The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: improving health status through functional food
Katherine
Bauer-Estrada
 ,
Camilo
Sandoval-Cuellar
,
Camilo
Sandoval-Cuellar
 ,
Yesica
Rojas-Muñoz
and
Maria Ximena
Quintanilla-Carvajal
,
Yesica
Rojas-Muñoz
and
Maria Ximena
Quintanilla-Carvajal
 *
*
Department of Engineering, Universidad de La Sabana, Chía, Colombia. E-mail: maria.quintanilla1@unisabana.edu.co; Tel: +57-1-8615555 ext 25216
First published on 24th November 2022
Abstract
The gut microbiota can be a determining factor of the health status of the host by its association with some diseases. It is known that dietary intake can modulate this microbiota through the consumption of compounds like essential oils, unsaturated fatty acids, non-digestible fiber, and probiotics, among others. However, these kinds of compounds can be damaged in the gastrointestinal tract as they pass through it to reach the intestine. This is due to the aggressive and changing conditions of this tract. For this reason, to guarantee that compounds arrive in the intestine at an adequate concentration to exert a modulatory effect on the gut microbiota, encapsulation should be sought. In this paper, we review the current research on compounds that modulate the gut microbiota, the encapsulation techniques used to protect the compounds through the gastrointestinal tract, in vitro models of this tract, and how these encapsulates interact with the gut microbiota. Finally, an overview of the regulatory status of these encapsulates is presented. The key findings are that prebiotics are the best modulators of gut microbiota fermentation metabolites. Also, probiotics promote an increase of beneficial gut microorganisms, which in some cases promotes their fermentation metabolites as well. Spray drying, freeze drying, and electrodynamics are notable encapsulation techniques that permit high encapsulation efficiency, high viability, and, together with wall materials, a high degree of protection against gastrointestinal conditions, allowing controlled release in the intestine and exerting a modulatory effect on gut microbiota.
1. Introduction
The gut microbiota can be defined as the microorganisms that inhabit the gastrointestinal tract. One of its main functions is the metabolization of food that cannot be digested by the host; thus, microorganisms can produce compounds that are further absorbed by the intestinal epithelium.1,2 The gut microbiota has a common core, and persistent alterations to this core can generate an imbalance in the microbial community structure; this is called dysbiosis. It has been found over the years that this dysbiosis is related to different types of disease in the body, from gastritis, inflammatory bowel disease, and colon cancer, to non-gastrointestinal diseases such as respiratory diseases, diabetes, obesity, and cardiovascular diseases.3–6 The study of this relationship between the alteration of the intestinal microbiota and different types of disease has been carried out through omics techniques such as transcriptomics, metagenomics, metabolomics, and genomics, among others. This has not only allowed the comparison of taxonomic microbiota composition but also the recognition of metabolites and enzyme expression, vitamins and essential amino acid biosynthesis pathways, and some specific genes that are involved in different biosynthetic pathways of interest.Thus, by using these techniques, it is possible to determine how certain bioactives (or their absence) modify the intestinal microbiota, stimulating the health of the person that consumes them in the recommended amounts. This modulation can be carried out by natural compounds that can be obtained from normal or specific types of diet. The mechanisms of action by which these compounds could help may be divided in two: by modulating the microbiota community composition, or by modulating the metabolites that the microbial community produces. An example of these bioactives is vitamins, which are micronutrients that promote the proper functioning of the body by keeping organs healthy, helping to harvest energy and promoting the appropriate functioning of the immune system, among other effects.7 Despite the beneficial effect of vitamins, the body is not capable of producing them on its own, so they must be acquired through the consumption of foods rich in these micronutrients on a daily basis.8
However, as much as people control their diet in order to feed the gut microbiota with the natural compounds that are needed to modulate it, there is no guarantee that these compounds are going to remain intact through the gastrointestinal tract. This is because of all the variations in pH or enzymatic activity in the digestive tract that can degrade some of the modulatory compounds, which would result in them not reaching the gut microbiota at an adequate concentration, or even in some cases not arriving in any proportion. Therefore, to guarantee that the modulatory compounds arrive in the intestine at an adequate concentration and have some effect on the gut microbiota, it is necessary to protect them from gastrointestinal conditions and enzymatic activity.
Encapsulation is a technique that allows modulatory compounds that can be damaged by gastrointestinal tract conditions to reach the gut microbiota in adequate concentrations to exert the effect needed. This technique has been demonstrated to protect biocompounds, increasing the amount that can be consumed and absorbed by the body.9 This protection is based on the wall materials of the capsule, which can be whey, gum arabic, alginate, cellulose, pectin, gelatin, and chitosan, among others. These shield biocompounds against different conditions in the gastrointestinal tract, ensuring their release in the intestine and their subsequent interaction with the gut microbiota.10,11 Also, encapsulation allows compounds and probiotics to be commercialized not only as supplements but as part of a food matrix and some processed foods, without altering their beneficial and nutritional content, giving in this way an additional value to processed products.
The encapsulation techniques used for the design of delivery systems can vary. There are four factors that guide the design: the physicochemical characteristics of the product that needs to be encapsulated; the delivery mechanism, which influences the selection of the wall material; the capsule size; and finally, the technique that is going to be used to encapsulate. However, when designing for a specific activity, like the modulation of the gut microbiota, the strategy is based not only on the compounds but on the whole panoramic view of the disease associated with the gut microbiota that requires modulation.
In this case, the regular diet of the person, the concentration needed to be loaded in each capsule, the effect of the wall material on the gut microbiota, and the bioaccesibility and bioavailability of the compounds by the intestinal epithelium and the intestinal microbial community are important factors to be considered when developing delivery systems.12 Bioaccesibility is measured as the fraction of the nutrient in food available to be absorbed by the intestinal walls, while bioavailability denotes the bioaccesibility of the nutrients and its bioactivity, as well as the process in which nutrients are absorbed and used by the epithelial cells. These factors (bioavailability and bioaccessibility) depend on the digestion of the encapsulates and how the wall material and the encapsulation technique used protect the core from the gastrointestinal conditions.13
The digestive process comprises a series of interdependent stages by which a food is reduced to simpler compounds that can be absorbed and used by the body. By understanding this process, it is possible to design foods with improved nutritional and health properties. In vitro digestion models have become a useful tool to determine the bioaccessibility of a nutrient or bioactive compound in the gastrointestinal tract; this is how they are increasingly being used to evaluate the possible effects of composition, structure, and processing of food on this parameter.12 Static methods are the simplest, most practical, and fastest models to carry out food digestion tests, and they allow the acquisition of preliminary evidence to make claims and test new hypotheses in future research.
This preliminary evidence helps to improve the design of encapsulates that later can be tested in in vivo systems. When working with in vivo models there are specific models of diseases, models with a particular and known microbiota, models that are germ-free, and healthy models. These systems allow the acquisition of more precise information on how the encapsulated compounds are going to be absorbed by the intestine and if they are going to exert the function expected. Furthermore, they permit the study of the behavior and modulation of the gut microbiota in its natural environment, by the encapsulated bioactives and probiotics.
Although in the literature there are several review articles about the modulation of gut microbiota and the current encapsulation techniques, there is no article reviewing the effect of encapsulated bioactives on the modulation of gut microbiota. Henceforth, in this article we review how the gut microbiota can be associated with different diseases, the modulatory effect of compounds on the gut microbiota, how it can be assured that these compounds arrive in the intestine in adequate concentrations through the encapsulation processes, the importance of analyzing these capsules through in vitro digestion systems, and how these encapsulated compounds have a modulatory effect on the gut microbiota. Finally, a general vision of the regulation of functional food additives around the globe is presented.
2. The gut microbiota and its association with disease
The human intestinal microbiota is understood to be the population of microorganisms that inhabits the intestine of each human being. It plays a very important role in metabolic, nutritional, physiological, and immunological processes.14 Diet and eating habits have been shown to impact the composition of the microbiota; for example, research on the gut microbiota of undernourished subjects has shown a low abundance of Bacteroidetes (a phylum of bacteria specialized in the conversion of carbohydrates into energy). Particularly, in the case of malnourished children, the abundance of Proteobacteria is higher compared with that in healthy children, who present a higher proportion of Bacteroidetes.15The gut microbiota plays a very important role in host metabolism. Among the mechanisms in which the microbiota is involved are functions such as the development of the immune16 and endocrine systems,17 degradation of components of the diet and toxins,18,19 growth of new intestinal veins, and modulation of bone mass density and fat storage.20,21 The microbiota of the human gut is made up of around 100 trillion archaea and bacterial cells. Furthermore, the amount of genetic information that this microbiota harbors is at least 100 times greater than the number of human genes.22 Metagenomic studies have determined that there are 3.3 million unique prokaryotic genes in our digestive system.23
The gut microbiota has the ability to alter the nutritional status of the host, as it can degrade components of the diet for which the host does not have the metabolic capacity. For example, bacteria in the phylum Firmicutes are efficient at extracting energy from carbohydrate- and fat-based diets.5 In a study in sterile mice which were inoculated with Bacteroides thetaiotaomicron (Bacteroidetes phylum) or co-inoculated with Methanobrevibacter smithii (methanogenic archaea), it was observed that B. thetaiotaomicron produced acetate and formate and that M. smithii used formate to produce methane. The interaction between the two promoted a more efficient fermentation of carbohydrates, increasing the absorption of energy in the intestine. This showed that the composition of the gut microbiota in the host affects digestion and energy harvesting.24
The host's diet has a profound effect on the microbial populations of the gastrointestinal tract as well. Given the observation that there are bacteria that can promote the health of the host, attempts have been made to modulate the intestinal microbiota with dietary compounds so that it has a greater abundance of beneficial bacteria that prevent the colonization of pathogenic and opportunistic bacteria.25 One of the ways to assess this modulation is through metagenomic analyses which determine changes in the composition of the microbiota due to dietary interventions.26 As an example, people who follow Mediterranean diets have more groups of microorganisms enriched such as Oscillospira and Butirycimonas,27 while people who have diets based on high levels of proteins have a community structure enriched in microorganisms of the Firmicutes phylum.28
One type of compound that has been seen to have a beneficial effect is high omega-3 oils, which increase the abundance of the probiotic bacterial genera Lactobacillus and Bifidobacterium.29 A high population of these bacteria can counteract gastrointestinal infections through different mechanisms, for example, through the production of organic acids, such as acetate and lactate, which can be used by butyrate-producing bacteria. Butyrate is a short-chain fatty acid which is the main energy source for colon cells; therefore, it maintains homeostasis in this part of the body.30 As an example, Bifidobacterium pseudolongum was administered in a mouse model of induced obesity. The mice showed a decrease in plasma triglycerides and higher abundance of Bacteroidetes while there was a decrease in Firmicutes; also, there was an increase in the proportion of Butyricimonas, Bifidobacterium, and Odoribacter.31
Greenblum et al.32 have stated how the intestinal bacteria interact with each other and the host at various levels, from single cells to populations of specific bacteria, to the entire community. The complexity of such multi-scale interactions presents a major challenge in understanding community functioning and its impact on host health. However, as Shetty et al. stated, the intestinal microbiota has a core composed of keystone species which have a profound influence on the microbial structure and function of this microbiota.33 These keystone species have different functions; as an example, some of them are in charge of the breakdown of complex carbon sources which would act as carbon source for the growth of other members of the microbial community. Different studies and comparisons of the intestinal microbiota from fecal samples have indicated the presence of a prevalent core microbiota; in Fig. 1 it can be observed how the core microbiota of colonic samples of healthy individuals is distributed (Fig. 1).6,34–36
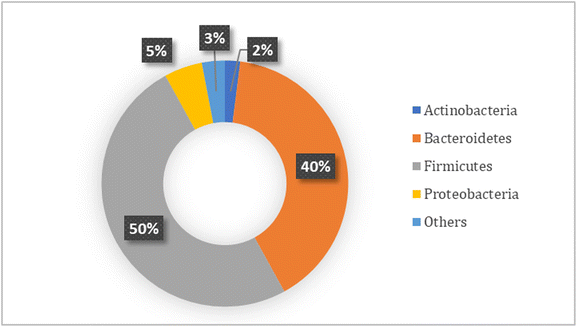 | ||
| Fig. 1 Relative phylum core composition of the human colonic microbiota.6,34–36 | ||
In this way, an alteration to the core of the intestinal microbiota could be associated with different diseases, and it would be expected that modulation of this imbalance to provide a microbiota more alike to the core of healthy people could benefit the host.37–40 Although the colonic microbiota is not representative of all the intestinal microbiota, as each part of the intestine has its own microbiota, it is important to note that the majority of studies that were carried out with this type of microbiota have shown that its composition and metabolism could be associated with some diseases and how its modulation led to an improvement of some disease symptoms. As mentioned in the introduction, this modulation could be carried out by different types of compound that present biological activities that are advantageous for the host, as well as by probiotics, which are microorganisms that will interact with the host microbiota, promoting positive changes and enhancing its usual activities.
