 Open Access Article
Open Access ArticleExploring innovative designs and heterojunctions in photocatalytic micromotors
Katherine
Villa

Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans, 16, Tarragona E-43007, Spain. E-mail: kvilla@iciq.es
First published on 14th June 2023
Abstract
Photocatalytic micromotors that convert light energy into mechanical energy have garnered increased interest due to their fast photoactivation, and potential for precise control and manipulation. This feature article provides key insights into the design of photocatalytic micromotors by using single semiconductors, and heterostructures. It also highlights the different strategies to develop efficient light-driven micromotors by minimizing electron–hole pair recombination and improving charge transfer among components. The remaining challenges and possible solutions are also discussed.
Introduction
Photoactive micromotors, made of semiconducting components, are a type of microscale device that can convert light energy into mechanical energy through photocatalytic reactions. Over the last few years, there has been an increased interest in these types of micromotors, due to their multiwavelength light responses, chemical stability, and ability to harvest both chemical and light energy to induce self-propulsion.1,2The photocatalytic material, usually an inorganic or organic semiconductor, absorbs light and generates electron–hole pairs. These charge carriers can then react with water or other chemicals in the environment, creating a gradient that propels the micromotor forward by different phoretic mechanisms.1 A more detailed explanation of the motion mechanisms can be found in previous reviews.1–3 By controlling the intensity, polarization, wavelength, and direction of the light, researchers can manipulate the motion of these devices, making them useful for a range of applications in fields such as biomedical engineering,3 environmental remediation, and energy harvesting. Moreover, the concentration and type of chemicals present in the liquid media can also influence their motion behaviors.
The robustness and variety of semiconductors make them ideal candidates for designing light-driven micromotors in comparison to photoactive organic compounds, such as azobenzene, that exhibit restricted light activation to specific wavelengths.4 Moreover, semiconductor-based micromotors offer the possibility of light absorption modulation by choosing materials with suitable bandgaps. Based on the position of the conduction and valence band potentials of the semiconducting components, the oxidative and reductive properties of these micromotors can be modulated as well. Therefore, light-driven photocatalytic micromotors with tuneable redox capabilities can be designed for specific target reactions.
The chassis of photocatalytic micromotors can be fabricated in various shapes and sizes, including spheres,5 rods,6 tubes,7 flowers,8 stars,9 butterfly-like,10 or more complex structures,11,12 to achieve specific movement patterns. Photocatalytic micromotors are typically composed of titanium dioxide. While this material has proven useful, its optical absorption limited to the UV range has led to the development of alternative micromotors composed of semiconductors that respond to visible and/or near-infrared wavelengths. In recent years, significant progress has been made in this field, resulting in the creation of more advanced and versatile light-driven micromotors.6,7,9,13,14
Since the pioneering works on amorphous AgCl and TiO2 micromotors by Sen and co-authors,15,16 a quite broad range of photocatalytic micromotors have been further designed, involving either single-component and/or multicomponent heterostructures. As can be seen in Fig. 1, the first examples of photocatalytic micromotors included UV-light responsive materials, in which the asymmetry required for their autonomous motion was provided by a heterogeneous rough surface. Then, more defined structures consisting of visible light-responsive photocatalysts were fabricated, including Fe2O3,17 CdS,18 and Ag3PO4.18 To enhance the electron–hole pair separation, Janus particles with metal–semiconductor heterostructures were further reported, including C3N4/Pt,19 Cu2O/Au,13 and BiOI/Au.20 The use of photocatalysts with hybridized orbitals and short bandgaps,9,21 such as BiVO4, also represented a promising strategy to design efficient visible-light-driven photocatalytic micromotors with enhanced speeds and improved motion capabilities, even in highly viscous glycerol media.9 Alternatively, the fabrication of heterostructured micromotors based on p–n junctions is also an attractive way of developing more sophisticated photoactive micromotors without the need of using noble metals. By focusing on the material properties, fabrication, and rational design of semiconductor structures, more efficient and robust photocatalytic micromotors can be developed in the future. Therefore, these different configurations along with their advantages and disadvantages are described in the following sections.
 | ||
| Fig. 1 Evolution of the design of photocatalytic micromotors from UV to visible-light responsive materials with different morphologies over the last years. | ||
Photocatalytic principles
The photoactivation of a semiconductor involves a series of processes that are influenced by the optical and morphological properties of the photocatalysts as well as the nature of the solid–liquid interfaces. The threshold wavelength required for inducing photocatalytic reactions can be estimated from the bandgap energy of the material (Egap), as shown in eqn (1). Where h corresponds to the Planck's constant (6.63 × 10−34 J s) and c is the speed of light (3.00 × 108 ms−1) and Egap is expressed in eV.22 Therefore, wide-bandgap semiconductors are commonly used to develop UV-light responsive micromotors, while intermediate and short-bandgap semiconductors are often used to design micromotors with light absorption in the visible and near-infrared, respectively.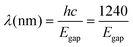 | (1) |
As represented in Fig. 2, the main steps of the photoactivation of the micromotors includes photon absorption (i), electron–hole pair separation (ii), diffusion and transport of the photogenerated charge carriers (ii), reaction efficiency, and mass transfer of reactants and products (iii).23 All the above factors have a direct influence on the self-propulsion capabilities of photocatalytic micromotors. Usually, generation, trapping, and recombination of the photogenerated charges occur in the femto-, pico-, and nano-seconds, while interfacial surface reactions take place at milli-second time scales.24 Therefore, the electron–hole pair recombination rates limit the overall photocatalytic motion efficiencies and is the bottleneck of photocatalytic processes. To overcome this limitation and enhance photocatalytic efficiencies, researchers have explored various strategies, e.g., incorporating noble metals and/or dual semiconductor components within the same structure of the micromotors. These approaches have been extensively investigated in recent years and show promise in improving the overall photocatalytic performance. In the following sections, we will discuss the most representative examples of current designs of photocatalytic micromotors. A summary of such works is also included in Table 1.
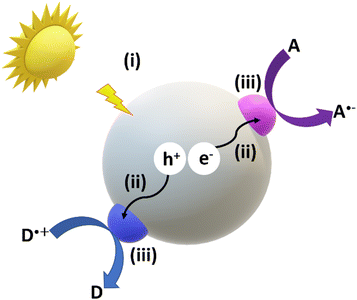 | ||
| Fig. 2 Photocatalytic mechanism illustrating the different steps that take place on a photocatalytic micro/nanomotor upon irradiation. | ||
| Type of micromotor | Material | Light source | Fuel type (% wt) | Motion mechanism | Size (μm) | Speed (μm s−1) | Fabrication method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Single semiconductor | AgCl | UV | Water | Self-diffusiophoresis | 1 | 100 | Co-precipitation | 15 |
| Single semiconductor | TiO2 | UV | Water | Self-diffusiophoresis | 0.2–2.5 | 10 | Commercial particles | 16 |
| Single semiconductor | Fe2O3 | Visible | H2O2 (1) | Self-diffusiophoresis | 1.5 | 4.5 | Sol–gel | 17 |
| Single semiconductor | BiVO4 | Visible | H2O2 (0.025) | Self-diffusiophoresis | 4–8 | 5 | Hydrothermal | 9 |
| Single semiconductor | Bi2WO6 | Visible | Water | Self-diffusiophoresis | 7 | 1.9 | Hydrothermal | 21 |
| Single semiconductor | C3N4 | Visible | H2O2 (30) | Bubble-recoil | 67 | 72 | Hydrothermal | 7 |
| Metal–semiconductor | Cu2O/Au | Visible | H2O2 (3) | Self-electrophoresis | 1.2 | 6 | Co-precipitation/thermal evaporator | 13 |
| Metal–semiconductor | TiO2/Au | UV | Water | Self-electrophoresis | 1 | 25 | Solvent extraction/evaporation/sputtering | 5 |
| Metal–semiconductor | C3N4/Pt | UV | Water | Self-electrophoresis | 2.6 | 23 | Calcination/metal reduction | 19 |
| Metal–semiconductor | BiOI/Au | Visible | Water | Self-electrophoresis | 2–4 | 1.62 | Self-assembly/sputtering | 20 |
| Metal–semiconductor | Si/Au | Visible | Water | Self-electrophoresis | 4 | 5 | Glancing angle deposition/thermal evaporator | 36 |
| Semiconductor–semiconductor | TiO2/Cu2O | UV and visible | H2O2 (1.5) | Self-phoresis/osmotic flow | 4–5 | — | Drop casting/thermal evaporator | 12 |
| Semiconductor–semiconductor | TiO2/CoO | UV and Visible | Water | Self-electrophoresis | 2–6 | 11.5 (visible) and 6.5 (UV) | Drop casting/sputtering | 48 |
| Semiconductor–semiconductor | p-Si/n+-Si | Visible | H2O2 (0.5) | Self-electrophoresis | 15 | 38 | Metal-assisted electroless chemical etching/photolithography/sputtering | 6 |
| Semiconductor–semiconductor | Si/TiO2 | UV | H2O2 (0.1) | Self-electrophoresis | 11 | 6 | wet etching/atomic layer deposition/hydrothermal | 11 |
| Semiconductor–semiconductor | Sb2S3/ZnO | Visible | 1,4-Benzoquinone/hydroquinone | Self-electrophoresis | 10 | 12 | Atomic layer deposition | 49 |
| Semiconductor–semiconductor | GaN/ZnO/Au | UV | H2O2 (10) | Self-diffusiophoresis | 4.6 | 5.5 | Epitaxial growth/sputtering | 51 |
| Semiconductor–semiconductor | TiO2/CdS | UV and visible | Water | Self-electrophoresis | 8 | 11.3 | Hydrothermal/ion-exchange/atomic layer deposition | 54 |
Single component micromotors
Single component photocatalytic micromotors have the advantage of requiring simple fabrication techniques based on wet chemical methods, such as hydrothermal, sol–gel, and co-precipitation reactions. Moreover, the yield of micromotors is usually higher than the obtained by large equipment, e.g., physical vapor deposition or sputtering techniques.25 The first examples of photocatalytic micromotors based on a single material consisted of UV-light responsive materials such as AgCl and TiO2, that were either unstable15 or required high concentrations of chemical fuels.16 Such drawbacks, combined with the necessity for a light source that was more biocompatible, have prompted the development of micromotors that respond to visible light. Therefore, semiconductors with a bandgap ranging from 2.1–2.8 eV are commonly employed as the chassis of visible-light-driven photocatalytic micromotors. Some of the most representative examples of single component photocatalytic micromotors, include the design of peanut-shaped hematite micromotors with a size of about 1.5 μm length and ∼0.6 μm diameter.17 Upon blue light irradiation, these micromotors self-propel along the surface in the presence of H2O2 as chemical fuel and tetramethylammonium hydroxide. Taking advantage of their magnetic responses, the motion direction was externally controlled by a weak magnetic field (∼1 mT). In addition, the direction was also steered by using a patterned surface. Moreover, the authors found that the etching of the surface of the micromotors was key to enhance their motion capabilities, due to an enhanced surface anisotropy. By increasing the pH of the solution, these micromotors were able to perform capture and cargo transportation of particles of sizes up to 20 μm with the aid of magnetic fields (Fig. 3(A)).17 This work illustrates the versatility that photocatalytic micromotors offer for motion control and manipulation.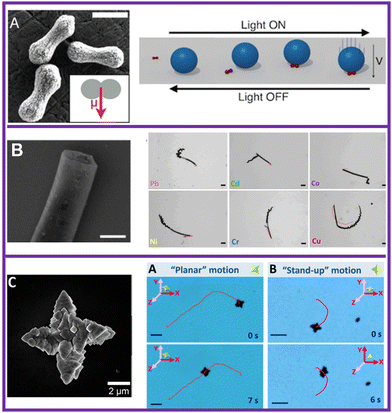 | ||
| Fig. 3 Most representative examples of single-component micromotors that are based on visible-light-responsive materials. (A) Hematite peanut-shaped micromotors for cargo transportation. Adapted with permission from ref. 17. Copyright 2013, American Chemical Society. (B) Metal-free C3N4 tubular micromotors for the removal of heavy metals. Adapted with permission from ref. 7. Copyright 2018, American Chemical Society. (C) Star-shaped BiVO4 micromotors for removal of microbial contamination. Adapted with permission from ref. 9. Copyright 2019, American Chemical Society. | ||
The development of totally metal-free C3N4 tubular micromotors that were prepared by a template-less method also represented an important milestone towards scalable fabrication methods for their potential application in the environmental field.7 This material possesses unique electronic and optical properties, making it an attractive candidate for various applications, ranging from biomedicine26 to environmental remediation. In the latter, it was found that C3N4 micromotors were able to capture different heavy metals present in the solution.7 This was attributed to metal complex formation and/or acid–base interactions between the surface of the micromotors and the metals. As a result, the speed of the micromotors increased up to 30%, requiring 20 times less amount of fuel for propulsion. On the other hand, owing to their intrinsic fluorescence, it was also possible to get an insight into the heavy metal removal rates by fluorescence quenching. Furthermore, taking advantage of their big sizes, it was possible to visualize the bubble formation rates (12–13 ms) and ejection (88 ms) over time. Overall, this study showed the opportunities that photocatalytic micromotors offer for pollutant removal with the concomitant visualization of such a decontamination process by optical means.
On the other hand, C3N4-derivates can store light energy by electron storage-release mechanisms and drive the motion of photoactive micromotors in the absence of light.27 Although this property is limited to this kind of materials and depends on the conditions of the liquid media, it is an interesting approach to further expand the applicability of these devices in highly scattering environments.
The introduction of star-shaped BiVO4 micromotors consolidated this material as a benchmark photocatalysts for fabricating visible-light responsive micromotors with a high efficiency and photostability. By changing the morphology from single-star to twin-star shaped, it was possible to modulate their motion capabilities from planar to stand-up motion modes.9 Moreover, these BiVO4 micromotors also showed smart features such as the autonomous ability to change their motion trajectories to capture and transport yeast cells. It should be noted that no magnetic guidance or surface modification was carried out. Moreover, the same yeast seeking behaviour was also confirmed in the presence of other microorganisms, such as E. coli. Further works have applied BiVO4 micromotors for preventing microbial contamination in alcoholic beverages,28 oxidation of benzylamine,29 as well as degradation of organic pollutants.10
To sum up, single component micromotors offer several advantages in terms of fabrication and high mass-production, allowing for large-scale manufacturing. Moreover, these micromotors can be produced using low-cost fabrication techniques,30 making them economically viable for various applications. Another advantage is the simplicity of their synthesis process, typically involving a one-step procedure. However, these type of micromotors often require the addition of chemical fuels for their propulsion, which can limit their autonomy and introduce additional complexity. Furthermore, their light absorption capability is usually limited to a specific wavelength region, which restricts their performance in broader light spectra. These types of micromotors usually suffer from high recombination rates of photogenerated electron–hole pairs, limiting their overall efficiency. To address some of these limitations, potential strategies such as crystal faceting show promise in enhancing the photoactivity of single component micromotors.31,32 Such configuration promotes electron–hole separation in each crystal facet within the same material, leading to enhanced motion efficiencies and photocatalytic performances.
Metal–semiconductor heterojunction
A metal–semiconductor interface is the junction between both materials. This interface has important properties for electronic devices and photovoltaic applications, as it can act as a barrier to the flow of electrical charge carriers or as a means of creating a junction for electronic devices. An optimal contact between the semiconductor and the metal is given by the characteristics of the materials and the type of contact formed between the metal and the semiconductor. Usually, ohmic junctions are formed with highly doped semiconductors, while Schottky junctions are established with slightly doped semiconductors.33Fig. 4 represents the band alignment of a metal–semiconductor (n-type) before and after contact. The vacuum level, denoted as E0, is the energy state of electrons with zero kinetic energy outside the material, either metal or semiconductor. The Fermi energy (EF) represents the highest occupied electron energy state at T = 0 K in a metal; in a non-degenerate semiconductor it lies in the gap between the valence and the conduction band. The energy difference between the Fermi energy and the vacuum level is defined as the work function, represented by Φ. On the other hand, the electron affinity, represented by χ, is defined as the energy required to move an electron from the vacuum level to the conduction band. Upon contact, the differences in the work functions lead to electron transfer from the semiconductor to the metal until the respective Fermi levels align at equilibrium. This results in the formation of a depletion region in the semiconductor, where W is the width of such a region and ΦB is the height of the potential barrier that an electron in the metal must overcome to move into the semiconductor.34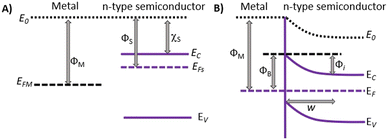 | ||
| Fig. 4 Schematic representation of the energy levels of a metal–semiconductor interface before (A) and after contact (B). | ||
The design of metal–semiconductor junctions is then a common strategy for minimizing electron–hole pair recombination. Usually, the decoration of semiconductors with co-catalysts, such as Pt and RuO2, leads to an enhancement of the reductive and oxidative pathways, respectively. In the field of photocatalytic micro/nanomotors, the design of Janus photocatalytic micromotors based on a metal–semiconductor heterojunction has been widely explored. One of the main advantages of these type of heterostructures in comparison with single-component semiconductors is their ability to achieve fast speeds in pure water. The first study based on this configuration included TiO2/Au micromotors that showed a high motion efficiency in pure water.5 The same heterostructure was also further applied to nanomotors, showing propulsion abilities in water as well.35 After this pioneering work, other type of Janus metal–semiconductor micromotors based on UV or visible-light responsive photocatalysts were further reported, including Cu2O/Au,13 Si/Au,36 Fe2O3/Au,37 BiOI/Au,20 ZnO/Pt,8 C3N4/Pt,19 and AgCl/Ag.38 However, some of these micromotors still require the addition of chemical fuels, such as H2O2 or organic solvents, to exhibit stronger propulsion abilities.
To illustrate the effect of the metal work function on the performance of photocatalytic micromotors, we considered Janus TiO2 micromotors as an example. As shown in Fig. 5(A), various Janus TiO2 micromotors with a spherical shape and containing different metallic counterparts have been reported, including TiO2/Pt,39 TiO2/Au,5 TiO2/Cu,40 TiO2/PtPd41 and TiO2/Fe.42 The differences between the metal work function values and the electron affinity of the semiconductor affect the formation of the resulting Schottky barrier, which has a direct influence on their motion capabilities (Fig. 5(B)).43 The reported velocities for Janus TiO2 micromotors based on Pt, Au, Cu, and Fe micromotors are 26.2, 25, 22.8, and 1.96 body length s−1, respectively. Therefore, the larger the work function difference with respect to the semiconductor, the higher the motion speeds. This can be attributed to the Schottky barrier junction, where the metallic counterpart acts as an electron sink and reduction active site. This contributes to minimize electron–hole pair recombination, increasing the overall photocatalytic performance. Thus, the work function value is an important parameter to consider when designing Janus micromotors based on metal–semiconductor junctions.
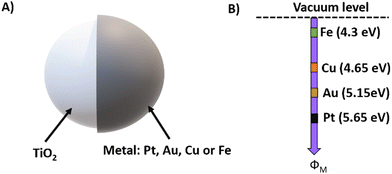 | ||
| Fig. 5 Janus photocatalytic TiO2 micromotors. (A) Schematic representation showing the different examples of Janus TiO2/metal micromotors. (B) Metal work functions of Fe, Cu, Au, and Pt. | ||
An additional advantage of Janus micromotors in comparison to single component micromotors is their ability to self-propel at a very low light intensity (2.5 × 10−3 W cm−2).5 Furthermore, as any photoresponsive micromotor, the motion speeds can be increased by modulating the light intensity (Fig. 6(A)–(E)).
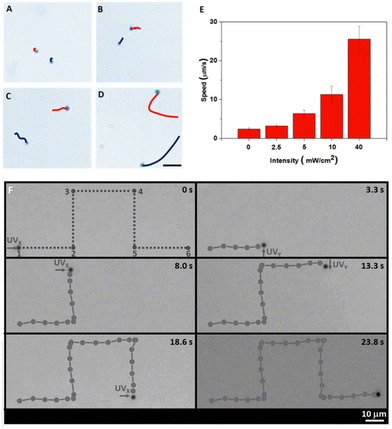 | ||
| Fig. 6 Light control of photocatalytic micromotors. (A) Motion trajectories and velocities of TiO2/Au Janus micromotors at different light intensities. Adapted with permission from ref. 5. Copyright 2016, American Chemical Society. (B) Motion control of TiO2 micromotors along X and Y light directions. Reproduced from ref. 18 with permission from John Wiley and Sons, copyright 2016. | ||
An alternative strategy to control the motion of photoactive micromotors is by taking advantage of the light penetration in semiconductors. When a light beam hits a semiconductor material, there is a decrease in the intensity as it traverses through the material. Therefore, the absorption coefficient of a semiconductor estimates how strongly the material absorbs light.18 For example, in silicon, the absorption coefficient is highest at shorter wavelengths and decreases as the wavelength increases. This means that blue light is absorbed more strongly than red light, and that light of shorter wavelengths can penetrate less deeply into the material than light of longer wavelengths.
Zhang et al. fabricated phototactic photocatalytic micromotors based on the limited light penetration depth in isotropic particles, including TiO2, CdS, and ZnO.18 By using a home-made experimental setup, it was possible to control the movement direction of the micromotors along X and Y axes. As can be seen from Fig. 6(F), TiO2 micromotors followed a predesigned motion trajectory, due to their phototactic behavior and sophisticated light control setup. Such motion control by the external light beam direction was greatly affected by the size of the micromotor. For sizes of about 1 μm, the micromotors showed phototactic behaviors, because the diameter was much larger than the penetration depth (δ). However, this was not observed for micromotors of 0.4 μm diameter since the light penetrated almost the whole body of the micromotor. On the other hand, when the size was increased up to 3.5 μm, the micromotor showed stronger friction with the glass hampering its motion.
Other factors, such as surface area or crystalline structure of the photocatalysts have also an influence on the motion efficiencies of photoactive micromotors. A more detailed discussion can be found in previous reviews.2,8
Overall, metal–semiconductor heterojunctions offer several advantages in their design and functionality. One notable advantage is their ability to self-propel in pure water under low light intensities, making them highly versatile for various environments and broadening their range of applications to biocompatible scenarios. Such metal–semiconductor-based micromotors also demonstrate high motion efficiencies and low recombination rates, resulting in improved overall performance. However, there are some disadvantages associated with their fabrication. One drawback is the low micromotor yield, which can limit their scalability and practical implementation. Additionally, the fabrication techniques required for creating these heterojunctions can be expensive and robust, posing challenges in terms of cost and feasibility. Moreover, the multistep fabrication process adds complexity and increases the chances of process-related issues. Potential strategies to overcome these challenges include using low-cost metals such as Fe, Cu, and Ni, or metal alloys, e.g., Cu/Ni and Fe/Ni. Furthermore, exploring alternative fabrication processes like emulsion polymerization, electrostatic assembly, and microfluidic techniques may offer cost-effective and accessible production of metal–semiconductor heterojunctions.
Semiconductor–semiconductor heterojunction
The development of light-driven micromotors by combining two semiconductors in the same structure is also an interesting approach to overcome the main limitations of single semiconductor-based microdevices, e.g., light absorption under specific wavelength region, limited redox potential and fast charge-pair recombination. Heterostructures are often used to facilitate charge transfer between the materials, which is necessary for the generation of a driving force. As shown in Fig. 7, there are two main types of heterostructure junctions: p–n junction and Z-scheme. The p–n heterojunction consists of the junction formed between a n-type semiconductor and a p-type semiconductor (Fig. 7(A)).44,45 Upon photoactivation, the photogenerated electrons from photocatalyst II (PS II) migrate to the conduction band of the photocatalyst I (PS I) to equilibrate the Fermi levels. In a similar fashion, the photogenerated holes from the valence band of PS I are transferred to the valence band of PS II. This results in the formation of an electrical field at the p–n interface that can drive the electrons and holes to migrate in opposite directions.43 Owing to this electron–hole separation, the charge pair recombination is greatly inhibited and the lifetime of the photogenerated carriers is extended.44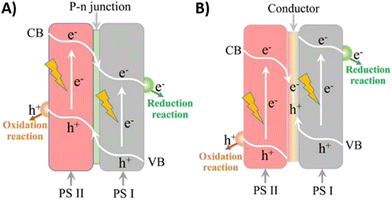 | ||
| Fig. 7 Schematic illustration of charge transfer in semiconductor heterojunctions. (A) P–n semiconductor junction and (B) Solid-state Z-scheme. Reproduced from refs. 44 and 45 with permission from John Wiley and Sons, copyright 2014, and licensee Beilstein-Institut, copyright 2018, respectively. | ||
In contrast to p–n configurations, where the redox potential is reduced compared to the bare semiconductors, the development of an all-solid-state Z-scheme heterostructure presents a promising approach to creating a photocatalytic system with a strong redox potential and minimal electron–hole pair recombination. This configuration allows for photogenerated electrons from PS II to combine with photogenerated holes from PS I, facilitated by the formed Ohmic contact (Fig. 7(B)).44 The resulting electrons, with a more negative potential, can then be utilized to carry out reductive reactions, while the holes, with a more positive potential, can be used for performing oxidative reactions. The formation of this heterostructure junction can be achieved through physical contact or with a solid electron mediator.46 A very well-known example of this solid Z-scheme configuration for the photocatalytic water splitting is the combination of Ru/SrTiO3:Rh and BiVO4.47 However, this configuration is yet to be explored for the construction of light-driven heterostructured micromotors.
Photocatalytic micromotors based on p–n heterojunctions have shown promising results in recent years. One example is the design of a TiO2/Cu2O micromotor, which has a unique chevron shape and is capable of self-propelling in both H2O2 and pure water (Fig. 8(A)).12 By combining materials that respond to both UV and visible light within a single motor structure, it became feasible to adjust the types of motion exhibited by the motor, shifting from translational to rotational, simply by altering the light wavelength from UV to blue. By following a similar approach, it was also possible to control the movement direction of TiO2/CoO Janus micromotors by switching from UV to visible wavelengths (Fig. 8(B)).48 In this configuration, TiO2 is only photoactivated under UV light, while visible light irradiation activates mostly the CoO counterpart. Therefore, the motion direction was changed by changing the electron transfer dynamics among components.
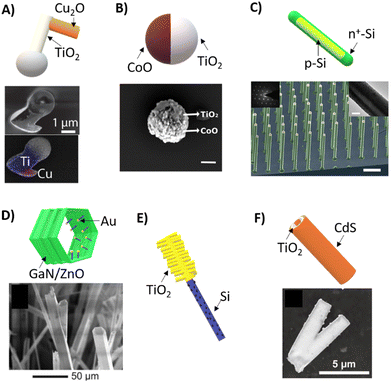 | ||
| Fig. 8 Photocatalytic micromotors based on semiconductor–semiconductor junctions. (A) TiO2/Cu2O micromotors and SEM/EDS images that show their chevron-like morphology and elemental mapping. Reproduced from ref. 12 with permission from John Wiley and Sons, copyright 2018. (B) TiO2/CoO micromotors with a spherical Janus morphology and SEM image. Adapted with permission from ref. 48. Copyright 2020, American Chemical Society. (C) Core–shell silicon-based nanomotors and SEM image showing the silicon nanowire array, scale bar 10 μm. Reproduced from ref. 6 with permission from John Wiley and Sons, copyright 2017. (D) GaN/ZnO tubular micromotors and SEM image showing the tubular structure with different diameters. Reproduced from ref. 51 with permission from John Wiley and Sons, copyright 2019. (E) Schematic representation of a programmable micromotor based on a TiO2/Si heterostructure.11 (F) TiO2/CdS micromotors and SEM image showing their a tubular structure. Reproduced from ref. 54 with permission from John Wiley and Sons, copyright 2021. | ||
Another example of heterostructuring in micromachines is the development of core–shell nanomotors with a p-Si core and an n+-Si shell using the vapor–liquid–solid growth method (Fig. 8(c)).6 These silicon nanomotors were able to move under visible and near-infrared illumination at ultralow light intensity (approximately 3 mW cm−2), making it a highly efficient device. Additionally, the motion trajectories of nanowires were altered by manipulating their end surface morphologies, resulting in various modes such as linear, circular, and rotational movements. By taking advantage of the strong optical resonance of the silicon nanowires, the spectral response of the nanomotors was also modulated by changing the diameter.
A less studied approach is the polarization of light to induce changes in the speed of the micro/nanomotors. In a recent study, a nanomotor made of core–shell Sb2Se3/ZnO nanowires with an anisotropic crystal structure was reported.49 When illuminated with light polarized parallel to the nanowires, the nanomotor absorbs the light preferentially, resulting in an enhanced motion speed compared to perpendicular polarized light. By combining two of these nanomotors, a polarotactic artificial microswimmer was created, allowing navigation by controlling the polarization of the incident light. This dichroic microswimmer offers a significant reduction in required light intensity compared to previous methods, making it suitable for large-scale nanomanipulation and heat-sensitive applications.
The formation of n–n semiconductor heterojunctions offers an alternative approach to achieving charge separation in photoactive micromotors. This can be accomplished by creating a sharp junction between n-type semiconductors with different electron affinities.50 One example of such a junction is the development of GaN/ZnO microtubes with a nanoarchitectured structure (Fig. 8(D)).51 These microtubes were decorated with (Ga1−xZnx)(N1−xOx) nanowires on their surface, which greatly enhanced their stability and photocatalytic efficiency in the UV region. The unique nanoarchitecture of these micromotors resulted in enhanced propulsion. Moreover, the inclusion of ZnO enabled fluorescence emission when excited by UV light, making them suitable for imaging and tracking applications at the microscale.
On the other hand, programmable phototactic behaviors in light-driven nanomotors have also been achieved Si/TiO2 Janus heterostructures (Fig. 8(E)).11 These micromotors consist of a nanostructured photocathode and photoanode at opposite ends that release cations and anions, respectively, thereby propelling the microdevice via self-electrophoresis. By adjusting the zeta potential of the photoanode, the motion dynamics of the micromotor were manipulated to provide them with sensing capabilities of light stimuli, by either showing negative or positive phototaxis. This feature is commonly displayed by natural photosynthetic microorganisms, which are able to move towards the light for harvesting nutrients or away from it to avoid damage. Therefore, this innovative approach based on artificial phototactic micromotors offers a promising method for incorporating biomimetic features in artificial photoactive nanomotors.
CdS is a widely explored material in photocatalysis, due to its short bandgap and optimal redox band potential for hydrogen generation.52,53 The coupling of this material with a wide bandgap photocatalyst is a common approach to avoid the photo-corrosion associated with sulfide-based semiconductors. In the field of photocatalytic micromotors, a heterojunction that might represent a Z-scheme heterostructure consists of the fabrication of TiO2/CdS tubular micromotors able to move under visible-light irradiation in water (Fig. 8(F)).54 To achieve this configuration, ZnO microrods were used as a sacrificial template. Moreover, the inclusion of magnetic nanoparticles enabled their external actuation under magnetic fields. Therefore, the use of semiconductor heterojunctions presents a promising approach to constructing efficient photocatalytic micromotors able to self-propel in pure water without requiring noble-metal components.
Overall, semiconductor–semiconductor heterojunctions offer several advantages in the design and functionality of heterostructured photocatalytic micromotors. Similar to metal–semiconductor heterojunctions, these types of micromotors can move under low light intensities and in pure water, making them versatile for various environmental conditions. Additionally, these heterojunctions also exhibit high motion efficiency and low recombination rates, ensuring reliable operation and enhanced functionality. However, there are certain disadvantages associated with semiconductor–semiconductor heterojunctions. Their fabrication often requires the use of templates and involves a multistep process, which can be complex and time-consuming. Moreover, sophisticated techniques are typically employed, adding to the complexity and cost of production.55 Depending on the specific heterojunction, the reductive or oxidative potential of the single material may be reduced, which can impact its overall performance. To address these challenges, potential strategies include engineering the heterojunctions with appropriate valence and conduction bands to optimize their properties and facilitate charge transfer. Additionally, incorporating chemical wet methods in the synthesis process can contribute to reduce the fabrication cost and scalability.
Conclusions
In conclusion, this feature article has provided a comprehensive overview of the latest advancements in the field of photoactive micromotors based on metal-free and inorganic metal oxide-based semiconductors. We have highlighted the evolution from UV- to visible-light responsive photocatalytic micromotors, as well as advancements in the type of morphologies from simple amorphous particles to more sophisticated designs. We have also discussed the progression of photocatalytic micromotors from single component structures to multiple-component heterojunctions, which have improved the performance and capabilities of these micromotors. This article also emphasizes the significance of heterojunctions in the design of photoactive micromotors, as they allow for the integration of multiple materials with distinct properties. The various examples of heterojunctions presented in the article, including Si/TiO2, TiO2/Cu2O, CoO/TiO2, TiO2/CdS, and GaN/ZnO, showcase the diverse range of configurations and possibilities of photoactive micromotors. Specifically, we have highlighted the potential for external control of motion behaviors by combining two types of semiconductors with different bandgaps, which enables control of the swimming direction (left and right) and/or motion modes under different wavelengths. Additionally, we have shown that the work function of metals in Janus photocatalytic micromotors can also influence their performance. Overall, these findings underscore the importance of a rational design approach that considers material properties such as bandgap, redox band potentials, Fermi levels, and stability. By optimizing the design of photocatalytic micromotors to minimize electron–hole pair recombination, we can fabricate devices with improved efficiency and performance.The research discussed in this article has significant implications for various fields, including microfluidics, biomedical applications, and environmental remediation. In the latter, the potential for harvesting direct sunlight to induce the motion of photocatalytic micromotors is an attractive prospect for developing sustainable technology in water treatment. However, the high fabrication costs and the lack of in-depth studies on the reusability of such devices for meeting industrial requirements currently limit their applicability, rendering it in a very premature phase. Moreover, it is crucial to consider aspects, such as life-cycle assessment and stability of photocatalytic micromotors in future investigations.
Meanwhile, a more practical approach would involve integrating these photocatalytic micromotors into water treatment plants as a polishing step after tertiary treatment. Their inclusion in advanced oxidative processes would facilitate the treatment without requiring external mechanical agitation. In particular, the micromotors would contribute to effectively eliminate persistent or highly toxic contaminants that cannot be removed through common microbiological processes.
However, it is important to note that the applicability of these devices in real-world applications, particularly in non-transparent media and turbid environments, is currently not envisioned due to the limitations of light actuation. To fully unlock the potential of these devices in diverse applications, such as biomedicine and microbiology, it is necessary to develop photoactive micromotors able to store optical energy to have motion autonomy regardless of the swimming environment. Furthermore, developing near-infrared responsive micromotors and possibly coupling optical fibers to reach desired areas are viable options worth considering in the biomedical field.
As research in this field continues to advance, it is likely that we will see even more sophisticated and efficient photoactive micromotors in the future. Therefore, it is crucial to continue exploring these materials and their capabilities and to investigate their potential for integration into practical applications. We hope that this feature article serves as a valuable resource and inspires further research in this exciting and rapidly developing area of science.
Author contributions
K. V. contributed to conceptualization, formal analysis, funding acquisition, writing – original draft, and review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. V. acknowledges the support from the Spanish Ministry of Science (MCIN/AEI/10.13039/501100011033) and the European Union (Next generation EU/PRTR) through the Ramón y Cajal grant, RYC2021-031075-I and the MCIN/AEI/10.13039/501100011033 (CEX2019-000925-S). This research has also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (GA no. 101076680; PhotoSwim).References
- L. Xu, F. Mou, H. Gong, M. Luo and J. Guan, Chem. Soc. Rev., 2017, 46, 6905–6926 RSC.
- K. Villa and M. Pumera, Chem. Soc. Rev., 2019, 48, 4966–4978 RSC.
- J. Wang, Z. Xiong, J. Zheng, X. Zhan and J. Tang, Acc. Chem. Res., 2018, 51, 1957–1965 CrossRef CAS PubMed.
- X. Xiong, X. Huang, Y. Liu, A. Feng, Z. Wang, X. Cheng, Z. He, S. Wang, J. Guo and X. Yan, Chem. Eng. J., 2022, 445, 136576 CrossRef CAS.
- R. Dong, Q. Zhang, W. Gao, A. Pei and B. Ren, ACS Nano, 2016, 10, 839–844 CrossRef CAS PubMed.
- J. Wang, Z. Xiong, X. Zhan, B. Dai, J. Zheng, J. Liu and J. Tang, Adv. Mater., 2017, 29, 1701451 CrossRef PubMed.
- K. Villa, C. L. Manzanares Palenzuela, Z. Sofer, S. Matějková and M. Pumera, ACS Nano, 2018, 12, 12482–12491 CrossRef CAS PubMed.
- A. M. Pourrahimi, K. Villa, C. L. Manzanares Palenzuela, Y. Ying, Z. Sofer and M. Pumera, Adv. Funct. Mater., 2019, 29, 1808678 CrossRef.
- K. Villa, F. Novotný, J. Zelenka, M. P. Browne, T. Ruml and M. Pumera, ACS Nano, 2019, 13, 8135–8145 CrossRef CAS PubMed.
- X. Yuan, R. Ferrer-Campos, F. A. Garcés-Pineda and K. Villa, Small, 2023, 19, 2207303 CrossRef CAS PubMed.
- B. Dai, J. Wang, Z. Xiong, X. Zhan, W. Dai, C.-C. Li, S.-P. Feng and J. Tang, Nat. Nanotechnol., 2016, 11, 1087–1092 CrossRef CAS PubMed.
- É. O’Neel-Judy, D. Nicholls, J. Castañeda and J. G. Gibbs, Small, 2018, 14, 1801860 CrossRef PubMed.
- D. Zhou, Y. C. Li, P. Xu, N. S. McCool, L. Li, W. Wang and T. E. Mallouk, Nanoscale, 2016, 9, 75–78 RSC.
- Q. Yang, H. Xu, H. Wen, H. Zhao, X. Liu, Y. Cai, H. Wang and R. Dong, Appl. Mater. Today, 2021, 22, 100943 CrossRef.
- M. Ibele, T. E. Mallouk and A. Sen, Angew. Chem., Int. Ed., 2009, 48, 3308–3312 CrossRef CAS PubMed.
- Y. Hong, M. Diaz, U. M. Córdova-Figueroa and A. Sen, Adv. Funct. Mater., 2010, 20, 1568–1576 CrossRef CAS.
- J. Palacci, S. Sacanna, A. Vatchinsky, P. M. Chaikin and D. J. Pine, J. Am. Chem. Soc., 2013, 135, 15978–15981 CrossRef CAS PubMed.
- C. Chen, F. Mou, L. Xu, S. Wang, J. Guan, Z. Feng, Q. Wang, L. Kong, W. Li, J. Wang and Q. Zhang, Adv. Mater., 2017, 29, 1603374 CrossRef PubMed.
- Z. Ye, Y. Sun, H. Zhang, B. Song and B. Dong, Nanoscale, 2017, 9, 18516–18522 RSC.
- R. Dong, Y. Hu, Y. Wu, W. Gao, B. Ren, Q. Wang and Y. Cai, J. Am. Chem. Soc., 2017, 139, 1722–1725 CrossRef CAS PubMed.
- K. Villa, L. Děkanovský, J. Plutnar, J. Kosina and M. Pumera, Adv. Funct. Mater., 2020, 30, 2007073 CrossRef CAS.
- A. L. Stanford and J. M. Tanner, in Physics for Students of Science and Engineering, ed. A. L. Stanford and J. M. Tanner, Academic Press, 1985, pp.691–716 Search PubMed.
- K. Takanabe, ACS Catal., 2017, 7, 8006–8022 CrossRef CAS.
- D. Friedmann, C. Mendive and D. Bahnemann, Appl. Catal., B, 2010, 99, 398–406 CrossRef CAS.
- J. Parmar, D. Vilela, K. Villa, J. Wang and S. Sánchez, J. Am. Chem. Soc., 2018, 140, 9317–9331 CrossRef CAS PubMed.
- V. Sridhar, F. Podjaski, Y. Alapan, J. Kröger, L. Grunenberg, V. Kishore, B. V. Lotsch and M. Sitti, Sci. Robot., 2022, 7, eabm1421 CrossRef PubMed.
- V. Sridhar, F. Podjaski, J. Kröger, A. Jiménez-Solano, B.-W. Park, B. V. Lotsch and M. Sitti, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 24748–24756 CrossRef CAS PubMed.
- K. Villa, J. Vyskočil, Y. Ying, J. Zelenka and M. Pumera, Chem. – Eur. J., 2020, 26, 3039–3043 CrossRef CAS PubMed.
- M. Wittmann, S. Heckel, F. Wurl, Z. Xiao, T. Gemming, T. Strassner and J. Simmchen, Chem. Commun., 2022, 58, 4052–4055 RSC.
- W. Liu, X. Chen, X. Lu, J. Wang, Y. Zhang and Z. Gu, Adv. Funct. Mater., 2020, 30, 2003195 CrossRef CAS.
- S. Heckel, C. Bilsing, M. Wittmann, T. Gemming, L. Büttner, J. Czarske and J. Simmchen, Adv. Sci., 2022, 9, 2105009 CrossRef CAS PubMed.
- W. Liu, X. Chen, X. Ding, Q. Long, X. Lu, Q. Wang and Z. Gu, Nanoscale Horiz., 2021, 6, 238–244 RSC.
- V. Chakrapani, in Encyclopedia of Applied Electrochemistry, ed. G. Kreysa, K. Ota and R. F. Savinell, Springer, New York, NY, 2014, pp.1882–1893 Search PubMed.
- G. Shao, Energy Environ. Mater., 2021, 4, 273–276 CrossRef CAS.
- X. Wang, V. Sridhar, S. Guo, N. Talebi, A. Miguel-López, K. Hahn, P. A. van Aken and S. Sánchez, Adv. Funct. Mater., 2018, 28, 1705862 CrossRef.
- D. Zhou, Y. C. Li, P. Xu, L. Ren, G. Zhang, T. E. Mallouk and L. Li, Nanoscale, 2017, 9, 11434–11438 RSC.
- D. Zhou, L. Ren, Y. C. Li, P. Xu, Y. Gao, G. Zhang, W. Wang, T. E. Mallouk and L. Li, Chem. Commun., 2017, 53, 11465–11468 RSC.
- X. Wang, L. Baraban, V. R. Misko, F. Nori, T. Huang, G. Cuniberti, J. Fassbender and D. Makarov, Small, 2018, 14, 1802537 CrossRef PubMed.
- F. Mou, L. Kong, C. Chen, Z. Chen, L. Xu and J. Guan, Nanoscale, 2016, 8, 4976–4983 RSC.
- L. Wang, M. N. Popescu, F. Stavale, A. Ali, T. Gemming and J. Simmchen, Soft Matter, 2018, 14, 6969–6973 RSC.
- F. Mushtaq, A. Asani, M. Hoop, X.-Z. Chen, D. Ahmed, B. J. Nelson and S. Pané, Adv. Funct. Mater., 2016, 26, 6995–7002 CrossRef CAS.
- Q. Wang, C. Wang, R. Dong, Q. Pang and Y. Cai, Inorg. Chem. Commun., 2018, 91, 1–4 CrossRef CAS.
- S. Hoang and P.-X. Gao, Adv. Energy Mater., 2016, 6, 1600683 CrossRef.
- P. Zhou, J. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- X. Huang, J. Wang, T. Li, J. Wang, M. Xu, W. Yu, A. E. Abed and X. Zhang, Beilstein J. Nanotechnol., 2018, 9, 30–41 CrossRef CAS PubMed.
- S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2, 1–17 Search PubMed.
- Y. Sasaki, H. Nemoto, K. Saito and A. Kudo, J. Phys. Chem. C, 2009, 113, 17536–17542 CrossRef CAS.
- V. Sridhar, B.-W. Park, S. Guo, P. A. van Aken and M. Sitti, ACS Appl. Mater. Interfaces, 2020, 12, 24149–24155 CrossRef CAS PubMed.
- X. Zhan, J. Zheng, Y. Zhao, B. Zhu, R. Cheng, J. Wang, J. Liu, J. Tang and J. Tang, Adv. Mater., 2019, 31, 1903329 CrossRef CAS PubMed.
- W. G. Oldham and A. G. Milnes, Solid-State Electron., 1963, 6, 121–132 CrossRef.
- N. Wolff, V. Ciobanu, M. Enachi, M. Kamp, T. Braniste, V. Duppel, S. Shree, S. Raevschi, M. Medina-Sánchez, R. Adelung, O. G. Schmidt, L. Kienle and I. Tiginyanu, Small, 2020, 16, 1905141 CrossRef CAS PubMed.
- K. Villa, X. Domènech, U. M. García-Pérez and J. Peral, Catal. Lett., 2016, 146, 100–108 CrossRef CAS.
- K. Villa, X. Domènech, U. M. García-Pérez and J. Peral, RSC Adv., 2016, 6, 36681–36688 RSC.
- Y. Ying, J. Plutnar and M. Pumera, Small, 2021, 17, 2100294 CrossRef CAS PubMed.
- Y. Zheng, H. Zhao, Y. Cai, B. Jurado-Sánchez and R. Dong, Nano-Micro Lett., 2022, 15, 20 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |

