Open Access Article
Open Access ArticleKinetics and products study of the reaction of Cl atoms with methyl dichloroacetate: reactivity, mechanism, and environmental implications
Vianni G.
Straccia C
a,
Cynthia B.
Rivela
ab,
María B.
Blanco
a and
Mariano A.
Teruel
 *a
*a
a(L. U. Q. C. A), Laboratorio Universitario de Química y Contaminación Del Aire, Instituto de Investigaciones en Fisicoquímica de Córdoba (I. N. F. I. Q. C.), Dpto. de Fisicoquímica. Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mariano.teruel@unc.edu.ar
bInstitute for Atmospheric and Environmental Research, University of Wuppertal, DE-42097 Wuppertal, Germany. Fax: (+54) 351-4334188
First published on 15th March 2023
Abstract
The relative rate coefficient of the gas-phase reaction of methyl dichloroacetate (CHCl2C(O)OCH3) with Cl atoms (kCl) was obtained at 298 K and atmospheric pressure. All the experiments were performed in a 480 L Pyrex glass atmospheric simulation reactor coupled to an “in situ” Fourier transform infrared (FTIR) spectrometer. The rate coefficient obtained from the average of different experiments was: kCl = (3.31 ± 0.88) × 10−13 cm3 per molecule per s. In addition, the product studies were performed in under similar conditions to those of the kinetic experiments in two different photoreactors by in situ FTIR spectroscopy and GC-MS/SPME. Dichloroacetic acid, phosgene, methyl trichloroacetate, and carbon monoxide were the main products identified and quantified. The obtained product yields for the reaction with Cl atoms were as follows: (24 ± 2), (19 ± 3), (16 ± 1), and (44 ± 2)% for Cl2CHCOOH, COCl2, CO, and CCl3C(O)OCH3, respectively. The initial pathway for the degradation of methyl dichloroacetate in the reaction with Cl atoms occurs via H-atom abstraction at the alkyl groups. The atmospheric implications of the reactions were assessed by the estimation of the tropospheric lifetime of τCl = 3 years. In addition, an acidification potential of 0.45 was estimated, suggesting a possible impact of the emission of methyl dichloroacetate on the rainfall acidification. On the other hand, significant global warming potentials of 8.2, 2.2, and 0.6 were calculated for the studied chloroester for the time horizons of 20, 100, and 500 years, respectively. Chlorinated persistent products, such as dichloroacetic acid, could have an impact on the atmosphere and other environmental matrixes as well as on human health and the biota.
Environmental significanceWhile kinetic data is essential for assessing the environmental impact of VOCs emissions and indicates the spatial extent of the spread of emissions, a thorough assessment also requires a complete understanding of the tropospheric degradation mechanism and the resulting products. In this work the rate coefficient of the Cl-initiated oxidation of methyl dichloroacetate, as an example of a polychlorinated ester, have been determined at atmospheric conditions. Complementary products studies were performed, where the molar yields of the reaction products were determined. The atmospheric lifetime of the dichloroester studied determines their contribution to the acid rain and global warming. Furthermore, dichloroacetic acid as other chlorinated products as emerging and persistent pollutants, could affect air quality and other environmental compartments. |
1 Introduction
A large amount of volatile organic compounds (VOCs), which are emitted by different sources, are released to the troposphere and stratosphere.1 This causes appreciable disturbances in the composition of the atmosphere, which can generate great changes in the future of the climate; some of these released gases increase the natural greenhouse effect, and as a consequence, an increase in the average temperature of the planet can occur.2 Once in the air, these compounds can react with the tropospheric oxidants (OH radicals, Cl atoms, NO3 radicals, or O3 molecules) to generate other oxygenated VOCs (OVOCs) as degradation products that possibly impact the environmental quality. In this sense, ethers, used as fuel additives and in chemical industries, are frequently released into the atmosphere. In addition, once in the air, methyl dichloroacetate (MDCA) could be generated by the atmospheric oxidation of some ethers3 and, as well as, stand out as volatilization products that are released from the earth's surface due to its wide range of use, as chemical products for disinfection, painting, varnishing, and as the starting reagents for pharmaceutical and agrochemical products3. The presence of MDCA in the atmosphere makes it necessary to study the reaction kinetics with different atmospheric oxidants in order to know the impact it produces at different scales. Reactions with Cl atoms under atmospheric conditions have great importance in the oxidative processes that occur in areas where the concentration of this oxidant can be competitive with the concentration of OH radicals.4,5This work carries out kinetics studies of the reaction of methyl dichloroacetate with Cl atoms under atmospheric conditions in a simulation chamber coupled with an in situ FTIR spectrometer. The relative overall rate coefficient of this reaction, using different reference compounds, was measured at 298 K and 1 atm.
Complementary, reaction products were identified and quantified by GC-MS/SPME and in situ FT-IR spectroscopy to postulate atmospheric chemical mechanisms at NOx free conditions.
Up to now, there are no kinetic and product studies reported for the reaction of methyl dichloroacetate with chlorine atoms. Consequently, this is the first reactivity and product distribution study of the title reaction. Furthermore, this work aims to clarify and contribute to the knowledge of the mechanisms through which polychlorinated esters are degraded by Cl atoms in the gas phase as indicated by the following reaction (eqn (1)):
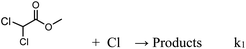 | (1) |
The rate coefficient obtained is compared with experimental and theoretical values reported previously for other VOCs with similar structures.
