Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Daily blueberry consumption for 12 weeks improves endothelial function in postmenopausal women with above-normal blood pressure through reductions in oxidative stress: a randomized controlled trial†
Emily K.
Woolf
a,
Janée D.
Terwoord
 b,
Nicole S.
Litwin
a,
Allegra R.
Vazquez
a,
Sylvia Y.
Lee
a,
Nancy
Ghanem
a,
Kiri A.
Michell
a,
Brayden T.
Smith
a,
Lauren E.
Grabos
a,
Nathaniel B.
Ketelhut
b,
Nate P.
Bachman
b,
Meghan E.
Smith
b,
Melanie
Le Sayec
d,
Sangeeta
Rao
c,
Christopher L.
Gentile
a,
Tiffany L.
Weir
a,
Ana
Rodriguez-Mateos
b,
Nicole S.
Litwin
a,
Allegra R.
Vazquez
a,
Sylvia Y.
Lee
a,
Nancy
Ghanem
a,
Kiri A.
Michell
a,
Brayden T.
Smith
a,
Lauren E.
Grabos
a,
Nathaniel B.
Ketelhut
b,
Nate P.
Bachman
b,
Meghan E.
Smith
b,
Melanie
Le Sayec
d,
Sangeeta
Rao
c,
Christopher L.
Gentile
a,
Tiffany L.
Weir
a,
Ana
Rodriguez-Mateos
 d,
Douglas R.
Seals
e,
Frank A.
Dinenno
b and
Sarah A.
Johnson
d,
Douglas R.
Seals
e,
Frank A.
Dinenno
b and
Sarah A.
Johnson
 *a
*a
aDepartment of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA. E-mail: sarah.johnson@colostate.edu
bDepartment of Health and Exercise Science, Colorado State University, Fort Collins, CO, USA
cDepartment of Clinical Sciences, Colorado State University, Fort Collins, CO, USA
dDepartment of Nutritional Sciences, School of Life Course and Population Sciences, King's College London, London, England, UK
eDepartment of Integrative Physiology, University of Colorado, Boulder, CO, USA
First published on 15th February 2023
Abstract
Estrogen-deficient postmenopausal women have oxidative stress-mediated suppression of endothelial function that is exacerbated by high blood pressure. Previous research suggests blueberries may improve endothelial function through reductions in oxidative stress, while also exerting other cardiovascular benefits. The objective of this study was to examine the efficacy of blueberries to improve endothelial function and blood pressure in postmenopausal women with above-normal blood pressure, and to identify potential mechanisms for improvements in endothelial function. A randomized, double-blind, placebo-controlled, parallel-arm clinical trial was performed, where postmenopausal women aged 45–65 years with elevated blood pressure or stage 1-hypertension (total n = 43, endothelial function n = 32) consumed 22 g day−1 of freeze-dried highbush blueberry powder or placebo powder for 12 weeks. Endothelial function was assessed at baseline and 12 weeks through ultrasound measurement of brachial artery flow-mediated dilation (FMD) normalized to shear rate area under the curve (FMD/SRAUC) before and after intravenous infusion of a supraphysiologic dose of ascorbic acid to evaluate whether FMD improvements were mediated by reduced oxidative stress. Hemodynamics, arterial stiffness, cardiometabolic blood biomarkers, and plasma (poly)phenol metabolites were assessed at baseline and 4, 8, and 12 weeks, and venous endothelial cell protein expression was assessed at baseline and 12 weeks. Absolute FMD/SRAUC was 96% higher following blueberry consumption compared to baseline (p < 0.05) but unchanged in the placebo group (p > 0.05), and changes from baseline to 12 weeks were greater in the blueberry group than placebo (+1.09 × 10−4 ± 4.12 × 10−5vs. +3.82 × 10−6 ± 1.59 × 10−5, p < 0.03, respectively). The FMD/SRAUC response to ascorbic acid infusion was lower (p < 0.05) at 12 weeks compared to baseline in the blueberry group with no change in the placebo group (p > 0.05). The sum of plasma (poly)phenol metabolites increased at 4, 8, and 12 weeks in the blueberry group compared to baseline, and were higher than the placebo group (all p < 0.05). Increases in several plasma flavonoid and microbial metabolites were also noted. No major differences were found for blood pressure, arterial stiffness, blood biomarkers, or endothelial cell protein expression following blueberry consumption. These findings suggest daily consumption of freeze-dried blueberry powder for 12 weeks improves endothelial function through reduced oxidative stress in postmenopausal women with above-normal blood pressure. The clinical trial registry number is NCT03370991 (https://clinicaltrials.gov)
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States (US) and globally. Each year, there are over 17.9 million CVD-related deaths which makes up approximately 32% of all deaths worldwide.1,2 Aging is the primary risk factor for CVD largely due to unfavorable changes to the vasculature, particularly the arteries. Vascular endothelial dysfunction is one major adverse change to the arteries characterized by impaired endothelium-dependent dilation due to an imbalance in endothelium-derived biologically active molecules.3 The endothelium is crucial for cardiovascular health as it is responsible for maintaining vascular tone through producing nitric oxide (NO) and other vasoactive molecules.4,5 NO is a key vasodilatory and vasoprotective molecule,6 and current knowledge indicates that reduced NO bioavailability secondary to excessive superoxide-driven oxidative stress is central to endothelial dysfunction.3,7–10 Superoxide radicals and other reactive oxygen species (ROS) decrease NO bioavailability by oxidizing the NO-producing enzyme endothelial nitric oxide synthase (eNOS) leading to further superoxide radical production instead of NO. ROS also react directly with NO which further promotes oxidative stress and endothelial dysfunction.11,12 Furthermore, ROS can stimulate pro-inflammatory processes, which perpetuates oxidative stress through a vicious cycle and contributes to endothelial dysfunction.12–15Postmenopausal women experience accelerated adverse changes to cardiovascular health, including endothelial dysfunction, which is an independent risk factor and a predictor of CVD events in this population.16,17 Indeed, reductions in endothelium-dependent dilation have been observed during the menopausal transition independent of age and other CVD risk factors.18–21 Estrogen exerts direct and indirect antioxidant and vasodilatory effects, and thus postmenopausal women experience oxidative stress-mediated endothelial dysfunction due to diminished estrogen production.22–24 Above-normal blood pressure (i.e. elevated blood pressure and hypertension), often present in postmenopausal women, is also a contributor to the increased risk for CVD.25,26 Menopause is associated with a 2-fold increase in hypertension, with approximately 75% of postmenopausal women estimated to have hypertension, which worsens endothelial function through mechanisms that include oxidative stress and inflammation.12,27 Therefore, intervention strategies targeted at improving endothelial function through reduced oxidative stress and inflammation are important for CVD risk reduction in postmenopausal women, and especially those with above-normal blood pressure.
Lifestyle modification, particularly diet and nutrition, is recommended for CVD risk reduction, and consuming a diet rich in (poly)phenol-rich plant foods is linked to lower CVD risk.28–30 (Poly)phenols are secondary plant metabolites characterized by their phenolic structures and hydroxyl moieties,31 and are broken into flavonoids and non-flavonoids. Flavonoids (e.g. anthocyanins) are the most studied (poly)phenols and are found in high concentrations in fruits such as berries.30,32 (Poly)phenols and their circulating derivatives of phase II enzyme and gut microbial metabolism (i.e. metabolites) can reduce oxidative stress and inflammation.33 For instance, (poly)phenol metabolites can directly interact with free radical-generating enzymes and antioxidant enzymes leading to reductions in oxidative stress.34–41 Blueberries are especially rich in (poly)phenols, namely anthocyanins and phenolic acids, and accumulating evidence supports their cardiovascular-protective effects, including improvements in endothelial function in healthy adult men, adults with metabolic syndrome, and cigarette smoking men with or without peripheral arterial dysfunction.42,43 We previously demonstrated that consuming 22 g freeze-dried blueberry powder (equal to 1 cup fresh blueberries) daily for 8 weeks led to improvements in systolic and diastolic blood pressure and brachial-ankle pulse wave velocity (PWV; a measure of arterial stiffness), and increased plasma concentrations of NO metabolites in postmenopausal women with above-normal blood pressure, suggestive of improvements in endothelial function.44 Other studies have also observed reductions in blood pressure and measures of arterial stiffness, but the results in this area are mixed.45
While promising, the effects of blueberry consumption on endothelial function in postmenopausal women with above-normal blood pressure remains unknown. Furthermore, determining underlying mechanisms is crucial to establish blueberries as a dietary intervention for improving endothelial function, and for identifying ways to increase clinical efficacy. Physiologically relevant cell and animal studies, and limited human studies, suggest that blueberries, their (poly)phenols, and/or resulting metabolites may improve endothelial function through reductions in oxidative stress.46–48 However, evidence demonstrating that improved endothelial function is directly linked to reductions in oxidative stress following chronic blueberry consumption in humans is needed. Therefore, the purpose of this randomized, double-blind, placebo-controlled, parallel-arm clinical trial was to examine the efficacy of chronic blueberry consumption to improve endothelial function, blood pressure, and other biomarkers of cardiometabolic health in postmenopausal women with above-normal blood pressure, and to gain insight into underlying mechanisms with a specific focus on oxidative stress-related mechanisms. We hypothesized that daily consumption of 22 g freeze-dried blueberry powder for 12 weeks would improve endothelium-dependent dilation through reductions in oxidative stress, blood pressure, and other cardiometabolic biomarkers in postmenopausal women with above-normal blood pressure.
Materials and methods
Study population and recruitment
Estrogen-deficient postmenopausal women (≥1 y free of menses, confirmed with blood measurement of estradiol <30 pg mL−1 and follicle-stimulating hormone (FSH) >30 mIU mL−1) aged 45–65 y with elevated blood pressure or stage-1 hypertension (systolic blood pressure 120–139 mmHg and/or diastolic blood pressure 80–89 mmHg) were recruited for the study. Individuals were excluded if they did not meet inclusion criteria, had a body mass index (BMI) <18.5 or >40 kg m−2, presence of clinical disease(s) (i.e. renal, respiratory, thyroid, diabetes, thrombosis, neuropathy, cancer, gastrointestinal, liver, kidney, pancreatic, or cardiovascular), cigarette smoking within past year prior to enrollment, taking >1 blood pressure medication or taking blood pressure medication for <3 months, use of sex hormone replacement, lipid-lowering, or phosphodiesterase-5 inhibitor medications, triglycerides (TG) >350 mg dL−1, and/or low-density lipoprotein cholesterol (LDL-C) ≥190 mg dL−1, body weight change ≥3 kg in the 3 months prior to study enrollment or actively trying to lose weight, unwilling to maintain normal eating, drinking, and/or physical activity habits throughout the study, heavy or binge drinking (>7 drinks per week or >3 drinks on any given occasion, respectively), and/or have allergies or contraindications to study treatment(s), pharmacological agents, and/or procedures.Participants were recruited from the greater Fort Collins, Colorado, area through flyer distribution, email, newspaper, Colorado State University (CSU) webpages, direct mailers, and ClinicalTrials.gov between December 2017 and January 2020. Individuals indicated their interest in study participation though email or phone call, and an initial phone screening was conducted for each interested participant where they were asked specific questions regarding their health history to determine study eligibility prior to their onsite screening visit. The onsite screening visit entailed reading and signing the informed consent form, confirmation of inclusion and exclusion criteria by measuring seated blood pressure in triplicate, calculating BMI, examining blood parameters (blood lipid profiles, estradiol, and FSH), and completing a medical health history questionnaire. Qualified participants were scheduled for their baseline visit. This study was approved by the CSU Institutional Review Board (protocol #2891), conducted in accordance with the Declaration of Helsinki, and is registered at ClinicalTrials.gov as NCT03370991.