3. Modulatory effect of selected biocompounds, probiotics, and prebiotics on the gut microbiota
Different kinds of biocompounds and probiotics have been described as potential modulators with important effects on the gut microbiota; some of the most recent studies are presented in Table 1. It can be observed that research is centered on the effect of pro- and prebiotics on the gut microbiota, followed by research on the modulatory potential of essential and unsaturated oils. In this section, we review the effect of food-grade essential oils, prebiotics, probiotics, synbiotics and unsaturated oils on gut microbiota.| Modulator compound | Example | Number of articles | Ref. |
|---|---|---|---|
| Essential oils | Cinnamon and lemon | 5 | 41–45 |
| Organic salts | Sodium acetate and sodium butyrate | 1 | 46 |
| Prebiotics | Xylooligosaccharides, galactooligosaccharides, human milk oligosaccharides, inulin, anthocyanins, flavonols, pectin, and different extracts | 18 | 47–65 |
| Probiotics | Bifidobacterium animalis, Lactobacillus acidophilus, Akkermansia muciniphila, Bifidobacterium longum, and Lactobacillus rhamnosus | 12 | 31 and 66–76 |
| Synbiotics | Lactobacillus plantarum–arabinoxylan, Lactobacillus plantarum–inulin, Lactobacillus casei–fish oil (omega 3), Lactobacillus casei–anthocyanins, and Bifidobacterium infantis–green tea extract | 4 | 77–79 |
| Unsaturated oils | Lard oil, fish oil, EPA, and DHA | 4 | 80–83 |
3.1. Essential oils
Some essential oils (EO), the ones which are non-toxic for humans and animals, have been recognized as a mix of biocompounds with strong health effects, from antioxidant to antimicrobial and anticancerogenic activity. In recent years it has been demonstrated that some EO can be used as antibiotics that have extra benefits for the gut microbiota when administered in adequate concentrations, as proved by several studies41–45,84,85 in which encapsulated cinnamon and ginger EO were fed to chickens as supplementation to their regular diet. In these studies, the use of EO helped to decrease the pathogens that affected the broilers’ health while increasing the relative abundance of Lactobacillus and probiotic strains in their gut. In some cases, it was proved that supplementation with fingered citron essential oils impacted the production of short-chain fatty acids (SCFAs), increasing the production of butyric and acetic acids by the gut microbiota.41,44,453.2. Prebiotics
Some compounds that have a direct modulatory effect on the gut microbiota community and the production of SCFAs are the prebiotics.86 These compounds are defined by ISAPP as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”87 and most are carbohydrate-type molecules, among them fructooligosaccharides (FOS), galactooligosaccharides (GOS), and starch- and glucose-derived oligosaccharides. However, there are prebiotics that are not cataloged in the carbohydrate-like group, i.e., flavanols and anthocyanins.88 The modulatory effect of these compounds has been proved through many studies. Daguet and collaborators56 described how arabinogalactan and FOS have a modulatory effect on the gut microbiota leading to higher SCFA production, the former being responsible for an increase in the production of butyric acid and the latter the promoter of an increase in the production of acetate in gut microbiota fermentations. In the distal colon, arabinogalactan increased the relative abundance of bifidobacteria, F. prausnitzii, and Roseburia spp., while FOS only had a strong modulatory effect on the proximal colon, leading to an increase of bifidobacteria, lactobacilli, and F. prausnitzii. Similar results were found by different authors (see Table 1). These studies confirm the positive modulatory effect of probiotics on the gut microbiota, increasing the relative abundance of beneficial bacteria while lowering the abundance of pathogenic bacteria. Also, the increase in the SCFAs produced in the fermentation of these prebiotics by the gut microbiota, in particular acetic, propionic, and butyric acids, make prebiotics good modulators to regulate the gut microbiota and help in the dysbiosis associated with some diseases.On the other hand, products containing prebiotics as oat flakes subjected to different treatments were digested in vitro and put in contact with the fecal microbiota. The butyrate concentration increased 2.6 times and it did not depend on the flake treatment; also, the pH of the digesta decreased, the ammonia levels were not altered, and the use of these flakes reduced the reproduction time of colon adenoma cells.51 Another example was investigated with the human milk oligosaccharide (HMO) 2′-fucosyllactose (2FL); it was put directly and after fermentation in an M-SHIME reactor to compare the process with lactose fermentation by the gut microbiota of infants and toddlers. In both cases, the pH level decreased considerably; 2FL did not present an increase in gas levels, while lactose presented a strong and immediate increase in gas formation. Also, the fermentation of lactose and 2FL showed an increase of acetate, but it was not statistically different between them; the same happened with the butyrate production. However, the production of propionate was higher with 2FL than lactose; also, butyrate production changed slightly but did not reach statistical significance. Furthermore, the relative abundance of Bifidobacterium increased in toddler gut microbiota samples after the fermentation.58
The above studies showed the modulatory potential of carbohydrate-type prebiotics. Besides that, non-carbohydrate-type prebiotics, such as anthocyanins, melanoids and flavanols, also have a modulatory effect on the gut microbiota. As an example, the use of anthocyanins from blueberry extract led to an increase in the production of acetic, butyric, and propionic acids by bacteria in comparison with the control and FOS treatments. Regarding the changes in microbial community caused by anthocyanins, there was a reduction of Clostridium histolyticum, which is known for its potential in tumor-promoting properties and inflammatory bowel disease.49,63 On the other hand, some melanoidins, which are the end product of Maillard reactions, were extensively used by gut microbes, increasing the production of SCFAs (mainly acetate and lactate) and favoring growth of the beneficial genera Bifidobacterium (bread crust, pilsner and black beers, chocolate and sweet wine melanoidins) and Faecalibacterium (biscuit melanoidins).61 Polyphenols found in green tea and coffee extracts are also modulators of the gut microbiota, significantly increasing the production of Bifidobacterium, Lactobacillus, Enterococcus spp., and SCFAs, while restraining the proliferation of Bacteroides, Prevotella, and C. histolyticum groups.52,54 These examples indicate prebiotics as compounds that interact directly with the gut microbiota with beneficial outcomes; these compounds can be consumed through extracts, fruits, vegetables and prepared foods, which is a great range of diverse sources; however, it is important to note that they should be consumed in an adequate quantity and regularly to exert their modulatory effect.
3.3. Probiotics and synbiotics
Probiotics have been identified as supplements to boost the health status of people. They are microorganisms which have a beneficial effect on health. Through the years, it has been proved that this beneficial effect emerges from their modulatory effect on the gut microbiota. Some bacterial species have been identified as probiotics, among them Bifidobacterium, Lactobacillus, and lately Akkermansia. Their interaction with the gut microbiota leads to an increase of beneficial bacteria in the intestine, as well as a reduction in the abundance of pathogenic species. This interaction can help to restore the gut microbiota from dysbiosis, as found by Bo and collaborators, where Bifidobacterium pseudolongum helped to recover the gut microbiota dysbiosis in obese mice, including the diversity of microbiota and the ratio of Firmicutes to Bacteroidetes. This treatment also increased the abundance of the bacterial genera Butyricimonas and Bifidobacterium.31 Similar results were obtained by Singh et al. in a study where old general-purpose mice were treated with Schizophyllum commune; this treatment significantly increased Bifidobacterium, Streptococcus, Lactobacillus, and unclassified species of the family Porphyromonadaceae, while decreasing diverse unclassified members of Lachnospiraceae and Porphyromonadaceae.75Supplementing the diet with probiotics will lead to an increase in the counts of probiotic microorganisms in the gut microbiota, as shown by several studies.67–71 However, it is important to note that probiotics alone cannot modulate the production of microbial metabolites.78 This finding led to the development of synbiotics, which are a mixture of probiotics and prebiotics whose synergic effect can benefit the gut microbiota, i.e. probiotics will help in the modulation of some species of gut microbiota while prebiotics will serve as “food” for that microbiota and modulate its production of metabolites, particularly SCFAs.
The above has been proved with some studies like the one carried out by Singh et al.,75 who not only evaluated the effect of probiotics but also that of prebiotics and synbiotics. In this research, they found that while the probiotic modulated the microbial community, it did not increase the metabolic production of SCFAs – on the contrary, it showed depleted metabolic activity in this regard – whereas in the treatments with prebiotics and synbiotics, the metabolic activity was not depleted. Another example of the activity of synbiotics in the intestine was described by Duysburgh et al.,77 who used a mixture of Bacillus spores with a blend of different prebiotics which were evaluated in vitro in a SHIME model. The synbiotic supplementation increased the gut microbial diversity of Bifidobacteriaceae, Lactobacillaceae, Prevotellaceae, Tannerellaceae, and Faecalibacterium prausnitzii; also, the synbiotic contributed to the stimulation of acetate, propionate, and butyrate production.77
Shinde et al. obtained similar results, where resistant starch from green banana was the prebiotic and Bacillus coagulans was the probiotic used for supplementing the diet of a mouse model of inflammatory bowel disease. They found that this synbiotic helped to reduce the damage score of the disease more than using the probiotic or prebiotic alone. Also, B. coagulans alone could not induce additional levels of SCFA production beyond the cecum, but the synbiotic combination with green banana resistant starch resulted in substantially increased SCFA levels across the whole length of the colon.78 The above supports the hypothesis that synbiotics are a better supplement than probiotics or prebiotics alone. Chaikham et al.79 found that using a synbiotic of Lactobacillus species and inulin or Tiliacora gum as a prebiotic enhanced the accumulation of lactic acid, SCFAs, and beneficial colon bacteria like Lactobacilli and Bifidobacteria, while decreasing the levels of toxic ammonia and the populations of harmful bacteria like Clostridia and fecal coliforms.
While the studies mentioned above have proved the modulatory effect of EO, prebiotics, probiotics, and unsaturated fatty acids, some of them omitted one important phase in the research: the digestion of these compounds. If there is no evaluation of the behavior of compounds and probiotics through the intestinal tract, it is not possible to conclude how to use them as possible treatments to modulate the gut microbiota to balance it when needed. This is mainly because the digestive tract is usually a wild environment, where enzymes and variation of pH affect the structure, viability, and activity of compounds and probiotics. For this reason, as expressed in the introduction, encapsulation is an essential process to protect bioactives from these digestive conditions, ensuring that their structure and viability are going to be as required when interacting with the gut microbiota.
4. Human digestion and in vitro digestion models
Human digestion is a complex biological process which comprises interdependent stages that allow the release of nutrients from the food matrix, for possible absorption and transportation via the circulatory system to the different tissues and organs where they will be used, either for growth and maintenance or as a source of energy. These stages occur within the gastrointestinal tract (GIT), which together with other organs and auxiliary glands make up the human digestive system.89 The GIT can be divided into two sections: the upper tract, composed of the mouth, esophagus, stomach, and small intestine (duodenum, ileum, and jejunum),90 and the lower tract composed of the cecum, colon, rectum, and anal canal, which make up the intestine.91 Each part of the GIT plays its own role in processing food; the mouth and stomach are primarily responsible for decreasing size and solubilizing nutrients, while the major absorption of water and nutrients occurs in the small intestine.90 However, the nutritional and functional performance of food during digestion is significantly moderated by its structure and composition, which have an important effect on postprandial metabolism and health.89,90 On the other hand, consumers are increasingly demanding novel high-quality food matrices that improve health and wellness.12To respond to consumer demand for health-beneficial food products, a common approach is to incorporate certain compounds with proven health benefits, called bioactive compounds. However, this is a current challenge due to their hydrophilic or lipophilic nature, site and mechanism of absorption and their chemical and thermal stability during processing, this due to their possible interaction with the food matrices in which they are going to be incorporated and their behavior through food production processes.12,92 In addition, gastrointestinal conditions of pH, temperature, and enzymatic activity can drastically reduce the fraction of bioactive compound released from the food matrix and available for absorption.91,93 For that reason, understanding and controlling digestion through the structure and composition of the food matrix is an area of interest for researchers.94,95
The term bioavailability is defined as the fraction of an ingested component that is available for use in the tissue of action in a normal physiological function as a result of three main steps: (i) digestion and solubilization of the elements in the GIT, (ii) the absorption of these elements and their transport via circulation, and (iii) their incorporation into the target tissue.96 Bioavailability, then, comprises two additional concepts: bioaccessibility and bioactivity.12 The first, bioaccessibility, is defined as the fraction of a nutrient that is released from the food matrix into the GIT and is therefore available for absorption through the intestinal walls.12,97 In this way, bioaccessibility includes events from food intake, solubilization in the GIT, and the release of bioactive compounds at the site where their absorption will occur. Bioactivity, on the other hand, includes events from the absorption of the bioactive compound, its transport via circulation to the site of action and its interaction with biomolecules, the transformations it may experience, and the physiological responses that can be induced98 (Fig. 2).
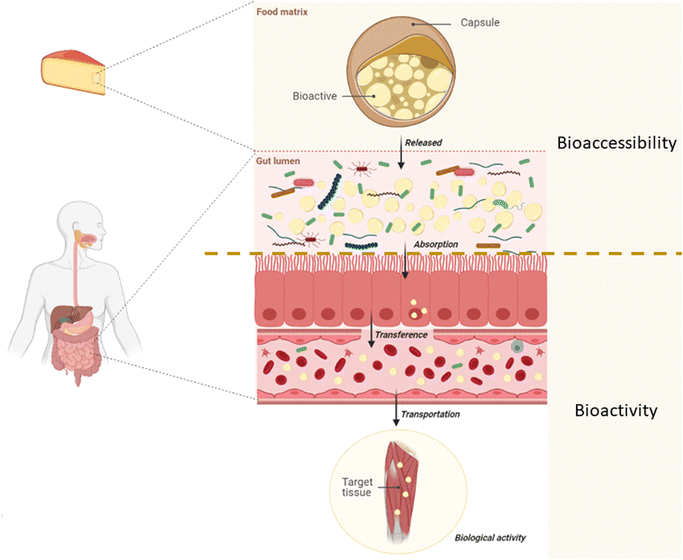 | ||
| Fig. 2 Bioavailability of a bioactive compound divided into the events that constitute bioaccessibility and bioactivity. | ||
Due to ethical and practical restrictions, the bioavailability of a nutrient is usually identified as the fraction that reaches the circulatory system, without considering its bioactivity;99 therefore, in vivo trials are required to measure it. Such tests are often costly, unsatisfactory, and involve important ethical considerations.90,100 On the other hand, many times the data obtained from these tests have high inter-subject variability and the conclusions are difficult to generalize.100 However, the bioaccessibility of a bioactive can be established using in vitro procedures, as an approximation to the behavior of food in the GIT, for predictive purposes and to establish trends that allow the hypothesis to be addressed in future research. These models seek to emulate gastrointestinal conditions such as salt concentration, the presence of enzymes, body temperature, and pH at the different stages of the digestive process, to study the effects of process conditions or matrix structure, for example, on the bioaccessibility of bioactive compounds and the stability of systems during their transit through the digestive system.100,101 In recent years, many in vitro digestion models have been developed and used to determine nutrient bioaccessibility, to estimate the bioavailability of the compounds tested. These models range from simple methods such as enzymatic reactions in a beaker to sophisticated dynamic models such as TIM-1, and the application of these models has been as broad as the diversity of existing models.13
4.1. In vitro digestion models
In vitro digestion methods are models of the GIT which try to mimic in vivo physiological conditions considering the presence and concentration of digestive enzymes, pH conditions, digestion times, and salt concentration, among other factors, generally considering three phases: the mouth, stomach, and small intestine.98 These methods are particularly relevant for determining the effects of the chemical structure of the bioactive compound, the morphology of the food, interactions with other components, and the physical and biological conditions of processing.102 They should be flexible, provide accurate results in a short time, and therefore allow quick analysis of food with different compositions and structures.97During the application of an in vitro digestion model, the bioactive nutrients or compounds of interest should be monitored to determine whether they are being affected by digestive conditions or whether, on the other hand, they are interacting with other components of the food.98 For this purpose, several methodologies can be used, such as the pH-Stat method during the intestinal or even gastric phase to monitor the release of fatty acids or amino acids.103–105 The use of in vitro models additionally allows the determination of the amount of nutrients or bioactive compounds that are released during digestion, which can be assimilated by the epithelial cells of the intestine (i.e. enterocytes), by incorporating in vivo models (e.g. intestinal infusion), ex vivo techniques, and cell culture models (e.g. Caco-2), after the intestinal phase.98
In vitro digestion models are divided into two types: static and dynamic. The difference between them is that while static ones start from initial conditions with averages and constant values, dynamic models include variation of parameters over time and try to approach in vivo conditions by simulating the flow of digestive fluids, gastric emptying rates, and peristaltic movements, among other aspects.106 In general, static methods are the most widely used models since they are simpler, more economical, practical, and allow a first approximation of the behavior of a functional food during digestion.97Table 2 shows a general comparison between the two types of human digestion models.
