Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of wide bandgap ZrS2 colloidal quantum dots for solution processed solar-blind UV photodetectors†
Zan
Wang
 abc,
Yunjiao
Gu
abc,
Yunjiao
Gu
 bcd,
Fenghua
Liu
bcd,
Fenghua
Liu
 bcd and
Weiping
Wu
bcd and
Weiping
Wu
 *bcd
*bcd
aDepartment of Optics and Optical Engineering, University of Science and Technology of China, Hefei, Anhui 230026, China
bLaboratory of Thin Film Optics, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, 201800, China. E-mail: wuwp@siom.ac.cn
cState Key Laboratory of High Field Laser Physics, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, 201800, China
dUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 24th October 2023
Abstract
We present a facile one-pot method for the successful synthesis of heavy metal-free ZrS2 colloidal quantum dots (QDs) with a wide bandgap. To achieve this, we employed 1-dodecanethiol (DT) as a sulfur precursor, enabling the controlled release of H2S in situ during the reaction at temperatures exceeding 195 °C. This approach facilitated the synthesis of small-sized ZrS2 QDs with precise control.
New blue quantum dot technology has the potential to enhance the energy efficiency of displays, light-emitting devices, and solar-blind UV photodetector devices. Therefore, the development of novel blue quantum dot materials becomes pivotal. Recently, there has been considerable interest in two-dimensional (2D) layered transition-metal dichalcogenides (TMDs) as a promising class of nanomaterials for various important applications, owing to their exceptional properties.1–4 As new nanomaterials, TMD quantum dots (QDs) display distinctive optical and electronic properties that surpass those of single or few-layer TMD nanosheets. This can be attributed to stronger edge effects and quantum confinement.5–8
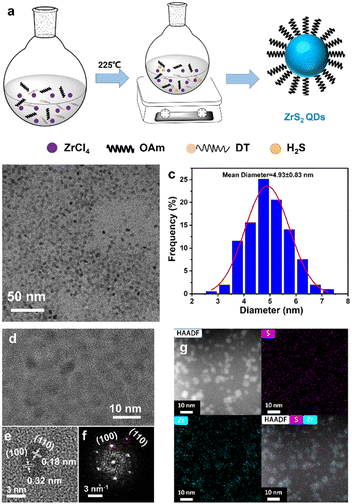
ZrS2 is an n-type indirect bandgap semiconductor with a typical layered sandwich structure and exhibits superior stability, excellent electrical properties and a larger bandgap of 1.7 eV.9–11 Recently, ZrS2 has been theoretically predicted to have acoustic phonon limited charge carrier mobility (∼1247 cm2 V−1 s−1) and high current density (800 μA μm−1).12,13 2D layered ZrS2 has been studied for various applications,14–17 whereas ZrS2 QDs have rarely been studied. The synthesis of the ZrS2 QDs poses significant challenges primarily due to the absence of methods for achieving the small size and high crystallinity of the ZrS2 QDs. Previous studies on ZrS2 nanostructures have been limited to relatively large sized ZrS2 nanocrystals, which may be derived from the highly active precursor S element. For example, Jang et al. reported a hot-injection route by injecting CS2 into a mixture of oleylamine and ZrCl4 to synthesize ultrathin ZrS2 nanodiscs and their lateral size can be tuned from 20 to 60 nm.18 Recently, Paul et al. have employed both heat up and hot injection methods to fabricate colloidal ZrS2 nanocrystals, and the average sizes of the particles are 15 nm and 41 nm, respectively.19 Despite the recent progress in the synthesis of the ZrS2 nanocrystals, it is still a challenge to prepare the ZrS2 QDs (less than 10 nm in size), especially through colloidal solution-based approaches. Herein, for the first time, we developed a facile colloidal solution-based synthetic method for the preparation of heavy metal-free, small blue ZrS2 QDs. 1-Dodecanethiol (DT) was chosen as a sulfur precursor to slowly and continuously release the influx of the chalcogen source (e.g., H2S) in situ at a high temperature of 225 °C by reacting with ZrCl4 in oleylamine. The reaction was continued for 1.5 h to generate small sized ZrS2 QDs (Fig. 1a).
TEM was performed to analyze the morphology and size of the as-synthesized ZrS2 QDs. The TEM image of the ZrS2 QDs is shown in Fig. 1b. It can be observed that the ZrS2 QDs were monodisperse and uniform. Fig. 1c shows the diameter distribution of the ZrS2 QDs (Fig. 1b), with an average size of 4.93 ± 0.83 nm. The HRTEM image revealed that the ZrS2 QDs are single-crystalline structures with parallel and ordered lattice fringes and the lattice distances of the particles are 0.32 nm and 0.18 nm, which are assigned to the (100) and (110) planes (Fig. 1d, e). The corresponding FFT pattern is consistent with the HRTEM results and revealed the hexagonal phase lattice structure of the ZrS2 QDs (Fig. 1f). In addition, the mapping of the elements and merging confirmed that the nanoparticles were composed of Zr and S elements characterized by HAADF-STEM (Fig. 1g).
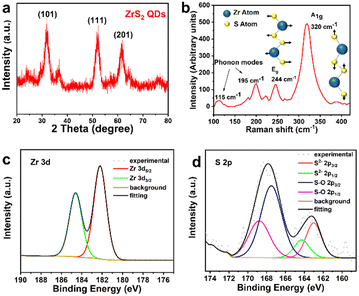
XRD was used to analyze the crystal structure of the as-synthesized ZrS2 QDs. As seen in Fig. 2a, the sample exhibits broad and diffuse diffraction peaks, and the major diffraction peaks at 2θ = 32.2°, 52.4° and 60.5° correspond to the (101), (111) and (201) lattice planes respectively, which can be assigned to the hexagonal phase with P3m1 ZrS2 (JCPDS no. 11-0679) and is in good agreement with the results of HRTEM. The structure of the ZrS2 QDs was further established by Raman spectra using a laser source operating at a wavelength of 532 nm, as shown in Fig. 2b, exhibiting the feature peaks of bulk zirconium disulfide crystals. The Raman spectra of the ZrS2 QDs were dominated by two rather broad, symmetric peaks at 320 and 244 cm−1. The stronger peak at 320 cm−1 can be attributed to the Zr–S bonds showing vertical out-of-plane vibration, corresponding to the A1g mode. In addition, a weaker non-degenerate in-of-plane vibrational Eg mode was observed at 244 cm−1 along with weak phonon modes at approximately 115 and 195 cm−1 in agreement with literature.20,21 In contrast to bulk ZrS2,22–24 both the dominant A1g and weak Eg modes of the ZrS2 QDs are slightly blue-shifted, which can be explained by small lateral dimensions and reduction in the number of layers. The blue-shift phenomenon is similar to the mode change observed for other TMD quantum dots.25–28
XPS was used to characterize the chemical compositions and the binding energies of the bonded elements of the ZrS2 QDs. The survey spectrum indicates that no obvious impurity is observed in the sample, which is consistent with EDS mapping results (Fig. S1, ESI†). In Fig. 2c, the high-resolution Zr 3d region spectra of the ZrS2 QDs clearly show that the two peaks of Zr 3d5/2 and Zr 3d3/2 correspond to the binding energies of 182.3 eV and 184.6 eV, respectively, indicating the oxidation state of Zr4+.17,29 In contrast, the high-resolution S 2p region spectra were analyzed to elucidate the nature of S element. Fig. 2d shows that the S 2p peaks can be fitted with two doublets of S 2p3/2 energy states and both of them are accompanied by 2p1/2 peaks (the binding energy is higher at about 1.2 eV than that of the corresponding 2p3/2 peak). One doublet appeared at around 163.1 eV, corresponding to intrinsic ZrS2, and the other peak in the high energy region (at around 167.6 eV) is indicative of sulfate ions, which may be caused by hydration and surface oxidation when the ZrS2 QDs are exposed to air.26,30
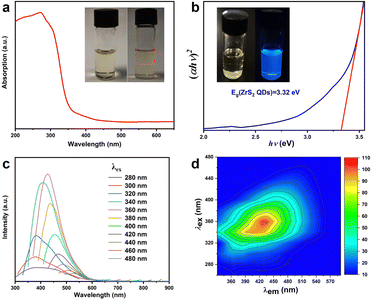
The optical properties of the ZrS2 QDs were investigated by UV-Vis absorption and PL spectroscopy techniques (Fig. 3). The ZrS2 QDs were dispersed in the cyclohexane solvent and appeared as a claybank colloidal solution under natural light (left insert in Fig. 3a) and the colloidal solution can remain stable for several months at room temperature in air, which was verified by the obvious Tyndall effect (the right inset in Fig. 3a). The absorption spectrum of the ZrS2 QDs solution is shown in Fig. 3a, which displays a strong absorption in the ultraviolet region with an absorption peak located at around 270 nm and a broad absorption tail in blue and green regions. The bandgap energy of the ZrS2 QDs is around 3.32 eV calculated by fitting the Tauc plot.31 The calculated bandgap energy of the ZrS2 QDs is much higher than that of bulk ZrS2 crystals (1.7 eV),32 which can be attributed to the significant quantum confined effects.33 As for the PL properties of ZrS2 QDs, the as-obtained ZrS2 QD solution shows strong luminescence under 365 nm excitation (inset of Fig. 3b). Furthermore, Fig. 3c shows the photoluminescence (PL) spectra of the ZrS2 QD solution at various excitation wavelengths ranging from 280 nm to 480 nm and an excitation-dependent PL behavior is observed for the ZrS2 QDs. In order to analyze the relationship between excitation and emission clearly, excitation–emission matrix (EEM) fluorescence spectra are plotted in Fig. 3d. The PL intensity of the ZrS2 QDs increases with increasing excitation wavelength, reaches a maximum at 435 nm at an excitation wavelength of 360 nm, and then decreases. However, it is observed that the PL peaks were redshifted from 360 nm to 540 nm and the PL intensity exhibits a Gaussian distribution as the excitation wavelength changes from 280 nm to 480 nm, respectively. For high-energy excitation at 280–320 nm, the ZrS2 QDs show a broad excitation-independent PL band behavior. At excitation wavelengths of 340–480 nm (low-energy), the PL peak shows an obvious red-shift with the increase of the excitation wavelength. A similar excitation-dependent PL phenomenon has also been observed for other TMD QDs.34–37 In such systems, the most comprehensively accepted reason for the excitation-dependent PL phenomenon can be attributed to the size effect (also known as the quantum confinement effect)38,39 and/or to edge states40,41 and surface traps.30,36
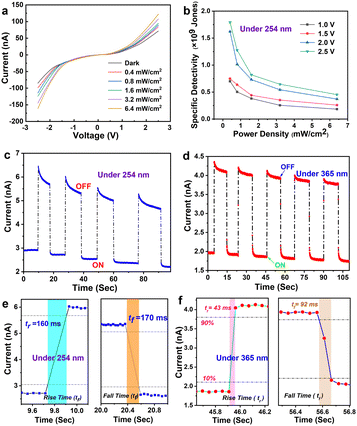
As a proof-of-concept application, the ZrS2 QDs were used as the active layer for ultraviolet and solar-blind photodetector (PD) devices with the structure of Au/ZrS2 QDs/Au. The photoelectric properties of the ZrS2 QD-based PD were then investigated (Fig. 4). The current–voltage (I–V) characteristic plot shows that the applied voltage ranges from −2.5 V to 2.5 V under dark and under 254 nm (Fig. 4a) and 365 nm (Fig. S3, ESI†) UV illumination at various light densities. The ZrS2 QD-based PD exhibits obvious photoresponse performance and nonlinear I–V characteristics, revealing that there may be a small barrier height at the metal–semiconductor (Au-ZrS2 QDs) junction.42 Therefore, the contact between Au and ZrS2 QDs is Schottky rather than Ohmic. In Fig. 4b, the specific detectivity of ZrS2 QD-based PD was described as the dependence of power density at various applied bias voltages under 254 nm UV illumination, and the specific detectivity decreases with the increase of power density and decrease of the applied bias voltage. This can be attributed to two reasons: on the one hand, the probability of carrier recombination increases when high illumination intensity is applied; on the other hand, the high applied bias voltage helps the carriers to transfer to the electrode.43 The specific detectivities of the PD were 1.8 × 109, 1.6 × 109, 0.75 × 109, and 0.7 × 109 Jones, corresponding to a power density of 0.4 mW/cm2 at applied bias voltages from 2.5 to 1 V, respectively. In addition, the specific detectivity of the PD also shows the same trend under 365 nm UV illumination.
Fig. 4c and d show the optical switching of the PD for five consecutive cycles at 1 V bias voltage under 254 nm and 365 nm UV illumination with a power density of 6.4 mW cm−2, revealing the stability and repeatability of the PD. It is worth noting that the photocurrent of the PD decreases with time under 254 nm and 365 nm UV illumination, which is associated with the fact that the photo-generated carriers easily recombine in the defect state, resulting in the decrease of the photocurrent.44Fig. 4e shows that the rise time was 160 ms and the fall time was 170 ms under 254 nm illumination. Besides, Fig. 4f shows that the rise time was 43 ms and the fall time was 92 ms under 365 nm illumination, indicating the potential application of the ZrS2 QD-based PD as a highly sensitive optical switch.
In summary, a facile one-pot method was used to successfully synthesize wide bandgap ZrS2 quantum dots (QDs) without the use of heavy metals. The sulfur precursor 1-dodecanethiol (DT) was selected to gradually release the chalcogen source (e.g., H2S) at high temperatures, enabling the synthesis of small-sized ZrS2 QDs. The resulting ZrS2 QDs, with an average size of 4.93 ± 0.83 nm, possess high crystallinity and demonstrate excellent dispersion and stability in the solvent. Additionally, the ZrS2 QDs exhibit a wide bandgap of 3.32 eV and photoluminescence behavior that depends on the excitation wavelength. As a proof-of-concept application, a solar-blind UV photodetector was fabricated using Au/ZrS2 QDs/Au on a glass substrate. The photodetector shows a low dark current (2 nA), high specific detectivity (1.8 × 109 Jones), and fast photoresponse with rise and fall times of 43 and 92 ms, respectively. These heavy metal-free, printable ZrS2 QDs possess remarkable optoelectronic properties and will have many important potential applications.
This work was supported by the National Natural Science Foundation of China (52273242, 22202231), the National Key R&D Program of China (2021YFB2800703, 2021YFB2800701) and the Chinese Academy of Sciences.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Li, H. L. Chen, Y. X. Guo, K. K. Wang, Y. Zhang, P. L. Lan, J. H. Guo, W. Zhang, H. Q. Zhong, Z. Y. Guo, Z. F. Zhuang and Z. M. Liu, Photonics Res., 2021, 9, 1039–1047 CrossRef CAS.
- A. Di Bartolomeo, A. Kumar, O. Durante, A. Sessa, E. Faella, L. Viscardi, K. Intonti, F. Giubileo, N. Martucciello, P. Romano, S. Sleziona and M. Schleberger, Mater. Today Nano, 2023, 24, 100382 CrossRef CAS.
- C. Patil, C. B. Dong, H. Wang, B. M. Nouri, S. Krylyuk, H. R. Zhang, A. V. Davydov, H. Dalir and V. J. Sorger, Photonics Res., 2022, 10, A97–A105 CrossRef.
- C. L. Liang, E. Z. Wang, X. Li, J. Wang, Y. J. Liu, B. Y. Chen, H. X. Chen, Y. Liu and X. F. Peng, Chin. Opt. Lett., 2022, 20, 021901 CrossRef.
- X. G. Ding, F. Peng, J. Zhou, W. B. Gong, G. Slaven, K. P. Loh, C. T. Lim and D. T. Leong, Nat. Commun., 2019, 10, 41 CrossRef CAS PubMed.
- R. A. Neville and B. L. Evans, Phys. Status Solidi B, 1976, 73, 597–606 CrossRef CAS.
- W. H. Dai, H. F. Dong, B. Fugetsu, Y. Cao, H. T. Lu, X. L. Ma and X. J. Zhang, Small, 2015, 11, 4158–4164 CrossRef CAS PubMed.
- S. Y. Cao, L. P. Hou, Q. F. Wang, C. Y. Li, W. X. Yu, X. T. Gan, K. H. Liu, M. Premaratne, F. J. Xiao and J. L. Zhao, Photonics Res., 2021, 9, 501–506 CrossRef.
- Y. Tian, Y. Cheng, J. D. Huang, S. Y. Zhang, H. Dong, G. K. Wang, J. R. Chen, J. L. Wu, Z. G. Yin and X. W. Zhang, Nano Res., 2022, 15, 6628–6635 CrossRef CAS.
- M. Moustafa, T. Zandt, C. Janowitz and R. Manzke, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 035206 CrossRef.
- T. Zhai, L. Li, X. Wang, X. S. Fang, Y. Bando and D. Golberg, Adv. Funct. Mater., 2010, 20, 4233–4248 CrossRef CAS.
- W. X. Zhang, Z. S. Huang, W. L. Zhang and Y. R. Li, Nano Res., 2014, 7, 1731–1737 CrossRef CAS.
- G. Fiori, F. Bonaccorso, G. Iannaccone, T. Palacios, D. Neumaier, A. Seabaugh, S. K. Banerjee and L. Colombo, Nat. Nanotechnol., 2014, 9, 768–779 CrossRef CAS PubMed.
- Z. Y. Zeng, Z. Y. Yin, X. Huang, H. Li, Q. Y. He, G. Lu, F. Boey and H. Zhang, Angew. Chem., Int. Ed., 2011, 50, 11093–11097 CrossRef CAS PubMed.
- L. Li, X. S. Fang, T. Y. Zhai, M. Y. Liao, U. K. Gautam, X. C. Wu, Y. Koide, Y. Bando and D. Golberg, Adv. Mater., 2010, 22, 4151–4156 CrossRef CAS PubMed.
- X. R. Zhang, Z. S. Meng, D. W. Rao, Y. H. Wang, Q. Shi, Y. Z. Liu, H. P. Wu, K. M. Deng, H. Y. Liu and R. F. Lu, Energy Environ. Sci., 2016, 9, 841–849 RSC.
- R. J. Toh, Z. Sofer and M. Pumera, J. Mater. Chem. A, 2016, 4, 18322–18334 RSC.
- J. T. Jang, S. Jeong, J. W. Seo, M. C. Kim, E. Sim, Y. Oh, S. Nam, B. Park and J. Cheon, J. Am. Chem. Soc., 2011, 133, 7636–7639 CrossRef CAS PubMed.
- P. Fadojutimi, Z. Tetana, J. Moma, N. Moloto and S. Gqoba, ChemistrySelect, 2022, 7, e202202293 CrossRef CAS.
- A. M. Stacy and D. T. Hodul, J. Phys. Chem. Solids, 1985, 46, 405–409 CrossRef CAS.
- M. Mattinen, G. Popov, M. Vehkamaki, P. J. King, K. Mizohata, P. Jalkanen, J. Raisanen, M. Leskela and M. Ritala, Chem. Mater., 2019, 31, 5713–5724 CrossRef CAS.
- S. Manas-Valero, V. Garcia-Lopez, A. Cantarero and M. Galbiati, Appl. Sci., 2016, 6, 264 CrossRef.
- N. Glebko, I. Aleksandrova, G. C. Tewari, T. S. Tripathi, M. Karppinen and A. J. Karttunen, J. Phys. Chem. C, 2018, 122, 26835–26844 CrossRef CAS.
- S. M. Oliver, J. J. Fox, A. Hashemi, A. Singh, R. L. Cavalero, S. Yee, D. W. Snyder, R. Jaramillo, H. P. Komsa and P. M. Vora, J. Mater. Chem. C, 2020, 8, 5732–5743 RSC.
- V. Stengl and J. Henych, Nanoscale, 2013, 5, 3387–3394 RSC.
- D. Dinda, M. E. Ahmed, S. Mandal, B. Mondal and S. K. Saha, J. Mater. Chem. A, 2016, 4, 15486–15493 RSC.
- X. Zhang, H. M. Xie, Z. D. Liu, C. L. Tan, Z. M. Luo, H. Li, J. D. Lin, L. Q. Sun, W. Chen, Z. C. Xu, L. H. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 3653–3657 CrossRef CAS PubMed.
- C. Wang, J. Gao, J. G. Zhao, D. J. Yan and X. D. Zhu, Small, 2020, 16, 1907091 CrossRef CAS PubMed.
- M. Zhang, Y. Zhu, X. Wang, Q. Feng, S. Qiao, W. Wen, Y. Chen, M. Cui, J. Zhang, C. Cai and L. Xie, J. Am. Chem. Soc., 2015, 137, 7051–7054 CrossRef CAS PubMed.
- B. Li, L. Jiang, X. Li, P. Ran, P. Zuo, A. D. Wang, L. T. Qu, Y. Zhao, Z. H. Cheng and Y. F. Lu, Sci. Rep., 2017, 7, 11182 CrossRef PubMed.
- P. Makula, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef CAS PubMed.
- E. Martino, D. Santos-Cottin, F. Le Mardele, K. Semeniuk, M. Pizzochero, K. Cernevics, B. Baptiste, L. Delbes, S. Klotz, F. Capitani, H. Berger, O. V. Yazyev and A. Akrap, ACS Mater. Lett., 2020, 2, 1115–1120 CrossRef CAS.
- T. Z. Wu, Y. Lin, Y. M. Huang, M. Liu, K. J. Singh, W. S. Lin, T. W. Lu, X. Zheng, J. Y. Zhou, H. C. Kuo and Z. Chen, Photonics Res., 2021, 9, 2132–2143 CrossRef.
- A. S. Sarkar, A. Kumari, Anchala, N. Nakka, R. Ray, E. Stratakis and S. K. Pal, Appl. Phys. Lett., 2021, 119, 241902 CrossRef CAS.
- W. Gu, Y. H. Yan, C. L. Zhang, C. P. Ding and Y. Z. Xian, ACS Appl. Mater. Interfaces, 2016, 8, 11272–11279 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien, B. Li, H. Gullappalli, V. K. Pillai, P. M. Ajayan and M. M. Shaijumon, Chem. Commun., 2015, 51, 6293–6296 RSC.
- R. K. Behera, L. Mishra, A. Panigrahi, P. K. Sahoo and M. K. Sarangi, ACS Appl. Mater. Interfaces, 2022, 14, 5750–5761 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297–5303 CrossRef CAS PubMed.
- H. D. Ha, D. J. Han, J. S. Choi, M. Park and T. S. Seo, Small, 2014, 10, 3858–3862 CrossRef CAS PubMed.
- H. F. Dong, S. S. Tang, Y. S. Hao, H. Z. Yu, W. H. Dai, G. F. Zhao, Y. Cao, H. T. Lu, X. J. Zhang and H. X. Ju, ACS Appl. Mater. Interfaces, 2016, 8, 3107–3114 CrossRef CAS PubMed.
- Y. Li, L. B. Tang, R. J. Li, J. Z. Xiang, K. S. Teng and S. P. Lau, Chin. Phys. B, 2019, 28, 037801 CrossRef CAS.
- A. Grillo and A. Di Bartolomeo, Adv. Electron. Mater., 2021, 7, 2000979 CrossRef CAS.
- C. Y. Wu, Z. Q. Pan, Y. Y. Wang, C. W. Ge, Y. Q. Yu, J. Y. Xu, L. Wang and L. B. Luo, J. Mater. Chem. C, 2016, 4, 10804–10811 RSC.
- Z. Wang, F. H. Liu, Y. J. Gu, Y. G. Hu and W. P. Wu, J. Mater. Chem. C, 2022, 10, 1097–1104 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03594h |
| This journal is © The Royal Society of Chemistry 2023 |