Platonic and Archimedean solids in discrete metal-containing clusters
Xi-Ming
Luo
 a,
Ya-Ke
Li
a,
Xi-Yan
Dong
a,
Ya-Ke
Li
a,
Xi-Yan
Dong
 ab and
Shuang-Quan
Zang
ab and
Shuang-Quan
Zang
 *a
*a
aCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, China. E-mail: zangsqzg@zzu.edu.cn
bCollege of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454003, China
First published on 19th December 2022
Abstract
Metal-containing clusters have attracted increasing attention over the past 2–3 decades. This intense interest can be attributed to the fact that these discrete metal aggregates, whose atomically precise structures are resolved by single-crystal X-ray diffraction (SCXRD), often possess intriguing geometrical features (high symmetry, aesthetically pleasing shapes and architectures) and fascinating physical properties, providing invaluable opportunities for the intersection of different disciplines including chemistry, physics, mathematical geometry and materials science. In this review, we attempt to reinterpret and connect these fascinating clusters from the perspective of Platonic and Archimedean solid characteristics, focusing on highly symmetrical and complex metal-containing (metal = Al, Ti, V, Mo, W, U, Mn, Fe, Co, Ni, Pd, Pt, Cu, Ag, Au, lanthanoids (Ln), and actinoids) high-nuclearity clusters, including metal-oxo/hydroxide/chalcogenide clusters and metal clusters (with metal–metal binding) protected by surface organic ligands, such as thiolate, phosphine, alkynyl, carbonyl and nitrogen/oxygen donor ligands. Furthermore, we present the symmetrical beauty of metal cluster structures and the geometrical similarity of different types of clusters and provide a large number of examples to show how to accurately describe the metal clusters from the perspective of highly symmetrical polyhedra. Finally, knowledge and further insights into the design and synthesis of unknown metal clusters are put forward by summarizing these “star” molecules.
1. Introduction
Metal-containing clusters whose main core or skeleton originates from the coordination-driven self-assembly of basic units such as metal atoms M0/n+, metal-oxo MOxm−, and secondary building blocks (SBBs, Fig. 1) are emerging as unique crystalline materials, and thus have attracted significant interest owing to their fascinating structures and great potential in numerous applications.1–23 Among the many types of metal-containing clusters, the highly symmetric structures are considered exceptional due to their complexity and beauty, which is inspired by the structure of the ‘star’ molecule, i.e., buckminsterfullerene (C60) discovered in 1985.24 The poly-nuclearity and high-nuclearity (with nuclearity of 30 or greater) metal clusters with orderly arrangements of metal atoms, especially those meeting well-known regular Platonic and/or Archimedean solids (Fig. 2 and 3), present aesthetically pleasing structures and intriguing physical properties.25–30 However, the synthesis of highly symmetric metal clusters has opened up a challenging field for chemists and materials scientists. These outcomes are usually serendipitous discoveries.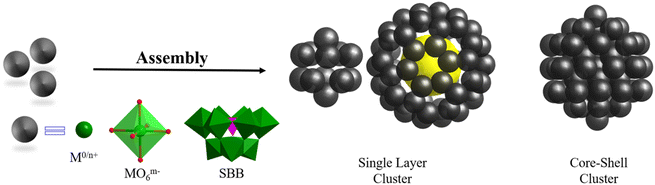 | ||
| Fig. 1 Regular packing of basic units (metal atoms M0/n+, metal-oxo MOxm−, and SBBs) to form highly symmetric metal clusters protected by additional ligands. For clarity, ligands were omitted. | ||
Over the last two decades, due to their continuing enthusiasm and efforts, synthetic chemists have proposed and designed multiple feasible synthesis strategies, developed new synthesis methods, and improved technology for the growth of single-crystals, resulting in several very exciting achievements in the synthesis and applications of metal clusters with different structures (especially high-nuclearity metal nanoclusters), such as cubic-box-shaped {Mo64Ni8Ln6},31 dodecahedra-shaped {Mo240},32 hedgehog-shaped {Mo368},33 tetrahedral {Dy30Co8W108},34 dodecameric {(Ta/Nb)3P2W15O62}12,35 protein-sized {Nb288},36 C60-shaped {U60},37 fullerene-like {Ti42},38 wheel {Mn84},39 {Fe168},40 {Co32},41 ligand-free {As@Ni12@As20},42 {Pt6Ni38},43–45 ring {Pd84},46 {(PtPd)165},47 {Zn14},48 {Cd66},49 {Hg32},50 {Ce100},51 {Gd140},52 cubic {Ln96Ni64} (Ln = Gd, Dy, Y),53 hexagon-shaped {Ni36Gd102},54 spherical {Ln104} (Ln = Gd, Nd),55 {Gd158Co38},56 {Cu179},57 {Ag374},58 {Ag490},59 {Au279},60 {Al77},61 {Ga84},62 {In68},63 {Sn34},64 {Pb18I44},65 and {Bi50}.66 Among them, highly symmetrical polyhedral structures (metal atoms or SBBs as vertices), ranging from simple tetrahedral ones to complex frameworks with multiple shells (Fig. 1), are always the most impressive structures, causing us to marvel at their nature.
The synthesis and structural chemistry of different giant metal-containing clusters and their related properties and applications have been previously reviewed.67 For example, Tasiopoulos and Christou et al. reviewed the synthesis, structures and magnetic properties of giant 3d (transition metal) and 3d–4f (lanthanide) paramagnetic metal clusters (including Mn, Fe, Co, Ni, Cu, and Ln) in moderate oxidation states.6 Long and Zheng et al. surveyed a wide variety of fullerene-like metal-containing molecular clusters.8
Recently, Scheer reviewed giant non-biological discrete metal-based species (molecules and ions) with nanometer size, including metal clusters, ligand-based metal–organic cages and spacer-based supramolecules.21
However, most of the metal cluster-related reviews focused on discussing metal clusters according to the metal type (Ti,2,68–70 3d metal,6 3d–4f metal,6,71–73 Ln,5 Cu,11,74 Ag,75,76 Au,12,15,77 polyoxometalates(POMs),4,78–80 Al, Ga,81,82etc.), the chemical properties (magnetism,6,73,83 optics,75 catalysis,1,13 biology,84etc.) of these metal clusters and the types of shell ligands (thiolate,85 phosphine,86–88 alkynyl,89,90 carbonyl,22 nitrogen91/oxygen92 donor ligands, etc.), with few summarizing their structural features. In 2005, Alvarez systematically summarized and described the chemistry of polyhedra (inorganic) and highlighted the geometrical relationships between different polyhedra.29 Zaworotko and co-workers summarized the design and synthesis of metal–organic frameworks (MOFs) using metal–organic polyhedra (Platonic and Archimedean solids) as super-molecular building blocks.93 Recently, Choe and Lah reviewed the topologies and the classification of hollow metal–organic polyhedra/cages.94 However, over the past two decades, different from MOFs and metal–organic polyhedra/cages, these exciting ‘star’ metal clusters have not been linked from a polyhedron perspective, in which the ordered arrangement of atoms or metal-cluster SBBs satisfy the regular polyhedron feature of Platonic and Archimedean solids. Thus, from the perspective of regular polyhedral geometry, taking highly symmetric crystal structures as examples, a systematic description of the recent development of poly-/high-nuclearity metal clusters in different fields of inorganic chemistry is urgently required. This will provide a highly valuable overview of what is possible and what approaches are successful for the formation of these poly-/high-nuclearity metal clusters. Meanwhile, a summary on the different properties resulting from different metal-type clusters with similar metal arrangements will help us reasonably predict the unknown structures of related metal clusters based on the existing results and obtain more excellent properties.
In this review, we summarize and introduce atomically precise discrete metal aggregates with structural characteristics of Platonic and Archimedean solids created in the field of chemistry (mainly inorganic chemistry), including metal clusters with metal–anionic cores (anionic: oxo/hydroxide/chalcogenide/halide, etc.), metal clusters with metal cores (with metal–metal binding) protected by thiolate, phosphine, alkynyl, carbonyl and nitrogen/oxygen donor ligands, and metal clusters with metal–anionic cores and metal–metal bonds. Herein, the polyhedral structural features of these metal clusters (special attention to complex high-nuclearity ones) will be discussed in the different chapters according to the metal type (polyoxometalates, group IV(Ti/Zr/Hf)/VII(Mn)/VIII(Fe/Co/Ni/Pd/Pt) metal, lanthanide, coinage metals (Cu/Ag/Au), and others), while their corresponding synthetic strategies and methods will be briefly introduced. By analyzing the examples from exciting achievements in the field of metal clusters over the past few decades, we show how different sets of atoms can be organized into different polyhedra, which can further combine into more complex or nested structures. In the following section, we discuss the synthesis (mainly from the perspective of shell ligands and templated anions) and structural prediction (from the perspective of the close packing of the underlying cells (metal atoms M0/n+, metal-oxo MOxm−, and SBBs) and the geometrical relationship between different Platonic and Archimedean polyhedra) of the giant ‘star’ clusters. The current research status including the major challenges will be elaborated at the end, together with some personal perspectives for future development.
All the metal clusters mentioned in this review were isolated as single-crystalline states, enabling their precise structural characterization by single-crystal X-ray diffraction. However, due to our limited knowledge, some metal clusters featuring Platonic and Archimedean solids, especially heterometallic clusters, may not be included in this review. Specifically, we searched the literature up to October 2022. It should be noted that metal–organic cages (metal atoms or metal clusters as vertexes and polydentate organic ligands as edges) with regular polyhedral features are beyond the scope of this review.
2. Platonic and Archimedean solids
2.1 Platonic solids
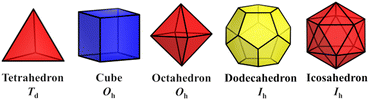
The simplest but best-known family of polyhedra, i.e., Platonic solids, contain five common polyhedra (tetrahedron, hexahedron (cube), octahedron, dodecahedron and icosahedron, Fig. 2 and Table 1), all of which are constructed from a single type of regular polygon (triangle, square or pentagon with equivalent vertices and equivalent edges). These five Platonic solids are regular polyhedra and belong to one of the highly symmetric point groups, i.e., tetrahedral (Td), octahedral (Oh), and icosahedral (Ih).| Platonic polyhedra | Vertices (V)a | Faces (F)b | Edges (E) | |
|---|---|---|---|---|
| a Where the numbers 3, 4, 5, 6, 8 and 10 represent the faces around that vertex. For instance, (3,6,6) represents one triangle, one hexagon, and another hexagon, respectively. b The index indicates the number and type of the polygon faces. For example, 43 + 46 represents four tetragonal and four hexagonal faces. c There are two enantiomeric versions of this polyhedron. Note: The listed polyhedron conforms to Euler's rule of V + F − E = 2. | ||||
| Tetrahedron | 4 | (3,3,3) | 43 | 6 |
| Cube | 8 | (4,4,4) | 64 | 12 |
| Octahedron | 6 | (3,3,3,3) | 83 | 12 |
| Dodecahedron | 20 | (5,5,5) | 125 | 30 |
| Icosahedron | 12 | (3,3,3,3,3) | 203 | 30 |
| Archimedean polyhedra | Vertices (V) | Faces (F) | Edges (E) | |
|---|---|---|---|---|
| Truncated tetrahedron | 12 | (3,6,6) | 43 + 46 = 8 | 18 |
| Cuboctahedron | 12 | (3,4,3,4) | 83 + 64 = 14 | 24 |
| Truncated cube | 24 | (3,8,8) | 83 + 68 = 14 | 36 |
| Truncated octahedron | 24 | (4,6,6) | 64 + 86 = 14 | 36 |
| Rhombicuboctahedron | 24 | (3,4,4,4) | 83 + 64 + 124 = 26 | 48 |
| Truncated cuboctahedron | 48 | (4,6,8) | 124 + 86 + 68 = 26 | 72 |
| Snub cubec | 24 | (3,3,3,3,4) | 243 + 83 + 64 = 38 | 60 |
| Icosidodecahedron | 30 | (3,5,3,5) | 203 + 125 = 32 | 60 |
| Truncated dodecahedron | 60 | (3,10,10) | 203 + 1210 = 32 | 90 |
| Truncated icosahedron | 60 | (5,6,6) | 125 + 206 = 32 | 90 |
| Rhombicosidodecahedron | 60 | (3,4,5,4) | 203 + 304 + 125 = 62 | 120 |
| Truncated icosidodecahedron | 120 | (4,6,10) | 304 + 206 + 1210 = 62 | 180 |
| Snub dodecahedronc | 60 | (3,3,3,3,5) | 603 + 203 + 125 = 92 | 150 |
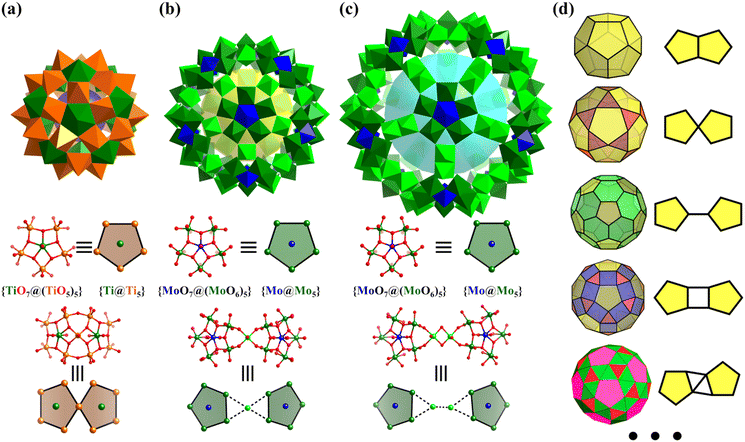
Numerous examples of discrete metal clusters featuring Platonic solids (tetrahedron, cube, octahedron, dodecahedron, and icosahedron) have been found in the literature (discussed in detail below).95 Among them, simple tetrahedral (M4) and hexahedral (M6) clusters are the most common ones and will not be discussed in detail in the subsequent chapters. In tetrahedral clusters, cubane-like (Fig. 4a–c) and adamantane-like (Fig. 4d–f) cores often appear, which can also be used as building blocks to build larger cluster structures.
 | ||
| Fig. 4 (a) Molecular structure of [Ti4(μ3-O)4(OiPr)4(Ph2PO2)4].96 (b) Cubane-like M4(μ3-O)4 core. (c) Cubane molecule. (d) Ball-and-stick representations of [(η-C5Me5)4V4(μ2-O)6].97 (e) Adamantane-like M4(μ2-O)6 core. (f) Adamantane molecule. | ||
2.2 Archimedean solids
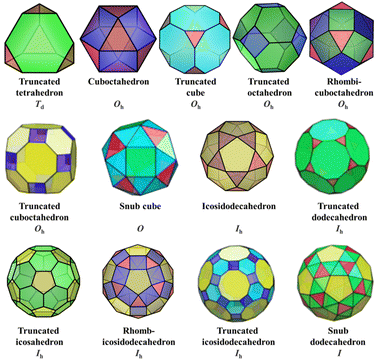
Archimedean solids are the second family of high symmetry polyhedrons, which are often called semiregular solids. There are 15 Archimedean solids (Fig. 3), two of which, the snub cube and snub dodecahedron, are chiral and have two pairs of enantiomers.29 Similar to Platonic solids, Archimedean solids feature a single type of vertex, namely, equivalent vertices surrounded by regular polygons (Table 1). All 13 Archimedean polyhedra can be obtained by simple manipulation of the Platonic polyhedrons.29 In addition to chiral Archimedean solids, other centrosymmetric ones have been found in atomically precise metal-containing clusters, mainly in truncated tetrahedron, cuboctahedron, truncated cube, truncated octahedron and rhombicuboctahedron. The specific cases are described in detail in the following sections.The term “keplerate” refers to a type of concentric nested structure that contains two or multi-Platonic and Archimedean solids, which was first proposed by Müller's group because of its similarity to Kepler's early cosmos model.25,98
The term “Mackay structure” refers a type of dense packing of equal-sized spheres, which can form concentric multi-shells of either regular icosahedra (Platonic solid) or cuboctahedra (Archimedean solid).30 The nth shell conforming to Mackay rules consists of (10n2 + 2) spheres. For example, the first shell can be a 12-atom icosahedron (Platonic solid) or cuboctahedron, either surrounding a central atom or a cavity, whereas the second shell, which is either a v2-icosahedron or v2-cuboctahedron (where νn denotes (n + 1) atoms along each edge of the polyhedron), consists of 42 atoms and encapsulates the 1st shell.
3. Platonic and Archimedean solids in POMs
Polyoxometalates (POMs) are a class of metal–oxygen clusters that have achieved rapid development due to their intriguing structural features and wide potential applications in the past two decades.3,4,78,79,99–109 These clusters contain highly symmetrical core assemblies of MOx units, in which M is the early transition metal with the highest oxidation state, such as V, Mo, W, Nb and Ta. POMs often adopt quasi-spherical structures based on Archimedean and Platonic solids of considerable topological interest, such as the α-Keggin cuboctahedral structure of {XM12O40} (X = P, Si, As; M = V, Mo, W, Nb, etc.) with Td symmetry (Fig. 5a and b) and Lindqvist-type octahedral structure of {M6O19} (M = V, Mo, W, Ta, Nb, etc.) with Oh symmetry (Fig. 5c and d). The related high-nuclearity and/or giant POMs with beautiful and complex polyhedral structures will be described below according to different metal elements (polyoxovanadates, polyoxomolybdates, polyoxotungstates, polyoxoniobates, and others). | ||
| Fig. 5 Polyhedral representation and corresponding polyhedral arrangement of the metal atoms of the α-Keggin {XM12O40} (a and b) and Lindqvist {M6O19} POMs (c and d). Structures shown correspond to K12[Ti2O2][SiNb12O40] (a and b)99 and Na7[HNb6O19] (c and d).110,111 | ||
3.1 Polyoxovanadates
In the small polyoxovanadates (POVs), in addition to the classic inorganic clusters (e.g., Lindqvist-type octahedron), some V–O clusters featuring Platonic and Archimedean solids are constructed by introducing organic composites.80,112,113 Furthermore, when other metals are present in the reaction system, unexpected structures can be obtained. For example, the reaction of [V2(μ-Cl)3(THF)6]2[Zn2Cl6] precursor with PhCOONa in THF solution yielded an octa-nuclear heterometal cluster [VIIIZnO(O2CC6H5)3(THF)]4, which contained a remarkable array of two concentric tetrahedra, i.e., V4 within Zn4 (V4Zn4, Fig. 6a),114 while the reaction of V2O5 with [(η-C5R5)Rh(OH)2]2 yielded a decanuclear heterometallic cluster [(C5Me5)Rh]4(V6O19) (V6Rh4, Fig. 6b), containing a remarkable array of two concentric polyhedra, i.e., V6 (octahedron) within Rh4 (tetrahedron).115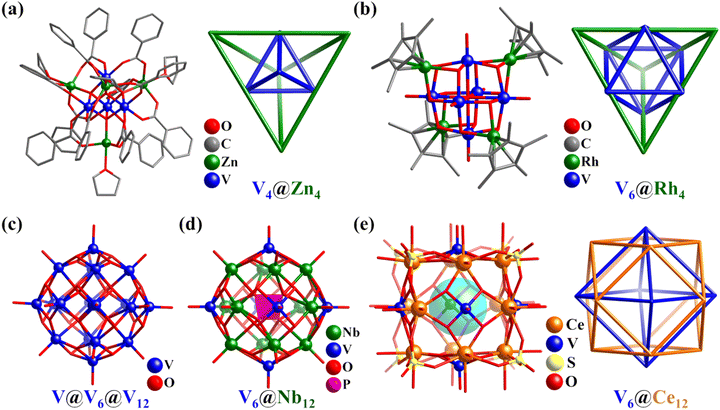 | ||
| Fig. 6 Ball-and-stick representation and nested metal skeletons of (a) [VIIIZnO(O2CC6H5)3(THF)]4,114 (b) [(C5Me5)Rh]4(V6O19),115 (c) [H8(VVO4)VIV18O42]7−,116 (d) {PNb12O40(VIVO)6}3−,117 and (e) [CeIV12(VVO)6O24(H2O)24(SO4)8]2+.118 | ||
In 2019, Das reported the preparation of two high-nuclearity POVs, i.e., [VVO4@VIV18O42] (V19, Fig. 6c) and [Cl@VIV18O42], exhibiting proton conductivity property.116 The spherical V19 is a classical “Keplerate” cluster, with the metal atoms in a polyhedral nested arrangement, VVO4(center)@V12(cuboctahedron)@V6(octahedron). Hu's group reported the preparation of a hexa-capped Keggin-type PONb, i.e., {PNb12O40(VIVO)6}3− (V6Nb12, Fig. 6d).117 The metal atoms in V6Nb12 can be simplified as a highly symmetrical core–shell structure, which consists of Nb12 (cuboctahedron) and V6 (octahedron), enveloping the center {PO4} group.
In 2022, Nyman and Farha synthesized three CeIV/VV oxo clusters with a [Ce12(VO)6O24]18+ cubane core capped by different ligands (SO42−, Ce12V6, Fig. 6e; toluenesulfonic acid; nitro-benzenesulfonic acid).118 In the Ce12V6 cube, the CeIV ions are located in the center of the 12 edges, while the vanadyl (VVO5) groups cap the six faces and SO42− anions are situated on the eight corners. The arrangement of the heterometallic atoms is similar to the above-mentioned V19, which is a nested structure (Fig. 6e), i.e., V6(octahedron)@Ce12(cuboctahedron). Interestingly, the functionalization of the [Ce12(VO)6O24]18+ core with organic ligands achieved adjustable catalytic reactivity.
Zhang and Schmitt prepared four high-nuclearity POV cages stabilized by organoarsonate ligands and provided a highly effective method using the template effect and modular self-assembly to realize the successive construction of nanoscopic symmetrical polyoxovanadate cages.119 In the biggest one of the four POV cages reported, i.e., {Cl6@[(VVO)16(VIVO)8O24(O3AsC6H5)8]}6− (Cl6@V24As8, Fig. 7a), the V atoms as the vertices of the hollow structure adopt a topology of truncated octahedron. The formation of the 24-metal Archimedean arrangement is the result of the assembly of 6 square tetramers {V4O8} induced by 6 Cl− ions as templates, in which the tetramers cap the vertices of the encapsulated Platonic {Cl6} octahedron. This cluster can be classified as a keplerate, with concentric arrangements of both Platonic and Archimedean solids (Cl6(octahedron)@V24(truncated octahedron)@As8(hexahedron), Fig. 7b).
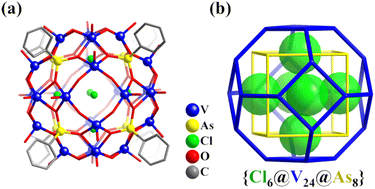 | ||
| Fig. 7 (a) Structure of Cl6@V24As8. (b) {V24As8} cluster topology with the six Cl− as anion templates highlighted using the space-filling representation.119 | ||
Besides, Streb reported the preparation of a tetrahedron-shaped 58-nuclear Ba–V–O cluster, H5[Br@Ba10(NMP)14(H2O)8[V12O33]4] (Ba10V48, NMP = N-methyl-2-pyrrolidone).120 In Ba10V48, the octahedral Ba10 shell (tetrahedron(Ba4)@octahedron(Ba6)) templated by a Br− anion is capped by four [V12O33]6− SBBs in a tetrahedral fashion.
3.2 Polyoxomolybdates
By introducing reducing agents, synthetic chemists achieved the preparation and isolation of many MoV-containing polyoxomolybdates (POMos, Fig. 8) and the self-assembly of metal-oxide building blocks (POM subunits, such as triangular {Mo3}, pentagonal {(Mo)Mo5}, and tripod-shaped {Mo6} groups generally), crossing the boundary of the small molecule world, as well as constructing multi-functional and highly complex molecular nano-objects.4 The main types of related giant polyoxomolybdates include (Fig. 9–11) core–shell-type {Mo60} (Fig. 9),121 cubic-box-shaped {Mo64Ni8Ln6} (Fig. 10),31 {Mo84} (Fig. 11a and b),122 spherical keplerates {M72M′30} (M = Mo, M′ = VIV, CrIII, FeIII, and MoV, Fig. 11c and d),123–125 {M72Mo60} (M = Mo, and W, Fig. 11e and f),126–128 dodecahedra-shaped {Mo240} (Fig. 11g and h),32 and hedgehog-shaped {Mo368}33 nanoclusters.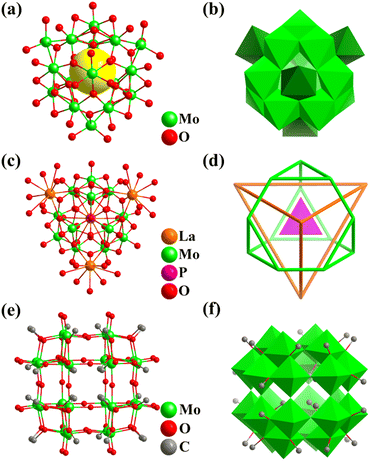 | ||
| Fig. 8 (a and b) Combined polyhedral/ball-and-stick representation of Mo16.129 (c and d) Ball-and-stick representation and nested structure of ε-La4PMo12, respectively.130 (e and f) Ball-and-stick and polyhedral representations of Mo24, and rhombicuboctahedral arrangement of 24 metal atoms, respectively.131 | ||
 | ||
| Fig. 9 (a) Ball-and-stick representation of Zn8Mo28 and simplified 36-metal-based skeleton with four regular polyhedrons nested together (Mo4(tetrahedron)@Zn4(tetrahedron)@Mo24(truncated octahedron)@Zn4(tetrahedron)).132 (b) Ball-and-stick representation of Mo60. (c) {MoV6O25}-based assembly of Mo60 from the inside out, an ε-Mo12 core (truncated tetrahedron), an Mo24 cage (truncated tetrahedron) and four Mo6 arranged in a tetrahedron. (d) Successive Archimedean polyhedral description featuring only equivalent vertex metal atoms of Mo60.121 | ||
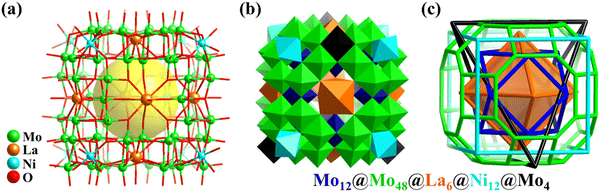 | ||
| Fig. 10 Ball-and-stick (a) and polyhedral (b) representations of Mo64Ni8La6. (c) Simplified 88-metal-based skeleton with five regular polyhedra nested together.31 | ||
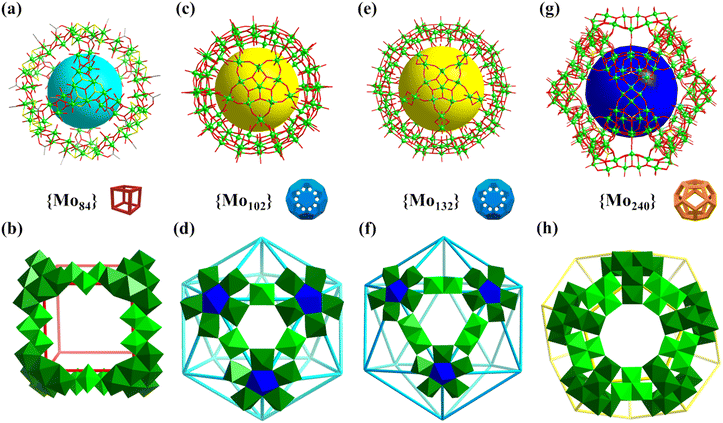 | ||
| Fig. 11 (a and b) Structure of Mo84 and the cubic arrangement with 8 {Mo6} (green) as vertexes and 12 {Mo3} (bright green) as linkers, respectively.122 (c and d) Structure of Mo102 and the icosahedral arrangement with 12 {(Mo)Mo5} as vertexes and 30 O = Mo(H2O) (bright green) as linkers, respectively.125 (e and f) Structure of Mo132 and the icosahedral arrangement with 12 {Mo6} (blue and green) as vertexes and 30 {MoV2} (bright green) as linkers, respectively.126 (g and h) Structure of Mo240 and the dodecahedral arrangement with 20 {Mo6} (green) as vertexes and 30 {Mo4} (bright green) as linkers, respectively.32 | ||
In 1993, Müller and co-workers reported the preparation of two 16-metal polyoxomolybdates with the same polyanion, i.e., [(MoVIO3)4MoV12O28 (OH)12]8− (Mo16, Fig. 8a).129Mo16 in full tetrahedral Td symmetry contains a truncated tetrahedral core (MoV12O28(OH)12) capped with four {MoVIO3} motifs with a tetrahedral arrangement, that is, Mo12(truncated tetrahedron)@Mo4(tetrahedron, Fig. 8b). In 2002, Sécheresse's group reported the first ε-Keggin cation (ε-PMo12O40) with a central {PO4}, stabilized by four {La(H2O)4}3− capping groups with a tetrahedral fashion, which has the detailed formula {ε-PMoV8MoVI4O36(OH)4{La(H2O)4}4]5− (ε-La4PMo12, Fig. 8c and d).130 The incorporation of four metal ions on the skeleton of ε-PMo12O40 (especially zinc-modified, ε-Zn4PMo12) provided the possibility to connect the ε-POM clusters into extended frameworks (1D, 2D and 3D) through coordination interactions between the metal ions and organic ligands.133,134
In 2022, Wang and co-workers reported a co-crystal example, (nBu4N)6H2[{MoV24O48(OCH3)32}{MoV/VI24O52(OCH3)28}2], assembled with an anionic [{MoV24O48(OCH3)32}]8− (Mo24, Fig. 8e) and two neutral [{MoV/VI24O52(OCH3)28}] high-nuclear iso-polymolybdates.131 The two iso-polymolybdates have the same metal frame, protected by different numbers of O2− linkers and CH3O− groups (Fig. 8f). Notably, this is the first 24-metal cluster with a rhombicuboctahedral character (Fig. 8e), although a rhombicuboctahedron can be observed as part of or a shell in many complex cluster structures. Recently, Zhang and Wang reported the preparation of three Td symmetry Mo4O4-cubane-templated heteropolyoxometalates, i.e., M8Mo28 (M = Zn or Co), M8MoV8MoVI20O88(H2O)4 (Fig. 9a), and Zn8MoV8MoVI20O88(H2O)(NH(CH3)2)3.132 In M8Mo28, the metal-oxo Mo4O4 core (Mo4: tetrahedron) acts as a template to form the larger truncated octahedral Mo24 shell, which is composed of six tetragons and eight 8-ring windows. Two additional sets of tetrahedrally arranged heterometal atoms (Zn or Co) are filled inside and outside the center of eight six-membered ring windows (Fig. 9a).
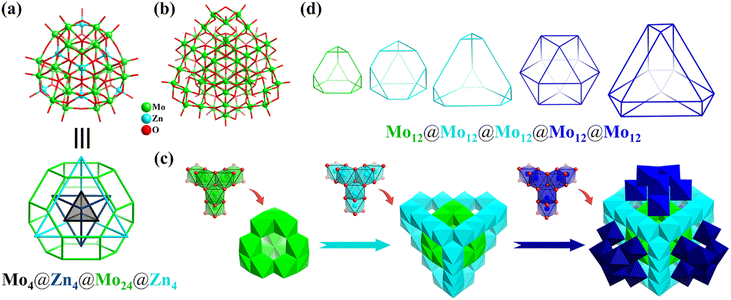
In the same year, under hydrothermal conditions, Wang obtained the largest fully reduced Na8[MoV60O140(OH)28] (Mo60, Fig. 9b) polyoxomolybdate to date, which is a good all-inorganic light-absorbing material with efficient improved photo-electronic performance.121Mo60 with Td symmetry can be described as one ε-Keggin Mo12 (truncated tetrahedron) encapsulated in an unprecedented Mo24 cage, which is further face-capped and surrounded by four tripod-shaped {Mo6} motifs. In addition, the whole structure of Mo60 (Fig. 9c) can be regarded as the splicing of tripod-shaped {Mo6} secondary building blocks (SBB), where ε-Mo12 can be viewed as consisting of four {Mo6} SBBs fused together. From the inside to the outside, the topologies of the 60 metals in the triple nesting structure contain five Td symmetrical Archimedean solids (three truncated tetrahedrons and two cuboctahedrons), abbreviated as Mo12@Mo12@Mo12@Mo12@Mo12 (Fig. 9d).
Besides, Cronin engineered highly reduced polyoxometalates with a super-cube structure by incorporating NiII and LnIII ions, i.e., Na9[MoV52MoVI12Ni8Ln6H26O200(H2O)30](ClO4) (Mo64Ni8Ln6, Ln = La, Fig. 10a; Ce, Pr).31 The super-cubic structure of Mo64Ni8Ln6 (Fig. 10b) is comprised of 5 different overlayed and co-centred Platonic or Archimedean structures, i.e., a primary truncated cuboctahedron embedding a {MoVI12} cuboctahedron and capped by different metal ions on its different faces to form an {NiII8} cube, an {LnIII6} octahedron, and a disordered {MoV4} tetrahedron, respectively (Fig. 10c).
In 2012, Cadot reported the preparation of a high-nuclearity polyoxothiomolybdate featuring a cubic box shape, i.e., [H8Mo84S48O188(H2O)12(CH3COO)24]32− (Mo84, Fig. 11a).122 The Mo84 cage is composed of 8 triangular {Mo6S6O6(OH)}5+ as 3 connected vertices and 12 {MoVI3O11}4− as edges, featuring a large inner void (diameter up to 9 Å) and 6 opening square windows (Fig. 11b).
In 1998, Müller's group prepared an inorganic giant fullerene-like 132-metal cluster based on {MoOx}, i.e., (NH4)42[MoVI72MoV60O372(CH3COO)30(H2O)72] (Mo132, Fig. 11e), and first mentioned the term “Keplerate”.126–128,135Mo132 can be regarded as the product of the assembly of 12 {Mo11} subunits ({(MoVI)(MoVI5)(MoV5)}) according to the regular icosahedron, wherein (MoVI)(MoVI5) are vertexes and {MoV2} are edges (Fig. 11f). The Mo atoms of {Mo11} with C5 symmetry can be divided into three parts (types I–III, Fig. 11f), including a central seven-coordinated Mo atom (type I, blue; 12 Mo: icosahedron) with pentagonal-bipyramid geometry, sharing its five equatorial edges with five {MoVIO6} octahedra (type II, green; 60 Mo (12 {Mo5}): rhombicosidodecahedron), and the other five {MoVO6} octahedra (type III, bright green; 60 Mo (30 {Mo2}): truncated icosahedron) share corners with that of the type-II {MoVIO6}. When red-brown solution of Mo132 is oxidized, the deep-blue rhombohedral crystals of smaller spherical Mo-clusters, i.e., [{(Mo)Mo5O21(H2O)4(CH3COO)}12{MoO(H2O)}30] (Mo102, Fig. 11c), were isolated after a few days.125 Similar to Mo132, Mo102 is composed of 12 {(Mo)Mo5} pentagonal units, but they are partially reduced. The units follow the same arrangement rule (icosahedron) as Mo132, which are interlinked through 30 O = Mo(H2O) species. Twelve five-membered {Mo5} rings ignore the center and build a twisted rhombicosidodecahedron skeleton (Mo atoms as equal (3,4,5,4) vertices), while 30 linkers as vertices (3,5,3,5) form another Archimedean solid (icosidodecahedron, Fig. 11d).
In 2020, Lan reported the preparation of a giant polyoxomolybdate featuring hollow opening dodecahedra, [Mo240(OH)60O620−x(SO3)20−x(SO4)x](80−2x)− (Mo240, Fig. 11g).32Mo240 is the highest-nuclearity metal cage with hollow opening dodecahedra reported to date. Mo240 consists of 20 tripodal {Mo6O21(SO4)}/{Mo6O22(SO3)} as three connected vertices and 30 {Mo4O16} as edges, featuring a large inner cavity (diameter of up to 18 Å, Fig. 11h). Due to the presence of rich terminal oxygen atoms, water molecules, and counter cations (H+ and NH4+), Mo240 exhibits ultrahigh proton conductivity (1.03 × 10−1 S cm−1 at 80 °C and 98% relative humidity (RH)), which is the highest among POM-based crystalline materials.
3.3 Polyoxotungstates
Metal-substituted polyoxometalates, especially metal-substituted polyoxotungstates (POWs) constitute one of the most exciting developments in POM chemistry.68,106,136,137 The motivation comes not only from their intriguing structural diversity but also their special properties, which are applicable to magnetism, catalysis, medicine, and materials science. Here, the metal includes transition-metals (TMs = Ti, Fe, Mn, Co, Ni, and Cu), lanthanides (Ln) and main group metals. Among the various structures, some exhibit regular polyhedral geometric arrangements based on metal atoms and SBBs, forming super-tetrahedral, cubic, octahedral, and icosahedral structures.In 2003, Kortz's group reported for the first time the structural characterization of TiIV-substituted polyoxoanions based on Wells–Dawson type and prepared the first tetrameric TiIV-substituted POMs, [{Ti3P2W15O57.5(OH)3}4]24− (Ti12P2W15, Fig. 12a).138 The 12-Ti-substituted polyoxotungstate is composed of four {Ti3P2W15} equivalent fragments, connected by terminal Ti–O–Ti bonds, leading to a tetrahedral POM cluster with Td symmetry. The central Ti12O46 core of Ti12P2W15 consists of four {Ti3O13} subgroups constructed by three edge-shared {TiO6} octahedra. With Ti metal as the vertices, 12 atoms can form a slightly distorted truncated tetrahedron (Fig. 12b), wherein the Ti⋯Ti separations in the four {Ti3P2W15} groups and between adjacent groups are 3.129(6)–3.208(6) Å and 3.488(6)–3.535(6) Å, respectively. In 2014, Kortz's group again reported the preparation of a tetrahedral Ti-substituted polyoxotungstate, [Ti6(TiO6)(AsW9O33)4]20− (Ti7AsW9, Fig. 12c).139Ti7AsW9 contains a novel Ti-center octahedral core, which consists of a central TiO6 (octahedron geometry) surrounded by six TiO5 (square-pyramid geometry) and capped by four {AsW9} tri-lacunary fragments (Fig. 12d). In 2018, Yang's group reported the synthesis of an Sb(V)-analogue of Ti7AsW9, [Ti7O6(SbW9O33)4]20− (Ti7SbW9).140Ti7SbW9 is a good heterogeneous catalyst for the catalytic oxidation of multiple thioethers, possessing high conversion and remarkable selectivity and exhibiting good stability and recyclability. Nomiya's group also reported the preparation of two types of TiIV-substituted polyoxotungstates, [(P2W15Ti3O60.5)4X]37− (X = Cl−,141 NH4+,142 similar to Ti12P2W15) and [Cl@(P2W15Ti3O62)4{μ3-Ti(OH)3}4]45− (Ti16P2W15, Fig. 12e).143Ti16P2W15 consists of four equivalent substructures of tri-TiIV-substituted POM {Ti3P2W15}, similar to Ti12P2W15, four extra bridging groups (Ti(OH)3), and one Cl− ion encapsulated in the center of the cluster. The four {Ti3} fragments of Ti16P2W15 form a twisted cuboctahedron geometry, where four Ti(OH)3 motifs capped at the triangles in a tetrahedral fashion (Fig. 12f). Some TM-substituted (TM = Fe,144–149 Mn,150 and Cr151) polyoxotungstate super-tetrahedral clusters with a truncated tetrahedral or cuboctahedron core have been published.
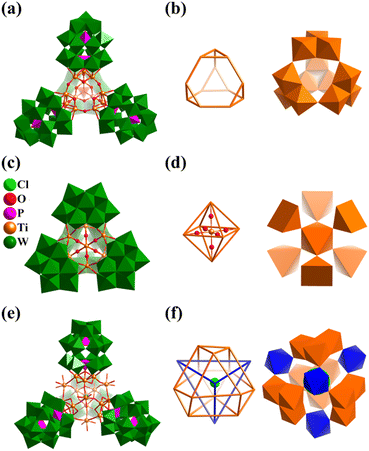 | ||
| Fig. 12 Combined polyhedral/ball-and-stick representation of Ti-substituted polyoxotungstates and their central Ti–O core: (a and b) [{Ti3P2W15O57.5(OH)3}4]24−;138 (c and d) [Ti6(TiO6)(AsW9O33)4]20−;139 and (e and f) [Cl@(P2W15Ti3O62)4{μ3-Ti(OH)3}4]45−.143 | ||
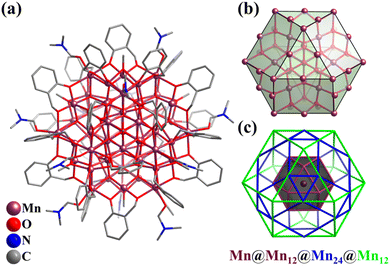
In 2016, Kortz's group reported the synthesis of two mixed-valent Mn16-substituted polyoxotungstate super-tetrahedra, [MnIII10MnII6O6(OH)6(PO4)4(SiW9O34)4]28− (Mn16P4SiW9, Fig. 13a) and [MnIII4MnII12(OH)12(PO4)4(SiW9O34)4]28−.152 The mixed-valent cationic {Mn16} cores are encapsulated by four {μ6-PO4} groups and four trivacant [SiW9O34]9− units in a tetrahedral fashion. Mn16P4SiW9 can also be regarded as four {Mn3SiW9} fragments connected by four {μ6-PO4} motifs, with an encapsulated {Mn4O4} cubane unit. The arrangement of the metal and bridged groups of Mn16P4SiW9 can be considered a complex nested structure (abbreviated as Mn4@P4@Mn12@{SiW9}4), contained in an innermost {Mn4O4} core, and moving outward, there are five regular polyhedrons, a tetrahedron, an elongated cuboctahedron and a tetrahedron (Fig. 13b). The synthesis of Co and Ni analogues with 16-metal-substituted polyoxotungstate was also successful.153,154
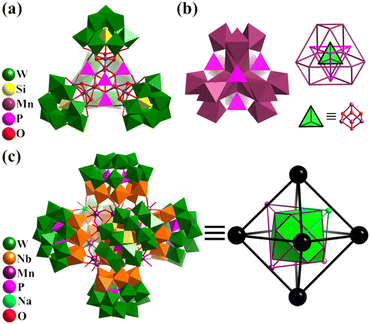 | ||
| Fig. 13 (a) Combined polyhedral/ball-and-stick representation of total structure and (b) central core of Mn-substituted polyoxotungstates [MnIII10MnII6O6(OH)6(PO4)4(SiW9O34)4]28−.152 (c) Combined polyhedral/ball-and-stick representation of super-octahedron {MnIII12MnII3Na(H2O)40(OH)12(P2W12Nb6 O62)6}41− and its simplified polyhedral model, MnIII12@MnII3Na@(P2W12Nb6)6.155 | ||
In addition to the above-mentioned TM16-substituted POM-based tetrahedral clusters, which are connected and stabilized by {XO4} (X = P, As, Si and Ge) groups in a tetrahedral fashion, there are TM16-containing POM clusters constructed by one central tetranuclear cluster unit and four tri-substituted POMs. In 1999, Hill's group reported the synthesis of an Nb16-substituted polyoxotungstate with an [Nb4O6]8− tetrahedral core, [Nb4O6(Nb3SiW9O40)4]20−.156 Four [Nb3SiW9O40]7− Keggin units are linked together by Nb–O–Nb–O–Nb bonds. This work realized the shape heredity of [Nb4O6]8− in overall tetrahedral POMs. In 2014, Wang's group reported two polytantalotungstate tetrahedrons with different cations, [Ta4O6(SiW9Ta3O40)4]20−.157
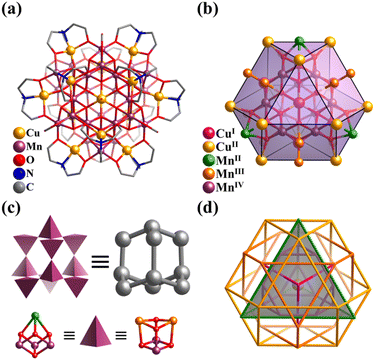
By introducing MnII in the metastable {(NbO2)6P2W12O56} in an acid aqueous solution of H2O2, Niu et al. synthesized a {P2W12Nb6}-based mixed-valence MnII/III cluster, K10{H31MnIII12MnII3Na(H2O)40(OH)12(P2W12Nb6O62)6} (Mn15Na(P2W12Nb6)6, Fig. 13c), exhibiting single-molecule magnet property.155 In the case of hexameric Mn15Na(P2W12Nb6)6, it is effective to clarify the complex architecture from a topological viewpoint involving Platonic and Archimedean solids. Four [MnIII3(OH)3(H2O)6]6+ motifs make up the basic Mn12 core, in which the distribution of 12 MnIII can be seen as an elongated cuboctahedron. Besides, four hinges (MnII and NaI) are distributed in a tetrahedral fashion and six [P2W12Nb6O62]9− SBBs arranged in an octahedral mode capping the outside of the Mn12 cuboctahedron.
In 2020, Chen and co-workers reported the organoboron-functionalization of polyoxotungstates ([M3P2W15O62]9− (M = TaV and NbV)), resulting in the hierarchical assembly of two tetrameric aggregates, {Na(3-PyB)4[M3P2W15O62]4}27− (3-PyBH2 = 3-pyridineboronic acid), and two giant polyoxometalate-based dodecameric nanocapsules containing up to twelve Dawson-type POM units, {K4(5-PymB)3(5-PymBH)12[M3P2W15O62]12}86− (5-PymBH2 = 5-pyrimidinylboronic acid, Fig. 14).35 In the super-tetrahedron, four Ta3-substituted Dawson-type building blocks are linked by four μ3-bridging organoboron (3-PyB) ligands into a tetrahedral aggregate (Ta12: cuboctahedron), similar to a tetrahedral tetramer through the inorganic unit (PO43−) mentioned above. Unexpectedly, when the organic linker was changed from 3-PyB to 5-PymB, a giant super-icosahedral based on {M3P2W15O62} was obtained. Twelve {M3P2W15O62} building blocks are linked by 12 μ2-5-PymBH and three μ3-5-PymB to form four trimers (Fig. 14a), resulting in the formation of a giant heteropolyoxotungstate icosahedral cage through 4 K+ ions arranged in tetrahedral mode (Fig. 14b).
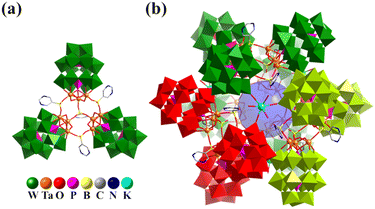 | ||
| Fig. 14 Combined polyhedral/ball-and-stick representation of trimer {(5-PymB)(5-PymBH)3[M3P2W15O62]3}32− (a) and nano-capsule {K4(5-PymB)3(5-PymBH)12[M3P2W15O62]12}86− (b).35 | ||
Main group metals also connected lacunary POM motifs to construct highly symmetric polyhedral structures. In 2005, Kortz's group reported two cases of Sn-containing heteropolytungstates in different works, i.e., tetrahedron [{Sn(CH3)2(H2O)}2{Sn(CH3)2}As3(AsW9O33)4]21− (Sn3As3AsW9)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 and icosahedron [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(XW9O34)12]36− (X = P, Sn36(PW9)12 (Fig. 15) and As).159Sn3As3AsW9 is composed of four {AsW9O33} POM units linked together by three As(III) atoms and two {Sn(CH3)2(H2O)} and one {Sn(CH3)2} group, resulting in a chiral polyoxoanion with C1 symmetry. Sn36(PW9)12 is composed of twelve tri-lacunary [PW9O34]9− Keggin units, which are linked by 12 inner {Sn(CH3)2} and 24 outer {Sn(CH3)2(H2O)} groups, resulting in a more 100-metal POMs with Th symmetry. If the [{Sn(CH3)2(H2O)}2{Sn(CH3)2}(XW9O34)]3− fragment is a vertex, Sn36(PW9)12 can be described as an almost perfect icosahedron (Fig. 15). The 12 inner and 24 outer Sn atoms also form a highly symmetrical arrangement, respectively.
158 and icosahedron [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(XW9O34)12]36− (X = P, Sn36(PW9)12 (Fig. 15) and As).159Sn3As3AsW9 is composed of four {AsW9O33} POM units linked together by three As(III) atoms and two {Sn(CH3)2(H2O)} and one {Sn(CH3)2} group, resulting in a chiral polyoxoanion with C1 symmetry. Sn36(PW9)12 is composed of twelve tri-lacunary [PW9O34]9− Keggin units, which are linked by 12 inner {Sn(CH3)2} and 24 outer {Sn(CH3)2(H2O)} groups, resulting in a more 100-metal POMs with Th symmetry. If the [{Sn(CH3)2(H2O)}2{Sn(CH3)2}(XW9O34)]3− fragment is a vertex, Sn36(PW9)12 can be described as an almost perfect icosahedron (Fig. 15). The 12 inner and 24 outer Sn atoms also form a highly symmetrical arrangement, respectively.