| Static model | Dynamic model |
|---|---|
| Study type | |
| - Suitable for limited digestion (gastric and/or intestinal stages) | - Suitable for total digestion studies |
| Food matrix | |
| - Simple/homogeneous foods | - Complex foods |
| - Isolated or purified compounds | |
| Major applications | |
| Macronutrients | Foods and pharmaceutics |
| - Protein hydrolysis | - Release and bioaccessibility of nutrients from complex food matrices |
| - Lipid hydrolysis | - Protein digestion |
| - Starch resistance | - Lipid breakout |
| - Peptide production | |
| Bioactive compounds | |
| - Release from simple food matrices | |
| - Solubility and bioaccessibility | |
| Main objectives | |
| - Enhance food properties | - Effects of food structure on the release of nutrients |
| - Preliminary evidence for nutritional and health claims | - Interactions between compounds |
| - Probiotic viability, release, etc. | |
| Main advantages | |
| - Fast and simple | - Greater accuracy in the dynamic intestinal environment |
| - Cheap | - Physical forces and shear stress are included |
| - Only requires validation considering its intended use | - Allows comparison with in vivo results |
| Main disadvantages | |
| - Absence of mechanical forces that contribute to digestion in vivo and in the change of the digestive environment | - Its ability to simulate gastrointestinal conditions must be validated |
| - Accumulation of metabolites that can interfere with digestion | |
In a review article published in 2018, Lucas-González and colleagues reviewed more than 2000 articles on in vitro food digestion, 89% of which were made using static methods. In these works, digestion was simulated in three successive stages (i.e., oral, gastric, and small intestine). At each stage, the temperature was maintained at a certain value and a specific simulated digestive fluid was used, while the pH was maintained at a fixed value by using a buffer solution of salts, the concentration of which varied depending on the method used in each study. McClements and Li103 published a review of several models that have been used to evaluate colloidal systems for the encapsulation of lipophilic bioactive compounds. It reports many different study conditions among the digestion models used. In another paper, Hur et al.107 reviewed about 80 works on in vitro digestion, reporting important differences between the digestion methods used, the composition of digestive fluids, and the number of stages (e.g., mouth, stomach, and intestine) being the most important. The authors also reported that the most used enzymes and biological molecules were pepsin, pancreatin, trypsin and chymotrypsin, amylase, lipase, bile salts, and mucin.107 Despite its apparent simplicity, the facts that each research team uses its own method of static in vitro digestion and that there is a lack of consensus on the physiological conditions used to carry out these studies have made progress in this field difficult, since the results are difficult to compare and general conclusions cannot be obtained. To try to solve this problem, the COST INFOGEST network has proposed a static protocol for harmonizing in vitro digestion based on physiological conditions considered relevant, which can be applied and adapted to various types of food.
5. Encapsulation techniques: an efficient strategy to deliver biocompounds, probiotics, and prebiotics to the gut microbiota
One of the ways of designing food that could modulate the gut microbiota in order to restore its balance is the formulation with beneficial bioactives that promote the appropriate balance of the intestinal microbiome. For this, a strategy that enhances the protection of these bioactives during their passage through the gastrointestinal tract is the technique of encapsulation; in this way, the compounds reach the intestine in an adequate concentration and can interact with the microbiota.Encapsulation involves the protection of one or more compounds using a material as a barrier against external conditions to increase the useful life of a product, improve solubility or retention, or control the release of the encapsulated compound. To achieve encapsulation various techniques have been used, such as emulsification, coacervation, liposomes, freeze drying, coating, ionic gelation, spray drying, electrospraying, and electrospinning, among others.108
However, the most used techniques are extrusion, freeze drying, and spray drying (Fig. 3). Additionally, it can be seen (Table 3) that probiotics are the most encapsulated modulator; this is done to increase their viability and ensure they reach the intestine in a functional concentration. Extrusion then is the technique used for the encapsulation of probiotics and synbiotics in this case, while techniques such as freeze drying and spray drying are more versatile for encapsulating different types of bioactives.
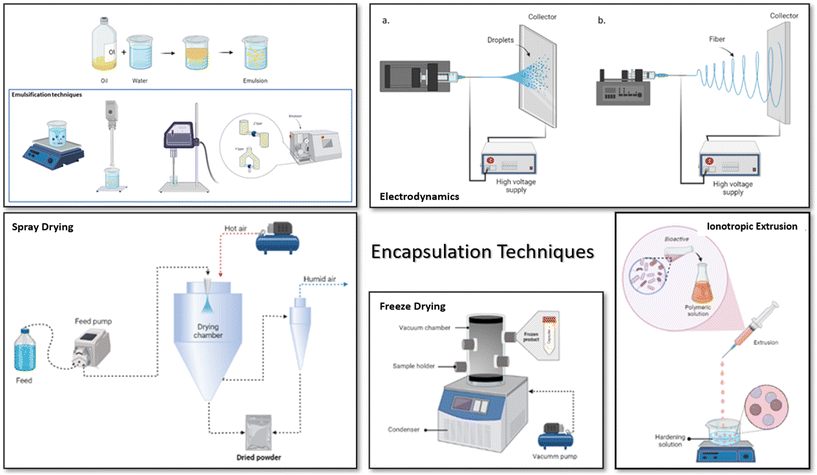 | ||
| Fig. 3 Most commonly used encapsulation techniques to encapsulate biocompounds, probiotics and prebiotics: emulsification, spray drying, electrodynamics, ionotropic extrusion and freeze drying. | ||
| Encapsulation technique | Core | Wall material | Encapsulation efficiency | Main results | Ref. |
|---|---|---|---|---|---|
| Ionotropic extrusion | Bifidobacterium longum | 2.5% whey protein concentrate + 1.5% PE | 93.23%; 8.95 log CFU per mL | More resistance to SGF and SIF. Resisted storage at 4 °C after 28 days with a final concentration greater than 107 log CFU per mL. | 109 |
| Pediococcus pentosaceus | 1% alginate + 1% gelatin + MgO nanoparticles | 78% | Microcapsules loaded with MgO nanoparticles were more stable than free cells and microcapsules without MgO, during exposure to SGF with a reduction of less than 2 log CFU. | 110 | |
| Lactobacillus salivarius Li01 (from fecal samples) | 1% alginate + 1.5% gelatin | ∼86.7%; 7.5 log CFU per mL | Reduction of 1.9 log CFU when exposed to SGF and a reduction of approximately 3 log CFU when exposed to SIF. | 111 | |
| Lactobacillus plantarum | Water-insoluble arabinoxylan (as a function of ferulic acid content) + 1.8% alginate | 79%; 8.89 log CFU per mL | Arabinoxylan materials enhanced the viability of cells after encapsulation compared with microcapsules of only alginate. The survival rate of the probiotic in arabinoxylan–alginate capsules was 51.1–74.0% and the bile salt survival rate was 70.6–81.6%. | 112 | |
| Lactobacillus plantarum + DHA-rich oil | 1.06% alginate + 0.55% pectin + 0.39% gelatin | 88.66% | Results from the optimization revealed that the highest survivability of encapsulated L. plantarum in gastrointestinal conditions was obtained by the incorporation of pectin and gelatin with alginate. The physicochemical properties of the product confirmed the suitability of the capsules. | 113 | |
| Lactobacillus fermentum L7 (from breast human milk) + FOS | 2% sodium alginate + 1.8% FOS | 89.75%; 9.5 log CFU per g | The co-encapsulation of L. fermentum L7 with oligosaccharides increased the survival rate for the encapsulation process and in the gastrointestinal environment, obtaining a concentration of 8.6 log CFU per g after 2 h in contact with SIF. | 114 | |
| Lactobacillus bulgaricus | 1% alginate/milk 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v 4 v/v |
100%; 9.98 log CFU per mL | The encapsulated L. bulgaricus resisted the conditions in the GIT, its viability being reduced by only 1.5 log cycles after 2 h of exposure to bile SIF. Also, these capsules protected the probiotic during storage at 4 °C for 1 month without cell loss. | 115 | |
| Ionotropic extrusion + freeze drying | Lactobacillus plantarum, Lactobacillus fermentum, and Bifidobacterium breve | 2% low methoxy pectin + skim milk, sucrose, and MnSO4 as cryoprotectant | 99% | In mice fed a high-fat diet, the body weight and cholesterol concentration in blood were lower than in control mice. Also, there was an increase in the abundance of Bifidobacterium and Lactobacillus in mice feces. | 76 |
| Ionotropic extrusion + freeze drying + coating | Lactobacillus acidophilus + ginger extract | Sodium alginate + PEG + Eudragit S100 coating material | 90% encapsulation efficiency; 92% entrapment of GE and 30% entrapment for LAB | The coating process protected the capsules from losses in the upper GIT. Regarding shelf life, powders should be stored at 4 °C so there is no significant reduction of LAB viability. In in vivo models of colitis, microcapsules showed an attenuation of oxidative stress and a downregulation of COX-2, iNOS, and c-Myc. | 116 |
| Ionotropic extrusion + coating | Lactobacillus acidophilus | 8.5% modified citrus pectin + 2% sodium alginate + chitosan as coating material | 83–93%; 8–9 log CFU per g | The effect of the capsules was proved in healthy mice following the count of lactobacilli in fecal samples. The microcapsules increased the count of lactobacilli significantly during the 28 days of treatment, with a significant increase in the first 7 days, augmenting from 7.6 log CFU per g on day 0 to 8.36 log CFU g at the end of the treatment. | 73 |
| Lactobacillus casei | 3% amidated pectin + 0.4% chitosan on acetic acid as coating material | 99%; 9.8 log CFU per mL | Pectin–chitosan capsules can protect L. casei from gastrointestinal conditions with no loss of cell viability when exposed to SGF and a disintegration of the capsule and release of probiotic in SIF. | 117 | |
| Bifidobacterium bifidum | 0.8% chitosan + 5% whey protein concentrate as coating material | 78.9%; 7.8 log CFU per mL | The coated microbeads had higher survival at pH 3.0 after 90 min of incubation time and at pH 7.0 after 3 h of exposure in comparison with free cells and encapsulated cells without coating. | 118 | |
| Spray drying | Lactobacillus rhamnosus GG (LGG) | 4.1% Eudragit + 10% mannitol (w/v) | 70% | The encapsulated probiotic had a reduction of 0.4 log on viable cells after 8 weeks of storage at 5 °C. When exposed to gastrointestinal conditions, after the last 2 h of contact with SIF there was only a 3-log reduction in cell viability, which corresponded to 4.5 × 107 CFU g−1. | 119 |
| 1,8-Cineole on 30% olive oil | Hydroxypropyl methyl cellulose + maltodextrin + colloidal silicon (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
7.98% entrapment of cineole on the oil; 7.89 g of 1,8-cineole per 100 g of powder | In vivo assessment of powders was carried out in broilers subjected to heat stress. Microcapsules of 1,8-cineole helped to recover the normal structure of the upper ileum and altered the ratio of the gut microbiota under heat stress. Also, they increased the ratio of Lactobacillus and Escherichia, while decreasing the abundance of Salmonella. | 45 | |
| Saccharomyces cerevisiae + dead yeast components (polysaccharides, peptides, essential amino acids, vitamins, and traces of mineral) | - | 1 × 109 CFU g−1 | The effect of a synbiotic on broilers was proved by in vivo assay. Supplementation of the normal diet with synbiotic increased the relative abundance of Lactobacillus and decreased Salmonella, Escherichia coli, and Clostridium in fecal samples compared with those fed control diet. Co-encapsulated Saccharomyces cerevisiae balanced the cecal microbial composition and improved the digestibility of nutrients in broilers compared with those fed control diet and diets supplemented with probiotics. | 70 | |
| Lactobacillus rhamnosus ATCC 7469 | 20% whey protein isolate + 4% inulin + 1% Persian gum | 90%; 10.88 log CFU per mL | The viability of encapsulated probiotics was reduced by 2 log cycles after simulated gastrointestinal conditions, allowing the microorganisms to arrive in the intestine in an adequate concentration. Also, microcapsules protected the probiotic against storage conditions (25 °C and 11% relative humidity); after 24 weeks, there was a 1.28 log reduction of probiotic viability compared with the initial concentration. | 120 | |
| Lactobacillus acidophilus LA5 | 10% maltodextrin + 10% inulin | 6.59 × 108 CFU g−1 | The capsules protected the probiotic against gastrointestinal conditions, allowing them to arrive in the intestine in an adequate concentration to colonize the colon, as shown by the results. Also, SCFA production was duplicated after 24 h and the count of lactobacilli in the fecal samples after 24 h increased compared with the control. | 79 | |
| Bifidobacterium infantis | 7.5% maltodextrin + 0.2% flaxseed mucilage + 7.5% flaxseed soluble protein | 98%; 11.08 log CFU per g | The capsules protected the probiotic; however, there was a 31% reduction of cell viability when exposed to SGF after 6 h of incubation. Regarding shelf life, the viability of probiotics was preserved at higher than 9 log CFU per g even though they were mixed with instant juice powder. | 121 | |
| Lactobacillus casei + omega 3-rich tuna oil | 12% whey protein isolate + 4% gum Arabic | 8 log CFU per mL | The co-encapsulation of L. casei and tuna oil allowed the probiotic to survive the gastrointestinal conditions more than the probiotic encapsulated individually and arrive in the intestine in a concentration of approximately 6.5 log CFU per mL. | 122 | |
| Bacillus clausii + resveratrol | Lactose | 82%; 8.6 log CFU per g | The microcapsules conserved the antioxidant activity of resveratrol. Also, the probiotic was successfully encapsulated; however, in capsules that did not have resveratrol, the viability was higher than in co-microcapsules. | 123 | |
| Freeze drying | Blueberry anthocyanin extract | 20% soy protein | Soy protein showed better protection against gastrointestinal conditions, allowing the anthocyanins to interact with the gut microbiota in colonic fermentations, which was proved by the phenolic compounds produced by the gut microbiota when the anthocyanins were degraded, and the changes in the gut microbial community. | 63 | |
| Anthocyanins | Beta-cyclodextrin 5 × 10−3 M | Malvidin-3-glucoside was the anthocyanin with the best concentration released from capsules. When interacting with the gut microbiota in colonic fermentations, this anthocyanin produced an increase in the production of SCFAs compared with the control fermentation. | 49 | ||
| Lactobacillus rhamnosus ATCC 7469 | 20% whey protein isolate + 4% inulin | 90%; 10.82 log CFU per mL | Encapsulated probiotics reduced their viability by 2 log cycles after simulated gastrointestinal conditions, allowing the microorganisms to arrive in the intestine in an adequate concentration. Also, microcapsules protected the probiotic against storage conditions (25 °C and 11% relative humidity); after 24 weeks, there was a 0.38 log reduction of probiotic viability compared with the initial concentration. | 120 | |
| Blackcurrant extract + Lactobacillus casei 431 | 2% whey protein isolate + 1% chitosan + 1% inulin | 95.46% for extract; 87.38% for probiotic | The viability of co-encapsulated probiotic was reduced from 8.13 to 6.35 log CFU per g after 90 days of storage. The release of anthocyanins was 94% after 2 h of intestinal digestion, showing that the capsules protected the anthocyanins and probiotic from the gastric conditions. | 124 | |
Through the process of reviewing the papers that involve the encapsulation of selected biocompounds, pre-, and probiotics, and the test of the resistance of the capsules through in vitro digestion systems, we found that the most used encapsulation technique to protect probiotics is ionotropic extrusion; however, this protection is always enhanced with a coating technique. The above is due to the survival rate of probiotics when subjected to the encapsulation process; ionotropic extrusion is one of the encapsulation techniques that exerts less stress on the microorganisms, providing in this way the best encapsulation yields, as mentioned at the beginning of this section. The next most used technique is spray drying; this is due to its versatility that allows the use of different encapsulation matrices, enabling adaptation of the wall material to the protected compound or microorganism; also, it is one of the easiest technologies to scale up and is an economical way to obtain micro- or nanocapsules in powder form which, as mentioned before, allows extension of the product's shelf life and represents an advantage in the food process industry. It is important to note that whatever encapsulation technique is used, emulsification is always part of the encapsulation process in each technology.
In this way, we review the findings on protection by the most commonly used encapsulation techniques against the simulation of in vitro digestion systems.
5.1. Emulsification
Emulsification can be achieved by energizing the mixture of two immiscible liquids through different methods to form droplets of the dispersed phase of between 10 and 100 micrometers; examples include ultrasound, Ultra-Turrax, microfluidization, and rotor stator, among others.125 For this, the use of an emulsifier to give stability to the formation of the particles is needed. Three types of emulsion can be formed, depending on what is to be encapsulated: water-in-oil (W/O), oil-in-water (O/W), and water-in-oil-in-water (W/O/W).126 The emulsification technique also crosslinks the materials of the emulsion to insolubilize the water-soluble polymers in the emulsion and in this way seal the capsules; this process can be carried out in different ways such as ionic, enzymatic, and thermal methods, among others. After being filtered and washed, the capsules formed (as in ionotropic extrusion) are further dried or coated in order to enhance the protection of the core.126–128Usually, emulsification is combined with other encapsulation techniques to seal the capsules to obtain powders. One of the advantages of this technique is that it helps to protect and overcome the problems of using oils (EO and fatty acids) in pharmaceutical and food applications. This was proved by Liu and collaborators;44 in their study, the encapsulation of fingered citron EO, which is highly valuable due to its antioxidant, antimicrobial, anti-inflammatory and insulin secretagogue activity, was carried out using emulsification to stabilize and enhance its solubility during transit through the GIT to reach the intestine and interact with the gut microbiota. In this case, an oil-in-water emulsion was made by stirring with different surfactants. The emulsions were put in contact with fecal slurries for the in vitro fermentations; SCFA production was promoted and the relative abundance of Lactococcus and Lactobacillus was augmented in comparison with the control.
Emulsification is an effective technique to protect biocompounds and probiotics from gastrointestinal conditions, as shown by Kumherová et al.129 In their study, they proved that emulsification provides the same protective effect for probiotics as ionic gelation. In this case, the emulsification was carried out in a water-in-oil emulsion of the fresh culture of probiotic Bifidobacterium animalis subsp. and with inulin and ascorbic acid added. The results showed that the viability of probiotics passing through the in vitro GIT simulation decreased by 0.9–1.8 orders, while the decrease in the survival of probiotics encapsulated by ionic gelation on alginate was in the range of 2.5–2.8 log cycles. In this case, the presence of inulin and ascorbic acid was only observable in the emulsified capsules, exerting a synergistic effect on the viability of the probiotic.
Another example of this advantage is shown by the encapsulation of Lactobacillus rhamnosus in different states of whey proteins carried out by Burgain and collaborators.130 This time, a water-in-oil emulsion was prepared containing the probiotic and the wall material (micellar casein, native and denatured whey proteins), then the microparticles formed were separated and tested in an in vitro simulation of the gastric phase. The results showed how the microparticles formulated with denatured whey protein and micellar casein allowed a probiotic encapsulation rate of 97% and a survival rate of approximately 99%.130 Similar results were obtained by van der Ark et al. in 2017;131 they encapsulated the probiotic Akkermansia muciniphila in a double emulsion (water-in-oil-in-water) with an encapsulation efficiency of 97.5%. After the encapsulation, in vitro digestion following the INFOGEST protocol was carried out, giving as a result a survival rate of approximately 99% with no significant difference between the probiotic concentration encapsulated in the double emulsion and that in the digesta after the intestinal phase. These studies allow emulsification to be marked as an effective technique to protect probiotics and compounds from gastrointestinal conditions, allowing these cores to reach the site of action at an adequate concentration to exert their function.
As mentioned before, emulsification is a technique that can be mixed with other techniques in order to seal the core components in different wall materials. This allows the protective effect of emulsification to be enhanced and confers different physicochemical properties to the encapsulates, such as an extended shelf life, controlled release in determinate sites of action, solubility of encapsulates, and stabilization, among others.
5.2. Ionotropic extrusion
Ionotropic extrusion or ionotropic gelation is one of the most simple and inexpensive encapsulation techniques. Its principle is based on the formation of a hydrocolloid solution with the microorganism or biocompounds; the mixture of the polymer and the core of the capsules is injected through a nozzle that defines the particle size of the capsule and then the capsule is put in contact with a hardening solution, typically an ionic solution that interacts electrostatically with the wall material of the capsule.132 Here, the electrostatic interaction between two ionic species, the wall material of the capsule and the hardening solution, allows the formation of microparticles. Depending on the conditions under which this technique is carried out, different types of microcapsules can be obtained, among them beads with one core or pearls with multiple cores.132,133 This technique is widely used in the pharmaceutical and food industries, and usually the capsules are dried or coated with another wall material after being filtered.In this sense, Iqbal et al.118 proved that encapsulating probiotics with ionic extrusion using chitosan and alginate, and coating with whey protein concentrate, presented high encapsulation efficiency and GIT tolerance compared with the unencapsulated (free) cells during the assay. Also, there was a significant difference in protection against gastrointestinal conditions between the capsules with and without the whey protein concentrate coating. Similar results were obtained by Bepeyeva et al.;117 here, pectin capsules without a chitosan coat were found to provide limited protection to L. casei in simulated gastric juice. Coating with chitosan effectively protected the bacterial cells from the acid in the simulated gastric and intestinal juices, allowing a survival concentration of 9.6 log CFU per mL. Related results were obtained by other authors,109,110,115,131 proving the efficacy of encapsulating probiotics by ionic gelation and coating the capsules to enhance the protection against the conditions of the GIT.
Encapsulation of synbiotics by the ionic gelation method enhances the survival of probiotics in the capsules when tested in in vitro digestions. In this way, the synergistic effect of the prebiotic and the probiotic allows the latter to survive longer in the capsule without the need to coat it, as studied by Wu et al.112 This time, the co-encapsulation of Lactobacillus plantarum with the prebiotics arabinoxylan and arabinoxylan oligosaccharides in microspheres of alginate allowed gastric stability of the capsules and resistance to bile salts such that 74.0% and 81.6% of cells survived, respectively, compared with the microcapsules made of only alginate. Liao et al.114 obtained similar results when encapsulating Lactobacillus fermentum with a mixture of oligosaccharides in alginate beads. The capsules were stable in gastric conditions and disintegrated when exposed to intestinal conditions compared with cells encapsulated without the prebiotic; the survival rates varied between 9.5 and 10 log CFU per mL in simulated gastric juice and 8 and 9.5 log CFU per mL when in contact with simulated intestinal juice. The survival concentrations of cells encapsulated without the prebiotics were 2 log cycles lower than those of the co-encapsulated ones, demonstrating the beneficial effect of co-encapsulation in the protection and survival of probiotics in the GIT.
5.3. Spray drying
One of the drying techniques that allows nano- and microcapsules to be obtained is spray drying. Its advantages, such as the prevention of the degradation of flavors and oils, economical operation, flexibility, and the use of water-soluble wall materials, make it a desirable technique to produce encapsulates for the food industry.108,134 Usually, it is used as a complement of emulsification; after acquisition of the emulsion with the core and wall material, it is atomized through different types of nozzles in a drying chamber which has a constant temperature. The microcapsules that are formed as a powder leave the chamber immediately, guaranteeing that the powder is not constantly at high temperatures.134,135Spray drying encapsulation protected the probiotic L. rhamnosus GG (LGG) from gastrointestinal conditions, with less than a 3 log reduction of viable cells during the whole time of exposure to gastrointestinal conditions.119 Also, this technique protected the probiotic L. rhamnosus more than the electrospraying technique when producing microcapsules, as presented by Moayyedi et al.120 One of the advantages of this technique is the easy process to obtain synbiotics because it allows the addition of water-soluble carbohydrates like prebiotics that will serve as an additional protection for the probiotics in the encapsulation mixture. In this way, the ability of L. casei to adhere to the intestinal wall, which is a desirable ability for microcapsules, was obtained by co-encapsulation with the omega-3 fatty acids contained in oil. These co-microcapsules had better retention of probiotics and oil when subjected to gastric conditions, and controlled and desirable release when exposed to intestinal conditions.122
The above is in correlation with the findings of Aragón-Rojas et al.136 In their study, the encapsulation of L. fermentum by spray drying in a whey:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) maltodextrin matrix protected the microorganisms through the GIT. The dynamic in vitro digestion showed that encapsulating L. fermentum with the above matrix allowed it to survive with a reduction of cycles of only 3.04 log CFU per g, in contrast with free cells which underwent a reduction of 5.8 log CFU per g cycles. In a similar study, dos Santos et al.137 encapsulated L. acidophilus La-5 using this technology, obtaining a survival rate after encapsulation of 85%. After the in vitro digestion process, microencapsulated La-5 had a reduction of 2 log CFU per g cycles, with a final concentration in the enteric phase II of 6.37 log CFU per g. Lately, these microencapsulates were added to the preparation of a mousse; the concentration of probiotics was lowered to 1.55 log CFU per g in the process of preparing the mousse but reduced by just 1.26 cycles in the digestion process, suggesting that the ingredients of the mousse had a protective effect on the encapsulates.
maltodextrin matrix protected the microorganisms through the GIT. The dynamic in vitro digestion showed that encapsulating L. fermentum with the above matrix allowed it to survive with a reduction of cycles of only 3.04 log CFU per g, in contrast with free cells which underwent a reduction of 5.8 log CFU per g cycles. In a similar study, dos Santos et al.137 encapsulated L. acidophilus La-5 using this technology, obtaining a survival rate after encapsulation of 85%. After the in vitro digestion process, microencapsulated La-5 had a reduction of 2 log CFU per g cycles, with a final concentration in the enteric phase II of 6.37 log CFU per g. Lately, these microencapsulates were added to the preparation of a mousse; the concentration of probiotics was lowered to 1.55 log CFU per g in the process of preparing the mousse but reduced by just 1.26 cycles in the digestion process, suggesting that the ingredients of the mousse had a protective effect on the encapsulates.
Another example of how the spray drying technique allows the acquisition of microcapsules that can be integrated in some food matrices is the results obtained by Chaikham et al.79 In this study, L. casei and L. acidophilus were encapsulated in Tiliacora triandra gum and inulin and added to maoluang juice to obtain a maoluang juice powder. The technique aimed to protect probiotic bacteria against the adverse conditions of the simulated gastrointestinal model. After 6 h of incubation in simulated gastrointestinal conditions, the survival rates of encapsulated probiotics were higher than those of the free cells. According to the in vitro gut microbiota experiments, the encapsulating material increased the accumulation of lactic acid and SCFAs and the abundance of lactobacilli and bifidobacteria in the colon.
The spray drying encapsulation technique has been proved to be valuable when encapsulating not only probiotics but also prebiotics. This is the case for the encapsulation of anthocyanins in a double emulsion of gelatin–acacia gum and chitosan–carboxymethylcellulose as wall materials. The encapsulation efficiency ranged from 90.4% to 94.7%, with an accumulative release of approximately 85% during the in vitro digestion, most of the release being in the intestinal phase through a diffusion-controlled release.138 The versatility of this technique allows the encapsulation of multiple oils, as Wang et al. did in 2018.139 In this study, the encapsulation of peony seed oil by spray drying was carried out with whey protein isolate and corn syrup as the wall materials, demonstrating that 80% of the oil released through in vitro digestion was in the intestinal phase.
