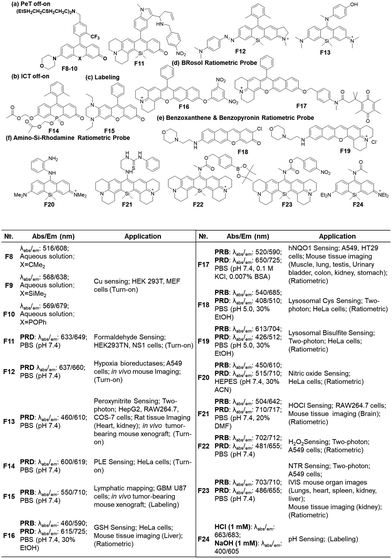
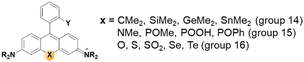Open Access Article
Open Access ArticleStrategies to convert organic fluorophores into red/near-infrared emitting analogues and their utilization in bioimaging probes
Mingchong
Dai
 *ab,
Yun Jae
Yang
a,
Sourav
Sarkar
a and
Kyo Han
Ahn
*ab,
Yun Jae
Yang
a,
Sourav
Sarkar
a and
Kyo Han
Ahn
 *a
*a
aDepartment of Chemistry, Pohang University of Science and Technology, Pohang, 37673, South Korea. E-mail: daimingchong@postech.ac.kr; ahn@postech.ac.kr
bCEDAR, Knight Cancer Institute, School of Medicine, Oregon Health & Science University, Portland, Oregon 97201, USA. E-mail: daimin@ohsu.edu
First published on 23rd August 2023
Abstract
Organic fluorophores aided by current microscopy imaging modalities are essential for studying biological systems. Recently, red/near-infrared emitting fluorophores have attracted great research efforts, as they enable bioimaging applications with reduced autofluorescence interference and light scattering, two significant obstacles for deep-tissue imaging, as well as reduced photodamage and photobleaching. Herein, we analyzed the current strategies to convert key organic fluorophores bearing xanthene, coumarin, and naphthalene cores into longer wavelength-emitting derivatives by focussing on their effectiveness and limitations. Together, we introduced typical examples of how such fluorophores can be used to develop molecular probes for biological analytes, along with key sensing features. Finally, we listed several critical issues to be considered in developing new fluorophores.
Key learning points1. Importance of red/NIR emitting fluorophores.2. Feasibility and challenges to convert conventional fluorophores into their red/NIR emitting analogues. 3. Molecular-level understanding of the correlation between the structural modifications and the bathochromic emission shifts. 4. Bio-relevant applications (utilization in bioimaging probes) of red/NIR emitting fluorophores. 5. Scope and limitations of conventional structural modification methods. |
1. Introduction
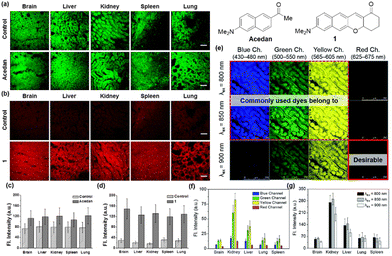
Organic fluorophores are essential for bioimaging applications due to their distinctive characteristics, such as non-invasiveness, high sensitivity and selectivity, and capability for real-time imaging. Such advantages also hold immense promise for clinical applications.1 Fluorophores emitting in the longer wavelengths, preferably in the red/near-infrared (NIR) region, are in great demand for bioimaging of tissues with reduced autofluorescence interference and at deeper imaging depths. The issue of autofluorescence, arising from innate biomolecules, presents a significant concern in tissue imaging at deeper depths. Due to the emission of fluorescent biomolecules, including NAD(P)H, flavins, lipofuscin, etc., mostly in the blue, green, and orange wavelengths,2 utilizing fluorophores that emit in the red (>630 nm) or near-infrared (NIR) region is essential for bioimaging applications (Fig. 1a–d). Failing to do so could lead to substantial autofluorescence interference in tissue imaging.3 The magnitude of autofluorescence varies across different tissue types, exhibiting notable levels in the green and yellow emission channels but diminishing beyond approximately 630 nm (Fig. 1e–g).4 Additionally, the excitation of fluorophores at longer wavelengths leads to reduced photo-bleaching of them and reduced photo-damaging of biological samples. Accordingly, great efforts have been made to develop longer-emitting dyes from typical organic fluorophores, leading to significant progress in this field.5 Hence, it is crucial to prioritize the development of fluorophores with emissions in the red/NIR region. At this point, it is worth noting that currently there is no consensus on using the terminology “NIR” among scientists working in the field of fluorescent probes. A dye is frequently claimed to be a NIR dye when its emission spectra cover some portion above 700 nm or so, even though the maximum is below 700 nm. The “red” wavelength region ranges from 620 to 750 nm and that for the NIR from 700 to 1500 nm (according to the IEC 60050-845:2007 standard), generating an overlapping region between red and NIR, from 700 to 750 nm. It may be acceptable to claim a NIR dye if its emission maximum is above 700 nm.In this tutorial review, we wish to introduce key strategies that convert several common organic fluorophores into analogues that emit in longer wavelengths, preferably in the red/NIR wavelength region. Commonly used strategies involve π-conjugation extension, modification of the electron-donor and -acceptor groups, and replacement of the central heteroatom in xanthene dyes, which can be best demonstrated with the fluorophores based on xanthene, coumarin, and naphthalene cores. Organic fluorophores having xanthene, coumarin, and naphthalene cores can be readily functionalized and thus have been widely used for biological applications. These fluorophores also provide good starting points for the development of longer-emitting derivatives. The structural modifications lead to variable emission bathochromic shifts, which are also dependent on the molecular geometry. Understanding the “effective” directions for the structural modifications toward the bathochromic emission spectral shift will provide valuable design guidance to new organic fluorophores with desirable features for bioimaging applications. Herein we analyzed the key structural modifications of the fluorophores for the bathochromic shift categorized by the core structures. Besides, we briefly introduced how these fluorophores can find their applications in studying biological issues.
2. Xanthene derivatives
The widely used rhodamine and fluorescein dyes are xanthene (10H-9-oxa-anthracene) derivatives with nitrogen and oxygen substitutions both at C-2 and C-7, respectively, together with the (o-carboxy)phenyl substituent at C-9. Rhodamine and fluorescein analogues simply with a phenyl substituent at C-9 are called rosamine and fluorone dyes, respectively (here denoted as CFPX). A hybrid form between rhodamine and fluorescein is named rhodol, and that between rosamine and fluorone is named rosol (Fig. 2a). Among them, the carboxyphenyl-containing dyes can exist in their spiro-lactone forms in equilibration with the ring-opened zwitterionic forms. The spiro-lactone ring-opening and -closing conversion process offers fluorescence on–off signalling in the fluorescence sensing of biological systems (Fig. 2b).6 The photophysical properties, particularly, the emission wavelengths, of the xanthene dyes are affected by: (a) the amino substituent (in red, Fig. 2c), (2) the C-9 central atom (in orange, Fig. 2c), and (3) the conjugation length (in blue, Fig. 2c).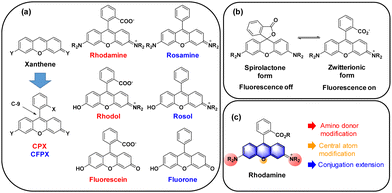 | ||
| Fig. 2 (a) Structures of C-9 phenyl-substituted xanthene dyes. (b) Spiro-lactone and zwitterionic forms of rhodamines. (c) Modifications to extend the emission wavelengths of rhodamines. | ||
2.1. o-Carboxyphenyl-substituted xanthene dyes
 | ||
| Fig. 3 The effect of the amino group on the absorption and emission wavelengths of rhodamine dyes,7 observed in ethanol. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (for example, –SiMe2, –P(
O bond (for example, –SiMe2, –P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OH, –P(
O)OH, –P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Me, and –P(
O)Me, and –P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Ph) and the π* orbital of the fluorophore nearby.29,30 For the atoms of group 16, the heteroatom lone pair occupancy and resonance stability are found to be the major determinants of the excitation energetics: the X atom of S, Se, or Te showed increased lone pair occupancy, which lowers the excitation energy and thus leads to longer absorption wavelengths.27
O)Ph) and the π* orbital of the fluorophore nearby.29,30 For the atoms of group 16, the heteroatom lone pair occupancy and resonance stability are found to be the major determinants of the excitation energetics: the X atom of S, Se, or Te showed increased lone pair occupancy, which lowers the excitation energy and thus leads to longer absorption wavelengths.27
A systematic variation of the central oxygen atom of rhodamines has been conducted by Lavis and co-workers.16 The maximum absorption and emission wavelengths of rhodamine analogues with amine (nitrogen), sulfur, sulfone, carbon, silicon, phosphinate, or phosphine oxide at the central C-9 are listed in Fig. 5. By the substitution of the central atom, the bathochromic emission wavelength shift increased in the order of N, O, S, C, Si, and P. Together with the known red-shift effect by the azetidine group, the maximum emission wavelength of 723 nm (pH 7.3 HEPES buffer) was realized with the –P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Ph analogue. Note that the replacement of the C-9 oxygen atom with silyl (–SiR2) groups to obtain deep-red/NIR-emitting dyes has received much attention, plausibly owing to the synthetic feasibility. The replacement of the C-9 oxygen atom with the dimethylsilyl group, for example, leads to increased electrophilicity at C-10. Accordingly, the spiro-lactone ring-opening takes place in media of rather higher polarities compared to the case of the corresponding rhodamine analogues. Recently, a rosamine analogue with a boron substituent at C-9 (B-(OH2)) was also reported; the rosamine borinic acid emits at around 650 nm.31
O)Ph analogue. Note that the replacement of the C-9 oxygen atom with silyl (–SiR2) groups to obtain deep-red/NIR-emitting dyes has received much attention, plausibly owing to the synthetic feasibility. The replacement of the C-9 oxygen atom with the dimethylsilyl group, for example, leads to increased electrophilicity at C-10. Accordingly, the spiro-lactone ring-opening takes place in media of rather higher polarities compared to the case of the corresponding rhodamine analogues. Recently, a rosamine analogue with a boron substituent at C-9 (B-(OH2)) was also reported; the rosamine borinic acid emits at around 650 nm.31
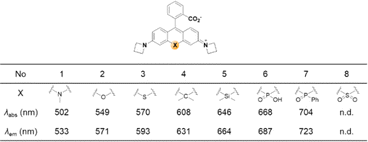 | ||
| Fig. 5 Effect of the replacement of the central atom (X) on the maximum absorption/emission wavelengths of a rhodamine dye,16 observed in HEPES at pH 7.3 (10 mM). | ||
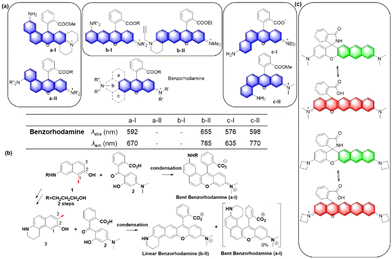
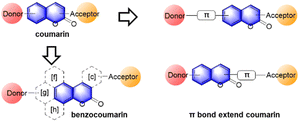
Up to now, four types of geometrically different benzorhodamines have been reported: benzorhodamine a-I,32b-II,33c-I34 and c-II35 types. These benzorhodamines showed significantly red-shifted emission wavelengths at more than 635 nm, reaching 785 nm in the case of linear b-II. Recently, Liu and co-workers disclosed a novel π-extension approach based on computational calculations, which led to dual-emission rhodamine analogues from single-emission rhodamines. The π-extended rhodamines composed of selected fragments with matched FMO energy levels avoided the otherwise possible inter-fragment PeT.36
At this point, it is worthwhile to mention a synthetic challenge encountered in the context of linear benzocoumarin dyes (Fig. 6b). A condensation reaction of an amino-naphthol such as 1 with keto-benzoic acid 2 does not lead to the linear type but the a-I type bent benzorhodamine exclusively because the C-1 site of compound 1 is more nucleophilic than the C-3 site toward electrophiles. The regioselectivity issue was solved by introducing steric hindrance near the electrophilic C-1 site, leading to linear benzorhodamines.33 Linearly conjugated dipolar dyes usually absorb and emit wavelengths longer than the corresponding bent analogues.37
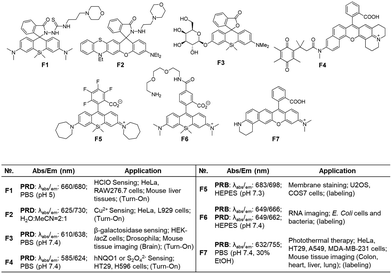
The fluorophores can be also applied to the labelling of the cell membrane (F5, Fig. 7d)19 and RNA (F6, Fig. 7d).21 As the dyes are in the fluorescence-on state, washing-out steps are required in such applications.
Recently, Dai and co-workers have developed a synthetic route to obtain linear benzorhodamines. In the free carboxylic acid form such as F7, it absorbs light efficiently but emits weaker fluorescence compared to its ester form, which has the potential for photothermal therapy.33 The linear benzorhodamine in the ester form is useful for NIR imaging.
2.2. Carboxy-free, phenyl-substituted xanthene fluorophores
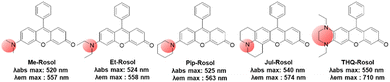 | ||
| Fig. 8 The amino donor effect on the photophysical properties of rosol dyes.41 The data were obtained in pH 7.4 phosphate buffer (50 mM, 150 mM NaCl). | ||
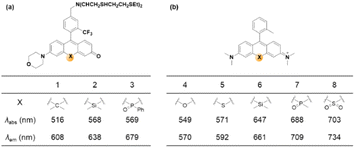 | ||
| Fig. 9 Effects of central atom replacement on the photophysical properties of rosol (left) and rosamine (right). Data for dyes 1–3 are measured in HEPES buffer (25 mM, pH 7.4),42 and those for dyes 4–8 are measured in PBS (20 mM, pH 7.4).43 | ||
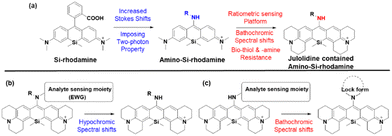
As noted above, the replacement of the central oxygen atom of rhodamines with silyl groups affords the corresponding sila-rhodamine analogues (Si-rhodamines), which offer several advantageous features for bioimaging applications, such as deep-red emission44 and enhanced two-photon (TP) properties.45,46 Si-rhodamines have been also used for super-resolution imaging.47 The replacement of the C-10 phenyl group of Si-rhodamines with an amine leads to the so-called amino-Si-pyronins that show unique optical properties. In contrast to the parent Si-rhodamines that show narrow Stokes shifts, amino-Si-pyronins exhibit large Stokes shifts. In addition, amino-Si-pyronins have excellent two-photon imaging capability, which is lacking in the case of Si-rhodamines. Si-rhodamines undergo a medium polarity-dependent spiro-lactone ring-opening and -closing equilibrium: they are highly fluorescent in the ring-opened form in highly polar media but nonfluorescent in the ring-closed form in nonpolar media. Amino-Si-pyronins can also exist in the fluorescence-on and -off states depending on the medium polarity. In highly polar media, amino-Si-pyronins are strongly fluorescent in the enamine form: contrarily, in nonpolar media, they are nonfluorescent in the imine form. This enamine–imine equilibrium is also dependent on the 2- and 7-amine substituents.46 A promising aspect of amino-Si-pyronins is that we can further functionalize the C-10 amino group, such as via the introduction of an acyl or analogous group onto it. In this case, the electrophilicity of C-10 also increases; hence, biothiols such as cysteine can react with some acylated amino-Si-pyronins. This chemical stability issue can be solved by the julolidine-derived amino-Si-pyronins that are stable in cells. The resulting acyl analogues now emit in the NIR region, leading to a novel type of NIR dyes. Notably, amino-Si-pyronins and their NIR analogues constitute a novel sensing platform for the development of activity-based (activatable or reaction-based) probes. For example, two-photon ratiometric probes can be developed by introducing H2O2- or NTR-responsive moieties onto the C-10 amino group. In this case, the emission maxima change from a NIR to a deep-red region (hypochromic shift, Fig. 10b). Also, ratiometric sensing systems that show signal changes from the deep-red to NIR region (bathochromic shift, Fig. 10c) can be conceivable, as exemplified by an HOCl probe (more examples are provided in the application section below).
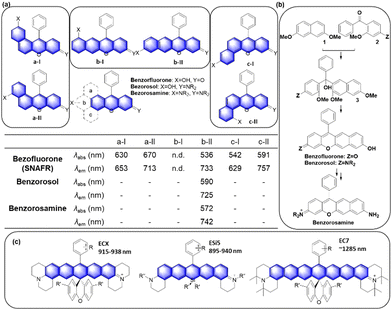
Another popular approach is to introduce an activatable group into the dyes in such a way that its introduction causes a change in intramolecular charge-transfer (ICT). The detachment of the reactive group by the target analyte can cause fluorescence signal changes. When the hydroxyl group of rosol and fluorone dyes is protected by the reactive group (Rg), the resulting probes mostly become nonfluorescent (ICT-off state). The activation/cleavage of Rg upon the interaction with the target analyte leads to a turn-on fluorescence response (ICT-on), as exemplified by an esterase probe F14 (Fig. 12b).57 The ICT modulation approach can be also explored for the labelling of biomolecules, as exemplified by rosol F15 (Fig. 12c), which allows lymphatic mapping.41 The commonly observed ICT- or PeT-off state of O-blocked rosol dyes can be tuned into the ICT- or PeT-on state by extending the conjugation length of dyes. The benzorosol (BRosol) dye system developed by Ahn and co-workers provides emissive derivatives even when the hydroxyl group is functionalized into its O-acyl or O-ethereal derivatives49 or connected with a typical PeT quencher (nitrophenyl or quinone groups).49,58 The further π-conjugation keeps the localized excited states of the O-functionalized derivatives still emissive. Also, π-conjugation causes a lowering of the LUMO energy, effectively prohibiting the otherwise feasible occurrence of the PeT process. Notably, there is a large difference in emission wavelength between the phenolic (λem = 590 nm) and phenolate (λem = 725 nm) forms of BRosol, which offers an excellent ratiometric sensing platform to sense GSH (F16, Fig. 12d)49 and hNQO1 (F17, Fig. 12d).58
In 2019, Gryko and co-workers reviewed the synthesis, photophysical properties and applications of rhodol (and rosol) as fluorescent probes. They predicted the development of a π-extended rhodol compound (Fig. 12e, benzoxanthene), which was successfully prepared in 2020, along with its amine analogue (Fig. 12e, benzopyronin). Taking advantage of the fact that the skeleton can be readily attacked by nucleophilic analytes through 1,6-conjugate addition, they were developed into fluorescent probes for cysteine (Cys) (F18, Fig. 12e) and59 bisulfite (F19, Fig. 13e).60
As we have mentioned above, the probes based on the amino-Si-rhodamine system can be developed into probes that show either hypsochromic (Fig. 10b) or bathochromic (Fig. 10c) emission spectral shifts, depending on the probe design: the probes for H2O2 (F20, Fig. 12f),46 NTR (F21, Fig. 12f),61 and pH (F22, Fig. 12f)62 exhibit hypsochromic signal changes, whereas those for nitric oxide (F23, Fig. 12f)63 and HOCl (F24, Fig. 12f) exhibit bathochromic signal changes.64
2.3. Xanthene derivatives: summary
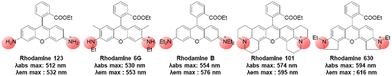
In the above subchapters, we have overviewed and discussed strategies toward the emission bathochromic shift applied to the xanthene fluorophores, such as rhodamine, fluorescein, and their analogues. Commonly used approaches are ring fusion, central atom replacement, and variation of the amino donor. The replacement of the primary amino group (rhodamine 123) with a tertiary amino group (rhodamine 630) results in the emission maximum shift from 532 nm to 616 nm (Δλ = 84 nm; ethanol). The central O atom replacement of a rhodamine dye to Si and P results in the emission maximum shift from 571 nm to 664 nm (Δλ = 93 nm) and 723 nm (Δλ = 152 nm) in pH 7.3 HEPES, respectively (Fig. 5). The linear fusion of one benzene ring to rhodamine B led to a benzorhodamine (b-II, Fig. 6), which resulted in the emission maximum shift from 576 nm to 785 nm (Δλ = 209 nm; rhodamine B in EtOH, benzorhodamine in 30% EtOH/PBS). The ring-fusion approach is highly effective for the emission bathochromic shift, as highlighted by the seven ring-fused EC7 dyes that emit at ∼1285 nm.3. Coumarin
Considering that the coumarins are typical ICT dyes, we can induce bathochromic shifts in their emission wavelength by increasing the donor/acceptor's ability, in addition to extending the π-conjugation length.In 1984, Jones and co-workers investigated the photophysical properties of coumarin dyes with several different electron-donor and -acceptor groups.65 However, the modification of the donor and acceptor alone is not sufficient to make the coumarin emit at more than 600 nm (λem). Therefore, π extension is required to generate coumarin derivatives emitting in the red/far-red or NIR region.
3.1. Benzocoumarins
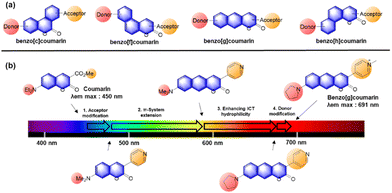
In 2017, Ahn and co-workers designed a novel strategy to modify a typical D–A coumarin dye to far-red emitting benzo[g]coumarins.4 The emission maximum data (Fig. 13b) indicate that the π-system extension causes a greater spectral bathochromic shift compared to donor/acceptor modifications. Through a series of modifications (Fig. 14b), a bathochromic emission spectral shift of 241 nm was obtained (in EtOH) compared to that of the original coumarin.
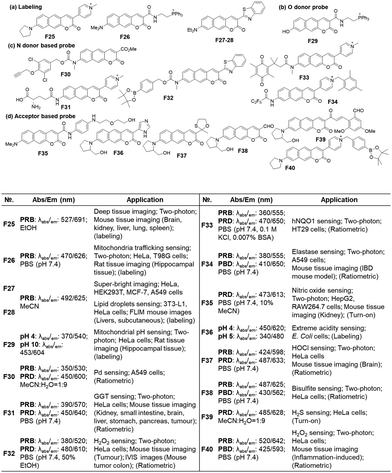 | ||
| Fig. 15 Applications of benzo[g]coumarins to develop fluorescent probes and their basic optical properties and target analytes summarized. | ||
Because the benzocoumarin core is rather difficult to functionalize, the probe design is mainly conducted by modification of the donor (hydroxyl or amino) or acceptor (carboxyl) groups. In 2016, a hydroxy-benzocoumarin dye was utilized for pH sensing, as its hydroxyl and alkoxy (–OH/–O−) forms exhibit different photophysical properties (F29, Fig. 15b).73
In the case of benzocoumarins bearing an amino donor (–NRR′) at the 7-position, modification of them into amide or carbamate derivatives commonly leads to a change in their ICT properties. These properties can be used to develop reaction-based ratiometric probes: accordingly, several ratiometric probes have been developed for sensing Pd (F30, Fig. 15c),74 GGT (F31, Fig. 15c),75 H2O2 (F32, Fig. 15c),76 hNQO1 (F33, Fig. 15c),77 and elastase (F34, Fig. 15c).78
Similarly, ratiometric probes can be developed by modifying the acceptor site, leading to probes for sensing nitric oxide (F35, Fig. 15d),79 extreme acidity (F36, Fig. 15d),80 HOCl (F37, Fig. 15d),81 bisulfite (F38, Fig. 15d),82 H2S (F39, Fig. 15d),83 and H2O2 (F40, Fig. 15d).84
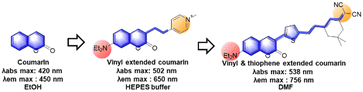
3.2 Vinyl-extended coumarins
As noted above, the vinyl-extension strategy (Fig. 16) can make the resulting electron-acceptor moiety vulnerable to both nucleophilic and electrophilic species, such as bisulfite, biothiols, hypochlorous acid, peroxynitrite, etc. The chemical and photochemical stability of the vinyl-containing acceptors including hemicyanine moieties seems to be dependent on the whole dye system: the vinyl-extended coumarins bearing 2-indolinum or 2-benzothiazolium groups are not chemically and photochemically stable, whereas those of the 4-pyridinium group have sufficient chemo- and photo-stability.85 Besides, the free bond rotation around the vinyl group provides the resulting dyes with viscosity- and polarity-sensitive emission behaviour, leading to fluorescent probes for these environmental parameters.92
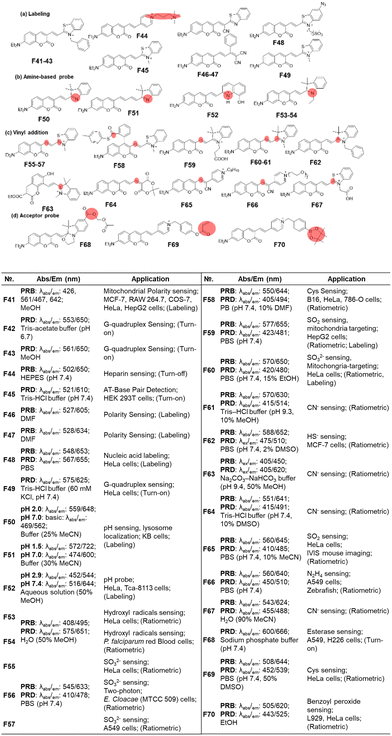 | ||
| Fig. 17 Vinyl-extended coumarins as fluorescent probes for bioanalytes: basic optical properties and target analytes. | ||
The most popular use of such vinyl-extended coumarins is found in sensing of nucleophilic species, such as bisulfite (F55–F57, F59, F60 & F65, Fig. 17c),105–110 cysteine (F58, Fig. 17c),111 cyanide (F61, F63, F64 & F67, Fig. 17c),112–115 hydrogen sulfide (F62, Fig. 17c),116 and hydrazine (F66, Fig. 17c).117 Note that these hemicyanine moieties may also undergo electrophilic reactions with hypochlorous acid or peroxynitrite,85 which warrant careful interference analysis against these species.
For the last type, it is also possible to cause a fluorescence signal change by further decorating the acceptor part (Fig. 17d), as shown in the detection of esterase (F68, Fig. 17d),118 cysteine (F69, Fig. 17d)119 and benzoyl peroxide (F70, Fig. 17d).120
4. Naphthalene
4.1. Extended naphthalene
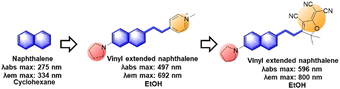
In previous work, we have disclosed how the donor group affects the emission properties of donor–acceptor type naphthalene compounds including ACEDAN: an amino donor that exerts little allylic 1,3-strain to the naphthalene ring and has high electron-donating capability. This endows the resulting dipolar dye with higher fluorescence in water as well as in cells.121 By fixing the amino donor with pyrrolidine, we have changed the acceptor part into a different heterocycle. Considering the chemo- and photo-stability of the resulting hemicyanine dyes, the dye with 4-pyridinium is the dye of choice for biological applications. With such modifications, the resulting napthylvinylpridinium (NVP) derivative emits at 692 nm (in ethanol), dramatically displaying a red-shift compared to naphthalene's emission at 334 nm in cyclohexane (Fig. 18). Later, Song replaced the pyridinium moiety of NVP with a tricyanofuranyl ring to form TCFAN (F75 in Fig. 19). This conversion even pushes the emission maximum up to 800 nm.92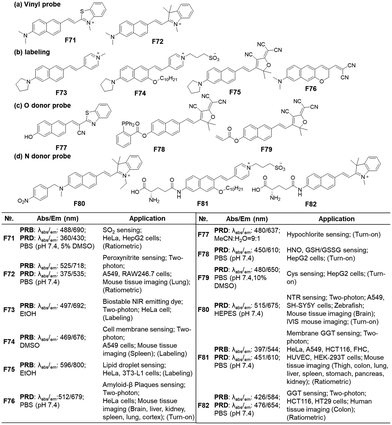 | ||
| Fig. 19 Vinylnaphthalene-based fluorescent probes for bioanalytes: basic optical properties and target analytes. | ||
4.2. Bioimaging probes based on vinylnaphthalene dyes
As mentioned above, the vinylnaphthalene-based hemicyanine dyes bearing an electron acceptor such as 2-indolinum or 2-benzothiazolium moieties have been widely used in monitoring nucleophilic analytes like bisulfite (an aqueous equilibrium species of SO2; F71, Fig. 19a),122 peroxynitrite (F72, Fig. 19a), etc.,123 which suggest that they are susceptible to these species. Also, such hemicyanine dyes are not photochemically stable. In contrast, their 4-pyridinium analogues (NVP dyes) have good stability against many bio-analytes, including Cys, GSH, H2O2, HSO3−, HOCl, and H2S (F73, Fig. 19b) as well as good photostability.85 The NVP system was used to develop a membrane-localizing fluorophore (F74, Fig. 19b) and other probes.124,125 Instead of the charged heterocycles, a charge-neutral but strong electron-withdrawing group, such as 2-(3-cyano-4,5,5-trimethyl-2(5H)-furanylidene)malononitrile (TCF), can be introduced to develop a NIR-emitting probe for lipid droplets, which provide a nonpolar and viscous environment to the dipolar dye (F75, Fig. 19b).92 Essentially, any dipolar dyes, which show polarity (and also viscosity) sensitive emission behaviour, can be used to sense lipid droplets. Also, environment-sensitive π-extended dipolar dyes can be used to detect amyloid-β (Aβ) plaques, which provide a much less polar and congested environment to the dipolar dye compared to the case where the dye resides outside the plaques. This effect is evidenced by a robust deep red-emitting Aβ probe (F76, Fig. 19b).3Instead of the amino donor, the hydroxyl group can act as an electron donor. Additionally, the dyes bearing such a hydroxyl donor show emission changes depending on the medium pH that governs their equilibrium between the hydroxyl and alkoxy forms. Accordingly, such 6-hydroxy-naphthalene dyes have been used to develop fluorescent probes for hypochlorite (F77, Fig. 19c),126 HNO + GSH/GSSG (F78, Fig. 19c),127 and Cys (F79, Fig. 19c).128 On the other hand, as the fluorescent probes based on aryl alcohol (ArOH) or phenolic dyes generally show pH-dependent emission behaviour, their use in the quantitative analysis should be conducted carefully by measuring the pH-dependent emission signal change.
In the case of dipolar dyes bearing an amino donor, we can develop activatable probes by introducing an analyte-reactive moiety onto the amino group. Such functionalization of the amino donor causes a significant change in the ICT of the dyes; this property allows us to develop activatable probes, as demonstrated for NTR (F80, Fig. 19d)129 and GGT (F81 & F82, Fig. 19d).130,131 When the reactive group is a fluorescence quencher, such as nitroaryl, we may observe a turn-on type fluorescence signal change. However, we may generate a ratiometric probe even in such a case wherein it could modulate the PeT energy levels by choosing an appropriate fluorophore.77
In the above three sections, we have described known strategies to have bathochromic emission shifts of the common fluorophores (benzene-substituted xanthene, coumarin, and naphthalene). Note that other hybrid dye systems are developed as well. Two important hemicyanine types are the CS dye132 and HD dye133 developed by Lin's group, which have been widely used in probe development as well as other bio-applications.134–136 Such a hybridization approach is also a useful method to obtain deep-red/NIR-emitting fluorophores; however, none of the original fluorophore's structure has been conserved in those dye systems and thus they are excluded in this review.
5. Molecular probe design
If we set aside the probes used to detect environmental parameters such as pH, polarity, viscosity, etc. (for example, F26, F48, F75), we can classify the molecular probes into two types based on the target detection mode: (1) binding-induced and (2) reaction/activity-induced. The latter detection mode has been widely explored recently, leading to a vast number of reaction-based/activatable probes.137–139 Note that such activatable probes provide time-lapsed accumulated concentration/activity levels, whereas the binding-based probes allow us to monitor the dynamic concentration/activity information on the target analyte. To develop the binding-based probes, we explore fluorescence signal changes (wavelength or intensity) upon binding with the target analyte. Accordingly, for developing such probes, fluorophores whose photophysical properties are sensitive to environmental changes, such as the electron push–pull type, dipolar dyes, are generally utilized. However, the rationale design of binding-based probes, in particular for enzymes, is challenging because we should consider a substrate that binds specifically to the target enzyme and the binding process should cause a fluorescence signal change. On the other hand, reaction-based probes have flourished recently, as benefited from a variety of chemical conversions that can be selectively executed by target analytes. We have provided selected examples of such activatable probes, which sense a variety of biologically important analytes. To develop activatable probes, we introduce a reactive group (Rg) typically at the donor or acceptor site (Fig. 20). The reactive group can be introduced to interrupt the spirolactone ring-opening process in the case of the CPX families (P1, P2 in Fig. 20). A part of a fluorophore, such as a vinyl unit, can also be the reactive site for certain analytes (P6, P7 in Fig. 20).When the reactive group undergoes an analyte-specific chemical conversion (cleavage or conversion into another structure), it involves an ICT change, thus resulting in fluorescence signal changes. The fluorescence change can be turn-off, turn-on, or ratiometric modes depending on the reactive group and fluorophore. Even if the reactive group acts as a quencher, we can realize turn-on or ratiometric signalling by choosing an appropriate fluorophore (for example, F4vs.F17, F32vs.F33).
Another general issue to be mentioned is that as the π-conjugation length increases (for example, P2 and P4), the probe may show aggregation-caused quenching (ACQ) behaviour even at the micromolar concentrations. Thus, it is always required to check the cellular aggregation behaviour of a probe for biological applications.
Also, it is necessary to secure the chemical stability of an activatable probe toward various biological analytes, particularly those containing “reactive” functional groups. For example, probes bearing a Michael acceptor are known to undergo conjugation addition reactions with nucleophilic biothiols (F62: hydrogen sulfide, F69: cysteine, F78: glutathione, etc.) and bisulfite anions (F55–F57, F59, F60, and F65). Also, probes containing an electron-rich vinyl unit are known to undergo oxidation reactions with reactive oxygen species, such as peroxynitrite (F72), hypochlorous acid (F77), etc.
6. Conclusion
We have analyzed the conversion strategies employed to achieve emission bathochromic shifts into the red/NIR region, starting from typical organic fluorophores based on three core structures—xanthene, coumarin, and naphthalene. Also, we have listed selected examples of how to apply these fluorophores to molecular probes for biological analytes. We believe that the strategies and their effectiveness compared here will motivate scientists to develop organic fluorophores even emitting in farther wavelengths, such as in the NIR-II region, and also serve as a valuable guide for designing red- and NIR-emitting fluorophores in the future, in addition to choosing a fluorophore in demand.7. Perspective
(1) The red/NIR emitting fluorophores shown here have the potential for imaging biological analytes at deeper depths with less autofluorescence interference as well as reduced photodamage.(2) We have analyzed the strategies to make three typical types of fluorophores emit in longer wavelengths, which involve electron-donor/-acceptor modifications, central atom replacement (for xanthene dyes), and conjugation length extension. A proper combination of these strategies to realize further emission wavelength shifts of known dyes and the design of new fluorophore skeletons will pave the way for the translation of fluorescent materials into medical practices in the future.
(3) Some of the known fluorophores emitting in the NIR-I or further in the NIR-II region have conformationally flexible vinyl units in their structures, which make them vulnerable to degradation by photons and chemicals. Also, the conformationally flexible nature makes them poorly emissive in aqueous media, as known with cyanine dyes. These issues should be carefully evaluated in developing fluorophores using the vinyl extension approach. In the pursuit of aromatic ring fusion, a key consideration lies in ensuring adequate solubility in biological media while mitigating the aggregation-caused quenching phenomenon. Furthermore, we must also explore cost-effective synthetic routes to complement these endeavours.
(4) A fluorophore's brightness is influenced by environmental factors, such as viscosity, polarity, pH, and hydrophobicity/hydrophilicity. As a result, it is inadequate to judge a fluorophore's potential for bioimaging application solely based on its quantum yield measured in solution. Indeed, it is not uncommon to see fluorophores with low quantum yields offering bright cellular images.
(5) In designing fluorescent probes, it is highly recommended to predict their optical behaviour (turn-on, turn-off or ratiometric response) through molecular orbital calculations before synthetic efforts. Even with a reactive group that can act as a PeT quencher, we can alleviate the quenching possibility by properly choosing a fluorophore with “mismatched” orbital energies for the PeT process.
(6) The selection of a fluorophore must consider compatibility with currently available optical devices. For instance, in the clinical instrument for fluorescence-guided surgery, lasers operating at 780–800 nm are extensively utilized (based on the FDA-approved ICG dye). Thus, fluorophores that can be excited within this spectral range and emit strong fluorescence have the potential for clinical experiments.
(7) Furthermore, our optimistic outlook for the future of this field encompasses advancements in multiplex imaging and sensing multiple analytes. Currently, multiplex imaging heavily relies on commonly-used emission channels, notably including wavelengths such as 488, 555, 647, and 750 nm. To enhance convenience and efficiency in this area, we envision the incorporation of one or two additional NIR-emitting fluorophores, alongside the utilization of compatible optical instruments. This progressive approach will significantly improve the efficiency of cyclic immunofluorescence imaging, while also enabling the simultaneous staining of a greater number of biomarkers for flow cytometry. As a result, the entire process will be streamlined. Another future direction will be the development of probes for sensing multiple analytes. The current probes mostly target single analytes. Looking ahead, our focus will be on designing probes capable of detecting multiple analytes in a sense of the AND logic gate, with which we could mitigate the false-positive or -negative errors in disease diagnosis. These advancements in probe technology hold immense potential for precise disease diagnosis and detection, facilitating more accurate and comprehensive assessments in clinical settings.
Author contributions
M. D.: writing original draft; writing review & editing; investigation. Y. J. Y.: investigation. S. S.: investigation. K. H. A.: supervision, writing review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2019R1A2C2085438); this research was also supported by funding from the Cancer Early Detection Advanced Research (CEDAR) centre at the Oregon Health & Science University's Knight Cancer Institute. M. D. gratefully acknowledges Professor Lei G. Wang (BME department, OHSU) for the helpful discussions.References
- R. Liu, Y. Xu, K. Xu and Z. Dai, Aggregate, 2021, 2, e23 CAS.
- M. Monici, Biotechnol. Annu. Rev., 2005, 11, 227–256 CrossRef CAS PubMed.
- D. Kim, H. Moon, S. H. Baik, S. Singha, Y. W. Jun, T. Wang, K. H. Kim, B. S. Park, J. Jung, I. Mook-Jung and K. H. Ahn, J. Am. Chem. Soc., 2015, 137, 6781–6789 CrossRef CAS PubMed.
- Y. W. Jun, H. R. Kim, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Sci., 2017, 8, 7696–7704 RSC.
- X. Zhang, S. Bloch, W. Akers and S. Achilefu, Curr. Protocol. Cytom., 2012, Chapter 12, 1–12 Search PubMed.
- M. Beija, C. A. Afonso and J. M. Martinho, Chem. Soc. Rev., 2009, 38, 2410–2433 RSC.
- J. Arden, G. Deltau, V. Huth, U. Kringel, D. Peros and K. H. Drexhage, J. Lumin., 1991, 48–49, 352–358 CrossRef CAS.
- G. Jiang, T. B. Ren, E. D'Este, M. Xiong, B. Xiong, K. Johnsson, X. B. Zhang, L. Wang and L. Yuan, Nat. Commun., 2022, 13, 2264 CrossRef CAS PubMed.
- T. B. Ren, W. Xu, W. Zhang, X. X. Zhang, Z. Y. Wang, Z. Xiang, L. Yuan and X. B. Zhang, J. Am. Chem. Soc., 2018, 140, 7716–7722 CrossRef CAS PubMed.
- G. Jiang, X. F. Lou, S. Zuo, X. Liu, T. B. Ren, L. Wang, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed. Engl., 2023, 62, e202218613 CrossRef CAS PubMed.
- J. B. Grimm, A. J. Sung, W. R. Legant, P. Hulamm, S. M. Matlosz, E. Betzig and L. D. Lavis, ACS Chem. Biol., 2013, 8, 1303–1310 CrossRef CAS PubMed.
- K. Kolmakov, V. N. Belov, C. A. Wurm, B. Harke, M. Leutenegger, C. Eggeling and S. W. Hell, Eur. J. Org. Chem., 2010, 3593–3610, DOI:10.1002/ejoc.201000343.
- Q. Zheng, A. X. Ayala, I. Chung, A. V. Weigel, A. Ranjan, N. Falco, J. B. Grimm, A. N. Tkachuk, C. Wu, J. Lippincott-Schwartz, R. H. Singer and L. D. Lavis, ACS Cent. Sci., 2019, 5, 1602–1613 CrossRef CAS PubMed.
- L. Wang, M. Tran, E. D'Este, J. Roberti, B. Koch, L. Xue and K. Johnsson, Nat. Chem., 2020, 12, 165–172 CrossRef CAS PubMed.
- J. B. Grimm, A. K. Muthusamy, Y. Liang, T. A. Brown, W. C. Lemon, R. Patel, R. Lu, J. J. Macklin, P. J. Keller, N. Ji and L. D. Lavis, Nat. Methods, 2017, 14, 987–994 CrossRef CAS PubMed.
- J. B. Grimm, A. N. Tkachuk, L. Xie, H. Choi, B. Mohar, N. Falco, K. Schaefer, R. Patel, Q. Zheng, Z. Liu, J. Lippincott-Schwartz, T. A. Brown and L. D. Lavis, Nat. Methods, 2020, 17, 815–821 CrossRef CAS PubMed.
- J. L. Bachman, C. I. Pavlich, A. J. Boley, E. M. Marcotte and E. V. Anslyn, Org. Lett., 2020, 22, 381–385 CrossRef CAS PubMed.
- J. Arden-Jacob, J. Frantzeskos, N. U. Kemnitzer, A. Zilles and K. H. Drexhage, Spectrochim. Acta, Part A, 2001, 57, 2271–2283 CrossRef CAS PubMed.
- J. B. Grimm, T. A. Brown, A. N. Tkachuk and L. D. Lavis, ACS Cent. Sci., 2017, 3, 975–985 CrossRef CAS PubMed.
- H. Ito, Y. Kawamata, M. Kamiya, K. Tsuda-Sakurai, S. Tanaka, T. Ueno, T. Komatsu, K. Hanaoka, S. Okabe, M. Miura and Y. Urano, Angew. Chem., Int. Ed. Engl., 2018, 57, 15702–15706 CrossRef CAS PubMed.
- R. Wirth, P. Gao, G. U. Nienhaus, M. Sunbul and A. Jaschke, J. Am. Chem. Soc., 2019, 141, 7562–7571 CrossRef CAS PubMed.
- G. Lukinavicius, K. Umezawa, N. Olivier, A. Honigmann, G. Yang, T. Plass, V. Mueller, L. Reymond, I. R. Correa, Jr., Z. G. Luo, C. Schultz, E. A. Lemke, P. Heppenstall, C. Eggeling, S. Manley and K. Johnsson, Nat. Chem., 2013, 5, 132–139 CrossRef CAS PubMed.
- L. G. Wang, A. R. Montano, J. R. Combs, N. P. McMahon, A. Solanki, M. M. Gomes, K. Tao, W. H. Bisson, D. A. Szafran, K. S. Samkoe, K. M. Tichauer and S. L. Gibbs, Nat. Chem., 2023, 15, 729–739 CrossRef CAS PubMed.
- X. Zhou, R. Lai, J. R. Beck, H. Li and C. I. Stains, Chem. Commun., 2016, 52, 12290–12293 RSC.
- T. C. Binns, A. X. Ayala, J. B. Grimm, A. N. Tkachuk, G. A. Castillon, S. Phan, L. Zhang, T. A. Brown, Z. Liu, S. R. Adams, M. H. Ellisman, M. Koyama and L. D. Lavis, Cell Chem. Biol., 2020, 27, 1063–1072 CrossRef CAS PubMed.
- T. M. McCormick, B. D. Calitree, A. Orchard, N. D. Kraut, F. V. Bright, M. R. Detty and R. Eisenberg, J. Am. Chem. Soc., 2010, 132, 15480–154803 CrossRef CAS PubMed.
- B. Calitree, D. J. Donnelly, J. J. Holt, M. K. Gannon, C. L. Nygren, D. K. Sukumaran, J. Autschbach and M. R. Detty, Organometallics, 2007, 26, 6248–6257 CrossRef CAS.
- A. Chmyrov, J. Arden-Jacob, A. Zilles, K. H. Drexhage and J. Widengren, Photochem. Photobiol. Sci., 2008, 7, 1378–1385 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, ACS Chem. Biol., 2011, 6, 600–608 CrossRef CAS PubMed.
- X. Chai, X. Cui, B. Wang, F. Yang, Y. Cai, Q. Wu and T. Wang, Chemistry, 2015, 21, 16754–16758 CrossRef CAS PubMed.
- Y. Komori, S. Sugimoto, T. Sato, H. Okawara, R. Watanabe, Y. Takano, S. Kitaoka and Y. Egawa, Sensors, 2023, 23, 1528 CrossRef CAS PubMed.
- M. Sibrian-Vazquez, J. O. Escobedo, M. Lowry and R. M. Strongin, Pure Appl. Chem., 2012, 84, 2443–2456 CrossRef CAS PubMed.
- M. Dai, H. Lee, Y. J. Yang, M. Santra, C. W. Song, Y. W. Jun, Y. J. Reo, W. J. Kim and K. H. Ahn, Chemistry, 2020, 26, 11549–11557 CrossRef CAS PubMed.
- L. G. Wang, I. Munhenzva, M. Sibrian-Vazquez, J. O. Escobedo, C. H. Kitts, F. R. Fronczek and R. M. Strongin, J. Org. Chem., 2019, 84, 2585–2595 CrossRef CAS PubMed.
- L. Wang, C. W. Barth, M. Sibrian-Vazquez, J. O. Escobedo, M. Lowry, J. Muschler, H. Li, S. L. Gibbs and R. M. Strongin, ACS Omega, 2017, 2, 154–163 CrossRef CAS PubMed.
- X. Wu, Y. Gao, W. Chi, C. Wang, Z. Xu and X. Liu, Mater. Chem. Front., 2023, 7, 1137–1145 RSC.
- D. Kim, Q. P. Xuan, H. Moon, Y. W. Jun and K. H. Ahn, Asian J. Org. Chem., 2014, 3, 1089–1096 CrossRef CAS.
- G. J. Mao, Z. Z. Liang, J. Bi, H. Zhang, H. M. Meng, L. Su, Y. J. Gong, S. Feng and G. Zhang, Anal. Chim. Acta, 2019, 1048, 143–153 CrossRef CAS PubMed.
- C. Liu, X. Jiao, Q. Wang, K. Huang, S. He, L. Zhao and X. Zeng, Chem. Commun., 2017, 53, 10727–10730 RSC.
- Q. A. Best, A. E. Johnson, B. Prasai, A. Rouillere and R. L. McCarley, ACS Chem. Biol., 2016, 11, 231–240 CrossRef CAS PubMed.
- K. S. Hettie, J. L. Klockow, T. E. Glass and F. T. Chin, Anal. Chem., 2019, 91, 3110–3117 CrossRef CAS PubMed.
- S. Jia, K. M. Ramos-Torres, S. Kolemen, C. M. Ackerman and C. J. Chang, ACS Chem. Biol., 2018, 13, 1844–1852 CrossRef CAS PubMed.
- F. Deng, L. Liu, W. Huang, C. Huang, Q. Qiao and Z. Xu, Spectrochim. Acta, Part A, 2020, 240, 118466 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680–5682 CrossRef CAS PubMed.
- Z. Mao, H. Jiang, X. Song, W. Hu and Z. Liu, Anal. Chem., 2017, 89, 9620–9624 CrossRef CAS PubMed.
- K. H. Kim, S. Singha, Y. W. Jun, Y. J. Reo, H. R. Kim, H. G. Ryu, S. Bhunia and K. H. Ahn, Chem. Sci., 2019, 10, 9028–9037 RSC.
- Y. Kushida, T. Nagano and K. Hanaoka, Analyst, 2015, 140, 685–695 RSC.
- Y. Yang, M. Lowry, X. Xu, J. O. Escobedo, M. Sibrian-Vazquez, L. Wong, C. M. Schowalter, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8829–8834 CrossRef CAS PubMed.
- M. Dai, Y. J. Reo, C. W. Song, Y. J. Yang and K. H. Ahn, Chem. Sci., 2020, 11, 8901–8911 RSC.
- Y. M. Poronik, K. V. Vygranenko, D. Gryko and D. T. Gryko, Chem. Soc. Rev., 2019, 48, 5242–5265 RSC.
- Z. Lei, X. Li, X. Luo, H. He, J. Zheng, X. Qian and Y. Yang, Angew. Chem., Int. Ed. Engl., 2017, 56, 2979–2983 CrossRef CAS PubMed.
- J. Li, Y. Dong, R. Wei, G. Jiang, C. Yao, M. Lv, Y. Wu, S. H. Gardner, F. Zhang, M. Y. Lucero, J. Huang, H. Chen, G. Ge, J. Chan, J. Chen, H. Sun, X. Luo, X. Qian and Y. Yang, J. Am. Chem. Soc., 2022, 144, 14351–14362 CrossRef CAS PubMed.
- R. Wei, Y. Dong, X. Wang, J. Li, Z. Lei, Z. Hu, J. Chen, H. Sun, H. Chen, X. Luo, X. Qian and Y. Yang, J. Am. Chem. Soc., 2023, 145, 12013–12022 CrossRef CAS PubMed.
- A. Roth, H. Li, C. Anorma and J. Chan, J. Am. Chem. Soc., 2015, 137, 10890–10893 CrossRef CAS PubMed.
- K. Hanaoka, Y. Kagami, W. Piao, T. Myochin, K. Numasawa, Y. Kuriki, T. Ikeno, T. Ueno, T. Komatsu, T. Terai, T. Nagano and Y. Urano, Chem. Commun., 2018, 54, 6939–6942 RSC.
- H. Zhang, J. Liu, L. Wang, M. Sun, X. Yan, J. Wang, J. P. Guo and W. Guo, Biomaterials, 2018, 158, 10–22 CrossRef CAS PubMed.
- Y. Fang, G. N. Good, X. Zhou and C. I. Stains, Chem. Commun., 2019, 55, 5962–5965 RSC.
- Y. J. Yang, M. Dai, Y. J. Reo, C. W. Song, S. Sarkar and K. H. Ahn, Anal. Chem., 2021, 93, 7523–7531 CrossRef CAS PubMed.
- U. Tamima, C. W. Song, M. Santra, Y. J. Reo, H. Banna, M. R. Islam and K. H. Ahn, Sens. Actuators, B, 2020, 322, 128588 CrossRef CAS.
- U. Tamima, M. Santra, C. W. Song, Y. J. Reo and K. H. Ahn, Anal. Chem., 2019, 91, 10779–10785 CrossRef CAS PubMed.
- S. Sarkar, H. Lee, H. G. Ryu, S. Singha, Y. M. Lee, Y. J. Reo, Y. W. Jun, K. H. Kim, W. J. Kim and K. H. Ahn, ACS Sens., 2021, 6, 148–155 CrossRef CAS PubMed.
- P. Horváth, P. Šebej, D. Kovář, J. Damborský, Z. Prokop and P. Klán, ACS Omega, 2019, 4, 5479–5485 CrossRef.
- J. Tang, Z. Guo, Y. Zhang, B. Bai and W. H. Zhu, Chem. Commun., 2017, 53, 10520–10523 RSC.
- K. H. Kim, S. J. Kim, S. Singha, Y. J. Yang, S. K. Park and K. H. Ahn, ACS Sens., 2021, 6, 3253–3261 CrossRef CAS PubMed.
- B. P. Czech and R. A. Bartsch, J. Org. Chem., 1984, 49, 4076–4078 CrossRef CAS.
- H. N. Lv, P. F. Tu and Y. Jiang, Mini. Rev. Med. Chem., 2014, 14, 603–622 CrossRef CAS.
- M. Tasior, D. Kim, S. Singha, M. Krzeszewski, K. H. Ahn and D. T. Gryko, J. Mater. Chem. C, 2015, 3, 1421–1446 RSC.
- Y. Jung, J. Jung, Y. Huh and D. Kim, J. Anal. Methods Chem., 2018, 2018, 5249765 Search PubMed.
- A. R. Sarkar, C. H. Heo, H. W. Lee, K. H. Park, Y. H. Suh and H. M. Kim, Anal. Chem., 2014, 86, 5638–5641 CrossRef CAS PubMed.
- Y. J. Reo, Y. W. Jun, S. W. Cho, J. Jeon, H. Roh, S. Singha, M. Dai, S. Sarkar, H. R. Kim, S. Kim, Y. Jin, Y. L. Jung, Y. J. Yang, C. Ban, J. Joo and K. H. Ahn, Chem. Commun., 2020, 56, 10556–10559 RSC.
- K. H. Ahn and Y. W. Jun, Phys. Sci., 2022, 126–129, DOI:10.26904/RF-141-2648143398.
- T. Yoshihara, R. Maruyama, S. Shiozaki, K. Yamamoto, S. I. Kato, Y. Nakamura and S. Tobita, Anal. Chem., 2020, 92, 4996–5003 CrossRef CAS PubMed.
- A. R. Sarkar, C. H. Heo, L. Xu, H. W. Lee, H. Y. Si, J. W. Byun and H. M. Kim, Chem. Sci., 2016, 7, 766–773 RSC.
- S. W. Cho, Y. J. Reo, S. Sarkar and K. H. Ahn, Bull. Korean Chem. Soc., 2020, 42, 135–139 CrossRef.
- Y. J. Reo, Y. W. Jun, S. Sarkar, M. Dai and K. H. Ahn, Anal. Chem., 2019, 91, 14101–14108 CrossRef CAS PubMed.
- S. W. Cho, Y. W. Jun, Y. J. Reo, S. Sarkar and K. H. Ahn, Results Chem., 2021, 3, 100117 CrossRef CAS.
- M. Dai, C. W. Song, Y. J. Yang, H. R. Kim, Y. J. Reo and K. H. Ahn, Sens. Actuators, B, 2021, 330, 129277 CrossRef CAS.
- S. Sarkar, A. Shil, M. Nandy, S. Singha, Y. J. Reo, Y. J. Yang and K. H. Ahn, Anal. Chem., 2022, 94, 1373–1381 CrossRef CAS PubMed.
- Z. Mao, H. Jiang, Z. Li, C. Zhong, W. Zhang and Z. Liu, Chem. Sci., 2017, 8, 4533–4538 RSC.
- H. Kim, S. Sarkar, M. Nandy and K. H. Ahn, Spectrochim. Acta, Part A, 2021, 248, 119088 CrossRef CAS PubMed.
- Y. W. Jun, S. Sarkar, S. Singha, Y. J. Reo, H. R. Kim, J. J. Kim, Y. T. Chang and K. H. Ahn, Chem. Commun., 2017, 53, 10800–10803 RSC.
- U. Tamima, S. Singha, H. R. Kim, Y. J. Reo, Y. W. Jun, A. Das and K. H. Ahn, Sens. Actuators, B, 2018, 277, 576–583 CrossRef CAS.
- M. Santra, S. Sarkar, Y. W. Jun, Y. J. Reo and K. H. Ahn, Tetrahedron Lett., 2018, 59, 3210–3213 CrossRef CAS.
- H. R. Kim, S. Sarkar and K. H. Ahn, Chem. – Asian J., 2022, 17, e202101317 CrossRef PubMed.
- H. J. Park, C. W. Song, S. Sarkar, Y. W. Jun, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Commun., 2020, 56, 7025–7028 RSC.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2002, 107, 597–606 CrossRef.
- É. Torres, S. Sequeira, P. Parreira, P. Mendes, T. Silva, K. Lobato and M. J. Brites, J. Photochem. Photobiol., A, 2015, 310, 1–8 CrossRef.
- L. Han, R. Kang, X. Zu, Y. Cui and J. Gao, Photochem. Photobiol. Sci., 2015, 14, 2046–2053 CrossRef CAS PubMed.
- K. C. Avhad, D. S. Patil, Y. K. Gawale, S. Chitrambalam, M. C. Sreenath, I. H. Joe and N. Sekar, ChemistrySelect, 2018, 3, 4393–4405 CrossRef CAS.
- K. D. Seo, I. T. Choi, Y. G. Park, S. Kang, J. Y. Lee and H. K. Kim, Dyes Pigm., 2012, 94, 469–474 CrossRef CAS.
- H. Feng, R. Li, Y. Song, X. Li and B. Liu, J. Power Sources, 2017, 345, 59–66 CrossRef CAS.
- C. W. Song, U. Tamima, Y. J. Reo, M. Dai, S. Sarkar and K. H. Ahn, Dyes Pigm., 2019, 171, 107718 CrossRef CAS.
- N. Jiang, J. Fan, F. Xu, X. Peng, H. Mu, J. Wang and X. Xiong, Angew. Chem., Int. Ed. Engl., 2015, 54, 2510–2514 CrossRef CAS PubMed.
- P. S. Deore and R. A. Manderville, Analyst, 2020, 145, 1288–1293 RSC.
- P. S. Deore, D. S. Coman and R. A. Manderville, Chem. Commun., 2019, 55, 3540–3543 RSC.
- N. Narayanaswamy, M. Kumar, S. Das, R. Sharma, P. K. Samanta, S. K. Pati, S. K. Dhar, T. K. Kundu and T. Govindaraju, Sci. Rep., 2014, 4, 6476 CrossRef CAS PubMed.
- A. Eordogh, J. Steinmeyer, K. Peewasan, U. Schepers, H. A. Wagenknecht and P. Kele, Bioconjugate Chem., 2016, 27, 457–464 CrossRef CAS PubMed.
- J. W. Yan, Y. G. Tian, J. H. Tan and Z. S. Huang, Analyst, 2015, 140, 7146–7149 RSC.
- P. Jana, M. Radhakrishna, S. Khatua and S. Kanvah, Phys. Chem. Chem. Phys., 2018, 20, 13263–13270 RSC.
- S. K. Lanke and N. Sekar, J. Fluoresc., 2016, 26, 497–511 CrossRef CAS PubMed.
- X. D. Liu, Y. Xu, R. Sun, Y. J. Xu, J. M. Lu and J. F. Ge, Analyst, 2013, 138, 6542–6550 RSC.
- S. Zhu, W. Lin and L. Yuan, Dyes Pigm., 2013, 99, 465–471 CrossRef CAS.
- L. Yuan, W. Lin and J. Song, Chem. Commun., 2010, 46, 7930–7932 RSC.
- F. Dubar, C. Slomianny, J. Khalife, D. Dive, H. Kalamou, Y. Guerardel, P. Grellier and C. Biot, Angew. Chem., Int. Ed. Engl., 2013, 52, 7690–7693 CrossRef CAS PubMed.
- Y. Q. Sun, J. Liu, J. Zhang, T. Yang and W. Guo, Chem. Commun., 2013, 49, 2637–2639 RSC.
- Y. Venkatesh, K. S. Kiran, S. S. Shah, A. Chaudhuri, S. Dey and N. D. P. Singh, Org. Biomol. Chem., 2019, 17, 2640–2645 RSC.
- K. A. Pardeshi, G. Ravikumar and H. Chakrapani, Org. Lett., 2018, 20, 4–7 CrossRef CAS PubMed.
- T. Zhang, C. Yin, Y. Zhang, J. Chao, G. Wen and F. Huo, Spectrochim. Acta, Part A, 2020, 234, 118253 CrossRef CAS PubMed.
- W. Xu, C. L. Teoh, J. Peng, D. Su, L. Yuan and Y. T. Chang, Biomaterials, 2015, 56, 1–9 CrossRef CAS PubMed.
- T. Zhang, L. Li, F. Huo, W. Zhang, J. Chao and C. Yin, Sens. Actuators, B, 2021, 342, 130041 CrossRef CAS.
- J. Liu, Y.-Q. Sun, H. Zhang, Y. Huo, Y. Shi, H. Shi and W. Guo, RSC Adv., 2014, 4, 64542–64550 RSC.
- X. Lv, J. Liu, Y. Liu, Y. Zhao, Y. Q. Sun, P. Wang and W. Guo, Chem. Commun., 2011, 47, 12843–12845 RSC.
- M.-J. Peng, Y. Guo, X.-F. Yang, F. Suzenet, J. Li, C.-W. Li and Y.-W. Duan, RSC Adv., 2014, 4, 19077–19085 RSC.
- H. Li, Z. Wen, L. Jin, Y. Kan and B. Yin, Chem. Commun., 2012, 48, 11659–11661 RSC.
- Z. Yang, Z. Liu, Y. Chen, X. Wang, W. He and Y. Lu, Org. Biomol. Chem., 2012, 10, 5073–5076 RSC.
- C. T. Yang, Y. Wang, E. Marutani, T. Ida, X. Ni, S. Xu, W. Chen, H. Zhang, T. Akaike, F. Ichinose and M. Xian, Angew. Chem., Int. Ed. Engl., 2019, 58, 10898–10902 CrossRef CAS PubMed.
- J. Li, Y. Cui, C. Bi, S. Feng, F. Yu, E. Yuan, S. Xu, Z. Hu, Q. Sun, D. Wei and J. Yoon, Anal. Chem., 2019, 91, 7360–7365 CrossRef CAS PubMed.
- H. Fujioka, S. N. Uno, M. Kamiya, R. Kojima, K. Johnsson and Y. Urano, Chem. Commun., 2020, 56, 5617–5620 RSC.
- D. Zhu, X. Yan, A. Ren, W. Xie and Z. Duan, Anal. Chim. Acta, 2019, 1058, 136–145 CrossRef CAS PubMed.
- X. Wu, L. Zeng, B. Q. Chen, M. Zhang, J. Rodrigues, R. Sheng and G. M. Bao, J. Mater. Chem. B, 2019, 7, 5775–5781 RSC.
- S. Singha, D. Kim, B. Roy, S. Sambasivan, H. Moon, A. S. Rao, J. Y. Kim, T. Joo, J. W. Park, Y. M. Rhee, T. Wang, K. H. Kim, Y. H. Shin, J. Jung and K. H. Ahn, Chem. Sci., 2015, 6, 4335–4342 RSC.
- Z. Zhan, R. Liu, L. Chai, Y. Dai and Y. Lv, Anal. Chem., 2019, 91, 11461–11466 CrossRef CAS PubMed.
- H. Huang, W. Liu, X. J. Liu, Y. Q. Kuang and J. H. Jiang, Talanta, 2017, 168, 203–209 CrossRef CAS PubMed.
- Y. J. Reo, S. Sarkar, M. Dai, Y. J. Yang and K. H. Ahn, ACS Appl. Bio Mater., 2021, 4, 2089–2096 CrossRef CAS PubMed.
- Y. J. Yang, M. Dai and K. H. Ahn, ACS Sens., 2023, 8(7), 2791–2798 CrossRef CAS PubMed.
- Z. Yu, W. Huang, S. Xu and S. Ke, Microchem. J., 2021, 164, 106009 CrossRef CAS.
- L. Nie, C. Gao, T. Shen, J. Jing, S. Zhang and X. Zhang, Anal. Chem., 2019, 91, 4451–4456 CrossRef CAS PubMed.
- J. Zhou, C. Yu, Z. Li, P. Peng, D. Zhang, X. Han, H. Tang, Q. Wu, L. Li and W. Huang, Anal. Methods, 2019, 11, 1312–1316 RSC.
- Y. Wang, X. Han, X. Zhang, L. Zhang and L. Chen, Analyst, 2020, 145, 1389–1395 RSC.
- Y. J. Reo, M. Dai, Y. J. Yang and K. H. Ahn, Anal. Chem., 2020, 92, 12678–12685 CrossRef CAS PubMed.
- Y. J. Kim, S. J. Park, C. S. Lim, D. J. Lee, C. K. Noh, K. Lee, S. J. Shin and H. M. Kim, Anal. Chem., 2019, 91, 9246–9250 CrossRef CAS PubMed.
- L. Yuan, W. Lin, Y. Yang and H. Chen, J. Am. Chem. Soc., 2012, 134, 1200–1211 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13513 CrossRef CAS PubMed.
- H. Chen, B. Dong, Y. Tang and W. Lin, Acc. Chem. Res., 2017, 50, 1410–1422 CrossRef CAS PubMed.
- Z. Zeng, S. S. Liew, X. Wei and K. Pu, Angew. Chem., Int. Ed. Engl., 2021, 60, 26454–26475 CrossRef CAS PubMed.
- Y. Wen, N. Jing, F. Huo and C. Yin, Analyst, 2021, 146, 7450–7463 RSC.
- M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583–7601 RSC.
- S. Singha, Y. W. Jun, S. Sarkar and K. H. Ahn, Acc. Chem. Res., 2019, 52, 2571–2581 CrossRef CAS PubMed.
- H. H. Han, H. Tian, Y. Zang, A. C. Sedgwick, J. Li, J. L. Sessler, X. P. He and T. D. James, Chem. Soc. Rev., 2021, 50, 9391–9429 RSC.
| This journal is © The Royal Society of Chemistry 2023 |