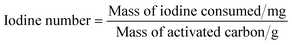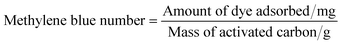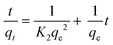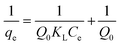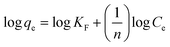Phosphate functionalized activated carbon sachet filters for drinking water purification†
Chamalki
Madhusha
a,
Kavindya
Weerasinghe
a,
Imalka
Munaweera
ab,
Chandani
Perera
c,
Gayan
Wijesinghe
d,
Manjula
Weerasekera
ef,
Yugantha
Idangodage
g,
C. S.
Kalpage
h and
Nilwala
Kottegoda
 *a
*a
aDepartment of Chemistry, Faculty of Applied Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka. E-mail: nilwala@sjp.ac.lk
bInstrument Center, Faculty of Applied Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka
cDepartment of Chemistry, University of Peradeniya, Peradeniya, Sri Lanka
dDepartment of Medical Laboratory Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka
eDepartment of Microbiology, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka
fSri Lanka Institute of Biotechnology (SLIBTEC), Pitipana, Homagama, Sri Lanka
gDepartment of Chemical Process and Engineering, University of Moratuwa, Sri Lanka
hDepartment of Chemical and Process Engineering, Faculty of Engineering, University of Peradeniya, Peradeniya, Sri Lanka
First published on 15th November 2022
Abstract
This study reports the synthesis and characterization of a phosphate functionalized porous activated carbon filter material suitable for sachet filters to remove fluoride, hardness and bacterial pathogens in drinking water. Phosphate functionalized activated carbon is prepared by treating waste coconut coir dust with phosphoric acid/water vapor followed by pyrolysis under an inert environment at 500 °C for 1 h. Unlike other reported chemical functionalization methods, the current approach allows controlling the amount of surface functional groups, thus facilitating the maintenance of pH and conductivity of treated water. Morphological studies and BET surface area analysis of phosphoric acid vapor activated carbon (FAC) suggest the presence of a highly porous network structure with pore sizes ranging from the micro to nano-range (2.2 μm–3.23 nm). Fourier transform infrared and X-ray photoelectron spectroscopy and the shifts in the D and G bands in Raman spectroscopy analysis confirm the functionalization of the activated carbon matrix with phosphate and hydroxyl groups. The isoelectric point of the filter material is 5.9. The hardness and fluoride removal efficiencies of 1.0 g of FAC in a sachet for a contact time of 20 min under static conditions are found to be approximately 45–60% and 45–50%, respectively, for natural water samples. Adsorption complies with the Freundlich isotherm model, and the adsorption kinetics is well described by the pseudo-second-order model. The laboratory scale method has been scaled up and the process parameters have been optimized.
Water impactThis study reports the synthesis and characterization of a functionalized porous activated carbon filter material suitable for sachet filters to remove fluoride, hardness and bacterial pathogens in drinking water. Simply, these domestic water filter/sachet bags can be used anytime, anywhere without the need for any high-tech involvement and they could be an alternative to plastic portable water bottles used worldwide, thus minimizing the use of plastics on the planet. |
1. Introduction
An adequate supply of safe drinking water is one of the major prerequisites for a healthy life and access to clean water resources has become a parameter ensuring that human basic needs are met. Poverty and water are closely linked since it is predominantly the poor who suffers from issues related to water. About 1 billion people in the world, mostly in developing countries, have no access to potable water while a further 2.6 billion people lack access to adequate sanitation.1 The world faces challenges in meeting the increasing demand for potable water, as available supplies of freshwater are decreasing due to extended droughts, population growth, more stringent health-based regulations and competing demands from a variety of users.2 According to the predicted global water scarcity, in the 21st century, many countries are entering an era of severe water shortage, increasing competition among agricultural,3–5 industrial and domestic users leading to significant increases in the real cost of water.6 As a result water will become the oil of the 21st century which may even lead to armed conflicts among nations.Currently, there is an increased interest in exploring the potential of advanced technology to solve or ameliorate many of the technical challenges involved in providing potable water to the world population. Most of the attempts focus on ensuring long term water quality, availability and viability. Currently, the main focus of water treatment is establishment of regional level treatment plants, in particular, most of the techniques are focused on removing turbidity, solid matter, and pathogens. The available technologies are mainly flocculation and coagulation, filtration, and disinfection.7 Flocculation and coagulation remove the turbidity in water, which reduces the supporting structure of microorganisms. Filtration removes microorganisms by size exclusion, whereby microorganisms larger than the pore size of the filter will be retained within the system. UV disinfection and chlorine further destroy the microorganisms in the system to a safe level.
Membrane filtration is a mature technology and is one of the most effective drinking water treatment processes. It provides an absolute barrier for microorganisms, retaining them within the water source. However, membrane filters require an external driving force such as electrical pumping. In the past decade, innovative designs and optimization allowed manufacturers to produce off-grid or non-electric driven membrane filter systems.8 Companies such as Wateroam, Icon Lifesaver, and Villagepump integrated a manual pump within the membrane system, making the system grid-independent. Grid-independent water filtration systems are crucial because many places around the world do not have continuous electricity supply. In South Asia, more than 50% of people living in rural areas do not have electricity.9 Biosand filtration (BSF) is a promising point-of-use (POU) treatment process with more than 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people using it worldwide.10 It can be easily constructed using raw materials that are locally sourced. The concept of BSF is similar to that of a conventional slow sand filter. However, BSF experiences varying flow rates and intermittent filtration through the sand layer. The outlet of the POU is located higher than the sand layer, allowing the sand layer to be saturated with water throughout the operation. This allows for microbial growth, developing biofilms around the filter media and sand particles.
000 people using it worldwide.10 It can be easily constructed using raw materials that are locally sourced. The concept of BSF is similar to that of a conventional slow sand filter. However, BSF experiences varying flow rates and intermittent filtration through the sand layer. The outlet of the POU is located higher than the sand layer, allowing the sand layer to be saturated with water throughout the operation. This allows for microbial growth, developing biofilms around the filter media and sand particles.
However, these techniques do not offer an efficient platform to remove fluoride, hardness and heavy metals, simultaneously. Over 70% of the water is inaccessible due to the presence of these ions and the most effective practically used technique to remove all these ions is reverse osmosis (RO). However, due to the complexity of the RO system, requirement for stable electricity supply, and maintenance, RO systems are not commonly employed in rural areas. In addition, the use of RO also increases the operation cost of the water treatment system.11
Functionalized activated carbon has been used as a water filtering medium for purification of drinking water for many years. It is widely used for the removal of contaminants in water due to its high capacity for adsorption of such compounds, arising from its large surface area and porosity. Generally, activated carbon can remove the total suspended solids (TSS) and the biological oxygen demand (BOD) effectively over 99%, to 1 mg l−1 and can also improve the tastes and odors of the drinking water.12 This is the reason for the use of activated carbon as a major filter medium in most of the home water filtration systems. However, activated carbon does not effectively remove water hardness, fluoride and pathogens.
The present study reveals a sachet filter based on phosphate-functionalized activated carbon derived from coconut coir dust. The requirement for such portable and convenient water purification systems particularly exists in remote/underprivileged areas where potable water supply is not readily available. Similarly, portable water systems are often required in the aftermath of disasters such as floods and droughts where the local water supply has been contaminated or disrupted. In addition, portable water availability is practically needed for field workers, school children, aerospace activities, recreational activities such as camping and hiking and any other activities which last for extended periods. Simply, this domestic water filter/sachet bag can be used anytime, anywhere without the need for any high-tech involvement and it could be an alternative to plastic portable water bottles used worldwide, thus minimizing the use of plastics on the planet.
2. Materials and methods
2.1 Materials
All chemicals and reagents used in this research were of analytical grade and were purchased from Sigma Aldrich, USA, and used as received. Raw coconut coir dust was obtained from a coconut farm in Sri Lanka. All sample preparation and experiments were carried out using deionized water.2.2 Methods
Pyrolyzed coconut coir dust without acid functionalization (AC) was prepared under the same conditions as a control material.
