Supramolecular carbohydrate-based hydrogels from oxidative hydroxylation of amphiphilic β-C-glycosylbarbiturates and α-glucosidase-induced hydrogelation†
Shun
Yao
a,
Robin
Brahmi
a,
Anaïs
Bouschon
a,
Jing
Chen
 *b and
Sami
Halila
*b and
Sami
Halila
 *a
*a
aUniv. Grenoble Alpes, CNRS, CERMAV, F-38000 Grenoble, France. E-mail: sami.halila@cermav.cnrs.fr
bThe Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250033, China. E-mail: phdchenj@gmail.com
First published on 5th December 2022
Abstract
We describe an ecofriendly two-step synthesis of glycoamphiphiles, capable of hierarchical self-assembly into supramolecular hydrogels, consisting of Knoevenagel condensation of barbituric acid derivatives with biobased carbohydrates (glucose and maltose) and selective H2O2-mediated oxidative hydroxylation of barbiturates in water. By modifying the molecular design of glycostructures and the hydrophilic/hydrophobic balance, various glyco-nanostructures (ribbons, tapes, vesicles, helices, fibers) have been created via supramolecular self-assembly. In addition, we found that a water-soluble glycoamphiphile underwent sol-to-gel phase transition upon the addition of α-glucosidase as a result of the hydrolysis of the non-reducing-end glucose in the maltose moiety.
Introduction
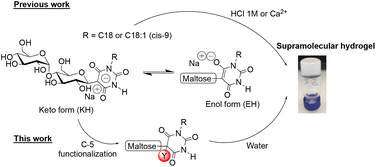
Glycoamphiphiles are basically defined as a hydrophilic carbohydrate moiety covalently bound via a glycosidic linkage to a hydrophobic building block. In nature, such amphiphilic species exist as glycolipids (glycosphingolipids, glycosylphosphatidylinositols, lipopolysaccharides, etc.) and are essential components of cell membranes and mediate diverse biological and pathological processes such as cell growth, fertility, immunity, metastasis, and viral and microbial invasion.1,2 On the other hand, because of their self-assembling properties, renewable raw materials, low toxicity and high biodegradability, glycoamphiphiles have found many applications in a huge variety of fields, from agriculture to biomedical applications.3,4 For instance, alkylpolyglucoside is used as a foaming agent, emulsifier, pharmaceutical granulating agent and cosmetic surfactant.5 Besides, glycoamphiphiles display advanced functions such as liquid crystal properties or self-assembly properties into various nanostructures ranging from micelles, vesicles and fibers in solution.6–8 A milestone was reached when we demonstrated that amphiphilic carbohydrate-based block copolymers could self-organize into periodic thin-film nanostructures of sub-10 nm resolution.9–11 In addition, glycoamphiphiles or glyco-hydrogelators are known to form supramolecular hydrogels resulting from the three-dimensional network of interpretated self-assembled nanofibers.12–14 Supramolecular hydrogels have gained tremendous attention in the development of soft materials with applications as drug releasing matrices, tissue engineering, removal of pollutants, sensors or templates for nanostructured materials, etc.15–17 Glyco-hydrogelators are mainly achieved through a multi-step synthesis including protection/functionalization/deprotection reactions in toxic and unsustainable solvents, which is a non-economical and non-eco-friendly strategy involving a time-consuming and labor-intensive process.12–14Very recently, we tackled these problems by reporting an efficient and versatile green synthetic method to construct glyco-hydrogelators through the Knoevenagel condensation of N,N′-substituted barbituric acids with protecting group-free carbohydrates in water.18 The resulting sodium salt of N-monosubstituted β-C-glycosylbarbiturates led to entangled nanostructured hydrogels either under strong acidic conditions or by adding calcium ions at neutral pH (Fig. 1). Because of the hydrogelation conditions that are inconsistent with biological applications, we investigated, once again, a sustainable chemical method to perform hydrogelation of β-C-glycosylbarbiturates at neutral pH and without adding divalent metal ions.
 | ||
| Fig. 1 Structures and conditions of hydrogelation of the sodium salt of amphiphilic β-C-glycosylbarbiturates. | ||
Starting from the observation that the exclusive presence of the keto tautomer form (KH) of barbiturates, which could be in equilibrium with the enol tautomer form (EH), is mandatory to promote hydrogelation, we decided, in this paper, to investigate the post-functionalization at the C-5 position of the barbituric moiety of the glycoamphiphiles, thus definitively blocking the KH form (Fig. 1). A survey of the literature indicated that reactions of (i) bromination,19 (ii) alkylation,20,21 (iii) hydroxymethylation,22 (iv) malonyl peroxide-mediated oxidation,23 and (v) oxidative hydroxylation could be envisioned. Keeping in mind that green chemistry approaches should be favored, we took the route of oxidative hydroxylation24 that occurs in water.