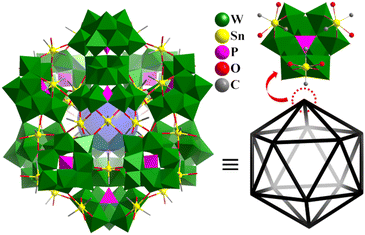 | ||
| Fig. 15 Combined polyhedral/ball-and-stick representation of [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(XW9O34)12]36− and its simplified icosahedron skeleton.159 | ||
In 2018, Zheng's group reported the synthesis of a giant ionic spherical alkali-metal-POM composite cluster, [K42Ge8W72O272(H2O)60]38− (K42Ge8W72, Fig. 16a).160K42Ge8W72 is not only a rare high-nuclearity K-H2O cluster {K42(H2O)60} (Fig. 16b), but also a new-type all-inorganic ionic porous material in which the cluster can be linked together through Na+, to construct a 3D framework possessing versatile properties (high stability, proton conductivity (6.8 × 10−2 S cm−1 at 98% RH and 85 °C), and water vapor adsorption 52 cm3 g−1). The rare {K42(H2O)60} cluster in K42Ge8W72 exhibits a core–shell framework (Fig. 16b) of K6(octahedron)@K24(truncated octahedron)@K12 (cuboctahedron), stabilized by eight C3v-symmetry tri-vacant Keggin-type [GeW9O34]10− with a cubic arrangement (Fig. 16c).
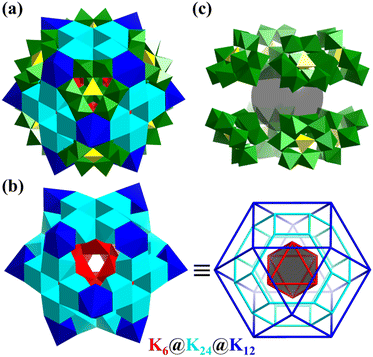 | ||
| Fig. 16 (a) Polyhedral representation of [K42Ge8W72O272(H2O)60]38−. (b) Core–shell {K42(H2O)60} cluster, K6(octahedron)@K24(truncated octahedron)@K12(cuboctahedron). (c) Eight C3v-symmetry tri-vacant [GeW9O34]10− with a cubic arrangement.160 | ||
Due to their luminescent and magnetic properties, the introduction of lanthanide (Ln) ions into POM systems to construct Ln-containing POM-based clusters and materials is an important research field in POM chemistry.109,161,162 In 2015, Powell and co-workers described the preparation of a giant tetrahedral 3d–4f heterometallic POM [{(GeW9O34)2Dy3(OH)3(H2O)}6{CoII2Dy3(OH)6(H2O)6}4]56− (Dy30Co8W108, Fig. 17a), which shows single-molecule magnet (SMM) behaviour.34Dy30Co8W108 (Fig. 17b) is composed of six sandwich-type building blocks ({(GeW9O34)2Dy3(OH)3(H2O)}14−), where a planar-triangular trinuclear cluster, {Dy3(OH)3(H2O)}, is sandwiched by a pair of tri-lacunary Keggin-type [GeW9O34]10− and four trigonal-bipyramidal 3d–4f five-metal clusters {Co2Dy3(μ3-OH)6(H2O)6}7+. Each dimeric POM {(GeW9O34)2Dy3(OH)3(H2O)} links two 3d–4f clusters, whilst each {Co2Dy3(μ3-OH)6(H2O)6} is coordinated by three dimeric moieties, resulting in an assembly (dimeric POMs as linkers and 3d–4f clusters as nodes) with idealized Td symmetry (Fig. 17b). In 2021, the analogues [{(GeW9O34)2Ln3 (CO3)(OH2)3}6{M2Ln3(OH)6(H2O)6}4]50−, where Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd and Y and M
Gd and Y and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn, Mn, and Co, were reported by the same research group.163
Zn, Mn, and Co, were reported by the same research group.163
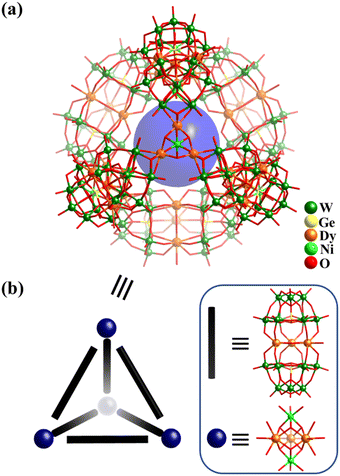 | ||
| Fig. 17 (a) Ball-and-stick representation of [Dy30Co8Ge12W108O408(OH)42(H2O)30]56−. (b) Tetrahedral geometry of Dy30Co8W108, based on 6 {(GeW9O34)2Dy3(OH)3 (H2O)}14− and 4 {Co2Dy3(μ3-OH)6(H2O)6}7+ building blocks.34 | ||
In 2021, Kong et al. reported the preparation of a giant 3d–4f polyoxometalate inorganic super-tetrahedron {[La6(SbO3)4(H2O)20]@[LaNi12W35Sb3P3O139(OH)6]4}86− (La6@(LaNi12)4, Fig. 18a) with high proton conductivity (6.8 × 10−2 S cm−1 at 100% RH and 295 K).164 The La6@(LaNi12)4 aggregate features a super-tetrahedral cluster@cluster framework (Fig. 18b), which is assembled from four [LaNi12W35Sb3P3O139(OH)6]23− SBBs (Fig. 18c) encapsulating a central [La6(SbO3)4(H2O)20] core. The SBB can be viewed as one WO4Ni12(OH)6 cluster stabilized by three tri-lacunary Keggin [PW9O34] and one [LaW7O24] anion.
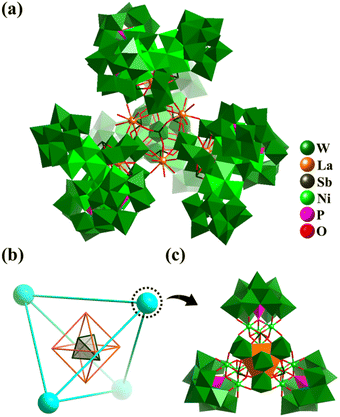 | ||
| Fig. 18 (a) Inorganic super-tetrahedron {[La6(SbO3)4(H2O)20]@[LaNi12W35Sb3P3O139(OH)6]4}86−. (b) Simplified nested skeleton Sb4@La6@(LaNi12)4 of La6@(LaNi12)4. (c) [LaNi12W35Sb3P3O139(OH)6] SBB (LaNi12), containing one [LaW7O24(SbO3)3], one [WO4Ni12(OH)6] and three [PW9O34].164 | ||
In 2015, Kortz prepared three hexameric super-octahedron, Ln12-containing 60-tungstogermanate-based six {GeW10O38}12− SBBs, [Na(H2O)6@Eu12(OH)12(H2O)18Ge2(GeW10O38)6]39− (Fig. 19), [Na(H2O)6@Gd12(OH)6(H2O)24Ge(GeW10O38)6]37−, and [(H2O)6@Dy12(H2O)24(GeW10O38)6]36−.165 The complex structures of these polyanions can be clearly elucidated from a topological polyhedral viewpoint. As shown in Fig. 19, six H2O coordinated with the central Na+ ion, 12 EuIII ions, and six {GeW10O38}12− SBBs are simplified as vertexes, which give rise to three Platonic solids arranged one inside the other, Na(H2O)6(octahedron)@Eu12(icosahedron)@(GeW10O38)6(octahedron). In 2019, Zheng and co-workers synthesized a series of octahedron-shaped Ln14-substituted polyoxotungstates, H27Na16[(Ln14(H2O)W4(OH)O14)(WO4)4 (GeW10O38)6] (Ln14W68, Ln = Eu, Gd, Tb, Dy, Ho, Er).166Ln14W68 possesses a core–shell structure, which is made up of a W4O15 core (tetrahedron), Ln14 tetrahedron (shell 1), W4 tetrahedron (shell 2) and Ge6W60 octahedron (shell 3). The Ln–O–W core is stabilized by 6 bi-vacant Keggin-type [GeW10O38]12− with an octahedral arrangement.
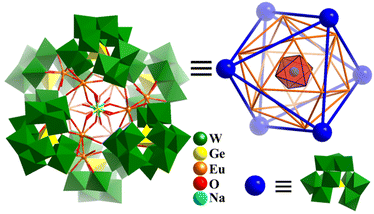 | ||
| Fig. 19 Combined polyhedral/ball-and-stick representation and Platonic solid-based shell structure of super-octahedron [Na(H2O)6@Eu12(OH)12(H2O)18Ge2(GeW10O38)6]39−.165 | ||
3.4 Polyoxoniobates
In contrast to the above-mentioned V/Mo/W-containing POMs, the development of polyoxoniobates (PONbs) is still in its infancy, owing to their very difficult synthesis. Besides the classical [Nb6O19]8− (octahedra)110,111 and [XNb12O40]10− (X = Si and Ge; cuboctahedra),167 PONbs with a regular polyhedron feature are rare. Great advancements in giant iso-polyoxoniobates and hetero-polyoxoniobates were achieved by Zheng et al., who synthesized a series of nanoscale discrete aggregates, such as dimer [H4Nb52O150]36−, trimer [Li3KNb81O225]41−, and tetramer [Li8Nb114O316]54− (Nb114, Fig. 20) based on {Nb26} or {Nb27} SBBs,168 as well as the protein-sized macromolecule [Nb288O768(OH)48(CO3)12]180−.36 The giant super-tetrahedral PONb Nb114 consists of two parts (Fig. 20), i.e., four 27-nuclear {LiNb27} SBBs with a tetrahedral arrangement and a slightly twisted tetrahedral kernel Li4Nb6(μ3-O)4 (Li4Nb2@Nb4).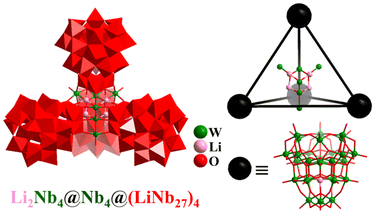 | ||
| Fig. 20 Combined polyhedral/ball-and-stick representation and simplified nested metal-SBB skeleton of super-tetrahedron [Li8Nb114O316]54−.168 | ||
The introduction of different metals into PONbs greatly enriched this family. In 2008, Casey's group reported a new v2-octahedral homoleptic polyoxotitanoniobate, [N(CH3)4]10[Ti12Nb6O44] (Ti12Nb6, Fig. 21), where νn denotes (n + 1) atoms along each edge.169Ti12Nb6 consists of a cuboctahedral Ti12-shell [Ti12O14]20+, of which six Ti4-square faces are capped by six {NbO5} units with an octahedral fashion. The nested type (or Keplerate type) makes the overall cluster hexagonal, and thus Ti12Nb6 is a type of “super-Lindqvist” ion (Fig. 21). Its Ta(V)-analogue, TMA10Ti12Ta6O44 (TBA = tetramethylammonium), was also successfully isolated in 2016.170
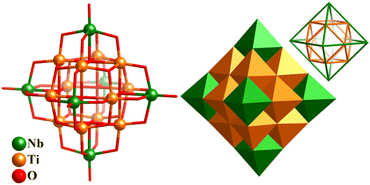 | ||
| Fig. 21 Ball-and-stick and polyhedron representation of v2-octahedral polyoxotitanoniobate cluster [Ti12Nb6O44]10− and Keplerate-type arrangement of the 12 Ti atoms (cuboctahedron) and 6 Nb atoms (octahedron).169 | ||
In 2015, Wang's group reported a Co8-containing PONb, Na6K12[H2Co8O4(Nb6O19)4] (Co8Nb24, Fig. 22a).171 The Lindqvist-type PONb [Nb6O19]8− fragments of Co8Nb24 show a tetrahedral arrangement and envelope an octa-nuclear cluster, {CoII4CoIII4}. The mixed-value cluster {CoII4CoIII4} is comprised of a central {CoIII4O4} cubane core, surrounded by four CoII ions in a tetrahedral fashion. The anion cluster of Co8Nb24 can be seen as three nested tetrahedrons of different sizes from the inside to the outside, denoted as CoIII4@CoII4@{Nb6}4. In 2017, this group also reported the preparation of a tetrahedral PONb with a larger kernel (Cu16Nb4) based on four [Nb6O19]8− SBBs, {Cu16Nb4O17 (H2O)12(AsO4)4(Nb6O19)4}26− (Cu16Nb28, Fig. 22b).172
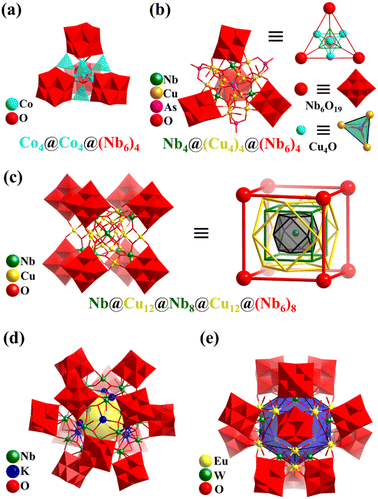 | ||
| Fig. 22 (a) Polyhedral/ball-and-stick representation of [H2Co8O4(Nb6O19)4]18− (Co8Nb24) with a triple tetrahedron nested framework, {CoIII4O4}@CoII4@{Nb6}4.171 (b) Polyhedral/ball-and-stick representation of {Cu16Nb4O17(H2O)12(AsO4)4(Nb6O19)4}26− (Cu16Nb28) and its simplified polyhedral architecture.172 (c) Polyhedral/ball-and-stick representation of [NbO8Cu24(Nb7O22)8]36− and simplified nested structure of Cu24Nb57.173 (d) Polyhedral/ball-and-stick representation of K12[Nb24O72H21]4.174 (e) Polyhedral/ball-and-stick representation of {Eu12W12O36(H2O)24(Nb6O19)12}.175 | ||
In 2010, Wang's group reported a cubic PONb, K12Na[H23NbO8Cu24(Nb7O22)8] (Cu24Nb57) with eight [Nb7O22] units enveloping a big regular core [NbO8Cu24] (Fig. 22c).173Cu24Nb57 with O point symmetry embodies the beauty of symmetry and can be regarded as a combination of multiple O-symmetry polyhedra from the inside to outside, i.e., NbO8(center)@Cu12(cuboctahedron)@Nb8(cube)@Cu12(cuboctahedron)@(Nb6)8(cube). In 2012, their group again reported the preparation of three new PONbs, KNa2[Nb24O72H21], K2Na2[Nb32O96H28], and K12[Nb24O72H21]4 (K12Nb96, Fig. 22d), showing triangle, molecular square, and cuboctahedral geometries, respectively.174 These PONbs can be used as catalysts for hydrogen evolution upon UV-light. Twelve Lindqvist-type PONb [Nb6O19]8− fragments of K12Nb96 show a slightly distorted cuboctahedral arrangement. K12Nb96 is assembled by four {KNb24O72} subunits joined by eight K ions. The {KNb24O72} cluster has three Lindqvist-type PONb [Nb6O19]8− fragments with a triangle distribution, held together by an Nb6 ring of six corner-sharing {NbO6} or {NbO5(H2O)}, in which one K+ ion is trapped in the center. Thus, K12Nb96 can be viewed as a huge tetrahedral cage.
In 2016, Zheng's group synthesized a giant hollow heterometallic PONb with sodalite-type 24-metal Ln12W12 cages, {Ln12W12O36(H2O)24(Nb6O19)12} (Ln12W12Nb72, Ln = Y, La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, and Yb, Fig. 22e).175 The overall structures of Ln12W12Nb72 can be viewed as bi-layered self-assembly, Ln12W12@(Nb6)12. The hollow structure of 24-metal Ln12W12 adopts a topology of the truncated octahedron, and the twelve Lindqvist-type PONb [Nb6O19]8− fragments in the outer layer form an icosahedral arrangement. Interestingly, Ln12W12Nb72 acts as a building block, bridged by [Cu(en)2]2+, resulting in a 3D framework, while Y12W12Nb72 is a discrete cluster. In 2019, this group first reported the preparation of two inorganic-organic hybrid polyoxoniobate cages, K4@{[Cu29(OH)7(H2O)2(en)8(trz)21][Nb24O67(OH)2(H2O)3]4} (K4Cu29Nb96, en = ethanediamine and Htrz = 1,2,4-triazole) and [Cu(en)2]@{[Cu2(en)2(trz)2]6(Nb68O188)} (Cu13Nb68).176 The cage materials exhibited high vapor adsorption capacity and hydrogen evolution activity upon visible light. K4Cu29Nb96 shows a giant tetrahedral nanocage, in which four Nb24 clusters ([H2Nb24O69(H2O)3]16−, like {KNb24O72} in K12Nb96) are assembled by a metal–organic polyhedron of K4@Cu29 ([K4Cu29(OH)7(H2O)2(en)8(trz)21]30+). Cu13Nb68 is a giant cubic cluster, in which eight Lindqvist-type PONb [Nb6O19]8− fragments with a cubic fashion are gathered by a spherical Nb20 polyoxoniobate cage ({Nb20O72N24}), resulting in a nanoscale high-nuclear PONb cage encapsulating an [Cu(en)2] complex and stabilized by six bi-Cu-complexes ([Cu2(trz)2(en)2]2+).
3.5 Other POMs
Since the beginning of this century, actinides have also been incorporated in polyoxometalates of transition metals,177–181 and the first uranium (U) derivative (U6O13(2,2′-bipyridine)2 (1,2,4-tBu3C5H2)2) with an octahedron [U6O13] core was prepared in 2001.180 Actinide-containing polyoxometalate clusters, which are mainly divided into two parts (U-containing heteropolyoxometalates and U-oxo-clusters),182 exhibit versatile and stunning structures, such as tetrameric, hexameric U-containing heteropolyoxometalates (Na32[(UO2)12O4(H2O)12(P2W15O56)4],183 Na24[U6(OH)4O4(AsW9O33)4],184 and (NH4)30[(UO2(H2O))6(UO2(H2O)6(AsW9O34)6]185), Keggin-type {U13},186 and cage-like U-oxo-clusters.The preparation of a series of cage-like structures (Fig. 23) has greatly enriched the large family of cage-like clusters.37,180,181,187–196 Burns’ group reported a series of U clusters with oxide (O2−), peroxide (O22−), and hydroxide (OH−) bridges, consisting of 20,194 24, 28, 32,181 36,37 40,196 44,37 50,196 60,37 84,197 and more metal atoms.178 In these U(IV) clusters, the U(IV) unit (usually hexagonal bipyramid) shares equatorial edges with its surroundings to form four-, five-, and six-membered ring-like subunits, corresponding to topological squares, pentagons, and hexagons, respectively. Further these metal polygons are integrated into regular structures, such as dodecahedron-shape U20 (Fig. 23a),194 truncated-octahedron-shape U24 (Fig. 23b),181 and truncated-icosahedron-shape U60 (like buckminsterfullerene, C60; Fig. 23c).37 By introducing additional inorganic and organic ligands in the U clusters, more cage aggregates with polyhedral characteristics can be constructed.187–189,198
 | ||
| Fig. 23 Polyhedral representation of (a) [(UO2)20(O2)30]20−, 20-metal framework presenting a dodecahedral feature.194 (b) [(UO2)24(O2)24(OH)24]24−, 24-metal framework, presenting a truncated octahedral feature.181 (c) [(UO2)60(O2)60(OH)60]60−, 60-metal framework, presenting a truncated icosahedral feature.37 | ||
In addition to the U-based clusters, Burns’ team also extensively studied other actinide elements, for example, plutonium(IV) oxide nanocluster [PuIV38O56Cl54(H2O)8]14− (Pu38, Fig. 24a).195 A detailed structural analysis showed that the metal arrangement of the [Pu38O56] core classifies it as typical Keplerate, i.e., a μ6-O2−-centered octahedron {Pu6}, which is encapsulated by a {Pu8} cube, and further encapsulated by a truncated octahedral {Pu24} shell (Fig. 24b). By controlling the H2O content and in the presence of PhCOOH in tetrahydrofuran (THF), Thierry Loiseau solvothermally synthesized an analogue of uranium, U38O56Cl18(THF)8(PhCOO)24·8THF.199 Subsequently, a series of U38 with the same core but different protection shells was synthesized and the cluster growth mechanism was deeply explored.200–203 In 2006, two research groups simultaneously published similar 38-nuclear Bi38 clusters, [Bi38O44(HSal)26(Me2CO)16(H2O)2]4+ (H2sal = (2-HO)C6H4COOH)204 and [Bi38O45(hfac)24] (hfac = hexafluoroacetylacetone)205 stabilized with different carboxylic acid ligands. Similar Keplerate Bi38 clusters can also be obtained with other carboxylic acid ligands.206–211 In 2022, Zhang et al. prepared a series of Tix-coated Bi38 heterometallic clusters, Bi38@Tix (x = 14, 16, 18 and 20). The sensitized outer shell (Tix) of Bi38@Tix clusters can regulate and influence the photocurrent response and CO2 cycloaddition reaction.212
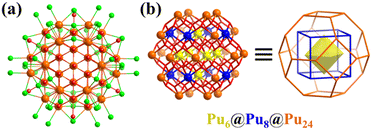 | ||
| Fig. 24 (a) Ball-and-stick representation of [Pu38O56Cl54(H2O)8]14−. Color codes: Pu, yellow, blue or orange; O, red; and Cl, green. (b) [Pu38O56] core exhibiting a nested structure, Pu6(octahedron)@Pu8(cube)@Pu24(truncated icosahedron).195 | ||
4. Platonic and Archimedean solids in clusters of group IV metals (Ti, Zr, and Hf)
Group IV metal (Ti, Zr, and Hf)-oxo clusters with unique structural stability, reactivity and electronic properties represent a diverse class of compounds with rapidly growing applications in catalysis, materials chemistry, electronics and optics.2,68–70,2134.1 Ti clusters
As one of the most widely applied photocatalytic materials, titanium dioxide (TiO2) and its molecular model compounds, titanium-oxo (Ti-O) clusters, are attracting increasing attention.2,68–70,214 Most Ti–O clusters are passivated with organic ligands or lacunary polyoxometalate fragments (discussed in detail in Section 3). Some regular Ti–O clusters have an intimate structural relationship with Platonic solids, such as four-metal tetrahedra (Ti4),215,216 six-metal octahedra (Ti6),217 eight-metal cube (Ti8),218,219 and 12-metal icosahedra (Ti12).220Recently, Jian Zhang and Lei Zhang team applied an effective coordination-delayed-hydrolysis strategy for the synthesis and structural modulation of atomically precise titanium-oxo clusters.2,68,221 Although there have been many examples of the construction of giant Ti–O clusters (with nuclearity of 30 or greater), with up to 52 metal-core,222 there is only one Ti–O cluster (Ti42) with polyhedral feature. Through the reaction of Ti(OiPr)4 with formic acid under solvothermal conditions, this group isolated the first fullerene-like Ti–O cage, H6[Ti42(μ3-O)60(OiPr)42(OH)12)] (Ti42, Fig. 25a and b) with an outer diameter of 1.53 nm, wherein the {Ti42O60} core exemplifies the highest possible symmetry for molecules (icosahedral symmetry, Ih, like C60).38 The structure of the 42-titanium framework, abbreviated as {Ti12}@{Ti30} (Fig. 25c), features a nested two-shell arrangement of 42 metal ions, comprised of one icosahedron (Platonic solid) and one icosidodecahedron (Archimedean solid), constructed by 12 seven-coordinated Ti atoms and 30 five-coordinated Ti atoms (Fig. 25b), respectively. Interestingly, the highly symmetrical arrangement of the 60 μ3-O units linking 42 Ti atoms forms a rare rhombicosidodecahedral cage, resulting from the polyhedral-like arrangement of 42 metals and the stabilization of a square({O4})/pentagon({O5}) O-ring by each six/seven-coordinate Ti atom (Fig. 25d).
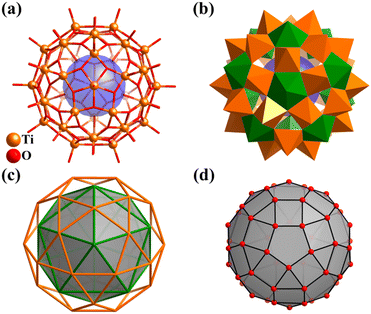 | ||
| Fig. 25 (a) Molecular structure of fullerene-like Ti42 cluster. (b) Polyhedral presentation of Ti42. Color codes: {TiO7}, green and {TiO5}, orange. (c) Nested two-shell arrangement of the 42 metal ions, {Ti12}@{Ti30} (icosahedron@icosidodecahedron). (d) Rhombicosidodecahedral cage {O60} of Ti42.38 | ||
4.2 Zr and Hf clusters
Compared with Ti–O clusters, the synthesis and studies of Zr–O and Hf–O clusters are still very scarce, especially with polyhedral characteristics, wherein mainly M4 tetrahedra,223 M6 octahedra,215,224 and simple M10 super-tetrahedra (M6(octahedron)@M4(tetrahedron), like V6Rh4 in Fig. 6b).225,226By mixing NaOH in methanol and the alkoxide of zirconium (Zr(OCH3)4), an unstable 13-metal cluster Zr13(μ4-O)8(OCH3)36 (Zr13) was obtained, which is similar to the α-Keggin POM structure (Fig. 5).227 The Hf-analogue Hf13(μ4-O)8(OCH3)36 (Hf13, Fig. 26a) was also prepared.228Hf13 features a highly symmetric molecule (Oh) similar to Keggin-type of POMs (Fig. 26b), comprised of an {Hf(μ4-O)8} cubic core and a cuboctahedral shell {Hf12(μ2-OCH3)24(μ-OCH3)12} (Hf⋯Hf distances: 3.527(2)–3.586(2) Å). In addition, the inorganic (8 μ4-O2−) and organic (24 μ2-OCH3− and 12 μ1-OCH3−) ligands that act as linkers and caps also exhibit highly ordered arrangements (Fig. 26c) and form a Russian nesting doll-like structure (Hf@O8@Hf12@O24@O12). Unexpectedly, Hf13 features chemical ultra-stability, keeping the original color, transparency, and morphology of the crystals without significant change in extremely harsh environments (both alkali (20 M boiling NaOH) and acid (10 M HNO3) aqueous solution), in sharp contrast to the isomorphic Zr13. Due to the excellent stability in acid-base aqueous solution, Hf13 can be used as a luminescence probe for the detection of high-concentration acid-based solution, exhibiting high selectivity and repeatability.
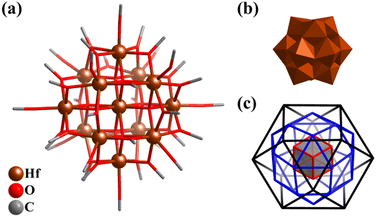 | ||
| Fig. 26 (a) Molecular structure of Hf13(μ4-O)8(OCH3)36 (Hf13). (b) Polyhedral presentation of Keggin-type Hf13. (c) Three-layer Matryoshka structure of 8 μ4-O2− (cube), 24 μ2-OCH3− (rhombicuboctahedron) and 12 μ1-OCH3− (cuboctahedron).228 | ||
5. Platonic and Archimedean solids in clusters of group VII metal (Mn)
In the past two decades, intense research efforts have been concentrated on manganese (Mn) cluster chemistry owing to their interesting magnetic properties, especially high-spin (S) ground state,229–232 magnetocaloric effects (MCE)83,233,234 and single-molecule magnets (SMMs).6,71,73,235 Mn-containing clusters have proven to be one of the most effective sources of SMMs, including the first one, [Mn12O12(O2CCH3)16(H2O)4].236 Meanwhile, the Mn atoms in multi-/high-nuclearity Mn-containing clusters can be stabilized in multiple oxidation states (such as MnII, MnIII, and MnIV), which are conducive to the formation and coordination of the bridging unit μ3/4/5/6-O2−, further promoting the construction of various multi-/high-nuclearity clusters.6Thus far, besides tetra-nuclear clusters with regular tetrahedra237,238 and hexa-nuclear clusters with regular octahedral arrangements,239–241 no metal skeleton of Mn clusters with only one of the other Platonic solids (cube, dodecahedron, and icosahedron) features has been found. In small Mn clusters, some simple nested structures were reported, such as Mn8 (Mn4@Mn4)242–249 and Mn10 (Mn6@Mn4).229,230,233,234,250–258
In addition to the simple clusters with Platonic solid feature described above, the Mn clusters exhibiting an Archimedean solid-type (mainly truncated tetrahedron and cuboctahedron) metal arrangement can also be found, such as Mn12 (truncated tetrahedral geometry),259 Mn13 with super-cubane {Mn13O14} (Mn@O6(octahedron)@Mn12(cuboctahedron)@O8(cube)),260–264 and α-Keggin-type Mn13 (Mn@Mn12(cuboctahedron)).265 The first Mn-based Keplerate cluster with high symmetry (Oh) and high-spin (S = 35/2) ([MnIIMnIII12(μ4-O)8(μ4-Cl)6(tert-butyl-PO3)8], Mn13, Fig. 27a) was formed by the reaction of MnCl2·4H2O, KMnO4 and tert-butylPO3H2 in the presence of Et3N.231 Keplerate Mn13 consists of concentric nested arrangements of both Platonic ({(μ4-O)8} cube, {P8} cube, and {Cl6} octahedron) and Archimedean ({Mn12} cuboctahedron) solids (Fig. 27b).
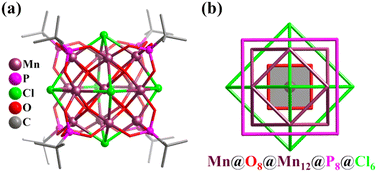 | ||
| Fig. 27 (a) Ball-and-stick representation of cluster [MnIIMnIII12(μ4-O)8(μ4-Cl)6(tert-butyl-PO3)8]. (b) Polyhedron arrangement of Mn-based keplerate.231 | ||
Through the reaction of Mn(NO3)2·4H2O, with triethanolamine (teaH3) and 6-chloro-2-hydroxypyridine (Hchp) in MeOH, an icosanuclear super-tetrahedral(v3) MnII/III cluster [MnII4MnIII16O12(OH)4(tea)8(chp)4] (Mn20, Fig. 28a) was obtained by Murray et al.266 The twenty Mn ions in Mn20 are primarily connected by ten μ4-O2− groups, giving rise to a distorted super-tetrahedral core {MnII4MnIII16O10}. Specifically, ten corner-sharing μ4-O-centered {(μ4-O)Mn4} tetrahedra self-organize to construct a larger v3-tetrahedron, consisting of a v1-tetrahedral Mn4 core encapsulated by a v3-tetrahedral Mn16 shell (Mn12(truncated tetrahedron) + Mn4(tetrahedron)). This Mn20v3-super-tetrahedron as an inner core also appeared in larger Mn26 clusters, [MnII4MnIII22(pdol)12(μ3-CH3O)12(μ3-O)6(μ4-O)10(N3)6] (Mn26, pdol2− = dipyridylketonediolate ion, Fig. 28b), which is identified as a single-molecule magnet according to low-temperature ac susceptibility data.267 The core of the Mn26 cluster consists of an ideal v3-tetrahedron Mn20 (Fig. 28c) and six external Mn ions in an octahedral manner, which are connected by six μ3-O2− groups. If the complex v3-tetrahedron is regarded as a combination of a tetrahedron and truncated tetrahedron featuring only equivalent vertex Mn atoms, then the metal core of Mn26 can be simplified as a combination of four Platonic/Archimedean polyhedrons, Mn4(tetrahedron)@Mn12(truncated tetrahedron)@Mn4(tetrahedron)@Mn6(octahedron) (Fig. 28d). Two similar 26-metal manganese clusters have also been prepared.235,268
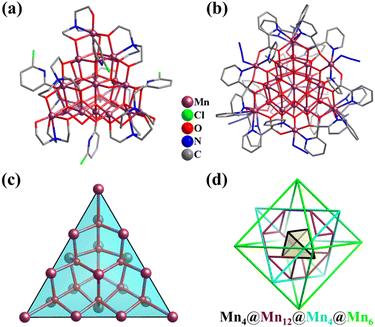 | ||
| Fig. 28 (a) Ball-and-stick representation of cluster [MnII4MnIII16O12(OH)4(tea)8(chp)4] (Mn20) with a distorted v3-tetrahedral {Mn20(μ4-O)10} core.266 (b) Ball-and-stick representation of cluster [MnII4MnIII22(pdol)12(μ3-OCH3)12(μ3-O)6(μ4-O)10(N3)6] (Mn26) with a v3-tetrahedral {Mn20(μ4-O)10(μ3-O)18} core capped by six external Mn ions. (c) v3-Tetrahedral {Mn20(μ4-O)10} framework, Mn4@O6@Mn12@O4@Mn4. (d) Arrangement of 26 Mn atoms and complementary successive Platonic/Archimedean solid description of Mn26 featuring only equivalent vertex metal atoms.267 | ||
In 2021, Huang and Li reported the preparation of a 38-metal high-nuclearity MnII cluster, [MnII38(CO3)9O6Cl24(bmpbt)12(H2bmpbt)6]2+ (Mn38, H2bmpbt = 5,5′-di(4-methyl-pyridin-2-yl)-3,3′-bi(1,2,4-triazole), Fig. 29a) with an inner inorganic core [Mn18(CO3)9] and metal–organic periphery.269 The core–shell is viewed as an interesting “Matryoshka doll”: Mn6(octahedron)@Mn12(truncated tetrahedron)@Mn8(cube)@{Mn2}6(octahedron) (Fig. 29b). Note that the vertices of the outermost octahedral shell are [Mn2OCl4] subunits rather than single metal atoms.
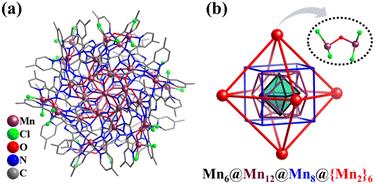 | ||
| Fig. 29 (a) Ball-and-stick representation of [Mn38(CO3)9O6Cl24(bmpbt)12(H2bmpbt)6]2+ (Mn38). (b) Matryoshka doll-like metal skeleton of Mn38.269 | ||
In 2016, Tasiopoulos's group reported the preparation of a magnetic nanosized cluster, [MnIII36MnII13(μ4-O)32(μ3-OCH3)8(μ3-hp)24(HCOO)6(DMF)12](OH)8 (Mn49, H2hp = 2-(hydroxymethyl)phenol, DMF = dimethylformamide, Fig. 30a), consisting of eight high-spin and high-symmetry (Td) [MnIII6MnII4(μ4-O)4] (S = 22) super-tetrahedral building blocks.232 The Mn–O core {MnIII36MnII13(μ4-O)32} of Mn49 (Fig. 30b) consists of an octa-coordinated MnII-centered v1-cuboctahedron {MnIIMnIII12}, encapsulated by an outer v2-cuboctahedral shell {MnII12MnIII24} (Fig. 30c). The giant mixed-valence Mn49 cluster features high virtual Oh symmetry and represents unique examples of metal-oxo clusters based on the assembly of a large number of edge-sharing high-nuclearity magnetic building blocks. Besides, Mn49 possesses high-spin ground-state values (S = 61/2) and displays SMM behavior.
 | ||
| Fig. 30 (a) Ball-and-stick representation of cationic cluster [Mn49O32(OCH3)8(hp)24(O2CH)6(DMF)12]8+. (b) Metal core {MnIII36MnII13(μ4-O)32} consisting of a centered v1-cuboctahedron {MnIIMnIII12(μ4-O)8} encapsulated by a larger v2-cuboctahedral shell {MnII12MnIII24(μ4-O)24}. (c) Arrangement of 49 Mn atoms and complementary successive Archimedean solid description of Mn49 featuring only equivalent vertex metal atoms, Mn@Mn12(cuboctahedron)@Mn24(rhombicuboctahedron)@Mn12(cuboctahedron).232 | ||
In 2007, Tong and co-workers reported the synthesis of a giant heterometallic neutral cluster with Td symmetry and unusual magnetic properties, [CuI4CuII13MnII4MnIII12MnIV12(μ3-O)12(μ4-O)28(tea)12(HCO2)6(H2O)4] (Cu17Mn28, Fig. 31a).270 Unprecedently, five metal oxidation states (CuI, CuII, MnII, MnIII, and MnIV) coexist in the Cu17Mn28 cluster (Fig. 31b). The most striking structural feature is that all the six-coordinated 28 Mn atoms are joined by forty bridged oxygen groups (12 μ3-O2− and 28 μ4-O2−) into four {MnIIMnIV3O4} and six {MnIII2MnIV2O4} cubanes, which are further bridged to be an adamantane-like Mn cage by sharing the inner 12 MnIV ions (Fig. 31c). Finally, the metallic arrangement of the metal core can be a complex Matryoshka structure (abbreviated as μ4-CuII@MnIV12@CuI4@MnIII12@CuII12@MnII4), contained in an innermost {CuII(μ4-O)4} core and moving outward, five regular polyhedra, i.e., a truncated tetrahedron, a tetrahedron, a distorted truncated tetrahedron, a cuboctahedron and a tetrahedron (Fig. 31d).
 | ||
| Fig. 31 (a) Ball-and-stick of Cu17Mn28. (b) Metal-oxo core {Cu17Mn28(μ4-O)28} of Cu17Mn28. (c) Adamantane-like MnII4MnIII12MnIV12O28 cluster containing ten sharing-vertices cubane-like units (four {MnIIMnIV3O4} and six {MnIII2MnIV12O4}). (d) Complementary successive Platonic/Archimedean polyhedral description of Cu17Mn28 core-geometry featuring only equivalent vertex metal atoms: {CuII(μ4-O)4}(tetrahedron)@MnIV12(truncated tetrahedron)@CuI4(tetrahedron)@MnIII12(distorted truncated tetrahedron)@CuII12(cuboctahedron)@MnII4(tetrahedron).270 | ||
6. Platonic and Archimedean solids in clusters of group VIII metals (Fe, Co, Ni, Pd, and Pt)
6.1 Fe clusters
Iron (Fe) clusters, especially high-nuclearity Fe aggregates, continue to be synthetic targets for synthetic chemists and materials scientists worldwide, not only because of their interesting structures and magnetic and catalytic properties but also their relevance to the Fe-storage ferritin proteins.271,272 Tetrahedral Fe4,273 octahedral Fe6,274,275 and cubic Fe8 clusters276,277 have been reported but are not discussed in detail in this review. In addition to cubic Fe8 clusters, some octa-nuclear Fe–O clusters with an inner Fe4(μ-O)4-cubane and four peripheral tetrahedral arranged Fe centers in an Fe4@Fe4 manner were reported.243,278–286The first open-shell Keggin-type 13-metal cluster, [FeIII13(μ4-O)4F24(μ2-OMe)12]5− (Fe13, Fig. 32a) containing 13 high-spin d5 FeIII atoms, was reported by Bino and co-workers.287 The anion of Fe13 has an ideal α-Keggin-type structure with Td symmetry (Fig. 32b), wherein the 12 surrounding FeIII atoms exhibit a cuboctahedron arrangement and a central tetrahedral {FeIII(μ4-O)4} core (Fig. 32c). Polyfluorometalate (Fe13) demonstrated for the first time the combination of transition-metal (TM) atoms with an open-shell configuration and F− ligands, leading to the isolation of a new Keggin-type cluster. The Keggin Fe13-oxo core is regarded as a basic structural unit for iron oxyhydroxide [FeO(OH)] cluster materials, such as ferrihydrite (Fe5HO8·4H2O) and magnetite (Fe3O4). Since then, with the efforts of synthetic chemists, not only a series of Fe–O/OH clusters containing α- or ε-Keggin-type Fe13-oxo cores (such as Fe17 (Fig. 32d–f),288,289Fe19 (Fig. 32j–l),290 Fe30,291 and Fe34 (Fig. 32m–o)292) were prepared, but also heterometal ions were used to capture and stabilize the basic motifs (like Bi3+-stabilized Bi6Fe13 (Fig. 32g–i)293–295 and Ln3+-shell-protected La6Fe13,296 Ln16Fe29 (Ln = Y, Gd),297 Ln12Fe33 (Ln = Y, Gd, Pr, and Dy).297,298 The synthesis and preparation of these metal oxyhydroxide clusters of different sizes can help us understand the formation mechanism of related natural minerals.
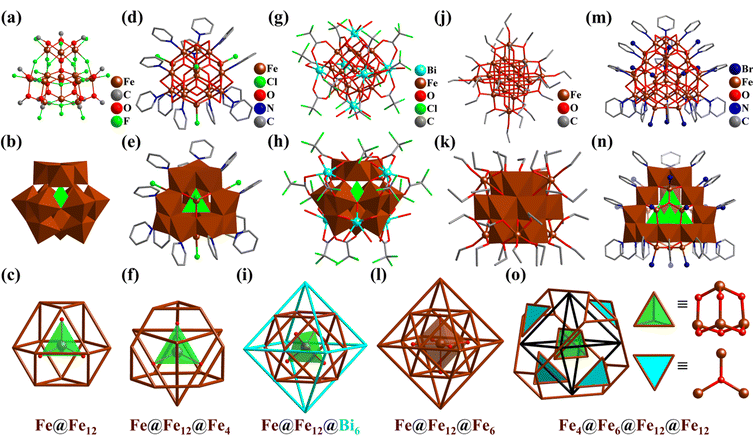 | ||
| Fig. 32 Ball-and-stick, polyhedral and metal arrangement representations of (a–c) [Fe13O4F24(OMe)12]5− (Fe13),287 (d–f) [Fe17O16(OH)12(py)12Cl4]3+ (Fe17),288 (g–i) Bi6[Fe13O16(OH)12(O2C(CCl3)12]+ (Bi6Fe13),293 (j–l) [Fe19O14(OEt)30]− (Fe19),290 and (m, n and o) [Fe34O38(μ2-OH)12Br12(py)18] (Fe34).292 | ||
By the simple reaction of anhydrous FeCl3 in pyridine (py), a 17-metal FeIII-oxo/hydroxy cluster [Fe17O16(μ4-OH)12(py)12Cl4]3+ (Fe17, Fig. 32d–f) was prepared by Heath et al.288,289 The core of Fe17 also has a central tetrahedral {FeIII(μ4-O)4} motif, then bridging twelve outer octahedral {FeIII(μ2-OH)2(μ3-O)(μ4-O)2(py)}. In contrast to Fe13, the twelve metal atoms form a truncated tetrahedron, further assembled with a central tetrahedron into a POM-like ε-Keggin architecture (Fig. 8 and 32e). Four hexagons of this truncated tetrahedron are capped by four additional {FeIII(μ3-O)3Cl} motifs with a tetrahedral arrangement (Fig. 32f). Using pyridine to control the slow hydrolysis of FeIII ions, Fe30291 and Fe34 (Fig. 32m–o)292 were successively synthesized.
In the presence of hexamethylenetetramine, the dissolution and reaction of anhydrous FeBr3 in a mixture solvents (py and CH3CN) resulted in the formation of a giant FeIII-oxyhydroxide cluster, [FeIII34(μ4-O)4(μ3-O)34(μ2-OH)12Br12(py)18]Br2 (Fe34, Fig. 32m).292 From the inside to the outside, there are alternating layers of tetrahedral (green) and hexahedral (brown) FeIII ions by oxide (μ3-O2− and μ4-O2−) and hydroxide (μ2-OH−) ions in Fe34 (Fig. 32n), similar to the enlarged version of Fe17. The metallic skeleton (Fig. 32o) described an adamantane-like {FeIII4(μ3-O)6} tetrahedron encapsulated within an elongated truncated tetrahedron, whose hexagons are capped by four {FeIII3(μ3-O)Br3} triangles. Interestingly, there is a regular octahedron inside the elongated truncated tetrahedron. Thus, the metallic skeleton of Fe34 can be viewed as the combination of concentric polyhedrons, Fe4(tetrahedron)@Fe6(octahedron)@Fe12(elongated truncated tetrahedron)@(Fe3)4(tetrahedron).
In 2015, Nyman's group isolated a discrete Bi6-coated Fe13-oxo/hydroxy cluster Bi6[FeO4Fe12O12(OH)12(O2C(CCl3)12]+ (Bi6Fe13, Fig. 32g), which has the same structural features as ferrihydrite.293–295Bi6Fe13 has an α-Keggin-type Fe–O core similar to Fe13 (Fig. 32h), which is comprised of a central {FeIIIO4} tetrahedron and a cuboctahedra shell {Fe12}, capped by six Bi atoms in an octahedral fashion (Fig. 32i). The FeIII-oxy/hydroxy aggregate of Bi6Fe13 may be an intermediate state from ion to iron-oxy/hydroxide precipitates or nanoparticles.
Besides, Spandl's group reported the preparation of a similar 19-metal v2-octahedral “Super-Lindqvist” Fe19 cluster, [HFe19O14(OEt)30] (Fe19, Fig. 32j).290 The Fe–O skeleton of Fe19 (without C atoms) exhibits an approximate Oh symmetry. In the center of Fe19, there are six μ6-O in a nearly perfect octahedral manner, surrounding a central six-coordinated FeIII atom. {Fe(μ6-O)6} is enveloped by an octahedral nested shell, {Fe18(μ3-O)8(OEt)6(μ2-OEt)24} (Fig. 32l, inner cuboctahedron {Fe12} and outer octahedron {Fe6}, like Ti12Nb6, Fig. 21). Although the 12-metals are arranged in a polyhedron (cuboctahedron) as in the α-Keggin structure, the spatial layout of the coordination atoms oxygen varies greatly (Fig. 32b, h and k). Fe19 features a core–shell arrangement of metal atoms and O bridges/ligands (μ3-O, μ6-O, μ2-OEt, and μ1-OEt) contained in an innermost FeIII atom and moving outward, six Platonic or Archimedean solids (μ6-Fe@(μ6-O)6@Fe12@(μ3-O)8@(μ2-OEt)24@Fe6@(μ-OEt)6), i.e., an octahedron, a cuboctahedron, a cube, a cuboctahedron and two octahedrons.
From Fe13, Fe17, and Fe19 to the most recently reported Fe34, exhibiting the structural similarity, these clusters are prepared by a simple bottom-up synthetic methodology. Thus, larger FeIII–O/OH clusters with high symmetry are likely to be successfully prepared soon.
Besides metal–O2−/OH− clusters with core–shell frameworks, some interesting hollow cluster-based cages were isolated and characterized.40,299–302 For example, a 64-nuclear cubic hollow cage, {[FeIII8O3(tea)(teaH)3(HCOO)6]8 (HCOO)12}12+ (Fe64).301 The cubic Fe64 consists of 8 corners of octanuclear [FeIII8O3(tea)(teaH)3(HCOO)6] subunits with a propeller-like FeIII8O3 core and 12 edges of anti–anti HCOO− ligands.
6.2 Co clusters
In numerous works, Co and Ni clusters are often studied together, owing to their same or similar skeleton protected by the same inorganic and organic ligands. The employment of organic oxygen-containing ligands has been proven to be a successful strategy for the synthesis of multi-/high-nuclearity Co and Ni clusters. Small Co/Ni-containing clusters featuring simple Platonic solids (tetrahedron,273,303–305 octahedron,241,306–308 and cube309–315), Archimedean solid (truncated tetrahedron),316–318 and common nested structure (M4(tetrahedron)@M4(tetrahedron),319–322 M6(octahedron)@M4(tetrahedron),323–325 and M6(octahedron)@M8(cube),326 M = Co or Ni) are not discussed here.In 2017, Bai and co-workers reported the preparation of a homochiral mixed-valence v3-supertetrahedral cobalt cluster from a racemic ligand, [CoIII4CoII16(μ6-O)4(μ3-OH)12(S-bme)12(OAc)6]6+ (Co20-T, Hbme = 1H-(benzimidazol-2-yl)ethanol, Fig. 33a).327Co20-T presents a beautiful mixed-valence Co-oxo supertetrahedral core, {CoIII4CoII16(μ6-O)4(μ3-OH)12} (Fig. 33b), constructed by an inner tetrahedron {CoIII4} and outer large v3-tetrahedron {CoII16} (Co12(truncated tetrahedron)@Co4(tetrahedron)). Alternatively, its 20 Co atoms are organized into 11 cubane-like units in three forms (one {CoIII4(μ6-O)4}, six {CoIII2CoII2(μ6-O)2(μ3-OH)2}, and four {CoIIICoII3(μ6-O)(μ3-OH)3}). Ten cubane groups on the periphery are connected by sharing corners, and the central {CoIII4(μ6-O)4} motif is encapsulated in the center by sharing edges with six neighbors {CoIII2CoII2(μ6-O)2(μ3-OH)2} (Fig. 33c). The chiral Co20-T is the largest super-tetrahedral Co cluster reported to date, which exhibits high photocatalytic activity and ferromagnetic behavior.