In complementary studies, the products were determined and quantified using two different environmental chambers coupled with FTIR spectroscopy and GC-MS, which gave information to postulate the atmospheric chemical mechanism for the reaction studied under NOx-free conditions.
With the kinetic and product data, the atmospheric implications of the interest reaction were assessed in terms of possible transport, acidification, and global warming potentials as well as the impact of the chlorinated products on the environment and the biosphere.
2 Methods and materials
2.1 Kinetics studies
The experiments were performed in a 480 L Pyrex glass atmospheric simulation reactor coupled to a Fourier Transform Infrared Spectrometer, Nicolet Magna 520 and the spectral range was 4000–700 cm−1. The chamber surrounded by 32 fluorescence lamps, emitting at a maximum of 360 nm, was used to photochemically initiate the experiments at 750 torr and 298 K. A full description of the reactor can be found in the literature.6 The system was evacuated by a pumping system consisting of a turbo-molecular pump backed by a double-stage rotary fore pump to 10−3 torr. The reactor has a support system for multiple reflection mirrors of type “White” with a base length of (48.11 ± 0.01) m. The mirrors allow reactions to be carried out at low concentrations simulating quasi-real atmospheres.Kinetic determinations were obtained by a relative method indirectly from their relationship with the rate coefficient of reference compounds. Methyl dichloroacetate and different reference compounds reacted competitively with the oxidants as the following:
| Methyl dichloroacetate + Cl → products | (2) |
| Reference + Cl → products | (3) |
Cl atoms were produced by photolysis at 360 nm of molecular Cl2 as (eqn (4)):
| Cl2 + hν (λ-360 nm) → 2Cl | (4) |
Considering that reactions (2) and (3) are the only reactions that deplete both compounds, it is possible to determine the relative rate coefficient of the reactions of interest as:
 | (5) |
Representing ln[MDCA]0/[MDCA]tversus ln[Reference]0/[Reference]t a straight line is observed whose slope is the ratios, kMDCA/kreference where the rate coefficient of reference with Cl atoms is known in the literature.7
Before kinetics experiments, some tests were performed to check that reactions (2) and (3) were the only reactions occurring inside the reactor. First, we made sure that the MDCA and the reference compound did not react with each other in the dark, and then with the lamps on to avoid some photolysis. On the other hand, the possible reaction of the organic compounds with the radical precursor in the dark and wall loss was checked.
2.2 FTIR product study
The reaction products between methyl dichloroacetate with Cl atoms were studied using the same experimental setup of the kinetic study. Mixtures of MDCA/Cl/air were irradiated with fluorescent lamps. With the residual spectrum, when possible, product identification and quantification were performed from a relationship with calibrated reference spectra stored in the IR spectral database of the laboratories at the University of Wuppertal, Germany.2.3 GC-MS product study
In addition, the reaction products under NOx-free conditions were monitored by gas-chromatography-mass spectrometry in LUQCA at Córdoba University. The experiments were carried out in an 80 L collapsible Teflon chamber at 296 ± 2 K and ∼750 torr of pressure situated in a wooden box with the internal walls protected by aluminium foil and four germicidal lamps (Philips 30W) with a UV emission at a maximum of 254 nm. Before and after each experiment, the collapsible Teflon chambers were cleaned by filling them with a mixture of O2 and N2 and photolyzed for 30 min using germicidal lamps to produce O3. The compounds and oxidant were flushed from the calibrated Pyrex bulbs into the Teflon chamber by a stream of zero grade N2 and then the chamber was filled with pure synthetic air.Cl atoms were generated by UV photolysis of oxalyl chloride (ClCOCOCl) (eqn (6))
| ClC(O)C(O)Cl + hν(254 nm) → 2Cl + 2CO | (6) |
The gas sample was taken from the Teflon bag using The Solid Phase Micro Extraction Technique (SPME), with the method of pre-concentration of the sample during 10 minutes of absorption for each measurement of the products. The organic compounds were monitored by gas chromatography coupled with a mass detector in a GC-MS VARIAN Saturn 2200 with column HP-5MS, Agilent (Part 19091S-433) of 30 meters in length, 0.25 mm internal diameter and film thickness 0.25 μm.
2.4 Materials
The initial concentrations used in the experiments were 4.92 ppm for methyl dichloroacetate and in a range of 6–32 ppm for the reference compounds (chloromethane and cyclopropane). (1 ppm = 2.46 × 1013 molecule per cm3 at 298 K and 760 torr of total pressure).The chemicals used in the experiments had the following purities as provided by the manufacturer and were used as supplied: synthetic air (air liquid, 99.999%), nitrogen (air lLiquid 99.999%), molecular chlorine (Messer Griesheim, 2.8), methyl dichloroacetate (Sigma-Aldrich, 99%), chloromethane (Sigma-Aldrich, 99%), cyclopropane (Sigma-Aldrich 99%) and oxalyl chloride (Aldrich, 99%).
3 Results and discussion
3.1 Kinetics
Eqn (5) was used to determine the relative rate coefficient for reactions of methyl dichloroacetate with Cl atoms employing the relative method with different reference compounds as follows (eqn (7) and (8)):| CH3Cl + Cl → products | (7) |
| C3H6 + Cl → products | (8) |
For the reaction studied, at least two experiments were performed for the rate coefficient determination for both oxidant reactions. Fig. 1 shows the plots ln[MDCA]0/[MDCA]tversus ln[Reference]0/[Reference]t of two examples for each reference. All the experiments were developed using N2. All plots showed linearity for the obtained straight lines, with correlation coefficients close to 1 and nearly zero intercepts indicating that secondary reactions are negligible.
