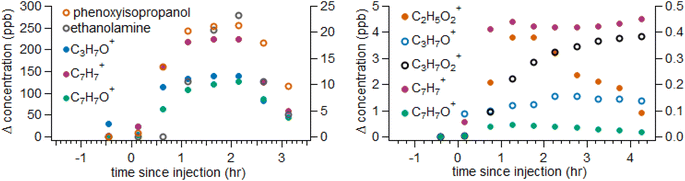Open Access Article
Open Access ArticleVolatile organic compound (VOC) emissions from the usage of benzalkonium chloride and other disinfectants based on quaternary ammonium compounds†
Leif G.
Jahn
 *a,
Mengjia
Tang
b,
Daniel
Blomdahl
*a,
Mengjia
Tang
b,
Daniel
Blomdahl
 b,
Nirvan
Bhattacharyya
b,
Nirvan
Bhattacharyya
 a,
Pearl
Abue
b,
Atila
Novoselac
b,
Lea Hildebrandt
Ruiz
a,
Pearl
Abue
b,
Atila
Novoselac
b,
Lea Hildebrandt
Ruiz
 a and
Pawel K.
Misztal
a and
Pawel K.
Misztal
 b
b
aMcKetta Department of Chemical Engineering, University of Texas at Austin, USA. E-mail: leif.jahn@austin.utexas.edu
bDepartment of Civil, Architectural, and Environmental Engineering, University of Texas at Austin, USA
First published on 14th December 2022
Abstract
Quaternary ammonium compounds (QACs) are a class of molecules commonly used as residential and industrial disinfectants whose prevalence has increased in recent years and during the COVID pandemic. QACs are typically considered relatively inert and nonvolatile; however, little is known about the propensity of QAC commercial products (CPs) to emit volatile organic compounds (VOCs) during usage. We performed a series of environmental chamber and solution headspace measurements using a Vocus proton transfer reaction time-of-flight mass spectrometer (PTR-ToF-MS) to examine VOC emissions during simulated spraying of a dilute solution of pure benzalkonium chloride (BAC), several CPs whose primary active ingredients include BAC and didecyl dimethyl ammonium chloride (DDAC), and a CP containing a novel silyl-based QAC. A number of VOCs were observed during spraying of pure BAC solution, including functionalized benzyl compounds, chlorotoluenes, and small functionalized hydrocarbons; these VOCs may be derived from the BAC synthesis process. Similar emission signatures were also detected from CPs, though specific source attribution was challenging due to the chemical complexity of commercial formulations, which include molecules to aid in solubilizing and stabilizing QACs (among other roles). Headspace measurements of the silyl-QAC suggest a functionalized propyl-silyl molecule is volatilized whose exact origin and structure could not be determined. Functionalized benzyl compounds are detected at the C7H7+ ion as well as at the protonated [M]H+ species or other ions, providing insight into the structures that can give rise to the C7H7+ ion signature that has commonly been detected during PTR-ToF measurements of indoor and urban environments. Within the pure BAC solution, the identified benzyl molecules may not account for the entirety of the measured C7H7+ signal, leaving open the possibility that unidentified VOCs are also present. Overall, the present measurements show that QAC solutions are not inherently inert or nonvolatile and will emit a variety of VOCs depending on the identity and purity of the contained QACs and product formulation.
Environmental significanceIndoor chemistry and air quality have substantial human health impacts due to the large amount of time typically spent within indoor spaces. Episodic events, such as disinfection or other cleaning, are significant potential contributors to indoor particle and VOC concentrations. Disinfectants based on quaternary ammonium compounds (QACs) are among the most common commercial disinfectants in use today; however, little is known about the potential for QAC disinfectants to emit VOCs during usage. This work examines VOC emissions from pure QAC solutions as well as commercial formulations and observes a variety of VOCs that relate to the active QAC ingredients as well as the inactive functional ingredients that stabilize and solubilize QACs, showing that QAC disinfectants can affect indoor air quality. |
Introduction
Quaternary ammonium compounds (QACs), commonly referred to as quats, are a class of molecule that possess biocidal properties and are the primary active ingredient in many commercial disinfectant formulations.1–6 The usage of commercial disinfectants including QAC-containing products has increased substantially in recent years, particularly during the COVID-19 pandemic,1,6–8 increasing the potential for human exposure to these molecules, associated additives, and their byproducts, especially within indoor environments.8 The QACs present in commercial disinfectants consist of a four-coordinate nitrogen atom that is typically bound to hydrocarbon ligands of differing lengths and structures and stabilized as a salt (Fig. 1), although ligands incorporating a variety of heteroatom- or silicon-based groups and functionalities are sometimes used.5 The hydrocarbon ligands typically consist of two methyl groups, an alkyl chain of variable length (–C8H17 to –C18H39), and then either a second alkyl chain, a benzyl group (–C7H7), or an alkyl group that is functionalized in a different manner.5,9 Many QACs that are common disinfectant ingredients incorporate the benzyl group functionality and are termed benzalkonium chloride (BAC) while another common QAC incorporates two C12 chains (didecyldimethylammonium chloride, DDAC); together, these two types of structures comprise the most common QACs in commercial products.2,3,5,9QAC disinfectants have traditionally been considered relatively safe and inert2,10 compared to other common disinfectants (such as hydrogen peroxide or chlorine-based solutions) as they tend to be large ionic salts. Research has suggested that QAC usage and exposure are linked to a variety of adverse health effects in humans,2,8,9,11,12 including increased asthma incidence, suggesting potential inhalation exposure.12,13 The stability of QACs has been highlighted in recent research demonstrating the potential for accumulation in terrestrial systems or indoor spaces.6–9 Additional concern has also arisen due to increased usage of QACs during the COVID-19 pandemic leading to QAC proliferation in natural systems6,7 and indoor spaces.8 QACs can degrade to tertiary amines or other compounds in solution through an alkyl-displacement reaction initiated by nucleophilic species.5 Such reactions do not appear to be sufficiently favorable to compete with biotic or disinfection-associated loss processes in natural systems and wastewater processing.6,9 These scenarios represent a bulk aqueous environment for the dissolved QAC; however, QAC application sometimes occurs through the generation and dispersal of microdroplets (such as with electrospray or misting devices).14 The reactivity of a variety of compounds within microdroplets has been observed to differ from that within bulk solutions due to a combination of potential factors including Laplace pressure, different solvation environments, and pH gradients,15–17 suggesting that different reaction pathways may be available for QACs during droplet-based application techniques compared to typical bulk environments.
In the present work, we conduct comprehensive measurements of the VOCs generated following injection of QAC solution droplets into a room-sized environmental chamber to simulate application in buildings as well as of the VOCs present in the headspace of commercial products. We compare laboratory-made pure QAC solutions with commercial products through these measurements.
