Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Resonance and structural assignment in (car)borane clusters using 11B residual quadrupolar couplings†
Franziska
Rüttger
 ,
Dietmar
Stalke
,
Dietmar
Stalke
 and
Michael
John
and
Michael
John
 *
*
Institute of Inorganic Chemistry, Georg-August-University of Göttingen, Germany. E-mail: mjohn@gwdg.de
First published on 22nd November 2023
Abstract
A new NMR method for structural verification and 11B resonance assignment in (car)borane clusters is presented, based on the measurement of 11B residual quadrupolar couplings (RQCs) in a stretched polystyrene (PS) gel. The method was applied to ortho-carborane (B10C2H12), a derivative thereof with reduced symmetry, meta-carborane and decaborane (B10H14).
Since carboranes were first discovered and classified in the 1960s, they have quickly gained popularity in various fields.1 The main body is usually derived from polyhedral structures that can be described by the Wade–Mingos rules as closo (closed), nido (nest) and arachno (spider web).2 With the inclusion of additional substituents, further heteroatoms and even metals, this leads to a nearly unlimited diversity of boron cage species.3 Due to the ease of functionalisation, carboranes enjoy high popularity for example in materials science, medicinal chemistry and coordination chemistry.4
For such highly specialised applications, reliable methods for the structural characterisation are needed, where X-ray crystallography and NMR spectroscopy5 are certainly the most versatile methods. Both naturally occurring boron isotopes, 10B (I = 3) and 11B (I = 3/2), are NMR active, but the heavier isotope 11B is usually preferred due to its (i) higher natural abundance (ca. 80% vs. ca. 20% for 10B), (ii) higher sensitivity and (iii) lower quadrupole moment resulting in narrower lines.
Simple 11B spectra provide basic information about the relative number of equivalent boron atoms and coupling to directly bound hydrogen atoms but give limited knowledge about the skeletal structure of the cage.6 Here, the implementation of homo- and heteronuclear two-dimensional experiments such as 11B–11B COSY present further possibilities in determining the arrangement and assignment of boron atoms.7 However, for many networks this remains ambiguous, and bonds to other heteroatoms such as halogens (except for F), carbon and oxygen cannot be observed that way.
The quadrupolar nature of the 11B nucleus can provide valuable information due to its coupling to the electric field gradient (EFG) which is highly sensitive to the closest coordination environment of the boron atom.8 Quadrupole coupling constants are most commonly derived from standard solid state NMR measurements, but very difficult to extract if more than 1–2 chemically distinct boron atoms are present in the cluster. Here, we present a strategy to obtain the correct structure and 11B resonance assignment for two closo-carboranes (B10C2H12), a derivative thereof and the nido-borane B10H14 (Scheme 1), using 11B residual quadrupolar couplings (RQCs), and/or one-bond 11B–1H and 13C–1H residual dipolar couplings (RDCs) obtained under weakly aligned conditions. The strategy comprises fitting of experimental couplings to model structures with DFT-derived EFG tensors using single molecular alignment tensors, related to previous work with 2H RQCs9,10 and 7Li RQCs.11
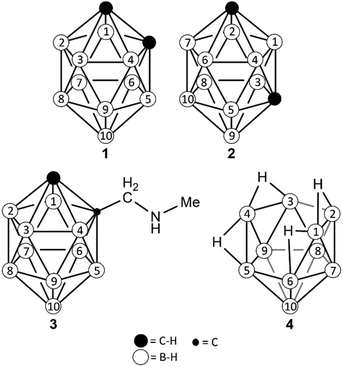 | ||
| Scheme 1 (Car)borane clusters used in this work. Numbering of the boron positions differs from the common numbering and was specifically adapted to simplify discussion in this work. | ||
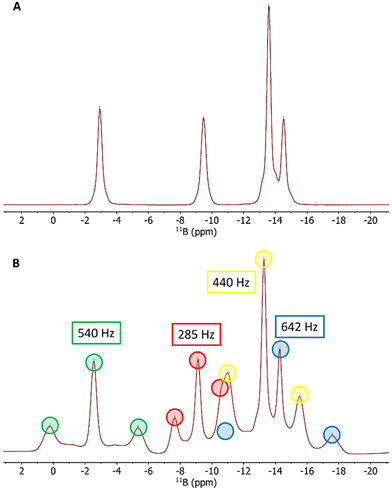
The isotropic 11B{1H} NMR spectrum of 1 in THF-d8 shows four 11B resonances in a small chemical shift range of ∼12 ppm with an intensity distribution of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is in agreement with the C2v symmetry of the compound (Fig. 1A). At this step, the largest signal can already be assigned to the four equivalent positions B2, B3, B5 and B6 while there are six possibilities to assign the remaining three signals to the boron pairs B1/B4, B7/B9 and B8/B10. Our first aim was to select the correct (and literature known)12 assignment using only 11B RQCs.
