tissue are needed as alternatives to autografts for successful clinical bone repair applications. In recent years, metals, polymers, and bioceramics have been used in preclinical practice. Metals such as titanium exhibit excellent corrosion resistance and mechanical properties. However, titanium also has relatively low osteoinductivity, and an excessive difference in the elastic moduli of bone tissue and titanium exists, which leads to a stress-shielding effect around surgical sites.8 Polymers have superior biocompatibility and biodegradability; however, their clinical applications are limited by poor osseointegration and uncontrollable degradation.9
Currently, bioceramics are widely applied in the field of bone repair as tissue engineering scaffolds due to their excellent biocompatibility, osteoconductive properties, and similarity to natural bone.10,11 These materials usually bind to soft and hard tissues by forming bone-like apatite on their surfaces; therefore, they are considered bioactive.12–15 However, biomaterials have poor mechanical properties compared to natural bone, which limits their use in filling bone defects in non-load-bearing areas, such as oral and maxillofacial areas. Furthermore, because bone defects have a variety of shapes and sizes, customizing existing biomaterials to satisfy the surgical requirements for certain bone defects is difficult.4,16,17
Specific structural features, such as pore size and geometry, can be controlled to a certain extent using conventional scaffold fabrication techniques; however, these features are still difficult to adapt to individualized wounds and precise scaffold structures.18,19 In recent years, 3D-printing technology, as a pioneering manufacturing method, has been intensely studied to address these structural challenges, providing an innovative method to create porous scaffolds with improved bioactive characteristics for bone regeneration.8–10,20,21 The preparation of bioceramic nanoparticles, their blending with other materials, or using 3D-printed customized structures allows for faster degradation of hard-to-degrade bioceramic materials such as hydroxyapatite (HA),22,23 while degradable bioceramic materials such as tricalcium phosphate (TCP) achieve better mechanical properties and a more controlled degradation rate.16,24 Degradable 3D-printed bioceramic scaffolds allow for the microscale structural design of customized scaffolds; when the bioceramic material degrades, a composition similar to that of bone tissue promotes bone regeneration and provides space for the growth of new bone. However, the inherently high brittleness and lack of mechanical properties of bioceramics, as well as the further weakening of the mechanical properties of the scaffold during degradation, make them unsuitable for application in bone defects in load-bearing areas4,20 (Table 1).
| Name | Materials | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| HA: hydroxyapatite; n-HA: nanosized hydroxyapatite; BG: bioactive glass; TCP: tricalcium phosphate; MgP: magnesium phosphate; CS: calcium silicate; PTMC: poly (trimethylene carbonate); PDLLA: poly (D, L-lactic acid); PCL: poly(epsilon-caprolactone); PLA: polylactide; SLA: stereolithography; DLP: digital light processing; SLS: selective laser sintering; FDM: fused deposition modelling. | ||||
| Solvent casting and salt leaching | BG | Porous 3D structure | Interconnected-pore structure | 19 and 25–27 |
| nHA | Able to control pore size and porosity | May lead to solvent residues | ||
| TCP | Irregular shaped pores | |||
| Direct Foaming | BG | Interconnected porosity | Irregular internal structure | 18 and 28 |
| HA | Easy production | Fast pyrolysis | ||
| Low cost | ||||
| Freeze-drying | CS | Preservation of the original physico-chemical and biological properties | Difficult to control porosity | 29–32 |
| HA | Long processing time | |||
| TCP | ||||
| SLA/DLP | HA/PTMC | High accuracy | The viscosity of paste can influence the scaffold's reliability | 33–40 |
| PDLLA/HA | Fine internal structure achieved | A long production time | ||
| TCP | Increased cell survivability achieved | |||
| CS | Material saving | |||
| BG | ||||
| SLS | nHA | Controllable mechanical properties | High processing temperature | 41–43 |
| PCL/HA | No post-processing required | Lower resolution | ||
| CS | ||||
| TCP | ||||
| FDM | PLA/nHA | Low cost | Limited shape and precision | 44–52 |
| PCL/HA | Easy to operate | Bioactive factor degradation and warping of the scaffold | ||
| β-TCP/HA | Controllable pore size and geometry | |||
| PCL/TCP | ||||
| PCL/BG | ||||
| BG | ||||
| CS | ||||
| MgP/SrHPO4 | ||||
| Inkjet printing technology | HA | Low cost | Risk of nozzle clogging | 53–60 |
| BG | Material saving | Limited choice of materials | ||
| BG/HA | High resolution | |||
| Fast printing speed | ||||
During the past five years, significant advances have been made in the preclinical studies of biodegradable bioceramics for bone repair and their clinical applications. Although the clinical application of bioceramics in load-bearing bones is currently infeasible, researchers have found that the mechanical properties of existing biodegradable bioceramics are suitable for application in oral and maxillofacial bones.61 Newly developed 3D-printing strategies and new degradable bioceramics have been used in preclinical trials for bone repair and clinical trials for bone defects, demonstrating that 3D-printed degradable bioceramics have excellent performance as substrate grafts and will have a positive impact on future treatment strategies for bone repair (Fig. 1).
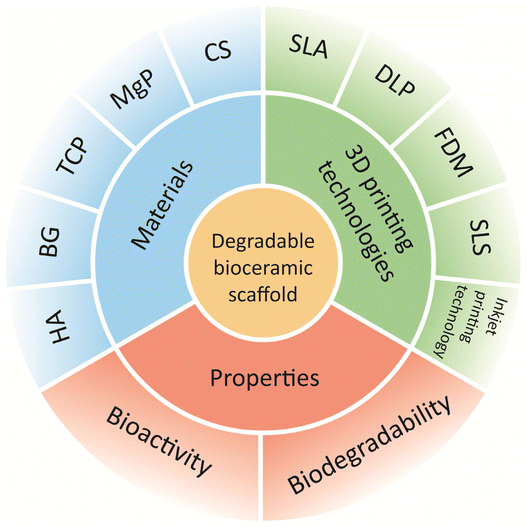 | ||
| Fig. 1 Benefits and applicable 3D printing technologies for 3D-printed degradable bio-ceramic scaffolds in bone repair. | ||
The aim of this review was to summarize the 3D-printed biodegradable bioceramic scaffolds for bone repair, with a focus on the osteogenic molecular mechanisms of the scaffolds and clinical applications in the treatment of bone defects. This review also briefly discusses the existing limitations of 3D printing technology and bioceramic materials as well as the potential future development of degradable bioceramic scaffolds.
2 3D-printing techniques for bioceramic scaffolds
2.1 Stereolithography (SLA) and digital light processing (DLP)
Manufacturing methods based on light irradiation through a vat containing photocurable and bioceramic materials, resulting in plasticity by the photopolymerization of scaffolds directly on the platform, are currently popular for manufacturing bone repair scaffolds. This strategy has translated into two technologies: SLA and DLP.33,62SLA is currently one of the most widely used 3D-printed technologies.8 SLA uses a highly viscous paste as the foundation material and then employs an ultraviolet beam to selectively cure the photosensitive paste. Meanwhile, DLP features a light source that illuminates each layer simultaneously, rather than using point-by-point exposure as in SLA.33,40 The raw materials for SLA and DLP printing scaffolds include resins (composed of monomers, photoinitiators) and additive materials.63 The curing of scaffolds is mainly dependent on the polymerisation reaction of the resin and is not significantly affected by the addition of inorganic substances. Therefore, a broad range of bioceramics, including HA, TCP, calcium silicate (CS), and bioactive glass (BG), is available for SLA and DLP.
The main printing parameters affecting the quality of SLA printed scaffolds are curing depth (CD) and post-cure processing. CD directly affects the efficacy of photocrosslinking within and between the printed scaffold layers. It is widely agreed that CD should reach an appropriate depth. A shallow CD can lead to defects in the scaffold, and unreacted resin may pose a higher risk of cytotoxicity.64 However, a high CD can also lead to over-curing and poor resolution of scaffold.65 Light intensity (LI) and irradiation time (IT) are essential factors in CD.36 The scaffolds need to be post-cured after printing to enhance the mechanical properties of the printed scaffolds, and there is a significant positive correlation between the mechanical properties and the curing time.66 In addition, resin viscosity is also closely related to the mechanical properties and printing accuracy of light-cured scaffolds.67 In order to ensure the precision of scaffolds in 3D printing, the viscosity of the resin needs to be reduced.64 Research demonstrates that the resin viscosity for DLP printing should be less than 10 Pa s to prevent structure breakage68 Adding ceramic components increases the viscosity of the resin and interfere with the light-curing process of the resin, leading to scaffold manufacturing defects.69 Therefore, incorporating bioceramic particles places high demands on the design of SLA or DLP printed bioceramic scaffolds (Table 2).
| Name | Material | Printing parameters | Physico-chemical characteristics | Ref. |
|---|---|---|---|---|
| SLA/DLP | β-TCP | IT: 6 seconds per layer | Significantly promotes angiogenesis and osteogenic activity. | 70 |
| Post-cure processing: dried overnight and sintered at 1150 °C for 3 h | ||||
| HA/poly (trimethylene carbonate) | LI: 180 mW dm−2 | Promotes cell adhesion, proliferation and differentiation. | 36 | |
| IT: 9 seconds per layer | ||||
Post-cure processing: The propylene carbonate diluent was removed using a gradual extraction process with propylene carbonate and ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then replaced with 100% ethanol. 1) and then replaced with 100% ethanol. |
Significantly promotes new bone formation. | |||
| SLS | n-HA | d: 1.0 mm | Scaffold created at 4 J mm−2 exhibit the highest Vickers hardness of 4 GPa and fracture toughness of 1.28 MPa. | 71 |
| LT: 0.2 mm | ||||
| ED: 2.0–5.0 J mm−2 | ||||
| HA/PA12 | d: 0.25 mm | Under the post-treatment at 1400 °C, the PA component disappeared from the samples and the size of the HA microcrystals was reduced to a minimum. | 72 | |
| LP: 8W | ||||
| LS: 380–900 mm s−1 | ||||
| LT: 0.1 mm | ||||
| ED: 11.26–2.1 J mm−2 | ||||
| CS | d: 0.8 mm | The hydrothermally treated CS scaffolds have a nanostructured surface that provides a suitable microenvironment for cell adhesion, spreading, proliferation and differentiation. | 73 | |
| LP: 5.5 W | ||||
| LS: 2 mm s−1 | ||||
| LT: 0.1 mm | ||||
| FDM | PLA/n-HA | Print Temperatures:165 °C | The scaffold is biocompatible and osteoinductive and can induce new bone growth in vivo. | 74 |
| LT: 0.2 mm | ||||
| Printing speed: 60 mm s−1 | ||||
| Diameter of filament: 1.75 ± 0.05 mm | ||||
| PDLGA/BG | Print temperatures:165 °C | Printed scaffolds containing BG have the fastest degradation rate and mechanical properties compatible with cancellous bone | 75 | |
| Diameter of filament: 1.75 mm | ||||
| LT: 0.25 mm | ||||
| Printing speed:22 mm s | ||||
| Inkjet printing technology | BG | Viscosity of ink: 5.5–10 Pa s | The ink retains the 3D structure of the printed filaments. | 76 |
| Nozzle diameter: 100 and 250 μm | The compressive strength of the BG scaffold is comparable to that of human cortical bone. | |||
| n-HA | Nozzle diameter: 210–840 μm | The reduction of the nozzle diameter reduces the number of defects in the material and improves the mechanical properties of the support. | 77 | |
| β-TCP | Viscosity of ink: 0.45 Pa s | β-TCP slurries extruded with a nozzle diameter of 210 μm show excellent viscoelastic behavior. | 78 | |
| Nozzle diameter: 210, 250 and 400 μm |
SLA and DLP can achieve an accuracy of 50 μm.37 The excellent accuracy allows scaffolds made using these processes contain complex internal structures and possess exceptionally high geometric resolution. β-TCP scaffolds with hollow tube structures prepared with DLP significantly promoted angiogenesis and osteogenesis.70 In addition, customized HA composite scaffolds prepared by SLA exhibited acceptable compressive and yield strengths under both walking and running conditions and could meet loading requirements.79 SLA and DLP bioceramic scaffolds offer unprecedented opportunities to design and fabricate high-precision internally structured bone repair scaffolds (Fig. 2).