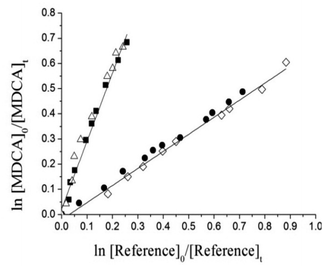 | ||
| Fig. 1 Plots of the kinetics data for the reaction of methyl dichloroacetate with Cl atoms using chloromethane (□ and ●) and cyclopropane (Δ and ■) as a reference at 298 K. | ||
Table 1 shows the rate coefficient ratios (kMDCA/kreference) obtained for each reference and the corresponding rate coefficient in absolute terms. These rate coefficient ratios are each from the average of three measurements and in some cases with a variation of the initial concentration. A good agreement between the results obtained with different reference compounds was observed. The recommended value for the rate coefficient after averaging, a minimum of 3 experiments is as follows:
| kMDCA+Cl = (3.31 ± 0.88) × 10−13 cm3 per molecule per s |
| Reference | k reference × 1013 cm3 per molecule per s | k MDCA/kreference | k MDCA × 1013 cm3 per molecule per s | |
|---|---|---|---|---|
| CHCl2C(O)OCH3 + Cl | CH3Cl | 5.20 ± 0.40 | 0.67 ± 0.02 | 3.48 ± 0.13 |
| 0.68 ± 0.02 | 3.54 ± 0.13 | |||
| C3H6 | 1.15 ± 0.17 | 2.75 ± 0.16 | 3.16 ± 0.65 | |
| 2.65 ± 0.10 | 3.05 ± 0.56 | |||
| Average | 3.31 ± 0.88 |
The errors shown are twice the standard deviation that results from the least squares fit of the straight lines and the corresponding error of the reference rate coefficient.
In the present study, the rate coefficient of the reaction of methyl dichloroacetate with Cl atoms was determined (in units of cm3 per molecule per s) to be (3.31 ± 0.88) × 10−13. This is the first kinetic determination of the reactions cited above and no comparison with previous work was possible.
The rate coefficient obtained for the reaction of Cl atoms with methyl dichloroacetate can be compared with the corresponding reaction of Cl with methyl chloroacetate, a similar structure compound reported previously of 8.5 × 10−13 cm3 per molecule per s. The addition of a second chlorine substituent in the ester molecule could generate a steric hindrance for H-atom abstraction that reduces the rate coefficient by a factor of three. A similar trend was previously observed in our laboratory for the reactivity of halogenated esters.11
A comparison between rate coefficients of the reactions of some chloro compounds towards OH radicals and with Cl atoms is shown in Table 2. It is possible to note that rate coefficients decrease with the number of chlorine substituents in the ester molecule.11,12 From Table 2, it is possible to observe that the rate coefficients for reactions of CH3C(O)OCH3, ClCH2C(O)OCH3, and Cl2CHC(O)OCH3 with Cl atoms are (22.00; 8.50 and 3.31) × 10−13 cm3 per molecule per s, respectively. In the same way, for some chloro alkanes the rate coefficient of CH3Cl, CH2Cl2, and CHCl3 with Cl atoms are 4.90, 3.60, and 0.76 × 10−13 cm3 per molecule per s, respectively.
| VOC | k Cl × 10−13 (cm3 per molecule per s) | k OH × 10−13 (cm3 per molecule per s) |
|---|---|---|
| a SAR-calculation. b This work. c (Ref. 10). d (Ref. 14). e (Ref. 13). f (Ref. 15). g (Ref. 12). h (Ref. 16). i (Ref. 17). j (Ref. 18). k (Ref. 19). l (Ref. 20). m (Ref. 21). n (Ref. 22). o (Ref. 23). p (Ref. 24). q (Ref. 16). r (Ref. 25). s (Ref. 26). | ||
| CH3C(O)OCH3 | 22.00 ± 0.30c | 3.26 ± 0.08f |
| ClCH2C(O)OCH3 | 8.50 ± 1.90c | — |
| Cl2CHC(O)OCH3 | 3.31 ± 0.88b | 1.956a |
| Cl3CC(O)OCH3 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Alkanes | ||
| CH3Cl | 4.90 ± 0.15d | 0.42 ± 0.10d |
| CH2Cl2 | 3.60 ± 0.15d | 1.20 ± 0.20d |
| CHCl3 | 0.76 ± 0.30d | 1.00 ± 0.01e |
| C2H5Cl | 57.60 ± 5.00h | 4.35 ± 0.35j |
| CH3CCl3 | 0.095 ± 0.001m | 0.40 ± 0.01m |
| CH3CHCl2 | 16.40 ± 0.80s | 2.59 ± 0.20r |
| CH2ClCH2CH3 | 490.0 ± 150i | 11.20 ± 2.80i |
| CH3CHClCH3 | 200.0 ± 60.0i | 9.20 ± 2.30i |
| C3H6Cl2 | 110.0 ± 30.0i | 7.80 ± 1.90i |
| CH2ClCH2Cl | 12.70 ± 3.80q | 2.55 ± 0.51p |
| C(CH3)3Cl | 130.0 ± 40.0i | 4.10 ± 1.00i |
| CH2ClCH2CH2CH3 | 110.0 ± 20.0k | 20.0 ± 1.50l |
| CH3CHClCH2CH3 | 700.0 ± 90.0k | 24.5 ± 3.00l |
| CH3CHClCH2Cl | 39.0 ± 6.00o | 4.59 ± 0.60n |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Ketones | ||
| CH3COCH3 | 22.0 ± 0.40g | 2.20 ± 0.50e |
| CH3COCH2Cl | 20.0 ± 0.20g | 4.20 ± 0.80g |
| CH3COCHCl2 | 1.70 ± 0.30g | 3.80 ± 0.80g |
| CH3COCCl3 | 0.17 ± 0.30g | 0.15 ± 0.30g |
Furthermore, the OH oxidation of ketones show the same trend, e.g., CH3COCH2Cl, CH3COCHCl2, and CH3COCCl3 with rate coefficients of (4.20; 3.80 and 0.15) × 10−13 cm3 per molecule per s, respectively.7,10,12–14
It is important to mention that the trends observed in the reactivity of methyl dichloroacetate cannot be explained only by the halogens substitution in the ester, other contributions that should be considered are the strength of the bonds and the steric effects.
Currently, there is not much information on the reactivity of chlorinated esters with different atmospheric oxidants. Therefore, it is not possible to make an extended evaluation of the dependence of the different contributions to the reactivity of chloroesters. Consequently, it is necessary to carry out more experiments and theoretical studies to increase the kinetic database of the atmospheric degradation reactions of chloroacetates and understand the reactivity changes associated with halogen substitution under different atmospheric conditions.
3.2 Free energy relationships
Many studies have reported a linear correlation between the rate coefficients of different compounds in reaction with tropospheric oxidants such as OH radicals, Cl atoms, O3 molecules, and NO3 radicals.14,27,28In this work, we present a correlation between kOH and kCl of a series of chlorine-containing compounds from the literature and included the kinetic data obtained for the methyl dichloroacetate through a free energy graph from Table 2.13–17
The correlations obtained between the rate coefficients for the reactions of OH radicals and Cl atoms obtained in this work with these compounds are shown in Fig. 2. An appreciable correlation was obtained and a least-squares treatment of the data points in Fig. 2 yielded the following expression (with the rate coefficients in the units of cm3 per molecule per s).
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH = 0.6013 kOH = 0.6013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kCl − 5.6451 (r2 = 0.88) kCl − 5.6451 (r2 = 0.88) | (9) |
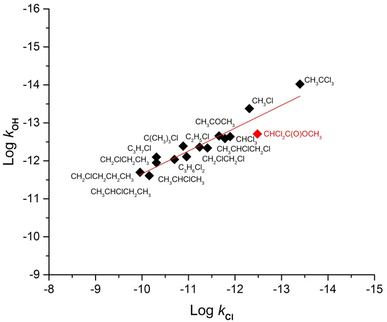 | ||
| Fig. 2 Free energy plots log(k(OH)) vs. log(k(Cl)) for the reactions with Cl and OH of esters, alkanes, and ketones, including chlorinated VOCs, reported in the previous work together with the chloroester studied in this work (Table 2). | ||
The free energy plot for the different chloro compounds shows a very good correlation between the rate coefficients with both oxidants. This indicates that the degradation mechanism by the reaction with Cl atoms is similar to the mechanism observed for their reactions with OH radicals, that is, by H-atoms abstraction.
Additionally, the rate coefficcient of the reaction of OH radicals with MDCA was calculated, using eqn (9) and the rate coefficient of MDCA with Cl atoms determined in this work. It was obtained as an estimated value of the rate coefficient for the reaction with OH radicals that have not yet been measured experimentally. The obtained value, in units of cm3 per molecule per s, was kOH = 7.24 × 10−13.
3.3 Products
To investigate the mechanism of Cl atoms initiating degradation of methyl dichloroacetate, mixtures of oxidant/methyl dichloroacetate/air/were photolyzed with fluorescent lamps under similar conditions of the kinetic experiments for approximately 20 minutes. Each measurement was performed at approximately 50% of the consumption of methyl dichloroacetate.The possible reaction pathway that can develop in the reaction of Cl atoms with methyl dichloroacetate will occur via H-atoms abstraction at the CH3 or CHCl2, followed by the addition of O2 to form peroxy radicals with further alkoxy radicals formation. The atmospheric sink of the alkoxy radicals formed can have several pathways of the reaction: decompose with a C–C or C–O bond cleavage, α-ester rearrangement with a further decomposition, or, react with O2.
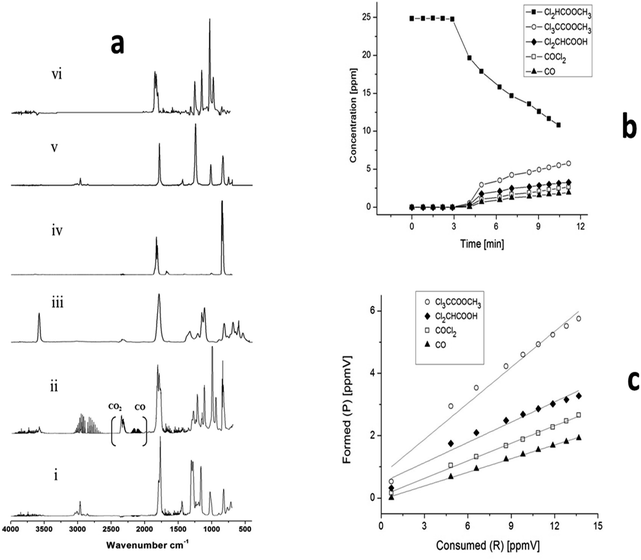
Fig. 3a shows IR spectra for the reaction of MDCA with Cl atoms before trace (i) and after trace (ii) UV irradiation with the lamps, dichloroacetic acid (Cl2CHCOOH) trace (iii), phosgene (COCl2) trace (iv), methyl trichloroacetate (Cl3CCOOCH3) trace (v), and carbon monoxide (CO) trace (ii). These compounds have been successfully identified as reaction products. Trace (vi) shows the residual spectrum after the subtraction of the features of the above products.