2, which is in agreement with the C2v symmetry of the compound (Fig. 1A). At this step, the largest signal can already be assigned to the four equivalent positions B2, B3, B5 and B6 while there are six possibilities to assign the remaining three signals to the boron pairs B1/B4, B7/B9 and B8/B10. Our first aim was to select the correct (and literature known)12 assignment using only 11B RQCs.
For this purpose, weak alignment was achieved by the use of cylindrical cross-linked polystyrene sticks swollen by a THF-d8 solution of 1.13 After the completion of the swelling process (typically 7–14 days after sample preparation), the anisotropic 11B{1H} spectrum now shows four quadrupolar triplets that are centred on the corresponding isotropic 11B{1H} singlets and with splittings between 285 Hz and 642 Hz (Fig. 1B).
The drawback of simple 1D spectra is that only the magnitudes of RQC values are obtained with no information about the absolute sign. We addressed this issue in three different ways (see below). First, we slightly modified the standard F2-coupled 1H,11B-HMQC experiment with a smaller (∼30°–45°) flip angle on the 11B channel (Fig. S1 in the ESI†), analogous to the reduced flip angles in the P.E.COSY,14 P.E.HSQC15 and Q.E.COSY16 experiments. This way, clean correlations between the 11B spin states observed in the 1H quartets along the F2 dimension and the transitions between these states in the 11B triplets along F1 is achieved (Fig. S2, ESI†). Since 1J(1H,11B) (or 1T(1H,11B) in weakly aligned samples) is known to be positive,17 the absolute sign of the 11B RQC can be derived from the tilt of the correlations in the 2D spectrum. An example 1H,11B-HMQC spectrum of 1 is shown in Fig. 2, clearly giving a positive sign for the 11B RQC in the triplets marked in yellow and red, and negative sign in the triplets marked in blue and green.
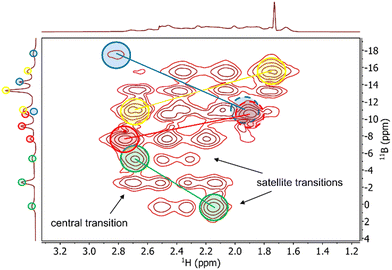 | ||
| Fig. 2 F2 coupled 1H,11B-HMQC spectrum of 1 in PS/THF-d8 after 10 days of swelling, recorded on a 600 MHz spectrometer and with a 11B flip angle of ∼45°. The correlations that are crucial for the 11B RQC sign determination are marked and linked in the same colours as in Fig. 1B. For the quadrupolar triplets marked in blue and red, one of the two correlations is overlapped (dashed circles), but the sign of the 11B RQC can be nevertheless deduced. The horizontal trace is taken from the isotropic 1H spectrum. | ||
The next step of the assignment procedure is the construction of structure models with geometry optimisation and calculation of EFG tensors. For this purpose we used the B3LYP method in Gaussian16,18 which is easy to set-up, quick and robust due to the EFG being a ground-state property.19 In 1, the EFG tensors at the non-carbon-bound boron atoms B7–B10 are nearly axially symmetric with the positive (blue) components aligned with the B–H bonds (Fig. 3B and Table S4, ESI†). This opens a second possibility to determine the absolute sign of the 11B RQC of these boron atoms via comparison with the sign of the respective 11B–1H RDC, similar to the analysis of 2H RQCs of C–2H groups.9,20 In 1, the 11B–1H RDC belonging to B7/B9 (B8/B10) is positive (negative, Table S1, ESI†), giving a positive (negative) 11B RQC with a RQC/RDC ratio of about +15. Of course, this method fails for the carbon-bound positions B1–B6, where the EFGs are up to 50% larger, rhombic and tilted away from the B–H axes (Fig. 3A). The EFGs correspond to quadrupole coupling constants between 1.4 and 2.1 MHz, which is in agreement with previous calculations or experimental data based on 11B relaxation or nuclear quadrupole resonance.8
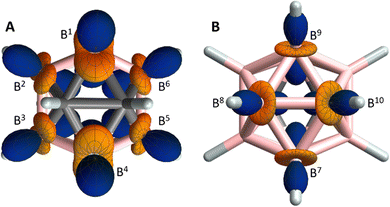 | ||
| Fig. 3 Geometry-optimised structure model of 1 with the EFG tensors shown at carbon-bound (A) and non-carbon-bound (B) boron positions (carbon: grey, boron: pink, hydrogen: light gray). The blue and orange lobes indicate directions of positive and negative EFG, respectively. The EFG tensor is given in the Gaussian sign convention11,21 which is opposite to the more commonly used sign convention used by Autschbach et al.22 | ||
For the fitting of experimental couplings to our models with calculated EFGs we used the software MSpin,23 which directly accepts the output from the EFG calculation and gives the molecular alignment and the Cornilescu Q factor24 as measure of agreement between experimental and calculated 11B RQCs. Due to the C2v symmetry the alignment tensor contains only two unknowns so that the Q factor determined from four 11B RQCs should become large for all except the correct assignment.25 Taking into account the sign information received from the 1H,11B-HMQC, the Q factors for the six possibilities to assign the green, red and blue 11B triplets to B1/B4, B7/B9 and B8/B10 are displayed in Fig. 4 (black bars).
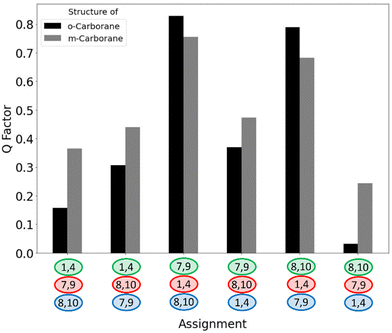 | ||
| Fig. 4 Possible assignments for the remaining three 11B resonances in the 11B spectrum of 1 plotted against the Q factor for using RQCs on a structure model of 1 (black) and 2 (gray). Colouring of the resonances is according to Fig. 1B. | ||
The correct assignment can clearly be identified with a Q factor of only 0.032, more than five times smaller than the next lowest value which belongs to swapping the assignment of the blue (−642 Hz) and green (−540 Hz) triplet. Even if the relative sign of all experimental couplings is left free floating, leading to overall 48 possibilities, 11B RQCs alone are able to provide the correct assignment along with the correct relative sign of all couplings (Fig. S61, ESI†). Note that the absolute sign of the couplings cannot be determined this way, but this can be achieved by comparing the RQC alignment tensors with that obtained from 11B–1H RDCs (Tables S2 and S3, ESI†). It should be mentioned that 11B–1H RDCs, extracted from 1H-coupled 11B or F2-coupled 1H,11B-HMQC spectra (Table S1, ESI†) are likewise able to achieve correct 11B assignment (Fig. S60, ESI†).
The procedure described above was applied identically to compound 2 (meta-carborane). In this case, three of the four signals show RQCs between 125 Hz and 533 Hz while there is no quadrupolar splitting visible in the 11B{1H} spectrum for the largest signal belonging to B2, B3, B5 and B6 (Fig. S20, ESI†). Despite that, the magnitude and sign (−25 Hz) of the 11B RQC can be estimated from the tilt of the respective signal in the 1H,11B-HMQC (Fig. S21, ESI†). Similar to 1, all boron signals could be assigned to the respective atoms in the cluster with the correct assignment giving the lowest Q factor (0.031), which is almost tenfold lower than the second lowest value. In this case, the use of RDCs gives more ambiguous results with Q factors of 0.073 and 0.114 for two assignments in which the opposite boron positions (B1/B4 and B8/B10) are swapped (Fig. S63, ESI†).
Because both 1 and 2 are structural isomers with the same symmetry, the 11B spectra of both compounds are very similar and cannot be easily distinguished (Fig. 1A and Fig. S16, ESI†). Hence, we also aimed at using 11B RQCs to discriminate between the two structures by cross-fitting the experimental couplings of 1 to the model of 2, and vice versa. Again, the assignment of the large 11B signal belonging to the positions B2, B3, B5 and B6 was fixed, while the other assignments were left free floating. Using the experimental couplings of 1, simultaneous structural and resonance assignment is unambiguous as none of the cross-fits gives Q factors below 0.2 (gray bars in Fig. 4). Using the experimental couplings of 2, there is a second low Q factor (0.051) for the wrong structure in combination with a wrong assignment (Fig. S64, ESI†), but with unrealistically high/low alignment tensors. Together with the data of 1 (Fig. 4), both spectra can be unambiguously assigned to the correct structure.
Compound 3 was chosen as an example for a partially functionalised carborane cage, in which the symmetry is reduced to Cs, resulting in now six 11B resonances with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. S25, ESI†). Apart from the problem of increased overlap, this compound suffers from ∼3 times weaker alignment compared to 1 and 2 (and at least 10 times weaker alignment compared to our previously investigated π systems).11,25 Hence, even after 15 days of swelling only three 11B resonances show a resolved quadrupolar splitting (up to 187 Hz) at all. As a consequence, there are not enough parameters for a 11B-RQC-only resonance assignment, and we instead had to pursue a strategy that included 11B RQCs and 13C-1H/11B-1H RDCs right from the beginning.
2 (Fig. S25, ESI†). Apart from the problem of increased overlap, this compound suffers from ∼3 times weaker alignment compared to 1 and 2 (and at least 10 times weaker alignment compared to our previously investigated π systems).11,25 Hence, even after 15 days of swelling only three 11B resonances show a resolved quadrupolar splitting (up to 187 Hz) at all. As a consequence, there are not enough parameters for a 11B-RQC-only resonance assignment, and we instead had to pursue a strategy that included 11B RQCs and 13C-1H/11B-1H RDCs right from the beginning.
First, the most downfield shifted 11B resonance at −3.36 ppm with the largest negative 11B–1H RDC (−14.5 Hz) can be assigned to B10 which is directly opposite to C11 with a similar 13C–1H RDC value (−16.3 Hz). Thus, B8 is automatically assigned to the single 11B resonance at −5.77 ppm. For the residual four 11B resonances of double intensity, 24 assignment possibilities remain (Fig. S66, ESI†). In total, there are four cases showing a significantly lower (<0.2) Q factor when using RDCs alone which is reduced to two when 11B RQCs are included in the analysis. The two possibilities only differ in the assignment of the two unsplit 11B resonances (red and orange in Fig. S30, ESI†), which accidentally show similar 11B–1H RDC values (3.3 vs. 2.4 Hz).
While a conventional 11B{1H} COSY spectrum (Fig. S27, ESI†) cannot discriminate between B5/B6/B10 in the C–H substituted half (upper half in Fig. S54, ESI†) and B2/B3/B8 in the unsubstituted half, B7/B9 are easily identified as their resonance (marked in red at −9.42 ppm) is the only one coupling to all other five 11B signals. This example thus shows that the information from 11B RQCs/RDCs in complementary to that from 11B{1H} COSY, and a combination of both methods is able to eliminate last doubts. The problem of increased overlap could also be addressed in the future using 2D methods such as Q-COSY or Q-resolved spectroscopy.26
The nido-borane 4 (decaborane) was chosen as an example for an air-sensitive compound, thus putting higher demands on the sample preparation. Formally, 4 is related to 1 by removal of two C–H groups to give a bowl-shaped structure with four additional bridging hydrogen atoms connecting the rim boron atoms B2–B1–B6 and B3–B4–B5, respectively. Hence, it has the same symmetry and number of 11B resonances as the closo-carboranes 1 and 2, but these are notably spread over a much larger range of ∼50 ppm (Fig. S36, ESI†). Also, the 1H resonances are well-dispersed, with a broad singlet appearing upfield at −1.72 ppm (bridging H) and a narrow quartet (with 10B satellites) at 0.58 ppm (Fig. S33, ESI†). In the aligned sample, all four 11B resonances appear as quadrupolar triplets with splittings between 122 Hz and 532 Hz (Fig. S40 and S41, ESI†).
Due to the opening of the icosahedron, the EFG tensors of 4 tend to be less uniform and more rhombic with the negative components elongated towards the opening of the bowl (Fig. S58 and S59, ESI†). When fitting the experimental 11B RQCs to the calculated EFGs, two assignment combinations with a low Q factor (0.039 and 0.057) are obtained that differ in the assignment of the 11B triplets with the smallest splittings (green, 155 Hz and red, 122 Hz, Fig. S40, ESI†). This ambiguity is easily lifted by including 11B–1H RDCs in the analysis, which are very different (−22.3 vs. −3.7 Hz) for the two sites. The boron atoms B7/B9 with the smallest EFG belong to the most upfield 11B resonance at −35.4 ppm and, notably, to the bound hydrogen with the narrow resonance at 0.58 ppm. All 11B and 1H assignments obtained this way are in agreement with the 11B{1H} COSY and with literature.27
In conclusion, using a method based on 11B RQCs and 11B–1H RDCs, we were able to discriminate between ortho- and meta-carborane (1 and 2) and assign the 11B resonances of all (car)boranes 1–4 unambiguously. The method relies on user-friendly software (Gaussian, MSpin) and should be applicable to a broad range of (functionalised) boron clusters.
This work has been funded by the Deutsche Forschungsgemeinschaft (Projects no. 428856821 and 405832858).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Bobinski, J. Chem. Educ., 1964, 41, 500 CrossRef CAS; (b) R. N. Grimes, Carboranes, Academic Press, New York, 1970 Search PubMed.
- (a) K. Wade, J. Chem. Soc. D, 1971, 792 RSC; (b) D. M. P. Mingos, Nat. Phys. Sci., 1972, 236, 99 CrossRef CAS; (c) K. Wade, Structural and Bonding Patterns in Cluster Chemistry, in Advances in Inorganic Chemistry and Radiochemistry, ed. H. J. Emeléus and A. G. Sharpe, Academic Press, 1976, ch. 1, vol. 18, pp. 1–66 Search PubMed; (d) D. M. P. Mingos, Acc. Chem. Res., 1984, 17, 311 CrossRef CAS.
- V. I. Bregadze, Chem. Rev., 1992, 92, 209 CrossRef CAS.
- (a) A. F. Armstrong and J. F. Valliant, Dalton Trans., 2007, 4240 RSC; (b) B. P. Dash, R. Satapathy, J. A. Maguire and N. S. Hosmane, New J. Chem., 1955, 2011, 35 Search PubMed; (c) M. F. Hawthorne and A. Maderna, Chem. Rev., 1999, 99, 3421 CrossRef CAS PubMed; (d) F. Issa, M. Kassiou and L. M. Rendina, Chem. Rev., 2011, 111, 5701 CrossRef CAS PubMed; (e) J. Plesek, Chem. Rev., 1992, 92, 269 CrossRef CAS; (f) B. B. Jei, L. Yang and L. Ackermann, Chem. – Eur. J., 2022, 28, e202200811 CrossRef CAS PubMed.
- D. Ellis, NMR of Carboranes, in Comprehensive Inorganic Chemistry III, ed. J. Reedijk, K. R. Poeppelmeier and D. L. Bryce, Elsevier, 2023, ch. 5, vol. 9, pp. 62–106 Search PubMed.
- (a) S. Hermanek, Chem. Rev., 1992, 92, 325 CrossRef CAS; (b) S. Heřmánek, Inorg. Chim. Acta, 1999, 289, 20 CrossRef.
- (a) T. L. Venable, W. C. Hutton and R. N. Grimes, J. Am. Chem. Soc., 1982, 104, 4716 CrossRef CAS; (b) T. L. Venable, W. C. Hutton and R. N. Grimes, J. Am. Chem. Soc., 1984, 106, 29 CrossRef CAS.
- (a) A. Lötz and J. Voitländer, J. Chem. Phys., 1991, 95, 3208 CrossRef; (b) Y. L. Pascal and O. Convert, Magn. Reson. Chem., 1991, 29, 308 CrossRef CAS; (c) J. Olliges, A. Lötz, D. Kilian, J. Voitländer and L. Wesemann, J. Chem. Phys., 1995, 103, 9568 CrossRef CAS.
- A. Navarro-Vázquez, P. Berdagué and P. Lesot, Chem. Phys. Chem., 2017, 18, 1252 CrossRef PubMed.
- P. Lesot, R. R. Gil, P. Berdagué and A. Navarro-Vázquez, J. Nat. Prod., 2020, 83, 3141 CrossRef CAS PubMed.
- F. Rüttger, T. Patten, J. Kretsch, A. Krawczuk, D. Stalke and M. John, Chem. – Eur. J., 2023, 29, e202203995 CrossRef PubMed.
- (a) J. A. Potenza, W. N. Lipscomb, G. D. Vickers and H. Schroeder, J. Am. Chem. Soc., 1966, 88, 628 CrossRef CAS; (b) F. P. Boer, R. A. Hegstrom, M. D. Newton, J. A. Potenza and W. N. Lipscomb, J. Am. Chem. Soc., 1966, 88, 5340 CAS.
- A.-C. Pöppler, H. Keil, D. Stalke and M. John, Angew. Chem., Int. Ed., 2012, 51, 7843 CrossRef PubMed.
- L. Mueller, J. Magn. Reson., 1987, 72, 191 CAS.
- P. Tzvetkova, S. Simova and B. Luy, J. Magn. Reson., 2007, 186, 193 CrossRef CAS PubMed.
- P. Tzvetkova and B. Luy, Magn. Reson. Chem., 2016, 54, 351 CrossRef CAS PubMed.
- B. Wrackmeyer, Z. Naturforsch. Pt. B, 2004, 59, 1192 CrossRef CAS.
- M. J. Frisch, et al., Gaussian16, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- W. C. Bailey, J. Mol. Spectrosc., 1997, 185, 403 CrossRef PubMed.
- P. Berdagué, B. Gouilleux, M. Noll, S. Immel, M. Reggelin and P. Lesot, Phys. Chem. Chem. Phys., 2022, 24, 7338 RSC.
- R. Bjornsson and M. Bühl, Dalton Trans., 2010, 39, 5319 RSC.
- J. Autschbach, S. Zheng and R. W. Schurko, Concepts Magn. Reson., Part A, 2010, 36, 84 CrossRef.
- A. Navarro-Vázquez, Magn. Reson. Chem., 2012, 50, S73–S79 CrossRef PubMed.
- G. Cornilescu, J. L. Marquardt, M. Ottiger and A. Bax, J. Am. Chem. Soc., 1998, 120, 6836 CrossRef CAS.
- T. Niklas, C. Steinmetzger, F. Rüttger, D. Stalke and M. John, Magn. Reson. Chem., 2017, 55, 1084 CrossRef CAS PubMed.
- D. Merlet, B. Ancian, J. Courtieu and P. Lesot, J. Am. Chem. Soc., 1999, 121, 5249 CrossRef CAS.
- D. F. Gaines, C. K. Nelson, J. C. Kunz, J. H. Morris and D. Reed, Inorg. Chem., 1984, 23, 3252 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05054h |
| This journal is © The Royal Society of Chemistry 2023 |