Therefore, herein, we describe the selective oxidative hydroxylation of a series of amphiphilic N-monosubstituted β-C-glycosylbarbiturates and investigate their hydrogelation properties in pure water.
Results and discussion
Our first attempts of selective oxidative hydroxylation at the C-5 position of the barbiturate ring were done with model substrates, such as β-C-glucosylbarbiturate 1 and N,N′-dimethyl β-C-glucosylbarbiturate 1′, which were easily synthesized and isolated from the aqueous reaction by precipitation into a mixture of EtOH/EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; v/v) with 96% and 98% yields, respectively (Scheme 1).25 A previous work from the L′Oréal group reported that H2O2-mediated oxidative hydroxylation of 1′ in water led in a one-pot procedure to β-methylamide C-glucoside derivative 4′ with the release of NaOH in the mechanism pathway.26
6; v/v) with 96% and 98% yields, respectively (Scheme 1).25 A previous work from the L′Oréal group reported that H2O2-mediated oxidative hydroxylation of 1′ in water led in a one-pot procedure to β-methylamide C-glucoside derivative 4′ with the release of NaOH in the mechanism pathway.26
Moreover, a patent from the previous authors showed that oxazolidine-2,4-dione intermediates 3 and 3′ could be favoured by controlling the reaction time or by using Oxone® as an oxidizing reagent for 16 h.27 For the latter, by reducing the reaction time from 16 to 2 h, the C-5 hydroxyl derivative 2′ was obtained in 71% yield. And compound 2 was not described. In this work, H2O2 was preferred because it is a very mild oxidant and relatively cheap and produces only water as the by-product, thus benefiting both the economic and green chemistry viewpoints. Since the basic conditions of H2O2-mediated oxidative hydroxylation led preferentially to rearrangements, we treated 1′ with 2 equivalents of 1 M KH2PO4 in order to control the pH (between 7 and 8) during the reaction. In spite of these precautions, we rapidly obtained a mixture of oxazolidinone 2′ and C-5 hydroxyl 3′ derivatives according to mass spectrometry analysis (data not shown). However, the same experimental conditions applied to 1 led to 2 in a satisfactory yield of 66%. This result highlights that N–H groups kinetically disfavor the rearrangement pathway, thereby allowing the control of oxidative hydroxylation at the C-5 position of barbituric acid.
As previously mentioned, we already demonstrated that MalB-3 and MalB-4 (Fig. 1), which feature a maltose disaccharide linked through an N-monosubstituted barbiturate to a C18 saturated aliphatic chain and a C18 mono-unsaturated C18:1 (cis-9) chain, respectively, were able to hierarchically self-assemble into supramolecular hydrogels either under strong acidic conditions or by adding calcium ions.18 We found, with others,28–30 that the hydrophobic–hydrophilic balance is a determinant parameter for exhibiting hydrogelation and since oxidative hydroxylation brings an additional hydroxyl function, we decided to screen a library of synthetic amphiphilic β-C-glycosylbarbiturates GlcB(OH) and MalB(OH) for their hydrogelation abilities (Scheme 2).
