Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible-light-induced bifunctionalisation of (homo)propargylic amines with CO2 and arylsulfinates†
Mandapati Bhargava
Reddy
 ab and
Eoghan M.
McGarrigle
ab and
Eoghan M.
McGarrigle
 *ab
*ab
aCentre for Synthesis & Chemical Biology, UCD School of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: eoghan.mcgarrigle@ucd.ie
bA2P CDT in Sustainable Chemistry and BiOrbic Bioeconomy SFI Research Centre, University College Dublin, Belfield, Dublin 4, Ireland
First published on 31st October 2023
Abstract
An unprecedented carboxylative sulfonylation of (homo)propargyl amines with CO2 and sodium arylsulfinates under visible light irradiation has been developed with high efficiency. This ruthenium-catalysed photochemical protocol offers broad substrate scope giving 2-oxazolidinones and 2-oxazinones bearing alkyl sulfones in good yields under ambient reaction conditions. An in situ double bond isomerisation occurs in tandem. A mechanistic rationale for these radical-initiated carboxylative cyclisations involving sulfinyl radicals is presented, supported by control and quenching experiments.
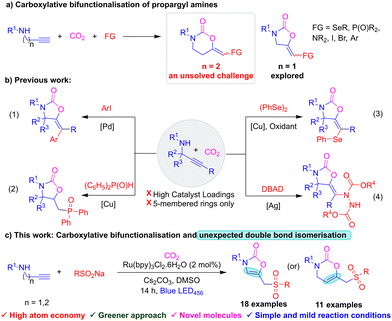
Over the past century atmospheric CO2 concentration has increased dramatically causing severe climate changes.1 In this context, utilisation of CO2 for the functionalisation of organic molecules has gained much attention.2 The conversion of CO2 typically requires high energy due to its thermodynamic stability and kinetic inertness.3 Nonetheless, various synthetic methods have been developed to transform CO2 into valuable organic compounds.4 Among them, multicomponent carboxylative bifunctionalisation of propargyl amines with CO2 and other functional groups represents a promising strategy for synthesising valuable functionalised heterocycles like 2-oxazolidines.5 2-Oxazolidine and 2-oxazinone motifs are frequently found in various bio-active molecules and chiral auxiliaries.6
Generally, carboxylative bifunctionalisation of propargyl amines affords functionalized vinyloxazolidinones, but the analogous conversion of homopropargyl amines to synthesise larger ring vinyloxazinones is still an unsolved challenge (Scheme 1a).7 Literature reported carboxylative bifunctionalisations of propargyl amines are depicted in Scheme 1b(1–4), including the synthesis of aryl, phosphono, selenyl and amino oxazolidinones.8–11 While all have potential advantages, the previous methods use high loadings of metal catalysts, oxidants and/or elevated temperatures and are limited to propargyl amines. Therefore, carboxylative bifunctionalisations of (homo)propargyl amines with other functional groups under mild conditions are highly sought after. Recently, visible light-promoted fixation of CO2 has played a promising role in organic synthesis because of the demonstrated complex bond constructions under mild reaction conditions and the environmentally benign nature of visible light.12 On the other hand sulfones are versatile building blocks in organic chemistry as they can be readily transformed into various useful functional groups.13 Furthermore, sulfones are frequently found in pharmaceuticals, agrochemicals and functional materials.14 Sulfur is found more often than fluorine in drug molecules and recently alkyl/vinyl sulfones have been found to act as radical precursors in synthetic organic chemistry.15 To the best of our knowledge, carboxylative sulfonylation of alkynyl amines to obtain sulfonylated oxazolidinones/oxazinones is unknown in the literature. Continuing our interest in photocatalysis and sulfonylation,16 herein we report an unprecedented double bond isomerised carboxylative sulfonylation of (homo)propargylamines with CO2 for the synthesis of oxazolidinones and oxazinones under mild photochemical conditions (Scheme 1c).
We began our investigation with amine 1aa and sulfinate 2a as model substrates to optimise photochemical carboxylative bifunctionalisation, and the results are shown in Table 1. Initially, the visible light irradiation of 1aa (0.14 mmol), 2a (0.21 mmol) and iodine (0.14 mmol) with MTBD as a base (0.28 mmol) under a CO2 balloon in DMF (2 mL) afforded 3a in 50% isolated yield (entry 1). We examined different organic and inorganic bases (Cs2CO3, DBU and DABCO) and photocatalysts (Eosin Y, 4-CzIPN and Ru(bpy)32+) (entries 2–8). After screening it was found that 2 mol% Ru(bpy)32+ and 2 equiv. of Cs2CO3 in DMSO under a CO2 atmosphere for 14 h were the best reaction conditions, yielding 3a in 84% yield (entry 8). Decreasing the loading of the catalyst and reaction time was found to lower the yield (entries 9 and 10). We screened different solvents: DMA was found to be less efficient than DMSO (entry 11) and other solvents such as MeCN, EtOH and H2O failed to afford 3a (entries 12–14). No product was observed in the absence of CO2 (entry 15) or blue light irradiation (entry 16).
| Entry | Solvent | Catalyst | Additive | Base | Time (h) | Yield 3ab (%) |
|---|---|---|---|---|---|---|
| a Standard reaction conditions: 1aa (0.14 mmol), 2a (0.21 mmol), base (0.28 mmol), catalyst (2 mol%) and additive (0.14 mmol) in solvent (2 mL) was irradiated with a blue LED (456 nm, 40 W) under a CO2 atmosphere. Ru(II) = Ru(bpy)3Cl2·6H2O. b Isolated yields. c 1 mol% Ru(II). d Reaction mixture was irradiated under N2. e Without light. | ||||||
| 1 | DMF | — | I2 | MTBD | 14 | 50 |
| 2 | DMF | — | I2 | Cs2CO3 | 14 | 48 |
| 3 | DMF | Eosin Y | I2 | Cs2CO3 | 14 | 55 |
| 4 | DMF | — | I2 | DABCO | 14 | 0 |
| 5 | DMF | — | I2 | DBU | 14 | 0 |
| 6 | DMF | 4-CzIPN | — | Cs2CO3 | 14 | 15 |
| 7 | DMF | Ru(II) | — | Cs2CO3 | 14 | 81 |
| 8 | DMSO | Ru(II) | — | Cs2CO3 | 14 | 84 |
| 9c | DMSO | Ru(II) | — | Cs2CO3 | 14 | 68 |
| 10 | DMSO | Ru(II) | — | Cs2CO3 | 10 | 71 |
| 11 | DMA | Ru(II) | — | Cs2CO3 | 14 | 51 |
| 12 | MeCN | Ru(II) | — | Cs2CO3 | 14 | 0 |
| 13 | EtOH | Ru(II) | — | Cs2CO3 | 14 | 0 |
| 14 | H2O | Ru(II) | — | Cs2CO3 | 14 | 0 |
| 15d | DMSO | Ru(II) | — | Cs2CO3 | 14 | 0 |
| 16e | DMSO | Ru(II) | — | Cs2CO3 | 14 | 0 |
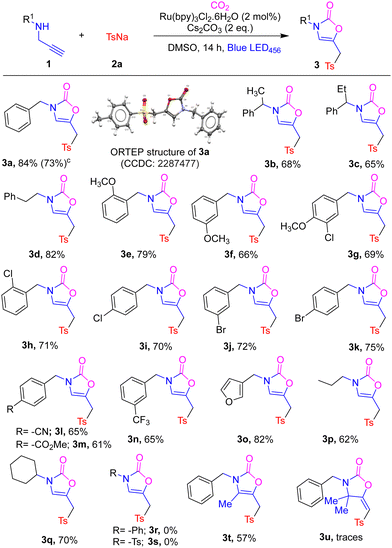
Having found suitable reaction conditions, we examined the generality of this photochemical carboxylative bifunctionalisation reaction and the results are shown in Table 2. Various propargyl amines with different N-substituents 1 and sulfinate 2a smoothly underwent photochemical carboxylative sulfonylation and afforded the corresponding oxazolidinones (3a–3t) in moderate to good yields. We established the structures of these novel double bond isomerized sulfonylative oxazolidinones from an X-ray crystal structure of 3a (see ESI† for details). α-Methyl and ethyl-substituted bulky propargyl amines 1ab and 1ac were well tolerated in this photochemical protocol giving the corresponding compounds 3b and 3c in 68% and 65% yields, respectively. N-Phenethyl-tethered propargyl amine 1ad afforded oxazolidone 3d in 82% yield. Furthermore, N-benzyl motifs bearing methoxyl groups at o/m/p-positions underwent photochemical carboxylative bifunctionalisation with sulfinate 2a smoothly, affording the corresponding sulfonylated oxazolidinones 3e–3g in 66–79% yield. Propargyl amines with halogen-substituents on the benzyl ring such as 2-Cl, 4-Cl, 3-Br and 4-Br were also suitable for this bifunctionalisation reaction giving the corresponding compounds 3g–3k in 69–75% yields. N-Benzyl motifs bearing electron-withdrawing groups such as 4-CN, 4-COOMe and 3-CF3 were well tolerated in this photochemical method giving compounds 3l–3o in good yields. Aromatic heterocyclic furan-3-ylmethyl and aliphatic cyclohexyl and propyl-substituted propargyl amines were also well tolerated in this protocol and afforded the corresponding oxalidones 3o–3q in 62–82% yield. In contrast, N-phenyl and N-tosyl substituted propargyl amines failed to produce the corresponding sulfonylated oxazolidinones 3r and 3s, presumably due to the poorer nucleophilicity of the amines. Notably, N-benzylbut-3-yn-2-amine 1at and N-benzyl-2-methylbut-3-yn-2-amine 1au afforded the corresponding products 3t and 3u in 57% yield and in trace amounts, respectively.
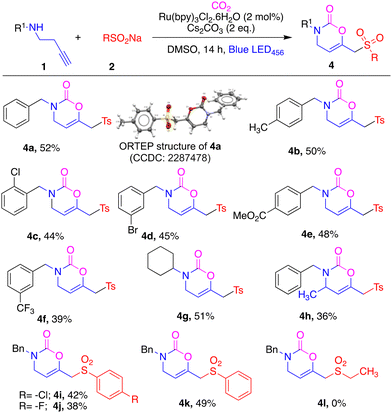
Then we addressed the unsolved challenge of the synthesis of vinyloxazinones using N-substituted homopropargyl amines under our optimised conditions and the results are depicted in Table 3. To our delight, N-benzylbut-3-yn-1-amine 1ba smoothly reacted with sulfinate 2a under this protocol and gave the corresponding oxazinone compound 4a in 52% yield. We established the structure of the novel sulfonylated oxazinone from an X-ray crystal structure of 4a (see ESI† for details). To the best of our knowledge, this is the first report of carboxylative functionalization of homopropargyl amines for the synthesis of 6-membered rings. Homopropargyl amines with electron-donating groups (4-Me), halogen-substituents (2-Cl and 3-Br) and electron-withdrawing groups (4-CO2Me and 3-CF3) on the benzyl ring smoothly reacted in this photochemical carboxylative sulfonylation reaction and afforded the corresponding compounds 4b–4f in 39–50% yield. N-Cyclohexyl homopropargyl amine 1bg and N-benzylpent-4-yn-2-amine 1bh were also well tolerated, giving the corresponding products 4g and 4h in moderate yields. Finally, we screened the scope of sulfinates in this photochemical bifunctionalisation. Halogen (4-Cl and 4-F) bearing sulfinates and simple benzenesulfinate 1bi–1bj reacted smoothly under this protocol affording the corresponding compounds 4i–4j in 38–49% yield. In contrast, alkyl sulfinate 1bl failed to produce compound 4l under these photochemical conditions.
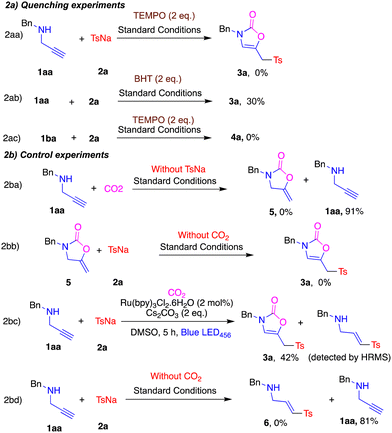
To gain insights into the reaction mechanism we performed quenching and control experiments (Scheme 2a and b). Addition of the radical scavenger TEMPO under standard reaction conditions stopped the production of 3a and 4a (Scheme 2aa and ab). Addition of BHT also lowered the yield of 3a (Scheme 2ac). Thus, both reactions are proposed to proceed via a radical pathway. When 1aa was subjected to the standard conditions without 2a the reaction failed to produce the carboxylative cyclization product 5 and the starting material 1aa was recovered in 91% yield (Scheme 2ba). The cyclized product 5 was synthesized17 and subjected to the standard conditions without CO2 but this failed to produce the product 3a (Scheme 2bb). Both these reaction outcomes suggest that the reaction is not proceeding via carboxylative cyclization intermediate 5. When the reaction mixture was quenched after 5 h and analysed by HRMS, target product 3a and intermediate 6 were observed (Scheme 2bc). When 1aa was subjected to the standard conditions without CO2 the reaction failed to produce intermediate 6, implying that in the case of free amine sulfinate 2a failed to react with propargyl amine (Scheme 2bd).
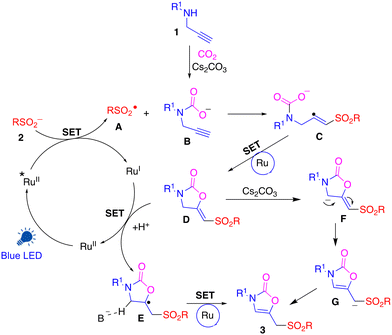
Based on the above experiments and earlier literature,18 we propose the reaction pathway of carboxylative sulfonylation (Scheme 3). Initially, a RuII complex is excited by the absorption of blue light, which then oxidises sulfinate 2 to form sulfinyl radical A and RuI species. Propargyl amine 1 reacts with CO2 and a base to form carbamate intermediate B.19 The addition of sulfinyl radical A to carbamate B produces vinyl radical intermediate C. Intermediate C then undergoes single electron transfer (SET) with Ru, followed by cyclization to afford cyclic intermediate D. Intermediate D might undergo SET with RuI followed by H+ addition to produce radical intermediate E and regenerate the RuII complex. The reaction of intermediate E in the presence of a base and photocatalyst can result in the loss of H+ affording the final product 3. Alternatively, intermediate D in the presence of a base could form anion F/G and the addition of H+ to the intermediate F/G affords the final product 3.20
In conclusion, we have demonstrated a sustainable carboxylative sulfonylation of propargylamines with CO2 and sodium arylsulfinates under photochemical conditions. This photochemical bifunctionalisation afforded a broad substrate scope of sulfonylated 5- and 6-membered heterocycles, 2-oxazolidinones (18 examples) and 2-oxazinones (11 examples), with good to moderate yields. A plausible mechanism was supported by control and quenching experiments. We anticipate that this methodology will enable further applications of carboxylative bifunctionalisation, especially with respect to homopropargyl amine bifunctionalisation with CO2, which was previously unknown in the literature.
M. B. R. thanks the Irish Research Council for a Postdoctoral Fellowship (GOIPD/2022/576). We thank Julia Bruno-Colmenárez of the UCD X-Ray Diffraction Laboratory for the crystal structure of 3a and 4a. We thank SFI (18/RI/5702) for MS infrastructure, and the A2P CDT which is supported by Science Foundation Ireland (SFI) and the Engineering and Physical Sciences Research Council (EPSRC) under Grant No. 18/EPSRC-CDT/3582 and BiOrbic, the Bioeconomy SFI Research Centre, which is funded by Ireland's European Structural and Investment Programmes, Science Foundation Ireland (16/RC/3889) and the European Regional Development Fund.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) https://climate.nasa.gov/vital-signs/carbon-dioxide ; (b) S. J. Davis, K. Caldeira and H. D. Matthews, Science, 2010, 329, 1330–1333 CrossRef CAS PubMed; (c) Atmospheric CO2 Levels Defy the Pandemic to Hit Record High. Available online: https://newatlas.com/environment/atmospheric-co2-pandemic-record-concentrations/.
- (a) M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed; (b) Y. Yang and J.-W. Lee, Chem. Sci., 2019, 10, 3905–3926 RSC; (c) A. Modak, P. Bhanja, S. Dutta, B. Chowdhury and A. Bhaumik, Green Chem., 2020, 22, 4002–4033 RSC.
- (a) J. Schneider, H. Jia, J. T. Muckerman and E. Fujita, Chem. Soc. Rev., 2012, 41, 2036–2051 RSC; (b) J. Wang, C.-S. Jia, C.-J. Li, X.-L. Peng, L.-H. Zhang and J.-Y. Liu, ACS Omega, 2019, 4, 19193–19198 CrossRef CAS PubMed.
- (a) R. Cauwenbergh, V. Goyal, R. Maiti, K. Natte and S. Das, Chem. Soc. Rev., 2022, 51, 9371–9423 RSC; (b) Z. Fan, Z. Zhang and C. Xi, ChemSusChem, 2020, 13, 6201–6218 CrossRef CAS PubMed.
- S.-F. Cai, H.-R. Li and L.-N. He, Green Chem., 2021, 23, 9334–9347 RSC.
- (a) F. Sun, E. V. Van der Eycken and H. Feng, Adv. Synth. Catal., 2021, 363, 5168–5195 CrossRef CAS; (b) K. Okuno, R. Nishiyori, T. Mori and S. Shirakawa, Chirality, 2022, 34, 915–924 CrossRef CAS PubMed; (c) M. M. Heravi, V. Zadsirjan and B. Farajpour, RSC Adv., 2016, 6, 30498–30551 RSC; (d) G. Chen, C. Fu and S. Ma, Org. Lett., 2009, 11, 2900 CrossRef CAS PubMed.
- (a) S. Wang and C. Xi, Chem. Soc. Rev., 2019, 48, 382–404 RSC; (b) J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Acc. Chem. Res., 2021, 54, 2518–2531 CrossRef CAS PubMed.
- P. García-Domínguez, L. Fehr, G. Rusconi and C. Nevado, Chem. Sci., 2016, 7, 3914–3918 RSC.
- W.-B. Huang, F.-Y. Ren, M.-W. Wang, L.-Q. Qiu, K.-H. Chen and L.-N. He, J. Org. Chem., 2020, 85, 14109–14120 CrossRef CAS PubMed.
- J. M. Chen, L. Qi, L. Zhang, L. J. Li, C. Y. Hou, W. Li and L. J. Wang, J. Org. Chem., 2020, 85, 10924–10933 CrossRef CAS PubMed.
- Y. Sadamitsu, K. Saito and T. Yamada, Chem. Commun., 2020, 56, 9517–9520 RSC.
- (a) S. Pradhan, S. Roy, B. Sahoo and I. Chatterjee, Chem. – Eur. J., 2021, 27, 2254–2269 CrossRef CAS PubMed; (b) X. He, L.-Q. Qiu, W.-J. Wang, K.-H. Chen and L.-N. He, Green Chem., 2020, 22, 7301–7320 RSC; (c) A. R. Nathan and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10116 CrossRef PubMed; (d) G. E. M. Crisenza and P. Melchiorre, Nat. Commun., 2020, 11, 803 CrossRef PubMed.
- (a) M. Mellah, A. Voituriez and E. Schulz, Chem. Rev., 2007, 107, 5133–5209 CrossRef CAS PubMed; (b) T. M. Monos, R. C. McAtee and C. R. J. Stephenson, Science, 2018, 361, 1369–1373 CrossRef CAS PubMed; (c) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701–8780 CrossRef CAS PubMed.
- (a) W. Guo, D.-Y. Wang, Q. Chen and Y. Fu, Adv. Sci., 2022, 9, 2103989 CrossRef CAS PubMed; (b) X. Zhang, K. Chen, Z. Sun, G. Hu, R. Xiao, H.-M. Cheng and F. Li, Energy Environ. Sci., 2020, 13, 1076–1095 RSC; (c) M. Feng, B. Tang, S. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed; (d) M. Yamada, T. Ichikawa, M. Ii, K. Itoh, N. Tamura and T. Kitazaki, Bioorg. Med. Chem., 2008, 16, 3941–3958 CrossRef CAS PubMed; R. Ahmadi and S. Emami, Eur. J. Med. Chem., 2022, 234, 114255–114279 Search PubMed.
- (a) M. Ociepa, J. Turkowska and D. Gryko, ACS Catal., 2018, 8, 11362–11367 CrossRef CAS; (b) Q. Q. Zhou, S. J. S. Düsel, L. Q. Lu, B. König and W. J. Xiao, Chem. Commun., 2019, 55, 107–110 RSC; (c) J. M. Smith, J. A. Dixon, J. N. DeGruyter and P. S. Baran, J. Med. Chem., 2019, 62, 2256–2264 CrossRef CAS PubMed.
- M. Bhargava Reddy and E. M. McGarrigle, Chem. Commun., 2023, 59, 7767–7770 RSC.
- M. Yoshida, T. Mizuguchi and K. Shishido, Chem. – Eur. J., 2012, 18, 15578–15581 CrossRef CAS PubMed.
- (a) Z. Yin, J. Ye, W. Zhou, Y. Zhang, L. Ding, Y. Gui, S. Yan, J. Li and D. Yu, Org. Lett., 2018, 20, 190–193 CrossRef CAS PubMed; (b) L. Lu, H. Wang, S. Huang, B. Xiong, X. Zeng, Y. Ling and X. Qiu, Chem. Commun., 2023, 59, 10420–10423, 10.1039/d3cc02740f.
- (a) X. He, X. Y. Yao, K. H. Chen and L. N. He, ChemSusChem, 2019, 12, 5081–5085 CrossRef CAS PubMed; (b) M. Y. Wang, Y. Cao, X. Liu, N. Wang, L. N. He and S. H. Li, Green Chem., 2017, 19, 1240–1244 RSC.
- (a) N. Collins, R. Connon, G. Sánchez-Sanz and P. Evans, Eur. J. Org. Chem., 2020, 6228–6235 CrossRef CAS; (b) Y. Seino, Y. Yamaguchi, A. Suzuki, M. Yamashita, Y. Kamei, F. Kamiyama, T. Yoshino, M. Kojima and S. Matsunaga, Chem. – Eur. J., 2023, 29, e202300804 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterisation, X-ray data for 3a and 4a and copies of NMR spectra. CCDC 2287477 and 2287478. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc04160c |
| This journal is © The Royal Society of Chemistry 2023 |