Open Access Article
Open Access ArticleExploring a Streptomyces wax synthase using acyl-SNACs as donor substrates†
Federica
Casolari
a,
Saad
Alrashdi
ab,
Reuben
Carr
c and
Hai
Deng
 *a
*a
aDepartment of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, UK. E-mail: h.deng@abdn.ac.uk
bCollege of Science and Arts in Gurayat, Jouf University, King Khaled Road, Saudi Arabia
cIngenza Ltd Scotland, UK
First published on 14th August 2023
Abstract
The demand of fragrance and food industries for short/branched wax esters is increasing due to their rich scent and low toxicity. Wax synthase and acyl-CoA:diacylglycerol O-acyltransferase (WS/DGAT) are a family of bacterial enzymes capable of catalysing the production of wax esters. Here, we report that a WS/DGAT from Streptomyces coelicolor is able to mediate the reactions between alcohol acceptors and synthetic acyl-donor mimics, acyl-SNACs. The enzyme displayed considerable substrate tolerance towards acyl-donors with structural diversity. Structural modelling-guided site directed mutagenesis resulted in a variant, L25F, the catalytic efficiency of which was improved toward aromatic, short-linear, and branched acyl-donors compared to the wild type.
Introduction
Wax esters (WEs) are considered key commercial products due to their numerous applications as biofuels, surface coatings, pharmaceuticals, and lubricants.1,2 While wax esters are primarily used in the solvent and biofuel industries, new applications are starting to emerge. The global flavours and fragrances market is expected to grow and was estimated at 29.15 billion US dollars in 2022. This spiking product market is being driven by the global demand and consumption of processed food and cosmetics.3 Interestingly, recent studies have demonstrated that branched wax esters can augment the skin-feel of lotions and other wax ester-containing cosmetics.4 Moreover, short chain esters, such as hexyl or ethyl acetate, can be utilised as both food additives and fragrances, while they can also function as drop-in fuels or lubricants.5–7 Long-chain wax esters are not only exploited as cosmetics or biodiesels, but also as commercial vegetable oils and fats.8,9 Additionally, both butyl and hexyl benzoate were reported as key esters responsible for the sweet aroma of different fruit species.10,11 The majority of these valuable wax esters are currently prepared from fossil derived feedstock which requires energy intensive processing and is considered by many as non-sustainable for the future. Therefore, biotransformation of WEs is an attractive route to a more sustainable supply of these chemicals under mild conditions.Generally, two enzyme families have been explored for the production of WEs. The first enzyme family is alcohol acyltransferases (AATs or ATFs, EC 2.3.1.84) reported mainly in plants and yeast. They are able to metabolise short-chain alcohols and acyl coenzyme As (acyl-CoA) to yield volatile esters for the fragrance profile of many fruits or flowers, as well as the flavour palate found in beer or wine production.12,13
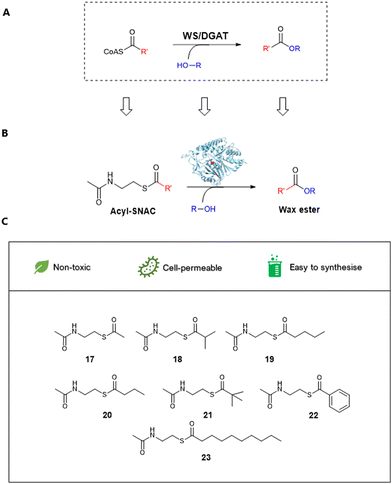
The second enzyme family is wax ester synthases. They are bifunctional enzymes behaving as wax synthase (EC 2.3.1.75) and acyl-CoA:diacylglycerol O-acyltransferase (EC 2.3.1.20) (WS/DGAT) having the ability to combine acyl-accepting compounds (i.e. fatty alcohol or diacylglycerol) and acyl-donors (i.e. acyl-CoA or acyl-ACP) while producing the corresponding wax esters or triacylglycerols (TAGs) (Scheme 1).14,15 It has been well-documented that WSs possess a conserved motif (HHXXXDG) in the binding pocket containing two catalytic histidine residues which are responsible for ester bond formation.16
Bacteria are among the most abundant sources of the WS/DGAT family, as wax esters are pivotal in their biosynthesis of storage lipids.17 As such, bacterial WS/DGATs have been extensively studied to explore their biocatalytic potential. For example, wax synthases from Marinobacter have shown substrate promiscuity and exhibit good selectivity towards short and medium chain acyl-CoA, using hexanol and decanol as acyl acceptors to generate medium to long acyl-chain wax esters, such as 6,10,14-trimethylpentadecan-2-one, an intrinsic taste compound present in celery, oregano and sweet basil.18–20 Structure-guided site mutagenesis leading a variant of the Marinobacter genus (Ma-WS-A144F) displayed significantly increased selectivity towards C6 acyl-CoA (caproyl-CoA) compared to palmitoyl-CoA (C7 acyl-CoA) since such mutation in the binding pocket was likely to decrease the binding affinity of longer chains.21 Analogous studies have also described how the selection of the mutation site was driven by its proximity to the conserved HHXXXDG motif of wax ester synthases, and how altering its surrounding could modify the enzyme substrate affinity.22,23
Streptomyces species are able to synthesize various types of WEs and TAGs. For example, WE and TAG contents occupy nearly 9% (wt/wt) of the cell dry weight (CDW) in S. lividans.24 As a result, WSs from Streptomyces have been well-studied to demonstrate their abilities to generate various DGATs.25 Notably, Streptomyces WS/DGAT naturally metabolise long aliphatic-chain substrates, catalysing the esterification reaction from C12 to C18 acyl-donors, such as myristoyl-CoA.26
In this study, we report characterization of a WS from Streptomyces coelicolor (WS-SCo) as a potential biocatalyst for WE production. We explore its catalytic capacity using different acyl-SNACs (17–23, Scheme 1) as acyl acceptors and C6 and C4 alcohols as acyl donors. To our knowledge, this is the first report to use synthetic mimics of CoAs for wax ester synthesis. The wild-type enzyme displays considerable substrate preference toward branched short chain and aromatic acyl-SNACs when hexanol is used, while showing substrate specificity toward straight acyl-SNACs after the addition of butanol, suggesting that different acyl donors affect substrate selectivity toward acyl acceptors, a similar phenomenon observed in other WS systems.27,28 Further structural modelling-guided site directed mutagenesis (SDM) allow generation of an enzyme variant, L25F, which displays a similar trend that the variant shows substrate selectivity toward aromatic acyl SNACs when hexanol is used, while toward straight chain acyl-SNACs when butanol is added.
Results and discussion
Considering that the catalytic capacities of WSs from Streptomyces as potential biocatalysts have been largely overlooked, we chose a putative WS (WS-SCo) (GenBank: QFI41200.1) from S. coelicolor A3(2), the model Streptomyces strain, to explore its substrate tolerance. In this regard, we first sought to overexpress the gene encoding a putative WS/DGAT (WS-SCo) in E. coli BL21 (pGro7). The enzyme was expressed as an N-terminal His6-recombinant protein that was purified to near homogeneity by Ni-NTA chromatography and gave an estimated molecular weight of 47 kDa, as observed in SDS-PAGE analysis (Fig. S1, ESI†). To assay the activity of the recombinant protein, the commercially available acetyl-CoA 24 was used for the initial examination. Incubation of the purified WS-SCo (10 μM) with acetyl CoA 24 (1 mM) and hexanol 25 (2.5 mM) as a common alcohol acceptor resulted in the production of hexyl acetate esters as observed in HR-LCMS analysis (Fig. S4, ESI†). In the control assay with the boiled enzyme, the formation of the ester was not observed. This indicated that WS-SCo is indeed a WS/DAG that can accept short-chain acyl-CoA species.To obtain the kinetic data of WS-SCo, we monitored the CoA release using the DTNB (5,5′-dithiobis(2-nitrobenzoic acid), Ellman's reagent) derivatisation assay.29 The kinetic constants were the values of Km = 73.294 ± 0.021 μM and kcat = 0.171 ± 0.022 min−1, suggesting that WS-SCo is a competent WS/DAG that has the capacity of transforming short-chain acyl-CoA with hexanol into the corresponding ester. Many WS/DAGs from other organisms have been examined for their substrate tolerances using various straight chain saturated or unsaturated acyl-CoAs as substrates.30,31 However, few have been tested with branch chain or aromatic acyl-CoAs due to their commercial unavailability.