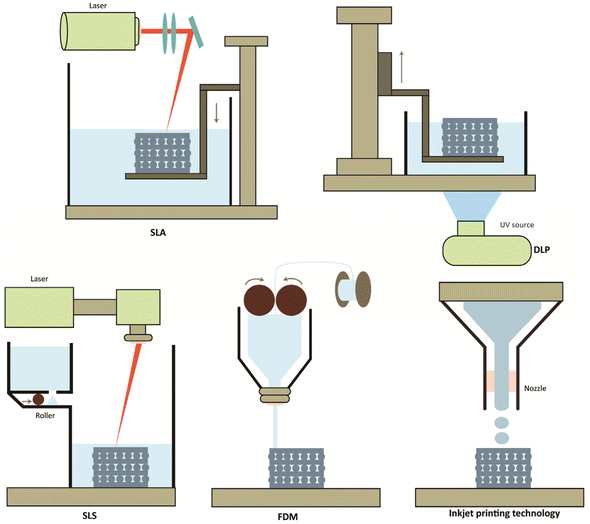 | ||
| Fig. 2 Schematic diagram of the 3D process commonly used for bone engineering with bio-ceramic materials: SLA, DLP, SLS, FDM, and ink printing technology. | ||
2.2 Selective laser sintering (SLS)
SLS is a 3D-printing technique in which a computer-assisted CO2 laser beam is used to selectively sinter thin layers of powder that are diffused from a powder container to the sintering platform via a roll, binding the materials together to form a solid structure.42,43 The formation of an object is enabled by layer-by-layer printing of the powder material and laser irradiation, which provides great flexibility in part design and manufacturing.12,43 However, the mechanical properties and structure of SLS-produced parts are influenced to some extent by the processing strategies and material properties.79 HA, BG and CS are currently popular bioceramics for SLS,73,80,81 but their printing is challenging. Research suggests that too high energy density of SLS may lead to additional phase formation or even decomposition of HA powder.71,82 Furthermore, the high processing temperature of SLS limits the incorporation of biomaterials into the scaffolds to fabricate bioceramic scaffolds that exhibit microstructural inhomogeneity.83 Therefore, the SLS printing parameters need to be improved to obtain bioceramic scaffolds with optimal sintering results. Laser scanning speed (LS) and laser power (LP) are critical printing parameters for SLS. The energy density (ED) of the material is directly affected by them, which in turn impacts its physico-chemical performance.84Previous studies have shown that the average grain size of pure granular n-HA powders sintered at a spot diameter (d) of 1 mm and a powder layer thickness (LT) of 0.2 mm increased when the ED of SLS was increased from 2.0 J mm−2 to 5.0 J mm−2. It is noteworthy that the sintered HA slightly decomposes and undergoes a phase transition at an energy density of 5.0 J mm−2, while the nano-HA powders sintered at 4.0 J mm−2 exhibit the best the mechanical properties that meet the requirements of cancellous bone.71 Recent studies have shown similar results in the fabrication of porous scaffolds using composites of HA and polyamide 12 (PA12) by SLS, where post-sintering treatments eliminated PA12 polymer from the scaffolds and improved the relative density, crystallinity, and microcrystalline size of the parts after treatment.72
The high-precision nature of SLS hints at the potential of SLS for fabricating scaffolds for bone repairs. However, the current variety of printing strategies makes it more difficult to form a consensus on the ideal bone repair scaffold for bone repair. Therefore, it is necessary to develop further regarding printing parameters and material preparation.
2.3 Fused deposition modelling (FDM)
FDM is a 3D-printing technique that enables the extrusion of molten thermoplastic material in the form of continuous filaments from a small nozzle and deposition on a surface at low temperature for rapid curing at the exit.8,52 In recent years, bioceramic materials such as HA, TCP, and BG have also been successfully used in FDM printing. FDM is more dependable and cost-effective when producing customized objects compared to other 3D-printing techniques and can be used to create scaffolds with controllable aperture sizes and morphologies.51,85The resolution and performance of FDM-fabricated scaffolds depend on machine parameters and printing parameters.65 Machine parameters include nozzle diameter, while printing parameters include nozzle temperature, extrusion speed and print thickness.75,86 Researchers have explored the relationship between layer height, number of layers, raster orientation and the mechanical properties of scaffolds. The results show the highest performance when the layer height decreases due to porosity decrease and when the filaments are deposited along the longitudinal direction.87 Moreover, the mechanical properties are lower when the number of layers increases due to increased bonding interfaces between filaments.16,50 As for the printing temperature, setting the level too high could result in the deterioration of cells or biologically active agents and warping of the printed object as it cools.49
Many studies have shown that FDM-based scaffolds have excellent tunable mechanical and biochemical properties, which can be exploited for bone regeneration.17,21,51,85 The degradable composite β-TCP scaffold formed using FDM exhibited excellent biological and mechanical properties, making it a promising candidate for bone tissue engineering.21 However, the viscosity of the molten material and nozzle size limit the printing resolution as well as shape and precision of the objects produced by FDM compared to those produced by SLA. In the future, more studies should be conducted to evaluate the effects of many parameters on printed scaffolds’ bioactivity and mechanical properties.
2.4 Inkjet printing technology
Inkjet printing is a promising method for manufacturing precision parts with customized geometries. The principle of inkjet printing is to use ceramic inks made of a mixture of non-metallic materials, dispersants, binders, and other auxiliary materials.53 Due to the ease of making ceramic inks, bioceramic materials such as TCP and BG are widely used in inkjet printing.76,78 Special computer-controlled nozzles eject the bioceramic ink, the individual cross-section layers are printed. These operations are then repeated in a three-dimensional manner until the required parts of all sections are bonded and stacked.49,54In inkjet printing, the accuracy of the scaffold is mainly affected by the bio-ink viscosity and the nozzle diameter.88 Studies have demonstrated that the viscosity of ink should be low enough (<10 Pa s) to form uniform droplets during the jetting process and have sufficient rheology.89 Meanwhile, nozzle diameter has been found to affect the mechanical properties.77 Recently, inkjet-based printing technologies have been used to prepare high-performance 3D-printed scaffolds. Combined with fast and efficient manufacturing, inkjet printing allows the production of complex-shaped ceramics with precise geometries and decreases material waste.55,56 Studies have shown that pure BG scaffolds or BG composite scaffolds for drug loading not only lead to favorable osteogenic quality but also to a significant increase in cellular alkaline phosphatase (ALP) activity.57 The main limitation of this printing technology is the need for excellent ceramic ink stability and the nozzles to remain unobstructed during the printing process. The ideal ink droplets must have a uniform shape and density, no droplet splash, and no “satellite droplets”.58,59 The non-metallic particles in the ceramic ink must be sufficiently small to avoid clogging of the nozzles during or after the jetting process.54,60
3 3D-printed degradable bioceramic scaffolds
3.1 HA scaffolds
HA, a bioceramic with an inorganic composition close to that of bone, has excellent biocompatibility and osteoconductivity; therefore, HA is frequently employed in clinical practice to repair bone defects.85 Despite the good osteogenic properties of HA scaffolds, their main drawback is that their biodegradation is difficult, which significantly limits the formation of new bone at the scaffold implantation site.4The degradation mechanisms of bioceramic scaffolds implanted in the body include solubility degradation and cell-mediated biodegradation.90 The solubility degradation of a scaffold primarily depends on its physico-chemical properties and structural design. Cells that participate in cell-mediated biodegradation, such as macrophages and osteoclasts, play a key role in determining the fate of bioceramics, especially calcium phosphate materials, as these cells play a vital role in material resorption and osteogenesis.12,14,91 Researchers have progressively investigated degradation strategies for HA scaffolds.
Biodegradable HA scaffolds not only produce molecules to regulate the microenvironment of bone regeneration but also allow new bone to grow inward.10,85,92 The current prevailing modification methods for increasing the biodegradability of HA scaffolds include mixing HA scaffolds with degradable substances or utilizing nanosized hydroxyapatite (n-HA) materials to prepare implant scaffolds.23,51,79 The combination of easily degradable materials with HA has resulted in satisfactory degradation rates.93,94 Owing to the nanoscale particle size (≤100 nm) and extensive surface area of the n-HA material, the n-HA scaffold can lead to relatively rapid degradation and accelerated substitution of the scaffold with bone tissue.23,95,96 In addition, n-HA is hydrophilic and promotes water penetration into the matrix, thereby accelerating scaffold degradation.51 Polylactide (PLA)/n-HA scaffolds with a homogeneous dispersion of n-HA particles prepared by FDM were confirmed to have good biocompatibility and drug loading. Rabbit model experiments showed that this scaffold has great potential for repairing critical bone defects.97 This result is supported by subsequent research studies, which revealed that increasing the HA content of the composite scaffold within an effective concentration range did not significantly affect the mechanical strength of the scaffold but enhanced osteogenesis in vivo.51,98 Applying degradable HA in 3D printing offers improved bioactive properties and more suitable degradation rates for tissue engineering solutions, demonstrating the great potential of degradable HA in applications for bone repair. However, most current research on degradable HA scaffolds is based on preclinical studies. Further investigations and clinical testing are required for the clinical application of HA scaffolds in bone repair.
3.2 BG scaffolds
Professor Hench developed the first BG material in the early 1970s based on a SiO2 (45%)–Na2O(24.5%)–CaO(24.5%)–P2O5(6%) system.8,99,100 BG is a promising material for clinical bone repair because of its excellent mechanical strength and biocompatibility. More importantly, the controlled degradation rate of BG also provides space and favorable factors for new bone formation101 (Table 3).| Material | Compressive strength (MPa) | Young's modulus (GPa) | Dissolution behavior | Ref. |
|---|---|---|---|---|
| HA | 4–60 | 20–55 | Very slow | 91, 92 and 102 |
| BG | 70 | 16.0–16.4 | Moderate | 47 and 101 |
| TCP | 2–17.94 | 1.48 | Moderate | 102 and 103 |
| MP | 5–6 | 11.9–12.1 | Rapid | 14, 104 and 105 |
| CS | 6.08–19.44 | 70.7–75 | Rapid | 106 and 107 |
| Cortical bone | 90–230 | 5–23 | — | 92 |
| Cancellous bone | 2–45 | 0.1–4.5 | — | 92 |
BG does not degrade in vivo via the same mechanism as that of HA materials; BG degrades mainly through dissolution. Partial dissolution of the glass surface occurs first because of ion dissolution, which triggers the formation of a silica-rich layer; finally, an apatite layer forms.108,109 The ions released by the dissolution of the BG surface control the phenotypic transformation of macrophages and their production of cytokines, chemokines, and growth factors, thus creating an ideal microenvironment for implant-site osteogenesis and angiogenesis.110
One of the major advantages of BG scaffolds is the use of hybrid scaffold materials consisting of BG and other materials. The 3D-printed composite scaffolds with different ratios (5, 10, and 20 wt%) of BG had higher hydrophilicity and compressive strength than pure poly(epsilon-caprolactone) (PCL) scaffolds, and the increased hydrophilicity of the scaffolds further enhanced cell adhesion and proliferation.48 Designing a new BG scaffold structure for bone repair is another common method for modifying BG scaffolds. Previous studies demonstrated that altering the scaffold pore geometry and porosity can regulate cell adhesion, proliferation, and differentiation.111 Pores with different sizes, such as macropores (>50 μm) and micropores (<10 μm), can facilitate cell migration and ion exchange between the scaffold and environment, respectively.111,112
Although BG materials show great promise for bone tissue regeneration, most current research on BG is based on preclinical investigations. Only a few clinical studies have reported the clinical application of BG materials, such as 45S5 BG, S53P4 BG, and borate-based BG compositions (13-93B3), in bone repair.113–119 Further investigations are required to understand the potential of 3D-printed BG scaffolds for application in repairing bone defects. Overall, the clinical application of 3D-printed BG scaffolds for bone defects remains limited. Overall, the use of 3D-printed BG scaffolds for bone defects in clinical applications is currently limited. Further translational studies are necessary.
