Benzophenone-containing phosphors with an unprecedented long lifetime of 1.8 s under ambient conditions†
Yuming
Su
ab,
Minjian
Wu
b,
Guangming
Wang
b,
Jiuyang
Li
b,
Xuefeng
Chen
b,
Xun
Li
b,
Guoxiang
Wang
*a and
Kaka
Zhang
 *b
*b
aSchool of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, People's Republic of China. E-mail: 11991397@hnist.edu.cn
bKey Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, People's Republic of China. E-mail: zhangkaka@sioc.ac.cn
First published on 13th January 2023
Abstract
It is well-known that benzophenone has a short phosphorescence lifetime of around 1 ms even at 77 K. Here we report a benzophenone-containing emitter with an unprecedented long phosphorescence lifetime of 1.8 s under ambient conditions, which can be attributed to its T1 state of localized excitation nature as revealed by detailed studies.
Manipulation of triplet excited states is of vital importance for fabricating high-performance room-temperature phosphorescence (RTP) materials.1–17 In organic systems, due to the spin-forbidden nature of the triplet population, transformation and decay, it is challenging to construct highly efficient and long-lived RTP materials under ambient conditions. Intersystem crossing and phosphorescence decay represent two of the most important photophysical processes in organic RTP systems, with rate constants of kISC and kP, respectively. Diverse strategies have been developed to enhance kISC or kP or both to increase RTP efficiency or RTP lifetime.18–25 Among these strategies, the heavy atom effect (HAE) and the introduction of n–π* transitions are the mostly used and very reliable methods to enhance both kISC and kP.26–31 For example, the internal HAE and external HAE have been reported to promote spin–orbit coupling, and facilitate S1-to-T1 ISC and T1-to-S0 phosphorescence.26–29 In some circumstance, the RTP efficiency can exceed 50%. The involvement of n–π* transition characters has been reported to reduce the fluorescence decay rate (kF), which is helpful for organic systems to achieve high ΦISC.30,31 On the other hand, T1 states with n–π* characters show large kP to harvest triplet energies. Despite the advantages of the triplet population and harvesting, the strategies based on HAE and n–π* transition show severe side effects of reducing the RTP lifetimes (τP).
The long-lived excited states are the defining characters of RTP materials, which endow the opportunity for their application in various fields.1–17 The present study focuses on RTP lifetimes in benzophenone-containing systems. It is known that benzophenone has high ΦISC close to unity because of two reasons. (1) The S1 state of benzophenone shows typical n–π* character and has small kF around 106 s−1. (2) The T2 state of π–π* character has an energy level close to the S1 state of n–π* character; according to the energy gap law and the El-Sayed rule, the S1-to-T2 ISC is very fast with kISC around 1011 s−1. However, benzophenone's T1 state is also of n–π* character to exhibit large kP and thus short RTP lifetime. Actually, even at low temperatures such as 77 K where nonradiative decay of the T1 state is sufficiently suppressed, the phosphorescence lifetime of benzophenone is still around 1 ms. To increase the RTP lifetimes, various benzophenone-containing systems have been reported, some of which exhibit RTP lifetimes longer than 0.1 s.4,20,32–34 The balance of RTP efficiency and RTP lifetime has also been sufficiently discussed in a recent study.31 It is found that most of the reported benzophenone-containing materials are based on single-component systems, with two-component systems being rarely explored. In addition, the RTP lifetimes in the reported benzophenone-containing systems are mostly several hundred ms or around 0.5 s. It remains a formidable task to achieve long RTP lifetimes in benzophenone-containing systems, for example, 0.8 s, 1.0 s and even longer.
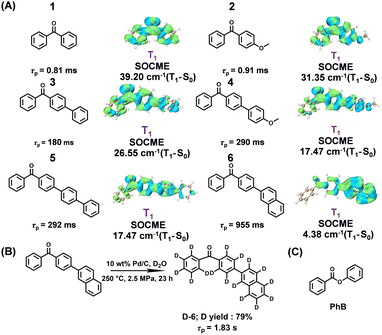
Here, we report the fabrication of a series of benzophenone-containing materials via a dopant-matrix strategy; benzophenone-containing compounds 1 to 6 are used as luminescent dopants while phenyl benzoate (PhB) serves as the organic matrix (Fig. 1). It is found that 1-PhB and 2-PhB materials show RTP properties, but have insignificant room-temperature afterglow (τP around 1 ms or several ms). 3-PhB, 4-PhB and 5-PhB materials exhibit afterglow with τP of several hundred ms under ambient conditions. Interestingly, 6-PhB materials have been found to display ultralong τP close to 1 s. Furthermore, after deuteration of 6, the deuterated 6-PhB materials exhibit τP up to 1.8 s, which is among the longest values in the reported benzophenone-containing systems.30–34 TD-DFT calculations show that the component of localized excitation in the T1 states increases from 1-PhB to 6-PhB, which is in line with the increase of τP from 1-PhB to 6-PhB (Fig. 1).
The synthetic procedures and structural characterization of the benzophenone-containing compounds 1–6 and deuterated 6 are attached in the ESI.† Solution UV-vis measurements and TD-DFT calculations show that the lower-energy absorption bands of compounds 1–6 at around 350 nm can be assigned as S0 to S1 transitions of mainly n–π* excitation characters, while the intense higher-energy bands can be attributed to S0 to Sn (n ≥ 2) transitions of π–π* or intramolecular charge transfer (ICT) characters (Table S1 and Fig. S1–S8, ESI†). 1–6 solids under ambient conditions show insignificant room-temperature afterglow (τP < 0.1 s) (Fig. S9, ESI†).
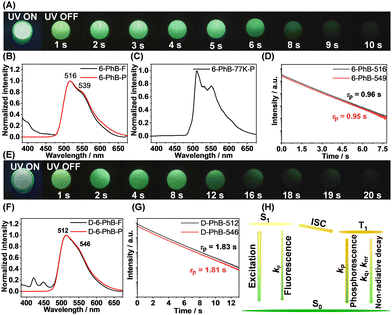
We apply the dopant-matrix strategy to fabricate afterglow materials and select the electronically inert PhB matrix to accommodate luminescent dopants. It is found that 1-PhB and 2-PhB materials still don’t show room-temperature afterglow, while 3-PhB to 6-PhB materials exhibit significant room-temperature afterglow that can last for 2 to 10 seconds in a dark room (Table S2, Fig. S10–S15, ESI† and Fig. 2A). Table S2 (ESI†) summarizes the photophysical data of these materials, among which the 6-PhB materials display very long afterglow lifetimes. The 6-PhB materials at room-temperature show a minor band in the range of 390 nm to 470 nm and a major band ranging from 470 nm to 650 nm (Fig. 2B) in their steady-state emission spectra. Their delayed emission spectra (1 ms delay) at room temperature are found to coincide with the 470–650 nm major band of the steady-state spectra, both of which exhibit emission maxima at 516 nm and a shoulder at 539 nm (Fig. 2B). Low temperature delayed emission spectra (1 ms delay) at 77 K display a clearly resolved phosphorescence band (Fig. 2C), which locates in the same region as the room-temperature delayed emission band (Fig. 2B). These results suggest that the 470–650 nm major band originates from phosphorescence, while the 390–470 nm minor band can be assigned as fluorescence (Fig. 2B); such kind of steady-state emission spectra indicate high ΦISC in the 6-PhB system.
The phosphorescence decay of 6-PhB materials follows single-exponential decay with RTP lifetimes of 0.96 s (Fig. 2D). It is known that deuteration of RTP molecules can reduce intramolecular motions and nonradiative decay of triplet states;35–37 the vibration of C–D bonds is much weaker than C–H bonds. However, deuteration of benzophenone-containing RTP molecules has been rarely reported. Here we perform deuteration of 6 to enhance the emission (Text S1, ESI†) and elongate the RTP lifetimes of 6-PhB systems (Fig. 1B). The deuterated 6-PhB materials show similar delayed emission spectra (Fig. 2F), while the RTP lifetimes from the phosphorescence decay profile are found to reach 1.8 s (Fig. 2G and Text S1, ESI†), which is among the longest values in the reported benzophenone-containing systems.
To investigate the afterglow mechanism in 6-PhB systems, several mechanisms should be ruled out. First, the reported studies showed that excited state energy transfer from matrix to dopant can give rise to room-temperature afterglow.38–40 This is not the case in 6-PhB afterglow systems excited by 350 nm light, because the PhB matrix cannot be excited by 350 nm light to serve as the donor of energy transfer (Fig. S16, ESI†). Second, PhB (HOMO = −6.96 eV, LUMO = −1.79 eV) has lower-lying HOMO and higher-lying LUMO than 6 (HOMO = −5.86 eV, LUMO = −1.84 eV), so the organic long persistent luminescence mechanism,41 which is based on intermolecular charge transfer between matrix and dopant, is not responsible for the afterglow in the 6-PhB system (Fig. S17, ESI†). Third, PhB's T1 levels are higher than 6's S1 and T1 levels, so the afterglow mechanism based on the matrix's T1 mediation13,42 is also not responsible for 6-PhB's afterglow (Fig. S18, ESI†). Fourth, HPLC of 6 and the match of the UV-vis/excitation spectra of the 6 systems can reject an impurity mechanism (Fig. S19 and S20, ESI†).43 Fifth, a thermally activated delayed fluorescence mechanism,44–47 which can also lead to room-temperature afterglow, has been ruled out by the low temperature delayed emission studies (Fig. 2C). Based on the above experiments and analyses, we propose that, upon excitation, singlet excited states of 6 form and subsequently undergo efficient ISC to reach triplet excited states (Fig. 2H). The PhB matrix suppresses nonradiative decay of 6's T1 and protects 6's T1 from oxygen quenching. The phosphorescence decay of 6's T1 in the rigid PhB matrix gives rise to the long-lived RTP of the 6-PhB materials under ambient conditions.
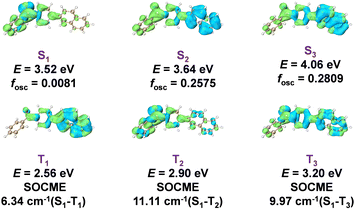
To further understand the triplet population and decay and the long-lived RTP property, we performed theoretical studies on the excited states of the 6 systems. TD-DFT calculations (Fig. 3) show that there are rich S1 to Tn channels with large spin–orbit coupling matrix elements (SOCMEs), which is consistent with the highly efficient intersystem crossing in the 6 systems. It is interesting to find that 6's T1 state has significant localized excitation (LE) character of the naphthalene group and small n–π* character of the benzophenone group, exhibiting T1 to S0 SOCME of only 4.38 cm−1 (Fig. 3 and 1A). In striking contrast, the T1 states of 1–5 show significant n–π* characters of the benzophenone groups with SOCME above 17 cm−1 (Fig. 1A). It is known that the kP values of 3n–π* states are much larger than those of 3LE states, so it is understandable that 6-PhB materials possess much longer RTP lifetimes than the 1-PhB to 5-PhB materials. These results indicate the important role of the manipulation of triplet excited states for fabricating high-performance RTP materials.
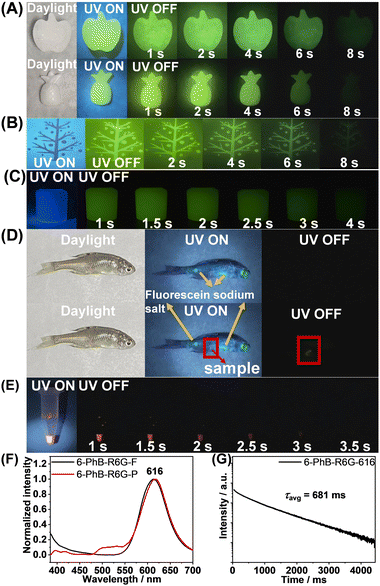
In view of the long RTP lifetimes and the excellent processability of the PhB matrix, the 6-PhB materials are selected for the demonstration of the functions of afterglow materials. The 6-PhB materials can be readily processed into fruit-shaped objects by melt casting with the aid of silicone molds (Fig. 4A). The 6-PhB materials can also be processed into aqueous afterglow dispersions in the presence of Pluronic F127 surfactant (Fig. 4C). Preliminary bioimaging studies in living fish show that the afterglow imaging mode has very clean background and can avoid the fluorescence interference (Fig. 4D). The 6-PhB materials have also been found to serve as a donor for energy transfer to fabricate long-lived red afterglow materials. By introducing rhodamine 6G (R6G) into the 6-PhB system, the resultant three-component materials exhibit red afterglow (Fig. 4E) under ambient conditions with a delayed emission band at 616 nm (Fig. 4F) and afterglow lifetime up to 681 ms (Fig. 4G and Fig. S21, ESI†). This provides an indirect pathway to achieve red afterglow materials, which can bypass the restriction of the energy gap law in directly constructing red RTP materials.
In conclusion, unlike the short RTP lifetimes reported in the benzophenone system, the present study exhibits a benzophenone-containing emitter with unprecedented long phosphorescence lifetime of 1.8 s under ambient conditions. Experimental and theoretical studies reveal that the 6-PhB materials inherit the advantage of highly efficient intersystem crossing of benzophenone-containing systems and generate a long-lived T1 state of significant 3LE nature from the naphthalene group. The present study demonstrates that it is still possible to achieve very long-lived RTP materials in benzophenone-containing systems despite the active nature of n–π* transitions in the organic systems. The detailed studies indicate that the key to achieving such long-lived RTP is to disrupt the contribution of n–π* transitions in the T1 states.
With this understanding, we believe that the incorporation of suitable functional groups of low T1 levels and 3LE characters into benzophenone-containing systems would give rise to afterglow materials with even higher performances. Therefore, we believe that the present study provides a new, robust and practical pathway for fabricating long-lived RTP materials, which would have a significant impact in the corresponding research and application fields.
We acknowledge the financial support from the National Natural Science Foundation of China (22175194), the Shanghai Scientific and Technological Innovation Project (20QA1411600, 20ZR1469200), and the Hundred Talents Program from the Shanghai Institute of Organic Chemistry (Y121078).
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed
.
- W. Zhao, Z. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869–885 CrossRef CAS
.
- N. Gan, H. Shi, Z. An and W. Huang, Adv. Funct. Mater., 2018, 28, 1802657 CrossRef
.
- X. Ma, J. Wang and H. Tian, Acc. Chem. Res., 2019, 52, 738–748 CrossRef CAS PubMed
.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef
.
- A. Forni, E. Lucenti, C. Botta and E. Cariati, J. Mater. Chem. C, 2018, 6, 4603–4626 RSC
.
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS PubMed
.
- M. Singh, K. Liu, S. Qu, H. Ma, H. Shi, Z. An and W. Huang, Adv. Opt. Mater., 2021, 9, 2002197 CrossRef CAS
.
- T. Zhang, X. Ma, H. Wu, L. Zhu, Y. Zhao and H. Tian, Angew. Chem., Int. Ed., 2020, 59, 11206–11216 CrossRef CAS PubMed
.
- W. Jia, Q. Wang, H. Shi, Z. An and W. Huang, Chem. – Eur. J., 2020, 26, 4437–4448 CrossRef CAS PubMed
.
- S. Guo, W. Dai, X. Chen, Y. Lei, J. Shi, B. Tong, Z. Cai and Y. Dong, ACS Mater. Lett., 2021, 3, 379–397 CrossRef CAS
.
- S. Hirata, Appl. Phys. Rev., 2022, 9, 011304 CAS
.
- X. Yan, H. Peng, Y. Xiang, J. Wang, L. Yu, Y. Tao, H. Li and W. Huang, Small, 2022, 18, e2104073 CrossRef PubMed
.
- H. Gao and X. Ma, Aggregate, 2021, 2, e38 Search PubMed
.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS PubMed
.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed
.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS
.
- Z. Yang, Z. Mao, X. Zhang, D. Ou, Y. Mu, Y. Zhang, C. Zhao, S. Liu, Z. Chi, J. Xu, Y.-C. Wu, P.-Y. Lu, A. Lien and M. R. Bryce, Angew. Chem., Int. Ed., 2016, 55, 2181–2185 CrossRef CAS PubMed
.
- Z. Yang, C. Xu, W. Li, Z. Mao, X. Ge, Q. Huang, H. Deng, J. Zhao, F. L. Gu, Y. Zhang and Z. Chi, Angew. Chem., Int. Ed., 2020, 59, 17451–17455 CrossRef CAS PubMed
.
- Y. Wang, J. Yang, M. Fang, Y. Yu, B. Zou, L. Wang, Y. Tian, J. Cheng, B. Z. Tang and Z. Li, Matter, 2020, 3, 449–463 CrossRef
.
- D. Li, F. Lu, J. Wang, W. Hu, X.-M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916–1923 CrossRef CAS PubMed
.
- Z.-Y. Zhang, Y. Chen and Y. Liu, Angew. Chem., Int. Ed., 2019, 58, 6028–6032 CrossRef CAS PubMed
.
- X.-F. Wang, H. Xiao, P.-Z. Chen, Q.-Z. Yang, B. Chen, C.-H. Tung, Y.-Z. Chen and L.-Z. Wu, J. Am. Chem. Soc., 2019, 141, 5045–5050 CrossRef CAS PubMed
.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed
.
- A. Fermi, G. Bergamini, R. Peresutti, E. Marchi, M. Roy, P. Ceroni and M. Gingras, Dyes Pigm., 2014, 110, 113–122 CrossRef CAS
.
- J. Wang, X. Gu, H. Ma, Q. Peng, X. Huang, X. Zheng, S. H. P. Sung, G. Shan, J. W. Y. Lam, Z. Shuai and B. Z. Tang, Nat. Commun., 2018, 9, 2963 CrossRef PubMed
.
- X.-K. Ma, W. Zhang, Z. Liu, H. Zhang, B. Zhang and Y. Liu, Adv. Mater., 2021, 33, 2007476 CrossRef CAS PubMed
.
- W. Z. Yuan, X. Y. Shen, H. Zhao, J. W. Y. Lam, L. Tang, P. Lu, C. Wang, Y. Liu, Z. Wang, Q. Zheng, J. Z. Sun, Y. Ma and B. Z. Tang, J. Phys. Chem. C, 2010, 114, 6090–6099 CrossRef CAS
.
- H. Ma, Q. Peng, Z. An, W. Huang and Z. Shuai, J. Am. Chem. Soc., 2019, 141, 1010–1015 CrossRef CAS PubMed
.
- W. Zhao, Z. He, J. W. Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao and B. Z. Tang, Chem, 2016, 1, 592–602 CAS
.
- Z. He, W. Zhao, J. W. Y. Lam, Q. Peng, H. Ma, G. Liang, Z. Shuai and B. Z. Tang, Nat. Commun., 2017, 8, 416 CrossRef PubMed
.
- S. M. A. Fateminia, Z. Mao, S. Xu, Z. Yang, Z. Chi and B. Liu, Angew. Chem., Int. Ed., 2017, 56, 12160–12164 CrossRef CAS PubMed
.
- Y. Sun, J. Liu, J. Li, X. Li, X. Wang, G. Wang and K. Zhang, Adv. Opt. Mater., 2022, 10, 2101909 CrossRef CAS
.
- X. Li, G. Wang, J. Li, Y. Sun, X. Deng and K. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 1587–1600 CrossRef CAS PubMed
.
- J. Liu, Y. Sun, G. Wang, X. Chen, J. Li, X. Wang, Y. Zou, B. Wang and K. Zhang, Adv. Opt. Mater., 2022, 10, 2201502 CrossRef CAS
.
- J.-X. Wang, H. Zhang, L.-Y. Niu, X. Zhu, Y.-F. Kang, R. Boulatov and Q.-Z. Yang, CCS Chem., 2020, 2, 1391–1398 CrossRef CAS
.
- S. Xu, W. Wang, H. Li, J. Zhang, R. Chen, S. Wang, C. Zheng, G. Xing, C. Song and W. Huang, Nat. Commun., 2020, 11, 4802 CrossRef CAS PubMed
.
- S. Kuila and S. J. George, Angew. Chem., Int. Ed., 2020, 59, 9393–9397 CrossRef CAS PubMed
.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed
.
- Y. Lei, W. Dai, J. Guan, S. Guo, F. Ren, Y. Zhou, J. Shi, B. Tong, Z. Cai, J. Zheng and Y. Dong, Angew. Chem., Int. Ed., 2020, 59, 16054–16060 CrossRef CAS PubMed
.
- C. Chen, Z. Chi, K. C. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Yang and B. Liu, Nat. Mater., 2021, 20, 175–180 CrossRef CAS PubMed
.
- X. Wang, Y. Sun, G. Wang, J. Li, X. Li and K. Zhang, Angew. Chem., Int. Ed., 2021, 60, 17138–17147 CrossRef CAS PubMed
.
- Y. Pan, J. Li, X. Wang, Y. Sun, J. Li, B. Wang and K. Zhang, Adv. Funct. Mater., 2022, 32, 2110207 CrossRef CAS
.
- G. Wang, J. Li, X. Li, X. Wang, Y. Sun, J. Liu and K. Zhang, Chem. Eng. J., 2022, 431, 134197 CrossRef CAS
.
- G. Wang, X. Chen, J. Liu, S. Ding and K. Zhang, Sci. China: Chem., 2022 DOI:10.1007/s11426-022-1432-y
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06601g |
| This journal is © The Royal Society of Chemistry 2023 |