Study design
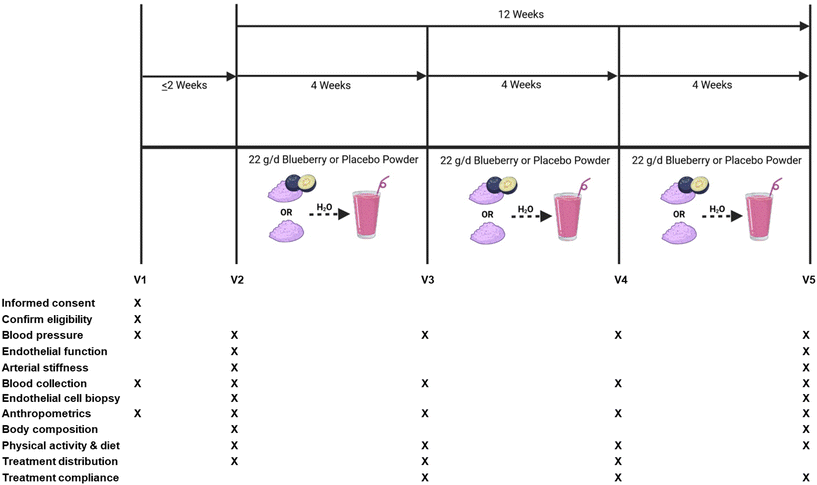
This study was a randomized, double-blind, placebo-controlled, parallel-arm clinical trial. The overall study design, schedule of study visits, and a schematic of study visits for data collection and measurements are presented in Fig. 1. The primary outcomes were brachial artery flow-mediated dilation (FMD; measure of endothelial function) and blood pressure. Secondary outcomes included the impact of intravenous ascorbic acid on oxidative stress-mediated suppression of endothelial function, endothelium-independent dilation, hemodynamics, arterial stiffness, plasma (poly)phenol metabolites, blood markers of cardiometabolic health, and venous endothelial cell protein expression related to NO production, oxidative stress, and inflammation.Laboratory measures were taken on 4 different occasions (i.e. study visits) separated by 4 weeks over a 12-week period, between 6:00 am and 11:00 am. All cardiovascular measures were conducted in a supine position, at room temperature, and with dimmed lighting. Participants were asked to be in a fasting state, which consisted of no food and drinks (other than water) for 8 h prior to the study visit, and no dietary supplements or prescription medication use was permitted within 24 h of the study visit. Participants were asked to maintain their usual diet and physical activity patterns, and to keep fresh and frozen blueberry consumption to ≤2 cups per week for the duration of the study (with the exception of the treatment intervention).
Treatment intervention and compliance
Eligible participants (n = 48) were randomly assigned to treatments using block randomization stratified by hypertension stage to receive either: (1) 22 g freeze-dried highbush blueberry powder (Tifblue/Rubel 50/50 blend; equivalent to about 1 cup fresh blueberries); or (2) 22 g isocaloric and carbohydrate-matched placebo powder (dextrose, maltodextrin, fructose, and artificial color and flavors) with a similar color, texture, and taste to the blueberry powder. The 22 g dose of blueberry powder contained 87 total kcals, <1 g fat, 21 g total carbohydrate, <1 g protein, 726 mg of total (poly)phenols, and 224 mg of anthocyanins, while the 22 g dose of placebo powder contained 83 total kcals, <1 g fat, 21 g total carbohydrate, <1 g protein, and was devoid of (poly)phenols. The US Highbush Blueberry Council provided the blueberry and placebo powders. Nutritional analysis of the treatment powders was performed by Medallion Laboratories (Minneapolis, MN, USA) and total (poly)phenol and anthocyanin analyses were performed by International Chemistry Testing (Hopkinton, MA, USA). Treatment powders were packaged in air-tight, opaque 11 g packets to further conceal treatment identity. Participants consumed 11 g of their assigned treatment 2× per day mixed in water in the morning and afternoon/evening, at least 6–8 h apart to decrease the risk for gastrointestinal distress, for 12 weeks.Treatment compliance was assessed by asking study participants to record the date and time their treatment packets were consumed each day in a daily treatment dosing log, and to document reasons for missing doses (i.e. sick, fell asleep, forgot). Non-compliance was defined as missing ≥ two 11 g doses per week or less than ∼86% treatment compliance on average, and was calculated using the following equation: ([dosages consumed − missed dosages/dosages required] × 100).
Endothelium-dependent and -independent dilation
Brachial artery FMD (endothelium-dependent dilation, n = 32) and brachial artery diameter responses to sublingual nitroglycerin (NTG) administration (endothelium-independent dilation, n = 20) were determined in a subset of participants as described previously49–53 and analyzed using commercially available software packages (Brachial Analyzer for Research, Medical Imaging Applications, LLC, Coralville, IA, USA and DATAQ Instruments, OH, USA).Brachial artery diameter and mean blood velocity (MBV) were measured with a 12 MHz linear-array ultrasound probe (Vivid7, GE Healthcare, Wauwatosa, WI, USA) clamped in place ∼3–6 cm proximal to the antecubital fossa with a <60° insonation angle. In brief, to assess FMD, a rapidly inflating blood pressure cuff (D.E. Hokanson, Inc., Bellevue, WA, USA) on the upper forearm (∼2 cm distal to the antecubital fossa) was inflated to 250 mmHg to occlude forearm blood flow. Ultrasound images were captured (Vascular Imager, Medical Imaging Applications, LLC, Coralville, IA, USA) at end-diastole for each cardiac cycle at baseline (30 s), during occlusion (5 min), and post-occlusion (5 min). Shear rate (SR) was calculated for each cardiac cycle as 8 × MBV − diameter, and the SR area under the curve (SRAUC) from cuff release to peak diameter was quantified as an index of the stimulus for FMD.54 FMD values are expressed as relative (%) changes from baseline to peak diameter and corrected for shear rate (FMD/SRAUC) to account for inter-individual variability in shear stress.55–57
To determine whether daily blueberry consumption improves endothelium-dependent dilation by suppressing oxidative stress, brachial artery FMD was measured before and after a supraphysiologic infusion of the antioxidant ascorbic acid (Mylan Institutional Inc., Rockford, IL, USA). Ascorbic acid was administered intravenously at a dose of 0.06 g kg−1 fat-free mass53,58 over a 20 min period, then brachial artery FMD was reassessed using the aforementioned procedures. Ascorbic acid is a potent antioxidant that scavenges ROS, including superoxide radicals. Thus, this approach has been used to evaluate the extent to which oxidative stress suppresses endothelium-dependent dilation.53,58,59
Lastly, sublingual NTG (0.4 mg) (Pfizer Inc., New York, NY, USA) was administered to assess endothelium-independent dilation in a subset of study participants (n = 20) following a 10 min rest period to reduce carry over effects. Briefly, baseline artery diameters were measured, a NTG tablet was placed under the participant's tongue, and brachial artery diameters were measured over 8 min to capture peak diameter. NTG values are expressed as relative (%) changes from baseline to peak diameter.
Hemodynamics and arterial stiffness
Hemodynamics and arterial stiffness were assessed after a 10 min rest in the supine position using the SphygmoCor XCEL (AtCor Medical Inc., Naperville, IL, USA) as previously described.60,61 Briefly, supine brachial blood pressure was measured, followed by pulse wave analysis for central aortic parameters (i.e. mean arterial pressure, pulse pressure, heart rate, augmentation index (AIx), AIx normalized to a heart rate of 75 bpm (AIx@75), systolic and diastolic pressure, and aortic pressure).After blood pressure measurements, carotid-femoral/aortic PWV was measured as previously described.51,60 In short, a blood pressure cuff was placed on the upper thigh at the femoral artery and a tonometer was placed on the carotid artery simultaneously to capture pulse waveforms. Three measurements were taken using calipers: (1) distance between carotid artery pulse and sternal notch, (2) sternal notch to the top of the blood pressure cuff, and (3) femoral artery pulse to the top of the blood pressure cuff. The distance traveled between the two sites and the time the waveform traveled was used to automatically calculate carotid-femoral/aortic PWV (distance/time) by the SphygmoCor system. All hemodynamic and arterial stiffness measurements were performed in triplicate.
Blood collection and biomarker analyses
Venous blood was collected using a standard butterfly needle (BD Vacutainer, Becton, Dickson and Company, Franklin Lakes, NJ, USA) or an intravenous catheter (Introcan Safety, Braun Medical Inc., Bethlehem, PA, USA). Blood lipids (high-density lipoprotein cholesterol (HDL-C), LDL-C, total cholesterol (TC), and TG), hemoglobin A1c, FSH, and estradiol were assessed using standard assays at the University of Colorado-Health/Poudre Valley Hospital Lab (Fort Collins, CO, USA). Remaining venous blood was processed for serum and plasma separation using VACUETTE® CAT Serum Sep Clot Activator tubes (Greiner Bio-One North America Inc., Monroe, NC, USA) and BD Vacutainer® K2 EDTA collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), respectively and stored at −80 °C until analysis. Plasma intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) levels were measured in duplicate using commercially available quantikine ICAM-1 and VCAM-1 enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Bio-Techne brand, Minneapolis, MN, USA). Plasma nitrate/nitrite was measured in duplicate using a commercially available colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA).Chromatography and mass spectrometry analysis of plasma (poly)phenols
The extraction of phenolic metabolites from plasma samples was performed by micro-elution solid phase extraction (μ-SPE) and measured by UPLC-Q-q-Q MS, as per the protocol of a validated method.62 Briefly, undiluted plasma samples were acidified with 4% phosphoric acid (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 1
v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This mix (total volume of 600 μL) was then loaded onto Oasis 96-well reversed-phase HLB (hydrophilic-lipophilic balanced) sorbent μ-SPE plates (Waters, Eschborn, Germany), washed with water and 0.2% acetic acid, and eluted using 90 μL of methanol. Identification and quantification of (poly)phenol metabolites were performed on a SHIMADZU Triple Quadrupole Mass Spectrometer (LCMS8060, SHIMADZU, Kyoto, Japan). Five microliters of the eluded samples were then injected through a Raptor Biphenyl column 2.1 × 50 mm, 1.8 μm (Restek, Bellefonte, USA) with a compatible Raptor Biphenyl Guard Cartridges 5 × 2.1 mm (Restek, Bellefonte, USA) in the UPLC system. A 14-minute gradient followed by a 2-minute equilibration was applied to the run under a flow rate of 0.5 mL min−1 at 30 °C. The identification of metabolites was performed by comparing retention times with authentic standards in corresponding multiple reaction monitoring (MRM) transitions and quantified by calibration curves made from standard mixes using SHIMADZU LabSolutions™ LCMS Software.
1). This mix (total volume of 600 μL) was then loaded onto Oasis 96-well reversed-phase HLB (hydrophilic-lipophilic balanced) sorbent μ-SPE plates (Waters, Eschborn, Germany), washed with water and 0.2% acetic acid, and eluted using 90 μL of methanol. Identification and quantification of (poly)phenol metabolites were performed on a SHIMADZU Triple Quadrupole Mass Spectrometer (LCMS8060, SHIMADZU, Kyoto, Japan). Five microliters of the eluded samples were then injected through a Raptor Biphenyl column 2.1 × 50 mm, 1.8 μm (Restek, Bellefonte, USA) with a compatible Raptor Biphenyl Guard Cartridges 5 × 2.1 mm (Restek, Bellefonte, USA) in the UPLC system. A 14-minute gradient followed by a 2-minute equilibration was applied to the run under a flow rate of 0.5 mL min−1 at 30 °C. The identification of metabolites was performed by comparing retention times with authentic standards in corresponding multiple reaction monitoring (MRM) transitions and quantified by calibration curves made from standard mixes using SHIMADZU LabSolutions™ LCMS Software.
Endothelial cell biopsy and protein quantification
Human venous endothelial cell biopsy was performed in a subset of participants (n = 24) as previously described.53,60,63–67 Venous endothelial cell protein expression has been shown to correlate with arterial endothelial cell expression65 and therefore was selected as a proxy for arterial endothelial cells. Briefly, endothelial cells were biopsied from the antecubital vein by inserting 2 sterile 0.025 mm J-shaped guidewires (GuideRight™, Abbott Medical, Plymouth, MN, USA) one at a time into the intravenous catheter ∼4 cm beyond the catheter tip. Wires were carefully withdrawn, and the distal portion of the wire was immediately transferred to a conical tube containing phosphate buffered saline solution for processing. Isolated endothelial cells were mounted on poly-L-lysine microscope slides, incubated for 5 h at 37 °C, and stored at −80 °C until immunofluorescence microscopy protein expression analyses.Previously described methods were used for quantitative immunofluorescence of endothelial cell protein expression with one modification (donkey serum was used in place of goat serum to view and confirm endothelial cells).60,65–67 Primary antibodies of interest included manganese superoxide dismutase (MnSOD) (Enzo Life Sciences, Inc., Farmingdale, NY, USA), phosphorylated endothelial nitric oxide synthase (eNOS) (Cell Signaling Technology, Inc., Danvers, MA, USA), phosphorylated nuclear factor kappa B (NFκB) (Cell Signaling Technology, Inc., Danvers, MA, USA), and NADPH oxidase/p47 subunit (MilliporeSigma, Burlington, MA, USA). Using a microscope (Olympus BX3-CBH Olympus Scientific Solutions Americas Corp., Waltham, MA, USA), cells were imaged by a DP73 digital camera (Olympus Scientific Solutions Americas Corp., Waltham, MA, USA) and automatically analyzed via cellSens Software (Olympus Scientific Solutions Americas Corp., Waltham, MA, USA) to quantify staining intensity. Human umbilical vein endothelial cells (HUVECs) were used as a control for each staining batch to account for between batch staining variability, and values are reported as a ratio of participant endothelial cell protein expression to HUVEC and expressed as arbitrary units (AU).
Anthropometrics, body composition, and energy intake and expenditure
Height without shoes was measured only at screening visits using a standard scale-mounted stadiometer and rounded to the nearest 0.5 cm, and body weight without shoes was measured using a scale and rounded to the nearest kg at all visits (Health o Meter Professional 500KL, Pelstar LLC, Countryside, IL, USA). BMI was calculated as weight (kg) divided by height (m2). Waist and hip circumferences were measured using a standard measuring tape and rounded to the nearest 0.5 cm, and ratios were calculated by dividing waist circumference by hip circumference. Body composition (fat mass, fat free mass, android fat, and gynoid fat) was assessed using dual-energy X-ray absorptiometry (DEXA) (Hologic, Mississauga, ON, Canada). Self-reported food intake on 3 separate days (2 weekdays and 1 weekend day) was collected prior to each study visit through the Automated Self-Administered 24-h (ASA24) Dietary Assessment Tool (National Institutes for Health, Bethesda, MD, USA) that calculated total calories (kcals) to assess energy intake throughout the study.68,69 Physical activity patterns were assessed using a validated 7-day physical activity recall at each study visit to estimate energy expenditure (kcal day−1).70,71Statistical analyses
Collected data were stored electronically using REDCap with double-data entry by two individuals and independent quality review by a third person. Missing data from continuous variables were imputed using the mean values of the outcomes within the groups. Data were evaluated for normality using Shapiro–Wilk tests (SAS Institute Inc., Cary, NC) and log-transformed prior to statistical analysis when appropriate. A linear mixed model approach was used to analyze the data and compare between the treatment groups as well as time with an interaction term, which were set as fixed effects in the model, while participants were set as random effects. Least square differences were calculated for comparisons and the adjustment of the p-value was performed using a Tukey–Kramer's correction for multiple comparisons. Univariate analyses was performed with each potential confounder (i.e. age, BMI, hypertension stage, hypertensive medication use, and years since menopause). The variables that met the criteria of a p-value of 0.25 were included in the multivariable linear mixed model. A backward elimination process was used to retain the variables that meet the p-value of 0.05 to finalize the model for each outcome variable. Spearman correlation analyses were performed to assess the relationship of improvements in endothelial function (FMD/SRAUC) with plasma (poly)phenol metabolites, and endothelial cell protein expression. Endothelial cell protein expression correlation analyses were performed using GraphPad (version 9.4.1, San Diego, CA, USA), and SAS v9.4 (SAS Institute Inc., Cary, NC) was used for all other statistical analyses. Results are presented as mean ± SEM, and a p-value of 0.05 was used to determine statistical significance. A p-value of less than 0.10 was used to determine a trend for statistical significance.Results
Study enrollment, attrition, compliance, and baseline characteristics
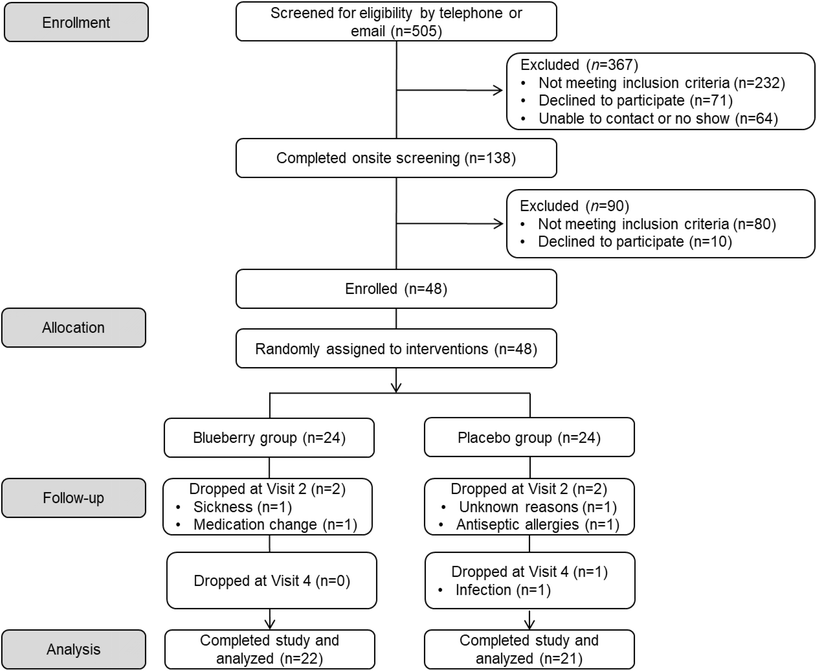
A detailed schematic of participant flow throughout the study (i.e. CONSORT flow diagram) is provided in Fig. 2. A total of 505 potential participants contacted the laboratory to express interest in participating in the study and were screened via telephone or email. Of these, 367 individuals were excluded due to not meeting inclusion criteria, subsequently declining to participate, or an inability to contact them, leaving 138 participants who completed an onsite screening visit. Of those, 90 people were excluded for not meeting inclusion criteria or declining to participate. As a result, 48 estrogen-deficient postmenopausal women were enrolled in the study and randomly assigned to an intervention (blueberry n = 24 and placebo n = 24). Throughout the 12-week intervention period, 5 participants dropped from the study due to sickness, medication change, antiseptic allergies, infection, or for unknown reasons, leaving 43 participants who completed the 12-week study (blueberry n = 22 and placebo n = 21). The overall attrition rate for the 12-week intervention study was approximately 10.4% (8.3% in the blueberry group and 12.5% in the placebo group). Overall self-reported treatment compliance was 97%. For the blueberry group, overall self-reported compliance was 97%, and was 98% between baseline and 4 weeks, 98% between 4 and 8 weeks, and 96% between 8 and 12 weeks. For the placebo group, self-reported compliance was 97%, and was 95% between baseline and 4 weeks, 98% between 4 and 8 weeks, and 97% between 8 and 12 weeks. Screening characteristics are presented in Table 1, and there were no differences (p > 0.05) between groups.| Variable | Blueberry (n = 22) | Placebo (n = 21) |
|---|---|---|
| Data are mean ± SEM (or %). Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FSH, follicle-stimulating hormone; HgbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein-cholesterol; HC, hip circumference; LDL-C, low-density lipoprotein-cholesterol; med, medication; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference. | ||
| Age (y) | 60 ± 1 | 61 ± 1 |
| Years after menopause | 10 ± 1 | 9 ± 1 |
| Estradiol (pg mL−1) | 17.0 ± 0.9 | 17.1 ± 1.0 |
| FSH (mIU mL−1) | 67.0 ± 5.0 | 67.0 ± 4.8 |
| Height (m) | 1.6 ± 0.0 | 1.6 ± 0.0 |
| Weight (kg) | 73.0 ± 3.2 | 74.6 ± 3.5 |
| BMI (kg m−2) | 27.6 ± 1.0 | 27.8 ± 1.1 |
| WC (cm) | 89 ± 3 | 89 ± 2 |
| HC (cm) | 108 ± 2 | 107 ± 2 |
WC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HC HC |
0.82 ± 0.01 | 0.83 ± 0.01 |
| SBP (mmHg) | 130 ± 1 | 128 ± 1 |
| DBP (mmHg) | 80 ± 1 | 82 ± 1 |
| TG (mg dL−1) | 121 ± 12 | 112 ± 14 |
| TC (mg dL−1) | 214 ± 5 | 220 ± 6 |
| HDL-C (mg dL−1) | 58 ± 3 | 58 ± 3 |
| LDL-C (mg dL−1) | 132 ± 5 | 139 ± 5 |
| HgbA1c (%) | 5.5 ± 0.1 | 5.5 ± 0.1 |
| BP med use, n (%) | 6 (27) | 4 (19) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 22 (100) | 20 (95) |
| Hispanic | 0 | 1 (5) |
Endothelium-dependent and -independent dilation
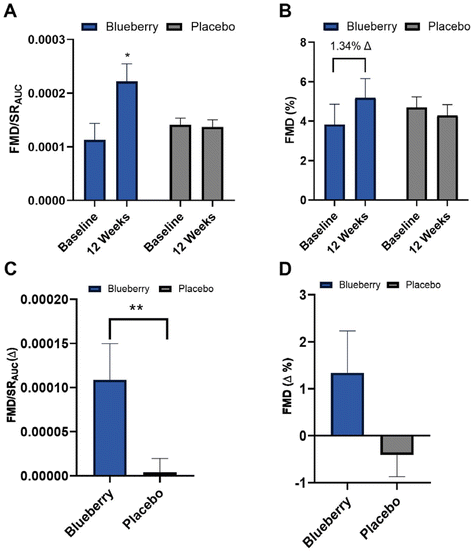
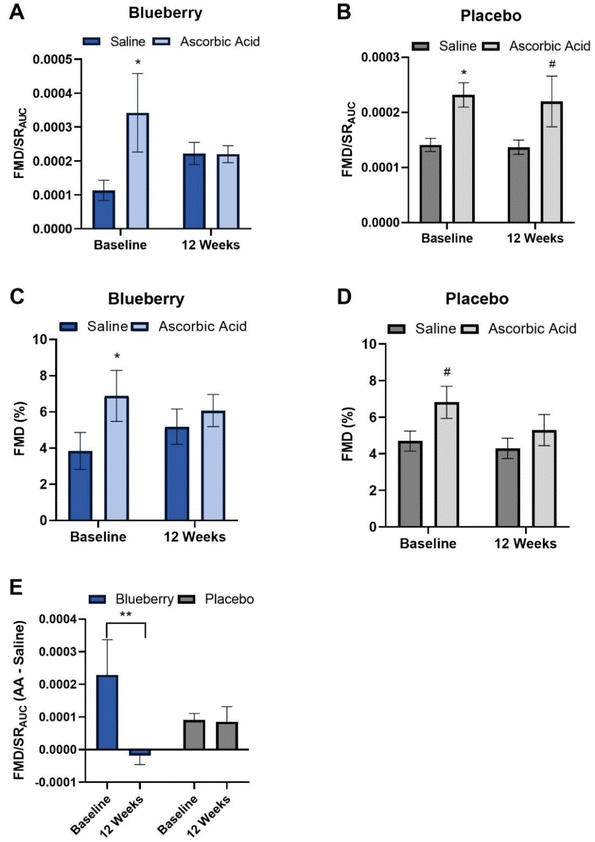
Absolute FMD/SRAUC was 96% higher (p < 0.01) at 12 weeks compared to baseline in the blueberry group with no change (p > 0.05) observed in the placebo group (Fig. 3A and ESI Table 1†), and the change in FMD/SRAUC from baseline to 12 weeks was higher (p < 0.03) than the placebo group (Fig. 3C and ESI Table 1†). Though there were no statistically significant differences (p > 0.05), there was a ∼1.34% unit increase in absolute FMD not normalized to SRAUC (35% higher) compared to baseline at 12 weeks in the blueberry group (Fig. 3B, D and ESI Table 1†). Individual FMD changes from baseline to 12 weeks are presented in ESI Fig. 1.† Brachial artery endothelium-independent dilation to sublingual NTG did not change (p > 0.05) within or between groups (ESI Table 2†), confirming the improvements in endothelial function were endothelium-dependent.To evaluate oxidative stress-mediated suppression of endothelial function and determine the impact of blueberries on its amelioration, endothelium-dependent dilation was assessed before and following intravenous infusion of the antioxidant ascorbic acid. At baseline (pre-blueberry or -placebo consumption), ascorbic acid administration acutely increased (p < 0.05) FMD/SRAUC in both groups (Fig. 4A and B). There was an increase (p < 0.01) in FMD not normalized to SRAUC in the blueberry group, with trending significance in the placebo group (p < 0.07) (Fig. 4C, D, and ESI Table 3†).
After 12 weeks of daily blueberry consumption, ascorbic acid administration did not alter (p > 0.05) FMD/SRAUC or FMD not normalized to SRAUC (Fig. 4A, C, and ESI Table 3†). The placebo group had a strong trend for an increase (p < 0.08) in FMD/SRAUC at 12 weeks (Fig. 4B and ESI Table 3†), but not FMD not normalized to SRAUC (p > 0.05) (Fig. 4C, D and ESI Table 3†). When assessed as the difference in the FMD/SRAUC response to ascorbic acid vs. saline, the blueberry group had a reduction in the FMD/SRAUC response (p < 0.04) to ascorbic acid at 12 weeks compared to baseline, while the placebo group had no change (p > 0.05) (Fig. 4E and ESI Table 3†).
Hemodynamics and arterial stiffness
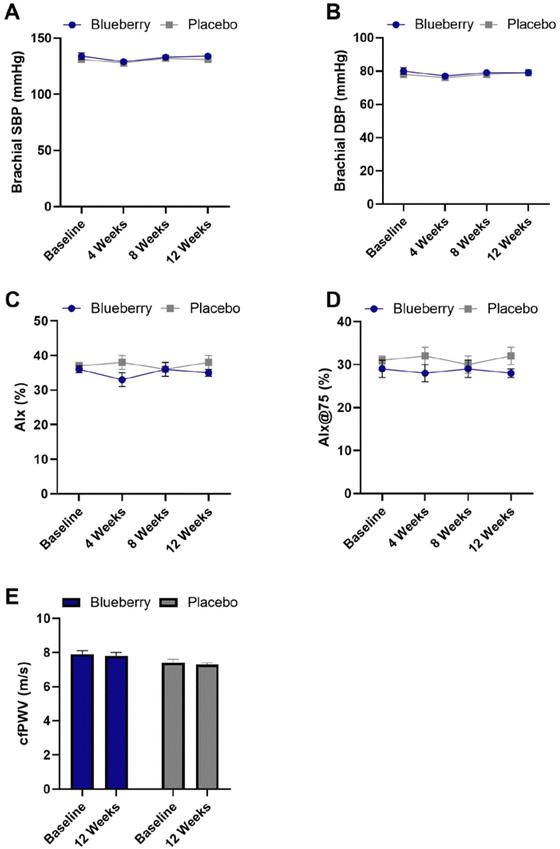
There were no significant differences (p > 0.05) within or between groups for hemodynamic and arterial stiffness measures except for a significant reduction in aortic pulse pressure in the blueberry group at 4 weeks compared to baseline (p < 0.04) (Fig. 5 and ESI Table 4†).Blood biomarkers
Blood biomarker results are presented in Table 2. Reductions (p < 0.05) in TG were observed at 12 weeks compared to baseline in the blueberry group, but while no changes (p > 0.05) were noted in the placebo group or for the change from baseline to 12 weeks. The change from baseline to 12 weeks for HDL-C was higher (p < 0.05) in the blueberry group than the placebo group, while there were no changes at 12 weeks compared to baseline (p > 0.05). Reductions in LDL-C (p < 0.05) were noted at 12 weeks compared to baseline in both groups, but not for the change from baseline to 12 weeks (p > 0.05). VCAM-1 was increased at 4 weeks (p < 0.004) and 8 weeks (p < 0.008) compared to baseline in the placebo group, with no changes (p > 0.05) in the blueberry group or for the change from baseline. No other effects (p > 0.05) were noted.| Blueberry (n = 22) | Placebo (n = 21) | |
|---|---|---|
| Data are mean ± SEM. *Different (p < 0.05) than baseline. **Different (p < 0.05) between groups at that time point. Abbreviations: HgbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein-cholesterol, LDL-C, low-density lipoprotein-cholesterol, TC, total cholesterol, TG, triglycerides, ICAM-1, intercellular adhesion molecule-1, VCAM-1, vascular cell adhesion protein-1. | ||
| TG (mg dL −1 ) | ||
| Baseline | 121 ± 12 | 114 ± 14 |
| 12 weeks | 100 ± 10* | 114 ± 18 |
| Δ 0 to 12 weeks | −21 ± 9 | 0 ± 9 |
| TC (mg dL −1 ) | ||
| Baseline | 214 ± 5 | 217 ± 5 |
| 12 weeks | 198 ± 5 | 201 ± 7 |
| Δ 0 to 12 weeks | −17 ± 4 | −16 ± 4 |
| HDL-C (mg dL −1 ) | ||
| Baseline | 58 ± 3 | 57 ± 3 |
| 12 weeks | 59 ± 4 | 54 ± 3 |
| Δ 0 to 12 weeks | 1 ± 3** | −3 ± 2 |
| LDL-C (mg dL −1 ) | ||
| Baseline | 132 ± 5 | 137 ± 4 |
| 12 weeks | 119 ± 6* | 125 ± 5* |
| Δ 0 to 12 weeks | −13 ± 5 | −12 ± 4 |
LDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) HDL HDL
|
||
| Baseline | 2.47 ± 0.20 | 2.57 ± 0.20 |
| 12 weeks | 2.28 ± 0.22 | 2.50 ± 0.18 |
| Δ 0 to 12 weeks | −0.19 ± 0.14 | −0.07 ± 0.10 |
| HgbA1c (%) | ||
| Baseline | 5.5 ± 0.1 | 5.5 ± 0.1 |
| 12 weeks | 5.5 ± 0.1 | 5.5 ± 0.1 |
| Δ 0 to 12 weeks | 0.0 ± 0.0 | 0.0 ± 0.0 |
| ICAM-1 (ng mL −1 ) | ||
| Baseline | 224.32 ± 16.27 | 224.88 ± 11.91 |
| 4 weeks | 234.42 ± 15.09 | 275.82 ± 30.07 |
| 8 weeks | 219.76 ± 12.60 | 273.46 ± 15.37 |
| 12 weeks | 246.19 ± 13.86 | 236.62 ± 12.19 |
| Δ 0 to 4 weeks | 10.10 ± 23.29 | 50.94 ± 34.97 |
| Δ 0 to 8 weeks | −1.39 ± 21.31 | 58.58 ± 15.15 |
| Δ 0 to 12 weeks | 21.87 ± 14.19 | 11.74 ± 18.28 |
| VCAM-1 (ng mL −1 ) | ||
| Baseline | 1275.76 ± 123.53 | 1001.81 ± 97.90 |
| 4 weeks | 1675.10 ± 153.41 | 1549.93 ± 140.86* |
| 8 weeks | 1567.96 ± 179.61 | 1497.26 ± 122.22* |
| 12 weeks | 1506.62 ± 104.77 | 1289.09 ± 126.88 |
| Δ 0 to 4 weeks | 399.33 ± 201.69 | 548.12 ± 192.96 |
| Δ 0 to 8 weeks | 238.92 ± 225.18 | 495.45 ± 160.61 |
| Δ 0 to 12 weeks | 230.86 ± 107.00 | 287.28 ± 435.23 |
| Nitrate/nitrite (μM) | ||
| Baseline | 9.99 ± 1.13 | 11.33 ± 1.51 |
| 4 weeks | 8.30 ± 1.24 | 12.37 ± 2.42 |
| 8 weeks | 7.93 ± 1.00 | 10.31 ± 2.01 |
| 12 weeks | 8.03 ± 1.31 | 9.76 ± 1.20 |
| Δ 0 to 4 weeks | −1.61 ± 1.09 | 1.04 ± 1.42 |
| Δ 0 to 8 weeks | −1.64 ± 1.18 | −0.51 ± 1.40 |
| Δ 0 to 12 weeks | −1.96 ± 1.35 | −1.58 ± 1.94 |
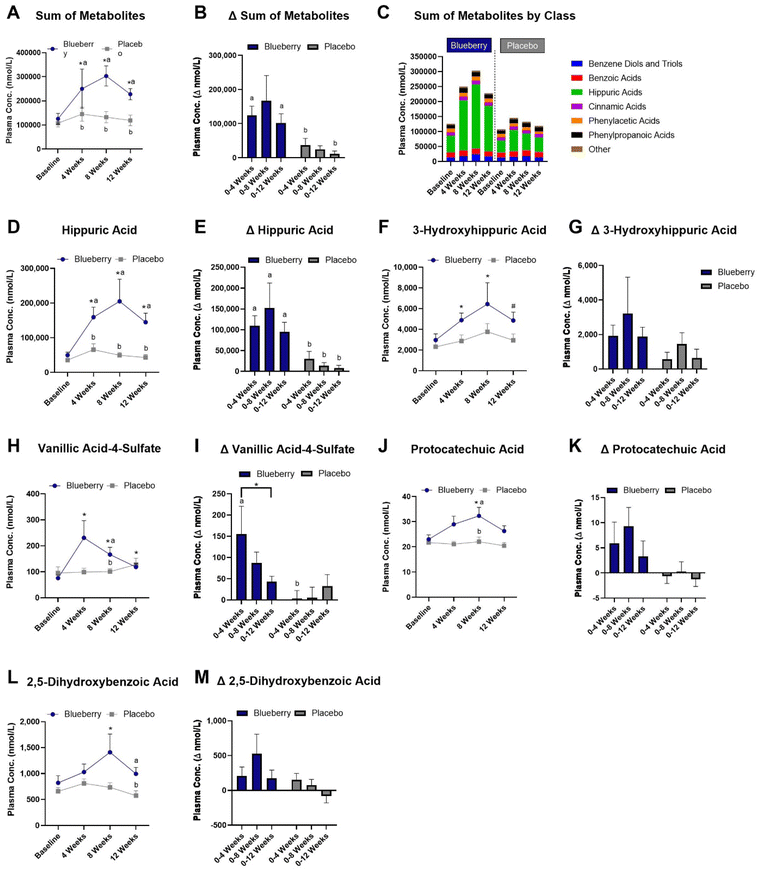
Plasma (poly)phenol metabolites
A total of 88 plasma (poly)phenol metabolites were detected (ESI Table 5†) and the sums of (poly)phenol metabolites grouped according to class are presented in Fig. 6C. In the blueberry group, the sum of plasma (poly)phenol metabolite concentrations (i.e. all detected plasma (poly)phenol metabolites) was increased at 4 weeks (p < 0.0001), 8 weeks (p < 0.0001), and 12 weeks (p < 0.0001) compared to baseline, higher than the placebo group at 4 (p < 0.02), 8 (p < 0.02), and 12 weeks (p < 0.004) (Fig. 6A and ESI Table 5†), and higher for the change from baseline to 4 (p < 0.02) and 12 (p < 0.05) weeks than the placebo group (Fig. 6A, B, and ESI Table 5†).With regard to individual metabolites (Fig. 6 and ESI Table 5†), hippuric acid was increased at 4 (p < 0.0001), 8 (p < 0.0001), and 12 (p < 0.0001) weeks in the blueberry group compared to baseline, and compared to the placebo group at 4 weeks (p < 0.05), 8 weeks (p < 0.02), and 12 weeks (p < 0.003) (Fig. 6D and ESI Table 5†) and for the change from baseline to 4 (p < 0.008), 8 (p < 0.03), and 12 (p < 0.007) weeks (Fig. 6E). The metabolite 3-hydroxyhippiuric acid was increased at 4 (p < 0.007) and 8 (p < 0.03) weeks, with a trend for an increase (p < 0.07) at 12 weeks compared to baseline in the blueberry group, but not (p > 0.05) compared to placebo (Fig. 6F and ESI Table 5†) or for the change from baseline (Fig. 6G). In the blueberry group, the metabolite 3-methoxybenzoic acid-4-sulfate (vanillic acid-4-sulfate) was increased at 4 (p < 0.0001), 8 (p < 0.0003), and 12 (p < 0.02) weeks compared to baseline, higher than placebo at 4 weeks (p < 0.04) (Fig. 6H and ESI Table 5†) and for the change from baseline to 4 weeks (p < 0.03) (Fig. 6I). The metabolite 3,4-dihydroxybenzoic acid (protocatechuic acid) was increased (p < 0.04) at 8 weeks compared to baseline following blueberry consumption (Fig. 6J and ESI Table 5†), and was higher (p < 0.03) than the placebo group at 8 weeks (Fig. 6K). The metabolite 2,5-dihydroxybenzoic acid was increased at 8 weeks (p < 0.03) compared to baseline in the blueberry group and higher (p < 0.05) than the placebo group at 12 weeks (Fig. 6L and ESI Table 5†), while no differences (p > 0.05) were noted for the change from baseline (Fig. 6M). The metabolite 2,3-dihydroxybenzene-1-sulfate was increased at 4 (p < 0.02) and 8 (p < 0.002) weeks in the blueberry group compared to baseline (ESI Table 5†), while no differences (p > 0.05) were noted for the change from baseline. The metabolite 3-hydroxy-4-methoxybenzoic acid-5-sulfate was higher at 4 (p < 0.01) and 8 (p < 0.004) weeks in the blueberry group compared to placebo, and the change from baseline to 8 weeks was higher (p < 0.05) than the placebo group (ESI Table 5†). The metabolite 2-hydroxybenzene-1-glucuronide was increased (p < 0.01) at 8 weeks compared to baseline in the blueberry group and the change from baseline to 4 weeks was higher (p < 0.02) than the placebo group (ESI Table 5†). The metabolite 2-hydroxy-3-(4′-hydroxyphenyl)propanoic acid was increased (p < 0.04) at 4 weeks compared to baseline in the blueberry group (ESI Table 5†). The metabolite 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide was increased (p < 0.007) at 8 weeks compared to baseline in the blueberry group (ESI Table 5†). Lastly, myricetin was higher (p < 0.05) in the blueberry group at 4 weeks compared to placebo at 4 weeks, and the change from baseline to 4 weeks was higher (p < 0.04) than placebo (ESI Table 5†).
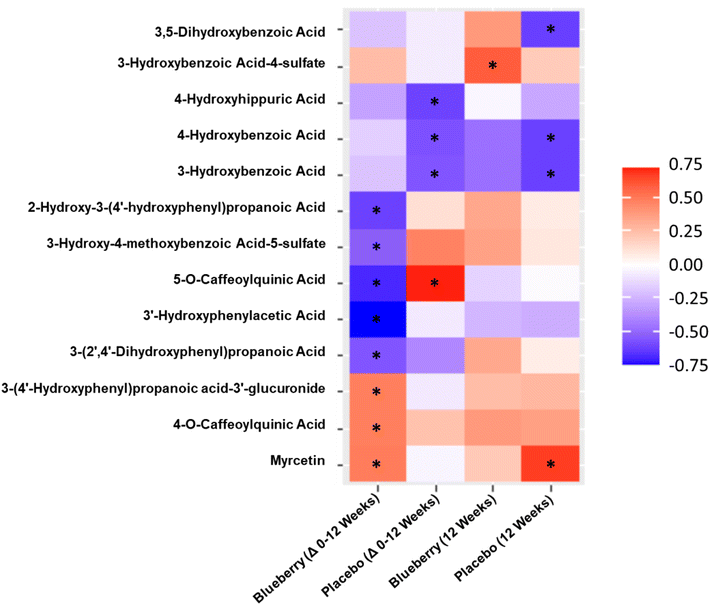
Correlations between endothelium-dependent dilation and plasma (poly)phenol metabolites
With the exception of flavonols (r = 0.57, p < 0.05), there were no correlations (p > 0.05) observed for the sum of (poly)phenol metabolites, specific metabolite classes, or in the sum of significantly increased metabolites with FMD/SRAUC at 12 weeks in the blueberry group. For correlation analysis for the change from baseline to 12 weeks in the blueberry group (Fig. 7), 3 metabolites had moderate positive correlations with improvements in FMD/SRAUC, specifically myricetin (p < 0.05), 4-O-caffeoylquinic acid (p < 0.05), and 3-(4′-hydroxyphenyl)propanoic acid-3′-glucuronide (p < 0.05). Five metabolites had moderate to moderately strong negative correlations with improvements in FMD/SRAUC (Fig. 7), specifically 5-O-caffeoylquinic acid (p < 0.003), 3′-hydroxyphenylacetic acid (p < 0.0007), 3-(2′,4′-dihydroxyphenyl)propanoic acid (p < 0.03), 3-hydroxy-4-methoxybenzoic acid-5-sulfate (p < 0.04), and 2-hydroxy-3-(4′-hydroxyphenyl)propanoic acid (p < 0.009). In the placebo group, 5-O-caffeoylquinic acid was positively correlated with the change in FMD/SRAUC (p < 0.004), while 3-hydroxybenzoic acid (0.55, p < 0.05), 4-hydroxybenzoic acid (p < 0.04), and 4-hydroxyhippuric acid (p < 0.03) were all negatively correlated with the change in FMD/SRAUC (Fig. 7).For correlation analysis between individual metabolites and FMD/SRAUC at 12 weeks (Fig. 7), 3-hydroxybenzoic acid-4-sulfate was positively correlated in the blueberry group (p < 0.02). In the placebo group, 3,5-dihydroxybenzoic acid (p < 0.02), 3-hydroxybenzoic acid (p < 0.02), and 4-hydroxybenzoic acid (r = −0.61, p < 0.02) were all negatively correlated with FMD/SRAUC, while only myricetin was positively correlated (r = 0.67, p < 0.009) (Fig. 7).
Endothelial cell protein expression
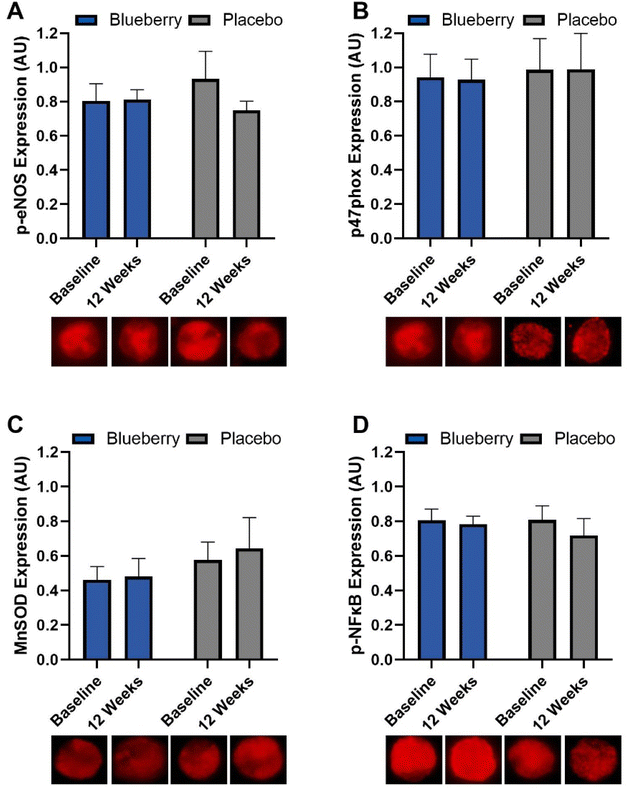
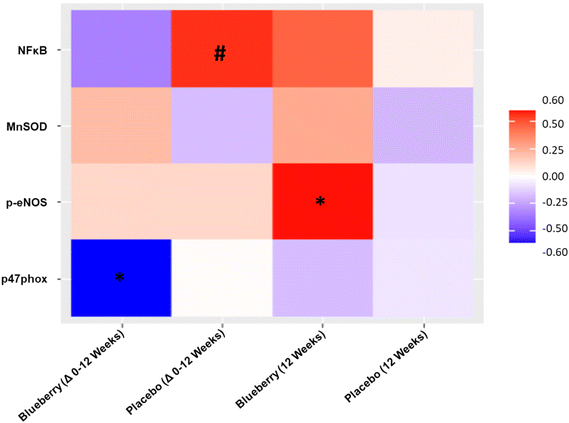
No differences (p > 0.05) were noted within or between groups at any time point for MnSOD, phosphorylated eNOS, phosphorylated NFκB, or p47phox (NADPH oxidase/p47 subunit) (Fig. 8 and ESI Table 6†).Correlations between endothelium-dependent dilation and endothelial cell protein expression
For the change from baseline to 12 weeks, FMD/SRAUC was moderately inversely correlated with NADPH oxidase/p47 subunit protein expression (p < 0.04) in the blueberry group but not placebo, and a trend for a moderately positive correlation with phosphorylated NFκB (p < 0.08) in the placebo group but not blueberry (Fig. 9). At 12 weeks, FMD/SRAUC had a moderately positive correlation with phosphorylated eNOS protein expression (p < 0.04) in the blueberry group but not placebo (Fig. 9). No other correlations (p > 0.05) were observed.Anthropometrics, body composition, energy intake and expenditure
Anthropometrics, body composition, energy intake and expenditure data are presented in ESI Table 7.† For anthropometrics, there were no differences (p > 0.05) in weight or BMI between or within groups at any time point. For body composition, android fat mass (%) was lower (p < 0.03) at 12 weeks compared to baseline in the placebo group but not in the blueberry group (p > 0.05), and no other differences (p > 0.05) were noted. No differences (p > 0.05) within or between groups were noted for energy intake or energy expenditure.Discussion
In this randomized, double-blind, placebo-controlled, parallel-arm clinical trial, we showed that consuming 22 g day−1 of freeze-dried highbush blueberry powder for 12 weeks significantly improved endothelial function, but not blood pressure, in postmenopausal women with above-normal blood pressure. These findings are clinically meaningful considering the prevalence of endothelial dysfunction in this population and its central role in CVD pathophysiology.72 We also found that improvements in endothelial function were due, at least in part, to reductions in oxidative stress, and that plasma (poly)phenol metabolites related to flavonoid and gut microbial metabolism were increased following blueberry consumption, providing insight into physiological mechanisms. Other measures of cardiometabolic health were largely unchanged following blueberry consumption. To our knowledge, this is the first study to assess the effects of blueberry consumption on endothelial function and oxidative stress-mediated suppression of endothelial function in postmenopausal women with above-normal blood pressure.The finding that daily blueberry consumption for 12 weeks improved macrovascular/conduit artery endothelial function in postmenopausal women with above-normal blood pressure is supported by previous randomized controlled trials performed with healthy adult men, adults with metabolic syndrome, and cigarette smoking men with or without peripheral arterial dysfunction.45,73,74 In our study, we found that endothelium-dependent dilation assessed as FMD corrected for shear stress (i.e. the stimulus for reactive hyperemia) was significantly improved compared to baseline, and the change from baseline to 12 weeks was significantly higher in the blueberry group than placebo. Though changes in FMD (not corrected for shear stress) were not statistically significant in our study, likely due to inter-individual variability in shear stress, a clinically significant increase of 1.34% was observed in the blueberry group compared to baseline. A 1% increase in FMD has been associated with a reduced risk for CVD and related events.52,75 Importantly, we observed no changes in endothelium-independent dilation, confirming that improvements in endothelial function observed were endothelium-dependent. An unexpected finding related to endothelial function was that plasma NO metabolites were unaffected by blueberry consumption, as we and others have demonstrated increases in these metabolites with blueberry consumption previously.44,76 However, several investigators have observed no change in plasma NO metabolites following blueberry consumption.73,77,78 In the longest randomized controlled trial to date with blueberries, Curtis et al. observed a 1.45% increase in FMD in middle-aged/older men and women with metabolic syndrome who consumed 26 g freeze-dried highbush blueberry powder daily for 6 months.73 In that study, circulating concentrations of cyclic guanosine monophosphate (GMP) were increased even though there were not concomitant increases in plasma NO metabolites, suggesting that blueberries exert vasodilatory effects through a NO-mediated mechanism.73 Rodriguez-Mateos et al.45 observed a 1.3% increase in FMD following consumption of 22 g freeze-dried lowbush blueberry powder daily for 1 month in healthy young males, but did not measure NO metabolites in their study. Overall, the findings of these 3 independent studies suggest that daily blueberry consumption can improve macrovascular/conduit artery endothelial function in high risk and healthy populations through an endothelium-dependent mechanism likely mediated by NO. Accurately assessing NO status is complex, and a single sampling of blood may not reflect tissue levels. The effects of blueberries on NO production and/or bioavailability, particularly as it relates to measures of endothelial function in humans is unknown at this time but should be evaluated in future randomized controlled trials to better understand mechanisms.
As with many human intervention studies, there was inter-individual variability in the direction and the magnitude of the response to treatment in our study.79,80 No studies with blueberries have reported the inter-individual variation in the magnitude and direction of the FMD response, but several have evaluated microvascular endothelial function measured through EndoPAT-assessed reactive hyperemic index (RHI),74,76,77 and inter-individual variability in responses was reported for a study in healthy males after consuming 25 g of freeze-dried wild blueberry powder daily for 6 weeks,77 and adults with metabolic syndrome after consuming 45 g of freeze-dried highbush blueberry powder daily for 6 weeks.74 In the case of FMD, values not corrected for shear stress may reflect conduit artery endothelial function as well as the magnitude of the hyperemic stimulus. To reduce variability and increase the utility of FMD as a measure of endothelial function, normalization of FMD to SRAUC has been validated in numerous research studies and has been recommended for use in research.56–58 Even with normalization of FMD to shear rate, variability in the direction and magnitude of the response was still noted in the current study. Nonetheless, our finding that daily freeze-dried blueberry consumption at a dose equivalent to about 1 cup (or 148 g) of fresh blueberries improved endothelial function in postmenopausal women with above-normal blood pressure is a clinically significant finding indicative of CVD risk reduction. Progressive impairments in endothelial function have been observed across the menopausal transition, and impairments in endothelial function are predictive of CVD development.16,52,75,81 Furthermore, sex differences exist for physiological responses to interventions aimed at improving endothelial function such that not all interventions in this population have been successful.82–85 For instance, endurance exercise training to improve endothelial function in postmenopausal women was only found to be effective in those receiving estrogen replacement therapy, whereas age-matched men consistently responded positively to endurance exercise training.86–88 The factors contributing to the lack of a response to interventions in postmenopausal women compared to men are not fully understood, but may be due to reduced estrogen receptor and eNOS activation, and oxidative stress. Our findings suggest that daily blueberry consumption may be an effective food-based intervention for reducing CVD risk in a high-risk population of estrogen-deficient postmenopausal women. Research aimed at understanding factors contributing to inter-individual variability in clinical endothelial function responses is needed, as well as ways to improve clinical responses to blueberry consumption.
Postmenopausal women have been demonstrated to have oxidative stress-mediated suppression of endothelium-dependent dilation,23 which is supported by an improvement in endothelium-dependent dilation observed at baseline in both groups following infusion of a supraphysiologic dose of ascorbic acid in our study. At 12 weeks, there were no improvements in endothelium-dependent dilation following ascorbic acid administration in the blueberry group, while there was a strong trend for an improvement in the placebo group. Importantly, the difference in the response to ascorbic acid administration versus saline was significantly reduced from baseline to 12 weeks in the blueberry group, while there was no change in the placebo group. Overall, these data indicate that this population of postmenopausal women with above-normal blood pressure has oxidative stress-mediated suppression of endothelial function that is improved by 12 weeks of daily blueberry consumption. The mechanisms contributing to improvements in oxidative stress-mediated suppression of endothelial function cannot be determined at this time, as included blood and endothelial cell measures related to NO production/bioavailability, oxidative stress, and inflammation were unchanged following blueberry consumption. It is possible that we were unable to detect subtle changes in protein expression and/or that blueberries reduce oxidative stress through alternative mechanisms. Interestingly, significant moderate correlations were observed for FMD/SRAUC such that it was inversely associated with NADPH oxidase protein expression and positively associated with phosphorylated eNOS expression in the blueberry group but not in the placebo group, and inversely associated with phosphorylated NFκB expression in the placebo group but not the blueberry group. These data suggest that improvements in endothelial function with blueberry consumption could be related to eNOS activation and/or NADPH oxidase and NFκB deactivation, but requires further investigation in humans. Rodriguez-Mateos et al.89 previously demonstrated acute blueberry consumption reduced neutrophil NADPH oxidase activity which was associated with increased FMD in healthy men. In diabetic db/db mice, Petersen et al.90 showed that blueberry consumption for 10 weeks improved endothelial function through reductions in NADPH oxidase 4 gene expression in aortic vessels and vascular endothelial cells. Several investigators have explored the effects of blueberry consumption on biomarkers of oxidative stress and antioxidant defense, and reported results have been mixed.91 We previously demonstrated reductions in blood concentrations of 8-hydroxydeoxyguanosine, a marker of DNA damage, following 4 weeks of daily consumption of 22 g freeze-dried blueberry powder in postmenopausal women with above-normal blood pressure;92 however, values returned to baseline levels at 8 weeks and other blood biomarkers of oxidative stress and antioxidant defense were unchanged. Basu et al.93 observed reductions in blood biomarkers of oxidative stress following 8 weeks of 50 g daily blueberry consumption in adults with metabolic syndrome. Studies evaluating oxidative stress in peripheral blood mononuclear cells have observed improvements following chronic daily blueberry consumption, one of which also observed improvements in microvascular endothelial function.74,78,94 As previously highlighted,92 circulating blood and static biomarkers of oxidative stress and inflammation, including those measured in our studies, may not always respond to dietary interventions in the same way that functional biomarkers do (e.g. evaluation of oxidative stress-mediated suppression of endothelial function, ex vivo cytokine release assays in peripheral blood mononuclear cells), and thus may be a factor contributing to discrepant findings in the field. Indeed, daily consumption of 45 g day−1 of freeze-dried blueberry powder for 6 weeks in adults with metabolic syndrome reduced markers of inflammation in circulating monocytes but not in the blood. The present study has identified reductions in oxidative stress as a mechanism for improvements in endothelial function in postmenopausal women with above-normal blood pressure. Though there are contradictory findings across randomized controlled trials with respect to oxidative stress, the present results and totality of the evidence suggests blueberries can reduce oxidative stress, and that reductions mediate the beneficial effects of blueberries on endothelial function and cardiovascular risk.
With respect to hemodynamic parameters, no significant changes were noted with the exception of a reduction in aortic pulse pressure at 4 weeks compared to baseline in the blueberry group. The finding that daily blueberry consumption did not reduce brachial systolic and/or diastolic blood pressure in the present study was unexpected and contrary to our previous findings demonstrating consumption of 22 g day−1 freeze-dried blueberry powder for 8 weeks led to statistically and clinically significant reductions in brachial systolic and diastolic blood pressure in postmenopausal women with above-normal blood pressure.44 However, the body of literature on the antihypertensive effects of blueberries is mixed such that equal numbers of studies have demonstrated benefits and null effects.44,45,73,74,76,77,95–97 The reason(s) contributing to the discrepancies between our studies cannot be determined at this time. However, one possible contributing factor is the difference in the study population as the present study was performed in Colorado vs. Florida previously,44 and there could be differences in the health status, physiology, and/or lifestyle factors. Additionally, effects on blood pressure may not have been sufficiently captured through measurement of “office” blood pressure, and future studies should also evaluate “out of office” blood pressure through 24-h ambulatory blood pressure monitoring and/or home-based blood pressure monitoring. Due to inconsistencies between office and out-of-office blood pressure and the “white coat” effect, it is increasingly being recommended that multiple methods be used.98–100 Overall, the body of evidence suggests that blueberries can reduce brachial blood pressure, but that factors impact their efficacy (e.g. health status, physiology, and lifestyle) and/or the ability to accurately and consistently detect their effects (e.g. measurement setting, device). Future research is needed to understand the antihypertensive effects of blueberries, particularly large randomized controlled trials that incorporate multiple methods for comprehensive assessment of blood pressure and approaches that will facilitate precision nutrition. Nonetheless, the current findings that endothelial function improved is a clinically meaningful finding considering endothelial dysfunction is a strong predictor of CVD events independent of blood pressure.
Arterial stiffness, as measured through PWV and AIx, has been shown to be a risk factor for CVD and predictive of hypertension progression.44,101–103 There were no improvements in measures of arterial stiffness in the present study. We previously found that systemic/peripheral arterial stiffness assessed as brachial-femoral PWV decreased by ∼1 m s−2 following daily consumption of 22 g freeze-dried highbush blueberry powder for 8 weeks in postmenopausal women with above-normal blood pressure,44 though no changes were observed in carotid-femoral/aortic PWV, suggesting that the current findings are not unexpected. Previously, AIx@75 (but not carotid-femoral/aortic PWV) significantly improved in men and women with metabolic syndrome who consumed 26 g freeze-dried highbush blueberry powder daily for 6 months,73 and in sedentary men and postmenopausal women who consumed 38 g of freeze-dried highbush blueberry powder daily for 6 weeks,95 supporting that blueberries may improve systemic/peripheral arterial stiffness. It is possible that the intervention duration in our study was not long enough to observe improvements in carotid-femoral/aortic PWV in our study population, and future longer-term intervention studies are needed.
We found that blueberry consumption led to increases in the sum of all measured plasma (poly)phenol metabolites, and individual metabolites present in blueberries and produced by gut microbial and flavonoid (e.g. anthocyanin) metabolism. These findings support self-reported compliance data provided by our study participants. Increased overall plasma (poly)phenol metabolite concentrations were largely driven by increases in hippuric acid. Several metabolites were positively and inversely linked to improvements in endothelial function. It is not entirely clear at this time how increases in (poly)phenol metabolites are related to improvements in endothelial function, but many of the increases in individual metabolites noted in our study (e.g. hippuric acid, 3-methoxybenzoic acid-4-sulfate, dihydroxybenzoic acids) are in line with those observed in previously performed chronic intervention trials with blueberries.45,73,104 (Poly)phenols and their metabolites have been shown to modulate endothelial function through mechanisms related to eNOS expression and coupling/uncoupling, NO bioavailability, oxidative stress, and inflammation.29,30,32 With respect to blueberries, anthocyanin metabolites have been demonstrated to be major mediators of improvements in endothelial function, though other blueberry-derived compounds play a role. (Poly)phenols also serve as prebiotics and the gut microbiota is responsible for metabolizing many (poly)phenols to form bioavailable and bioactive metabolites, suggesting the gut microbiome plays an essential role in determining the cardiovascular-protective effects of blueberries. In our study, we observed increases in metabolites of phase II metabolism (i.e. sulfates and glucuronides), which further supports that the gut microbiome plays an important role in blueberry (poly)phenol metabolism. The gut microbiome is being increasingly linked to CVD as a central mediator, though exact mechanisms are complex. Evidence in animal models and limited human studies indicate blueberries modulate the gut microbiota, and emerging evidence suggests beneficial effects on endothelial function may be mediated by the gut microbiota.90 Research evaluating the impacts on the gut microbiota and the extent to which and how the gut microbiome mediates beneficial health effects of blueberries in humans is needed.
There are several strengths of the present study including the robust randomized, double-blind, placebo-controlled study design and the 12-week length of the dietary intervention. The amount of freeze-dried blueberry powder used in our study is equivalent to about 1 cup of fresh blueberries (or 148 g), which is an achievable dietary dose for daily consumption and supports the translational potential of our findings. We used gold-standard methodologies to directly assess endothelium-dependent and -independent dilation, and to gain mechanistic insight through assessment of oxidative stress-mediated suppression of endothelial function, endothelial cell protein expression, and plasma (poly)phenol metabolites. The inclusion of a high-risk (i.e. above-normal blood pressure) and understudied population of postmenopausal women highlights the translational potential of a food-based intervention for improving cardiovascular health. There are also limitations to our research that have not been previously discussed and are worth mentioning. First, this study was conducted in Northern Colorado and may not reflect the greater United States and/or international population of postmenopausal women. Unfortunately, the majority of our study participants identified as being White/Caucasian which limits the generalizability to other races and ethnicities. Another limitation is the reduced sample size for some of the analyses, such as endothelial function and endothelial cell biopsy due to various reasons such as the inability to place an intravenous catheter and contraindications to sublingual NTG.
Conclusions
In conclusion, our study showed for the first time that blueberry consumption improved endothelial function in postmenopausal women with above-normal blood pressure, and that improvements were mediated by reductions in oxidative stress. The underlying mechanisms contributing to these results cannot be determined at this time, but our results suggest that improvements may be related to gut-derived circulating (poly)phenol metabolites and endothelial cell protein expression related to NO production, oxidative stress, and inflammation. Future studies evaluating blueberry consumption in humans from diverse populations are needed to provide further insight into physiological mechanisms, and to understand factors contributing to inter-individual variability in clinical responses to support a precision nutrition approach. With respect to blood pressure, the current findings do not suggest that blueberries exert antihypertensive effects in this population. However, considering our previous findings in this population, and the mixed body of evidence surrounding blueberries and blood pressure such that about half demonstrated beneficial effects, further research is needed to better understand the antihypertensive effects of blueberries, and factors that can be modified and/or implemented to improve efficacy.Abbreviations
| DEXA | Dual-energy X-ray absorptiometry |
| FMD/SRAUC | Flow-mediated dilation normalized to shear rate area under the curve |
| FMD | Flow-mediated dilation |
| FSH | Follicle-stimulating hormone |
| PWV | Pulse wave velocity |
| Aix | Augmentation index |
| AIx@75 | Augmentation index at 75 beats per minute |
| CVD | Cardiovascular disease |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| eNOS | Endothelial nitric oxide synthase |
| MnSOD | Manganese superoxide dismutase |
| NFκB | Nuclear factor kappa B |
| NTG | Nitroglycerin |
Author contributions
DRS, FAD, and SAJ designed the research. CLG, TLW, ARM, DRS, FAD, and SAJ secured funding to support the research. EKW, JDT, NSL, ARV, SYL, NG, KAM, BTS, LEG, NBK, NPB, MES, and SAJ were involved in data collection, management, and/or analyses. MLS and ARM performed plasma (poly)phenol metabolite analyses. SR performed statistical analyses. EKW, ARM, CLG, TLW, DRS, FAD, and SAJ interpreted the data. EKW and SAJ wrote the initial manuscript. All authors read, provided edits and suggestions, and approved the final manuscript. SAJ had primary responsibility for final content.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the US Highbush Blueberry Council, the USDA National Institute of Food and Agriculture [Grant No. 2020-67017-30833/Project Accession No. 1021875], and the College of Health and Human Sciences at Colorado State University, and Kerry L. Hildreth at the University of Colorado Anschutz Medical Campus for their assistance in the study, as well as all of our volunteer participants.References
- X. Ma, O. Laaksonen, J. Zheng, W. Yang, M. Trepanier, H. Kallio and B. Yang, Food Chem., 2016, 200, 189–198 CrossRef CAS PubMed.
- W. Yang, O. Laaksonen, H. Kallio and B. Yang, J. Agric. Food Chem., 2016, 64, 1274–1282 CrossRef CAS PubMed.
- D. R. Seals, K. L. Jablonski and A. J. Donato, Clin. Sci., 2011, 120, 357–375 CrossRef CAS PubMed.
- P. M. Vanhoutte, H. Shimokawa, M. Feletou and E. H. Tang, Acta Physiol., 2017, 219, 22–96 CrossRef CAS PubMed.
- T. D. Giles, G. E. Sander, B. D. Nossaman and P. J. Kadowitz, J. Clin. Hypertens., 2012, 14, 198–205 CrossRef CAS PubMed.
- C. Bleakley, P. K. Hamilton, R. Pumb, M. Harbinson and G. E. McVeigh, J. Clin. Hypertens., 2015, 17, 651–654 CrossRef PubMed.
- H. Cai and D. G. Harrison, Circ. Res., 2000, 87, 840–844 CrossRef CAS PubMed.
- M. M. Bachschmid, S. Schildknecht, R. Matsui, R. Zee, D. Haeussler, R. A. Cohen, D. Pimental and B. Loo, Ann. Med., 2013, 45, 17–36 CrossRef CAS PubMed.
- E. G. Lakatta, Circulation, 2003, 107, 490–497 CrossRef PubMed.
- C. A. Hamilton, M. J. Brosnan, M. McIntyre, D. Graham and A. F. Dominiczak, Hypertension, 2001, 37, 529–534 CrossRef CAS PubMed.
- K. H. Park and W. J. Park, J. Korean Med. Sci., 2015, 30, 1213–1225 CrossRef CAS PubMed.
- Q. N. Dinh, G. R. Drummond, C. G. Sobey and S. Chrissobolis, BioMed Res. Int., 2014, 2014, 406960 Search PubMed.
- M. A. Incalza, R. D'Oria, A. Natalicchio, S. Perrini, L. Laviola and F. Giorgino, Vasc. Pharmacol., 2018, 100, 1–19 CrossRef CAS PubMed.
- H. N. Siti, Y. Kamisah and J. Kamsiah, Vasc. Pharmacol., 2015, 71, 40–56 CrossRef CAS PubMed.
- S. A. Johnson, N. S. Litwin and D. R. Seals, J. Acad. Nutr. Diet., 2019, 119, 1785–1796 CrossRef PubMed.
- R. Rossi, A. Nuzzo, G. Origliani and M. G. Modena, J. Am. Coll. Cardiol., 2008, 51, 997–1002 CrossRef PubMed.
- M. G. Modena, L. Bonetti, F. Coppi, F. Bursi and R. Rossi, J. Am. Coll. Cardiol., 2002, 40, 505–510 CrossRef PubMed.
- K. L. Moreau and K. L. Hildreth, Adv. Vasc. Med., 2014, 204390 Search PubMed.
- K. L. Moreau, K. L. Hildreth, A. L. Meditz, K. D. Deane and W. M. Kohrt, J. Clin. Endocrinol. Metab., 2012, 97, 4692–4700 CrossRef CAS PubMed.
- D. S. Celermajer, K. E. Sorensen, D. J. Spiegelhalter, D. Georgakopoulos, J. Robinson and J. E. Deanfield, J. Am. Coll. Cardiol., 1994, 24, 471–476 CrossRef CAS PubMed.
- S. Taddei, A. Virdis, L. Ghiadoni, P. Mattei, I. Sudano, G. Bernini, S. Pinto and A. Salvetti, Hypertension, 1996, 28, 576–582 CrossRef CAS PubMed.
- Y. B. Somani, J. A. Pawelczyk, M. J. De Souza, P. M. Kris-Etherton and D. N. Proctor, Am. J. Physiol.: Heart Circ. Physiol., 2019, 317, H395–H404 CrossRef CAS PubMed.
- K. L. Moreau, K. L. Hildreth, J. Klawitter, P. Blatchford and W. M. Kohrt, GeroScience, 2020, 42, 1699–1714 CrossRef CAS PubMed.
- A. Iorga, C. M. Cunningham, S. Moazeni, G. Ruffenach, S. Umar and M. Eghbali, Biol. Sex Differ., 2017, 8, 33 CrossRef PubMed.
- D. Mozaffarian, E. J. Benjamin, A. S. Go, D. K. Arnett, M. J. Blaha, M. Cushman, S. de Ferranti, J. P. Després, H. J. Fullerton, V. J. Howard, M. D. Huffman, S. E. Judd, B. M. Kissela, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, S. Liu, R. H. Mackey, D. B. Matchar, D. K. McGuire, E. R. Mohler 3rd, C. S. Moy, P. Muntner, M. E. Mussolino, K. Nasir, R. W. Neumar, G. Nichol, L. Palaniappan, D. K. Pandey, M. J. Reeves, C. J. Rodriguez, P. D. Sorlie, J. Stein, A. Towfighi, T. N. Turan, S. S. Virani, J. Z. Willey, D. Woo, R. W. Yeh and M. B. Turner, Circulation, 2015, 131, e29–e322 Search PubMed.
- L. L. Yanes and J. F. Reckelhoff, Am. J. Hypertens., 2011, 24, 740–749 CrossRef PubMed.
- J. Hsia, K. L. Margolis, C. B. Eaton, N. K. Wenger, M. Allison, L. Wu, A. Z. LaCroix and H. R. Black, Circulation, 2007, 115, 855–860 CrossRef PubMed.
- L. Santos, Eur. J. Intern. Med., 2022, 97, 18–25 CrossRef PubMed.
- R. Andriantsitohaina, C. Auger, T. Chataigneau, N. Étienne-Selloum, H. Li, M. C. Martínez, V. B. Schini-Kerth and I. Laher, Br. J. Nutr., 2012, 108, 1532–1549 CrossRef CAS PubMed.
- M. H. Oak, C. Auger, E. Belcastro, S. H. Park, H. H. Lee and V. B. Schini-Kerth, Free Radicals Biol. Med., 2018, 122, 161–170 CrossRef CAS PubMed.
- L. Zhang, Z. Han and D. Granato, Adv. Food Nutr. Res., 2021, 98, 1–33 CrossRef CAS PubMed.
- K. B. Pandey and S. I. Rizvi, Oxid. Med. Cell. Longevity, 2009, 2, 270–278 CrossRef PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. Spencer, M. Tognolini, G. Borges and A. Crozier, Antioxid. Redox Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- X. Xie, R. Zhao and G. X. Shen, Int. J. Mol. Sci., 2012, 13, 15867–15880 CrossRef CAS PubMed.
- M. Edwards, C. Czank, G. M. Woodward, A. Cassidy and C. D. Kay, J. Agric. Food Chem., 2015, 63, 2423–2431 CrossRef CAS PubMed.
- W. Gan, Y. Dang, X. Han, S. Ling, J. Duan, J. Liu and J. W. Xu, Mol. Nutr. Food Res., 2016, 60, 266–277 CrossRef CAS PubMed.
- X. Wu, J. Kang, C. Xie, R. Burris, M. E. Ferguson, T. M. Badger and S. Nagarajan, J. Nutr., 2010, 140, 1628–1632 CrossRef CAS PubMed.
- J. Li, D. Ruzhi, X. Hua, L. Zhang, F. Lu, T. G. Coursey, S. C. Pflugfelder and D. Q. Li, Sci. Rep., 2016, 6, 19408 CrossRef CAS PubMed.
- T. Mačičková, J. Pečivová, J. Harmatha, K. Sviteková and R. Nosáľ, Interdiscip. Toxicol., 2012, 5, 71–75 Search PubMed.
- A. Speciale, S. Anwar, R. Canali, J. Chirafisi, A. Saija, F. Virgili and F. Cimino, Mol. Nutr. Food Res., 2013, 57, 1979–1987 CrossRef CAS PubMed.
- Y. J. Cao, Y. M. Zhang, J. P. Qi, R. Liu, H. Zhang and L. C. He, Int. Immunopharmacol., 2015, 28, 1018–1025 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, M. Le Sayec, G. Istas and S. A. Johnson, Berries and Berry Bioactive Compounds in Promoting Health, 2022, vol. 33, p. 120 Search PubMed.
- B. R. Cutler, C. Petersen and P. V. Anandh Babu, Mol. Nutr. Food Res., 2017, 61(6) DOI:10.1002/mnfr.201600271.
- S. A. Johnson, A. Figueroa, N. Navaei, A. Wong, R. Kalfon, L. T. Ormsbee, R. G. Feresin, M. L. Elam, S. Hooshmand, M. E. Payton and B. H. Arjmandi, J. Acad. Nutr. Diet., 2015, 115, 369–377 CrossRef PubMed.
- A. Rodriguez-Mateos, G. Istas, L. Boschek, R. P. Feliciano, C. E. Mills, C. Boby, S. Gomez-Alonso, D. Milenkovic and C. Heiss, J. Gerontol., Ser. A, 2019, 74, 967–976 CrossRef CAS PubMed.
- W. Huang, Y. Zhu, C. Li, Z. Sui and W. Min, Oxid. Med. Cell. Longevity, 2016, 2016, 1591803 Search PubMed.
- D. Bharat, R. R. M. Cavalcanti, C. Petersen, N. Begaye, B. R. Cutler, M. M. A. Costa, R. K. L. G. Ramos, M. R. Ferreira, Y. Li, L. P. Bharath, E. Toolson, P. Sebahar, R. E. Looper, T. Jalili, N. S. Rajasekaran, Z. Jia, J. D. Symons and P. V. Anandh Babu, Mol. Nutr. Food Res., 2018, 62(2) DOI:10.1002/mnfr.201700601.
- S. H. Park, S. O. Jeong, H. T. Chung and H. O. Pae, Plant Foods Hum. Nutr., 2015, 70, 263–268 CrossRef CAS PubMed.
- I. Eskurza, L. A. Myerburgh, Z. D. Kahn and D. R. Seals, J. Physiol., 2005, 568, 1057–1065 CrossRef CAS PubMed.
- P. E. Gates, M. L. Boucher, A. E. Silver, K. D. Monahan and D. R. Seals, J. Appl. Physiol. (1985), 2007, 102, 63–71 CrossRef CAS PubMed.
- N. P. Bachman, J. D. Terwoord, J. C. Richards, B. Braun, C. P. Green, G. J. Luckasen and F. A. Dinenno, Atherosclerosis, 2021, 320, 105–111 CrossRef CAS PubMed.
- D. H. J. Thijssen, R. M. Bruno, A. van Mil, S. M. Holder, F. Faita, A. Greyling, P. L. Zock, S. Taddei, J. E. Deanfield, T. Luscher, D. J. Green and L. Ghiadoni, Eur. Heart J., 2019, 40, 2534–2547 CrossRef PubMed.
- V. E. Brunt, R. A. Gioscia-Ryan, A. G. Casso, N. S. VanDongen, B. P. Ziemba, Z. J. Sapinsley, J. J. Richey, M. C. Zigler, A. P. Neilson, K. P. Davy and D. R. Seals, Hypertension, 2020, 76, 101–112 CrossRef CAS PubMed.
- B. A. Parker, T. L. Trehearn and J. R. Meendering, J. Appl. Physiol. (1985), 2009, 107, 1357–1359 CrossRef PubMed.
- K. E. Pyke, E. M. Dwyer and M. E. Tschakovsky, J. Appl. Physiol. (1985), 2004, 97, 499–508 CrossRef PubMed.
- J. Padilla, B. D. Johnson, S. C. Newcomer, D. P. Wilhite, T. D. Mickleborough, A. D. Fly, K. J. Mather and J. P. Wallace, Cardiovasc. Ultrasound, 2008, 6, 44 CrossRef PubMed.
- J. Padilla, B. D. Johnson, S. C. Newcomer, D. P. Wilhite, T. D. Mickleborough, A. D. Fly, K. J. Mather and J. P. Wallace, J. Vasc. Res., 2009, 46, 592–600 CrossRef CAS PubMed.
- I. Eskurza, K. D. Monahan, J. A. Robinson and D. R. Seals, J. Physiol., 2004, 556, 315–324 CrossRef CAS PubMed.
- G. L. Pierce, L. A. Lesniewski, B. R. Lawson, S. D. Beske and D. R. Seals, Circulation, 2009, 119, 1284–1292 CrossRef CAS PubMed.
- N. S. Litwin, H. J. Van Ark, S. C. Hartley, K. A. Michell, A. R. Vazquez, E. K. Fischer, C. L. Melby, T. L. Weir, Y. Wei, S. Rao, K. L. Hildreth, D. R. Seals, M. J. Pagliassotti and S. A. Johnson, Curr. Dev. Nutr., 2019, 3(11), nzz113 CAS.
- R. E. Trotter, A. R. Vazquez, D. S. Grubb, K. E. Freedman, L. E. Grabos, S. Jones, C. L. Gentile, C. L. Melby, S. A. Johnson and T. L. Weir, Benefic. Microbes, 2020, 11, 621–630 CrossRef CAS PubMed.
- R. P. Feliciano, E. Mecha, M. R. Bronze and A. Rodriguez-Mateos, J. Chromatogr. A, 2016, 1464, 21–31 CrossRef CAS PubMed.
- I. Eskurza, Z. D. Kahn and D. R. Seals, J. Physiol., 2006, 571, 661–668 CrossRef CAS PubMed.
- P. C. Colombo, A. W. Ashton, S. Celaj, A. Talreja, J. E. Banchs, N. B. Dubois, M. Marinaccio, S. Malla, J. Lachmann, J. A. Ware and T. H. Le Jemtel, J. Appl. Physiol. (1985), 2002, 92, 1331–1338 CrossRef PubMed.
- A. E. Silver, D. D. Christou, A. J. Donato, S. D. Beske, K. L. Moreau, K. A. Magerko and D. R. Seals, J. Vasc. Res., 2010, 47, 1–8 CrossRef CAS PubMed.
- A. J. Donato, I. Eskurza, A. E. Silver, A. S. Levy, G. L. Pierce, P. E. Gates and D. R. Seals, Circ. Res., 2007, 100, 1659–1666 CrossRef CAS PubMed.
- A. E. Silver, S. D. Beske, D. D. Christou, A. J. Donato, K. L. Moreau, I. Eskurza, P. E. Gates and D. R. Seals, Circulation, 2007, 115, 627–637 CrossRef CAS PubMed.
- A. F. Subar, S. I. Kirkpatrick, B. Mittl, T. P. Zimmerman, F. E. Thompson, C. Bingley, G. Willis, N. G. Islam, T. Baranowski, S. McNutt and N. Potischman, J. Acad. Nutr. Diet., 2012, 112, 1134–1137 CrossRef PubMed.
- S. I. Kirkpatrick, A. F. Subar, D. Douglass, T. P. Zimmerman, F. E. Thompson, L. L. Kahle, S. M. George, K. W. Dodd and N. Potischman, Am. J. Clin. Nutr., 2014, 100(1), 233–240 CrossRef CAS PubMed.
- J. F. Sallis, W. L. Haskell, P. D. Wood, S. P. Fortmann, T. Rogers, S. N. Blair and R. S. Paffenbarger Jr., Am. J. Epidemiol., 1985, 121, 91–106 CrossRef CAS PubMed.
- H. A. Hayden-Wade, K. J. Coleman, J. F. Sallis and C. Armstrong, Med. Sci. Sports Exercise, 2003, 35, 801–809 CrossRef PubMed.
- B. Abramson, K. Srivaratharajah, L. Davis and B. Parapid, Evaluation, and Management of High Blood Pressure in Adults. An Expert Analysis, 2018 Search PubMed.
- P. J. Curtis, V. van der Velpen, L. Berends, A. Jennings, M. Feelisch, A. M. Umpleby, M. Evans, B. O. Fernandez, M. S. Meiss, M. Minnion, J. Potter, A. M. Minihane, C. D. Kay, E. B. Rimm and A. Cassidy, Am. J. Clin. Nutr., 2019, 109, 1535–1545 CrossRef PubMed.
- A. J. Stull, K. C. Cash, C. M. Champagne, A. K. Gupta, R. Boston, R. A. Beyl, W. D. Johnson and W. T. Cefalu, Nutrients, 2015, 7, 4107–4123 CrossRef CAS PubMed.
- Y. Inaba, J. A. Chen and S. R. Bergmann, Int. J. Cardiovasc. Imaging, 2010, 26, 631–640 CrossRef PubMed.
- K. S. Stote, M. I. Sweeney, T. Kean, D. J. Baer, J. A. Novotny, N. L. Shakerley, A. Chandrasekaran, P. M. Carrico, J. A. Melendez and K. T. Gottschall-Pass, BMC Nutr., 2017, 3, 45 CrossRef CAS PubMed.
- P. Riso, D. Klimis-Zacas, C. Del Bo, D. Martini, J. Campolo, S. Vendrame, P. Møller, S. Loft, R. De Maria and M. Porrini, Eur. J. Nutr., 2013, 52, 949–961 CrossRef CAS PubMed.
- C. Del Bó, P. Riso, J. Campolo, P. Møller, S. Loft, D. Klimis-Zacas, A. Brambilla, A. Rizzolo and M. Porrini, Nutr. Res., 2013, 33, 220–227 CrossRef PubMed.
- D. Milenkovic, C. Morand, A. Cassidy, A. Konic-Ristic, F. Tomás-Barberán, J. M. Ordovas, P. Kroon, R. De Caterina and A. Rodriguez-Mateos, Adv. Nutr., 2017, 8, 558–570 CrossRef PubMed.
- M. T. García-Conesa, K. Chambers, E. Combet, P. Pinto, M. Garcia-Aloy, C. Andrés-Lacueva, S. de Pascual-Teresa, P. Mena, A. Konic Ristic, W. J. Hollands, P. A. Kroon, A. Rodríguez-Mateos, G. Istas, C. A. Kontogiorgis, D. K. Rai, E. R. Gibney, C. Morand, J. C. Espín and A. González-Sarrías, Int. J. Mol. Sci., 2018, 19(3), 694 CrossRef PubMed.
- R. T. Ras, M. T. Streppel, R. Draijer and P. L. Zock, Int. J. Cardiol., 2013, 168, 344–351 CrossRef PubMed.
- Y. A. Al-Dashti, R. R. Holt, J. G. Carson, C. L. Keen and R. M. Hackman, J. Med. Food, 2019, 22, 982–992 CrossRef CAS PubMed.
- J. R. Santos-Parker, T. R. Strahler, V. M. Vorwald, G. L. Pierce and D. R. Seals, J. Appl. Physiol. (1985), 2017, 122, 11–19 CrossRef CAS PubMed.
- V. Habauzit, M. A. Verny, D. Milenkovic, N. Barber-Chamoux, A. Mazur, C. Dubray and C. Morand, Am. J. Clin. Nutr., 2015, 102, 66–74 CrossRef CAS PubMed.
- M. Abshirini, M. Omidian and H. Kord-Varkaneh, Menopause, 2020, 27, 1425–1433 CrossRef PubMed.
- C. Ozemek, K. L. Hildreth, P. J. Blatchford, K. J. Hurt, R. Bok, D. R. Seals, W. M. Kohrt and K. L. Moreau, J. Appl. Physiol. (1985), 2020, 128, 739–747 CrossRef CAS PubMed.
- K. L. Moreau, B. L. Stauffer, W. M. Kohrt and D. R. Seals, J. Clin. Endocrinol. Metab., 2013, 98, 4507–4515 CrossRef CAS PubMed.
- D. R. Seals, E. E. Nagy and K. L. Moreau, J. Physiol., 2019, 597, 4901–4914 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Am. J. Clin. Nutr., 2013, 98, 1179–1191 CrossRef CAS PubMed.
- C. Petersen, D. Bharat, U. D. Wankhade, J. S. Kim, B. R. Cutler, C. Denetso, S. Gholami, S. Nelson, J. Bigley, A. Johnson, S. V. Chintapalli, B. D. Piccolo, A. K. Satheesh Babu, H. A. Paz, K. Shankar, J. D. Symons and P. V. Anandh Babu, Mol. Nutr. Food Res., 2022, 66(8), e2100784 CrossRef PubMed.
- D. Martini, M. Marino, S. Venturi, M. Tucci, D. Klimis-Zacas, P. Riso, M. Porrini and C. Del Bo, J. Nutr. Biochem., 2023, 111, 109154 CrossRef CAS PubMed.
- S. A. Johnson, R. G. Feresin, N. Navaei, A. Figueroa, M. L. Elam, N. S. Akhavan, S. Hooshmand, S. Pourafshar, M. E. Payton and B. H. Arjmandi, Food Funct., 2017, 8, 372–380 RSC.
- A. Basu, M. Du, M. J. Leyva, K. Sanchez, N. M. Betts, M. Wu, C. E. Aston and T. J. Lyons, J. Nutr., 2010, 140, 1582–1587 CrossRef CAS PubMed.
- A. R. Nair, N. Mariappan, A. J. Stull and J. Francis, Food Funct., 2017, 8, 4118–4128 RSC.
- L. S. McAnulty, S. R. Collier, M. J. Landram, D. S. Whittaker, S. E. Isaacs, J. M. Klemka, S. L. Cheek, J. C. Arms and S. R. McAnulty, Nutr. Res., 2014, 34, 577–584 CrossRef CAS PubMed.
- S. Vendrame, T. E. Adekeye and D. Klimis-Zacas, Nutrients, 2022, 14(13), 2701 CrossRef CAS PubMed.
- Y. Wang, J. L. Gallegos, C. Haskell-Ramsay and J. K. Lodge, Nutrients, 2022, 14(13), 2562 CrossRef CAS PubMed.
- K. Asayama, T. Ohkubo and Y. Imai, J. Hum. Hypertens., 2021, 1–9, DOI:10.1038/s41371-021-00486-8.
- P. K. Whelton, R. M. Carey, W. S. Aronow, D. E. Casey Jr., K. J. Collins, C. Dennison Himmelfarb, S. M. DePalma, S. Gidding, K. A. Jamerson, D. W. Jones, E. J. MacLaughlin, P. Muntner, B. Ovbiagele, S. C. Smith Jr., C. C. Spencer, R. S. Stafford, S. J. Taler, R. J. Thomas, K. A. Williams Sr., J. D. Williamson and J. T. Wright Jr., Hypertension, 2018, 71, e13–e115 CAS.
- P. K. Whelton, R. M. Carey, G. Mancia, R. Kreutz, J. D. Bundy and B. Williams, Circulation, 2022, 146, 868–877 CrossRef PubMed.
- A. N. Lyle and U. Raaz, Arterioscler., Thromb., Vasc. Biol., 2017, 37, e1–e11 CrossRef CAS PubMed.
- M. A. Said, R. N. Eppinga, E. Lipsic, N. Verweij and P. van der Harst, J. Am. Heart Assoc., 2018, 7(2), e007621 CrossRef PubMed.
- Q. Zhong, M. J. Hu, Y. J. Cui, L. Liang, M. M. Zhou, Y. W. Yang and F. Huang, Angiology, 2018, 69, 617–629 CrossRef PubMed.
- R. P. Feliciano, G. Istas, C. Heiss and A. Rodriguez-Mateos, Molecules, 2016, 21(9), 1120 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00157a |
| This journal is © The Royal Society of Chemistry 2023 |