Open Access Article
Open Access ArticleBenign catalytic oxidation of potato starch using a homogeneous binuclear manganese catalyst and hydrogen peroxide†
J. O. P.
Broekman
 a,
Homer C.
Genuino
a,
Hero J.
Heeres
a,
Homer C.
Genuino
a,
Hero J.
Heeres
 a,
Jelle
Brinksma
b,
Thomas
Wielema
b and
Peter J.
Deuss
a,
Jelle
Brinksma
b,
Thomas
Wielema
b and
Peter J.
Deuss
 *a
*a
aGreen Chemical Reaction Engineering, Engineering and Technology institute Groningen, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: p.j.deuss@rug.nl
bAvebe Innovation Center, Zernikelaan 8, 9747 AW, Groningen, The Netherlands
First published on 10th January 2023
Abstract
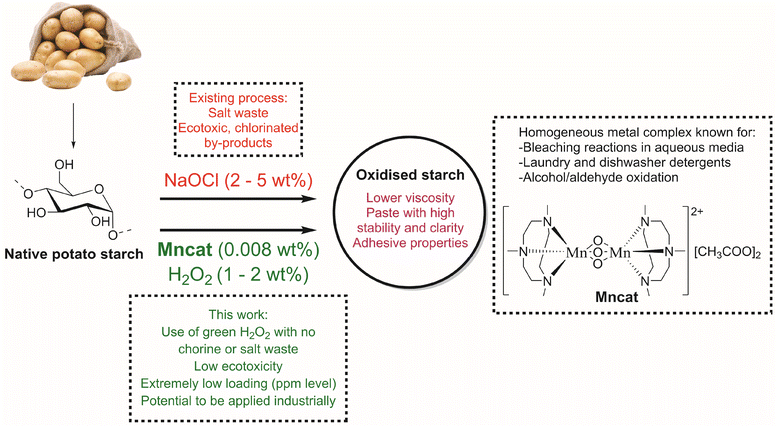
Oxidation is an excellent way to improve the properties of native starches. After oxidation, products are easier to handle due to a lowered paste viscosity in water, an improved stability and enhanced adhesive properties. Currently, oxidation by sodium hypochlorite (NaOCl) is the dominant commercial process for oxidized starches, which allows for oxidation of hydroxyl groups into carboxylic acids. Here, we show that by using a commercial homogeneous binuclear manganese catalyst ([MnIV2(μ-O)3(tmtacn)2][(CH3COO)2] (Mncat), with tmtacn = 1,4,7-trimethyl-1,4,7-triazacyclononane), and H2O2 as oxidant, starch can be oxidised without the cogeneration of ecotoxic chlorinated waste products. Although oxidation with H2O2 and other catalysts (mainly iron-based) has been done, high loadings were needed and the starch pasting properties were not yet on par with NaOCl oxidised starches. Starch granules suspended in water can be oxidized at room temperature with 0.0021 mol% Mncat and 1 wt% H2O2 yielding starch with similar properties (DSCOOH, yield, pasting properties) as those achieved by NaOCl oxidation. This catalytic oxidation of starch with an earth-abundant metal catalyst at ppm loadings, which is widely applied in detergents, highlights the potential for the development of a more sustainable process to produce oxidized starches.
1. Introduction
Starch is a cheap abundant resource derived from natural sources at a million tonne scale every year.1–3 As such, its utilization for chemical products fits well in the trend in both industry and academia to move towards greener, and more sustainable products and processes. To give starch desirable (pasting) properties (DSCOOH, viscosity stability) for its use in a wide range of applications requires (bio)chemical or physical modification. For example, native potato starch forms pastes with a high viscosity and retrogrades upon cooling or storage. This retrogradation is a crystallisation process of the chains in the paste which causes undesirable property changes such as hardening, expulsion of water after freezing (syneresis) or altering the taste and texture of food.4–6 A well-known example is the staling of bread.7 To improve upon these pasting properties (lower viscosity and retrogradation tendency), modifications such as etherification, acetylation, cross-linking, hydrolysis or oxidation, and thermal, mechanical or high-pressure treatment are performed.8–11 One such modification method is oxidation, which is a well-established practise using aqueous NaOCl or bleach dating back about 150 years ago.9By introducing carboxylic acid groups, starches with improved properties such as a higher paste clarity, water holding capacity and storage stability, are obtained. Additionally, the anionic groups make the starches suitable to be used as adhesive or for surface sizing, both used extensively in the paper industry. Furthermore, oxidative cleavage helps to lower the viscosity of the final starch paste, which improves handling and ease of application.12–15 In spite of the low cost and versatility offered by the NaOCl oxidation process, it has drawbacks in terms of sustainability and storage safety. Not only does it produce copious amounts of waste in the form of brine, chlorination of organic substrates takes place during the process.16–18 These issues lead to a foreseen reduction in its use due to upcoming stricter legislation for waste disposal in surface water.
To oxidise insoluble starch in a more sustainable way, we envision the use of catalysts with environmentally friendlier oxidants. Using hydrogen peroxide (H2O2) as oxidant, which decomposes to water and oxygen only, has clear benefits over the wastes generated by NaOCl oxidation.19 This has been used in several studies (for an overview, see Table S1†), such as with iron salt catalysts (Fenton chemistry) which showed mainly depolymerisation with only limited formation of carboxylic acid groups and required high metal loadings (0.29 mol%, Table S1,† entry 1) indicating more specific and efficient catalysts bearing protective ligands might be needed.17,20,21 In recent years, an iron(III) tetrasulfonatophthalocyanine complex (FePcS) was studied for starch oxidation and allowed the more efficient use of H2O2 at lower metal loadings (0.0042–0.017 mol%, entries 2–7).22–26 However, the catalyst complex was found to decompose rapidly at optimal reaction conditions.26 Furthermore, the loadings were still quite high (H2O2 loading >5.6 wt%) and long reaction times were required (>420 min), while the starch products only reached moderate degree of oxidation (<0.016) and yields (<91%) which would make them economically unfeasible at an industrial scale. We aimed to improve upon these shortcomings by changing the catalytic system for starch oxidation in order to reach higher yields and DSCOOH at lower loadings and reaction times.