3.3 TCP scaffolds
TCP is a representative bioceramic material that has an inorganic composition similar to that of bone11,120 as well as ideal structural, mechanical, biocompatibility, and osteoinductive qualities.10,121 The TCP degradation process includes both solution degradation and cell-mediated biodegradation. Foam-like TCP scaffolds with concave pores have been found to have a faster degradation rate because more osteoclasts can be formed and collected.122 This result agrees with the opinions of subsequent researchers.91Improving 3D-printed technology and developing new composite materials for applications are central goals for promoting the bone regeneration potential of TCP scaffolds. A PCL/β-TCP cross-scale scaffold created by combining meltelectrowriting and FDM 3D-printed methods provides a stable environment for cell growth in the thick fibers as well as a connecting bridge for cell growth in the thin fibers, allowing cells to form bone in the pores of the scaffold. Furthermore, in vitro experiments revealed that the Ca2+ and PO43− provided by β-TCP lysis in the composite significantly encouraged osteogenic differentiation of the bone marrow mesenchymal stromal cells (BMSCs) on the scaffold, significantly improving its osteogenic performance.46
Recently, the drug-delivery potential of CP scaffolds has attracted considerable attention. A 3D-printed novel drug-loaded porous scaffold allowed for controlled drug loading and release curves and was found to have antimicrobial and bone-regenerative properties with low cytotoxicity.120 TCP scaffolds with dual drug delivery capabilities have also attracted attention. A release system consisting of the rapid release of one drug and sustained release of another improved in vitro angiogenesis and enhanced osteogenic differentiation.123
The biodegradable behaviour of TCP has received increasing attention. However, bone tissue engineering scaffolds built from pure TCP alone have thus far been unable to achieve the desired degradation rates and mechanical qualities necessary for bone regeneration despite their good biocompatibility and osteoconductivity.103 Therefore, creating composite TCP scaffolds with equivalent degradation and bone formation rates as well as other additional features is needed for the regeneration of bone defects.
3.4 Magnesium phosphate (MgP) scaffold
Recently, fully bioresorbable MgP-based bioceramic materials have attracted significant interest. MgP materials are biocompatible and biodegradable, allowing bone healing while delivering magnesium ions to the bone repair microenvironment. MgP can be used as a component in composite implants for bone lifting. Histological investigations have shown that MgP/PCL scaffold are embedded and integrated into newly formed bone, demonstrating that this biphasic ceramic material can be successfully used as a graft substitute.45The in vivo degradation of MgP materials can be broadly divided into dissolution degradation and cell-mediated degradation; however, in recent years, researchers have found the presence of osteoclasts in animal experiments, suggesting that biolysis may occupy a more critical position.124 Overall, the degradation process and osteogenesis of MgP scaffolds in vivo are still poorly understood compared with those of other biodegradable bioceramic materials. Studies have shown that high concentrations of Mg ions are cytotoxic and prevent the formation of new bone.44,124 Recent studies have shown that 3D-printed MgP scaffolds exhibit higher cell activity than TCP in vitro experiments, suggesting a far-reaching future for MgP scaffolds in bone repair applications.14 However, in vivo degradation is a complex process that depends on various factors such as pore size, porosity and side of implantation. In vitro experiments can only be approximated due to the lack of cellular presence. Therefore, future in vivo studies are needed to confirm the experimental results further.
3.5 CS scaffold
CS materials have emerged as promising bone substitute materials. CS ceramic materials possess better mechanical properties and faster degradation rates than those of HA and phosphate-based materials.11,107,125 The in vivo degradation rate of CS materials is similar to those of the bioceramic materials mentioned above and involves both dissolution and cell-mediated degradation processes. However, some researchers have found that dissolution degradation may be more prevalent in vivo given that CS ceramics have better solubility than other potential scaffold materials.126,127In recent years, there has been increasing research on composite scaffolds made from CS doped with other biomaterials, many of which have shown promise for bone repair, good biocompatibility, and excellent degradability.126 Magnesium-doped CS 3D-printed scaffolds exhibited superior mechanical properties to those of pure CS scaffolds. Histological analysis of cranial bone specimens in vivo showed that the CS composite scaffold had a higher osteogenic capacity than the pure CS scaffold at 8 and 12 weeks.128
CS-based ceramics have been shown to have good osteogenic and mechanical properties, similar to those of human bone tissue. However, their faster degradation rate than the regeneration rate of bone tissue may limit their application on bone repair. The researchers prepared ZrO2/CS composite scaffolds using the DLP technique. Their findings indicated that as the CS content increased, the aggregated CS interconnected and formed a stable support structure, increasing the composite scaffolds’ compressive strength. Also, the CS spread area on the scaffold surface increased, and the degradation rate increased.129 Hence, exploit the beneficial effects of the released calcium and silica ions, the design of calcium silicate ceramic materials with controlled degradation may be a future development direction.
4. Immune-inflammatory response to 3D-printed degradable bioceramic scaffolds
4.1 Acute inflammatory stage
In body-implant-mediated immune and osteogenic responses, macrophages have received the most attention because of their plasticity and multiple roles in the overall process.130 During the initial stage of bioceramic scaffold placement at the site of bone trauma, a hematoma forms near the scaffold, and the interaction between blood and scaffold triggers an acute inflammation characterized by neutrophil and macrophage recruitment.131,132 Macrophages are polarized towards the pro-inflammatory phenotype, M1 macrophages, by lipopolysaccharide and interferon-γ (IFN-γ) in the inflammatory microenvironment. Researchers generally believe that M1 macrophages regulate osteoclast activation and kill pathogens by releasing pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-12 (IL-12), thus acting as anti-infective and removing excess matrix.131,133–136 Research claimed that β-TCP can drive macrophage differentiation towards the M1 phenotype, whereas HA drives macrophages more towards the M2 phenotype. The high pro-inflammatory factors secreted by β-TCP-induced M1 macrophages may prolong inflammation and enhance the immune response. In contrast, HA may result in lower levels of inflammation compared to β-TCP, suggesting the superiority of HA in providing a local environment suitable for initiating bone formation.137 A dose-dependent effect of BG was also observed on macrophage polarization. Low concentrations of BG promoted macrophage conversion to the M2 phenotype, whereas high concentrations of BG particles increased the number of the M1 macrophage, thereby slowing wound healing.138 However, in recent years, it has been shown that M1 macrophages not only induce mesenchymal stem cells (MSCs) to form osteoblasts by producing oncostatin M (OSM) and cyclooxygenase-2 (COX-2) but also promote endothelial cells to upregulate genes associated with sprouting angiogenesis,139–141 suggesting that an initial inflammatory response, predominantly by M1 macrophages, is necessary for optimal fracture healing.4.2 Bone formation stage
The phases of bone repair are consecutive but partially overlapping.142 Thus, before the end of the inflammatory phase, the site of degradable bioceramic scaffold placement also initiates the process of cartilage formation and replacement by bone trabeculae. Anti-inflammatory phenotype M2 macrophages are actively involved in the post-inflammatory repair phase at the site of bioceramic scaffold implantation.143 On the one hand, M2 macrophages can secrete platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and related enzymes to enhance early angiogenesis and the coupling between angiogenesis and osteogenesis.144,145 On the other hand, M2 macrophages induce the secretion of transforming growth factor-β (TGF-β) and bone morphogenetic protein-2 (BMP-2), which in turn induces osteogenic Differentiation.146 It has been shown that silica ions produced by CS degradation can directly induce M2 polarization.147 Furthermore, CS ion products significantly enhance the secretion of immunosuppressive factors in hBMSCs, which are activated to stimulate M2 macrophage polarization.148Notably, during the bone repair phase, it is one-sided to assume that M1 macrophages inhibit new osteogenesis while M2 macrophages promote osteogenesis. The interaction between different phenotypes of macrophages and the organism can effectively regulate the behavior of osteogenesis-associated cells, which play a crucial role in bone remodeling. Research showed that M1 macrophages can promote the proliferation and differentiation of osteoclasts through the secretion of TNF-α,149 whereas M2 macrophages are associated with matrix mineralization of BMSCs.150 Therefore, it is possible to achieve optical bone repair by properly regulating macrophage polarization during implanting of a bioceramic scaffold.
5 Bioceramic scaffold-induced pathway modulation for bone repair
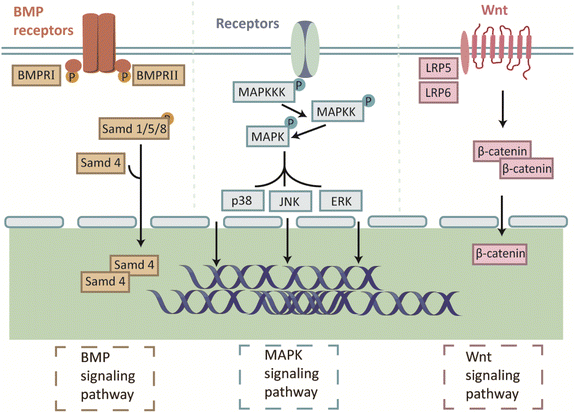
5.1 Mitogen-activated protein kinase (MAPK) signaling pathway
MAPKs are a group of serine/threonine kinases primarily involved in cell proliferation in response to external stimuli. Extracellular stimulation leads to the activation of a signaling cascade consisting of MAPK, MAPK kinase, and MAPK kinase kinase.151 These three kinases are sequentially activated and regulate the downstream pathways. There are three well-studied MAPK osteogenic branching pathways, extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38.152 The JNK and p38 pathways have similar functions in inflammation, apoptosis, and growth, while the ERK pathway mainly regulates cell growth and differentiation.153,154The role of HA in promoting osteogenic differentiation at the defect site during bone repair has been uncovered gradually.90 n-HA was shown to accelerate the expression of osteogenic genes in osteoblast lineage cells compared with micron-sized HA.155,156 The application of n-HA promoted osteopontin (OPN) expression and decreased ALP expression in BMSCs and MC3T3-E1 cells. The ERK signaling pathway mediated these effects by stimulating the expression of the specific signaling proteins fibroblast growth factor receptor substrate 2 (Frs2α) and ERK1/2 through the cell surface, that is, phosphate transporters and fibroblast growth factor (Fgf) receptors, and subsequently stimulated the downstream protein expression of OPN and ALP.157
Many studies have suggested that Mg2+ may be essential to promoting bone regeneration through MAPK signaling pathways, including the ERK1/2 and p38 signaling pathways.158 Researchers found that Mg2+ activates the ERK/MAPK and p38/MAPK pathways, stimulating osteogenic differentiation.159,160 Li et al. also showed that Mg2+ could upregulate Runx2 transcription in BMSCs by targeting the ERK1/2 and p38 MAPK pathways.161 This is consistent with the findings of Lin, who found that Mg2+ selectively activated the ERK/MAPK pathway.159
Researchers have found that a critical characteristic of CS is its tendency to release ions at concentrations that promote osteoblast proliferation and differentiation.107 Further studies found that CS can promote osteogenesis through the MAPK pathway (including the p38, ERK, and JNK subfamilies), especially encouraging the expression of Collagen I (COL-I), a2b1 sub integrin, ALP, and osteocalcin (OCN), and silicon ions increased ERK and p38 activity in a dose-dependent manner.162–165 Interestingly, some researchers have suggested that CS does not affect JNK activity.162 However, a subsequent study showed that CS induced a pro-inflammatory response via a the toll-like receptor 2 (TLR2)-mediated JNK pathway in a mouse RAW 264.7 macrophage line.166
5.2 Bone morphogenetic protein (BMP) signaling pathway
BMPs are members of the TGF-beta superfamily and play an important role in bone formation and stem cell differentiation.167 Two types of BMPs exist: receptor type I (BMPR-I) and type II (BMPR-II). BMPs bind to receptors on the cell membrane and initiate signaling.168 BMPR-II phosphorylates BMPR-I, which in turn phosphorylates the small mother against decapentaplegic (Smad) protein.169 The Smad protein then initiates a series of phosphorylation events in the Smad complex and forms a complex with runt-related transcription factor 2 (Runx2), a key osteogenic transcription factor, controlling osteogenesis-related genes such as osterix (OSX) and OCN to direct transcriptional activity.170,171Bioceramics are thought to activate the BMP signaling pathway. BMSCs cultured with HA showed significantly increased mRNA expression levels of BMP2 and BMP4. Smad1, Smad4, Smad5, and distal-free homologous frame 5 (Dlx5), macromolecules of the Smad signaling pathway downstream of BMP signaling, were also upregulated.172 This result is consistent with that of Wang et al., who found that n-HA facilitates cell adhesion and osteogenic differentiation of BMSC, which can be attributed to the activation of the BMP/Smad signaling pathway.173 Similar osteogenic mechanisms have been identified and β-TCP has been shown to regulate BMP2 expression in macrophages. The released BMP2 binds to BMPR2, thereby activating Smad5. Activated Smad5 forms a complex with Smad4 and translocates to the nucleus. The complex then promotes the expression of Dlx5 and induces the expression of Runx2, leading to osteogenic differentiation of BMSCs.174
CS ceramics have been found to significantly enhance osteoblast migration and differentiation. In vitro and in vivo experiments revealed that CS bioceramics activate BMP signaling pathways and induce downstream cascades of bone morphogenetic proteins (OCN, OPN, and Runx2).175 CS induces the accumulation of phosphorylated Smad1/5.100 Follow-up experiments further revealed that CS mediated migration and osteoblast differentiation. Furthermore, downstream cascades of bone morphogenetic proteins are significantly downregulated by the inhibition of BMP2 activity.176
5.3 Wingless/integrated (Wnt)/β-catenin signaling pathway
The Wnt signaling pathway is important for osteoblast differentiation and bone development in MSCs. In the classical Wnt signaling pathway, Wnt ligands bind to the Frizzled receptor and low-density lipoprotein receptor-related protein 5 (LRP5) and low-density lipoprotein receptor-related protein 6 (LRP6), which prevent the phosphorylation and degradation of β-linked proteins and thus promote osteogenic gene expression.100,177 Degradable bioceramics can activate the classical Wnt/β-catenin signaling pathway by releasing corresponding ions, thereby facilitating bone repair. Activation of the Wnt/β-catenin signaling pathway may occur during the degradation of bio-ceramics containing calcium, phosphate, and silicate ions, thereby regulating the expression of ALP, OPN, COL1, and OCN.178–180A significant increase in cellular β-catenin-related mRNA and protein levels was observed after culturing hBMSCs with strontium-substituted BG. In contrast, samples pretreated with Wnt signaling pathway inhibitors showed complete blockage of β-catenin, dickkopf-1 (DKK1), and Wnt5a expression.181 A more in-depth study was conducted to explore the molecular processes involved in activating the Wnt/β-catenin signaling pathway for osteogenesis using boron-containing mesoporous BG scaffolds. Researchers found that transcription factor 7-like 2, which is located downstream of the Wnt/β-catenin signaling pathway, could induce the upregulation of lipocalin-2 (LCN2) expression in osteoblasts, thereby improving osteogenic differentiation182 (Fig. 3).