Fig. 3b shows plots of the concentration–time performance of MDCA with Cl atoms and the products were identified. Fig. 3c shows that the plots of the formation of the product versus the loss of MDCA are linear with near zero intercepts and least squares analyses of the slopes of these plots show yields of (24 ± 2; 19 ± 3; 16 ± 1 and 44 ± 2) % for Cl2CHCOOH, COCl2, CO, and CCl3C(O)OCH3, respectively. The first five points were considered to fit the line and to calculate the product's yield of dichloroacetic acid and trichloroacetate (Table 3). The errors are a combination of 2σ statistical errors from the regression.
| Reaction | Products | Yields (%) |
|---|---|---|
| Cl2CHC(O)OCH3 + Cl | Cl2CHCOOH | 24 ± 2 |
| COCl2 | 19 ± 3 | |
| Cl3CCOOCH3 | 44 ± 2 | |
| CO | 16 ± 1 |
A condensed reaction mechanism for the reaction of MDCA with Cl atoms in the absence of NOx is shown in Scheme 1. Degradation of MDCA was initiated by Cl atoms occurring via H-atoms abstraction from the alkyl groups. Therefore, there are two possible routes for the reaction. According to SAR (US Environmental Protection Agency), H-atoms abstraction is estimated to account for 55% and 45% at –CH3 and –Cl2HC– groups, respectively, in the overall reaction.29 The probability of H-atom abstraction for both groups is similar, and this fact was observed in the yields calculated for phosgene and dichloroacetic acid formation.
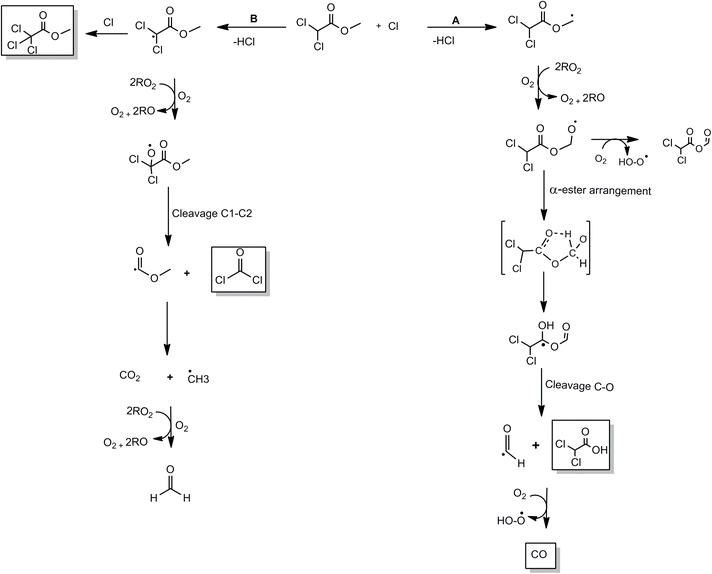 | ||
| Scheme 1 The mechanism of the Cl-atoms initiated oxidation of methyl dichloroacetate at NOx-free conditions via H-abstraction. | ||
Route A shows H-atoms abstraction from the ester CH3 group followed by the addition of O2 and further formation of an alkoxy radical. These Cl2CHC(O)OCH2(O˙) radicals can: (a) react with O2 to produce the polyfunctional compound Cl2CHC(O)OC(O)H (b) undergo an α-ester rearrangement followed by C–C bond cleavage to give the carboxylic acid Cl2CHC(O)OH and ˙C(O)H radicals. These radicals can further react with O2 to give carbon monoxide. Both compounds (Cl2CHC(O)OC(O)H and Cl2CHC(O)OH) were identified and positively quantified. No evidence was observed for the formation of formaldehyde (HCHO) in the product spectra, supporting that the reaction route involving the C–O bond cleavage in the alkoxy Cl2CHC(O)OCH2(O˙), is negligible in our experimental conditions.
Route B will occur if the H-atoms abstraction is from the CCl2H group to form ˙CCl2C(O)OCH3 radicals. These radicals could add Cl atoms to produce methyl trichloroacetate Cl3CC(O)OCH3. This compound was effectively identified and quantified by FTIR. On the other hand, the alkyl radicals will add O2 followed by the decomposition with C–C bond cleavage with phosgene, COCl2, and ˙C(O)OCH3 radical formation. These ˙C(O)OCH3 radicals can be decarboxylated to form CO2 and ˙CH3 radicals. HCHO could not be detected in the experiments; however, formaldehyde could be an important source of the CO observed. Methyl radicals produce formaldehyde by the reaction with O2 with further CO and CO2 production by its degradation.
Complementary studies were performed concerning the products of the reactions of methyl dichloroacetate with Cl atoms monitoring the nascent products by GC-MS at LUQCA in the Córdoba, University.
