Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison between dietary assessment methods and biomarkers in estimating dietary (poly)phenol intake†
Yifan
Xu
a,
Yong
Li
a,
Xuemei
Ma
 b,
Wafa
Alotaibi
a,
Melanie
Le Sayec
a,
Alex
Cheok
b,
Wafa
Alotaibi
a,
Melanie
Le Sayec
a,
Alex
Cheok
 a,
Eleanor
Wood
a,
Sabine
Hein
ac,
Paul
Young Tie Yang
a,
Wendy L.
Hall
a,
Chiara
Nosarti
de,
Paola
Dazzan
bf,
Rachel
Gibson
a and
Ana
Rodriguez-Mateos
a,
Eleanor
Wood
a,
Sabine
Hein
ac,
Paul
Young Tie Yang
a,
Wendy L.
Hall
a,
Chiara
Nosarti
de,
Paola
Dazzan
bf,
Rachel
Gibson
a and
Ana
Rodriguez-Mateos
 *a
*a
aDepartment of Nutritional Sciences, School of Life Course and Population Sciences, Faculty of Life Sciences and Medicine, King's College London, 150 Stamford Street, London SE1 9NH, UK. E-mail: ana.rodriguez-mateos@kcl.ac.uk; Tel: +44 (0)20 7848 4349
bDepartment of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), King's College London, London, UK
cSchool of Psychology and Clinical Language Sciences, University of Reading, Reading, UK
dDepartment of Child and Adolescent Psychiatry, Institute of Psychiatry, Psychology, and Neuroscience, King's College London, London, UK
eCentre for the Developing Brain, Department of Perinatal Imaging & Health, School of Biomedical Engineering & Imaging Sciences, King's College London, London, UK
fNational Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, London, UK
First published on 9th January 2023
Abstract
Background: although widely used, there is limited understanding of the suitability of different dietary assessment tools to estimate (poly)phenol intake. This study aims to compare the agreement between a food frequency questionnaire (FFQ) and a 7-day food diary (7DD) in assessing (poly)phenol intake and explore their associations with the urinary and plasma (poly)phenol metabolites. Methods: healthy free-living participants aged 18–80 years (n = 413) completed a 7DD and an FFQ (EPIC-Norfolk) and provided a 24 h urine and a fasting plasma sample. A comprehensive in-house (poly)phenol database was used to estimate (poly)phenol intake. The phenolic metabolite levels were analysed using a validated LC-MS method. The agreement between dietary assessment methods and biomarkers were evaluated by intraclass correlation coefficients (ICC), weighted kappa, quartile classification, Bland–Altman plots and correlations. Results: the total (poly)phenol intake estimated from FFQ was higher than from 7DD (median 1463 and 1042 mg d−1, respectively). The agreement between FFQ and 7DD were moderate (ICC 0.51–0.59) for total (poly)phenols, flavan-3-ols, total phenolic acids, hydroxycinnamic acids and alkylmethoxyphenols, and were poor for all the other classes and subclasses (ICC 0.00–0.48). Positive correlations with total urine phenolic metabolites were found in FFQ estimated anthocyanins, dihydroflavonols, total lignans, tyrosols, alkylmethoxyphenols, total phenolic acids, and total stilbenes and the 7DD estimated theaflavins and thearubigins (all FDR adjusted p values < 0.1). No significant correlations were found between total plasma phenolic metabolites and (poly)phenol intake. Conclusion: agreements between dietary assessment tools were moderate for the major classes of (poly)phenols, while agreements between (poly)phenol intake and biomarkers were poor. Future research using biomarker approaches to increase the accuracy of estimating (poly)phenol exposure in larger populations is needed.
Introduction
Diet is one of the most important modifiable factors that influence human health. It is well evidenced that a healthy and balanced diet is composed of an adequate intake of plant-based foods such as fruits, vegetables, whole grains, and seeds.1 Some studies have also linked beneficial effects to the consumption of specific food items for example, cocoa products,2 coffee,3 and tea.4 Beside micronutrients, fibre and healthy fatty acids, those foods are also rich in (poly)phenols, a large group of phytochemical compounds that occur naturally in plants. Evidence from both epidemiological studies and clinical trials have been accumulating on the health benefits of (poly)phenol consumption, in particular on improving cardiovascular function5,6 and age-related cognitive decline.7 However, there is still not enough evidence to give suitable dietary advice to the general population regarding (poly)phenol consumption for optimal health benefits.6In epidemiological studies, the relationship between diet and health outcomes is usually evaluated using dietary assessment tools, such as food frequency questionnaires (FFQs) and food records. The accuracy of dietary assessment is key to the validity of the evidence derived from these studies. However, when it comes to estimating (poly)phenol intake, although FFQs have been widely used, only very few of them have been validated for estimating (poly)phenol intake.8 There is also very limited understanding of the performance of various dietary assessment tools in capturing the main food sources of different classes and subclasses of (poly)phenols.9,10 The lack of validation, together with limited availability of (poly)phenol composition data, makes it difficult to interpret the current research evidence linking (poly)phenols and health.
Unlike urinary nitrogen, sodium, or potassium,11,12 and serum carotenoids or vitamin C,11 which have been used to validate dietary assessment tools to estimate nutrient or bioactive intake, no gold standard biomarkers have been established for estimating (poly)phenol exposure. One of the challenges to establish biomarkers associated with (poly)phenol intake is that many metabolites are not specific to a certain parent compound and could come from multiple sources, or even from non-polyphenol sources, such as food additives,13 drugs14 or endogenous metabolism, such as the metabolites coming from the dopamine pathway.15 Besides, the short half-life of some of these metabolites makes it especially challenging to reflect long-term intake. Very few biomarkers have been partially validated to reflect the intake of a single (poly)phenol group, such as isoflavones16 and flavan-3-ols.17,18 Total urinary (poly)phenols analysed using a modified Folin–Ciocalteu assay has been proposed to reflect total (poly)phenol intake.19 However, doubts still arise regarding their specificity to phenolic compounds20 and no information on the different subclasses of (poly)phenols can be obtained with this method. Currently, no method exists to estimate (poly)phenol intake using fully validated biomarkers, however, a number of studies have used a combination of different (poly)phenol metabolites as surrogate markers for estimating (poly)phenol intakes in both epidemiological21–24 and randomized controlled studies.25,26
To address the above gaps in knowledge, we aimed to assess (poly)phenol intake in a group of free-living participants in the UK using an FFQ, a 7-day food diary (7DD) and a combination of 110 phenolic metabolites measured in 24 h urine and fasting plasma samples, representing most of the major subclasses of dietary (poly)phenols. We compared the differences and agreements between dietary assessment methods and plasma and urinary (poly)phenol metabolites in evaluating (poly)phenol intake levels and sources.
Methods
Study population
The POLYNTAKE cohort consists of participants from a series of clinical trials conducted from 2015 to 2021 at the Metabolic Research Unit of the Department of Nutritional Sciences, King's College London, UK. These studies applied the same dietary assessment protocols and tools, which include an FFQ and a 7DD, to obtain the baseline dietary intake of the participants. The baseline dietary assessment data, plasma and urine biomarkers and cardiovascular risk markers were used to study the relationships between dietary (poly)phenol intake and cardiovascular health in a cross-sectional design. Participants aged 18–80 years old from 7 clinical trials (n = 515) were involved in this work (Trial registration number/registration date: NCT03434574/2018-01-11, NCT03041961/2017-02-02, NCT04084457/2019-09-02, NCT04179136/ 2019-11-21, NCT03553225/2018-05-29, NCT03995602/2019-06-20, and NCT03573414/2018-06-01). These studies were approved by the Research Ethics Committee of King's College London (Ethics numbers: RESCM-17/18-5283, HR-15/16-3739, HR-18/19-9091, HR-18/19-8999, HR-17/18-5703, RESCM-18/19-9036, and HR-17/18-5353) and conducted following the Declaration of Helsinki. All participants gave written informed consent before participation. The included participants were healthy men and women whose BMI were 18–35 kg m−2. The participants were excluded if there was no available dietary assessment data from either FFQ or 7-day food records (n = 93), FFQs with >10 missing ticks (n = 5) or food diaries with >3 days of missing logs (n = 3). One participant was excluded for the high consumption of (poly)phenols (12 g d−1) estimated from 7DD due to the high consumption of cloves (68.5 g d−1). In the end, 413 participants with both FFQ and 7-day food diaries were included in the analysis.Dietary assessment
A self-administered FFQ (the EPIC-Norfolk FFQ version 6, CAMB/PQ/6/1205)27 was completed by the participants at the baseline visit to reflect their habitual diet before intervention. The FFQ (accessed from https://www.epic-norfolk.org.uk/wp-content/uploads/2020/11/CAMB-PQ-6-1205a_front.pdf) was designed and validated12 for estimating nutrient and food intake in the past 1 year in UK adults and was applied in the EPIC-Norfolk study. The FFQ collects the dietary intake of 130 food items that are consumed in the UK diet with 9 frequency options ranging from “Never or less than once a month” to “more than 6 times per day”. Details about the types of milk, cereals, cooking fat and amount of visible fat consumed in meals were also investigated in a separate section of the FFQ.One week prior to the baseline visit, participants were given a 7-day food diary (the EPIC-Norfolk 7DD)27 to record habitual food or drinks consumed in a consecutive 7 days. The food diary was a paper-based booklet with sections of 6 different time slots: before breakfast, breakfast, mid-morning, lunch, tea, evening meal, and later evening on each day. Participants were asked to record the type and amount of foods and drinks in as much detail as possible. Standard photos for portion sizes and instructions and examples were given at the beginning pages of the food diaries to help with the recording.
Estimation of (poly)phenol intake
The FFQs were coded with the Microsoft Access software (Access 2019, Microsoft, USA) and transformed into daily food and nutrient intake levels by the FFQ EPIC Tool for Analysis (FETA) software.28 Nutrient composition was calculated from the McCance and Widdowson's “The Composition of Foods (5th edition)” and supplementary materials.29 The 7-day food records were coded into standard food codes and portions by trained coders using the Nutritics software (Nutritics Research Edition v 5.76, Nutritics, Dublin, Ireland). A standard protocol was followed by all coders to minimize coding error and improve the quality and consistency of the data.An in-house database involving food (poly)phenol content data from Phenol-Explorer30 and USDA databases,31–33 and published papers34–53 was applied to calculate daily (poly)phenol intake. This database was developed to cover as many food items consumed by the study population as possible and incorporate comprehensive (poly)phenol content data and specific recipes that could represent (poly)phenol intake in the UK diet. In this database, the (poly)phenol content of some foods, for example mushrooms, certain seeds (quinoa, chia seeds, and hempseeds), fruits (goji berry, jujube, juniper berry, lychee, acai berry, and barberry), and oils (linseed oil, avocado oil) were obtained from published papers since they were not available in either Phenol-Explorer or USDA databases. Only data analysed by high-performance liquid chromatography (HPLC), gas chromatography (GC), capillary electrophoresis (CE) and mass spectrometry (MS) methods were included. All (poly)phenol content data with compounds attached to a sugar moiety were transformed into the corresponding amount in aglycones so that they could be summarized with data from other sources. The proanthocyanidin content data analysed using normal phase HPLC methods was used and if not available, the data from reverse phase HPLC was applied instead. For processed foods, if the only available food content data was from raw food, a process yield factor obtained from Phenol-Explorer30 was multiplied by the unprocessed content to determine the content of cooked food. When there was no available yield factor, a factor of a similar food item (e.g., common cabbage for spring greens, pigeon peas for mung beans) or similar processing method (e.g., boiled for blanched, fried for roasted) of the same item was applied instead. Recipes for composite foods, if not indicated by participants, were obtained from the supplementary materials in McCane and Widdowson's The Composition of Foods29,54 and the internet such as BBC Good Food (https://www.bbcgoodfood.com/recipes). Foods with no (poly)phenol content (e.g., animal products) were removed from the calculation. (Poly)phenol intake (mg d−1) was calculated using the daily food intake (g d−1) multiplied by the corresponding (poly)phenol content in the database (mg per 100 g) and divided by 100. Total or subclasses of (poly)phenol intakes were calculated by summarising all compounds under the group. The classification of (poly)phenols followed the one in Phenol-Explorer. In addition to the subtotals of classes and subclasses, extra subtotals were calculated for flavan-3-ol monomers, theaflavins, proanthocyanidins, tyrosols, and ellagitannins.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min at 4 °C. An aliquot of 100 μL urine sample was diluted with 400 μL HPLC water (Sigma-Aldrich, Steinheim, Germany) to reach 5-fold dilution prior to processing while plasmas were processed directly. Then, 350 μL of diluted urine or plasma samples were with the same volume of 4% phosphoric acid acidified (85% HPLC grade, Yorlab, Fluka, York, UK) and vortexed. An aliquot of 600 μL of the acidified sample was loaded onto the Oasis 96-well reversed-phase HLB μ-SPE plate (Waters, Eschborn, Germany). The plates were then washed with HPLC water (200 μL) and 0.2% acetic acid (200 μL) (glacial HPLC grade, Thermo Fisher Scientific, Loughborough, UK) before being eluded with 30 μL of methanol (HPLC grade, Sigma-Aldrich, Steinheim, Germany) containing 0.1% formic acid and 10 nM ammonium formate (HPLC grade, Sigma-Aldrich, Steinheim, Germany) for 3 times. A total volume of 90 μL eluded samples were collected in the 96-well collection plates and then spiked with internal standard taxifolin (final concentration 0.25 mg ml−1) and added 35 μL of HPLC water to make a 130 μL of the final volume. A pooled sample was prepared by mixing all the samples in the batch with the same volume and it is processed in the same way as the other samples. To calculate the recoveries of different compounds, the pooled sample was also loaded into 2 wells, with one spiked with a mix of standards (30 μL) in the loaded sample and the other one fortified with the same volume of mixed standards in the collected sample.
000g for 15 min at 4 °C. An aliquot of 100 μL urine sample was diluted with 400 μL HPLC water (Sigma-Aldrich, Steinheim, Germany) to reach 5-fold dilution prior to processing while plasmas were processed directly. Then, 350 μL of diluted urine or plasma samples were with the same volume of 4% phosphoric acid acidified (85% HPLC grade, Yorlab, Fluka, York, UK) and vortexed. An aliquot of 600 μL of the acidified sample was loaded onto the Oasis 96-well reversed-phase HLB μ-SPE plate (Waters, Eschborn, Germany). The plates were then washed with HPLC water (200 μL) and 0.2% acetic acid (200 μL) (glacial HPLC grade, Thermo Fisher Scientific, Loughborough, UK) before being eluded with 30 μL of methanol (HPLC grade, Sigma-Aldrich, Steinheim, Germany) containing 0.1% formic acid and 10 nM ammonium formate (HPLC grade, Sigma-Aldrich, Steinheim, Germany) for 3 times. A total volume of 90 μL eluded samples were collected in the 96-well collection plates and then spiked with internal standard taxifolin (final concentration 0.25 mg ml−1) and added 35 μL of HPLC water to make a 130 μL of the final volume. A pooled sample was prepared by mixing all the samples in the batch with the same volume and it is processed in the same way as the other samples. To calculate the recoveries of different compounds, the pooled sample was also loaded into 2 wells, with one spiked with a mix of standards (30 μL) in the loaded sample and the other one fortified with the same volume of mixed standards in the collected sample.
The (poly)phenol metabolites were analysed with UHPLC-triple-quadrupole mass spectrometry (UHPLC-Q-q-Q MS) on a SHIMADZU 8060 (Shimadzu, Kyoto, Japan). The samples (5 μL) were injected through a Raptor Biphenyl Column 2.1 × 50 mm, 1.8 μm (Restek, Bellefonte, USA) coupled with a compatible guard column 5 × 2.1 mm, 2.7 μm (Restek, Bellefonte, USA) before reaching the HESI source. The reverse-phase chromatography was performed under a 0.5 ml min−1 flow rate at 30 °C with mobile phases composed of water (phase A) and acetonitrile (phase B) both acidified with 0.1% formic acid. The gradient was 14 minutes joined with a 2-minute equilibration. Details about the UHPLC and MS parameters were described previously.55
A total of 110 phenolic metabolites were identified and quantified by authentic standards in the samples. The targeted compounds were identified with 1–3 transitions at the specific retention times and quantified with dilutions of mixed authentic standards analysed in the same run. We used the peak areas of the compounds relative to the taxifolin internal standards for the quantifications to minimize the influence of device performance variances during the run. The pooled and spiked pooled samples were used to calculate the recovery rate of the compounds and used as quality controls throughout the run. The collected raw data was analysed with LabSolutions software (SHIMADZU, Kyoto, Japan) and calculated with Microsoft Excel (Excel 2020, Microsoft, USA). The limit of quantification (LOQ), inter- and intra-batch coefficient of variation (CV%) of the analysis were presented in ESI Table 1.†
Agreements between the estimated (poly)phenol intake from the two dietary assessment tools were presented as two-way mixed effects intraclass correlation coefficients (ICC). ICCs from both the consistency (ICC-C) and agreement (ICC-A) models were calculated. The consistency model ignores the systematic difference between FFQ and 7DD while the agreement model compares the absolute values of estimated intake. To estimate the agreements between the two methods in ranking participants into quartiles, weighted Kappas were calculated. The linear weights (Cicchetti–Allison weights) were applied in the model. The 95% confidence intervals (CI) were calculated for ICC and kappa values. (Poly)phenol intake was adjusted for self-reported energy intake by the residual method and the ICCs and kappa values were also calculated for the energy-adjusted (poly)phenol intake. The ICC values lower than 0.5 were considered poor agreement, and between 0.50 to 0.75 were considered moderate agreement, between 0.75 and 0.90 were considered good agreement, and above 0.90 were considered excellent agreement.57 The weighted kappa followed the same criteria as the unweighted kappa,58 which means kappa values over 0.75 were considered excellent agreement, 0.40–0.75 were considered fair to good agreement, and lower than 0.40 were considered poor agreement. The percentages of participants grouped into the same or opposite quartiles were also calculated to show agreements between the two methods. Bland–Altman plots were used to present the agreements between the two dietary assessment tools on the absolute estimated intakes of total and different types of (poly)phenols.
Correlations between the energy-adjusted dietary (poly)phenols and the levels of urine and plasma (poly)phenol metabolites were calculated. The Spearman's rank correlation coefficients and significant levels were presented in heatmaps. The significant levels were adjusted for multiple comparisons using the false discovery rate (FDR) method and p < 0.1 was used as the significant level after adjustment. Agreements between the intake of total (poly)phenol, total flavonoids, flavonols, flavanones, isoflavonoids, total lignans, total stilbenes, and tyrosols estimated by FFQ and 7DD and the corresponding (poly)phenol metabolite levels in urine and plasma were assessed by their abilities in ranking participants in quartiles. These classes or subclasses were chosen because the metabolites were relatively representative of the intakes of the same group. Weighted Kappa and percentages in the same or opposite quartiles were calculated.
To test the effect of misreporting on the results, sensitivity analysis was conducted in a subgroup of participants reporting plausible energy intake (EI) by 7DD (n = 242). The plausible reporting was defined by EI to basal metabolic rate (BMR) ratio within the 95% confidence interval (CI) calculated from the Goldberg equation59,60 according to their physical activity levels (PAL). The participants were classified as having low, moderate or high physical activity levels according to a self-reported long-form international physical activity questionnaire (IPAQ) and estimated PALs of 1.4, 1.6, and 1.8 were assigned to each level, respectively according to previous UK studies.60 Besides, men participants with energy intake levels <800 kcal d−1 or >4000 kcal d−1, and women participants with energy intake levels <500 kcal d−1 or >3500 kcal d−1 were also considered as misreporting. The results of sensitivity analysis are shown in ESI.†
Results
Participant characteristics
A total of 413 participants were included in the analysis. The average age of the study population was 43.2 ± 18.6 years old. Among them, there were 231 (55.9%) women and 182 (44.1%) men. The average BMI of the participants was 23.8 ± 3.4 kg m−2. Most of the participants reported high physical activity levels (68.6% of the 392 available data), non-smoking (70.9%) and alcohol consumption lower than 14 units per week (95.4%). The average energy intake obtained from FFQ was 1746 ± 785 kcal and the fibre intake was 17.5 ± 10.6 g d−1. Participants had an average fruit intake of 267.5 ± 247.8 g d−1 and vegetable intake (excluding white potato) of 299.3 ± 300.6 g d−1 estimated from the FFQ. The food groups and nutrient intake levels of the study population are detailed in the ESI Table 2.†FFQ and 7DD estimated (poly)phenol intake
The median (IQR) of total (poly)phenol intake estimated from FFQs was 1463 (1407) mg d−1, and the median (IQR) intake estimated from 7DD was 1042 (1178) mg d−1. The FFQ estimated total (poly)phenol intake was significantly higher than the 7DD estimated intake in nearly all subgroups of participants stratified by sex, age groups, ethnicity, BMI levels, physical activity levels, smoking status, and alcohol consumption levels (all p values < 0.05), except for subgroups with age ≥65 years old and alcohol consumption >14 units per week, in which the difference was not significant (p = 0.077 and 0.066, respectively) (Table 1). The FFQ estimated intakes were higher than 7DD estimated intake in most of the classes and subclasses of (poly)phenols (all p values < 0.05), except for anthocyanins, dihydroflavonols, flavones, total lignans, total other (poly)phenols, tyrosols, hydroxyphenylacetic acids, hydroxyphenylpropanoic acids, and total stilbenes, the 7DD estimated intakes were higher than the FFQ estimated intakes (all p values < 0.001) (Table 2). Among these (poly)phenol subclasses where 7DD showed a higher estimation of intake than FFQ, the main food sources from 7DDs that were not reported in the FFQs include blueberries, red wine and aubergine for anthocyanins, red wine for dehydroflavonols (where for FFQ the default item used in (poly)phenol estimation is rose wine), soups for flavones, sesame oil and seeds for total lignans, herbs and spices for total other (poly)phenols, and olives for tyrosols, hydroxyphenylacetic acids and hydroxyphenylpropanoic acids (Table 3). No significant difference was found between the FFQ and 7DD in the estimated intakes of proanthocyanidins (p = 0.934) and alkylphenols (p = 0.162).| Characteristics | N | FFQ | 7DD | P | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| 7DD: 7-day food diary, BMI: body mass index, FFQ: food frequency questionnaire, IQR: interquartile range, SD: standard deviation. N: number of participants in each group. P values were from the paired-sample Wilcoxon signed-rank test. | |||||||
| Sex | Men | 182 | 1367 (1023) | 1194 (1355) | 1101 (874) | 870 (1120) | <0.001 |
| Women | 231 | 1653 (973) | 1642 (1234) | 1297 (844) | 1163 (1146) | <0.001 | |
| Age group | 18–34 | 184 | 1138 (792) | 944 (1225) | 813 (581) | 697 (712) | <0.001 |
| 35–49 | 64 | 1784 (1327) | 1525 (1453) | 1434 (1122) | 1121 (1180) | 0.016 | |
| 50–64 | 83 | 1862 (1005) | 1810 (1613) | 1419 (828) | 1368 (1182) | <0.001 | |
| ≥65 | 82 | 1860 (835) | 1760 (600) | 1719 (797) | 1642 (970) | 0.077 | |
| Ethnicity | White | 269 | 1640 (950) | 1629 (1333) | 1356 (858) | 1197 (1052) | <0.001 |
| Black | 18 | 1886 (1715) | 1252 (1552) | 1080 (762) | 865 (1432) | 0.010 | |
| Asian | 85 | 1108 (877) | 870 (1285) | 790 (722) | 527 (712) | 0.005 | |
| Mixed | 14 | 1211 (968) | 939 (1303) | 866 (951) | 586 (223) | 0.042 | |
| BMI | <25 | 275 | 1463 (962) | 1409 (1462) | 1204 (870) | 1027 (1145) | <0.001 |
| ≥25 | 138 | 1655 (1076) | 1495 (1049) | 1225 (849) | 1067 (1184) | <0.001 | |
| Physical activity | High | 296 | 1578 (1015) | 1600 (1386) | 1255 (911) | 1060 (1209) | <0.001 |
| Moderate | 106 | 1388 (880) | 1271 (1292) | 1157 (803) | 989 (1116) | <0.001 | |
| Low | 17 | 1241 (797) | 1188 (1309) | 840 (467) | 738 (544) | 0.045 | |
| Smoking | Never | 293 | 1446 (968) | 1309 (1306) | 1193 (885) | 1003 (1098) | <0.001 |
| Former | 96 | 1693 (1042) | 1705 (1350) | 1288 (765) | 1182 (994) | <0.001 | |
| Current | 24 | 1856 (1177) | 1890 (1648) | 1120 (947) | 806 (1605) | 0.001 | |
| Alcohol consumption | Not drinking | 134 | 1679 (1212) | 1524 (1667) | 1279 (1086) | 1040 (1362) | <0.001 |
| ≤14 unit per w | 260 | 1460 (886) | 1453 (1319) | 1191 (711) | 1053 (1030) | <0.001 | |
| >14 unit per w | 19 | 1363 (819) | 1317 (1085) | 1006 (960) | 688 (576) | 0.066 | |
| (Poly)phenols | FFQ | 7DD | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | % | Mean (SD) | Median (IQR) | % | |
| 7DD: 7-day food diary, BMI: body mass index, FFQ: food frequency questionnaire, IQR: inter-quartile range, SD: standard deviation. %: percentage of contribution to the total (poly)phenol intake. Significant values were from paired-sample Wilcoxon signed-rank test.a p < 0.001.b 0.001 < p < 0.05. | ||||||
| Total (poly)phenols | 1527.0 (1004.3) | 1463.4 (1406.9) | 100.0 | 1210.8 (861.7) | 1041.9 (1178.1)a | 100.0 |
| Total Flavonoids | 709.1 (553.1) | 492.5 (714.3) | 46.4 | 534.5 (520.3) | 400.6 (470.0)a | 44.1 |
| Anthocyanins | 8.4 (8.3) | 6.5 (7.6) | 0.6 | 27.7 (50.9) | 14.0 (32.6)a | 2.3 |
| Chalcones | 0.0 (0.0) | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 (0.0)a | 0.0 |
| Dihydroflavonols | 0.1 (0.1) | 0.0 (0.1) | 0.0 | 0.8 (1.6) | 0.0 (0.8)a | 0.1 |
| Dihydrochalcones | 2.8 (3.5) | 2.1 (3.2) | 0.2 | 1.7 (2.3) | 1.0 (2.5)a | 0.1 |
| Total flavan-3-ols | 595.4 (522.3) | 383.5 (697.3) | 39.0 | 436.7 (495.8) | 277.8 (432.8)a | 36.1 |
| Flavan-3-ol monomers | 159.4 (155.9) | 101.9 (227.0) | 10.4 | 144.1 (183.2) | 70.4 (193.8)b | 11.9 |
| Theaflavins | 36.0 (38.6) | 23.6 (55.8) | 2.4 | 16.2 (36.4) | 0.0 (14.7)a | 1.3 |
| Thearubigins | 235.4 (252.3) | 154.5 (364.5) | 15.4 | 107.0 (237.3) | 3.9 (96.9)a | 8.8 |
| Proanthocyanidins | 164.5 (136.7) | 133.4 (117.8) | 10.8 | 169.4 (164.0) | 131.4 (156.3) | 14.0 |
| Flavanones | 34.3 (46.2) | 23.6 (36.7) | 2.2 | 12.1 (23.6) | 3.0 (10.5)a | 1.0 |
| Flavones | 4.5 (3.0) | 3.9 (2.8) | 0.3 | 9.2 (17.4) | 6.1 (7.1)a | 0.8 |
| Flavonols | 55.9 (46.7) | 48.4 (32.8) | 3.7 | 43.4 (31.5) | 36.2 (38.1)a | 3.6 |
| Isoflavonoids | 7.8 (17.4) | 1.6 (5.7) | 0.5 | 2.9 (8.5) | 0.3 (1.8)a | 0.2 |
| Total Phenolic acids | 793.5 (744.9) | 609.0 (1155.7) | 52.0 | 621.2 (592.7) | 473.8 (741.3)a | 51.3 |
| Hydroxybenzoic acids | 65.8 (55.2) | 44.3 (73.5) | 4.3 | 52.3 (61.4) | 34.4 (53.2)a | 4.3 |
| Ellagitannins | 3.6 (5.6) | 1.5 (3.8) | 0.2 | 3.3 (16.9) | 0.0 (0.0)a | 0.3 |
| Hydroxycinnamic acids | 727.7 (740.5) | 556.0 (1205.6) | 47.7 | 568.0 (586.3) | 383.7 (734.5)a | 46.9 |
| Hydroxyphenylacetic acids | 0.0 (0.1) | 0.0 (0.0) | 0.0 | 0.8 (3.7) | 0.1 (0.4)a | 0.1 |
| Hydroxyphenylpropanoic acids | 0.0 (0.0) | 0.0 (0.0) | 0.0 | 0.1 (0.3) | 0.0 (0.0)a | 0.0 |
| Total Stilbenes | 0.1 (0.1) | 0.1 (0.1) | 0.0 | 0.6 (1.2) | 0.1 (0.7)a | 0.1 |
| Total Lignans | 1.9 (1.1) | 1.6 (1.4) | 0.1 | 6.9 (14.7) | 2.4 (4.2)a | 0.6 |
| Other (poly)phenols | 22.3 (26.5) | 16.2 (15.4) | 1.5 | 47.6 (89.7) | 26.5 (36.3)a | 3.9 |
| Tyrosols | 0.7 (0.8) | 0.4 (0.8) | 0.0 | 5.5 (12.6) | 2.5 (6.0)a | 0.5 |
| Alkylmethoxyphenols | 2.5 (2.6) | 1.8 (4.2) | 0.2 | 1.9 (2.2) | 1.3 (2.8)a | 0.2 |
| Alkylphenols | 16.3 (25.5) | 9.7 (11.3) | 1.1 | 22.3 (35.5) | 10.0 (19.2) | 1.8 |
| (Poly)phenols | FFQ estimated (poly)phenol food sources (% to total) | 7DD estimated (poly)phenol food sources (% to total) |
|---|---|---|
| Total (poly)phenols | Coffee (42.4%), Tea (31.9%), Apples (5.6%), Oranges (1.7%), Brown rice (1.4%) | Coffee (39.7%), Tea (26.0%, black tea 24.0%, green tea 1.9%), Apples (4.3%), Chocolates (2.4%), Cocoa powder and drinks (2.1%) |
| Total flavonoids | Tea (62.2%), Apples (10.4%), Oranges (3.7%), Tomatoes (2.6%), Hazelnuts (2.6%) | Tea (52.0%, black tea 48.1%, green tea 3.6%), Apples (8.4%), Chocolates (5.5%), Cocoa powder and drinks (4.6%), tomatoes (1.6%) |
| Anthocyanins | Fruit squash drink (26.1%), Strawberries (18.0%), Raspberries (14.5%), Fruit jam (10.5%), Fruit yogurt (8.2%) | Blueberries (12.0%), Strawberries (11.5%), Red wine (9.5%), Fruit squash drink (8.6%), Aubergine (8.1%) |
| Chalcones | Broad beans (86.9%), Lager (13.1%) | Ale (53.9%), Lager (33.9%), Broad beans (8.8%), Bitter beers (2.6%), Ginger ale (0.4%) |
| Dihydroflavonols | Wine (100%) | Red wine (90.0%), White wine (8.6%), Rose wine (0.7%), Meat dishes (0.5%), Tiramisu (0.1%) |
| Dihydrochalcones | Apples (85.5%), Apple juice (13.5%), Apple chutney (1.0%), Breakfast cereals (0.02%) | Apples (87.3%), Apple juice (10.9%), Mixed fruit juice (1.0%), Fruit smoothie (0.6%), Meat dishes (0.1%) |
| Total flavan-3-ols | Tea (75.5%), Apples (11.8%), Hazelnuts (3.1%), Grapes (2.0%), Drinking chocolate powder (1.6%) | Tea (62.1%, black tea 57.6%, green tea 4.3%), Apples (9.6%), Chocolates (6.6%), Cocoa powder and drinks (5.7%), Strawberries (2.4%) |
| Flavan-3-ol monomers | Tea (89.1%), Apples (3.9%), Broad beans (2.4%), Apple juices (0.7%), Bananas (0.45%) | Tea (89.0%, black tea 76.3%, green tea 11.9%, herb tea 0.8%), Apples (2.5%), Red wine (1.5%), Cocoa powder and drinks (1.4%), Chocolates (1.4%) |
| Theaflavins | Tea (100%) | Black tea (100%) |
| Thearubigins | Tea (100%) | Black tea (99.8%), Green tea (0.2%) |
| Proanthocyanidins | Apples (37.0%), Tea (18.6%), Hazelnuts (10.4%), Grapes (6.9%), Drinking chocolate powder (5.0%) | Apples (22.7%), Chocolates (15.9%), Cocoa powder and drinks (13.4%), Tea (11.7%, black tea 11.0%, green tea 0.7%), Strawberries (5.8%) |
| Flavanones | Oranges (72.3%), Orange juice (14.1%), Grapefruit (11.3%), Tomatoes (0.9%), Trifle (0.2%) | Oranges (51.0%), Orange juice (16.9%), Grapefruit (15.5%), Tomatoes (5.1%), Lemon juice (2.9%) |
| Flavones | Brown bread (22.8%), Pizza (12.2%), Celery (9.7%), Orange juice (7.6%), Spinach (5.0%) | Soups (25.8%), White breads (11.84%), Brown breads (10.2%), Vegetable dishes (5.3%), Pizza (5.2%) |
| Flavonols | Tomatoes (30.3%), Spinach (28.4%), Tea (13.3%), Onions (7.6%), Vegetable soup (6.3%) | Tomatoes (38.2%), Tea (15.2%, black tea 13.2%, green tea 2.0%), Spinach (10.0%), Soups (4.6%), Vegetable dishes (4.2%) |
| Isoflavonoids | Tofu (51.6%), Soya milk (27.6%), Beansprouts (16.0%),Vegeburger (2.9%), Soya mince (1.5%) | Soya milk (28.4%), Tofu (12.3%), Soya beans (7.8%), Black bean sauce (7.5%), Beansprouts (7.4%) |
| Total Phenolic acids | Coffee (80.0%), Tea (6.5%), Brown rice (2.6%), Apples (1.5%), White rice (1.4%) | Coffee (76.7%), Tea (5.9%, black tea 5.4%, green tea 0.5%), White rice (1.4%), Apples (1.2%), Blueberries (0.9%) |
| Hydroxybenzoic acids | Tea (69.7%), Raspberries (7.6%), Garlic (5.8%), Strawberries (2.4%), White rice (1.5%) | Tea (58.5%, black tea 53.5%, green tea 4.9%), Raspberries (7.9%), Strawberries (6.0%), Lager (2.7%), Red wine (2.4%) |
| Ellagitannins | Raspberries (99.2%), Fruit flavoured ice-cream (0.8%) | Raspberries (96.5%), Mixed berries (4.5%), Fruit yogurt (1.8%), Fruit smoothie (1.4%), Pomegranate juice (0.2%) |
| Hydroxycinnamic acids | Coffee (87.0%), Brown rice (2.8%), Apples (1.6%), White rice (1.4%), Tea (1.0%) | Coffee (83.9%), White rice (1.4%), Apples (1.3%), Tea (1.0%, black tea 0.9%, green tea 0.1%), Blueberries (0.9%) |
| Hydroxyphenylacetic acids | Lager (99.4%), Olive oil (0.5%), Fat spread (20–25% fat not polyunsaturated)(0.1%) | Olives (84.1%), Lager (7.9%), Red wine (3.5%), White wine (1.9%), Vegetable dishes (1.4%) |
| Hydroxyphenylpropanoic acids | Not estimated from diet | Olives (96.5%), Vegetable dishes (3.5%) |
| Total Stilbenes | Wine (58.4%), Grapes (20.0%), Strawberries (10.3%), Fruit yogurt (4.7%), Mousse (3.4%) | Red wine (79.0%), White wine (12.3%), Strawberries (3.8%), Grapes (1.5%), Rose wine (0.8%) |
| Total Lignans | Boiled potatoes (30.0%), Roast potatoes (11.0%), Broccoli (10.5%), Potato chips (7.5%), Tea (5.9%) | Sesame oil (36.5%), Flaxseeds (9.1%), Sesames (6.2%), Mixed seeds (4.9%), Nut bar (4.6%) |
| Other (poly)phenols | Coffee (23.1%), Wholemeal bread (19.6%), Breakfast cereals (19.2%), Spaghetti, wholemeal (15.6%), Brown bread (6.7%) | Herbs and spices (27.7%, dried cloves 11.4%, ground turmeric 9.2%), Breakfast cereals (23.8%), Coffee (8.3%), Wholemeal breads (8.0%), Olives (5.5%) |
| Tyrosols | Wine (43.3%), Olive oil (31.7%), Lager (18.0%), Fat spread (20–25% fat, not polyunsaturated) (3.6%), Sherry (2.8%) | Olives (47.2%), Olive oil (25.4%), Red wine (12.21%), Sauces (4.2%, pesto sauce 3.9%), Lager (3.64%) |
| Alkylmethoxyphenols | Coffee (91.2%), Fat spread (70% fat, polyunsaturated) (5.6%), Lager (2.3%), Rapeseed oil (0.4%), Fat spread (40% fat, not polyunsaturated) (0.3%) | Coffee (90.4%), Lager (4.7%), Rapeseed oil (1.2%), Vegetable dishes (0.8%), Soups (0.5%) |
| Alkylphenols | Wholemeal bread (26.5%), Breakfast cereals (26.1%), Spaghetti, wholemeal (21.1%), Brown bread (9.1%), Spaghetti, white (9.1%) | Breakfast cereals (50.8%), Wholemeal breads (17.1%), Rye bread (7.0%), Brown bread (6.1%), White breads (4.0%) |
In terms of the contribution of individual subclasses, phenolic acids were the major type of (poly)phenols estimated from both FFQs (52.0%) and 7DDs (51.3%), followed by flavonoids (46.4% and 44.1%, respectively). Besides, lignans, stilbenes and other (poly)phenols all had a higher percentage of contribution in 7DD estimated intakes compared to FFQ (Table 2).
Regarding food sources, FFQ and 7DD derived similar results in most of the (poly)phenol classes and subclasses (Table 3). Non-alcoholic drinks were the major food sources of total (poly)phenols estimated from both tools (75.6% and 67.0% for FFQ and 7DD, respectively) followed by fruits and products (10.8%, 10.9%), vegetables (5.8%, 5.6%) and cereals and products (4.1%, 5.7%). As to individual food items, coffee contributed the most to the total intake in both FFQ and 7DD data (42.4% and 39.7%, respectively) followed by tea (31.9% and 26.0%, respectively). The average coffee intake was 1.2 ± 1.4 cups per d (234.1± 271.3 g d−1) from FFQ and 0.8 ± 1.0 cups per d (162.2± 188.3 g d−1) from 7DD. The average tea intake was 1.5 ± 1.6 cups per d (289.6 ± 310.4 g d−1) from FFQ and 1.3 ± 1.8 cups per d (250.5± 349.8) g d−1 from 7DD (standardized as 190 g per cup for both coffee and tea according to default portion size in the EPIC-FFQ). The intakes of both coffee and tea estimated from FFQs were higher than the amount estimated from 7DDs (both p < 0.001). Apples contributed 5.6% and 4.3% to the total intake in FFQ and 7DD. Chocolates, cocoa powder and drinks presented 2.4% and 2.1% of contribution in 7DD while in FFQ they presented 0.03% and 0.6% to the total intake, respectively. Oranges contributed 1.7% in FFQs, compared to 0.5% in 7DD. The 7DD yielded 1783 types of foods and among them, 975 were not measured in the FFQ. These foods contributed 10.1% of the total (poly)phenol intake, 11.3% of the flavonoids intake, 6.0% of the phenolic acids intake, 1.5% of the stilbenes intake, 37.9% of the lignans intake, and 46.0% of the other (poly)phenols intake estimated from the 7DDs.
Agreements between the FFQ and 7DD estimated intakes
There was moderate reliability between FFQ and 7DD estimated total (poly)phenol in absolute values (ICC-A: 0.53, 95% CI: 0.41–0.62). As for (poly)phenol classes and subclasses intakes, moderate agreements were found between FFQ and 7DD estimated total flavan-3-ols (ICC-A: 0.51, 95% CI: 0.41–0.60), flavan-3-ol monomers (ICC-A: 0.59, 95% CI: 0.52–0.65), total phenolic acids (ICC-A: 0.59, 95% CI: 0.50–0.66), hydroxycinnamic acids (ICC-A: 0.59, 95% CI: 0.51–0.66) and alkymethoxyphenols (ICC-A: 0.60, 95% CI: 0.52–0.66). Poor reliabilities were found for the rest of the (poly)phenol classes and subclasses, although they were significantly correlated (rho 0.11–0.65, all p < 0.05), as shown in Table 4. For some types of (poly)phenols, the reliabilities between FFQ and 7DD estimated intakes were extremely poor, for example, anthocyanins, chalcones, dihydroflavonols, flavones, ellagitannins, hydroxyphenylacetic acids, hydroxyphenylpropanoic acids, total stilbenes, total lignans, total other (poly)phenols, and tyrosols. The ICCs were lower than 0.1 in these (poly)phenol groups. After adjusting for energy intake, the reliabilities between FFQ and 7DD improved slightly for total and all subclasses of (poly)phenols (data is shown in the ESI Table 3†). The estimated total flavonoids showed moderate reliabilities (ICC-A: 0.53, 95% CI: 0.46–0.60).| (Poly)phenols | ICC-A | (95% CI) | ICC-C | (95% CI) | Kappa | (95% CI) | Same quartile (%) | Opposite quartile (%) | Spearman's Rho |
|---|---|---|---|---|---|---|---|---|---|
| ICC-C: intraclass correlation coefficient-consistency model: when systematic difference between FFQ and 7-day food record estimated (poly)phenol intakes were not relevant. ICC-A: intraclass correlation coefficient-agreement model: when systematic difference between FFQ and 7-day food record estimated (poly)phenol intakes were relevant. Kappa: weighted kappa coefficient (linear weights). 95% CI: 95% confidence interval.a p < 0.001.b p = 0.021.c p = 0.003.d There was no reported value from FFQ for hydroxyphenylpropanoic acids, so only agreement on the absolute estimated values was assessed. | |||||||||
| Total (poly)phenols | 0.53 | (0.41, 0.62) | 0.56 | (0.49, 0.62) | 0.46 | (0.40, 0.52) | 47.94 | 2.42 | 0.63a |
| Total Flavonoids | 0.48 | (0.37, 0.57) | 0.50 | (0.43, 0.57) | 0.39 | (0.32, 0.45) | 42.37 | 2.42 | 0.55a |
| Anthocyanins | 0.03 | (−0.05, 0.12) | 0.03 | (−0.06, 0.13) | 0.15 | (0.08, 0.22) | 31.48 | 7.99 | 0.24a |
| Chalcones | 0.06 | (−0.03, 0.16) | 0.07 | (−0.03, 0.16) | 0.15 | (0.08, 0.22) | 32.93 | 9.93 | 0.23a |
| Dihydroflavonols | 0.06 | (−0.03, 0.14) | 0.07 | (−0.03, 0.16) | 0.48 | (0.42, 0.54) | 49.64 | 2.91 | 0.65a |
| Dihydrochalcones | 0.33 | (0.23, 0.42) | 0.34 | (0.26, 0.43) | 0.33 | (0.26, 0.39) | 39.23 | 4.36 | 0.48a |
| Total flavan-3-ols | 0.51 | (0.41, 0.60) | 0.54 | (0.46, 0.60) | 0.41 | (0.35, 0.47) | 43.34 | 2.91 | 0.58a |
| Flavan-3-ol monomers | 0.59 | (0.52, 0.65) | 0.59 | (0.52, 0.65) | 0.46 | (0.40, 0.52) | 48.67 | 3.15 | 0.61a |
| Theaflavins | 0.41 | (0.23, 0.54) | 0.47 | (0.39, 0.54) | 0.22 | (0.15, 0.29) | 33.66 | 4.12 | 0.42a |
| Thearubigins | 0.41 | (0.24, 0.55) | 0.47 | (0.39, 0.54) | 0.31 | (0.25, 0.38) | 37.05 | 2.18 | 0.54a |
| Proanthocyanidins | 0.26 | (0.17, 0.35) | 0.26 | (0.17, 0.35) | 0.30 | (0.23, 0.37) | 38.50 | 5.57 | 0.39a |
| Flavanones | 0.20 | (0.08, 0.31) | 0.23 | (0.14, 0.32) | 0.26 | (0.20, 0.33) | 35.35 | 5.33 | 0.38a |
| Flavones | 0.00 | (−0.09, 0.09) | 0.00 | (−0.10, 0.10) | 0.09 | (0.02, 0.16) | 29.54 | 10.65 | 0.11b |
| Flavonols | 0.12 | (0.03, 0.22) | 0.13 | (0.03, 0.22) | 0.14 | (0.08, 0.21) | 29.06 | 7.02 | 0.23a |
| Isoflavonoids | 0.19 | (0.10, 0.28) | 0.20 | (0.11, 0.30) | 0.21 | (0.14, 0.28) | 33.90 | 6.54 | 0.32a |
| Total Phenolic acids | 0.59 | (0.50, 0.66) | 0.61 | (0.54, 0.66) | 0.51 | (0.45, 0.57) | 51.57 | 2.42 | 0.66a |
| Hydroxybenzoic acids | 0.44 | (0.35, 0.51) | 0.45 | (0.37, 0.52) | 0.38 | (0.32, 0.45) | 43.10 | 3.15 | 0.58a |
| Ellagitannins | 0.08 | (−0.02, 0.17) | 0.08 | (−0.02, 0.17) | 0.14 | (0.07, 0.21) | 31.96 | 7.99 | 0.26a |
| Hydroxycinnamic acids | 0.59 | (0.51, 0.66) | 0.61 | (0.54, 0.66) | 0.50 | (0.44, 0.56) | 48.91 | 1.69 | 0.67a |
| Hydroxyphenylacetic acids | 0.00 | (−0.09, 0.09) | 0.00 | (−0.09, 0.10) | 0.20 | (0.13, 0.27) | 31.72 | 6.78 | 0.32a |
| Hydroxyphenylpropanoic acids | 0.00 | (−0.09, 0.09) | — | — | — | — | — | — | — |
| Total Stilbenes | 0.08 | (−0.01, 0.17) | 0.10 | (0.00, 0.19) | 0.37 | (0.30, 0.43) | 42.13 | 2.91 | 0.54a |
| Total Lignans | 0.00 | (−0.09, 0.09) | 0.00 | (−0.10, 0.09) | 0.07 | (0.00, 0.15) | 27.60 | 11.86 | 0.15c |
| Other (poly)phenols | 0.05 | (−0.04, 0.14) | 0.06 | (−0.04, 0.15) | 0.23 | (0.16, 0.30) | 35.35 | 5.81 | 0.35a |
| Tyrosols | 0.01 | (−0.08, 0.09) | 0.01 | (−0.09, 0.10) | 0.20 | (0.13, 0.27) | 31.96 | 7.02 | 0.28a |
| Alkylmethoxyphenols | 0.60 | (0.52, 0.66) | 0.61 | (0.55, 0.67) | 0.47 | (0.40, 0.53) | 49.15 | 3.15 | 0.64a |
| Alkylphenols | 0.23 | (0.13, 0.32) | 0.23 | (0.14, 0.32) | 0.28 | (0.21, 0.35) | 38.50 | 5.08 | 0.40a |
In the ability of ranking participants according to (poly)phenol intake levels, the reliabilities between FFQ and 7DD were poor to moderate. Similarly, the estimated total (poly)phenol intake (ICC-C: 0.56, 95% CI:0.49–0.62), total flavonoid intake (ICC-C: 0.50, 95% CI: 0.43–0.57), total flavan-3-ol intake (ICC-C: 0.54, 95% CI:0.46–0.60), flavan-3-ol monomer intake (ICC-C: 0.59, 95% CI: 0.52–0.65), total phenolic acid intake (ICC-C: 0.61, 95% CI: 0.54–0.66), hydroxycinnamic acid intake (ICC-C: 0.61, 95% CI: 0.54–0.66) and alkymethoxyphenol intake (ICC-C: 0.61, 95% CI: 0.55–0.67) showed moderate reliability between the two methods. Similarly, when sorting participants into quartiles by intakes, fair agreement between the FFQ and 7DD were seen for total (poly)phenols (kappa: 0.46, 95% CI: 0.40–0.52), dihydroflavonols (kappa: 0.48, 95% CI: 0.42–0.54), total flavan-3-ols (kappa: 0.41, 95% CI: 0.35–0.47), flavan-3-ol monomers (kappa: 0.46, 95% CI: 0.40–0.52), total phenolic acids (kappa: 0.51, 95% CI: 0.45–0.57), hyroxycinnamic acids (kappa: 0.50, 95% CI: 0.44–0.56) and alkylmethoxyphenols (kappa: 0.47, 95% CI: 0.40–0.53). The agreements between the estimated intake of all other classes and subclasses were poor (Table 4). When comparing the estimated (poly)phenol intake after adjusting for energy intake, the ICC-C did not improve while the kappa values decreased slightly (ESI Table 3†).
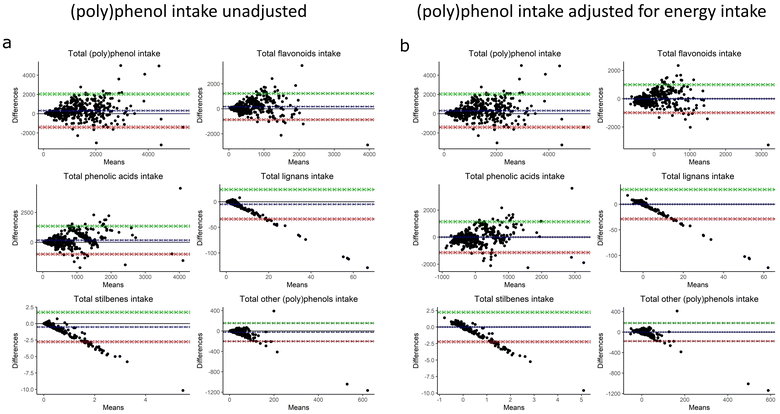
Bland–Altman plots showed that the agreement between the estimated total (poly)phenol intake from FFQ and 7DD varied along with the levels of intake (Fig. 1a). The bias increased proportionally with the levels of intake in total and all classes of (poly)phenols. For total (poly)phenol intake, the bias of FFQ estimated intake to the 7DD estimated intake was 316.2 mg d−1 (95% CI: 231.1–401.2) and the agreement range was −1406.9 to 2039.3 mg d−1 (95% CI: −1552.4∼-1261.5, 1893.8–2184.7, respectively). However, for total lignans, total stilbenes and total other (poly)phenols, the biases were negative (−4.9 mg d−1, −0.5 mg d−1, −25.3 mg d−1, respectively) and presented in the same direction. This means that the 7DD estimated intakes were higher than the FFQ estimated intakes for total lignans, stilbenes, and other (poly)phenols while the differences increased with the levels of intake in a proportional manner. When adjusted for energy intakes, the bias of FFQ to the 7DD estimated intakes get closer to 0 mg d−1 for total and all (poly)phenol classes (Fig. 1b). The bias of FFQ estimated total (poly)phenol intake to the 7DD estimated intake turned to 3.3 × 10−14 mg d−1 (95% CI: −78.3–78.3) after energy adjustment. The Bland–Altman plots of individual subclasses of (poly)phenols are shown in the supplementary materials (ESI Fig. 1–4†).
 | ||
| Fig. 1 Bland–Altman plots on estimated total and major classes of (poly)phenol intake by FFQ and 7DD (n = 413). FFQ: food frequency questionnaire; 7DD: 7-day food diary. | ||
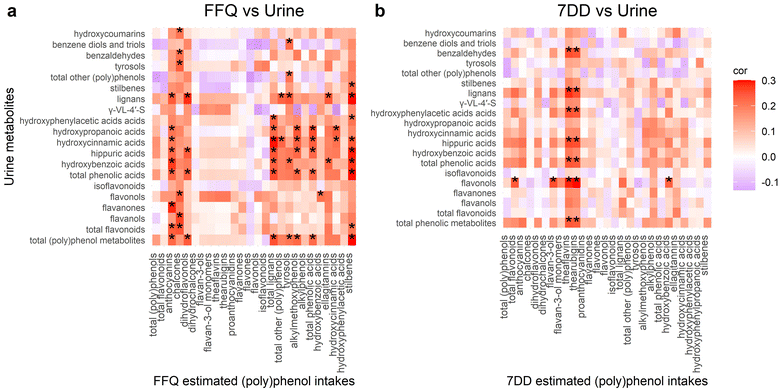
Correlations with (poly)phenol metabolites in urine and plasma
Heatmaps in Fig. 2 shows the Spearman's correlations between estimated intakes from FFQ and 7DD with urinary (poly)phenol metabolites. FFQ estimated intake showed positive correlations with total urine phenolic metabolites and intake of anthocyanins, dihydroflavonols, total lignans, tyrosols, alkylmethoxyphenols, total phenolic acids, and total stilbenes (all FDR adjusted p values < 0.1) (Fig. 2a). Similar correlations were seen for individual urine phenolic metabolite subgroups. Additionally, FFQ estimated chalcones intake was positively correlated with urinary total flavonoids, flavanols, flavonols, tyrosols, benzaldehydes, and hydroxycoumarins, while FFQ estimated ellagitannins intake was positively correlated with urinary hydroxybenzoic acids and lignans (all FDR adjusted p values < 0.1). FFQ estimated hydroxycinnamic acids was positively correlated with urinary hydroxycinnamic acids and hydroxypropanoic acids (FDR adjusted p = 0.088, 0.064, respectively).In comparison, for the 7DD estimated intake, the estimated theaflavins and thearubigins were positively correlated with urine total metabolites (FDR adjusted p values were 0.067 and 0.072, respectively) (Fig. 2b). The 7DD estimated total flavonoid intake was positively correlated with urinary flavonols (rho = 0.243, FDR adjusted p = 0.067). Besides, 7DD estimated flavan-3-ols and hydroxybenzoic acids were positively correlated with urinary flavonols (rho = 0.256, 0.234, FDR adjusted p = 0.067, 0.008, respectively). Multiple significant correlations were seen between the 7DD estimated theaflavins and thearubigins intake and urinary metabolite subclasses such as flavonols, total phenolic acids, hippuric acids, hydroxyphenylacetic acids, lignans, and benzaldehydes (all FDR adjusted p values < 0.1). Among them, the correlations between 7DD estimated theaflavins and thearubigins intake, which were derived only from tea, and urinary flavonols were stronger than the others (rho = 0.290, 0.300, both FDR adjusted p = 0.042).
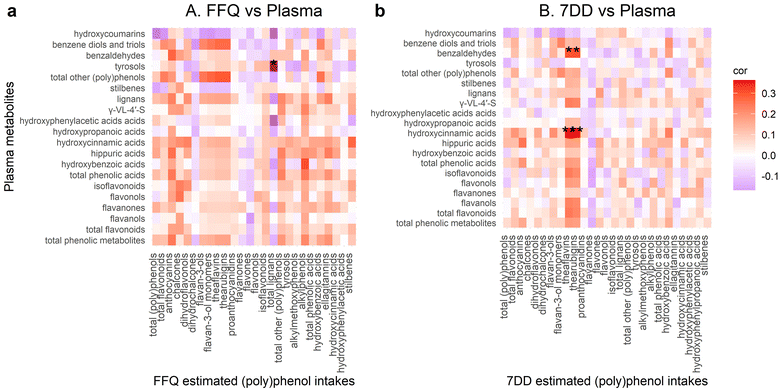
Regarding plasma phenolic metabolites, no significant correlations were found between FFQ estimated (poly)phenol intakes and plasma phenolic metabolites after adjusting for multiple comparisons (Fig. 3a). The 7DD estimated dietary theaflavins and thearubigins were positively correlated with plasma hydroxycinnamic acids (rho = 0.363, 0.354, respectively, both FDR adjusted p = 0.003 and urinary benzaldehydes (rho = 0.301, 0.305, respectively, both FDR adjusted p = 0.026) (Fig. 3b).
Agreements between dietary assessment methods and biomarkers
The agreements between dietary assessment and metabolites in ranking participants in quartiles of (poly)phenol intake levels are shown in Table 5. Poor agreements were seen for all groups of (poly)phenols including total (poly)phenols, total flavonoids, flavanols, flavanones, isoflavonoids, total lignans, total stilbenes, and tyrosols between biomarkers in either specimen type (urine or plasma) and either dietary assessment (FFQ or 7DD) (kappa 0–0.12). Regarding the agreement between total (poly)phenol metabolite levels and the total estimated (poly)phenol intakes, 7DD (kappa = 0.09) showed a slightly better agreement than FFQ (kappa = 0.06) with urine total metabolites, while FFQ (kappa = 0.10) showed a slightly better agreement than 7DD (kappa = 0.08) with plasma total metabolites, although all these agreements were poor.| Metabolite levels | Questionnaires | Groups | Kappa | (95% CI) | Same quartile (%) | Opposite quartile (%) |
|---|---|---|---|---|---|---|
| FFQ: food frequency questionnaires. 7DD: 7-day food diaries. Kappa: weighted kappa coefficient (linear weights). 95% CI: 95% confidence interval. Urine with dietary intake n = 165, plasma with dietary intake n = 150. | ||||||
| Urine (n = 164) | FFQ | Total (poly)phenols | 0.06 | (−0.04, 0.17) | 23.64 | 4.85 |
| Total flavonoids | 0.05 | (−0.06, 0.17) | 30.91 | 13.33 | ||
| Flavonols | 0.09 | (−0.02, 0.20) | 26.67 | 7.88 | ||
| Flavanones | 0.06 | (−0.05, 0.17) | 25.45 | 7.27 | ||
| Isoflavonoids | 0.00 | (−0.11, 0.12) | 27.88 | 15.15 | ||
| Total lignans | 0.09 | (−0.02, 0.20) | 27.88 | 9.09 | ||
| Total stilbenes | 0.12 | (0.01, 0.23) | 29.09 | 6.67 | ||
| Tyrosols | -0.03 | (−0.13, 0.08) | 18.18 | 7.27 | ||
| 7DD | Total (poly)phenols | 0.09 | (−0.02, 0.20) | 26.67 | 6.67 | |
| Total flavonoids | 0.01 | (−0.10, 0.13) | 27.27 | 13.94 | ||
| Flavonols | 0.04 | (−0.07, 0.15) | 27.88 | 11.52 | ||
| Flavanones | 0.09 | (−0.02, 0.20) | 29.70 | 12.12 | ||
| Isoflavonoids | 0.03 | (−0.08, 0.14) | 27.27 | 11.52 | ||
| Total lignans | 0.02 | (−0.08, 0.13) | 18.18 | 7.27 | ||
| Total stilbenes | 0.01 | (−0.10, 0.12) | 22.42 | 9.70 | ||
| Tyrosols | 0.03 | (−0.08, 0.14) | 24.24 | 11.52 | ||
| Plasma (n = 155) | FFQ | Total (poly)phenols | 0.10 | (−0.02, 0.22) | 30.00 | 10.67 |
| Total flavonoids | 0.01 | (−0.11, 0.12) | 26.00 | 13.33 | ||
| Flavonols | 0.09 | (−0.02, 0.21) | 27.33 | 11.33 | ||
| Flavanones | 0.07 | (−0.04, 0.19) | 28.00 | 10.67 | ||
| Isoflavonoids | 0.02 | (−0.10, 0.13) | 26.00 | 15.33 | ||
| Total lignans | −0.10 | (−0.21, 0.01) | 22.00 | 18.00 | ||
| Total stilbenes | 0.01 | (−0.10, 0.12) | 23.33 | 9.33 | ||
| Tyrosols | −0.05 | (−0.17, 0.07) | 26.67 | 15.33 | ||
| 7DD | Total (poly)phenols | 0.08 | (−0.04, 0.20) | 28.00 | 10.00 | |
| Total flavonoids | 0.09 | (−0.02, 0.21) | 28.67 | 11.33 | ||
| Flavonols | −0.01 | (−0.13, 0.11) | 30.00 | 13.33 | ||
| Flavanones | 0.01 | (−0.11, 0.12) | 26.00 | 11.33 | ||
| Isoflavonoids | 0.08 | (−0.03, 0.20) | 30.67 | 11.33 | ||
| Total lignans | 0.09 | (−0.02, 0.21) | 29.33 | 10.00 | ||
| Total stilbenes | −0.03 | (−0.15, 0.08) | 24.67 | 14.00 | ||
| Tyrosols | −0.03 | (−0.15, 0.08) | 28.00 | 16.67 | ||
Sensitivity analysis on main results
A total of 242 participants who had plausible energy intake reported from 7DDs were included in the sensitivity analysis. Robust results were seen in the comparisons between FFQ and 7DD estimated (poly)phenols (ESI Table 4†), agreements between the two dietary assessment methods (ESI Table 5†) and agreements between dietary (poly)phenol intake and (poly)phenol biomarkers (ESI Table 5†). The correlations between estimated (poly)phenol intake and urinary and plasma (poly)phenol biomarkers showed similar patterns (ESI Fig. 5 and 6†), although the correlations between dietary intake and urinary biomarkers were not significant after adjustment for multiple comparisons using the FDR method.Discussion
Estimating dietary (poly)phenol intake is challenging. Beyond the well-established misreporting bias from self-reported dietary data, there are more challenges to be addressed before an accurate estimation of (poly)phenol intake can be made. The first is the limited food (poly)phenol content data and the limited information on factors affecting the (poly)phenol content of foods such as species, harvesting, storage, and processing. Besides, the (poly)phenol content of food can be highly variable even when the nutrient profiles are similar,61 and it requires more detailed questionnaires to capture all the food sources accurately.Our recent systematic review investigating methods used for estimating (poly)phenol intake8 showed that dietary assessment questionnaires used to estimate (poly)phenol intake were usually validated for a number of macro and micronutrients and energy, but in over 80% of the studies they were not further validated for (poly)phenols. Among the very few tools validated for (poly)phenols, most of them (74%) compared their method (in most cases FFQ) to other types of dietary assessment such as food records. Only 37% of them (n = 17) analysed the corresponding levels of (poly)phenols in plasma or urine, which are considered objective to the misreporting bias derived from dietary assessment. The lack of validation and the use of different types of questionnaires makes it difficult to yield consistent results from the current evidence and therefore hard to draw a conclusion on a suitable estimated intake of (poly)phenols for optimal health benefits.6 There is an urgent need to test the suitability of commonly used dietary assessment tools in estimating (poly)phenol intake. This study compared the estimated dietary intakes estimated from two different tools, a widely used FFQ and a 7-day food diary and a biomarker approach, using a quantitative targeted metabolomics method which includes a large list of phenolic metabolites, representing the most common dietary (poly)phenols in a UK-based population.
The estimated total (poly)phenol intake levels in this study were different between FFQ and 7DDs, with a higher intake derived from FFQs. Compared to other studies which have reported (poly)phenol intake levels in the UK population, the FFQ estimated results were in accordance with the intakes reported by the EPIC main study, using the same FFQ, with estimated median total (poly)phenol intake of 1443 mg d−1 for women and 1509 mg d−1 for men.62 The 7DD estimated intake was lower than the amount reported by the EPIC-calibration study (around 1750 mg d−1 for men and 1600 mg d−1 for women), which was estimated using 24 h recalls.63 Besides, the 7DD estimated results were higher compared to the results by age groups in the UK National Diet and Nutrition Survey (NDNS) (2008–2014),64 which were around 600 to 1100 mg d−1 estimated from 4-day food records. However, the NDNS data only used Phenol-Explorer as the data source and did not report the intake of lignans and other (poly)phenols in the total (poly)phenol intake, which could explain the differences in the estimation compared to our results. On the contrary to flavonoids as the major (poly)phenol from diet reported in the NDNS and EPIC study,62,64 the major dietary (poly)phenol found in our study was phenolic acids. This could probably be explained by the high proportion of coffee consumers in our cohort and the different (poly)phenol database used. In our population, there were 80.4% and 72.9% of coffee consumers measured from FFQ or 7DD, respectively, which is higher than the 62% reported in the UK adults in the NDNS study.65
In terms of comparisons between FFQ and food records on estimating (poly)phenol intake, in agreement with our data Kent et al. found that FFQ significantly overestimated total and subclasses of flavonoids9 while Yue et al. observed slightly higher FFQ estimated flavonoid intake in women but not in men.10 Although different FFQs may have different validities in estimating (poly)phenols, and the population characteristics might also influence the results, one possible explanation for the discrepancies between FFQs and food records is that FFQs tend to overestimate healthy food intake such as fruits and vegetables, which are important sources of (poly)phenols.66 We also found a higher estimation of coffee and tea consumption from FFQs compared to 7DDs, which is in accordance with previous studies.67,68 Besides, one study that measured caffeine intake in 259 women found that there was a significantly higher amount of coffee intake estimated from FFQs compared to 24 h recalls.69 The different estimations for tea and coffee intake could be due to the difference in the default portion size in the FFQ and the actual portion size measured in the 7DD. The portion size for coffee and tea are both 190 g in the EPIC FFQ and they were considered as “coffee, infusion, average” and “tea, infusion, average” by default. In 7DD, the portion size varies by participants according to the size of their cups and the amount of milk added. Although the default portion size for tea and coffee was the same (190 g, 225 g in total including 35 g milk) in 7DD, when participants had more milk added, the amount of coffee and tea was lowered accordingly. These differences were not reflected in the FFQ and therefore derived higher estimated intakes. One limitation in estimating (poly)phenols from tea and coffee in this study and possibly many other studies is that the (poly)phenols were calculated based on the portions of made-up drinks assuming they were in the same default concentrations. However, this is not true in real life, where the (poly)phenol content depends more on the amount of tea bags or coffee powder or beans, the amount of water added, and the time of brewing according to the habits of participants. The current existing FFQs and food diaries were not able to reflect this information and there is limited food content data available addressing this issue. It is also worth noticing that in the validation studies of FFQs, coffee and tea intakes could be easily neglected because they contribute a negligible amount to the total energy or nutrient levels. However, for (poly)phenol intake estimations, they could represent up to 70% of the total (poly)phenols and any small systematic errors in estimation could result in considerable misreport of the final result. This emphasises again the importance of validating tools for (poly)phenol assessment and developing specialized tools to estimate (poly)phenol intake.
In 7DD estimated intakes, anthocyanins, dihydroflavonols, flavones, total lignans, stilbenes, and other (poly)phenols showed a higher estimation of intake compared to FFQs. This could be explained by the different food sources of (poly)phenols that had been captured by the two different tools. Firstly, the FFQ used in this study did not include several important food sources of (poly)phenols, such as blueberries, aubergine, olives, herbs and spices and seeds. Besides, some food items with distinct (poly)phenol levels or profiles were grouped in one question, such as “tea” (including black, green, and herbal tea), “wine” (including white, rose, or red wine), “strawberries, raspberries, kiwi fruit”, “peanuts or other nuts”, “dried lentils, beans, peas” and so on. Different participants may interpret the questions differently, while in data analysis those foods were transformed into a certain item or combinations of default items. For example, the “wine” in FFQ was represented by “rose wine” in the calculation process of nutrients and (poly)phenols by default, which has less anthocyanidin content than red wine. These could all result in potential underestimation of the (poly)phenol intake by FFQs.
The agreement between FFQ and 7DD was moderate to poor in general. Moderate agreements were seen in subclasses of (poly)phenols that came from food sources consumed every day and contributed greatly to the total intake, including tea, coffee, and apples. Agreements were extremely poor for the groups contributing a small percentage of the total intake, such as anthocyanins, chalcones, dihydroflavonols, flavones, hydroxyphenylacetic acids, total other (poly)phenols and tyrosols. Besides the limited food sources of these subclasses included in the EPIC-Norfolk FFQ as discussed earlier, the disagreement between FFQ and 7DD could also be due to the fact that it is hard to capture the food sources that were less frequently consumed by food diary when the consumption was only collected once during a relatively short period (7 days) in this study. Therefore, if the research aimed to measure intakes of these subclasses of (poly)phenols, the length of measurement needs to be longer and more detailed dietary assessment tools need to be designed.
In most of the validation studies that have compared FFQ against 7DDs in measuring (poly)phenol intake, the reliability was only obtained by correlation coefficients between the methods,8 while the real agreement between absolute values was not measured. Our results were consistent with previous findings from some validation studies which compared estimated (poly)phenols from FFQ and food records (3 days). Vian et al. validated an FFQ against 3-day food records and reported an ICC of 0.489 for total (poly)phenol intakes.70 Besides, cross-classification tests showed 23–37% of the same quartiles for total (poly)phenols70,71 and flavonoids subclasses,72 which is a bit lower than our results (48% for total (poly)phenol and 29%-50% for flavonoids). Although significant correlations were seen between the estimated (poly)phenol intakes from FFQ and 7DD, a moderate correlation (0.4 < rho < 0.6) does not always mean a fair agreement (0.50 < ICC < 0.75 or 0.40 < kappa < 0.75) between the two measurements. This suggests a more cautious interpretation of the validation results only represented by correlations.
The dietary assessments from FFQ and food diaries are prone to misreporting bias due to their self-reported nature. Therefore, sensitivity analysis was conducted on participants with plausible reported energy intake from 7DDs compared to the estimated BMR and physical activity levels. Energy intakes are widely used as a measurement for misreporting errors. Therefore, adjusting for energy intake in the comparisons could partially remove the influence of misreporting. In our study, the energy-adjusted intakes showed slightly better agreements in the absolute estimated values between FFQ and 7DD but did not change the conclusion of the findings. The improvement of adjusting energy intake on agreement of estimation was weak and the sensitivity analysis on the subgroup of participants with plausible energy reports showed similar results as all participants, indicating a limited impact of misreporting on our results.
Regarding correlations between intake and phenolic metabolites, although more significant correlations were seen between urinary (poly)phenols and intake estimated from FFQ than from 7DD, significant relationships were found in different subclasses of (poly)phenols for FFQ and 7DD. (Poly)phenol metabolites showed stronger correlations with 7DD estimated flavonoid intake and FFQ estimated phenolic acid, other (poly)phenols and stilbenes intake. We could not draw a convincing conclusion on which dietary assessment tool is better from these results. Dietary (poly)phenols undergo extensive metabolism after ingestion, including phase II metabolisms into glucuronides, sulfates or methoxy conjugates, as well as cleavage and ring fissions into smaller molecules by the gut microbiota.73 Some metabolites are produced specifically from the aglycone of the same structure, such as most of the flavonoid phase II metabolites. However, many phenolic compounds with small molecular weight such as phenolic acids, benzaldehydes and benzenes could not only be present in food but also be generated by the gut-microbiota from various types of (poly)phenol molecules. Therefore, they are not specific biomarkers for dietary intake or exposure to specific (poly)phenols. On the other hand, the different half-lives of the various (poly)phenols and the sample collection time in relation to the dietary assessment could also influence the relationships between the (poly)phenol metabolites and intake. The 7DD captures recent intakes and the FFQ captures habitual intakes, while the 24 h urine and fasting plasma were both related to the (poly)phenol intakes in the past 24–48 hours. The correlations with (poly)phenol biomarkers in this study indicated that 7DDs might be better at estimating short-term flavonoid intake, especially flavan-3-ol intakes over the FFQ we used, while the habitual intake of phenolic acids and other (poly)phenols could also be reflected by the short-term urine biomarkers. However, this needs further exploration and validation in bigger cohorts.
The correlations between estimated intake and plasma metabolites were not as strong as the urinary metabolite excretion levels. This could be due to the fact that plasma samples in this study were taken after at least 8 hours of fasting when many metabolites have been removed from circulation. Despite more significant correlations being seen between total urinary metabolites than total plasma metabolites with dietary (poly)phenol intake, the agreements between urine and dietary intakes in ranking participants did not show clear advantages over plasma. Overall, the correlations between estimated dietary (poly)phenol intake and metabolites in urine and plasma were weak for both FFQ and 7DD (Spearman's rho < 0.4). This could be due to many factors such as the limited reporting accuracy of the dietary assessment methods, the inter-individual variability in (poly)phenol metabolism, in particular gut microbial metabolism, other exposure sources of (poly)phenols (such as food additives), phenolic metabolites being produced from endogenous pathways, and the short half-life of most of the metabolites.
To our knowledge, this is the first study to evaluate the agreement between dietary assessed data from two well-established dietary assessment tools and compare them against a large list of different types of phenolic metabolites. However, it has the following limitations, and the results should be interpreted with caution. First, the study was conducted on a small cohort of participants based in London, UK and the study population had high levels of fruits and vegetables intake and healthy lifestyles. Therefore, the conclusions might not be generalizable to other populations. Besides, the food diary was collected once, which might not reflect participants’ actual habitual intake, and the ICCs could not be deattenuated for the intra-person variance. However, the reproducibility analysis on a subgroup of participants in our cohort74,26 showed that there was no significant difference between the 7DD estimated total (poly)phenol intakes after 5 or 10 weeks. Besides, participants were instructed not to have vigorous exercise on the day before the study visit, which might also influence the habitual intake and the (poly)phenol metabolite levels in 24 h urine and fasting plasma. Furthermore, the questionnaires and biospecimens were collected and analysed by different researchers at different times. The batch effect might influence the results. However, standard protocols were applied in all the processes, including data collection, coding, and analysis to keep consistency and eliminate human errors. Finally, every FFQ is different in terms of the ability to capture (poly)phenol intake. The results from the Norfolk-EPIC FFQ used in this study might not be comparable to other existing FFQs and need to be evaluated prior to their use.
To date, although some (poly)phenol-specific dietary assessment tools were designed in other populations,70,75–77 there is no available tool designed to estimate (poly)phenol intake in the UK diet, and no gold standard method in (poly)phenol intake assessment has been established. In this work, we used two well-established tools to measure the UK diet and accurate measurements of more than 100 phenolic metabolites representing the major (poly)phenol groups present in the diet in two different types of biofluids to explore the suitability of these different methods in estimating dietary (poly)phenol intakes. If we consider the short-term biomarkers to be the gold standard objective measurement, FFQ and 7DD have their own advantages in measuring different subclasses of (poly)phenols. The FFQ we used might be better in estimating intakes of phenolic acids, other (poly)phenols and stilbenes while 7DD might be preferable in estimating the intake of flavonoids. If we consider 7DD as a more reliable method in capturing food sources of (poly)phenol intake using dietary assessment methods, the FFQ we used might not be accurate in measuring all (poly)phenol subclasses especially for the ones contributing less to the total intake and from food sources that were not included in the questionnaires. FFQ also tend to overestimate total (poly)phenol intake. Every assessment method has advantages and flaws, and it is hard to select the best tool just from these comparisons, but it is essential to keep in mind the limitations and potential bias in the interpretation of the results derived from them.
To conclude, the findings of this study suggest that the agreements between dietary assessment tools were moderate but the agreements with biomarkers measured in plasma and urine for the estimation of (poly)phenol intake were poor. To develop a standardized and accurate approach to measure dietary (poly)phenol exposure levels in the free-living population, many research questions still need to be answered. A better understanding of the relationship between (poly)phenol intake and exposure levels needs to be reached with more research needed on the bioaccessibility and bioavailability of dietary (poly)phenols, as well as their inter-individual variability. Better food content databases are needed, with information coming from validated accurate methods for estimating the (poly)phenol content of foods. The single use of a dietary assessment tool or biomarker may not be sufficient to reflect intake levels due to its own potential limitations and bias. More accurate and specific dietary assessment tools need to be developed for measuring (poly)phenol intake. Validation of biomarkers78 needs to be conducted and tested in larger populations with sufficient consideration of inter-individual variability in bioavailability and metabolism. The combination of validated tailored dietary assessment methods and biomarkers may be the best approach to increase the accuracy of (poly)phenol intake estimation in the future.
Conflicts of interest
There are no conflicts to declare.Author contributions
WA, MLS, AC, EW, SH, PYTY and YX conducted dietary assessment data collection and laboratory analysis and data analysis of the (poly)phenol biomarkers in biological samples. YX, YL, XM, and WA conducted data harmonization. YX and YL conducted (poly)phenol analysis from the dietary assessment data. YX conducted the statistical analysis and visualization. ARM and RG supervised the data collection and analysis. YX wrote the original draft, and all authors were involved in the review and editing of the final manuscript.Acknowledgements
YX, YL, and XM are funded by the King's-China Scholarship Council (K-CSC) joint scholarship. WA is funded by a scholarship from King Faisal University. The authors thank Anthony Sullivan, Chris Titman, and Neil Loftus from Shimadzu UK Ltd for their support on the LC-MS/MS instrument.References
- World Health Organization, Diet, nutrition, and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation, World Health Organization, 2003 Search PubMed.
- L. Hooper, C. Kay, A. Abdelhamid, P. A. Kroon, J. S. Cohn, E. B. Rimm and A. Cassidy, Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials, Am. J. Clin. Nutr., 2012, 95, 740–751 CrossRef CAS PubMed.
- G. Grosso, A. Micek, J. Godos, S. Sciacca, A. Pajak, M. A. Martinez-Gonzalez, E. L. Giovannucci and F. Galvano, Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis, Eur. J. Epidemiol., 2016, 31, 1191–1205 CrossRef CAS PubMed.
- M. Chung, N. Zhao, D. Wang, M. Shams-White, M. Karlsen, A. Cassidy, M. Ferruzzi, P. F. Jacques, E. J. Johnson and T. C. Wallace, Dose-Response Relation between Tea Consumption and Risk of Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis of Population-Based Studies, Adv. Nutr., 2020, 11, 790–814 CrossRef PubMed.
- A. Micek, J. Godos, D. Del Rio, F. Galvano and G. Grosso, Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis, Mol. Nutr. Food Res., 2021, 65, e2001019 CrossRef PubMed.
- C. Del Bo, S. Bernardi, M. Marino, M. Porrini, M. Tucci, S. Guglielmetti, A. Cherubini, B. Carrieri, B. Kirkup, P. Kroon, R. Zamora-Ros, N. H. Liberona, C. Andres-Lacueva and P. Riso, Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern?, Nutrients, 2019, 11, 1355 CrossRef CAS PubMed.
- D. J. Lamport and C. M. Williams, Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses, Brain Plast., 2021, 6, 139–153 Search PubMed.
- Y. Xu, M. Le Sayec, C. Roberts, S. Hein, A. Rodriguez-Mateos and R. Gibson, Dietary Assessment Methods to Estimate (Poly)phenol Intake in Epidemiological Studies: A Systematic Review, Adv. Nutr., 2021, 12, 1781–1801 CrossRef PubMed.
- K. Kent and K. E. Charlton, Development, validation and reproducibility of a food frequency questionnaire to measure flavonoid intake in older Australian adults, Nutr. Diet., 2018, 75, 106–116 CrossRef PubMed.
- Y. Yue, J. Petimar, W. C. Willett, S. A. Smith-Warner, C. Yuan, S. Rosato, L. Sampson, B. Rosner, A. Cassidy, E. B. Rimm and K. L. Ivey, Dietary flavonoids and flavonoid-rich foods: validity and reproducibility of FFQ-derived intake estimates, Public Health Nutr., 2020, 23, 3295–3303 CrossRef PubMed.
- S. A. Bingham, C. Gill, A. Welch, A. Cassidy, S. A. Runswick, S. Oakes, R. Lubin, D. I. Thurnham, T. J. Key, L. Roe, K. T. Khaw and N. E. Day, Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers, Int. J. Mol. Epidemiol., 1997, 26(Suppl 1), S137–S151 CrossRef PubMed.
- N. M. McKeown, N. E. Day, A. A. Welch, S. A. Runswick, R. N. Luben, A. A. Mulligan, A. McTaggart and S. A. Bingham, Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort, Am. J. Clin. Nutr., 2001, 74, 188–196 CrossRef CAS PubMed.
- A. Del Olmo, J. Calzada and M. Nunez, Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy, Crit. Rev. Food Sci. Nutr., 2017, 57, 3084–3103 CrossRef CAS PubMed.
- M. Bojic, C. A. Sedgeman, L. D. Nagy and F. P. Guengerich, Aromatic hydroxylation of salicylic acid and aspirin by human cytochromes P450, Eur. J. Pharm. Sci., 2015, 73, 49–56 CrossRef CAS PubMed.
- R. Carecho, D. Carregosa and C. N. Dos Santos, Low Molecular Weight (poly)Phenol Metabolites Across the Blood-Brain Barrier: The Underexplored Journey, Brain Plast., 2021, 6, 193–214 Search PubMed.
- M. R. Ritchie, M. S. Morton, A. M. Thompson, N. Deighton, A. Blake, J. H. Cummings and C. M. Steel, Investigation of the reliability of 24 h urine excretion as a biomarker of isoflavone exposure over time and over a wide range of isoflavone intakes, Eur. J. Clin. Nutr., 2004, 58, 1286–1289 CrossRef CAS PubMed.
- J. I. Ottaviani, R. Fong, J. Kimball, J. L. Ensunsa, N. Gray, A. Vogiatzoglou, A. Britten, D. Lucarelli, R. Luben, P. B. Grace, D. H. Mawson, A. Tym, A. Wierzbicki, A. D. Smith, N. J. Wareham, N. G. Forouhi, K. T. Khaw, H. Schroeter and G. G. C. Kuhnle, Evaluation of (-)-epicatechin metabolites as recovery biomarker of dietary flavan-3-ol intake, Sci. Rep., 2019, 9, 13108 CrossRef PubMed.
- J. I. Ottaviani, R. Fong, J. Kimball, J. L. Ensunsa, A. Britten, D. Lucarelli, R. Luben, P. B. Grace, D. H. Mawson, A. Tym, A. Wierzbicki, K. T. Khaw, H. Schroeter and G. G. C. Kuhnle, Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies, Sci. Rep., 2018, 8, 9859 CrossRef PubMed.
- A. Medina-Remon, A. Barrionuevo-Gonzalez, R. Zamora-Ros, C. Andres-Lacueva, R. Estruch, M. A. Martinez-Gonzalez, J. Diez-Espino and R. M. Lamuela-Raventos, Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake, Anal. Chim. Acta, 2009, 634, 54–60 CrossRef CAS.
- R. Zamora-Ros, M. Touillaud, J. A. Rothwell, I. Romieu and A. Scalbert, Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits, Am. J. Clin. Nutr., 2014, 100, 11–26 CrossRef CAS.
- R. Zamora-Ros, D. Achaintre, J. A. Rothwell, S. Rinaldi, N. Assi, P. Ferrari, M. Leitzmann, M. C. Boutron-Ruault, G. Fagherazzi, A. Auffret, T. Kuhn, V. Katzke, H. Boeing, A. Trichopoulou, A. Naska, E. Vasilopoulou, D. Palli, S. Grioni, A. Mattiello, R. Tumino, F. Ricceri, N. Slimani, I. Romieu and A. Scalbert, Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study, Sci. Rep., 2016, 6, 26905 CrossRef CAS.
- L. I. Mennen, D. Sapinho, H. Ito, P. Galan, S. Hercberg and A. Scalbert, Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects - variability and possible use as biomarkers of polyphenol intake, Eur. J. Clin. Nutr., 2008, 62, 519–525 CrossRef CAS PubMed.
- J. Radtke, J. Linseisen and G. Wolfram, Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake, Eur. J. Nutr., 2002, 41, 203–209 CrossRef CAS PubMed.
- J. Cao, Y. Zhang, W. Chen and X. Zhao, The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake, Br. J. Nutr., 2010, 103, 249–255 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. Dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60, 2130–2140 CrossRef CAS PubMed.
- G. Istas, E. Wood, M. Le Sayec, C. Rawlings, J. Yoon, V. Dandavate, D. Cera, S. Rampelli, A. Costabile, E. Fromentin and A. Rodriguez-Mateos, Effects of aronia berry (poly)phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men, Am. J. Clin. Nutr., 2019, 110, 316–329 CrossRef PubMed.
- S. A. Bingham, A. A. Welch, A. McTaggart, A. A. Mulligan, S. A. Runswick, R. Luben, S. Oakes, K. T. Khaw, N. Wareham and N. E. Day, Nutritional methods in the European Prospective Investigation of Cancer in Norfolk, Public Health Nutr., 2001, 4, 847–858 CrossRef CAS PubMed.
- A. A. Mulligan, R. N. Luben, A. Bhaniani, D. J. Parry-Smith, L. O'Connor, A. P. Khawaja, N. G. Forouhi, K. T. Khaw and E. P.-N. F. Study, A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability, BMJ Open, 2014, 4, e004503 CrossRef PubMed.
- R. A. McCance, E. M. Widdowson and B. Holland, McCance and Widdowson's The composition of foods, Royal Society of Chemistry, Cambridge, 5th revised and extended edn, 1991 Search PubMed.
- J. A. Rothwell, J. Perez-Jimenez, V. Neveu, A. Medina-Remon, N. M'Hiri, P. Garcia-Lobato, C. Manach, C. Knox, R. Eisner, D. S. Wishart and A. Scalbert, Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content, Database, 2013, 2013, bat070 CrossRef PubMed.
- S. Bhagwat and D. B. Haytowitz, USDA Database for the Flavonoid Content of Selected Foods, 2016, DOI:10.15482/USDA.ADC/1324465 (Accessed June 2022).
- S. Bhagwat and D. B. Haytowitz, USDA Database for the Isoflavone Content of Selected Foods, 2015, DOI:10.15482/USDA.ADC/1324538 (Accessed June 2022).
- S. Bhagwat and D. B. Haytowitz, USDA Database for the Proanthocyanidin Content of Selected Foods, 2015, DOI:10.15482/USDA.ADC/1324621 (Accessed June 2022).
- J. I. Alonso-Esteban, J. Pinela, A. Ćirić, R. C. Calhelha, M. Soković, I. C. F. R. Ferreira, L. Barros, E. Torija-Isasa and M. d. C. Sánchez-Mata, Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds, Food Chem., 2022, 374, 131754 CrossRef CAS PubMed.
- L. Alvarez-Jubete, H. Wijngaard, E. K. Arendt and E. Gallagher, Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking, Food Chem., 2010, 119, 770–778 CrossRef CAS.
- R. L. Bertin, L. V. Gonzaga, G. d. S. C. Borges, M. S. Azevedo, H. F. Maltez, M. Heller, G. A. Micke, L. B. B. Tavares and R. Fett, Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC–ESI-MS/MS, Food Res. Int., 2014, 55, 404–411 CrossRef CAS.
- H. Cai, Q. Zhang, L. Shen, J. Luo, R. Zhu, J. Mao, M. Zhao and C. Cai, Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters, LWT, 2019, 111, 226–234 CrossRef CAS.
- A. V. Carvalho, T. F. F. Da Silveira, R. A. Mattietto, M. D. P. de Oliveira and H. T. Godoy, Chemical composition and antioxidant capacity of acai (Euterpe oleracea) genotypes and commercial pulps, J. Sci. Food Agric., 2017, 97, 1467–1474 CrossRef CAS PubMed.
- N. Cicero, A. Albergamo, A. Salvo, G. D. Bua, G. Bartolomeo, V. Mangano, A. Rotondo, V. Di Stefano, G. Di Bella and G. Dugo, Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market, Food Res. Int., 2018, 109, 517–525 CrossRef CAS PubMed.
- Q. H. Gao, C. S. Wu, J. G. Yu, M. Wang, Y. J. Ma and C. L. Li, Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections, J. Food Sci., 2012, 77, C1218–C1225 CrossRef CAS PubMed.
- M. Gundogdu, Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits, Adv. Environ. Biol., 2013, 7, 344–348 CAS.
- M. A. Hassan, T. Xu, Y. Tian, Y. Zhong, F. A. Z. Ali, X. Yang and B. Lu, Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review, Phytomedicine, 2021, 93, 153771 CrossRef PubMed.
- A. N. Karunasiri, M. Gunawardane, C. M. Senanayake, N. Jayathilaka and K. N. Seneviratne, Antioxidant and Nutritional Properties of Domestic and Commercial Coconut Milk Preparations, Int. J. Food Sci., 2020, 2020, 3489605 Search PubMed.
- M. Kaspar, T. Bajer, P. Bajerova and P. Cesla, Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry, Molecules, 2022, 27, 1356 CrossRef CAS PubMed.
- M. Y. Kim, P. Seguin, J. K. Ahn, J. J. Kim, S. C. Chun, E. H. Kim, S. H. Seo, E. Y. Kang, S. L. Kim, Y. J. Park, H. M. Ro and I. M. Chung, Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea, J. Agric. Food Chem., 2008, 56, 7265–7270 CrossRef CAS PubMed.
- Q. Lv, F. Luo, X. Zhao, Y. Liu, G. Hu, C. Sun, X. Li and K. Chen, Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity, PLoS One, 2015, 10, e0120480 CrossRef PubMed.
- N. Miceli, A. Trovato, A. Marino, V. Bellinghieri, A. Melchini, P. Dugo, F. Cacciola, P. Donato, L. Mondello, A. Güvenç, R. De Pasquale and M. F. Taviano, Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey, Food Chem. Toxicol., 2011, 49, 2600–2608 CrossRef CAS.
- A. Mocan, F. Cairone, M. Locatelli, F. Cacciagrano, S. Carradori, D. C. Vodnar, G. Crisan, G. Simonetti and S. Cesa, Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation, Antioxidants, 2019, 8, 562 CrossRef CAS PubMed.
- W. C. B. Muala, Z. S. C. Desobgo and N. E. Jong, Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract, Heliyon, 2021, 7, e06744 CrossRef CAS PubMed.
- P. S. Prasanthi, N. Naveena, M. Vishnu Vardhana Rao and K. Bhaskarachary, Compositional variability of nutrients and phytochemicals in corn after processing, J. Food Sci. Technol., 2017, 54, 1080–1090 CrossRef CAS PubMed.
- M. J. Rahman, A. C. de Camargo and F. Shahidi, Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities, J. Funct. Foods, 2017, 35, 622–634 CrossRef CAS.
- A. Rueda, C. Samaniego-Sanchez, M. Olalla, R. Gimenez, C. Cabrera-Vique, I. Seiquer and L. Lara, Combination of Analytical and Chemometric Methods as a Useful Tool for the Characterization of Extra Virgin Argan Oil and Other Edible Virgin Oils. Role of Polyphenols and Tocopherols, J. AOAC Int., 2016, 99, 489–494 CrossRef CAS PubMed.
- L. Xu, B. Du and B. Xu, A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China, Food Chem., 2015, 174, 202–213 CrossRef CAS PubMed.
- R. A. McCance and E. M. Widdowson, McCance and Widdowson's The composition of foods, Royal Society of Chemistry, Cambridge, 6th summary edn, 2002 Search PubMed.
- M. Dominguez-Fernandez, Y. Xu, P. Young Tie Yang, W. Alotaibi, R. Gibson, W. L. Hall, L. Barron, I. A. Ludwig, C. Cid and A. Rodriguez-Mateos, Quantitative Assessment of Dietary (Poly)phenol Intake: A High-Throughput Targeted Metabolomics Method for Blood and Urine Samples, J. Agric. Food Chem., 2021, 69, 537–554 CrossRef CAS PubMed.
- R Core Team, R: A language and environment for statistical computing, Vienna, Austria, 2022. https://www.R-project.org/ Search PubMed.
- T. K. Koo and M. Y. Li, A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research, J. Chiropr. Med., 2016, 15, 155–163 CrossRef PubMed.
- J. L. Fleiss, B. Levin and M. C. Paik, Statistical methods for rates and proportions, Wiley, Newy York, 3rd edn, 2003 Search PubMed.
- A. E. Black, Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations, Int. J. Obes. Relat. Metab. Disord., 2000, 24, 1119–1130 CrossRef CAS PubMed.
- R. Gibson, R. Eriksen, K. Lamb, Y. McMeel, A. C. Vergnaud, J. Spear, M. Aresu, Q. Chan, P. Elliott and G. Frost, Dietary assessment of British police force employees: a description of diet record coding procedures and cross-sectional evaluation of dietary energy intake reporting (The Airwave Health Monitoring Study), BMJ Open, 2017, 7, e012927 CrossRef PubMed.
- G. G. C. Kuhnle, Nutrition epidemiology of flavan-3-ols: The known unknowns, Mol. Aspects Med., 2018, 61, 2–11 CrossRef CAS PubMed.
- R. Zamora-Ros, V. Cayssials, M. Jenab, J. A. Rothwell, V. Fedirko, K. Aleksandrova, A. Tjonneland, C. Kyro, K. Overvad, M. C. Boutron-Ruault, F. Carbonnel, Y. Mahamat-Saleh, R. Kaaks, T. Kuhn, H. Boeing, A. Trichopoulou, E. Valanou, E. Vasilopoulou, G. Masala, V. Pala, S. Panico, R. Tumino, F. Ricceri, E. Weiderpass, M. Lukic, T. M. Sandanger, C. Lasheras, A. Agudo, M. J. Sanchez, P. Amiano, C. Navarro, E. Ardanaz, E. Sonestedt, B. Ohlsson, L. M. Nilsson, M. Rutegard, B. Bueno-de-Mesquita, P. H. Peeters, K. T. Khaw, N. J. Wareham, K. Bradbury, H. Freisling, I. Romieu, A. J. Cross, P. Vineis and A. Scalbert, Dietary intake of total polyphenol and polyphenol classes and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, Eur. J. Epidemiol., 2018, 33, 1063–1075 CrossRef CAS PubMed.
- R. Zamora-Ros, V. Knaze, J. A. Rothwell, B. Hemon, A. Moskal, K. Overvad, A. Tjonneland, C. Kyro, G. Fagherazzi, M. C. Boutron-Ruault, M. Touillaud, V. Katzke, T. Kuhn, H. Boeing, J. Forster, A. Trichopoulou, E. Valanou, E. Peppa, D. Palli, C. Agnoli, F. Ricceri, R. Tumino, M. S. de Magistris, P. H. Peeters, H. B. Bueno-de-Mesquita, D. Engeset, G. Skeie, A. Hjartaker, V. Menendez, A. Agudo, E. Molina-Montes, J. M. Huerta, A. Barricarte, P. Amiano, E. Sonestedt, L. M. Nilsson, R. Landberg, T. J. Key, K. T. Khaw, N. J. Wareham, Y. Lu, N. Slimani, I. Romieu, E. Riboli and A. Scalbert, Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study, Eur. J. Nutr., 2016, 55, 1359–1375 CrossRef CAS PubMed.
- N. Ziauddeen, A. Rosi, D. Del Rio, B. Amoutzopoulos, S. Nicholson, P. Page, F. Scazzina, F. Brighenti, S. Ray and P. Mena, Dietary intake of (poly)phenols in children and adults: cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014), Eur. J. Nutr., 2019, 58, 3183–3198 CrossRef CAS PubMed.
- R. Poole, S. Ewings, J. Parkes, J. A. Fallowfield and P. Roderick, Misclassification of coffee consumption data and the development of a standardised coffee unit measure, BMJ Nutr. Prev. Health, 2019, 2, 11–19 CrossRef PubMed.
- M. Jessri, W. Y. Lou and M. R. L'Abbe, Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey, Br. J. Nutr., 2016, 115, 147–159 CrossRef CAS PubMed.
- M. Ferraroni, A. Tavani, A. Decarli, S. Franceschi, M. Parpinel, E. Negri and C. La Vecchia, Reproducibility and validity of coffee and tea consumption in Italy, Eur. J. Clin. Nutr., 2004, 58, 674–680 CrossRef CAS PubMed.
- R. Takechi, H. Alfonso, A. Harrison, N. Hiramatsu, A. Ishisaka, A. Tanaka, L. Tan and A. H. Lee, Assessing self-reported green tea and coffee consumption by food frequency questionnaire and food record and their association with polyphenol biomarkers in Japanese women, Asia Pac. J. Clin. Nutr., 2018, 27, 460–465 CAS.
- K. C. Schliep, E. F. Schisterman, S. L. Mumford, N. J. Perkins, A. Ye, A. Z. Pollack, C. Zhang, C. A. Porucznik, J. A. VanDerslice, J. B. Stanford and J. Wactawski-Wende, Validation of different instruments for caffeine measurement among premenopausal women in the BioCycle study, Am. J. Epidemiol., 2013, 177, 690–699 CrossRef PubMed.
- I. Vian, P. Zielinsky, A. M. Zilio, A. Mello, B. Lazzeri, A. Oliveira, K. V. Lampert, A. Piccoli, L. H. Nicoloso, G. B. Bubols and S. C. Garcia, Development and validation of a food frequency questionnaire for consumption of polyphenol-rich foods in pregnant women, Matern. Child Nutr., 2015, 11, 511–524 CrossRef PubMed.
- S. Shahar, C. H. Lin and H. B. Haron, Development and Validation of Food Frequency Questionnaire (FFQ) for Estimation of the Dietary Polyphenol Intake Among Elderly Individuals in Klang Valley, Malaysian J. Health Sci., 2014, 12, 33–40 Search PubMed.
- S. Somerset and K. Papier, A food frequency questionnaire validated for estimating dietary flavonoid intake in an Australian population, Nutr. Cancer, 2014, 66, 1200–1210 CrossRef CAS PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases, Antioxid. Redox Signal., 2013, 18, 1818–1892 CrossRef CAS PubMed.
- A. Cheok, Y. Xu, Z. Zhang, P. W. Caton and A. Rodriguez-Mateos, Betalain-rich dragon fruit (pitaya) consumption improves vascular function in men and women: a double-blind, randomized controlled crossover trial, Am. J. Clin. Nutr., 2022, 115, 1418–1431 CrossRef PubMed.
- Y. Zhang, J. Cao, W. Chen, J. Yang, D. Hao, Y. Zhang, P. Chang and X. Zhao, Reproducibility and relative validity of a food frequency questionnaire to assess intake of dietary flavonol and flavone in Chinese university campus population, Nutr. Res., 2010, 30, 520–526 CrossRef CAS PubMed.
- S. Ranka, J. M. Gee, L. Biro, G. Brett, S. Saha, P. Kroon, J. Skinner, A. R. Hart, A. Cassidy, M. Rhodes and I. T. Johnson, Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake, Eur. J. Clin. Nutr., 2008, 62, 1131–1138 CrossRef CAS PubMed.
- N. D. Fisher, S. Hurwitz and N. K. Hollenberg, Habitual flavonoid intake and endothelial function in healthy humans, J. Am. Coll. Nutr., 2012, 31, 275–279 CrossRef CAS PubMed.
- L. O. Dragsted, Q. Gao, A. Scalbert, G. Vergeres, M. Kolehmainen, C. Manach, L. Brennan, L. A. Afman, D. S. Wishart, C. Andres-Lacueva, M. Garcia-Aloy, H. Verhagen, E. J. M. Feskens and G. Pratico, Validation of biomarkers of food intake-critical assessment of candidate biomarkers, Genes Nutr., 2018, 13, 14 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02755k |
| This journal is © The Royal Society of Chemistry 2023 |