Iodine number. The iodine number is defined as the amount of iodine adsorbed (mg) by 1 g of carbon. It is a measure of the micropore (0–20 Å) content of the activated carbon by adsorption of iodine from solution. The iodine number was determined according to Saka et al.13 and calculated using the following equation,
Methylene blue number. The methylene blue number is defined as the maximum amount of dye adsorbed (mg) on 1.0 g of adsorbent. In this assay, 10.0 mg of activated carbon was placed in contact with 10.0 mL of a methylene blue solution (1000 mg L−1) for 24 h at 25 °C. The remaining concentration of methylene blue was analyzed using a UV/vis spectrophotometer at 645 nm.14 The amount of methylene blue adsorbed from each solution was calculated by the following equation,
Determination of phosphate functional groups present in FAC. The Boehm approach was used to identify the relevant surface functional groups.15 Thus, under a nitrogen (N2, 99.999%) atmosphere, 0.5 g of FAC was added to 50 mL of 0.05 N NaOH, sodium bicarbonate (NaHCO3, 99.9%), sodium carbonate (Na2CO3, 99.9%), or hydrochloric acid (HCl). The heterogeneous mixture was agitated (200 rpm) in a shaker at 25 ± 1 °C for 24 hours. Following that, the solution was vacuum filtered, and an aliquot was back titrated with standard 0.1 N NaOH or HCl solutions to determine the density of acidic and basic groups, respectively. By this approach, the acid functional groups available on the surface of the activated carbon samples were determined.15
2.3 Morphological and structural characterization of FAC
The surface morphology of the products was studied using a scanning electron microscope (SEM) of model Hitachi SU 6600 using the secondary electron mode at an accelerating voltage of 20 kV. The atomic arrangement of the activated carbon materials was observed through a transmission electron microscope (TEM), JEOL JEM-2100, operating at 200 kV. Powder X-ray diffraction (PXRD) patterns were recorded using a Rigaku Ultima IV X-ray diffractometer (Japan) equipped with a scintillation counter detector. The diffractometer was operated with Cu Kα (1.141 Å) radiation in continuous mode at 40 kV and 30 mA, and the PXRD patterns were recorded over a 2θ range of 4° to 70° with a scan speed of 2 deg min−1 and a step width of 0.02°. PDXL2 software was used for the analysis. All the Fourier transform infrared (FTIR) spectra were recorded on a Bruker Vertex 80 instrument coupled with a Ram-FT module (RAM II) FTIR spectrophotometer in a range from 400 to 4000 cm−1 using 100 scans at 4 cm−1 resolution. A Thermo Scientific DXR2 Smart Raman spectrometer was used for the Raman analysis. Adsorption experiments were carried out using an atomic absorption spectrophotometer (AAS) of model Thermo Scientific iCE 3000 series AAS in the flame mode. The surface area measurements were obtained from an automated gas adsorption analyzer, AUTOSORB-1 (QuantaChrome Instruments, USA), with adsorption–desorption isotherms of nitrogen at −196 °C. For each analysis, 0.2 g of sample was used. The samples were degassed at 150 °C under nitrogen for 6 h. The specific surface area of the sample was calculated by the BET (Brunauer, Emmett, and Teller) method while the pore volume was directly calculated from the volume of nitrogen held at the highest relative pressure (P/Po = 0.99). An X-ray photoelectron spectroscopic (XPS) analysis was conducted using a Thermo Scientific TM ESCALAB Xi+ instrument. A monochromatic X-ray beam generated from an Al Kα (1486.6 eV) source was used as the X-ray source. All the samples were tested after incubating them inside an argon purged desiccator. An ESCALAB 250Xi spectrophotometer (Thermo Fisher, USA) was used to carry out the XPS analysis. The background pressure of the analysis chamber was 2 × 10−9 mbar, whereas the sample insertion chamber pressure was 1 × 10−7 mbar in the standby mode. A flood gun was used to correct the charging effect on the surface. The background pressure of the analysis chamber was recorded to be 5 × 10−8 mbar in the presence of a flood gun. The charge comp standard mode was selected considering that the samples are non-magnetic. Al Kα radiation was used with a spot size of 900 μm with monitored beam values of 14.4 kV (anode HT) and 18.08 mA (beam current). All XPS measurements were performed in constant analyzer energy (CAE) mode. Here, an ion energy of 2000 eV with mid-range current has been used for 30 s to clean the surface. XPS test parameters for survey and narrow scans are given in Table S1.†Thermo Avantage (version-5.982) software was used to process the obtained spectra. The spectra were corrected for charge compensation effects by offsetting the binding energy relative to the intrinsic aliphatic component of the C1s spectrum to 284.8 eV, which is a known carbon status for the activated carbon sample. High-resolution spectra were resolved by fitting each peak with a combined Gaussian/Lorentzian function after subtracting the background (maximum iterations – 100, convergence – 1 × 10−5, fitting algorithm – Powell).
The thermal stability of AC and FAC was investigated via a thermogravimetric analyzer (TA Instruments SDT 650) in the thermogravimetric analyzer temperature range from room temperature to 700 °C under a nitrogen atmosphere at 10 °C min−1 ramp.
2.4 Hardness and fluoride removal capacity
For the adsorption experiments, natural water samples from North Central province in Sri Lanka (Ipalogama, Galkulama, Mahailluppallama, Kiribathwehera, Galpalama, Alapathwewa, Thuruwila, Kahatagasdigiliya and Hadagaswewa) were collected and were used for the measurement of hardness and fluoride removal levels using the synthesized activated carbon. The atomic absorption spectroscopic method and fluoride ion selective electrode method were used to determine the hardness and fluoride levels in natural water samples, respectively, according to the ASTM D511-14 and ASTM D1179-16.16 A HACH 40 DQ pH meter was used for measuring the pH of the water samples. All the experiments were repeated three times and the results are reproducible.2.5 Testing of phosphate and carbon leaching into water from FAC sachet filters
In order to check whether there was any phosphate leaching to water samples, the molybdenum blue colorimetric method was used in which a blue phosphomolybdate complex is formed. The absorbance of the samples was then measured again at 885 nm and the P concentration was calculated.18 Furthermore, leaching of phosphates in basic (pH = 9) and acidic water (pH = 3) samples and natural water samples (pH = 6.5–7.5) was also tested.The carbon particle leaching into water was tested by examining the water samples (after treating with FAC sachet filters) under a high resolution light microscope at different time intervals, up to 24 h.
2.6 Point of zero charge
The point of zero charge (pHpzc) of the adsorbents was determined by the solid addition method.19 A volume of 25 mL of 0.01 N NaCl solution was transferred into a series of conical flasks. The pH value of the solutions was adjusted in the range between 2 and 12 using 0.01 N HCl and 0.01 N NaOH. Then, 0.1 g of adsorbent was added to each flask and placed in a constant temperature water bath shaker. After keeping for 24 h to reach equilibrium under continuous shaking, the solutions were filtered, and their respective final pH values were obtained. The point where pHinitial = pHfinal was considered as the pHpzc of activated carbon.192.7 Adsorption kinetics
The experimental data obtained for the adsorption of calcium, magnesium and fluoride onto FAC were fitted to pseudo-first order (PFO) and pseudo second order (PSO) kinetic models to study the dynamics of the adsorption process.The linear form of the PFO model is given by the following equation,
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t |
The linear form of the PSO model is expressed by the following equation,
2.8 Isothermal studies
The equilibrium data obtained for the adsorption of calcium, magnesium and fluoride onto FAC were fitted into two different isotherm models namely the Langmuir and Freundlich models.The linear form of the Langmuir isotherm equation is given as follows,
The logarithmic form of the Freundlich isotherm is given by the following equation,
3. Results and discussion
The objective of this study was to synthesize functionalized activated carbon using a vapor based method which could be used as a filter material in sachet water filters (Fig. 1). Sachet water filters have not been reported before and here we disclose the use of phosphate functionalized activated carbon for removal of fluoride, hardness and water pathogens. The adsorption properties of activated carbon depend significantly on the chemical composition of the final product, especially, the presence of functional groups on the surface. Therefore, the nature of activated carbon depends greatly on the raw materials used, the functionalization and the activation process.24 The vapor based functionalization method reported here allows controlling the amount of functional groups present on the surface and also controlling the pH and conductivity of the treated water. Consequently, the effects of the pyrolysis temperature and activation time are of major concern in determining the optimum conditions of the activation process. Vapor phase functionalization is carried out using a mixture of water and phosphoric acid (50% v/v) above the boiling point of phosphoric acid. According to the vapor phase diagram of phosphoric acid reported by Brown et al., the temperature of the acid solution increases, while the vapor evolved will contain increasing amounts of P2O5.25 At 350 °C temperature, the P2O5 concentration in the vapor phase is 38.29%. These P2O5 molecules react with both aliphatic and aromatic hydroxyl groups in the lignocellulosic material.| P2O5(l) + H2O(l) ↔ 2H3PO4(l) |
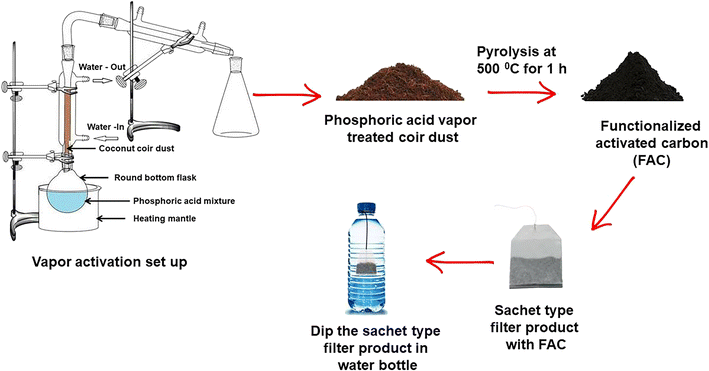 | ||
| Fig. 1 Schematic representation of the synthesis procedure of FAC and its application on water purification. | ||
| Type of activated carbon material | Iodine number (mg g−1) | Methylene blue number (mg g−1) | ||||
|---|---|---|---|---|---|---|
| 300 °C | 500 °C | 700 °C | 300 °C | 500 °C | 700 °C | |
| AC | 501 ± 2 | 572 ± 1 | 588 ± 1 | 138 ± 2 | 197 ± 1 | 199 ± 1 |
| FAC | 915 ± 1 | 918 ± 1 | 900 ± 2 | 154 ± 1 | 230 ± 2 | 212 ± 2 |
Based on the above results, further investigations of adsorption characteristics were carried out using activated carbon by selecting FAC obtained after 1 h vapor treatment and pyrolyzed at 500 °C.
The morphological and structural characteristics of FAC were studied using SEM, TEM, PXRD, Raman spectroscopy, FT-IR and XPS.
The micrograph of AC without acid treatment (Fig. 2(A)) shows that the surface is heterogeneous and porous forming a network structure. Functionalization with phosphoric acid catalyzes the oxidation and bond cleavage reactions of the interior structure of coir dust where most of the organic volatiles are removed creating an abundance of pores with different sizes. This is clearly demonstrated by the development of the well-defined porous structure with tunnel-shaped pores of different sizes as shown in the SEM image of FAC (Fig. 2(B)). Such observations were reported by Xu et al., 2014, and Oginni et al., 2019 for activated carbon prepared from reedy grass leaves by chemical activation with H3PO4 and activated carbon from Kanlow switchgrass and public miscanthus by two-step H3PO4 activation, respectively.27,28 The inner structure of activated carbon with and without acid functionalization is viewed by TEM. Fig. 2(C) reveals a layered like carbon morphology probably due to the formation of micropores in activated carbon. The carbon frame/pore walls and pores of activated carbon are visualized as dark and light areas, respectively, in the TEM image.29,30 Light shading from the TEM results indicates the development of large amounts of pores after phosphoric acid functionalization, in comparison with the virgin AC. It also confirms the defective nature of graphene layers indicating the non-planarity and microporosity.31
 | ||
| Fig. 2 SEM images of (A) AC and (B) FAC at 1000× magnification, and TEM images of (C) AC and (D) FAC. | ||
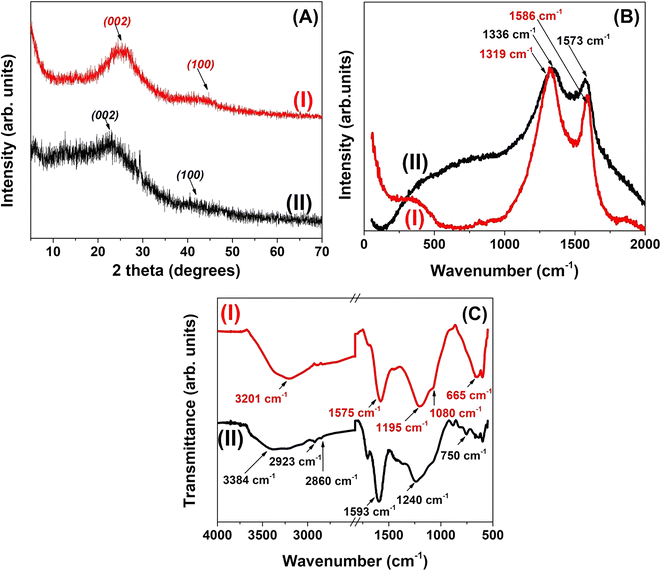
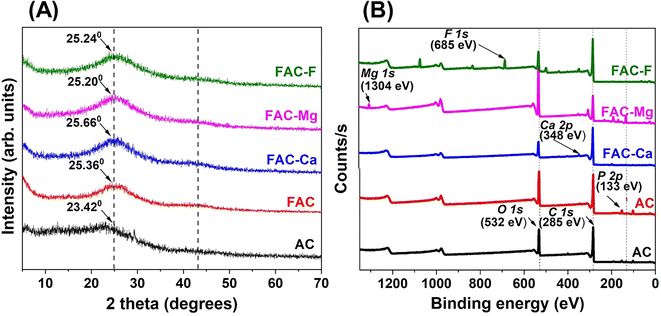
The change of crystallinity or extent of graphitization of AC and FAC is revealed by the PXRD characterization. As depicted in Fig. 3(A), two typical diffraction peaks of activated carbon appear at 2θ = 23.04° and 2θ = 41.44° for AC, corresponding to the (002) and (100) planes, respectively, which is in good agreement with literature data.32,33 According to Xu and coworkers, the strong and broad diffraction peak indicates the presence of several disordered graphitic crystals whereas the weak peak (100 plane) reflects the partial graphitization of activated carbon and the random turbostratic stacking of layers.27,34 Functionalization of activated carbon with phosphate groups has resulted in PXRD patterns with relatively increased 2θ values (25.66° and 43.94°) compared to the AC. Xie et al. suggested that the degree of graphitization can be related to the interplanar spacing d002 of carbon materials so that the degree of graphitization becomes higher with lowering the value of the diffraction angle.35 Therefore, functionalization of the carbon network has led to a higher degree of disorder.
Raman spectroscopy reveals the information on the structure and the bonding types of different carbon based materials. The Raman spectra of AC and FAC are shown in Fig. 3(B). Both spectra are dominated by the two intense bands, D and G, which are the two signature bands for typical graphitic carbon materials.36 The G band corresponds to the stretching vibrations of sp2 carbon pairs in the chain and the ring structures, whereas the D band is attributed to the vibrations of the breathing mode in the aromatic carbon ring.37 As shown in Fig. 3(B), the presence of D and G bands clearly demonstrates the heterogeneous microstructure of the activated carbon material. Accordingly, the D band is observed at 1336 cm−1 and 1319 cm−1 for AC and FAC, respectively, while the G band appears at 1573 cm−1 and 1586 cm−1 for AC and FAC, respectively. Since the D mode becomes active only in the presence of disorder, it confirms the disordered graphitic structure of both AC and FAC.38 Moreover, there is a blue shift in the G band of FAC compared to AC (1573 to 1586 cm−1) which is probably due to merging of the G peak with a second peak D′ (around 1620 cm−1) resulting in a net increase of the G band position. According to Elcey and Manoj, such peaks around 1620 cm−1 become well resolved as the extent of disorder in the crystal lattice increases.39 In addition, the blue shift is attributed to the consequences of the electron transfer between carbon and phosphate groups inducing strong modifications to the carbon material.40 On the other hand, the red shift in the D band of FAC compared to AC (from 1336 to 1319 cm−1) also demonstrates the structural changes to the aromatic carbon ring with the introduction of phosphate groups. The Raman spectrum of FAC consists of an additional broad band in the region of 300–500 cm−1 which is less broadened. According to Gupta and co-workers, such peaks are assigned to PO43− in a triply degenerate bending mode.41 However, observations by Sun et al. suggested that the region around 300–500 cm−1 indicates the presence of phosphorus bonds and probably P–C bonds in the activated carbon.42 The relative ratio of intensities of D and G bands (ID/IG) represents the degree of structural disorder43 or inversely, the degree of graphitization where a lower ID/IG ratio indicates a higher degree of graphitization.32 Therefore, the graphitization degree has decreased in FAC (ID/IG = 0.82) compared to AC (ID/IG = 0.84) attributed to the effect of phosphoric acid functionalization on the carbon material, thus resulting in increased defects on the graphitic structure.28
FTIR spectra (Fig. 3(C)) were obtained to further characterize the surface groups as they provide information about the chemical structure of activated carbon. Assigning of functional groups is mentioned in Table S2.† The broad absorption band at 3600–3200 cm−1 is characteristic of the stretching vibration of hydrogen bonded –OH groups. AC shows low intensity bands at 2923 and 2860 cm−1 corresponding to stretching vibrations of aliphatic C–H, which have disappeared in the spectrum of FAC. This indicates the increased aromaticity of activated carbon upon functionalization. The low intense band around 1700 cm−1 in AC is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of ketones, aldehydes, lactones or carboxyl groups. The weak intensity of this peak in FAC suggests that phosphate functionalized carbon contains only a small amount of carboxyl groups.44,45 The spectrum of AC also shows a strong band at 1600–1580 cm−1 attributed to C
O stretching vibrations of ketones, aldehydes, lactones or carboxyl groups. The weak intensity of this peak in FAC suggests that phosphate functionalized carbon contains only a small amount of carboxyl groups.44,45 The spectrum of AC also shows a strong band at 1600–1580 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations in aromatic rings. The red shift in FAC (from 1593 to 1575 cm−1) suggests an enlargement of the aromatic ring system after acid activation.44 The broad band at around 1240 cm−1 in AC is assigned to the C–O asymmetric stretching of aromatic ethers, esters and phenols.46 Interestingly, this band has shifted from 1240 to 1195 cm−1 in FAC due to the overlapping of the C–O asymmetric stretching band with the newly appeared band of stretching vibrations of hydrogen bonded P
C vibrations in aromatic rings. The red shift in FAC (from 1593 to 1575 cm−1) suggests an enlargement of the aromatic ring system after acid activation.44 The broad band at around 1240 cm−1 in AC is assigned to the C–O asymmetric stretching of aromatic ethers, esters and phenols.46 Interestingly, this band has shifted from 1240 to 1195 cm−1 in FAC due to the overlapping of the C–O asymmetric stretching band with the newly appeared band of stretching vibrations of hydrogen bonded P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups from phosphates or polyphosphates, O–C stretching vibration in the P–O–C (aromatic) linkage and P
O groups from phosphates or polyphosphates, O–C stretching vibration in the P–O–C (aromatic) linkage and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OOH.47 Furthermore, the presence of the weak and overlapped band located at 1080 cm−1 in FAC is caused by the stretching vibrations of P–O–P in polyphosphates or the ionized linkage P+–O− in acid phosphate esters.48 Even though the assignment of peaks in this region is difficult due to overlapped absorption bands, similar observations have been reported in previous research work.44,49 Thus, this confirms the existence of chemically bonded phosphorus containing groups on activated carbon after impregnating with phosphoric acid, successfully supporting the previously explained Raman spectral data. In addition, some weak bands also appeared in AC in the range of 600–900 cm−1 associated with the out of plane bending mode of the C–H or O–H groups.49 The disappearance of the C–H band in FAC at 750 cm−1 suggests the substitution of C–H bonds in the aromatic system to form new C–R bonds.44 This observation is in line with the strong band around 665 cm−1 probably due to increased aromatic ring vibrations.17 Although, according to Puziy et al., the C–P bond also falls into the region of 670 cm−1, it is not confirmed by the other characterization data obtained in the present study.50 This is further supported by the observations by Lee and Radovic showing that the structures with C–O–P bonding are more stable than the structures with C–P–O bonding.51
OOH.47 Furthermore, the presence of the weak and overlapped band located at 1080 cm−1 in FAC is caused by the stretching vibrations of P–O–P in polyphosphates or the ionized linkage P+–O− in acid phosphate esters.48 Even though the assignment of peaks in this region is difficult due to overlapped absorption bands, similar observations have been reported in previous research work.44,49 Thus, this confirms the existence of chemically bonded phosphorus containing groups on activated carbon after impregnating with phosphoric acid, successfully supporting the previously explained Raman spectral data. In addition, some weak bands also appeared in AC in the range of 600–900 cm−1 associated with the out of plane bending mode of the C–H or O–H groups.49 The disappearance of the C–H band in FAC at 750 cm−1 suggests the substitution of C–H bonds in the aromatic system to form new C–R bonds.44 This observation is in line with the strong band around 665 cm−1 probably due to increased aromatic ring vibrations.17 Although, according to Puziy et al., the C–P bond also falls into the region of 670 cm−1, it is not confirmed by the other characterization data obtained in the present study.50 This is further supported by the observations by Lee and Radovic showing that the structures with C–O–P bonding are more stable than the structures with C–P–O bonding.51
XPS is used to analyze the surface functional groups of the activated carbon and phosphoric acid functionalized activated carbon. The survey scan spectra of AC and FAC are shown in Fig. S2.† They indicate the presence of C 1s, O 1s and P 2p in FAC at the peak positions of approximately 285 eV, 532 eV and 133 eV, respectively, thus confirming the carbon, oxygen and phosphorus containing functional groups in synthesized phosphoric acid activated carbon.
The high resolution XPS spectra of C 1s excitation for both AC and FAC showed a complex envelope exhibiting several carbon species at the carbon surface (Fig. 4(A) and (B)). The most intense band of AC, located at around 286.25 eV, is assigned to carbon species in alcohol, phenol, and ether groups, and C–O–P and/or C–O–C linkages and the peak at 284.75 eV is attributed to graphitic sp2 carbon.43 These peaks have shifted to higher binding energies of 286.45 eV and 284.8 eV, respectively, after phosphoric acid functionalization (Table S3†). Such shifts to higher binding energies arise due to the interaction of phosphorus species with the graphitic carbon matrix. The peak at 288.3 eV is usually assigned to carbon species in carboxylic groups or esters. More interestingly, a shake-up satellite due to π–π* transitions in aromatic rings (291.4 eV) has appeared on the carbon surface of FAC.52 In addition, the contribution of the 286.45 eV peak is considerably higher than the 288.3 eV peak, reflecting the significant presence of C–O–P and/or C–O–C bonds.27 However, according to the literature, phosphorus compounds cannot be clearly distinguished from the C 1s spectral region because the binding energy of C–O–P bonding is similar to the binding energy in alcohol and ether groups. Moreover, the C 1s electron binding energy of phosphonates (compounds with direct C–P bonding) lies between graphitic and oxygenated carbon.50
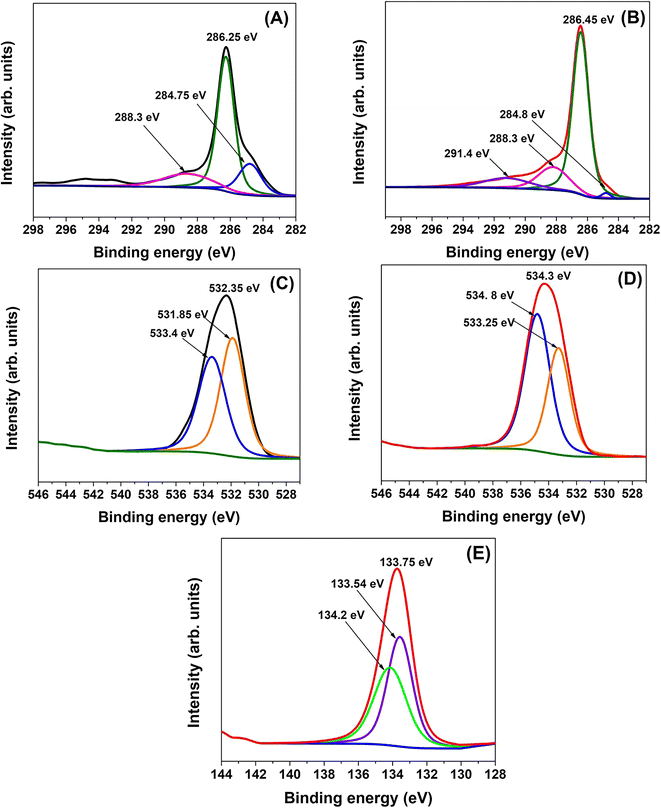 | ||
| Fig. 4 High-resolution XPS spectra of (A) C 1s of AC, (B) C 1s of FAC, (C) O 1s of AC, (D) O 1s of FAC and (E) P 2p of FAC. | ||
The O 1s spectra of AC and FAC give further evidence to support the conclusion regarding the changes in carbon surface composition. Accordingly, the O 1s spectra of both AC and FAC are shown in Fig. 4(C) and (D), indicating broad peaks as a result of different chemical states of oxygen present. In FAC, the peak at 533.25 eV is attributed to singly bonded oxygen in the C–OH, C–O–C and/or C–O–P groups. The peak at 534.8 eV is ascribed to non-carbonyl oxygen in carboxylic groups. These bands are positioned at higher binding energies compared to AC implying changes in electron density around oxygen atoms due to interaction of phosphorus with the surface functional groups.50
Fig. 4(E) shows the high-resolution spectrum of P 2p in FAC. The major peak at 133.54 eV is attributed to the formation of phosphates, pyrophosphates and phosphonates, while the peak at 134.2 eV is assigned to metaphosphates and phenyl-phosphate.53 The P 2p peak was fitted with the two components P1/2 and P3/2, corresponding to spin–orbit coupling. The P 2p peak of P2O5 has been reported to have a binding energy of 133.54 eV, while unoxidized P has a binding energy of 134.2 eV. The peaks were fitted with a spin–orbit coupling of 0.66 eV. It can be suggested that the interaction of phosphoric acid with the surface phenolic and carbonyl groups may be the reason for the formation of P-containing carbonaceous species. Thus, the high resolution spectrum of P 2p proves the impregnation of the carbon precursor by phosphoric acid supporting the FT-IR spectra results and successful chemical activation of coir dust.
Furthermore, the profiles of thermogravimetric analysis of AC and FAC are shown and explained in Fig. S3.† Three stages of weight loss have taken place in both activated carbon materials. The thermal stability of FAC is high up to 450 °C compared to that of AC.
Hardness and fluoride removal efficiency of FAC and AC in sachet filters
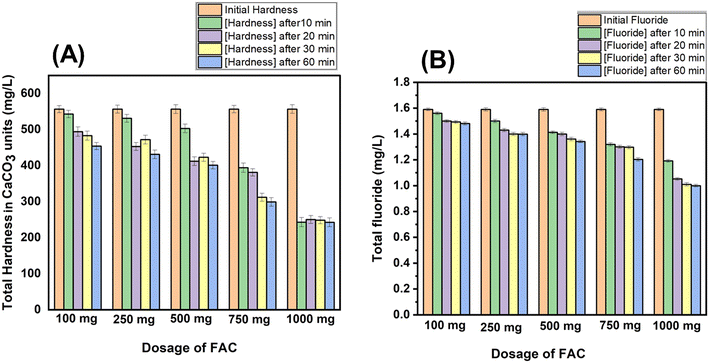
The presence of carboxyl and hydroxyl groups on the surface of the activated carbon influences the adsorption characteristics of the material and the location and number of functional groups on the pore structure of activated carbon play a significant role in the adsorption process.28 Most importantly, studies have shown that the phosphorus compounds impregnated on the surface of activated carbon are responsible for enhanced cation exchange properties in addition to their role in developing porosity of carbon materials during the synthesis process.50 In a similar manner, activated carbon possesses the ability to adsorb anions such as fluoride ions depending on the surface chemistry of activated carbon along with its well-developed porosity, in particular, the presence of OH and phosphate groups leads to such favorable interactions with fluoride.Therefore, in the preliminary step, the optimization of the FAC dosage and contact time period of FAC sachet filters with water samples was carried out. According to Fig. 5(A) and (B), when the dosage of FAC was increased, the hardness and fluoride removal was increased. This can be explained by the availability of adsorption sites. When there is a higher amount of adsorbent, there are more adsorption sites for cations/anions. In this regard, the optimized dosage was 1000 mg for 250 ml of the water sample. The time optimization was carried out for both hardness and fluoride removal and 20–60 min showed better removal results. Here we are going to use FAC sachet filters; therefore it should have a minimum contact time. As longer contact times are not practical, we concluded that the optimized contact time is 20 min.
Natural water samples collected from different areas in Sri Lanka were used in adsorption experiments after testing for their initial pH, hardness and fluoride levels. In the first step, the hardness and fluoride removal efficiency in the water samples of the FAC loaded sachet filters was determined and compared with that of AC which was synthesized without acid activation. According to the results (Table 2), FAC demonstrates better hardness and fluoride removal efficiency than the AC material for all the samples tested. Therefore, it can be concluded that FAC has a higher adsorption capacity than AC, with the potential to be used in water softening applications and reduction of fluoride levels.
| Selected sample location | Latitudes and longitudes | Initial pH (±0.01) | Final pH (±0.01) | Hardness removal (mg L−1) (±0.01) | Fluoride removal (mg L−1) (±0.001) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final (AC treated) | Final (FAC treated) | Initial | Final (AC treated) | Final (FAC treated) | ||||
| Ipalogama | 8°06′03.4′′N 80°30′35.0′′E | 7.56 | 7.52 | 413.50 | 305.34 | 170.80 | 0.598 | 0.442 | 0.305 |
| Galkulama | 8°16′18.5′′N 80°29′54.8′′E | 7.88 | 7.93 | 446.22 | 367.43 | 209.96 | 2.430 | 1.921 | 1.584 |
| Mahailluppallama | 8°06′10.5′′N 80°28′19.0′′E | 7.59 | 7.63 | 556.59 | 417.01 | 250.23 | 1.590 | 1.398 | 1.052 |
| Kiribathwehera | 8°23′57.1′′N 80°23′59.6′′E | 7.69 | 7.60 | 327.87 | 285.32 | 142.23 | 0.808 | 0.665 | 0.419 |
| Galpalama | 8°23′56.4′′N 80°24′21.6′′E | 6.91 | 6.84 | 485.13 | 401.50 | 313.82 | 1.710 | 1.691 | 1.512 |
| Alapathwewa | 8°23′32.8′′N 80°48′14.0′′E | 7.24 | 7.21 | 338.82 | 302.87 | 251.69 | 1.010 | 0.981 | 0.530 |
| Hadagaswewa | 8°29′42.1′′N 80°11′19.9′′E | 7.55 | 7.58 | 295.97 | 223.45 | 132.64 | 1.390 | 1.276 | 1.010 |
| Thuruwila | 8°13′27.1′′N 80°26′21.0′′E | 8.39 | 8.32 | 579.33 | 442.21 | 258.62 | 1.312 | 1.213 | 0.650 |
| Kahatagasdigiliya | 8°35′12.4′′N 80°41′10.4′′E | 8.27 | 8.24 | 253.83 | 242.40 | 136.56 | 1.480 | 1.282 | 0.821 |
Additionally, a statistical analysis was carried out using ANOVA to evaluate the performance of FAC in the present study and activated carbon synthesized by the phosphoric acid soaking method (liquid phase) in the previous study done by Hettiarachchi et al., 2016.54 All the experiments were conducted under similar conditions and detailed results obtained for hardness and fluoride removal using activated carbon synthesized by the phosphoric acid soaking method are shown in Table S4.† The data reveal a statistically significant correlation between the two types of activated carbon materials for hardness removal (p < 0.05) and a significant difference for fluoride removal (p < 0.05). This indicates that the adsorption behavior of both types of activated carbon materials is similar irrespective of the synthesis method, in terms of hardness removal. However, there is a significant difference in fluoride removal. Overall, the phosphoric acid vapor functionalization method can be suggested as a potential economically suitable procedure which uses a lower amount of phosphoric acid, fewer washing steps, and easy maintenance of water pH and conductivity reaching the ASTM standards and being more towards a greener process.
When FAC sachets are used, after 20 minutes of contact time, a hardness removal percentage of 45–60% was achieved for several samples while a fluoride removal percentage of 45–50% was achieved except for certain higher initial fluoride levels (Table 2). These values are well within the WHO recommended levels of hardness and fluoride in drinking water.55 The use of handy sachet bags containing FAC would be useful for consumers in need of clean water as they are convenient and small in size. Rural areas lacking water treatment facilities may benefit from the use of these water treatment sachets containing FAC to remove excess hardness and fluoride levels in their drinking water. On the other hand, there were not any carbon particles leaching into the water samples confirming the suitability of FAC sachet filters in purification of drinking water (Fig. S4†). The percentage of acidic phosphate functional group concentration was 23.3% which was determined by the Boehm method. It was found that 0.001 ± 0.001 mg L−1 phosphate ions leached into water after 24 h at neutral pH values (pH 6.5–7). It was below the threshold value (0.1 mg L−1) of recommended phosphate ions in drinking water. Under acidic (pH = 3) conditions, phosphate leaching into water was 0.023 ± 0.001 mg L−1 compared to neutral pH values. At basic pH values (pH = 9), phosphate leaching into water was 0.012 ± 0.001 mg L−1. In this work, the recommended time period for FAC sachet filters is 20 min. During this 20 min, phosphate leaching was not observed at basic and neutral pH values while at highly acidic pH values, 0.001 ± 0.01 mg phosphate leaching was observed. In this regard, FAC proved its suitability to be used in drinking water purification at acidic, basic or neutral pH values. A pre-packed sachet technique using a controlled dose would facilitate maintaining an exact amount of adsorbent for treatment. An appropriate dose of adsorbent in multiple sachets could be used if necessary. Thus, FAC in sachet filters would be ideal in delivering the activated carbon material into water for the adsorption of cations and anions present in water.
Hardness and fluoride removal mechanism
The pHpzc value determines the pH at which the charge of an adsorbent surface becomes zero. It is a fundamental factor indicating the adsorption ability and thus the adsorption mechanism of an adsorbent. The adsorbent surface is predominantly net positively charged at solution pH < pHpzc as the functional groups are protonated and predominantly net negatively charged at solution pH > pHpzc as the groups are deprotonated.56 Hence, at higher solution pH, adsorption of cations onto the surface increases whereas at lower solution pH, anions adsorb onto the surface increasingly.57According to the experimental results, the pHpzc value obtained for FAC is 5.9 ± 0.1 while that for AC is 7.4 ± 0.1 (Fig. 6). As the pH values of the tested water samples (Table 2) are higher than the pHpzc of phosphoric acid functionalized activated carbon, FAC possesses a net negative charge on its surface under the conditions adopted in the adsorption experiments. Thus, this favors the higher adsorption ability of FAC towards the cations by electrostatic interactions compared to AC. In addition, the presence of oxygen and phosphorus containing functional groups further facilitates the adsorption of cations into the large number of pores present in FAC.54
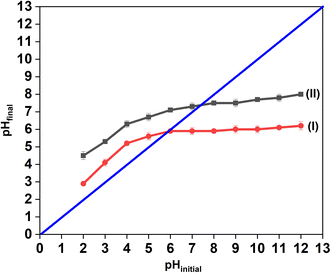 | ||
| Fig. 6 Determination of the point of zero charge for activated carbon materials (I) FAC and (II) AC. | ||
However, the fluoride removal may become hindered in solutions with higher pH due to the formation of aqua complexes followed by the adsorbent surface becoming net negatively charged.58 Therefore, anions like fluoride will have electrostatic repulsive forces with the activated carbon surface. Furthermore, there would be a competition between hydroxide and fluoride ions for the adsorption onto the activated carbon surface. But some positively charged sites due to the presence of protonated groups may still be available at the pH values of the tested water samples.56 Therefore, the adsorption of fluoride under these conditions into FAC is likely related to the lesser extent of electrostatic interaction, hydrogen bonding and anion exchange. A similar explanation has been given by Araga et al., 2017 supporting such interactions.59 In addition, the highly porous nature of FAC facilitates the trapping and adsorption of fluoride ions into the pores present.
More interestingly, Souza et al., 2014 have shown and supported another hypothesis describing the synergistic effect of Ca2+ ions on adsorption of negatively charged ions onto the activated carbon surface.56 It is proposed that the interaction of Ca2+ ions with acid groups available on the activated carbon (especially carboxyl and phenol groups) generates an excess of positive charges on the surface of the adsorbent, decreasing the overall negative charge of carbon particles and, therefore, creating favorable sites for adsorption of the negatively charged species, even when the net surface charge is negative. A similar model could be applied to fluoride adsorption in natural water samples analyzed in this study where the solution pH exceeds the pHpzc of FAC. However, further experimental data would be necessary to confirm such mechanisms.
Characterization of FAC after adsorption experiments
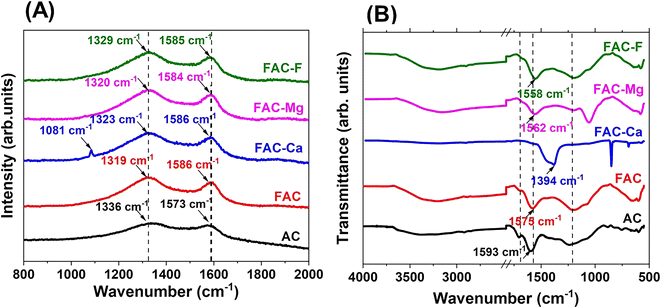
As shown in Fig. 7(A), a blue shift has occurred in the D band of FAC after the adsorption of calcium, magnesium and fluoride ions. This clearly demonstrates the structural changes in the graphitic system as a result of the adsorption mechanism where the intrinsic D band moves to higher frequencies. In contrast, the G band shows a red shift (except for FAC-Ca) after the adsorption process. However, only the FAC-Ca spectrum shows a 1081 cm−1 peak which corresponds to the asymmetric P–O stretching.60After calcium, magnesium and fluoride adsorption, the FTIR bands of the functional groups in FAC have been shifted, especially for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations, P
C vibrations, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups from phosphates or polyphosphates, O–C stretching vibration in the P–O–C (aromatic) linkage and P
O groups from phosphates or polyphosphates, O–C stretching vibration in the P–O–C (aromatic) linkage and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OOH (Fig. 7(B)). Probably, these functional groups provide the active sites for adsorption of ions. The shifts in the functional group bands suggest the successful adsorption of adsorbents on the active sites of FAC.
OOH (Fig. 7(B)). Probably, these functional groups provide the active sites for adsorption of ions. The shifts in the functional group bands suggest the successful adsorption of adsorbents on the active sites of FAC.
Fig. 8(A) depicts the PXRD analysis of FAC after adsorption experiments. The 2θ values of the two major diffraction peaks of activated carbon have decreased after calcium, magnesium and fluoride ion adsorption. The degree of graphitization has also increased slightly, probably due to structural changes in activated carbon after the adsorption process and this phenomenon is consistent with the Raman spectral analysis of the carbon materials.
According to XPS analysis, calcium, magnesium and fluoride ion adsorption has led to the emergence of new peaks related to Ca 2p, Mg 1s and F 1s, respectively, along with shifts in binding energies of existing peaks relevant to C 1s, O 1s and P 2p in FAC (Fig. 8(B), Tables S5 and S6†) indicating the adsorption behavior of activated carbon. The spectrum of FAC-Ca exhibits two peaks at 351.3 eV and 347.65 eV assigned to Ca 2p1/2 and Ca 2p3/2, respectively, indicating the presence of calcium ions on the activated carbon surface. The only peak observed at 1306.1 eV in FAC-Mg is attributed to Mg 1s which indicates the presence of magnesium on activated carbon after the adsorption process. The spectrum of FAC-F depicts two peaks related to F 1s at 685.7 eV and 686.65 eV suggesting the fluoride adsorption onto activated carbon. Binding energy shifts in the regions of C 1s, O 1s and P 2p in FAC are due to the alterations in bonding and changes in electron density of atoms in the surface functional groups after the adsorption process. A change in each region reveals the interaction of calcium, magnesium and fluoride ions with phosphorus containing functional groups in addition to carbon oxygen species.
Antimicrobial studies
The qualitative antimicrobial screening test was done for commonly found three water pathogens: E. coli, S. typhi and S. flexneri. According to the qualitative antimicrobial screening test, AC did not exhibit antimicrobial activity against all three test strains whereas FAC exhibited antibacterial activity on all three test strains (Table 3). The antimicrobial susceptibility testing methodology for FAC and AC is mentioned in detail in the ESI.†| Organism | Treatment | |
|---|---|---|
| AC | FAC | |
| E. coli (ATCC 25922) | − | + |
| S. typhi (ATCC 19430) | − | + |
| S. flexneri (ATCC 9199) | − | + |
The FAC MIC values ranged from 2.5 to 10.0 mg mL−1 whereas the MBC values ranged from 10.0 to 40.0 mg mL−1 for the three test strains (Table 4). Importantly, the MBC/MIC ratio indicates the bactericidal activity of the analyzed antibacterial compound FAC. In the current study, FAC exerted a bactericidal effect against E. coli, S. typhi and S. flexneri since the MBC/MIC ratio was equal to or lower than 4.0 (MBC/MIC ≤ 4.0). Furthermore, how these water pathogens destroyed with the treatment of FAC was examined by SEM and the images are shown in Fig. S5.† This can be explained that the initial adhesion of the bacteria on the activated carbon surface is considered to be a physicochemical process described by colloid chemical theories. In the presence of different cations/anions in water the repulsive electrostatic free energy would be reduced, and depending on the extent of this reduction, the van der Waal's attractive interaction may exceed the repulsive electrostatic interaction, therefore favoring the bacterial adsorption.61
| Organism | Treatment (mg mL−1) | ||
|---|---|---|---|
| FACFAC | |||
| MIC | MBC | MBC/MIC | |
| E. coli (ATCC 25922) | 5.0 | 20.0 | 4.0 |
| S. typhi (ATCC 19430) | 2.5 | 10.0 | 4.0 |
| S. flexneri (ATCC 9199) | 10.0 | 40.0 | 4.0 |
Kinetic and isothermal studies
The kinetic studies of adsorption on the functionalized activated carbon were studied by applying two different and commonly used kinetic models namely PFO and PSO models. The kinetic data for the two models, PFO and PSO, are shown in Fig. 9, and the kinetic model parameters obtained are listed in Table S7.† The results indicate that the adsorption process of calcium, magnesium and fluoride follows the PSO model which involves the chemisorption interactions.62 The pseudo-second-order kinetic model is based on the assumption that the rate-limiting step is chemisorption and predicts the behavior over the whole range of adsorption. Under these conditions, the adsorption rate is dependent on the adsorption capacity not on the concentration of the adsorbate. According to Ho et al., it can be suggested that the overall rate of the adsorption process is controlled by chemisorption where valency forces are involved through sharing or exchange of electrons between the adsorbent and adsorbate.21 Furthermore, a better fit to the PSO model implies that the adsorption rate depends more on the available adsorption sites on activated carbon rather than the adsorbate concentration in the solution.63 The correlation coefficient R2 was found to be between 0.997 and 0.999 for the pseudo second order fitting of all three adsorption processes. Also the calculated qe values agree well with the experimental data. Similar kinetics fittings related to activated carbon have been reported in the literature.54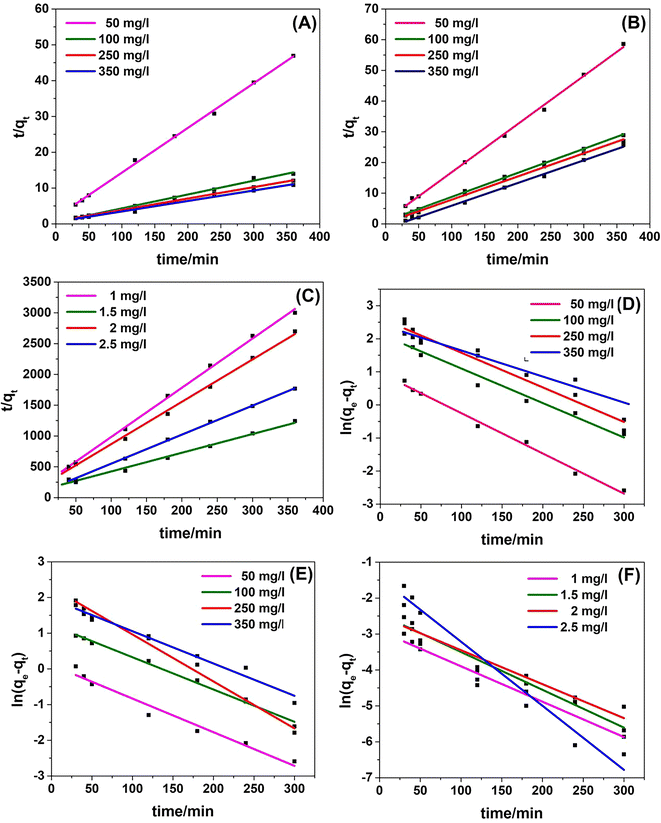 | ||
| Fig. 9 Kinetic study curves of the PSO model for (A) calcium, (B) magnesium, and (C) fluoride and of the PFO model for (D) calcium, (E), magnesium, and (F) fluoride. | ||
Adsorption isotherms offer explanation about how the adsorbate interacts with the adsorbent and describe the nature of the adsorption process. They indicate the distribution of adsorbate molecules between the solid phase and the liquid phase when the adsorption process reaches the equilibrium state.64 In the present work, Langmuir and Freundlich isotherms were employed to investigate the adsorption behavior by evaluating the applicability of isotherm equations to the adsorption process. The fitting of the experimental data for the two isotherm models and related parameters are shown in Fig. 10 and Table S8.†
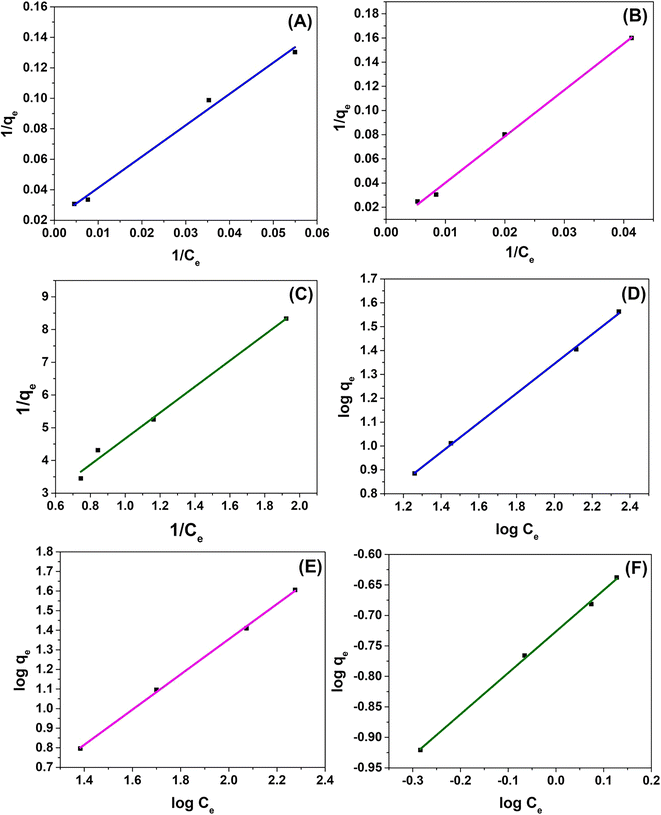 | ||
| Fig. 10 Isotherm curves of the Langmuir model for (A) calcium, (B) magnesium, and (C) fluoride and of the Freundlich model for (D) calcium, (E) magnesium, and (F) fluoride. | ||
The results indicate that the adsorption process is more fitted to the Freundlich isotherm than the Langmuir isotherm, as it possesses higher R2 values of 0.9989, 0.9988 and 0.9980 for calcium, magnesium and fluoride, respectively. This implies multi-layer adsorption on the heterogeneous surface of the activated carbon. KF is a Freundlich constant that shows the adsorption capacity on heterogeneous sites with non-uniform distribution of the energy level, and the n value shows the intensity between the adsorbate and adsorbent. According to Hettiarachchi et al., the adsorption becomes favorable if n is between 1 and 10.54 The calculated values of n prove that the adsorption of all three ions on FAC is favorable as the magnitude lies between 1 and 10.
Engineering design for the activated carbon manufacturing pilot plant using coconut coir dust
The process flow chart (Fig. 11) of the pilot plant was developed based on the laboratory experiment. The first step of the process is to wash the coir dust to remove dust and other foreign particles in a concrete tank which has a volume of 1 m3 as a batch process. Then the washed coir is dewatered using a mechanical press. The vapor activation is the most complex and critical step of the process. The vapor activation unit consists of four major pieces of equipment, namely, acid boiler, activation column, condenser and buffer tank as mentioned in Fig. S6.† The operations of the vapor activation unit start with a mixture of phosphoric acid and water being loaded to the acid boiler and then being heated using the built-in electrical heater to create phosphoric acid vapor. The produced vapor is allowed to flow through the activation column and react with the pre-loaded washed coir dust. The spent vapor is directed through the condenser and the condensed vapor is collected in the buffer tank. The vapor activation unit was designed to handle a large range of concentrations of acid (maximum 85 w/w%), operational temperatures (maximum 300 °C) and pressures (maximum 2 bar). The drying step is carried out next to remove the water that has been absorbed by the coir dust during vapor activation. The coir dust is kept in the adsorption column and hot air is introduced into the column prior to the pyrolysis step. An external compressor is used for air supply and a drained acid boiler is used to heat the air to a maximum of 140 °C before sending it through the coir dust. The pyrolysis step is carried out at a maximum of 500 °C in a modified muffle furnace which consists of purge lines for nitrogen to create an inert environment. The last step is to grind the produced activated carbon to any desired size and a variety of techniques are available for the grinding step.Conclusion
A sachet water filter is developed using phosphate-functionalized activated carbon (FAC) prepared in the vapor phase. Unlike other functionalized activated carbon materials, the pH and conductivity maintenance of purified water was easier when the vapor functionalization method was used. FAC exhibits a better porous structure, rich in surface functional groups including phosphorus containing groups, and higher adsorption capacity when compared to AC. FAC (1 g) in a sachet packet shows better adsorption characteristics towards removal of excessive hardness (>60%) and fluoride levels (>45%) in water within a 20 min time period bringing them down to the WHO recommended levels for drinking water. FAC demonstrates a bactericidal effect against E. coli, S. typhi and S. flexneri. The presence of functional groups on the vapor activated carbon allows it to adsorb inorganic cations and fluorides present in water. The process has been successfully scaled up as a mini-pilot plant and the most economical conditions for the production have been explored. It is noteworthy that phosphoric acid is reused in the process to minimize wastage.Abbreviations
| AC | Activated carbon without phosphoric acid vapor activation |
| FAC | Phosphoric acid vapor activated carbon |
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This project was funded by the National Research Council, Grant Number NRC Private Public Partnership 19-04 (NRC PPP 19-04) and Anunine Holdings Pvt Ltd. The authors acknowledge the Department of Chemistry and Instrument Center, Faculty of Applied Sciences, and the Sri Lanka Institute of Nanotechnology for their assistance in conducting the characterization studies.References
- C. L. Moe and R. D. Rheingans, Global challenges in water, sanitation and health, J. Water Health, 2006, 4, 41–57 CrossRef CAS PubMed.
- N. Savage and M. S. Diallo, Nanomaterials and water purification: opportunities and challenges, J. Nanopart. Res., 2005, 7, 331–342 CrossRef CAS.
- T. Amarasinghe, C. Madhusha, I. Munaweera and N. Kottegoda, Review on Mechanisms of Phosphate Solubilization in Rock Phosphate Fertilizer, Commun. Soil Sci. Plant Anal., 2022, 53, 944–960 CrossRef CAS.
- C. Madhusha, K. Rajapaksha, I. Munaweera, M. de Silva, C. Perera, G. Wijesinghe, M. Weerasekera, D. Attygalle, C. Sandaruwan and N. Kottegoda, A Novel Green Approach to Synthesize Curcuminoid-Layered Double Hydroxide Nanohybrids: Adroit Biomaterials for Future Antimicrobial Applications, ACS Omega, 2021, 6, 9600–9608 CrossRef CAS PubMed.
- C. Madhusha, I. Munaweera, V. Karunaratne and N. Kottegoda, Facile mechanochemical approach to synthesizing edible food preservation coatings based on alginate/ascorbic acid-layered double hydroxide bio-nanohybrids, J. Agric. Food Chem., 2020, 68, 8962–8975 CrossRef CAS PubMed.
- D. Zetland, The role of prices in managing water scarcity, Water Secur., 2021, 12, 100081 CrossRef.
- T. Leiknes, The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review, J. Environ. Sci., 2009, 21, 8–12 CrossRef CAS PubMed.
- C. K. Pooi and H. Y. Ng, Review of low-cost point-of-use water treatment systems for developing communities, npj Clean Water, 2018, 1, 1–8 CrossRef.
- R. A. Khanna, Y. Li, S. Mhaisalkar, M. Kumar and L. J. Liang, Comprehensive energy poverty index: Measuring energy poverty and identifying micro-level solutions in South and Southeast Asia, Energy Policy, 2019, 132, 379–391 CrossRef.
- D. A. Duran Romero, M. C. de Almeida Silva, B. J. M. Chaúque and A. D. Benetti, Biosand filter as a point-of-use water treatment technology: influence of turbidity on microorganism removal efficiency, Water, 2020, 12, 2302 CrossRef.
- S. Indika, Y. Wei, D. Hu, J. Ketharani, T. Ritigala, T. Cooray, M. Hansima, M. Makehelwala, K. Jinadasa and S. K. Weragoda, Evaluation of performance of existing ro drinking water stations in the north central province, Sri Lanka, Membranes, 2021, 11, 383 CrossRef CAS PubMed.
- Y. Siong, J. Idris and M. Atabaki, Performance of activated carbon in water filters, Water Resour., 2013, 1–19 CrossRef.
- C. Saka, Iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2, J. Anal. Appl. Pyrolysis, 2012, 95, 21–24 CrossRef CAS.
- R. M. Shrestha, Effect of preparation parameters on methylene blue number of activated carbons prepared from a locally available material, Journal of the Institute of Engineering, 2016, 12, 169–174 CrossRef.
- H. Boehm, Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons, Carbon, 1994, 32, 759–769 CrossRef CAS.
- A. Rana, S. M. S. Jillani and K. Alhooshani, Water Quality Characterization Using ASTM Methods in an Undergraduate Advanced Instrumental Analysis Laboratory Course, J. Chem. Educ., 2021, 98, 2919–2926 CrossRef CAS.
- E. Hettiarachchi, N. Kottegoda and A. Perera, Activated coconut coir for removal of water hardness, Desalin. Water Treat., 2017, 66, 103–110 CrossRef CAS.
- J. Murphy and J. Riley, Colorimetric method for determination of P in soil solution, Anal. Chim. Acta, 1962, 27, 31–36 CrossRef CAS.
- E. Cristiano, Y.-J. Hu, M. Siegfried, D. Kaplan and H. Nitsche, A comparison of point of zero charge measurement methodology, Clays Clay Miner., 2011, 59, 107–115 CrossRef CAS.
- J. F. Corbett, Pseudo first-order kinetics, J. Chem. Educ., 1972, 49, 663 CrossRef CAS.
- Y.-S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- A. W. Marczewski, Analysis of kinetic Langmuir model. Part I: integrated kinetic Langmuir equation (IKL): a new complete analytical solution of the Langmuir rate equation, Langmuir, 2010, 26, 15229–15238 CrossRef CAS PubMed.
- C.-h. Yang, Statistical mechanical study on the Freundlich isotherm equation, J. Colloid Interface Sci., 1998, 208, 379–387 CrossRef CAS PubMed.
- A. Dąbrowski, P. Podkościelny, Z. Hubicki and M. Barczak, Adsorption of phenolic compounds by activated carbon—a critical review, Chemosphere, 2005, 58, 1049–1070 CrossRef PubMed.
- E. H. Brown and C. D. Whitt, Vapor pressure of phosphoric acids, Ind. Eng. Chem., 1952, 44, 615–618 CrossRef CAS.
- J. Guo and A. C. Lua, Textural and chemical properties of adsorbent prepared from palm shell by phosphoric acid activation, Mater. Chem. Phys., 2003, 80, 114–119 CrossRef CAS.
- J. Xu, L. Chen, H. Qu, Y. Jiao, J. Xie and G. Xing, Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4, Appl. Surf. Sci., 2014, 320, 674–680 CrossRef CAS.
- O. Oginni, K. Singh, G. Oporto, B. Dawson-Andoh, L. McDonald and E. Sabolsky, Effect of one-step and two-step H3PO4 activation on activated carbon characteristics, Bioresour. Technol. Rep., 2019, 8, 100307 CrossRef.
- X. Wang, D. Li, W. Li, J. Peng, H. Xia, L. Zhang, S. Guo and G. Chen, Optimization of mesoporous activated carbon from coconut shells by chemical activation with phosphoric acid, BioResources, 2013, 8, 6184–6195 Search PubMed.
- R. Radhakrishnan, K. E. Gubbins, A. Watanabe and K. Kaneko, Freezing of simple fluids in microporous activated carbon fibers: comparison of simulation and experiment, J. Chem. Phys., 1999, 111, 9058–9067 CrossRef CAS.
- H. Marsh and F. Rodriguez-Reinoso, SEM and TEM Images of Structures in Activated Carbons, Activated Carbon, 2006, 1, 366–382 Search PubMed.
- J. Zhao, L. Yu, F. Zhou, H. Ma, K. Yang and G. Wu, Synthesis and characterization of activated carbon from sugar beet residue for the adsorption of hexavalent chromium in aqueous solutions, RSC Adv., 2021, 11, 8025–8032 RSC.
- T.-H. Liou, Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation, Chem. Eng. J., 2010, 158, 129–142 CrossRef CAS.
- F.-T. You, G.-W. Yu, Z.-J. Xing, J. Li, S.-Y. Xie, C.-X. Li, G. Wang, H.-Y. Ren and Y. Wang, Enhancement of NO catalytic oxidation on activated carbon at room temperature by nitric acid hydrothermal treatment, Appl. Surf. Sci., 2019, 471, 633–644 CrossRef CAS.
- Z. Xie, W. Guan, F. Ji, Z. Song and Y. Zhao, Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology, J. Chem., 2014, 2014, 491912 Search PubMed.
- A. C. Ferrari and J. Robertson, Interpretation of Raman spectra of disordered and amorphous carbon, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- M. Zięzio, B. Charmas, K. Jedynak, M. Hawryluk and K. Kucio, Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid (V), Appl. Nanosci., 2020, 10, 4703–4716 CrossRef.
- A. C. Ferrari, Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects, Solid State Commun., 2007, 143, 47–57 CrossRef CAS.
- C. Elcey and B. Manoj, Graphitization of coal by bio-solubilization: structure probe by Raman spectroscopy, Asian J. Chem., 2016, 28, 1557 CrossRef CAS.
- C. Marino, A. Debenedetti, B. Fraisse, F. Favier and L. Monconduit, Activated-phosphorus as new electrode material for Li-ion batteries, Electrochem. Commun., 2011, 13, 346–349 CrossRef CAS.
- A. Gupta, A. Prasad, N. Mulchandani, M. Shah, M. Ravi Sankar, S. Kumar and V. Katiyar, Multifunctional nanohydroxyapatite-promoted toughened high-molecular-weight stereocomplex poly (lactic acid)-based bionanocomposite for both 3D-printed orthopedic implants and high-temperature engineering applications, ACS Omega, 2017, 2, 4039–4052 CrossRef CAS PubMed.
- J. Sun, G. Zheng, H.-W. Lee, N. Liu, H. Wang, H. Yao, W. Yang and Y. Cui, Formation of stable phosphorus–carbon bond for enhanced performance in black phosphorus nanoparticle–graphite composite battery anodes, Nano Lett., 2014, 14, 4573–4580 CrossRef CAS PubMed.
- A. Lazzarini, A. Piovano, R. Pellegrini, G. Leofanti, G. Agostini, S. Rudić, M. R. Chierotti, R. Gobetto, A. Battiato and G. Spoto, A comprehensive approach to investigate the structural and surface properties of activated carbons and related Pd-based catalysts, Catal. Sci. Technol., 2016, 6, 4910–4922 RSC.
- A. Puziy, O. Poddubnaya and A. Ziatdinov, On the chemical structure of phosphorus compounds in phosphoric acid-activated carbon, Appl. Surf. Sci., 2006, 252, 8036–8038 CrossRef CAS.
- S. Yakout and G. S. El-Deen, Characterization of activated carbon prepared by phosphoric acid activation of olive stones, Arabian J. Chem., 2016, 9, S1155–S1162 CrossRef CAS.
- R. H. Hesas, A. Arami-Niya, W. M. A. W. Daud and J. Sahu, Preparation and characterization of activated carbon from apple waste by microwave-assisted phosphoric acid activation: application in methylene blue adsorption, BioResources, 2013, 8, 2950–2966 Search PubMed.
- N. Sych, S. Trofymenko, O. Poddubnaya, M. Tsyba, V. Sapsay, D. Klymchuk and A. Puziy, Porous structure and surface chemistry of phosphoric acid activated carbon from corncob, Appl. Surf. Sci., 2012, 261, 75–82 CrossRef CAS.
- Y. Guo and D. A. Rockstraw, Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation, Bioresour. Technol., 2007, 98, 1513–1521 CrossRef CAS PubMed.
- Q.-S. Liu, T. Zheng, P. Wang and L. Guo, Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation, Ind. Crops Prod., 2010, 31, 233–238 CrossRef CAS.
- A. Puziy, O. Poddubnaya, R. Socha, J. Gurgul and M. Wisniewski, XPS and NMR studies of phosphoric acid activated carbons, Carbon, 2008, 46, 2113–2123 CrossRef CAS.
- Y.-J. Lee and L. R. Radovic, Oxidation inhibition effects of phosphorus and boron in different carbon fabrics, Carbon, 2003, 41, 1987–1997 CrossRef CAS.
- Y. Liu, C. Hou, T. Jiao, J. Song, X. Zhang, R. Xing, J. Zhou, L. Zhang and Q. Peng, Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment, Nanomaterials, 2018, 8, 35 CrossRef PubMed.
- R. Fu, L. Liu, W. Huang and P. Sun, Studies on the structure of activated carbon fibers activated by phosphoric acid, J. Appl. Polym. Sci., 2003, 87, 2253–2261 CrossRef CAS.
- E. Hettiarachchi, R. Perera, A. Chandani Perera and N. Kottegoda, Activated coconut coir for removal of sodium and magnesium ions from saline water, Desalin. Water Treat., 2016, 57, 22341–22352 CrossRef CAS.
- G. S. Bilotta and R. E. Brazier, Understanding the influence of suspended solids on water quality and aquatic biota, Water Res., 2008, 42, 2849–2861 CrossRef CAS PubMed.
- C. Souza, D. Majuste and V. Ciminelli, Effects of surface properties of activated carbon on the adsorption mechanism of copper cyanocomplexes, Hydrometallurgy, 2014, 142, 1–11 CrossRef CAS.
- A. M. Cardenas Peña, J. G. Ibáñez Cornejo and R. C. Vásquez Medrano, Determination of the point of zero charge for electrocoagulation precipitates from an iron anode, Int. J. Electrochem. Sci., 2012, 7, 6142–6153 Search PubMed.
- R. Bhaumik and N. K. Mondal, Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: thermally and chemically activated coconut fibre dust, Clean Technol. Environ. Policy, 2015, 17, 2157–2172 CrossRef CAS.
- R. Araga, S. Soni and C. S. Sharma, Fluoride adsorption from aqueous solution using activated carbon obtained from KOH-treated jamun (Syzygium cumini) seed, J. Environ. Chem. Eng., 2017, 5, 5608–5616 CrossRef CAS.
- C. S. Ciobanu, S. L. Iconaru, P. Le Coustumer and D. Predoi, Vibrational investigations of silver-doped hydroxyapatite with antibacterial properties, J. Spectrosc., 2013, 2013, 471061 Search PubMed.
- J. Rivera-Utrilla, I. Bautista-Toledo, M. A. Ferro-García and C. Moreno-Castilla, Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption, J. Appl. Chem. Biotechnol., 2001, 76, 1209–1215 CrossRef CAS.
- R. Baby, M. Z. Hussein, Z. Zainal and A. H. Abdullah, Functionalized Activated Carbon Derived from Palm Kernel Shells for the Treatment of Simulated Heavy Metal-Contaminated Water, Nanomaterials, 2021, 11, 3133 CrossRef CAS PubMed.
- J. Salman, V. Njoku and B. Hameed, Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium, Chem. Eng. J., 2011, 173, 361–368 CrossRef CAS.
- I. Tan, A. Ahmad and B. Hameed, Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies, J. Hazard. Mater., 2008, 154, 337–346 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00623e |
| This journal is © The Royal Society of Chemistry 2023 |