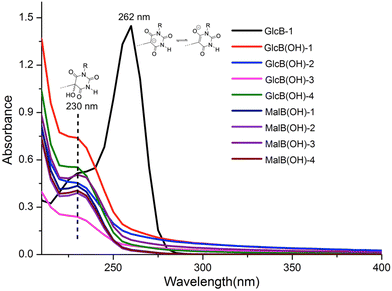
Briefly, the syntheses of GlcB and MalB were performed through the Knoevenagel condensation in water by reacting chemo-selectively N,N′-alkyl barbituric acid derivatives with unprotected Glc and Mal at the reducing-end anomeric position, as already reported.18 Next, H2O2-mediated oxidative hydroxylation of GlcB and MalB in water led successfully to GlcB(OH) and MalB(OH), respectively, with moderate to good yields (54–72%) as shown in Table 1. While thin-layer chromatography analyses indicated an almost complete reaction, a chromatographic separation step was required, lowering the yields in isolated products. All the structures were characterized and confirmed by high resolution mass spectrometry, NMR, FTIR and UV-visible spectroscopy (see the ESI and Fig. S1–S8†). The formation of 5,5-disubstituted barbiturates induces a significant upfield shift of the H-anomeric involved in β-C-glycoside formation, which appears as a clean doublet at 4.5 ppm in GlcB and MalB (Fig. S9†).18 The keto tautomer form for the series of GlcB(OH) and MalB(OH) was also confirmed by UV-visible spectroscopy with an expected λmax at around 230 nm and nothing at 262 nm, such as for GlcB-1, which is the sign of the enol tautomer form (Fig. 2).18
To investigate the self-assembling properties of GlcB(OH) and MalB(OH), tube-inversion experiments were performed, which involved cooling to induce gelation (self-assembly) after dissolution into aqueous solution via heating at 80 °C (Table 2). In contrast to GlcB samples that didn't dissolve in water even after heating, GlcB(OH) samples dissolved upon heating and more importantly slightly opaque hydrogels were formed after cooling-down at room temperature (rt) with a minimal gelation weight concentration (MGC) of 2 wt% for GlcB(OH)-2, and 3 wt% for GlcB(OH)-3 and GlcB(OH)-4. GlcB(OH)-1 dissolved in hot water and upon cooling formed sedimented large spherical aggregates and could not be considered as hydrogel. For GlcB(OH)-4, the formation of the hydrogel occurred after 3 days at rest, highlighting that particularly low self-assembly kinetics and aging helped for improving packing over time. In summary, these results clearly indicated that the additional hydroxyl function changed dramatically the solubility parameter in pure water and induced hierarchical self-assembly into supramolecular hydrogels. For MalB(OH) samples, opaque and transparent hydrogels were only observed for MalB(OH)-3 at 4 wt% and MalB(OH)-4 at 5 wt%, respectively. The obtention of a stable transparent gel for MalB(OH)-4 compared to MalB(OH)-3 reveals the formation of thinner aggregates that are shorter than the visible wavelength region. Decreasing the aliphatic chain from C18 (MalB(OH)-3) to C16 (MalB(OH)-2) and C14 (MalB(OH)-1) resulted in complete solubility in water. While MalB(OH)-3 is stable at room temperature for several months, MalB(OH)-4 becomes liquid after 1 day at rt. The hydrogels were all thermoreversibles, as confirmed by alternating heating (solution) and cooling (gel) cycles, which is one of the characteristics of supramolecular hydrogels owing to the nature of weak interconnected bonds. The gel-to-sol transition (Tgel) of the hydrogel at the MGC was investigated by differential scanning calorimetry (DSC) which showed endothermic peaks at 64, 61, and 42 °C for GlcB(OH)-2 to -4, respectively and at 38 °C for both MalB(OH)-3 and -4, supporting the disruption of the gel phase (Table 2).
To gain deeper insight into the morphology of self-assembled glyco-hydrogels, transmission electron microscopy observations were performed. As shown in Fig. 3, various well-structured nanoscale architectures entrapping solvent water molecules in the 3-D network were observed which feature carbohydrates exposed at the periphery. GlcB(OH)-2 self-assembled into ribbons constituted of fine fibrils (white arrow in Fig. 3A) across their width, while GlcB(OH)-3 revealed uneven giant multilamellar vesicles ranging from 0.3 to 1 μm in diameter (Fig. 3B) as already observed for phospholipids.31 For the latter, the matrix of the hydrogel seems to be formed by the closely packed structures of multilamellar vesicles as already reported in our recent publication.18 For GlcB(OH)-4, TEM showed several micrometer-long semi-rigid helical nanofibers with a uniform lateral diameter (17 ± 1 nm) close to the lateral arrangement of four GlcB(OH)-4 molecules (Fig. 3C). MalB(OH)-3 displayed a plate-like morphology of about hundred nanometers in diameter (Fig. 3D),32 while MalB(OH)-4 self-assembled into 5 ± 0.5 nm-wide and several micrometer-long tortuous wormlike cylinders consistent with the model of bilayer structures of interdigitated oleyl (C18:1(cis-9)) chains (Fig. 3E), which physically entangled into a network that forms the hydrogel matrix. These small wormlike aggregate dimensions confirmed our previous assumption related to the transparency of the hydrogel.
