 Open Access Article
Open Access ArticleRecent advances in the production of oligogalacturonides and their biological properties
Sergio
Martínez-Gómez
ab,
Marcos
Fernández-Bautista
ab,
Sandra
Rivas
ab,
Remedios
Yáñez
 *bc and
José L.
Alonso
*bc and
José L.
Alonso
 ab
ab
aUniversidade de Vigo, Facultade de Ciencias, As Lagoas s/n, 32004 Ourense, Spain
bCINBIO, Universidade de Vigo, 36310 Vigo, Spain. E-mail: reme@uvigo.es
cUniversidade de Vigo, Escola de Enxeñaría Industrial, Campus Lagoas-Marcosende, 36310 Vigo, Spain
First published on 10th April 2023
Abstract
The human population is becoming old and ageing, which is related to a variety of health issues, such as Alzheimeŕs disease, obesity, diabetes, hypercholesterolemia, and some types of cancers like colorectal cancer. Furthermore, diet is a determining factor in the appearance of some of these diseases due to its direct effect at the systemic level (for instance, increase in glucose and LDL-cholesterol levels in the serum) and its influence on the composition and activity of the gut microbiota. In this context, the use of functional ingredients can be a useful strategy to prevent or even treat (in combination with drugs) some of the above-mentioned pathologies. Among the variety of functional ingredients, prebiotics have received significant attention by the scientific community. Although the already commercialized FOS are the most studied prebiotics, some efforts have been devoted to the search and evaluation of new prebiotic candidates with additional properties. In particular, in the last decade, a variety of in vitro and in vivo assays have been carried out using well isolated and characterized oligogalacturonides, demonstrating that some of them have interesting biological properties, including anticancer, antioxidant, antilipidemic, anti-obesity and anti-inflammatory activities besides prebiotic effects. This work reviews the scientific literature published recently on the production of oligogalacturonides with a special focus on their biological properties.
1. Introduction
Several human health issues, such as Alzheimer's disease, Parkinson, obesity, diabetes, hypercholesterolemia, IBD, arthrosis, osteoporosis, and even some types of cancers, such as the colorectal cancer, are associated with ageing. Alternatively, in the last few years, it has been suggested that diet (quantity and quality) is a determining factor in the appearance of some diseases due to its influence on the composition and activity of the gut microbiota and/or its direct effects at the systemic level (for instance, increase in glucose and LDL-cholesterol levels in the serum).In this context, functional ingredients can be useful tools to prevent or even treat (in combination with other drugs) some of the above-mentioned pathologies given that they can be easily added to several common foods, such as dairy products.
Among the functional ingredients, prebiotics have received significant attention by the scientific community, among which the most studied ones are the already commercialized FOS. According to ISAPP, a prebiotic is a substrate that is selectively utilized by host microorganisms and confers a health benefit.1 In recent years, many efforts have been devoted to developing new prebiotic candidates with additional properties, including pectin-derived oligosaccharides. However, as summarized in previous papers, these studies mainly used complex mixtures (made up of oligogalacturonides, arabinooligosaccharides, galactooligosaccharides, and rhamnooligogalacturonides, among others).2–4 The use of these mixtures of carbohydrates hinders the advancement in the knowledge of the structure–function relationships, which will enable tighter control of substances and doses.5 Therefore, studies using well-defined products should be carried out.
In this context, during the last decade, some in vitro and in vivo assays have been performed using well isolated and characterized oligogalacturonides, demonstrating a variety of interesting biological properties, including anti-inflammatory,6,7 anticancer,8–11 antioxidant,10,12–15 antilipidemic,12,16–19 antidiabetic,20 antiobesity,16,17,20,21 and antibacterial,22–24 together with prebiotic effects.25–29 Moreover, although anti-inflammatory and anticancer effects have also been reported for pectin,30 its poor solubility together with its difficultly in penetrating the body barrier to reach cells affect its biological functions.7 Therefore, depolymerization can compensate for these drawbacks, thus expanding their application range and increasing the interest in OGalA as functional ingredients.7
In this work, we review the scientific literature published mainly in the last 15 years on the production of oligogalacturonides, focusing on their biological properties. Therefore, only experimental works dealing with isolated oligogalacturonides or highly enriched mixtures of this type of product are included herein.
To the best of our knowledge, no previous reviews focused on these types of oligomers and their properties to date.
2. Pectin and oligogalacturonides
Herein, oligogalacturonides (OGalA) are defined as mixtures of oligomers (mainly, DP2-10) made up of α(1–4)-linked galacturonic acid residues (GalA), which can be saturated or unsaturated and randomly methylated and/or acetylated.OGalA mixtures can be obtained via the depolymerization of pectin, a complex and ramified heteropolysaccharide present in a wide variety of fruits and vegetables. Fig. 1 shows a simplified structure of pectin. As can be seen, this polymer is mainly made up of three components, as follows:2
• Homogalacturonan (HG), a polysaccharide made up of GalA units containing free and/or methylated carboxyl groups, which can be acetylated at O-2 and/or O-3 positions.
• Rhamnogalacturonan type I (RG-I), a polymer made up of alternating residues of GalA and rhamnose (Rha), which contains branches of arabinan, galactan and arabinogalactans I and II.
• Rhamnogalacturonan type II (RG-II), a polymer of GalA with a variety of complex ramifications, which is made up of a number of sugars and other compounds.
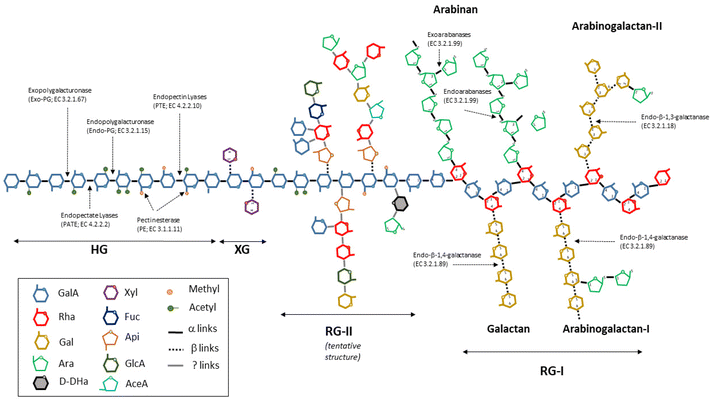 | ||
| Fig. 1 Simplified pectin structure and the mode of action of the main enzymes involved in the degradation of pectin. | ||
Moreover, short fragments of xylogalacturonan (XG), a chain of GalA units partially substituted by D-xylose linked by β-(1,3) bonds at the C-3 and/or C-2 positions can also be found in the structure of pectin.
In this context, it is important to note that SBP pectin contains a significant amount of ferulic acid, which can be related to its antioxidant activity.2
During the last few years, several raw materials (mainly fruits, agroindustrial byproducts and pectins derived from them) have been used for production of OGalA, including haw,12,19–22,31 apple pectin,7,9,10 artichoke,13 mango peel wastes,24 lemon peels,27 mandarin peels,11,32 finger citron pomace,15 pomelo peels,29 sugar beet pulp,33,34 citrus pectin,8,23,25 sunflower plate pectin35,36 and Lonicera japonica Thunb.14 With the same purpose, commercial polygalacturonic acid (PGalA) was also tested.25,37Table 1 summarizes the relevant information provided in all the reviewed studies about OGalA production, including raw materials, treatments and refining strategies as well as product characteristics.
