Open Access Article
Open Access ArticleHigh efficiency of nitric acid controls in alleviating particulate nitrate in livestock and urban areas in South Korea†
Haeri
Kim
 a,
Junsu
Park
a,
Seunggi
Kim
a,
Junsu
Park
a,
Seunggi
Kim
 a,
Komal Narayan
Pawar
a,
Komal Narayan
Pawar
 a and
Mijung
Song
a and
Mijung
Song
 *ab
*ab
aDepartment of Environment and Energy, Jeonbuk National University, Jeonju 54896, Korea
bDepartment of Earth and Environmental Sciences, Jeonbuk National University, Jeonju 54896, Korea
First published on 18th January 2023
Abstract
Remarkably, enhanced particulate nitrate (NO3−) concentrations occur in many environments during particulate matter (PM) pollution; however, information on the formation mechanism and alleviation strategies is still limited. Herein, to explore the NO3− formation mechanism and conditions, we measured the concentrations of water-soluble inorganic ions in PM1.0 as well as the inorganic gas concentrations of HNO3, NO2, and NH3 in Gimje, a highly dense livestock area, from June to July 2020 and January to February 2021. At the monitoring site, extremely high atmospheric NH3 was measured with an hourly average of 96.9 ± 48.1 ppb, and the daily average of HNO3 and PM1.0 was 0.7 ± 0.7 ppb, and 20.1 ± 8.8 μg m−3, respectively. A clear increase in the NO3− concentration in PM1.0 was observed on high pollution days (PM1.0 ≥ 20 μg m−3), suggesting that HNO3 and NH3 contributed to NO3− formation. Moreover, we applied the thermodynamic model ISORROPIA-II to predict the NO3− response to the reduction of total HNO3 (TN), total NH3 (TA), and SO42−. The results showed that controlling TN could be more effective in alleviating particulate NO3− than controlling SO42− and TA in the livestock area. We also compared this result to that of a nearby urban area, Jeonju. A similar result was observed, with efficient HNO3 control, which reduced the NO3− concentration in Jeonju. These measurements and simulations indicated that NOx control could be the most effective approach to reduce particulate NO3− concentrations in both livestock and urban areas. Our results provide a significant contribution to developing a strategy for alleviating particulate NO3− pollution.
Environmental significanceThe particulate nitrate (NO3−) concentration is often enhanced during particulate matter (PM) pollution; however, there is limited information on the formation mechanism and reduction strategies of particulate NO3−. Using thermodynamic model ISORROPIA-II and the measurements of inorganic gaseous species and water-soluble ions of PM1.0, we show that controlling total nitrate could be more effective in reducing particulate NO3− and PM concentrations than controlling SO42− and total ammonia in a livestock area. Moreover, a similar result was observed with efficient HNO3 control, which reduced NO3− and PM concentrations in a nearby urban area. This suggests that NOx control is an effective approach to reduce particulate NO3− in both livestock and urban areas. In the future, a strategy can be developed to reduce NO3− pollution by using the results of this study. |
Introduction
Particulate matter (PM) below 2.5 μm in aerodynamic diameter (PM2.5) significantly impacts the air quality, global climate, and human health.1–3 PM2.5 is emitted directly into the atmosphere. PM2.5 mainly contains ammonium sulfate ((NH4)2SO4), ammonium nitrate (NH4NO3), and organic aerosols formed via photochemical reactions of precursor gaseous species such as nitrogen oxides (NOx), ammonia (NH3), sulfur dioxide (SO2), and volatile organic compounds.4 In PM2.5, secondary inorganic aerosols (SIAs) account for more than 80% by mass, depending on the location and season.5,6Among SIAs, the concentration of NH4NO3 remarkably increases during PM2.5 pollution in various environments.7–9 NH4NO3 can be produced by a series of chemical reactions of NOx and NH3, as follows:4
| NO2 (g) + OH (g) → HNO3 (g) | (R1) |
| NH3 (g) + HNO3 (g) → NH4NO3 (s, aq) | (R2) |
In the atmosphere, nitrogen dioxide (NO2) reacts with hydroxyl radicals (OH) to produce nitric acid (HNO3). HNO3 then reacts with NH3 to form NH4NO3. NH4NO3 is a semi-volatile species that can partition primarily from the gas-phase to the particle-phase, depending on the temperature.4
Studies on night-time chemistry have addressed nitrate (NO3−) formation by heterogeneous reactions.10 During night-time, nitrogen trioxide (NO3) is produced by the reaction of NO2 and ozone (O3) (R3), and the NO3 reacts with NO2 to form dinitrogen pentoxide (N2O5) (R4). Heterogeneous uptake of N2O5 on aerosol surfaces produces HNO3via hydrolysis (R5).4
| NO2 (g) + O3 (g) → NO3 (g) + O2 (g) | (R3) |
| NO3 (g) + NO2 (g) → N2O5 (g) | (R4) |
| N2O5 (g) + H2O (l) → 2HNO3 (aq) | (R5) |
These reactions produce particulate NO3− under NH3-rich conditions.4 However, under NH3-poor conditions, HNO3 can be formed through the reaction of other alkaline species.11
NOx and NH3, the main precursor gases of NH4NO3, are emitted into the atmosphere by various sources. NOx is emitted not only by anthropogenic activities such as combustion of oil and coal, energy generation, on-road transportation, and agricultural activities, but also by natural sources such as soil, biomass burning, and lightning.12,13 In California14 and the North China Plain,15 where there are active agricultural areas, the total NOx emissions accounted for more than 30% in 2013 and 50% in 2017. NH3 is mainly emitted from agricultural sources, such as fertilizer application, soil, and livestock, accounting for approximately 90% of the total global NH3 emission,12,16 which has dramatically increased by 80% from 1970 to 2017.12
Studies have been conducted to understand the formation mechanism and develop effective reduction strategies for NH4NO3 pollution. Some modeling studies have suggested that reduction of total NH3 (TA) could be the most effective method to reduce PM concentrations.9,17 For instance, Franchin et al. (2018) used ISORROPIA-II modeling showing that a reduction in the NH3 concentration by approximately 50% could decrease the PM1.0 concentration by ∼36% in a rural area in Utah, United States. Moreover, in central China, which is surrounded by rural and urban areas, GEOS-Chem modeling results showed that a reduction in NH3 emissions by 47% reduced the PM2.5 concentrations by ∼9% in July and ∼11% in January.17 By contrast, other modeling studies have shown that reducing NOx emissions would be more effective than reducing NH3 emissions for controlling PM.18–20 Guo et al. (2018) showed that controlling NOx emission would effectively reduce NO3− concentrations in an agricultural area in Cabauw, Netherlands. Furthermore, Chen et al. (2014) suggested that a 50% reduction in NOx emissions would decrease PM2.5 and NO3− concentrations by more than 24% and 42%, respectively, in an urban area in Bakersfield, USA. Analysis of ground-based data showed that NH4NO3 formation in the Salt Lake Valley is likely to be total HNO3 (TN)-limited, and, thus, reduction of NOx is effective in improving the air quality.20 However, scientific data on highly efficient control strategies of precursor gas in alleviating particulate NO3− in different areas are still lacking.
In this study, to determine NH4NO3 formation and related PM pollution conditions, two intensive field measurements of NH3, NO2, HNO3, and water-soluble inorganic ions (WSIIs) in PM1.0 were performed in the summer in Gimje, a rural area in South Korea. Gimje is a highly dense livestock area with populated livestock facilities and farms.21 Extremely high NH3 emission22 and PM pollution23 have been reported in this region. There are almost no studies on the temporal distribution of WSIIs and their precursor gases. In this study, the WSIIs and inorganic gas-particle-phase partitioning of NH4NO3 were analyzed by exploring the WSIIs and inorganic gases. Based on the measurement dataset and thermodynamic model ISORROPIA-II, we discuss the effective implementation of PM and NO3− reduction strategies in livestock-dense areas. Moreover, we compared the results acquired from the rural area with those from a nearby urban area, Jeonju, via thermodynamic modeling by using the NH3 and WSII concentrations in PM2.5 data measured from May 2019 to April 2020 by Park et al. (2021).8
Methods
Measurements
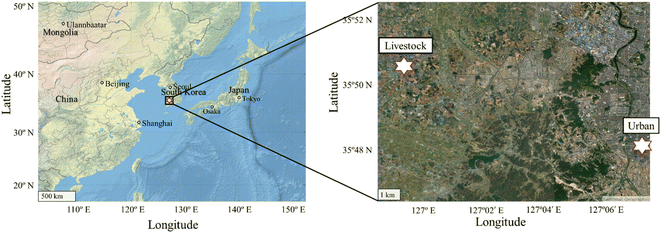
Particulate WSIIs and inorganic gases were measured in Gimje, Jeollabuk-do, South Korea (35.8395° N, 126.9889° E) (Fig. 1). The monitoring site has livestock complexes for raising pigs and chickens and large-scale farms with open manure storage facilities,21,24 resulting in high NH3 emissions.22Two intensive field measurements were performed during the summer (June to July 2020) and winter (January to February 2021). In the livestock area, concentrations of inorganic gases such as NH3, NOx (NO, and NO2), and HNO3, and WSIIs in PM1.0 (Na+, NH4+, Mg2+, Ca2+, K+, NO3−, SO42−, and Cl−) were measured. Atmospheric NH3 was measured every 1 s using a real-time NH3 instrument (DLT-100, Los Gatos Research, USA) using off-axis integrated cavity output spectroscopy. In principle, the NH3 instrument using specific wavelengths does not require external calibration.25 However, we performed calibration to confirm the performance of the instrument by mixing standard NH3 (9.2 ppm, with an accuracy of ±2%, Air Korea, Korea) and N2 (99.999%, Air Korea, Korea) gases before and after field measurements, resulting in an R2 of 0.9999 (Fig. S1†). The detailed procedure for the calibration and measurement of atmospheric NH3 is described by Park et al. (2021). For the analysis, we used hourly averaged concentrations of atmospheric NH3. The NOx concentration was also monitored every 1 m using a NOx instrument (Serinus 40 Oxides of Nitrogen, Ecotech, Australia), based on the chemiluminescence method. Before and after conducting the field measurements, calibration was conducted by mixing standard NO (4.9 ppm, with an accuracy of ±10%, Air Korea, Korea) and N2 (99.999%, Air Korea, Korea) gases at four different concentrations (20, 15, 12, and 0 ppb), which resulted in an error concentration of ∼3%. The hourly averaged concentration of atmospheric NOx was used for the analysis.
PM1.0 and HNO3 were collected using a two-stage sequential air sampler (APM Engineering, PMS-114, Korea) which has been widely used.26,27 The sequential air sampler consisted of two stages for collection of PM1.0 on a Teflon filter (PTFE, 66155, PALL, USA) at the first stage, and of HNO3 on a nylon filter (Nylon, 1213776, GVS, USA) at the second stage. It was operated at a flow rate of 16.7 L min−1 for 24 h from 00:00 am to 00:00 am the following day during the measurement period. During the sampling, some semi-volatile species (i.e. NO3−, NH4+ and HNO3) could be evaporated, leaving approximately ∼10% of their total mass on the filters.28,29 A total of 84 samples for PM1.0 (42 samples) and HNO3 (42 samples) were collected during all measurements. Subsequently, all filter samples were stored at −4 °C in a freezer and then analyzed within two weeks after collection. The PM1.0 mass concentration collected from the Teflon filter was observed based on the procedure of the method of the USA Environmental Protection Agency.30 To analyze the HNO3 and WSIIs of PM1.0, each filter sample was extracted in purified water (18.2 MΩ cm, Merck Milli-Q®, Millipore, Burlington, MA, USA). A detailed extraction procedure was described previously.31 The extracts from nylon filters were analyzed to quantify the concentration of HNO3, and the extracts from Teflon filters were analyzed to determine five cations (Na+, NH4+, Mg2+, Ca2+, and K+) and three anions (NO3−, SO42−, and Cl−) using ion chromatography (Aquion, Thermo Scientific, USA). The method detection limits (MDLs) of Na+, NH4+, Ca2+, Mg2+, K+, NO3−, SO42−, and Cl− were 0.01, 0.01, 0.03, 0.01, 0.02, 0.02, 0.03 and 0.02 μg m−3, respectively. For gaseous species, the MDLs of HNO3, NOx, and NH3 were 0.01, 0.4,32 and 0.5 (ref. 25) ppb, respectively. Hourly averaged meteorological parameters, including relative humidity (RH), temperature, wind direction, precipitation, and wind speed, were obtained during the measurement period from the Korea Meteorological Administration in Jeonju.33
Thermodynamic modeling
To estimate whether HNO3 or NH3 is the limiting factor for particulate NO3− formation and to calculate aerosol liquid water content (ALWC), we used ISORROPIA-II, an extensively applied thermodynamic equilibrium model based on a Na+–Cl−–Ca2+–K+–Mg2+–SO42−–NH4+–NO3−–H2O aerosol system.18,34,35 As model inputs, the measured concentrations of TA (particle-phase NH4+ + gas-phase NH3), TN (particle-phase NO3− + gas-phase HNO3), Na+, K+, Ca2+, Mg2+, Cl−, and SO42− and meteorological parameters in the rural area were used. ALWC was also calculated using measured data of inorganic gases, WSIIs, temperature, and RH.35 It was reported that a correlation coefficient between the simulated ISORROPIA-II ALWC and the measured hygroscopic growth factors agreed well at ∼0.89; however, the uncertainty of the predicted ALWC in aerosols would be ∼20% compared to the observed ALWC.36 This underestimation might be due to the unidentified organic species information in ISORROPIA-II.35,36In this study, we used the “forward” mode, which calculates gas-particle equilibrium partitioning concentrations using the total concentration of species because this mode provides substantially better computational results for the concentrations of inorganic gases than the reverse mode.18,37 The mean RH of 74.7 ± 11.3% observed throughout the monitoring site was below the deliquescence relative humidity (DRH) for pure (NH4)2SO4.4 However, PM1.0 could still absorb water below the DRH of the pure salt because PM is a complex mixture of various inorganic salts and organic compounds.37–39 Therefore, we used the “metastable state” solution, assuming that the aerosol compositions are an aqueous supersaturated solution that prevents the formation of a solid precipitate.35,37
To compare the phenomena of gas-particle partitioning between rural and urban areas, we also calculated the limiting factor of particulate NO3− formation and gas-particle partitioning using previous data (measured from May 2019 to April 2020) of WSIIs in PM2.5 and NH3 measured in Jeonju, an urban area situated approximately 10 km from Gimje.8 In this study, HNO3 measurement data were unavailable for the urban area; thus, the concentration was estimated using the method of Seo et al. (2020).40 The uncertainty in the estimated TN was confirmed by comparing the measured TN at the rural site (Fig. S2†). To confirm the thermodynamic equilibrium, we also compared the measured and predicted values of the SNA (SO42−, NO3−, NH4+) species (Fig. S1 and S3†). Fig. S3† shows the scatter plots of observations against model predictions of the major secondary inorganic aerosol (SIA) species (NH4+, NO3− and SO42−) in livestock and urban areas during measurement periods. A good correlation was found between observations and model predictions, suggesting the good performance of ISORROPIA-II.
Conditional probability function (CPF) analysis
To identify the potential regional-scale transport and local emission sources, a conditional probability function (CPF) analysis was conducted using the wind direction, wind speed, and NH3 and NO2 concentrations. The CPF estimates a probability that the given source contribution from the given wind speed and wind direction will exceed a threshold criterion. The threshold criterion was set at the 80th percentile to indicate the directionality of the source.Results and discussion
Overview of gaseous species and WSIIs
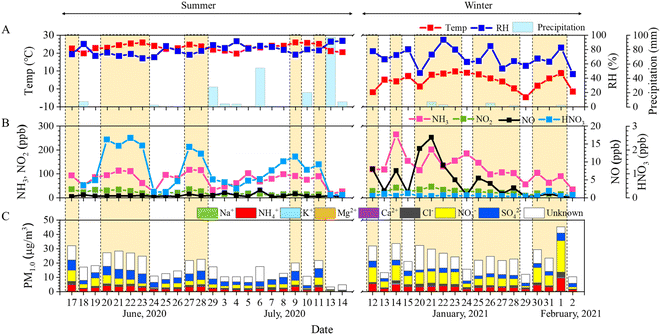
Two intensive measurements of inorganic gaseous species (namely, HNO3, NO, NO2, and NH3) and WSIIs in PM1.0 were conducted in the highly dense livestock area of Gimje during the summer and winter of 2020–2021. Fig. 2 shows the daily averaged variations in meteorological parameters, gaseous species, and WSIIs in PM1.0. Over the study period, the daily average temperature was 15.1 ± 9.8 °C, and RH was 74.7 ± 11.3% (Fig. 2A). The mean concentrations of NH3, HNO3, NO, and NO2 were 96.9, 0.7, 2.3, and 21.3 ppb, respectively (Fig. 2B). The atmospheric NH3 concentration in the populated livestock area was significantly (2–35 times) higher than that in other livestock and urban areas listed in Table 1.8,27,41–45 This is because of livestock activities, open manure storage facilities, and livestock waste disposal in the region. The HNO3 concentration at the monitoring site was lower than that reported in Seoul,27 Quzhou, Beijing,41 and New Delhi42 but higher than that reported in Jeonju,8 Seolseongmyeon,27 Gucheng,43 Niangon Adjamé44 and San Diego45 (Table 1).| Location | Period | NH3 (unit: ppb) | HNO3 (unit: ppb) | References |
|---|---|---|---|---|
| a Estimation based on an equation from Seo et al., 2020. | ||||
| Livestock area | ||||
| Gimje, Korea | 2020.6–2020.7 | 96.9 | 0.7 | This study |
| 2021.1–2021.2 | ||||
| Seolseongmyeon, Korea | 2009.1–2018.12 | 50.0 | 0.4 | Sung et al. (2020)27 |
| Quzhou, China | 2011.1–2014.12 | 31.2 | 2.7 | Xu et al. (2016)41 |
| Gucheng, China | 2013.5–2013.9 | 36.2 | 0.2 | Meng et al. (2018)43 |
| Niangon Adjamé, Côte d’Ivoire | 2015.12–2016.2 | 44.0 | 0.2 | Bahino et al. (2018)44 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Urban area | ||||
| San Diego, United States | 2007.2–2012.3 | 3.3 | 0.2 | Li et al. (2014)45 |
| Seoul, Korea | 2009.1–2018.12 | 7.5 | 0.7 | Sung et al. (2020)27 |
| Beijing, China | 2011.1–2014.12 | 17.2 | 3.2 | Xu et al. (2016)41 |
| New Delhi, India | 2017.12–2018.2 | 33.9 | 1.0 | Acharja et al. (2020)42 |
| Jeonju, Korea | 2019.5–2020.4 | 10.5 | 0.2a | Park et al. (2021)8 |
The daily concentrations of PM1.0 and its WSIIs are shown in Fig. 2C. The daily averaged PM1.0 mass concentration ranged from 3.3 to 45.2 μg m−3, with an average of 20.1 ± 8.8 μg m−3. NO3−, SO42−, and NH4+ were the major components of PM1.0, and the daily concentration of NO3− ranged from 0.4 to 22.0 μg m−3 with an average of 4.8 ± 3.9 μg m−3. The daily SO42− concentrations fluctuated between 0.1 and 7.1 μg m−3, with an average of 3.5 ± 1.8 μg m−3. The daily NH4+ concentrations varied from 0.2 to 9.4 μg m−3 and showed an average of 2.8 ± 1.6 μg m−3. Among all WSII species, a significant dominance of NO3− was observed at the livestock site during the measurement period (Fig. 2C).
All WSIIs, PM1.0, and inorganic gaseous species, except for SO42− and HNO3, exhibited higher concentrations in the winter (Table S1†). Among the WSIIs, the NO3−concentration was remarkably enhanced from 2.9 μg m−3 in the summer to 7.3 μg m−3 in the winter (Table S1 and Fig. S4E†). However, the HNO3 concentration dramatically decreased to an average of 0.1 ppb in the winter (summer: 1.1 ppb) (Table S1 and Fig. S4C†). Theoretically, NH3 and HNO3 phases are dependent on temperature and RH.4 NH4NO3 partitions can occur from the particle-phase to the gas-phase at low RH and high temperature.4,46 For this reason, NO3− is mostly present in gaseous HNO3 during the summer and has low concentrations, whereas NO3− is partitioned into particle-phase NO3− during the winter, resulting in higher atmospheric NO3− concentrations. Our results, which revealed low HNO3 levels in the summer and high NO3− levels in the winter, are consistent with previously reported ones.27,47 NH3 is generally temperature-dependent. Thus, high NH3 levels have been reported in the summer.8,27,48 However, the atmospheric NH3 concentration was significantly higher in the winter (124.6 ppb) than in the summer (76.0 ppb) (Table S1 and Fig. S4D†). The plausible reasons for this observation are (1) active manure production at the open storage from the livestock farms and (2) dense NH3 emissions from mechanically ventilated farms with a low ventilation rate in winter in the study area.31 Higher NH3 concentrations have been reported previously in the winter than in other seasons in livestock areas.31,49
Enhancements of nitrate during PM pollution
To investigate the phenomenon of PM1.0 pollution in the livestock area, we compared the chemical characteristics of PM1.0 on clean and pollution days. Based on PM1.0 mass concentrations, we classified days into clean (daily average PM1.0 < 20 μg m−3) and pollution days (daily average PM1.0 ≥ 20 μg m−3), as suggested in previous studies.50,51 On clean days, the average concentration of PM1.0 was 12.1 ± 4.0 μg m−3, and WSIIs accounted for 58.2% (7.1 μg m−3) of PM1.0 (Fig. 3A). SO42− was the most dominant ion during the clean days, accounting for 37.1% of PM1.0, followed by NO3− (29.9%) and NH4+ (21.8%). Out of 42 days, 23 were defined as PM1.0-pollution days at the monitoring site. During pollution days, the daily averaged concentration of PM1.0 was 26.7 ± 5.6 μg m−3 with a remarkably enhanced NO3− fraction (41.7%) in PM1.0 (Fig. 3A), followed by SO42− (25.3%) and NH4+ (23.0%). Moreover, the daily average concentrations of NH3 and HNO3 increased significantly to 118.9 ppb and 0.8 ppb, respectively, during pollution days when compared with those during clean days (70.1 ppb and 0.5 ppb, respectively) (Fig. 3B and C). These findings suggest that NH3 and HNO3 play a critical role in NO3− formation during high-PM1.0 events.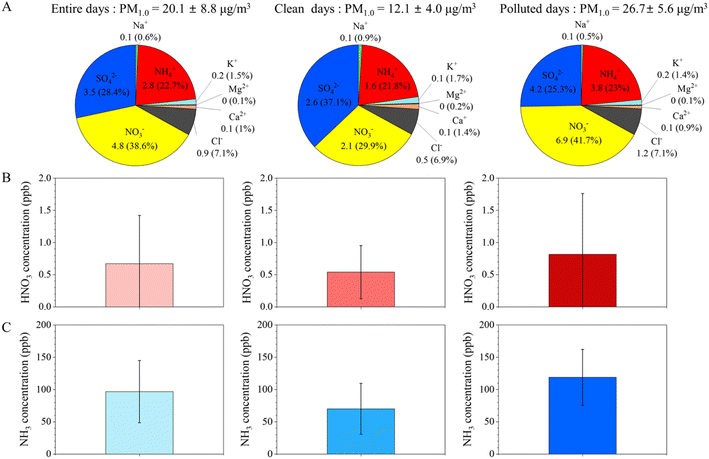 | ||
| Fig. 3 Comparison of (A) water-soluble ions in PM1.0, (B) HNO3 concentration, and (C) NH3 concentrations for entire, clean (PM1.0 < 20 μg m−3), and high pollution (PM1.0 ≥ 20 μg m−3) days. | ||
Fig. S5† illustrates the CPF result during pollution days. A high probability of NH3 concentration (179 ppb at the 80th percentile) was observed around the measurement site during pollution days, suggesting that the high levels of NH3 were mostly affected by local sources around the livestock complexes rather than long-range transport.
PM1.0 nitrate formation
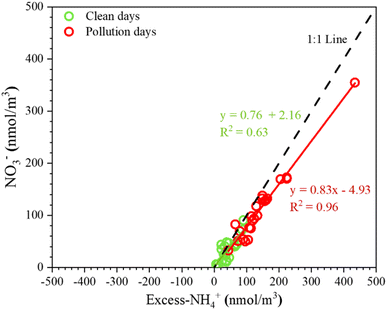
Fig. 4 shows the relationship between NO3− and excess-NH4+ molar concentrations. The excess-NH4+ concentration ranged from 6.0 to 434.3 nmol m−3, and NO3− molar concentrations increased with excess-NH4+ molar concentrations during both clean and pollution days, supporting that NO3− formation always occurred at the monitoring site. The excess-NH4+ and NO3− molar concentrations showed a significant correlation, with a slope close to 1.0, thereby indicating that the formation of NH4NO3 was primarily through the reaction between NH3 and HNO3. This is because of the extremely high levels of atmospheric NH3 at the livestock site during the study period.52 Moreover, a shallower slope for both clean (0.76) and pollution (0.83) days was observed for a livestock site, indicating that most of the measured PM1.0 samples were residual NH4+ associated with species other than NO3−, such as ammonium chloride (NH4Cl). This implies that there is enough excess NH4+ that it can drive the partitioning of other semi-volatile acids such as HNO3 and HCl.53HNO3/NH3 limitation of particulate nitrate formation
In the livestock area, PM pollution with a drastic enhancement of NO3− occurred under NH3-rich conditions, indicating that both NH3 and HNO3 are critical precursors for PM1.0 nitrate formation. To suggest a reduction of such high NO3− concentrations in the livestock area, we calculated the limiting factor for NO3− formation using the ISORROPIA-II model.35,54 The measurement data of the average WSII concentrations, temperature, and RH were used as the model input data (Table S1†).Fig. 5A shows the contour plots of simulated NO3− concentrations depending on the TA and TN levels at the minimum SO42− (∼1 μg m−3) and maximum SO42− (∼10 μg m−3) concentrations, respectively. In this figure, the left side is the TA-limited area and the right side is the TN-limited area, which were calculated using eqn (1) (unit of each species: μmol m−3) as follows:55
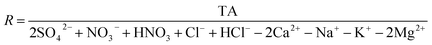 | (1) |
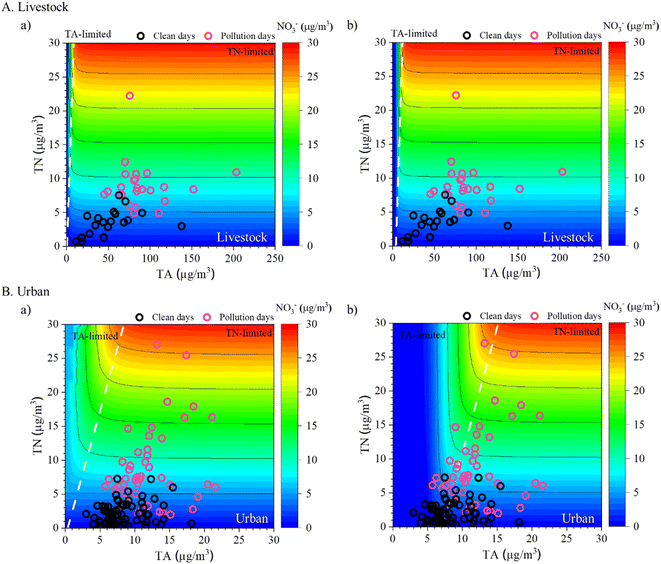 | ||
| Fig. 5 NO3− concentrations calculated using the ISORROPIA-II model depending on total nitrate (TN) and total ammonia (TA) concentrations under (a) SO42− = 1 μg m−3 and (b) SO42− = 10 μg m−3 in the (A) livestock area and under (a) SO42− = 1 μg m−3 and (b) SO42− = 20 μg m−3 in (B) the urban area8 during clean and pollution days. Measurement data of TN and TA concentrations during the monitoring periods are shown by black circles (clean days) and pink circles (pollution days). | ||
The measurement data of TA and TN during clean (black circles) and pollution days (pink circles) are shown in Fig. 5. All measured data were TN-limited on both clean and pollution days in all cases of minimum and maximum SO4− concentrations, given the NH3-rich concentration in the study area (Fig. 5A). NO3− formation in livestock can be controlled through HNO3. Hence, to reduce HNO3 concentrations, controlling NOx emissions might be an important approach to reduce NO3− concentrations and thus improve air quality in livestock areas.
Park et al. (2021) observed remarkable NH4NO3 formation during high PM2.5 events at nearby urban sites. To compare the results of the limiting factor for NO3− formation in an urban area, we calculated the limiting factor in Jeonju, a nearby urban area, using measurement data from May 2019 to April 2020.8 The method and procedure applied for ISORROPIA-II simulation were used for this step as well. The measurement values of the WSIIs in PM2.5, NH3, temperature, and RH in the nearby urban area were used as the input data (Table S1†);8 however, the HNO3 concentration was estimated using ISORROPIA-II because of the absence of data (details are given in S1 in the ESI†). The simulation results showed that under low-SO42− conditions (∼1 μg m−3), all observed data were affected by the TN-limited regime on both clean and pollution days in the urban area (Fig. 5B). In contrast, although under high-SO42− (∼20 μg m−3), the measured data fell into both TA-limited and TN-limited regimes during clean days, and most of the data fell into the TN-limited regime during pollution days (Fig. 5B). As the daily SO42− concentration in the urban area was ∼4.3 μg m−3, the TN-limited regime was more reliable in most cases. This shows that high NO3− production in the urban area during PM2.5-pollution days was also mainly controlled by HNO3. Therefore, to alleviate particulate NO3− in PM in both livestock and nearby urban areas, control of NOx emissions (which reduces the HNO3 concentration) could be crucial.
The efficiency of PM reduction via precursor controls
To understand the alleviating efficiency of particulate NO3− in PM in livestock and urban areas, we performed a sensitivity analysis using ISORROPIA-II, for which the measurement-averaged data were used as input data (Table S1†). Given the abundance of inorganic salts in both areas, we assumed that the SO42−–NH4+–NO3−–H2O system is in equilibrium and that particulate NO3− is formed through the gas-particle partitioning of NH3 and HNO3.First, we attempted to control the TN concentration to study the decrease in particulate NO3− and PM concentrations caused by HNO3 reduction in both livestock and urban areas. In Korea, HNO3 and NOx are mainly locally contributed rather than long-range transported to form a particle-phase given their lifetimes and concentrations.56 During the measurement periods, the CPF results for NO2 also showed a high probability of high NO2 concentrations in both livestock and urban areas occurring locally, supporting that the high NO2 concentration originated near the study area rather than during long-range transport (Fig. S6†).
Fig. 6 shows the sensitivity results for TN control in livestock and urban areas. The PM mass concentrations decreased linearly as TN decreased in both livestock and urban areas (Fig. 6A and B). In the livestock area, a 50% decrease in the TN resulted in a 50% decrease in the particulate NO3− and an approximately 33% decrease in the PM1.0 mass concentrations during the entire period as well as during pollution periods (Fig. 6A). Significant responses in NO3− (50%) and PM2.5 (∼23%) were observed during an entire day in the urban area, but a higher reduction in NO3− (50%) and PM2.5 (31%) was predicted by a 50% decrease in the TN during pollution days (Fig. 6B). Therefore, to decrease particulate NO3− and PM concentrations in both areas, controlling HNO3 is a highly efficient approach.
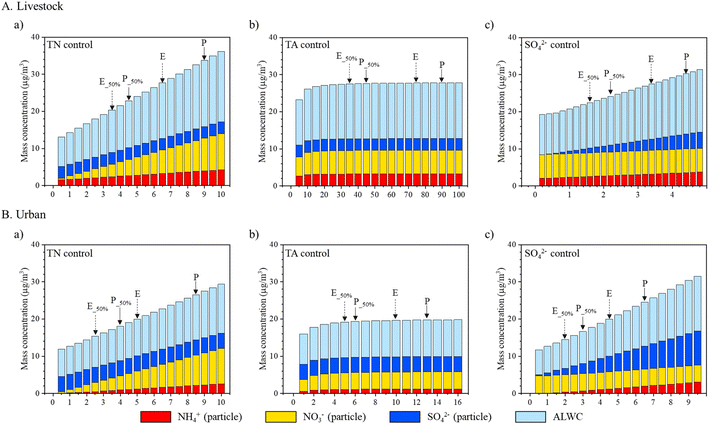 | ||
| Fig. 6 Thermodynamic ISORROPIA-II model simulations for SO42−, NO3−, and NH4+ (SNA) concentrations and aerosol liquid water content (ALWC) varied by (a) total nitrate (TN), (b) total ammonia (TA) and (c) SO42− concentration inputs in (A) livestock and (B) urban areas. “E” indicates the wet particulate matter (PM) mass concentration during entire days and “P” indicates the wet PM mass concentration during pollution days. “E_50%” and “P_50%” indicate the wet PM mass concentration when TN, TA, and SO42− are reduced by up to 50%. The following inputs were used in the model: temperature (livestock: 288.3 K; urban: 288.2 K); relative humidity (livestock: 75%; urban: 64%); and TN, TA, and SO42− concentrations (livestock: 6.5, 73.1, and 3.5 μg m−3; urban: 4.8, 9.9, and 4.4 μg m−3) (Table S1†). | ||
Second, a sensitivity analysis was performed by controlling TA (Fig. 6). Atmospheric NH3 is generally considered a local contributor because of its short lifetime.57 As shown in Fig. 6A and B, a 50% decrease in TA did not respond to the NO3− and PM concentrations (only ∼1% in the livestock site and ∼2% in the urban site). An ∼87% (livestock) and ∼78% (urban) reduction in TA would be required to effectively decrease PM mass concentrations.
Finally, SO42− was controlled during the simulation. SO42− does not undergo gas-particle partitioning and is favored in the particle-phase because of its low volatility.4 Reportedly, SO2 can be transported from China to Korea, and therefore, we cannot rule out the possibility of the transported SO42−.58
Illustrated in Fig. 6A and B are the results of SO42− control in the two regions. If we assume that SO42− is not transported at all to the receptor areas and formed locally by applying to the actual measured concentration of SO42− (livestock: 3.5 μg m−3 and urban: 4.3 μg m−3), only the PM concentration can be found to decrease in the livestock (PM1.0: ∼20%, NO3−: ∼0.1%, Fig. 6A) and urban areas (PM2.5: ∼30%, NO3−: ∼0.0%, Fig. 6B) during PM pollution. A similar reduction in PM was observed during the entire period in both areas. However, some SO42− may have been transported from outside Korea. A modeling study showed that approximately half of the SO42− in PM2.5 was transported from China to South Korea during 2012–2016.58 Accordingly, if we assume that only half of the SO42− was locally produced, then a 50% reduction in SO42− leads to a reduction in the PM1.0 and PM2.5 concentrations in the livestock (PM1.0: ∼12%, NO3−: ∼0.1%, Fig. 6A) and urban areas (PM2.5: ∼20%, NO3−: ∼0.0%, Fig. 6B) during PM pollution. On an entire day, a 50% decrease in the SO42− concentration could lead to a ∼13% decrease in PM concentrations and ∼0.0% decrease in the particulate NO3− concentration in the livestock and urban areas. For SO42− control, a linear reduction in the SO42− concentration, regardless of whether SO42− was transported, resulted in a linear decrease in PM mass concentrations but a non-linear decrease in the particulate NO3− concentration.
Additionally, we investigated the effect of TN, TA, and SO42− control on the gas-particle partitioning of HNO3 and NH3. Almost ∼96% of the TA remains in the gas-phase in a livestock area when the TN, TA, and SO42− were reduced (Fig. S7A, S8A, and S9A†); however, a decrease in the TN and SO42− led to more evaporation of particulate NH4+ into more gaseous NH3 in an urban area (Fig. S7B and S9B†). However, the particle-phase of NO3− was always the dominant form in both sites (Fig. S7–S9†).
Conclusions
We monitored NH3, HNO3, and WSII concentrations in PM1.0, in a livestock area in Gimje, from June to July 2020 and January to February 2021 to characterize NO3− formation. During the study periods, the average concentrations of NH3 and HNO3 were 96.9 and 0.7 ppb, respectively, and the daily average of PM1.0 concentration was 20.1 μg m−3, with averages of 2.8 μg m−3 for NH4+, 4.8 μg m−3 for NO3−, and 3.5 μg m−3 for SO42−. On pollution days (PM1.0 ≥ 20 μg m−3), a remarkable increase in the fraction of NO3− in PM1.0 and the daily NH3 and HNO3 concentrations was observed, suggesting the critical role of NH3 and HNO3 in NO3− formation. The measurements and ISORROPIA-II-simulated results showed that NO3− formation in the livestock area was always TN-limited owing to its NH3-rich environment. Recent studies showed that some amount of the water content of organic materials (∼30%) could influence the water content and pH of aerosol particles.36,59 Further studies are needed to confirm the effect of organic materials on aerosol water content. Recent studies reported that VOC reduction would be efficient in reducing NH4NO3 aerosol in some areas.60–62 To confirm this, further studies are needed with more measurement datasets.In comparing the effect of reducing particulate NO3− and PM concentrations by controlling TN, TA, and SO42− from two different environments (i.e., livestock and urban areas), we found that reducing TN is more effective than reducing TA and SO42− in alleviating particulate NO3− and PM in both areas. Collectively, our results provide an understanding of the characteristics and formation of NO3−—the most serious aerosol pollutants in Korea—as well as provide scientific data to develop effective PM reduction policies for livestock and urban areas.
Author contributions
M. S. designed this study. H. K., J. P., S. G. K. and M. S. conducted measurements and analysed the data. M. S., H. K., K. P. and S. G. K prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) funded by the Korea government (MSIT) (NRF-2019M1A2A2103956), and by the Fine Particle Research Initiative in East Asia Considering National Differences (FRIEND) Project (NRF-2020M3G1A1114548). We thank Sangmin Oh and Junghyeong Ryu for technical support.Notes and references
- B. B. Booth, N. J. Dunstone, P. R. Halloran, T. Andrews and N. Bellouin, Aerosols implicated as a prime driver of twentieth-century North Atlantic climate variability, Nature, 2012, 484, 228–232, DOI:10.1016/j.envint.2016.02.003.
- G. Bhattarai, J. B. Lee, M.-H. Kim, S. Ham, H.-S. So, S. Oh, H.-J. Sim, J.-C. Lee, M. Song and S.-H. Kook, Maternal exposure to fine particulate matter during pregnancy induces progressive senescence of hematopoietic stem cells under preferential impairment of the bone marrow microenvironment and aids development of myeloproliferative disease, Leukemia, 2020, 34, 1481–1484, DOI:10.3390/ijerph14040440.
- G. Myhre, D. Shindell and F. M. Breon, Anthropogenic and natural radiative forcing, in IPCC, Climate Change 2013: The Physical Science Basis, Working Group I Contribution to the IPCC Fifth Assessment Report, https://www.ipcc.ch/report/ar5/wg1/anthropogenic-and-natural-radiative-forcing/, accessed on 1 April 2022 Search PubMed.
- J. Seinfeld and S. Pandis, Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, Wiley, Hoboken, NJ, USA, 3rd edn, 2016, ISBN 978-1-118-94740-1 Search PubMed.
- J. L. Jimenez, M. Canagaratna, N. Donahue, A. Prevot, Q. Zhang, J. H. Kroll, P. F. DeCarlo, J. D. Allan, H. Coe and N. Ng, Evolution of organic aerosols in the atmosphere, science, 2009, 326, 1525–1529, DOI:10.1126/science.1180353.
- Z. Cheng, L. Luo, S. Wang, Y. Wang, S. Sharma, H. Shimadera, X. Wang, M. Bressi, R. M. de Miranda and J. Jiang, Status and characteristics of ambient PM2.5 pollution in global megacities, Environ. Int., 2016, 89, 212–221, DOI:10.1016/j.envint.2016.02.003.
- Y.-C. Lin, Y.-L. Zhang, M.-Y. Fan and M. Bao, Heterogeneous formation of particulate nitrate under ammonium-rich regimes during the high-PM2.5 events in Nanjing, China, Atmos. Chem. Phys., 2020, 20, 3999–4011, DOI:10.5194/acp-20-3999-2020.
- J. Park, E. Kim, S. Oh, H. Kim, S. Kim, Y. P. Kim and M. Song, Contributions of Ammonia to High Concentrations of PM2.5 in an Urban Area, Atmosphere, 2021, 12, 1676, DOI:10.3390/atmos12121676.
- A. Franchin, D. L. Fibiger, L. Goldberger, E. E. McDuffie, A. Moravek, C. C. Womack, E. T. Crosman, K. S. Docherty, W. P. Dube and S. W. Hoch, Airborne and ground-based observations of ammonium-nitrate-dominated aerosols in a shallow boundary layer during intense winter pollution episodes in northern Utah, Atmos. Chem. Phys., 2018, 18, 17259–17276, DOI:10.5194/acp-18-17259-2018.
- S. S. Brown and J. Stutz, Nighttime radical observations and chemistry, Chem. Soc. Rev., 2012, 41, 6405–6447, 10.1039/C2CS35181A.
- R.-J. Huang, J. Duan, Y. Li, Q. Chen, Y. Chen, M. Tang, L. Yang, H. Ni, C. Lin and W. Xu, Effects of NH3 and alkaline metals on the formation of particulate sulfate and nitrate in wintertime Beijing, Sci. Total Environ., 2020, 717, 137190, DOI:10.1016/j.scitotenv.2020.137190.
- E. E. McDuffie, S. J. Smith, P. O'Rourke, K. Tibrewal, C. Venkataraman, E. A. Marais, B. Zheng, M. Crippa, M. Brauer and R. V. Martin, A global anthropogenic emission inventory of atmospheric pollutants from sector-and fuel-specific sources (1970–2017): an application of the Community Emissions Data System (CEDS), Earth Syst. Sci. Data, 2020, 12, 3413–3442, DOI:10.5194/essd-12-3413-2020.
- P. Oikawa, C. Ge, J. Wang, J. Eberwein, L. Liang, L. Allsman, D. Grantz and G. Jenerette, Unusually high soil nitrogen oxide emissions influence air quality in a high-temperature agricultural region, Nat. Commun., 2015, 6, 1–10, DOI:10.1038/ncomms9753.
- M. Almaraz, E. Bai, C. Wang, J. Trousdell, S. Conley, I. Faloona and B. Z. Houlton, Agriculture is a major source of NOx pollution in California, Sci. Adv., 2018, 4, eaao3477, DOI:10.1126/sciadv.aao3477.
- X. Lu, X. Ye, M. Zhou, Y. Zhao, H. Weng, H. Kong, K. Li, M. Gao, B. Zheng and J. Lin, The underappreciated role of agricultural soil nitrogen oxide emissions in ozone pollution regulation in North China, Nat. Commun., 2021, 12, 1–9, DOI:10.1038/s41467-021-25147-9.
- M. A. Sutton, S. Reis, S. N. Riddick, U. Dragosits, E. Nemitz, M. R. Theobald, Y. S. Tang, C. F. Braban, M. Vieno and A. J. Dore, Towards a climate-dependent paradigm of ammonia emission and deposition, Philos. Trans. R. Soc., B, 2013, 368, 20130166, DOI:10.1098/rstb.2013.0166.
- Z. Zhang, Y. Yan, S. Kong, Q. Deng, S. Qin, L. Yao, T. Zhao and S. Qi, Benefits of refined NH3 emission controls on PM2.5 mitigation in Central China, Sci. Total Environ., 2022, 814, 151957, DOI:10.1016/j.scitotenv.2021.151957.
- H. Guo, R. Otjes, P. Schlag, A. Kiendler-Scharr, A. Nenes and R. J. Weber, Effectiveness of ammonia reduction on control of fine particle nitrate, Atmos. Chem. Phys., 2018, 18, 12241–12256, DOI:10.5194/acp-18-12241-2018.
- J. Chen, J. Lu, J. C. Avise, J. A. DaMassa, M. J. Kleeman and A. P. Kaduwela, Seasonal modeling of PM2.5 in California's San Joaquin Valley, Atmos. Environ., 2014, 92, 182–190, DOI:10.1016/j.atmosenv.2014.04.030.
- R. Kuprov, D. J. Eatough, T. Cruickshank, N. Olson, P. M. Cropper and J. C. Hansen, Composition and secondary formation of fine particulate matter in the Salt Lake Valley: Winter 2009, J. Air Waste Manage. Assoc., 2014, 64, 957–969, DOI:10.1080/10962247.2014.903878.
- Korean Statistical Information Service (KOSIS), https://kosis.kr, accessed on 1 April 2022.
- Clean Air Policy Support System (CAPSS). 2017 Korea National Air Pollutants Emission. 2019, https://airemiss.nier.go.kr, accessed on 1 April 2022 Search PubMed.
- C. Han, S. Kim, Y.-H. Lim, H.-J. Bae and Y.-C. Hong, Spatial and temporal trends of number of deaths attributable to ambient PM2.5 in the Korea, J. Korean Med. Sci., 2018, 33, 1–14, DOI:10.3346/jkms.2018.33.e193.
- J.-S. Hwang, Y.-K. Park and C.-H. Won, Runoff characteristics of non-point source pollution in lower reaches of livestock area, J. Korean Soc. Environ. Eng., 2012, 34, 557–565, DOI:10.4491/KSEE.2012.34.8.557.
- ABB, ABB-Los Gatos Research Inc., Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS), http://www.lgrinc.com/advantages/, accessed on 1 April 2022 Search PubMed.
- E. S. Edgerton, Y.-M. Hsu, E. M. White, M. E. Fenn and M. S. Landis, Ambient concentrations and total deposition of inorganic sulfur, inorganic nitrogen and base cations in the Athabasca Oil Sands Region, Sci. Total Environ., 2020, 706, 134864, DOI:10.1016/j.scitotenv.2019.134864.
- M. Sung, J. Park, J. Lim, H. Park and S. Cho, A long term trend of gaseous and particulate acid/base species and effects of ammonia reduction on nitrate contained in PM2.5, 2009∼ 2018, J. Korean Soc. Atmos, 2020, 36, 249–261, DOI:10.5572/KOSAE.2020.36.2.249.
- C. J. Tsai, C. H. Huang and H. H. Lu, Adsorption capacity of a nylon filter of filter pack system for HCl and HNO3 gases, Sep. Sci. Technol., 2005, 39, 629–643, DOI:10.1081/SS-120027998.
- W. Nie, T. Wang, X. Gao, R. K. Pathak, X. Wang, R. Gao, Q. Zhang, L. Yang and W. Wang, Comparison among filter-based, impactor-based and continuous techniques for measuring atmospheric fine sulfate and nitrate, Atmos. Environ., 2010, 44, 4396–4403, DOI:10.1016/j.atmosenv.2010.07.047.
- Environmental Protection Agency (EPA), http://www.epa.gov/aqs, accessed on 1 April 2022.
- S. Oh, S.-G. Kim, J. B. Lee, J. Park, J.-B. Jee, S.-W. Hong, K.-S. Kwon and M. Song, Spatial distributions of atmospheric ammonia in a rural area in south Korea and the associated impact on a nearby urban area, Atmosphere, 2021, 12, 1411, DOI:10.3390/atmos12111411.
- ECOTECH, Environmental monitoring, Serinus 40 oxides of Nitrogen analyzer, user manual, version 3.5, https://www.ecotech.com/, accessed on 1 April 2022 Search PubMed.
- Korea Meteorological Administration (KMA), https://data.kma.go.kr/cmmn/main.do, accessed on 1 April 2022.
- ISORROPIA, version 2.1, http://nenes.eas.gatech.edu/ISORROPIA, accessed on 1 April 2022 Search PubMed.
- C. Fountoukis and A. Nenes, ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+–NH4+–Na+–SO42−–NO3−–Cl−–H2O aerosols, Atmos. Chem. Phys., 2007, 7, 4639–4659, DOI:10.5194/acp-7-4639-2007.
- X. Jin, Y. Wang, Z. Li, F. Zhang, W. Xu, Y. Sun, X. Fan, G. Chen, H. Wu and J. Ren, Significant contribution of organics to aerosol liquid water content in winter in Beijing, China, Atmos. Chem. Phys., 2020, 20, 901–914, DOI:10.5194/acp-20-901-2020.
- Y. B. Lim, J. Seo, J. Y. Kim, Y. P. Kim and H. C. Jin, Local formation of sulfates contributes to the urban haze with regional transport origin, Environ. Res. Lett., 2020, 15, 084034, DOI:10.1088/1748-9326/ab83aa.
- W. Li, X. Teng, X. Chen, L. Liu, L. Xu, J. Zhang, Y. Wang, Y. Zhang and Z. Shi, Organic coating reduces hygroscopic growth of phase-separated aerosol particles, Environ. Sci. Technol., 2021, 55, 16339–16346, DOI:10.1021/acs.est.1c05901.
- J. Sun, L. Liu, L. Xu, Y. Wang, Z. Wu, M. Hu, Z. Shi, Y. Li, X. Zhang and J. Chen, Key role of nitrate in phase transitions of urban particles: implications of important reactive surfaces for secondary aerosol formation, J. Geophys. Res.: Atmos., 2018, 123, 1234–1243, DOI:10.1002/2017JD027264.
- J. Seo, Y. B. Lim, D. Youn, J. Y. Kim and H. C. Jin, Synergistic enhancement of urban haze by nitrate uptake into transported hygroscopic particles in the Asian continental outflow, Atmos. Chem. Phys., 2020, 20, 7575–7594, DOI:10.5194/acp-20-7575-2020.
- W. Xu, Q. Wu, X. Liu, A. Tang, A. J. Dore and M. R. Heal, Characteristics of ammonia, acid gases, and PM2.5 for three typical land-use types in the North China Plain, Environ. Sci. Pollut. Res., 2016, 23, 1158–1172, DOI:10.1007/s11356-015-5648-3.
- P. Acharja, K. Ali, D. K. Trivedi, P. Safai, S. Ghude, T. Prabhakaran and M. Rajeevan, Characterization of atmospheric trace gases and water soluble inorganic chemical ions of PM1 and PM2.5 at Indira Gandhi International Airport, New Delhi during 2017–18 winter, Sci. Total Environ., 2020, 729, 138800, DOI:10.1016/j.scitotenv.2020.138800.
- Z. Meng, X. Xu, W. Lin, B. Ge, Y. Xie, B. Song, S. Jia, R. Zhang, W. Peng and Y. Wang, Role of ambient ammonia in particulate ammonium formation at a rural site in the North China Plain, Atmos. Chem. Phys., 2018, 18, 167–184, DOI:10.5194/acp-18-167-2018.
- J. Bahino, V. Yoboué, C. Galy-Lacaux, M. Adon, A. Akpo, S. Keita, C. Liousse, E. Gardrat, C. Chiron and M. Ossohou, A pilot study of gaseous pollutants' measurement (NO2, SO2, NH3, HNO3 and O3) in Abidjan, Côte d'Ivoire: contribution to an overview of gaseous pollution in African cities, Atmos. Chem. Phys., 2018, 18, 5173–5198, DOI:10.5194/acp-18-5173-2018.
- Y. Li, F. M. Schwandner, H. J. Sewell, A. Zivkovich, M. Tigges, S. Raja, S. Holcomb, J. V. Molenar, L. Sherman and C. Archuleta, Observations of ammonia, nitric acid, and fine particles in a rural gas production region, Atmos. Environ., 2014, 83, 80–89, DOI:10.1016/j.atmosenv.2013.10.007.
- M. Mozurkewich, The dissociation constant of ammonium nitrate and its dependence on temperature, relative humidity and particle size, Atmos. Environ., Part A, 1993, 27, 261–270, DOI:10.1016/0960-1686(93)90356-4.
- J. Neuman, J. Nowak, C. Brock, M. Trainer, F. Fehsenfeld, J. Holloway, G. Hübler, P. Hudson, D. Murphy and D. Nicks Jr, Variability in ammonium nitrate formation and nitric acid depletion with altitude and location over California, J. Geophys. Res.: Atmos., 2003, 108, 4557, DOI:10.1029/2003JD003616.
- Y. Chang, Z. Zou, Y. Zhang, C. Deng, J. Hu, Z. Shi, A. J. Dore and J. L. Collett Jr, Assessing contributions of agricultural and nonagricultural emissions to atmospheric ammonia in a Chinese megacity, Environ. Sci. Technol., 2019, 53, 1822–1833, DOI:10.1021/acs.est.8b05984.
- C. Loftus, M. Yost, P. Sampson, E. Torres, G. Arias, V. B. Vasquez, K. Hartin, J. Armstrong, M. Tchong-French and S. Vedal, Ambient ammonia exposures in an agricultural community and pediatric asthma morbidity, Epidemiology, 2015, 26, 794, DOI:10.1097/EDE.0000000000000368.
- Y. Sun, Q. Jiang, Z. Wang, P. Fu, J. Li, T. Yang and Y. Yin, Investigation of the sources and evolution processes of severe haze pollution in Beijing in January 2013, J. Geophys. Res.: Atmos., 2014, 119, 4380–4398, DOI:10.1002/2014JD021641.
- H. Zhang, S. Cheng, X. Wang, S. Yao and F. Zhu, Continuous monitoring, compositions analysis and the implication of regional transport for submicron and fine aerosols in Beijing, China, Atmos. Environ., 2018, 195, 30–45, DOI:10.1016/j.atmosenv.2018.09.043.
- G. Zheng, H. Su, S. Wang, M. O. Andreae, U. Pöschl and Y. Cheng, Multiphase buffer theory explains contrasts in atmospheric aerosol acidity, Science, 2020, 369, 1374–1377, DOI:10.1126/science.aba3719.
- M. Tian, H. Wang, Y. Chen, F. Yang, X. Zhang, Q. Zou, R. Zhang, Y. Ma and K. He, Characteristics of aerosol pollution during heavy haze events in Suzhou, China, Atmos. Chem. Phys., 2016, 16, 7357–7371, DOI:10.5194/acp-16-7357-2016.
- C. Fountoukis, A. Nenes, A. Sullivan, R. Weber, T. V. Reken, M. Fischer, E. Matías, M. Moya, D. Farmer and R. Cohen, Thermodynamic characterization of Mexico City aerosol during MILAGRO 2006, Atmos. Chem. Phys., 2009, 9, 2141–2156, DOI:10.5194/acp-9-2141-2009.
- Z. Xu, M. Liu, M. Zhang, Y. Song, S. Wang, L. Zhang, T. Xu, T. Wang, C. Yan and T. Zhou, High efficiency of livestock ammonia emission controls in alleviating particulate nitrate during a severe winter haze episode in northern China, Atmos. Chem. Phys., 2019, 19, 5605–5613, DOI:10.5194/acp-19-5605-2019.
- A. H. Souri, Y. Choi, W. Jeon, J. H. Woo, Q. Zhang and J. i. Kurokawa, Remote sensing evidence of decadal changes in major tropospheric ozone precursors over East Asia, J. Geophys. Res.: Atmos., 2017, 122, 2474–2492, DOI:10.1002/2016JD025663.
- C. Hendriks, R. Kranenburg, J. Kuenen, B. Van den Bril, V. Verguts and M. Schaap, Ammonia emission time profiles based on manure transport data improve ammonia modelling across north western Europe, Atmos. Environ., 2016, 131, 83–96, DOI:10.1016/j.atmosenv.2016.01.043.
- C. Bae, B.-U. Kim, H. C. Kim, C. Yoo and S. Kim, Long-range transport influence on key chemical components of PM2.5 in the Seoul metropolitan area, South Korea, during the years 2012–2016, Atmosphere, 2019, 11, 48, DOI:10.3390/atmos11010048.
- S. Lv, F. Wang, C. Wu, Y. Chen, S. Liu, S. Zhang, D. Li, W. Du, F. Zhang and H. Wang, Gas-to-Aerosol Phase Partitioning of Atmospheric Water-Soluble Organic Compounds at a Rural Site in China: An Enhancing Effect of NH3 on SOA Formation, Environ. Sci. Technol., 2022, 56, 3915–3924, DOI:10.1021/acs.est.1c06855.
- M. Li, Z. Zhang, Q. Yao, T. Wang, M. Xie, S. Li, B. Zhuang and Y. Han, Nonlinear responses of particulate nitrate to NOx emission controls in the megalopolises of China, Atmos. Chem. Phys., 2021, 21, 15135–15152, DOI:10.5194/acp-21-15135-2021.
- X. Fu, T. Wang, J. Gao, P. Wang, Y. Liu, S. Wang, B. Zhao and L. Xue, Persistent heavy winter nitrate pollution driven by increased photochemical oxidants in northern China, Environ. Sci. Technol., 2020, 54, 3881–3889, DOI:10.1021/acs.est.9b07248.
- C. Womack, E. McDuffie, P. Edwards, R. Bares, J. de Gouw, K. Docherty, W. Dubé, D. Fibiger, A. Franchin and J. Gilman, An odd oxygen framework for wintertime ammonium nitrate aerosol pollution in urban areas: NOx and VOC control as mitigation strategies, Geophys. Res. Lett., 2019, 46, 4971–4979, DOI:10.1029/2019GL082028.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ea00051b |
| This journal is © The Royal Society of Chemistry 2023 |