 | ||
| Fig. 3 TEM image of the nanostructures of hydrogels formed by GlcB(OH)-2 (A), GlcB(OH)-3 (B), GlcB(OH)-4 including a rectangle zoom (C), MalB(OH)-4 (D) and MalB(OH)-5 (E). | ||
If the control of noncovalent interactions with DNA, peptides or phospholipids is well mastered, the known difficulties in controlling the precise structures of carbohydrate-based amphiphiles can be attributed to the stereochemistry of carbohydrates that greatly impacts hydroxyl-based hydrogen bonding (carbohydrate–carbohydrate interactions or CCIs) along with steric effects. However, it is well-established that the self-assembly process is roughly governed by geometrical constraints impacting the surface curvature of individual uncharged amphiphile molecules (packing parameter),33 in addition to the attractive hydrophobic interactions between the lipophilic segments and repulsive steric interactions between the hydrophilic headgroups.34,35 The different morphologies observed between Glc-B(OH)-3vs. MalB(OH)-3 and GlcB(OH)-4vs.MalB(OH)-4 are undoubtedly attributed to the molecular geometric considerations and subsequent molecular packing, since Mal disaccharide possesses one additional bulky glucose unit compared to Glc monosaccharide. Moreover, from dissipative particle dynamics simulations with amphiphilic carbohydrate-based metallacycles, G. Chen and coll.36 revealed that a disaccharide displays stronger CCIs than a monosaccharide by four times, driving the self-assembly along the CCI direction. Therefore, besides the simple molecular geometric considerations, the nature of the carbohydrate headgroup also plays an important role in driving hierarchical self-assembly into diversiform glyco-nanostructures. Likewise, the morphologies drastically changed from stearyl-containing glycoamphiphiles (GlcB(OH)-3 or MalB(OH)-3) to their counterpart monounsaturated oleyl-containing glycoamphiphiles (GlcB(OH)-4 or MalB(OH)-4) only differing in the absence or presence of a cis double bond in the middle of the alkyl chain moiety. Once again, intermolecular packing plays a significant role because one can expect that oleyl chains, due to the higher chain mobility (Tm = 13 °C) than the stearyl chain (Tm = 69 °C) at RT, will form a tighter intermolecular packing promoting low mean-curvature aggregates such as fiber- or worm-like nanostructures.37 After analyzing individually all the morphologies from the series of GlcB(OH)-2 to 4, which vary slightly in two C–C bonds in the hydrophobic chain length, it seems difficult to find direct relationships with the molecular packing parameters. Above all, our results emphasize that HLB still plays a major role in driving their self-assembly behavior regardless of the discrete molecular architectures.
Carbohydrate-based hydrogelators have found many applications in the biomedical field. For instance, supramolecular glyco-hydrogels exhibited inhibition towards biofilm formation and bacterial growth of P. aeruginosa,38 served as biomimetic scaffolds for cell growth and adhesion,39,40 promoted wound healing,41 and selectively inhibited cancer cells.42 For the latter, Pashkuleva et al.43–45 originally proposed a simple N-Fmoc glucoamine-6-phosphate derivative as a precursor of self-assembly and hydrogelation triggered by the overexpressed alkaline phosphatase-catalyzed dephosphorylation and produced by an osteosarcoma cell line. This innovative supramolecular-based therapeutic approach stems from pioneering works of Bing Xu's group with peptide-based hydrogelator precursors and it is commonly called “Enzyme-Instructed Self-Assembly” or EISA.46,47
By carefully studying the gelation tests of our amphiphilic β-C-glycosylbarbiturates, we identified a potential precursor of hydrogelation that could be induced by the action of α-glucosidases. Indeed, while we demonstrated that MalB(OH)-2 is fully soluble in water, the monosaccharide analogue GlcB(OH)-2 formed an opaque hydrogel with a MGC of 2 wt%. So, we evaluated MalB(OH)-2 as a precursor to turn into a hydrogelator, GlcB(OH)-2, upon Aspergillus niger amyloglucosidase-catalyzed non-reducing D-glucosyl residue hydrolysis. We started our study using 3 wt% of MalB(OH)-2, which should result in 2.3 wt% of GlcB(OH)-2 if the hydrolysis is total, therefore slightly above the MGC. After overnight enzymatic hydrolysis at 45 °C, we were pleased to see that the clear solution turned into an opaque gel. TEM images revealed uniform self-assembled nanofibers (Fig. 4) that differ from the expected ribbons for GlcB(OH)-2 (Fig. 3A). In addition, if the weight concentration is divided by 5 to reach 0.6 wt% of MalB(OH)-2, the α-glucosidase-induced gelation of the resulting GlcB(OH)-2 occurred and at theoretically 0.46 wt%, which is much lower than the MGC. These results pointed out that nanostructure morphologies resulting from self-assembled hydrogelators are clearly dictated by the kinetics of supramolecular polymerization (production rate of the hydrogelator) and not only by the three-dimensional structure of hydrogelators.48 This work reports for the first time the use of α-glucosidase for a sol-to-gel phase transition while it was only used for a gel-to-sol phase transition.49
The in vivo preparation of self-assembled hydrogelators for selective inhibition of cancer cells by taking advantage of overexpressed endogenous enzymes could be envisioned for treating pancreatic cancer, a devastating gastrointestinal cancer, since a recent study demonstrated that an elevated level of lysosomal acid α-glucosidase was observed in response to gemcitabine treatment, an anticancer nucleoside.50
Conclusions
In conclusion, following our observation from a previous study that the sodium salt of glycosylbarbiturate hydrogelators was effective if the keto form of barbiturate is predominant, we successfully developed a green chemistry derivatization to stabilize the keto form through the H2O2-mediated oxidative hydroxylation of the barbiturate ring in water. Thus, we synthesized a series of hydroxylated glycosylbarbiturates and formed hydrogelators enabling the supramolecular gelation of water at neutral pH and without the need for strong acids or divalent cations such as for the sodium salt of glycosylbarbiturates. Glucose and maltose-based hydrogelators have been discovered resulting in various self-assembled glyco-nanostructures due to their different intermolecular interactions and packing parameters. And in the context of EISA, we showed that α-glucosidase could trigger the formation of supramolecular nanofibers to result in hydrogels even at a concentration below the minimal concentration of gelation. Indeed, a maltose-based precursor was hydrolyzed into a glucose-based hydrogelator with the use of Aspergillus niger amyloglucosidase. Since EISA found relevant applications in cancer therapy by selectively generating in situ self-assembled nanostructures or at the periphery of cancer cells, it can be anticipated that these C-glycosylbarbiturates have promising applications in medicine.Author contributions
S. Y., R. B. and A. B. synthesized and optimized the carbohydrate-based hydrogelators and interpretated the experimental data. J. C. brought his expertise and knowledge in hydrogels. S. H. proposed and supervised the project and wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from CNRS, Université Grenoble Alpes, LabEx ARCANE and CBH-EUR-GS (ANR-17-EURE-0003), the Glyco@Alps program (ANR-15-IDEX-02), Institut Carnot PolyNat (No. 16-CARN-025-01), the National Natural Science Foundation of China (51803227), the S&T Innovation 2025 Major Special Program of Ningbo (2019B10063), and CAS President's International Fellowship for Visiting Scientists (2019VBA0016). All authors acknowledge J. L. Putaux for his help in TEM images and the NanoBio-ICMG Platform (UAR 2607, Grenoble) for granting access to the electron microscopy, NMR and mass spectrometry facilities. S. Y. thanks the China Scholarship Council (CSC) for his scholarship support (201908320428).References
- B. L. Stocker and M. S. M. Timmer, ChemBioChem, 2013, 14, 1164–1184 CrossRef CAS PubMed.
- B. Kalisch, P. Doermann and G. Hoelzl, Subcell. Biochem., 2016, 86, 51–83 CAS.
- B. N. Paulino, M. G. Pessoa, M. C. R. Mano, G. Molina, I. A. Neri-Numa and G. M. Pastore, Appl. Microbiol. Biotechnol., 2016, 100, 10265–10293 CrossRef CAS PubMed.
- I. Mnif and D. Ghribi, J. Sci. Food Agric., 2016, 96, 4310–4320 Search PubMed.
- B. Gutsche and A. Behler, Handbook of Detergents, Part F: Production, ed. U. Zoller and P. Sosis, CRC Press, Boca Raton, FL, 2008, pp. 239–246 Search PubMed.
- A. Brito, S. Kassem, R. L. Reis, R. V. Ulijn, R. A. Pires and I. Pashkuleva, Chem, 2021, 7, 2943–2964 CAS.
- S. de Medeiros Modolon, I. Otsuka, S. Fort, E. Minatti, R. Borsali and S. Halila, Biomacromolecules, 2012, 13, 1129–1135 CrossRef CAS PubMed.
- H. Li, M. Mumtaz, T. Isono, T. Satoh, W.-C. Chen and R. Borsali, Polym. J., 2022, 54, 455–464 Search PubMed.
- K. Aissou, I. Otsuka, C. Rochas, S. Fort, S. Halila and R. Borsali, Langmuir, 2011, 27, 4098–4103 Search PubMed.
- J. D. Cushen, I. Otsuka, C. M. Bates, S. Halila, S. Fort, C. Rochas, J. A. Easley, E. L. Rausch, A. Thio, R. Borsali, C. Grant Willson and C. J. Ellison, ACS Nano, 2012, 6, 3424–3433 Search PubMed.
- Y. Sakai-Otsuka, S. Zaioncz, I. Otsuka, S. Halila, P. Rannou and R. Borsali, Macromolecules, 2017, 50, 3365–3376 CrossRef CAS.
- W. Wang, H. Wang, C. Ren, J. Wang, M. Tan, J. Shen, Z. Yang, P. G. Wang and L. Wang, Carbohydr. Res., 2011, 346, 1013–1017 CrossRef CAS PubMed.
- T. Tsuzuki, M. Kabumoto, H. Arakawa and M. Ikeda, Org. Biomol. Chem., 2017, 15, 4595–4600 RSC.
- M. J. Clemente, P. Romero, J. L. Serrano, J. Fitremann and L. Oriol, Chem. Mater., 2012, 24, 3847–3858 Search PubMed.
- J. Y. C. Lim, Q. Lin, K. Xue and X. J. Loh, Mater. Today Adv., 2019, 3, 100021 CrossRef.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed, 2008, 47, 8002–8018 Search PubMed.
- S. Datta and S. Bhattacharya, Chem. Soc. Rev., 2015, 44, 5596–5637 RSC.
- S. Yao, R. Brahmi, F. Portier, J.-L. Putaux, J. Chen and S. Halila, Chem. – Eur. J., 2021, 27, 16716–16721 CrossRef CAS PubMed.
- A. Faust, B. Waschkau, J. Waldeck, C. Hötlke, H.-J. Breyholz, S. Wagner, K. Kopka, W. Heindel, M. Scäfers and C. Bremer, Bioconjugate Chem., 2008, 19, 1001–1008 Search PubMed.
- G. Wulff and G. Clarkson, Carbohydr. Res., 1994, 257, 81–95 CrossRef CAS.
- F. Portier, A. Imberty and S. Halila, Bioconjugate Chem., 2019, 30, 647–656 CrossRef CAS PubMed.
- H. Böhme and H. P. Teltz, Arch. Pharm., 1955, 288, 349–352 CrossRef PubMed.
- A. O. Terent'ev, V. A. Vil, E. S. Gorlov, O. N. Rusina, A. A. Korlyukov, G. I. Nikishin and W. Adam, ChemistrySelect, 2017, 2, 3334–3341 CrossRef.
- W. Stadlbauer and T. Kappe, Monatsh. Chem., 1985, 116, 1005–1015 Search PubMed.
- M. Bueno Martinez, F. Zamora Mata, A. Munoz Ruiz and J. A. Galbis Perez, Carbohydr. Res., 1990, 199, 235–238 Search PubMed.
- M.-C. Frantz, S. Dropsit-Montovert, F. Pic, A. Prévot-Guéguiniat, C. Aracil, Y. Ding, M. Lima, F. Alvarez, S. Ramos, L. Mao, L. Lu, J. Xu, X. Marat and M. Dalko-Csiba, Org. Lett., 2019, 21, 2684–2687 CrossRef CAS PubMed.
- M. Dalko, A. Prevot-Gueguiniat, M.-C. Frantz and S. Dropsit, Pat, WO2020002076A1, 2020 Search PubMed.
- M. J. Clemente, R. M. Tejedor, P. Romero, J. Fitremann and L. Oriol, New J. Chem., 2015, 39, 4009–4019 Search PubMed.
- S. A. Holey, K. P. C. Sekhar, D. K. Swain, S. Bojja and R. R. Nayak, ACS Biomater. Sci. Eng., 2022, 8, 1103–1114 CrossRef CAS PubMed.
- T. Xiong, X. Li, Y. Zhou, Q. Song, R. Zhang, L. Lei and X. Li, Acta Biomater., 2018, 73, 275–284 CrossRef CAS PubMed.
- K. Tallo, M. Bossch, T. Pons, M. Cocera and O. Lopez, J. Mater. Chem. B, 2020, 8, 161–167 RSC.
- P. Chakraborty, B. Das, P. Pal, S. Datta, S. Bera and P. Dastidar, Chem. Commun., 2020, 56, 5251–5254 Search PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. J. Ninham, Chem. Soc., Faraday Trans., 1976, 2, 1525–1568 RSC.
- J. C. Stendahl, M. S. Rao, M. O. Guler and S. I. Stupp, Adv. Funct. Mater., 2006, 16, 499–508 Search PubMed.
- H. Jiang, M. O. Guler and S. I. Stupp, Soft Matter, 2007, 3, 454–462 RSC.
- G. Yang, W. Zheng, G. Tao, L. Wu, Q.-F. Zhou, Z. Kochovski, T. Ji, H. Chen, X. Li, Y. Lu, H.-M. Ding, H.-B. Yang, G. Chen and M. Jiang, ACS Nano, 2019, 13, 13474–13485 CrossRef CAS PubMed.
- N. Baccile, M. Selmane, P. Le Griel, S. Prévost, J. Perez, C. V. Stevens, E. Delbeke, S. Zibek, M. Guenther, W. Soetaert, I. N. A. Van Bogaert and S. Roelants, Langmuir, 2016, 32, 6343–6359 CrossRef CAS PubMed.
- S. Liu, H. Li, J. Zhang, X. Tian and X. Li, RSC Adv., 2020, 10, 33642–33650 RSC.
- J. Liu, Z. Sun, Y. Yuan, X. Tian, X. Liu, G. Duan, Y. Yang, L. Yuan, H.-C. Lin and X. Li, ACS Appl. Mater. Interfaces, 2016, 8, 6917–6924 Search PubMed.
- A. Chalard, L. Vaysse, P. Joseph, L. Malaquin, S. Souleille, B. Lonetti, J.-C. Sol, I. Loubinoux and J. Fitremann, ACS Appl. Mater. Interfaces, 2018, 10, 17004–17017 CrossRef CAS PubMed.
- Z. Yang, G. Liang, M. Ma, A. S. Abbah, W. W. Lu and B. Xu, Chem. Commun., 2007, 8, 843–845 Search PubMed.
- J. Gao, J. Zhan and Z. Yang, Adv. Mater., 2020, 32, 1805798 CrossRef CAS PubMed.
- R. A. Pires, Y. M. Abul-Haija, D. S. Costa, R. Novoa-Carballal, R. L. Reis, R. V. Ulijn and I. Pashkuleva, J. Am. Chem. Soc., 2015, 137, 576–579 CrossRef CAS PubMed.
- A. Brito, P. M. Pereira, R. L. Reis, R. V. Ulijn, J. S. Lewis, R. A. Pires and I. Pashkuleva, Nanoscale, 2020, 12, 19088–19092 RSC.
- A. Brito, P. M. Pereira, D. S. da Costa, R. L. Reis, R. V. Ulijn, J. S. Lewis, R. A. Pires and I. Pashkuleva, Chem. Sci., 2020, 11, 3737–3744 RSC.
- Z. Yang, H. Gu, D. Fu, P. Gao, J. Lam and B. Xu, Adv. Mater., 2004, 16, 1440–1444 Search PubMed.
- Z. M. Yang, K. M. Xu, Z. F. Guo, Z. H. Guo and B. Xu, Adv. Mater., 2007, 19, 3152–3156 CrossRef CAS.
- J. Baillet, A. Gaubert, J. Verget, L. Latxague and P. Barthélémy, Soft Matter, 2020, 16, 7648–7651 RSC.
- R. Yoshisaki, S. Kimura, M. Yokoya and M. Yamanaka, Chem. – Asian J., 2021, 16, 1937–1941 CrossRef CAS PubMed.
- R. Hamura, Y. Shirai, Y. Shimada, N. Saito, T. Taniai, T. Horiuchi, N. Takada, Y. Kanegae, T. Ikegami, T. Ohashi and K. Yanga, Cancer Sci., 2021, 112, 2335–2348 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc04180d |
| This journal is © The Royal Society of Chemistry 2023 |