| Raw materials | Pretreatment | Main treatment | Separation and refining stages | Products | Ref. |
|---|---|---|---|---|---|
| a Mainly OGalA; PGalA: polygalacturonic acid; C: centrifugation, EP: ethanol precipitation; UF: ultrafiltration; NF: nanofiltration; DL: dialysis; FD: freeze-drying; IE: ion exchange; E: evaporation; D: drying; G: grinding; and S: sieving. | |||||
| Pomelo peels | Pectin extraction with acids + filtration + concentration + EP + DL + FD | Electron beam irradiation | Concentration + FD | DP9 | 29 |
| PGalA | No pretreatment | Free hydroxyl radical hydrolysis (H2O2/Cu2+) | Cu2+ chelation (EDTA) + methanol precipitation + chromatographic separation | DP2–6 (47%) + DP>6 | 38 |
| Lemon peels | Aqueous extraction of free sugars | Autohydrolysis | NF | DP1–13 (22%) | 26 |
| DP>13 (70%) | |||||
| DM = 61% | |||||
| OgalA content>62%a | |||||
| PGalA | No pretreatment | Autohydrolysis | EP + C + E + DL + FD | DP1–10 (DPav = 5) | 25 |
| DP 4–23 (DPav = 9) | |||||
| No pretreatment | Subcritical water with citric acid as catalyst | No downstream processing | DP2–7 | 37 | |
| Finger citron pomace | Ultrasonic extraction of pectin under acidic conditions + EP | Enzymatic hydrolysis | Activated carbon decolourization + yeast fermentation + UF + FD | M w < 2.15 kDaa | 15 |
| Mandarin peels | G + Ultrasound-assisted extraction with oxalic acid-ammonium oxalate + EP + Acetone washing + HPSEC purification | Enzymatic hydrolysis with pectate-lyases | FD | DP2–3 unsaturated (methylated and non-methylated) | 11 |
| Lemon peels | Aqueous extraction of free sugars | Enzymatic hydrolysis | NF | DP2–10 (72%) | 46 |
| DM = 37% | 27 | ||||
| OGalA content>53%a | |||||
| Haw pectin | No pretreatment | Enzymatic hydrolysis | UF, NF | M w 0.2–6 kDa | 22 |
| Sugar beet pulp | No pretreatment | Enzymatic hydrolysis | Ion exchange chromatography | DP2, DP4 | 33 |
| Citrus pectin | No pretreatment | Enzymatic hydrolysis | FD | M w < 1 kDa | 8 |
| Pectin | No pretreatment | Enzymatic hydrolysis | Separation of high Mw fragments from OGalA (no more details) + FD or atomization | DP1–5 | 6,75 |
| Methylated citrus pectin | No pretreatment | Enzymatic hydrolysis | DL + E + C + UF + FD | DP1–10 (DPav = 5) | 25 |
| Sunflower plate pectin | No pretreatment | Enzymatic hydrolysis | C + Spray drying | DP2–7 (Av. Mw = 770 Da) | 36 |
| Spray drying | DP2–10 | 35 | |||
| Citrus pectin | No pretreatment | Enzymatic hydrolysis | FD | M w < 1 kDa | 23 |
| OGalA < 1 kDa | |||||
| DE = 11.6% | |||||
| Artichoke pectin | No pretreatment | Enzymatic hydrolysis | UF | DP2–5 partially methyl- and/or acetyl-esterified (+some neutral sugars and ferulic acid) | 13 |
| Haw fruit | Pectin extraction with hot water | Enzymatic hydrolysis | Chromatography (DEAE-Sephadex A-25) + deionization + FD | DP5 | 12,20,21 |
| 16–18 | |||||
| Flax stems | Pectin extraction with acetate buffer + ethanol washing + HCl treatment + neutralization + EP + FD | Enzymatic hydrolysis with pectate lyases | Semipreparative chromatography | Methylated and acetylated, saturated and unsaturated (DPav = 15) | 44 |
| Unsaturated, with low substitution degree (DPav = 5) | |||||
| Mango peels | D + G + S + Pectin extraction with HCl + EP | Enzymatic hydrolysis with immobilized pectinases | C | DP2, DP4 | 24 |
| Haw fruit | Pectin extraction with hot water | Enzymatic hydrolysis with immobilized pectinases | UF + Chromatography (DEAE-Sephadex A-25) | DP2–11 | 31 |
| Apple pectin | No pretreatment | Enzymatic hydrolysis + High hydrostatic pressure | C + IE | DP3 | 7 |
| Lonicera japonica Thunb. | Polysaccharide water extraction + concentration + EP + C + D + chromatography (DEAE-Cellulose and Sepharose CL-6B) | Enzymatic hydrolysis + chemical de-esterification | Chromatographic separation (Sephadex G-75) + DL or Sephadex G-10 + FD | DP1–8 | 14 |
| Apple pectin | No pretreatment | Fermentation with S. hydrogenans YAM1 | No downstream processing | DP2–3 unsaturated methylated and/or acetylated | 10 |
| Orange peels | Sterilization by UV + FD + G + S | Fermentation with an engineered Pichia pastoris | C | DP2–6 | 32 |
| Mandarin peels | Sterilization by UV + FD + G + S | Fermentation with an engineered Pichia pastoris | C | DP2–7 | 32 |
| Sugar beet pulp | Sterilization | Fermentation with an engineered E. coli KO11 | EP | DP2–6 | 34 |
3. Manufacture and refining
Several approaches have been described in the literature for pectin depolymerization, including physical treatments, such as electron beam irradiation,29 free-radical hydrolysis,38 physico-chemical processes,25,37 enzymatic hydrolysis either in submerged systems8,11,12,15,21–23,35 or with immobilized enzymes24,31 and even microbial fermentation using wild10 or genetically modified microorganisms.32,34 In addition, combined technologies such as enzymatic hydrolysis and high hydrostatic pressure7 or enzymatic hydrolysis and chemical de-esterification14 were also employed for the production of di- and tri-galacturonides and mixtures of OGalA with DP1–8, respectively.As can be seen in Table 1, enzymatic hydrolysis was the preferred alternative given that it allows mixtures of OGalA to be obtained in a narrow range of DP under mild operational conditions. Employing this technology, when pectins or PGA were used as raw materials, no pretreatments were applied before enzymatic hydrolysis, whereas starting from agroindustrial byproducts, one or several sequential pretreatments were required (see Table 1 for details). However, mixtures of pectic oligosaccharides have also been obtained by direct enzymatic hydrolysis or hydrothermal processing of a variety of byproducts such as orange peel wastes,39,40 lemon peels26,41 and sugar beet pulp.33,42
Unlike PGalA hydrolysis, the enzymatic saccharification of pectin or agroindustrial byproducts resulted in mixtures of oligosaccharides containing OGalA and other type of oligomers made up of combinations of rhamnose, arabinose, galactose, xylose and other minority sugars. In this context, in the function of the type of pectin (which depends on the raw material and the ripening degree) and the activities of the selected enzymes, several oligosaccharides differing in the degree of methylation, acetylation or saturation can be obtained. For instance, the hydrolytic action of endopolygalacturonases (Endo-PG) results in the formation of saturated OGalA, whereas pectin- and pectate-lyases generate unsaturated products by β-transelimination.43 Alternatively, side-enzymes, which are also present in commercial preparations, such as galactanases and arabanases, provoke the breakdown of neutral sugar chains attached to RGI, generating oligosaccharides made up of arabinose and/or galactose units. Fig. 1 also depicts the mode of action of the main enzymes involved in the hydrolysis of pectin.
Concerning the chemical pretreatments, the combination of sequential aqueous extraction and hydrothermal treatment resulted in the formation of both saturated and unsaturated OGalA.26
As stated before, to advance in the knowledge of the structure–function interrelationships, well-isolated and characterized products need to be used in laboratory assays. In an effort to delve deeper into this issue, the isolation of OGalA from streams containing mixtures of oligomers has been assessed by several techniques, among which membrane filtration15,22,25,31 and/or chromatographic separation12,14,20,21,31,33,44 are the most used. However, other types of refining operations such as ion exchange7 and ethanol precipitation25 were also tested.
In conclusion, the combination of some of these processing stages led to the manufacture of isolated OGalA with DP3,7 DP512,21 and DP929 from apple pectin, haw and pomelo peels, respectively. Moreover, mixtures with DP2 and/or DP3,10,11 mixtures with DP in the range of 2–513 or 2–732,37 were also recovered from other raw materials (see summary in Table 1). Fig. 2 shows a general scheme for the production of OGalA, including in each block, a list of techniques that can be potentially employed.
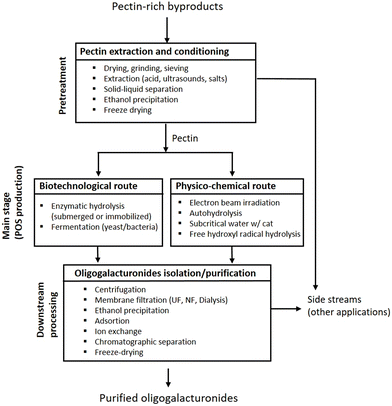 | ||
| Fig. 2 General scheme for the production of OGalA including a list of techniques that can be potentially employed in the main stages. | ||
4. Chemical characterization
In 2008, FAO published a roadmap for scientists to follow to demonstrate the prebiotic potential of a given product, where its chemical characterization is the first step.45 Moreover, complete knowledge of its composition (type of sugars and bonds, degree of esterification or the presence of substituents) is necessary for determining its structure–function relationships.The breakdown of pectin, a complex polymer, as depicted in Fig. 1, gives mixtures of oligomers, the identification and quantification of which are difficult, thereby needing the combination of various analytical techniques. Moreover, only OGalA standards with DP2 and DP3 are commercially available.
One of the most used instruments for the characterization of OGalA is HPSEC, but it just allows an estimation of the average Mw of OGalA mixtures.13,46,47 In this context, Wang et al.47 recently proposed a method based on multistep ethanol precipitation for fractionating complex mixtures of OGalA as a prior step to HPSEC analysis. However, additional and more advanced instrumental techniques are usually applied to obtain more detailed information. For instance, MALDI-TOF-MS10,13 and ESI-MS7,11,33 have been used to identify structures containing neutral sugars with or without methyl and acetyl groups in mixtures of OGalA. Moreover, HPAEC-PAD/UV was often proposed for determining the content of saturated and unsaturated OGalA,24–26 whereas HILIC-ELSD was employed by Gómez et al.26 and Valdivieso-Ramírez et al.37 to separate, identify and quantify mixtures of OGalA. Although this technique provided satisfactory outcomes in determining the presence of methyl and acetyl groups, its application may be hindered by its limit of detection (DP10).46 Alternatively, 13C and 1H NMR were used for a deeper product identification, including information about the type of linkages.24 GC-FID can also be used for the determination of DP2 and DP3 oligogalacturonides13 and the neutral sugars and uronic acid distribution can be determined by GC-FID,26 HPLC-UV7 or HPAEC/PAD29 after quantitative enzymatic or chemical hydrolysis of oligomers (using, for instance, TFA). For more information, the degree of esterification (DE) can be determined by a titrimetric method,48 the degree of acetylation (DA) by using the Megazyme acetate kit49 and the degree of methylation (DM) by a colorimetric method50 or by FTIR analysis,11,13,29 whereas ferulic acid substituents can be quantified by RP-HPLC/DAD.51
Finally, regarding the OGalA structure at the molecular level, two possible conformations (3.1 and 2.1) for homogalacturonan (the oligogalacturonide precursor) based on molecular dynamic simulations as well as a detailed view of a DP2 OGalA (including its 1,4 glycosidic bond) can be found in the article by Zdunek et al.52 Conformations 3.1 and 3.2 indicate that there can be three or two GalA units per helical turn, respectively.
5. Biological properties of oligogalacturonides
The determination of the biological properties of OGalA and products enriched in OGalA has recently attracted the attention of the scientific community. Table 2 summarizes the studies found on this subject in the literature as well as their main conclusions and Table 3 shows the results obtained when these types of products were compared to other prebiotics or medications.| Biological property | Raw material | Product characteristics | Conclusions | Ref. |
|---|---|---|---|---|
| a No data about DP range available. b In vivo study. c Clinical trial; HFD: high-fat diet; DP: degree of polymerization; DE: degree of esterification; DM: degree of methylation; HCD: high-cholesterol diet; BAs = bile acids; NS = neutral sugars; FA = ferulic acid; and N.D. = no data. | ||||
| Prebiotic | PGalA | DP1–10 (DPav = 5) | Promotion the growth of F. prausnitzii | 25 |
| Methylated | ||||
| Pomelo peels | DP9 (approx.) | Promotion of E. maltosivorans M-W14, C. eutactus y Lb. plantarum 299v but not C. perfringens | 29 | |
| DE = 72% | ||||
| Lemon peels | DP2–10 (mainly) | Bifidogenic effect towards Bif. lactis Bb-12. | 27 | |
| DM = 37% | ||||
| [OGalA]>53% | ||||
| DP1–13 22% | Increases in F. prausnitzii and R. intestinalis. | 26 | ||
| DP>13 70% | ||||
| DM = 61% | ||||
| [OGalA]>62% | ||||
| N.D. | DP2–3b | Increases in bifidobacteria and lactobacilli, Decreases in E. coli | 28 | |
| N.D. | Unsaturated, methylated OGalA + GOS + FOSc | Minimizes the alteration of microbiota after breast-feeding of full-term babies and increases the bifidobacteria proportion | 58 | |
| N.D. | OGalA + GOS + FOSc | Lower incidence in endogen infections in preterm infants (probably due to the modulation of the microbiota or the intestinal inflammatory response) | 60 | |
| N.D. | OGalA + GOS + FOSc | Improvement in the microbiota composition (increases in bifidobacteria and decreases in fecal pathogens) in HIV-infected adults | 59 | |
| Citrus pectin | OGalAc | Acidic oligosaccharides did not affect to the bifidobacteria and lactobacilli numbers but reduced the stool consistency in infants | 57 | |
| Sunflower pectin | HG (12.5 kDa)[GalA = 78%] | Significant increases in bifidobacteria and lactobacilli as well as in Eubacterium rectale numbers. | 55 | |
| Sugar beet pulp | DP4, DP5 | DP5 resulted in significantly higher Bacteroidetes densities and DP4 resulted in significantly higher Firmicutes densities | 56 | |
| Lemon peels | HG (<70% GalA, DE < 70%) | Increases, mainly, the number of F. prausnitzii and Ruminococcaceae family. | 54 | |
| Anti-inflammatory, Immunomodulatory activity | Apple pectin | DP3 | Attenuation of the release of hexosaminidase B and histamine Reduction in the production of IL-4 proinflammatory cytokine | 7 |
| Pectin | DP1–5 | Reduction in skin inflammation and diapedesis | 6 | |
| N.D. | OGalA + GOS + FOSc | Reduction in sCD14 and LPS levels, CD4 + T-cell activation (CD25), and improved NK cell cytolytic activity in HIV-infected adults | 59 | |
| Green tea leaves | HG (20 KDa) partially methylated | High phagocytosis-enhancing activity in HL-60 cells | 66 | |
| Antioxidant | Apple pectin | DP2–3 unsaturated methylated and/or acetylated | Strong antioxidant activity (DPPH test) | 10 |
| Haw | OGalA (0.2–6 kDa) | Increases in SOD and reduction in MDA levels in serum of mice fed with HFD | 68 | |
| DP5b | High antioxidant activity Increases in SOD, CAT and GSH-Px, TAC and GSH and decreases in MDA | 12 | ||
| Artichoke pectin | OGalA (DP2–5) partially methyl- and/or acetyl-esterified(+some NS and FA) | Higher antioxidant activity than original pectin | 13 | |
| Finger citron pomace | 2.15 kDa, (DP11 approx.) | Strong antioxidant activity (DPPH test) | 15 | |
| Lonicera japonica Thunb. | DP1–8 de-esterified and partially methyl-and/or acetyl-esterified | De-esterified OGalA reduced H2O2-induced reactive oxygen species production in HEK-283T cells | 14 | |
| Better antioxidant activities than RG-I y RG-II fragments | ||||
| Antibacterial | Mango peels | DP2–4 | Antimicrobial effect against E. coli, S. aureus, B. subtilis and S. Typhimurium | 24 |
| Citrus pectin | OGalA < 1 kDa; (DP < 5 approx.) DE = 11.6% | Bactericide effect against S aureus, P. aeruginosa, L monocytogenes and S Typhimurium. | 23 | |
| Haw | OGalA (0.2–6 kDa)a | Strong antibacterial activity against E. coli, which is enhanced by combination with lactic acid or sodium lactate | 22 | |
| Cranberry | DP3–4 unsaturated, methylated | Inhibit the quiescence of the uropathogenic strain E. coli CFT073 and reduced the population of the persister cells which are tolerant to antibiotics. | 69 | |
| Anticancer | Mandarin peels | DP2–3 unsaturated methylated and not methylated | Biocompatibility with normal cells of human kidney (HEK293) Cytotoxicity against colon cancer cells HT29, higher effect in mOGalA in comparison with non-methylated OGalA, or polysaccharide | 11 |
| Apple | OGalA (pectic acid)a | Inhibition of growth of breast cancer cells (MDA-MB-231) | 9 | |
| No inhibition of growth of healthy cells (HUVEC), Induction of cancer cell apoptosis | ||||
| Apple pectin | DP2–3 unsaturated methylated and/or acetylated | Inhibition of growth of breast cancer cells (MCF-7) with mOGalA and higher than that observed for pectins | 10 | |
| Citrus pectin | OGalA Mw < 1 kDaa | Inhibition to the growth of several cancer cells (HepG2, A549 and Colo205) | 8 | |
| (DP < 5 approx.) | Cytotoxic effect against cancer cells by increasing the membrane permeability and galectin-3 release | |||
| PGalA | OGalA DP7–23 | No apoptotic effects on human prostate cancer cells | 70 | |
| Antidiabetic | Haw | DP5b | Reductions of Glc and insulin contents in plasma, increased by HFD Improves insulin sensitivity | 20 |
| DP 5, 6, 7, 10, 12 (85% GalA) | High and dose-dependent in vitro antiglycation activity | 73 | ||
| Antilipidemic | Haw | DP5b (average) | Adiponectin activation in mice, Reductions in hepatic TC, TG and total lipids in mice in comparison with HFC diet | 19 |
| DP5b | Decrease in TG level in the liver and in the accumulation of epididymal and perirenal fat | 12 | ||
| Decreases in plasma and hepatic TC and increases in plasma HDL-c, Reduces TG levels (similar to standard diet), Promotes the removal of bile acids in feces | 17 | |||
| Haw | DP5b | Suppresses intestinal bile acids absorption and promotes the biosynthesis of hepatic BAs and the output in feces, reducing the hepatic cholesterol | 18 | |
| Reductions in serum TC, serum LDL-c and hepatic TC Increases in serum HDL-c | 16 | |||
| 0.2–6 kDa | Decreases of lipids in serum and perirenal fat accumulation | 68 | ||
| Antiobesity | Haw | DP5b | OGalA addition to a HFD reduces liver and animal weights in mice with respect to animals fed just with HFD | 16 |
| OGalA addition to a HCD reduces the weight gain in mice compared to animals fed just with HCD | 17 | |||
| OGalA reduces weight gains and food efficiency ratios in mice fed with HFD with respect to animal fed just with HFD | 20 | |||
| Suppresses weight gain and decreased serum TG level by promoting fatty acid oxidation and lipid excretion in feces | 21 | |||
| Reproductive performance | Pectin | DP2–3b | Increase in the number of born rats per litter and the progesterone, NO and VEGF levels in serum | 28 |
| Anti-aging | Pectin | DP1–5 | Prevents the skin aging by stimulation adherence of basal keratinocytes to laminin V and/or collagen IV | 75 |
| Apple pectin | DP2–5 (50%) | OGalA (0.01%) stimulates epidermal growth and differentiation and promotes keratinocytes attachment to basement membrane by reorganizing the cytoskeleton | 76 | |
| Biological property | OGalA | Alternative product | Results | Ref. |
|---|---|---|---|---|
| a No data about DP range available. b In vivo study. c Clinical trial; BAs = bile acids; AG = aminoguanidine; CHO: cholestyramine; GOS: galactooligosaccharides; and FOS: fructooligosaccharides. | ||||
| Prebiotic | OGalA DP1–10 (DPav = 5) | Inulin | Increases in F. prausnitzii with OGalA but not with inulin. Increases in bifidobacteria and lactobacilli with inulin but not with OGalA | 25 |
| OGalA + GOS + FOSc | GOS + FOS | The addition of OGalA results in stronger positive effects on bifidobacteria proportion in full-term infants | 58 | |
| OGalAc | GOS + FOS + OGalA | Higher increases in bifidobacteria and lactobacilli and decreases in stool pH. Similar decreases in stool consistency | 57 | |
| HG (12.5 kDa) [GalA = 78%] | FOS or inulin | Similar increases in Bifidobacteria, Lactobacilli and Eubacterium rectale numbers and similar SCFA profiles and concentrations | 55 | |
| DP9 (approx) | Inulin | Higher increases in Lb plantarum 299v, Coprococcus eutactus MP-16 and Eubacterium maltosivorans M-W14 and similar results for Bif. animalist subsp lactis | 29 | |
| Higher butyrate production when using OGalA in E. maltosivorans assays | ||||
| DP1–13 22% | FOS | Both substrates resulted in similar microbiota profiles and butyrate and propionate productions. | 26 | |
| DP > 13 70% | ||||
| DM = 61% [OGalA] > 62% | FOS led to higher lactate productions but OGalA led to higher acetate and total SCFA concentrations. | |||
| Anti-inflammatory, Immunomodulatory activity | HG (20 kDa) partially methylated | Lipopolysaccharides | Similar phagocytosis effect | 66 |
| Antioxidant | M w 0.2–6 kDa | Zhibituo (Chinese medicinal herb) | Higher SOD activity and lower MDA accumulation in serum when using OGalA | 68 |
| OGalA (DP2–5) partially methyl- and/or acetyl-esterified (+some NS and FA) | Ascorbic acid | Lower antioxidant activities (DPPH and superoxide radical scavenging activities) than ascorbic acid | 15 | |
| DP1–8 de-esterified and partially methyl-and/or acetyl-esterified | Ascorbic acid | Lower antioxidant activities (DPPH, ABTS and hydroxyl superoxide radicals scavenging activities) than ascorbic acid | 14 | |
| Antibacterial | OGalA < 1 kDa (DP < 5 approx.) DE = 11.6% | Penicillin | Oligogalacturonides exhibited higher antibacterial activities against S aureus, P. aeruginosa, L monocytogenes and S Typhimurium than penicillin at the same concentration | 23 |
| OGalA (0.2–6 kDa)a | Sodium lactate or lactic acid | OGalA showed higher antibacterial effect against E. coli than sodium lactate or lactic acid | 22 | |
| Anticancer | OGalA DP7–23 | Commercial fractionated pectin powder (FPP) | OGalA have no apoptotic effects on human prostate cancer cells, whereas FPP induced apoptosis in this cell line | 70 |
| OGalA Mw < 1 kDaa (DP < 5 approx.) | Citrus pectin | Higher growth inhibition and higher cytotoxic effects on several cancer cell lines (HepG2, A549 and Colo205) in comparison with citrus pectin. Similar values in normal cells with both carbohydrates. | 8 | |
| Antidiabetic | DP 5, 6, 7, 10, 12 (85% GalA) | Aminoguanidine | In vitro antiglycation activity similar or higher than that observed for AG | 73 |
| Antilipidemic | M w 0.2–6 kDa | Zhibituo (Chinese medicinal herb) | No differences in TG, TC or HDL-c levels in the serum. Lower accumulation of perirenal fat when OGalA used at higher doses | 68 |
| DP5b | Cholestyramine | OGalA and CHO led to similar values of ileal, gallbladder, small intestine, hepatic and total BAs, whereas CHO resulted in higher fecal BAs | 18 | |
Some of the more relevant findings will be discussed in the next sections of this review. However, it necessary to note that acidic oligosaccharides are not mutagenic, making them suitable for human consumption under the conditions of their intended usage.53
5.1 Prebiotic potential: in vitro and in vivo effects on microbiota
The prebiotic potential of pectic oligosaccharides was probably the first biological property evaluated by several research groups, but mainly using mixtures of neutral and acidic oligomers (previous literature on this subject has been reviewed by Gullón et al.,2 Singh et al.3 and Míguez et al.4). However, to date, little information is available on the evaluation of the prebiotic potential of pure OGalA or products highly enriched in OGalA. In this field, it was reported that acidic oligosaccharides are non-digestible carbohydrates,53 and thus they can reach the colon where they are fermented by the gut microbiota resulting, among others, in changes in the microbiota composition.For instance, it can be stated that some authors demonstrated that OGalA mainly promote the growth of some butyrate-producing bacteria,25,26,29 as well as some bifidobacteria27,28 and lactobacilli,27–29 while they decrease or do not affect the population of some pathogenic species.28,29 In 2011, Onumpai et al. produced, among others, mixtures of OGalA with DP1–10 (DPav = 5) and DP4–23 (DPav = 9) from PGalA and mixtures of methylated OGalA (mOGalA) with DP1–10 (DPav = 5; DM = 29%) using methylated citrus pectin as the raw material. In their study, the experiments conducted with fecal cultures confirmed that mOGalA promote the growth of F. prausnitzii species to a higher extent compared to the parent pectin. This is an interesting finding given that butyrate-producing bacteria are considered a marker of intestinal health, where low numbers are correlated with the recurrence of IBD.25 In the same work, the authors also observed that mOGalA and OGalA with DPav = 9 resulted in an increase in Bacteroides spp. and total bacteria. Similar results were obtained by Gómez et al.26 by comparing different types of POS, demonstrating that lemon peel oligosaccharides (made up of >62% OGalA with a wide DP range and DM = 61%) resulted in higher increases in F. prausnitzii and R. intestinalis than that observed for sugar beet pectin, a substrate rich in neutral sugars (GalA/(Ara + Gal) ≈ 1). Significant increases in F. prausnitzii and the Ruminococcaceae family were also observed by Larsen et al. when fermented HG-rich pectins (>70% GalA) highly sterified (DE = 70%) were obtained from lemon peels in a TIM-2 colon model.54 In another work, Ferreira-Lazarte et al. observed that similar increases in bifidobacteria, lactobacilli and Eubacterium rectale and similar amounts of acetate, propionate and butyrate could be produced using FOS, inulin or partially methylated HG (GalA 78%, DM = 17%, Mw = 12.5 kDa) as the substrates.55
Regarding the effects of the OGalA structure on the gut microbiota, it is also necessary to note the changes observed at the phylum level by Holck et al., who carried out in vitro fermentation assays using unsaturated DP4 and DP5 OGalA (two similar OGalA). Surprisingly, DP5 OGalA resulted in significantly higher Bacteroidetes densities than that obtained with DP4, whereas DP4 OGalA resulted in significantly higher Firmicutes densities.56
In another work, in vitro fermentation assays performed with individual strains of several bacteria demonstrated that mixtures with a high OGalA content (mainly with DP2–10 and DM = 37%) promote the growth of the probiotic strain Bif. lactis Bb-12, and to a minor extent, Lb. rhamnosus GG, thus opening the possibility for the formulation of new symbiotics.27 Gamonpilas et al.29 carried out a study with concentrates rich in OGalA of DP ≈ 9 and DE = 72% and observed an increase in E. maltosivorans M-W14 (a butyrate- and acetate-producing bacteria), as well as in C. eutactus and Lb. plantarum 299v (a commercial probiotic). Moreover, this substrate inhibited the growth of C. perfringens, an opportunist pathogen found in gut microbiota, thereby indicating its good selectivity.
Alternatively, in an in vivo study with pregnant Wistar rats, Liu et al.28 demonstrated that supplementation of the diet with DP2 and DP3 OGalA resulted in a significant increase in the Bifidobacterium spp. and Lactobacillus spp. counts and decrease in the population of E. coli.
Regarding human studies, it should be noted that there are no reported clinical assays for evaluating the effects of pure OGalA intake, but some studies were carried out to assess the effects of several combinations of FOS, GOS and acidic oligosaccharides on the gut microbiota and other parameters.57–60 In a clinical trial with infants, Fanaro et al.57 demonstrated that acidic oligosaccharides were well tolerated and their addition to infant formula did not affect the bifidobacteria and lactobacilli numbers but reduced the stool consistency. Magne et al.58 demonstrated that the intake of mixtures of GOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOS
FOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mOGalA minimized the alteration of fecal microbiota after cessation of breast-feeding in full-term infants and promoted bifidobacteria proportions with a stronger effect when acidic oligosaccharides were present. Westerbeek et al.60 evaluated the effect of diet supplementation with mixtures FOS + GOS
mOGalA minimized the alteration of fecal microbiota after cessation of breast-feeding in full-term infants and promoted bifidobacteria proportions with a stronger effect when acidic oligosaccharides were present. Westerbeek et al.60 evaluated the effect of diet supplementation with mixtures FOS + GOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acidic oligosaccharides (80
acidic oligosaccharides (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio) on the intestinal permeability in preterm infants, observing a lower trend of endogen infections, which may be due to the gut microbiota or inflammatory intestinal response modulations. Also, Gori et al.59 demonstrated that when HIV-infected adults consumed mixtures of GOS
20 ratio) on the intestinal permeability in preterm infants, observing a lower trend of endogen infections, which may be due to the gut microbiota or inflammatory intestinal response modulations. Also, Gori et al.59 demonstrated that when HIV-infected adults consumed mixtures of GOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOS
FOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acidic oligosaccharides (9
acidic oligosaccharides (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio), their gut microbiota composition improved (bifidobacteria increased, whereas fecal pathogens decreased).
10 ratio), their gut microbiota composition improved (bifidobacteria increased, whereas fecal pathogens decreased).
Alternatively, in parallel to the shifts in the gut microbiota, OGalA (and other carbohydrates) fermentation resulted in pH reductions and SCFA generation. Acetate, propionate and butyrate are the most abundant SFCA in the colon (up to 90–95% of the total SCFA) but they are readily absorbed, exerting a variety of effects with a significant impact on human health.61 For example, propionate and butyrate improve the barrier effect of the colonic epithelium and exert anti-inflammatory effects. Additionally, butyrate is the major energy source for the epithelium cells, and shows anti-tumor activity.62–64 Conversely, some of the SCFA reach the liver through the portal vein, where they are metabolized. Propionate is mainly incorporated in gluconeogenesis, whereas acetate and butyrate are mostly introduced in lipid biosynthesis,61 regulating the cholesterol levels. Moreover, it was reported that butyrate and propionate, but not acetate, can induce the production of gut hormones, thus reducing the food intake, thereby exhibiting protective effects against diet-induced obesity,65 and that SCFA, mainly butyrate, can protect against the development of colorectal cancer.61
Finally, it is necessary to remark that the gut microbiota is a complex ecosystem, in which a variety of cross-feeding mechanisms (including substrate and metabolic cross-feedings) are continuously present.
5.2 Anti-inflammatory/anti-allergic effects
The anti-inflammatory effect and regulation of the immune system associated with OGalA mixtures have also been evaluated in a few articles, showing some promising results. For instance, Ma et al.7 demonstrated, using the model cell line RBL-2H3, that doses of 75–150 μg mL−1 of trigalacturonic acid (DP3) inhibit the cell degranulation and significantly reduce the release of β-hexosaminidase and histamine as well as the production of IL-4 proinflammatory cytokine. Therefore, this product can act as an inhibitor in the regulation of mast cell-mediated allergic inflammatory responses and serve as a potential therapeutic agent for the treatment of allergic diseases.Alternatively, in an interesting in vivo study, it was proven that the addition of DP2 and DP3 OGalA to the diet improved the production of Th cytokines (decreased IL-2 and increased IL-10 levels) in pregnant rats, reducing the immunological rejection during the middle gestation phase.28
Also, in a clinical trial using mixtures of GOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOS
FOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acidic oligosaccharides (9
acidic oligosaccharides (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio), Gori et al.59 observed a significant reduction in sCD14 and LPS levels and CD4 + T-cell activation (CD25), and an improvement in NK cell cytolytic activity in HIV-1-infected adults after the prebiotic supplementation. Finally, Wang et al.66 demonstrated that partially methylated (DM ≈ 27%) HG with Mw = 20 kDa (DP ≈ 100) obtained from green tea leaves showed high phagocytosis-enhancing activity in HL-60 cells.
10 ratio), Gori et al.59 observed a significant reduction in sCD14 and LPS levels and CD4 + T-cell activation (CD25), and an improvement in NK cell cytolytic activity in HIV-1-infected adults after the prebiotic supplementation. Finally, Wang et al.66 demonstrated that partially methylated (DM ≈ 27%) HG with Mw = 20 kDa (DP ≈ 100) obtained from green tea leaves showed high phagocytosis-enhancing activity in HL-60 cells.
Finally, regarding the dermatology field, Lubrano et al. patented the use of OGalA mixtures (DP1–5) as agents for limiting the expansion of the immune response to a variety of external attacks suffered by sensitive skin, in which those responses are aggravated. The authors demonstrated that this type of product can reduce the expression of cellular adhesion factors to the endothelial cells of the dermis layer, thereby limiting the recognition of circulating cells responsible for inflammation expansion and diapedesis, and exerting cytoprotector and anti-stress effects.6
5.3 Antioxidant activity
It is well known that carboxyl, hydroxyl and methoxy groups can enhance the antioxidant activity of lignin and phenolic compounds,67 and thus the presence of carbohydrates, especially, galacturonic acid with several electron-donating groups can be associated with the improved antioxidant properties of pectin and its derivatives.10 In fact, some papers dealing with the evaluation of the antioxidant potential of OGalA have been recently published, including in vitro10,12,13,15 and in vivo studies.12,28In particular, in vitro assays carried out with 5 mg mL−1 of OGalA with DP5 resulted in free-radical scavenging activities against superoxide anion (O2−), DPPH radicals (DPPH) and hydroxyl radicals (OH) in the range of 80–90%, and even higher when the concentration was increased up to 10 mg mL−1.12 In this same study, in vivo experiments confirmed that high-fat diets (HFD) decreased several antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) as well as the levels of total antioxidant activity (T-AOC) and glutathione (GSH), whereas increased the malondialdehyde (MDA) content (an oxidative stress marker) in the liver of mice. Interestingly, these effects were reverted by the supplementation of the diet with DP5 OGalA at doses in the range of 50–300 mg kg−1 animal. These results are in agreement with that reported by Liu et al., who in an in vivo study with pregnant rats, demonstrated that dietary supplementation with DP2–3 OGalA improved the T-AOC and total SOD and reduced the products derived from lipid peroxidation (mainly MDA) in the serum and placenta. This fact can be one possible explanation for the reduced fetal losses observed in comparison with the control group.28 Changes in the SOD and MDA levels in the serum were also observed by Li et al.68 when OGalA (Mw 200–6000 Da) were used as a supplement in mice fed with HFD.
Abari et al.10 reported that unsaturated OGalA of DP2–3, with or without methoxyl and acetyl groups, showed high antioxidant activity (DPPH radical scavenging values of 93% with 20 mg mL−1), mainly due to the electron-donating groups on the GalA residues. Moreover, the antioxidant activity in these OGalA was higher than that observed for pectin (for instance, at 0.6 mg mL−1, OGalA showed antioxidant activity, whereas pectin did not). These results demonstrated that the degradation of pectin boosts the antioxidant effect due to the increase in low molecular weight chains, which have more reducing hydroxyl groups available to remove free radicals. Similar conclusions were published by Yu et al.15 using a mixture of oligomers with an average Mw = 2.15 kDa (approx. DP = 11), mainly made up of GalA, mannose, galactose and arabinose at a molar ratio of 11.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.72
2.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.26
1.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, obtained by enzymatic hydrolysis of finger citron pectin.
1, obtained by enzymatic hydrolysis of finger citron pectin.
Finally, Sabater et al.13 confirmed that mixtures of DP2–5 OGalA methyl and/or acetyl esterified containing some neutral sugars and ferulic acid as substituents showed a higher antioxidant activity than the original artichoke pectin, concluding that enzymatic hydrolysis promotes the antioxidant activity.
5.4 Antibacterial activity
Citrus pectin has no antimicrobial activity;23 however, some studies have reported that when pectin is hydrolyzed, the oligomers obtained can exhibit significant antibacterial activity. For instance, Wu et al.23 demonstrated that several mixtures of OGalA with Mw < 1 kDa (approx. DP < 5) and DE = 11.6% showed high antibacterial activity, being classified as bactericide against selected foodborne pathogens including S. aureus, P. aeruginosa, L. monocytogenes and S. typhimurium. In this case, the antibacterial activity values were even higher than that obtained with penicillin at the same concentration. Therefore, this product can be used not only as an antibacterial agent in the food industry (for increasing the lifetime) but also as a novel pharmaceutical.23 Strong pH and dose-dependent antibacterial activity against E. coli was also observed by Li et al.22 using high-purity OGalA with Mw in the range of 200–6000 Da (approx. DP1–30). The results also indicated that the combination of OGalA with lactic acid, sodium lactate or tea polyphenols improved the activity values,22 hypothesizing that lactic acid could permeabilize the outer membrane of the bacteria, thus facilitating oligogalacturonide attack.Alternatively, in a study dealing with the enzymatic hydrolysis of mango pectin, Xue et al.24 demonstrated that POS mixtures rich in DP2 and DP4 OGalA showed higher antibacterial activity in assays with Gram-positive and Gram-negative bacteria (E. coli, S. aureus, B. subtilis and S. Typhimurium), which was even higher than that observed for high Mw pectin or GalA. The authors suggested that mixtures of these OGalA can be used as a natural antibacterial agent in the food industry.
In this context, Sun et al. demonstrated that samples of highly methylated unsaturated DP3 an DP4 OGalA inhibited the quiescence of the uropathogenic strain E. coli CFT073 and reduced the population of persister cells, which are tolerant to antibiotics, concluding that additional investigations should be carried out to include this type of product as a part of an “active” cocktail of chemical compounds.69
5.5 Anticancer activity
The use of pectin as a food supplement has been associated with a decreased risk of colon cancer. Pectin- and POS-rich diets may block the ability of caspase-3 to promote tumor cell migration, and they also decrease anti-apoptotic Bcl2 protein expression.10Several papers on the anticancer potential of pectins and their hydrolysis products can be found in literature. For instance, Delphi et al. reported that pectins and their oligomers may exert apoptotic effects in cancer cells. They found that a commercial pectic acid (a product made up of OGalA) inhibited the growth of cancer cells (MDA-MB-231), whereas it did not have any effect on healthy ones (HUVEC). At a concentration of 2.5 mg mL−1, the cell viability was 20–25% in cancer cells but 90–95% in healthy ones, thereby demonstrating that this product has cytotoxic effects on cancer cells, hypothesizing that it triggers apoptosis by interfering with proteins that take part in apoptotic pathways, such as caspase-3.9 Likewise for other previous commented properties such as prebiotic, antioxidant and antibacterial activity,8,10,11 significant differences were observed in the antitumoral effect of oligogalacturonides in comparison with pectins.
In a previous study, Huang et al. determined the antitumoral activity of oligogalacturonides (Mw < 1 kDa; approx. DP < 5) and their effects on the cell membrane permeability on three human cancer cell lines (HepG2 (liver), A549 (lung) and Colo205 (colon)) and on a healthy one (HEK293). This study demonstrated that these OGalA inhibited the growth of cancer cells (up to 60%) without almost any effects on normal cells, with the inhibition low with pectin, whereas no differences were observed between pectin and oligomers in the normal cells. As an additional result, the authors found a significant increase in the membrane permeability in cancer cells (measured as released LDH) and negligible effects in the normal ones. Moreover, there was an increase in galectin-3 release, which participates in tumor development as well as an increase in OGalA in the serum in BALB/c mice when fed with this type of carbohydrate but not when pectin was used as a supplement, indicating that these oligomers can be absorbed in the small intestine.8
These results are in agreement with that reported by Rajulapati et al., who demonstrated that di- and trigalacturonic acids (unsaturated and methylated or unmethylated) show high biocompatibility with normal cells of the human kidney (HEK293) and cytotoxicity against colon cancer cells (HT29), with the anticancer effect higher in the methylated than in unmethylated OGalA or in polysaccharides.11
Abari et al. carried out an in vitro study using unsaturated, methylated and/or acetylated DP2–3 OGalA to assess their effects on the viability of MCF-7 breast cancer cells, demonstrating that their anticancer properties were significantly higher than that obtained for pectin or GalA (monomer). For instance, at 20 mg mL−1, the reduction in cell viability reached values of up to 84%, whereas this parameter was just 10% when pectin or GalA was used. Moreover, higher antiproliferation activity was observed for methylated oligomers and both methylated and unmethylated showed higher effects than non-methylated pectin, thus demonstrating that size and structure changes modify the biological properties.10
By contrast, Jackson et al.70 demonstrated that commercial fractionated pectin powder (FPP) induced apoptosis in human prostate cancer cells, whereas OGalA DP7–23 (from PGA) did not have apoptotic effects, concluding that the specific structural characteristics of pectin are the responsible for inducing apoptosis in cancer cells.
5.6 Antidiabetic effects
There is scientific evidence demonstrating that the probability of developing type 2 diabetes increases drastically after 45 years old, becoming an important health problem in the elderly.71 To the best of our knowledge, only one article regarding the potential of OGalA as antidiabetic agent exists in the literature. In this study, in vivo assays with mice confirmed the increased levels of insulin and glucose in the plasma after HFD intake compared with a standard diet. However, when the HFD was supplemented with DP5 OGalA, these values decreased below that observed with no supplemented HFD. The authors concluded that the HFD increased the levels of hepatic lipids, which provoked an increase in insulin, glucose and leptin in plasma, an effect counteracted by pentagalacturonic acid supplementation after 10 weeks, therefore confirming that these oligomers have some antidiabetic activity.20Alternatively, it is well-known that proteins are modified by glucose, resulting in the formation of AGEs (advanced glycation end products), which are one of the major risk factors for many chronic diseases including aging, arteriosclerosis and diabetic complications.72 In this context, Zhu et al.73 demonstrated that a mixture of medium molecular weight OGalA (Mw 700–3000 Da, 85% GalA) showed high antiglycation activity in vitro in a dose-dependent manner, which can be attributed to its high GalA content.
5.7 Antilipidemic effects
Hypercholesterolemia has been widely recognized as an important risk factor for atherosclerosis and coronary heart disease.16 Several works demonstrated the effectiveness of different OGalA preparations in decreasing the lipid levels in the serum12,16,17,19,68 and perirenal fat accumulation.12,68 Li et al.68 observed the above-mentioned effects using haw pectic oligogalacturonides with Mw in the range of 200–6000 Da as a supplement in the HFD of mice and Li et al.12 assessed the effect of DP5 OGalA consumption in mice fed with an HFD, demonstrating that this supplement provokes a significant decrease in hepatic triglycerides and reduces the weight gain and accumulation of epididymal and perirenal fat. The authors concluded that this specific type of OGalA could block the accumulation of adipose tissue in the liver of mice, thereby protecting against liver diseases such as fatty liver, cirrhosis and oxidative stress caused by a hyperlipidemic diet.In this context, it was also reported that DP5 OGalA could act as an adiponectin (ADPN) activator in mice, improving the lipid metabolism. The results of a recent in vivo study with mice confirmed that the intake of OGalA DP5 resulted in a reduction in the hepatic total cholesterol (TC), triglycerides (TG) and total lipids in comparison with mice fed with an HFD. Moreover, the morphological changes observed in hepatic tissue were partially counteracted by the intake of oligogalacturonides at higher doses (0.75–1.5 g kg−1).19
Zhu et al. investigated the effects of OGalA with DP5 on mice with hypercholesterolemia induced by an HFD. In this case, supplementation with OGalA (mainly at high doses and long times) returned the high LDL-c, serum TC and hepatic TC levels previously caused by HFD to normal values, in addition to increasing the serum HDL-c content, compared with HFD or standard diet groups.16
In 2017, similar behavior was found in mice fed a high-cholesterol diet (HCD) and later supplemented with haw pectin penta-oligogalacturonide, which lowered the plasma and hepatic TC and increased the plasma HDL-c levels. However, no significant differences were detected in the TG contents in the liver and plasma between HCD and DP5 OGalA-supplemented HCD.17
Alternatively, it was proven that OGalA administration led to significant increases in the bile acid concentration in the feces16–18 by suppressing the absorption of intestinal bile acids and promoting the biosynthesis of hepatic bile acids,18 thereby reducing the hepatic and plasma cholesterol.
Therefore, all these results revealed that DP5 OGalA have great potential for the development of functional foods that can improve the metabolism of cholesterol.
5.8 Antiobesity effects
Obesity is a major problem in industrialized countries. It was reported that 1/6 of Europeans with an age of ≥18 suffered from obesity in 2014, which means a BMI of ≥30 kg m−2.74 Among the various strategies to reduce body weight and obesity, the intake of prebiotics can play a remarkable role, given that it is known that the diet affects the gut microbiota and there seems to be a close relationship between the gut microbiota composition and obesity. In this context, some in vivo works have been reported on the positive effects of oligogalacturonide consumption on obesity. In 2013, Li et al. demonstrated that when mice were fed with an HFD supplemented with DP5 OGalA, their weight gain was significantly moderated and the serum TG levels decreased by promoting the fatty acid oxidation and lipid excretion in the feces.21In a later study, Li et al.20 also confirmed lower weight gains after adding DP5 OGalA to an HFD, as well as lower food efficiency ratios and lighter livers. These findings are in accordance with the results obtained by Zhu et al., who evaluated the effects of OGalA DP5 on mice with hypercholesterolemia induced by an HFD, demonstrating that this supplement resulted in a significant decrease in the liver and individual weight in comparison with the HFD group.16 In a later study, Zhu et al. observed that mice fed with an HCD supplemented with DP5 OGalA achieved, with a similar food intake, lower weight gains (and lower liver weights) than that just fed with HCD,17 demonstrating that this type of oligomer can have antiobesity effects.
5.9 Effects on the reproductive performance
In this field, an in vivo study28 was recently published on the effects of di- and trigalacturonic acid dietary supplementation on the reproductive capacity of Wistar rats. The results revealed that the addition of this type of oligosaccharide (at a dose of 80 mg kg−1) during the gestation period significantly increased (p < 0.05) the number of rats per litter, in both total born and born alive. Moreover, the authors observed higher progesterone, nitric oxide and vascular endothelial growth factor (VEGF) levels in the serum in comparison with the control group, concluding that this type of carbohydrate can boost the generation of blood vessels in the fetus and in the placenta during the middle gestation phase.5.10 Anti-aging potential
Lubrano et al. patented the use of a mixture of DP1–5 OGalA as a method to prevent aging of the skin by applying a therapeutically effective amount that stimulates the adherence of basal keratinocytes to laminin V and/or collagen IV.75 Furthermore, Lebreton-decoster et al.76 assessed the effect of oligogalacturonide mixtures (mainly DP2–5) obtained via the enzymatic hydrolysis of apple pectin on normal human keratinocytes in in vitro models and demonstrated that 0.01% OGalA treatment stimulated epidermal growth and differentiation and promoted keratinocyte attachment to the basement membrane components by reorganizing the cytoskeleton and modulating integrin recruitment, concluding that these OGalA can be considered a new anti-aging ingredient.6. Conclusions and future perspectives
Pectin is a type of fiber that can exert positive effects on human health but recent studies, such as the ones included and discussed in this review, demonstrated that these beneficial effects can be improved when pectin is broken into oligomers.In particular, recent research works have shown that OGalA exhibit great potential as functional ingredients given that they possess a variety of biological properties such as antioxidant, anticancer, antimicrobial, antiobesity, antilipidemic and anti-inflammatory, and thus they can be used to treat or at least prevent several diseases including cancer, diabetes, inflammatory bowel disease, obesity, hypercholesterolemia and dermatological problems, among others.
However, although these results are promising, the number of studies carried out using pure OGalA or mixtures rich in OGalA is still limited, and thus more in vitro and in vivo assays are needed, and of course, clinical assays (no clinical study using only OGalA was found in the literature) to confirm the results as a prior step to their commercialization as food ingredients with health claims.
In addition, other biological properties should be assessed to expand the knowledge of these types of carbohydrates and the scope of their application in several areas.
Alternatively, in an in vivo study, it was found that DP2–5 are absorbed before reaching the colon, and thus if this behavior is confirmed in clinical assays and a prebiotic effect is desired, methods such as product encapsulation should be assayed to guarantee this objective.
Finally, there is a need to focus research efforts on the development of sustainable, cheaper and cleaner technologies for the production of OGalA, in which agroindustrial byproducts can be directly used as raw materials instead of previously extracted pectin. To achieve this goal, harmful chemical-free and energy-saving processes will be required as well as easily scalable purification steps instead of expensive preparative chromatography.
All these measures can reduce the production costs, making the final product more accessible to the population (mainly to elderly) as dietetic supplements or bioactive ingredients in functional foods.
Abbreviations
| CAT | Catalase |
| DPav | Average degree of polymerization |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase (GSH-Px) |
| HCD | High-cholesterol diet |
| HEK293 | Human embryonic kidney 293 |
| HFD | High-fat diet |
| HT-29 | Human colon adenocarcinoma |
| HUVEC | Human Umbilical Vein Endothelial cells |
| IBD | Inflammatory bowel disease |
| LDL-c | Low density lipoproteins-cholesterol |
| HDL-c | High density lipoproteins-cholesterol |
| MCF-7 | Michigan Cancer Foundation-7 (breast cancer cell) |
| MDA | Malondialdehyde |
| MDA-MB-231 | MD Anderson-Metastatic Breast |
| mOGalA | Methylated oligogalacturonides |
| NO | Nitrogen oxide |
| OGalA | Oligogalacturonides |
| PGalA | Polygalacturonic acid |
| RBL | Rat Basophilic Leukemia |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglycerides |
| VEGF | Vascular Endothelial Growth Factor |
Author contributions
Conceptualization: SM, RY and JLA; investigation SM, RY and JLA; formal analysis: SM, RY and JLA; writing – original draft: SM, RY and JLA; review & editing: SM, MF, SR, RY and JLA; supervision: JLA; project administration: RY and JLA; funding acquisition: RY and JLA.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Project PID2020-116717RB-I00 (acronym BIOPLATFUN) funded by MCIN/AEI /10.13039/501100011033 and Project GRC-ED431C 2022/08 supported by Xunta de Galicia and by Ministry of Universities. Grant IJC2018-037665 funded by MCIN/AEI /10.13039/501100011033 and Grants RYC2021-031964-I and PRE2021-098927 funded by MCIN/AEI /10.13039/501100011033 and by “ESF + Investing in your future”.References
- G. R. Gibson, R. Hutkins, M. E. Sanders, S. L. Prescott, R. A. Reimer, S. J. Salminen, K. Scott, C. Stanton, K. S. Swanson, P. D. Cani, K. Verbeke and G. Reid, Nat. Rev. Gastroenterol. Hepatol., 2017, 14, 491–502 CrossRef PubMed.
- B. Gullón, B. Gómez, M. Martínez-Sabajanes, R. Yáñez, J. C. Parajó and J. L. Alonso, Trends Food Sci. Technol., 2013, 30, 153–161 CrossRef.
- R. P. Singh and J. M. R. Tingirikari, Biocatal. Agric. Biotechnol., 2021, 31, 101910 CrossRef CAS.
- B. Míguez, B. Gómez, P. Gullón, B. Gullón and J. L. Alonso, in Probiotics and Prebiotics in Human Nutrition and Health, ed. V. Rao and L. G. Rao, IntechOpen, Rijeka, 2016 Search PubMed.
- Isapp, International Scientific Association for Probiotics and Prebiotics (ISAPP), https://isappscience.org/for-scientists/resources/prebiotics/, (accessed 30 March 2023).
- C. Lubrano and G. Saintigny, Pat., WO/2004/082583, 2004.
- J. Ma, P. Tong, Y. Chen, Y. Wang, H. Ren, Z. Gao, T. Yue and F. Long, Food Chem., 2022, 371, 131097 CrossRef CAS PubMed.
- P.-H. Huang, L.-C. Fu, C.-S. Huang, Y.-T. Wang and M.-C. Wu, Food Chem., 2012, 132, 1987–1995 CrossRef CAS.
- L. Delphi, H. Sepehri, M. Khorramizadeh and F. Mansoori, Asian Pac. J. Cancer Prev., 2015, 16, 5265–5271 CrossRef PubMed.
- A. H. Abari, H. A. Rourani, S. M. Ghasemi, H. Kim and Y. G. Kim, Sci. Rep., 2021, 11, 8491 CrossRef PubMed.
- V. Rajulapati, A. Dhillon and A. Goyal, Bioresour. Technol. Rep., 2021, 15, 100740 CrossRef CAS.
- T. Li, S. Li, Y. Dong, R. Zhu and Y. Liu, Food Chem., 2014, 145, 335–341 CrossRef CAS PubMed.
- C. Sabater, A. Blanco-Doval, A. Montilla and N. Corzo, Food Hydrocolloids, 2021, 110, 106161 CrossRef CAS.
- X. Qi, Y. Yu, X. Wang, J. Xu, X. Wang, Z. Feng, Y. Zhou, H.-X. Xiao and L. Sun, Front. Nutr., 2022, 9, 998462 CrossRef PubMed.
- M. Yu, Y. Xia, W. Xie, Y. Li, X. Yu, J. Zheng and Y. Zhang, Food Funct., 2021, 12, 9855–9865 RSC.
- R. Zhu, T. Li, Y. Dong, Y. Liu, S. Li, G. Chen, Z. Zhao and Y. Jia, Food Res. Int., 2013, 54, 262–268 CrossRef CAS.
- R.-G. Zhu, Y.-D. Sun, Y.-T. Hou, J.-G. Fan, G. Chen and T.-P. Li, Chem.-Biol. Interact., 2017, 272, 153–159 CrossRef CAS PubMed.
- R. Zhu, Y. Hou, Y. Sun, T. Li, J. Fan, G. Chen and J. Wei, Lipids, 2017, 52, 489–498 CrossRef CAS PubMed.
- Q. Yu, X. Chen, X. Sun, W. Li, T. Liu, X. Zhang, Y. Li, T. Li and S. Li, Mol. Nutr. Food Res., 2021, 65, 2100167 CrossRef CAS PubMed.
- S. Li, Z. Huang, Y. Dong, R. Zhu and T. Li, J. Funct. Foods, 2017, 34, 440–446 CrossRef CAS.
- T. P. Li, R. G. Zhu, Y. P. Dong, Y. H. Liu, S. H. Li and G. Chen, J. Agric. Food Chem., 2013, 61, 7599–7605 CrossRef CAS PubMed.
- S. Li, T. Li, R. Zhu, N. Wang, Y. Song, S. Wang and M. Guo, Int. J. Food Prop., 2013, 16, 706–712 CrossRef CAS.
- M.-C. Wu, H. Li, P.-H. Wu, P.-H. Huang and Y.-T. Wang, J. Food Sci., 2014, 79, M1541–M1544 CrossRef CAS PubMed.
- L. Xue, J. Long, C. Lu, X. Li, X. Xu and Z. Jin, Food Biosci., 2021, 39, 100837 CrossRef CAS.
- C. Onumpai, S. Kolida, E. Bonnin and R. A. Rastall, Appl. Environ. Microbiol., 2011, 77, 5747–5754 CrossRef CAS PubMed.
- B. Gómez, B. Gullón, R. Yáñez, H. Schols and J. L. Alonso, J. Funct. Foods, 2016, 20, 108–121 CrossRef.
- B. Gómez, C. Peláez, M. C. Martínez-Cuesta, J. C. Parajó, J. L. Alonso and T. Requena, LWT–Food Sci. Technol., 2019, 109, 17–25 CrossRef.
- M. Liu, X. Mao, D. Chen, B. Yu, J. He, P. Zheng, J. Yu, J. Luo, Y. Luo, J. Wang, Q. Wang and H. Wang, Anim. Nutr., 2020, 6, 210–216 CrossRef PubMed.
- C. Gamonpilas, C. Buathongjan, W. Sangwan, M. Rattanaprasert, K. C. Weizman, M. Klomtun, N. Phonsatta and P. Methacanon, Food Hydrocolloids, 2021, 113, 106551 CrossRef CAS.
- S. Kapoor and S. M. Dharmesh, Carbohydr. Polym., 2017, 160, 52–61 CrossRef CAS PubMed.
- S. Li, T. Li, Y. Jia, R. Zhu, N. Wang, S. Jin and M. Guo, Eur. Food Res. Technol., 2011, 233, 731–734 CrossRef CAS.
- G. Yang, H. Tan, S. Li, M. Zhang, J. Che, K. Li, W. Chen and H. Yin, Bioresour. Technol., 2020, 300, 122645 CrossRef CAS PubMed.
- B. Quéméner, J. C. C. Pino, M.-C. Ralet, E. Bonnin and J.-F. Thibault, J. Mass Spectrom., 2003, 38, 641–648 CrossRef PubMed.
- M. C. Edwards, E. D. C. Henriksen, L. P. Yomano, B. C. Gardner, L. N. Sharma, L. O. Ingram and J. D. Peterson, Appl. Environ. Microbiol., 2011, 77, 5184–5191 CrossRef CAS PubMed.
- G. Yang, S. Li, H. Tan, K. Li, W. Chen and H. Yin, ACS Food Sci. Technol., 2021, 1, 338–346 CrossRef CAS.
- C. Zhao, C. Wu, K. Li, J. Kennedy, M. Wisniewski, L. Gao, C.-G. Han, J. Liu, H. Yin and X. Wu, J. Fungi, 2022, 8, 716 CrossRef CAS PubMed.
- C. S. Valdivieso-Ramírez, J. E. Sánchez-Gallego, M. Gänzle, F. Temelli and M. D. A. Saldaña, J. Supercrit. Fluids, 2021, 169, 105103 CrossRef.
- R. Elboutachfaiti, C. Delattre, P. Michaud, B. Courtois and J. Courtois, Int. J. Biol. Macromol., 2008, 43, 257–261 CrossRef CAS PubMed.
- M. Martínez, R. Yáñez, J. L. Alonsó and J. C. Parajó, Ind. Eng. Chem. Res., 2010, 49, 8470–8476 CrossRef.
- M. Martínez, R. Yáñez, J. Alonso and J. Parajó, Int. J. Food Sci. Technol., 2012, 47, 747–754 CrossRef.
- B. Gómez, B. Gullón, R. Yáñez, J. C. Parajó and J. L. Alonso, J. Agric. Food Chem., 2013, 61, 10043–10053 CrossRef PubMed.
- M. Martínez, B. Gullón, H. A. Schols, J. L. Alonso and J. C. Parajó, Ind. Eng. Chem. Res., 2009, 48, 4681–4687 CrossRef.
- H. W. Uhlig and E. M. Linsmaier-Bednar, Industrial enzymes and their applications, John Wiley and Sons, Inc. New York, USA, 1998 Search PubMed.
- L. Bédouet, B. Courtois and J. Courtois, Biotechnol. Lett., 2005, 27, 33–40 CrossRef PubMed.
- M. Pineiro, N.-G. Asp, G. Reid, S. Macfarlane, L. Morelli, O. Brunser and K. Tuohy, J. Clin. Gastroenterol., 2008, 42, 156–159 CrossRef PubMed.
- B. Gómez, R. Yáñez, J. C. Parajó and J. L. Alonso, J. Chem. Technol. Biotechnol., 2016, 91, 234–247 CrossRef.
- T. Wang, Y. Tao, C. Lai, C. Huang, Z. Ling and Q. Yong, Int. J. Biol. Macromol., 2021, 188, 343–349 CrossRef CAS PubMed.
- T. Jiang, F. He, S. Han, C. Chen, Y. Zhang and H. Che, J. Funct. Foods, 2019, 60, 103414 CrossRef CAS.
- C. Remoroza, S. Cord-Landwehr, A. G. M. Leijdekkers, B. M. Moerschbacher, H. A. Schols and H. Gruppen, Carbohydr. Polym., 2012, 90, 41–48 CrossRef CAS PubMed.
- S. E. Guillotin, A. Van Loey, P. Boulenguer, H. A. Schols and A. G. J. Voragen, Food Hydrocolloids, 2007, 21, 85–91 CrossRef CAS.
- J. Holck, A. Lorentzen, L. Vigsnaes, T. Licht, J. D. Mikkelsen and A. Meyer, J. Agric. Food Chem., 2011, 59, 6511–6519 CrossRef CAS PubMed.
- A. Zdunek, P. Pieczywek and J. Cybulska, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1101–1117 CrossRef CAS PubMed.
- J. A. Garthoff, S. Heemskerk, R. A. Hempenius, B. A. R. Lina, C. A. M. Krul, J. H. Koeman and G. J. A. Speijers, Regul. Toxicol. Pharmacol., 2010, 57, 31–42 CrossRef CAS PubMed.
- N. Larsen, C. B. de Souza, L. Krych, T. Cahu, M. Wiese, W. Kot, K. Hansen, A. Blennow, K. Venema and L. Jespersen, Front. Microbiol., 2019, 10, 223 CrossRef PubMed.
- A. Ferreira-Lazarte, V. Kachrimanidou, M. Villamiel, R. A. Rastall and F. J. Moreno, Carbohydr. Polym., 2018, 199, 482–491 CrossRef CAS PubMed.
- J. Holck, K. Hjernø, A. Lorentzen, L. K. Vigsnæs, L. Hemmingsen, T. R. Licht, J. D. Mikkelsen and A. S. Meyer, Process Biochem., 2011, 46, 1039–1049 CrossRef CAS.
- S. Fanaro, J. Jelinek, B. Stahl, G. Boehm, R. Kock and V. Vigi, J. Pediatr. Gastroenterol. Nutr., 2005, 41, 186–190 CrossRef CAS PubMed.
- F. Magne, W. Hachelaf, A. Suau, G. Boudraa, K. Bouziane-Nedjadi, L. Rigottier-Gois, M. Touhami, J.-F. Desjeux and P. Pochart, J. Pediatr. Gastroenterol. Nutr., 2008, 46, 580–588 CrossRef CAS PubMed.
- A. Gori, G. Rizzardini, B. van't Land, K. B. Amor, J. van Schaik, C. Torti, T. Quirino, C. Tincati, A. Bandera, J. Knol, K. Benlhassan-Chahour, D. Trabattoni, D. Bray, A. Vriesema, G. Welling, J. Garssen and M. Clerici, Mucosal Immunol., 2011, 4, 554–563 CrossRef CAS PubMed.
- E. A. M. Westerbeek, A. van den Berg, H. N. Lafeber, W. P. F. Fetter and R. M. van Elburg, Br. J. Nutr., 2011, 105, 268–274 CrossRef CAS PubMed.
- D. Ríos-Covián, P. Ruas-Madiedo, A. Margolles, M. Gueimonde, C. G. de los Reyes-Gavilán and N. Salazar, Front. Microbiol., 2016, 7, 185 Search PubMed.
- J. Fernández, S. Redondo-Blanco, I. Gutiérrez-del-Río, E. M. Miguélez, C. J. Villar and F. Lombó, J. Funct. Foods, 2016, 25, 511–522 CrossRef.
- D. Parada-Venegas, M. K. la Fuente, G. Landskron, M. J. González, R. Quera, G. Dijkstra, H. J. M. Harmsen, K. N. Faber and M. A. Hermoso, Front. Immunol., 2019, 10, 277 CrossRef PubMed.
- A. Rivière, M. Selak, D. Lantin, F. Leroy and L. De Vuyst, Front. Microbiol., 2016, 7, 979 Search PubMed.
- H. V. Lin, A. Frassetto, E. J. K. Jr, A. R. Nawrocki, M. M. Lu, J. R. Kosinski, J. A. Hubert, D. Szeto, X. Yao, G. Forrest and D. J. Marsh, PLoS One, 2012, 7, e35240 CrossRef CAS PubMed; H. Wang, G. Wei, F. Liu, G. Banerjee, M. Joshi, S. W. A. Bligh, S. Shi, H. Lian, H. Fan, X. Gu and S. Wang, Int. J. Mol. Sci., 2014, 15, 9963–9978 CrossRef PubMed.
- H. Wang, G. Wei, F. Liu, G. Banerjee, M. Joshi, S. W. A. Bligh, S. Shi, H. Lian, H. Fan, X. Gu and S. Wang, Int. J. Mol. Sci., 2014, 15, 9963–9978 CrossRef PubMed.
- J. Chen, J. Yang, L. Ma, J. Li, N. Shahzad and C. Kim, Sci. Rep., 2020, 10, 2611 CrossRef CAS PubMed.
- T. Li, S. Li, L. Du, N. Wang, M. Guo, J. Zhang, F. Yan and H. Zhang, Food Chem., 2010, 121, 1010–1013 CrossRef CAS.
- J. Sun, R. W. Deering, Z. Peng, L. Najia, C. Khoo, P. S. Cohen, N. P. Seeram and D. C. Rowley, Sci. Rep., 2019, 9, 19590 CrossRef CAS PubMed.
- C. L. Jackson, T. M. Dreaden, L. K. Theobald, N. M. Tran, T. L. Beal, M. Eid, M. Y. Gao, R. B. Shirley, M. T. Stoffel, M. V. Kumar and D. Mohnen, Glycobiology, 2007, 17, 805–819 CrossRef CAS PubMed.
- J. Huizen, Medical News Today, https://www.medicalnewstoday.com/articles/317477 (accessed March 2023).
- M. W. Poulsen, R. V. Hedegaard, J. M. Andersen, B. de Courten, S. Bügel, J. Nielsen, L. H. Skibsted and L. O. Dragsted, Food Chem. Toxicol., 2013, 60, 10–37 CrossRef CAS PubMed.
- R. Zhu, X. Zhang, Y. Wang, L. Zhang, C. Wang, F. Hu, C. Ning and G. Chen, Food Chem., 2019, 286, 129–135 CrossRef CAS PubMed.
- L. Agafitei, J. Hrkal and V. Bourgeais, European Health Interview Survey, Eurostat Press Office, 2016, pp. 1–4 Search PubMed.
- C. Lubrano, L. Flavet, G. Saintigny and J.-R. Robin, US Pat, US20040224892A1, 2004 Search PubMed.
- C. Lebreton-decoster, P. Rousselle, C. Laperdrix, C. Lubrano, J.-R. Robin and B. Coulomb, Int. J. Cosmet. Sci., 2011, 33, 455–461 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