[MnIV2(μ-O)3(tmtacn)2][(CH3COO)2], with tmtacn = 1,4,7-trimethyl-1,4,7-triazacyclononane (Mncat, Fig. 1) is a well-known catalyst for epoxidation, cis-dihydroxylation,27–30 and for alcohol or aldehyde oxidation31,32 at room temperature or even 0 °C. These transformations are typically done in acetonitrile after modification of Mncat with two acetic acid-bridges. The carboxylic acid additives function both as co-catalysts and in suppressing undesired catalase-type activity.28,29,33,34 More interesting perhaps is that Mncat has been used for bleaching of cotton in aqueous media and was commercialized in laundry and dishwasher detergents highlighting the non-toxic nature of the catalyst.35,36 However, Mncat was shown to cause fabric damage due to depolymerisation of the cellulose, causing the catalyst to be withdrawn for these applications.36,37 The molecular similarities of cotton and starch, which are both composed of glucose units, make this catalytic system interesting for starch oxidation. Furthermore, some depolymerisation is actually a vital part of the starch oxidation process and thus some structural deterioration by this catalytic system could actually aid reaching desired pasting properties. Oxidation of starch by Mncat/H2O2 was studied in a patent from Solvay38 and mentioned in another patent from Avebe along with dinuclear copper complexes,39 but an in-depth investigation of the starch properties after oxidation and an optimisation of the oxidation conditions as well as insight into the mode of action of this catalytic system under alkali conditions in water remains unexplored.
Here, we report [MnIV2(μ-O)3(tmtacn)2][(CH3COO)2] as a non-toxic, earth abundant metal catalyst for benign starch oxidation utilising H2O2 as oxidant to yield starch with similar pasting properties and yields as those achieved by NaOCl oxidation. Reaction conditions and modes of addition of peroxide are explored to increase the efficiency of the oxidation of starch in terms of DSCOOH. Furthermore, the mode of action of this catalyst is studied. All of this to improve upon the current benchmark of catalytic oxidation of starch with H2O2, which is FePcS and to provide a catalytic alternative for industrial NaOCl oxidation of starch.
2. Experimental
2.1 Materials and methods
Avebe (The Netherlands) kindly provided native, granular potato starch (moisture content ∼15 wt%, ∼20% amylose) which was used as received, and the [MnIV2(μ-O)3(tmtacn)2][(CH3COO)2] complex Mncat (WeylChem Catexel, The Netherlands) provided in a 3.5 wt% solution, which was diluted to a 1000 ppm solution with deionized water before use. H2O2 (30 wt% in water), NaOH and hydroxylamine hydrochloride (reagent grade) were purchased from Merck. Phenolphthalein (reagent grade) was purchased from Sigma-Aldrich. Concentrated H2SO4 and HCl were purchased from different commercial suppliers and used as received.2.2 Mncat/H2O2 catalytic oxidation of starch
This is a general procedure for the oxidation of starch. Detailed reaction conditions (amounts of peroxide/catalyst/etc.) and reproducibility experiments are specified in the results and discussion and can be found in the ESI† (Tables S4 and S5).Native potato starch (39 g dry basis, 0.241 mol) was added to deionized water (∼55 g) while stirring vigorously with an overhead stirrer to obtain a 39 wt% suspension (dry basis) and brought to the desired pH by addition of a 1.1 M NaOH solution. The suspension was heated and kept at the selected temperature using a Julabo 4 thermostat bath (Fig. S1†). Next, the desired amount of Mncat was added from a 1000 ppm stock solution (e.g. 3.11 mL/0.0021 mol% to dry starch weight). The reaction was initiated by the addition of H2O2 (30 wt% in water) either added in batch or semi-batch fashion using a New Era NE-1000 syringe pump. In case of batch addition, the first dose was 30% of the total peroxide amount (e.g. 351 μL/0.3 wt% to dry starch weight), the next two additions consisted of 35% of the total peroxide amount. After every addition, consumption of peroxide was confirmed by peroxide test strips. For semi-batch addition a constant rate of 2 wt% H2O2 per h was used unless specified otherwise. During the reaction, temperature and pH were monitored by a WTW InoLab pH 7310 pH meter with a WTW SenTix 81 Precision pH electrode with temperature sensor. The pH was kept constant at the desired pH by an Arduino Uno with an Atlas Scientific EZO circuit and Atlas Scientific pH electrode which controlled a Watson Marlow 101 dosing pump supplying 1.1 M NaOH. After complete consumption of the last added hydrogen peroxide, the reaction had concluded and the suspension was acidified to pH 5 using conc. H2SO4 or HCl followed by filtration over a Büchner funnel and washing using 1 L deionized water. The starch residue was dried overnight in a fume hood under air. The gravimetrically determined product yield was corrected for the moisture content to determine the starch loss and final recovered solid starch yield. The moisture content was determined using a Kern moisture analyser DBS 60-3.
2.3 NaOCl oxidation of starch
Detailed results can be found in the ESI† (Tables S2 and S3). The procedures were adopted from patented procedures.39,40Native potato starch (39 g dry basis, 0.241 mol) was added to deionized water (∼55 g) while stirring vigorously with an overhead stirrer to obtain a 39 wt% suspension (dry basis) and brought to pH 8.3 by addition of a 1.1 M NaOH solution. The suspension was heated to 35 °C and kept at this temperature using a Julabo 4 thermostat bath (Fig. S1†). Next, a NaOCl solution (15% active chlorine) was added over 10 min (8.08 mL total) for a total of 3.7 wt% (active Cl to dry weight starch). During addition the pH was kept at 8.3 by addition of dilute sulfuric acid. After addition and for an additional 2 h reaction time, temperature and pH were monitored by a WTW InoLab pH 7310 pH meter with a WTW SenTix 81 Precision pH electrode with temperature sensor. The pH was kept constant at 8.3 by an Arduino Uno with an Atlas Scientific EZO circuit and Atlas Scientific pH electrode which controlled a Watson Marlow 101 dosing pump supplying 1.1 M NaOH. After 2 h, the consumption of all NaOCl was verified by a test strip, after which the temperature was decreased to 30 °C and the pH increased to 10.5. The reaction was kept at these values for 1 h and 2 mL additional NaOCl (15% active Cl) was added for bleaching purposes. After complete consumption in 10 min, the reaction had concluded and the suspension was acidified to pH 5 using conc. H2SO4 followed by filtration over a Büchner funnel and washing using 1 L deionized water. The starch residue was dried overnight in a fume hood under air. The gravimetrically determined product yield was corrected for the moisture content to determine the starch loss and final recovered solid starch yield (97%). The moisture content was determined using a Kern moisture analyser DBS 60-3.
2.4 Determination of the degree of oxidation
The carboxyl content was determined titrimetrically according to the modified procedure of Chattopadhyay et al.41 Dried starch (2.5 g, wet weight) was suspended in 50 mL of 0.1 M HCl with stirring for 30 min at room temperature. The product was filtered, washed with deionized water (1 L), and dissolved in 200 mL of boiling water. The starch solution was cooled to 40–50 °C and titrated with 0.1 M NaOH using phenolphthalein as indicator. The degree of carboxyl substitution was expressed as the amount of mol of carboxyl group per mol of glucose units calculated using eqn (1):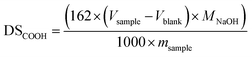 | (1) |
2.5 Determination of the pasting properties
The pasting properties of the oxidised starches was determined using a Rapid Visco-Analyzer (RVA) from Newport Scientific. An RVA sample was prepared by mixing 8.4 g of oxidised starch (dry basis) with deionised water to a total weight of 28 g. During analysis, the sample was spun and heated from 50 °C to 95 °C in 3.7 min, followed by a hold stage at 95 °C for 2.5 min, cooling to 50 °C in 3.7 min, and a final hold stage at 50 °C for 2 min. From the resulting RVA pasting curves the following parameters were obtained and noted down (see ESI†): peak viscosity (maximum viscosity in the curve), hold viscosity (lowest viscosity after cooling), breakdown (peak – hold viscosities), final viscosity (viscosity at the end of the measurement) and setback (final – hold viscosities).2.6 Starch structure characterization
High-performance size-exclusion chromatography (HPSEC) was performed on a Agilent Technologies 1200 Series instrument using 3 PSS Suprema columns in series (100, 1000 and 3000 Å, 300 × 8 mm, 10 μm), PSS WinGPC UniChrom software and a RID detector. Calibration was done using pullulan standards. The eluent was 50 mM NaNO3 in water with a flow rate of 1 mL min−1, a column temperature of 40 °C and an injection volume of 10 μL. Sample preparation was done by suspending 60–70 mg of sample (oxidised starch) in ∼20 mL solvent which was then heated in pressure vials for 60 min at 130 °C followed by filtration over 0.2 μm filters.Scanning electron microscope (SEM) images were acquired on a Fei NovaNanoSEM 650 using a CBS detector and an accelerating voltage of 5 kV. Samples were loaded onto a sample holder and then sputter-coated with a layer of gold (∼20 nm) prior to analysis.
IPC-MS analysis was performed on a Thermo Scientific iCAP Q spectrometer according to NEN-EN 15763 and NEN-EN-ISO 17294-2 standard norms. A calibration curve for the amount of Mn was made to be able to accurately determine the Mn content in the starch and water samples.
3. Results and discussion
3.1 Starch oxidation using H2O2 catalysed by Mncat
Initially, the performance of the Mncat/H2O2 system was screened at temperatures ranging from 10 to 40 °C, a pH of 11, 0.0021 mol% Mncat (to anhydroglucose units), and 1 wt% H2O2 (to dry starch weight) monitoring the yield and degree of oxidation (DSCOOH, Table S4,†Fig. 2a). Reactions were run in batch via addition of H2O2 in three batches so as to prevent a high concentration of peroxide to decompose the catalyst (vide infra). The consumption of H2O2 was monitored by test strips and a subsequent batch was added upon complete consumption. pH dependent bleaching performance was recognised early on in the development of Mncat,35 compelling us to carefully monitor and control the pH by addition of a solution of NaOH. For industrial applications the recovered yield of starch should be as high as possible, so for this work our aim was to stay above 95%. The DSCOOH for starches suitable to be used industrially have a range of 0.01–0.2 mol mol−1.24,26 Our interest is purely in granular starch, however, and a high DSCOOH is typically coupled to a high solubility meaning we cannot reach our desired yield of granular starch. Therefore, we aimed for a DS of approximately 0.02–0.04 mol mol−1.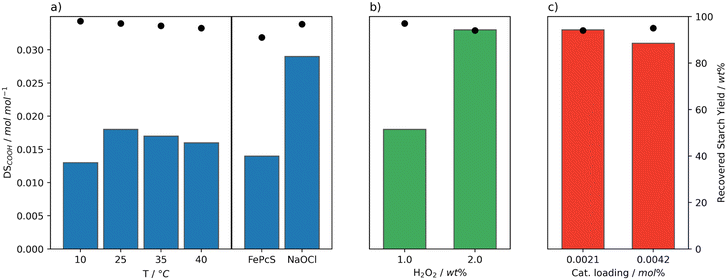 | ||
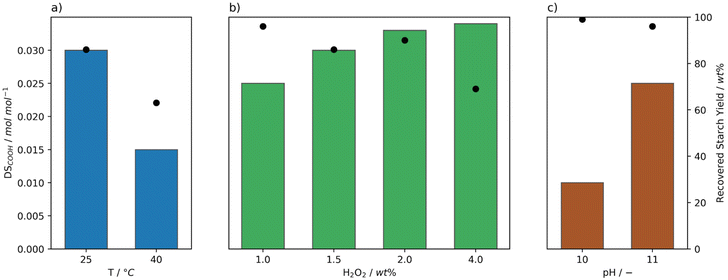
| Fig. 2 Degree of oxidation (DSCOOH, bars) and yield (dots) of starch after oxidation at pH 11 by Mncat with H2O2 added in batch, at varying reaction conditions. a) Reaction temperature (1 wt% H2O2, 0.0021 mol% Mncat). The values are compared to the results using the optimal reaction conditions for FePcS oxidation (0.0042 mol% cat., 50 °C, pH 10, 5.6 wt% H2O2)26 and for oxidation using NaOCl (3.7 wt% active Cl, pH 8.3, 35 °C, our work). b) Amounts of oxidant (0.0021 mol% Mncat, 25 °C). c) Catalyst loadings (2 wt% H2O2, 25 °C). | ||
Mncat is known to oxidise alcohols at 25 °C or below31 and our results on starch indicate that indeed significant oxidation was found at 25 °C (DSCOOH = 0.018 mol mol−1), at a high granular starch recovery yield of 98% (Fig. 2a and Table S4,† entry 2). Comparing results at other temperatures, it was found that at 10 °C (Fig. 2a, entry 1) the reaction time increased significantly (i.e. 32 ≥ 360 min) and the DSCOOH decreased to 0.013 mol mol−1. At the same time, the average molecular weight was 360 kDa compared to 96 kDa for the reaction at 25 °C. These results indicate overall lower oxidation and depolymerization activity. Increasing the temperature (to 35 °C and 40 °C) caused a slight lowering in both the degree of oxidation and starch recovery yield (Fig. 2a, entries 3–4). This indicated that the catalytic activity was too high in terms of starch breakdown releasing soluble fragments that are not recovered by filtration combined with overoxidation to CO2. We can see from this screening that at a mild 25 °C the catalytic system has the best performance.
The performance of our system can be further illustrated by comparison with the best known catalytic oxidation system for starch using H2O2, which is FePcS that reached a DSCOOH of 0.014 mol mol−1 at a moderate recovery yield of 91% at a higher reaction temperature of 50 °C (Fig. 2a).26 Using our system, apart from the ability to reach a higher DSCOOH combined with a higher recovery yield, the oxidant is used far more efficiently requiring less than a fifth of the amount of H2O2 (1 and 5.6 wt%, respectively) and half of the catalyst loading (0.0021 and 0.0042 mol%, respectively) used by the FePcS system. Comparing to the oxidation using NaOCl, a higher DSCOOH (0.029 mol mol−1) at a yield of 97% was obtained when using the batch conditions and 1 wt% H2O2 (Fig. 2a). Thus, further optimisation, of the catalytic procedure was required to reach desirable DS (see below).
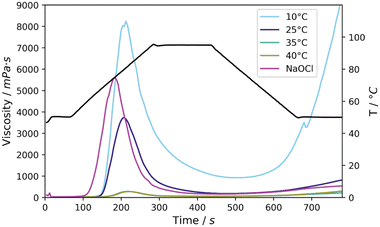
First, a more detailed look was given at the pasting properties of the starches oxidised at various temperatures and to be able to compare these properties after oxidation by NaOCl, analysis was done by a Rapid Visco Analyser (RVA). RVA analysis makes it possible to gain insight into the storage stability of processed starches and more generally the viscosity of the starches in water. A low stability is signified by high retrogradation, in other words an increase in viscosity upon cooling of the starch paste. Furthermore, viscosity build-up is required for food applications, signified by the peak viscosity, as this improves the texture of the foods.11
After oxidation at 10 °C large setback was observed (8277 mPa s) together with a high peak viscosity of 8231 mPa s (Fig. 3, Table S5† entry 1). The peak viscosities decreased to 5344, 282 and 275 mPa s for the reactions at 25, 35 and 40 °C, respectively. A higher stability was attained at these temperatures, seen from the setback of 669 mPa s, 153 mPa s and 232 mPa s, respectively (Fig. 3, entries 2–4). At 10 °C, instability of the paste was observed and a tendency of the starch to retrograde upon cooling and storage. This can be ascribed to the high molecular weight and relatively low DSCOOH, which do not provide enough chain repulsion to prevent retrogradation from occurring. Conversely, at 25 °C we noticed much improved stability of the paste, provided by a higher DSCOOH and more oxidative cleavage causing the molecular weight to be lower. Comparison to NaOCl oxidised starch shows that by oxidation with Mncat/H2O2 at 25 °C similar properties were achieved with a high peak viscosity (5595 mPa s and 5344 mPa s, respectively, Table S3†) and viscosity stability as signified by the low setback (381 mPa s and 669 mPa s, respectively). A key difference was the 10 °C lower gelatinisation temperature (57 °C and 68 °C, respectively, Fig. 3) for NaOCl oxidised starch. We hypothesise that the location or higher homogeneity of oxidation within the granule causes the faster swelling compared to the Mncat oxidised granules which SEM images show were oxidised more on the outside of the granules (section 3.3). In spite of the difference, the high stability combined with the high peak viscosity was achieved by using Mncat/H2O2 for the oxidation of native starch, which makes it viable for replacement of NaOCl.
Increasing the temperature during oxidation led to dramatically lower peak viscosities and little viscosity build-up, which in part could be ascribed to a further increase in oxidative cleavage. At the high concentration used for RVA analysis (30 wt% in H2O) native starch could not be analysed as the viscosity became too high during heating. Therefore, it became apparent that the oxidation decreased the overall viscosity of the starches, and that a higher temperature had a more significant effect (Fig. 3, Table S5† entries 1–4).
Next, the effect of increasing the amount of peroxide and loading of Mncat was explored. To keep the concentration of H2O2 over the whole reaction consistent, H2O2 was added in 6 batches instead of 3. Using a higher loading of H2O2, the degree of oxidation could be increased markedly to 0.033 mol mol−1 with only a minor increase in solid starch losses (98 and 94%, Fig. 2b). The additional H2O2 further changed the pasting properties of the recovered starch, decreasing the peak viscosity (Table S5† entries 2 and 6, 5344 mPa s and 156 mPa s respectively). At this higher H2O2 concentration, it is possible that most catalyst had degraded by the end of the reaction. For this reason, the reaction was run again with double the loading of Mncat, to see whether we could further oxidise the starch (Fig. 2c). No increase in the DSCOOH was observed (0.029 mol mol−1) and the final properties of the recovered starch were similar. Overall, from these results we can see that oxidation by Mncat with H2O2 provides the means necessary to obtain an oxidised starch with excellent stability, and the possibility to be used for applications in the food and paper industries.
3.2 Guiding starch properties by careful reaction control
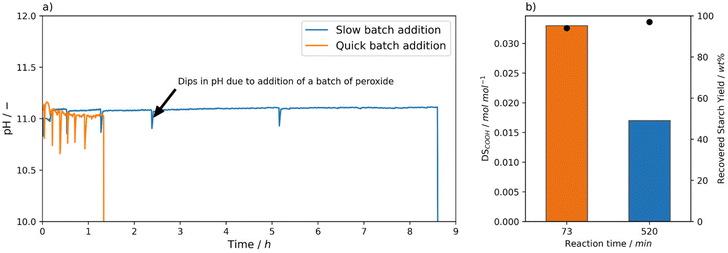
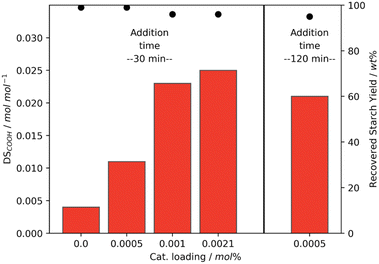
Above, it was established that using Mncat with H2O2 starch with relatively high DSCOOH can be obtained. From this initial set of experiments and related reactions in literature, we realised that pH,35 peroxide dosing,26 and reaction time (also after complete H2O2 consumption) have a large influence on the obtained starch properties. To exemplify, the oxidation using 1 wt% H2O2 and 0.0021 mol% Mncat at 25 °C the degree of oxidation (0.018 ± 0.0002 mol mol−1) and recovery yield (98 ± 1) were reproducible, but the pasting behaviour between repeat experiments showed a variation in the viscosities (i.e. peak viscosity = 5344 ± 2495 mPa s, Tables S4 and S5† entry 2). Since conditions were kept as identical as possible by controlling the pH and H2O2 dosing it was surprising to see these differences. We hypothesized that small changes in pH and time between addition of batches caused these changes. These differences can arise because after every batch the depletion of H2O2 was checked manually using peroxide test strips. Due to the inherent differences arising from the timing of this manual control, we imagined this could cause the differences in pasting properties.A set of experiments was performed with a clear differentiation in the time between addition of the next batch of H2O2 to see whether this had an effect on the DSCOOH and final starch properties (Fig. 4, Tables S4 and S5† entries 5–6). The starches were oxidised using 0.0021 mol% Mncat, at 25 °C and 2 wt% H2O2. As was discussed before, when a short time between batches was taken and the checking by test strips was carefully done (i.e. 10 min, Fig. 4a) a high DSCOOH of 0.033 mol mol−1 was reached at a moderate recovery yield of 94% (vide supra, Fig. 2b). However, when the time of addition of the 2nd batch was increased to 30 min a final starch with a DSCOOH of 0.017 mol mol−1 at 97% yield was obtained (Fig. 4b). Moreover, the time for all H2O2 to be depleted increased for every subsequent batch added, until 3.5 h was not enough to deplete all oxidant after the 5th batch was added and a 6th batch was not added (Fig. 4a). We concluded that the catalyst had degraded at this point, indicating that the catalyst degradation is dependent on the exact timing of the H2O2 additions. We observed that the starch oxidised with 2 wt% H2O2 with a longer batch addition time had a similar DSCOOH and recovery yield as the starches oxidised with 1 wt% H2O2 (cf. Table S4,† entry 2, Fig. 2b), showing that the additional 0.65 wt% H2O2 was not utilised effectively but converted to water and oxygen via catalase-type reactions. This also shows that using test strips to monitor the progress of the reaction is not ideal, as it can cause the reaction times to fluctuate slightly depending on the testing frequency, resulting in small differences between starch batches.
The results show that the catalytic activity of the catalyst drops for longer reaction times (Fig. 4b). We tested whether this was also the case for shorter reactions. After a batch reaction (1 wt% H2O2, 3 batches, 25 °C) new batches of starch and H2O2 were added to the filtered reaction solution and the depletion of H2O2 was monitored. Even the first batch of H2O2 (of three, 0.3 wt%) was not depleted after more than 1 h. This showed that the catalytic activity was lost and the catalyst could not be effectively recovered and reused this way. To investigate the fate of the catalyst, a reaction was done under representative batch conditions (3 batches, 1.25 wt% H2O2, 30 °C) and followed by ICP-MS analysis. Parts of the final starch were washed 1–6 times and the Mn content for all samples was determined. The Mn content remained the same irrespective of the amount of washing steps at an average of 8.1 ppm compared to a Mn content of 0.3 ppm for native starch. 8.1 ppm constitutes 60% of the 13.4 ppm Mn added as Mncat. In the first wash, 4.6 ppm Mn was found (34% of added Mn), meaning that most Mn added was accounted for. From this analysis we can see that ∼60% of the Mn from the catalyst stays trapped on the starch after oxidation. We assume this is as Mn ions, which readily bind to carboxylic acid groups formed during oxidation. The loss of catalytic activity is ascribed to degradation of the Mncat complex. We hypothesise that the ligand is oxidised itself, which was seen by Gilbert et al. under similar conditions.42
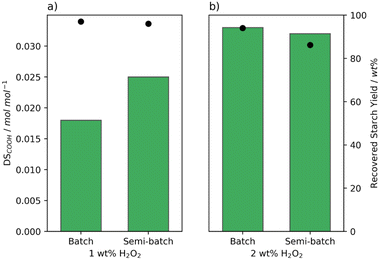
After observation of the differences brought about by changes in the batch addition, we aimed to better control the addition speed and chose to use a syringe pump for semi-batch addition of the H2O2. This eliminates the differences in addition time between batches as the addition speed is kept constant. Moreover, the concentration of H2O2 is kept low and constant which was shown for the oxidation with FePcS to limit the decomposition of the catalyst.26 Indeed an improvement in the oxidation efficiency was achieved reaching a higher degree of oxidation using the same amount of H2O2 (Fig. 5a). Likewise, the pasting properties changed with a lowering in peak viscosity observed from 5344 to 674 mPa s (Table S5† entries 2 and 14). On the other hand, at 2 wt% H2O2, the semi-batch addition did not lead to an increase in DS with a reduction in recovery yield (Fig. 5b). Semi-batch addition seemed to make the oxidation more efficient, however the additional efficiency caused oxidised starch to be lost either due to over-oxidation or dissolution of highly oxidised starch chains.
The oxidation was influenced by temperature as observed for the batch addition experiments (Fig. 2a), and a similar observation was done for the semi-batch addition experiments. When the experiment was done at 40 °C with 0.0042 mol% Mncat and 2 wt% H2O2 the DSCOOH halved (0.030 to 0.015 mol mol−1) and the yield dropped to 63% compared to 86% for the reaction done at 25 °C (Fig. 6a, Table S4† entries 19–20). Although the lower DSCOOH mirrored the batch results, the loss of recovered starch was surprisingly large showing that at these higher catalyst and oxidant loadings it is also undesirable to perform experiments above 25 °C. We wondered whether altering the loading of the oxidant would allow the starches to be oxidised further. At 0.0021 mol% Mncat, a trend was observed of an increase in DSCOOH with increasing loading, although at the same time the yield of recovered starch was lowered from 96 to 69% (Fig. 6b, Table S4† entries 14–17).
Another aspect which was inherent to the batch addition was that drops in pH occur when the slightly acidic peroxide solution was added (Fig. 4a). We were curious to what extend these fluctuations could impact the oxidation results. At a pH of 10, the activity of Mncat was greatly diminished, resulting in a much lower degree of oxidation of 0.010 mol mol−1 (Fig. 6c). Furthermore, the pasting properties were barely improved compared to native starch with a high viscosity and a high retrogradation being observed (Table S5,† entries 14 and 18). From this, we can conclude that the dips in pH brought about by the batch addition of H2O2 can influence the outcome of the reactions. Still, since the pH is quickly returned to the set value by automatic addition of a NaOH solution, the impact is not expected to be that large compared to the differences brought about by changes in the time between addition of H2O2 batches.
In contrast to the batch addition of H2O2, the semi-batch addition led to more efficient oxidation and the loading of Mncat could be lowered. Experiments with 1 wt% H2O2 at 25 °C and lower catalyst loading were performed in which the addition time of H2O2 was kept constant (Fig. 7, left). Gratifyingly, the obtained starch had similar pasting properties (Table S5,† entry 13) and recovery yield using 0.0010 mol% loading of Mncat with only a small decrease in DSCOOH. When the loading was decreased further to 0.0005 mol%, however, the DSCOOH dropped to just 0.011 mol mol−1 and a starch with a high final viscosity of 9393 mPa s was obtained (Tables S4 and S5,† entry 11). Possibly, the degradation of the catalyst is too fast here and only a marginal increase compared to the reaction without catalyst is achieved (DSCOOH of 0.004 mol mol−1 due to the relatively high content of phosphate groups in native starch4).
From these experiments, we hypothesised that the improved oxidation efficiency seen when going from batch to semi-batch oxidation (Fig. 5a) is attributed to the decreased degradation of Mncat due to the lower peroxide concentration during the course of the reaction. By lowering the concentration even further, i.e. by lowering the peroxide addition speed, we corroborated this theory. We observed a higher DSCOOH when the peroxide addition time was increased from 30 min to 120 min with 0.0005 mol% catalyst (Fig. 7, right). Thus when the degradation of Mncat was limited more of the H2O2 can be used efficiently for starch oxidation. This further emphasises that controlling the H2O2 addition speed is essential for tuning the starch modification process, both for addition in batch and semi-batch. Moreover, this means that the process as described here may be optimised further to reach the desired properties at even lower levels of Mncat and H2O2. For example, by also dosing catalyst or changing catalyst, H2O2 and base feed concentrations.
3.3 Hydrolysis and location of oxidation within the granule
When looking in more detail at the oxidation results discussed previously (sections 3.1 and 3.2), what can be observed is that a high oxidation efficiency (i.e. a high DSCOOH) is typically accompanied by decrease in the amount of recovered starch. We were interested in this effect and decided to further analyse these starches by looking into the molecular weight as obtained by Gel Permeation Chromatography (GPC). When taking the molecular weight for all starches discussed in this work (Table S4†) and relating it to the DSCOOH for these starches we can gain more insight into the general action of the catalytic system on the starches (Fig. 8). Immediately striking is the trend observed for the starches oxidised at 25 °C, which shows that at this temperature, the molecular weight and oxidation to carboxylic acids are closely correlated. Based on this we propose a mechanism in which partial hydrolysis precedes oxidation to acid groups. Alkaline hydrolysis is well-known for polysaccharides since at high pH conditions the glycosidic linkages are unstable. The formed reducing end can be oxidised in its open form.43,44 Thus, we hypothesise that under our reaction conditions, in analogy to FePcS oxidation,26 partial hydrolysis forms reducing ends which can subsequently be oxidised to the carboxylic acids (Scheme S1†). We would not expect such a clear trend when the Mncat freely oxidises all alcohol groups in the anhydroglucose units. The same trend (Fig. 8) could hold at higher temperatures, but for these experiments the loss of starch is higher due to over-oxidation and loss of highly oxidised chains causes them to deviate from the trend. To support our hypothesis, we performed the oxidation of glucose at similar reaction conditions to those for starch oxidation. We found that no glucuronic acid (oxidation of glucose at C6) was formed, but instead only the open form was oxidised to gluconic acid (Fig. S3,† oxidation at C2/C3/C4 not observed). This shows that formed aldehydes (most likely reducing ends) are oxidised, and that MnCat does not oxidise alcohols groups of starch in water under our conditions.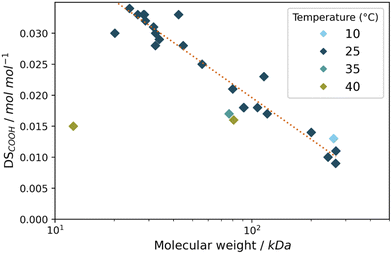 | ||
| Fig. 8 Plot of the log of the average molecular weight (kDa) and DSCOOH (mol mol−1) of the starches used in this study after oxidation, with the various temperatures during the reaction shown in colour. The 25 °C values were fitted to a logarithmic model with r2 = 0.93. For additional data of shown experiments see Tables S4 and S5.† | ||
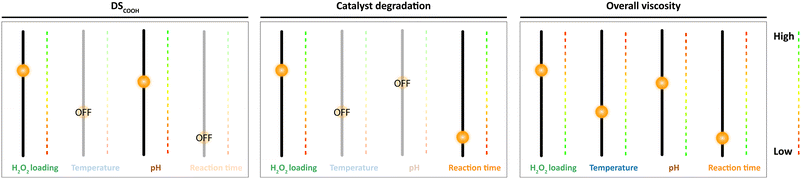
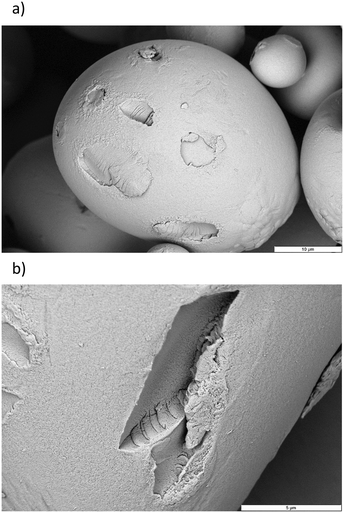
The intimate relation of the oxidative cleavage and the oxidation to carboxylic acids makes oxidation by Mncat/H2O2 distinct from oxidation by NaOCl, which allows tuning of the DSCOOH and extent of oxidative depolymerisation by making changes in the pH.45 Although tuning in this way might not be as direct for our system as for NaOCl, we have gained important insight in this work on the way the reaction parameters influence the starch properties (Fig. 9). We were further interested in the influence of the oxidation on the granule structure which we studied by taking electron microscope images of the oxidised products (Fig. 10). Damage on the outside of the granules in the form of shallow pitting can be observed in contrast to native starch which has smooth granules (Fig. S2†). Similarly, oxidation by FePcS led to granules being damaged solely on the outside.25 In contrast, NaOCl oxidised starch granules remain mostly smooth.46 The most likely explanation for this effect is the size of the catalytic species (dimeric Mncat has an approximate end-to-end size of 1 nm) compared to the small −OCl species, which can enter the granule more easily and oxidise more homogeneously while Mncat might preferentially oxidise the granule periphery. One could expect that localised oxidation could lead to a low stability with high retrogradation potential. However, the pasting properties of the starches do have a high stability according to RVA analysis. Thus, potentially the oxidation also takes place on the inside of the granule in spite of what is observed by SEM images. Further research into this phenomenon is taking place in our laboratory.
4. Conclusions
It has been shown in this work that efficient oxidation of starch can be achieved using homogeneous water-soluble metal complex [MnIV2(μ-O)3(tmtacn)2][(CH3COO)2]. With this catalyst and a low amount of H2O2 (1–2 wt%) the starches can be modified in water to achieve desirable pasting properties with a high stability (peak viscosity ∼5000 mPa s, setback <700 mPa s), making them suitable for applications in the paper and food industries. The process conditions allow the good recovery of starch with a desired DSCOOH (0.02–0.04 mol mol−1) at low loadings of catalyst (0.0005–0.0021 mol%), short reaction times (0.5–2 h) and at a mild temperature (25 °C). Furthermore we have shown that careful control over the reaction conditions in terms of pH, temperature and H2O2 addition timing and dosing is essential. Using semi-batch dosing of H2O2, an oxidised starch with a DSCOOH of 0.025 mol mol−1 at 96% yield could be obtained in 34 min at a pH of 11 and at 25 °C, which showed limited setback indicating a high stability. A correlation was found between the molecular weight and the degree of carboxylation, which shows the importance of oxidative cleavage in this process. The developed system allows for attaining modified starches that can be used as replacement for ubiquitous NaOCl, which struggles with waste and toxicity concerns. Moreover, the successful application of this metal complex shows that the modification of starch with homogeneous catalysts and benign oxidants has significant promise to make existing processes more sustainable.Author contributions
J. O. P. Broekman: investigation, methodology, conceptualisation, visualisation, writing – original draft. Homer C. Genuino: investigation, conceptualisation. Hero J. Heeres: supervision, conceptualisation. Jelle Brinksma: supervision, conceptualisation, validation, writing – review & editing. Thomas Wielema: supervision, resources, validation, conceptualisation. Peter J. Deuss: supervision, validation, methodology, conceptualisation, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Note added after first publication
This article replaces the version published on 19/01/2023, which contained an error in equation 1. The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.Acknowledgements
This chlorine-free starch oxidation work is part of a collaborative project between the University of Groningen and Avebe. Financial support from Samenwerkingsverband Noord-Nederland (SNN), as part of the project Transitie II, Pieken in de delta, project T3003 Duurzaam & kostenefficiënt productieproces zetmeel, is gratefully acknowledged. P. J. D. and J. O. P. B. acknowledge funding via the carbobased research program “A greener starch oxidation”. The carbobased research program is co-financed by the “SNN, Ruimtelijk Economisch Programma” and coordinated by the Carbohydrate Competence Center (https://www.cccresearch.nl). The authors furthermore acknowledge the financial support of Avebe for this research program. Lisa Langelaan is acknowledged for analytical measurements (RVA). Tim Meinds is acknowledged for useful initial experimentation. Zhenlei Zhang is acknowledged for fruitful discussions.References
- H. W. Maurer, in Starch, ed. J. BeMiller and R. Whistler, Academic Press, San Diego, 3rd edn, 2009, pp. 657–713 Search PubMed.
- S. Jobling, Curr. Opin. Plant Biol., 2004, 7, 210–218 CrossRef CAS PubMed.
- A. Behr and T. Seidensticker, in Chemistry of Renewables, Springer, Berlin, Heidelberg, 2020, pp. 161–176 Search PubMed.
- V. Vamadevan and E. Bertoft, Starch/Staerke, 2015, 67, 55–68 CrossRef CAS.
- S. Wang, C. Li, L. Copeland, Q. Niu and S. Wang, Compr. Rev. Food Sci. Food Saf., 2015, 14, 568–585 CrossRef CAS.
- S. Srichuwong, N. Isono, H. Jiang, T. Mishima and M. Hisamatsu, Carbohydr. Polym., 2012, 87, 1275–1279 CrossRef CAS.
- H. Goesaert, K. Brijs, W. S. Veraverbeke, C. M. Courtin, K. Gebruers and J. A. Delcour, Trends Food Sci. Technol., 2005, 16, 12–30 CrossRef CAS.
- B. C. Maniglia, N. Castanha, M. L. Rojas and P. E. Augusto, Curr. Opin. Food Sci., 2021, 37, 26–36 CrossRef CAS.
- C. Chiu and D. Solarek, in Starch, ed. J. BeMiller and R. Whistler, Academic Press, San Diego, 3rd edn, 2009, pp. 629–655 Search PubMed.
- J. N. BeMiller, in Carbohydrate Chemistry for Food Scientists, ed. J. N. BeMiller, AACC International Press, Duxford, 3rd edn, 2019, pp. 191–221 Search PubMed.
- R. N. Tharanathan, Crit. Rev. Food Sci. Nutr., 2005, 45, 371–384 CrossRef CAS PubMed.
- A. O. Ashogbon and E. T. Akintayo, Starch/Staerke, 2014, 66, 41–57 CrossRef CAS.
- O. S. Lawal, K. O. Adebowale, B. M. Ogunsanwo, L. L. Barba and N. S. Ilo, Int. J. Biol. Macromol., 2005, 35, 71–79 CrossRef CAS PubMed.
- D. Kuakpetoon and Y.-J. Wang, Starch/Staerke, 2001, 53, 211–218 CrossRef CAS.
- D. Kuakpetoon and Y. J. Wang, Carbohydr. Res., 2006, 341, 1896–1915 CrossRef CAS PubMed.
- B. C. Maniglia, N. Castanha, P. Le-Bail, A. Le-Bail and P. E. D. Augusto, Crit. Rev. Food Sci. Nutr., 2021, 61, 2482–2505 CrossRef CAS PubMed.
- K. Sangseethong, N. Termvejsayanon and K. Sriroth, Carbohydr. Polym., 2010, 82, 446–453 CrossRef CAS.
- H. Vogt, J. Balej, J. E. Bennett, P. Wintzer, S. A. Sheikh, P. Gallone, S. Vasudevan and K. Pelin, Chlorine Oxides and Chlorine Oxygen Acids, in Ullmann’s Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd, 2010, vol. 8, pp. 624–677, ISBN 978-3-527-30673-2 Search PubMed.
- R. Ciriminna, L. Albanese, F. Meneguzzo and M. Pagliaro, ChemSusChem, 2016, 9, 3374–3381 CrossRef CAS PubMed.
- Y. R. Zhang, X. L. Wang, G. M. Zhao and Y. Z. Wang, Carbohydr. Polym., 2012, 87, 2554–2562 CrossRef CAS.
- P. Parovuori, A. Hamunen, P. Forssell, K. Autio and K. Poutanen, Starch/Staerke, 1995, 47, 19–23 CrossRef CAS.
- P. Tolvanen, P. Mäki-Arvela, A. B. Sorokin, T. Salmi and D. Y. Murzin, Chem. Eng. J., 2009, 154, 52–59 CrossRef CAS.
- P. Tolvanen, A. Sorokin, P. Mäki-Arvela, S. Leveneur, D. Y. Murzin and T. Salmi, Ind. Eng. Chem. Res., 2011, 50, 749–757 CrossRef CAS.
- P. Tolvanen, A. Sorokin, P. Mäki-Arvela, D. Y. Murzin and T. Salmi, Ind. Eng. Chem. Res., 2013, 52, 9351–9358 CrossRef CAS.
- A. B. Sorokin, S. L. Kachkarova-Sorokina, C. Donzé, C. Pinel and P. Gallezot, Top. Catal., 2004, 27, 67–76 CrossRef CAS.
- H. C. Genuino, T. G. Meinds, J. O. P. Broekman, M. Staal, J. Brinksma, T. Wielema, F. Picchioni, W. R. Browne, P. J. Deuss and H. J. Heeres, ACS Omega, 2021, 6, 13847–13857 CrossRef CAS PubMed.
- P. Saisaha, J. W. de Boer and W. R. Browne, Chem. Soc. Rev., 2013, 42, 2059–2074 RSC.
- J. W. De Boer, J. Brinksma, W. R. Browne, A. Meetsma, P. L. Alsters, R. Hage and B. L. Feringa, J. Am. Chem. Soc., 2005, 127, 7990–7991 CrossRef CAS PubMed.
- J. W. De Boer, P. L. Alsters, A. Meetsma, R. Hage, W. R. Browne and B. L. Feringa, Dalton Trans., 2008, 6283–6295 RSC.
- J. Brinksma, L. Schmieder, G. Van Vliet, R. Boaron, R. Hage, D. E. De Vos, P. L. Alsters and B. L. Feringa, Tetrahedron Lett., 2002, 43, 2619–2622 CrossRef CAS.
- P. Saisaha, L. Buettner, M. van der Meer, R. Hage, B. L. Feringa, W. R. Browne and J. W. de Boer, Adv. Synth. Catal., 2013, 355, 2591–2603 CrossRef CAS.
- C. Zondervan, R. Hage and B. L. Feringa, Chem. Commun., 1997, 419–420 RSC.
- J. Bonvoisin, M. Corbella, S. E. Vitols, J. J. Girerd, K. Wieghardt, U. Bossek, B. Nuber and J. Weiss, J. Am. Chem. Soc., 1988, 110, 7398–7411 CrossRef.
- C. B. Woitiski, Y. N. Kozlov, D. Mandelli, G. V. Nizova, U. Schuchardt and G. B. Shul'Pin, J. Mol. Catal. A: Chem., 2004, 222, 103–119 CAS.
- R. Hage, J. E. Iburg, J. Kerschner, J. H. Koek, E. L. M. Lempers, R. J. Martens, U. S. Racherla, S. W. Russell, T. Swarthoff, M. R. P. Van Vliet, J. B. Warnaar, L. Van Der Wolf and B. Krijnen, Nature, 1994, 369, 637–639 CrossRef CAS.
- R. Hage and A. Lienke, Angew. Chem., Int. Ed., 2005, 45, 206–222 CrossRef PubMed.
- K. Shastri, E. W. C. Cheng, M. Motevalli, J. Schofield, J. S. Wilkinson and M. Watkinson, Green Chem., 2007, 9, 996–1007 RSC.
- P. Dournel, US20120070554A1, 2012.
- A. Vrieling-smit, T. Wielema and J. Beugeling, EP3205673A1, 2018.
- A. Delnoye, J. Beugeling, P. Slor and J. A. Bleeker, WO2018186736, 2018.
- S. Chattopadhyay, R. S. Singhal and P. R. Kulkarni, Carbohydr. Polym., 1997, 34, 203–212 CrossRef CAS.
- B. C. Gilbert, J. R. L. Smith, M. S. Newton, J. Oakes and R. P. Prats, Org. Biomol. Chem., 2003, 1, 1568–1577 RSC.
- T. C. Taylor and G. M. Salzmann, J. Am. Chem. Soc., 1933, 55, 264–275 CrossRef CAS.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351–1390 RSC.
- N. L. Vanier, S. L. M. El Halal, A. R. G. Dias and E. da Rosa Zavareze, Food Chem., 2017, 221, 1546–1559 CrossRef CAS PubMed.
- S. Pietrzyk, T. Fortuna, L. Juszczak, D. Gałkowska, M. Bączkowicz, M. Łabanowska and M. Kurdziel, Int. J. Biol. Macromol., 2018, 106, 57–67 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cy01629j |
| This journal is © The Royal Society of Chemistry 2023 |