Experimental
VOC measurements
VOC measurements were performed with a Vocus 2R proton transfer reaction time-of-flight mass spectrometer (Vocus 2R PTR-ToF-MS, hereafter abbreviated as Vocus; Tofwerk AG/Aerodyne Research, Inc.). The Vocus detects gas-phase analytes primarily as protonated adducts following reaction with H3O+ and has been described in detail in prior work.18 The Vocus was operated with an ion molecular reactor pressure of 2.3 mbar, 15 sccm flow rate from the water reservoir (H3O+ source), and IMR front and back voltages of 650 V and 25 V, respectively. The E/N ratio was calculated to be 150 Td. The Vocus draws sample flow at 2.5 Lpm through an approximately 2 m PTFE tube (1/8′′ ID, 1/4′′ OD) and overflows much of this while sub-sampling from this flow at a rate of 0.1 Lpm. Vocus data was analyzed in Igor Pro (version 8.0.4.2, Wavemetrics) using TofWare (version 3.2.2.1, Tofwerk AG/Aerodyne Research, Inc.). Data was acquired at a rate of 1 Hz and was averaged over 10 second intervals. Explicit calibrations were performed with a stepwise increasing flow from a mixed calibration gas cylinder (Apel Riemer Environmental) that was diluted with clean air from the internal generator on the Vocus. Calibrations were used to construct a correlation plot between calibrant sensitivities (cps ppb−1) and proton-transfer reaction rate coefficients (kPTR) which was used to estimate measured analyte concentrations for species that were not included in the calibration gas cylinder (Section S2 and Fig. S1†). Analyte sensitivities were estimated based on previously measured kPTR values19 or previously described methods based on analyte molecular formula, polarizability, and dipole moment when measurements are unavailable.19,20 These methods generally yielded sensitivities that agreed within 20–50% during prior work (with a small number of exceptions).20 The uncertainty in concentration measurements of analytes without an explicit calibration is expected to be ∼30%. Relative humidity was measured in the chamber using an LI-840 monitor (LI-COR Environmental).Chamber and headspace measurements
Environmental chamber experiments will be described in more detail in a future publication21 and characterization of the chamber and sampling system will be described only briefly here. Chamber experiments were conducted in a 67 m3 (5.5 × 4.5 × 2.7) stainless steel chamber operating with an air change rate of approximately 2.3 h−1. For each experiment 500 mL of QAC solution was placed in a commercial ultrasonic misting device (Holmes HM827TG-FCA) that was placed on a laminate table and turned on to inject QAC solution as small droplets at a rate of approximately 0.25 L h−1 until the reservoir was low (approximately 2 hours). Injection through the commercial misting device is intended to distribute a large volume of QAC solution droplets throughout the chamber during the measurement period. This method is similar in principle to the injections achieved through commercial disinfectant fogging devices. Electrostatic, fogging, and manual disinfectant spraying will generate droplets of a range of sizes with the size distribution dependent on the technique.22 It is not known how the droplet size distribution generated in this work compares to size distributions generated via other techniques and a measurement of the droplet size distribution generated during this work was not feasible. A thermal manikin breathing system23 was placed in the chamber on a wooden chair and “inhaled” at a rate of 0.50 L per inhalation with 12 inhalations occurring each minute. A new KN95 mask (BYD Electronics, Cueva) was placed over the manikin mouth and secured with a plastic zip tie during each experiment. The manikin is intended to represent the potential exposure a person (wearing a mask) may experience while in the chamber during disinfectant application. Mask usage is common in many healthcare and disinfection settings, increasingly so due to the COVID-19 pandemic. The Vocus sampled from four separate locations during each chamber experiment with the sample location controlled by a valve switching system (Valco Instruments Co., Inc.) at a flow rate of 2.5 Lpm, while lines from the location not being actively sampled pulled at 1 Lpm. The four sampling locations were the chamber inflow, the chamber outflow, a location within the chamber approximately 20 cm in front of the manikin, and subsampling the manikin breathing line located approximately 15 cm behind the manikin mask. Measurement at each chamber location lasted 5 minutes while chamber inflow was sampled for a total of 15 minutes. Measurements at each location are reported as an average taken without the first minute of sampling to allow VOC concentrations to stabilize. Headspace measurements were performed by opening the commercial product bottle and placing a capillary sampling line directly attached to the Vocus near the headspace above the product liquid and sampling for 5–15 minutes.QAC solutions and products
QAC solutions were either a laboratory-made benzalkonium chloride solution or a QAC-containing commercial product. BAC was purchased at >95% purity from Millipore-Sigma (#12060). The BAC content is listed as ∼70% dodecyl (–C12H25) dimethyl benzyl ammonium chloride and ∼30% tetradecyl (–C14H29) dimethyl benzyl ammonium chloride and potential impurities are stated to be water (<10%). BAC was weighed, added to a 500 mL volumetric flask, and then dissolved while diluting to 500 mL with deionized water. This led to solutions which ranged from 0.40–0.45 wt% (assuming 100% of weighed mass is BAC), comparable to the concentrations typically present in commercial over-the counter formulations. The laboratory-made BAC solutions do not contain compounds such as pH buffering agents or other additives that are typically present in commercial products and may act to stabilize QACs in solution. Four readily available over-the-counter commercial products marketed as “ready to use” (i.e., no dilution or mixing is necessary) disinfectant cleaners were purchased for this work. Commercial products tested are referred to as CP #1–4 through the rest of the manuscript and have the following total QAC contents: CP #1 0.11%, CP #2 0.073%, CP #3 0.50%, and CP #4 1.44%; a detailed description of each QAC ingredient is listed in the ESI Section S1.† Environmental chamber experiments were performed with the laboratory-made BAC solutions (two times) and CP #1–3 (once each). Headspace measurements were performed with each commercial product (CP #1–4). Chamber injection of CP #3 resulted in bubbling of the solution within the misting device rather than injection of droplets, apparently due to viscosity differences between CP #3 and the other aqueous solutions used. For this reason, measurements made with CP #3 are treated in a purely qualitative manner. Blank experiments were also performed in the same manner as the QAC experiments where 500 mL of deionized water was injected to the chamber via the misting device.Results and discussion