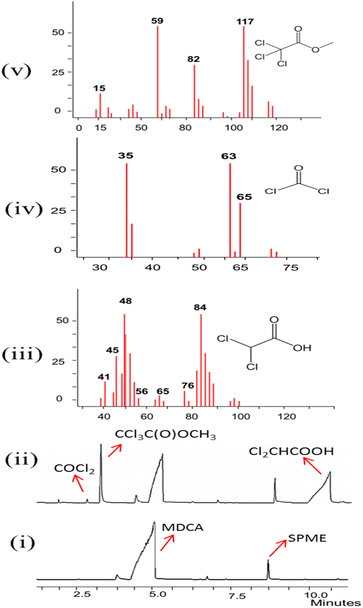
Fig. 4 shows the chromatograms before (i) and after (ii) photolysis, where MDCA was observed at the retention time of 4.9 min, together with the characteristic fragments (m/z) for the 3 main products found: dichloroacetic acid (iii) at 10 min, phosgene (iv) at 2.5 min and methyl trichloroacetate (v) at 3 min.
Dichloroacetic acid and phosgene were observed with ions with m/z = 45, 48, 76, 84, 35, 63, and 65, respectively. Methyl trichloroacetate showed the fragments m/z = 15, 59, 82, and 117. All of these fragments m/z are characteristic of these cited compounds.
Products identified by Fourier transform infrared spectroscopy and gas chromatography coupled to a mass detector were matched.
4 Atmospheric chemistry implications
Some species can last a long time in the atmosphere. The residence time can be calculated from the kinetic data of the oxidation reactions with tropospheric oxidants such as OH radicals, Cl atoms, O3 molecules, or NO3 radicals. The tropospheric lifetime (τ) was calculated using the determined rate coefficients and the average tropospheric oxidant concentrations.From Table 4, the atmospheric lifetimes obtained was τCl = 2.96 years for methyl dichloroacetate. This value was calculated with the expression τ = 1/kMDCA × [oxidants], where the concentrations of Cl atoms are reported as follows [Cl] = (3.3 ± 1.1) × 104 atoms cm−3 for 24 hours30 This chlorine atoms concentration value is an average obtained in marine areas of the North Atlantic as determined by Oliver W. Wingenter et al., 1996.
Using eqn (9), of the free energy relationship, the rate coefficient for the reaction with OH radicals was estimated to be kOH = 7.24 × 10−13 cm3 per molecule per s. With this kinetic value, and the concentration of [OH]31 = 2.0 × 106 radicals cm−3 for about 12 hours, the tropospheric lifetimes for the reaction with OH radicals could be estimated.
Unfortunately, there are no kinetic data available in the literature for the reactions of this compound with O3 molecules and NO3 radicals. Photolysis studies performed before the kinetics experiments for MDCA did not show an important decrease in the signals of the FTIR that is, the MDCA was stable to actinic radiation. This leaves the solar photolysis of the compound. MDCA has a low solubility in water, so the wet deposition is negligible.
The stability of methyl dichloroacetate in the atmosphere is considerably high. This compound survives a long time before it is transferred from its source of emission to other areas and can contribute to tropospheric ozone formation or it can ascend to the stratosphere and contribute to destroying the ozone layer depletion.
Rainfall acidification is a well-known environmental problem caused by the presence of acids in the atmosphere, the most important of which are HNO3, H2SO4, and HCl. However, compounds with Cl, F, N, and S substituents may contribute to acidification. AP is defined as the number of acid equivalent potentials (H+) per unit mass of a compound relative to the number of H+ per unit mass of a reference compound, (SO2 = 1).
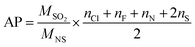 | (10) |
AP was estimated using the empirical eqn (10) given by Frank A. A. M. de Leeuw.32 The number of equivalent potentials (H+) per molecule is obtained by adding the number of substituents of nitrogen (N), chlorine (Cl), fluorine (F), and double sulfur (2 × S).
Table 4 shows that the potential of acidifying MDCA is 0.45, it is almost half that of the reference compound. This value indicates that methyl dichloroacetate degradation could be involved in acidifying rainwater and increasing environmental problems. However, since the time scale between emission and washout, as acidic species is not known, these values should be regarded as upper limits for the acidification potential of the studied compound.
To evaluate the contribution to greenhouse warming, the Global Warming Potential (GWP) using the method by Ø. Hodnebrog et al. with the eqn (11).33
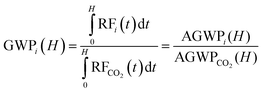 | (11) |
The GWP of a specific gas is calculated as the ratio of the time-integrated radiative forcing from a pulse emission of a unit mass of gas (1 kg) of that gas relative to that of 1 kg of a reference gas, normally CO2 where H is the time horizon, this is for 20, 100, and 500 years.
GWPs for MDCA were calculated to be 8.2; 2.2, and 0.6 for a time horizon of 20, 100, and 500 years, respectively. These potentials were estimated using the kOH value calculated by SAR. Its impact on global warming could be significant compared with carbon dioxide CO2 = 1 due to its long lifetime. However, as we mentioned before since the exact emissions of MDCA to the troposphere are not possible to be determined yet in comparison with CO2 emissions, these values must be considered higher limits for the GWP of the MDCA.
Atmospheric degradation of methyl dichloroacetate can produce phosgene gas (COCl2). Phosgene is an extremely toxic gas, which can be gradually degraded through UV radiation to produce ClOx, which has an important impact on the depletion of the ozone layer.34 This gas can react with the humidity of the rains and form hydrochloric acid that can cause acid rains.31–33
Dichloroacetic acid as a product of methyl dichloroacetate degradation is a stable tropospheric product and it can dissolve in the water droplets of the clouds.35 This persistent acid is a potentially hazardous compound since it is still more toxic than the other chlorinated compounds such as trichloroethylene36.
5 Conclusions
There are no previous kinetic and product distribution data for the reactions of Cl atoms with methyl dichloroacetate under atmospheric conditions. Consequently, this work reports the first kinetic and product distribution data for the photodegradation reaction of the mentioned chloroester initiated by atmospheric oxidants.The correlation between kOH and kCl gives evidence that the degradation mechanisms of both reactions occur in the same way, the abstraction of hydrogen atoms. On the other hand, these results can be useful for predictive reaction modelers and further laboratory studies, in case of atmospheric reactions not yet studied.
The residence time of methyl dichloroacetate around 3 three years, will have a regional and global impact. Loss by photolysis can be considered negligible since MDCA is photolytically stable in the actinic region of the electromagnetic spectrum.
The acidification potential suggests that the degradation of MDCA could contribute to the acidification of rainwater and since the GWPs, calculated as 8.2, 2.2, and 0.6 (with times horizon of 20, 100, and 500 years, respectively), are higher than the reference of CO2, this compound could contribute to the positive radiative forcing as a greenhouse gas.
Furthermore, the reaction products formed on the oxidation of the chloroester in the air, such as phosgene and dichloroacetic acid could have a negative impact on the atmosphere-biosphere in different environmental matrixes and on the ozone layer.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge EUROCHAMP 2020, FONCyT, CONICET, and SECyT UNC, Argentina. C. B. R and M. B. B. wish to acknowledge the Alexander von Humboldt Foundation for financial support. V. G. S. C wishes to acknowledge CONICET for a doctoral fellowship and support.References
- B. J. Finlayson-Pitts and J. N. Pitts Jr, Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications, Elsevier, 1999 Search PubMed.
- I. S. A. Isaksen, C. Granier, G. Myhre, T. Berntsen, S. B. Dalsøren, M. Gauss, Z. Klimont, R. Benestad, P. Bousquet, W. Collins, T. Cox, V. Eyring, D. Fowler, S. Fuzzi, P. Jöckel, P. Laj, U. Lohmann, M. Maione, P. Monks, A. S. H. Prevot, F. Raes, A. Richter, B. Rognerud, M. Schulz, D. Shindell, D. Stevenson, T. Storelvmo, W.-C. Wang, M. van Weele, M. Wild and D. J. Wuebbles, in The Future of the World's Climate, eds A. Henderson-Sellers and K. McGuffie, Elsevier, Boston, 2nd edn, 2012, pp. 309–365 Search PubMed.
- K. Terao, α-Chlorocarbonyl compounds: their synthesis and applications, Bull. Inst. Chem. Res., Kyoto Univ., 1992, 70, 338–377 CAS.
- Y. Ren, J. Wang, B. Grosselin, V. Daële and A. Mellouki, Kinetic and product studies of Cl atoms reactions with a series of branched Ketones, J. Environ. Sci., 2018, 71, 271–282 CrossRef CAS PubMed.
- J. A. Thornton, J. P. Kercher, T. P. Riedel, N. L. Wagner, J. Cozic, J. S. Holloway, W. P. Dubé, G. M. Wolfe, P. K. Quinn, A. M. Middlebrook, B. Alexander and S. S. Brown, A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry, Nature, 2010, 464, 271–274 CrossRef CAS PubMed.
- I. Barnes, K. H. Becker and N. Mihalopoulos, An FTIR product study of the photooxidation of dimethyl disulfide, J. Atmos. Chem., 1994, 18, 267–289 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, M. J. Rossi and J. Troe, Evaluated Kinetic, Photochemical and Heterogeneous Data for Atmospheric Chemistry: Supplement V. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 1997, 26, 521–1011 CrossRef CAS.
- IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation – Data Sheet IV.A2.91 oClOx17.
- M. D. Hurley, W. F. Schneider, T. J. Wallington, D. J. Mann, J. D. DeSain and C. A. Taatjes, Kinetics of Elementary Reactions in the Chain Chlorination of Cyclopropane, J. Phys. Chem. A, 2003, 107, 2003–2010 CrossRef CAS.
- L. K. Christensen, J. C. Ball and T. J. Wallington, Atmospheric Oxidation Mechanism of Methyl Acetate, J. Phys. Chem. A, 2000, 104, 345–351 CrossRef CAS.
- M. B. Blanco and M. A. Teruel, Atmospheric degradation of fluoroesters (FESs): Gas-phase reactivity study towards OH radicals at 298K, Atmos. Environ., 2007, 41, 7330–7338 CrossRef CAS.
- S. Carr, D. E. Shallcross, C. E. Canosa-Mas, J. C. Wenger, H. W. Sidebottom, J. J. Treacy and R. P. Wayne, A kinetic and mechanistic study of the gas-phase reactions of OH radicals and Cl atoms with some halogenated acetones and their atmospheric implications, Phys. Chem. Chem. Phys., 2003, 5, 3874 RSC.
- W. DeMore, S. Sander, D. Golden, R. Hampson, M. Kurylo, C. Howard, A. R. Ravishankara, C. Kolb, M. Molina, Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling, JPL Publication, 1985 Search PubMed.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, M. J. Rossi and J. Troe, Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement VI. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 1997, 26, 1329–1499 CrossRef CAS.
- A. El Boudali, S. Le Calvé, G. Le Bras and A. Mellouki, Kinetic Studies of OH Reactions with a Series of Acetates, J. Phys. Chem., 1996, 100, 12364–12368 CrossRef CAS.
- E. Tschuikow-Roux and J. Niedzielski, Secondary kinetic isotope effects in hydrogen and deuterium abstraction by chlorine atoms from the chloromethyl group in gaseous ethyl chlorides: effects of isotopic substitution in the adjacent methyl group, J. Photochem., 1984, 27, 141–150 CrossRef CAS.
- T. Donaghy, I. Shanahan, M. Hande and S. Fitzpatrick, Rate constants and atmospheric lifetimes for the reactions of OH radicals and Cl atoms with haloalkanes, Int. J. Chem. Kinet., 1993, 25, 273–284 CrossRef CAS.
- J. H. Kasner, P. H. Taylor and B. Dellinger, Laser photolysis/laser induced fluorescence study of hydroxyl-chloroethane rate constants from 294 to 789 K, J. Phys. Chem., 1990, 94, 3250–3253 CrossRef CAS.
- G. S. Tyndall, J. J. Orlando, T. J. Wallington, M. Dill and E. W. Kaiser, Kinetics and mechanisms of the reactions of chlorine atoms with ethane, propane, and n-butane, Int. J. Chem. Kinet., 1997, 29, 43–55 CrossRef CAS.
- J. C. Loison, L. Ley and R. Lesclaux, Kinetic study of OH radical reactions with chlorobutane isomers at 298K, Chem. Phys. Lett., 1998, 296, 350–356 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr and J. Troe, Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement IV. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 1992, 21, 1125–1568 CrossRef CAS.
- M. Yujing and A. Mellouki, Rate constants for the reactions of OH with chlorinated propanes, Phys. Chem. Chem. Phys., 2001, 3, 2614–2617 RSC.
- E. W. Kaiser and T. J. Wallington, Pressure Dependence of the Reaction Cl + C 3 H 6, J. Phys. Chem., 1996, 100, 9788–9793 CrossRef CAS.
- S.-B. Xing, S.-H. Shi and L.-X. Qiu, Kinetics studies of reactions of OH radicals with four haloethanes part I. Experiment and BEBO calculation, Int. J. Chem. Kinet., 1992, 24, 1–10 CrossRef CAS.
- N. Cohen and K. R. Westberg, Chemical Kinetic Data Sheets for High-Temperature Reactions. Part II, J. Phys. Chem. Ref. Data, 1991, 20, 1211–1311 CrossRef CAS.
- M. G. Bryukov, I. R. Slagle and V. D. Knyazev, Kinetics of Reactions of Cl Atoms with Ethane, Chloroethane, and 1,1-Dichloroethane, J. Phys. Chem. A, 2003, 107, 6565–6573 CrossRef CAS.
- M. T. Baumgartner, R. A. Taccone, M. A. Teruel and S. I. Lane, Theoretical study of the relative reactivity of chloroethenes with atmospheric oxidants (OH, NO3, O(3P), Cl(2P) and Br(2P)), Phys. Chem. Chem. Phys., 2002, 4, 1028–1032 RSC.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, Temperature-dependent rate coefficients for the reactions of Cl atoms with methyl methacrylate, methyl acrylate and butyl methacrylate at atmospheric pressure, Atmos. Environ., 2009, 43, 5996–6002 CrossRef CAS.
- E. Kwok and R. Atkinson, Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update, Atmos. Environ., 1995, 29, 1685–1695 CrossRef CAS.
- O. W. Wingenter, M. K. Kubo, N. J. Blake, T. W. Smith, D. R. Blake and F. S. Rowland, Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine, and vertical mixing obtained during Lagrangian flights, J. Geophys. Res., 1996, 101, 4331–4340 CrossRef CAS.
- R. Hein, P. J. Crutzen and M. Heimann, An inverse modeling approach to investigate the global atmospheric methane cycle, Global Biogeochem. Cycles, 1997, 11, 43–76 CrossRef CAS.
- F. A. A. M. de Leeuw, Assessment of the atmospheric hazards and risks of new chemicals: Procedures to estimate “hazard potentials”, Chemosphere, 1993, 27, 1313–1328 CrossRef CAS.
- Ø. Hodnebrog, M. Etminan, J. S. Fuglestvedt, G. Marston, G. Myhre, C. J. Nielsen, K. P. Shine and T. J. Wallington, Global warming potentials and radiative efficiencies of halocarbons and related compounds: A comprehensive review: HALOCARBON REVIEW, Rev. Geophys., 2013, 51, 300–378 CrossRef.
- D. Fu, C. D. Boone, P. F. Bernath, K. A. Walker, R. Nassar, G. L. Manney and S. D. McLeod, Global phosgene observations from the Atmospheric Chemistry Experiment (ACE) mission, Geophys. Res. Lett., 2007, 34, L17815 CrossRef.
- J. H. Hu, J. A. Shorter, P. Davidovits, D. R. Worsnop, M. S. Zahniser and C. E. Kolb, Uptake of gas-phase halogenated acetic acid molecules by water surfaces, J. Phys. Chem., 1993, 97, 11037–11042 CrossRef CAS.
- C. S. Zalazar, M. D. Labas, R. J. Brandi and A. E. Cassano, Dichloroacetic acid degradation employing hydrogen peroxide and UV radiation, Chemosphere, 2007, 66, 808–815 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |