Open Access Article
Open Access ArticleBioorthogonal 4H-pyrazole “click” reagents†
Nile S.
Abularrage
 ,
Brian J.
Levandowski
,
Brian J.
Levandowski
 ,
JoLynn B.
Giancola
,
JoLynn B.
Giancola
 ,
Brian J.
Graham
,
Brian J.
Graham
 and
Ronald T.
Raines
and
Ronald T.
Raines
 *
*
Department of Chemistry, Massachusetts institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: rtraines@mit.edu
First published on 23rd March 2023
Abstract
4H-Pyrazoles are emerging as useful click reagents. Fluorinating the saturated center enables 4H-pyrazoles to react rapidly as Diels–Alder dienes without a catalyst but compromises the stability of these dienes under physiological conditions. To identify more stable 4H-pyrazoles for bioorthogonal chemistry applications, we investigated the Diels–Alder reactivity and biological stability of three 4-oxo-substituted 4H-pyrazoles. We found that these dienes undergo rapid Diels–Alder reactions with endo-bicyclo[6.1.0]non-4-yne (BCN) while being much more stable to biological nucleophiles than their fluorinated counterparts. We attribute the rapid Diels–Alder reactivity of the optimal oxygen-substituted pyrazole to a combination of antiaromaticity, predistortion, and spirocyclization. Their reactivity and stability suggest that 4-oxo-4H-pyrazoles can be useful bioorthogonal reagents.
Reactions that proceed rapidly and selectively under mild conditions have been termed “click reactions”.1 These reactions have had transformative effects on diverse fields, ranging from chemical biology2–6 to materials chemistry.7–9 Reactions that fit these stringent criteria have primarily been cycloadditions, such as the copper-catalysed azide–alkyne cycloaddition.7,10 To enable rapid reactivity without catalysis, creative strategies have been employed that include the use of strained reagents that are predisposed to react.
A balance of reactivity and stability is necessary for reagents that are effective in living organisms. Click reactions that can be carried out in physiological media are known as “bioorthogonal” reactions.11 Strain-promoted azide–alkyne cycloadditions transformed the field of bioorthogonal chemistry because they do not require toxic metal catalysts and can proceed without interfering with biological processes.12 These attributes have enabled unprecedented analyses of living systems.2–6
The Diels–Alder reaction has desirable attributes for click chemistry.13 Diels–Alder reactions of 4H-pyrazoles generally require acid catalysis, even with strained dienophiles, and are therefore incompatible with living systems.14–17 Recently, we found that fluorinated 4H-pyrazoles react as Diels–Alder dienes without the need for acid catalysis.18,19 Fluorination of the saturated center induces hyperconjugative antiaromaticity, which destabilizes the diene and increases its Diels–Alder reactivity.20–23 Regrettably, however, fluorination also made the dienes highly reactive towards biological nucleophiles, such as reduced glutathione, repressing biological applications.19
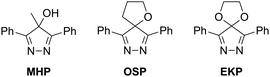
Here, we turn our attention to 4-oxo-substituted 4H-pyrazoles. We reasoned that replacing the fluoro groups with less electron-withdrawing oxo groups could maintain high Diels–Alder reactivity without compromising biological stability. Onto this scaffold, we graft another motif. Recently, we showed that geminal repulsion in the transition state is the basis for the surprisingly low reactivity of 4,4-dimethyl-4H-pyrazoles and 5,5-dimethylcyclopentadienes as Diels–Alder dienes.24 Accordingly, we chose to study three 4-oxo-4H-pyrazoles: 4-methyl-3,5-diphenyl-4H-pyrazol-4-ol (MHP), 6,9-diphenyl-1-oxa-7,8-diazaspiro[4.4]nona-6,8-diene (OSP), and 6,9-diphenyl-1,4-dioxa-7,8-diazaspiro[4.4]nona-6,8-diene (EKP) (Scheme 1). Notably, OSP and EKP are spirocyclic and will not suffer from an increase in geminal repulsion.25
We began by investigating the hyperconjugative antiaromaticity of MHP, OSP and EKP computationally with the isodesmic equation in Scheme 2.26 This equation is useful for predicting reactivity amongst a similar series of 4H-pyrazoles, such as 4,4-dimethyl-3,5-diphenyl-4H-pyrazole (DMP) and EKP (which are symmetrical and planar) or MHP and OSP (which are asymmetrical and puckered). As expected, switching the substituents from fluorine to oxygen reduced the destabilization due to antiaromaticity. The facially symmetrically substituted dienes, 4,4-difluoro-3,5-diphenyl-4H-pyrazole (DFP), EKP, and DMP were destabilized by ΔH = 12.8, 7.8, and 1.7 kcal mol−1, respectively, showing a decrease in antiaromaticity with decreasing electronegativity of the substituents. The facially asymmetrically substituted dienes 4-fluoro-4-methyl-3,5-diphenyl-4H-pyrazole (MFP), MHP, and OSP were destabilized by ΔH = 6.4, 3.2, and 2.7 kcal mol−1, respectively. The diminished antiaromatic destabilization in the 4-oxo-4H-pyrazoles suggested that they would be less reactive as Diels–Alder dienes but more stable than their fluorinated counterparts.
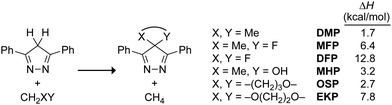 | ||
| Scheme 2 Reaction enthalpies of isodesmic equations to estimate the hyperconjugative antiaromaticity in DMP, MFP, DFP, MHP, OSP, and EKP. | ||
We synthesized EKP according to literature procedures.27 We developed synthetic routes to MHP and OSP (Fig. S1, ESI†). Briefly, these pyrazoles were synthesized by condensing the corresponding 1,3-diketone with hydrazine.28 The diketone in the route toward MHP was synthesized by the oxidation of an oxadiazole, whereas the diketone in the route toward EKP was synthesized by the reaction of 2-bromoethanol with the corresponding 1,2,3-triketone.
With the three 4-oxo-substituted 4H-pyrazoles in hand, we assessed their Diels–Alder reactivity towards a common strained dienophile, endo-bicyclo[6.1.0]non-4-yne (BCN).29 We measured second-order rate constants of these reactions in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/water. This solvent system is identical to that used to previously assess the reactivity of other 4H-pyrazoles,18,19 allowing for the direct comparison of rate constants. OSP, with a second-order rate constant of 0.17 M−1 s−1, was the most reactive of the three 4-oxo-4H-pyrazoles (Fig. S2 (ESI†) and Fig. 1). Its Diels–Alder reactivity is on the order of commonly employed azide–alkyne bioorthogonal cycloadditions.30,31 For example, the reaction of BCN and an aliphatic azide has a second-order rate constant of 0.14 M−1 s−1.31 Both MHP and EKP reacted with rate constants of 0.030 M−1 s−1.
1 methanol/water. This solvent system is identical to that used to previously assess the reactivity of other 4H-pyrazoles,18,19 allowing for the direct comparison of rate constants. OSP, with a second-order rate constant of 0.17 M−1 s−1, was the most reactive of the three 4-oxo-4H-pyrazoles (Fig. S2 (ESI†) and Fig. 1). Its Diels–Alder reactivity is on the order of commonly employed azide–alkyne bioorthogonal cycloadditions.30,31 For example, the reaction of BCN and an aliphatic azide has a second-order rate constant of 0.14 M−1 s−1.31 Both MHP and EKP reacted with rate constants of 0.030 M−1 s−1.
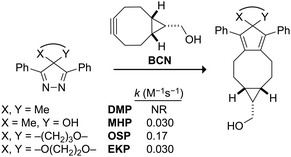 | ||
Fig. 1 Reactivity of 4H-pyrazoles in a Diels–Alder reaction. Second-order rate constants were measured in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/water at 26 °C by HPLC. Values are the mean from triplicate experiments. The value for DMP is from ref. 18; NR, no reaction. 1 methanol/water at 26 °C by HPLC. Values are the mean from triplicate experiments. The value for DMP is from ref. 18; NR, no reaction. | ||
Why is the reactivity of OSP greater than that of MHP and EKP? Asymmetric substitution in a 4H-pyrazole results in a puckering of the saturated center into an envelope-like geometry. In their energy-minimized structure, the saturated centers of MHP and OSP are puckered to be 2.6° and 4.0° above the plane of the diene π-system, respectively. EKP, even though it is symmetrically substituted, has a slight pucker of 1.3°. The puckering of OSP enhances its reactivity by reducing the conformational distortion needed to access the geometry of the syn Diels–Alder transition state.19,23 The decreased reactivity of MHP relative to OSP is likely the result of MHP being less puckered in its ground state and its experiencing geminal repulsion in its transition state.24 EKP suffers from being less puckered than either OSP or MHP. The reactivity of the 4-oxo-4H-pyrazoles is also likely to be affected by their solvation.32 Specifically, water molecules hydrogen-bonded to the 4-oxo group(s) could hinder access to the diene carbons. This solvation is expected to increase in the order: OSP < EKP < MHP,33 favoring the reactivity of OSP.
Bioorthogonal reagents must be stable in a physiological environment. For example, a difluorinated cyclooctyne (DIFO) is highly reactive in azide–alkyne cycloadditions (k = 0.076 M−1 s−1 in acetonitrile31,34) but has little stability in biological contexts35 and is thus of limited utility. To test the physiological stability of the 4-oxo-4H-pyrazoles, we incubated them for 8 h at 37 °C in a biomimetic solution of glutathione36 that contains sulfhydryl, amino, and carboxyl groups as well as a disulfide bond. As shown in Fig. 2, we detected no degradation of MHP, OSP, or EKP. These high stabilities contrast markedly with the instabilities of the fluorinated dienes MFP and DFP, which degraded completely. OSP also displayed negligible degradation when incubated for 24 h at 37 °C in cell culture medium, and high stability upon incubation for 24 h at 37 °C in a HeLa cell lysate. Together, these conditions mimic the inside and outside of human cells.
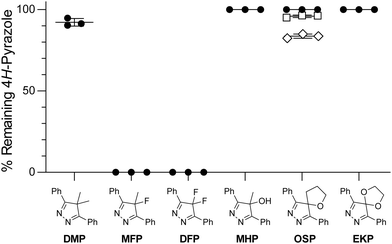 | ||
| Fig. 2 Stability of 4H-pyrazoles in physiological environments. Remaining 4H-pyrazole was measured by HPLC. ●, 8 h incubation at 37 °C in PBS containing reduced glutathione (1.0 mM) and oxidized glutathione (0.2 mM),36 along with DMSO (2% v/v for solubility). □, 24 h incubation at 37 °C in full Dulbecco's modified Eagle's medium. ◇, 24 h incubation at 37 °C in undiluted HeLa cell lysate. Values are the mean ± SD from triplicate experiments. Data for MFP and DFP are from ref. 19. | ||
To validate further the utility of 4-oxo-4H-pyrazoles as bioorthogonal reagents, we assessed the toxicity of OSP for human cells. After both 24 and 48 h incubations, we observed robust cellular viability at OSP concentrations up to 100 μM (Fig. 3). This concentration is greater than or equal to those used in typical experiments in which mammalian cells are exposed to bioorthogonal reagents.34,37–40 Thus, these cell viability data provide additional support for the utility of OSP in biological contexts.41
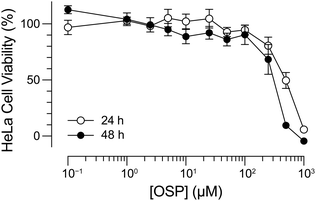 | ||
| Fig. 3 Graph of HeLa cell viability in the presence of OSP. Cells were treated with OSP (0.1 μM–1 mM) for 24 or 48 h at 37 °C. Viability was assessed with a tetrazolium-based assay.42 Values are the mean ± SE from two independent experiments, each performed with three technical replicates. | ||
In conclusion, we used principles of physical organic chemistry to derive a new bioorthogonal reagent: OSP. This diene has high Diels–Alder reactivity as a result of its antiaromaticity, predistortion and reduction of geminal repulsion through spirocyclization. The Diels–Alder reactivity of OSP is the highest of all known 4H-pyrazoles that are stable in the presence of biological nucleophiles and not toxic at relevant concentrations to human cells. Its reaction with a cyclooctyne proceeds with a second-order rate constant (0.17 M−1 s−1) that is comparable to those of commonly used bioorthogonal reagents. Synthetic handles for conjugation could be installed on either of its phenyl groups or on the spirocycle. Thus, we put forth spirocyclic 4-oxo-4H-pyrazoles as an attractive scaffold for the development of a new class of highly reactive and physiologically stable click reagents.
N. S. A. was supported by a Graduate Research Fellowship from the NSF. B. J. L. was supported by postdoctoral fellowship F32 GM137543 from the NIH. This work was supported by Grants R01 GM044783 and R35 GM148220 from the NIH. Computational resources were provided by the Extreme Science and Engineering Discovery Environment (XSEDE) Bridges at the Pittsburgh Supercomputing Center through allocation TG-CHE190066. XSEDE is supported by Grant ACI-1548562 from the NSF.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- C. S. McKay and M. G. Finn, Chem. Biol., 2014, 21, 1075–1101 CrossRef CAS PubMed.
- N. K. Devaraj, ACS Cent. Sci., 2018, 22, 952–959 CrossRef PubMed.
- R. D. Row and J. A. Prescher, Acc. Chem. Res., 2018, 51, 1073–1081 CrossRef CAS PubMed.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. Am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Nat. Rev. Methods Primers, 2021, 1, 30 CrossRef CAS PubMed.
- R. E. Bird, S. A. Lemmel, X. Yu and Q. A. Zhou, Bioconjugate Chem., 2021, 32, 2457–2479 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- Z. Geng, J. J. Shin, Y. Xi and C. J. Hawker, J. Polym. Sci., 2021, 59, 963–1042 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- B. J. Levandowski and R. T. Raines, Chem. Rev., 2021, 121, 6777–6801 CrossRef CAS PubMed.
- K. Beck, A. Höhn, S. Hünig and F. Prokschy, Chem. Ber., 1984, 117, 517–533 CrossRef CAS.
- K. Beck, S. Hünig, F.-G. Klärner, P. Kraft and U. Artschwager-Perl, Chem. Ber., 1987, 120, 2041–2051 CrossRef CAS.
- W. Adam, H. M. Harrer, W. M. Nau and K. Peters, J. Org. Chem., 1994, 59, 3786–3797 CrossRef CAS.
- W. Adam, H. Ammon, W. M. Nau and K. Peters, J. Org. Chem., 1994, 59, 7067–7071 CrossRef CAS.
- B. J. Levandowski, N. S. Abularrage, K. N. Houk and R. T. Raines, Org. Lett., 2019, 21, 8492–8495 CrossRef CAS PubMed.
- N. S. Abularrage, B. J. Levandowski and R. T. Raines, Int. J. Mol. Sci., 2020, 21, 3964 CrossRef CAS PubMed.
- L. Nyulászi and P. V. R. Schleyer, J. Am. Chem. Soc., 1999, 121, 6872–6875 CrossRef.
- I. Fernández, J. I. Wu and P. von Ragué Schleyer, Org. Lett., 2013, 15, 2990–2993 CrossRef PubMed.
- B. J. Levandowski, L. Zou and K. N. Houk, J. Comput. Chem., 2016, 37, 117–123 CrossRef CAS PubMed.
- B. J. Levandowski, L. Zou and K. N. Houk, J. Org. Chem., 2018, 83, 14658–14666 CrossRef CAS PubMed.
- B. J. Levandowski, N. S. Abularrage and R. T. Raines, Tetrahedron, 2021, 91, 132160 CrossRef CAS PubMed.
- B. J. Levandowski, N. S. Abularrage and R. T. Raines, J. Phys. Org. Chem., 2023, 36, e4478 CAS.
- An alternative method to assess antiaromaticity, nuclease-independent chemical shift (NICS) calculations, requires that the ring be planar, as in a cyclopentadienyl anion Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- M. Abe, W. Adam, W. T. Borden, M. Hattori, D. A. Hrovat, M. Nojima, K. Nozaki and J. Wirz, J. Am. Chem. Soc., 2004, 126, 574–582 CrossRef CAS PubMed.
- M. P. Sammes and A. R. Katritzky, in Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Academic Press, 1983, vol. 34, pp. 53–78 Search PubMed.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422–9425 CrossRef CAS PubMed.
- K. Lang and J. W. Chin, ACS Chem. Biol., 2014, 9, 16–20 CrossRef CAS PubMed.
- J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef PubMed.
- In contrast to oxo groups, fluoro groups do not form strong hydrogen bonds ( J. D. Dunitz and R. Taylor, Chem. – Eur. J., 1997, 3, 89–98 CrossRef CAS; J. D. Dunitz, ChemBioChem, 2004, 5, 614–621 CrossRef PubMed; D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308–319 RSC ). Accordingly, aqueous solvation is less likely to affect the Diels–Alder reactivity or stability of MFP and DFP.
- The c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of OSP, EKP, and MHP are 3.65, 3.09, and 3.01, respectively (Molinspiration, Slovenský Grob, Slovak Republic).
P values of OSP, EKP, and MHP are 3.65, 3.09, and 3.01, respectively (Molinspiration, Slovenský Grob, Slovak Republic). - J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- E. J. Kim, D. W. Kang, H. F. Leuck, M. R. Bond, S. Ghosh, D. C. Love, J.-S. Ahn, D.-O. Kang and J. A. Hanover, Carbohydr. Res., 2013, 377, 18–27 CrossRef CAS PubMed.
- M. M. Lyles and H. F. Gilbert, Biochemistry, 1991, 30, 613–619 CrossRef CAS PubMed.
- N. K. Devaraj and R. Weissleder, Acc. Chem. Res., 2011, 44, 816–827 CrossRef CAS PubMed.
- I. Nikić, J. H. Kang, G. E. Girona, I. V. Aramburu and E. A. Lemke, Nat. Protoc., 2015, 10, 780–791 CrossRef PubMed.
- K. A. Andersen, M. R. Aronoff, N. A. McGrath and R. T. Raines, J. Am. Chem. Soc., 2015, 137, 2412–2415 CrossRef CAS PubMed.
- H. E. Murrey, J. C. Judkins, C. W. Am Ende, T. E. Ballard, Y. Fang, K. Riccardi, L. Di, E. R. Guilmette, J. W. Schwartz, J. M. Fox and D. S. Johnson, J. Am. Chem. Soc., 2015, 137, 11461–11475 CrossRef CAS PubMed.
- The four measures of physiological stability and biocompatibility deployed herein provide a comprehensive assessment of reagent bioorthogonality: an incubation with millimolar glutathione, an incubation in cell culture medium and a human cell extract, and an assay of cytotoxicity.
- A. H. Cory, T. C. Owen, J. A. Barltrop and J. G. Cory, Cancer Commun., 1991, 3, 207–212 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00112a |
| This journal is © The Royal Society of Chemistry 2023 |