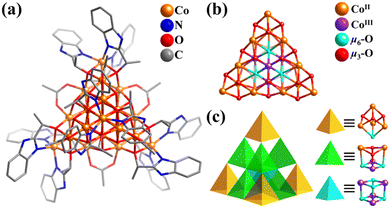 | ||
| Fig. 33 (a) Ball-and-stick representation of cationic cluster [Co20O4(OH)12(S-bme)12(OAc)6]6+. (b) Co–O core structure, {CoIII4CoII16(μ6-O)4(μ3-OH)12}. (c) Illustration of the assembly of 11 vertex-sharing or edge-sharing cubane units ({CoIII4(μ6-O)4} + 6{CoIII2CoII2(μ6-O)2(μ3-OH)2} + 4{CoIIICoII3(μ6-O)(μ3-OH)3}) into a v3-tetrahedron.327 | ||
In 2012, Huang's group reported the preparation of an eight-cobalt-capped 20-metal isopolyoxometalate cluster with an α-Keggin-type structure, [CoII20(μ3-OH)24(MMT)12(SO4)]2+ (Co20-1, MMT = 2-mercapto-5-methyl-1,3,4-thiadiazole, Fig. 34a).328 A 20-metal Ni analogue has also been reported.329Co20-1 has a non-crystallographic Td symmetry and possesses an open-shell SO42−-centered α-Keggin core [SO4@Co12(μ3-OH)24N12]2+, with a cuboctahedral arrangement. The α-Keggin-like core was capped by eight metal atoms in a cubic fashion (Fig. 34b). Clusters (Mn13Cu8,330 Cu20,331,332 Cu12Zn8,332 and Cu12Mg8331) similar to the metal framework (cuboctahedron(M@M12 or M12)@cube(M8)) have also been reported.
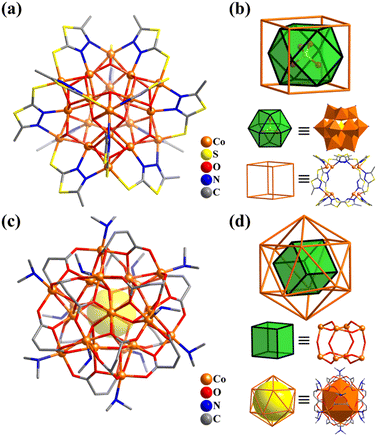 | ||
| Fig. 34 Ball-and-stick representation and metal arrangement of (a and b) [Co20(OH)24(MMT)12(SO4)]2+ (Co20-1)328 and (c and d) [Co20(OH)12(maleate)12(Me2NH)12]4+ (Co20-2).333 | ||
Liang and co-workers also reported two isostructural 20-metal clusters, [M20(μ3-OH)12(maleate)12(Me2NH)12](BF4)3(OH) (M = CoII (Co20-2) or NiII; H2maleate = fumaric acid, Fig. 34c),333 different from Co20-1. The spherical Co20-2 with Th symmetry possesses an ideal cubic {M8(μ3-OH)12} core, where the M atoms as vertices are bridged by μ3-OH− groups as the edges, while these μ3-OH− groups are coordinated further by outer identical 12 metal atoms, which build a distorted M12 icosahedral shell to encapsulate the cubic core (Fig. 34d).
In 2011, Liao's group obtained p-tert-butylthiacalix[4]arene(TC4A)-capped high-nuclearity bi-metallic {CoII24M8} (M = Mo or W) nanospheres, [CoII24(TC4A)6(MO4)8Cl6]2+ (Co24, Fig. 35a).334 In Co24, each Cl− anion is encapsulated by a different metal–organic super calix, resulting in the constructing of six square-like Co4Cl-TC4A SBBs. Each Co4Cl-TC4A linked with four MoO42− or WO42− anions, and each MoO42− or WO42− as a template connects with three Co4Cl-TC4A motifs, forming a flat hexagonal arrangement (Fig. 35b). Consistent with Cl6@V24As8 described above (Fig. 7), Co24 with Oh symmetry has the same topology (keplerate structure: Cl6(octahedron)@Co24(truncated octahedron)@Mo8/W8(cube)).
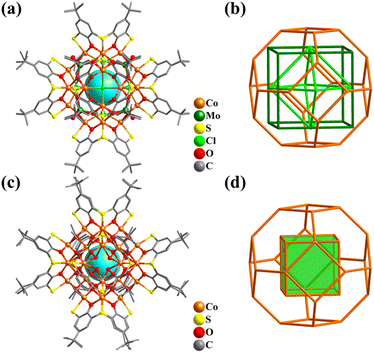 | ||
| Fig. 35 Ball-and-stick representation and metal frameworks of (a and b) [Co24(TC4A)6(MoO4)8Cl6]2+ (Co24)334 and (c and d) {Co32O24(H2O)24(TC4A)6} (Co32).41 | ||
In 2009, Liao and co-workers reported the preparation of a giant spherical Co cluster capped by TC4A ligands, {CoII24CoIII8(μ3-O)24(H2O)24(TC4A)6} (Co32, Fig. 35c).41 A 32-metal Ni analogue had also been reported.335Co32 is also a nested structure, possessing a CoIII8 cubic core encapsulated by a 24-metal shell with a truncated octahedral arrangement (Fig. 35d). Interestingly, the SBBs (M4-TC4A) of Co24 and Co32 all exhibit a regular octahedral arrangement. By introducing suitable polydentate ligands to assemble thiacalixarene-based SBBs, a series of metal cluster–organic polyhedra with a regular arrangement (such as tetrahedra, octahedra, and icosahedra) were obtained.336–342
In 2013, the dimethylmalonate(Me2Mal2−)-protected anion cage cluster [Co36(H2O)12(μ3-OH)20(HMe2Mal)2(Me2Mal)28]6− (Co36) was prepared.343 The isostructural 36-Ni cluster was also obtained.344 The metal skeleton of Co36 can be seen as an arrangement of two shells, in which the 24 metals inside form eight triangles with a cubic fashion or a distorted truncated cube, and the other twelve metals on the outside can form a slightly distorted cuboctahedron.
6.3 Ni clusters
Given that the coordination forms of Ni and Co are similar, the Ni–O/OH clusters featuring metal frameworks of Platonic and Archimedean solids are highly similar with the above-mentioned cobalt clusters. The related structures have already been discussed and will not be repeated here.Due to the existence of the original state of the metal bonds, atomically precise Ni-containing clusters with direct metal–metal bonding have been widely synthesized and studied. Nuckolls and co-workers described a series of solid-state materials constructed from the binary assembly of atomically precise molecular clusters featuring direct metal–metal bonding and activated electronic transport, [Co6Se8(PEt3)6][C60]2 (Co6Se8P6), [Cr6Te8(PEt3)6][C60]2 (Cr6Te8P6), and [Ni9Te6(PEt3)8][C60] (Ni9Te6P8, Fig. 36a).307 These clusters with Oh symmetry show the classic Keplerate structure, as follows: (i) Co6Se8P6 and Cr6Te8P6 have three-shell topology, i.e., Co6/Cr6(octahedron)@Se8/Te8(cube)@P6(octahedron) and (ii) Ni9Te6P8 (Fig. 36b) exhibits a core–shell structure, i.e., (μ8-Ni)@Ni8(cube)@Te6(octahedron)@P8(cube).
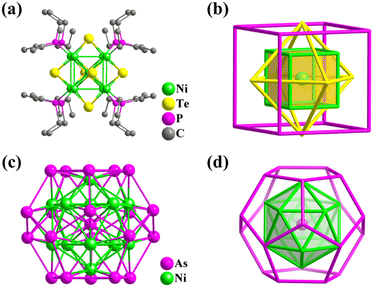 | ||
| Fig. 36 Ball-and-stick and atomic arrangement representation of (a and b) [Ni9Te6(PEt3)8] with Ni@Ni8@Te6@P8307 and (c and d) [As@Ni12@As20]3−.42 | ||
In 2003, Eichhorn's group prepared a ligand-free intermetalloid cluster, [As@Ni12@As20]3− (Fig. 36c).42 This cluster exhibits interpenetrating reciprocal Platonic polyhedrons and contains an icosahedral Ni12 fragment with a central μ12-As atom, encapsulated by an As20 dodecahedral (fullerene-like) shell, resulting in the formation of an onion-skin-like [As@Ni12@As20]3− with nearly perfect Ih point symmetry (Fig. 36d). The structures of the same ligand-free core–shell-type intermetalloid cluster built from other elements A@B12@A20 (A = As, Sn, Pb, Sb; B = Ni, Mg, Zn, Cd, Mn, and Pd) have been synthesized.345–348
Recently, Zacchini and Berben reviewed the advances in metal carbonyl clusters involving groups VIII (Fe, Co, Ni, Pd, Pt, etc.) elements, with emphasis on their synthesis, electronic structures and catalysis.22 Nickel carbonyl clusters featuring Platonic and Archimedean solids are summarized and discussed here. In 2015, Zacchini's group reported the preparation of some Ni poly-carbide carbonyl nanoclusters.349 During the optimization experiment, a by-product ([Ni32C6(CO)36]6−, Ni32C6, Fig. 37a) with low yield was isolated. The hexacarbide Ni32C6 exhibits the classical Keplerate-type structure and can be viewed as a combination of three Platonic or Archimedean solids (Fig. 37b), abbreviated as Ni8(cube)@C6(octahedron)@Ni24(truncated octahedron).
 | ||
| Fig. 37 (a) Ball-and-stick representation of [Ni32C6(CO)36]6−. (b) Nested structure of [Ni32C6(CO)36]6−, Ni8(octahedron)@C6(hexahedron)@Ni24(truncated octahedron).349 | ||
In addition to the preparation of homopolymetallic carbonyl clusters, Ni-containing heterometallic clusters have also been extensively studied, such as Au6Ni12,350Ag16Ni24,351Pt14Ni24,352 and super-octahedron Pt6Ni38.43–45 Dahl's group reported the synthesis of the first bimetallic Au-Ni carbonyl cluster, [Au6Ni12(CO)24]2− (Au6Ni12).350Au6Ni12 contained an ideal Td configuration and its metal core can be regarded as an Au6 octahedron capped by four tetrahedral {Ni3(CO)8} triangular units (12 Ni atoms: elongated cuboctahedron). The first high-nuclearity bimetallic Ag–Ni carbonyl cluster [Ag16Ni24(CO)40]4− (Ag16Ni24, Fig. 38a) was also reported by Dahl's group.351Ag16Ni24 possesses a 40-metal cubic pseudo-Td closed-packed geometry (Fig. 38b), which can be described as a central Ag16 kernel (v2-truncated tetrahedron: Ag4(tetrahedron)@Ag12(truncated tetrahedron)) linked by direct Ag–Ni bonding to four triangular {Ni6(CO)10} units featuring a v2-cuboctahedron, i.e., Ni12(truncated tetrahedron)@Ni12(cuboctahedron). Each triangular {Ni6(CO)10} can be viewed as a hexagonal close-packing (hcp) extension of the inner Ag16 core along one of the four symmetry-equivalent 3-fold axes, causing each of the Ag atoms of Ag4(tetrahedron) to be encapsulated by 12 hcp metal atoms.
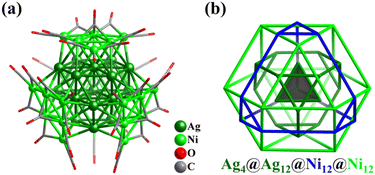 | ||
| Fig. 38 (a) Ball-and-stick representation of [Ag16Ni24(CO)40]4− (Ag16Ni24). (b) Four-layered nested 40-metal core Ag4(tetrahedron)@Ag12(truncated tetrahedron)@Ni12(truncated tetrahedron)@Ni12(cuboctahedron) of Ag16Ni24.351 | ||
With the reaction of [NnBu4]2[Ni6(CO)12] in THF solution with K2PtCl4, Giuliano Longoni and co-workers obtained hexagonal black crystals of the heterometal Pt–Ni truncated octahedral cluster [Pt14Ni24(CO)44]4− (Pt14Ni24, Fig. 39a).352Pt14Ni24 with a core–shell structure is comprised of a face-centre cubic (fcc) Pt14 core and Ni24 truncated octahedral shell (Fig. 39b). The fcc-Pt14 core geometry can be viewed as the combination of an octahedron (Pt6) and cube (Pt8). The structural polyhedron of the 38-metal Pt–Ni core can be abbreviated as Pt6@Pt8@Ni24, featuring a core–shell arrangement of metal atoms contained in an octahedron and moving outward, a hexahedron and a truncated octahedron.
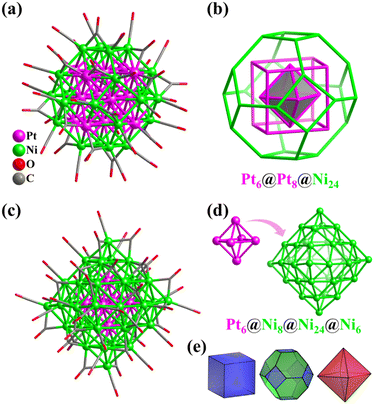 | ||
| Fig. 39 (a) Ball-and-stick representation of [Pt14Ni24(CO)44]4− (Pt14Ni24). (b) Core–shell structure of Pt14Ni24, Pt6(octahedron)@Pt8(cube)@Ni24(truncated octahedron).352 (c) Ball-and-stick representation of [Pt6Ni38(CO)48]6− (Pt6Ni38). (d) Pt6 octahedral core and v3-octahedral Ni38 shell.44 (e) 38-metal-based v3-octahedron consists of three components, cube, truncated octahedron and octahedron. | ||
Manassero and co-workers first synthesized four v3-octahedral heterometal Pt–Ni clusters with 44 metal atoms, [Pt6Ni38(CO)48]6− (Pt6Ni38, Fig. 39c).43–45 The 44-metal v3-octahedral structure is comprised of an octahedral Pt6 core and a v3-octahedral Ni38 shell (Fig. 39d). The v3-octahedral Ni38 shell can be viewed as the combination of three polyhedra (cube Ni8 + truncated octahedron Ni24 + octahedron Ni6, Fig. 39e) with the metal as the vertex. Thus, the metal core of the carbonyl cluster can be viewed as the assembly of four Platonic and Archimedean solids (Pt6@Ni8@Ni24@Ni6).
6.4 Pd clusters
In the field of the metal-based catalysts, palladium-containing nanoparticles and materials occupy a privileged position, mainly due to their excellent catalytic performance, such as catalysts in coupling and hydrogenation reactions, oxidation catalysts in automobile emission-control systems, and reforming catalysts in the production of high-octane gasoline.353–356 As ideal models, the precise structure of polyoxopalladates (POPs)356 and Pd clusters with direct metal–metal bonding15 can help researchers understand the underlying catalytic mechanisms.Controlled hydrolysis-condensation processes of {PdIIO4} square-planar units in the presence of oxyacid groups (such as PO43−, AsO43−, and SeO32−) induce the self-assembly of these discrete units to obtain polynuclear PdII-oxo clusters.356 Since the first cubic POPs [H6PdII13O8(AsO4)8]8− were reported by Kortz and co-workers in 2008,357 more than seventy POPs have been obtained, which encompass a large structural variety. Among them, only in the family of cubic clusters, with the general formula of MPd12L8 (M = NaI, CaII, ScIII, CrIII, MnII, FeIII, CoII, NiII, CuII, ZnII, GaIII, SrII, YIII, PdII, InIII, and LnIII; L = PO43−, AsO43−, SeO32−, PhPO33−, and PhAsO33−), the twelve peripheral Pd-metal atoms are arranged in the form of a cuboctahedron (an Archimedean solid), in which the degree of deviation from the ideal polyhedron is related to the nature of the selected capping group (such as charge, X–O bond length (X = P, As, Se), steric hindrance, and geometry).356 Here, we choose [PdII13(AsVPh)8O32]6− with Oh symmetry (without phenyl substituents) to introduce the cuboid-shaped POP cluster.357 The structure of {Pd13As8O32} can be described as Keplerate type, featuring Platonic and Archimedean solids of different sizes, similar to a complicated “Matryoshka doll sequence”, i.e., Pd@O8(cube)@Pd12(cuboctahedron)@As8(cube)@O24(truncated cube).
In addition to POPs, palladium can also form an interesting family of highly condensed carbonyl/phosphine-protected high-nuclearity homopalladium/heteropalladium clusters.22,45 Lawrence F. Dahl and co-workers reported the preparation of two Pd- and one Pd–Pt-carbonyl cluster with the icosahedral Mackay hard-sphere model, Pd55(P(iPr)3)12(μ3-CO)20 (Pd55, Fig. 40a),358 Pd145(CO)x(PEt3)30 (x ≈ 60, Pd145, Fig. 40b),359 and (μ12-Pt)Pd164−xPtx(CO)72(PPh3)20 (x ≈ 7, (PtPd)165, Fig. 40c).47Pd55 is the first crystallographically documented example of a 55-metal cluster with Mackay two-shell icosahedron. Pd55 features pseudo-Ih symmetry (without isopropyl groups), and the 55-Pd core (ideally Ih symmetry) can be viewed as simply a “Matryoshka doll sequence”. The center μ12-Pd atom (color code: black) is encapsulated by the icosahedron in shell 1 (Pd12, Fig. 40d), which in turn is encapsulated by the icosahedron in shell 2 (Pd42, Fig. 40e). The 42-metal shell can be regarded as a combination of an icosidodecahedron and icosahedron (Fig. 40h). Meanwhile, the peripheral ligands (12 P(iPr)3 and 20 μ3-CO) are arranged in the form of icosahedron and pentagonal dodecahedron, respectively. In addition to the same interior core (Pd55), Pd145 has a third-shell (shell 3) that possesses 60 equivalent vertices with the arrangement of rhombicosidodecahedra (Fig. 40f). 30 additional Pd atoms with the icosidodecahedral arrangement cap the thirty square faces of shell 3 and each Pd is attached by phosphine ligands (PEt3). The structure of the 145-palladium core, which can be abbreviated as μ12-Pd@Pd12@Pd42(Pd12 + Pd30)@Pd60@Pd30, features a core–shell arrangement of metal atoms contained in the innermost μ12-Pd atom, and moving outward, five Platonic or Archimedean solids, i.e., an icosahedron, icosidodecahedron, icosahedron, rhombicosidodecahedron, and icosidodecahedron. The (PtPd)165 cluster features a Pt-centered four-shell 165-atom Pt-Pd core with interesting intershell bridging carbonyl ligands (Fig. 40c). The same interior 115-metal-atom three-shell (Pdcenter@Pd12@Pd42@Pd60) polyhedral geometries of Pd145 can be observed in the 165-metal-atom four-shell Pt-centered (PtPd)165 nanoclusters (Ptcenter@Pd12−xPtx (x ≈ 1.2)@Pd42−xPtx (x ≈ 3.5)@Pd60−xPtx (x ≈ 2.2)@Pd50). In (PtPd)165, the outermost 50 Pd–metal atoms construct shell 4, featuring an arrangement of pentagonal dodecahedra (Fig. 40g). Shell 4 can be regarded as a combination of Pd30(icosidodecahedron) and Pd20(pentagonal dodecahedron) if it is described with only equivalent vertex metal atoms (Fig. 40i). Thus, the structure of the 165-atom Pt–Pd core, which can be abbreviated as μ12-Pt@(PtPd)12@(PtPd)42((PtPd)12 + (PtPd)30)@(PtPd)60@Pd50(Pd20 + Pd30), features a core–shell arrangement of metal atoms contained in the innermost μ12-Pt atom, and moving outward, six Platonic or Archimedean solids, i.e., an icosahedron, icosidodecahedron, icosahedron, rhombicosidodecahedron, icosidodecahedron, and pentagonal dodecahedron.
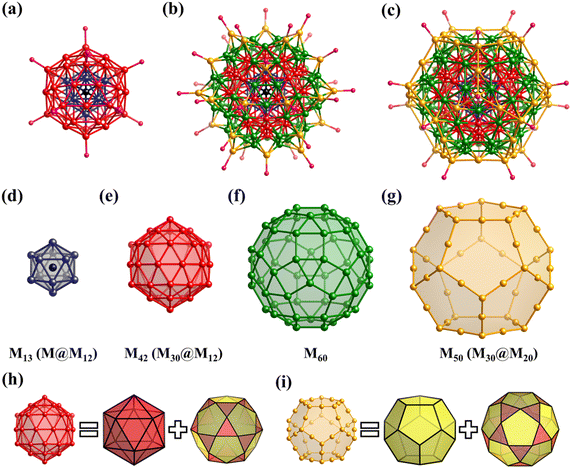 | ||
| Fig. 40 (a) Two-shell structure of Pd55(P(iPr)3)12(μ3-CO)20 containing a Mackay icosahedral cluster core.358 (b) Three-shell structure of Pd145(PEt3)30.359 (c) Four-shell structure of (μ12-Pt)Pd164−xPtx(CO)72(PPh3)20 (x ≈ 7). (d–g) Shells 1–4 of (PtPd)165: (PtPd)12 icosahedron with a central (μ12-Pt) atom, (PtPd)42 icosahedron, (PtPd)60 rhombicosidodecahedron, and Pd50 pentagonal dodecahedron. (h) (PtPd)42(icosahedron) is transformed into (PtPd)12(icosahedron) and (PtPd)30(icosidodecahedron). (i) Pd50(pentagonal dodecahedron) is transformed into Pd20(pentagonal dodecahedron) and Pd30(icosidodecahedron).47 For clarity, the C, O, and H atoms are omitted. Color codes: Pd(Pt), black, indigo, red, green, yellow; and P, pink. | ||
In addition to the above-mentioned Pd clusters protected by carbonyl and phosphine ligands (Fig. 40), through the reduction of an organometallic complex [Pd3(μ3-C7H7)2]2+ with tetraarylborate, Murahashi afforded an interesting cationic organometallic cluster, [Pd13(μ4-Tr)6]2+ (Tr = cycloheptatrienyl), containing a Pd-centered cuboctahedral Pd13 core protected by an octahedral ligand–shell (μ4-Tr)6.360
6.5 Pt clusters
No platinum (Pt) analogues featuring Platonic and/or Archimedean solids of polyoxopalladates have been reported to date. Wickleder and Pley discovered the first noble metal-based POMs (NH4)4[PtIII12O8(SO4)12] in 2004.361 In the anion cluster, the 12 PtIII ions form a {PtIII12} skeleton with a remarkably distorted icosahedral arrangement, owing to the shorter metal–metal bond (Pt⋯Pt: ca. 2.53 Å) in the six {Pt2} groups and longer Pt⋯Pt separations (ca. 3.45 Å) between the adjacent {Pt2} groups.Besides Pt–O clusters, platinum carbonyl (Ptx(CO)y) clusters have been reported to be an ideal model to understand the CO adsorption on surfaces of ultrasmall metal crystallites and nanoparticles.22,362 Researchers synthesized a series of carbonyl-protected homoplatinum/heteroplatinum clusters with direct metal–metal bonding, and some Ptx(CO)y clusters featuring classical Platonic or Archimedean solids.43,44,47,352,363 Masciocchi and co-workers first reported the single-crystal structure of a cubic close-packed (ccp) Pt38 cluster, [N(PPh3)2]2[Pt38(CO)44] (Pt38), which disclosed the exact stereochemistry of 44 carbonyl ligands that had never been determined before.364Pt38 possessed a ccp metal core of idealized Oh symmetry. The 38 platinum atoms constitute a truncated octahedron (like Pt14Ni24, Fig. 39) with three chemically distinct types of metal atoms, exhibiting different polyhedron geometries, Pt6(hexahedron)@Pt8(octahedron)@Pt24(truncated octahedron). In addition, Pt38 bears 32 terminal carbonyl groups and 12 edge-bridging CO ligands. Thus, the overall ideal symmetry is lowered to D2d.365,366
7. Platonic and Archimedean solids in lanthanide-containing clusters
The chemistry of lanthanide clusters has become one of the most interesting research frontiers, and lanthanide-containing clusters (including lanthanide-exclusive clusters and heterometallic clusters containing lanthanide elements) are attracting widespread current interest due to their fantastic architecture (especially high-nuclearity lanthanide clusters,5,6,8 such as wheel Ce70,367 Gd140,52 cubic Ln96Ni64,53 hexagon-shaped Ni36Gd102,54 tubular Dy72,368 spherical Ln104,55 and Gd158Co3856), and intriguing properties in the fields of single-molecular magnets (SMMs),73 magnetocaloric effects (MCEs),7,72,83 and catalysis.369,3707.1 Lanthanide-exclusive clusters
Among the reported lanthanide-exclusive clusters, a series of tetrahedral Ln4,71,371–377 octahedral Ln6,378–381 cubic Ln8,382 and Keggin-type Eu13 (cuboctahedron)383 clusters has been obtained, while tetrahedral, octahedral and cubic cores have been widely used as building blocks in high-nuclearity Ln clusters.384Utilizing linear-like multi-coordinated 1-methyl-3,5-bis[3-(pyrid-2-yl)-1,2,4-triazolyl]pyridine (H2MebptpO) ligands reacting with LnIII salt under solvothermal reaction, Tong's group obtained a series of supertetrahedral clusters, [Ln20(μ4-O)11(μ3-OMe)12(μ2-OMe)8(MebptpO)4(PhCOO)8(H2O)4]2+ (Ln20, Ln = Tb, Dy, Ho, and Er, Fig. 41a).373 In the super-tetrahedral Ln20 cluster, the two perpendicular edges opposite the super-tetrahedron are bridged by two pairs of MebptpO2− ligands (Fig. 41b) and the remaining four edges are protected by eight PhCOO− and eight μ2-OMe− ligands. Additionally, each face and vertex of the v3-tetrahedron is capped by three μ3-OMe− groups and one H2O molecule, respectively. Eleven μ4-O2− are all located at the center of the vertex-sharing {Dy4(μ4-O)} units and the outer ten μ4-O2− atoms form a v2-tetrahedron, encapsulating an inner μ4-O2− (Fig. 41c). The metal arrangement of the v3-tetrahedral cluster can be described as a large Dy16 tetrahedral shell (Dy12(truncated tetrahedron)@Dy4(tetrahedron)) wrapping a small Dy4 tetrahedral core.
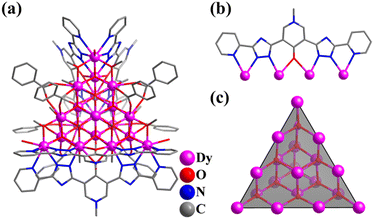 | ||
| Fig. 41 (a) Structure of [Dy20(μ4-O)11(μ3-OMe)12(μ2-OMe)8(MebptpO)4(PhCOO)8(H2O)4]2+. (b) Coordination mode of linear-like MebptpO2− of Ln20. (c) {Dy20(μ4-O)11} super-tetrahedral core with nested arrangement, Dy4(tetrahedron)@Dy16(tetrahedron).373 | ||
Using different hydroxyl-containing small organic molecules (Hchp (2-chloro-6-hydroxypyridine), mdaH2 (N-methyl-diethanolamine), and Hdmp (2,2-dimethylol propionic acid)), Zheng and co-workers synthesized a series of highly symmetric spherical gadolinium(GdIII) clusters with polyhedral characteristics ranging from small to large (Fig. 42), [Gd9(μ4-OH)2(μ3-OH)8(mda)4(mdaH)2(mdaH2)2(NO3)7(CH3OH)4] (Gd9), [Gd15(μ5-Cl)(μ3-OH)20(dmp)10(H2O)10](ClO4)8Cl6 (Gd15), [Gd20(μ5-CO3)12(chp)30(NO3)6(H2O)6] (Gd20), [Gd32(μ4-OH)6(μ3-OH)48(mda)12(NO3)12(H2O)24]6+ (Gd32), [Gd50(μ5-Cl)12(μ3-OH)80(dmp)30(H2O)]28+ (Gd50), and [Gd60(μ6-CO3)8(μ3-OH)96(dmp)28(NO3)8(H2O)8]32+ (Gd60).385 Magnetization analyses revealed the magneto-structural correlation in the GdIII clusters and this correlation can be used as a “fingerprint” to identify these aggregates. The structural analysis revealed that four high-symmetry spherical GdIII clusters can be viewed as the self-assembly of polymetallic lanthanide fragments that contain different topological polygons and polyhedrons, such as {Gd3(μ3-OH)} triangle, {Gd4(μ4-OH)} square, {Gd4(μ3-OH)4} tetrahedron, {Gd5(μ5-CO3)} pentagon, {Gd5(μ5-Cl)} pentagon, and {Gd6(μ6-CO3)} hexagon (Fig. 42e). Simple anion templates (such as OH−, Cl−, and CO32−) facilitate the formation of these fragments and complete spherical clusters.386,387
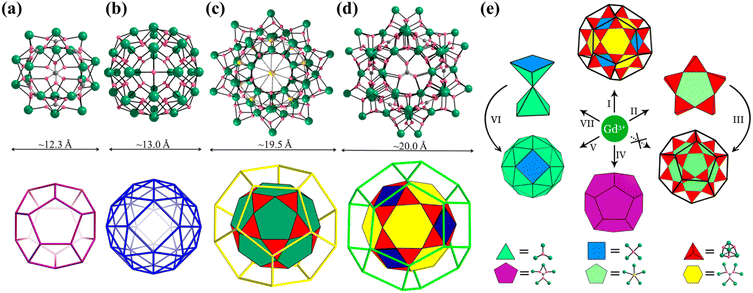 | ||
| Fig. 42 Core structures and topological representation of the four spherical GdIII clusters Gd20 (a), Gd32 (b), Gd50 (c), and Gd60 (d). (e) Synthetic strategy of the four spherical GdIII clusters and two intermediates (Gd9 and Gd15).385 Color codes: Gd, dark green; Cl, yellow; O, pink; and C, gray. | ||
Gd20 with approximate Ih symmetry, resembles a pentagonal dodecahedron with the GdIII atoms lying on the vertices and twelve μ5-CO32− anions on the slightly distorted hexagonal faces {Gd5(μ5-CO3)} (Fig. 42a). Similar dodecahedral Ln20 clusters were also reported.388,389Gd32 polyhedron features six {Gd4(μ4-OH)} squares and 32 {Gd3(μ3-OH)} triangles and its 24 GdIII sites construct a truncated octahedron with each of regular hexagonal faces capped by a GdIII atom (Fig. 42b). Gd32 can be viewed as a Keplerate, Gd24(truncated octahedron)@Gd8(octahedron), similar to Co24 (Co24@Mo8/W8, Fig. 35). Interestingly, Gd32 is the first giant GdIII cluster core with Oh symmetry. Gd50 displays an unprecedented core–shell Keplerate structure with approximate Ih symmetry, in which an icosidodecahedral core Gd30 (12 {Gd5(μ5-Cl)} pentagons and 20 {Gd3(μ3-OH)} triangles) is encapsulated by an outer dodecahedral shell Gd20 (Fig. 42c). The 30 GdIII atoms inside Gd50 cluster construct a topology of Archimedean solid (icosidodecahedron) with each of the regular triangle faces capped by a GdIII atom, resulting in 20 vertex-sharing {Gd4(μ3-OH)4} tetrahedrons/cubanes with a dodecahedral arrangement.
Gd60 also displays a core–shell structure and its metal core exhibits approximately Oh symmetry (Fig. 42d). The 36 GdIII atoms inside the Gd60 cluster construct a truncated topology of truncated octahedra, containing twenty-four {Gd3(μ3-OH)} triangles, six {Gd4(μ3-OH)8} squares, and eight {Gd6(μ6-CO3)} hexagons. Each of regular triangle faces of the doubly truncated octahedron is capped by a GdIII atom, resulting in 24 vertex-sharing {Gd4(μ3-OH)4} tetrahedra/cubanes with a truncated octahedral arrangement. Similar 60-metal (Er, Ho, and Y) core–shell clusters were first reported by Zhang and co-workers in 2009.390 Subsequently, other Ln60 clusters with a similar metal framework were also reported by Xu's group.391,392 In addition, two different research groups revealed two different ways to generate similar Ln60 clusters.393,394
By controlling the hydrolysis of Ln(ClO4)3 (Ln = Nd and Gd) in the presence of CH3COO− and N-acetyl-D-glucosamine, Kong and co-workers prepared three beautiful highly symmetric cage-like clusters containing 104 lanthanide atoms, i.e., [Nd104(μ4-O)30(μ3-OH)168(CH3COO)60(H2O)112(ClO4)6](ClO4)18 (Nd104-1, Fig. 43) and [Ln104(μ4-O)30(μ3-OH)168(CH3COO)56 (H2O)112(ClO4)6]·(ClO4)22 (Ln = Nd, Nd104-2; Gd, Gd104).55 These clusters represent the second largest lanthanide-exclusive cluster to date, while the largest is the {Gd140} wheel.52 These huge Ln oxyhydroxide clusters (Fig. 43a) can be seen as being assembled from square-pyramidal [Nd5(μ4-O)(μ3-OH)4]9+ units (Fig. 43b) and [Nd(μ3-OH)6]3− linkers (Fig. 43d). Four square-pyramidal SBBs are clustered together by corner sharing to generate a 16-metal tetramer centered on a μ4-O2− group, [Nd16(μ4-O)5(μ3-OH)20]18+ (Fig. 43c). Six of these tetrameric assemblies are arranged as vertices in an octahedral form, and eight [Nd(μ3-OH)6]3− linkers further fill the center of the eight faces of the octahedron, resulting in the formation of the giant hollow structure. It is a typical Keplerate structure with its 104 Ln3+ ions organized into four distinct shells (Fig. 43e). From the inside to the outside, the topologies of the 104 metal core contain an inner Platonic solid (octahedron) and outer three Archimedean solids (a truncated cuboctahedron, truncated octahedron, and rhombicuboctahedron), abbreviated as Ln8@Ln48@Ln24@Ln24. Gd104 possesses the second largest magnetic entropy change (−ΔSm = 46.9 J kg−1 K−1 at 2 K for ΔH = 7 T) among the known Ln-exclusive clusters.
 | ||
| Fig. 43 (a) Molecule structure of cationic cluster Nd104-1. (b) Square-pyramidal [Nd5(μ4-O)(μ3-OH)4]9+ unit. (c) Tetramer [Nd16(μ4-O)5(μ3-OH)20]18+. (d) Linker [Nd(μ3-OH)6]3−. (e) Arrangement of the 104 Nd atoms in the cluster core of Nd104-1 with nested four-shell structure.55 | ||
The research group led by Professor George Christou developed a simple bottom-up method, where CeIII/V salt and small organic acid molecules react in solutions containing pyridine or its derivatives, to prepare ultra-small CeO2 nanoparticles in Ce-oxo cluster form (from Ce6,395,396 Ce10,396 Ce16, Ce19,397 Ce20,395 Ce24, Ce38, and Ce40,398 to the largest Ce100 with a size of 2.4 nm51). These Ce–O clusters possess a regular metal core with the fluorite structure of bulk CeO2 and reflect critical information at atomic resolution of important surface features, helping CeO2 nanoparticles to be better used in catalysis and other fields. Among them, the metal skeletons of Ce6 (octahedron), Ce10 (Ce6@Ce4, octahedron@tetrahedron), Ce16 (Ce4@Ce12, tetrahedron@truncated tetrahedron), and Ce38 (octahedron@cube@truncated icosahedron, such as Pu38, Fig. 24) feature Platonic and Archimedean solids.
7.2 Heterometallic clusters containing lanthanide
Owing to the diverse aesthetic structures, existence of significant d–d, d–f, and f–f magnetic exchange, and intriguing magnetocaloric effect (MCE) and single-molecule magnet (SMM) properties of d–f high-nuclearity heterometallic clusters containing lanthanide (Ln) and other metal ions, especially transition metals (TM), are of continuing interest.72 In particular, Tong et al. summarized the recent advances in heterometallic clusters based on cubane {TMxLn4−x} subunits, focusing on their structure and magnetic properties.71 Thus, here we will not discuss simple heterometallic clusters containing lanthanides, only a few examples of typical high-nuclearity clusters. For example, the v2-tetrahedral cluster M10 with an {Fe4Ln6(μ4-O)4} (Ln = Dy and Ho) core,399v2-octahedral cluster (M18:M12@M6, [(ClO4)@Cu12Ln6(OH)24(H2O)18(Pyb)12]17+ (Ln6Cu12, Ln = Y, Nb, and Gd, and Pyb = pyridine betaine)) with an α-Keggin-type Cu–O core),331,400 and truncated tetrahedron ([Ln12(MoO4)4(HL)6(μ3-OH)4(OOCCH3)12] (Ln12Mo4, Ln = Gd, Eu, and Sm; H3L = (E)-2-(2,3-dihydroxypropylimino)methyl)-phenol)401 and (2-MepyH)5[(μ4-Cl)Pr4Sb12(μ3-O)12(μ4-O)6Cl16] (Pr4Sb12, 2-MepyH+ = monoprotonated 2-methylpyridine)).402With the development and improvement of synthetic strategies, e.g., controlling the hydrolysis of metal ions in the presence of small organic molecules containing carboxyl and/or hydroxyl groups, significant progress in the preparation of high-nuclearity Ln-containing clusters has been achieved, including the above-mentioned Ln clusters (Gd9, Gd15, Gd20, Gd32, Gd50, Gd60 (Fig. 42), and Gd104 (Fig. 43)) and various heterometallic (Ln–Ti, Ln–Fe, Ln–Co, Ln–Ni, Ln–Cu, Ln–Zn, etc.) clusters. Long and Kong's team made a great contribution in this regard, preparing a series of representative clusters, many of which fit the theme of this review, such as Ln24Zn4 (Fig. 44), Gd30Co12, Ln12Fe33 (Fig. 45), and La20Ni30 (Fig. 45).
 | ||
| Fig. 44 (a) Molecule structure of [Gd24Zn4O5(OH)44(CH3COO)12 (H2O)48]14+ (Gd24Zn4). (b) Metal core Zn4@{Gd6}4, wherein four octahedrons encapsulate a tetrahedron. (c) Nested arrangement of 28 metals, Zn4(tetrahedron)@Gd12(truncated tetrahedron)@Gd12(elongated cuboctahedron).403 | ||
 | ||
| Fig. 45 (a) Ball-and-stick representation of [Pr12Fe33(NO3)6(L-van)4(D-van)5(TEOA)12(μ3-OH)12(μ4-OH)12(μ4-O)28(H2O)4]4+ (Pr12Fe33). (b) ε-Keggin-type Fe13-oxo core encapsulated by Pr12Fe20 shell (Fe4@Pr12@Fe4@Fe12). (c) [Fe(μ4-OH)3(μ4-O)3]6− linking the inner Fe13-oxo core and outer PrIII shell. (d) Hexagon [Pr6Fe4(TEOA)3]21+.298 (e) Molecule structure of [La20Ni30(IDA)30(CO3)6(NO3)6(OH)30(H2O)12]12+ (La20Ni30). (f) La20 core enveloped by an outer Ni-IDA shell, in which 50 metal atoms present a nested arrangement, La20(dodecahedron)@Ni30(icosidodecahedron).404 | ||
Through the self-assembly of Ln(ClO4)3 (Ln = Gd and Sm) and Zn(OAc)2 in a mixed solution containing NaOH, two rare high-nuclearity heterometallic Ln–Zn clusters [Ln24Zn4(μ6-O)4(μ4-O)(μ3-OH)44(CH3COO)12(H2O)48] (ClO4)14 (Ln24Zn4, Ln = Gd and Sm, Fig. 44a) were obtained,403 where the Gd-containing cluster exhibited a good magnetocaloric effect (−ΔSm = 31.4 J kg−1 K−1 at 2 K for ΔH = 7 T). Ln24Zn4 with ideal Td symmetry features four octahedra {(μ6-O)@Ln6(μ3-OH)8} encapsulating a {(μ4-O)@Zn4} tetrahedron (Fig. 44b). Alternatively, the metal core of Ln24Zn4 can be viewed as a three-shell Keplerate, as shown in Fig. 44c. From the inside to the outside, the topologies of the 28-metal core possess an innermost tetrahedron, followed by a truncated tetrahedron and elongated cuboctahedron with four small triangles, four big triangles and six rectangle faces.
By replacing Zn(OAc)2 with other transition metal salts and adjusting the types of LnIII salts, other high-symmetry clusters ([Gd30CoII/III12(OH)56(NO3)12(CH3COO)30(H2O)30]·(NO3)22 (Gd30Co12)405 and [Pr12Fe33(NO3)6(L-van)4(D-van)5(TEOA)12(μ3-OH)12(μ4-OH)12(μ4-O)28(H2O)4]·(ClO4)3·(NO3)·10H2O (Pr12Fe33, L/D-van = L/D-valine, TEOA = triethanolamine, Fig. 45a)298) were obtained in a similar manner. Gd30Co12 features a double-shell structure, possessing a twisted icosahedron (Co12) encapsulating a slightly distorted icosidodecahedron (Gd30).405 Meanwhile, Gd30Co12 exhibits a good magnetocaloric effect, i.e., −ΔSm = 44.7 J kg−1 K−1 at 2 K for ΔH = 7 T. High-symmetry Pr12Fe33 (Fig. 45a) is a typical example of capturing Keggin Fe13-oxo clusters by using LnIII ions, and their Ln–organic shells make the functionalization of Fe13-oxo clusters convenient (Fig. 45b).298 The cationic core of Pr12Fe33 can be described from the inside to outside as follows: (1) the ε-Keggin Fe13-oxo capped by four [Fe(μ4-OH)3(μ4-O)3]6− (Fig. 45c) in the hexagonal “window” of the truncated tetrahedron generates a Fe17-oxo core (Fe@Fe12@Fe4) and (2) the Fe17-oxo unit further capped by 12 PrIII and 4 FeIII forms a Fe21Pr12-oxo core (Fe@Fe12@Fe4@Pr12@Fe4), which is further capped by four [FeIII4(TEOA)3]3+ motifs (Fig. 45d), producing a total lanthanide-Fe oxyhydroxide core (Fe(center)@Fe12(truncated tetrahedron)@Fe4(tetrahedron)@Pr12(truncated tetrahedron)@Fe4(tetrahedron)@Fe12(cuboctahedron)) of Pr12Fe33. These lanthanide-containing clusters stabilized and constructed by small ligands possess beautiful symmetry and high magnetocaloric properties.7
Iminodiacetate (IDA), possessing five coordinating atoms (one N and four O atoms), has achieved major success in the construction and assembly of heterometallic 3d–4f nanoclusters. A series of high-nuclear Ln–Ni/Co clusters with core–shell or giant hollow structures has been synthesized using IDA and its derivatives, as well as an IDA-containing multi-ligand strategy, such as Ln18Ni24(23.5) (Ln = Gd and Eu),406 Ln20Ni21 (Ln = Gd,407 Pr and Nd408), Gd22Ni21,409 Ln23Ni20 (Ln = Gd and Eu),410 La20Ni30,404,408 Nd38Ni42.5,411 Ln40Ni44 (Ln = Gd and Eu),412 Pr42Ni38,411 Ln52Ni52 (Ln = Gd and Dy),413 Ln52Ni56 (Ln = Eu, Pr, Nd and Gd),414,415 Gd54Ni54,416 La60Ni76,417 La68Ni90, La76Ni88,418 Gd78Ni64, Eu78Ni62,419 Ln96Ni64 (Ln = Gd, Dy, Y),53 Gd44Co28, Gd95Co60,420 and Gd158Co38.56 In these Ln–Ni/Co clusters, simple 3d/3d–4f complexes with IDA ligands assemble into a peripheral shell connected by carboxylate O atoms, which greatly control the degree of lanthanide hydrolysis. This controlled hydrolysis and additional anions (such as NO3−, CO32−, Cl−, Br− and I−) afford polyhedral Ln–OH or Ln–OH–Ln/TM cores of the eventual complex heterometallic clusters.
The first high-nuclearity heterometallic Ln–Ni cluster protected by IDA ligands is [La20(OH)30(CO3)6(NiIDA)30(NO3)6 (H2O)12](CO3)6 (La20Ni30, Fig. 45e).404 Keplerate La20Ni30 features a double-sphere structure with an outer spherical shell constructed by 30 Ni-IDA metal-complexes, encapsulating the inner one of 20 La atoms. The topologies of the double-shell structure can be viewed as an inner dodecahedron encapsulated by an icosidodecahedron, La20@Ni30 (Fig. 45f). The nested arrangement of the two distinct sets of metal atoms exhibits symmetrical beauty as both ideally possess Ih symmetry (dodecahedron and icosidodecahedron).
Based on a mixed-ligand (IDA and 2,2-dimethylol propionic acid (DMPA)) approach, Zheng's group isolated three isostructural gigantic Ln–Ni clusters, [Ln96Ni64(μ3-OH)156(IDA)66 (DMPA)12(CH3COO)48(NO3)24(H2O)64]Cl24 (Ln = Gd, Gd96Ni64; Dy, Y).53Gd96Ni64 with a unique porous cube-like structure represents the second highest nuclearity heterometallic Ln-TM clusters. The cation of Gd96Ni64 is a cube-like aggregate of [Gd96Ni60(μ3-OH)156(IDA)60(DMPA)12(CH3COO)48(NO3)24(H2O)52]24+ (abbreviated as {Gd96Ni60}) with four add-on {Ni(IDA)(H2O)3} fragments. {Gd96Ni60} consists of eight propeller-like {Ni6Gd9} fragments on each vertex of its cubic framework and twelve triangular {NiGd2} units as edges. These hollow structures exhibit highly selective adsorption for CO2 over N2 or CH4 at ambient temperature and Gd96Ni64 exhibits a large magnetocaloric effect (−ΔSm = 42.8 J kg−1 K−1 at 3 K for ΔH = 7 T).
Through the ‘multi-anions-template’ strategy and controlling the hydrolysis of metal ions (Gd and Co) in the presence of the IDA-derivative N-methyliminodiacetic acid (H2MIDA), the largest 3d–4f heterometallic cluster [Gd158Co38Cl12(μ3-OH)236(CO3)90(MIDA)42(Sar)6(CH3COO)18(H2O)84]·Cl24·144H2O (Gd158Co38Cl12, HSar = sarcosine, Fig. 46a) to date was obtained, which exhibits a large magnetocaloric effect (−ΔSm = 46.95 J kg−1 K−1 at 2 K for ΔH = 7 T).56 Different from the reported giant hollow Ln-containing clusters (wheel Ce70,367 Gd140,52 cubic Ln96Ni64,53 cage-like Gd95Co60,420 hexagon-shaped Ni36Gd102,54 tubular Dy72,368 and spherical Ln10455), the giant Gd158(μ3-OH)236(CO3)90 templated by twelve halide ions is a solid assembly, and 38 Co complexes (30 Co(μ3-OH)3(MIDA) and 8 Co(μ3-OH)3(H2O)3) cap its surface. As shown in Fig. 46b, the cationic Gd158Co38Cl12(μ3-OH)236(CO3)90 core protected by multiple organic small molecules and water molecules can be described (from the inside out, Fig. 46b) as follows: (1) the 20 GdIII ions in the center form a dodecahedron framework by 12 CO32− and 36 μ3-OH− groups (like Gd20 in Fig. 42a), whose 12 pentagonal “windows” are capped by 12 templates (Cl−) in an icosahedron fashion. (2) Gd20@Cl12 with Ih symmetry further is encapsulated by a cuboctahedron-like heterometallic framework (Gd6Co4)8, built by eight propeller-like Gd6Co4 SBBs with a cubic arrangement. (3) Twelve GdIII are packed outside the junction between the Gd6Co4 SBBs and present an icosahedral arrangement. (4) Eight saddle-like Gd12 SBBs are capped on the hexagonal “windows” of the cuboctahedron-like (Gd6Co4)8 framework, forming the cubic structure of the main body. (5) In addition, there are 6 GdIII (near-octahedral configuration) and 6 CoII/III (hexagonal geometry) at the periphery of the cubic Gd152Co32Cl12 cluster.