BAC solution: gas-phase composition and signal attribution
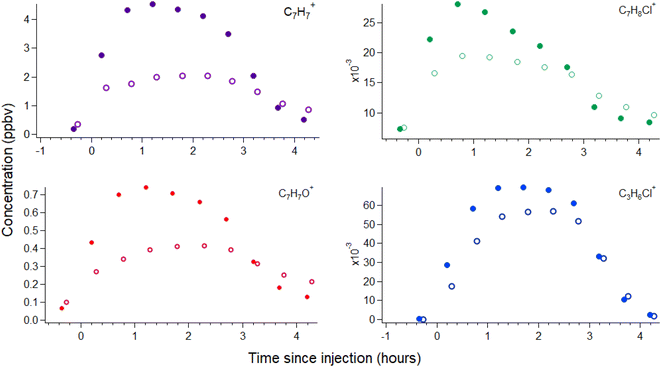
A number of VOCs are detected during the injection of the laboratory-made BAC solution into the environmental chamber that are not detected during the blank experiments. An increase in water vapor was observed during blank experiments, as expected because water droplets were injected into the chamber and no significant changes were observed in VOC concentrations (ESI Section S2 and Fig. S2†). The VOCs detected during BAC solution injection are summarized in Table 1 along with maximum concentrations reached within the chamber. A representative timeseries showing the concentration profiles of several of these VOCs during and following injection at the inside-chamber and behind-mask valve sampling positions is shown in Fig. 2. Background concentrations are higher for many VOCs in the behind-mask position due to VOC off-gassing from the manikin apparatus.21 VOC concentrations rise in the chamber air during injection; however, concentrations rise more slowly at the behind-mask position and reach a lower maximum concentration before decreasing. Stable concentrations of a majority of VOCs appear to be reached within the chamber approximately 60–80 minutes after injection and then begin to decrease as the liquid level drops and after injection stops, approximately 100–120 minutes. Periods of stable concentrations are used to calculate analyte concentrations (reported per g of BAC in Table 1) in solution using a mass-balance approach that is described in more detail in the ESI Section S3† and is based on prior work.24 The ion signals C7H8Cl+ and C7H7Cl2+ decrease more quickly, suggesting potentially unique loss mechanisms besides ventilation or wall losses (such as hydrolysis25,26). VOC concentrations inside the chamber then slowly return to background levels or drop below detection limits, with concentrations at the behind-mask position decaying at a slower rate and returning to pre-injection levels over longer timescales. This suggests that a mask may serve as a temporary sink for some VOCs that can deposit during exposure and then acts as a lower-level exposure source over longer timescales. In the hours following injection, we do not observe any further increases in any VOC signals, indicating that BAC deposited to surfaces within the chamber does not readily degrade to produce detectable VOCs over this timescale. Potential VOC production from BAC degradation may be detectable under other conditions, for example in a smaller chamber with lower air change rate or under different relative humidity, oxidant, and lighting conditions.| Analyte | Compound | Approx. steady state conc. | mganalyte gBAC−1 | Proposed origin |
|---|---|---|---|---|
| a Ethanol is not effectively quantified due to a low-m/z mass filter applied during Vocus operation.18 b Some amount of signal at C6H7+ and C7H7O+ likely originates from benzyl alcohol. c The benzyl and benzene cations originate from several functionalized benzyl molecules, as discussed in the main text. d Calculation not feasible due to high background concentration. | ||||
| C2H7O+ | Ethanol | n/aa | n/aa | Solvent |
| C3H8N+ | Allylamine | 10 pptv | 0.003 | |
| C3H6Cl+ | Chloropropene | 66 pptv | 0.16 | |
| C6H7+ | Benzene ionb,c | 980 pptv | 2.1 | Fragmentation product |
| C3H6ClO+ | Epichlorohydrin/chloroacetone | 31 pptv | 0.081 | |
| C6H7O+ | Phenol | 430 pptv | 0.39 | |
| C7H7+ | Benzyl ionc | 4200 pptv | 14 | Fragmentation product |
| C7H7O+ | Benzaldehydeb | 390 pptv | 2.0 | Synthesis byproduct27 |
| C7H10N+ | Benzylamine/undetermined | 70 pptv | 0.076 | |
| C7H9O+ | Undetermined/benzyl alcohol | 58 pptv | N/Ad | Synthesis byproduct27 |
| C7H8Cl+ | Chlorotoluene/benzyl chloridec | 16 pptv | 0.069 | Synthetic reagent27 or byproduct28 |
| C7H7Cl2+ | Dichlorotoluene/benzal chloride | 7 pptv | 0.035 | Synthesis byproduct28,29 |
The proposed most likely origin for several of the compounds detected during BAC injection is also listed in Table 1. Several of the VOCs that increase in concentration during injection (benzaldehyde, benzyl alcohol, and chlorotoluenes) have previously been observed in BAC solutions and were identified as unintended impurities originating as byproducts of common industrial synthetic routes.27,28 If an alkyl replacement reaction producing a tertiary amine were to occur,5 a likely co-product would be a functionalized benzyl molecule (such as benzyl alcohol). However, as benzyl alcohol may also be present as a synthesis byproduct, it is not clear whether BAC degradation might be occurring within droplets. We use chlorotoluenes to refer broadly to molecules with the molecular formula C7H6–7Cl1–2, whose isomers we cannot readily distinguish through PTR-MS. These structures comprise α-chlorotoluene (where Cl is located on the methyl group; also referred to as benzyl chloride), α-dichlorotoluene (benzal chloride) and (2–6)-chlorotoluenes (where at least one Cl is located on the benzyl ring). Benzyl chloride is the typical alkylating agent for attaching the benzyl group to the amine during BAC synthesis, so residual benzyl chloride could be present due to incomplete synthesis.3,27 Benzyl alcohol, benzaldehyde, benzal chloride, and chlorotoluene can be produced during the synthesis of benzyl chloride27–29 and may persist through the remainder of the BAC synthesis process to be present in the final product. The molecules C3H5Cl and C3H5ClO have multiple possible structures, 1- or 3-chloropropene for the former and epichlorohydrin or chloroacetone for the latter. 3-Chloropropene (allyl chloride) and epichlorohydrin appear to be the more likely structures, as these molecules are used in a variety of industrial synthetic processes. The molecules C7H10N+ and C3H8N+ are consistent with benzyl and propyl amine; however, analysis of pure benzyl amine solution (discussed in the following paragraph) suggests the C7H10N+ ion may instead correspond to a compound of undetermined structure (given the several possible structures) and origin. If benzyl, benzal, and allyl chloride are present in solution, they may be short-lived, as hydrolysis reactions can occur for benzyl (t1/2 ∼ 15 h), benzal (t1/2 ∼ 0.1 h), and allyl chloride (t1/2 ∼ 69 days), resulting in the formation of benzyl alcohol, benzaldehyde, and allyl alcohol.25 The hydrolysis rate measurements for these three halocarbons suggest that, if present, then some degree of hydrolytic degradation would have occurred over the ∼24 h that BAC solutions rested before chamber injection. Ethanol, benzene, and phenol were also detected during injection and may be residual solvents or side reaction products from the synthetic process.
A relatively substantial signal increase is observed for the C7H7+ ion during BAC injection. C7H7+ may conceivably correspond to a protonated C7H6 molecule. Such structures (e.g., cycloheptatetraene or norcaratriene) would not reasonably be present, however, and we do not consider this a plausible explanation. Rather, we believe the most probable origin for the C7H7+ ion is production through the fragmentation of a larger molecule containing the benzyl functionality (i.e., C6H6–CH2–R, where the CH2–R bond is to an electronegative atom such as X, N, O), as outlined in the reaction scheme below (R1).
| C7H7–R + H3O+ → [C7H7–R]H+ + H2O → C7H7+ + RH, R = X, NR2, OR | (R1) |
Prior PTR work has observed a variety of functionalized benzyl molecules to undergo fragmentation to C7H7+, as compiled in recent work.19 Some of these prior studies were performed using SIFT-MS,30,31 a related PTR technique whose reactor conditions and therefore molecular fragmentation patterns differ from the Vocus PTR-ToF used in the present work. Nevertheless, these studies provide insight into potential molecular structures that may fragment to C7H7+. We conducted headspace analysis of benzyl alcohol, benzyl amine, and benzyl chloride solutions to assess whether these analytes could contribute to the observed C7H7+ ion signal. This analysis is discussed in greater detail in the ESI Section S3 and Fig. S3† and shows that the C7H7+ ion is produced from each molecule, with >99% of benzyl chloride, ∼85% of benzyl alcohol, and ∼74% of benzyl amine detected as the C7H7+ ion (at the relatively high E/N ratio of 150 Td used in the present experiments). Benzyl amine signal is also detected as C7H8N+ (12%) and C7H10N+ (14%), consistent with other amines,19 while benzyl alcohol is also detected as the ions C6H7+ (10%) and C7H7O+ (5%), consistent with other alcohols.19 An ion signal corresponding to C7H8N+ does not increase during BAC solution injection, suggesting observed C7H10N+ may not originate from benzyl amine. Ion signal corresponding to the protonated [M + H]+ species is <0.5% for both benzyl chloride and benzyl alcohol. Given the stated purity of the analytical standards (>99%), the extent to which observed [M + H]+ signal is due to primary analyte or solution impurities cannot be reliably determined. For benzyl alcohol, an assumption that the C6H7+ and C7H7O+ ion signals observed during BAC injection arise solely from benzyl alcohol leads to an expected C7H7+ ion signal higher than observed, presumably due to the presence of both benzaldehyde and other compounds that may contribute to the C6H7+ ion signal. Therefore, it is possible that benzyl alcohol, amine, and chloride account for the entirety of the C7H7+ signal, but it also remains possible that unknown VOCs or BAC reaction products are present and undergo fragmentation to the C7H7+ ion during analysis.
Benzyl alcohol is likely not the only species in the present measurements that undergoes fragmentation to produce the C6H7+ ion. This ion is consistent with protonated benzene; however, a variety of functionalized benzyl compounds and single-ring aromatics have been observed to undergo fragmentation to C6H7+.19 This includes pure hydrocarbons such as ethylbenzene32 as well as functionalized oxygenates such as benzyl alcohol (Fig. S3†). Based on these fragmentation processes, we suspect the majority of the signal appearing at the C6H7+ ion (Table 1) is due to fragmentation of larger molecules, either functionalized benzylic or other single-ring aromatic molecules.
We do not observe a peak corresponding to protonated chlorotoluene (i.e., [C7H7Cl]H+ at m/z 127.0314), likely because it is obscured in the mass spectrum by a nearby overlapping peak from C6H7O3+ (exact mass 127.0395). However, we observe a new peak to develop during injection with a best-fit exact mass of 129.0282 that we attribute to the 37Cl isotope of C7H8Cl+ (exact mass 129.0285). Given the intensity of the proposed 37Cl peak, we would not expect the 35Cl peak to be clearly discernible from the C6H7O3+ peak. C7H8Cl+ signal may be due to either protonated chlorotoluene or benzyl chloride. Chlorotoluene is the more likely origin due to the significant fragmentation of benzyl chloride observed during headspace measurements. Another new peak we observe during injection is consistent with the C7H7Cl+ ion, originating from O2+ charge-transfer to C7H7Cl, which can again be due to benzyl chloride or chlorotoluene. This peak is only used as a qualitative identifier, as detection via O2+ charge-transfer is not quantified in this work.
Commercial products
The CPs we examine are composed of a mixture of QACs and a variety of other compounds as inactive ingredients rather than just BAC. We take the VOCs observed during injection of laboratory-made BAC solution (summarized in Table 1) as those potentially originating from BAC (as opposed to other ingredients) and summarize the presence of these compounds in the commercial products in Table 2, as measured during chamber injection and headspace sampling. Analyte mass concentrations were calculated using a mass-balance approach that is described in more detail in the ESI Section S4† and is based on prior work24 and are displayed as mganalyte gBAC−1 (and as ganalyte kgproduct−1 in the ESI Table S1†). Concentration differences within the chamber may arise due to formulation differences between solutions (including differing BAC concentrations: laboratory solution 0.41%, CP #1 0.11%, and CP #2 0.034%) as well as physical differences (such as viscosity) that may affect droplet size or VOC volatilization behavior. Headspace measurements directly analyze the VOCs in a small volume above the product and represent a more concentrated VOC measurement, while during chamber measurements VOCs may volatilize differently after solution is dispersed in small droplets that slowly evaporate. CPs may also contain the molecules summarized in Table 1 for reasons not related to QAC content. Ethanol is excluded from Table 2 because it is not reliably quantified.| Sigma BAC (mg gBAC−1) | CP #1 (mg gBAC−1) | CP #2 (mg gBAC−1) | CP #3a | CP #4b | |
|---|---|---|---|---|---|
| a Injection of CP #3 did not produce droplets and measurements are only presented qualitatively; see also discussion in the main text. b Chamber experiments with CP #4 were not feasible due to instrument and scheduling constraints. c Calculation not feasible because background air concentrations could not be accurately determined. d Partial attribution to the inactive ingredient phenoxyisopropanol or other species is likely; see discussion in text. | |||||
| C3H8N+ | 0.003 (n/a) | 2.4 (Y) | 0.12 (Y) | Y (n/a) | n/a (Y) |
| C3H6Cl+ | 0.16 (n/a) | N (N) | N (N) | N (n/a) | n/a (N) |
| C3H6ClO+ | 0.081 (n/a) | 0.068 (N) | N (N) | N (n/a) | n/a (Y) |
| C6H7+ | 2.1 (n/a) | 175d (Y) | 1.8 (Y) | Y (n/a) | n/a (Y) |
| C6H7O+ | 0.39 (n/a) | 1320d (Y) | Yc (Y) | Y (n/a) | n/a (Y) |
| C7H7+ | 14 (n/a) | 208 (Y) | 15 (Y) | Y (n/a) | n/a (Y) |
| C7H7O+ | 2.0 (n/a) | 1340d (Y) | 0.74 (Y) | Y (n/a) | n/a (Y) |
| C7H10N+ | 0.076 (n/a) | 0.087 (Y) | 0.41 (Y) | Y (n/a) | n/a (Y) |
| C7H9O+ | Yc (n/a) | Y (Y) | Y (Y) | Y (n/a) | n/a (Y) |
| C7H8Cl+ | 0.069 (n/a) | 0.19 (Y) | 0.62 (Y) | Y (n/a) | n/a (Y) |
| C7H7Cl2+ | 0.035 (n/a) | N (Y) | N (Y) | N (n/a) | n/a (Y) |
Most of the molecular ions that are observed during BAC injection are also detected during measurements of the commercial products. The ion C3H6Cl+ is not observed in any of the products and C3H6ClO+ is detected during only a few measurements. These molecules may never have been present in the other commercial products or may have undergone reaction in the aqueous solution following initial formulation. The several previously identified benzyl derivative are detected during headspace and/or chamber measurements of each product, with dichlorotoluene absent in several measurements. VOCs are diluted during chamber measurements relative to headspace measurements, meaning that some VOCs present at low concentrations may be detected within the product headspace but not during chamber injection.
Several ions are observed during chamber injection of CP #1 that may relate to the BAC present in this product, with the caveat that apportioning signal to a specific origin within the commercial products is complex. These ions appear to be large amines with the formulae C14H32N+ and C16H36N+, representing fully saturated molecules (Fig. S4†). There are multiple possible structures for these ions but two potential candidates are N,N-dimethyl dodecylamine and N,N-dimethyl tetradecylamine, the likely starting materials for the synthesis of the two most abundant BAC compounds within CP #1 (Methods and Section S1†). These ions are not clearly detected during CP #1 headspace measurements or measurements of the pure BAC solution, suggesting different volatilization behavior between the bulk solution within the bottle and suspended droplets. The large amines are expected to have low volatilities and may condense to the bottle during headspace analysis but are also expected to have low Henry's law solubility constants, which would drive volatilization from a suspended droplet as it evaporates. The low volatility of these compounds also leads to condensation within the unheated sampling lines and complicates any attempts at quantification, so we only discuss the qualitative detection of these compounds.
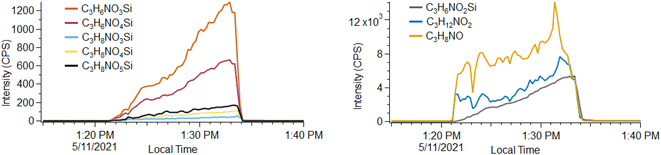
Unique ion signatures are observed during headspace measurements of the silyl-QAC containing CP #3, characterized by a gradual increase in concentration over the duration of the measurement. This is in contrast to most compounds observed during headspace measurements where the concentration initially spikes and then remains steady. Eight such ions are clearly observed and appear to relate to one another, with all ions consisting of a C3N1 group and then differing by the number of H (6, 8, or 12), O (2–5) and Si (0–1) atoms. We attribute one of the most intense signals to an ion with the formula C3H6NO2Si+, with several similar ions also observed (Fig. 3). We also observe two C3HyNiOz ions that display similar behavior, with C3H12NO2+ the most intense. We base these formulae attributions on excellent fits to the masses of the unknown peaks (<5 ppm for each peak), consistency with the natural isotopic distribution of Si (92.2% 28Si, 4.7% 29Si, 3.1% 30Si), and similarity to the functionality of the silyl QAC ligand (that consists of an –Si(OH)3 head group connected by a propyl (–C3H6–) chain to the four-coordinate N atom). A variety of fragmentation, water clustering, and/or ligand switching processes appear to be possible for molecules with the C–Si–O functionality during PTR-ToF analysis, based on analysis of Si3–Si5 cyclic methylsiloxane molecules present in the calibration gas used (see Fig. S6†). Given these possibilities and the range of molecular formulae observed, we are unsure which molecule(s) constitute a primary analyte and which are fragmentation or reaction products or clusters. The unique timeseries profiles of these molecules suggest that they either volatilize slowly from solution (e.g., due to a low vapor pressure) or are produced from the degradation of another solution component after the product is unsealed and exposed to air. A variety of heavy molecules (e.g., m/z > 150) that are presumably less volatile than the majority of solution components are observed and do not exhibit similar time-dependent behavior, so we suspect this behavior does not depend solely upon volatility.
The inactive ingredients in a disinfectant product can have non-functional roles such as providing fragrance or functional roles that affect product efficacy such as solvating or stabilizing active ingredients, controlling pH, or acting as a surfactant. Ingredient lists are available from the manufacturers of CP #1 and CP #3 and are provided in the ESI Section S1.† VOC emission profiles during injection of CP #1 and CP #3 are broadly consistent with the provided ingredient lists. Inactive ingredients will possess a range of structures and properties and may volatilize during or following application. Any VOCs may then be inhaled, undergo reaction to produce more oxygenated compounds and potentially secondary aerosols, or be ultimately lost to outdoor transport. The potential emission of inactive ingredients is an unavoidable part of the usage of any product, and we therefore choose to also discuss these VOCs that do not directly relate to BAC or QACs. We observe a large number of VOCs during chamber injection and headspace measurements, which is unsurprising given the ingredients lists (ESI Section S1†) and the variety of small oxygenated VOCs (such as acetone) that are present in many products.33 Many of these small oxygenated VOCs are present in a wide array of volatile chemical products33,34 and are not expected to contribute substantially to indoor air quality under most circumstances. Therefore, we do not focus on providing a complete accounting of these VOCs and limit our discussion to a select few compounds for each commercial product that either appear relevant to product efficacy (based on the labelled function) or are relatively novel among VOC sources.33 We estimate a total VOC emission factor of approximately 9.6 gTVOC kgproduct−1 (1.4 ppbvTVOC mL−1) for CP #1 and 0.16 gTVOC kgproduct−1 (0.03 ppbvTVOC mL−1) for CP #2. These emission factors are in a similar range or lower than those measured for a variety of cleaning agents examined in prior work.33,35 This may reflect the low concentrations of QAC active ingredients required for commercial disinfectants as well as the application method used in this work where droplets may be lost due to air change prior to complete evaporation of water-soluble VOCs or S/IVOCs. VOC emissions are driven mostly by non-BAC ingredients that comprise the majority (>90%) of detected signals. The large difference in TVOC emissions between CP #1 and CP #2 results from relatively high emissions of ethanolamine and phenoxyisopropanol (emission factors 2.6 and 4.6 gVOC kgproduct−1, respectively) from CP #1, which are shown below in Fig. 4 and discussed further in the following paragraphs. CP #3 could not be injected in the same manner as other solutions as injection resulted in bubbling without droplet formation, so VOC observations are only discussed qualitatively and not shown in Fig. 4.
Substantial concentration increases to levels of ∼250 ppb within the chamber are observed for the ions C9H11O+ and C2H8NO+ during injection of CP #1 (Fig. 4), which likely correspond to phenoxyisopropanol (derived from C9H12O2 following loss of water during analysis;19 a solubilizing agent) and ethanolamine (C2H7NO; to control pH as a buffering agent) from the ingredients list. Both of these compounds are present in the ingredients list (ESI Section S1†) and their observation during injection is unsurprising. Fragmentation of phenoxyisopropanol during analysis may contribute to other ion signals as well, potentially C6H7O+ (alternatively phenol), C7H7O+ (alternatively benzaldehyde or benzyl alcohol), and C6H7+ (alternatively due to the fragmentation of other aromatic compounds or benzene).19 Aromatic molecules (as a general class, not specifically phenoxyisopropanol) have been observed to form SOA efficiently through atmospheric oxidation,36 so phenoxyisopropanol could ultimately contribute to SOA formation. Ethanolamine has been shown to be an efficient base in the formation and growth of new particles37,38 and is also a respiratory irritant,39 and may therefore be relevant to indoor air quality.
VOC concentrations rise less substantially during injection of CP #2 (Fig. 4): apart from small oxygenated hydrocarbons and ion signals consistent with monoterpenes (a common fragrance component of VCPs33), few substantial concentration increases are observed. The concentrations of these VOCs are still substantially greater than those of the VOCs likely attributed to QACs. Monoterpene oxidation can lead to particle formation and growth within indoor environments,40,41 though monoterpene concentrations peak at <1 ppb during measurements. VOCs also appear to peak over different timescales, in contrast to the BAC solution and CP #1, for reasons that are not clear. Lower VOC concentrations potentially indicate that the inactive ingredients of CP #2 are present at lower concentrations or are less volatile relative to those in the other products. Qualitatively, emissions from CP #3 mainly consist of small oxygenated VOCs, similar to CP #2, as well as some larger compounds that are likely solvents or fragrance compounds, including C10H23O3+ (likely dipropylene glycol butyl ether, listed as a solvent) and C10H17+ (monoterpenes, fragrance). Also detected are a pair of ion signals consistent with highly saturated hydrocarbons, C10H11+ and C11H11O+. Whether these last two ions represent protonated molecules or mass spectral fragments of larger structures, these ions with high double bond equivalents (DBE; 6 and 7, respectively) suggest molecules with a C6 aromatic ring and then 2–3 additional C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds or rings, potentially consistent with functionalized polycyclic aromatic hydrocarbons (PAHs). These molecules do not have a clear origin in the ingredients list for CP #3. Aromatic molecules, and PAHs in particular, can lead to negative health effects following inhalation42 and can efficiently forming SOA through oxidation.36 CP #3 also contains a pH-adjusting buffer, triethanolamine, that is observed during injection at lower levels than ethanolamine in CP #1; this may be due to the fact that CP #3 could not be injected as droplets into the chamber, the lower KH of triethanolamine compared to ethanolamine, or that a smaller concentration of pH buffering agent was present in CP #3 than CP #1.
C double bonds or rings, potentially consistent with functionalized polycyclic aromatic hydrocarbons (PAHs). These molecules do not have a clear origin in the ingredients list for CP #3. Aromatic molecules, and PAHs in particular, can lead to negative health effects following inhalation42 and can efficiently forming SOA through oxidation.36 CP #3 also contains a pH-adjusting buffer, triethanolamine, that is observed during injection at lower levels than ethanolamine in CP #1; this may be due to the fact that CP #3 could not be injected as droplets into the chamber, the lower KH of triethanolamine compared to ethanolamine, or that a smaller concentration of pH buffering agent was present in CP #3 than CP #1.
Significance and implications
A number of VOCs are generated during chamber injections of solutions made with pure BAC. Some VOCs appear to relate to different aspects of the BAC synthesis process, being either synthetic reagents, synthesis byproducts, or residual solvents, while the precise origin of other VOCs remains undetermined. We are also unable to determine whether any BAC degradation may occur within droplets prior to deposition or removal and contribute to the observed VOCs. The strongest signal detected during chamber measurements is from the C7H7+ ion, which appears to originate predominately from benzyl alcohol but may also originate from benzyl chloride, benzyl amine, and/or unidentified molecules. The C7H7+ ion has been detected during a variety of indoor VOC measurements without specific source attribution, and the present measurements suggest that these or similarly structured molecules may contribute to these prior observations.43,44 We do not observe any additional compounds to volatilize in the chamber after injection concludes, indicating that under these experimental conditions and timescales BAC remains relatively stable after depositing to surfaces within the chamber.We observe VOCs with the same molecular formulae during commercial product measurements as during BAC solution measurements; however, we cannot determine whether these compounds relate to BAC/QACs or other ingredients or whether isomeric compounds are observed as well (though this would be unlikely for some of the ions detected). Substantially higher concentrations of some ions in commercial products compared to the BAC solution suggest multiple origins. A variety of other VOCs are also observed during commercial product injection with large differences in concentrations between some products (9.6 vs. 0.16 gTVOC kgproduct−1 for CP #1 vs. CP #2), likely due to differences in formulation and/or ingredient volatilities. VOCs observed include aromatic molecules and small amines, which are potentially relevant for indoor particle growth and SOA formation.37,38 Some of these compounds are inactive ingredients (not conferring disinfecting action) that fulfill important roles such as stabilizing or solubilizing QAC compounds within solution and are therefore an integral part of product functionality. Signals related to non-QAC ingredients appear to comprise the bulk (>90%) of commercial product VOC emissions, illustrating that disinfectant contributions to indoor air quality depend strongly on the product formulation and the specific active and inactive ingredients. The potential effects of these compounds on indoor health and air quality should therefore also be considered when evaluating the effects of using QACs as well as other disinfectants and VCPs. During analysis of the commercial products containing traditional alkyl-based QACs we did not consistently observe any additional VOCs that could be definitively attributed to BAC or the other QACs. Ions consistent with the amines C14H31N and C16H35N were observed during chamber injection of CP #1 and may be residual N,N-dimethylamine synthetic reagents but were unable to be quantified due to their low volatility. During analysis of a product containing a QAC incorporating a silyl functionality, we observe the volatilization of a compound that appears to be a derivative of the propyl-silyl ligand of the QAC and may be a degradation product of the QAC or other solution components.
The VOCs we observe are expected to be reactive towards common oxidants and in low-ventilated spaces may contribute to indoor secondary organic aerosol formation or growth.45 The benzyl and other aromatic VOCs are semivolatile and may partition to indoor surfaces, including particles, where they may accumulate or undergo further reaction.45,46 VOC concentrations during chamber measurements are well below acute exposure limits for an indoor environment representing a well-ventilated space; however, the chronic effects of low-level inhalation exposure to a number of these VOCs (primarily the halocarbons) during QAC disinfectant usage or solution dilution12 are not currently well quantified and may contribute to previously observed negative health effects.2,12,13 Personal exposure would be expected to increase with decreasing ventilation volume and air exchange rates. Some VOCs we observe appear to relate to the BAC synthesis process, suggesting that the nature of the synthesis (i.e., the synthetic route, overall efficiency, and purification) will have a significant effect on the QAC-related VOCs that may be emitted during disinfectant usage and that different compounds may be present in different products containing the same or similar active ingredients. Halocarbons are common reagents for QAC synthesis,5 so the potential for halocarbon exposure will likely exist for many QAC-containing commercial products. While BAC is one of the most common QACs currently used in household cleaners,2,3,5,6,9 a variety of other QAC structures have been developed and may see increasing usage if antibiotic resistance to BAC (or the other most commonly used QAC, didecyl dimethyl ammonium chloride) become of concern.5–7,9 QACs of varying structures will necessarily require a variety of synthetic reagents, leading to potential exposure to a wider array of VOCs. These observations suggest that measures to reduce or separate impurities and byproducts generated during QAC synthesis could be a means toward reducing QAC-associated VOC emissions. Overall, the present work challenges the traditional assumption that QAC solutions are composed of non-volatile and inert molecules and shows that further work is needed to evaluate potential exposure risks related to usage of QAC disinfectant solutions. Future experiments utilizing a combination of gas chromatography and/or online mass spectrometry with other reagent ion chemistries would be useful in further speciating QAC-related VOC emissions and calculating emission factors under different application scenarios.
Author contributions
LGJ conceived of the measurements, conducted the analysis, and wrote the manuscript. MT assisted in data analysis. LGJ, MT, DB, NB, PA, AN, LHR, and PKM set up and performed experiments. All authors contributed to review and revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based on work supported by the National Science Foundation under grant no. 2027420. This material is based in part on work supported by the Alfred P. Sloan Foundation under grant no. G-2018-11240. LGJ was supported by the Camille and Henry Postdoctoral Program in Environmental Chemistry. We thank all sponsors for their generous support.References
- List N: EPA's Registered Antimicrobial Products for Use against Novel Coronavirus SARS-CoV-2, the Cause of COVID-19, United States Environmental Protection Agency Office of Chemical Safety and Pollution Prevention, 2020, available at https://www.epa.go Search PubMed.
- T. C. Hrubec, R. P. Seguin, L. Xu, G. A. Cortopassi, S. Datta, A. L. Hanlon, A. J. Lozano, V. A. McDonald, C. A. Healy, T. C. Anderson, N. A. Musse and R. T. Williams, Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure, Toxicol. Rep., 2021, 8, 646–656 CrossRef CAS PubMed.
- B. M. P. Pereira and I. Tagkopoulos, Benzalkonium chlorides: Uses, regulatory status, and microbial resistance, Appl. Environ. Microbiol., 2019, 85, 1–13 Search PubMed.
- Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC), US Environmental Protection Agency, Office of Prevention, Pesticides, Toxic Substances, Washington, D.C., 2006 Search PubMed.
- F. Bureš, Quaternary Ammonium Compounds: Simple in Structure, Complex in Application, Top. Curr. Chem., 2019, 377, 14 CrossRef PubMed.
- O. W. Barber and E. M. Hartmann, Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies, Crit. Rev. Environ. Sci. Technol., 2021, 1–30 Search PubMed.
- P. Hora, S. G. Pati, P. J. McNamara and W. A. Arnold, Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications, Environ. Sci. Technol. Lett., 2020, 7, 622–631 CrossRef CAS.
- G. Zheng, G. M. Filippelli and A. Salamova, Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic, Environ. Sci. Technol. Lett., 2020, 7, 760–765 CrossRef CAS.
- C. Zhang, F. Cui, G. ming Zeng, M. Jiang, Z. zhu Yang, Z. gang Yu, M. ying Zhu and L. qing Shen, Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment, Sci. Total Environ., 2015, 518–519, 352–362 CrossRef CAS PubMed.
- G. Vincent, M. C. Kopferschmitt-Kubler, P. Mirabel, G. Pauli and M. Millet, Sampling and analysis of quaternary ammonium compounds (QACs) traces in indoor atmosphere, Environ. Monit. Assess., 2007, 133, 25–30 CrossRef CAS PubMed.
- A. Bernstein, T. Stauder, D. Bernstein and I. Bernstein, A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines, J. Allergy Clin. Immunol., 1994, 94, 257–259 CrossRef PubMed.
- M. Gonzalez, J. Jégu, M.-C. Kopferschmitt, C. Donnay, G. Hedelin, F. Matzinger, M. Velten, L. Guilloux, A. Cantineau and F. de Blay, Asthma among workers in healthcare settings: role of disinfection with quaternary ammonium compounds, Clin. Exp. Allergy, 2014, 44, 393–406 CrossRef CAS PubMed.
- I. Folletti, A. Siracusa and G. Paolocci, Update on asthma and cleaning agents, Curr. Opin. Allergy Clin. Immunol., 2017, 17, 90–95 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency (EPA), About List N: Disinfectants for Coronavirus (COVID-19), U.S. Environ. Prot. Agency Search PubMed.
- M. I. Jacobs, R. D. Davis, R. J. Rapf and K. R. Wilson, Studying Chemistry in Micro-compartments by Separating Droplet Generation from Ionization, J. Am. Soc. Mass Spectrom., 2019, 30, 339–343 CrossRef CAS PubMed.
- X. Yan, R. M. Bain and R. G. Cooks, Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry, Angew. Chem., Int. Ed., 2016, 55, 12960–12972 CrossRef CAS PubMed.
- H. Wei, E. P. Vejerano, W. Leng, Q. Huang, M. R. Willner, L. C. Marr and P. J. Vikesland, Aerosol microdroplets exhibit a stable pH gradient, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 7272–7277 CrossRef CAS PubMed.
- J. Krechmer, F. Lopez-Hilfiker, A. Koss, M. Hutterli, C. Stoermer, B. Deming, J. Kimmel, C. Warneke, R. Holzinger, J. Jayne, D. Worsnop, K. Fuhrer, M. Gonin and J. de Gouw, Evaluation of a New Reagent-Ion Source and Focusing Ion–Molecule Reactor for Use in Proton-Transfer-Reaction Mass Spectrometry, Anal. Chem., 2018, 90, 12011–12018 CrossRef CAS PubMed.
- D. Pagonis, K. Sekimoto and J. de Gouw, A Library of Proton-Transfer Reactions of H3O+ Ions Used for Trace Gas Detection, J. Am. Soc. Mass Spectrom., 2019, 30, 1330–1335 CrossRef CAS PubMed.
- K. Sekimoto, S.-M. Li, B. Yuan, A. Koss, M. Coggon, C. Warneke and J. de Gouw, Calculation of the sensitivity of proton-transfer-reaction mass spectrometry (PTR-MS) for organic trace gases using molecular properties, Int. J. Mass Spectrom., 2017, 421, 71–94 CrossRef CAS.
- M. Tang, D. C. Blomdahl, N. Bhattacharyya, L. G. Jahn, P. Abue, L. H. Ruiz, P. K. Misztal and A. Novoselac, Chamber Study on the Dynamics of Water and Primary Compounds in the Gas Phase Following Disinfection, to be submitted.
- J. Wood, M. Magunson, A. Touati, J. Gilberry, J. Sawyer, T. Chamberlain, S. McDonald and D. Hook, Evaluation of electrostatic sprayers and foggers for the application of disinfectants in the era of SARS-CoV-2, PLOS One, 2021, 16, 9 Search PubMed.
- D. Rim and A. Novoselac, Transport of particulate and gaseous pollutants in the vicinity of a human body, Build. Environ., 2009, 44, 1840–1849 CrossRef.
- X. Tang, P. K. Misztal, W. W. Nazaroff and A. H. Goldstein, Volatile Organic Compound Emissions from Humans Indoors, Environ. Sci. Technol., 2016, 50, 12686–12694 CrossRef CAS PubMed.
- W. Mabey and T. Mill, Critical review of hydrolysis of organic compounds in water under environmental conditions, J. Phys. Chem. Ref. Data, 1978, 7, 383–415 CrossRef CAS.
- K. Tanabe and T. Sato, The Mechanism of the Hydrolysis of Benzyl Chloride, J. Res. Inst. Catal., Hokkaido Univ., 1962, 10, 173–182 CAS.
- M. C. Prieto-Blanco, P. López-Mahía and D. Prada-Rodríguez, Analysis of residual products in benzalkonium chloride by high-performance liquid chromatography, J. Chromatogr. Sci., 1999, 37, 295–299 CAS.
- M. C. Prieto-Blanco, P. López-Mahía and D. Prada-Rodríguez, Analysis of residual products in benzyl chloride used for the industrial synthesis of quaternary compounds by liquid chromatography with diode-array detection, J. Chromatogr. Sci., 2009, 47, 121–126 CAS.
- K.-A. Lipper and E. Löser, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011 Search PubMed.
- P. Španěl and D. Smith, Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several aromatic and aliphatic hydrocarbons, Int. J. Mass Spectrom., 1998, 181, 1–10 CrossRef.
- P. Španěl and D. Smith, Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with some chloroalkanes and chloroalkenes, Int. J. Mass Spectrom., 1999, 184, 175–181 CrossRef.
- A. R. Koss, K. Sekimoto, J. B. Gilman, V. Selimovic, M. M. Coggon, K. J. Zarzana, B. Yuan, B. M. Lerner, S. S. Brown, J. L. Jimenez, J. Krechmer, J. M. Roberts, C. Warneke, R. J. Yokelson and J. de Gouw, Non-methane organic gas emissions from biomass burning: identification, quantification, and emission factors from PTR-ToF during the FIREX 2016 laboratory experiment, Atmos. Chem. Phys., 2018, 18, 3299–3319 CrossRef CAS.
- B. C. McDonald, J. A. De Gouw, J. B. Gilman, S. H. Jathar, A. Akherati, C. D. Cappa, J. L. Jimenez, J. Lee-Taylor, P. L. Hayes, S. A. McKeen, Y. Y. Cui, S. W. Kim, D. R. Gentner, G. Isaacman-VanWertz, A. H. Goldstein, R. A. Harley, G. J. Frost, J. M. Roberts, T. B. Ryerson and M. Trainer, Volatile chemical products emerging as largest petrochemical source of urban organic emissions, Sci. 80, 2018, 359, 760–764 CAS.
- R. U. Shah, M. M. Coggon, G. I. Gkatzelis, B. C. McDonald, A. Tasoglou, H. Huber, J. Gilman, C. Warneke, A. L. Robinson and A. A. Presto, Urban Oxidation Flow Reactor Measurements Reveal Significant Secondary Organic Aerosol Contributions from Volatile Emissions of Emerging Importance, Environ. Sci. Technol., 2020, 54, 714–725 CrossRef CAS PubMed.
- B. C. Singer, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids, Indoor Air, 2006, 16, 179–191 CrossRef CAS PubMed.
- D. R. Gentner, S. H. Jathar, T. D. Gordon, R. Bahreini, D. A. Day, I. El Haddad, P. L. Hayes, S. M. Pieber, S. M. Platt, J. de Gouw, A. H. Goldstein, R. A. Harley, J. L. Jimenez, A. S. H. Prévôt and A. L. Robinson, Review of Urban Secondary Organic Aerosol Formation from Gasoline and Diesel Motor Vehicle Emissions, Environ. Sci. Technol., 2017, 51, 1074–1093 CrossRef CAS PubMed.
- J. Shen, H.-B. Xie, J. Elm, F. Ma, J. Chen and H. Vehkamäki, Methanesulfonic Acid-driven New Particle Formation Enhanced by Monoethanolamine: A Computational Study, Environ. Sci. Technol., 2019, 53, 14387–14397 CrossRef CAS PubMed.
- H.-B. Xie, J. Elm, R. Halonen, N. Myllys, T. Kurtén, M. Kulmala and H. Vehkamäki, Atmospheric Fate of Monoethanolamine: Enhancing New Particle Formation of Sulfuric Acid as an Important Removal Process, Environ. Sci. Technol., 2017, 51, 8422–8431 CrossRef CAS PubMed.
- Ethanolamine; MSDS No. E9508, Sigma-Aldrich, Inc., St. Louis, Missouri, United States, January 28th, 2020, https://www.sigmaaldrich.com/US/en/sds/sial/e9508, accessed 03/31/2022 Search PubMed.
- C. Wang, D. B. Collins and J. P. D. Abbatt, Indoor Illumination of Terpenes and Bleach Emissions Leads to Particle Formation and Growth, Environ. Sci. Technol., 2019, 53, 11792–11800 CrossRef CAS PubMed.
- C. M. F. Rosales, J. Jiang, A. Lahib, B. P. Bottorff, E. K. Reidy, V. Kumar, A. Tasoglou, H. Huber, S. Dusanter, A. Tomas, B. E. Boor and P. S. Stevens, Chemistry and human exposure implications of secondary organic aerosol production from indoor terpene ozonolysis, Sci. Adv., 2022, 8, 1–17 Search PubMed.
- K. H. Kim, S. A. Jahan, E. Kabir and R. J. C. Brown, A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects, Environ. Int., 2013, 60, 71–80 CrossRef CAS PubMed.
- C. Arata, P. K. Misztal, Y. Tian, D. M. Lunderberg, K. Kristensen, A. Novoselac, M. E. Vance, D. K. Farmer, W. W. Nazaroff and A. H. Goldstein, Volatile organic compound emissions during HOMEChem, Indoor Air, 2021, 31, 2099–2117 CrossRef CAS PubMed.
- Y. Liu, P. K. Misztal, J. Xiong, Y. Tian, C. Arata, R. J. Weber, W. W. Nazaroff and A. H. Goldstein, Characterizing sources and emissions of volatile organic compounds in a northern California residence using space- and time-resolved measurements, Indoor Air, 2019, 29, 630–644 CAS.
- J. P. D. Abbatt and C. Wang, The atmospheric chemistry of indoor environments, Environ. Sci.: Processes Impacts, 2020, 22, 25–48 RSC.
- C. Wang, D. B. Collins, C. Arata, A. H. Goldstein, J. M. Mattila, D. K. Farmer, L. Ampollini, P. F. DeCarlo, A. Novoselac, M. E. Vance, W. W. Nazaroff and J. P. D. Abbatt, Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents, Sci. Adv., 2020, 6, eaay8973 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ea00054g |
| This journal is © The Royal Society of Chemistry 2023 |