Structurally simplified CoA analogues such as N-acetyl-cysteamine thioesters (SNACs) have been developed to study thiolate-templated enzymes such as biosynthetic pathways to fatty acids, polyketides and non-ribosomal peptides.32 Therefore, we decided to examine whether WS-SCo could accept acyl-SNACs, the acyl-CoA synthetic mimic, as its substrates. To this end, we synthesized seven acyl-SNACs (acetyl-17, isobutyryl-18, valeryl-19, butyryl-20, tert-butyl-21, phenyl-22 and decanoyl-23) with structural diversity using our previously described method.33 Briefly, the one-pot synthesis started with the commercially available stable cystamine reacting with acetic anhydride under alkali conditions to provide N,N-diacetyl-cystamine. In situ reduction by TCEP and S-acylation using chosen anhydrides gave the corresponding acyl-SNACs (Fig. S5, ESI†). The products were confirmed by HR-LCMS, 1H-NMR spectroscopy and 13C-NMR spectroscopy analyses (Fig. S6–S29, ESI†). To validate this, kinetic data were obtained as previously described. WS-SCo displayed a much larger affinity toward acetyl-SNAC compared to its CoA analogue, as indicated by a low Km value of 28.261 ± 0.104 μM and a kcat/Km constant (4.897 ± 1.093 mM min−1) which was twice that of acetyl-CoA (2.392 ± 0.394 mM min−1). This suggested that acyl-SNACs have the potential to replace to acyl-CoAs as enzymatic substrates for wax ester bio-catalysis.
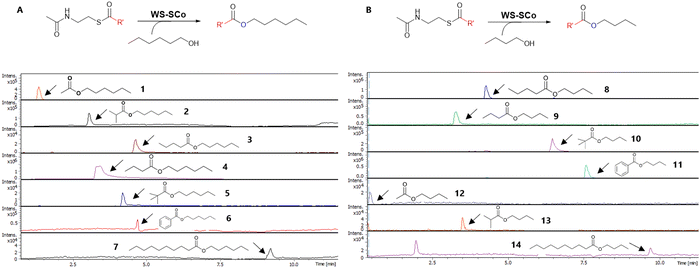
With acyl-SNACs available, we then set out to probe the overall substrate tolerance of WS-SCo. Incubation of the purified enzyme (10 μM) with acyl-SNACs (1 mM) and alcohol (2.5 mM) (hexanol 25 and butanol 26) resulted in the production of the corresponding esters, 1–14, as shown by HR-LCMS analysis (Fig. 1). This is the first report to demonstrate that bacterial WS-SCo is able to process acyl-CoA synthetic mimics, acyl-SNACs, thus allowing us to evaluate various acyl-donors with structural diversity. Interestingly, WS-SCo appears to display similar reactivity towards aromatic and branched chain acyl-SNACs compared to straight-chain SNACs when 25 or 26 was used as acyl-acceptors. However, when decanol 27 was used, no corresponding esters were observed, indicating that WS-SCo is unable to process C10 or longer-alcohols.
Encouraged by this observation, detailed kinetic studies of WS-SCo were performed. We measured the reactions by monitoring SNAC release using the DTNB derivatisation assay. Incubation of WS-SCo (10 μM) with hexanol or butanol (2.5 mM) and various concentrations (25 μM to 1 mM) of acyl-SNACs provided the corresponding hexyl or butyl esters (Table 1).
When hexanol is present, the enzyme exhibits decreasing selectivity from longer (decanoyl-SNAC Km = 15.206 ± 0.001 μM) to shorter linear chains while appearing to favour small-branched substrates (isobutyryl-SNAC Km = 5.229 ± 0.003 μM) in comparison to aromatics. Similar conclusions are supported by the catalytic efficiency (kcat/Km) data, which exhibit highest values for long (40.548 ± 0.343 mM min−1) and branched (23.333 ± 5.072 mM min−1) acyl chains. This reveals a degree of variance in substrate acceptance which can be explained by the promiscuous nature of bacterial WS.34
When butanol was added in the reactions, the preference of selectivity and efficiency of WS-SCo is less apparent because it lacks the ability to selectively convert long, short linear (acetyl-SNAC) and short branched (isobutyryl-SNAC) acyl-donors into butyl esters. The enzyme still displays good enzymatic efficiency towards branched (tert-butyl-SNAC kcat/Km = 6.019 ± 2.654 mM min−1), aromatic (phenyl-SNAC kcat/Km = 6.284 ± 0.872 mM min−1) and linear chains (butyryl-SNAC kcat/Km = 5.041 ± 2.648 mM min−1), indicating its ability to efficiently produce tert-butyl, phenyl and butyryl C4-esters.
Clearly, there is a discrepancy of kinetic profiles between hexanol and butanol as acyl-acceptors, which has been previously reported.27 This may be due to a key conformational change in the active site when an acyl-donor is bound in its corresponding binding pocket, as observed in previous studies.35,36
To the best of our knowledge, this is the first instance of a bacterial WS/DGAT demonstrating its catalytic capacity toward branched/aromatic acyl-SNACs to process the corresponding esters. To further investigate whether the key residues in the acyl-CoA pocket of WS-SCo can affect the length of acyl-SNACs, we generated a site-specific variant and compared its activity with that of the wild type of WS-SCo.
Previous crystallography and mutagenesis studies of bacterial WS/DGATs allowed the identification of key residues that can influence substrate selectivity.21,30 For example, it was found that mutations of Ala144 to phenylalanine, Gly25 to valine and Ala148 to isoleucine improved the selectivity of a WS/DGAT from a Marinobacter sp. towards shorter acyl-CoAs. This is because these residues are positioned in the acyl-CoA pocket and increasing the amino acid side chain would decrease the binding affinity of longer-chain acyl-CoAs.
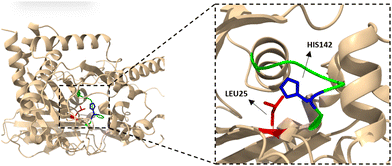
With this in mind, we performed structural modelling analysis of WS-SCo using AlphaFold (DeepMind), searching for the potential location of the acyl-donor active site.37,38 WS-SCo displays high structural similarity to Ma-WS/DGAT (Fig. S30, ESI†). Interestingly, structural comparison with WT-Ma-WS/DGAT indicated that, although enzymes contain an Ala residue in positions 143 and 144, respectively, WS-SCo possesses Phe138 in the acyl-CoA pocket while Ma-WS/DGAT has Thr132. In the latter, these two residues (Ala144 and Thr132) act as gatekeepers to interact with the hydrocarbon chains of acyl-CoAs. Changing Ala144 to a bulky side chain such as phenylalanine resulted in good selectivity toward short chain acyl-CoA. Additionally, WS-SCo possesses Ala27, which is equivalent to Gly25 in Ma-WS/DGAT. The variations of Phe138 and Ala27 in WS-SCo may explain the reason for the good selectivity of WS-SCo toward branched short chain/aromatic acyl-SNACs. However, WS-SCo still displays good catalytic efficiency toward longer-chain acyl-SNACs (i.e. decanoyl-SNAC), suggesting that, although possibly not fully extended, the acyl chain can still be folded into the pocket for the esterification. Another interesting observation is that WS-SCo possesses Leu25 (Fig. 2), instead of Val23 in Ma-WS/DGAT, which is intrinsically close to His142, responsible for the WS/DGAT mechanism. It was hypothesized that changing this residue to one with a “bulkier” and hydrophobic side chain (such as phenylalanine) may affect the binding of acyl-chains. For this reason, a WS-SCo-L25F variant was generated and expressed in the same system.
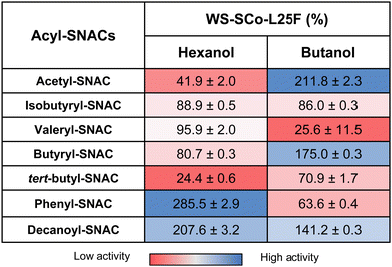
To assess the impact of L25F mutation on enzymatic activity, we conducted a comparison analysis of both wild type and L25F variant using available acyl-SNACs and alcohols. Again, WS-SCo activity seems to be varying according to the alcohol used. When hexanol is present, Leu25Phe mutation improves the enzyme activity to bind with aromatic (285.5%, phenyl-SNAC) and long chains (207.6%, decanoyl-SNAC), while it decreases for all other substrates. This behaviour could be attributed to stacked aromatic interactions between the rings of phenyl-SNAC and Phe25 and with the long chain of decanoyl-SNAC being not fully extended. Significant low activity (24.4%) for “bulkier” branched acyl chains, such as tert-butyl-SNAC, suggests that the third methyl group could be blocked by the aromatic ring of Phe25, inhibiting its binding.
Conversely, WS-SCo-L25F betters its activity towards linear chains, from longer (C10) to shorter (C1) linear substrates (acetyl-SNAC > butyryl-SNAC > decanoyl-SNAC), when butanol is used. Under the same conditions, a significant decrease in activity for branched acyl-SNACs, such as tert-butyl (70.9%) and aromatic (63.6%) also becomes apparent (Table 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 M−1 cm−1), of the wild type (100%) and the mutant for each substrate tested. Results are shown as percentage (%) of at least duplicates samples
150 M−1 cm−1), of the wild type (100%) and the mutant for each substrate tested. Results are shown as percentage (%) of at least duplicates samples
Although previously reported mutations in the acyl-CoA binding pocket achieved higher selectivity towards smaller acyl-donors, an active preference for longer alcohol chains could still be noticed, which could support our reported substrate variation.21 Additionally, a recent study described a change in the structural configuration occurring in the pocket during the binding of the acyl-donor, preventing the correct reactivity with the alcohol.28 Therefore, WS-SCo-L25F could potentially lose the selective binding to the alcohol if the binding site is too narrow. In conclusion, our findings indicate that although the Leu25Phe mutation does not improve activity towards branched acyl chains, it does significantly increase WS-SCo reactivity with linear and aromatic SNACs. Future crystallography studies may help us improve our understanding of WS-SCo towards branched and aromatic acyl-SNACs.