5.4. Freeze drying
Freeze drying as an encapsulation technique allows enhancement of the stability of microencapsulated compounds and probiotics.140 Its principle is the dehydration of the capsules by sublimation in a first drying step, followed by a process of dehydration by desorption. This combination allows the production of microcapsules that protect the core, conferring the ability to resist without altering its biological and physicochemical characteristics over long periods of time, which gives the final powder a potential shelf life longer than with other techniques.140,141 However, when encapsulating probiotics, inadequate rates of freezing may result in the formation of crystals that could damage the membrane of the cells, in this way decreasing the viable concentration of probiotics in the powder.Freeze drying shows little cell loss when encapsulating probiotic L. rhamnosus; also, it maintains cell viability during storage and when exposed to GIT conditions.120 When combining freeze drying with other encapsulation techniques, such as dehydrating capsules prepared initially by ionic gelation, this technique enhances the protective effect of the capsules, allowing the maintenance of cell viability upon storage for long periods and enhancing its ability to colonize the colon, as studied by Haffner et al.69 This technology allows the acquisition of a satisfactory concentration of phytochemicals encapsulated along with probiotics, without affecting their biological activity. Also, the co-encapsulation of probiotics with phytochemicals that have potent inhibitory effects on alpha-amylase and alpha-glucosidase will enhance the protective effect of the encapsulation technique; Enache et al. demonstrated its remarkable action in protecting probiotics from the gastric environment.124
5.5. Electrospinning and electrospraying
An emergent technology for encapsulating bioactives and probiotics is the use of electrodynamic processes such as electrospraying and electrospinning to form nanofibers and powders composed of nanoparticles. This is an alternative to encapsulation techniques that include thermal processes, such as spray drying and freeze drying. The principle of this technique is the use of high voltage to generate dried nano- and microparticles without the need to subject them to a thermal process, in this way protecting the bioactives or probiotics from thermal damage.142,143 In this technique, an external electric field is imposed on a polymeric solution which contains the compounds or probiotics. Depending on the arrangement of the equipment, electrospraying or electrospinning is carried out. The first one allows the acquisition of powders of microencapsulated compounds and probiotics, the latter the encapsulation of compounds and probiotics in nanofibers.144 The advantage of this technique is the high efficiency rate of encapsulation and the high survival rate of the probiotics when subjected to this process.Moayyedi et al.120 demonstrated how the encapsulation process affects the viability of L. rhamnosus; in this study, microcapsules obtained by electrospinning showed higher viability after encapsulation than those produced using freeze and spray drying technologies. Electrospraying enhances the survival of probiotic L. plantarum BL011 during storage at 4 °C for 21 days, as proved by Coghetto et al.145 Also, the microcapsules produced by this technique protected L. plantarum BL011 when exposed to simulated gastric and intestinal juices.
The challenges that encapsulation techniques face regarding their use as a delivery mechanism for biocompounds and probiotics to the gut microbiota include the reduction of the particle size, especially regarding compounds. The smaller the particles of delivery, the higher the probability of interaction between the particles and the microbiota. Regarding probiotics, there are studies that have used nanoencapsulation techniques to encapsulate probiotics; however, it is important to remember that the size of microorganisms is in the micrometers range, thus research on the delivery of probiotics should not be directed to reducing the size of delivery particles but to developing capsules that also have a positive impact on the gut microbiota, and co-encapsulation systems with compounds that not only feed the gut microbiota but that also facilitate interaction between the probiotic and this microbiota.
Another challenge that encapsulation still is facing is to develop dry products that can be incorporated in different types of food matrices. The most common technologies to obtain such powder encapsulates involve heat or freeze treatments, and in this way the encapsulation efficiency of thermally sensitive compounds and probiotics can be reduced. In this sense the use of technologies such as electrospinning and electrospraying becomes an excellent alternative as they do not exert thermal damage on the compounds and probiotics. Additionally, this technology allows nano and macro fibers and powders that can interact effectively with the gut microbiota to be obtained, as discussed in this section.
6. Effect of encapsulated compounds and probiotics on the gut microbiota during in vitro fermentations
Bioactives and probiotics that could play the role of modulating the gut microbiota can accomplish this modulation by promoting the production of different metabolites such as SCFAs, by modifying the community structure of the gut microbiota, or doing both at the same time. Evaluation of this activity can be carried out through in vitro fermentations with the gut microbiota of donors or in clinical trials; however, the latter presents ethical concerns when researching in human disease and with children. For the in vitro analysis of modulation, the digested compounds and probiotics that are going to exert the modulatory activity are mixed with the fermentation medium inoculated with the gut microbiota obtained from the donor's fecal sample.53,59 After fermentation of the gut microbiota with the digested probiotics and biocompounds, many techniques could be employed to analyze the changes in the gut microbiota, such as 16s next-generation sequencing, whole metagenomic shotgun sequencing or the use of different omics; for this purpose, the DNA of the sample should be extracted.59,63,64Table 4 shows how different studies have evaluated the effect on the gut microbiota of microencapsulated compounds and probiotics and their co-encapsulation.| Type of core | Detail | Gut microbiota donors | Modulation | Main results | Ref. |
|---|---|---|---|---|---|
| Essential oils | Blend of EO and organic acids encapsulated in Ca–alginate and whey protein microcapsules | 288 one-day-old male Arbor Acres broiler chicks, some of them infected with Eimeria spp./C. perfringens | Gut microbiota community | Alleviation of necrotic enteritis symptoms. Increase in relative abundance of unclassified Lachnospiraceae and a significant decrease in relative abundance of Erysipelotrichaceae. | 41 |
| Fingered citron EO | 6 healthy humans between 20 and 25 years old. Chinese food diet and no antibiotic treatment | Gut microbiota community and SCFA production | Promoted an increase of Lactobacillus, Lactococcus, and Firmicutes when increasing the concentration of nanoemulsions. Regarding SCFA production, acetate and propionate were the ones promoted by the nanoemulsions. | 44 | |
| 1,8-Cineole encapsulated by spray drying in hydroxypropyl methyl cellulose and maltodextrin | 30-Day-old Ebayka broilers, treated under heat stress | Gut microbiota community | The 1,8-cineole capsules increased the proportion of Lactobacillus and decreased the relative abundance of Salmonella in the gut. Also, they inhibited the activity of Salmonella, Escherichia coli, and Staphylococcus aureus. | 45 | |
| Prebiotics | FOS, GOS, xylo-oligosaccharides, and antioxidant vitamin blend | 6 healthy human donors between 26 and 36 years old | Gut microbiota community and SCFA production | The prebiotics increased the levels of lactate, acetate, and butyrate, and decreased the proteolytic markers. They reduced the relative abundance of F. nucleatum and increased the levels of B. wexlerae. | 59 |
| Anthocyanins | 6 healthy adult donors between 20 and 30 years old, and with no intake of antibiotics in the last 6 months | Gut microbiota community and antioxidant activity | The microcapsules decreased the Firmicutes/Bacteroidetes ratio, and the relative abundance of Lachnospiraceae and Ruminococcaceae. They also increased the relative abundance of Bacteroidaceae. | 63 | |
| Sucrose–alginate beads | 3 healthy donors, one female and two males, with a mean age of 34.2 years and not taking antibiotics | Antioxidant activity and SCFA production | Antioxidant values for gut microbiota fermentation increased after exposure to microcapsules and SCFA production was enhanced. | 64 | |
| Probiotics | Lactobacillus acidophilus in calcium alginate beads coated with Eudragit® | Male Wistar rats | Gut microbial community and SCFA production | Treatment of mice with induced colitis with encapsulated L. acidophilus showed how microcapsules increased the butyrate levels; the treatment also increased the abundance of Bacteroidetes and the genera Lactobacillus and Roseburia, which were unbalanced before the treatment with the microcapsules. | 68 |
| Lactobacillus plantarum, Lactobacillus fermentum, and Bifidobacterium breve in low-methoxyl pectin capsules | 48 SPF male Kunming mice, between 6 and 8 weeks old | Gut microbial community | After treating mice with a high-fat diet and the microcapsules, there was a reduction in the Firmicutes/Bacteroidetes ratio. Also, treatment with microcapsules increased the abundance of Bifidobacterium and Lactobacillus in fecal samples. | 76 | |
| Lactobacillus acidophilus NCFM and Bifidobacterium longum BB534 in a bilayer of gelatin and pectin | 12 healthy adult volunteers | The counts of lactobacilli were increased 7 days after stopping the treatment on healthy humans. Bifidobacterium longum was not able to colonize the intestine, but its presence increased the levels of bifidobacteria in fecal samples. | 71 | ||
| Shewanella putrefaciens in alginate beads | 48 fish specimens of gilthead seabream (S. aurata L.) | Gut microbial community | Fish supplemented with the capsules containing probiotics showed high levels of Lactococcus and Lactobacillus strains and a reduction of Proteobacteria. | 67 | |
| Synbiotics | Bifidobacterium longum CICC 6259 in alginate beads containing oligosaccharides coated with chitosan | 72 eight-week-old SPF mice | Gut microbial community | Fecal samples of mice treated with the encapsulated synbiotic showed a significant increase in the content of Bifidobacterium and Lactobacillus, as well as a reduction in the abundance of Enterococcus and Escherichia. | 66 |
| Beta-(1/3,1/6)-glucan as prebiotic and blend of eight probiotic strains | 24 five-week-old ICR mice (a general-purpose mouse model) | Gut microbial community and SCFA production | Mice were treated with the capsules containing probiotics, prebiotics, and synbiotics. Prebiotic dietary intervention increased the total bacterial abundance and metabolisms related to host immune strengthening, represented in an active metabolic activity of SCFAs production. Also, while probiotics showed a modification of gut microbiota, the results showed a depletion of metabolic activities related to SCFA production. | 75 | |
An example of this is the administration of encapsulated CIN and citral (CIT) with the normal diet to broilers. In this study, the encapsulated CIN and CIT showed a modulatory effect on the gut microbiota of broilers, increasing the relative abundance of the genus Lactobacillus and decreasing that of Clostridium and Enterococcus, which are known to cause different types of infection in broilers.43,45 Also, microparticles encapsulating LGG by freeze drying allowed the probiotic to survive in an adequate concentration to colonize the colon; this was proved by the results obtained for the microbial community structure, which showed that LGG outcompeted other species of lactobacilli.69
Another example of the modulatory effect of microencapsulated probiotics is the study carried out by Chang et al.,66 where Bifidobacterium longum was co-encapsulated with a marine oligosaccharide and administered to a group of mice after proving in vitro that the probiotic survives in an adequate concentration in gastrointestinal conditions. When administered to mice, these microcapsules significantly increased the content of Bifidobacterium and Lactobacillus and reduced the content of Enterococcus and Escherichia in the gut microbiome of the mice.
Coated microcapsules containing ginger extract and Lactobacillus acidophilus were incorporated into the diet of rats with induced colitis after optimization through resistance to in vitro digestion conditions. The results showed how the optimized capsules attenuated oxidative stress and inflammatory burden and helped in the downregulation of COX-2, iNOS, and c-Myc. Also, the consumption of ginger extract and probiotic helped to restore colonic permeability and modulated the gut bacteria, restoring the genera Lactobacillus and Roseburia and phylum Bacteroidetes, and normalized SCFA production.68
Spores of the Bacillus family were co-encapsulated with a commercial blend of dietary fiber; simulation of the GIT showed how encapsulation protected the probiotic spores, allowing them and the prebiotics to interact with the simulated gut microbiota. Enhancement of Bacillaceae levels was observed, which probably resulted in a stimulatory effect on Bifidobacteriaceae, Lactobacillaceae, Prevotellaceae, Tannerellaceae, and Faecalibacterium prausnitzii, among others, contributing directly or indirectly to the stimulation of SCFA production.68,77,78
Another similar situation is the encapsulation of Lactobacillus acidophilus by spray drying with malouang juice and guar gum, which protected the probiotic from the gastrointestinal conditions. The intestinal digesta obtained at the end of in vitro digestion was assessed in in vitro colon experiments, which resulted in enhanced production of lactic acid and SCFAs and modulation of the gut microbial community, increasing the abundance of lactobacilli and bifidobacteria, while the levels of toxic ammonia and the populations of harmful bacteria such as clostridia and fecal coliforms were diminished.79
7. Regulation and laws to control the administration of supplements and functional additives in food
The growing demand for products that benefit health by more health-conscious consumers has led regulatory agencies to enforce their existing laws about the claims of food products and supplements. Around the world, probiotics and biocompounds which may be integrated in different types of food matrix or sold alone as supplements are regulated by different entities and laws.As an example, the European Union, through the European Parliament and the Council, regulates the claims and labeling of food products regarding nutritional and health value; regulations EC No107/2008 and 109/2008, which are amendments to regulation Bo1924/2006, stipulate the requirements that must be met by food products in order to make different functional claims for their nutritional or health value. In this case, these requirements are defined for the active compounds and probiotics independently of the food matrix in which they may be included. One of the main requirements for making the claims is that there should be sufficient scientific evidence, which in most cases should be clinical evidence of the functionality of the food product. Finally, if the product bearing the claim misleads consumers, the company can be penalized and the product prohibited.146
On the other hand, in the United States of America, the entity in charge of regulating these kinds of products is the Food and Drug Administration. In this case, probiotics are controlled and regulated as a supplement or food additive first in order to be an ingredient of food products. The document “Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials: Guidance for Industry” provides guidance with respect to the declaration of the quantity of living microbials that should be met to obtain the claim of functional probiotics.147 Another agency that controls the labeling and declaration of food claims is the Federal Trade Commission. Since 2001, its guide has shown how the benefits of dietary supplements should be advertised; in this, they remark how a product without sufficient evidence supported by expert professionals in the field should not be labeled with the health benefits that will be deceptive to consumers.148
In Latin America, products containing probiotics or claims that confer a health benefit for the consumer should undergo an extract process to be approved before commercialization.149 This occurs in countries like Argentina, Brazil, Mexico, and Colombia, among others. For example, in Colombia, the regulatory entity that is in charge of the labeling and verification of food products is the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA).150 In the case of products containing probiotics or with health claims, companies that want to obtain permission to commercialize their products should provide scientific information and demonstrate under clinical trials that their products meet their health objective and claims. Without accurate proof, the product should not be commercialized under that claim, and the label should not contain anything related that may misinform the consumer. Similar occurs in Brazil, where claims and their application must be registered with the Agência Nacional de Vigilância Sanitária (ANVISA).151
Finally, in Asia, Japan is the country that has commercialized and regulated nutraceutical and probiotics since 1995. To obtain approval to commercialize food under the label of probiotics or nutraceuticals that have an impact on the health of the consumer, a reviewing process in which scientific evidence should be present has to be undertaken, so an expert committee can approve the labeling.151 Within the scientific evidence, clinical trials and epidemiological studies are mandatory. On the other hand, in China there is a list of specific microorganisms that can be used in foods; however, a strong regulation on the safety requirements in the usage of these foods is not explicit.151
Although the regulatory process regarding probiotics and nutraceuticals is being observed in some parts of the world, there is no evidence of regulation regarding products that can directly modulate the gut microbiota. Also, regulation about the information on food labels should be stricter, as the labels could contain confusing information that consumers could read as health claims.
8. Conclusion
The gut microbiota can be modulated through different compounds, balancing the microbial community and promoting the production of molecules that will have a positive effect on the health of the host. However, it is important to encapsulate these effective compounds adequately, so they are bioavailable and bioaccessible to the gut microbiota and the intestinal cells. Spray drying, freeze drying, and electrodynamics are notable encapsulation techniques that permit high rates of encapsulation efficiency, high viability of microorganisms, and, together with wall materials, a high degree of protection against gastrointestinal conditions, allowing controlled release in the intestine. Although ionotropic extrusion is one of the most commonly used techniques to encapsulate probiotics because it is a soft technique that allows a high survival rate of the microorganisms during the encapsulation process, it is not recommended to use it alone when designing microcapsules with the purpose of modulating the gut microbiota, due to the porosity of wall materials with which this technique works. If it is going to be used for this purpose, it should be coupled with a coating technique to ensure that the capsules can resist the conditions in the GIT and protect the probiotics and bioactives.The information reviewed above indicates choosing prebiotics as the best modulators of gut microbiota fermentation metabolites. On the other hand, probiotics always promote an increase of beneficial gut microorganisms, which in some cases promotes their fermentation metabolites as well; however, that is not a rule. From in vivo studies, like those mentioned before with mice and broilers, the effect of this modulation is clearer, not only at the level of microbial community structure or fermentation metabolites, but at the level of typical symptoms of disease and in the improvement of some physical conditions. This give us an insight into the importance of designing encapsulates that in the later stages of research can be assessed in these kinds of in vivo studies that reflect the systemic bioactivity of the bioactives and probiotics encapsulated.
It is important to evaluate the effect of different encapsulated or non-encapsulated compounds as a process with different factors that must be carried out to achieve modulation of the microbiota and the production of beneficial compounds that improve the health of consumers in the long and short term.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Author Katherine Bauer is grateful to the Universidad de La Sabana and the Engineering Department for the Carlos Jordana scholarship to develop her Doctoral studies. This review was supported by the project ING-261-2020 funded by Universidad de La Sabana in the call for projects of 2020.References
- H. Verma, S. Phian, P. Lakra, J. Kaur, S. Subudhi, R. Lal and C. D. Rawat, Human Gut Microbiota and Mental Health: Advancements and Challenges in Microbe-Based Therapeutic Interventions, Indian J. Microbiol., 2020, 60, 405–419 CrossRef PubMed.
- E. Thursby and N. Juge, Introduction to the human gut microbiota, Biochem. J., 2017, 474, 1823–1836 CrossRef CAS PubMed.
- C. Duvallet, S. M. Gibbons, T. Gurry, R. A. Irizarry and E. J. Alm, Meta-analysis of gut microbiome studies identifies disease-specific and shared responses, Nat. Commun., 2017, 8, 1–10 CrossRef CAS.
- R. L. Guerrant, R. B. Oriá, S. R. Moore, M. O. B. Oriá and A. A. A. M. Lima, Malnutrition as an enteric infectious disease with long-term effects on child development, Nutr. Rev., 2008, 66, 487–505 CrossRef PubMed.
- P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis and J. I. Gordon, An obesity-associated gut microbiome with increased capacity for energy harvest, Nature, 2006, 444, 1027–1031 CrossRef.
- G. Wu, N. Zhao, C. Zhang, Y. Y. Lam and L. Zhao, Guild-based analysis for understanding gut microbiome in human health and diseases, Genome Med., 2021, 13, 22 CrossRef PubMed.
- R. Thirumdas, A. Kothakota, R. Pandiselvam, A. Bahrami and F. J. Barba, Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: A review, Trends Food Sci. Technol., 2021, 110, 66–77 CrossRef CAS.
- M. Abolfathi, Y. Pasdar, M. Kheiri, S. Irandoost and F. Darabi, The effect of consuming multivitamin/mineral supplements on elderly quality of life: Based on randomized control trial, J. Educ. Health Promot., 2021, 10, 63 CrossRef PubMed.
- Y. Wu, Y. Han, Y. Tao, D. Li, G. Xie, P. L. Show and S. Y. Lee, In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract, Food Res. Int., 2020, 132, 109098 CrossRef CAS PubMed.
- M. Yao, J. Wu, B. Li, H. Xiao, D. J. McClements and L. Li, Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota, Food Hydrocolloids, 2017, 72, 228–236 CrossRef CAS.
- Y. Shi, S. Zhou, S. Fan, Y. Ma, D. Li, Y. Tao and Y. Han, Encapsulation of bioactive polyphenols by starch and their impacts on gut microbiota, Curr. Opin. Food Sci., 2021, 38, 102–111 CrossRef CAS.
- A. Gonçalves, B. N. Estevinho and F. Rocha, Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds, Trends Food Sci. Technol., 2021, 114, 510–520 CrossRef.
- K. Verhoeckx and P. D. Cotter, in The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models, ed. K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka and H. Wichers, Springer International Publishing, Cham, 2015, pp. VII–XII Search PubMed.
- L. Putignani, F. del Chierico, A. Petrucca, P. Vernocchi and B. Dallapiccola, The human gut microbiota: A dynamic interplay with the host from birth to senescence settled during childhood, Pediatr. Res., 2014, 76, 2–10 CrossRef PubMed.
- S. Monira, S. Nakamura, K. Gotoh, K. Izutsu, H. Watanabe, N. H. Alam, H. P. Endtz, A. Cravioto, S. I. Ali, T. Nakaya, T. Horii, T. Iida and M. Alam, Gut microbiota of healthy and malnourished children in Bangladesh, Front. Microbiol., 2011, 8, 228 Search PubMed.
- H. J. Wu and E. Wu, The role of gut microbiota in immune homeostasis and autoimmunity, Gut Microbes, 2012, 3, 4 CrossRef PubMed.
- G. Clarke, S. Grenham, P. Scully, P. Fitzgerald, R. D. Moloney, F. Shanahan, T. G. Dinan and J. F. Cryan, The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner, Mol. Psychiatry, 2013, 18, 666–673 CrossRef CAS PubMed.
- H. J. Flint, K. P. Scott, S. H. Duncan, P. Louis and E. Forano, Microbial degradation of complex carbohydrates in the gut, Gut Microbes, 2012, 3(4), 289–306 CrossRef PubMed.
- C. F. Maurice, H. J. Haiser and P. J. Turnbaugh, Xenobiotics shape the physiology and gene expression of the active human gut microbiome, Cell, 2013, 152, 39–50 CrossRef CAS PubMed.
- S. Cheng, X. Qi, M. Ma, L. Zhang, B. Cheng, C. Liang, L. Liu, P. Li, O. P. Kafle, Y. Wen and F. Zhang, Assessing the Relationship Between Gut Microbiota and Bone Mineral Density, Front. Genet., 2020, 11, 6 CrossRef CAS PubMed.
- K. Sjögren, C. Engdahl, P. Henning, U. H. Lerner, V. Tremaroli, M. K. Lagerquist, F. Bäckhed and C. Ohlsson, The gut microbiota regulates bone mass in mice, J. Bone Miner. Res., 2012, 27, 1357–1367 CrossRef.
- R. E. Ley, D. A. Peterson and J. I. Gordon, Ecological and evolutionary forces shaping microbial diversity in the human intestine, Cell, 2006, 124, 837–848 CrossRef CAS PubMed.
- J. Qin, R. Li, J. Raes, M. Arumugam, K. S. Burgdorf, C. Manichanh, T. Nielsen, N. Pons, F. Levenez, T. Yamada, D. R. Mende, J. Li, J. Xu, S. Li, D. Li, J. Cao, B. Wang, H. Liang, H. Zheng, Y. Xie, J. Tap, P. Lepage, M. Bertalan, J. M. Batto, T. Hansen, D. le Paslier, A. Linneberg, H. B. Nielsen, E. Pelletier, P. Renault, T. Sicheritz-Ponten, K. Turner, H. Zhu, C. Yu, S. Li, M. Jian, Y. Zhou, Y. Li, X. Zhang, S. Li, N. Qin, H. Yang, J. Wang, S. Brunak, J. Doré, F. Guarner, K. Kristiansen, O. Pedersen, J. Parkhill, J. Weissenbach, P. Bork, S. D. Ehrlich, J. Wang, M. Antolin, F. Artiguenave, H. Blottiere, N. Borruel, T. Bruls, F. Casellas, C. Chervaux, A. Cultrone, C. Delorme, G. Denariaz, R. Dervyn, M. Forte, C. Friss, M. van de Guchte, E. Guedon, F. Haimet, A. Jamet, C. Juste, G. Kaci, M. Kleerebezem, J. Knol, M. Kristensen, S. Layec, K. le Roux, M. Leclerc, E. Maguin, R. Melo Minardi, R. Oozeer, M. Rescigno, N. Sanchez, S. Tims, T. Torrejon, E. Varela, W. de Vos, Y. Winogradsky and E. Zoetendal, A human gut microbial gene catalogue established by metagenomic sequencing, Nature, 2010, 464, 59–65 CrossRef CAS.
- B. S. Samuel and J. I. Gordon, A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10011–10016 CrossRef CAS.
- R. J. Gøbel, N. Larsen, M. Jakobsen, C. Mølgaard and K. F. Michaelsen, Probiotics to Adolescents With Obesity, J. Pediatr. Gastroenterol. Nutr., 2012, 55, 673–678 CrossRef.
- N. Ottman, H. Smidt, W. M. de Vos and C. Belzer, The function of our microbiota: who is out there and what do they do?, Front. Cell. Infect. Microbiol., 2012, 2, 104 Search PubMed.
- I. Garcia-Mantrana, M. Selma-Royo, C. Alcantara and M. C. Collado, Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population, Front. Microbiol., 2018, 890 CrossRef.
- R. K. Singh, H. W. Chang, D. Yan, K. M. Lee, D. Ucmak, K. Wong, M. Abrouk, B. Farahnik, M. Nakamura, T. H. Zhu, T. Bhutani and W. Liao, Influence of diet on the gut microbiome and implications for human health, J. Transl. Med., 2017, 15(1), 1–17 CrossRef PubMed.
- T. S. Ghosh, S. Sen Gupta, T. Bhattacharya, D. Yadav, A. Barik, A. Chowdhury, B. Das, S. S. Mande and G. B. Nair, Gut microbiomes of Indian children of varying nutritional status, PLoS One, 2014, 9(4), e95547 CrossRef PubMed.
- N. T. Baxter, A. W. Schmidt, A. Venkataraman, K. S. Kim, C. Waldron and T. M. Schmidt, Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers, mBio, 2019, 10(1), e02566-18 CrossRef PubMed.
- T. B. Bo, J. Wen, Y. C. Zhao, S. J. Tian, X. Y. Zhang and D. H. Wang, Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food, J. Steroid Biochem. Mol. Biol., 2020, 198, 105602 CrossRef CAS.
- S. Greenblum, H. C. Chiu, R. Levy, R. Carr and E. Borenstein, Towards a predictive systems-level model of the human microbiome: Progress, challenges, and opportunities, Curr. Opin. Biotechnol, 2013, 24, 810–820 CrossRef CAS.
- S. A. Shetty, F. Hugenholtz, L. Lahti, H. Smidt and W. M. de Vos, Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies, FEMS Microbiol. Rev., 2017, 41, 182–199 CrossRef CAS PubMed.
- M. Arumugam, J. Raes, E. Pelletier, D. Le Paslier, T. Yamada, D. R. Mende, G. R. Fernandes, J. Tap, T. Bruls, J. M. Batto, M. Bertalan, N. Borruel, F. Casellas, L. Fernandez, L. Gautier, T. Hansen, M. Hattori, T. Hayashi, M. Kleerebezem, K. Kurokawa, M. Leclerc, F. Levenez, C. Manichanh, H. B. Nielsen, T. Nielsen, N. Pons, J. Poulain, J. Qin, T. Sicheritz-Ponten, S. Tims, D. Torrents, E. Ugarte, E. G. Zoetendal, J. Wang, F. Guarner, O. Pedersen, W. M. De Vos, S. Brunak, J. Doré, J. Weissenbach, S. D. Ehrlich and P. Bork, Enterotypes of the human gut microbiome, Nature, 2011, 473, 174–180 CrossRef CAS PubMed.
- A. Almeida, A. L. Mitchell, M. Boland, S. C. Forster, G. B. Gloor, A. Tarkowska, T. D. Lawley and R. D. Finn, A new genomic blueprint of the human gut microbiota, Nature, 2019, 568, 499–504 CrossRef CAS.
- S. M. Jandhyala, R. Talukdar, C. Subramanyam, H. Vuyyuru, M. Sasikala and D. N. Reddy, Role of the normal gut microbiota, World J. Gastroenterol., 2015, 21, 8836–8847 CrossRef.
- M. Sgritta, S. W. Dooling, S. A. Buffington, E. N. Momin, M. B. Francis, R. A. Britton and M. Costa-Mattioli, Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder, Neuron, 2019, 101, 246–259 CrossRef CAS PubMed.
- P. W. O'Toole and I. B. Jeffery, Microbiome–health interactions in older people, Cell. Mol. Life Sci., 2018, 75, 119–128 CrossRef PubMed.
- R. Martín, L. G. Bermúdez-Humarán and P. Langella, Gnotobiotic rodents: An in vivo model for the study of microbe-microbe interactions, Front. Microbiol., 2016, 7, 409 Search PubMed.
- N. W. Hansen and A. Sams, The microbiotic highway to health—New perspective on food structure, gut microbiota, and host inflammation, Nutrients, 2018, 10, 1590 CrossRef PubMed.
- V. H. Pham, L. Kan, J. Huang, Y. Geng, W. Zhen, Y. Guo, W. Abbas and Z. Wang, Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis, J. Anim. Sci. Biotechnol., 2020, 11, 1–18 CrossRef.
- X. Yang, Y. Liu, F. Yan, C. Yang and X. Yang, Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens, Poult. Sci., 2019, 98, 2858–2865 CrossRef CAS.
- C. Yang, Y. M. Kennes, D. Lepp, X. Yin, Q. Wang, H. Yu, C. Yang, J. Gong and M. S. Diarra, Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis, Poult. Sci., 2020, 99, 936–948 CrossRef CAS PubMed.
- W. Liu, Z. Li, K. Yang, P. Sun and M. Cai, Effect of nanoemulsion loading finger citron (Citrus medica L. var. Sarcodactylis) essential oil on human gut microbiota, J. Funct. Foods, 2021, 77, 104336 CrossRef CAS.
- Z. Jiang, M. Luo, W. Ma, S. Ma, Y. Wang and K. Zhang, Protective Effects of 1,8-Cineole Microcapsules Against Inflammation and Gut Microbiota Imbalance Associated Weight Loss Induced by Heat Stress in Broiler Chicken, Front. Pharmacol., 2021, 11, 1–11 Search PubMed.
- O. Safari, M. Paolucci and H. Ahmadniaye Motlagh, Effect of dietary encapsulated organic salts (Na-acetate, Na-butyrate, Na-lactate and Na-propionate) on growth performance, haemolymph, antioxidant and digestive enzyme activities and gut microbiota of juvenile narrow clawed crayfish, Astacus leptodactylu, Aquacult. Nutr., 2021, 27, 91–104 CrossRef CAS.
- R. Gough, R. Cabrera Rubio, P. M. O'Connor, F. Crispie, A. Brodkorb, S. Miao, C. Hill, R. P. Ross, P. D. Cotter, K. N. Nilaweera and M. C. Rea, Oral Delivery of Nisin in Resistant Starch Based Matrices Alters the Gut Microbiota in Mice, Front. Microbiol., 2018, 9, 1186 CrossRef PubMed.
- W. S. F. Chung, A. W. Walker, P. Louis, J. Parkhill, J. Vermeiren, D. Bosscher, S. H. Duncan and H. J. Flint, Modulation of the human gut microbiota by dietary fibres occurs at the species level, BMC Biol., 2016, 14, 3 CrossRef.
- G. Flores, M. L. Ruiz del Castillo, A. Costabile, A. Klee, K. B. Guergoletto and G. R. Gibson, In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth, J. Funct. Foods, 2015, 16, 50–57 CrossRef CAS.
- P. Van Den Abbeele, A. Kamil, L. Fleige, Y. Chung, P. De Chavez and M. Marzorati, Different Oat Ingredients Stimulate Specific Microbial Metabolites in the Gut Microbiome of Three Human Individuals in Vitro, ACS Omega, 2018, 3, 12446–12456 CrossRef CAS.
- M. Glei, S. Zetzmann, S. Lorkowski, C. Dawczynski and W. Schlörmann, Chemopreventive effects of raw and roasted oat flakes after in vitro fermentation with human faecal microbiota, Int. J. Food Sci. Nutr., 2021, 72, 57–69 CrossRef CAS PubMed.
- P. Van den Abbeele, C. Duysburgh, T. A. Jiang, M. Rebaza, I. Pinheiro and M. Marzorati, A combination of xylooligosaccharides and a polyphenol blend affect microbial composition and activity in the distal colon exerting immunomodulating properties on human cells, J. Funct. Foods, 2018, 47, 163–171 CrossRef CAS.
- P. Van Den Abbeele, B. Taminiau, I. Pinheiro, C. Duysburgh, H. Jacobs, L. Pijls and M. Marzorati, Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells, J. Agric. Food Chem., 2018, 66, 1121–1130 CrossRef CAS PubMed.
- H. Sun, Y. Chen, M. Cheng, X. Zhang, X. Zheng and Z. Zhang, The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro, J. Food Sci. Technol., 2018, 55, 399–407 CrossRef CAS PubMed.
- Z. Yang, S. Huang, D. Zou, D. Dong, X. He, N. Liu, W. Liu and L. Huang, Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice, Amino Acids, 2016, 48, 2731–2745 CrossRef CAS PubMed.
- D. Daguet, I. Pinheiro, A. Verhelst, S. Possemiers and M. Marzorati, Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem, J. Funct. Foods, 2016, 20, 369–379 CrossRef CAS.
- N. Reichardt, M. Vollmer, G. Holtrop, F. M. Farquharson, D. Wefers, M. Bunzel, S. H. Duncan, J. E. Drew, L. M. Williams, G. Milligan, T. Preston, D. Morrison, H. J. Flint and P. Louis, Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production, ISME J., 2018, 12, 610–622 CrossRef CAS PubMed.
- P. Van den Abbeele, N. Sprenger, J. Ghyselinck, B. Marsaux, M. Marzorati and F. Rochat, A comparison of the in vitro effects of 2′fucosyllactose and lactose on the composition and activity of gut microbiota from infants and toddlers, Nutrients, 2021, 13, 1–23 CrossRef PubMed.
- V. T. Pham, M. Calatayud, C. Rotsaert, N. Seifert, N. Richard, P. van den Abbeele, M. Marzorati and R. E. Steinert, Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro, Nutrients, 2021, 13, 1125 CrossRef CAS PubMed.
- M. A. Farag, A. Abdelwareth, I. E. Sallam, M. el Shorbagi, N. Jehmlich, K. Fritz-Wallace, S. Serena Schäpe, U. Rolle-Kampczyk, A. Ehrlich, L. A. Wessjohann and M. von Bergen, Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model, J. Adv. Res., 2020, 23, 47–59 CrossRef CAS PubMed.
- S. Pérez-Burillo, S. Rajakaruna, S. Pastoriza, O. Paliy and J. Ángel Rufián-Henares, Bioactivity of food melanoidins is mediated by gut microbiota, Food Chem., 2020, 316, 126309 CrossRef PubMed.
- R. B. Canani, F. De Filippis, R. Nocerino, M. Laiola, L. Paparo, A. Calignano, C. De Caro, L. Coretti, L. Chiariotti, J. A. Gilbert and D. Ercolini, Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow’s milk containing heat-killed Lactobacillus paracasei CBA L74, Appl. Environ. Microbiol., 2017, 83(19), e01206-17 CrossRef PubMed.
- Y. Wu, Y. Han, Y. Tao, D. Li, G. Xie, P. L. Show and S. Y. Lee, In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract, Food Res. Int., 2020, 132, 109098 CrossRef CAS PubMed.
- T. R. Aguirre-Calvo, S. Molino, M. Perullini, J. Á. Rufián-Henares and P. R. Santagapita, Effect of in vitro digestion-fermentation of Ca(II)-alginate beads containing sugar and biopolymers over global antioxidant response and short chain fatty acids production, Food Chem., 2020, 333, 127483 CrossRef CAS PubMed.
- M. A. Azcarate-Peril, A. J. Ritter, D. Savaiano, A. Monteagudo-Mera, C. Anderson, S. T. Magness and T. R. Klaenhammer, Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E367–E375 CrossRef CAS PubMed.
- L. Chang, P. Wang, S. Sun, Z. Shen and X. Jiang, A synbiotic marine oligosaccharide microcapsules for enhancing Bifidobacterium longum survivability and regulatory on intestinal probiotics, Int. J. Food Sci. Technol., 2021, 56, 362–373 CrossRef CAS.
- H. Cordero, F. A. Guardiola, S. T. Tapia-Paniagua, A. Cuesta, J. Meseguer, M. C. Balebona, M. Á. Moriñigo and M. Á. Esteban, Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.), Fish Shellfish Immunol., 2015, 45, 608–618 CrossRef CAS PubMed.
- P. K. Deol, P. Khare, D. P. Singh, M. Bishnoi, K. K. Kondepudi and I. P. Kaur, Pharmabiotic beads with improved encapsulation and gut survival for management of colonic inflammation associated gut derangements, J. Drug Targeting, 2020, 28, 1053–1062 CrossRef CAS PubMed.
- F. B. Haffner, T. van de Wiele and A. Pasc, Original behavior of: L. rhamnosus GG encapsulated in freeze-dried alginate-silica microparticles revealed under simulated gastrointestinal conditions, J. Mater. Chem. B, 2017, 5, 7839–7847 RSC.
- A. H. Khalid, K. S. Ullah, S. Naveed, F. Latif, T. N. Pasha, I. Hussain and S. N. Qaisrani, Effects of spray dried yeast (Saccharomyces cerevisiae) on growth performance and carcass characteristics, gut health, cecal microbiota profile and apparent ileal digestibility of protein, amino acids and energy in broilers, Trop. Anim. Health Prod., 2021, 53, 252 CrossRef PubMed.
- V. Mai, S. Waugh, D. Byrd, D. Simpson and M. Ukhanova, Novel encapsulation improves recovery of probiotic strains in fecal samples of human volunteers, Appl. Microbiol. Biotechnol., 2017, 101, 1419–1425 CrossRef CAS PubMed.
- A. M. Neyrinck, J. Rodriguez, B. Taminiau, C. Amadieu, F. Herpin, F. A. Allaert, P. D. Cani, G. Daube, L. B. Bindels and N. M. Delzenne, Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: a double-blind randomized placebo-controlled trial, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- F. Odun-Ayo, J. Mellem and L. Reddy, The effect of modified citrus pectin-probiotic on faecal lactobacilli in Balb/c mice, Food Sci. Technol., 2017, 37, 478–482 CrossRef.
- C. O’Reilly, Ó. O’Sullivan, P. D. Cotter, P. M. O’Connor, F. Shanahan, A. Cullen, M. C. Rea, C. Hill, I. Coulter and R. P. Ross, Encapsulated cyclosporine does not change the composition of the human microbiota when assessed ex vivo and in vivo, J. Med. Microbiol., 2020, 69, 1–10 CrossRef.
- V. Singh, K. Muthuramalingam, Y. M. Kim, S. Park, S. H. Kim, J. Lee, C. Hyun, T. Unno and M. Cho, Synbiotic supplementation with prebiotic Schizophyllum commune derived β-(1,3/1,6)-glucan and probiotic concoction benefits gut microbiota and its associated metabolic activities, Appl. Biol. Chem., 2021, 64, 7 CrossRef CAS.
- Q. Sun, X. Liu, Y. Zhang, Y. Song, X. Ma, Y. Shi and X. Li, L. plantarum, L. fermentum, and B. breve Beads Modified the Intestinal Microbiota and Alleviated the Inflammatory Response in High-Fat Diet–Fed Mice, Probiotics Antimicrob. Proteins, 2020, 12, 535–544 CrossRef CAS PubMed.
- C. Duysburgh, P. Van den Abbeele, K. Krishnan, T. F. Bayne and M. Marzorati, A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro, Int. J. Pharm.: X, 2019, 1, 100021 CAS.
- T. Shinde, A. P. Perera, R. Vemuri, S. V. Gondalia, D. J. Beale, A. V. Karpe, S. Shastri, W. Basheer, B. Southam, R. Eri and R. Stanley, Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases, Eur. J. Nutr., 2020, 59, 3669–3689 CrossRef CAS PubMed.
- P. Chaikham, V. Kemsawasd and P. Seesuriyachan, Spray drying probiotics along with maoluang juice plus Tiliacora triandra gum for exposure to the in vitro gastrointestinal environments, LWT, 2017, 78, 31–40 CrossRef CAS.
- R. C. Robertson, C. Seira Oriach, K. Murphy, G. M. Moloney, J. F. Cryan, T. G. Dinan, R. P. Ross and C. Stanton, Deficiency of essential dietary n-3 PUFA disrupts the caecal microbiome and metabolome in mice, Br. J. Nutr., 2017, 118, 959–970 CrossRef CAS PubMed.
- H. Li, Y. Zhu, F. Zhao, S. Song, Y. Li, X. Xu, G. Zhou and C. Li, Fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- E. Y. Huang, V. A. Leone, S. Devkota, Y. Wang, M. J. Brady and E. B. Chang, Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue, JPEN, J. Parenter. Enteral Nutr., 2013, 37, 746–754 CrossRef CAS PubMed.
- R. Caesar, V. Tremaroli, P. Kovatcheva-Datchary, P. D. Cani and F. Bäckhed, Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling, Cell Metab., 2015, 22, 658–668 CrossRef CAS PubMed.
- Y. H. Ma, Q. Wang, J. Gong and X. Y. Wu, Formulation of Granules for Site-Specific Delivery of an Antimicrobial Essential Oil to the Animal Intestinal Tract, J. Pharm. Sci., 2016, 105, 1124–1133 CrossRef CAS PubMed.
- Y. Yang, S. Cui, J. Gong, S. S. Miller, Q. Wang and Y. Hua, Stability of citral in oil-in-water emulsions protected by a soy protein-polysaccharide Maillard reaction product, Food Res. Int., 2015, 69, 357–363 CrossRef CAS.
- H. J. Flint, K. P. Scott, P. Louis and S. H. Duncan, The role of the gut microbiota in nutrition and health, Nat. Rev. Gastroenterol. Hepatol., 2012, 9, 577–589 CrossRef CAS PubMed.
- G. R. Gibson, R. Hutkins, M. E. Sanders, S. L. Prescott, R. A. Reimer, S. J. Salminen, K. Scott, C. Stanton, K. S. Swanson, P. D. Cani, K. Verbeke and G. Reid, Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics, Nat. Rev. Gastroenterol. Hepatol., 2017, 14(8), 491–502 CrossRef PubMed.
- D. Davani-Davari, M. Negahdaripour, I. Karimzadeh, M. Seifan, M. Mohkam, S. J. Masoumi, A. Berenjian and Y. Ghasemi, Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications, Foods, 2019, 8(3), 92 CrossRef CAS PubMed.
- I. Sensoy, A review on the food digestion in the digestive tract and the used in vitro models, Curr. Res. Food Sci., 2021, 4, 308–319 CrossRef PubMed.
- C. Li, W. Yu, P. Wu and X. D. Chen, Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract, Trends Food Sci. Technol., 2020, 96, 114–126 CrossRef CAS.
- K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka and H. Wichers, The impact of food bioactives on health: In vitro and Ex Vivo models, Springer International Publishing, 2015 Search PubMed.
- M. J. Rein, M. Renouf, C. Cruz-Hernandez, L. Actis-Goretta, S. K. Thakkar and M. da Silva Pinto, Bioavailability of bioactive food compounds: a challenging journey to bioefficacy, Br. J. Clin. Pharmacol., 2013, 75, 588 CrossRef CAS PubMed.
- A. Rezaei, M. Fathi and S. M. Jafari, Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers, Food Hydrocolloids, 2019, 88, 146–162 CrossRef CAS.
- D. J. McClements, The biophysics of digestion: lipids, Curr. Opin. Food Sci., 2018, 21, 1–6 CrossRef.
- M. J. Rein, M. Renouf, C. Cruz-Hernandez, L. Actis-Goretta, S. K. Thakkar and M. da Silva Pinto, Bioavailability of bioactive food compounds: a challenging journey to bioefficacy, Br. J. Clin. Pharmacol., 2013, 75, 588 CrossRef CAS PubMed.
- A. Alegría, G. Garcia-Llatas and A. Cilla, in The Impact of Food Bioactives on Health, Springer International Publishing, Cham, 2015, pp. 3–12 Search PubMed.
- R. Lucas-González, M. Viuda-Martos, J. A. Pérez-Alvarez and J. Fernández-López, In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges, Food Res. Int., 2018, 107, 423–436 CrossRef PubMed.
- E. Fernández-García, I. Carvajal-Lérida and A. Pérez-Gálvez, In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency, Nutr. Res., 2009, 29, 751–760 CrossRef PubMed.
- M. T. Ariza, P. Reboredo-Rodríguez, L. Cervantes, C. Soria, E. Martínez-Ferri, C. González-Barreiro, B. Cancho-Grande, M. Battino and J. Simal-Gándara, Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs, Food Chem., 2018, 248, 155–165 CrossRef CAS PubMed.
- C. E. Sandoval-Cuellar, M. de Jesus Perea-Flores and M. X. Quintanilla-Carvajal, In vitro digestion of whey protein- and soy lecithin-stabilized High Oleic Palm Oil emulsions, J. Food Eng., 2020, 278, 109918 CrossRef CAS.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 CrossRef CAS PubMed.
- A. Guerra, L. Etienne-Mesmin, V. Livrelli, S. Denis, S. Blanquet-Diot and M. Alric, Relevance and challenges in modeling human gastric and small intestinal digestion, Trends Biotechnol., 2012, 30, 591–600 CrossRef CAS PubMed.
- D. J. McClements and Y. Li, Review of in vitro digestion models for rapid screening of emulsion-based systems, Food Funct., 2010, 1, 32–59 RSC.
- D. J. L. Mat, T. Cattenoz, I. Souchon, C. Michon and S. Le Feunteun, Monitoring protein hydrolysis by pepsin using pH-stat: In vitro gastric digestions in static and dynamic pH conditions, Food Chem., 2018, 239, 268–275 CrossRef CAS PubMed.
- D. J. L. Mat, S. Le Feunteun, C. Michon and I. Souchon, In vitro digestion of foods using pH-stat and the INFOGEST protocol: Impact of matrix structure on digestion kinetics of macronutrients, proteins and lipids, Food Res. Int., 2016, 88, 226–233 CrossRef CAS.
- L. de Souza Simões, D. A. Madalena, A. C. Pinheiro, J. A. Teixeira, A. A. Vicente and Ó. L. Ramos, Adv. Colloid Interface Sci., 2017, 243, 23–45 CrossRef PubMed.
- S. J. Hur, B. O. Lim, E. A. Decker and D. J. McClements, In vitro human digestion models for food applications, Food Chem., 2011, 125, 1–12 CrossRef CAS.
- E. Assadpour and S. M. Jafari, Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules, Annu. Rev. Food Sci. Technol., 2019, 10, 103–131 CrossRef CAS PubMed.
- I. Yasmin, M. Saeed, I. Pasha and M. A. Zia, Development of Whey Protein Concentrate-Pectin-Alginate Based Delivery System to Improve Survival of B. longum BL-05 in Simulated Gastrointestinal Conditions, Probiotics Antimicrob. Proteins, 2019, 11, 413–426 CrossRef CAS PubMed.
- M. Yao, B. Li, H. Ye, W. Huang, Q. Luo, H. Xiao, D. J. McClements and L. Li, Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles, Food Hydrocolloids, 2018, 83, 246–252 CrossRef CAS.
- M. Yao, J. Wu, B. Li, H. Xiao, D. J. McClements and L. Li, Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota, Food Hydrocolloids, 2017, 72, 228–236 CrossRef CAS.
- Y. Wu and G. Zhang, Synbiotic encapsulation of probiotic Latobacillus plantarum by alginate -arabinoxylan composite microspheres, LWT, 2018, 93, 135–141 CrossRef CAS.
- A. S. Vaziri, I. Alemzadeh, M. Vossoughi and A. C. Khorasani, Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites, Carbohydr. Polym., 2018, 199, 266–275 CrossRef CAS PubMed.
- N. Liao, B. Luo, J. Gao, X. Li, Z. Zhao, Y. Zhang, Y. Ni and F. Tian, Oligosaccharides as co-encapsulating agents: effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract, Biotechnol. Lett., 2019, 41, 263–272 CrossRef CAS PubMed.
- L. E. Shi, Z. H. Li, D. T. Li, M. Xu, H. Y. Chen, Z. L. Zhang and Z. X. Tang, Encapsulation of probiotic lactobacillus bulgaricus in alginate'milk microspheres and evaluation of the survival in simulated gastrointestinal conditions, J. Food Eng., 2013, 117, 99–104 CrossRef CAS.
- P. K. Deol, P. Khare, D. P. Singh, G. Soman, M. Bishnoi, K. K. Kondepudi and I. P. Kaur, Managing colonic inflammation associated gut derangements by systematically optimised and targeted ginger extract-Lactobacillus acidophilus loaded pharmacobiotic alginate beads, Int. J. Biol. Macromol., 2017, 105, 81–91 CrossRef CAS PubMed.
- A. Bepeyeva, J. M. S. de Barros, H. Albadran, A. K. Kakimov, Z. K. Kakimova, D. Charalampopoulos and V. V. Khutoryanskiy, Encapsulation of Lactobacillus casei into Calcium Pectinate-Chitosan Beads for Enteric Delivery, J. Food Sci., 2017, 82, 2954–2959 CrossRef CAS PubMed.
- R. Iqbal, T. Zahoor, N. Huma, A. Jamil and G. Ünlü, In vitro GIT Tolerance of Microencapsulated Bifidobacterium bifidum ATCC 35914 Using Polysaccharide-Protein Matrix, Probiotics Antimicrob. Proteins, 2019, 11, 830–839 CrossRef CAS PubMed.
- E. Akanny, S. Bourgeois, A. Bonhommé, C. Commun, A. Doleans-Jordheim, F. Bessueille and C. Bordes, Development of enteric polymer-based microspheres by spray-drying for colonic delivery of Lactobacillus rhamnosus GG, Int. J. Pharm., 2020, 584, 119414 CrossRef CAS PubMed.
- M. Moayyedi, M. H. Eskandari, A. H. E. Rad, E. Ziaee, M. H. H. Khodaparast and M. T. Golmakani, Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469, J. Funct. Foods, 2018, 40, 391–399 CrossRef CAS.
- M. Bustamante, B. D. Oomah, M. Rubilar and C. Shene, Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying, Food Chem., 2017, 216, 97–105 CrossRef CAS PubMed.
- D. Eratte, K. Dowling, C. J. Barrow and B. P. Adhikari, In vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates, Food Chem., 2017, 227, 129–136 CrossRef CAS PubMed.
- D. Vázquez-Maldonado, V. Espinosa-Solis, C. Leyva-Porras, P. Aguirre-Bañuelos, F. Martinez-Gutierrez, M. Román-Aguirre and M. Z. Saavedra-Leos, Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol, Processes, 2020, 8, 849 CrossRef.
- I. M. Enache, A. M. Vasile, E. Enachi, V. Barbu, N. Stănciuc and C. Vizireanu, Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices, Molecules, 2020, 25, 1700 CrossRef CAS PubMed.
- T. Huppertz, Homogenization of Milk: Low Pressure Homogenization (High-Speed Mixing, Ultrasonics, Microfluidizers, Membrane Emulsification), Encyclopedia of Dairy Sciences, 2022, pp. 691–694 Search PubMed.
- M. Â. P. R. Cerqueira, A. C. B. Pinheiro, C. V. S. do Carmo, C. M. M. Duarte, M. D. G. C. da Cunha and A. A. M. de Oliveiros Soares, in Nutrient Delivery, Elsevier, 2017, pp. 43–85 Search PubMed.
- S. Pund, A. Joshi and V. Patravale, Improving bioavailability of nutraceuticals by nanoemulsification, Nutraceuticals, 2016, 481–534 CAS.
- L. Ricaurte, M. de J. Perea-Flores, A. Martinez and M. X. Quintanilla-Carvajal, Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization), Innovative Food Sci. Emerging Technol., 2016, 35, 75–85 CrossRef CAS.
- M. Kumherová, K. Veselá, K. Jokešová, I. Klojdová and Š. Horáčková, Influence of co-encapsulation of bifidobacterium animalis subsp. lactis Bb12 with inulin and ascorbic acid on its viability, Czech J. Food Sci., 2020, 38, 57–62 CrossRef.
- J. Burgain, C. Gaiani, C. Cailliez-Grimal, C. Jeandel and J. Scher, Encapsulation of Lactobacillus rhamnosus GG in microparticles: Influence of casein to whey protein ratio on bacterial survival during digestion, Innovative Food Sci. Emerging Technol., 2013, 19, 233–242 CrossRef CAS.
- K. C. H. van der Ark, A. D. W. Nugroho, C. Berton-Carabin, C. Wang, C. Belzer, W. M. de Vos and K. Schroen, Encapsulation of the therapeutic microbe Akkermansia muciniphila in a double emulsion enhances survival in simulated gastric conditions, Food Res. Int., 2017, 102, 372–379 CrossRef CAS PubMed.
- P. Lai, W. Daear, R. Löbenberg and E. J. Prenner, Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate, Colloids Surf., B, 2014, 118, 154–163 CrossRef CAS PubMed.
- S. P. Ahirrao, P. S. Gide, B. Shrivastav and P. Sharma, Ionotropic Gelation: A Promising Cross Linking Technique for Hydrogels, Res. Rev.: J. Pharm. Nanotechnol., 2013, 2, 1–6 CrossRef.
- K. N. Dyvelkov and J. Sloth, New Advances in Spray-Drying Processes, Microencapsulation Food Ind., 2014, 57–63 CAS.
- D. G. Burke, P. D. Cotter, R. P. Ross and C. Hill, Microbial production of bacteriocins for use in foods, Microbial Production of Food Ingredients, Enzymes and Nutraceuticals, 2013, pp. 353–384 Search PubMed.
- S. Aragón-Rojas, A. J. Hernández-Álvarez, I. Mainville, Y. Arcand and M. X. Quintanilla-Carvajal, Effect of the carrier material, drying technology and dissolution media on the viability of: Lactobacillus fermentum K73 during simulated gastrointestinal transit, Food Funct., 2020, 11, 2339–2348 RSC.
- D. Xavier dos Santos, A. A. Casazza, B. Aliakbarian, R. Bedani, S. M. I. Saad and P. Perego, Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying, LWT, 2019, 99, 404–410 CrossRef CAS.
- N. Kanha, J. M. Regenstein, S. Surawang, P. Pitchakarn and T. Laokuldilok, Properties and kinetics of the in vitro release of anthocyanin-rich microcapsules produced through spray and freeze-drying complex coacervated double emulsions, Food Chem., 2021, 340, 127950 CrossRef CAS PubMed.
- S. Wang, Y. Shi and L. Han, Development and evaluation of microencapsulated peony seed oil prepared by spray drying: Oxidative stability and its release behavior during in vitro digestion, J. Food Eng., 2018, 231, 1–9 CrossRef CAS.
- Z. Berk, Freeze drying (lyophilization) and freeze concentration, Food Process Engineering and Technology, 2018, pp. 567–581 Search PubMed.
- R. B. Waghmare, A. B. Perumal, J. A. Moses and C. Anandharamakrishnan, Recent Developments in Freeze Drying of Foods, Innovative Food Processing Technologies, 2021, pp. 82–99 Search PubMed.
- S. M. Jafari, in Nanoencapsulation of Food Bioactive Ingredients, 2017, pp. 1–62 Search PubMed.
- J. A. Tapia-Hernández, F. Rodríguez-Félix and I. Katouzian, Nanocapsule formation by electrospraying, Nanoencapsulation Technologies for the Food and Nutraceutical Industries, 2017, pp. 320–345 Search PubMed.
- L. Ricaurte and M. X. Quintanilla-Carvajal, Use of electrospinning technique to produce nanofibres for food industries: A perspective from regulations to characterisations, Trends Food Sci. Technol., 2019, 85, 92–106 CrossRef.
- C. C. Coghetto, G. B. Brinques, N. M. Siqueira, J. Pletsch, R. M. D. Soares and M. A. Z. Ayub, Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids, J. Funct. Foods, 2016, 24, 316–326 CrossRef CAS.
- European Comission, Nutrition and Health Claims, https://ec.europa.eu/food/safety/labelling-and-nutrition/nutrition-and-health-claims_en, (accessed 22 October 2021).
- FDA, Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials: Guidance for Industry Draft Guidance, 2018.
- Dietary Supplements: An Advertising Guide for Industry | Federal Trade Commission, https://www.ftc.gov/tips-advice/business-center/guidance/dietary-supplements-advertising-guide-industry#Application, (accessed 22 October 2021).
- A. Binetti, P. Burns, D. Tomei, J. Reinheimer and G. Vinderola, Probiotics Regulation in Latin American Countries, Lactic Acid Bact., 2020, 683–692 Search PubMed.
- INVIMA, Normograma del Instituto Nacional de Vigilancia de Medicamentos y Alimentos – INVIMA [RESOLUCION 333 de 2011 Ministerio de la Protección Social], 2011 Search PubMed.
- S. Koirala and A. K. Anal, Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims, Future Foods, 2021, 3, 100013 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |