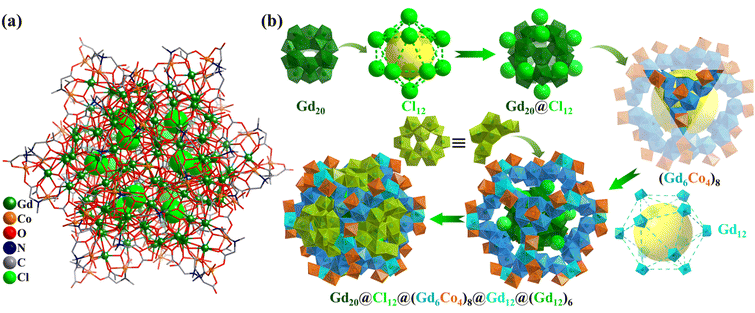 | ||
| Fig. 46 (a) Ball-and-stick representation of cationic cluster of Gd158Co38Cl12. (b) Metal (152 Gd and 32 Co) framework templated by 12 Cl− in Gd158Co38Cl12.56 | ||
8. Platonic and Archimedean solids in coin metals (Cu, Ag, and Au) clusters
Atomically precise coinage metal (Cu, Ag, and Au) cluster crystalline materials are new categories and have been comprehensively studied owing to their aesthetically beautiful molecular structures and fascinating properties such as quantized electronic absorption, fluorescence, and catalysis.1,11,12,15,21,75–77,85,89–92,421 With the diversification of synthetic methods and ligands, especially the introduction of numerous reducing agents, an increasing number of coinage metal clusters have been obtained. Due to the unique metal–metal bonds, polyhedral cores with Platonic and Archimedean solids can commonly appear in these clusters, such as regular tetrahedron, cube, octahedron, and icosahedron.8.1 Cu clusters
In 2016, Xu's group reported the preparation of three cubic 20-metal clusters containing CuII, [(CO3)@CuII20(μ3-OH)24(CH3COO)8(n-propylamine)10](CH3COO)6, [(CO3)@CuII20(μ3-OH)24(CH3COO)6(iso-propylamine)8](CH3COO)8, and [(CO3)@CuII12ZnII8(μ3-OH)24(CH3COO)12](CH3COO)2.332 Although there are some differences in their peripheral ligands, the core of these clusters is the same, and a double-shell metal skeleton with carbonate as the anion template, {(CO3)@Cu12M8(μ3-OH)24} (M = Cu and Zn), is similar to the above-mentioned Co20-1 (Fig. 34a and b). The {Cu12M8} core can be further viewed as a cuboctahedron shell {Cu12} with each triangular face capped by CuII or ZnII ions from an outer cube. It should be noted that two other analogous 20-metal clusters, {Cu20} and {Cu12Mg8}, were revealed by Winpenny et al.331
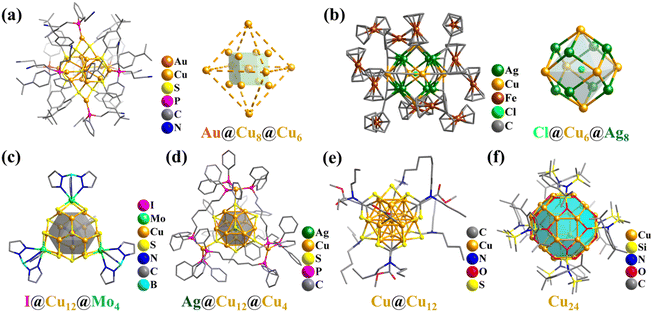 | ||
Fig. 47 Ball-and-stick representation of (a) [Au@Cu14(SPhtBu)12(PPh(C2H4CN)2)6]+ with an Au@Cu14 (Cu8@Cu6: cube@octahedron) framework,466 (b) [Cl@Cu6Ag8(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc)12]+ with a Cl@Cu6(octahedron)@Ag8(cube) framework,462 (c) [I@{TpMoS4Cu3}4]−,464 (d) [Ag@Cu12S8@Cu4(dpph)6]+,465 (e) [Cu13(S2CNnBu2)6(C CFc)12]+ with a Cl@Cu6(octahedron)@Ag8(cube) framework,462 (c) [I@{TpMoS4Cu3}4]−,464 (d) [Ag@Cu12S8@Cu4(dpph)6]+,465 (e) [Cu13(S2CNnBu2)6(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(O)OMe)4]+,446 and (f) Cu24(O3Si(2,6-iPr2C6H3)N(SiMe3))8.453 CC(O)OMe)4]+,446 and (f) Cu24(O3Si(2,6-iPr2C6H3)N(SiMe3))8.453 | ||
In 2021, Zhu's group reported the preparation of an ultrabright Au–Cu cluster with two free valence electrons (2e), [Au@Cu14(SPhtBu)12(PPh(C2H4CN)2)6]+ (Au@Cu14, Fig. 47a), with the record phosphorescence quantum yield (71.3%) in non-degassed solution at room temperature.466Au@Cu14 has a single-Au-atom center encapsulated by a rigid Cu(I) complex cage (Cu8@Cu6: cube@octahedron). Numerous clusters of core–shell structures (M(center)@M8(cube)@M6(octahedron)) protected by other ligands have also been reported.467,468
Anion-templated 14-metal clusters with a rhombic dodecahedral metal framework MI6AgI8 (M = Cu, Ag, and Au) fall in the theme of our review. MI6AgI8 (M = Cu, Ag, and Au) clusters protected by different ligands have been continuously reported, such as [Cl@MI6AgI8(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc)12]+ (
CFc)12]+ (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc− = ferrocenylacetylide, Fig. 47b).462 The MI6AgI8 cluster forms a rhombic dodecahedron made up of 12 MI2AgI2 quadrangles. Alternatively, six MI atoms occupy the corners of a regular octahedron, whereas eight AgI atoms are located at the corners of a regular cube. Interestingly, each of the triangular faces in the MI6 octahedron is capped with an AgI atom and each of the square faces in the AgI8 cube is capped with an MI atom. In 2020, Zang's group showed that o-carboranealkynyl-protected metal clusters, Cu6Ag8 and Ag14 (octahedron@cube), can serve as catalysts, enabling spontaneous ignition to achieve one-off combustion reactions.463
CFc− = ferrocenylacetylide, Fig. 47b).462 The MI6AgI8 cluster forms a rhombic dodecahedron made up of 12 MI2AgI2 quadrangles. Alternatively, six MI atoms occupy the corners of a regular octahedron, whereas eight AgI atoms are located at the corners of a regular cube. Interestingly, each of the triangular faces in the MI6 octahedron is capped with an AgI atom and each of the square faces in the AgI8 cube is capped with an MI atom. In 2020, Zang's group showed that o-carboranealkynyl-protected metal clusters, Cu6Ag8 and Ag14 (octahedron@cube), can serve as catalysts, enabling spontaneous ignition to achieve one-off combustion reactions.463
Lang's group reported the preparation of a unique tetracubane cluster, [PyH][(μ12-I){TpMo(μ3-S)4Cu3}4] ({MoCu3}4, Tp = hydridotris(pyrazol-1-yl)borate, Py = pyridine, Fig. 47c).464 The [I@{TpMo(μ3-S)4Cu3}4]− anion can be viewed as a μ12-I− (as anionic template)-centred tetrahedral cage, in which four corners are occupied by four incomplete cubane-like [TpMo(μ3-S)4Cu3}4] fragments. The twelve CuI atoms form a slightly distorted cuboctahedron cage. Sun isolated two cluster-in-cage structures with an Ag@Cu12 cuboctahedron (Archimedean solid) in a Cu4(dpph)6 tetrahedral (Platonic solid) cage, [Ag@CuI12S8@Cu4(dpph)6]X (X = OH or PF6, dpph = bis(diphenylphosphino)hexane, Fig. 47d).465 Xin and co-workers synthesized a bi-metallic CuI–MoVI–S cluster, [nBu4N]4[Cu12Mo8S32] (Cu12Mo8).469 The anionic icosanuclear cluster [Cu12Mo8S32]4− of Cu12Mo8 exhibits an octameric supra-cubane-like architecture. In the supra-cubane-like/cubic cage, eight MoVI atoms are located at the corners, whereas the midpoints of the twelve edges are occupied by CuI atoms, exhibiting a cuboctahedral arrangement. The anion cluster [Cu12Mo8S32]4− stabilized by different counter cations was also prepared.470–472
In addition to the above-mentioned monovalent copper cluster, Liu and co-workers reported the first structurally characterized two-electron superatom Cu0/I cluster, [Cu13(S2CNnBu2)6(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)4](PF6) (Cu13, R = C(O)OMe, or C6H4F; Fig. 47e), with a centered cuboctahedral arrangement Cu13 (as an ideal model of the bulk copper fcc structure).446 Besides, Liu and co-workers also reported the synthesis of the first rhombicuboctahedral CuI–H clusters, [H15Cu28{S2CNR}12]PF6 (H15Cu28, NR = NnPr2 or aza-15-crown-5, Fig. 48a).473 The H15Cu28 cluster is air- and moisture-stable both in the solid state and in solution. The cation cluster of H15Cu28 with D2d symmetry contains a central irregular Cu4 tetrahedron with an interstitial hydride and an outer slightly distorted Cu24 rhombicuboctahedron (Fig. 48b), in which the Cu⋯Cu separation is 2.603(1)–2.824(1) Å. The outer shell Cu24 is further encapsulated in an array of twelve {S2CNPr2}− (dtc = di-n-propyldithiocarbamate) ligands. Each dtc ligand in a μ4-η2,η2 binding mode bridges four CuI atoms (Fig. 48c) and the 24 S atoms of the twelve ligands are arranged in a truncated octahedral cage (Fig. 48d). The hydrides of H15Cu28 are distributed in three locations, playing an important role in the following connections: (i) one central hydride; (ii) six hydrides with an octahedral array, located inside square faces of Cu24 rhombicuboctahedron and bridging between the inner and outer CuI polyhedrons (Fig. 48f); and (iii) eight hydrides with a cubic manner, located outside the triangular faces of the Cu24 rhombicuboctahedron (Fig. 48e). The aesthetically pleasing nested-structure of H15Cu28 can be expressed as μ4-H@Cu4(tetrahedron)@H6(octahedron)@Cu24(rhombicuboctahedron)@H8(cube)@S24(truncated octahedron), and the geometric entity is reminiscent of a Chinese puzzle ball (Fig. 48g). Intriguingly, limited H2 evolution was observed upon the exposure of H15Cu28 solutions to mild conditions at room temperature.
CR)4](PF6) (Cu13, R = C(O)OMe, or C6H4F; Fig. 47e), with a centered cuboctahedral arrangement Cu13 (as an ideal model of the bulk copper fcc structure).446 Besides, Liu and co-workers also reported the synthesis of the first rhombicuboctahedral CuI–H clusters, [H15Cu28{S2CNR}12]PF6 (H15Cu28, NR = NnPr2 or aza-15-crown-5, Fig. 48a).473 The H15Cu28 cluster is air- and moisture-stable both in the solid state and in solution. The cation cluster of H15Cu28 with D2d symmetry contains a central irregular Cu4 tetrahedron with an interstitial hydride and an outer slightly distorted Cu24 rhombicuboctahedron (Fig. 48b), in which the Cu⋯Cu separation is 2.603(1)–2.824(1) Å. The outer shell Cu24 is further encapsulated in an array of twelve {S2CNPr2}− (dtc = di-n-propyldithiocarbamate) ligands. Each dtc ligand in a μ4-η2,η2 binding mode bridges four CuI atoms (Fig. 48c) and the 24 S atoms of the twelve ligands are arranged in a truncated octahedral cage (Fig. 48d). The hydrides of H15Cu28 are distributed in three locations, playing an important role in the following connections: (i) one central hydride; (ii) six hydrides with an octahedral array, located inside square faces of Cu24 rhombicuboctahedron and bridging between the inner and outer CuI polyhedrons (Fig. 48f); and (iii) eight hydrides with a cubic manner, located outside the triangular faces of the Cu24 rhombicuboctahedron (Fig. 48e). The aesthetically pleasing nested-structure of H15Cu28 can be expressed as μ4-H@Cu4(tetrahedron)@H6(octahedron)@Cu24(rhombicuboctahedron)@H8(cube)@S24(truncated octahedron), and the geometric entity is reminiscent of a Chinese puzzle ball (Fig. 48g). Intriguingly, limited H2 evolution was observed upon the exposure of H15Cu28 solutions to mild conditions at room temperature.
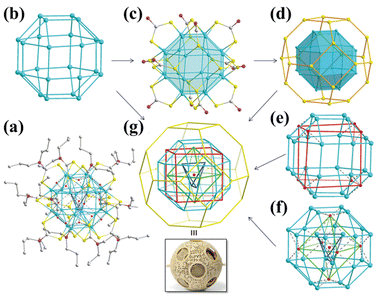 | ||
| Fig. 48 (a) Ball-and-stick representation of [H15Cu28{S2CNPr2}12]+ cation. (b) Rhombicuboctahedral arrangement of 24 CuI atoms. (c) Each of the 12 square faces of the Cu24 shell is capped by an {S2CNPr2}− ligand. (d) Cu24 shell is enclosed by a truncated octahedron of 24 S atoms from twelve {S2CNPr2}− ligands. (e) Eight hydrides μ3-H− on triangular faces of the Cu24 shell. (f) Six hydrides with an octahedron array inside square faces of the Cu24 shell. (g) Core assembly: μ4-H@Cu4(tetrahedron)@H6(octahedron)@Cu24(rhombicuboctahedron)@H8(cube)@S24(truncated octahedron).473 Colour codes: C, grey; Cu, cyan; H, red; N, pink; and S, yellow. | ||
In 2018, Fischer and co-workers prepared a paramagnetic and all-hydrocarbon ligand-stabilized heterometallic cluster, [Cu43Al12](Cp*)12 (Cu43Al12, Fig. 49a, Cp* = η5-C5Me5), obtained from the reaction of [AlCp*]4 and [CuMes]5 (Mes = mesityl).474Cu43Al12 possesses a magic atom-number (55) and features a Mackay-type nested icosahedral structure, similar to Pd55 (Fig. 40a), and a unique open-shell 67 electron super-atom configuration. The Mackay-type structure of Cu43Al12 is composed of a μ12-Cu-centered Cu13 icosahedral core and a heterometallic Cu30Al12v2-icosahedral shell. The 44-metallic shell can also be divided into two parts, i.e., icosidodecahedron Cu30 (Fig. 49b) and (AlCp*)12 icosahedral shell (Fig. 49c). The complete structure of Cu43Al12 can be described by a concentric core–shell, Cu@Cu12@Cu30@(AlCp*)12. A similar M43 metal kernel containing Cu was also observed in the bimetallic Au19Cu30 nanocluster,475 featuring a core–shell structure of Au@Au12@Cu30@Au6 and consisting of an Au-centered icosahedral core Au13 encapsulated by an icosidodecahedral shell Cu30 and an outmost hexagonal Au6. Zhu and Wang et al. also reported a bimetallic Ag61Cu30 nanocluster, which is composed of an Ag@Ag12@Cu30 kernel, capped by a peripheral Ag48(SAdm)38S3 shell.476
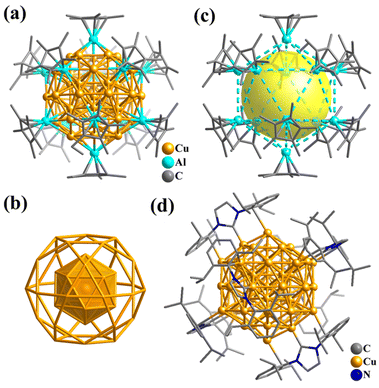 | ||
| Fig. 49 (a) Ball-and-stick representation of [Cu43Al12](Cp*)12. (b) Core–shell arrangement of 43 Cu atoms, μ12-Cu@Cu12(icosahedron)@Cu30(icosidodecahedron). (c) (AlCp*)12 icosahedral shell.474 (d) Ball-and-stick representation of [(IDipp)6Cu55].57 | ||
Accidentally, two unprecedented low-valent copper clusters [(IDipp)6Cu55] (Cu55, Fig. 49d) and [(IDipp)12Cu179] were obtained as minor products from the reductive decomposition of the unstable N-heterocyclic carbine (NHC) copper-boryl complex [(IDipp)Cu-Bneop] (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, neop = (OCH2)2CMe2)) by Kleeberg's team.57 The copper atoms in the remarkable metalloid Cu55 cluster adopt approximately icosahedral symmetry and a Cu@Cu12@Cu30@Cu12 arrangement similar to Cu43Al12 (Fig. 49a) and Pd55 (Fig. 40a).
8.2 Ag clusters
Similar to CuI-containing clusters, a large number of multi-/high-nuclearity clusters with metal cores featuring Platonic and Archimedean solids (such as tetrahedron,477 cube,478–480 octahedron,481 tetra-capped tetrahedron (M4@M4),455,482,483 cuboctahedron,478,484,485 and v2-tetrahedron (M6@M4)486) has been obtained in atomically precise silver clusters but will not be discussed in detail here. A distinct feature has been demonstrated in CuI and CuI/0 clusters, which is more vividly manifested in the silver cluster, where the anions play an important role ((i) template and directing agent to control the shape and size of the structures; (ii) stabilizing and balancing the local positive charges from metal ions; and (iii) improving the cluster functionality) in the formation of the structure (Fig. 47b and c) in CuI clusters, while that (the metal occupies the position of the anion, Fig. 47a and e) in the CuI/0 clusters requires additional free valence electrons.76 For example, hexa-capped body-centered cubic (bcc) silver clusters (X/Ag@Ag8(cube)@Ag6(octahedron)): [X@AgI14(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)12]Y (X = F−, Cl−, Br−; Y = OH−, BF4−)487,488 and [Ag15(Ntriphos)4(Cl4)](NO3)3 (Ntriphos = tris((diphenylphosphino)methyl)amine) with 8 free electrons.489 Interestingly, a homoleptic alkynyl-protected 2e Ag15 cluster [Ag@Ag14(C
CtBu)12]Y (X = F−, Cl−, Br−; Y = OH−, BF4−)487,488 and [Ag15(Ntriphos)4(Cl4)](NO3)3 (Ntriphos = tris((diphenylphosphino)methyl)amine) with 8 free electrons.489 Interestingly, a homoleptic alkynyl-protected 2e Ag15 cluster [Ag@Ag14(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)12](CH3O) with almost the same composition and structure as [X@AgI14(C
CtBu)12](CH3O) with almost the same composition and structure as [X@AgI14(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)12]+ was also successfully prepared, which exhibited high activity for the CO2 reduction reaction (FE(CO) = 95% at −0.6 V and TOFmax = 6.37 s−1).490 A series of similar alloy M15+ (Ag9Cu6, Au7Ag8, and Au2Ag8Cu5) is also available.491–493
CtBu)12]+ was also successfully prepared, which exhibited high activity for the CO2 reduction reaction (FE(CO) = 95% at −0.6 V and TOFmax = 6.37 s−1).490 A series of similar alloy M15+ (Ag9Cu6, Au7Ag8, and Au2Ag8Cu5) is also available.491–493
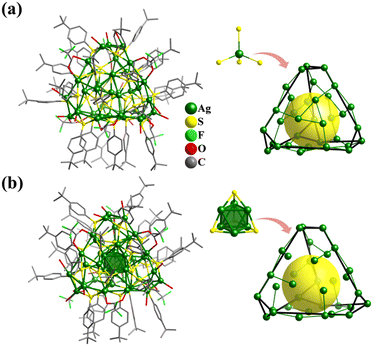 | ||
Fig. 50 Crystallographic structures of (a) [Ag37S4(SC6H4tBu)24(CF3COO)6(H2O)12]}3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 and (b) [Ag6@Ag36S4(SC6H4tBu)24(CF3COO)6(H2O)12]4+.495 494 and (b) [Ag6@Ag36S4(SC6H4tBu)24(CF3COO)6(H2O)12]4+.495 | ||
In 2020, Zhang prepared a series of silver clusters with various combinations of Platonic and/or Archimedean solid arrangements, induced by a flexible trifurcate metallo-ligand Ti(Sal/5-FSal)3 (SalH2 = salicylic acid, 5-FSalH2 = 5-fluorosalicylic acid): Ag8{Ti(Sal)3)}4 (Ag8Ti4, Ag4@Ag4@Ti4, Fig. 51a), H2Ag12(SiPr)6{Ti(Sal)3)}4 (Ag12Ti4, Ag12@Ti4, Fig. 51b), SO4@Ag22(SiPr)12{Ti(Sal)3)}4 (Ag22Ti4, Ag4@Ag6@Ag12@Ti4, Fig. 51c), (SO4)2@Ag36(SiPr)24{Ti(5-FSal)3)}4 (Ag36Ti4, Ag12@Ag12@Ag12@Ti4, Fig. 51d), and Ag42S4(SiPr)18(SO4)4{Ti(Sal)3)}4 (Ag42Ti4, Ag6@Ag24@Ag12@Ti4, Fig. 51e).483 The Ag/Ag–S clusters (number of metal atoms ranging from 8 to 42) are each capped by four metallo-ligands Ti(Sal/5-FSal)3, thereby forming supertetrahedra {Ti4} with different edge lengths (1–2 nm). These supertetrahedra {Ti4} further induce a tetrahedral geometry (approximate T symmetry) in the protected Ag/Ag–S aggregates. Owing to the chiral feature of the propeller-shaped metallo-ligand Ti(Sal/5-FSal)3, the Ag/Ag–S cores in these silver clusters exhibit distinctly distorted polyhedral structures. In Ag8Ti4, there is one tetrahedral Ag4 inside and 4 Ag atoms in the tetrahedral pattern outside (Fig. 51a). The Ag12 core in Ag12Ti4 forms a distorted cuboctahedron (Fig. 51b), often as nodes in Ag cluster-based assembled materials.497–499 In the limited space of {Ti(Sal)3)}4 in Ag22Ti4, a 22-metal truncated tetrahedral cage templated by SO42− exists. In this Archimedean solid (Ag12, truncated tetrahedron), two Platonic solids exist (Fig. 51c), an Ag6 octahedron (metal atoms located on edges of truncated tetrahedron Ag22) and an Ag4 tetrahedron (metal atoms from the center of four faces of Ag22). The larger SO42−-templated truncated tetrahedral metal cage in Ag36Ti4 is comprised of three Archimedean solids, two Ag12 truncated tetrahedra and one Ag12 cuboctahedron (Fig. 51d), similar to the outer Ag36 shell in Ag37S4 and Ag42S4 (Fig. 50). The largest silver cluster Ag42Ti4 in this system (Fig. 51e) is similar to Ag42S4 (Fig. 50b) in terms of metal number and core–shell (Ag6S4@Ag36) structure. Comparing the two 42-metal Ag–S structures, they basically have the same tetrahedral core capping an Ag6 octahedron, but different truncated tetrahedral Ag36 shells (Fig. 50b and Fig. 51e), which may have originated from the template effect of SO42− in Ag42Ti4. From the inside to the outside, the topologies of the 46-metal framework contain an inner Platonic solid (octahedron) and outer three Platonic and/or Archimedean solids (a truncated octahedron, truncated tetrahedron, and tetrahedron), abbreviated as Ag6@Ag24@Ag12@Ti4. Subsequently, Zang et al. achieved restricted growth from single Ag(I) atoms (Ag@Ti5) to all-carboxylate-protected superatomic Ag cluster (Ag6@Ti6) by controlling the size of titanium–organic cages.482
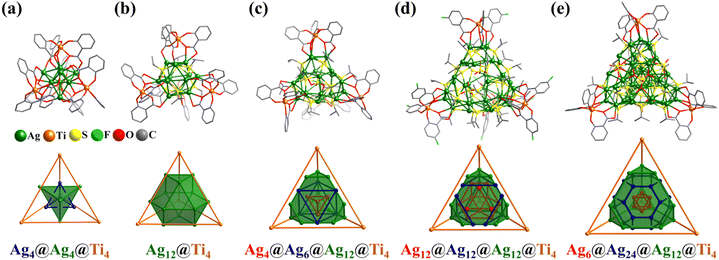 | ||
| Fig. 51 Crystallographic structures and metal arrangements of (a) Ag8{Ti(Sal)3)}4, (b) Ag12(SiPr)6{Ti(Sal)3)}42−, (c) SO4@Ag22(SiPr)12{Ti(Sal)3)}4, (d) Ag36(SiPr)24{Ti(5-FSal)3)}4, and (e) Ag42S4(SiPr)18(SO4)4{Ti(Sal)3)}4.483 | ||
Next is the AgI cluster with higher symmetry. In 2016, Zang and co-workers reported the preparation of a novel spherical AgI–S cluster, [Ag30(tBuS)20VV10VIV2O34] (V12@Ag30, Fig. 52a), featuring a dodecahedrane-like shell {Ag30(tBuS)20}10+ with approximately Oh symmetry (Fig. 52b), which tightly wraps an unusual C2h polyoxovanadate (POV) anion core {VV10VIV2O34}10−.500 In the Ag–S shell of V12@Ag30, each tBuS− ligand adopts the μ3-η1,η1,η1 coordination mode to cap an Ag triangle. Each AgI atom connects two neighboring S atoms to construct an almost linear S–Ag–S motif. Five vertex-sharing motifs form a quasi-regular pentagon, and then twelve vertex-sharing pentagons come together to constitute a Platonic polyhedron (regular dodecahedron, Fig. 52b). Twenty silver triangles capped by tBuS− ligands share Ag vertices to form a slightly distorted icosidodecahedron. It is worth noting that V12@Ag30 is the first reported spherical cluster featuring an AgI–organic dodecahedral nano-cage and an icosidodecahedra silver skeleton. In addition to the semiregular Archimedean icosidodecahedral metal arrangement found in single-shell silver clusters, a similar Ag30 metal framework was also observed in multi-shell silver clusters. In 2012, Mak's group constructed two high-nuclearity silver ethynide clusters, [Cl6Ag8@Ag30(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20(ClO4)12] (Ag38Cl6) and [Cl6Ag8@Ag30(chxC
C)20(ClO4)12] (Ag38Cl6) and [Cl6Ag8@Ag30(chxC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20(ClO4)10](ClO4)2 (chx = cyclohexyl), bearing the same novel central core Cl6Ag8 (octahedron@cube, like Cu6Ag8 in Fig. 47b) and a similar shell Ag30 (icosidodecahedra).501 In the synthesis of Ag38Cl6, a rhombohedral silver ethynide cluster [Cl@Ag14(tBuC
C)20(ClO4)10](ClO4)2 (chx = cyclohexyl), bearing the same novel central core Cl6Ag8 (octahedron@cube, like Cu6Ag8 in Fig. 47b) and a similar shell Ag30 (icosidodecahedra).501 In the synthesis of Ag38Cl6, a rhombohedral silver ethynide cluster [Cl@Ag14(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)12]OH487 was used as the Ag precursor to react with other silver salts (AgClO4) in CH2Cl2, resulting in a structural transformation and increase in nuclearity from 14 to 38. In 2017, Shen and Mizuta reported the preparation of an alkynyl-protected Pt-doped 8-electron Ag superatomic nanocluster, [PtAg42(C
C)12]OH487 was used as the Ag precursor to react with other silver salts (AgClO4) in CH2Cl2, resulting in a structural transformation and increase in nuclearity from 14 to 38. In 2017, Shen and Mizuta reported the preparation of an alkynyl-protected Pt-doped 8-electron Ag superatomic nanocluster, [PtAg42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4CH3)28](SbF6)6.502 In 2020, Zhang et al. synthesized and characterized a trifluoromethanesulfonate and tert-butylacetylene co-protected Ag43 nanocluster, Ag43(tBuC
CC6H4CH3)28](SbF6)6.502 In 2020, Zhang et al. synthesized and characterized a trifluoromethanesulfonate and tert-butylacetylene co-protected Ag43 nanocluster, Ag43(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)24(CF3SO3)8 (Ag43, Fig. 52c).503 The 43-metal kernel can be viewed as a concentric core–shell structure (Fig. 52d), which is abbreviated as Pt/Ag@Ag12(icosahedron)@Ag30(slightly distorted icosidodecahedron).
C)24(CF3SO3)8 (Ag43, Fig. 52c).503 The 43-metal kernel can be viewed as a concentric core–shell structure (Fig. 52d), which is abbreviated as Pt/Ag@Ag12(icosahedron)@Ag30(slightly distorted icosidodecahedron).
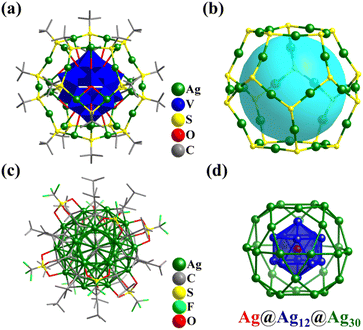 | ||
Fig. 52 (a) Crystallographic structure of [Ag30(tBuS)20V12O34] cluster. (b) Dodecahedrane-like shell {Ag30(tBuS)20}10+.500 (c and d) Crystallographic structure and concentric metal core–shell framework of Ag43(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)24(CF3SO3)8, respectively.503 C)24(CF3SO3)8, respectively.503 | ||
In 2015, Xie and co-workers reported the preparation of an MoO42−-templating high-nuclearity silver(I) cluster co-protected by phosphonate and thiolate ligands, [CH3OH2]6{MoO4@Ag12@(nBuPO3)8S6@Ag36(tBuS)24} (Ag48, Fig. 53), featuring a phosphonate-functionalized cubic silver(I) cluster core (Ag12@(nBuPO3)8S6, Fig. 53c) encapsulated by a silver(I)-thiolate shell with a truncated octahedral geometry (Ag36(tBuS)24, Fig. 53d).504 The template anion MoO42− in μ12-η3,η3,η3,η3 ligation mode is bound to its 12 neighboring silver atoms, resulting in the formation of an MoO42−-centered Ag12 core with slightly distorted cuboctahedron (Fig. 53b). In the Ag12 cuboctahedron, the six square faces are capped by six S2− ions in an octahedral fashion, and the eight triangular faces are stabilized by eight cubic arranged nBuPO32− ligands (Fig. 53c). The MoO42−-centered Ag12 core is further attached to the outer truncated v3-octahedron shaped silver(I)-thiolate shell by the S2− ions and the phosphonate ligands (Fig. 53d). Meanwhile, the S2− ions and the phosphonate ligands are located inside the center of the six square and eight hexagon faces of the truncated octahedron, respectively. Ag48 can be described as an arrangement of multiple concentric shells with polyhedral features, MoO4(tetrahedron)@Ag12(cuboctahedron)@(nBuPO3)8(cube)@S6(octahedron)@Ag36(tBuS)24(truncated octahedron). In 2017, this group again prepared an S2−-templated high-nuclearity silver(I) cluster co-protected by phosphonate, ethynide and thiolate ligands, {S@Ag12S6@Ag36(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)12(tBuS)12(nBuPO3)2(nBuPO3H)6}, possessing a nested polyhedron framework similar to Ag48.505
C)12(tBuS)12(nBuPO3)2(nBuPO3H)6}, possessing a nested polyhedron framework similar to Ag48.505
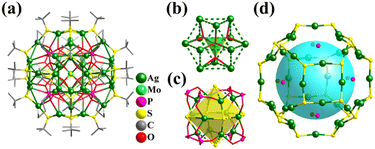 | ||
| Fig. 53 (a) Crystallographic structure of anionic cluster {MoO4@Ag12@(nBuPO3)8S6@Ag36(tBuS)24}6− (Ag48). (b) Cuboctahedral Ag12 core encapsulating an MoO42− anion as a template. (c) Phosphonate-functionalized cubic silver(I) cluster core, Ag12@(nBuPO3)8S6. (d) Truncated v3-octahedron-shaped silver(I)–thiolate shell, Ag36(tBuS)24 (without C atoms), where its eight hexagonal centers are occupied by nBuPO32− ligands (without C and O atoms).504 | ||
In 2020, Sun and co-workers reported the preparation of two giant globular AgI nanoclusters with a sodalite-type silver orthophosphate skeleton {(Ag3PO4)8}, [(PO4)8@Ag90S6(tBuS)24(PhPO3)12 (PhPO3H)6] (Ag90, Fig. 54a–c) in pseudo-Th symmetry506 and [(PO4)8@Ag102S6(CyS)30(H2PO4)6(HPO4)6(CF3COO)18] (Ag102, Cy = cyclohexyl, Fig. 54d–f) in D3h symmetry.507 The ball-shaped Ag90 (Fig. 54a) contains three concentric polyhedra (octahedron(Ag6, Oh)@truncated octahedron(Ag24, Oh)@rhombicosidodecahedron(Ag60, Ih)) with apparently incompatible symmetry (Oh and Ih, Fig. 54b). The arrangement of 90 metals is similar to that in the model devised by the mathematician John Conway called Kepler's Kosmos. The polyhedra of the inner (Ag6, octahedron) and middle (Ag24, truncated octahedron) shells have been observed in many structures. A rhombicosidodecahedron is rarely seen, where this polyhedral feature was previously observed in the third shell in Pd145 and (PtPd)165 (Fig. 40), mainly because its formation requires more atoms (60) and can only appear in huge complex nanoclusters or nanocages. This multi-shell structure (Ag6@Ag24@Ag60, Fig. 54b) with polyhedral features but distinctly different symmetries (symmetry raises from the inside out) can be attributed to the role of the small anions inside. These anions (six S2− and eight PO43−, Fig. 54c) are important templates, charge balancers and structural support materials. The S2− anions are located between the middle layer (Ag24) and the outermost layer (Ag60), presenting an octahedral arrangement (Fig. 54c). Six S2− play an important role in the formation and stabilization of 12 Ag4 quadrilaterals in the truncated octahedron and rhombicosidodecahedron (Fig. 54c). The PO43− tetrahedra in a cubic fashion penetrate the hexagonal Ag6 windows of the middle truncated octahedral layer, connecting the inner octahedron and the outer rhombicosidodecahedron (Fig. 54c). PO43− is essential for the formation of the hexagon of the truncated octahedron (Fig. 54c), which is observed in many truncated octahedral structures (Fig. 35 and 53). Ag102 (Fig. 54d) features an analogous multi-shell inner core of Ag6@Ag24@Ag60. Furthermore, six linear Ag2 staples in octahedral mode further surround this core (Fig. 54e). The difference in the outer shells of the structures in Ag90 and Ag102 comes from the difference in their protecting ligands (tBuS−, PhPO32−, and PhPO3H− in Ag90 and CyS−, H2PO4−, HPO42−, and CF3COO− in Ag102). Interestingly, the high symmetry of the overall structure makes the arrangement of peripheral ligands also have polyhedral characteristics, i.e., 12 PhPO32− with icosahedral arrangement and 6 PhPO3H− exhibiting an octahedral framework in Ag90 and 6 H2PO4− and 6 HPO42− with icosahedral mode in Ag102 (Fig. 54f).
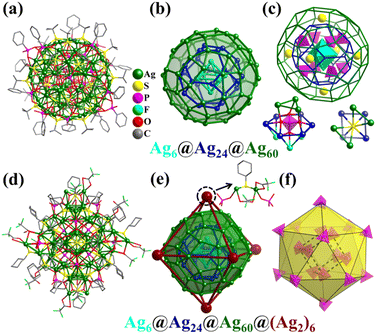 | ||
| Fig. 54 (a) Crystallographic structure of [(PO4)8@Ag90S6(tBuS)24(PhPO3)12(PhPO3H)6]. (b) Topologies of 90-metal framework of Ag90. (c) Distribution of eight PO43− and six S2− anions as templates in the cluster and their detailed coordination towards Ag atoms in different shells.506 (d) Crystallographic structure of [(PO4)8@Ag102S6(CyS)30(H2PO4)6(HPO4)6(CF3COO)18]. (e) 102 metal framework topologies of Ag102. (f) Distribution of eight PO43− anions as templates with cubic fashion and 12 HxPO4(3−x)− anions (6 H2PO4− and 6 (HPO4)62−) as peripheral protection ligands in icosahedral mode.507 | ||
In 2021, Sun's group prepared the largest and highly symmetric silver chalcogenide cluster reported thus far, (H3O)2[S22(SO4)36@Ag192(CyS)66(NO3)12]·4CH3OH (Ag192, Fig. 55a) with 6 silver (Ag6@Ag30@Ag12@Ag12@Ag60@Ag72) and 14 chalcogenide ((SO4)8((SO4)4@(SO4)4)@S6@S4@S12@(SO4)12@(SO4)12@(SO4)4@(CyS)12@(CyS)6@(CyS)24@(CyS)12@(CyS)12@(NO3)12) octahedral and tetrahedral shells, which are assigned to Platonic or Archimedean solids.508 The metal framework was analyzed first. From the inside out, the 192 silver atoms can be divided into several parts (Fig. 55b and c), as follows: v1-octahedron (turquoise), Ag6 (shell 1); v3-octahedron (blue, truncated octahedron@octahedron), Ag30 (Ag24@Ag6, shell 2); truncated tetrahedron (pink), Ag12 (shell 3); cuboctahedron or doubly truncated tetrahedron (dark blue),509 Ag12 (shell 4); spherical hollow cage (orange), Ag60 (shell 5); and four bowl-like subunits (Ag18, green) with tetrahedral arrangement, Ag72 (shell 6). Shell 5 containing 60 metal atoms can be subdivided into several non-standard Archimedean solids (Fig. 55c), i.e., two truncated octahedra with 24 (4,6,6) vertices and one cuboctahedron with 12 (3,4,3,4) vertices. In shell 6, the bowl-like subunit (Ag18) can be seen as a part of the rhombicosidodecahedron. The 72-Ag atoms can be split into three truncated octahedra with 24 (4,6,6) vertices (Fig. 55c). Next, the arrangement of a large number of anions (22 S2− and 36 SO42−) inside Ag192 is discussed. Six μ6-S2− (octahedral arrangement, Fig. 55d) and eight SO42− in cubic mode (penetrated in the center of the v3-triangle in shell 2, Fig. 55e) connect adjacent shells, forming an {Ag6@(Ag3SO4)8@S6} core, similar to {Ag6@(Ag3PO4)8@S6} in Ag90 and Ag102 (Fig. 54c). However, the difference in the position of the S2− atoms (on the quadrilateral face for Ag192 and outside the quadrilateral face for Ag90 and Ag102) and more anion intercalation make the arrangement of the outer silver atoms very different. The remaining 16 S2− anions are distributed between shell 2 (or 3) and shell 5 (Fig. 55d), of which four μ6-S2− templates with a tetrahedral arrangement connect the two triangles (from shell 3 and 5) and the other 12 (truncated tetrahedral arrangement) connect shells 2, 3, and 5. The remaining 28 SO42− anions are all in four Ag18 bowls (Fig. 55e) and function as glue to connect and consolidate the outer silver shells 2, 4, 5, and 6. 28 SO42− can also be arranged to form three Platonic or Archimedean solids, i.e., a truncated tetrahedron, cuboctahedron and tetrahedron (Fig. 55e). In addition, the distribution of the peripheral protective ligands (66 CyS− and 12 NO3−) also exhibits polyhedral characteristics (Fig. 55a), i.e., truncated tetrahedron (12 CyS−), octahedron (6 CyS−), truncated octahedron (24 CyS−), cuboctahedron (12 CyS−), truncated tetrahedron (12 CyS−), and cuboctahedron (12 NO3−).
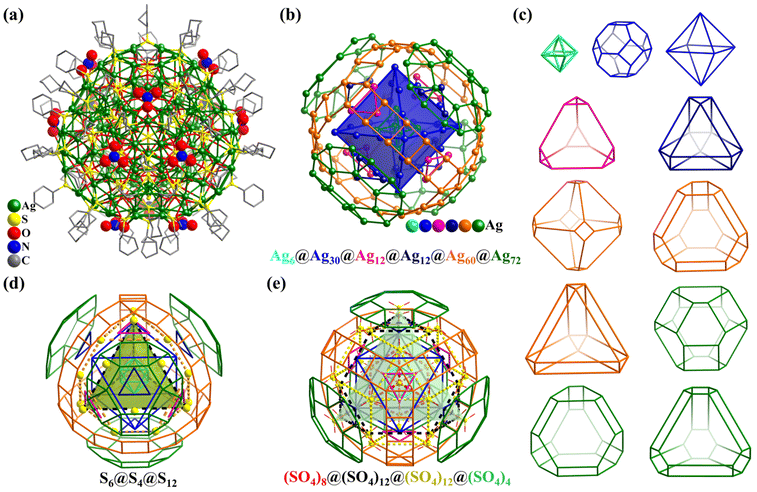 | ||
| Fig. 55 (a) Crystallographic structure of [S22(SO4)36@Ag192(CyS)66(NO3)12]2− (Ag192). (b) Six Ag shells with Td or Oh symmetry in Ag192. (c) Successive Platonic/Archimedean polyhedral description featuring only equivalent vertex metal atoms. (d) Distribution of 22 S2− anions in three shells. (e) Distribution of 36 SO42− anions in four shells.508 | ||
In addition to these silver clusters with inner cores, Sun et al. prepared an unprecedented buckyball-like Ag180 nanocage ([Ag180(iPrS)90(CH3SO3)44]·(CH3SO3)46·34CH3OH) with icosahedral (Ih) topology via the solvothermal reaction of [Ag(iPrS)]n and CH3SO3Ag in methanol.510 180 Ag atoms can be divided into two types of 4-valent ((3,4,5,4) or (3,4,6,4)) vertices to constitute 360 edges and 182 faces (sixty trigons, ninety tetragons, twelve pentagons, and twenty hexagons). Ag180 can be regarded as the product of the perfect combination of chemistry and geometric mathematics, which can be viewed as an assembly composed of 60 equivalent silver trigons or 12 equivalent pentagons and 20 equivalent hexagons. If the silver trigons are viewed as vertices, the topology (truncated icosahedron) will be similar to C60. Excitingly, a careful split reveals that Ag180 can be understood as a combination of two Archimedean solids (truncated icosahedron built by 12 silver pentagons and truncated icosidodecahedron constructed by 20 silver hexagons) with icosahedral (Ih) point-group symmetry.
In 2011, Huang et al. reported the first reductive AgI/0 cage-like cluster, [Ag34(CO3)12Cl4(dppm)12] (Ag34, dppm = 1,1′-bis(diphenylphosphine)-methane, Fig. 56a), with perfect Th symmetry.511 All the Ag atoms are connected by twelve CO32− anions originating from atmospheric CO2. The 34 Ag atoms of Ag34 can be divided into two parts (Ag10@Ag24, Fig. 56b), as follows: (i) the 24 AgI atoms on the surface construct a distorted truncated tetrahedral framework, in which four triangles are capped by μ3-Cl− ions and (ii) the ten AgI/0 atoms in the interior form an v2-tetrahedron core with an octahedral Ag6. In 2020, Xie and co-workers reported the preparation of an array of phosphine and oxometallate ligand co-protected superatomic Ag nanoclusters, Ag28(dppb)6(MoO4)4 (Ag28Mo4, dppb = 1,4-bis(diphenylphosphino)butane, Fig. 56c), Ag28(dppb)6(WO4)4 and Ag32(dppb)12(MoO4)4(NO3)4, which all feature a core–shell structure of Ag4@Ag24 (Fig. 56d) protected by four oxometallate ligands (MoO42− or WO42−) and six phosphine ligands (dppb).512 The Ag4@Ag24 metal skeleton of Ag28Mo4 can be described as an ideal ν1-tetrahedral Ag4 core capped on all its triangle faces by four ν2-triangular Ag6 fragments. Alternatively, the twenty-four-metal silver shell of Ag28Mo4 can also be viewed as the fusion of two distorted truncated tetrahedrons, including an inner Ag12 skeleton capped by four tetrahedral-arranged MoO42− anions, and an outer Ag12 framework, which is consolidated by peripheral phosphine ligands (Fig. 56d).
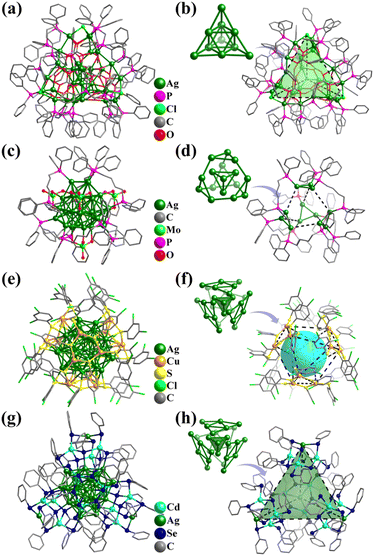 | ||
| Fig. 56 Crystallographic and core–shell structures of (a and b) [Ag34(CO3)12Cl4(dppm)12] with Ag10@Ag24,511 (c and d) Ag28(dppb)6(MoO4)4 with Ag4@Ag12@Ag12,512 (e and f) [Cu12Ag28(2,4-SPhCl2)24]4− with Ag4@Ag12@Ag12@Cu12,513 and (g and h) Cd12Ag32(SePh)36 with Ag4@Ag12@Ag12@Cd12@Ag4, respectively.514 | ||
A similar core–shell structure of Ag4@Ag24 was observed in the heterometallic [Cu12Ag28(2,4-SPhCl2)24]4− (Cu12Ag28, 2,4-SPhCl2 = 2,4-dichlorobenzenethiol, Fig. 56e and f)513 and Cd12Ag32(SePh)36 (Cd12Ag32, Fig. 56g and h)514 clusters. Different from Ag28Mo4 co-protected by phosphine and oxometallate ligands, the Ag4@Ag24 skeleton of Cu12Ag28 and Cd12Ag32 is capped by four Cu3(SPhCl2)6 (Cu12: distorted cuboctahedron, Fig. 56f) and Cd3Ag(SePh)9 (Cd12(distorted cuboctahedron)@Ag4(tetrahedron), Fig. 56h) fragments, respectively. Revisiting the geometry of the core–shell structure containing an Ag4@Ag24 skeleton (Fig. 56c–h), a series of distortions from the core to the shells lowers the point-group symmetry (from centrosymmetric Td to chiral T), turning them into chiral clusters. Due to the strong ion–pair interactions between the [Cu12Ag28(2,4-SPhCl2)24]4− anion clusters and counter-cations (TBA+ = tetrabutylammonium), chiral quaternary ammonium cations can be used to successfully separate the enantiomers.513 In 2021, Zhu and co-workers reported the preparation of a 44-metal racemic nanocluster with the same metal arrangement (Ag4@Ag12@Ag12@Cu12@Ag4) as Cd12Ag32, [Ag32Cu12(CH3COO)12(SAdm)12(P(CH3OPh)3)4] (HSAdm = 1-adamantanethiol).515 The deracemization process was achieved by ligand exchange (CH3COO− → 2-chloropropionic acid). The optical activity was conserved after further exchange (2-chloropropionic acid → Br−), to give two new optically active nanoclusters, R/S-[Ag28Cu16Br12(SAdm)12(P(CH3OPh)3)4] with Ag4@Ag12@Ag12@Cu12@Cu4 core.
Giant T-symmetric reducing Ag nanoclusters with a dense metal core (>50 metal atoms) have not been reported to date, whereas two larger ones were successfully constructed by introducing additional anionic templates. In 2022, Zang presented a nearly perfectly-symmetrical doubly truncated tetrahedral 70-Ag nanocluster with pseudo-Td symmetry, {Ag70S4(SiPr)24(CF3COO)20(DMF)3}2− (Ag70, Fig. 57a), containing a very rare geometric configuration among atomically-precise clusters.509,516 The core of this cluster consists of an unprecedented fcc-based (fcc = face centered cubic) Ag4@Ag12S4 with tetrahedron symmetry, while all-tetrahedral symmetries Ag4@Ag12S4@Ag12@Ag42 develop its multi-shells (Fig. 57b). Overall, high-symmetry makes the arrangement of atoms in Ag70 more regular and attractive, and the overall skeleton (including metal, anions, organic ligands) can be transformed into a combination of several Platonic or Archimedean solids (Fig. 57b), which is denoted as Ag4@Ag12@S4@Ag12@Ag6@Ag24@Ag12@(SiPr)12@(SiPr)12, i.e., from the inside to the outside, a tetrahedron (Ag4), truncated tetrahedron (Ag12), tetrahedron (S4), truncated tetrahedron (Ag12), octahedron (Ag6, located in the center of the rectangular face of outer Ag42 shell), truncated octahedron (Ag24, consisting of four six-membered rings from the Ag42 shell), cuboctahedron (or say doubly truncated tetrahedron, Ag12), truncated tetrahedron ((SiPr)12), and cuboctahedron ((SiPr)12).
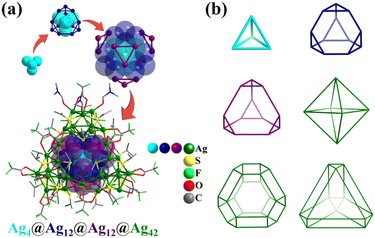 | ||
| Fig. 57 (a) Crystallographic structure of {Ag70S4(SiPr)24(CF3COO)20(DMF)3}2− (Ag70). (b) Successive Platonic/Archimedean polyhedral description featuring only equivalent vertex metal atoms.509 | ||
In 2021, Sun successfully isolated a reductive silver thiolate nanocluster, [Ag89S16(CyS)42(p-NH2PhAsO3)4](NO3)3 (Ag89, Fig. 58a), containing an icosahedral μ12-Ag@Ag12 kernel passivated by 4 tetrahedra {AgS4} and 4 ligands (p-NH2PhAsO32−), and further encapsulated by an outer Ag72 shell (Fig. 58b).517 Comparing the two pseudo-Td-symmetric reductive Ag–S nanoclusters (Ag70 and Ag89, Fig. 57 and 58), the interior (Ag4@Ag12@S4 in Ag70 and Ag@Ag12@(AgS4)4 in Ag89) expands due to the change in the templated anions (S2− in Ag70 and AgS47− in Ag89), and thus the outer shell also expands (Ag12@Ag42 in Ag70 and Ag24@Ag48 in Ag89). Similar to Ag70, the overall Ag–S skeleton can be transformed into a combination of multiple Platonic or Archimedean solids (Fig. 58c), denoted as Ag@Ag12@S12@Ag4@S4@Ag24@Ag12@Ag24@Ag12, including one icosahedron, two tetrahedra, three truncated tetrahedra, and two truncated octahedra.