Conclusions
In summary, we have demonstrated that WS-SCo from Streptomyces coelicolor A32 is a considerably promiscuous wax ester synthase capable of catalysing the production of numerous small wax esters. To our knowledge, WS-SCo is the first enzyme of its family to be examined towards CoA analogues, synthetic acyl-SNACs, where its substrate affinity varies according to the alcohol tested (butanol or hexanol). Moreover, the enzymatic activity assay revealed that the L25F mutated enzyme favoured aromatic and linear acyl-donors. When butanol was used as the acyl-acceptor, WS-SCo-L25F also displayed improved activity towards aromatic/long-chain acyl acceptors. Overall, WS-SCo is capable of yielding a multitude of small wax esters, such as hexyl and butyl acetate (1 and 12), isobutyrate (2 and 13), tert-butyrate (5 and 10) and benzoate (6 and 11), which are esters commonly exploited by the food and fragrance industries.Author contributions
FC carried out all experiments, designed the study and drafted the manuscript. SA participated in the design of the study and synthesised all acyl-SNACs. HD conceived the study, participated in its design and helped in drafting the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The research was funded by the IBioIC PhD studentship (F. C.), the College of Science and Arts, Jouf University, King Khaled Road, Kingdom of Saudi Arabia, and the Royal Embassy of Saudi Arabia Cultural Bureau in the UK for the PhD scholarship (S. A.). H. D. is grateful to the Royal Society – NSFC International Exchanges Award (IEC\NSFC\211349), Biotechnology and Biological Sciences Research Council UK (BB/R00479X/1), the Royal Society of Edinburgh-NSFC Joint Project (947_Deng) and the National Natural Science Foundation of China (31929001).References
- J. Nielsen and S. Shi, Methods and products for production of wax esters, WO2012/017083A1, 2012 Search PubMed.
- A. J. Kruis, A. C. Bohnenkamp, C. Patinios, Y. M. Van Nuland, M. Levisson and A. E. Mars, et al., Microbial production of short and medium chain esters: Enzymes, pathways, and applications, Biotechnol. Adv., 2019, 37(7), 107407, DOI:10.1016/j.biotechadv.2019.06.006.
- Grand View Research 2023, Flavors And Fragrances Market Size, Share & Trends Analysis Report By Product (Aroma Chemicals, Natural), By Application (Flavors, Fragrances), By Region (Asia Pacific, North America), And Segment Forecasts, 2023–2030 [Internet]. 2021 [cited 2023 May 5]. Available from: https://www.grandviewresearch.com/industry-analysis/flavors-fragrances-market/methodology.
- F. Wilson and D. Loughran, The link between function and structure of esters, 2017.
- E. G. Dennis, R. A. Keyzers, C. M. Kalua, S. M. Maffei, E. L. Nicholson and P. K. Boss, Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must, J. Agric. Food Chem., 2012, 60(10), 2638–2646 CrossRef CAS PubMed.
- N. Kushwaha, D. Banerjee, K. A. Ahmad, N. P. Shetti, T. M. Aminabhavi and K. K. Pant, et al., Catalytic production and application of bio-renewable butyl butyrate as jet fuel blend – A review, J. Environ. Manage., 2022, 310, 114772, DOI:10.1016/j.jenvman.2022.114772.
- F. Domergue and M. Miklaszewska, The production of wax esters in transgenic plants: towards a sustainable source of bio-lubricants, J. Exp. Bot., 2022, 73(9), 2817–2834 CrossRef CAS PubMed.
- R. Kalscheuer, T. Stölting and A. Steinbüchel, Microdiesel: Escherichia coli engineered for fuel production, Microbiology, 2006, 152(9), 2529–2536 CrossRef CAS PubMed.
- A. Röttig and A. Steinbüchel, Acyltransferases in Bacteria, Microbiol. Mol. Biol. Rev., 2013, 77(2), 277–321 CrossRef PubMed.
- J. Adedeji, T. G. Hartman, R. T. Rosen and C. T. Ho, Free and Glycosidically Bound Aroma Compounds in Hog Plum (Spondias mombins L.), J. Agric. Food Chem., 1991, 39, 1494–1497 CrossRef CAS . Available from https://pubs.acs.org/sharingguidelines.
- O. Lasekan and S. P. Yap, Characterization of the aroma compounds in fresh and dried sapodilla (Manikara zapota, L.) by the application of aroma extract dilution analysis, CyTA--J. Food, 2018, 16(1), 801–806 CrossRef CAS.
- G. M. Rodriguez, Y. Tashiro and S. Atsumi, Expanding ester biosynthesis in Escherichia coli, Nat. Chem. Biol., 2014, 10(4), 259–265 CrossRef CAS PubMed.
- J. Beekwilder, M. Alvarez-Huerta, E. Neef, F. W. A. Verstappen, H. J. Bouwmeester and A. Aharoni, Functional characterization of enzymes forming volatile esters from strawberry and banana, Plant Physiol., 2004, 135, 1865–1878 CrossRef CAS PubMed.
- T. Stöveken, R. Kalscheuer and A. Steinbüchel, Both histidine residues of the conserved HHXXXDG motif are essential for wax ester synthase/acyl-CoA:Diacylglycerol acyltransferase catalysis, Eur. J. Lipid Sci. Technol., 2009, 111(2), 112–119 CrossRef.
- T. Stöveken, R. Kalscheuer, U. Malkus, R. Reichelt and A. Steinbüchel, The wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: Characterization of a novel type of acyltransferase, J. Bacteriol., 2005, 187(4), 1369–1376 CrossRef PubMed.
- J. Buglino, K. C. Onwueme, J. A. Ferreras, L. E. N. Quadri and C. D. Lima, Crystal structure of PapA5, a phthiocerol dimycocerosyl transferase from Mycobacterium tuberculosis, J. Biol. Chem., 2004, 279(29), 30634–30642 CrossRef CAS PubMed.
- A. Radunz and G. H. Schmid, Wax esters and triglycerides as storage substances in seeds of Buxus sempervirens, Eur. J. Lipid Sci. Technol., 2000, 102(12), 734–738 CrossRef CAS.
- J. F. Rontani, P. C. Bonin and J. K. Volkman, Production of Wax Esters during Aerobic Growth of Marine Bacteria on Isoprenoid Compounds, Appl. Environ. Microbiol., 1999, 65(1), 221–230 CrossRef CAS PubMed . Available fromhttps://journals.asm.org/journal/aem.
- E. Holtzapple and C. Schmidt-Dannert, Biosynthesis of isoprenoid wax ester in Marinobacter hydrocarbonoclasticus DSM 8798: Identification and characterization of isoprenoid coenzyme a synthetase and wax ester syethases, J. Bacteriol., 2007, 189(10), 3804–3812 CrossRef CAS PubMed.
- The Good Scents Company (2009). Flavor and fragrance information catalog, 2023 [cited 2023 Jun 6]. Available from: http://www.thegoodscentscompany.com/data/rw1309781.html.
- N. Petronikolou and S. K. Nair, Structural and Biochemical Studies of a Biocatalyst for the Enzymatic Production of Wax Esters, ACS Catal., 2018, 8(7), 6334–6344 CrossRef CAS PubMed.
- M. G. Chacón, E. G. Kendrick and D. J. Leak, Engineering Escherichia coli for the production of butyl octanoate from endogenous octanoyl-CoA, PeerJ, 2019, 2019(7), e6971, DOI:10.7717/peerj.6971.
- L. Lucchetta, D. Manriquez, I. El-Sharkawy, F. B. Flores, P. Sanchez-Bel and M. Zouine, et al., Biochemical and catalytic properties of three recombinant alcohol acyltransferases of melon. Sulfur-containing ester formation, regulatory role of CoA-SH in activity, and sequence elements conferring substrate preference, J. Agric. Food Chem., 2007, 55(13), 5213–5220 CrossRef CAS PubMed.
- E. R. Olukoshi and N. M. Packter, Importance of stored triacylglycerols in Streptomyces: possible carbon source for antibiotics, Microbiology, 1994, 140, 931–943, DOI:10.1099/00221287-140-4-931.
- R. Kalscheuer Genetics of Wax Ester and Triacylglycerol Biosynthesis in Bacteria, Handbook of Hydrocarbon and Lipid Microbiology, 2010, pp. 527–535 Search PubMed.
- A. Arabolaza, E. Rodriguez, S. Altabe, H. Alvarez and H. Gramajo, Multiple pathways for triacylglycerol biosynthesis in Streptomyces coelicolor, Appl. Environ. Microbiol., 2008, 74(9), 2573–2582 CrossRef CAS PubMed.
- S. Shi, J. O. Valle-Rodríguez, S. Khoomrung, V. Siewers and J. Nielsen, Functional expression and characterization of five wax ester synthases in Saccharomyces cerevisiae and their utility for biodiesel production, Biotechnol. Biofuels, 2012, 5, 7, DOI:10.1186/1754-6834-5-7.
- K. Vollheyde, K. Kühnel, F. Lambrecht, S. Kawelke, C. Herrfurth and I. Feussner, Crystal Structure of the Bifunctional Wax Synthase 1 from Acinetobacter baylyi Suggests a Conformational Change upon Substrate Binding and Formation of Additional Substrate Binding Sites, ACS Catal., 2022, 12(15), 9753–9765 CrossRef CAS.
- G. L. Ellman, Tissue Sulfhydryl Groups, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS PubMed.
- N. C. Mancipe, K. M. Mulliner, M. H. Plunkett and B. M. Barney, Canvasing the Substrate-Binding Pockets of the Wax Ester Synthase, Biochemistry, 2022, 61(10), 922–932, DOI:10.1021/acs.biochem.2c00076.
- M. Wältermann, T. Stöveken and A. Steinbüchel, Key enzymes for biosynthesis of neutral lipid storage compounds in prokaryotes: Properties, function and occurrence of wax ester synthases/acyl-CoA:diacylglycerol acyltransferases, Biochimie, 2007, 89, 230–242 CrossRef PubMed.
- J. Franke and C. Hertweck, Biomimetic Thioesters as Probes for Enzymatic Assembly Lines: Synthesis, Applications, and Challenges, Cell Chem. Biol., 2016, 23, 1179–1192 CrossRef CAS PubMed.
- F. Maglangit, S. Alrashdi, J. Renault, L. Trembleau, C. Victoria and M. H. Tong, et al., Characterization of the promiscuous: N-Acyl CoA transferase, LgoC, in legonoxamine biosynthesis, Org. Biomol. Chem., 2020, 18(12), 2219–2222 RSC.
- R. Kalscheuer, T. Stöveken, H. Luftmann, U. Malkus, R. Reichelt and A. Steinbüchel, Neutral lipid biosynthesis in engineered Escherichia coli: Jojoba oil-like wax esters and fatty acid butyl esters, Appl. Environ. Microbiol., 2006, 72(2), 1373–1379 CrossRef CAS PubMed.
- S. Galaz, L. Morales-Quintana, M. A. Moya-Leõn and R. Herrera, Structural analysis of the alcohol acyltransferase protein family from Cucumis melo shows that enzyme activity depends on an essential solvent channel, FEBS J., 2013, 280(5), 1344–1357 CrossRef CAS PubMed.
- B. M. Barney, J. M. Ohlert, J. G. Timler and A. M. Lijewski, Altering small and medium alcohol selectivity in the wax ester synthase, Appl. Microbiol. Biotechnol., 2015, 99(22), 9675–9684 CrossRef CAS PubMed.
- A. W. Senior, R. Evans, J. Jumper, J. Kirkpatrick, L. Sifre and T. Green, et al., Improved protein structure prediction using potentials from deep learning, Nature, 2020, 577(7792), 706–710 CrossRef CAS PubMed.
- M. Mirdita, K. Schütze, Y. Moriwaki, L. Heo, S. Ovchinnikov and M. Steinegger, ColabFold: making protein folding accessible to all, Nat. Methods, 2022, 19(6), 679–682 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cb00107e |
| This journal is © The Royal Society of Chemistry 2023 |