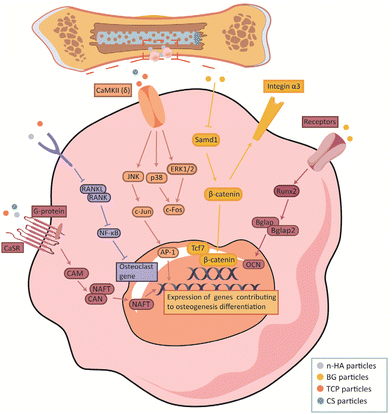
5.4 Other discovered signaling pathways
Recently, new pathways through which BG materials promote bone repair have been identified. Li-MBG reverses high glucose-induced proliferation, migration, and osteogenic differentiation of BMSCs by upregulating Integrin α3 and activating the β-catenin/transcription factor 7 (Tcf7)/cellular communication network factor 4 (Ccn4) signaling pathway.183 Some researchers have found that BG cross-linked to hydrogels can promote osteogenesis by activating the Runx2/Bone gamma-carboxyglutamate protein (Bglap) pathway. However, the mechanism by which this material activates the Runx2 upstream signaling pathway is not clear and further research is needed.184Studies have shown that dissolved calcium and phosphorus ions are non-toxic to the human body and encourage bone cell proliferation and differentiation, enabling bone defect repair.185,186 Therefore, one possible mechanism by which TCP regulates osteogenesis is that TCP can promote osteoblast differentiation via the release of ionic components. Calcium ions have been proposed to activate the calmodulin and calmodulin-dependent kinase II (CaM-CaMKII) pathways, thereby promoting osteoblast differentiation.187 ERK, JNK, p38, and cAMP response element binding Protein (CREB) are important CaMKII (δ) downstream molecules. Calcium ions activate CaMKII (δ), leading to the phosphorylation of ERK, JNK, and p38. ERK and JNK activate activator protein-1 (AP-1) through fos proto-oncogene (c-Fos) and c-Jun, respectively. AP-1 activates the nuclear factor of activated T cells c1 (NFATc1) expression, leading to osteoclast formation in many osteoblasts.188 In addition, calcium ions can also flow into the downstream MAPK, Wnt, and BMP-2 signaling pathways, thereby promoting the expression of osteogenic differentiation-related genes.174,189,190 This result is consistent with subsequent experiments.179 Recently, a novel mechanism by which calcium phosphate promotes osteogenesis was proposed. Ca/P ratios in calcium phosphate can effectively promote the receptor activator of nuclear factor-kappaB ligand (RANKL)-RANK binding and trigger activated nuclear factor-kappaB (NF-κB) signaling, leading to dramatic osteoclast differentiation.191 Researchers have also suggested that another Ca2+/CaN/NFAT signaling pathway plays an important role in bone resorption, formation, and pathophysiology. However, the exact upstream and downstream relationships between this signaling pathway remain unclear and need to be explored further192 (Fig. 4).
6 Applications of 3D-printed degradable bioceramic scaffolds in clinical settings
6.1 Neurosurgery
In addition to properties such as biocompatibility shared by all 3D-printed scaffolds used in the medical field, site-specific requirements must be addressed for grafts applied to different sites. Scaffolds with multiple functions have been developed for different critical defects, such as maxillofacial, cranial, and long bone. Cranial reconstruction is often used to preserve brain tissue, restore normal contours of the skull, and normalize neurological deficits.193 Bioceramics are attractive bone grafts, and their osteoconductivity, osteoinductivity, and ability to form artifacts in high-field MRI at shallow levels have led to their increased use in cranioplasty.194–196 3D-printed bioceramic scaffolds enable precise reconstruction of the skull; thus, the implanted scaffold and defect margins can be well anastomosed during surgery, with satisfactory aesthetic and functional reconstructions and fewer complications observed during the postoperative and follow-up periods.197The use of 3D-printed HA scaffolds in cranial reconstruction has begun to be clinically studied by surgeons.198,199 In a recent study, the process of repairing the cranium with a 3D-printed scaffold was observed during a 6-month follow-up. Further evidence that the mechanical properties of PCL/β-TCP implants are adequate for cranioplasty is that cranial symmetry was attained immediately following surgery and that neither implant dislocation nor fracture was seen during postoperative follow-up.200
6.2 Oral and maxillofacial surgery
Currently, the low degradation rate of bone substitutes and difficulty in matching patient-specific trauma remain clinical and experimental challenges in bone repair.201 As mentioned above, 3D printing techniques have been used to create patient-specific models for surgeons to visualize bone models and surgical guidelines with precise and predictable results.202 Simultaneously, the strength of existing bioceramic materials has been proven suitable by researchers for the fabrication of maxillofacial graft scaffolds. Therefore, the development of biodegradable 3D-printed bioceramic materials is an emerging trend in the clinical treatment of oral and maxillofacial bone defects203 (Table 4).| Sites | Number of patients | Materials | Prognosis | Ref. |
|---|---|---|---|---|
| Mandible | 3 patients (1 females and 2 males) | β-TCP | No post operative complications, and no adverse events | 204 |
| Maxilla, mandible and frontal bone | 20 patients (14 females and 6 males) | α-TCP | One case of infection and three cases of adverse reactions | 61 |
| Maxilla and mandible | 20 patients (14 females and 6 males) | HA/α-TCP | Four cases of infection | 202 |
| Frontal and occipital bone | 7 patients (7 males) | PCL/β-TCP | One case of hematoma | 200 |
| Cranial bone | 25 patients (7 females and 18 males) | HA | No post operative complications, and no adverse events | 198 |
| Cranial bone | 8 patients (2 females and 6 males) | HA | Two cases of pain and one cases of itching | 199 |
| Frontal, temporal and cranial bones | 57 patients (2 females and 6 males) | HA | Five cases of infection and two cases of infection | 205 |
| Alveolar bone | 15 patients (13 females and 2 males) | HA | No post operative complications, and no adverse events | 206 |
| Zygomatic bone | 10 patients (9 females and 1 males) | CaOSiO2-P2O5-B2O3 glass-ceramics (BGS-7) | No post operative complications, and no adverse events | 203 |
| Zygomatic bone | 1 patient (male) | BGS-7 | No post operative complications, and no adverse events | 207 |
Although bioceramic scaffolds have shown good properties in repairing maxillofacial bone defects in animal models,208 few products are currently available in clinical practice. Encouragingly, several combinations of bioceramic and 3D printing technologies appear promising for clinical use, particularly 3D-printed TCP and HA scaffolds that have been successfully tested in humans, suggesting that biodegradable bioceramic scaffolds can be used as reliable bone graft materials to facilitate the bone repair and bone remodeling.206,209 Researchers have demonstrated a digital approach for creating customized clinical-scale CP scaffolds that can be transplanted into alveolar bone defects using 3D imaging, design, and printing. In addition, a strong foundation for future studies to evaluate personalized medical approaches to treat alveolar bone defects was established.210 Further studies have demonstrated promising results in terms of the aesthetics and postoperative prognosis of bioceramic scaffolds.203,204,206,209 Complications such as infection and hematoma have also been reported in some cases, but optimistic results have been achieved with proper preoperative care and attention.205 Although researchers have highlighted the bone regeneration potential of biodegradable scaffolds, follow-up studies addressing the clinical sustainability of the scaffold degradation processes are still lacking.
6.3 Orthopedics
The most typical applications of 3D printing technology in bone regeneration of the extremities and joints include bone defects and fracture nonunions. With the development of new equipment and materials, although bioceramic scaffold applications in orthopedic surgery are not yet clinically available, several studies have been conducted to improve the interaction between grafts and their surroundings by tuning the degradability, bioactivity, and other properties of biomaterials.211 TCP/BG scaffolds with gyroid structures fabricated using DLP effectively induced inward bone growth and fusion in a rabbit femur model.212 3D-printed CS composite scaffolds also induced active bone formation and remodeling in a goat tibia critical bone defect model.213 Numerous preclinical experiments based on animal models have laid the theoretical and practical foundations for the clinical application of bioceramic scaffolds to load-bearing bones. Although only a few studies have reported the use of 3D biodegradable bioceramic scaffolds for upper and lower limb bone repair, they have achieved a low risk of complications and surgical failure in the postoperative period207,214 (Table 5).| Animal | Site | Materials | Fabrication methods | Ref. |
|---|---|---|---|---|
| New Zealand rabbit | The femur condyle | TCP/BG | DLP | 212 |
| The femoral trochanter | PLA/BG | FDM | 215 | |
| The radial diaphysis | TCP | Inkjet printing technology | 216 | |
| Mandible | TCP | Inkjet printing technology | 217 | |
| Tibia | PTMC/HA | SLA | 218 | |
| Belgian white rabbit | Cranial bone | PTMC/TCP | SLA | 218 |
| Sheep | Tibia | Sr-Ca2ZnSi2O7/Al2O3 | Inkjet printing technology | 213 |
| Equine | Tuber coxae bone | MgP/PCL | FDM | 219 |
| Beagle dogs | Mandible | HA/TCP | DLP | 220 |
| The dorsal muscles | HA/TCP | DLP | 221 | |
| Rat | Femoral | TCP | Inkjet printing technology | 222 |
| Femoral | CS | Inkjet printing technology | 223 | |
| Cranial bone | HA/TCP | SLA | 224 | |
| Pig | Tibia | Calcium phosphate | Inkjet printing technology | 225 |
| Mandible | Calcium phosphate | Inkjet printing technology | 225 |
7 Conclusions and perspectives
As discussed above, 3D-printed degradable bioceramic scaffolds have great potential for the treatment of critical bone defects. Bioceramic scaffolds based on advanced 3D printing technologies can be adapted to individual wounds and enable the biomimetic design of bone tissue. In addition, the degradation of the bioceramics effectively promotes cell proliferation and osteogenic differentiation. A series of clinical and preclinical experiments have demonstrated that bioceramics can improve bone repair. However, according to the literature reviewed, the mechanism by which 3D-printed biodegradable bioceramic scaffolds promote bone healing is complex and involves numerous regulatory factors intertwined with signalling networks, and much knowledge remains to be investigated in depth. In addition, current studies about 3D-printed biodegradable bioceramic scaffolds mainly focus on investigating factors and signaling pathways that have been proven to be involved in bone repair. A comprehensive and pioneering understanding of how bioceramic scaffolds promote bone healing remains to be improved. Another fact to consider is that research on biodegradable bioceramic scaffolds is still in the preclinical stage, and there has yet to be a consensus on the printing parameters and feedstocks for using bioceramics for different printing techniques. It is also worth noting that existing clinical reports need more controlled trials and more cases. It is foreseeable that the commercial clinical application of biodegradable bioceramic scaffolds has potential for bone repair, but only after complete testing and clinical trials have demonstrated the safety and efficacy of these scaffolds.Author contributions
G. Y. and Q. W. conceived the concept of this review. H. L. prepared the original draft. L. Z., Q. Z. and X. W. contributed to the review and editing of the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Ning Wang for the revision of our figures. We would like to thank Editage for English language editing.References
- E. H. Schemitsch, J. Orthop. Trauma, 2017, 31, S20–S22 CrossRef PubMed.
- F. A. Shah, M. Jolic, C. Micheletti, O. Omar, B. Norlindh, L. Emanuelsson, H. Engqvist, T. Engstrand, A. Palmquist and P. Thomsen, Bioact. Mater., 2023, 19, 103–114 CrossRef CAS PubMed.
- J. X. Hao, B. S. Bai, Z. Ci, J. C. Tang, G. H. Hu, C. X. Dai, M. Y. Yu, M. Li, W. Zhang, Y. X. Zhang, W. J. Ren, Y. J. Hua and G. D. Zhou, Bioact. Mater., 2022, 14, 97–109 CrossRef CAS PubMed.
- H. S. Sohn and J. K. Oh, Biomater. Res., 2019, 23, 9 CrossRef PubMed.
- R. Fairag, D. H. Rosenzweig, J. L. Ramirez-Garcialuna, M. H. Weber and L. Haglund, ACS Appl. Mater. Interfaces, 2019, 11, 15306–15315 CrossRef CAS PubMed.
- P. Baldwin, D. J. Li, D. A. Auston, H. S. Mir, R. S. Yoon and K. J. Koval, J. Orthop. Trauma, 2019, 33, 203–213 CrossRef PubMed.
- S. Tharakan, S. Khondkar and A. Ilyas, Sensors, 2021, 21, 7477 CrossRef CAS PubMed.
- Z. Wang, Y. C. Wang, J. Q. Yan, K. S. Zhang, F. Lin, L. Xiang, L. F. Deng, Z. P. Guan, W. G. Cui and H. B. Zhang, Adv. Drug Delivery Rev., 2021, 174, 504–534 CrossRef CAS PubMed.
- C. Garot, G. Bettega and C. Picart, Adv. Funct. Mater., 2021, 31, 2006967 CrossRef CAS PubMed.
- X. Liu, Y. Miao, H. Liang, J. Diao, L. Hao, Z. Shi, N. Zhao and Y. Wang, Bioact. Mater., 2022, 12, 120–132 CrossRef CAS PubMed.
- R. G. Ribas, V. M. Schatkoski, T. L. D. Montanheiro, B. R. C. de Menezes, C. Stegemann, D. M. G. Leite and G. P. Thim, Ceram. Int., 2019, 45, 21051–21061 CrossRef CAS.
- M. A. A. Ansari, A. A. Golebiowska, M. Dash, P. Kumar, P. K. Jain, S. P. Nukavarapu, S. Ramakrishna and H. S. Nanda, Biomater. Sci., 2022, 10, 2789–2816 RSC.
- M. Vallet-Regi, Pure Appl. Chem., 2019, 91, 687–706 CrossRef CAS PubMed.
- G. Eugen, M. Claus, S. Anna-Maria, D. Niklas, S. Philipp, E. Andrea, M.-L. Andrea and V. Elke, Bioact. Mater., 2023, 19, 376–391 CrossRef CAS PubMed.
- A. D. Dalgic, A. Z. Alshemary, A. Tezcaner, D. Keskin and Z. Evis, J. Biomater. Appl., 2018, 32, 1392–1405 CrossRef CAS PubMed.
- L. Zhang, G. J. Yang, B. N. Johnson and X. F. Jia, Acta Biomater., 2019, 84, 16–33 CrossRef CAS PubMed.
- L. M. Ulbrich, G. d. S. Balbinot, G. L. Brotto, V. C. B. Leitune, R. M. D. Soares, F. M. Collares and D. Ponzoni, J. Tissue Eng. Regener. Med., 2022, 16, 267–278 CrossRef CAS PubMed.
- F. Dehghani and N. Annabi, Curr. Opin. Biotechnol, 2011, 22, 661–666 CrossRef CAS PubMed.
- P. K. Juan, F. Y. Fan, W. C. Lin, P. B. Liao, C. F. Huang, Y. K. Shen, M. Ruslin and C. H. Lee, Polymers, 2021, 13, 2718 CrossRef CAS PubMed.
- C. X. Lin, Y. C. Wang, Z. Y. Huang, T. T. Wu, W. K. Xu, W. M. Wu and Z. B. Xu, Int. J. Bioprint., 2021, 7, 43–64 Search PubMed.
- X. Ye, Y. Zhang, T. Liu, Z. Chen, W. Chen, Z. Wu, Y. Wang, J. Li, C. Li, T. Jiang, Y. Zhang, H. Wu and X. Xu, Int. J. Biol. Macromol., 2022, 209, 1553–1561 CrossRef CAS PubMed.
- Z. H. Dong, Y. B. Li and Q. Zou, Appl. Surf. Sci., 2009, 255, 6087–6091 CrossRef CAS.
- A. T. Xu, C. Zhou, W. T. Qi and F. M. He, Int. J. Oral Maxillofac. Implants, 2019, 34, 434–442 CrossRef PubMed.
- J. B. Vella, R. P. Trombetta, M. D. Hoffman, J. Inzana, H. Awad and D. S. W. Benoit, J. Biomed. Mater. Res., Part A, 2018, 106, 663–667 CrossRef CAS PubMed.
- S. Biscaia, M. V. Branquinho, R. D. Alvites, R. Fonseca, A. C. Sousa, S. S. Pedrosa, A. R. Caseiro, F. Guedes, T. Patrício, T. Viana, A. Mateus, A. C. Maurício and N. Alves, Int. J. Mol. Sci., 2022, 23, 2318 CrossRef CAS PubMed.
- J. Sonatkar and B. Kandasubramanian, Eur. Polym. J., 2021, 160, 110801 CrossRef CAS.
- X. J. Zhang, W. Chang, P. Lee, Y. H. Wang, M. Yang, J. Li, S. G. Kumbar and X. J. Yu, PLoS One, 2014, 9, e85871 CrossRef PubMed.
- X. M. Shi, A. Nommeots-Nomm, N. M. Todd, A. Devlin-Mullin, H. Geng, P. D. Lee, C. A. Mitchell and J. R. Jones, Appl. Mater. Today, 2020, 20, 100770 CrossRef.
- T. Johnson, R. Bahrampourian, A. Patel and K. Mequanint, Bio-Med. Mater. Eng., 2010, 20, 107–118 CAS.
- M. Xu, H. Li, D. Zhai, J. Chang, S. Chen and C. Wu, J. Mater. Chem. B, 2015, 3, 3799–3809 RSC.
- X. M. Dong, A. Heidari, A. Mansouri, W. S. Hao, M. Dehghani, S. Saber-Samandari, D. Toghraie and A. Khandan, J. Mech. Behav. Biomed. Mater., 2021, 121, 104643 CrossRef CAS PubMed.
- J. Z. Yang, X. Z. Hu, R. Sultana, R. E. Day and P. Ichim, Biomed. Mater., 2015, 10, 045006 CrossRef PubMed.
- M. Dehurtevent, L. Robberecht, J.-C. Hornez, A. Thuault, E. Deveaux and P. Behin, Dent. Mater., 2017, 33, 477–485 CrossRef CAS PubMed.
- L. Breideband, K. N. Waechtershaeuser, L. Hafa, K. Wieland, A. S. Frangakis, E. H. K. Stelzer and F. Pampaloni, Adv. Mater. Technol., 2022, 7, 2200029 CrossRef CAS.
- A. Smirnov, S. Chugunov, A. Kholodkova, M. Isachenkov, A. Tikhonov, O. Dubinin and I. Shishkovsky, Materials, 2022, 15, 960 CrossRef CAS PubMed.
- O. Guillaume, M. A. Geven, C. M. Sprecher, V. A. Stadelmann, D. W. Grijpma, T. T. Tang, L. Qin, Y. Lai, M. Alini, J. D. de Bruijn, H. Yuan, R. G. Richards and D. Eglin, Acta Biomater., 2017, 54, 386–398 CrossRef CAS PubMed.
- S. Derakhshanfar, R. Mbeleck, K. Xu, X. Zhang, W. Zhong and M. Xing, Bioact. Mater., 2018, 3, 144–156 CrossRef PubMed.
- C. Yu, J. Schimelman, P. Wang, K. L. Miller, X. Ma, S. You, J. Guan, B. Sun, W. Zhu and S. Chen, Chem. Rev., 2020, 120, 10748–10796 CrossRef PubMed.
- S. B. Hua, X. Yuan, J. M. Wu, J. Su, L. J. Cheng, W. Zheng, M. Z. Pan, J. Xiao and Y. S. Shi, Ceram. Int., 2022, 48, 3020–3029 CrossRef CAS.
- A. Bagheri and J. Y. Jin, ACS Appl. Polym. Mater., 2019, 1, 593–611 CrossRef CAS.
- S. L. Sing, W. Y. Yeong, F. E. Wiria, B. Y. Tay, Z. Q. Zhao, L. Zhao, Z. L. Tian and S. F. Yang, Rapid Prototyp J., 2017, 23, 611–623 CrossRef.
- Y. Du, H. Liu, Q. Yang, S. Wang, J. Wang, J. Ma, I. Noh, A. G. Mikos and S. Zhang, Biomaterials, 2017, 137, 37–48 CrossRef CAS PubMed.
- F. Lupone, E. Padovano, M. Pietroluongo, S. Giudice, O. Ostrovskaya and C. Badini, eXPRESS Polym. Lett., 2021, 15, 177–192 CrossRef CAS.
- J. Shen, B. Chen, X. Zhai, W. Qiao, S. Wu, X. Liu, Y. Zhao, C. Ruan, H. Pan, P. K. Chu, K. M. C. Cheung and K. W. K. Yeung, Bioact. Mater., 2021, 6, 503–519 CrossRef CAS PubMed.
- B. C. Lei, X. B. Gao, R. Zhang, X. Yi and Q. Zhou, Mater. Des., 2022, 215, 110477 CrossRef CAS.
- Q. F. Wang, W. J. Ye, Z. Y. Ma, W. J. Xie, L. N. Zhong, Y. Wang and Q. Rong, Mater. Sci. Eng., C, 2021, 127, 112197 CrossRef CAS PubMed.
- H. Wang, Z. W. Deng, J. Chen, X. Qi, L. B. Pang, B. C. Lin, Y. T. Y. Adib, N. Miao, D. P. Wang, Y. D. Zhang, J. S. Li and X. Q. Zeng, Int. J. Biol. Sci., 2020, 16, 1821–1832 CrossRef CAS PubMed.
- C. L. Wang, C. Y. Meng, Z. Zhang and Q. S. Zhu, Ceram. Int., 2022, 48, 7491–7499 CrossRef CAS.
- C. M. Gonzalez-Henriquez, M. A. Sarabia-Vallejos and J. Rodriguez-Hernandez, Prog. Polym. Sci., 2019, 94, 57–116 CrossRef CAS.
- S. Singh, S. Ramakrishna and R. Singh, J. Manuf. Process, 2017, 25, 185–200 CrossRef.
- W. Wang, B. Zhang, M. Li, J. Li, C. Zhang, Y. Han, L. Wang, K. Wang, C. Zhou, L. Liu, Y. Fan and X. Zhang, Composites, Part B, 2021, 224, 109192 CrossRef CAS.
- L. Zhao, X. Wang, H. Xiong, K. Zhou and D. Zhang, J. Eur. Ceram. Soc., 2021, 41, 5066–5074 CrossRef CAS.
- J. H. Park, J. Jang, J. S. Lee and D. W. Cho, Tissue Eng. Regener. Med., 2016, 13, 612–621 CrossRef CAS PubMed.
- D. Graf, A. Qazzazie and T. Hanemann, Materials, 2020, 13, 2587 CrossRef CAS PubMed.
- Z. J. Peng, X. D. Luo, Z. P. Xie, D. An and M. M. Yang, Ceram. Int., 2018, 44, 16766–16772 CrossRef CAS.
- X. Wang, M. Zhang, L. Zhang, J. Xu, X. Xiao and X. Zhang, Mater. Today Commun., 2022, 31, 103263 CrossRef CAS.
- G. F. Gao and X. F. Cui, Biotechnol. Lett., 2016, 38, 203–211 CrossRef CAS PubMed.
- M. A. Shah, D. G. Lee, B. Y. Lee and S. Hur, IEEE Access, 2021, 9, 140079–140102 Search PubMed.
- J. Chang, M. Chi, T. Shen and Z. Liang, J. Adhes. Sci. Technol., 2020, 34, 1128–1143 CAS.
- H. C. Ji, X. J. Zhang, W. C. Pei, Y. G. Li, L. Zheng, X. M. Ye and Y. H. Lu, Cailiao Gongcheng, 2018, 46, 19–28 Search PubMed.
- H. Saijo, Y. Fujihara, Y. Kanno, K. Hoshi, A. Hikita, U.-i. Chung and T. Takato, Regen. Ther., 2016, 5, 72–78 CrossRef PubMed.
- D. Dong, H. J. Su, X. Li, G. R. Fan, D. Zhao, Z. L. Shen, Y. Liu, Y. N. Guo, C. B. Yang, L. Liu and H. Z. Fu, J. Manuf. Process, 2022, 81, 433–443 CrossRef.
- A. J. Guerra, H. Lara-Padilla, M. L. Becker, C. A. Rodriguez and D. Dean, Curr. Drug Targets, 2019, 20, 823–838 CrossRef CAS PubMed.
- Y. Luo, G. Le Fer, D. Dean and M. L. Becker, Biomacromolecules, 2019, 20, 1699–1708 CrossRef CAS PubMed.
- A. Kafle, E. Luis, R. Silwal, H. M. Pan, P. L. Shrestha and A. K. Bastola, Polymers, 2021, 13, 3101 CrossRef CAS PubMed.
- I. K. Cingesar, M. P. Markovic and D. Vrsaljko, Addit. Manuf., 2022, 55, 102813 CAS.
- A. J. Guerra, J. Lammel-Lindemann, A. Katko, A. Kleinfehn, C. A. Rodriguez, L. H. Catalani, M. L. Becker, J. Ciurana and D. Dean, Acta Biomater., 2019, 97, 154–161 CrossRef CAS PubMed.
- T. Zandrini, S. Florczak, R. Levato and A. Ovsianikov, Trends Biotechnol., 2023, 41, 604–614 CrossRef CAS PubMed.
- Q. Lian, F. Yang, H. Xin and D. Li, Ceram. Int., 2017, 43, 14956–14961 CrossRef CAS.
- Y. Tian, H. Ma, X. Yu, B. Feng, Z. Yang, W. Zhang and C. Wu, Biomed. Mater., 2023, 18, 034102 CrossRef PubMed.
- C. J. Shuai, P. Feng, C. D. Cao and S. P. Peng, Biotechnol. Bioprocess Eng., 2013, 18, 520–527 CrossRef CAS.
- A. Alahnoori, M. Badrossamay and E. Foroozmehr, Mater. Chem. Phys., 2023, 296, 127316 CrossRef CAS.
- C. J. Shuai, H. Sun, C. D. Gao, P. Feng, W. Guo, W. J. Yang, H. Xu, Q. Li, Y. W. Yang and S. P. Peng, Appl. Surf. Sci., 2018, 455, 1150–1160 CrossRef CAS.
- W. Z. Wang, B. Q. Zhang, L. H. Zhao, M. X. Li, Y. L. Han, L. Wang, Z. D. Zhang, J. Li, C. C. Zhou and L. Liu, Nanotechnol. Rev., 2021, 10, 1359–1373 CrossRef CAS.
- R. Han, F. Buchanan, L. Ford, M. Julius and P. J. Walsh, Mater. Sci. Eng., C, 2021, 120, 111755 CrossRef CAS PubMed.
- Q. Fu, E. Saiz and A. P. Tomsia, Acta Biomater., 2011, 7, 3547–3554 CrossRef CAS PubMed.
- D. Mondal and T. L. Willett, J. Mech. Behav. Biomed. Mater., 2020, 104, 103653 CrossRef CAS PubMed.
- J. J. Diao, J. OuYang, T. Deng, X. Liu, Y. T. Feng, N. R. Zhao, C. B. Mao and Y. J. Wang, Adv. Healthcare Mater., 2018, 7, e1800441 CrossRef PubMed.
- M. Ramu, M. Ananthasubramanian, T. Kumaresan, R. Gandhinathan and S. Jothi, Bio-Med. Mater. Eng., 2018, 29, 739–755 CAS.
- H. Schappo, G. V. Salmoria, A. Magnaudeix, A. Dumur, E. Renaudie, K. Giry, C. Damia and D. Hotza, Powder Technol., 2022, 412, 117966 CrossRef CAS.
- S. F. S. Shirazi, S. Gharehkhani, M. Mehrali, H. Yarmand, H. S. C. Metselaar, N. A. Kadri and N. A. A. Osman, Sci. Technol. Adv. Mater., 2015, 16, 033502 CrossRef PubMed.
- N. Kamboj, A. Ressler and I. Hussainova, Materials, 2021, 14, 5338 CrossRef CAS PubMed.
- S. Chaudhary, S. K. Avinashi, J. T. D. Rao and C. Gautam, ACS Biomater. Sci. Eng., 2023, 9, 3987–4019 CrossRef CAS PubMed.
- K. Senthilkumaran, P. M. Pandey and P. V. M. Rao, Mater. Des., 2009, 30, 2946–2954 CrossRef CAS.
- H. Park, J. Ryu, S. Jung, H. Park, H. Oh and M. Kook, Materials, 2022, 15, 827 CrossRef CAS PubMed.
- B. Q. Zhang, L. Wang, P. Song, X. Pei, H. Sun, L. A. Wu, C. C. Zhou, K. F. Wang, Y. J. Fan and X. D. Zhang, Mater. Des., 2021, 201, 109490 CrossRef CAS.
- S. Garzon-Hernandez, A. Arias and D. Garcia-Gonzalez, Composites, Part B, 2020, 201, 108373 CrossRef CAS.
- B. Derby, J. Eur. Ceram. Soc., 2011, 31, 2543–2550 CrossRef CAS.
- C. F. Marques, G. S. Diogo, S. Pina, J. M. Oliveira, T. H. Silva and R. L. Reis, J. Mater. Sci.: Mater. Med., 2019, 30, 32 CrossRef CAS PubMed.
- K. B. Devi, B. Tripathy, P. N. Kumta, S. K. Nandi and M. Roy, ACS Biomater. Sci. Eng., 2018, 4, 2126–2133 CrossRef CAS PubMed.
- M. S. Attia, H. M. Mohammed, M. G. Attia, M. A. Hamid and E. A. Shoeriabah, J. Periodontol., 2019, 90, 281–287 CrossRef CAS PubMed.
- Z. B. Liu, H. X. Liang, T. S. Shi, D. Q. Xie, R. Y. Chen, X. Han, L. D. Shen, C. J. Wang and Z. J. Tian, Ceram. Int., 2019, 45, 11079–11086 CrossRef CAS.
- H. Y. Chang, W. H. Tuan and P. L. Lai, Mater. Sci. Eng., C, 2021, 118, 111421 CrossRef CAS PubMed.
- H. L. Li, L. L. Li, Y. Q. He, W. Mao, H. F. Ni, A. L. Yang, F. Z. Lyu and Y. H. Dong, Stem Cells Int., 2021, 2021, 8359582 Search PubMed.
- Y. R. Cai, Y. K. Liu, W. Q. Yan, Q. H. Hu, J. H. Tao, M. Zhang, Z. L. Shi and R. K. Tang, J. Mater. Chem., 2007, 17, 3780–3787 RSC.
- G. Balasundaram, M. Sato and T. J. Webster, Biomaterials, 2006, 27, 2798–2805 CrossRef CAS PubMed.
- X. B. Chen, C. X. Gao, J. W. Jiang, Y. P. Wu, P. Z. Zhu and G. Chen, Biomed. Mater., 2019, 14, 065003 CrossRef CAS PubMed.
- Z. Geng, X. P. Li, L. L. Ji, Z. Y. Li, S. L. Zhu, Z. D. Cui, J. Wang, J. Y. Cui, X. J. Yang and C. S. Liu, J. Mater. Sci. Technol., 2021, 79, 35–45 CrossRef CAS.
- O. Demir-Oguz, A. R. Boccaccini and D. Loca, Bioact. Mater., 2023, 19, 217–236 CrossRef CAS PubMed.
- S. H. Rao, B. Harini, R. P. K. Shadamarshan, K. Balagangadharan and N. Selvamurugan, Int. J. Biol. Macromol., 2018, 110, 88–96 CrossRef CAS PubMed.
- Z. Fan, J. Wang, F. Liu, Y. Nie, L. Ren and B. Liu, Colloids Surf., B, 2016, 145, 438–446 CrossRef CAS PubMed.
- H. Jodati, B. Yilmaz and Z. Evis, Ceram. Int., 2020, 46, 15725–15739 CrossRef CAS.
- E. B. Montufar, K. Slámečka, M. Casas-Luna, P. Skalka, E. Ramírez-Cedillo, M. Zbončák, J. Kaiser and L. Čelko, J. Eur. Ceram. Soc., 2020, 40, 4923–4931 CrossRef CAS.
- F. P. He, X. Y. Yuan, T. L. Lu, Y. Wang, S. H. Feng, X. T. Shi, L. Wang, J. D. Ye and H. Yang, J. Mater. Chem. B, 2022, 10, 4040–4047 RSC.
- Y. X. Li, W. Liao, T. Taghvaee, C. L. Wu, H. Y. Ma and N. Leventis, Mater. Lett., 2019, 237, 274–277 CrossRef CAS.
- F. S. Shirazi, M. Mehrali, A. A. Oshkour, H. S. Metselaar, N. A. Kadri and N. A. A. Osman, J. Mech. Behav. Biomed. Mater., 2014, 30, 168–175 CrossRef CAS PubMed.
- P. Srinath, P. A. Azeem and K. V. Reddy, Int. J. Appl. Ceram. Technol., 2020, 17, 2450–2464 CrossRef CAS.
- J. Bejarano, A. R. Boccaccini, C. Covarrubias and H. Palza, Materials, 2020, 13, 2908 CrossRef CAS PubMed.
- J. R. Jones, Acta Biomater., 2013, 9, 4457–4486 CrossRef CAS PubMed.
- C. Zhao, W. Liu, M. Zhu, C. Wu and Y. Zhu, Bioact. Mater., 2022, 18, 383–398 CrossRef CAS PubMed.
- K. C. R. Kolan, Y.-W. Huang, J. A. Semon and M. C. Leu, Int. J. Bioprint., 2020, 6, 274 CrossRef CAS PubMed.
- J. Zhang, W. Wei, L. L. Yang, Y. K. Pan, X. H. Wang, T. L. Wang, S. C. Tang, Y. Yao, H. Hong and J. Wei, Colloids Surf., B, 2018, 164, 347–357 CrossRef CAS PubMed.
- G. S. Polymeris, V. Giannoulatou, A. Kyriakidou, I. K. Sfampa, G. S. Theodorou, E. Sahiner, N. Meric, G. Kitis and K. M. Paraskevopoulos, Mater. Sci. Eng., C, 2017, 70, 673–680 CrossRef CAS PubMed.
- G. Gomez and L. E. Westerlund, J Spine Surg., 2021, 7, 124–131 CrossRef PubMed.
- K. E. Kojima, E. S. F. B. de Andrade, M. C. Leonhardt, V. C. de Carvalho, P. R. D. de Oliveira, A. Lima, P. R. D. Reis and J. D. S. Silva, Injury, 2021, 52(Suppl 3), S23–S28 CrossRef PubMed.
- D. G. Armstrong, D. P. Orgill, R. D. Galiano, P. M. Glat, L. A. DiDomenico, M. J. Carter and C. M. Zelen, Int Wound J., 2022, 19, 791–801 CrossRef PubMed.
- A. C. Profeta and G. M. Prucher, Open Dent. J., 2017, 11, 164–170 CrossRef CAS PubMed.
- R. S. Pereira, J. D. Menezes, J. P. Bonardi, G. L. Griza, R. Okamoto and E. Hochuli-Vieira, Int. J. Oral Maxillofac. Surg., 2018, 47, 665–671 CrossRef CAS PubMed.
- P. Stoor, S. Apajalahti and R. Kontio, J. Craniofac. Surg., 2017, 28, 1197–1205 CrossRef PubMed.
- H. Sun, C. Hu, C. C. Zhou, L. N. Wu, J. X. Sun, X. D. Zhou, F. Xing, C. Long, Q. Q. Kong, J. Liang, Y. Fan and X. Zhang, Mater. Des., 2020, 189, 108540 CrossRef CAS.
- I. Uiiah, L. Cao, W. Cui, Q. Xu, R. Yang, K. L. Tang and X. Zhang, J. Mater. Sci. Technol., 2021, 88, 99–108 CrossRef.
- A. Barba, Y. Maazouz, A. Diez-Escudero, K. Rappe, M. Espanol, E. B. Montufar, C. Ohman-Magi, C. Persson, P. Fontecha, M. C. Manzanares, J. Franch and M. P. Ginebra, Acta Biomater., 2018, 79, 135–147 CrossRef CAS PubMed.
- C. Wang, J. H. Lai, K. Li, S. K. Zhu, B. H. Lu, J. Liu, Y. J. Tang and Y. Wei, Bioact. Mater., 2021, 6, 137–145 CrossRef CAS PubMed.
- M. Nabiyouni, T. Bruckner, H. Zhou, U. Gbureck and S. B. Bhaduri, Acta Biomater., 2018, 66, 23–43 CrossRef CAS PubMed.
- A. Zheng, L. Y. Cao, Y. Liu, J. N. Wu, D. L. Zeng, L. W. Hu, X. K. Zhang and X. Q. Jiang, Carbohydr. Polym., 2018, 199, 244–255 CrossRef CAS PubMed.
- H. Zhang, C. Jiao, Z. He, M. Ge, Z. Tian, C. Wang, Z. Wei, L. Shen and H. Liang, Ceram. Interfaces, 2021, 47, 27032–27041 CrossRef CAS.
- C. Wang, Y. Xue, K. Lin, J. Lu, J. Chang and J. Sun, Acta Biomater., 2012, 8, 350–360 CrossRef CAS PubMed.
- H. F. Shao, X. R. Ke, A. Liu, M. Sun, Y. He, X. Y. Yang, J. Z. Fu, Y. M. Liu, L. Zhang, G. J. Yang, S. Z. Xu and Z. R. Gou, Biofabrication, 2017, 9, 025003 CrossRef PubMed.
- Z. J. He, C. Jiao, H. X. Zhang, D. Q. Xie, M. X. Ge, Y. W. Yang, G. F. Wu, H. X. Liang, L. D. Shen and C. J. Wang, Ceram. Interfaces, 2022, 48, 25923–25932 CrossRef CAS.
- Z. Chen, T. Klein, R. Z. Murray, R. Crawford, J. Chang, C. Wu and Y. Xiao, Mater. Today, 2016, 19, 304–321 CrossRef CAS.
- A. Schindeler, M. M. McDonald, P. Bokko and D. G. Little, Semin. Cell Dev. Biol., 2008, 19, 459–466 CrossRef CAS PubMed.
- S. Franz, S. Rammelt, D. Scharnweber and J. C. Simon, Biomaterials, 2011, 32, 6692–6709 CrossRef CAS PubMed.
- A. Shapouri-Moghaddam, S. Mohammadian, H. Vazini, M. Taghadosi, S.-A. Esmaeili, F. Mardani, B. Seifi, A. Mohammadi, J. T. Afshari and A. Sahebkar, J. Cell. Physiol., 2018, 233, 6425–6440 CrossRef CAS PubMed.
- K. Hu and B. R. Olsen, Bone, 2016, 91, 30–38 CrossRef CAS PubMed.
- H. Newman, Y. V. Shih and S. Varghese, Biomaterials, 2021, 277, 121114 CrossRef CAS PubMed.
- K. Zheng, W. Niu, B. Lei and A. R. Boccaccini, Acta Biomater., 2021, 133, 168–186 CrossRef CAS PubMed.
- X. Chen, M. Wang, F. Chen, J. Wang, X. Li, J. Liang, Y. Fan, Y. Xiao and X. Zhang, Acta Biomater., 2020, 103, 318–332 CrossRef CAS PubMed.
- W. Xie, X. Fu, F. Tang, Y. Mo, J. Cheng, H. Wang and X. Chen, J. Mater. Chem. B, 2019, 7, 940–952 RSC.
- P. Guihard, Y. Danger, B. Brounais, E. David, R. Brion, J. Delecrin, C. Richards, S. Chevalier, F. Redini, D. Heymann, H. Gascan and F. Blanchard, Bone, 2012, 50, S83–S83 CrossRef.
- P. L. Graney, S. Ben-Shaul, S. Landau, A. Bajpai, B. Singh, J. Eager, A. Cohen, S. Levenberg and K. L. Spiller, Sci. Adv., 2020, 6, eaay6391 CrossRef CAS PubMed.
- L. Y. Lu, F. Loi, K. Nathan, T.-h. Lin, J. Pajarinen, E. Gibon, A. Nabeshima, L. Cordova, E. Jamsen, Z. Yao and S. B. Goodman, J. Orthop. Res., 2017, 35, 2378–2385 CrossRef CAS PubMed.
- T. A. Einhorn and L. C. Gerstenfeld, Nat. Rev. Rheumatol., 2015, 11, 45–54 CrossRef PubMed.
- S. D. Dutta, T. V. Patil, K. Ganguly, A. Randhawa and K.-T. Lim, Bioact. Mater., 2023, 28, 284–310 CrossRef CAS PubMed.
- S. Hoshikawa, K. Shimizu, A. Watahiki, M. Chiba, K. Saito, W. Wei, S. Fukumoto and H. Inuzuka, FASEB J., 2020, 34, 14930–14945 CrossRef CAS PubMed.
- K. Hu and B. R. Olsen, J. Clin. Invest., 2016, 126, 509–526 CrossRef PubMed.
- Q.-L. Ma, L. Fang, N. Jiang, L. Zhang, Y. Wang, Y.-M. Zhang and L.-H. Chen, Biomaterials, 2018, 154, 234–247 CrossRef CAS PubMed.
- T. Li, M. Peng, Z. Yang, X. Zhou, Y. Deng, C. Jiang, M. Xiao and J. Wang, Acta Biomater., 2018, 71, 96–107 CrossRef CAS PubMed.
- H. Li, W. Wang and J. Chang, Regener. Biomater., 2021, 8, rbab056 CrossRef PubMed.
- B. Liang, H. Wang, D. Wu and Z. Wang, J. Leukocyte Biol., 2021, 110, 433–447 CrossRef CAS PubMed.
- W. Qiao, H. Xie, J. Fang, J. Shen, W. Li, D. Shen, J. Wu, S. Wu, X. Liu, Y. Zheng, K. M. C. Cheung and K. W. K. Yeung, Biomaterials, 2021, 276, 121038 CrossRef CAS PubMed.
- K. Arvidson, B. M. Abdallah, L. A. Applegate, N. Baldini, E. Cenni, E. Gomez-Barrena, D. Granchi, M. Kassem, Y. T. Konttinen, K. Mustafa, D. P. Pioletti, T. Sillat and A. Finne-Wistrand, J. Cell. Mol. Med., 2011, 15, 718–746 CrossRef CAS PubMed.
- L. Voisin, M. K. Saba-El-Leil, C. Julien, C. Fremin and S. Meloche, Mol. Cell. Biol., 2010, 30, 2918–2932 CrossRef CAS PubMed.
- M. Majidinia, A. Sadeghpour and B. Yousefi, J. Cell. Physiol., 2018, 233, 2937–2948 CrossRef CAS PubMed.
- W. Zhang and H. T. Liu, Cell Res., 2002, 12, 9–18 CrossRef PubMed.
- W. Hatakeyama, M. Taira, N. Chosa, H. Kihara, A. Ishisaki and H. Kondo, Int. J. Mol. Med., 2013, 32, 1255–1261 CrossRef CAS PubMed.
- Y. Huang, G. Zhou, L. S. Zheng, H. F. Liu, X. F. Niu and Y. B. Fan, Nanoscale, 2012, 4, 2484–2490 RSC.
- S. W. Ha, J. Park, M. M. Habib and G. R. Beck, ACS Appl. Mater. Interfaces, 2017, 9, 39185–39196 CrossRef CAS PubMed.
- N. Wang, S. Yang, H. X. Shi, Y. P. Song, H. Sun, Q. Wang, L. L. Tan and S. Guo, J. Magnesium Alloys, 2022, 10, 3327–3353 CrossRef CAS.
- S. H. Lin, G. Z. Yang, F. Jiang, M. L. Zhou, S. Yin, Y. M. Tang, T. T. Tang, Z. Y. Zhang, W. J. Zhang and X. Q. Jiang, Adv. Sci., 2019, 6, 1900209 CrossRef PubMed.
- Z. T. Wang, Q. Liu, C. C. Liu, W. Tan, M. Y. Tang, X. H. Zhou, T. S. Sun and Y. W. Deng, J. Cell. Physiol., 2020, 235, 5182–5191 CrossRef CAS PubMed.
- M. Li, P. He, Y. H. Wu, Y. Zhang, H. Xia, Y. F. Zheng and Y. Han, Sci. Rep., 2016, 6, 32323 CrossRef CAS PubMed.
- M. Y. Shie and S. J. Ding, Biomaterials, 2013, 34, 6589–6606 CrossRef CAS PubMed.
- C. H. Liu, C. J. Hung, T. H. Huang, C. C. Lin, C. T. Kao and M. Y. Shie, Mater. Sci. Eng., C, 2014, 43, 359–366 CrossRef CAS PubMed.
- B. C. Wu, C. T. Kao, T. H. Huang, C. J. Hung, M. Y. Shie and H. Y. Chung, J. Endod., 2014, 40, 1105–1111 CrossRef PubMed.
- E. Rathinam, S. Rajasekharan, R. T. Chitturi, H. Declercq, L. Martens and P. De Coster, J. Endod., 2016, 42, 1713–1725 CrossRef PubMed.
- S. X. Lai, L. J. Chen, W. Cao, S. M. Cui, X. Y. Li, W. C. Zhong, M. Y. Ma and Q. B. Zhang, Mediators Inflammation, 2018, 2018, 8167932 Search PubMed.
- G. Luther, E. R. Wagner, G. H. Zhu, Q. Kang, Q. Luo, J. Lamplot, Y. Bi, X. J. Luo, J. Y. Luo, C. Teven, Q. Shi, S. H. Kim, J. L. Gao, E. Y. Huang, K. Yang, R. Rames, X. Liu, M. Li, N. Hu, H. Liu, Y. X. Su, L. Chen, B. C. He, G. W. Zuo, Z. L. Deng, R. R. Reid, H. H. Luu, R. C. Haydon and T. C. He, Curr. Gene Ther., 2011, 11, 229–240 CrossRef CAS PubMed.
- L. F. Hu, C. Yin, F. Zhao, A. Ali, J. H. Ma and A. R. Qian, Int. J. Mol. Sci., 2018, 19, 360 CrossRef PubMed.
- C. H. Heldin, M. Landstrom and A. Moustakas, Curr. Opin. Cell Biol., 2009, 21, 166–176 CrossRef CAS PubMed.
- S. Vermeulen, Z. T. Birgani and P. Habibovic, Biomaterials, 2022, 283, 121431 CrossRef CAS PubMed.
- M. Bruderer, R. G. Richards, M. Alini and M. J. Stoddart, Eur. Cells Mater., 2014, 28, 269–286 CrossRef CAS PubMed.
- Z. R. Tang, Z. Wang, F. Z. Qing, Y. L. Ni, Y. J. Fan, Y. F. Tan and X. D. Zhang, J. Biomed. Mater. Res., Part A, 2015, 103, 1001–1010 CrossRef PubMed.
- J. Wang, M. L. Wang, F. Y. Chen, Y. H. Wei, X. N. Chen, Y. Zhou, X. Yang, X. D. Zhu, C. Q. Tu and X. D. Zhang, Int. J. Nanomed., 2019, 14, 7987–8000 CrossRef CAS PubMed.
- Z. T. Chen, C. T. Wu, W. Y. Gu, T. Klein, R. Crawford and Y. Xiao, Biomaterials, 2014, 35, 1507–1518 CrossRef CAS PubMed.
- D. Zhai, M. C. Xu, L. Q. Liu, J. Chang and C. T. Wu, J. Mater. Chem. B, 2017, 5, 7297–7306 RSC.
- F. Han, T. Li, M. M. Li, B. J. Zhang, Y. F. Wang, Y. F. Zhu and C. T. Wu, Bioact. Mater., 2023, 20, 29–40 CrossRef CAS PubMed.
- C. Y. Janda, D. Waghray, A. M. Levin, C. Thomas and K. C. Garcia, Science, 2012, 337, 59–64 CrossRef CAS PubMed.
- L. Mao, J. Liu, J. Zhao, J. Chang, L. Xia, L. Jiang, X. Wang, K. Lin and B. Fang, Int. J. Nanomed., 2015, 10, 7031–7044 CrossRef CAS PubMed.
- M. T. Zheng, M. J. Weng, X. Y. Zhang, R. M. Li, Q. Tong and Z. Q. Chen, Biomed. Mater., 2021, 16, 025005 CrossRef CAS PubMed.
- R. L. Wang, L. J. Chen and L. Q. Shao, J. Nanobiotechnol., 2020, 18, 119 CrossRef CAS PubMed.
- X. Cui, Y. Zhang, J. Wang, C. Huang, Y. Wang, H. Yang, W. Liu, T. Wang, D. Wang, G. Wang, C. Ruan, D. Chen, W. W. Lu, W. Huang, M. N. Rahaman and H. Pan, Bioact. Mater., 2020, 5, 334–347 CrossRef PubMed.
- C. C. Yin, X. S. Jia, Q. Zhao, Z. F. Zhao, J. Y. Wang, Y. F. Zhang, Z. Li, H. C. Sun and Z. B. Li, Mater. Sci. Eng., C, 2020, 110, 110671 CrossRef CAS PubMed.
- Y. Chen, L. Chen, Y. T. Wang, K. L. Lin and J. Q. Liu, Composites, Part B, 2022, 230, 109550 CrossRef CAS.
- F. J. Zhao, Z. Yang, H. C. Xiong, Y. Yan, X. F. Chen and L. Q. Shao, Bioact. Mater., 2023, 22, 201–210 CrossRef CAS PubMed.
- P. Feng, P. Wu, C. Gao, Y. Yang, W. Guo, W. Yang and C. Shuai, Adv. Sci., 2018, 5, 1700817 CrossRef PubMed.
- M. Bohner and R. J. Miron, Mater. Today, 2019, 22, 132–141 CrossRef CAS.
- H. P. Lu, Y. H. Zhou, Y. P. Ma, L. Xiao, W. J. Ji, Y. Zhang and X. Wang, Front. Mater., 2021, 8, 698915 CrossRef.
- D. Z. Lu, W. Dong, X. J. Feng, H. Chen, J. J. Liu, H. Wang, L. Y. Zang and M. C. Qi, Mol. Cell. Endocrinol., 2020, 508, 110791 CrossRef CAS PubMed.
- S. Kanaya, B. L. Xiao, Y. Sakisaka, M. Suto, K. Maruyama, M. Saito and E. Nemoto, J. Appl. Oral Sci., 2018, 26, e20170231 Search PubMed.
- A. De, Acta Biochim. Biophys. Sin., 2011, 43, 745–756 CrossRef CAS PubMed.
- X. G. Wang, Y. M. Yu, L. L. Ji, Z. Geng, J. Wang and C. S. Liu, Bioact. Mater., 2021, 6, 4517–4530 CrossRef CAS PubMed.
- R. Y. Ren, J. C. Guo, Y. M. F. Chen, Y. Y. Zhang, L. X. Chen and W. Xiong, Cell Proliferation, 2021, 54, e13122 CrossRef CAS PubMed.
- J. C. Lee and E. J. Volpicelli, Adv. Healthcare Mater., 2017, 6, 1700232 CrossRef PubMed.
- M. Maroulakos, G. Kamperos, L. Tayebi, D. Halazonetis and Y. J. Ren, J. Dent., 2019, 80, 1–14 CrossRef PubMed.
- H. Matsuura, T. Inoue, H. Konno, M. Sasaki, K. Ogasawara and A. Ogawa, J. Neurosurg., 2002, 97, 1472–1475 Search PubMed.
- V. Siracusa, G. Maimone and V. Antonelli, Polymers, 2021, 13, 1452 CrossRef CAS PubMed.
- J. Brie, T. Chartier, C. Chaput, C. Delage, B. Pradeau, F. Caire, M. P. Boncoeur and J. J. Moreau, J Craniomaxillofac. Surg., 2013, 41, 403–407 CrossRef PubMed.
- P. C. Whitfield, Acta Neurochirurgica, 2007, 149, 170–170 Search PubMed.
- J. Brie, T. Chartier, C. Chaput, C. Delage, B. Pradeau, F. Caire, M. P. Boncoeur and J. J. Moreau, J Craniomaxillofac. Surg., 2013, 41, 403–407 CrossRef PubMed.
- H. Park, J. W. Choi and W. S. Jeong, J. Craniofac. Surg., 2022, 33, 1394–1399 CrossRef PubMed.
- A. Hikita, U. I. Chung, K. Hoshi and T. Takato, Tissue Eng., Part A, 2017, 23, 515–521 CrossRef PubMed.
- Y. Kanno, T. Nakatsuka, H. Saijo, Y. Fujihara, H. Atsuhiko, U. I. Chung, T. Takato and K. Hoshi, Regen. Ther., 2016, 5, 1–8 CrossRef PubMed.
- U. L. Lee, J. Y. Lim, S. N. Park, B. H. Choi, H. Kang and W. C. Choi, Materials, 2020, 13, 4515 CrossRef CAS PubMed.
- J. Wolff, G. K. Sandor, A. Miettinen, V. J. Tuovinen, B. Mannerstrom, M. Patrikoski and S. Miettinen, Ann. Maxillofac. Surg., 2013, 3, 114–125 CrossRef PubMed.
- H. B. Chen, J. M. Sun and J. C. Wang, J. Craniofac. Surg., 2018, 29, 618–621 CrossRef PubMed.
- P. Kijartorn, J. Wongpairojpanich, F. Thammarakcharoen, J. Suwanprateeb and B. Buranawat, J. Dent. Sci., 2022, 17, 194–203 CrossRef PubMed.
- H. Jo and U. L. Lee, J. Craniofac. Surg., 2022, 33, E521–E523 CrossRef PubMed.
- F. F. Xu, H. Ren, M. J. Zheng, X. X. Shao, T. Q. Dai, Y. L. Wu, L. Tian, Y. Liu, B. Liu, J. Gunster, Y. X. Liu and Y. P. Liu, J. Mech. Behav. Biomed. Mater., 2020, 103, 103532 CrossRef CAS PubMed.
- C. Mangano, A. Giuliani, I. De Tullio, M. Raspanti, A. Piattelli and G. Iezzi, Front. Bioeng. Biotechnol., 2021, 9, 614325 CrossRef PubMed.
- M. Anderson, N. Dubey, K. Bogie, C. Cao, J. Y. Li, J. Lerchbacker, G. Mendonca, F. Kauffmann, M. C. Bottino and D. Kaigler, Dent. Mater., 2022, 38, 529–539 CrossRef CAS PubMed.
- M. Navarro, A. Michiardi, O. Castano and J. A. Planell, J. R. Soc., Interface, 2008, 5, 1137–1158 CrossRef CAS PubMed.
- H. Zhu, M. Li, X. L. Huang, D. H. Qi, L. P. Nogueira, X. Yuan, W. B. Liu, Z. H. Lei, J. W. Jiang, H. L. Dai and J. Xiao, Appl. Mater. Today, 2021, 25, 101166 CrossRef.
- J. J. Li, C. R. Dunstan, A. Entezari, Q. Li, R. Steck, S. Saifzadeh, A. Sadeghpour, J. R. Field, A. Akey, M. Vielreicher, O. Friedrich, S. Roohani-Esfahani and H. Zreiqat, Adv. Healthcare Mater., 2019, 8, e1801298 CrossRef PubMed.
- Y. J. Lu, G. J. Chen, Z. Y. Long, M. H. Li, C. L. Ji, F. W. Wang, H. Z. Li, J. X. Lu, Z. Wang and J. Li, J. Bone Oncol., 2019, 16, 100220 CrossRef PubMed.
- Y. R. Ding, X. L. Liu, J. Zhang, Z. C. Lv, X. C. Meng, Z. G. Yuan, T. Long and Y. Wang, Front. Bioeng. Biotechnol., 2022, 10, 947521 CrossRef PubMed.
- L. Witek, A. M. Alifarag, N. Tovar, C. D. Lopez, B. N. Cronstein, E. D. Rodriguez and P. G. Coelho, J. Orthop. Res., 2019, 37, 2499–2507 CrossRef CAS PubMed.
- C. D. Lopez, J. R. Diaz-Siso, L. Witek, J. M. Bekisz, B. N. Cronstein, A. Torroni, R. L. Flores, E. D. Rodriguez and P. G. Coelho, J. Surg. Res., 2018, 223, 115–122 CrossRef CAS PubMed.
- A. K. Teotia, K. Dienel, I. Qayoom, B. van Bochove, S. Gupta, J. Partanen, J. Seppala and A. Kumar, ACS Appl. Mater. Interfaces, 2020, 12, 48340–48356 CrossRef CAS PubMed.
- N. Golafshan, E. Vorndran, S. Zaharievski, H. Brommer, F. B. Kadumudi, A. Dolatshahi-Pirouz, U. Gbureck, R. van Weeren, M. Castilho and J. Malda, Biomaterials, 2020, 261, 120302 CrossRef CAS PubMed.
- J. W. Kim, B. E. Yang, S. J. Hong, H. G. Choi, S. J. Byeon, H. K. Lim, S. M. Chung, J. H. Lee and S. H. Byun, Int. J. Mol. Sci., 2020, 21, 4837 CrossRef CAS PubMed.
- Y. H. Wei, D. Y. Zhao, Q. L. Cao, J. Wang, Y. H. Wu, B. Yuan, X. F. Li, X. N. Chen, Y. Zhou, X. Yang, X. D. Zhu, C. Q. Tu and X. D. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 1787–1797 CrossRef CAS PubMed.
- S. Bose, D. Banerjee, S. Robertson and S. Vahabzadeh, Ann. Biomed. Eng., 2018, 46, 1241–1253 CrossRef PubMed.
- C. T. Wu, W. Fan, Y. H. Zhou, Y. X. Luo, M. Gelinsky, J. Chang and Y. Xiao, J. Mater. Chem., 2012, 22, 12288–12295 RSC.
- L. Le Guehennec, V. Dorien, E. Plougonven, G. Nolens, B. Verlee, M. C. De Pauw and F. Lambert, J. Biomed. Mater. Res., Part A, 2020, 108, 412–425 CrossRef CAS PubMed.
- I. I. Bozo, R. V. Deev, I. V. Smirnov, A. Y. Fedotov, V. K. Popov, A. V. Mironov, O. A. Mironova, A. Y. Gerasimenko and V. S. Komlev, Int. J. Bioprint., 2020, 6, 275 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |