Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hexagon based Mn(II) rod metal–organic framework – structure, SF6 gas sorption, magnetism and electrochemistry†
Francoise M.
Amombo Noa
 *a,
Ocean
Cheung
*a,
Ocean
Cheung
 b,
Michelle
Åhlén
b,
Elisabet
Ahlberg
b,
Michelle
Åhlén
b,
Elisabet
Ahlberg
 c,
Priyanka
Nehla
b,
Germán
Salazar-Alvarez
c,
Priyanka
Nehla
b,
Germán
Salazar-Alvarez
 b,
Soheil
Ershadrad
d,
Biplab
Sanyal
b,
Soheil
Ershadrad
d,
Biplab
Sanyal
 d and
Lars
Öhrström
d and
Lars
Öhrström
 *a
*a
aChemistry and Biochemistry, Department of Chemistry and Chemical Engineering, Chalmers University of Technology, Gothenburg SE-41296, Sweden. E-mail: mystere@chalmers.se; ohrstrom@chalmers.se
bDepartment of Materials Science and Engineering, Ångström Laboratory, Uppsala University, Uppsala SE-751 03, Sweden
cDepartment of Chemistry & Molecular Biology, University of Gothenburg, Gothenburg 405 30, Sweden
dDepartment of Physics & Astronomy, Uppsala University, Box-516, Uppsala 75120, Sweden
First published on 25th January 2023
Abstract
A manganese(II) metal–organic framework based on the hexatopic hexakis(4-carboxyphenyl)benzene, cpb6−: [Mn3(cpb)(dmf)3], was solvothermally prepared showing a Langmuir area of 438 m2 g−1, rapid uptake OF sulfur hexafluoride (SF6) as well as electrochemical and magnetic properties, while single crystal diffraction reveals an unusual rod-MOF topology.
Metal–Organic Frameworks, colloquially known as MOFs,1 are coming of age with advanced2 and basic3 textbooks published, and gas storage devices on the market with other applications close to commercialization.4 An emerging application is the capture of SF6, a greenhouse gas some 22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times more potent than CO2, used in industrial settings because of its dielectric properties, its non-toxicity, and thermal stabilty.5,6
000 times more potent than CO2, used in industrial settings because of its dielectric properties, its non-toxicity, and thermal stabilty.5,6
Manganese(II) is an attractive choice for such MOF construction as it is ubiquitous, non-toxic and present in relatively high amounts in most living systems. It is also a less common MOF metal ion, compared to i.e. Zn(II).7 In contrast to zinc(II) it is also magnetically and electrochemically active, properties that have recently been investigated.8 Higher stability may be induced in such MOFs by having the metal secondary building units (SBUs) forming an infinite rod.9,10
Here we explore Mn(II) with the hexatopic linker hexakis(4-carboxyphenyl)benzene, cpb6−, Fig. 1. This hexagon shaped linker,11 has earlier led us to investigate unique topological properties,12 and recently we implicated the concerted movement of the six carboxyphenyl groups in gate opening CO2 gas sorption dynamics in a rod-MOF.13 We noted that the size of the rhombic channels formed by pairwise stacks of cpb linkers would probably conform to the 7 Å diameter suggested as optimal for SF6 capture,14 and therefore targeted this greenhouse gas in the present gas sorption study.
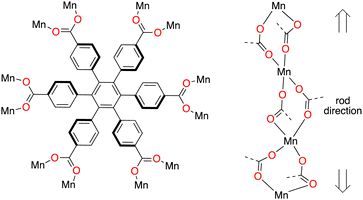 | ||
| Fig. 1 The linker 1,2,3,4,5,6-hexakis(4-carboxyphenyl) benzene, cpb6−, and how it is connected to a MOF in [Mn3(cpb)(dmf)3], CTH-18. | ||
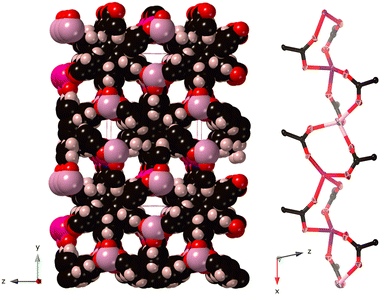
Solvothermal synthesis in dimethylformamide (dmf) at 120 °C gave the MOF [Mn3(cpb)(dmf)3], CTH-18, in good yield. Contrary to some other M(II) cpb MOFs that form 2D kgd-nets with counter ions partly blocking the pores12 the single crystal structure of CTH-18 shows an infinite rod metal SBU pairwise connecting the six-connected cpb linkers creating two types of channels running parallel with the rods having approximate minimal dimensions 4.3 × 6.0 Å and 3.4 × 4.7 Å when the van der Waals radii have been excluded. Thus, every Mn(II) only connects to four cpb linkers thereby maintaining a neutral framework, see Fig. 2 and Fig. S1 (ESI†).
CTH-18 is stable in organic solvents including ethanol, unstable in 1 M NaOH(aq) and transforms to other crystalline phases in 1 M HCl and deionized water. Thermogravimetric studies in air indicated gradual solvent loss from the framework below 300 °C, and rapid breakdown of the MOF at 425 °C.
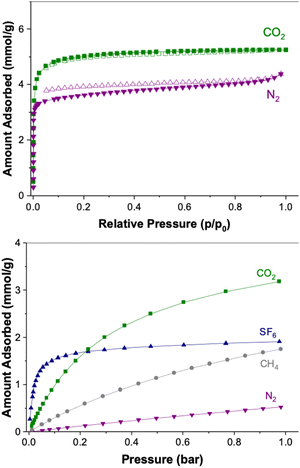
Taking the TGA results into account, the material was activated in dynamic vacuum at 250 °C prior to gas sorption analysis. N2 sorption isotherms recorded at 77 K demonstrated the microporous nature of the material, shown by the steep N2 adsorption isotherm shape at the very low-pressure regime, Fig. 3 top. However, we noted a very slow N2 adsorption kinetics on CTH-18, especially at low relative pressures. In fact, repeated N2 sorption experiments yielded different equilibrium uptakes due to this kinetic effect (and the equilibrium N2 uptake points at low pressures would take several days to reach). The apparent hysteresis shown by the N2 desorption isotherm was likely to be an artefact of this slow N2 adsorption rather than N2 entrapment, i.e. N2 adsorption had not reached equilibrium when recording the adsorption isotherm.
The slow diffusion of N2 is most likely related to the dimensions of the pore channels being very close to the kinetic diameter of N2 (3.6 Å). We therefore carried out CO2 sorption experiments at −78 °C as this molecule has a smaller kinetic diameter (3.3 Å) and thus faster diffusion within these narrow pore channels. Indeed, we did not observe slow CO2 adsorption kinetics on CTH-18 and neither hysteresis upon desorption. The specific BET and Langmuir surface area of CTH-18 estimated using the CO2 adsorption isotherm were 354 and 438 m2 g−1, respectively (values estimated using the N2 isotherm were 289 and 356 m2 g−1).
The pore size distributions (PSD) were estimated using the CO2 adsorption isotherm by Density Functional Theory (DFT), with the slit pore model. The DFT PSD of CTH-18 are plotted in Fig. S2 (ESI†) and showed two distinct types of pores with estimated diameters of ∼4.3 and ∼5.1 Å. The estimated pore diameters should not be taken as accurate numbers but they were in a comparable range to the crystallographic pore sizes of 4.3 × 6.0 Å and 3.4 × 4.7 Å.
The 20 °C N2, CH4, SF6 and CO2 adsorption isotherms are shown in Fig. 3. CTH-18 showed selective uptake towards greenhouse gases over N2. The CO2 uptake at 1 bar (20 °C) reached over 3.0 mmol g−1 with an isosteric heat of CO2 sorption of around 30–35 kJ mol−1 at up to 2.5 mmol g−1 loading (Fig. S3, ESI†).
The SF6 adsorption isotherm showed very sharp increase at low pressures. This sharp increase also indicated that the pore size was close to the kinetic diameter of SF6 (5.5 Å). As the pore size of CTH-18 was similar to the size of the kinetic diameter of the adsorbate gas (SF6), enhanced van der Waals interaction between the pore surface and the adsorbate gas was expected.6,14,15 The enhanced interaction was reflected by the high isosteric heat of SF6 adsorption (∼40–50 kJ mol−1).
The greenhouse gas selectivity of CTH-18 was estimated using s = (q1/q2)/(p1/p2). The greenhouse gas selectivities were: for CO2/N2 ∼ 16 (15% CO2, 85% N2), for SF6/N2 ∼ 29 (10% SF6, 90% N2) and CH4/N2 ∼ 3.9 (50% CH4, 50% N2). The SF6/N2 selectivity of CTH-18 is thus slightly lower than some highly SF6 selective MOFs such as Yb-TBAPy or UU-200.6,14 However, the SF6 uptake on CTH-18 at 0.1 bar was higher than many other MOFs, including Zn-MOF-74 (∼1.35 mmol g−1 at 298 K)16 UiO-66-Zr (∼0.75 mmol g−1 at 298 K)17 or SU-100 (∼1.08mmol g−1 at 298 K).18
The SF6 uptake kinetics on CTH-18 is shown in Fig. S3 (ESI†) to be reasonably fast, and over 80% of the equilibrium uptake was reached within 600 s. Moreover, CTH-18 showed high cyclic SF6 uptake stability (Fig. 4 and Fig. S4, ESI†) with the BET/Langmuir specific area remained unchanged after a number of adsorption/desorption cycles (both with vacuum swing or temperature swing SF6 adsorption).
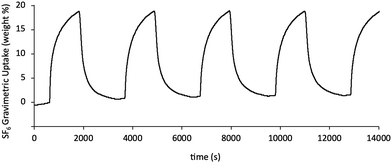 | ||
| Fig. 4 TGA cyclic SF6 uptake on CTH-18. Adsorption was carried out at 30 °C over 20 minutes, regeneration by heat (desorption) was carried out at 100 °C for 20 minutes. | ||
Water adsorption/desorption isotherms are shown in Fig. S3 (ESI†) indicating that CTH-18 has low water uptake at low relative pressures (p/p0). Two steps in the water uptake were observed at p/p0 between 0.1 and 0.3. The shape of the water sorption isotherms are comparable to some MOFs that have been investigated for water harvesting,19CTH-18 may thus be interesting for water harvesting applications, although this is beyond the scope of the present study.
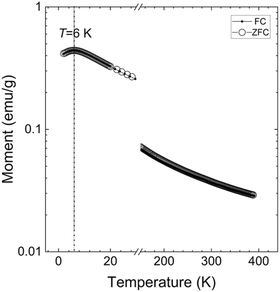
Magnetic measurements on Mn(II) MOFs more often than not reveals antiferromagnetic couplings with a few notable exceptions like [Mn12O12(O2CR)16(H2O)4] (R = Me, Et, etc.) and other so-called single molecule magnets.20 There is no reason to anticipate strong coupling through the cpb linker, and in the chain one would predict antiferromagnetic coupling by the relative close proximity of the d5 Mn(II) ions (ca. 4 Å), either directly or through spin polarization of the carboxylate linkers.21
This is also what we find for CTH-18, the temperature dependence of the magnetisation shows a Néel-like transition at around TN ≈ 6 K, Fig. 5. The field-dependent magnetisation curves show the expected linear paramagnetic response in a wide temperature interval, as can be found in Fig. S4 (ESI†).
Calculations based on density functional theory (DFT) reveals an intra-chain antiferromagnetic coupling (5.7 meV Mn−1 lower than the ferromagnetic state) between the Mn moments along the x-axis whereas the magnetic coupling between the chains is negligibly small (tens of μeV). The calculated Mn moments were around 4.5μB, establishing the oxidation state of Mn as 2+.
Electrochemistry has also, but less frequently, been investigated for Mn(II) MOFs.22–24 We note that an easily accessible Mn(IV) state may be beneficial for water oxidation,25 but we found no evidence of this in the cyclic voltammetry of CTH-18 sweeping up to potentials of 1.5 V vs. Ag/AgCl (Fig. S7, ESI†). The sweep rate dependence in a restricted potential region is shown in Fig. 6.
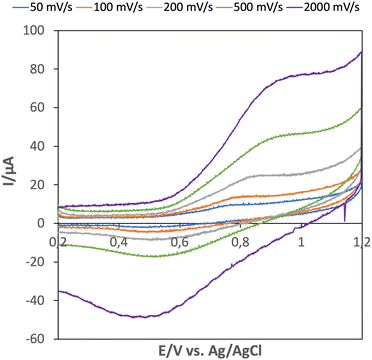 | ||
| Fig. 6 Cyclic voltammetry at different sweep rates, see legends in phosphate buffer pH = 7. The electrode consisted of carbon paste and [Mn3(cpb)(dmf)3], CTH-18. | ||
The redox couple is related to the oxidation of Mn(II) to Mn(III) in the MOF. To simulate the voltammetric response a mechanism separating the electrochemical oxidation and reduction steps was necessary. This is reasonable since the oxidation of Mn(II) to Mn(III) will require that an anion enter the structure.20 The resulting Mn(III)-anion site is reduced on the negative going scan and the anion leaves. Simulated voltammograms are shown in Fig. S6 (ESI†).
The detailed structure of CTH-18 shows no unusual features as far as Mn(II) coordination and cpb conformations are concerned, but in contrast to [La2(cpb)], CTH-1713 the cpb linkers are stacked with a longer spacing (5.7 Å vs. 5.5 Å) and linkers with opposite conformational chirality alter in the stacks, see Fig. S8 (ESI†).
The network topology on the other hand, merits a few words. CTH-18 is an example of a rod-MOF,26–28 because the metal secondary building unit cannot be reduced to a 0D point forming a dot-MOF, instead it extends in one dimension. However, rod-MOFs pose a descriptive problem as they are commonly analyzed in a different way from dot-MOFs.26,29,30
The straight rod, STR, approach is the simplest but even that in this case gives an unusual topology. Each cpb connects pairwise to the same points on the rod giving a three nodal 4- and 6-connected net with point symbol {52.63.7}{4.52.62.7}{4.54.66.74} (Fig. S9, ESI†). The more elaborate points-of-extension method that also considers the metal SBU gives a four-nodal six-connected net. Details of the topology assignment can be found in the ESI.†
In conclusion, CTH-18 shows high SF6 uptake at 0.1 bar, a pressure practically relevant for SF6 sorbents, combined with relatively fast kinetics. The SF6 selectivity compared to N2 is also higher than for CO2 and CH4, and the thermal stability, judged from TGA, is up to 425 °C. It therefore shows some promise for practical applications, shown to be needed by recent reports of industrial safety issues.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡
‡
We thank the Swedish Research Council (F. M. A. N, L. Ö.), the Olle Engqvist Foundation (F. M. A. N., L. Ö.), Chalmers areas of Advance Energy, Materials, Health and Nano (F. M. A. N.), the Swedish Research Council for Sustainable Development, #2018-00651 and the Swedish Research Council, #2020-04029 (O. C., M. Å.) and #2016-06959 (P. N., G. S. A.), the Chalmers Materials Analysis Laboratory, Chalmers students Mr A. Jonsson and Mr V. Engdahl for initial synthesis, and Prof. Peter Svedlindh at Uppsala University for discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS.
- O. M. Yaghi, M. J. Kalmutzki and C. S. Diercks, Introduction to Reticular Chemistry – Metal–Organic Frameworks and Covalent Organic Frameworks, Wiley-VCH, Weinheim, 2019 Search PubMed.
- L. Öhrström and F. M. Amombo Noa, Metal–Organic Frameworks, American Chemical Society, 2021 DOI:10.1021/acs.infocus.7e4004.
- Z. Chen, M. C. Wasson, R. J. Drout, L. Robison, K. B. Idrees, J. G. Knapp, F. A. Son, X. Zhang, W. Hierse, C. Kühn, S. Marx, B. Hernandez and O. K. Farha, Faraday Discuss., 2021, 225, 9–69 RSC.
- M. Chang, T. Yan, Y. Wei, J.-X. Wang, D. Liu and J.-F. Chen, Chem. Mater., 2022, 34, 9134–9143 CrossRef CAS.
- M. Åhlén, F. M. Amombo Noa, L. Öhrström, D. Hedbom, M. Strømme and O. Cheung, Microporous Mesoporous Mater., 2022, 343, 112161 CrossRef.
- S. G. Dunning, N. K. Gupta, J. E. Reynolds, III, M. Sagastuy-Breña, J. G. Flores, E. Martínez-Ahumada, E. Sánchez-González, V. M. Lynch, A. Gutiérrez-Alejandre, J. Aguilar-Pliego, K.-S. Kim, I. A. Ibarra and S. M. Humphrey, Inorg. Chem., 2022, 61, 15037–15044 CrossRef CAS PubMed.
- Q.-X. Jia, Y.-Q. Wang, Q. Yue, Q.-L. Wang and E.-Q. Gao, Chem. Commun., 2008, 4894–4896, 10.1039/B811865E.
- C. Healy, K. M. Patil, B. H. Wilson, L. Hermanspahn, N. C. Harvey-Reid, B. I. Howard, C. Kleinjan, J. Kolien, F. Payet, S. G. Telfer, P. E. Kruger and T. D. Bennett, Coord. Chem. Rev., 2020, 419, 20 CrossRef.
- F. M. Amombo Noa, M. Abrahamsson, E. Ahlberg, O. Cheung, C. R. Göb, C. J. McKenzie and L. Öhrström, Chem, 2021, 7, 2491–2512 CAS.
- P. T. K. Nguyen, H. T. D. Nguyen, H. Q. Pham, J. Kim, K. E. Cordova and H. Furukawa, Inorg. Chem., 2015, 54, 10065–10072 CrossRef CAS PubMed.
- F. M. Amombo Noa, E. Svensson Grape, S. M. Brülls, O. Cheung, P. Malmberg, A. K. Inge, C. J. McKenzie, J. Mårtensson and L. Öhrström, J. Am. Chem. Soc., 2020, 142, 9471–9481 CrossRef CAS PubMed.
- F. M. Amombo Noa, E. S. Grape, M. Åhlén, W. E. Reinholdsson, C. R. Göb, F.-X. Coudert, O. Cheung, A. K. Inge and L. Öhrström, J. Am. Chem. Soc., 2022, 144, 8725–8733 CrossRef CAS PubMed.
- M. Åhlén, A. Jaworski, M. Strømme and O. Cheung, Chem. Eng. J., 2021, 422, 130117 CrossRef.
- S.-M. Wang, X.-T. Mu, H.-R. Liu, S.-T. Zheng and Q.-Y. Yang, Angew. Chem., Int. Ed., 2022, 61, e202207066 CAS.
- M.-B. Kim, S.-J. Lee, C. Y. Lee and Y.-S. Bae, Microporous Mesoporous Mater., 2014, 190, 356–361 CrossRef CAS.
- M.-B. Kim, T.-U. Yoon, D.-Y. Hong, S.-Y. Kim, S.-J. Lee, S.-I. Kim, S.-K. Lee, J.-S. Chang and Y.-S. Bae, Chem. Eng. J., 2015, 276, 315–321 CrossRef CAS.
- E. S. Grape, H. Xu, O. Cheung, M. Calmels, J. Zhao, C. Dejoie, D. M. Proserpio, X. Zou and A. K. Inge, Cryst. Growth Des., 2020, 20, 320–329 CrossRef CAS.
- Z. Zheng, N. Hanikel, H. Lyu and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144(49), 22669–22675 CrossRef CAS PubMed.
- R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011–1026 RSC.
- V. Baron, B. Gillon, O. Plantevin, A. Cousson, C. Mathoniere, O. Kahn, A. Grand, L. Öhrström and B. Delley, J. Am. Chem. Soc., 1997, 119, 3500 CrossRef CAS.
- X. Wang, X. Liu, H. Rong, Y. Song, H. Wen and Q. Liu, RSC Adv., 2017, 7, 29611–29617 RSC.
- H. Yuan, G. Yuming and L. Jiang, New J. Chem., 2022, 46, 5741–5750 RSC.
- C. Yin, C. Pan, X. Liao, Y. Pan and L. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 35837–35847 CrossRef CAS PubMed.
- K. Singh, J. d J. Guillen Campos, F. Dinic, Z. Hao, T. Yuan and O. Voznyy, ACS Mater. Lett., 2020, 2, 798–800 CrossRef CAS.
- A. Schoedel, M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2016, 116, 12466–12535 CrossRef CAS PubMed.
- T. Grancha, X. Qu, M. Julve, J. Ferrando-Soria, D. Armentano and E. Pardo, Inorg. Chem., 2017, 56, 6551–6557 CrossRef CAS PubMed.
- T. Grancha, J. Ferrando-Soria, D. M. Proserpio, D. Armentano and E. Pardo, Inorg. Chem., 2018, 57, 12869–12875 CrossRef CAS PubMed.
- L. S. Xie, E. V. Alexandrov, G. Skorupskii, D. M. Proserpio and M. Dincă, Chem. Sci., 2019, 10, 8558–8565 RSC.
- P. Tshuma, B. C. E. Makhubela, L. Öhrström, S. A. Bourne, N. Chatterjee, I. N. Beas, J. Darkwa and G. Mehlana, RSC Adv., 2020, 10, 3593–3605 RSC.
- A. Lawson, World's ‘most potent greenhouse gas’ escaped during work on UK windfarm, The Guardian, 2022. https://www.theguardian.com/business/2022/nov/08/greenhouse-gas-uk-windfarm-seagreen-project-scotland.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 CrossRef CAS.
- O. Delgado-Friedrichs and M. O’Keeffe, Acta Crystallogr., Sect. A: Found. Crystallogr., 2003, 59, 351–360 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic information files, details of synthesis, characterisation, DFT calculations and topology analysis. CCDC 2226584. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06916d |
| ‡ CTH-18 was prepared by solvothermal methods in dmf, and characterised by SCXRD, PXRD, TGA, SEM, gas sorption, magnetic and electrochemical measurements and elemental analysis. Topologies were analysed with ToposPro32 and SYSTRE33 and chemical stability tests were performed. |
| This journal is © The Royal Society of Chemistry 2023 |