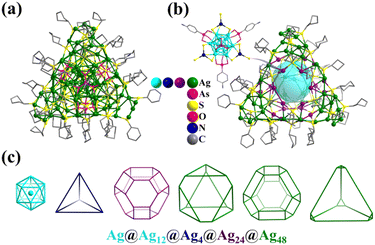 | ||
| Fig. 58 (a and b) Crystallographic and core–shell structure of [Ag89S16(CyS)42(p-NH2PhAsO3)4]3+, respectively. (c) Successive Platonic/Archimedean polyhedral description featuring only equivalent vertex metal atoms.517 | ||
In addition to the above-mentioned T-symmetric multi-shell structures, there is a striking family of polyhedral with octahedral (Oh) topology, i.e., box-shaped fcc Ag nanoclusters with the same vertex ([Ag(SR)3(PR′3)], as shown in Fig. 59).518–531 In 2013, Zheng and co-workers reported the preparation of a thiolate and phosphine co-protected Ag14 cluster containing two zero-valent Ag atoms, [Ag14(3,4-SPhF2)12(PPh3)8] (Ag14, 3,4-SPhF2 = 3,4-difluorothiophenol, Fig. 59a), exhibiting excellent yellow luminescence.518Ag14 is a neutral cluster possessing a nearly cubic shape cluster and it had two important structural features, significantly different from that of the reported thiolate-protected Au clusters, as follows: (i) all thiolate (SPhF2−) ligands bond to three Ag atoms, and thus no classical staple motifs are found in Ag14 and (ii) the metal core of Ag14 is an Ag64+ octahedron with two zero-valent Ag atoms, representing one of the basic structural fragments of single-crystalline face-centered-cubic (fcc) bulk metals (e.g., Au and Ag). The Ag64+ octahedral core is encapsulated by a cubic {Ag8(3,4-SPhF2)12(PPh3)8} cage, consisting of eight vertex-sharing [Ag(3,4-SPhF2)3(PPh3)] tetrahedra with a cubic arrangement (Fig. 59a). Alternatively, Ag14 can be described as an octa-capped octahedron, Ag6@Ag8. In 2018, Zang's group reported the preparation of an analogous Ag14 superatomic silver cluster, [Ag14(C2B10H10S2)6(CH3CN)8] (NC-1, C2B10H10S2 = 1,2-dithiolate-o-carborane).525 Given that NC-1 with labile CH3CN is unstable, monodentate pyridine ligands (such as pyridine (Py) and p-methylpyridine (p-MePy)) were used to site-specifically replace these solvent molecules, obtaining two new Ag14 clusters [Ag14(C2B10H10S2)6(Py/p-MePy)8] (NCs-2,3), which exhibited ultrastability up to 150 °C in air. Moreover, by introducing bidentate N-containing ligands to bridge the Ag14 building blocks, a hierarchical series of 1D-to-3D superatomic Ag cluster-assembled materials (SCAM-1,2,3,4) was successfully obtained, which exhibited tunable dual emission with wide-range thermochromism. Thereafter, a series of similar Ag14 (Ag6@Ag8 with octahedron@cube) superatomic clusters was prepared.519–524
 | ||
| Fig. 59 Crystallographic structures of cubic box-like Ag nanoclusters: (a) [Ag14(3,4-SPhF2)12(PPh3)8] (Ag14);518 (b) [Ag40(2,4-SPh(CH3)2)24(PPh3)8] (Ag40);529 (c) [Ag46(2,5-SPh(CH3)2)24(PPh3)8]2+ (Ag46);529 (d) [Ag63(3,4-SPhF2)36(PnBu3)8]+ (Ag63);524 (e) [Ag100(3,4-SPhF2)48(PPh3)8]− (Ag100);530 (f) outer eight [Ag(SR)3(PR′3)] tetrahedron motifs with cubic fashion; and (g) inner cores with nested structures: octahedron (Ag6 core in Ag14), cube@truncated octahedron (Ag8@Ag24 core in Ag40), octahedron@cube@truncated octahedron (Ag6@Ag8@Ag24 core in Ag46), v1-cuboctahedron@v2-cuboctahedron (Ag@Ag12@Ag42 core in Ag63), and v1-octahedron@v3-octahedron@rhombicuboctahedron (Ag6@Ag38@Ag48 core in Ag100), respectively. | ||
In 2018, utilizing a common mixed-ligand strategy, Zhu and co-workers synthesized a unique pair of new cube-shaped superatomic nanoclusters, [Ag40(2,4-SPh(CH3)2)24(PPh3)8] (Ag40, 2,4-SPh(CH3)2 = 2,4-dimethylbenzenethiolate, Fig. 59b) and [Ag46(2,5-SPh(CH3)2)24(PPh3)8]2+ (Ag46, 2,5-SPh(CH3)2 = 2,5-dimethylbenzenethiolate, Fig. 59c), representing two new members of the high-nuclearity cubic cluster family.529 The crystal structures of Ag40 and Ag46 show that the skeleton of both clusters have a common Ag32S24P8 protecting framework, in which a unique Ag24 shell with a truncated octahedron arrangement encapsulated by eight cubic-arranged tetrahedron units [Ag(SPh(CH3)2)3(PPh3)]. Differently, Ag40 has an unprecedented simple-cubic Ag8 core, whereas Ag46 has a common face-centered cubic Ag14 core. The metal skeleton of Ag40 and Ag46 can be described by the form of nested polyhedrons, abbreviated as Ag8(cube)@Ag24(truncated octahedron)@Ag8(cube) for Ag40 and Ag6(octahedron)@Ag8(cube)@Ag24(truncated octahedron)@Ag8(cube) for Ag46. Simultaneously, Pradeep reported two similar superatomic clusters, [Ag40(2,4-SPh(CH3)2)24(PPh3)8](NO3)2 and [Ag46(2,4-SPh(CH3)2)24(PPh3)8](NO3)2, co-crystallized in the same crystal.528 In 2019, Zheng and co-workers reported the preparation of a hydride-rich Ag–S–P nanocluster, [Ag40(2,4-SPh(CH3)2)24(PPh3)8H12]2+ (Ag40H12).526 Similarly, the metal framework of Ag40H12 consists of three concentric shells (abbreviated as Ag8@Ag24@Ag8). The presence of 12 hydrides (H−) in Ag40H12 was systematically identified using various characterization techniques and DFT calculations, with H− located outside the midpoints of the 12 edges of the inner Ag8 cube.
Through the optimization of experiments for the synthesis of Ag14, Zheng's group successfully prepared a pair of nearly perfect half-cubic-shape superatomic clusters ([Ag38(3,4-SPhF2)26(PR3)8], R = Ph or nBu) and a pair of larger nearly perfect cubic superatomic clusters ([Ag63(3,4-SPhF2)36(PR3)8]+X−, R = Ph, X = Br; or R = nBu, X = BPh4, denoted as Ag63, Fig. 59d) in 2017.524 The metal framework of Ag63 is formed by eight face-sharing fcc Ag14 units arranged to form a larger cube and made up of a v2-cuboctahedral Ag55 core and cubic [Ag(3,4-SPhF2)3(PR3)] (R = Ph or nBu) shell. Its Ag55 metal core consists of a central μ12-Ag atom surrounded by twelve Ag atoms with a cuboctahedral arrangement (Ag12), which in turn are encapsulated by 42 Ag atoms with a distorted v2-cuboctahedral fashion (Ag42). The v2-cuboctahedron Ag42 can be viewed as a combination of Ag6 (octahedron, made up of 6 Ag atoms from the centers of the six rectangles Ag9), Ag24 (rhombicuboctahedron, consisting of 8 Ag3 triangles from the centers of the eight v2-triangles Ag6) and Ag12 (cuboctahedron, constituted by 12 Ag atoms from vertices). Thus, the metal framework of Ag63 can be abbreviated as μ12-Ag@Ag12(v1-cuboctahedron)@Ag42(v2-cuboctahedron, Ag6(octahedron) + Ag24 (rhombicuboctahedron) + Ag12(cuboctahedron))@Ag8(cube). In 2020, Zhu and Yu reported the largest known fcc Ag nanocluster, [Ag100(3,4-SPhF2)48(PPh3)8] (Ag100, Fig. 59e).530Ag100 is the first all-octahedral symmetric four-layered nesting structure, similar to a Russian doll. From the inside to the outside, the topologies of the 100 Ag atoms contain an inner Platonic solid (v1-octahedron, Ag6) enveloped by outer three Platonic/Archimedean solids (v3-octahedron, Ag38; rhombicuboctahedron, Ag48; and cube, Ag8), abbreviated as Ag6@Ag38@Ag48@Ag8. The v3-octahedron Ag38 can be viewed as a combination of Ag8 (cube, made up of 8 Ag atoms from the centers of the eight v3-triangles Ag10), Ag24 (truncated octahedron, consisting of 8 edge-shared Ag5 triangles from the centers of the eight v3-triangles Ag10) and Ag6 (octahedron, constituted by 6 Ag atoms from vertices). The rhombicuboctahedron shell (Ag48) is made up of eight v2-triangles Ag6 with a cubic arrangement, which can be split into a truncated cube (Ag24) constituted by 8 Ag3 triangles from the centers of the eight v2-triangles Ag6 and a rhombicuboctahedron (Ag24). Interestingly, the surface ligands of all the fcc silver nanoclusters share similar structural features, in which the phosphines serve as terminal ligands to bridge the eight vertices of the polyhedron (Fig. 59f), while the thiolate ligands occupy the twelve edges and eight faces of half-cube or cube. The cubic nanoclusters with the same Ag8S24P8 surface but different cores (Fig. 59g) provide an ideal platform for the study of structure–property correlation.
Bigioni et al. reported an ultra-stable silver nanocluster with Ih-symmetrical Ag32 (icosahedron@dodecahedron, Ag12@Ag20) kernel (Ag44(p-MBA)304−, p-MBA = p-mercaptobenzoic acid, Fig. 60a; denoted as Ag44) produced by a simple synthetic protocol, which is a single size product without further size sorting.532 The purity, yield, and stability of Ag44 were substantially better than that of other gold or metal nanoparticles at that time, owing to its ultra-stable 32-silver-atom dodecahedral core (Fig. 60b), suitable silver-sulfur protective shell and unique electronic structure (closed-shell 18 free valence electrons with a large energy gap). Ag44 consists of a hollow icosahedron Ag12 encapsulated by a dodecahedron Ag22, and further stabilized by six {Ag2(p-MBA)5} units with an octahedral arrangement. During the same period, Zheng reported the preparation of similar structures (Ag44 and Au12Ag32).533 After extensive literature research, we found that numerous high-nuclearity silver clusters have similar icosahedral cores, but the outer metal shells of most of them do not have polyhedral features, such as Ag20,534 Ag21,535–537 Ag22,536 Ag25,538–541 Ag34,542 Ag35,543 Ag61,544 and Ag112.545 However, there are some examples of icosahedral cores encapsulated by shells with a regular polyhedral arrangement in Ag-containing clusters. It should be noted that 12 or 13-metal silver clusters with icosahedral configuration have not been successfully isolated to date.
 | ||
| Fig. 60 Crystallographic structure and core–shell framework of Ag44(p-MBA)304−.532 | ||
In 2017, Zhu and co-workers reported the preparation of a novel 8-electron Au-Ag alloy cluster, Au4Ag13(DPPM)3(2.5-SPhMe2)9 (Au4Ag13, DPPM = bis(diphenylphosphino)methane, Fig. 61a), exhibiting interesting crystallization-induced emission enhancement.546Au4Ag13 consists of four-Au-doped icosahedral Au4Ag9 kernel protected by three AgS2P and one AgS3 motif with a tetrahedral arrangement (Au@(AuAg)12@Ag4, Fig. 61b). In the same year, Liu and co-workers isolated the first atomically precise superatomic Ag nanoclusters stabilized by Se-donor ligands, [Ag20{Se2P(OiPr)2}12] and [Ag21{Se2P(OEt)2}12]+ (Ag21, Fig. 61c), through a common ligand-exchange strategy.547 Furthermore, the reaction of Ag21 with Au precursor resulted in the formation of a heteroatom-doped analogue [AuAg20{Se2P(OEt)2}12]+. The Ag skeleton of Ag21, possessing an idealized T symmetry, can be described as a μ12-Ag-centered icosahedron Ag13 inscribed in a large cube formed by eight Ag atoms (Ag@Ag12@Ag8, Fig. 61d).
 | ||
| Fig. 61 (a) Crystallographic structure of Au4Ag13(DPPM)3(2,5-SPhMe2)9. (b) Four-Au-doped icosahedral Au@Au3Ag9 kernel enveloped by outer Ag4 tetrahedron.546 (c) Crystallographic structure of [Ag21{Se2P(OEt)2}12]+. (d) Centered icosahedral Ag@Ag12 unit inscribed in an outer Ag8 cube.547 | ||
In 2015, Bakr's group reported the synthesis of an atomically precise 8-electron superatomic Ag nanocluster, [Ag29(BDT)12(TPP)4]3− (Ag29, BDT = 1,3-benzenedithiol and TPP = triphenylphosphine, Fig. 62a), featuring four tetrahedrally symmetrical sites formed by the supramolecular self-assembly of twelve wide footprint bidentate thiolate ligands (BDT) on an Ag13 core.548 The overall core–shell structure of Ag29 consists of a common center icosahedral 13-metal core inscribed in an exterior shell Ag16S24P4 (Fig. 62b). In the Ag16S24P4 shell, the remaining 16 Ag atoms can be divided into two categories, as follows: (i) 12 Ag atoms cap all 12 Ag atoms of the icosahedral core, leading to four silver triangles in a distorted tetrahedral arrangement and (ii) each of the additional four Ag atoms is protected and stabilized by one P atom and three S atoms, resulting in the formation of four Ag1S3P1 motifs in a tetrahedral fashion (Fig. 62b). Thus, the Ag16S24P4 shell is composed of four pairs of S atom-sharing Ag3S6 and Ag1S3P1 motifs, providing the complete passivation of Ag29. Alloy nanoclusters with the same structure have also been prepared, such as [AuAg28(BDT)12(TPP)4],549 [PtAg28(BDT)12(TPP)4],550 [Ag17Cu12(BDT)12(TPP)4], and [AuAg16Cu12(BDT)12(TPP)4].551 These M29 clusters have high stability and excellent optical and catalytic properties.
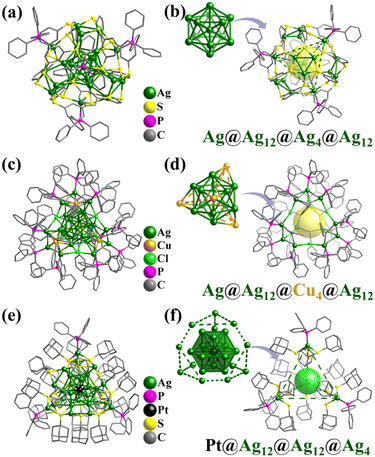 | ||
Fig. 62 Crystallographic structures and core–shell frameworks of (a and b) [Ag29(BDT)12(TPP)4]3−,548 (c and d) [Ag25Cu4(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)12(PPh3)12Cl6]11+,467 and (e and f) PtAg28(S-Adm)18(PPh3)4, respectively.552 C)12(PPh3)12Cl6]11+,467 and (e and f) PtAg28(S-Adm)18(PPh3)4, respectively.552 | ||
In 2021, Zheng reported the synthesis of a hydride-rich Ag–Cu nanocluster, [Ag25Cu4(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)12(PPh3)12Cl6H8]3+ (Ag25Cu4, Fig. 62c), using (PPh3)2CuBH4 as a new reducing agent.467Ag25Cu4 is made up of an icosahedral Ag13 core capped by a mixed Ag–Cu–Cl shell, exhibiting a tetra-stratified configuration (Fig. 62d), i.e., μ12-Ag(center)@Ag12(icosahedron)@Cu4(tetrahedron)@Cl4(tetrahedron)@Ag12(truncated tetrahedron). Ag25Cu4 exhibited high stability and no decomposition was observed after exposure to CH2Cl2 for 7 days by UV-Vis detection. In addition, Zhu reported a case of M29 nanocluster (PtAg28(S-Adm)18(PPh3)4, HS-Adm = 1-adamantanethiol, PtAg28, Fig. 62e) with different core–shell configurations in 2017.552 The 29 metal atoms of PtAg28 present a typical v3-supertetrahedral configuration, containing a tetra-stratified arrangement (Fig. 62f), i.e., μ12-Pt(center)@Ag12(cuboctahedron)@Ag12(truncated tetrahedron)@Ag4(tetrahedron). Since then, this research group conducted in-depth research on related scientific issues such as alloying and functionalization based on the M29 structure.553 It should be noted that these three types of M29 clusters have similarities, but are not identical (Fig. 62), and it can be seen that shell engineering (i.e., peripheral protection ligands) has a great influence on their structure.
C)12(PPh3)12Cl6H8]3+ (Ag25Cu4, Fig. 62c), using (PPh3)2CuBH4 as a new reducing agent.467Ag25Cu4 is made up of an icosahedral Ag13 core capped by a mixed Ag–Cu–Cl shell, exhibiting a tetra-stratified configuration (Fig. 62d), i.e., μ12-Ag(center)@Ag12(icosahedron)@Cu4(tetrahedron)@Cl4(tetrahedron)@Ag12(truncated tetrahedron). Ag25Cu4 exhibited high stability and no decomposition was observed after exposure to CH2Cl2 for 7 days by UV-Vis detection. In addition, Zhu reported a case of M29 nanocluster (PtAg28(S-Adm)18(PPh3)4, HS-Adm = 1-adamantanethiol, PtAg28, Fig. 62e) with different core–shell configurations in 2017.552 The 29 metal atoms of PtAg28 present a typical v3-supertetrahedral configuration, containing a tetra-stratified arrangement (Fig. 62f), i.e., μ12-Pt(center)@Ag12(cuboctahedron)@Ag12(truncated tetrahedron)@Ag4(tetrahedron). Since then, this research group conducted in-depth research on related scientific issues such as alloying and functionalization based on the M29 structure.553 It should be noted that these three types of M29 clusters have similarities, but are not identical (Fig. 62), and it can be seen that shell engineering (i.e., peripheral protection ligands) has a great influence on their structure.
In addition to being encapsulated in simple low-symmetric Platonic and Archimedean solids (such as tetrahedra (Fig. 61a and b), cubes (Fig. 61c and d), and truncated tetrahedra (Fig. 62)), Ag-centered icosahedra (Ag@Ag12) can also appear in high-symmetric dodecahedra (Ih). In 2021, Zang reported the preparation of a fullerene-like Ag nanocluster Ag33(C2B10H10S2)12 (Ag33, C2B10H10S2H2 = 9,12-dimercapto-1,2-closo-carborane, Fig. 63a) with an Ag@Ag12(icosahedron)@Ag20(dodecahedron) core–shell framework (Fig. 63b).554 Interestingly, continuous Cu-doping in the outer Ag20 dodecahedral shell first induced lower symmetry to generate an Ag@Ag12@Ag20−nCun (6 ≥ n ≥ 2) chiral analog, which shows clearly chiroptical activity, and then produced a highly symmetrical achiral cluster, Ag@Ag12@Ag20−nCun (20 ≥ n ≥ 13).
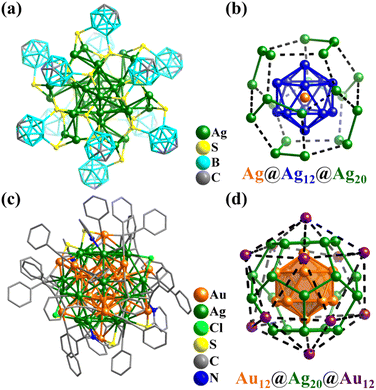 | ||
Fig. 63 (a) Crystallographic structure of Ag33(C2B10H10S2)12. (b) Tri-stratified configuration of Ag33: Ag(center)@Ag12(icosahedron)@Ag20(dodecahedron).554 (c) Crystallographic structure of Au24Ag20(2-SPy)4(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20Cl2. (d) Hollow concentric three-shell of Au24Ag20: Au12(icosahedron)@Ag20(dodecahedron)@Au12(icosahedron).555 C)20Cl2. (d) Hollow concentric three-shell of Au24Ag20: Au12(icosahedron)@Ag20(dodecahedron)@Au12(icosahedron).555 | ||
In 2015, Zheng and co-workers reported the synthesis of a bi-metallic 18-electron superatomic nanocluster, Au24Ag20(2-SPy)4(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20Cl2 (Au24Ag20, 2-SPy = 2-pyridylthiolate, Fig. 63c), and it was found for the first time that three different ligands (2-SPy−, PhC
C)20Cl2 (Au24Ag20, 2-SPy = 2-pyridylthiolate, Fig. 63c), and it was found for the first time that three different ligands (2-SPy−, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−, Cl−) coexist on the surface of Au24Ag20.555 The metal skeleton of Au24Ag20 can be described as a Keplerate (Fig. 63d), featuring a concentric three-shell Au12(icosahedron)@Ag20(dodecahedron)@Au12(icosahedron). Due to the synergy of multiple ligands, the outer metal–ligand shell has the characteristics of regular polyhedra (icosahedrons), which cannot be formed in 44-/45-metal clusters (Fig. 60) protected by single ligands.556
C−, Cl−) coexist on the surface of Au24Ag20.555 The metal skeleton of Au24Ag20 can be described as a Keplerate (Fig. 63d), featuring a concentric three-shell Au12(icosahedron)@Ag20(dodecahedron)@Au12(icosahedron). Due to the synergy of multiple ligands, the outer metal–ligand shell has the characteristics of regular polyhedra (icosahedrons), which cannot be formed in 44-/45-metal clusters (Fig. 60) protected by single ligands.556
In 2018, Zheng and co-workers reported the co-crystallization of two atomically precise, different-size metal aggregates in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. One is an atomically closed-shell intermetallic spherical nanoparticle (AuAg)267(2,4-SPhMe2)80 ((AuAg)267, Fig. 64a) and the other is a smaller electronically closed-shell trigonal-prismatic (AuAg)45(2,4-SPhMe2)27(PPh3)6 nanocluster.557 The larger (AuAg)267 has four notable features, as follows: (i) a concentric four-shell icosahedral structure of Ag@M12@M42@M92@Ag120(2,4-SPhMe2)80 (M = Au or Ag) with an inner-core M147v3-icosahedron (Fig. 64b–f); (ii) an open electron shell possessing 187 delocalized-electrons; (iii) fully metallic, plasmonic behavior; and (iv) zero HOMO–LUMO energy gap. The 147-metal inner-core M147 (M= Au or Ag) exhibits an unprecedented high-symmetry structure of three-layer Mackay icosahedron mode (Fig. 64c–e), encapsulated by a metal Ag120 shell in anti-Mackay configuration (Fig. 64f) and an outmost fullerene-like thiolate-ligand (2,4-SPhMe2)80 shell. The anti-Mackay fourth shell Ag120 consists of twenty v2-triangles Ag6 (Fig. 64g), sixty squares Ag4 (Fig. 64h), and twelve pentagons Ag5, and these polyhedra form a semi-regular polyhedron by sharing edges. The center of each Ag6 triangular and Ag4 square face is capped by a 2,4-SPhMe2− ligand. Interestingly, the 60 S atoms from the peripheral protective ligands can be arranged as a slightly distorted fullerene C60, and 12 additional S atoms are located at the center of twenty hexagons of the fullerene ball, building a dodecahedral framework.
1 ratio. One is an atomically closed-shell intermetallic spherical nanoparticle (AuAg)267(2,4-SPhMe2)80 ((AuAg)267, Fig. 64a) and the other is a smaller electronically closed-shell trigonal-prismatic (AuAg)45(2,4-SPhMe2)27(PPh3)6 nanocluster.557 The larger (AuAg)267 has four notable features, as follows: (i) a concentric four-shell icosahedral structure of Ag@M12@M42@M92@Ag120(2,4-SPhMe2)80 (M = Au or Ag) with an inner-core M147v3-icosahedron (Fig. 64b–f); (ii) an open electron shell possessing 187 delocalized-electrons; (iii) fully metallic, plasmonic behavior; and (iv) zero HOMO–LUMO energy gap. The 147-metal inner-core M147 (M= Au or Ag) exhibits an unprecedented high-symmetry structure of three-layer Mackay icosahedron mode (Fig. 64c–e), encapsulated by a metal Ag120 shell in anti-Mackay configuration (Fig. 64f) and an outmost fullerene-like thiolate-ligand (2,4-SPhMe2)80 shell. The anti-Mackay fourth shell Ag120 consists of twenty v2-triangles Ag6 (Fig. 64g), sixty squares Ag4 (Fig. 64h), and twelve pentagons Ag5, and these polyhedra form a semi-regular polyhedron by sharing edges. The center of each Ag6 triangular and Ag4 square face is capped by a 2,4-SPhMe2− ligand. Interestingly, the 60 S atoms from the peripheral protective ligands can be arranged as a slightly distorted fullerene C60, and 12 additional S atoms are located at the center of twenty hexagons of the fullerene ball, building a dodecahedral framework.
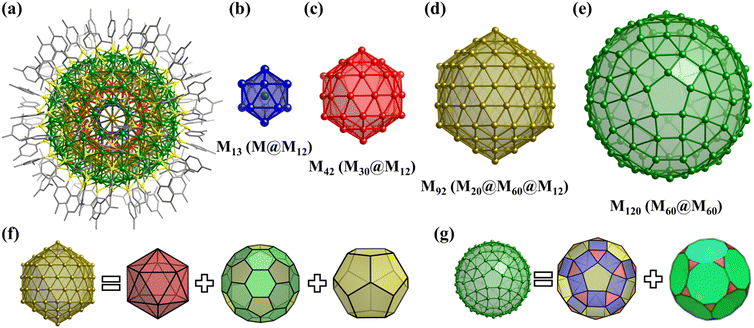 | ||
| Fig. 64 (a) Ball-and-stick representation of spherical (AuAg)267(2,4-SPhMe2)80. (b) First Mackay ν1-icosahedral (Au/Ag)12 shell with a central Ag atom. (c) Second Mackay ν2-icosahedral (Au/Ag)42 shell. (d) Third Mackay v3-icosahedral (Au/Ag)92 shell. (e) Fourth anti-Mackay Ag120 shell. (f) v3-icosahedron (Au/Ag)92 including three polyhedrons, where the metal atoms are only at the vertices (icosahedron, truncated icosahedron, and dodecahedron). (g) Anti-Mackay Ag120 with spherical geometry including two polyhedra (rhombicosidodecahedron and truncated dodecahedron).557 Color codes: Au/Ag, green, blue, red, and dark yellow; S, yellow; and C, gray. | ||
In the past few decades, two-layer M55 Mackay icosahedra have been found in a diversity of atomically precise nanosized metal clusters, characterized fully by X-ray crystallographic analysis. For example, two-shell clusters Pd55 (Fig. 40a) and Cu43Al12 (Fig. 49), three-shell cluster Pd145 (Fig. 40b), four-shell clusters (PtPd)165 (Fig. 40c); and some clusters (such as Au133,558,559 and Ag112545) without a complete regular polyhedral shell. In (AuAg)267, the next Mackay overlayer based on the M55 icosahedron is the first to be crystallographically observed, giving rise to three well-known complete Mackay metal shells of 147 atoms in contrast to the embryonic growth of decahedral (Au102,560 Au130,561 Au246,562 Ag112,545 Ag136,58 Ag307,563 and Ag37458) and fcc cuboctahedral Au146564 and Au27960 clusters possessing a partial irregular shell without polyhedral feature.
Among the numerous silver-containing metal carbonyl clusters, there are also examples of polyhedral features, such as [Ag16Ni24(CO)40]4− (Ag16Ni24, Fig. 38d–f). In 1992, Longoni's group reported the synthesis of a paramagnetic tetra-anion, [(μ12-Ag)Ag12{Fe(CO)4}8]4− (Ag13Fe8, Fig. 65), behaving as a 4-electron donor.565–567 The molecular structure of Ag13Fe8 consists of an (μ12-Ag)Ag12-centered cuboctahedron capped on eight triangular faces by Fe(CO)4 motifs, displaying C3v symmetry, and the eight Fe atoms form a slightly distorted cube. Also, its suggested molecular symmetry is Oh.
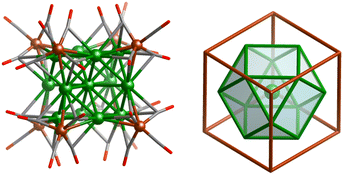 | ||
| Fig. 65 Ball-and-stick representation and metal framework of [(μ12-Ag)Ag12{Fe(CO)4}8]4− (Ag13Fe8).565 | ||
8.3 Au clusters
Although Cu, Ag and Au belong to the same group and their mono-value ions possess a similar valence-electron configuration, the structures of their corresponding clusters exhibit significant differences.12,15,77,421As mentioned above, cubic copper or silver clusters can be easily built through suitable ligands, but similar cubic gold clusters constructed with similar ligands have not be observed to date. Recently, Zheng and co-workers obtained a metastable nona-nuclear superatomic cubic Au cluster, [Au9(PPh3)8]+ (Fig. 66a), featuring a rare, nearly perfect body-centered cubic (bcc) metal core, capped by eight cubic-arranged phosphine ligands.87 Thereafter, Schnepf and Clayborne reported a 20-electron superatomic gold cluster Au20(tBu3P)8 (Au20, Fig. 66b) in the oxidation state ±0, resembling the Au bulk structure.88 The metal atoms of Au20 show a highly symmetric hollow cubic arrangement, Au12(cuboctahedron)@Au8(cube).
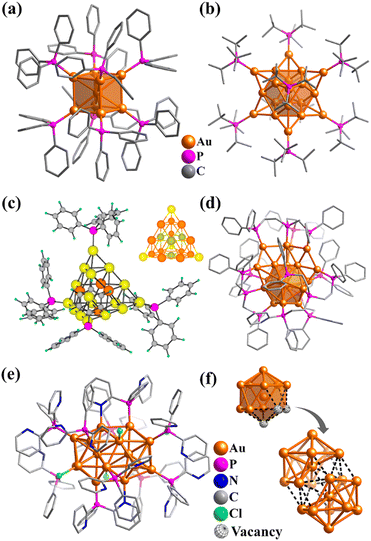 | ||
| Fig. 66 (a) Crystallographic structure of [Au9(PPh3)8]+ with cubic core Au@Au8.87 (b) Crystallographic structure of [Au20(tBu3P)8] with bigger cubic core Au12@Au8.88 (c) Computed structure of Au20(PPh3)4. Inset: Tetrahedral structure of the Au20− cluster.568,569 (d) Crystallographic structure of [Au20(P(P(CH2)2Ph2)3)4]4+ with distorted icosahedral unit Au@Au12.570 (e and f) Crystallographic structure of [Au20(PPhpy2)10Cl4]2+ with Au20 kernel (e), generated from the fusion of two tri-vacant icosahedral Au@Au9 subunits (f).571 | ||
The preparation and successful structure elucidation of Au20 remind people of the ligand-free Td-Au20 pyramidal cluster found by Wang et al. in 2003, which is highly stable in the gas phase (Fig. 66c).568 The geometries of the ligand-free metal clusters experimentally found in the gas phase were assigned by combining experimental data (such as ion-mobility spectrometry, trapped ion electron diffraction photoelectron spectroscopy, and infrared photodissociation spectroscopy), with related quantum chemical calculations. These structures have been extensively studied by M. M. Kappesm,572 L.-S. Wang,568,573,574 and others.19,575–577 Comparing the structure of the ligand-free systems with that of the corresponding ligand-protected atomically precise metal clusters will help shed light on the influence of ligands on the formation of cluster structures. This is clearly reflected in the efforts to synthesize Td-Au20 clusters in single crystal solids.568–571,573,578–581 To maintain the integrity of the pyramidal structure, phosphine ligands were selected to synthesize Td-Au20 (Fig. 66c).573 Wang synthesized a 20-metal cluster using tetraphosphine ligands (tris[2-(diphenylphosphino)ethyl]phosphine = P(P(CH2)2Ph2)3), [Au20(P(P(CH2)2Ph2)3)4]Cl4, with a distorted icosahedral kernel Au@Au12 and Au(Au2)3 motif (Fig. 66d);570 as well as a new Au22 nanocluster protected by diphosphine ligands (1,8-bis(diphenylphosphino)octane).579 Besides, Wang reported a phosphine-protected Au20 nanocluster (Fig. 66e) in which its core can be viewed as being generated from the fusion of two tri-vacant icosahedral Au@Au9 subunits (Fig. 66f).571 Unfortunately, the crystal structures of these Au20 cores did not reveal the expected pyramid feature, possibly reflecting that the introduction of protected ligands dramatically altered the electronic and geometrical structure of the metal clusters.
In the reported 12- or 13-nuclear gold clusters, a cuboctahedron arrangement similar to that of copper and silver clusters was found, such as [Ph4As]4[Au12S8]582 and [NaAu12Se8].583 More interestingly, an individual icosahedral metal framework was never found in copper and silver clusters, while the first atomically precise centered icosahedral gold cluster, [Au13(PMe2Ph)10Cl2](PF6)3 (Fig. 67a), was reported by Mingos et al. in 1981.584 Some 13-nuclear centered icosahedral gold clusters protected by different ligands have been reported.585 Commonly, Au13 icosahedron as part of the metal core often appears in larger metal clusters (such as Au18, Au20, Au25, Au36, Au37, Au38, Au55, and Au60), which is critical for cluster growth and formation.77,421 In 2013, Zheng and co-workers reported the preparation of a set of mixed-ligand-stabilized Au13Cux (x = 2, 4, and 8) bimetallic 8-electron superatomic nanoclusters, featuring centered icosahedral Au13 core and parts of triangle faces capped by 2, 4, or 8 Cu atoms.586 Specifically, the four Cu atoms of [Au13Cu4(PPh2Py)4(SC6H4-tert-C4H9)8]+ (Au13Cu4) exhibit a slightly distorted tetrahedral arrangement.
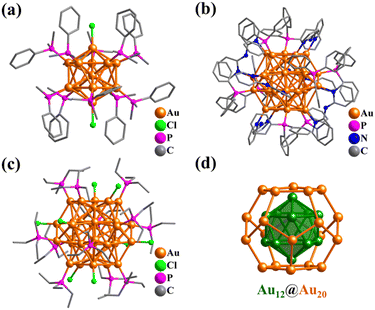 | ||
| Fig. 67 (a) Crystallographic structure of the cationic cluster [Au13(PMe2Ph)10Cl2]3+.584 (b) Crystallographic structure of [Au32(Ph3P)8(dpa)6]2+ cationic cluster.587 (c) Crystallographic structure of Au32(Et3P)12Cl8. (e) Au12@Au20 two-shell structure.588 | ||
In addition to these single-shell gold clusters with Platonic/Archimedean polyhedral features, multi-shell gold clusters with similar characteristics have been also prepared. Wang's group prepared a golden fullerene Au32 cluster employing a bi-ligand strategy, [Au32(Ph3P)8(dpa)6](SbF6)2 (Hdpa = 2,2′-dipyridylamine, Fig. 67b),587 consisting of a hollow Au12@Au20 Keplerate cage (two concentric Platonic solids: icosahedron@dodecahedron; Fig. 67d) co-protected by six amido and eight phosphine ligands, similar to the above-mentioned Ag33(C2B10H10S2)12 (Ag33, Fig. 62a). Interestingly, the arrangement of six amido (dpa) ligands can be viewed as an octahedron, while eight phosphine (Ph3P) ligands bridging at eight vertexes of the dodecahedral Au20 shell form a cubic coordination polyhedron. Simultaneously, Schnepf et al. reported the preparation of three 32-metal gold clusters with the same Au12@Au20 kernel but different protective ligands, Au32(R3P)12Cl8 (R = Et, Fig. 67c; nPr, nBu).588
The reduction of the Au precursor ((Ph3P)AuCl) with NaBH4 in the presence of a sulfur (S2−) source (HSC(SiMe3)3), resulting in the formation of one of the largest metalloid Au clusters, Au108S24(PPh3)16 (Au108, Fig. 68), was reported by Schnepf et al. In Au108 (Fig. 68a), the v3-octahedral Au44 (Au6@Au38(Au8@Au24@Au6), like Pt6Ni38 in Fig. 39) core is surrounded by a rhombicuboctahedron-shaped Au48S24 shell, where the “hollow window” of the Au48S24 shell (Au24(rhombicuboctahedron)@S24(rhombicuboctahedron)@Au24(truncated octahedron), Fig. 68c) is capped by 16 additional Au(PPh3) units (Au12(cuboctahedron)@Au4(tetrahedron), Fig. 68d), leading to the complete protection (Au64S24(PPh3)16 shell) of the Au44 core (Fig. 68b).
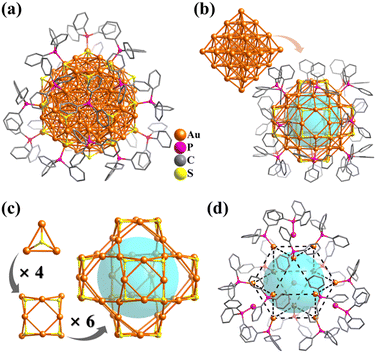 | ||
| Fig. 68 (a) Molecular structure of Au108S24(PPh3)16 (Au108). (b) v3-octahedron Au44 (Au6@Au38) core and spherical Au64S24(PPh3)16 shell. (c) Rhombicuboctahedron-shaped Au48S24 framework containing six Au8S4 motifs (assembled by four corner-sharing Au3S). (d) Outer PPh3-coordinated 16 Au atoms.86 | ||
In 2018, Wu and Jin et al. reported the preparation of an all-thiolate ligand-protected gold nanocluster Au144(SCH2Ph)60 (Fig. 69a),589 while Wang's group isolated an all alkynyl protected Au144(2-FC6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)60 nanocluster.590 Two 144-Au clusters exhibit the same spherical four-shell metal core architectures, abbreviated as Au12@Au42(Au30@Au12)@Au60@Au30. Taking Au144(SCH2Ph)60 as an example for structural analysis, the innermost shell (shell 1, Fig. 69b) is a hollow 12-atom Mackay v1-icosahedron, observed in thiolate-protected gold nanoclusters for the first time. Shell 1 is further enclosed by shell 2 of an Au42 Mackay v2-icosahedron (Fig. 69c), comprising a hollow complete two-shell Mackay icosahedron Au54 (Au12@Au42). The two-shell Mackay icosahedron is encapsulated by an Au60 rhombicosidodecahedral shell, also known as an anti-Mackay icosahedron (Fig. 69d). The three-shell metal kernel is wrapped by the outmost metal-ligand shell (Fig. 69f), consisting of thirty linear staple motifs (Fig. 69e). Also, 30 additional Au atoms in Shell 4 are arranged in an icosidodecahedral manner.
C)60 nanocluster.590 Two 144-Au clusters exhibit the same spherical four-shell metal core architectures, abbreviated as Au12@Au42(Au30@Au12)@Au60@Au30. Taking Au144(SCH2Ph)60 as an example for structural analysis, the innermost shell (shell 1, Fig. 69b) is a hollow 12-atom Mackay v1-icosahedron, observed in thiolate-protected gold nanoclusters for the first time. Shell 1 is further enclosed by shell 2 of an Au42 Mackay v2-icosahedron (Fig. 69c), comprising a hollow complete two-shell Mackay icosahedron Au54 (Au12@Au42). The two-shell Mackay icosahedron is encapsulated by an Au60 rhombicosidodecahedral shell, also known as an anti-Mackay icosahedron (Fig. 69d). The three-shell metal kernel is wrapped by the outmost metal-ligand shell (Fig. 69f), consisting of thirty linear staple motifs (Fig. 69e). Also, 30 additional Au atoms in Shell 4 are arranged in an icosidodecahedral manner.
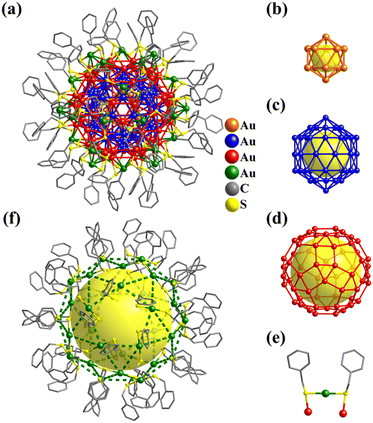 | ||
| Fig. 69 (a) Crystallographic structure of Au144(SCH2Ph)80. (b) Shell 1: hollow Au12 icosahedron. (c) Shell 2: Au42 Mackay v2-icosahedron (icosahedron + icosidodecahedron). (d) Shell 3: Au60 rhombicosidodecahedron. (e) S–Au–S linear staple motif. (f) Shell 4: icosidodecahedral arrangement of 30 Au atoms of linear S–Au–S staple motifs.589 | ||
9. Platonic and Archimedean solids in other metal clusters
Compared with transition metal clusters, the main group metal (Al,61,81,82,591,592 Ga,62,81,82,593 In,63 Sn,64,594 Pb,65 Sb,595,596 Bi,66,204–212etc.) clusters that meet the theme of this review are relatively scarce. Herein, we briefly summarize the aluminium (Al) clusters with polyhedral characteristics and some T-symmetry metal-chalcogenide clusters.9.1 Al clusters
In 1988, Werner Uhl reported the first bi-nuclear complex, [Al2{CH(SiMe3)2}4], containing Al–Al (metal–metal) bonding.597 Subsequently, Al clusters with metal–metal bonding have attracted widespread attention due to their unique structures.81,82,591 A series of Al clusters with Platonic polyhedral characteristics has been reported, such as Al4 tetrahedra,598–600 Al6 octahedra,593,601,602 Al8 cubes (Fig. 70a),603–609 and Al12 icosahedra (Fig. 70b).610,611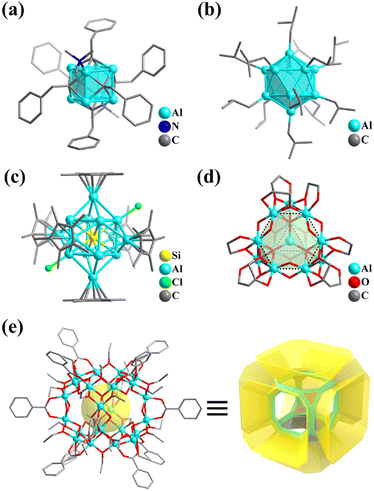 | ||
| Fig. 70 (a) Cubic Al8 cluster, [(AlH)6(AlNMe3)2(CCH2Ph)6].603 (b) Icosahedral Al12 cluster, [Al12iBu12]2−.610 (c) Face-centered cubic Al14 cluster, Si@Al8(AlCp*)6.612 (d) ε-Keggin-type Al13 cluster, [AlO4Al(OH)12(OC2H4OH)12], with a truncated tetrahedral metal skeleton.613 (e) Crystallographic structure of cationic core–shell Al24 cluster, [Al24(OH)32(PhCOO)12(EtO)24]4+, with inorganic truncated cubic Al-hydroxy core and calixarene-like organic shell.614 | ||
Great advancements in giant Al clusters were reported by Schnöckel and co-workers. Their works on core–shell structures that satisfy the subject of this review are listed here, including Al14 (Si@Al8@Al6, Fig. 70c),612,615 fullerene-like Al38(AlCp*)12 (Al50),616 Si@Al56[N(2,6-iPr2C6H3)SiMe3]12 (Al56, Fig. 71a)592 and [Al77(N(SiMe3)2)20]2−. Al50 has an irregular Al8 core and a slightly distorted icosidodecahedral Al30 shell, where 12 pentagons on the shell are covered by 12 AlCp* units with a regular icosahedral arrangement, similar to the peripheral framework of Cu43Al12 (Fig. 49).
 | ||
| Fig. 71 (a) Ball-and-stick representation of Si@Al56[N(2,6-iPr2C6H3)SiMe3]12. (b) Si@Al32 core (Si@Al4@Al4@Al24) enveloped in the {Al6[N(2,6-iPr2C6H3)SiMe3]3}4 (Al12(truncated tetrahedron)@Al12(cuboctahedron)) shell.592 | ||
In the center of the Al56 cluster (Fig. 71a), a μ8-silicon atom is surrounded by 8 Al atoms, forming two distinct Al4 tetrahedra (Fig. 71b). Its core is surrounded by a truncated octahedral shell containing 24 Al atoms. These 33 atoms (1 Si + 32Al) are all members of the kernel (Si@Al4@Al4@Al24, Fig. 71b). Four no-adjacent pentagons of the Al24 truncated octahedron are capped by four triangles {Al6[N(2,6-iPr2C6H3)SiMe3]3} in a tetrahedral manner (Fig. 71b). The four Al3 triangles in the center of {Al6[N(2,6-iPr2C6H3)SiMe3]3} can form a truncated tetrahedron, while 12 additional periphery Al atoms bound by organic ligands can construct a distorted cuboctahedron (Fig. 71b).
In addition to metal clusters with metal–metal bonding, the synthesis and properties of Al–O clusters are also flourishing.617–619 Since the discovery of the first polyoxyaluminum cluster, Al13, a series of Al–O clusters has been produced via the hydrolysis of Al salt, which is widely used in catalysis, water treatment and other fields.591 There are five isomers (α, β, γ, δ and ε) in Keggin Al13 clusters.591,620 In 2018, He and Sun et al. reported the preparation of a glycol-coordinated ε-Keggin-type Al13 cluster, [AlO4Al(OH)12(OC2H4OH)12]Cl7 (Fig. 70d).613
In recent years, Zhang and Fang's team has made a series of groundbreaking advances in the study of Al-oxo clusters, including the synthesis high-nuclearity Al–O clusters, Al24 (Fig. 70e)614 and Al32618 and the discovery, size expansion, supramolecular assembly and the application of iodine capture of wheel-like clusters.617,619,621–623 Recently, this group reported the preparation of a series of water-stable porous Al24 crystalline materials ([Al24(OH)32(PhCOO)12(EtO)24]4+, Fig. 70e), which remove trace iodine from I2/KI aqueous solution effectively (down to 4 ppm concentration). The structure of Al24 shows an inorganic and organic “core–shell” feature and two-tier cavity arrangement. The inorganic “core” Al24(OH)32 is a rare Archimedean solid (truncated cube), consisting of eight Al3 trigons and six Al8 octagons. The organic “shell” is a large calixarene-like annular cavity with 6 face centers. Although there are reports of 24 metal-centered truncated cube-like metal–organic cages,624–631 this is the first metal-oxo cluster possessing a pure inorganic metal framework.
9.2 Supertetrahedrons in metal chalcogenides
Among the clusters with polyhedral features introduced above, tetrahedral or supertetrahedral clusters (M4, M10, and M20) appear frequently, which exist in various metal clusters.632–640 They can all be represented by Tn, where T represents a tetrahedron and n is the number of metal layers in the cluster or the number of {ME4} (E = group 16 element, O, S and Se) tetrahedra along each edge.445,632–650 The main-group metal (Ga, In, Ge, and Sn) chalcogenides and their heterometallic doped counterparts based on supertetrahedral clusters have been widely explored due to their interesting properties, such as ion exchange and fast-ion conductivity.635 The supertetrahedral T6 clusters are the largest reported in this category.651 A T1 unit is a single tetrahedron like InS45−, whilst the T2 cluster possesses an adamantane-like core, whose metal corners are capped by four non-metal atoms (Fig. 72a).641 Alternatively, Tn (n = 3–6) can be described as the self-assembly of corner-sharing tetrahedra (Fig. 72a–d, T2: 4 {ME4};641 T3: 10 {ME4};642,647,648,652 T4: 20 {ME4};445 T5: 35 {ME4}643,644,648 and T6: 56 {ME4}651). These supertetrahedral clusters can be assembled into a network structure or larger clusters (Fig. 72e).645 | ||
| Fig. 72 (a) T2 supertetrahedron, In4S108−.641 (b) T3 supertetrahedron, In10S2010−.642 (c) T4 supertetrahedron, Mn4In16S3514−.643 (d) T5 supertetrahedron, Cu5In30S54Cl215−.644 (e) Cd16In64S13444− supertetrahedron with a large cavity.645 | ||
10. Synthesis and structural prediction of giant ‘star’ clusters with Platonic and Archimedean solids
10.1 Summary and consideration for the synthesis of giant ‘star’ clusters
Most of the highly symmetrical metal clusters described in this review were prepared via wet chemistry and their synthetic methods vary widely due to the variety of different types of metal clusters involved (I: metal clusters with metal–anionic cores; II: metal clusters with metal cores (with metal–metal binding); III: metal clusters with metal–anionic cores and metal–metal bonds). Thus, the deliberation and selection of the organic/inorganic ligands, metal sources, and reaction conditions (metal ion/ligand ratio, pH, solvents, anionic templates, reducing agents, etc.) play crucial roles in the synthesis of metal clusters. Due to the unpredictability of the inner metal core, based on the relevant typical cases mentioned in this review, here we mainly discuss the effect of ligands and anionic templates on the construction of high-symmetry metal clusters with Platonic and Archimedean solid features. In this section, we attempt to summarize the common structural features and sources of high symmetry in metal clusters, especially high-nuclearity metal clusters with core–shell structures. It should be noted that we will not discuss the synthesis of SBB-based multimeric structures here, mainly giant inorganic POMs.The formation of metal clusters (especially high-nuclearity) involves the organization of a large number of disordered metal ions into a complex aggregate in an ordered manner. Undoubtedly, a suitable shell-ligand is crucial, or even the most important, for the construction of high-symmetry metal clusters with a metal core or metal–anionic core. This directly affects the arrangement of the outermost metal atoms. In many cases where the metal cores exhibit polyhedral characteristics, the framework of the outermost metal atoms loses its regularity and symmetry due to the surface ligands (such as Ag44 (Fig. 60), and Au279(SPhtBu)84 with a cuboctahedron Au147 kernel in an fcc arrangement (Au@Au12(v1-cuboctahedron)@Au42(v2-cuboctahedron)@Au92(v3-cuboctahedron)), and irregular Au132(SPhtBu)84 shell). The ligands (mainly organic) distributed on the surface of the high-nuclearity metal clusters listed in the text are mainly divided into two categories, as follows:
(1) Simple or highly symmetric ligands with little or no geometrical restrictions in the inner metal cores or metal–anionic cores, e.g., X− (X = F (Fe13, Fig. 32a), Cl (Pu38, Fig. 24a), Br (Fe34, Fig. 32), I (super-octahedron Pb18I448−)65); E2− (E = O, S or Se); CO (metal carbonyl clusters, such as Ni32C6 (Fig. 37), Ag16Ni24 (Fig. 38), Pt14Ni24, Pt6Ni38 (Fig. 39), Pd55, Pd145, (PtPd)165 (Fig. 40), Ag13Fe8 (Fig. 65)); H2O (Mo64Ni8Ln6 (Fig. 10)); RO− (R groups mainly are simple alkyl, such as –CH3 (Mo24 (Fig. 8e), Zr13, Hf13 (Fig. 26)), –CH2CH3 (Fe19 (Fig. 32)), and –CH(CH3)2 (Ti42, Fig. 25)); R′COO−, where the R′ groups can be simple (–H, Fe64; –CH3 (Nd104 (Fig. 43), Ln24Zn4 (Fig. 44), Gd30Co12) or phenyl moieties; R′′SO3−/R′′PO32−/R′′AsO32− inducing the formation of a regular M3 triangle (Ce12V6 (Fig. 6e), Mn13 (Fig. 27)) or M6 hexagon motifs (Cl6@V24As8 (Fig. 7), Ag89 (Fig. 58)); cyclopentadiene and its derivatives (Cp* in Cu43Al12 (Fig. 49), Al14 (Fig. 70)); pyridine (Fe17, Fe34 (Fig. 32); Ce38) and its derivatives (pdol2− in Mn26 (Fig. 28b)); simple polycarboxylic acid (such as IDA2− in La20Ni30 (Fig. 45) and Gd96Ni64, MIDA2− in Gd158Co38Cl12 (Fig. 46)), polyol ligands (such as triethanolamine in Mn20 (Fig. 28a) and Cu17Mn28 (Fig. 31a); 2-(hydroxymethyl)phenol in Mn49 (Fig. 30)), and oxygen-rich ligands containing carboxylic acids and hydroxyls (dmp− in Gd60 (Fig. 42)); simple thiolate (iPrS− (AgxTi4 (x = 12, 24, 36, 42, Fig. 51), Ag70 (Fig. 57)), tBuS− (V12@Ag30, Fig. 52; Ag48, Fig. 53; Ag90, Fig. 54), CyS− (Ag192, Fig. 55; Ag89, Fig. 58), thiophenol and its derivatives (tBuPhS−: Ag37S4, Ag42S4 in Fig. 50; 2,4-SPhMe2: Ag40 in Fig. 59b, (AuAg)267 in Fig. 64; 3,4-SPhF2: Ag14, Ag63 and Ag100 in Fig. 59); phosphine (PEt3 in Ni9Te6P8 (Fig. 36a); PtBu3 in Au20 (Fig. 66); PPh3 in Ag29 (Fig. 62), Agx (x = 14, 40, 46, 63, 100, Fig. 59), Au9 (Fig. 66), Au108 (Fig. 68)); and alkynyl ligands (tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− in Ag38Cl6 and Ag43 (Fig. 52c)).
C− in Ag38Cl6 and Ag43 (Fig. 52c)).
(2) Ligands inducing specific structures. For example, the linear-like multidentate organic ligand MebptpO2− with seven coordination atoms on the same side in Ln20 (Fig. 41), thiacalix[4]arene and its derivatives (p-tert-butylthiacalix[4]arene (H4TCA), inducing the formation of regular M4 square modules in Co24, Co32 (Fig. 35) and other giant clusters).336
In addition to shell-ligands, the important role of the anions in the formation, passivation and protection of the metal core cannot be ignored.76 According to most of the examples in this review, it is not difficult to find that anions as templates exhibit unique advantages in the control of the structure of highly symmetrical nanoclusters and the preparation of giant metal nanoclusters.
The anion templates can be roughly divided into four categories according to their shape including simple spherical (like halogen anions, C4−, Si4−, S2−, and Se2−), triangular (NO3− and CO32−), tetrahedral-shaped (ClO4−, SO42−, CrO42−, MoO42−, WO42−, PO43−, AsO43−, SiO44−, etc.) and complex POM anions ({V12O34}10− in V12@Ag30 (Fig. 52)).653 The role of anions is mainly reflected in three aspects, as follows: (1) anions as structure-directing agents can influence and control the shape and size of the structures, especially in small clusters (α-Keggin POMs (Fig. 5), Cl@Cu6Ag8 (Fig. 47b)) and POM-templated high-nuclear Ag clusters.653 (2) Anions can effectively stabilize the metal core and balance the local positive charges from the metal cations. (3) Anions with intrinsic properties can improve or endow cluster functionality.
The progress over the past few decades has witnessed the transition from the initial single anion template strategy for the preparation of small metal clusters (XO4-templated α-Keggin POMs, Cl@Cu6Ag8 (Fig. 47b), Cl@Ag14, SO4-templated Co20-1 (Fig. 34), etc.) to the now multiple homo- (e.g., 6Cl− in Cl6@V24As8 (Fig. 7) and Mn13 (Fig. 27); 12Cl− in Gd50 (Fig. 42); 4S2− in Ag37S4, Ag42S4 (Fig. 50), and Ag70 (Fig. 57); 16S2− in Ag89 (Fig. 58); 6C4− in Ni32C6 (Fig. 37), etc.) or hetero-anion templates (e.g., 12Cl− + 90CO32− in Gd158Co38Cl12 (Fig. 46), MoO42− + 6S2− in Ag48 (Fig. 53), 8PO43− + 6S2− in Ag90 (Fig. 54), and 22SO42− + 36S2− in Ag192 (Fig. 55), etc.) approach to achieve the preparation of high-nuclearity metal clusters. Here, we mainly discuss the role of the anions in the construction of regular metallic polygons and polyhedrons in local space (Fig. 73), and the size expansion of metal-oxo/hydroxide/chalcogenide clusters. In the introduction of Ln (Fig. 42) and Ag clusters (Fig. 54), the construction of topological polygons and polyhedra based on anionic templates is briefly discussed. The polygons and polyhedra constructed with different shapes of anions (sphere, triangle, and tetrahedron) as templates (Fig. 73) are summarized in detail here. With the exception of octagons and decagons, almost all the faces (trigon, square, pentagon, and hexagon) used to form a Platonic or Archimedean solid are covered here (Table 1). In addition to the polygons directly induced by anions or surface ligands, more are formed during the assembly of multiple small modules in high-nuclearity metal clusters.
 | ||
| Fig. 73 Polygonal motifs (trigon, square, pentagon, and hexagon) or polyhedral modules (tetrahedron, octahedron, cube, and cuboctahedron) constructed from different types of anion templates. Initial examples: O@Mo3, Mo60; O@Co4, [Co8(μ4-O)(8-hydroxyquinoline)12]2+;320 O@Nd4, Nd104; O@V6, V6Rh4; Cl@V4, Cl6@V24As8; Cl@Gd5, Gd50; S@Ag6, Ag192; S@Ag8, Ag90; Si@Al8, Al14; CO3@Gd5, Gd20; CO3@Gd6, Gd60; SO4@Ce3, Ce12V6; MoO4@Co6, Co24; SO4@Ag9, Ag192; SO4@Ag12, Ag192; and SO4@Ag12, Ag90. | ||
Notably, most of the anion-templated metal-containing clusters surveyed in this review are kinetic products rather than thermodynamic ones, and the incorporation of anions in many cases is inadvertently an accidental result rather than rationally designed.654–656 In recent years, with some exploration on the role of various anion templates in directing the multi-step assembly process of high-nuclearity metal clusters, the guiding role of the homo-/hetero-anions in the “dynamic” process becomes clearer, which is not limited to the somewhat “static” role for directing structure or supporting construction.387,393,394,501,516,657 Recently, the study by Zheng and Feng provided a rare groundbreaking example, i.e., how to generate a large target–lanthanide hydroxide cluster (Ln60 cluster with an individual sodalite cage structure, Fig. 42d) through hetero-anion-guided rational stepwise assembly (Fig. 74).393 A reasonable “dynamic” route starting from the Er(ClO4)3 metal salt to Er60 ([Er60(μ6-CO3)8(μ4-I)7(μ2-NO3)12(μ3-OH)96(His)24(H2O)36](ClO4)5I4-(NO3)16, H-His = L-histidine) was outlined (Fig. 74a) by capturing three key intermediates (Er12 ([Er12(μ4-I)2(μ3-OH)16(His)8(H2O)20](ClO4)10), Er34 ([Er34(μ6-CO3)2(μ5-CO3)2(μ4-I)2(μ3-OH)48(His)20(H-His)8(H2O)24](ClO4)15I9), and Er48 ([Er48(μ6-CO3)4(μ5-CO3)3(μ4-I)4(μ2-NO3)3(μ3-OH)72(His)24(H-His)6(H2O)24](ClO4)11I6(NO3)10) with the guidance of I−, I−/CO32−, I−/CO32−/NO3−, respectively, Fig. 74b) in this process. The simplified geometries based on the cubane-like [Er4(μ3-OH)4] subunits of the lower-degree Er12, Er34, and Er48 represent 1/6, 1/2, and 3/4 of the complete sodalite solid of Er60, respectively.
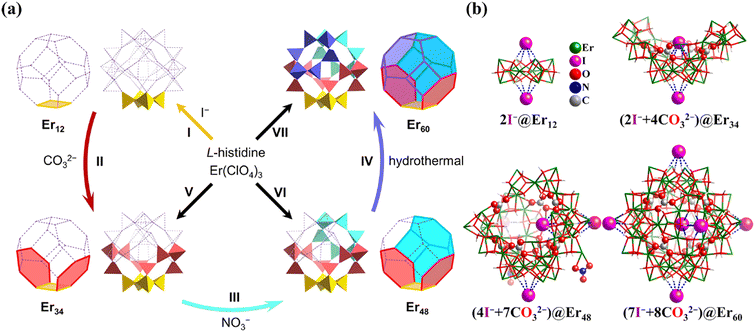 | ||
| Fig. 74 (a) Synthetic strategies (stepwise assembly (I → II → III → IV) and direct synthesis (I, V, VI, VII)) of the target Er60 cluster and three intermediates (Er12, Er34 and Er48). (b) Positions of anion templates (I− and CO32−) in the lanthanide hydroxide Lnn(μ3-OH)m frameworks of Er12, Er34, Er48 and Er60.393 | ||
In addition to the structural template effect, the strategy of intercalating a large number of anions has shown increasing advantages in the construction of high-nuclearity clusters. The typical examples include the largest atomically precise metal cluster [Ag490S188(SC5H11)114]59 and the largest 3d–4f heterometallic cluster Gd158Co38Cl12 (Fig. 46). The progress of the rich structural variety of Cu and Ag chalcogenide clusters was reviewed by Fenske,11 and the preparation of clusters from polynuclear to nanosized molecules consisting of hundreds of metal atoms can be achieved with the introduction of S2− or Se2− anions.
Furthermore, two representative examples are given to introduce the role of anions in the structural expansion (Fig. 75). Gd20, La20Ni30 and Gd158Co38Cl12 have similar Ln20(μ5-NO3/CO3)12 kernels, while the number of metal atoms and size of their clusters differ greatly (Fig. 75a–c). In contrast to Gd20 (Fig. 75a), the expansion (20 → 50) of La20Ni30 (Fig. 75b) originates from the introduction of Ni-IDA metallo-ligands (marked in blue). Gd158Co38Cl12 with nuclearity of 196 (Fig. 75c) is a perfect product of the ‘metallo-ligand-controlled hydrolysis’ and ‘multi-anions-template’ strategies. The chloride ions outside the Gd20(μ5-CO3)12 kernel construct 12 anion (OH− and CO32−)-rich cavities, which greatly balance the charges from the GdIII cations and grow a Gd–OH–CO3 layer outward (marked in sky blue, Fig. 75c). Meanwhile, the metallo-ligands (Co-MIDA and Gd-MIDA) well-stabilize the total cluster structure. Ag48, Ag90, and Ag192 demonstrate the role of anions in the core expansion and epitaxial growth of Ag clusters (Fig. 75d–f), respectively. With the same octahedral arrangement of six S2− anions, the expansion of the core and increase in the size of the peripheral silver shell are realized by the insertion of more tetrahedral-shaped anions (from one MoO42− in Ag48 (Fig. 75d) to eight PO43− in Ag90 (Fig. 75e)). In contrast to Ag90 with a similar core ([Ag30(PO4)8S6], Fig. 75e), the expansion (90 → 192) of Ag192 (Fig. 75f) is attributed to the intermediate anionic layer [Ag30(SO4)28S16] located between the [Ag30(SO4)8S6] core and surface shell of [Ag132(CyS)66(NO3)12].
The specific classical and various innovative synthesis methods (for example, ‘ligand-controlled hydrolysis’ strategy for metal-oxo/hydroxide clusters; ‘multi-anion-template’ strategy; ‘building-block’ method; ‘reduction of metal precursor’ for metalloid clusters; ‘disproportionation reaction’ for main group metal clusters such as Al and Ga; and hetero-metal strategy) of metal clusters were summarized and discussed in detail in the above main text and relevant reviews, and will not be repeated here.
10.2 Structural prediction of new ‘star’ clusters with Platonic and Archimedean solids
Based on the close-packing principle (such as face-centre cubic (fcc) close packing, hexagonal close packing (hcp) and body-centered cubic (bcc) close packing) of the equal sphere-like elements (metal atoms M0/n+, metal-oxo MOxm−, or SBBs, Fig. 1), and the relevant examples mentioned herein, and the geometric relationships between different Platonic and Archimedean solids (Fig. 2 and 3), we attempt to make some predictions for non-synthesized ‘star’ molecules that satisfy the topological features of Platonic and Archimedean solids. There are mainly two cases, as follows: (1) the existing metal frameworks of metal clusters may also appear in other systems in the future and (2) some reasonable speculations about unknown structures with polyhedron features are made based on the current examples.The first type of structure prediction is based on the large number of successful examples, where the family of α-Keggin-type molecules (XM12, M = V, Mo, W, Nb, etc.Fig. 76) is a typical representative. A variety of elements as the central atoms (X), from the original main group atoms (B, Al, Si, Ge, P, and As) to the later transition metals (Fe, Co, and Cu), and more recently the alkali/alkaline earth metals (Na, Ca), was successfully encapsulated in inorganic structures. Besides traditional inorganic POMs, similar α-Keggin structures have been found in metal clusters protected by organic ligands (U13,186Fe13,287Zr13,227Hf13,228 Mn13,265 and Eu13,383Fig. 76) and Keggin-type cores also appear in larger metal clusters (such as Co20-1, Fig. 34). Other isomers (like γ-Keggin AgSn12)658 have also been reported. Under suitable synthetic conditions and the protection of ligand, more Keggin and existing polyhedral structures based on other metals will be synthesized in the near future. Similarly, more polyhedral cores (such as v1-icosahedron Ag12/Ag13, Cu12/Cu13, and v2-icosahedron Ag55 and Au55) appearing in large metalloid clusters can theoretically be isolated separately based on the effective protection of ligands, and there are many examples to prove this (such as Pd55/Pd145/(PdPt)165 (Fig. 40) and icosahedron Au13 (Fig. 67 and 69)).
 | ||
| Fig. 76 Polyhedral mode of α-Keggin-type metal clusters: classical inorganic POM, {Fe13(μ4-O)4F24(μ2-OMe)12} (Fe13), Zr13(μ4-O)8(OCH3)36 (Zr13), Hf13(μ4-O)8(OCH3)36 (Hf13), {Mn13O6(OH)2(OMe)4(bemp)6} (H3bemp = 2,6-bis[N-(2-hydroxyethyl)iminomethyl]-4-methylphenol),265 and {Eu13O8(Ph4Si4O8)6(DMF)12}.383 | ||
The second category is to predict unknown structures based on existing structural features, which are undoubtedly quite difficult, and only a general trend is discussed here. As the number of metal atoms increases, to adjust the charge balance inside the metal-containing aggregates, the structures generally have the following three forms: (1) the presence of a large number of anion-rich holes (such as Gd158Co38Cl12, Fig. 46 and 74; Ag192, Fig. 55 and 74); (2) hollow structures with more surfaces, which facilitate the coordination of more negatively charged ligands (such as Mo24, Fig. 8; Mo84, Mo102, Mo132, Mo240, Fig. 11; Ti42, Fig. 25; and Ag180510); and (3) the internal metal atoms are reduced to form a metalloid core. The first approach is based on the use of multi-anions, and anions with high symmetry help promote the local symmetry, thereby affecting the molecular symmetry.
The second case is especially common in aggregates containing more than 50 metal atoms, and in addition to cage-like structures, there are also wheel-like (such as Ce70,367 Mn84,39 and Gd14052), tubular (such as Dy72),368 and various hollow structures (such as Ni36Gd102).54 In the case of cage-like structures based on M-O/OH/S (non-metal organic cage), their constituent units have certain regularity. For example, U20 (Fig. 23a), Gd20 (Fig. 42a), Ti42 (Fig. 77a), U60 (Fig. 23c), Mo102 (Fig. 77b), and Mo132 (Fig. 77c) can be regarded as different forms of an Ih symmetry assembly of 12 pentagon basic units (M@M5 or M5). We believe that these pentagon-motifs can also be used to construct cage structures with hitherto unknown metal frameworks featuring Platonic and Archimedean solids and may be a species (M60 (12 M5) or M72 (12 M@M5) with rhombicosidodecahedron or snub dodecahedron feature) between Ti42 (Fig. 77a) and Mo102 (Fig. 77b) or a larger hollow monolayer structure (Fig. 77d).
The third way is fully reflected in the synthesis of giant gold and silver clusters in recent years. Compared with small clusters, surface ligands have less effect on the arrangement of the metal atoms inside high-nuclearity clusters with multi-shell structures, showing the same packing form (fcc) as bulk materials. In many cases, the metalloid kernels have high symmetry, but the metal-ligand layer symmetry on the surface may be reduced. Among the many reported structures, there are several families of Ag/Au clusters with regular size expansion, such as tetrahedral (Fig. 50, 51, 57 and 58) and cubic shape (Fig. 59 and 78). There are obviously many missing members of these metal cluster families, and the cubic silver clusters with Oh-symmetry metal cores (Fig. 59 and 78) are taken as an example for structural analysis and prediction. Ag14 (Ag6@Ag8), Ag46 (Ag6@Ag8@Ag24@Ag8), and Ag100 (Ag6@Ag8@Ag24@Ag6@Ag24@Ag24@Ag8) with the same Ag6 in their center clearly show the manner and regularity of the fcc Ag core growth. The appearance of Ag40 (Ag8@Ag24@Ag8) suggests that the internal metal atomic arrangement can be tuned under the same symmetry. The successful synthesis of Ag63 (Ag@Ag12@Ag6@Ag24@Ag12@Ag8) proves that regular growth with the cuboctahedron Ag13 (Ag@Ag12) as the core can also be achieved. In the reported Au clusters, some structures (Au20 (Au12@Au8, Fig. 66b) and Au279(SPhtBu)84 (Au@Au12@Au42@Au92@Au54@Au48@Au30)) with cuboctahedral kernels were observed. Thus, we expect that Ag clusters with similar cuboctahedral kernels will be successfully synthesized.
 | ||
| Fig. 78 Structure (analogous [Ag(SR)3(PR′3)] motifs) of equal vertices of cubic box-like Ag nanoclusters: (a) [Ag14(3,4-SPhF2)12(PPh3)8] (Ag14); (b) [Ag40(2,4-SPh(CH3)2)24(PPh3)8] (Ag40) and [Ag46(2,5-SPh(CH3)2)24(PPh3)8]2+ (Ag46); (c) [Ag63(3,4-SPhF2)36(PnBu3)8]+ (Ag63); and (d) [Ag100(3,4-SPhF2)48(PPh3)8]− (Ag100). Metal-framework with eight [Ag(S/SeR)3] motifs arranged in cubes around the periphery: (e) [Ag21{Se2P(OEt)2}12]+ (Ag@Ag12@Ag8@(Se3)8, Ag21); (f) Ag33(C2B10H10S2)12 (Ag@Ag12@Ag20@(S3)8, Ag33) and (g) [Ag39(SPhF5)24(PPh3)8]2− (Ag@Ag12@Ag18@(Ag(SPhF5)3(PPh3))8, Ag39). Some Ag⋯Ag interactions and organic ligands have been omitted for clarity. (h) Intermediate vertex-deficient dodecahedral Ag18 shell of Ag39.659 | ||
In addition to the regularity of the inner metal kernels, they also have similar eight [Ag(SR)3(PR′3)] vertices at the periphery (Fig. 78a–d), which are also observed in some non-Oh-symmetry clusters (Fig. 78e–g). We present the bold speculation that in the future, Platonic and Archimedean polyhedra belonging to different symmetry groups will exist in the same multi-shell Ag cluster, which may be the following structures: Ag28 (Ag12(icosahedron)@Ag8(cube)@Ag8(cube)), Ag29 (Ag(center)@Ag12(icosahedron)@Ag8(cube)@Ag8(cube)), Ag40(Ag12(icosahedron)@Ag20(dodecahedron)@Ag8(cube)) and Ag41(Ag(center)@Ag12(icosahedron)@Ag20(dodecahedron)@Ag8(cube)). Some recent experimental results also confirm the rationality of our conjecture. Zang's group synthesized a distorted cubic silver cluster, Na2[Ag39(SPhF5)24(PPh3)8] (Ag39, Fig. 78g), co-protected by phosphine (PPh3) and thiolate (HSPhF5 = pentafluorobenzenethiol).659 The metal arrangement (Ag(center)@Ag12(icosahedron)@Ag18@Ag8(cube)) of Ag39 is consistent with our speculation, but the Ag18 shell reveals vertex vacancy defects, deviating from the dodecahedron constructed by 20 vertices (Fig. 78h).
11. Conclusions and future prospective
In this review, we discussed more than 100 examples of high-nuclearity metal clusters with metal frameworks featuring Platonic and Archimedean solids. It is not difficult to recognize that some polyhedral arrangements, for example, five Platonic solids (tetrahedron, cube, octahedron, dodecahedron, and icosahedron) and most parts of 13 Archimedean solids (truncated tetrahedron, cuboctahedron, truncated cube, truncated octahedron, rhombicuboctahedron, icosidodecahedron, and rhombicosidodecahedron), appear in these clusters. The discovery of these highly symmetric metal clusters will undoubtedly guide synthetic chemists to explore new structures and giant molecules with similar characteristics, not only to pursue structural aesthetics, but more importantly to create materials with unique properties and useful applications.However, it should be noted that the characteristics (including nucleus, shape, and configuration) of the crystal structures of metal clusters, especially giant metal clusters are mostly unpredictable and frequently the result of serendipitous discovery. Their acquisitions are affected by a large number of factors, which may depend on the specific structural characteristics of the surface ligands, reaction conditions and other factors. Thus, high-throughput synthetic workstations combined with machine learning and the application of artificial intelligence techniques in cluster synthesis will be helpful for the rapid development of this field. Although there are great difficulties in the synthesis process, the research on these clusters has provided an in-depth understanding of the possible mechanisms that cause their assembly, the establishment of bridges from atoms to nanoparticles, and new systems to study new physical and chemical properties. These interesting polyhedral cluster structures have shown excellent properties and applications in many fields, but whether similar polyhedral structures have parallel properties and the relationships between different polyhedral structures are not clear. Many studies have shown that small cubane-like M4 clusters have similar properties, but other high-nuclearity polyhedral metal clusters have not been studied to date.
We summarized and analyzed the metal clusters from the perspective of polyhedra (Platonic and Archimedean solids), hoping that more researchers will pay attention to these fascinating molecules with high symmetry. Simultaneously, it is hoped that the polyhedral approach for describing and understanding the complex structures can bring some new insights into related research, and stimulate research on higher-level relationships, such as the influence of the electronic properties on the geometric structure and pointing out the possible systematics in the dependence of the structure on metal and ligand.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 92061201, 21825106, U21A20277, 21975065) and Zhengzhou University.References
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- W.-H. Fang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2018, 47, 404–421 RSC.
- J.-X. Liu, X.-B. Zhang, Y.-L. Li, S.-L. Huang and G.-Y. Yang, Coord. Chem. Rev., 2020, 414, 213260 CrossRef CAS.
- A. Müller and P. Gouzerh, Chem. Soc. Rev., 2012, 41, 7431–7463 RSC.
- X.-Y. Zheng, J. Xie, X.-J. Kong, L.-S. Long and L.-S. Zheng, Coord. Chem. Rev., 2019, 378, 222–236 CrossRef CAS.
- C. Papatriantafyllopoulou, E. E. Moushi, G. Christou and A. J. Tasiopoulos, Chem. Soc. Rev., 2016, 45, 1597–1628 RSC.
- X.-Y. Zheng, X.-J. Kong, Z. Zheng, L.-S. Long and L.-S. Zheng, Acc. Chem. Res., 2018, 51, 517–525 CrossRef CAS PubMed.
- X.-J. Kong, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, Acc. Chem. Res., 2010, 43, 201–209 CrossRef CAS PubMed.
- S. Scharfe, F. Kraus, S. Stegmaier, A. Schier and T. F. Fässler, Angew. Chem., Int. Ed., 2011, 50, 3630–3670 CrossRef CAS PubMed.
- M. Shieh, C.-Y. Miu, Y.-Y. Chu and C.-N. Lin, Coord. Chem. Rev., 2012, 256, 637–694 CrossRef CAS.
- O. Fuhr, S. Dehnen and D. Fenske, Chem. Soc. Rev., 2013, 42, 1871–1906 RSC.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- Z. Luo, A. W. Castleman and S. N. Khanna, Chem. Rev., 2016, 116, 14456–14492 CrossRef CAS PubMed.
- F. K. Sheong, J.-X. Zhang and Z. Lin, Coord. Chem. Rev., 2017, 345, 42–53 CrossRef CAS.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- K. Mayer, J. Weßing, T. F. Fässler and R. A. Fischer, Angew. Chem., Int. Ed., 2018, 57, 14372–14393 CrossRef CAS PubMed.
- C. Healy and W. Schmitt, Coord. Chem. Rev., 2018, 371, 67–85 CrossRef CAS.
- P. Jena and Q. Sun, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS PubMed.
- R. J. Wilson, N. Lichtenberger, B. Weinert and S. Dehnen, Chem. Rev., 2019, 119, 8506–8554 CrossRef CAS PubMed.
- Y. Jia and Z. Luo, Coord. Chem. Rev., 2019, 400, 213053 CrossRef CAS.
- A. V. Virovets, E. Peresypkina and M. Scheer, Chem. Rev., 2021, 121, 14485–14554 CrossRef CAS PubMed.
- C. Cesari, J.-H. Shon, S. Zacchini and L. A. Berben, Chem. Soc. Rev., 2021, 50, 9503–9539 RSC.
- J. Kobylarczyk, E. Kuzniak, M. Liberka, S. Chorazy, B. Sieklucka and R. Podgajny, Coord. Chem. Rev., 2020, 419, 213394 CrossRef CAS.
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- A. Müller, Science, 2003, 300, 749–750 CrossRef PubMed.
- S. Schein and J. M. Gayed, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 2920 CrossRef CAS PubMed.
- S. Torquato and Y. Jiao, Nature, 2009, 460, 876–879 CrossRef CAS PubMed.
- B. K. Teo and N. J. A. Sloane, Inorg. Chem., 1985, 24, 4545–4558 CrossRef CAS.
- S. Alvarez, Dalton Trans., 2005, 2209–2233 RSC.
- A. Mackay, Acta Cryst., 1962, 15, 916–918 CrossRef CAS.
- E. G. Ribó, N. L. Bell, D.-L. Long and L. Cronin, Angew. Chem., Int. Ed., 2022, 61, e202201672 CrossRef PubMed.
- J. Lin, N. Li, S. Yang, M. Jia, J. Liu, X.-M. Li, L. An, Q. Tian, L.-Z. Dong and Y.-Q. Lan, J. Am. Chem. Soc., 2020, 142, 13982–13988 CrossRef CAS PubMed.
- A. Müller, E. Beckmann, H. Bögge, M. Schmidtmann and A. Dress, Angew. Chem., Int. Ed., 2002, 41, 1162–1167 CrossRef.
- M. Ibrahim, V. Mereacre, N. Leblanc, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2015, 54, 15574–15578 CrossRef CAS PubMed.
- S. Li, Y. Zhou, N. Ma, J. Zhang, Z. Zheng, C. Streb and X. Chen, Angew. Chem., Int. Ed., 2020, 59, 8537–8540 CrossRef CAS PubMed.
- Y.-L. Wu, X.-X. Li, Y.-J. Qi, H. Yu, L. Jin and S.-T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572–8576 CrossRef CAS PubMed.
- G. E. Sigmon, D. K. Unruh, J. Ling, B. Weaver, M. Ward, L. Pressprich, A. Simonetti and P. C. Burns, Angew. Chem., Int. Ed., 2009, 48, 2737–2740 CrossRef CAS PubMed.
- M.-Y. Gao, F. Wang, Z.-G. Gu, D.-X. Zhang, L. Zhang and J. Zhang, J. Am. Chem. Soc., 2016, 138, 2556–2559 CrossRef CAS PubMed.
- A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117–2121 CrossRef CAS PubMed.
- Z.-M. Zhang, S. Yao, Y.-G. Li, R. Clérac, Y. Lu, Z.-M. Su and E.-B. Wang, J. Am. Chem. Soc., 2009, 131, 14600–14601 CrossRef CAS PubMed.
- Y. Bi, X.-T. Wang, W. Liao, X. Wang, X. Wang, H. Zhang and S. Gao, J. Am. Chem. Soc., 2009, 131, 11650–11651 CrossRef CAS PubMed.
- M. J. Moses, J. C. Fettinger and B. W. Eichhorn, Science, 2003, 300, 778–780 CrossRef CAS PubMed.
- A. Ceriott, F. Demartin, G. Longoni, M. Manassero, M. Marchionna, G. Piva and M. Sansoni, Angew. Chem., Int. Ed. Engl., 1985, 24, 697–698 CrossRef.
- N. de Silva and L. F. Dahl, Inorg. Chem., 2006, 45, 8814–8816 CrossRef CAS PubMed.
- C. Cesari, T. Funaioli, B. Berti, C. Femoni, M. C. Iapalucci, F. M. Vivaldi and S. Zacchini, Inorg. Chem., 2021, 60, 16713–16725 CrossRef CAS PubMed.
- F. Xu, H. N. Miras, R. A. Scullion, D.-L. Long, J. Thiel and L. Cronin, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11609–11612 CrossRef CAS PubMed.
- E. G. Mednikov, M. C. Jewell and L. F. Dahl, J. Am. Chem. Soc., 2007, 129, 11619–11630 CrossRef PubMed.
- S. S. Mondal, A. Bhunia, A. Kelling, U. Schilde, C. Janiak and H.-J. Holdt, J. Am. Chem. Soc., 2014, 136, 44–47 CrossRef CAS PubMed.
- S. P. Argent, A. Greenaway, M. D. C. Gimenez-Lopez, W. Lewis, H. Nowell, A. N. Khlobystov, A. J. Blake, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2012, 134, 55–58 CrossRef CAS PubMed.
- S. Behrens, M. Bettenhausen, A. C. Deveson, A. Eichhöfer, D. Fenske, A. Lohde and U. Woggon, Angew. Chem., Int. Ed. Engl., 1996, 35, 2215–2218 CrossRef CAS.
- B. Russell-Webster, J. Lopez-Nieto, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2021, 60, 12591–12596 CrossRef CAS PubMed.
- X.-Y. Zheng, Y.-H. Jiang, G.-L. Zhuang, D.-P. Liu, H.-G. Liao, X.-J. Kong, L.-S. Long and L.-S. Zheng, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- W.-P. Chen, P.-Q. Liao, Y. Yu, Z. Zheng, X.-M. Chen and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 9375–9379 CrossRef CAS PubMed.
- W.-P. Chen, P.-Q. Liao, P.-B. Jin, L. Zhang, B.-K. Ling, S.-C. Wang, Y.-T. Chan, X.-M. Chen and Y.-Z. Zheng, J. Am. Chem. Soc., 2020, 142, 4663–4670 CrossRef CAS PubMed.
- J.-B. Peng, X.-J. Kong, Q.-C. Zhang, M. Orendáč, J. Prokleška, Y.-P. Ren, L.-S. Long, Z. Zheng and L.-S. Zheng, J. Am. Chem. Soc., 2014, 136, 17938–17941 CrossRef CAS PubMed.
- N.-F. Li, X.-M. Luo, J. Wang, J.-L. Wang, H. Mei, Y. Song and Y. Xu, Sci. China Chem., 2022, 65, 1577–1583 CrossRef CAS.
- W. Drescher, C. Borner and C. Kleeberg, New J. Chem., 2021, 45, 14957–14964 RSC.
- H. Yang, Y. Wang, X. Chen, X. Zhao, L. Gu, H. Huang, J. Yan, C. Xu, G. Li, J. Wu, A. J. Edwards, B. Dittrich, Z. Tang, D. Wang, L. Lehtovaara, H. Häkkinen and N. Zheng, Nat. Commun., 2016, 7, 12809 CrossRef CAS PubMed.
- C. E. Anson, A. Eichhöfer, I. Issac, D. Fenske, O. Fuhr, P. Sevillano, C. Persau, D. Stalke and J. Zhang, Angew. Chem., Int. Ed., 2008, 47, 1326–1331 CrossRef CAS PubMed.
- N. A. Sakthivel, S. Theivendran, V. Ganeshraj, A. G. Oliver and A. Dass, J. Am. Chem. Soc., 2017, 139, 15450–15459 CrossRef CAS PubMed.
- A. Ecker, E. Weckert and H. Schnöckel, Nature, 1997, 387, 379–381 CrossRef CAS.
- A. Schnepf and H. Schnöckel, Angew. Chem., Int. Ed., 2001, 40, 711–715 CrossRef PubMed.
- A. V. Protchenko, J. Urbano, J. A. B. Abdalla, J. Campos, D. Vidovic, A. D. Schwarz, M. P. Blake, P. Mountford, C. Jones and S. Aldridge, Angew. Chem., Int. Ed., 2017, 56, 15098–15102 CrossRef CAS PubMed.
- Y. Zhu, L. Zhang and J. Zhang, Chem. Sci., 2019, 10, 9125–9129 RSC.
- H. Krautscheid and F. Vielsack, Angew. Chem., Int. Ed. Engl., 1995, 34, 2035–2037 CrossRef CAS.
- M. Mehring, D. Mansfeld, S. Paalasmaa and M. Schürmann, Chem. – Eur. J., 2006, 12, 1767–1781 CrossRef CAS PubMed.
- G. E. Kostakis, A. M. Ako and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238–2271 RSC.
- Y.-J. Liu, W.-H. Fang, L. Zhang and J. Zhang, Coord. Chem. Rev., 2020, 404, 213099 CrossRef CAS.
- Y. Zhang, F. de Azambuja and T. N. Parac-Vogt, Coord. Chem. Rev., 2021, 438, 213886 CrossRef CAS.
- Q.-Y. Zhu and J. Dai, Coord. Chem. Rev., 2021, 430, 213664 CrossRef CAS.
- J. Wang, M. Feng, M. N. Akhtar and M.-L. Tong, Coord. Chem. Rev., 2019, 387, 129–153 CrossRef CAS.
- A. Dey, P. Bag, P. Kalita and V. Chandrasekhar, Coord. Chem. Rev., 2021, 432, 213707 CrossRef CAS.
- K. Liu, W. Shi and P. Cheng, Coord. Chem. Rev., 2015, 289–290, 74–122 CrossRef CAS.
- L. L.-M. Zhang and W.-Y. Wong, Aggregate, 2022, e266 Search PubMed.
- Y. Jin, C. Zhang, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Soc. Rev., 2021, 50, 2297–2319 RSC.
- Q.-M. Wang, Y.-M. Lin and K.-G. Liu, Acc. Chem. Res., 2015, 48, 1570–1579 CrossRef CAS PubMed.
- S. Kenzler and A. Schnepf, Chem. Sci., 2021, 12, 3116–3129 RSC.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009–6048 CrossRef CAS PubMed.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- K. Y. Monakhov, W. Bensch and P. Kögerler, Chem. Soc. Rev., 2015, 44, 8443–8483 RSC.
- A. Schnepf and H. Schnöckel, Angew. Chem., Int. Ed., 2002, 41, 3532–3554 CrossRef CAS PubMed.
- H. Schnöckel, Chem. Rev., 2010, 110, 4125–4163 CrossRef PubMed.
- S.-J. Liu, S.-D. Han, J.-P. Zhao, J. Xu and X.-H. Bu, Coord. Chem. Rev., 2019, 394, 39–52 CrossRef CAS.
- X. Zhao, S.-Q. Zang and X. Chen, Chem. Soc. Rev., 2020, 49, 2481–2503 RSC.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS PubMed.
- S. Kenzler, C. Schrenk and A. Schnepf, Angew. Chem., Int. Ed., 2017, 56, 393–396 CrossRef CAS PubMed.
- H. Shen, E. Selenius, P. Ruan, X. Li, P. Yuan, O. Lopez-Estrada, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, Chem. – Eur. J., 2020, 26, 8465–8470 CrossRef CAS PubMed.
- F. Fetzer, N. Pollard, N. C. Michenfelder, M. Strienz, A. N. Unterreiner, A. Z. Clayborne and A. Schnepf, Angew. Chem., Int. Ed., 2022, 61, e202206019 CrossRef CAS PubMed.
- M.-M. Zhang, X.-Y. Dong, Y.-J. Wang, S.-Q. Zang and T. C. W. Mak, Coord. Chem. Rev., 2022, 453, 214315 CrossRef CAS.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- S.-F. Yuan, W.-D. Liu, C.-Y. Liu, Z.-J. Guan and Q.-M. Wang, Chem. – Eur. J., 2022, 28, e202104445 CAS.
- X. Fan, S. Chen, L. Zhang and J. Zhang, Chem. – Eur. J., 2021, 27, 15563–15570 CrossRef CAS PubMed.
- J. J. Perry, Iv, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- S. Lee, H. Jeong, D. Nam, M. S. Lah and W. Choe, Chem. Soc. Rev., 2021, 50, 528–555 RSC.
- S. Alvarez, Inorg. Chim. Acta, 2010, 363, 4392–4398 CrossRef CAS.
- G. Guerrero, M. Mehring, P. Hubert Mutin, F. Dahan and A. Vioux, Dalton Trans., 1999, 1537–1538 RSC.
- F. Bottomley, C. P. Magill and B. Zhao, Organometallics, 1990, 9, 1700–1701 CrossRef CAS.
- A. Müller, P. Kögerler and A. W. M. Dress, Coord. Chem. Rev., 2001, 222, 193–218 CrossRef.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- N. V. Izarova, M. T. Pope and U. Kortz, Angew. Chem., Int. Ed., 2012, 51, 9492–9510 CrossRef CAS PubMed.
- H.-Y. Zhao, Y.-Z. Li, J.-W. Zhao, L. Wang and G.-Y. Yang, Coord. Chem. Rev., 2021, 443, 213966 CrossRef CAS.
- L. Chen, W.-L. Chen, X.-L. Wang, Y.-G. Li, Z.-M. Su and E.-B. Wang, Chem. Soc. Rev., 2019, 48, 260–284 RSC.
- D.-Y. Du, L.-K. Yan, Z.-M. Su, S.-L. Li, Y.-Q. Lan and E.-B. Wang, Coord. Chem. Rev., 2013, 257, 702–717 CrossRef CAS.
- D.-Y. Du, J.-S. Qin, S.-L. Li, Z.-M. Su and Y.-Q. Lan, Chem. Soc. Rev., 2014, 43, 4615–4632 RSC.
- X.-X. Li, D. Zhao and S.-T. Zheng, Coord. Chem. Rev., 2019, 397, 220–240 CrossRef CAS.
- S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623–7646 RSC.
- A. V. Anyushin, A. Kondinski and T. N. Parac-Vogt, Chem. Soc. Rev., 2020, 49, 382–432 RSC.
- L. Vilà-Nadal and L. Cronin, Nat. Rev. Mater., 2017, 2, 17054 CrossRef.
- V. Das, R. Kaushik and F. Hussain, Coord. Chem. Rev., 2020, 413, 213271 CrossRef CAS.
- T. M. Anderson, M. A. Rodriguez, F. Bonhomme, J. N. Bixler, T. M. Alam and M. Nyman, Dalton Trans., 2007, 4517–4522 RSC.
- C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 8251–8254 CrossRef CAS PubMed.
- C. Aronica, G. Chastanet, E. Zueva, S. A. Borshch, J. M. Clemente-Juan and D. Luneau, J. Am. Chem. Soc., 2008, 130, 2365–2371 CrossRef CAS PubMed.
- J. Wang, X. Liu, Z. Du and Y. Xu, Dalton Trans., 2021, 50, 7871–7886 RSC.
- F. A. Cotton, S. A. Duraj and W. J. Roth, Inorg. Chem., 1984, 23, 4042–4045 CrossRef CAS.
- H. K. Chae, W. G. Klemperer and V. W. Day, Inorg. Chem., 1989, 28, 1423–1424 CrossRef CAS.
- S. Mulkapuri, S. K. Kurapati, S. Mukhopadhyay and S. K. Das, New J. Chem., 2019, 43, 17670–17679 RSC.
- J. Hu, Y. Xu, D. Zhang, B. Chen, Z. Lin and C. Hu, Inorg. Chem., 2017, 56, 10844–10847 CrossRef CAS PubMed.
- X. Wang, K. Brunson, H. Xie, I. Colliard, M. C. Wasson, X. Gong, K. Ma, Y. Wu, F. A. Son, K. B. Idrees, X. Zhang, J. M. Notestein, M. Nyman and O. K. Farha, J. Am. Chem. Soc., 2021, 143, 21056–21065 CrossRef CAS PubMed.
- L. Zhang and W. Schmitt, J. Am. Chem. Soc., 2011, 133, 11240–11248 CrossRef CAS PubMed.
- K. Kastner, B. Puscher and C. Streb, Chem. Commun., 2013, 49, 140–142 RSC.
- X.-X. Li, T. Ji, J.-Y. Gao, W.-C. Chen, Y. Yuan, H.-Y. Sha, R. Faller, G.-G. Shan, K.-Z. Shao, X.-L. Wang and Z.-M. Su, Chem. Sci., 2022, 13, 4573–4580 RSC.
- F. Bannani, S. Floquet, N. Leclerc-Laronze, M. Haouas, F. Taulelle, J. Marrot, P. Kögerler and E. Cadot, J. Am. Chem. Soc., 2012, 134, 19342–19345 CrossRef CAS PubMed.
- A. Müller, S. Sarkar, S. Q. N. Shah, H. Bögge, M. Schmidtmann, S. Sarkar, P. Kögerler, B. Hauptfleisch, A. X. Trautwein and V. Schünemann, Angew. Chem., Int. Ed., 1999, 38, 3238–3241 CrossRef.
- A. Müller, E. Krickemeyer, S. K. Das, P. Kögerler, S. Sarkar, H. Bögge, M. Schmidtmann and S. Sarkar, Angew. Chem., Int. Ed., 2000, 39, 1612–1614 CrossRef.
- A. Müller, S. Q. N. Shah, H. Bögge, M. Schmidtmann, P. Kögerler, B. Hauptfleisch, S. Leiding and K. Wittler, Angew. Chem., Int. Ed., 2000, 39, 1614–1616 CrossRef.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann and F. Peters, Angew. Chem., Int. Ed., 1998, 37, 3359–3363 CrossRef.
- C. Schäffer, A. M. Todea, P. Gouzerh and A. Müller, Chem. Commun., 2012, 48, 350–352 RSC.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, S. Roy and A. Berkle, Angew. Chem., Int. Ed., 2002, 41, 3604–3609 CrossRef.
- M. I. Khan, A. Müller, S. Dillinger, H. Bögge, Q. Chen and J. Zubieta, Angew. Chem., Int. Ed. Engl., 1993, 32, 1780–1782 CrossRef.
- P. Mialane, A. Dolbecq, L. Lisnard, A. Mallard, J. Marrot and F. Sécheresse, Angew. Chem., Int. Ed., 2002, 41, 2398–2401 CrossRef CAS PubMed.
- Y. Wang, X. Ma, G. Li, H. Li, Q. Wang, W. Chen, P. Ma, S. Li, J. Niu and J. Wang, Chem. – Eur. J., 2022, e202200637 Search PubMed.
- X.-Y. Zhou, F. Wang and J. Zhang, J. Solid State Chem., 2023, 317, 123647 CrossRef CAS.
- V. Baskar, M. Shanmugam, M. Helliwell, S. J. Teat and R. E. P. Winpenny, J. Am. Chem. Soc., 2007, 129, 3042–3043 CrossRef CAS PubMed.
- W.-W. He, S.-L. Li, H.-Y. Zang, G.-S. Yang, S.-R. Zhang, Z.-M. Su and Y.-Q. Lan, Coord. Chem. Rev., 2014, 279, 141–160 CrossRef CAS.
- A. Müller, S. Polarz, S. K. Das, E. Krickemeyer, H. Bögge, M. Schmidtmann and B. Hauptfleisch, Angew. Chem., Int. Ed., 1999, 38, 3241–3245 CrossRef.
- O. Oms, A. Dolbecq and P. Mialane, Chem. Soc. Rev., 2012, 41, 7497–7536 RSC.
- Z.-J. Liu, X.-L. Wang, C. Qin, Z.-M. Zhang, Y.-G. Li, W.-L. Chen and E.-B. Wang, Coord. Chem. Rev., 2016, 313, 94–110 CrossRef CAS.
- U. Kortz, S. S. Hamzeh and N. A. Nasser, Chem. – Eur. J., 2003, 9, 2945–2952 CrossRef CAS.
- K.-Y. Wang, B. S. Bassil, Z.-G. Lin, A. Haider, J. Cao, H. Stephan, K. Viehweger and U. Kortz, Dalton Trans., 2014, 43, 16143–16146 RSC.
- H.-L. Li, C. Lian, D.-P. Yin, Z.-Y. Jia and G.-Y. Yang, Cryst. Growth Des., 2019, 19, 376–380 CrossRef CAS.
- Y. Sakai, K. Yoza, C. N. Kato and K. Nomiya, Dalton Trans., 2003, 3581–3586 RSC.
- Y. Sakai, S. Ohta, Y. Shintoyo, S. Yoshida, Y. Taguchi, Y. Matsuki, S. Matsunaga and K. Nomiya, Inorg. Chem., 2011, 50, 6575–6583 CrossRef CAS PubMed.
- Y. Sakai, K. Yoza, C. N. Kato and K. Nomiya, Chem. – Eur. J., 2003, 9, 4077–4083 CrossRef CAS PubMed.
- C. P. Pradeep, D.-L. Long, P. Kögerler and L. Cronin, Chem. Commun., 2007, 4254–4256 RSC.
- J.-W. Zhao, J. Zhang, S.-T. Zheng and G.-Y. Yang, Inorg. Chem., 2007, 46, 10944–10946 CrossRef CAS PubMed.
- J.-W. Zhao, H.-P. Jia, J. Zhang, S.-T. Zheng and G.-Y. Yang, Chem. – Eur. J., 2007, 13, 10030–10045 CrossRef CAS PubMed.
- C. Pichon, A. Dolbecq, P. Mialane, J. Marrot, E. Rivière and F. Sécheresse, Dalton Trans., 2008, 71–76 RSC.
- P. I. Molina, H. N. Miras, D.-L. Long and L. Cronin, Dalton Trans., 2014, 43, 5190–5199 RSC.
- M. Ibrahim, A. Haider, Y. Xiang, B. S. Bassil, A. M. Carey, L. Rullik, G. B. Jameson, F. Doungmene, I. M. Mbomekallé, P. D. Oliveira, V. Mereacre, G. E. Kostakis, A. K. Powell and U. Kortz, Inorg. Chem., 2015, 54, 6136–6146 CrossRef CAS PubMed.
- Q. Wu, Y.-G. Li, Y.-H. Wang, E.-B. Wang, Z.-M. Zhang and R. Clérac, Inorg. Chem., 2009, 48, 1606–1612 CrossRef CAS PubMed.
- P. Huang, X.-L. Wang, D.-Q. He, H.-Y. Wu, C. Qin, M. Du, C. Lai and Z.-M. Su, Dalton Trans., 2017, 46, 13345–13348 RSC.
- A. Haider, M. Ibrahim, B. S. Bassil, A. M. Carey, A. N. Viet, X. Xing, W. W. Ayass, J. F. Miñambres, R. Liu, G. Zhang, B. Keita, V. Mereacre, A. K. Powell, K. Balinski, A. T. N’Diaye, K. Küpper, H.-Y. Chen, U. Stimming and U. Kortz, Inorg. Chem., 2016, 55, 2755–2764 CrossRef CAS PubMed.
- M. Ibrahim, Y. Lan, B. S. Bassil, Y. Xiang, A. Suchopar, A. K. Powell and U. Kortz, Angew. Chem., Int. Ed., 2011, 50, 4708–4711 CrossRef CAS PubMed.
- M. Ibrahim, A. Haider, Y. Lan, B. S. Bassil, A. M. Carey, R. Liu, G. Zhang, B. Keita, W. Li, G. E. Kostakis, A. K. Powell and U. Kortz, Inorg. Chem., 2014, 53, 5179–5188 CrossRef CAS PubMed.
- D. Zhang, F. Cao, P. Ma, C. Zhang, Y. Song, Z. Liang, X. Hu, J. Wang and J. Niu, Chem. – Eur. J., 2015, 21, 17683–17690 CrossRef CAS PubMed.
- G.-S. Kim, H. Zeng, D. VanDerveer and C. L. Hill, Angew. Chem., Int. Ed., 1999, 38, 3205–3207 CrossRef CAS PubMed.
- T.-Z. Zhang, S. Yao, Z.-M. Zhang, Y. Lu, Y.-G. Li and E.-B. Wang, ChemPlusChem, 2014, 79, 1153–1158 CrossRef CAS.
- F. Hussain and U. Kortz, Chem. Commun., 2005, 1191–1193 RSC.
- U. Kortz, F. Hussain and M. Reicke, Angew. Chem., Int. Ed., 2005, 44, 3773–3777 CrossRef CAS PubMed.
- Z. Li, L.-D. Lin, H. Yu, X.-X. Li and S.-T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 15777–15781 CrossRef CAS PubMed.
- C. Boskovic, Acc. Chem. Res., 2017, 50, 2205–2214 CrossRef CAS PubMed.
- J.-W. Zhao, Y.-Z. Li, L.-J. Chen and G.-Y. Yang, Chem. Commun., 2016, 52, 4418–4445 RSC.
- M. Ibrahim, Y. Peng, E. Moreno-Pineda, C. E. Anson, J. Schnack and A. K. Powell, Small Struct., 2021, 2, 2100052 CrossRef CAS.
- S.-R. Li, H.-Y. Wang, H.-F. Su, H.-J. Chen, M.-H. Du, L.-S. Long, X.-J. Kong and L.-S. Zheng, Small Methods, 2021, 5, 2000777 CrossRef CAS PubMed.
- K.-Y. Wang, B. S. Bassil, Z. Lin, I. Römer, S. Vanhaecht, T. N. Parac-Vogt, C. Sáenz de Pipaón, J. R. Galán-Mascarós, L. Fan, J. Cao and U. Kortz, Chem. – Eur. J., 2015, 21, 18168–18176 CrossRef CAS PubMed.
- Y.-J. Wang, S.-Y. Wu, Y.-Q. Sun, X.-X. Li and S.-T. Zheng, Chem. Commun., 2019, 55, 2857–2860 RSC.
- M. Nyman, F. Bonhomme, M. Alam Todd, A. Rodriguez Mark, R. Cherry Brian, L. Krumhansl James, M. Nenoff Tina and M. Sattler Amy, Science, 2002, 297, 996–998 CrossRef CAS PubMed.
- L. Jin, Z.-K. Zhu, Y.-L. Wu, Y.-J. Qi, X.-X. Li and S.-T. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16288–16292 CrossRef CAS PubMed.
- C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 5634–5636 CrossRef CAS PubMed.
- J.-H. Son and W. H. Casey, Chem. – Eur. J., 2016, 22, 14155–14157 CrossRef CAS PubMed.
- Z. Liang, D. Zhang, P. Ma, J. Niu and J. Wang, Chem. – Eur. J., 2015, 21, 8380–8383 CrossRef CAS PubMed.
- L. Li, K. Dong, P. Ma, C. Zhang, J. Niu and J. Wang, Chem. – Eur. J., 2017, 23, 16957–16960 CrossRef CAS PubMed.
- H. Tan, W. Chen, D. Liu, Y. Li and E. Wang, Inorg. Chem. Commun., 2010, 13, 1354–1356 CrossRef CAS.
- P. Huang, C. Qin, Z.-M. Su, Y. Xing, X.-L. Wang, K.-Z. Shao, Y.-Q. Lan and E.-B. Wang, J. Am. Chem. Soc., 2012, 134, 14004–14010 CrossRef CAS PubMed.
- L. Jin, X.-X. Li, Y.-J. Qi, P.-P. Niu and S.-T. Zheng, Angew. Chem., Int. Ed., 2016, 55, 13793–13797 CrossRef CAS PubMed.
- Z.-K. Zhu, Y.-Y. Lin, H. Yu, X.-X. Li and S.-T. Zheng, Angew. Chem., Int. Ed., 2019, 58, 16864–16868 CrossRef CAS PubMed.
- C. Walther and M. A. Denecke, Chem. Rev., 2013, 113, 995–1015 CrossRef CAS PubMed.
- J. Qiu and P. C. Burns, Chem. Rev., 2013, 113, 1097–1120 CrossRef CAS PubMed.
- M. Nyman and P. C. Burns, Chem. Soc. Rev., 2012, 41, 7354–7367 RSC.
- P. B. Duval, C. J. Burns, D. L. Clark, D. E. Morris, B. L. Scott, J. D. Thompson, E. L. Werkema, L. Jia and R. A. Andersen, Angew. Chem., Int. Ed., 2001, 40, 3357–3361 CrossRef CAS PubMed.
- P. C. Burns, K.-A. Kubatko, G. Sigmon, B. J. Fryer, J. E. Gagnon, M. R. Antonio and L. Soderholm, Angew. Chem., Int. Ed., 2005, 44, 2135–2139 CrossRef CAS PubMed.
- G.-P. Yang, K. Li and C.-W. Hu, Inorg. Chem. Front., 2022, 9, 5408–5433 RSC.
- A. J. Gaunt, I. May, D. Collison, K. Travis Holman and M. T. Pope, J. Mol. Struct., 2003, 656, 101–106 CrossRef CAS.
- S. Duval, S. Sobanska, P. Roussel and T. Loiseau, Dalton Trans., 2015, 44, 19772–19776 RSC.
- M. Mohadeszadeh, J. Clust. Sci., 2011, 22, 183–192 CrossRef CAS.
- S. Fichter, T. Radoske and A. Ikeda-Ohno, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2021, 77, 847–852 CrossRef CAS PubMed.
- M. Xu, H. Traustason, F. D. Bo, S. Hickam, S. Chong, L. Zhang, A. G. Oliver and P. C. Burns, J. Am. Chem. Soc., 2019, 141, 12780–12788 CrossRef CAS PubMed.
- J. Qiu, M. Dembowski, J. E. S. Szymanowski, W. C. Toh and P. C. Burns, Inorg. Chem., 2016, 55, 7061–7067 CrossRef CAS PubMed.
- J. Ling, F. Hobbs, S. Prendergast, P. O. Adelani, J.-M. Babo, J. Qiu, Z. Weng and P. C. Burns, Inorg. Chem., 2014, 53, 12877–12884 CrossRef CAS PubMed.
- J. Qiu, K. Nguyen, L. Jouffret, J. E. S. Szymanowski and P. C. Burns, Inorg. Chem., 2013, 52, 337–345 CrossRef CAS PubMed.
- J. Qiu, J. Ling, A. Sui, J. E. S. Szymanowski, A. Simonetti and P. C. Burns, J. Am. Chem. Soc., 2012, 134, 1810–1816 CrossRef CAS PubMed.
- J. Ling, J. Qiu and P. C. Burns, Inorg. Chem., 2012, 51, 2403–2408 CrossRef CAS PubMed.
- J. Ling, J. Qiu, G. E. Sigmon, M. Ward, J. E. S. Szymanowski and P. C. Burns, J. Am. Chem. Soc., 2010, 132, 13395–13402 CrossRef CAS PubMed.
- G. E. Sigmon, J. Ling, D. K. Unruh, L. Moore-Shay, M. Ward, B. Weaver and P. C. Burns, J. Am. Chem. Soc., 2009, 131, 16648–16649 CrossRef CAS PubMed.
- L. Soderholm, P. M. Almond, S. Skanthakumar, R. E. Wilson and P. C. Burns, Angew. Chem., Int. Ed., 2008, 47, 298–302 CrossRef CAS PubMed.
- T. Z. Forbes, J. G. McAlpin, R. Murphy and P. C. Burns, Angew. Chem., Int. Ed., 2008, 47, 2824–2827 CrossRef CAS PubMed.
- I. Colliard, G. Morrison, H.-C. Z. Loye and M. Nyman, J. Am. Chem. Soc, 2020, 142, 9039–9047 CrossRef CAS PubMed.
- J. Qiu, S. Dong, J. E. S. Szymanowski, M. Dobrowolska and P. C. Burns, Inorg. Chem., 2017, 56, 3738–3741 CrossRef CAS PubMed.
- C. Falaise, C. Volkringer, J.-F. Vigier, A. Beaurain, P. Roussel, P. Rabu and T. Loiseau, J. Am. Chem. Soc., 2013, 135, 15678–15681 CrossRef CAS PubMed.
- C. Falaise, C. Volkringer, C. Hennig and T. Loiseau, Chem. – Eur. J., 2015, 21, 16654–16664 CrossRef CAS PubMed.
- N. P. Martin, C. Volkringer, N. Henry, X. Trivelli, G. Stoclet, A. Ikeda-Ohno and T. Loiseau, Chem. Sci., 2018, 9, 5021–5032 RSC.
- L. Chatelain, R. Faizova, F. Fadaei-Tirani, J. Pécaut and M. Mazzanti, Angew. Chem., Int. Ed., 2019, 58, 3021–3026 CrossRef CAS PubMed.
- N. A. Vanagas, R. F. Higgins, J. N. Wacker, D. R. C. Asuigui, E. Warzecha, S. A. Kozimor, S. L. Stoll, E. J. Schelter, J. A. Bertke and K. E. Knope, Chem. – Eur. J., 2020, 26, 5872–5886 CrossRef CAS PubMed.
- E. V. Dikarev, H. Zhang and B. Li, Angew. Chem., Int. Ed., 2006, 45, 5448–5451 CrossRef CAS PubMed.
- P. C. Andrews, G. B. Deacon, C. M. Forsyth, P. C. Junk, I. Kumar and M. Maguire, Angew. Chem., Int. Ed., 2006, 45, 5638–5642 CrossRef CAS PubMed.
- V. André, A. Hardeman, I. Halasz, R. S. Stein, G. J. Jackson, D. G. Reid, M. J. Duer, C. Curfs, M. T. Duarte and T. Friščić, Angew. Chem., Int. Ed., 2011, 50, 7858–7861 CrossRef PubMed.
- D. Mansfeld, L. Miersch, T. Rüffer, D. Schaarschmidt, H. Lang, T. Böhle, R. W. Troff, C. A. Schalley, J. Müller and M. Mehring, Chem. – Eur. J., 2011, 17, 14805–14810 CrossRef CAS PubMed.
- L. Miersch, T. Rüffer and M. Mehring, Chem. Commun., 2011, 47, 6353–6355 RSC.
- L. Miersch, M. Schlesinger, R. W. Troff, C. A. Schalley, T. Rüffer, H. Lang, D. Zahn and M. Mehring, Chem. – Eur. J., 2011, 17, 6985–6990 CrossRef CAS PubMed.
- P. C. Andrews, M. Busse, P. C. Junk, C. M. Forsyth and R. Peiris, Chem. Commun., 2012, 48, 7583–7585 RSC.
- V. Chandrasekhar, R. K. Metre and D. Sahoo, Eur. J. Inorg. Chem., 2014, 164–171 CrossRef CAS.
- Q.-R. Ding, Y. Yu, C. Cao, J. Zhang and L. Zhang, Chem. Sci., 2022, 13, 3395–3401 RSC.
- D. Van den Eynden, R. Pokratath and J. De Roo, Chem. Rev., 2022, 122, 10538–10572 CrossRef CAS PubMed.
- B. Chakraborty and I. A. Weinstock, Coord. Chem. Rev., 2019, 382, 85–102 CrossRef CAS.
- T. Shima, S. Hu, G. Luo, X. Kang, Y. Luo and Z. Hou, Science, 2013, 340, 1549–1552 CrossRef CAS PubMed.
- A. Radtke, P. Piszczek, T. Muzioł and A. Wojtczak, Inorg. Chem., 2014, 53, 10803–10810 CrossRef CAS PubMed.
- C. Lorber and L. Vendier, Inorg. Chem., 2013, 52, 4756–4758 CrossRef CAS PubMed.
- J. Ali, T. Navaneetha and V. Baskar, Inorg. Chem., 2020, 59, 741–747 CrossRef CAS PubMed.
- G. Zhang, J. Hou, M. Li, C.-H. Tung and Y. Wang, Inorg. Chem., 2016, 55, 4704–4709 CrossRef CAS PubMed.
- V. W. Day, T. A. Eberspacher, W. G. Klemperer and C. W. Park, J. Am. Chem. Soc., 1993, 115, 8469–8470 CrossRef CAS.
- L. Zhang, X. Fan, X. Yi, X. Lin and J. Zhang, Acc. Chem. Res., 2022, 55, 3150–3161 CrossRef CAS PubMed.
- W.-H. Fang, L. Zhang and J. Zhang, J. Am. Chem. Soc., 2016, 138, 7480–7483 CrossRef CAS PubMed.
- J. A. Sommers, D. C. Hutchison, N. P. Martin, K. Kozma, D. A. Keszler and M. Nyman, J. Am. Chem. Soc., 2019, 141, 16894–16902 CrossRef CAS PubMed.
- C. Hennig, S. Weiss, W. Kraus, J. Kretzschmar and A. C. Scheinost, Inorg. Chem., 2017, 56, 2473–2480 CrossRef CAS PubMed.
- G.-H. Chen, Y.-P. He, S.-H. Zhang and L. Zhang, Inorg. Chem. Commun., 2018, 97, 125–128 CrossRef CAS.
- C. Artner, M. Czakler and U. Schubert, Inorg. Chim. Acta, 2015, 432, 208–212 CrossRef CAS.
- B. Morosin, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 303–305 CrossRef.
- X.-M. Kang, H.-S. Hu, Z.-L. Wu, J.-Q. Wang, P. Cheng, J. Li and B. Zhao, Angew. Chem., Int. Ed., 2019, 58, 16610–16616 CrossRef CAS PubMed.
- T. C. Stamatatos, K. A. Abboud, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2006, 45, 4134–4137 CrossRef CAS PubMed.
- G. Wu, J. Huang, L. Sun, J. Bai, G. Li, E. Cremades, E. Ruiz, R. Clérac and S. Qiu, Inorg. Chem., 2011, 50, 8580–8587 CrossRef CAS PubMed.
- L. Zhang, R. Clérac, P. Heijboer and W. Schmitt, Angew. Chem., Int. Ed., 2012, 51, 3007–3011 CrossRef CAS PubMed.
- M. Manoli, S. Alexandrou, L. Pham, G. Lorusso, W. Wernsdorfer, M. Evangelisti, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2016, 55, 679–684 CrossRef CAS PubMed.
- M. Manoli, R. D. L. Johnstone, S. Parsons, M. Murrie, M. Affronte, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2007, 46, 4456–4460 CrossRef CAS PubMed.
- M. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti and E. K. Brechin, J. Am. Chem. Soc., 2008, 130, 11129–11139 CrossRef CAS PubMed.
- C. M. Zaleski, E. C. Depperman, C. Dendrinou-Samara, M. Alexiou, J. W. Kampf, D. P. Kessissoglou, M. L. Kirk and V. L. Pecoraro, J. Am. Chem. Soc., 2005, 127, 12862–12872 CrossRef CAS PubMed.
- R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS.
- T. A. Hudson, K. J. Berry, B. Moubaraki, K. S. Murray and R. Robson, Inorg. Chem., 2006, 45, 3549–3556 CrossRef CAS PubMed.
- G. C. Dismukes, R. Brimblecombe, G. A. N. Felton, R. S. Pryadun, J. E. Sheats, L. Spiccia and G. F. Swiegers, Acc. Chem. Res., 2009, 42, 1935–1943 CrossRef CAS PubMed.
- F. A. Stokes, L. Kloo, Y. Lv, P. J. Harford, A. E. H. Wheatley and D. S. Wright, Chem. Commun., 2012, 48, 11298–11300 RSC.
- A. R. Fout, Q. Zhao, D. J. Xiao and T. A. Betley, J. Am. Chem. Soc., 2011, 133, 16750–16753 CrossRef CAS PubMed.
- Y.-L. Miao, S.-D. Li, J.-L. Liu, F.-S. Guo and M.-L. Tong, Inorg. Chem. Commun., 2015, 52, 77–79 CrossRef CAS.
- M. R. Razali, A. S. R. Chesman, N. F. Chilton, S. K. Langley, B. Moubaraki, K. S. Murray, G. B. Deacon and S. R. Batten, Dalton Trans., 2013, 42, 1400–1405 RSC.
- B. F. Abrahams, T. A. Hudson and R. Robson, Chem. – Eur. J., 2006, 12, 7095–7102 CrossRef CAS PubMed.
- Z. Chen, Z. Hu, Y. Li, Y. Liang, X. Wang, L. Ouyang, Q. Zhao, H. Cheng and F. Liang, Dalton Trans., 2016, 45, 15634–15643 RSC.
- A. Masello, M. Murugesu, K. A. Abboud and G. Christou, Polyhedron, 2007, 26, 2276–2280 CrossRef CAS.
- K. Babic-Samardzija, P. Baran, R. Boča, R. Herchel, R. G. Raptis and C. L. Vélez, Polyhedron, 2018, 149, 142–147 CrossRef CAS.
- A. G. Flamourakis, D. Tzimopoulos, M. Siczek, T. Lis, J. R. O'Brien, P. D. Akrivos and C. J. Milios, Dalton Trans., 2011, 40, 11371–11373 RSC.
- S. Heinl, K. Kiefer, G. Balázs, C. Wickleder and M. Scheer, Chem. Commun., 2015, 51, 13474–13477 RSC.
- T. Shiga, L. Han, M. Nihei, N. Hoshino, T. Ito and H. Oshio, Chem. Lett., 2006, 35, 440–441 CrossRef CAS.
- D. P. Goldberg, A. Caneschi and S. J. Lippard, J. Am. Chem. Soc., 1993, 115, 9299–9300 CrossRef CAS.
- K. Skordi, C. Papatriantafyllopoulou, S. Zartilas, K. M. Poole, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Polyhedron, 2018, 151, 433–440 CrossRef CAS.
- D. P. Goldberg, A. Caneschi, C. D. Delfs, R. Sessoli and S. J. Lippard, J. Am. Chem. Soc., 1995, 117, 5789–5800 CrossRef CAS.
- T. C. Stamatatos, K. A. Abboud, W. Wernsdorfer and G. Christou, Polyhedron, 2007, 26, 2042–2046 CrossRef CAS.
- S. M. Taylor, R. D. McIntosh, J. Rezé, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2012, 48, 9263–9265 RSC.
- J. Utko, A. B. Canaj, C. J. Milios, M. Sobocińska and T. Lis, Inorg. Chem. Commun., 2014, 45, 71–74 CrossRef CAS.
- S. Nayak, M. Evangelisti, A. K. Powell and J. Reedijk, Chem. – Eur. J., 2010, 16, 12865–12872 CrossRef CAS PubMed.
- S. Stuiber, G. Wu, J. Nehrkorn, J. Dreiser, Y. Lan, G. Novitchi, C. E. Anson, T. Unruh, A. K. Powell and O. Waldmann, Chem. – Eur. J., 2011, 17, 9094–9106 CrossRef CAS PubMed.
- C.-M. Liu, R.-G. Xiong, D.-Q. Zhang and D.-B. Zhu, J. Am. Chem. Soc., 2010, 132, 4044–4045 CrossRef CAS PubMed.
- S. Tandon, F. W. Steuber, A. C. Kathalikkattil, M. Venkatesan, G. W. Watson and W. Schmitt, Inorg. Chem., 2021, 60, 8388–8393 CrossRef CAS PubMed.
- Z. Sun, P. K. Gantzel and D. N. Hendrickson, Inorg. Chem., 1996, 35, 6640–6641 CrossRef CAS PubMed.
- C. Lampropoulos, M. Murugesu, K. A. Abboud and G. Christou, Polyhedron, 2007, 26, 2129–2134 CrossRef CAS.
- A. Ferguson, K. Thomson, A. Parkin, P. Cooper, C. J. Milios, E. K. Brechin and M. Murrie, Dalton Trans., 2007, 728–730 RSC.
- M. Kondo, R. Shinagawa, M. Miyazawa, M. K. Kabir, Y. Irie, T. Horiba, T. Naito, K. Maeda, S. Utsuno and F. Uchida, Dalton Trans., 2003, 515–516 RSC.
- C. Lampropoulos, C. Koo, S. O. Hill, K. Abboud and G. Christou, Inorg. Chem., 2008, 47, 11180–11190 CrossRef CAS PubMed.
- G. N. Newton, S. Yamashita, K. Hasumi, J. Matsuno, N. Yoshida, M. Nihei, T. Shiga, M. Nakano, H. Nojiri, W. Wernsdorfer and H. Oshio, Angew. Chem., Int. Ed., 2011, 50, 5716–5720 CrossRef CAS PubMed.
- S. K. Langley, B. Moubaraki, K. J. Berry and K. S. Murray, Dalton Trans., 2010, 39, 4848–4855 RSC.
- C. Dendrinou-Samara, M. Alexiou, C. M. Zaleski, J. W. Kampf, M. L. Kirk, D. P. Kessissoglou and V. L. Pecoraro, Angew. Chem., Int. Ed., 2003, 42, 3763–3766 CrossRef CAS PubMed.
- T. C. Stamatatos, V. Nastopoulos, A. J. Tasiopoulos, E. E. Moushi, W. Wernsdorfer, G. Christou and S. P. Perlepes, Inorg. Chem., 2008, 47, 10081–10089 CrossRef CAS PubMed.
- T.-X. Wu, Y. Tao, Q.-J. He, H.-Y. Li, H.-D. Bian and F.-P. Huang, Chem. Commun., 2021, 57, 2732–2735 RSC.
- W.-G. Wang, A.-J. Zhou, W.-X. Zhang, M.-L. Tong, X.-M. Chen, M. Nakano, C. C. Beedle and D. N. Hendrickson, J. Am. Chem. Soc., 2007, 129, 1014–1015 CrossRef CAS PubMed.
- A. K. Dutta and R. Ghosh, Inorg. Chem. Commun., 2011, 14, 337–342 CrossRef CAS.
- R. H. Holm and W. Lo, Chem. Rev., 2016, 116, 13685–13713 CrossRef CAS PubMed.
- F. Jian, H. Xiao, Z. Bai and P. Zhao, J. Mater. Chem., 2006, 16, 3746–3752 RSC.
- O. Botezat, J. van Leusen, P. Kögerler and S. G. Baca, Inorg. Chem., 2018, 57, 7904–7913 CrossRef CAS PubMed.
- M. Ashafaq, M. Khalid, M. Raizada, A. Ali, M. Faizan, M. Shahid and M. Ahmad, Polyhedron, 2019, 163, 131–143 CrossRef CAS.
- H. Kalyvas and D. Coucouvanis, Inorg. Chem., 2006, 45, 8462–8464 CrossRef CAS PubMed.
- S. Pohl and W. Saak, Angew. Chem., Int. Ed. Engl., 1984, 23, 907–908 CrossRef.
- B. F. Abrahams, T. A. Hudson and R. Robson, J. Am. Chem. Soc., 2004, 126, 8624–8625 CrossRef CAS PubMed.
- P. Baran, R. Boča, I. Chakraborty, J. Giapintzakis, R. Herchel, Q. Huang, J. E. McGrady, R. G. Raptis, Y. Sanakis and A. Simopoulos, Inorg. Chem., 2008, 47, 645–655 CrossRef CAS PubMed.
- I. Chakraborty, P. Baran, Y. Sanakis, A. Simopoulos, E. Fachini and R. G. Raptis, Inorg. Chem., 2008, 47, 11734–11737 CrossRef CAS PubMed.
- S. Das, I. Chakraborty, D. Skachkov, M. Ahmadi, Y. Ishikawa, P. Baran and R. G. Raptis, Eur. J. Inorg. Chem., 2012, 3704–3711 CrossRef CAS PubMed.
- J. Deutscher, T. Corona, K. Warm, X. Engelmann, S. Sobottka, B. Braun-Cula, B. Sarkar and K. Ray, Eur. J. Inorg. Chem., 2018, 4925–4929 CrossRef CAS.
- I. A. Gass, C. J. Milios, A. G. Whittaker, F. P. A. Fabiani, S. Parsons, M. Murrie, S. P. Perlepes and E. K. Brechin, Inorg. Chem., 2006, 45, 5281–5283 CrossRef CAS PubMed.
- K. A. Lazarou, K. González-Nieves, I. Chakraborty and R. G. Raptis, Angew. Chem., Int. Ed., 2019, 58, 7324–7328 CrossRef CAS PubMed.
- R. G. Raptis, I. P. Georgakaki and D. C. R. Hockless, Angew. Chem., Int. Ed., 1999, 38, 1632–1634 CrossRef CAS PubMed.
- E. M. Zueva, W. M. C. Sameera, D. M. Piñero, I. Chakraborty, E. Devlin, P. Baran, K. Lebruskova, Y. Sanakis, J. E. McGrady and R. G. Raptis, Inorg. Chem., 2011, 50, 1021–1029 CrossRef CAS PubMed.
- A. Bino, M. Ardon, D. Lee, B. Spingler and S. J. Lippard, J. Am. Chem. Soc., 2002, 124, 4578–4579 CrossRef CAS PubMed.
- G. W. Powell, H. N. Lancashire, E. K. Brechin, D. Collison, S. L. Heath, T. Mallah and W. Wernsdorfer, Angew. Chem., Int. Ed., 2004, 43, 5772–5775 CrossRef CAS PubMed.
- M. Evangelisti, A. Candini, A. Ghirri, M. Affronte, G. W. Powell, I. A. Gass, P. A. Wood, S. Parsons, E. K. Brechin, D. Collison and S. L. Heath, Phys. Rev. Lett., 2006, 97, 167202 CrossRef CAS PubMed.
- O. Nachtigall, M. Kusserow, R. Clérac, W. Wernsdorfer, M. Menzel, F. Renz, J. Mrozinski and J. Spandl, Angew. Chem., Int. Ed., 2015, 54, 10361–10364 CrossRef CAS PubMed.
- A. E. Dearle, D. J. Cutler, M. Coletta, E. Lee, S. Dey, S. Sanz, H. W. L. Fraser, G. S. Nichol, G. Rajaraman, J. Schnack, L. Cronin and E. K. Brechin, Chem. Commun., 2022, 58, 52–55 RSC.
- A. E. Dearle, D. J. Cutler, H. W. L. Fraser, S. Sanz, E. Lee, S. Dey, I. F. Diaz-Ortega, G. S. Nichol, H. Nojiri, M. Evangelisti, G. Rajaraman, J. Schnack, L. Cronin and E. K. Brechin, Angew. Chem., Int. Ed., 2019, 58, 16903–16906 CrossRef CAS PubMed.
- O. Sadeghi, N. Zakharov Lev and M. Nyman, Science, 2015, 347, 1359–1362 CrossRef CAS PubMed.
- O. Sadeghi, C. Falaise, P. I. Molina, R. Hufschmid, C. F. Campana, B. C. Noll, N. D. Browning and M. Nyman, Inorg. Chem., 2016, 55, 11078–11088 CrossRef CAS PubMed.
- O. Sadeghi, M. Amiri, E. W. Reinheimer and M. Nyman, Angew. Chem., Int. Ed., 2018, 57, 6247–6250 CrossRef CAS PubMed.
- X.-Y. Zheng, M.-T. Chen, M.-H. Du, R.-J. Wei, X.-J. Kong, L.-S. Long and L.-S. Zheng, Chem. – Eur. J., 2020, 26, 11985–11988 CrossRef CAS PubMed.
- X.-Y. Zheng, M.-H. Du, M. Amiri, M. Nyman, Q. Liu, T. Liu, X.-J. Kong, L.-S. Long and L.-S. Zheng, Chem. – Eur. J., 2020, 26, 1388–1395 CrossRef CAS PubMed.
- M.-T. Chen, X.-Y. Zheng, X.-J. Kong, L.-S. Long and L.-S. Zheng, Inorg. Chem., 2022 DOI:10.1021/acs.inorgchem.2c01018.
- Z.-M. Zhang, Y.-G. Li, S. Yao, E.-B. Wang, Y.-H. Wang and R. Clérac, Angew. Chem., Int. Ed., 2009, 48, 1581–1584 CrossRef CAS PubMed.
- C. M. Beavers, A. V. Prosverin, J. D. Cashion, K. R. Dunbar and A. F. Richards, Inorg. Chem., 2013, 52, 1670–1672 CrossRef CAS PubMed.
- T. Liu, Y.-J. Zhang, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2008, 130, 10500–10501 CrossRef CAS PubMed.
- S. Kang, H. Zheng, T. Liu, K. Hamachi, S. Kanegawa, K. Sugimoto, Y. Shiota, S. Hayami, M. Mito, T. Nakamura, M. Nakano, M. L. Baker, H. Nojiri, K. Yoshizawa, C. Duan and O. Sato, Nat. Commun., 2015, 6, 5955 CrossRef PubMed.
- K. Dimitrou, K. Folting, W. E. Streib and G. Christou, J. Am. Chem. Soc., 1993, 115, 6432–6433 CrossRef CAS.
- L. Deng, E. Bill, K. Wieghardt and R. H. Holm, J. Am. Chem. Soc., 2009, 131, 11213–11221 CrossRef CAS PubMed.
- P. F. Smith, L. Hunt, A. B. Laursen, V. Sagar, S. Kaushik, K. U. D. Calvinho, G. Marotta, E. Mosconi, F. De Angelis and G. C. Dismukes, J. Am. Chem. Soc., 2015, 137, 15460–15468 CrossRef CAS PubMed.
- Q. Chen, M.-H. Zeng, L.-Q. Wei and M. Kurmoo, Chem. Mater., 2010, 22, 4328–4334 CrossRef CAS.
- X. Roy, C.-H. Lee, C. Crowther Andrew, L. Schenck Christine, T. Besara, A. Lalancette Roger, T. Siegrist, W. Stephens Peter, E. Brus Louis, P. Kim, L. Steigerwald Michael and C. Nuckolls, Science, 2013, 341, 157–160 CrossRef CAS PubMed.
- G. A. Seisenbaeva, T. Mallah and V. G. Kessler, Dalton Trans., 2010, 39, 7774–7779 RSC.
- G. Christou, K. S. Hagen, J. K. Bashkin and R. H. Holm, Inorg. Chem., 1985, 24, 1010–1018 CrossRef CAS.
- J.-Y. Xu, X. Qiao, H.-B. Song, S.-P. Yan, D.-Z. Liao, S. Gao, Y. Journaux and J. Cano, Chem. Commun., 2008, 6414–6416 RSC.
- L. D. Lower and L. F. Dahl, J. Am. Chem. Soc., 1976, 98, 5046–5047 CrossRef CAS.
- V. Ovcharenko, E. Fursova, G. Romanenko, I. Eremenko, E. Tretyakov and V. Ikorskii, Inorg. Chem., 2006, 45, 5338–5350 CrossRef CAS PubMed.
- H. Brunner, D. Lucas, T. Monzon, Y. Mugnier, B. Nuber, B. Stubenhofer, A. C. Stückl, J. Wachter, R. Wanninger and M. Zabel, Chem. – Eur. J., 2000, 6, 493–503 CrossRef CAS PubMed.
- M. Bencharif, O. Cador, H. Cattey, A. Ebner, J.-F. Halet, S. Kahlal, W. Meier, Y. Mugnier, J.-Y. Saillard, P. Schwarz, F. Z. Trodi, J. Wachter and M. Zabel, Eur. J. Inorg. Chem., 2008, 1959–1968 CrossRef CAS.
- A. Ebner, J. Wachter and M. Zabel, J. Clust. Sci., 2004, 15, 163–174 CrossRef CAS.
- N. P. Burkovskaya, M. E. Nikiforova, M. A. Kiskin, A. S. Lermontov, A. S. Bogomyakov, V. S. Mironov, Z. V. Dobrokhotova, V. I. Pekhnyo, A. A. Sidorov, V. M. Novotortsev and I. L. Eremenko, Polyhedron, 2011, 30, 2941–2949 CrossRef CAS.
- J. A. Sheikh, S. Goswami, A. Adhikary and S. Konar, Inorg. Chem., 2013, 52, 4127–4129 CrossRef CAS PubMed.
- B. A. Breeze, M. Shanmugam, F. Tuna and R. E. P. Winpenny, Chem. Commun., 2007, 5185–5187 RSC.
- S. P. Argent, I. Tarassova, A. Greenaway, H. Nowell, S. A. Barnett, M. R. Warren, C. C. Tang, C. G. Morris, W. Lewis, N. R. Champness, M. Schröder and A. J. Blake, Polyhedron, 2016, 120, 18–29 CrossRef CAS.
- X.-N. Cheng, W. Xue, J.-B. Lin and X.-M. Chen, Chem. Commun., 2010, 46, 246–248 RSC.
- K. Dimitrou, A. D. Brown, K. Folting and G. Christou, Inorg. Chem., 1999, 38, 1834–1841 CrossRef CAS PubMed.
- K. Dimitrou, J.-S. Sun, K. Folting and G. Christou, Inorg. Chem., 1995, 34, 4160–4166 CrossRef CAS.
- L. Lisnard, F. Tuna, A. Candini, M. Affronte, R. E. P. Winpenny and E. J. L. McInnes, Angew. Chem., Int. Ed., 2008, 47, 9695–9699 CrossRef CAS PubMed.
- R. Shaw, I. S. Tidmarsh, R. H. Laye, B. Breeze, M. Helliwell, E. K. Brechin, S. L. Heath, M. Murrie, S. Ochsenbein, H.-U. Güdel and E. J. L. McInnes, Chem. Commun., 2004, 1418–1419 RSC.
- A. W. Cook, G. Wu and T. W. Hayton, Inorg. Chem., 2018, 57, 8189–8194 CrossRef CAS PubMed.
- Y. Zhang, R. Lv, J. Wang, L. Yang, S. Liao, J. Tian, W. Gu and X. Liu, Dalton Trans., 2016, 45, 3247–3250 RSC.
- W. Du, Y.-L. Bai, X. Yin, J. Fang, S. Zhu and J. Tao, Chem. – Eur. J., 2017, 23, 8025–8031 CrossRef CAS PubMed.
- L. Dong, X. Li, J. Cao, W. Chu and R. Huang, Dalton Trans., 2013, 42, 1342–1345 RSC.
- L. Dong, R. Huang, Y. Wei and W. Chu, Inorg. Chem., 2009, 48, 7528–7530 CrossRef CAS PubMed.
- Z. Peng, S. Li, A. Li, J. Liao, Y. Wang, X. Li, W. Meng and J. Zhang, Dalton Trans., 2022, 51, 2652–2655 RSC.
- M. A. Palacios, E. Moreno Pineda, S. Sanz, R. Inglis, M. B. Pitak, S. J. Coles, M. Evangelisti, H. Nojiri, C. Heesing, E. K. Brechin, J. Schnack and R. E. P. Winpenny, ChemPhysChem, 2016, 17, 55–60 CrossRef CAS PubMed.
- J. Chen, H. Zhou and F. Xu, Inorg. Chem., 2016, 55, 4695–4697 CrossRef CAS PubMed.
- L.-C. Gui, X.-J. Wang, Q.-L. Ni, M. Wang, F.-P. Liang and H.-H. Zou, J. Am. Chem. Soc., 2012, 134, 9827 CrossRef CAS.
- Y. Bi, S. Du and W. Liao, Chem. Commun., 2011, 47, 4724–4726 RSC.
- A. Bilyk, J. W. Dunlop, R. O. Fuller, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. A. Koutsantonis, I. W. Murray, B. W. Skelton, R. L. Stamps and A. H. White, Eur. J. Inorg. Chem., 2010, 2106–02126 CrossRef CAS.
- Y. Bi, S. Du and W. Liao, Coord. Chem. Rev., 2014, 276, 61–72 CrossRef CAS.
- H. Han, L. Kan, P. Li, G. Zhang, K. Li, W. Liao, Y. Liu, W. Chen and C. T. Hu, Sci. China Chem., 2021, 64, 426–431 CrossRef CAS.
- D. Geng, X. Han, Y. Bi, Y. Qin, Q. Li, L. Huang, K. Zhou, L. Song and Z. Zheng, Chem. Sci., 2018, 9, 8535–8541 RSC.
- S. J. Dalgarno, J. L. Atwood and C. L. Raston, Chem. Commun., 2006, 4567–4574 RSC.
- M. Liu, W. Liao, C. Hu, S. Du and H. Zhang, Angew. Chem., Int. Ed., 2012, 51, 1585–1588 CrossRef CAS PubMed.
- S. Du, C. Hu, J.-C. Xiao, H. Tan and W. Liao, Chem. Commun., 2012, 48, 9177–9179 RSC.
- F.-R. Dai and Z. Wang, J. Am. Chem. Soc., 2012, 134, 8002–8005 CrossRef CAS PubMed.
- E. N. Zorina, N. V. Zauzolkova, A. A. Sidorov, G. G. Aleksandrov, A. S. Lermontov, M. A. Kiskin, A. S. Bogomyakov, V. S. Mironov, V. M. Novotortsev and I. L. Eremenko, Inorg. Chim. Acta, 2013, 396, 108–118 CrossRef CAS.
- E. N. Zorina-Tikhonova, N. V. Gogoleva, A. A. Sidorov, G. G. Aleksandrov, M. A. Kiskin, A. V. Vologzhanina, L. I. Demina, A. S. Bogomyakov, N. N. Efimov, V. S. Mironov, V. M. Novotortsev and I. L. Eremenko, Polyhedron, 2017, 130, 67–74 CrossRef CAS.
- S. Stegmaier and T. F. Fässler, J. Am. Chem. Soc., 2011, 133, 19758–19768 CrossRef CAS PubMed.
- X. Huang, J. Zhao, Y. Su, Z. Chen and R. B. King, Sci. Rep., 2014, 4, 6915 CrossRef CAS PubMed.
- Z. Li, H. Ruan, L. Wang, C. Liu and L. Xu, Dalton Trans., 2017, 46, 3453–3456 RSC.
- Y. Wang, M. Moses-DeBusk, L. Stevens, J. Hu, P. Zavalij, K. Bowen, B. I. Dunlap, E. R. Glaser and B. Eichhorn, J. Am. Chem. Soc., 2017, 139, 619–622 CrossRef PubMed.
- A. Bernardi, I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, J. Organomet. Chem., 2016, 812, 229–239 CrossRef CAS.
- A. J. Whoolery and L. F. Dahl, J. Am. Chem. Soc., 1991, 113, 6683–6685 CrossRef CAS.
- J. Zhang and L. F. Dahl, Dalton Trans., 2002, 1269–1274 RSC.
- C. Femoni, M. Carmela Iapalucci, G. Longoni and P. H. Svensson, Chem. Commun., 2001, 1776–1777 RSC.
- V. M. Chernyshev, O. V. Khazipov, D. B. Eremin, E. A. Denisova and V. P. Ananikov, Coord. Chem. Rev., 2021, 437, 213860 CrossRef CAS.
- I. Favier, D. Pla and M. Gómez, Chem. Rev., 2020, 120, 1146–1183 CrossRef CAS PubMed.
- Y. Shi, Z. Lyu, M. Zhao, R. Chen, Q. N. Nguyen and Y. Xia, Chem. Rev., 2021, 121, 649–735 CrossRef CAS PubMed.
- P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed.
- E. V. Chubarova, M. H. Dickman, B. Keita, L. Nadjo, F. Miserque, M. Mifsud, I. W. C. E. Arends and U. Kortz, Angew. Chem., Int. Ed., 2008, 47, 9542–9546 CrossRef CAS PubMed.
- J. D. Erickson, E. G. Mednikov, S. A. Ivanov and L. F. Dahl, J. Am. Chem. Soc., 2016, 138, 1502–1505 CrossRef CAS PubMed.
- N. T. Tran, D. R. Powell and L. F. Dahl, Angew. Chem., Int. Ed., 2000, 39, 4121–4125 CrossRef CAS PubMed.
- M. Teramoto, K. Iwata, H. Yamaura, K. Kurashima, K. Miyazawa, Y. Kurashige, K. Yamamoto and T. Murahashi, J. Am. Chem. Soc., 2018, 140, 12682–12686 CrossRef CAS PubMed.
- M. Pley and M. S. Wickleder, Angew. Chem., Int. Ed., 2004, 43, 4168–4170 CrossRef CAS PubMed.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, J. Clust. Sci., 2014, 25, 115–146 CrossRef CAS.
- M. Bortoluzzi, C. Cesari, I. Ciabatti, C. Femoni, M. C. Iapalucci and S. Zacchini, Inorg. Chem., 2017, 56, 6532–6544 CrossRef CAS PubMed.
- A. Ceriotti, N. Masciocchi, P. Macchi and G. Longoni, Angew. Chem., Int. Ed., 1999, 38, 3724–3727 CrossRef CAS PubMed.
- C. Femoni, F. Kaswalder, M. C. Iapalucci, G. Longoni, M. Mehlstäubl and S. Zacchini, Chem. Commun., 2005, 5769–5771 RSC.
- E. Cattabriga, I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Inorg. Chim. Acta, 2018, 470, 238–249 CrossRef CAS.
- I. Colliard, J. C. Brown, D. B. Fast, A. K. Sockwell, A. E. Hixon and M. Nyman, J. Am. Chem. Soc., 2021, 143, 9612–9621 CrossRef CAS PubMed.
- L. Qin, Y.-Z. Yu, P.-Q. Liao, W. Xue, Z. Zheng, X.-M. Chen and Y.-Z. Zheng, Adv. Mater., 2016, 28, 10772–10779 CrossRef CAS PubMed.
- Z.-H. Pan, Z.-Z. Weng, X.-J. Kong, L.-S. Long and L.-S. Zheng, Coord. Chem. Rev., 2022, 457, 214419 CrossRef CAS.
- W. Huang, Q. Liu, W. Chen, M. Feng and Z. Zheng, Magnetochemistry, 2021, 7, 161 CrossRef CAS.
- C.-M. Liu, D.-Q. Zhang, X. Hao and D.-B. Zhu, Cryst. Growth Des., 2012, 12, 2948–2954 CrossRef CAS.
- S.-Y. Lin, B. Sun and Z. Xu, Inorg. Chim. Acta, 2017, 464, 119–124 CrossRef CAS.
- W.-Q. Lin, X.-F. Liao, J.-H. Jia, J.-D. Leng, J.-L. Liu, F.-S. Guo and M.-L. Tong, Chem. – Eur. J., 2013, 19, 12254–12258 CrossRef CAS PubMed.
- N. Kato, T. Mita, M. Kanai, B. Therrien, M. Kawano, K. Yamaguchi, H. Danjo, Y. Sei, A. Sato, S. Furusho and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 6768–6769 CrossRef CAS PubMed.
- M. Nishiura and Z. Hou, Nat. Chem., 2010, 2, 257–268 CrossRef CAS PubMed.
- J. Wu, X.-L. Li, L. Zhao, M. Guo and J. Tang, Inorg. Chem., 2017, 56, 4104–4111 CrossRef CAS PubMed.
- M. Yousufuddin, M. J. Gutmann, J. Baldamus, O. Tardif, Z. Hou, S. A. Mason, G. J. McIntyre and R. Bau, J. Am. Chem. Soc., 2008, 130, 3888–3891 CrossRef CAS PubMed.
- G. Calvez, F. Le Natur, C. Daiguebonne, K. Bernot, Y. Suffren and O. Guillou, Coord. Chem. Rev., 2017, 340, 134–153 CrossRef CAS.
- B.-K. Ling, J. Li, Y.-Q. Zhai, H.-K. Hsu, Y.-T. Chan, W.-P. Chen, T. Han and Y.-Z. Zheng, Chem. Commun., 2020, 56, 9130–9133 RSC.
- N. Mahé, O. Guillou, C. Daiguebonne, Y. Gérault, A. Caneschi, C. Sangregorio, J. Y. Chane-Ching, P. E. Car and T. Roisnel, Inorg. Chem., 2005, 44, 7743–7750 CrossRef PubMed.
- R. Wang, M. D. Carducci and Z. Zheng, Inorg. Chem., 2000, 39, 1836–1837 CrossRef CAS PubMed.
- J. H. Melman, T. J. Emge and J. G. Brennan, Chem. Commun., 1997, 2269–2270 RSC.
- K. Sheng, W.-D. Si, R. Wang, W.-Z. Wang, J. Dou, Z.-Y. Gao, L.-K. Wang, C.-H. Tung and D. Sun, Chem. Mater., 2022, 34, 4186–4194 CrossRef CAS.
- J.-N. Li, N.-F. Li, J.-L. Wang, X.-M. Liu, Q.-D. Ping, T.-T. Zang, H. Mei and Y. Xu, Dalton Trans., 2021, 50, 13925–13931 RSC.
- L. Qin, G.-J. Zhou, Y.-Z. Yu, H. Nojiri, C. Schröder, R. E. P. Winpenny and Y.-Z. Zheng, J. Am. Chem. Soc., 2017, 139, 16405–16411 CrossRef CAS PubMed.
- W. Huang, Z. Zhang, Y. Wu, W. Chen, D. A. Rotsch, L. Messerle and Z. Zheng, Inorg. Chem. Front., 2021, 8, 26–34 RSC.
- G.-J. Zhou, W.-P. Chen, Y. Yu, L. Qin, T. Han and Y.-Z. Zheng, Inorg. Chem., 2017, 56, 12821–12829 CrossRef CAS PubMed.
- D. A. Gálico and M. Murugesu, ACS Appl. Mater. Interfaces, 2021, 13, 47052–47060 CrossRef PubMed.
- D. A. Gálico, A. A. Kitos, J. S. Ovens, F. A. Sigoli and M. Murugesu, Angew. Chem., Int. Ed., 2021, 60, 6130–6136 CrossRef PubMed.
- X.-J. Kong, Y. Wu, L.-S. Long, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918–6919 CrossRef CAS PubMed.
- X.-M. Luo, N.-F. Li, Q.-F. Lin, J.-P. Cao, P. Yuan and Y. Xu, Inorg. Chem. Front., 2020, 7, 2072–2079 RSC.
- X.-M. Luo, Z.-B. Hu, Q.-F. Lin, W. Cheng, J.-P. Cao, C.-H. Cui, H. Mei, Y. Song and Y. Xu, J. Am. Chem. Soc., 2018, 140, 11219–11222 CrossRef CAS PubMed.
- W. Huang, W. Chen, Q. Bai, Z. Zhang, M. Feng and Z. Zheng, Angew. Chem., Int. Ed., 2022, 61, e202205385 CAS.
- S.-S. Chen, H.-F. Su, L.-S. Long, L.-S. Zheng and X.-J. Kong, Inorg. Chem., 2021, 60, 16922–16926 CrossRef CAS PubMed.
- B. Russell-Webster, K. A. Abboud and G. Christou, Chem. Commun., 2020, 56, 5382–5385 RSC.
- B. Russell-Webster, J. Lopez-Nieto, K. A. Abboud and G. Christou, Dalton Trans., 2021, 50, 15524–15532 RSC.
- K. J. Mitchell, J. L. Goodsell, B. Russell-Webster, U. T. Twahir, A. Angerhofer, K. A. Abboud and G. Christou, Inorg. Chem., 2021, 60, 1641–1653 CrossRef CAS PubMed.
- K. J. Mitchell, K. A. Abboud and G. Christou, Nat. Commun., 2017, 8, 1445 CrossRef PubMed.
- M. N. Akhtar, X.-F. Liao, Y.-C. Chen, M. A. AlDamen, G.-Z. Huang, J.-L. Liu, J. Khan and M.-L. Tong, Inorg. Chim. Acta, 2018, 482, 240–245 CrossRef CAS.
- X.-M. Chen, S. M. J. Aubin, Y.-L. Wu, Y.-S. Yang, T. C. W. Mak and D. N. Hendrickson, J. Am. Chem. Soc., 1995, 117, 9600–9601 CrossRef CAS.
- Y. Zheng, Q.-C. Zhang, L.-S. Long, R.-B. Huang, A. Müller, J. Schnack, L.-S. Zheng and Z. Zheng, Chem. Commun., 2013, 49, 36–38 RSC.
- B. Hu, M.-L. Feng, J.-R. Li, Q.-P. Lin and X.-Y. Huang, Angew. Chem., Int. Ed., 2011, 50, 8110–8113 CrossRef CAS PubMed.
- X.-Y. Zheng, S.-Q. Wang, W. Tang, G.-L. Zhuang, X.-J. Kong, Y.-P. Ren, L.-S. Long and L.-S. Zheng, Chem. Commun., 2015, 51, 10687–10690 RSC.
- X.-J. Kong, Y.-P. Ren, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, J. Am. Chem. Soc., 2007, 129, 7016–7017 CrossRef CAS PubMed.
- H.-J. Lun, L. Xu, X.-J. Kong, L.-S. Long and L.-S. Zheng, Inorg. Chem., 2021, 60, 10079–10083 CrossRef CAS PubMed.
- N. Li, Q. Lin, Y. Han, Z. Du and Y. Xu, Chinese Chem. Lett., 2021, 32, 3803–3806 CrossRef CAS.
- W.-P. Chen, J. Singleton, L. Qin, A. Camón, L. Engelhardt, F. Luis, R. E. P. Winpenny and Y.-Z. Zheng, Nat. Commun., 2018, 9, 2107 CrossRef PubMed.
- X.-J. Kong, Y.-P. Ren, L.-S. Long, Z. Zheng, G. Nichol, R.-B. Huang and L.-S. Zheng, Inorg. Chem., 2008, 47, 2728–2739 CrossRef CAS PubMed.
- N.-F. Li, Y.-M. Han, J.-N. Li, Q. Chen and Y. Xu, Dalton Trans., 2022, 51, 2669–2673 RSC.
- N.-F. Li, Q. Wang, J.-N. Li, Y.-T. Yu and Y. Xu, Inorg. Chem., 2022, 61, 7180–7187 CrossRef CAS PubMed.
- Q. Lin, Y. Zhang, W. Cheng, Y. Liu and Y. Xu, Dalton Trans., 2017, 46, 643–646 RSC.
- N.-F. Li, Q.-F. Lin, X.-M. Luo, J.-P. Cao and Y. Xu, Inorg. Chem., 2019, 58, 10883–10889 CrossRef CAS PubMed.
- Q. Lin, J. Li, Y. Dong, G. Zhou, Y. Song and Y. Xu, Dalton Trans., 2017, 46, 9745–9749 RSC.
- D.-P. Liu, X.-P. Lin, H. Zhang, X.-Y. Zheng, G.-L. Zhuang, X.-J. Kong, L.-S. Long and L.-S. Zheng, Angew. Chem., Int. Ed., 2016, 55, 4532–4536 CrossRef CAS PubMed.
- R. Chen, Z.-H. Yan, X.-J. Kong, L.-S. Long and L.-S. Zheng, Angew. Chem., Int. Ed., 2018, 57, 16796–16800 CrossRef CAS PubMed.
- X.-J. Kong, Y.-P. Ren, W.-X. Chen, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, Angew. Chem., Int. Ed., 2008, 47, 2398–2401 CrossRef CAS PubMed.
- X.-J. Kong, L.-S. Long, R.-B. Huang, L.-S. Zheng, T. D. Harris and Z. Zheng, Chem. Commun., 2009, 4354–4356 RSC.
- M.-H. Du, X.-Y. Zheng, X.-J. Kong, L.-S. Long and L.-S. Zheng, Matter, 2020, 3, 1334–1349 CrossRef.
- Q.-f Lin, J. Li, X.-m Luo, C.-h Cui, Y. Song and Y. Xu, Inorg. Chem., 2018, 57, 4799–4802 CrossRef CAS PubMed.
- M.-H. Du, D.-H. Wang, L.-W. Wu, L.-P. Jiang, J.-P. Li, L.-S. Long, L.-S. Zheng and X.-J. Kong, Angew. Chem., Int. Ed., 2022, 61, e202200537 CAS.
- Y. Li, M. Zhou and R. Jin, Adv. Mater., 2021, 33, 2006591 CrossRef CAS PubMed.
- A. Kondinski and K. Y. Monakhov, Chem. – Eur. J., 2017, 23, 7841–7852 CrossRef CAS PubMed.
- F. Schax and C. Limberg, Z. Anorg. Allg. Chem., 2015, 641, 2060–2064 CrossRef CAS.
- X.-Q. Zhao, J. Wang, D.-X. Bao, S. Xiang and D.-B. Luo, Inorg. Chem. Commun., 2015, 62, 77–80 CrossRef CAS.
- N. Yoshinari, A. Igashira-Kamiyama and T. Konno, Chem. – Eur. J., 2010, 16, 14247–14251 CrossRef CAS PubMed.
- N. Yoshinari and T. Konno, Dalton Trans., 2011, 40, 12191–12200 RSC.
- H. J. Schugar, C.-C. Ou, J. A. Thich, J. A. Potenza, T. R. Felthouse, M. S. Haddad, D. N. Hendrickson, W. Furey and R. A. Lalancette, Inorg. Chem., 1980, 19, 543–552 CrossRef CAS.
- H. Suzuki, C. Otani, N. Yoshinari and T. Konno, Inorg. Chem. Front., 2016, 3, 274–278 RSC.
- C. Yue, C. Yan, R. Feng, M. Wu, L. Chen, F. Jiang and M. Hong, Inorg. Chem., 2009, 48, 2873–2879 CrossRef CAS PubMed.
- C. W. Liu, H.-C. Chen, C. W. Liu, J.-C. Wang and T.-C. Keng, Chem. Commun., 1998, 1831–1832 RSC.
- C. W. Liu, C.-M. Hung, B. K. Santra, H.-C. Chen, Hsueh and J.-C. Wang, Inorg. Chem., 2003, 42, 3216–3220 CrossRef CAS PubMed.
- C. W. Liu, C.-M. Hung, B. K. Santra, Y.-H. Chu, J.-C. Wang and Z. Lin, Inorg. Chem., 2004, 43, 4306–4314 CrossRef CAS PubMed.
- C. W. Liu, C.-M. Hung, H.-C. Chen, J.-C. Wang, T.-C. Keng and K. Guo, Chem. Commun., 2000, 1897–1898 RSC.
- C. W. Liu, B. Sarkar, Y.-J. Huang, P.-K. Liao, J.-C. Wang, J.-Y. Saillard and S. Kahlal, J. Am. Chem. Soc., 2009, 131, 11222–11233 CrossRef CAS PubMed.
- C. W. Liu, M. D. Irwin, A. A. Mohamed and J. P. Fackler, Inorg. Chim. Acta, 2004, 357, 3950–3956 CrossRef CAS.
- G. F. Manbeck, A. J. Lipman, R. A. Stockland, A. L. Freidl, A. F. Hasler, J. J. Stone and I. A. Guzei, J. Org. Chem., 2005, 70, 244–250 CrossRef CAS PubMed.
- N. Assavathorn, R. P. Davies and A. J. P. White, Polyhedron, 2008, 27, 992–998 CrossRef CAS.
- S. Perruchas, K. Boubekeur and P. Auban-Senzier, J. Mater. Chem., 2004, 14, 3509–3515 RSC.
- C. W. Liu, C.-M. Hung, B. K. Santra, J.-C. Wang, H.-M. Kao and Z. Lin, Inorg. Chem., 2003, 42, 8551–8556 CrossRef CAS PubMed.
- F. J. Hollander and D. Coucouvanis, J. Am. Chem. Soc., 1977, 99, 6268–6280 CrossRef CAS.
- D. Cardell, G. Hogarth and S. Faulkner, Inorg. Chim. Acta, 2006, 359, 1321–1324 CrossRef CAS.
- S. A. Baudron, M. W. Hosseini and N. Kyritsakas, New J. Chem., 2006, 30, 1083–1086 RSC.
- C. Xu, X.-Y. Yi, T.-K. Duan, Q. Chen and Q.-F. Zhang, Polyhedron, 2011, 30, 2637–2643 CrossRef CAS.
- L.-J. Liu, Z.-Y. Wang, Z.-Y. Wang, R. Wang, S.-Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2022, 61, e202205626 CAS.
- X.-W. Zhao, L.-W. Qian, H.-C. Su, C.-J. Mo, C.-J. Que, Q.-Y. Zhu and J. Dai, Cryst. Growth Des., 2015, 15, 5749–5753 CrossRef CAS.
- K. K. Chakrahari, J.-H. Liao, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2016, 55, 14704–14708 CrossRef CAS PubMed.
- K. K. Chakrahari, R. P. B. Silalahi, J.-H. Liao, S. Kahlal, Y.-C. Liu, J.-F. Lee, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Chem. Sci., 2018, 9, 6785–6795 RSC.
- P. Betz, B. Krebs and G. Henkel, Angew. Chem., Int. Ed. Engl., 1984, 23, 311–312 CrossRef.
- X.-X. Yang, I. Issac, S. Lebedkin, M. Kühn, F. Weigend, D. Fenske, O. Fuhr and A. Eichhöfer, Chem. Commun., 2014, 50, 11043–11045 RSC.
- A. Eichhöfer, G. Buth, S. Lebedkin, M. Kühn and F. Weigend, Inorg. Chem., 2015, 54, 9413–9422 CrossRef PubMed.
- N. Wiberg, A. Wörner, D. Fenske, H. Nöth, J. Knizek and K. Polborn, Angew. Chem., Int. Ed., 2000, 39, 1838–1842 CrossRef CAS PubMed.
- U. Riese, N. Faza, W. Massa and K. Dehnicke, Angew. Chem., Int. Ed., 1999, 38, 528–531 CrossRef CAS PubMed.
- G. Tan, Y. Yang, C. Chu, H. Zhu and H. W. Roesky, J. Am. Chem. Soc., 2010, 132, 12231–12233 CrossRef CAS PubMed.
- K. Freitag, H. Banh, C. Gemel, R. W. Seidel, S. Kahlal, J.-Y. Saillard and R. A. Fischer, Chem. Commun., 2014, 50, 8681–8684 RSC.
- P.-K. Liao, K.-G. Liu, C.-S. Fang, C. W. Liu, J. P. Fackler and Y.-Y. Wu, Inorg. Chem., 2011, 50, 8410–8417 CrossRef CAS PubMed.
- P.-K. Liao, B. Sarkar, H.-W. Chang, J.-C. Wang and C. W. Liu, Inorg. Chem., 2009, 48, 4089–4097 CrossRef CAS PubMed.
- P.-K. Liao, C.-S. Fang, A. J. Edwards, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2012, 51, 6577–6591 CrossRef CAS PubMed.
- K. Tang, T. Xia, X. Jin and Y. Tang, Polyhedron, 1993, 12, 2895–2898 CrossRef CAS.
- Y.-L. Li, J. Wang, P. Luo, X.-H. Ma, X.-Y. Dong, Z.-Y. Wang, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Adv. Sci., 2019, 6, 1900833 CrossRef CAS PubMed.
- B. P. Johnson, F. Dielmann, G. Balázs, M. Sierka and M. Scheer, Angew. Chem., Int. Ed., 2006, 45, 2473–2475 CrossRef CAS PubMed.
- F. Dielmann, E. V. Peresypkina, B. Krämer, F. Hastreiter, B. P. Johnson, M. Zabel, C. Heindl and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 14833–14837 CrossRef CAS PubMed.
- Q.-H. Wei, G.-Q. Yin, L.-Y. Zhang and Z.-N. Chen, Organometallics, 2006, 25, 4941–4944 CrossRef CAS.
- Q.-Y. Wang, J. Wang, S. Wang, Z.-Y. Wang, M. Cao, C.-L. He, J.-Q. Yang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2020, 142, 12010–12014 CrossRef CAS PubMed.
- Z.-H. Wei, C.-Y. Ni, H.-X. Li, Z.-G. Ren, Z.-R. Sun and J.-P. Lang, Chem. Commun., 2013, 49, 4836–4838 RSC.
- B.-L. Han, Z. Wang, R. K. Gupta, L. Feng, S. Wang, M. Kurmoo, Z.-Y. Gao, S. Schein, C.-H. Tung and D. Sun, ACS Nano, 2021, 15, 8733–8741 CrossRef CAS PubMed.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Jin, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- H. Shen, Y.-Z. Han, Q. Wu, J. Peng, B. K. Teo and N. Zheng, Small Methods, 2021, 5, 2000603 CrossRef CAS PubMed.
- H. Li, F. Song, D. Zhu, Y. Song, C. Zhou, F. Ke, L. Lu, X. Kang and M. Zhu, J. Am. Chem. Soc., 2022, 144, 4845–4852 CrossRef CAS PubMed.
- L. Jiguo, X. Xinquan, Z. Zhongyuan and Y. Kaibei, Chem. Commun., 1991, 249–250 RSC.
- J. Zhang, W. Chen, Y. Fang, D. Jia, Y. Wang, Y. Song and C. Zhang, Polyhedron, 2015, 101, 1–5 CrossRef CAS.
- J. Zhang, J. Feng, G. Tang, J. Wu, F. Luo, B. Xu and C. Zhang, J. Clust. Sci., 2018, 29, 385–390 CrossRef CAS.
- J.-F. Zhang, W.-T. Chen, Y.-L. Wang and C. Zhang, J. Clust. Sci., 2015, 26, 973–982 CrossRef CAS.
- A. J. Edwards, R. S. Dhayal, P.-K. Liao, J.-H. Liao, M.-H. Chiang, R. O. Piltz, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2014, 53, 7214–7218 CrossRef CAS PubMed.
- J. Weßing, C. Ganesamoorthy, S. Kahlal, R. Marchal, C. Gemel, O. Cador, A. C. H. Da Silva, J. L. F. Da Silva, J.-Y. Saillard and R. A. Fischer, Angew. Chem., Int. Ed., 2018, 57, 14630–14634 CrossRef PubMed.
- X.-K. Wan, X.-L. Cheng, Q. Tang, Y.-Z. Han, G. Hu, D.-E. Jiang and Q.-M. Wang, J. Am. Chem. Soc, 2017, 139, 9451–9454 CrossRef CAS PubMed.
- X. Zou, Y. Li, S. Jin, X. Kang, X. Wei, S. Wang, X. Meng and M. Zhu, J. Phys. Chem. Lett., 2020, 11, 2272–2276 CrossRef CAS PubMed.
- D. Fenske and T. Langetepe, Angew. Chem., Int. Ed., 2002, 41, 300–304 CrossRef CAS PubMed.
- J.-H. Liao, C. Latouche, B. Li, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2014, 53, 2260–2267 CrossRef CAS PubMed.
- J.-H. Liao, H.-W. Chang, Y.-J. Li, C.-S. Fang, B. Sarkar, W. E. van Zyl and C. W. Liu, Dalton Trans., 2014, 43, 12380–12389 RSC.
- C. W. Liu, I.-J. Shang, J.-C. Wang and T.-C. Keng, Chem. Commun., 1999, 995–996 RSC.
- Z. Han, X.-Y. Dong, P. Luo, S. Li, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Sci. Adv., 2020, 6, eaay0107 CrossRef CAS PubMed.
- X.-M. Luo, C.-H. Gong, X.-Y. Dong, L. Zhang and S.-Q. Zang, Nano Res., 2021, 14, 2309–2313 CrossRef CAS.
- M.-Y. Gao, K. Wang, Y. Sun, D. Li, B.-Q. Song, Y. H. Andaloussi, M. J. Zaworotko, J. Zhang and L. Zhang, J. Am. Chem. Soc., 2020, 142, 12784–12790 CrossRef CAS PubMed.
- C.-C. Li, S. Zhang, J. Tang, R. Jian, Y. Xia and L. Zhao, Chem. Sci., 2022, 13, 8095–8103 RSC.
- S. Li, Z.-Y. Wang, G.-G. Gao, B. Li, P. Luo, Y.-J. Kong, H. Liu and S.-Q. Zang, Angew. Chem., Int. Ed., 2018, 57, 12775–12779 CrossRef CAS PubMed.
- G.-G. Luo, H.-F. Su, A. Xiao, Z. Wang, Y. Zhao, Q.-Y. Wu, J.-H. Wu, D. Sun and L.-S. Zheng, Chem. – Eur. J., 2017, 23, 14420–14424 CrossRef CAS PubMed.
- D. Rais, J. Yau, D. M. P. Mingos, R. Vilar, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2001, 40, 3464–3467 CrossRef CAS PubMed.
- D. Rais, D. M. P. Mingos, R. Vilar, A. J. P. White and D. J. Williams, J. Organomet. Chem., 2002, 652, 87–93 CrossRef CAS.
- X.-T. Shen, X.-L. Ma, Q.-L. Ni, M.-X. Ma, L.-C. Gui, C. Hou, R.-B. Hou and X.-J. Wang, Nanoscale, 2018, 10, 515–519 RSC.
- L. Qin, F. Sun, X. Ma, G. Ma, Y. Tang, L. Wang, Q. Tang, R. Jin and Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 26136–26141 CrossRef CAS PubMed.
- X. Ma, L. Xiong, L. Qin, Y. Tang, G. Ma, Y. Pei and Z. Tang, Chem. Sci., 2021, 12, 12819–12826 RSC.
- Y. Wang, H. Su, L. Ren, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, Angew. Chem., Int. Ed., 2016, 55, 15152–15156 CrossRef CAS PubMed.
- X. Ma, F. Sun, L. Qin, Y. Liu, X. Kang, L. Wang, D.-E. Jiang, Q. Tang and Z. Tang, Chem. Sci., 2022, 13, 10149–10158 RSC.
- X.-Y. Li, H.-F. Su, K. Yu, Y.-Z. Tan, X.-P. Wang, Y.-Q. Zhao, D. Sun and L.-S. Zheng, Nanoscale, 2015, 7, 8284–8288 RSC.
- Z. Wang, J.-W. Liu, H.-F. Su, Q.-Q. Zhao, M. Kurmoo, X.-P. Wang, C.-H. Tung, D. Sun and L.-S. Zheng, J. Am. Chem. Soc., 2019, 141, 17884–17890 CrossRef CAS PubMed.
- Z. Wang, M.-D. Li, J.-Y. Shi, H.-F. Su, J.-W. Liu, L. Feng, Z.-Y. Gao, Q.-W. Xue, C.-H. Tung, D. Sun and L.-S. Zheng, CCS Chemistry, 2021, 4, 1788–1795 CrossRef.
- R.-W. Huang, X.-Y. Dong, B.-J. Yan, X.-S. Du, D.-H. Wei, S.-Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2018, 57, 8560–8566 CrossRef CAS PubMed.
- R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- J.-Y. Wang, R.-W. Huang, Z. Wei, X.-J. Xi, X.-Y. Dong and S.-Q. Zang, Chem. – Eur. J., 2019, 25, 3376–3381 CrossRef CAS PubMed.
- H. Liu, C.-Y. Song, R.-W. Huang, Y. Zhang, H. Xu, M.-J. Li, S.-Q. Zang and G.-G. Gao, Angew. Chem., Int. Ed., 2016, 55, 3699–3703 CrossRef CAS PubMed.
- S. C. K. Hau, P.-S. Cheng and T. C. W. Mak, J. Am. Chem. Soc., 2012, 134, 2922–2925 CrossRef CAS PubMed.
- H. Shen and T. Mizuta, Chem. – Asian J., 2017, 12, 2904–2907 CrossRef CAS PubMed.
- T. Li, X. Cui, L. Liang, C. Luo, H. Li and X.-M. Zhang, RSC Adv., 2020, 10, 19397–19400 RSC.
- Y.-P. Xie, J.-L. Jin, X. Lu and T. C. W. Mak, Angew. Chem., Int. Ed., 2015, 54, 15176–15180 CrossRef CAS PubMed.
- J.-L. Jin, Y.-P. Xie, H. Cui, G.-X. Duan, X. Lu and T. C. W. Mak, Inorg. Chem., 2017, 56, 10412–10417 CrossRef CAS PubMed.
- Y.-M. Su, Z. Wang, S. Schein, C.-H. Tung and D. Sun, Nat. Commun., 2020, 11, 3316 CrossRef CAS PubMed.
- C. Deng, C. Sun, Z. Wang, Y. Tao, Y. Chen, J. Lin, G. Luo, B. Lin, D. Sun and L. Zheng, Angew. Chem., Int. Ed., 2020, 59, 12659–12663 CrossRef CAS PubMed.
- Y.-M. Su, Z. Wang, C.-H. Tung, D. Sun and S. Schein, J. Am. Chem. Soc., 2021, 143, 13235–13244 CrossRef CAS PubMed.
- X.-M. Luo, C.-H. Gong, F. Pan, Y. Si, J.-W. Yuan, M. Asad, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Nat. Commun., 2022, 13, 1177 CrossRef CAS PubMed.
- Z. Wang, H.-F. Su, Y.-Z. Tan, S. Schein, S.-C. Lin, W. Liu, S.-A. Wang, W.-G. Wang, C.-H. Tung, D. Sun and L.-S. Zheng, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12132–12137 CrossRef CAS PubMed.
- D. Sun, G.-G. Luo, N. Zhang, R.-B. Huang and L.-S. Zheng, Chem. Commun., 2011, 47, 1461–1463 RSC.
- G.-X. Duan, J. Han, B.-Z. Yang, Y.-P. Xie and X. Lu, Nanoscale, 2020, 12, 1617–1622 RSC.
- J. Yan, H. Su, H. Yang, C. Hu, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2016, 138, 12751–12754 CrossRef CAS PubMed.
- M. S. Bootharaju, H. Chang, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2019, 141, 8422–8425 CrossRef CAS PubMed.
- J. Chai, S. Yang, T. Chen, Q. Li, S. Wang and M. Zhu, Inorg. Chem., 2021, 60, 9050–9056 CrossRef CAS PubMed.
- X.-M. Luo, S. Huang, P. Luo, K. Ma, Z.-Y. Wang, X.-Y. Dong and S.-Q. Zang, Chem. Sci., 2022, 13, 11110–11118 RSC.
- Y.-M. Su, B.-Q. Ji, Z. Wang, S.-S. Zhang, L. Feng, Z.-Y. Gao, Y.-W. Li, C.-H. Tung, D. Sun and L.-S. Zheng, Sci. China Chem., 2021, 64, 1482–1486 CrossRef CAS.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300–302 RSC.
- A. K. Das, R. Mekkat, S. Maity, A. S. Nair, S. Bhandary, R. Bhowal, A. Patra, B. Pathak, D. Chopra and S. Mandal, Inorg. Chem., 2021, 60, 19270–19277 CrossRef CAS PubMed.
- G. Deng, S. Malola, P. Yuan, X. Liu, B. K. Teo, H. Häkkinen and N. Zheng, Angew. Chem., Int. Ed., 2021, 60, 12897–12903 CrossRef CAS PubMed.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- X.-Q. Liang, Y.-Z. Li, Z. Wang, S.-S. Zhang, Y.-C. Liu, Z.-Z. Cao, L. Feng, Z.-Y. Gao, Q.-W. Xue, C.-H. Tung and D. Sun, Nat. Commun., 2021, 12, 4966 CrossRef CAS PubMed.
- Q. Sun, H.-H. Nie, H.-F. Su, S.-Y. Yang and B. K. Teo, Inorg. Chem., 2020, 59, 8836–8845 CrossRef CAS PubMed.
- H. Yang, J. Yan, Y. Wang, H. Su, L. Gell, X. Zhao, C. Xu, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2017, 139, 31–34 CrossRef CAS PubMed.
- Z.-Y. Wang, M.-Q. Wang, Y.-L. Li, P. Luo, T.-T. Jia, R.-W. Huang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- X. Yuan, C. Sun, X. Li, S. Malola, B. K. Teo, H. Häkkinen, L.-S. Zheng and N. Zheng, J. Am. Chem. Soc., 2019, 141, 11905–11911 CrossRef CAS PubMed.
- B. K. Teo, H. Yang, J. Yan and N. Zheng, Inorg. Chem., 2017, 56, 11470–11479 CrossRef CAS PubMed.
- M. Bodiuzzaman, A. Ghosh, K. S. Sugi, A. Nag, E. Khatun, B. Varghese, G. Paramasivam, S. Antharjanam, G. Natarajan and T. Pradeep, Angew. Chem., Int. Ed., 2019, 58, 189–194 CrossRef CAS PubMed.
- J. Chai, S. Yang, Y. Lv, T. Chen, S. Wang, H. Yu and M. Zhu, J. Am. Chem. Soc., 2018, 140, 15582–15585 CrossRef CAS PubMed.
- X. Ma, Y. Bai, Y. Song, Q. Li, Y. Lv, H. Zhang, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 17234–17238 CrossRef CAS PubMed.
- M. J. Alhilaly, M. S. Bootharaju, C. P. Joshi, T. M. Besong, A.-H. Emwas, R. Juarez-Mosqueda, S. Kaappa, S. Malola, K. Adil, A. Shkurenko, H. Häkkinen, M. Eddaoudi and O. M. Bakr, J. Am. Chem. Soc., 2016, 138, 14727–14732 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 2422 CrossRef PubMed.
- R. S. Dhayal, Y.-R. Lin, J.-H. Liao, Y.-J. Chen, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Chem. – Eur. J., 2016, 22, 9943–9947 CrossRef CAS PubMed.
- R. S. Dhayal, J.-H. Liao, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702–3706 CrossRef CAS PubMed.
- S.-F. Yuan, Z.-J. Guan, W.-D. Liu and Q.-M. Wang, Nat. Commun., 2019, 10, 4032 CrossRef PubMed.
- S. K. Barik, T.-H. Chiu, Y.-C. Liu, M.-H. Chiang, F. Gam, I. Chantrenne, S. Kahlal, J.-Y. Saillard and C. W. Liu, Nanoscale, 2019, 11, 14581–14586 RSC.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578–11581 CrossRef CAS PubMed.
- Q. Li, S. Wang, K. Kirschbaum, K. J. Lambright, A. Das and R. Jin, Chem. Commun., 2016, 52, 5194–5197 RSC.
- X. Liu, J. Yuan, C. Yao, J. Chen, L. Li, X. Bao, J. Yang and Z. Wu, J. Phys. Chem. C, 2017, 121, 13848–13853 CrossRef CAS.
- X.-J. Xi, J.-S. Yang, J.-Y. Wang, X.-Y. Dong and S.-Q. Zang, Nanoscale, 2018, 10, 21013–21018 RSC.
- Z.-J. Guan, J.-L. Zeng, Z.-A. Nan, X.-K. Wan, Y.-M. Lin and Q.-M. Wang, Sci. Adv., 2016, 2, e1600323 CrossRef PubMed.
- S.-F. Yuan, C.-Q. Xu, W.-D. Liu, J.-X. Zhang, J. Li and Q.-M. Wang, J. Am. Chem. Soc., 2021, 143, 12261–12267 CrossRef CAS PubMed.
- F. Hu, J.-J. Li, Z.-J. Guan, S.-F. Yuan and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 5312–5315 CrossRef CAS PubMed.
- T. Chen, S. Yang, J. Chai, Y. Song, J. Fan, B. Rao, H. Sheng, H. Yu and M. Zhu, Sci. Adv., 2017, 3, e1700956 CrossRef PubMed.
- W.-T. Chang, P.-Y. Lee, J.-H. Liao, K. K. Chakrahari, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2017, 56, 10178–10182 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. Del Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-E. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970–11975 CrossRef CAS PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A.-H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, M. Harb, M. R. Parida, M. N. Hedhili, O. F. Mohammed, O. M. Bakr, L. Cavallo and J.-M. Basset, Nanoscale, 2017, 9, 9529–9536 RSC.
- X. Kang, H. Abroshan, S. Wang and M. Zhu, Inorg. Chem., 2019, 58, 11000–11009 CrossRef CAS PubMed.
- X. Kang, M. Zhou, S. Wang, S. Jin, G. Sun, M. Zhu and R. Jin, Chem. Sci., 2017, 8, 2581–2587 RSC.
- X. Kang, X. Wei, S. Jin, Q. Yuan, X. Luan, Y. Pei, S. Wang, M. Zhu and R. Jin, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18834–18840 CrossRef CAS PubMed.
- J.-H. Huang, Y. Si, X.-Y. Dong, Z.-Y. Wang, L.-Y. Liu, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2021, 143, 12439–12444 CrossRef CAS PubMed.
- Y. Wang, H. Su, C. Xu, G. Li, L. Gell, S. Lin, Z. Tang, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 4324–4327 CrossRef CAS PubMed.
- Y. Tang, F. Sun, X. Ma, L. Qin, G. Ma, Q. Tang and Z. Tang, Dalton Trans., 2022, 51, 7845–7850 RSC.
- J. Yan, S. Malola, C. Hu, J. Peng, B. Dittrich, B. K. Teo, H. Häkkinen, L. Zheng and N. Zheng, Nat. Commun., 2018, 9, 3357 CrossRef PubMed.
- A. Dass, S. Theivendran, P. R. Nimmala, C. Kumara, V. R. Jupally, A. Fortunelli, L. Sementa, G. Barcaro, X. Zuo and B. C. Noll, J. Am. Chem. Soc., 2015, 137, 4610–4613 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. Appavoo, M. Y. Sfeir and R. Jin, Sci. Adv., 2015, 1, e1500045 CrossRef PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- Y. Chen, C. Zeng, C. Liu, K. Kirschbaum, C. Gayathri, R. R. Gil, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2015, 137, 10076–10079 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS PubMed.
- M.-X. Ma, X.-L. Ma, G.-M. Liang, X.-T. Shen, Q.-L. Ni, L.-C. Gui, X.-J. Wang, S.-Y. Huang and S.-M. Li, J. Am. Chem. Soc., 2021, 143, 13731–13737 CrossRef CAS PubMed.
- S. Vergara, D. A. Lukes, M. W. Martynowycz, U. Santiago, G. Plascencia-Villa, S. C. Weiss, M. J. de la Cruz, D. M. Black, M. M. Alvarez, X. López-Lozano, C. O. Barnes, G. Lin, H.-C. Weissker, R. L. Whetten, T. Gonen, M. J. Yacaman and G. Calero, J. Phys. Chem. Lett., 2017, 8, 5523–5530 CrossRef CAS PubMed.
- V. G. Albano, L. Grossi, G. Longoni, M. Monari, S. Mulley and A. Sironi, J. Am. Chem. Soc., 1992, 114, 5708–5713 CrossRef CAS.
- V. G. Albano, F. Calderoni, M. C. Iapalucci, G. Longoni, M. Monari and P. Zanello, J. Clust. Sci., 1995, 6, 107–123 CrossRef CAS.
- D. Collini, C. Femoni, M. C. Iapalucci and G. Longoni, C. R. Chim., 2005, 8, 1645–1654 CrossRef CAS.
- J. Li, X. Li, H.-J. Zhai and L.-S. Wang, Science, 2003, 299, 864–867 CrossRef CAS PubMed.
- H.-F. Zhang, M. Stender, R. Zhang, C. Wang, J. Li and L.-S. Wang, J. Phys. Chem. B, 2004, 108, 12259–12263 CrossRef CAS.
- J. Chen, Q.-F. Zhang, P. G. Williard and L.-S. Wang, Inorg. Chem., 2014, 53, 3932–3934 CrossRef CAS PubMed.
- X.-K. Wan, Z.-W. Lin and Q.-M. Wang, J. Am. Chem. Soc., 2012, 134, 14750–14752 CrossRef CAS PubMed.
- D. Schooss, P. Weis, O. Hampe and M. M. Kappes, Phil. Trans. R. Soc. A, 2010, 368, 1211–1243 CrossRef CAS PubMed.
- Q.-F. Zhang, X. Chen and L.-S. Wang, Acc. Chem. Res., 2018, 51, 2159–2168 CrossRef CAS PubMed.
- L.-M. Wang and L.-S. Wang, Nanoscale, 2012, 4, 4038–4053 RSC.
- Z. Li, H.-Y. T. Chen, K. Schouteden, T. Picot, T.-W. Liao, A. Seliverstov, C. Van Haesendonck, G. Pacchioni, E. Janssens and P. Lievens, Sci. Adv., 2020, 6, eaay4289 CrossRef CAS PubMed.
- Y.-K. Li, F. Müller, W. Schöllkopf, K. R. Asmis and J. Sauer, Angew. Chem., Int. Ed., 2022, 61, e202202297 CAS.
- Y.-K. Li, S. Debnath, M. Schlangen, W. Schöllkopf, K. R. Asmis and H. Schwarz, Angew. Chem., Int. Ed., 2019, 58, 18868–18872 CrossRef CAS PubMed.
- J. Dong, Z.-H. Gao, Q.-F. Zhang and L.-S. Wang, Angew. Chem., Int. Ed., 2021, 60, 2424–2430 CrossRef CAS PubMed.
- J. Chen, Q.-F. Zhang, T. A. Bonaccorso, P. G. Williard and L.-S. Wang, J. Am. Chem. Soc., 2014, 136, 92–95 CrossRef CAS PubMed.
- J. Dong, J. R. Robinson, Z.-H. Gao and L.-S. Wang, J. Am. Chem. Soc., 2022, 144, 12501–12509 CrossRef CAS PubMed.
- Z.-H. Gao, K. Wei, T. Wu, J. Dong, D.-E. Jiang, S. Sun and L.-S. Wang, J. Am. Chem. Soc., 2022, 144, 5258–5262 CrossRef CAS PubMed.
- G. Marbach and J. Strähle, Angew. Chem., Int. Ed. Engl., 1984, 23, 715–716 CrossRef.
- S.-P. Huang and M. G. Kanatzidis, Angew. Chem., Int. Ed. Engl., 1992, 31, 787–789 CrossRef.
- C. E. Briant, B. R. C. Theobald, J. W. White, L. K. Bell, D. M. P. Mingos and A. J. Welch, Chem. Commun., 1981, 201–202 RSC.
- H. Shen, S. Xiang, Z. Xu, C. Liu, X. Li, C. Sun, S. Lin, B. K. Teo and N. Zheng, Nano Res., 2020, 13, 1908–1911 CrossRef CAS.
- H. Yang, Y. Wang, J. Lei, L. Shi, X. Wu, V. Mäkinen, S. Lin, Z. Tang, J. He, H. Häkkinen, L. Zheng and N. Zheng, J. Am. Chem. Soc., 2013, 135, 9568–9571 CrossRef CAS PubMed.
- S.-F. Yuan, C.-Q. Xu, J. Li and Q.-M. Wang, Angew. Chem., Int. Ed., 2019, 58, 5906–5909 CrossRef CAS PubMed.
- S. Kenzler, F. Fetzer, C. Schrenk, N. Pollard, A. R. Frojd, A. Z. Clayborne and A. Schnepf, Angew. Chem., Int. Ed., 2019, 58, 5902–5905 CrossRef CAS PubMed.
- N. Yan, N. Xia, L. Liao, M. Zhu, F. Jin, R. Jin and Z. Wu, Sci. Adv., 2018, 4, eaat7259 CrossRef CAS PubMed.
- Z. Lei, J.-J. Li, X.-K. Wan, W.-H. Zhang and Q.-M. Wang, Angew. Chem., Int. Ed., 2018, 57, 8639–8643 CrossRef CAS PubMed.
- W. H. Casey, Chem. Rev., 2006, 106, 1–16 CrossRef CAS PubMed.
- M. Huber, A. Schnepf, C. E. Anson and H. Schnöckel, Angew. Chem., Int. Ed., 2008, 47, 8201–8206 CrossRef CAS PubMed.
- C. C. Landry, C. J. Harlan, S. G. Bott and A. R. Barron, Angew. Chem., Int. Ed. Engl., 1995, 34, 1201–1202 CrossRef CAS.
- Y. Lin, W. Massa and S. Dehnen, J. Am. Chem. Soc., 2012, 134, 4497–4500 CrossRef CAS PubMed.
- C. Liu, N. V. Tkachenko, I. A. Popov, N. Fedik, X. Min, C.-Q. Xu, J. Li, J. E. McGrady, A. I. Boldyrev and Z.-M. Sun, Angew. Chem., Int. Ed., 2019, 58, 8367–8371 CrossRef CAS PubMed.
- Z.-F. Wu, B. Hu, Z.-H. Fu, H. Wang, G. Xu, L.-K. Gong, G.-D. Zou, X.-Y. Huang and J. Li, Chem. Commun., 2019, 55, 7442–7445 RSC.
- W. Uhl, Z. Naturorsch. B, 1988, 43, 1113 CrossRef CAS.
- M. Schiefer, N. D. Reddy, H. W. Roesky and D. Vidovic, Organometallics, 2003, 22, 3637–3638 CrossRef CAS.
- C. Schnitter, H. W. Roesky, C. Röpken, R. Herbst-Irmer, H.-G. Schmidt and M. Noltemeyer, Angew. Chem., Int. Ed., 1998, 37, 1952–1955 CrossRef CAS.
- H. Sitzmann, M. F. Lappert, C. Dohmeier, C. Üffing and H. Schnöckel, J. Organomet. Chem., 1998, 561, 203–208 CrossRef CAS.
- C. Y. Tang, A. R. Cowley, A. J. Downs and S. Parsons, Inorg. Chem., 2007, 46, 5439–5446 CrossRef CAS PubMed.
- S. D. Waezsada, F.-Q. Liu, C. E. Barnes, H. W. Roesky, M. L. Montero and I. Usón, Angew. Chem., Int. Ed. Engl., 1997, 36, 2625–2626 CrossRef CAS.
- A. Stasch, M. Ferbinteanu, J. Prust, W. Zheng, F. Cimpoesu, H. W. Roesky, J. Magull, H.-G. Schmidt and M. Noltemeyer, J. Am. Chem. Soc., 2002, 124, 5441–5448 CrossRef CAS PubMed.
- S. S. Kumar, J. Rong, S. Singh, H. W. Roesky, D. Vidovic, J. Magull, D. Neculai, V. Chandrasekhar and M. Baldus, Organometallics, 2004, 23, 3496–3500 CrossRef CAS.
- A. Stasch, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 2005, 44, 5854–5857 CrossRef CAS PubMed.
- A. Stasch, H. W. Roesky, D. Vidovic, J. Magull, H.-G. Schmidt and M. Noltemeyer, Inorg. Chem., 2004, 43, 3625–3630 CrossRef CAS PubMed.
- W. Uhl and F. Breher, Angew. Chem., Int. Ed., 1999, 38, 1477–1479 CrossRef CAS PubMed.
- W. Uhl, F. Breher, J. Grunenberg, A. Lützen and W. Saak, Organometallics, 2000, 19, 4536–4543 CrossRef CAS.
- H. Wessel, H.-S. Park, P. Müller, H. W. Roesky and I. Usón, Angew. Chem., Int. Ed., 1999, 38, 813–815 CrossRef CAS PubMed.
- K.-W. Klinkhammer, W. Uhl, J. Wagner and W. Hiller, Angew. Chem., Int. Ed. Engl., 1991, 30, 179–180 CrossRef.
- P. Henke, N. Trapp, C. E. Anson and H. Schnöckel, Angew. Chem., Int. Ed., 2010, 49, 3146–3150 CrossRef CAS PubMed.
- A. Purath, C. Dohmeier, A. Ecker, R. Köppe, H. Krautscheid, H. Schnöckel, R. Ahlrichs, C. Stoermer, J. Friedrich and P. Jutzi, J. Am. Chem. Soc., 2000, 122, 6955–6959 CrossRef CAS.
- B. Gu, C. Sun, J. C. Fettinger, W. H. Casey, A. Dikhtiarenko, J. Gascon, K. Koichumanova, K. Babu Sai Sankar Gupta, H. Jan Heeres and S. He, Chem. Commun., 2018, 54, 4148–4151 RSC.
- Y.-J. Liu, Y.-F. Sun, S.-H. Shen, S.-T. Wang, Z.-H. Liu, W.-H. Fang, D. S. Wright and J. Zhang, Nat. Commun., 2022, 13, 6632 CrossRef CAS PubMed.
- M. Huber, J. Hartig, K. Koch and H. Schnöckel, Z. Anorg. Allg. Chem., 2009, 635, 423–430 CrossRef CAS.
- J. Vollet, J. R. Hartig and H. Schnöckel, Angew. Chem., Int. Ed., 2004, 43, 3186–3189 CrossRef CAS PubMed.
- L. Geng, C.-H. Liu, S.-T. Wang, W.-H. Fang and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 16735–16740 CrossRef CAS PubMed.
- Y.-J. Liu, Q.-H. Li, D.-J. Li, X.-Z. Zhang, W.-H. Fang and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 4849–4854 CrossRef CAS PubMed.
- Y. Li, C. Zheng, S.-T. Wang, Y.-J. Liu, W.-H. Fang and J. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202116563 CAS.
- C. André Ohlin, J. R. Rustad and W. H. Casey, Dalton Trans., 2014, 43, 14533–14536 RSC.
- C.-H. Liu, W.-H. Fang, Y. Sun, S. Yao, S.-T. Wang, D. Lu and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 21426–21433 CrossRef CAS PubMed.
- S. Yao, W.-H. Fang, Y. Sun, S.-T. Wang and J. Zhang, J. Am. Chem. Soc., 2021, 143, 2325–2330 CrossRef CAS PubMed.
- S.-T. Wang, Y.-J. Liu, C.-C. Feng, W.-H. Fang and J. Zhang, Aggregate, 2022, e264 Search PubMed.
- R. M. McKinlay, G. W. V. Cave and J. L. Atwood, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5944–5948 CrossRef CAS PubMed.
- H. Kumari, A. V. Mossine, S. R. Kline, C. L. Dennis, D. A. Fowler, S. J. Teat, C. L. Barnes, C. A. Deakyne and J. L. Atwood, Angew. Chem., Int. Ed., 2012, 51, 1452–1454 CrossRef CAS PubMed.
- A. S. Rathnayake, C. L. Barnes and J. L. Atwood, Cryst. Growth Des., 2017, 17, 4501–4503 CrossRef CAS.
- C. Zhang, R. S. Patil, T. Li, C. L. Barnes and J. L. Atwood, Chem. Commun., 2017, 53, 4312–4314 RSC.
- C. Zhang, R. S. Patil, C. Liu, C. L. Barnes and J. L. Atwood, J. Am. Chem. Soc., 2017, 139, 2920–2923 CrossRef CAS PubMed.
- A. S. Rathnayake, K. A. Feaster, J. White, C. L. Barnes, S. J. Teat and J. L. Atwood, Cryst. Growth Des., 2016, 16, 3562–3564 CrossRef CAS.
- K. Su, M. Wu, D. Yuan and M. Hong, Nat. Commun., 2018, 9, 4941 CrossRef PubMed.
- Y. Domoto, M. Abe and M. Fujita, J. Am. Chem. Soc., 2021, 143, 8578–8582 CrossRef CAS PubMed.
- S. Dehnen and M. Melullis, Coord. Chem. Rev., 2007, 251, 1259–1280 CrossRef CAS.
- K. F. Hsu, S. Loo, F. Guo, W. Chen, J. S. Dyck, C. Uher, T. Hogan, E. K. Polychroniadis and M. G. Kanatzidis, Science, 2004, 303, 818–821 CrossRef CAS PubMed.
- H. Li, A. Laine, M. O'Keeffe and O. M. Yaghi, Science, 1999, 283, 1145–1147 CrossRef CAS PubMed.
- J. Zhang, X. Bu, P. Feng and T. Wu, Acc. Chem. Res., 2020, 53, 2261–2272 CrossRef CAS PubMed.
- N. Zheng, X. Bu and P. Feng, Nature, 2003, 426, 428–432 CrossRef CAS PubMed.
- N. Zheng, X. Bu, B. Wang and P. Feng, Science, 2002, 298, 2366–2369 CrossRef CAS PubMed.
- P. Feng, X. Bu and N. Zheng, Acc. Chem. Res., 2005, 38, 293–303 CrossRef CAS PubMed.
- X. Bu, N. Zheng and P. Feng, Chem. – Eur. J., 2004, 10, 3356–3362 CrossRef CAS PubMed.
- P. Vaqueiro, Dalton Trans., 2010, 39, 5965–5972 RSC.
- C. L. Cahill and J. B. Parise, Dalton Trans., 2000, 1475–1482 RSC.
- Z.-X. Lei, Q.-Y. Zhu, X. Zhang, W. Luo, W.-Q. Mu and J. Dai, Inorg. Chem., 2010, 49, 4385–4387 CrossRef CAS PubMed.
- D.-D. Yang, W. Li, W.-W. Xiong, J.-R. Li and X.-Y. Huang, Dalton Trans., 2018, 47, 5977–5984 RSC.
- W.-W. Xiong, J.-R. Li, B. Hu, B. Tan, R.-F. Li and X.-Y. Huang, Chem. Sci., 2012, 3, 1200–1204 RSC.
- H. Li, J. Kim, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2003, 42, 1819–1821 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, B. Han, X. Wang, Z. Wang, C. Xue, G. Bian, D. Hu, R. Zhou, D.-S. Li, Z. Wang, Z. Ouyang, M. Li and T. Wu, J. Am. Chem. Soc., 2020, 142, 6649–6660 CrossRef CAS PubMed.
- N.-N. Shen, B. Hu, C.-C. Cheng, G.-D. Zou, Q.-Q. Hu, C.-F. Du, J.-R. Li and X.-Y. Huang, Cryst. Growth Des., 2018, 18, 962–968 CrossRef CAS.
- T. Wu, X. Bu, P. Liao, L. Wang, S.-T. Zheng, R. Ma and P. Feng, J. Am. Chem. Soc., 2012, 134, 3619–3622 CrossRef CAS PubMed.
- T. Wu, X. Bu, X. Zhao, R. Khazhakyan and P. Feng, J. Am. Chem. Soc., 2011, 133, 9616–9625 CrossRef CAS PubMed.
- T. Wu, L. Wang, X. Bu, V. Chau and P. Feng, J. Am. Chem. Soc., 2010, 132, 10823–10831 CrossRef CAS PubMed.
- X. Xu, W. Wang, D. Hu, T. Wu, X. Bu and P. Feng, J. Am. Chem. Soc., 2018, 140(3), 888–891 CrossRef CAS PubMed.
- Y.-P. Zhang, X. Zhang, W.-Q. Mu, W. Luo, G.-Q. Bian, Q.-Y. Zhu and J. Dai, Dalton Trans., 2011, 40, 9746–9751 RSC.
- R. Ge, X.-X. Li and S.-T. Zheng, Coord. Chem. Rev., 2021, 435, 213787 CrossRef CAS.
- T. J. Hubin and D. H. Busch, Coord. Chem. Rev., 2000, 200–202, 5–52 CrossRef CAS.
- A. Sauer-Masarwa, L. D. Dickerson, N. Herron and D. H. Busch, Coord. Chem. Rev., 1993, 128, 117–137 CrossRef CAS.
- D. H. Busch and N. A. Stephenson, Coord. Chem. Rev., 1990, 100, 119–154 CrossRef CAS.
- L. Yang, X.-Y. Wang, X.-Y. Tang, M.-Y. Wang, C.-Y. Ni, H. Yu, Y.-L. Song, B. F. Abrahams and J.-P. Lang, Sci. China Chem., 2022, 65, 1094–1099 CrossRef CAS.
- Y. Zhu, Z. Wang, D. Li, Y.-D. Zhu, Q.-H. Li, D.-S. Li and L. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202202853 CAS.
- X.-H. Ma, Y. Si, L.-L. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, ACS Nano, 2022, 16, 5507–5514 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |