Future bioenergy source by microalgae–bacteria consortia: a circular economy approach
Shir Reen
Chia†
 a,
Jing
Ling†
a,
Wen Yi
Chia
a,
Saifuddin
Nomanbhay
*b,
Tonni Agustiono
Kurniawan
c and
Kit Wayne
Chew
*a
a,
Jing
Ling†
a,
Wen Yi
Chia
a,
Saifuddin
Nomanbhay
*b,
Tonni Agustiono
Kurniawan
c and
Kit Wayne
Chew
*a
aSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459 Singapore. E-mail: kitwayne.chew@ntu.edu.sg; kitwayne.chew@gmail.com
bInstitute of Sustainable Energy, Universiti Tenaga Nasional (UNITEN), Jalan IKRAM-UNITEN, 43000 Kajang, Selangor Darul Ehsan, Malaysia. E-mail: saifuddin@uniten.edu.my; saifuddin1963@gmail.com
cCollege of the Environment and Ecology, Xiamen University, Xiamen, 361102, Fujian, PR China
First published on 20th October 2023
Abstract
The potentiality of microalgae–bacteria consortia applied in a circular economy is acknowledged and explored; however, the commercialization of biofuel from microbial technology remains complex and controversial regarding the practicability of the technology. This review provides a concise and comprehensive analysis of various extraction techniques of the microalgae-bacteria consortium, algae cultivation methods, and biofuel production processes. The conversion processes are critically reviewed along with the challenges faced to lay the foundation of in-depth microalgae–bacteria biotechnology. The yield of biohydrogen produced and the lipid content of the obtained biomass can be greatly improved through the cultivation of a microalgae–bacteria consortium compared to the pure culture of microalgae. The consumption of nutrients in wastewater by the microalgae–bacteria consortium protects the environment as the effluent returned to nature is of minimum toxicity. The symbiotic relationship between microalgae and bacteria has enhanced the lipid productivity of biomass in previous studies. In contrast to other recent reviews, the linkage of the circular economy with the microalgae–bacteria consortium was critically reviewed and discussed. Uncertainties under culturing conditions and techno-economics are the concerns and factors that impede the development of microalgae–bacteria consortia in energy commercialization.
Introduction
The increase in human population and world globalization leads to the high consumption of energy, which is estimated to increase by 50% or more by 2030.1 The extensive applications of energy for human activities will result in the depletion of non-renewable sources (fossil fuels) in the near future.2 Therefore, an alternative approach that utilizes renewable and environmentally friendly feedstocks to replace fossil fuels is essential. Moreover, the heavy usage of petroleum has caused air pollution worldwide. The scorching of fossil fuels emits many potentially hazardous pollutants, such as carbon monoxide and nitrogen oxides, in the air, that affect health and cause global warming. The production of bioenergy via symbiotic interaction between microalgae and bacteria is yet to be explored owing to the rapid growth rate of microalgae and the “give-and-take” of nutrients among microalgae and bacteria. In an ecosystem, heterotrophic microorganisms, mainly bacteria, are always found in the consortia with microalgae, taking O2 to oxidize organic substrates and produce CO2.3It has been reported that symbiotic interactivity between algae and bacteria appears favorable in biotechnology as their interaction influences physiology and metabolism. Intensive interaction between microalgae and bacteria can be observed in the removal of biodegradable organic matter in wastewater treatment ponds, as well as in the remediation of hazardous pollutants, such as phenolics, organic solvents, and polycyclic aromatic hydrocarbons.4 In the microalgae–bacteria consortium, microalgae can generate numerous organic substances that the bacteria assimilate and vice versa.5 The microalgae–bacteria consortium is likely a possible substitution of the current biofuel feedstock. The use of a microalgae–bacteria consortium is an excellent approach because the production of algae is ten-fold higher compared to those of higher plants and more biomass can be generated for biofuel production.6 It was reported in the study by Do et al. (2020) that higher biomass productivity was achieved in a semi-continuous mode for nutrient removal.7 This shows that the microalgae–bacteria consortium has the potential to enhance both the production of microalgae-based biofuel and biomass in the future.
This review paper aims to evaluate the fundamental aspects related to the condition and prospective of algal biomass as a feedstock for bioenergy production. Several extended and consecutive overviews related to the interaction between microalgae and bacteria for bioenergy and biofuel production were analyzed. In addition, investigations of several suitable technological methods for converting microalgae biomass into liquid fuel and gas using biochemical or thermochemical processes were also comprehensively studied.
Interactions between microalgae and bacteria
It is obvious that microalgae and bacteria live together and interact in complex microbial communities in their natural habitats or industrial processes. Most microalgae and bacteria form microecosystems in nature, and their growth is influenced by each other in various ways.8 Without their partnership, concerted activities performed by these microbial communities are impossible in many cases.9 Hence, the perception of bacteria as contaminants in microalgal culture has changed over the last few years, and the positive effects of interactions between microalgae and bacteria on growth and flocculation are promising for algal biotechnology. Despite several studies conducted on microalgal–bacterial partnerships in the last few decades, there has been a complete exploration of various kinds of interactions in the planktonic zone. This is most likely because of the difficulty of separating partners that are naturally bound to each other. Additionally, the interactions between microalgae and bacteria are extremely species-specific and sophisticated because each alga has a unique microenvironment.10Numerous studies have shown that heterotrophic bacteria have a favorable impact on microalgal development, biomass composition, cell aggregation and other associated activities.11,12 An increment in microalgae biomass productivity was observed owing to the prevention of exotic and pathogenic bacteria by growth-promoting bacteria from invading the microalgae culture.13 The whole range of symbiotic relationships is covered by algae–bacteria interactions, such as commensalism, mutualism, parasitism and competition.10 For commensalism, which only one partner benefits, heterotrophic bacteria deliver vitamin B12 for Chlamydomonas reinhardtii to use, but they do not take the organic carbon emitted by the algae.14 However, it has been reported that microalgae infrequently engage with bacteria via commensalism. More commonly, their interaction is rooted in mutualism.15 In the beginning, the interaction between microalgae and bacteria takes the form of mutualism, leading to mutual benefits. However, in a subsequent phase, an imbalance could arise as one of these partners outpaces the other in growth rate, resulting in one benefiting more through commensalism. The transition from mutualism to commensalism is triggered by shifts in growth conditions.16
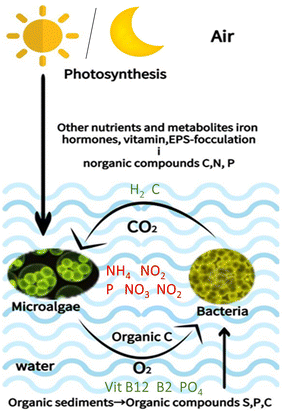
An example of mutualism where partners of different species profit from each other is that bacteria supply vitamins, nitrogen as well as organic and/or inorganic compounds to the algal partner, and in exchange, algae provide fixed organic carbon to the consortia. The mutualistic interaction of bacteria with microalgae is significant as the bacteria provide a conducive environment for microalgae to produce more carbon dioxide and eliminate excess oxygen. Moreover, bacteria supply vitamins (B12, B9, B7/H or B1), trace elements and inorganic nutrients as well as contribute to the chelators, growth factors, and phytohormones; for example, auxins and indole acetic acid that stimulate and promote microalgal growth.17 This kind of exchange between biotic communities plays an important role in carbon, sulfur, phosphorus (P) and nitrogen (N) cycling in the ecosystems.18 For instance, microalga generates cysteine, methionine, and dimethyl sulphoniopropionate (DMSP) when fixing carbon dioxide through photosynthesis.15Phaeobacter gallaeciensis can digest DMSP into volatile dimethylsulfide (DMS) and generate growth promoters as well as antibiotic compounds to protect microalgae from bacterial infections.19 Nitrogen-mediated interactions were observed between heterotrophic bacteria and microalgae because bacteria improved nitrogen assimilation in Dunaliella.20
Furthermore, parasitism is where only one species benefits and causes negative effects on another, and it happens when parasitic bacteria cause cell lysis of algae and use intracellular compounds as nutrients. In addition, a healthy kelp can regulate bacterial colonization on its surface and possess mechanisms that prevent bacterial overgrowth. Signal transduction, a type of algae–bacteria interaction, utilizes chemicals for activating/inhibiting the gene expression or physiological activity rather than for nutrient purposes, which alter their behaviour and growth. It has been reported that chemical signals are secreted by bacteria to induce morphogenesis in algae during their interactions.21,22 Furthermore, excessive biofilm formation on the macroalgae surface is inhibited by healthy macroalgae through the secretion of specific chemicals to prevent bacterial quorum sensing.23 Gene transfer is the horizontal transfer of genes between adjacent microorganisms, such as algae and bacteria, in phycospheres, which is also known as an evolutionary process. Some horizontally transferred genes allow algae to survive better in a changing environment, such as facilitation of the metabolic response of the diatom genes to the availability of episodic nitrogen, in which the diatom genes encode enzymes in the ornithine–urea cycle (from bacteria).24
Moreover, competition is present, as it pertains to one of the community relationships within the phycosphere where neither benefits. First, one group of organisms can lead to a decrease in another community by depleting nutrients from the environment, thereby causing nutrient deficiencies. With the proliferation of algae, they assimilate nutrients and vitamins from aquatic environments. If the available nutrients in the environment fail to meet the requirements of both algae and bacteria engaged in a mutualistic association, this gives rise to a competitive dynamic between them.25 For example, P serves as a crucial element for adenosine triphosphate (ATP) synthesis in cells, prompting competition between microalgae and bacteria. Bacteria can use phosphate more efficiently than microalgae when cultured in low-phosphate mediums. Conversely, under phosphorus-rich conditions, microalgae thrive more than bacteria. Similar competition occurs for N in water. However, bacterial growth is favoured in high N concentrations. Conversely, microalgae exhibit a faster growth rate than bacteria when N is limited. Another type of competition is that both microalgae and bacteria can suppress each other's growth and potentially even cause each other's demise through the release of metabolites. For instance, alginolytic bacteria, such as Myxobacteria, Pseudomonas, and Vibrio release cyclin, that are lethal to Microcystis microalgae.25,26 Specific extracellular substances generated by microalgae, such as soluble amino acids and antibiotics, can exert inhibitory or toxic effects on bacteria and pathogens.27 However, although algal and bacterial communities strive for consistent stability and abundance, the mutual suppression of one community by the other engenders a counter-inhibition pattern.
In brief, various microalgae–bacteria interactions can be controlled and utilized as a highly helpful tool. Mutualism, for instance, enhances consortium growth, sewage treatment effectiveness, and biomass output. The competitive interaction between these organisms is widely used to maintain a balanced community structure for wastewater treatment; for example, the utilization of alginolytic bacteria to manage freshwater blooms. The utilization of consortia also helps to tackle the situation where a single strain can be dead or inefficient when there is variation in conditions by providing organism strength against environmental fluctuations, enabling the stability of species, allowing the sharing of metabolites and preventing the invasion of undesirable species.8 Simultaneously, certain types of bacteria can stimulate algal cell aggregation and subsequently enhance the efficiency of microalgae biomass collection. Microalgal-related bacteria play a significant role in the flocculation of Chlorella vulgaris by increasing the floc size to aid in the settling process of microalgae.28 The integrated microalgae–bacteria culture is more efficient than the pure culture of microalgae and may yield a greater titer of microbial biomass.29
Cultivation of microalgae and bacteria consortia
The process of chemical exchange varies according to species because the microenvironment around each microalga is unique. It is also influenced by the environment.17 Microalgae include both autotrophic and heterotrophic species. Autotrophic microalgae harness sunlight for photosynthetic food production, offering potential use in wastewater treatment because of their oxygen supply to aerobic bacteria aiding in organic pollutant degradation.30 Their photosynthesis also boosts biomass productivity (although low) and nutrient recovery. Conversely, heterotrophic microalgae thrive in toxic wastewater and grow well in darkness, displaying robust tolerance to various environments and utilizing wastewater's organic content to produce valuable substances, thus serving dual purposes of wastewater treatment and product, which improves the effectiveness of wastewater treatment.31 The utilization of an advanced substrate feed control strategy by Jin et al. (2020) has enabled the achievement of ultrahigh-cell-density heterotrophic cultivation.32 Jin et al. (2021) successfully developed an efficient and industrially scalable technology for the production strain C. sorokiniana GT-1; under optimized culturing conditions, the substantial starch accumulation ability of C. sorokiniana GT-1 cells plays a crucial role in achieving ultrahigh-cell density under heterotrophic conditions.33The main interaction between photosynthetic microorganisms and heterotrophic organisms is the gas exchange cycle, which is the coexistence of microalgae with bacteria and is applicable in both open raceway systems and closed systems.3 The metabolite exchange occurs in a symbiotic microalgae–bacterial relationship based on the bacteria absorbing extracellular organic carbon produced by algae during photosynthesis, as shown in Fig. 1. In exchange, it can (i) promote bacterial growth to remove oxygen and create carbon dioxide; (ii) feed microalgae with nutrients, vitamins, and trace elements; and (iii) produce growth stimulating factors, chelating agents, and plant hormones.17,34
To begin the cultivation of microalgae–bacteria consortia, the isolates were characterized in artificial wastewater to screen potential strains that thrive in wastewater and treat wastewater efficiently. Parameters such as biomass titer, nutrient clearance percentage, and chemical oxygen demand (COD) of the strains were examined. The optimum microalgae–bacterial combination is chosen based on its performance in COD removal efficiency, nutrients, and total biomass titer (TBT). Finally, by tweaking the process parameters, the TBT of the optimum combination was further enhanced.35 The reduction of organic compounds through the synergistic interaction of microalgae and bacteria is even applied in microbial desalination cells to produce maximum powder density and save up to 1.24 kW h m−3 of power in the net energy output of study.36
Several pathways of the microalgae–bacteria consortium are listed below.
• Auto-phototrophic mechanisms: microalgae utilize solar energy directly and perform CO2 fixation for growth. Microalgae absorb CO2 in the sequence of carboxylation, reduction and regeneration, which is known as the Calvin cycle or Calvin–Benson–Bassham (CBB) cycle.37
• Heterotrophic metabolism: microalgae change their metabolic derivatives in response to changes in organic carbon sources. Heterotrophs derive their substrate and energy requirements from organic compounds synthesized by other organisms. The basic medium composition of heterotrophic culture is distinguished by the addition of organic carbon sources. Organic carbon sources include sugars, acetate and organic acids. This metabolism often enhances the culture density of biomass along with higher lipid and protein output bypassing the light constraint observed in auto-phototrophic metabolism.38
• Mixotrophic metabolism: light energy and inorganic carbon sources, such as CO2, are used as the main sources, supplemented by organic carbon sources. The advantage is to improve the culture density of microalgae and eliminate photoinhibition. However, this method requires a high cost of instruments and strict experimental conditions, making it difficult to be commercialized.39,40
It was found that more lipids accumulated in C. reinhardtii through co-cultivation with bacteria under nitrogen deficiency conditions.41 The co-culture led to a 2.4-fold increase in lipid content, a 5.9-fold increase in lipid synthesis, and a 19.4-fold increase in lipid productivity compared with axenic microalgae. This was due to an increase in the genetic expression that positively controls lipid metabolism, while the genetic expression that negatively regulates lipid metabolism decreased.42 In mixotrophic conditions, the co-culture of Chlorella minutissima with E. coli generates more starch (glucose, glycerol, and acetate substrates).43
Harvesting of algal biomass
Microalgae biomass usually comprises a high water content. Therefore, the removal of water content is essential during downstream processing. Several methods of harvesting microalgae by separating the microalgae biomass from water have been identified, such as bulk-harvesting methods comprising flocculation and floatation, natural gravity sedimentation, and thickening methods, including centrifugation and filtration.44 The bulk-harvesting method usually focuses on the separation of microalgae biomass from the suspension to retrieve a low percentage (2%–7%) of solid matter, while thickening is performed to concentrate the slurry.45 These methods are necessary to increase the biomass concentration and lessen the volume of biomass to be processed. Moreover, the most recent advanced method for harvesting cultured biomass in wastewater is ozone flotation by controlling ozone exposition. Exposure to ozone for more than 19 min resulted in less efficient collision and adhesion stages in microalgae-bubble complexes, thereby lowering the recovery of targeted biomolecules and microalgae biomass.46In general, flocculation and sedimentation are commercialized harvesting methods used to extract algae.44 According to Menegazzo and Fonseca (2019), the sedimentation method has high energy efficiency even though it is a comparatively slow process, while the density and size of microalgae cells regulate the sedimentation process associated with sedimentation velocity.47 Flocculation involves the use of chemicals to form the aggregation of the microalgae. According to Tan et al. (2018), the mechanism of flocculation begins when negatively charged microalgae cells repel each other, resulting in cell suspension.45 However, the addition of metal salts, such as ferric chloride, causes the charges to turn into a neutral state and cell aggregation occurs. Coagulants are categorized into organic and inorganic coagulants. The aluminium-based inorganic coagulants were used to harvest Chlorella sp. and Scenedesmus sp. when biodegradable organic coagulants, such as chitosan, were applied to enlarge the microalgae floc size (>100 μm) and enhance the settling of microalgae.48 It was reported that interparticle bridging and charge neutralization were attributed to the synergistic effect of using inorganic coagulants and chitosan for dual flocculation, leading to an improvement of 24 to 57% harvesting efficiency.49
The work on the harvesting efficiency of biofuel-producing algae can be intensified owing to particular types of bacteria that can facilitate the aggregation of algal cells.50 Bacteria produce large polysaccharides or proteins that promote microalgae aggregation because the ionisable functional groups on the algal cell surface deprotonate or protonate based on pH values, which triggers the surface charge.15 The flocculating activity increases with an increase in the extracellular polymeric substance content released by bacteria.51 Therefore, the use of bacteria in the aggregation of algal cells is an alternative approach to chemical flocculation in harvesting microalgae biomass.
Bacteria-based flocculation, a technique involving the simultaneous cultivation of microalgae and bacteria, is commonly referred to direct bio-flocculation. Although the mechanism varies across different microalgae–bacteria combinations, this approach appears to be more feasible in industrial-scale applications.52 The inherent presence of bacteria in wastewater substantially enhances both the efficacy of wastewater treatment and the effectiveness of microalgae harvesting; in particular, 92% efficiency of flocculation was achieved in the study of Nguyen et al. (2019).51 Enhancing harvest efficiency is notably achieved through the prominent mechanisms of the inherent bacterial structure, intercellular communication, and the formation of extracellular polymeric substances (EPS) released by bacteria.52 For example, Vu et al. (2019) found that axenic Ettlia sp. flocculation was facilitated by small, dust-like EPS particles operating through a patching mechanism, and a long filamentous EPS structure created by the bacterial community additionally enhanced flocculation efficiency through bridging, ultimately bolstering aggregate integrity by maintaining a compact EPS structure.53 A study conducted by Li et al. (2018) demonstrated that the bacterium HSN08, a Micrococcus genus member, can effectively induce the flocculation of C. vulgaris biomass through direct contact, with a pivotal role attributed to cell wall amino acids, highlighting potential applications for algal biomass harvesting.54 Furthermore, Li et al. (2019) reported that the bacterial cells of Bacillus sp. y3 and y6 exhibited flocculation activity on C. vulgaris and the microalgal growth was enhanced for those cultivated in the recycled BG11 media after harvest of microalgae biomass by these strains.55 Overall, the introduction of bacteria frequently necessitates supplementary nutrients, making wastewater an optimal medium for both cost-effective harvesting and the establishment of symbiotic bacterial populations.
However, the method of cell immobilization involves immobilizing microalgae and bacteria on specific supports, such as agar polyacrylamide, carrageenan, and calcium alginate, at defined ratios to capitalize on bacterial synergy, boosting biomass yield per unit area while simplifying microalgae harvesting; this immobilized algae–bacteria system not only enhances biomass harvesting but also augments wastewater treatment efficiency through the microalgae–bacteria consortium system.56 Moreover, sewage and wastewater treatment using attached growth systems relies on the development of algal–bacterial biofilms, with a straightforward harvesting method involving biomass scraping from the systems and solar energy-assisted drying.57 Despite their cost-effectiveness, these systems have not yet been widely applied on a large scale.10
Co-harvesting and separating microalgae and bacteria are required for the extraction of valuable components, such as high-value protein amino acids, which can be further processed into animal feed, baits, and soil amendments, thereby maximizing economic benefits. However, the potential risk of bacterial contamination was alleviated by utilizing the final biomass for bioenergy production.52
Pre-treatment
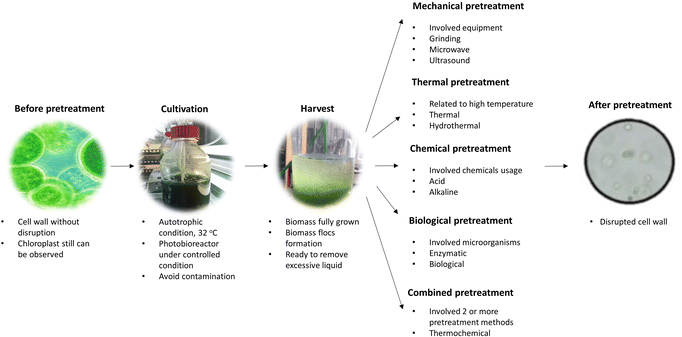
The cell wall of algal cells comprises tri-layered frameworks of hemicellulose, cellulose, algaenan and glycoproteins, making the process of extracting lipid or producing biodiesel in large-scale difficult.58–60 Cell disruption is particularly crucial for releasing more lipids before lipid extraction to maximize the obtained yield. In addition to mechanical, physical, and chemical activities, enzymatic processes can also be applied for cell disruption.61,62 Scientists have discovered that the enzymes generated by biomass-degrading bacteria can deteriorate and destroy the algae cell walls and their inner components, thereby promoting the release of lipids. The conventional method for pretreating algae biomass is shown in Fig. 2.Several algicidal bacteria are yet to be identified; however, those identified are lytic to phytoplankton. These algicidal bacteria generally follow coccolithophores, Phaeocystis spp., algal blooms of diatoms, as well as quite often poisonous dinoflagellates and raphidoflagellates.63 Although these bacteria may interact with freely floating algae, it is likely that active algicidal metabolite excretion would only be a viable technique at high cell densities. The majority of known algicidal bacteria are Alphaproteo bacteria, Bacteroidetes or Gammaproteo bacteria.64,65 Preliminary research suggests that particle-associated bacteria have a lower host specificity and a higher hit rate than free-living bacteria, but comprehensive screening is currently lacking.66 This is consistent with the finding that these microbial interactions are based on nutrient shuttling between algae and bacteria. For the synthesis of algicides, high nutrient availability is required to be present in the phycosphere. Algicides are frequently released at high cell densities under optimized culture conditions in laboratory circumstances.67,68
Microalgae–bacteria consortia in the conversion process and biofuel production
Biodiesel has been proposed as a potential replacement for fossil fuels owing to its high oxygen ratio and good combustion properties.69,70 Corn, rapeseed, soybeans, sunflower, jatropha and oil palm are the most common sources of traditional biodiesel and are cultivated alongside food crops. Consequently, their long-term significance as a source of global energy is limited.71–73 Microalgal biomass is a viable feedstock for the synthesis of lipids and elevated compounds, such as pharmaceuticals and cosmetics, with the leftover biomass being utilized for bioenergy production via anaerobic digestion or hydrothermal liquefaction to bio-crude oil.74,75After concentrating the biomass, thermochemical liquefaction, solvent-aided methods or ultrasonic techniques were applied for the oil extraction. The conversion of microalgae to biofuels was done through a biochemical or thermochemical conversion process. Biogas, bioethanol and biodiesel are generated through biochemical conversion, whereas syngas and bio-oil are produced through thermochemical conversion.76 A specific strain of microalgae produces specific types of biofuels. For example, Schizochytrium sp. and Haematococcus pluvialis, known as lipid producers, are suitable strains used to produce biodiesel.77 Similar reports have also demonstrated that Spirogyra sp. yielded a higher biomass (after oil extraction) compared to Oedogonium sp.78 The carbohydrate and protein analyses might be useful in determining which type of microalgae consortia can be utilized for different biofuel productions. For example, in anaerobic dark fermentation, carbohydrate concentration is exactly proportional to hydrogen production.79 Moreover, the protein and lipid content of microalgae might be directly converted to methane and biodiesel; new research has shown that lipid-extracted biomass can be a viable feedstock for increasing methane production.80
Microalgal biodiesel
Transesterification is the process of biodiesel production from microalgal biomass. It is estimated that the conversion of triglycerides or oil to biodiesel is up to 98% as a substitute fuel for diesel engines.81 This process occurs when triglycerides react with mono-alcohol in the presence of acidic-, alkaline- or enzymes-based catalyst to produce a mixture of fatty acid methyl ester (FAME) and glycerol.82 Tan et al. (2018) reported that microalgae and vegetable oils are incompatible for direct usage in diesel engines owing to their high viscosity compared to diesel and gasoline.45 Hence, the conversion of vegetable and microalgae oil to FAME is necessary through a transesterification reaction.Chia et al. (2018) found that some conditions can be applied to enhance feedstock conversion or to make the alcohol phase more miscible.83 From an economic perspective, wet algal biomass is suggested to be used directly for transesterification to eliminate dewatering costs. One of the factors known to induce high lipid content is strain isolation.84 However, researchers have also analyzed the use of Chlorella vulgaris in the transesterification process, where the lipids were heated to 48 °C before reacting with the methanol and the catalyst, NaOH. Hence, FAME and glycerol contents were 20 times greater than the initial lipid weights.
The interaction of algae and bacteria has the potential to enhance the production of biodiesel. Yao et al. (2019) reported that a total increase from 2- to 6-fold was observed in the growth of biomass and doubled the natural lipid content compared to the axenic growth observed in the symbiotic relationship between Auxenochlorella protothecoids and E. coli.4 The total lipid content of biomass grown with various leachate spike ratios was in the range of 14.5–20.8%, which was lower than the highest value of 30% reported on Chlorella species in a previous work.85 The bacteria element in the microalgae–bacteria consortium could be one of the primary reasons negatively affecting lipid accumulation in algal cells, thereby limiting the total lipid content of the biomass.86
However, size and cost are primary industrialization bottlenecks, and there is a fundamental conflict between oil content and growth rate that must be addressed to obtain high oil output, which is also the most significant technological obstacle faced in microalgae and bacterial bio-oil production.87,88 The procedure for improvement is as follows: (1) obtain highly efficient engineered algae by genetic engineering, (2) efficient and low-cost cultivation of microalgae on a large scale, (3) recycling waste, and (4) biological refining. The cultivation of microalgae–bacteria consortia using wastewater can recycle the waste and minimize the overall cost of cultivation, where significant lipid yield can be obtained from the studies listed in Table 1.
| Type of algae | Type of bacteria | Culture media | Biomass concentration | Lipid content | Lipid yield | Ref. |
|---|---|---|---|---|---|---|
| Chlorella pyrenoidosa | Ammonia-oxidizing strain FN5 (Kluyvera genus) | Municipal wastewater | 0.35 g L−1 | 39% | 0.14 g L−1 | 91 |
| Scenedesmus sp. | Azospirillum brasilense | N-deficient media | 103 mg L−1 | Total fatty acid: 51.4% | — | 92 |
| Algae consortium | Rhodobacter sphaeroides, Rhodococcus | Treated dairy effluent | 2.3 g L−1 | 42% | 501 mg g−1 | 93 |
| Chlorella sp. MA1, Coelastrella sp. KE4 | Advenella sp., Arcobacter sp., Bacillus sp., Staphylococcus sp. | Swine wastewater | Chlorella sp.: 6.27 g L−1, Coelastrella sp.: 7.63 g L−1 | Chlorella sp.: 10.63%, Coelastrella sp.: 16.23% | — | 94 |
| Chlorella sorokiniana | Streptomyces thermocarboxydus | Cassava wastewater | 2.11 g L−1 under sterilized wastewater | 54.11–61.52% saturated fatty acids | — | 95 |
| Chlorella sp. GZQ001 | Lysinibacillus sp. SJX05 | Biogas slurry | 113.3 mg L−1 d−1 | — | 19.2 mg L−1 d−1 | 96 |
| FAME: 3.7 mg L−1 d−1 | ||||||
| Scenedesmus obliquus | Bacillus megaterium | Biogas slurry | 50 mg L−1 d−1 | 22.06–30.32% | 12.27–15.32 mg L−1 d−1 | 97 |
Biohydrogen
Biohydrogen, which is derived from carbohydrate content in biomass through bio-photolysis or dark fermentation reaction, has attracted significant attention because it is a type of renewable energy, and there is the release of a large amount of energy per unit weight in combustion without the emergence of CO2. Hence, the conversion of biohydrogen to electricity by fuel cells can be done effortlessly.1Some photosynthetic microorganisms, such as C. reindhardtii, have developed the ability to utilize light energy to emit hydrogen gas produced from water.47 This is the mechanism of bio-photolysis. Behera et al. (2019) stated that microalgal biomass produced from Anabaena sp. and Scenedesmus obliquus could be utilized directly as a substrate for dark fermentation to produce biohydrogen.89 According to Hankamer et al. (2007), certain types of green algae and cyanobacteria have developed the ability to exploit solar energy for the extraction of protons and electrons from water through the water-splitting reaction of PSll.90 Afterwards, these protons and electrons are recombined by a chloroplast hydrogenase to form molecular hydrogen. Moreover, these researchers explained the use of H2 as one of the biofuels in the US, the European Union and Japan, as these countries have already started constructing H2 fuel stations and developing H2 fuel cell-powered cars.
The types of microalgae–bacteria consortium and cultivation media used for the hydrogen production are listed in Table 2 along with the hydrogen yield obtained. Differences in hydrogen yield were observed using different types of bacteria, and similar types of microalgae and culture medium were utilized in the study by Fakhimi & Tavakoli, 2019.98 With mixed cultures of bacteria,99,100 optimized condition allows the hydrogen yield to be obtained up to 4700 mL L−1 for the first study and around 150 mL H2 L−1 d−1 for the second study. According to Yao et al. (2019), it was found that some species of bacterial symbionts, Brevundimonas sp., Leifsonia sp., and Rhodococcus sp. improve the bio-hydrogen production of the microalgae, Chlamydomonas.4 The bacterial respiration performed by bacterial symbionts in eliminating oxygen is of utmost importance to activating the Fe-dependent hydrogenase in Chlamydomonas.4 It has also been reported that the synergistic cooperation of the microalgae–bacteria consortium is advantageous in converting solar energy into electricity in microalgal fuel cells (MFCs) consisting of G. sulfurreducens and photo-grown microalgae cells.50
| Type of algae | Type of bacteria | Culture medium | Hydrogen yield | Remarks | Ref. |
|---|---|---|---|---|---|
| Chlorella vulgaris | Activated sludge bacteria | Z-medium | 1246 mL L−1 gas volume | Hydrogen obtained with least 57 mL O2 per L during 6 days of incubation | 106 |
| Chlorella vulgaris MACC360 | Sludge from beer brewing factory (with reduced methanogenic Archaea) | Non-diluted dark fermentation effluent | 154 mL H2 per L per d | Condition: 100% effluent concentration with 5% microbial and 10% Chlorella inoculum | 100 |
| Chlamydomonas reinhardtii | Escherichia coli | Tris-acetate-phosphate | 24% | Produce hydrogen at low light intensity | 98 |
| Pseudomonas stutzeri | 46% | ||||
| Pseudomonas putida | 32% | ||||
| Mineral-deprived Chlorella sp. | Mixed cultures of Rhodobacter sp. and Rhodopseudomonas palustris | Hydrogen evolving cocktails | 4700 mL L−1 | Culture medium: 10% phosphate buffer, 10% early log phase bacteria containing algal supernatant | 99 |
| Chlamydomonas sp. and Scenedesmus sp. | Escherichia coli (a pleiotropic hydrogenase mutant strain) | Tris-acetate-phosphate | 1.52 mL H2 L−1 | Reduced O2 level from 21% to 4% in 2 h with addition of E. coli and acetate | 107 |
| Chlamydomonas reinhardtii | Methylobacterium oryzae | Minimal mineral medium without nitrogen source supplemented with NH4Cl, potassium acetate, ethanol and methanol | 33 mL L−1 | Biomass generation: 1.22 g L−1 d−1 | 108 |
| Inhibitory occurs if the medium is only supplemented with ethanol | |||||
| Chlamydomonas reinhardtii | Pseudomonas putida | Tris-acetate-phosphate | 40.8 mL H2 L−1 | Enhanced capacity to prolong the hypoxia phase (favoring the H2 production phase) | 109 |
| Chlamydomonas reinhardtii | Escherichia coli | Tris-acetate-phosphate | 35.1 mL H2 L−1 | 60% more H2 production when co-cultures supplemented with glucose | 109 |
The produced biohydrogen has to be handled carefully owing to its inflammability. It can be stored via geological storage, which is stored under compressed or liquefied conditions using large and highly pressurized containers, and material-based storage. Geological storage is one of the cost-effective options in which biohydrogen is restricted to salt caverns.101 A company that stores biohydrogen using such a technique is Mitsubishi Power, where the salt caverns are constructed deep underground with a diameter of 67 m and a height of 580 m.102 In the case of storing in pressurized containers, biohydrogen has to be compressed or liquefied or can be both compressed and liquefied (to achieve a significant reduction in hydrogen volume after compression). However, the storage cost for storing in containers is high due to the complex process, in which the gas has to be cooled to −253 °C and maintained at such a low temperature for liquefied biohydrogen. For material-based storage, several types of elements, such as palladium, aluminium, and magnesium, are suggested for their potentiality as they can react with or absorb biohydrogen. Ammonia is proposed as a carrier because of its convenience in storing and transporting, with the additional advantage of its higher energy density compared to liquefied biohydrogen.102 However, the development of ammonia as a biohydrogen carrier is still at an early stage and requires deeper study to improve its conversion rate.
The drawback of using microalgal biomass as feedstock is that microalgae contain a considerable amount of protein, which is less conducive to hydrogen production than carbohydrates. An ammonia buildup can occur in the fermentation system owing to protein-rich substrates, which can be harmful to hydrogen-producing bacteria. Another issue is the decomposition efficiency influenced by insufficient carbon, which varies from the ideal C/N ratio.103,104 These issues can be solved by co-digesting microalgal biomass with other substrates and optimizing the C/N ratio of the substrates.105
Bioelectricity
Microalgal–bacterial bioelectricity
New developments in MFCs have opened up numerous possibilities for generating bioelectricity through microbial metabolism. MFCs are devices that create clean bioelectricity through a biocatalytic process performed by electrochemically active microorganisms, such as bacteria or yeast.110 Most oxygen generating bioactive microalgae can be used in the place of bacteria-assisted MFCs, while the photosynthetic performed by microalgae offers electrons to produce current on the anode of the MFCs, and the O2 produced acts as a long-term electron acceptor on the cathode. Microalgae-based MFCs have shown encouraging outcomes in terms of electricity generation and energy consumption compared to bacteria.111MFCs can convert the chemical energy held in the organic matter of biomass into electrical energy. MFCs commonly comprise a cathode acting as the positive pole, an anode acting as the negative pole, and an electrolyte; an ion exchange membrane may be included to separate the anodic and cathodic compartments. The oxidation of organic substrates is carried out by the microorganisms in liquid or those adhered to as biofilm. End products, such as protons, electrons, and other metabolites, are produced in the anodic chamber. A range of organic substrates, such as sugar acetate, alcohols, organic compounds and wastewaters, are required by MFCs in their most basic form.112 Protons pass via a separator/ion exchange membrane to react with electrons and subsequently reduce O2 into water. The electrons produced in the oxidation process flow through an external circuit for current generation.113
MFC is a cutting-edge technology known for its compatibility with low-cost substrates and high efficiency without external energy application under low-temperature conditions.114,115 The environmental toxicity of MFC, the final electron acceptor's availability at the cathode and the cost of electrodes are the challenges to be addressed. These challenges could be tackled using bio-electrolytes, in which an oxygenator organism is involved in the biocathode chamber to act as the final electron acceptor and aid in reducing the costs.111 Many bacteria and yeast species can degrade biomass at anodic MFCs, including microalgae. Microalgae consist of proteins, carbohydrates, and lipids.116 These components are believed to be high-energy compounds that can release sufficient electrons to generate bioelectricity in MFCs. The treated algal biomass has previously been shown to be suitable for bioelectricity output.117 Microalgal-assisted MFCs rely on autotrophic microalgae that utilize photosynthesis for the conversion of solar energy to electricity. Microalgae can be applied either as an electron acceptor at the cathode or as an electron generator at the anode by consuming the O2 produced from photosynthesis.111,118
Photosynthetic microbial fuel cells (PMFC)
An implanted algal-assisted photosynthesis microbial fuel cell (PMFC) comprise an anode and cathode, where the first is inoculated with bacteria consortium and the latter is microalgae. Both chambers are separated by an ion exchange membrane. The cathode analyte is the culture media for microalgae, and the electrons are produced in the anode chamber by organic matter generated from the replacement sample water of microorganisms. O2 is generated in a PMFC by photosynthesis performed by Chlorella sp., and the electrons are transferred via an external circuit to produce bioelectricity.119 The results showed that Chlorella sp. grew well when it was grown in an MFC cathode chamber.Several parameters have been investigated owing to their effects on PMFC performance, biomass yield, and power generation. One of the parameters is the type of electron donor utilized in the PMFC. It was reported that the application of sodium acetate as an electron donor allowed higher maximum power and voltage output than glucose as an electron donor, with a total amount of 1.8 g L−1 biomass obtained for the PMFC using sodium acetate.120 As the distribution and characteristics of microbiota are closely related to moisture, an appropriate operating temperature may significantly increase the survival of electrogenic bacteria and affect their dynamic and thermomechanical properties. A power density of 2572.8 mW m−3 was reported at a temperature of 25 °C, which agrees with the statement of Muñoz and Guieysse regarding the increased activities of microalgae within temperatures ranging from 25 °C to 30 °C.121,122 Light is also an important environmental factor in photosynthesis. Various studies have shown that increasing light intensity subsequently increases the PMFC power, which promotes photosynthetic activity and oxygen production for the PMFC cathodic reaction. Hence, a higher voltage output is obtained.119
In general, bacteria can survive only within a particular pH range. Microorganisms are inhibited if their growth, reproduction, and metabolism are performed beyond that particular pH range, while the properties of energy generation are better in an acidic environment. However, the value of PMC anode fluid changes throughout the development, reproduction, and metabolism of microorganisms owing to the breakdown of organic matter and the creation, and accumulation of products and intermediates in the anode liquid.
Roles of the microalgae–bacteria consortium in the circular economy
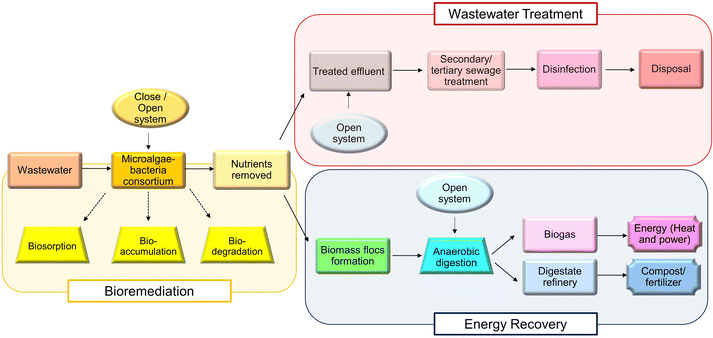
The current climate condition, pollution severity, and resource depletion have alerted the world to design a sustainable system to overcome the aforementioned issues, and the circular economy is the designed closed system to restore and stop further damage to the biotic and abiotic components of ecosystems. Circular economy is noticed as a solution owing to its principles, which eliminate waste and pollution, circulate products and materials (at their highest value) and regenerate nature. The circular economy aims to minimize the usage of raw materials, eliminate the application of toxic chemicals, replace them with renewable materials, and terminate waste generation. Microalgae–bacteria consortia play an important role in the circular economy through the consumption of nutrients, biomass as the feedstock of bioenergy production and the remaining can be utilized as fertilizers, as shown in Fig. 3.In the concept of the integration of wastewater treatment and energy production, wastewater is treated without additives and extra steps (e.g. adsorption, ion exchange or membrane filtration) for heavy metal removal in a low-cost and eco-friendly method. The wastewater treatment performed using the microalgae–bacteria consortium is biosorption. The bacteria aid in the settling efficiency of biomass to facilitate the convenience in harvesting the biomass, while the nutrient uptake for the growth of microalgae–bacteria consortium removes the “pollutants”, such as P, nitrate and trace metals, and serves as wastewater treatment while growing the biomass for energy production. The mechanism of biosorption involves a passive process that is independent of energy sources and is predominantly performed by microalgal cells. It occurs through interactions, including molecular forces, electrostatic forces and covalent bonding.123 Another mechanism involved is bioaccumulation, which begins with biosorption on the surface of the microalgal cell wall. It is driven by energy from the microalgae and enzymatic reaction to stimulate bioaccumulation, biosedimentation and biotransformation in the cell bodies.124 Open systems, such as a high-rate algal pond (HRAP) or raceway pond, can be utilized for wastewater treatment, and a settling tank can be included for sediment removal. The high efficiency of productivity and harvesting was enhanced using a combination culture system. A study by Rodrigues et al. (2020) reported that the hybrid system combining the closed system (biofilm reactor) for algal biomass production and the open system (HRAP) for effluent treatment has a higher efficiency in harvesting biomass, and the biomass productivity is 2.6 times better than the conventional system.125 An additional advantage is the activity of bacteria towards the degradation of antibiotics, and other pollutants can be enhanced and improved through the symbiotic relationship.124 Treated effluent can be released back into the environment without harming living things and even used for irrigation, while biomass flocs are subjected to anaerobic digestion for biogas production. It is noteworthy that the flocs/solid sludges are not removed from the integration system, but they are used as raw materials for biogas production, and the digestate can finally be used as compost or fertilizer. Using digestate as fertilizer is safer than using synthetic fertilizer because the digestate possesses less toxicity and is natural in nature. The generated energy can be used to power the plant that grow the microalgae–bacteria consortium as well. A study has reported that the use of immobilized microalgae-activated sludge bacterial symbiosis integrated with ozone pretreatment allows enhanced biodegradable efficiency and good phosphorus removal efficiency.126 Biomass flocs can be utilized for lipid extraction to convert the extracted lipid into biodiesel or subjected to biomolecule extraction for valuable components, such as natural pigments or nutraceuticals.127 By minimizing the pollutants released into the environment using the microalgae–bacteria consortium, green nature is believed to be restored with proper handling of waste and effective production of renewable energy aids to overcome the depletion of energy sources.
The concept of a circular economy is an ideal case for treating wastewater and generating bioenergy simultaneously, with the aforementioned advantages. However, the development of technology is immature or not established compared to conventional techniques, which may require a higher cost for the overall process. In addition, the economic analysis is significantly varied depending on the cultivation condition, the type of consortium involved, harvesting expenditure, cultivation mode, transportation, etc. It is reported by Chia et al. (2021) that the production costs of microalgae biomass, bio-oil, bio-crude oil, green diesel, hydrocarbon and methane vary depending on the cultivation systems used and subsequently lead to different yields obtained in the approach of circular economy.128 The production cost considers capital cost and operational cost, in which the harvesting cost is reported to be around 20–40% of the total cost.129 The high contribution of harvesting costs is a major challenge due to the dewatering process of biomass.
The capital cost for a closed system in obtaining only microalgae biomass ranges from $94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 to $1675k per year, while it costs around $1.2M to $3001M for open systems.128 From the cost reviewed, the low capital cost of the closed system does not lead to a low price of production cost, but a higher production cost is observed owing to the low productivity capacity. Factors affecting cost, including the location, cultivation system, the areal productivity of the biomass and the production capacity. However, the incorporation of wastewater into the system might lower the price of algal biomass. In the analysis conducted by Feng et al. (2011), the production cost of biomass can be deducted to around $231 if wastewater treatment accounts for credit.129,130 The utilization of wastewater may reduce the usage of fresh water and the consumption of commercial fertilizers, which leads to reduced overall cultivation costs. With the aid of bacteria, the settling efficiency of biomass is enhanced, allowing for the efficient recovery of biomass. For products such as bio-oil, hydrocarbons or green diesel, further cost is required after harvesting the biomass to extract the desired components from the harvested biomass. The cost is subjected to the type of extraction technique; hence, further study is required to estimate the overall cost. The production cost for bio-oil is $1.75 L−1 for microalgae cultivated in a raceway pond (open system) and $1.85 per gallon for a multi-layer photobioreactor (closed system).131,132
356 to $1675k per year, while it costs around $1.2M to $3001M for open systems.128 From the cost reviewed, the low capital cost of the closed system does not lead to a low price of production cost, but a higher production cost is observed owing to the low productivity capacity. Factors affecting cost, including the location, cultivation system, the areal productivity of the biomass and the production capacity. However, the incorporation of wastewater into the system might lower the price of algal biomass. In the analysis conducted by Feng et al. (2011), the production cost of biomass can be deducted to around $231 if wastewater treatment accounts for credit.129,130 The utilization of wastewater may reduce the usage of fresh water and the consumption of commercial fertilizers, which leads to reduced overall cultivation costs. With the aid of bacteria, the settling efficiency of biomass is enhanced, allowing for the efficient recovery of biomass. For products such as bio-oil, hydrocarbons or green diesel, further cost is required after harvesting the biomass to extract the desired components from the harvested biomass. The cost is subjected to the type of extraction technique; hence, further study is required to estimate the overall cost. The production cost for bio-oil is $1.75 L−1 for microalgae cultivated in a raceway pond (open system) and $1.85 per gallon for a multi-layer photobioreactor (closed system).131,132
Challenges and future prospects
Microalgae-bacterial biotechnology is one of the components of modern biotechnology, and its main research object is single-celled microalgae.133,134 Since the large-scale production of Chlorella in Japan in the 1960s, microalgal–bacterial biotechnology has reached its initial scale and has shown great potential through development in recent decades.135 In the context of rising fossil energy prices and global warming caused by excessive greenhouse gas emissions, research on microalgal and bacterial biotechnology has attracted great attention around the world.136There are several enduring areas of limited understanding concerning algal–bacterial wastewater treatment. The basic work of the microalgae biotechnology industry is to obtain high-quality algae species. However, basic biological research on microalgae is the prerequisite for obtaining high-quality algae species. It is estimated that there are hundreds of thousands or more microalgal species around the world, but only 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of them have been discovered and documented.137,138 In addition, the basic biological research of known microalgae species is very backward, and effective technical means to improve the quality of microalgae are still very scarce.139 Although Chlamydomonas rheiniscens has been used as a model species for photosynthesis studies globally, and the whole genome has been sequenced, there is little in-depth research on the species that can be used for microalgal biotechnology development.140,141 The foremost concern involves managing microbial communities, which presents a significant obstacle to the widespread implementation of microalgae–bacteria consortia systems for large-scale wastewater treatment. The interactions between microalgae and bacteria are intricate, with limited molecular-level insights currently available. Enhanced comprehension of these interactions could equip engineers with novel means of manipulating these communities effectively. Future research also needs to transcend mere application-oriented approaches and delve into elucidating aspects, such as metabolite exchange, enzymatic reactions, biochemical pathways, and gene expression.
000 of them have been discovered and documented.137,138 In addition, the basic biological research of known microalgae species is very backward, and effective technical means to improve the quality of microalgae are still very scarce.139 Although Chlamydomonas rheiniscens has been used as a model species for photosynthesis studies globally, and the whole genome has been sequenced, there is little in-depth research on the species that can be used for microalgal biotechnology development.140,141 The foremost concern involves managing microbial communities, which presents a significant obstacle to the widespread implementation of microalgae–bacteria consortia systems for large-scale wastewater treatment. The interactions between microalgae and bacteria are intricate, with limited molecular-level insights currently available. Enhanced comprehension of these interactions could equip engineers with novel means of manipulating these communities effectively. Future research also needs to transcend mere application-oriented approaches and delve into elucidating aspects, such as metabolite exchange, enzymatic reactions, biochemical pathways, and gene expression.
Even though numerous published studies on specific applications of algae–bacteria consortia for biomass generation and/or wastewater treatment exist, the available information about microalgae–bacteria consortia in wastewater contexts remains rudimentary and limited primarily owing to the intricate interplay of biological and non-biological factors within wastewater environments. Limited comprehensive knowledge is available regarding how microalgae and bacteria react to intricate variations in conditions as well as to the microbial communities naturally present in authentic wastewater environments, especially in scenarios where algae are cultured for biofuel generation alongside nutrient recovery.142 Additionally, the complexities of operational parameters, including factors such as inhibition or light constraints, pH, temperature, microalgae–bacteria interactions, hydraulic retention time (HRT), and dissolved oxygen levels, often exhibit significant deviations from controlled laboratory settings. Therefore, conducting pilot studies is recommended to examine the complexities of contaminants under multifaceted environmental conditions.
The engineering technology foundation of the microalgal–bacterial biological industry is weak. Compared with the industrial microbial fermentation culture, the technical facilities of the microalgal–bacterial industrial culture are not mature. At present, the main facility for the large-scale culture of microalgae and bacteria is the racetrack culture tank.134,143 The disadvantages of a racetrack culture tank are its low yield per unit area and an open culture environment, causing the microalgal bacteria culture to be easily contaminated by other organisms (such as rotifers) during cultivation. One effective strategy to improve yield is to ensure that there is a minimal possibility of biomass sedimentation by reducing the presence of dead zones and the residence time of the biomass in different sections of the reactor. The implementation of strategies such as baffle partition design, adjusting rotational velocities of mixing devices, and maintaining an ideal flow regime can lead to improved reactor performance and higher yields.144
In general, the incorporation of organic substrates and the need for sterilization to counteract resource competition from other microorganisms in wastewater pose a constraint in adopting heterotrophic cultures for wastewater treatment and biomass generation, with the associated expenses rendering the process economically unfeasible.145 Glucose has been introduced as an organic carbon source in most heterotrophic cultures documented in the existing literature. For example, cultivating C. vulgaris in wastewater supplemented with glucose and sodium acetate (NaAc) resulted in high biomass, lipid, and carbohydrate productivities, surpassing those achieved under photoautotrophic conditions. The higher biomass productivity also correlated with improved nutrient removal in glucose and NaAc-enriched wastewaters. Interestingly, unlike glucose and NaAc, protein in wastewater did not notably impact C. vulgaris growth and nutrient removal.146 Glucose can be derived from cost-effective sources, such as lignocellulosic biomass, which serves as a secondary feedstock for biorefineries. Other cost-effective alternatives involve utilizing industrial waste products and waste fluids, including diverse wastewater forms, as nutrient sources to replace traditional nutrient media, thus enabling economical and environmentally sound support for algae growth.147 Microalgal–bacterial consortia offer an alternative approach by employing non-sterile microalgae heterotrophic processes to treat wastewater with high concentrations of organic matter, leading to reduced operational demands such as illuminated cultivation areas compared to phototrophic cultures reliant on light as well as the requirement for an external organic substrate.145
Despite several benefits associated with suspended microalgal–bacterial consortia, challenges persist because of the complexities involved in effectively separating and harvesting dispersed microalgal biomass to achieve satisfactory effluent quality. Moreover, the low biomass concentration of microalgae in the culture medium and the small individuals require complex biochemical engineering operations to enrich and harvest the microalgae biomass, subsequently increasing the production cost of microalgae biomass.148 The absence of such an effective harvesting solution constitutes a significant obstacle to the industrial adoption of algal-based wastewater treatments. In current practices, the harvesting process is simplified and involves an initial flocculation step succeeded by filtration through methods such as membrane, ultrafiltration, centrifugal sedimentation, or gravity settling.149 Although certain researchers have attempted to overcome this constraint through the implementation of diverse biomass retention methods (such as biomass immobilization and membrane bioreactors), there remains a need for further research in this area. It has been suggested to prioritize the advancement of biofilm-based cultivation techniques, aiming to immobilize microalgae and bacteria onto specific solid substrates, thereby reducing the potential for contamination risks.150
The microalgal–bacterial biotechnology industry has advantages in the development of high-value-added products, but it lacks competitiveness in the areas of low-value-added food, feed and agriculture.151,152 Microalgal–bacterial biotechnology is mainly oriented to the fields of energy, environment, food and medical health. In the field of energy, microalgae and bacteria are expected to become the feedstock of the third-generation biofuel after bioethanol of food crops, cellulosic bioethanol and biodiesel of land crops.153,154 From the environmental perspective, microalgal–bacterial has the potential to significantly reduce greenhouse gas emissions and has broad application prospects in treating domestic and industrial sewage.155–157 Moreover, the production of bioplastics is possible using carbohydrate and protein-based polymers from microalgae that can further reduce the generation of synthetic plastics and lighten the environmental burden.158 In the food industry, microalgal–bacterial has the potential to provide many food additives, such as monocellular proteins, vegetable oils, carotenoids and omega-3 long-chain unsaturated fatty acids.159,160 In the field of medicine and health, the search for new antibiotics, anticancer and antiviral drugs from microalgae–bacterial biological resources is often reported.161
Conclusions
Microalgae are still the focus of ongoing research arguments because they are economically viable. The accessibility and feasibility of microalgae are the key attributes that are much preferred compared to other types of feedstock in biofuel production. The microalgae–bacteria consortium is complex but beneficial to their growth and biotechnological outcomes. Microalgae–bacterial flora can serve as a powerful biological system that survives under varied cultivation settings and nutrient availability owing to their various metabolic activities and tolerance to harsh environmental conditions. Increasing the demand for microalgae in industrial applications will be the key factor in developing the integration of bacteria, particularly microalgal production processes. Numerous studies are needed to explore the potential applications of microalgae, and further investigations of high-value-added products must be performed for higher revenues. As a suggestion for further studies, the integration of microalgae cultivation systems and wastewater treatment should be performed to minimize the water and carbon footprint as well as enhance the financial income of biomass production for biofuel production and wastewater treatment.Author contributions
SR Chia: writing – original draft preparation, writing – review & editing, visualization; J Ling: writing – original draft preparation, writing – review & editing, visualization; WY Chia: writing – review & editing, visualization; S Nomanbhay: supervision, project administration; TA Kurniawan: conceptualization, writing – review & editing; KW Chew: conceptualization, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Tenaga Nasional Berhad (TNB) and UNITEN through the BOLD Refresh Publication Fund under the project code of J510050002-IC-6, BOLDREFRESH2025-Centre of Excellence. Also acknowledged AAIBE Chair of Renewable Energy (ChRE) – UNITEN under project code [202006KETTHA] for postdoctoral researcher and publication funding. The authors would like to thank Nanyang Technological University (Singapore) for the facilities and resources provided for the completion of this work.References
- E. S. Shuba and D. Kifle, Renewable Sustainable Energy Rev., 2018, 81, 743–755 CrossRef CAS.
- W.-H. Leong, J.-W. Lim, M.-K. Lam, Y. Uemura and Y.-C. Ho, Renewable Sustainable Energy Rev., 2018, 91, 950–961 CrossRef CAS.
- G. Zuccaro, J.-P. Steyer and R. van Lis, Bioresour. Technol., 2019, 273, 608–617 CrossRef CAS PubMed.
- S. Yao, S. Lyu, Y. An, J. Lu, C. Gjermansen and A. Schramm, J. Appl. Microbiol., 2019, 126, 359–368 CrossRef CAS PubMed.
- H. Jia and Q. Yuan, Cogent Environ. Sci., 2016, 2, 1275089 CrossRef.
- D. Hernández, B. Riaño, M. Coca and M. García-González, Bioresour. Technol., 2013, 135, 598–603 CrossRef PubMed.
- T. C. V. Do, T. N. T. Nguyen, D. T. Tran, T. G. Le and V. T. Nguyen, Environ. Technol. Innovation, 2020, 20, 101172 CrossRef CAS.
- S. R. Subashchandrabose, B. Ramakrishnan, M. Megharaj, K. Venkateswarlu and R. Naidu, Biotechnol. Adv., 2011, 29, 896–907 CrossRef CAS PubMed.
- T. J. Donohue and R. J. Cogdell, Nat. Rev. Microbiol., 2006, 4, 800–800 CrossRef CAS PubMed.
- R. Ramanan, B.-H. Kim, D.-H. Cho, H.-M. Oh and H.-S. Kim, Biotechnol. Adv., 2016, 34, 14–29 CrossRef CAS PubMed.
- T. J. Samo, J. A. Kimbrel, D. J. Nilson, J. Pett-Ridge, P. K. Weber and X. Mayali, Environ. Microbiol., 2018, 20, 4385–4400 CrossRef CAS PubMed.
- R. Zhao, G. Chen, L. Liu, W. Zhang, Y. Sun, B. Li and G. Wang, Environ. Pollut., 2020, 259, 113924 CrossRef CAS PubMed.
- J. Park, B. S. Park, P. Wang, S. K. Patidar, J. H. Kim, S.-H. Kim and M.-S. Han, Front. Plant Sci., 2017, 8, 289 Search PubMed.
- E. Kazamia, H. Czesnick, T. T. V. Nguyen, M. T. Croft, E. Sherwood, S. Sasso, S. J. Hodson, M. J. Warren and A. G. Smith, Environ. Microbiol., 2012, 14, 1466–1476 CrossRef CAS PubMed.
- J. L. Fuentes, I. Garbayo, M. Cuaresma, Z. Montero, M. González-del-Valle and C. Vílchez, Mar. Drugs, 2016, 14, 100 CrossRef PubMed.
- N. Rashid, W.-K. Park and T. Selvaratnam, Chemosphere, 2018, 194, 67–75 CrossRef CAS PubMed.
- H. Wang, R. T. Hill, T. Zheng, X. Hu and B. Wang, Crit. Rev. Biotechnol., 2016, 36, 341–352 CrossRef CAS PubMed.
- D.-H. Cho, R. Ramanan, J. Heo, Z. Kang, B.-H. Kim, C.-Y. Ahn, H.-M. Oh and H.-S. Kim, Bioresour. Technol., 2015, 191, 481–487 CrossRef CAS PubMed.
- M. R. Seyedsayamdost, G. Carr, R. Kolter and J. Clardy, J. Am. Chem. Soc., 2011, 133, 18343–18349 CrossRef CAS PubMed.
- M. Le Chevanton, M. Garnier, G. Bougaran, N. Schreiber, E. Lukomska, J. B. Bérard, E. Fouilland, O. Bernard and J. P. Cadoret, Algal Res., 2013, 2, 212–222 CrossRef.
- D. T. Hughes and V. Sperandio, Nat. Rev. Microbiol., 2008, 6, 111–120 CrossRef CAS PubMed.
- Y. Matsuo, H. Imagawa, M. Nishizawa and Y. Shizuri, Science, 2005, 307, 1598–1598 CrossRef CAS PubMed.
- B. Jha, K. Kavita, J. Westphal, A. Hartmann and P. Schmitt-Kopplin, Mar. Drugs, 2013, 11, 253–265 CrossRef PubMed.
- A. E. Allen, C. L. Dupont, M. Oborník, A. Horák, A. Nunes-Nesi, J. P. McCrow, H. Zheng, D. A. Johnson, H. Hu and A. R. Fernie, Nature, 2011, 473, 203–207 CrossRef CAS PubMed.
- K. Yang, Q. Chen, D. Zhang, H. Zhang, X. Lei, Z. Chen, Y. Li, Y. Hong, X. Ma, W. Zheng, Y. Tian, T. Zheng and H. Xu, Sci. Rep., 2017, 7, 7750 CrossRef PubMed.
- L. Qixin, F. Xuan, S. Zhiya, S. Wenxin, W. Shuo and L. Ji, Bioresour. Technol., 2022, 354, 127161 CrossRef PubMed.
- R. Mu, Y. Jia, G. Ma, L. Liu, K. Hao, F. Qi and Y. Shao, Water Environ. Res., 2021, 93, 1217–1230 CrossRef CAS PubMed.
- J. Lee, D.-H. Cho, R. Ramanan, B.-H. Kim, H.-M. Oh and H.-S. Kim, Bioresour. Technol., 2013, 131, 195–201 CrossRef CAS PubMed.
- R. J. Powell and R. T. Hill, Appl. Environ. Microbiol., 2014, 80, 4042–4050 CrossRef PubMed.
- C. González-Fernández, B. Molinuevo-Salces and M. C. García-González, Bioresour. Technol., 2011, 102, 960–966 CrossRef PubMed.
- F. G. A. Fernández, A. Reis, R. H. Wijffels, M. Barbosa, V. Verdelho and B. Llamas, New Biotechnol., 2021, 61, 99–107 CrossRef PubMed.
- H. Jin, H. Zhang, Z. Zhou, K. Li, G. Hou, Q. Xu, W. Chuai, C. Zhang, D. Han and Q. Hu, Biotechnol. Bioeng., 2020, 117, 96–108 CrossRef CAS PubMed.
- H. Jin, W. Chuai, K. Li, G. Hou, M. Wu, J. Chen, H. Wang, J. Jia, D. Han and Q. Hu, Biotechnol. Bioeng., 2021, 118, 4138–4151 CrossRef CAS PubMed.
- T. Sayara, S. Khayat, J. Saleh, N. Abu-Khalaf and P. van der Steen, Environ. Technol. Innovation, 2021, 23, 101548 CrossRef CAS.
- B. B. Makut, D. Das and G. Goswami, Algal Res., 2019, 37, 228–239 CrossRef.
- V. R. V. Ashwaniy, M. Perumalsamy and S. Pandian, Environ. Technol. Innovation, 2020, 19, 100926 CrossRef.
- J. O. Ighalo, K. Dulta, S. B. Kurniawan, F. O. Omoarukhe, U. Ewuzie, S. O. Eshiemogie, A. U. Ojo and S. R. S. Abdullah, CLCE, 2022, 3, 100044 Search PubMed.
- S. Venkata Mohan, M. V. Rohit, P. Chiranjeevi, R. Chandra and B. Navaneeth, Bioresour. Technol., 2015, 184, 169–178 CrossRef CAS PubMed.
- T. Roach, A. Sedoud and A. Krieger-Liszkay, Biochim. Biophys. Acta, Bioenerg., 2013, 1827, 1183–1190 CrossRef CAS PubMed.
- R. Ganesh Saratale, V. K. Ponnusamy, R. B. Jeyakumar, R. Sirohi, G. Piechota, S. Shobana, J. Dharmaraja, C. H. Lay, G. Dattatraya Saratale, H. Seung Shin and V. Ashokkumar, Bioresour. Technol., 2022, 361, 127691 CrossRef CAS PubMed.
- L. Xu, X. Cheng and Q. Wang, Front. Plant Sci., 2018, 9, 741 CrossRef PubMed.
- L. A. Leyva, Y. Bashan and L. E. de-Bashan, Ann. Microbiol., 2015, 65, 339–349 CrossRef CAS.
- B. T. Higgins and J. S. VanderGheynst, PLoS One, 2014, 9, e96807 CrossRef PubMed.
- D. Sarkar and K. Shimizu, Bioresour. Bioprocess., 2015, 2, 17 CrossRef.
- X. B. Tan, M. K. Lam, Y. Uemura, J. W. Lim, C. Y. Wong and K. T. Lee, Chin. J. Chem. Eng., 2018, 26, 17–30 CrossRef CAS.
- E. V. Hernández, I. Monje-Ramírez, S. B. Velásquez-Orta, J. Gracia-Fadrique and M. T. Orta Ledesma, Environ. Technol. Innovation, 2022, 26, 102354 CrossRef.
- M. L. Menegazzo and G. G. Fonseca, Renewable Sustainable Energy Rev., 2019, 107, 87–107 CrossRef CAS.
- C.-Y. Chen, K.-L. Yeh, R. Aisyah, D.-J. Lee and J.-S. Chang, Bioresour. Technol., 2011, 102, 71–81 CrossRef CAS PubMed.
- H. P. Vu, L. N. Nguyen, G. Lesage and L. D. Nghiem, Environ. Technol. Innovation, 2020, 17, 100622 CrossRef CAS.
- A. Kouzuma and K. Watanabe, Curr. Opin. Biotechnol., 2015, 33, 125–129 CrossRef CAS PubMed.
- T. D. P. Nguyen, T. V. A. Le, P. L. Show, T. T. Nguyen, M. H. Tran, T. N. T. Tran and S. Y. Lee, Bioresour. Technol., 2019, 272, 34–39 CrossRef CAS PubMed.
- C. Zhang, S. Li and S.-H. Ho, Bioresour. Technol., 2021, 342, 126056 CrossRef CAS PubMed.
- C. H. T. Vu, S.-J. Chun, S.-H. Seo, Y. Cui, C.-Y. Ahn and H.-M. Oh, Bioresour. Technol., 2019, 281, 56–65 CrossRef CAS PubMed.
- Y. Li, Y. Xu, T. Zheng and H. Wang, Bioresour. Technol., 2018, 249, 417–424 CrossRef CAS PubMed.
- Y. Li, Z. Zhang, Y. Duan and H. Wang, Bioresour. Technol., 2019, 280, 188–198 CrossRef CAS PubMed.
- M. Lu, H. Zhang, Y. Tian, W. Yao, J. Wang and Y. Wang, J. Hazard. Mater., 2023, 132364 CrossRef CAS PubMed.
- S.-H. Lee, H.-M. Oh, B.-H. Jo, S.-A. Lee, S.-Y. Shin, H.-S. Kim, S.-H. Lee and C.-Y. Ahn, J. Microbiol. Biotechnol., 2014, 24, 1566–1573 CrossRef CAS PubMed.
- B. Allard and J. Templier, Phytochemistry, 2000, 54, 369–380 CrossRef CAS PubMed.
- G. Ciudad, O. Rubilar, L. Azócar, C. Toro, M. Cea, Á. Torres, A. Ribera and R. Navia, J. Biosci. Bioeng., 2014, 117, 75–80 CrossRef CAS PubMed.
- Z. A. Popper, G. Michel, C. Hervé, D. S. Domozych, W. G. Willats, M. G. Tuohy, B. Kloareg and D. B. Stengel, Annu. Rev. Plant Biol., 2011, 62, 567–590 CrossRef CAS PubMed.
- J.-Y. Lee, C. Yoo, S.-Y. Jun, C.-Y. Ahn and H.-M. Oh, Bioresour. Technol., 2010, 101, S75–S77 CrossRef CAS PubMed.
- A. K. Lee, D. M. Lewis and P. J. Ashman, Biomass Bioenergy, 2012, 46, 89–101 CrossRef CAS.
- M. Demuez, C. González-Fernández and M. Ballesteros, Biotechnol. Adv., 2015, 33, 1615–1625 CrossRef CAS PubMed.
- F. Goecke, V. Thiel, J. Wiese, A. Labes and J. F. Imhoff, Phycologia, 2013, 52, 14–24 CrossRef CAS.
- D. L. Kirchman, FEMS Microbiol. Ecol., 2002, 39, 91–100 CAS.
- J.-H. Park, I. Yoshinaga, T. Nishikawa and I. Imai, Aquat. Microb. Ecol., 2010, 60, 151–161 CrossRef.
- X. Mayali and G. J. Doucette, Harmful Algae, 2002, 1, 277–293 CrossRef.
- P. B. Roth, M. J. Twiner, C. M. Mikulski, A. B. Barnhorst and G. J. Doucette, Harmful Algae, 2008, 7, 682–691 CrossRef.
- J. Hill, E. Nelson, D. Tilman, S. Polasky and D. Tiffany, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11206–11210 CrossRef CAS PubMed.
- D. Qi, H. Chen, L. Geng and Y. Z. Bian, Energy Convers. Manage., 2010, 51, 2985–2992 CrossRef CAS.
- A. Demirbas, Appl. Energy, 2011, 88, 3541–3547 CrossRef CAS.
- C. S. Jones and S. P. Mayfield, Curr. Opin. Biotechnol., 2012, 23, 346–351 CrossRef CAS PubMed.
- S. Singh and D. Singh, Renewable Sustainable Energy Rev., 2010, 14, 200–216 CrossRef CAS.
- M. Šoštarič, D. Klinar, M. Bricelj, J. Golob, M. Berovič and B. Likozar, New Biotechnol., 2012, 29, 325–331 CrossRef PubMed.
- Y. Zhou, L. Schideman, G. Yu and Y. Zhang, Energy Environ. Sci., 2013, 6, 3765–3779 RSC.
- Y. Guo, T. Yeh, W. Song, D. Xu and S. Wang, Renewable Sustainable Energy Rev., 2015, 48, 776–790 CrossRef CAS.
- K. Dutta, A. Daverey and J.-G. Lin, Renewable Energy, 2014, 69, 114–122 CrossRef CAS.
- S. Khan, R. Siddique, W. Sajjad, G. Nabi, K. M. Hayat, P. Duan and L. Yao, HAYATI J. Biosci., 2017, 24, 163–167 CrossRef.
- G. Kumar, P. Sivagurunathan, N. B. D. Thi, G. Zhen, T. Kobayashi, S.-H. Kim and K. Xu, Int. J. Hydrogen Energy, 2016, 41, 21628–21640 CrossRef CAS.
- G. Cea-Barcia, G. Buitrón, G. Moreno and G. Kumar, Bioresour. Technol., 2014, 163, 370–373 CrossRef CAS PubMed.
- S. Amin, Energy Convers. Manage., 2009, 50, 1834–1840 CrossRef CAS.
- J. Milano, H. C. Ong, H. H. Masjuki, W. T. Chong, M. K. Lam, P. K. Loh and V. Vellayan, Renewable Sustainable Energy Rev., 2016, 58, 180–197 CrossRef.
- S. R. Chia, H. C. Ong, K. W. Chew, P. L. Show, S.-M. Phang, T. C. Ling, D. Nagarajan, D.-J. Lee and J.-S. Chang, Renewable Energy, 2018, 129, 838–852 CrossRef CAS.
- O. M. Adeniyi, U. Azimov and A. Burluka, Renewable Sustainable Energy Rev., 2018, 90, 316–335 CrossRef.
- Z.-Y. Liu, G.-C. Wang and B.-C. Zhou, Bioresour. Technol., 2008, 99, 4717–4722 CrossRef CAS PubMed.
- Y. Zhang, H. Su, Y. Zhong, C. Zhang, Z. Shen, W. Sang, G. Yan and X. Zhou, Water Res., 2012, 46, 5509–5516 CrossRef CAS PubMed.
- R. Craggs, Pond treatment technology, 2005, p. 282–310 Search PubMed.
- W. J. Oswald, Algae biomass: production and use, [sponsored by the National Council for Research and Development, Israel and the Gesellschaft fur Strahlen-und Umweltforschung (GSF), Munich, Germany], ed. G. Shelef and C. J. Soeder, 1980 Search PubMed.
- B. Behera, A. Acharya, I. A. Gargey, N. Aly and B. P, Bioresour. Technol. Rep., 2019, 5, 297–316 CrossRef.
- B. Hankamer, F. Lehr, J. Rupprecht, J. H. Mussgnug, C. Posten and O. Kruse, Physiol. Plant., 2007, 131, 10–21 CrossRef CAS PubMed.
- X. Zhou, W. Jin, Q. Wang, S. Guo, R. Tu, S.-f. Han, C. Chen, G. Xie, F. Qu and Q. Wang, Renewable Energy, 2020, 151, 598–603 CrossRef CAS.
- J. R. Contreras-Angulo, T. M. Mata, S. P. Cuellar-Bermudez, N. S. Caetano, R. Chandra, J. S. Garcia-Perez, K. Muylaert and R. Parra-Saldivar, Sustainability, 2019, 11, 707 CrossRef CAS.
- T. Biswas, S. Bhushan, S. K. Prajapati and S. Ray Chaudhuri, J. Environ. Manage., 2021, 286, 112196 CrossRef CAS PubMed.
- W. Qu, C. Zhang, X. Chen and S.-H. Ho, J. Hazard. Mater., 2021, 418, 126264 CrossRef CAS PubMed.
- M. Padri, N. Boontian, N. Teaumroong, P. Piromyou and C. Piasai, Bioresour. Technol., 2022, 347, 126732 CrossRef CAS PubMed.
- H. Yan, R. Lu, Y. Liu, X. Cui, Y. Wang, Z. Yu, R. Ruan and Q. Zhang, Bioresour. Technol., 2022, 354, 127187 CrossRef CAS PubMed.
- D. Li, R. Liu, X. Cui, M. He, S. Zheng, W. Du, M. Gao and C. Wang, J. Water Process Eng., 2021, 41, 102014 CrossRef.
- N. Fakhimi and O. Tavakoli, Mater. Sci. Energy Technol., 2019, 2, 1–7 Search PubMed.
- H. A. A. Abdel-Kader, R. Abdel-Basset and A. W. Danial, Int. J. Hydrogen Energy, 2022, 47, 1516–1528 CrossRef CAS.
- P. Shetty, I. Z. Boboescu, B. Pap, R. Wirth, K. L. Kovács, T. Bíró, Z. Futó, R. A. White and G. Maróti, Front. Energy Res., 2019, 7, 52 CrossRef.
- K. Alms, B. Ahrens, M. Graf and M. Nehler, Front. Energy Res., 2023, 11, 1172003 CrossRef.
- A. Willige, 4 ways of storing hydrogen from renewable energy, https://spectra.mhi.com/4-ways-of-storing-hydrogen-from-renewable-energy, (accessed 16 August 2023, 2023).
- A. Xia, A. Jacob, M. R. Tabassum, C. Herrmann and J. D. Murphy, Bioresour. Technol., 2016, 205, 118–125 CrossRef CAS PubMed.
- J. Xu, T. Upcraft, Q. Tang, M. Guo, Z. Huang, M. Zhao and W. Ruan, Energy Fuels, 2019, 33, 1279–1289 CrossRef CAS.
- A. Salakkam, S. Sittijunda, C. Mamimin, O. Phanduang and A. Reungsang, Bioresour. Technol., 2021, 322, 124533 CrossRef CAS PubMed.
- M. A. Javed, A. M. Zafar and A. Aly Hassan, Algal Res., 2022, 63, 102649 CrossRef.
- R. Wirth, G. Lakatos, G. Maróti, Z. Bagi, J. Minárovics, K. Nagy, É. Kondorosi, G. Rákhely and K. L. Kovács, Biotechnol. Biofuels, 2015, 8, 59 CrossRef PubMed.
- M. J. Torres, D. González-Ballester, A. Gómez-Osuna, A. Galván, E. Fernández and A. Dubini, Bioresour. Technol., 2022, 352, 127088 CrossRef CAS PubMed.
- N. Fakhimi, A. Dubini, O. Tavakoli and D. González-Ballester, Bioresour. Technol., 2019, 289, 121648 CrossRef CAS PubMed.
- N. S. Malvankar and D. R. Lovley, ChemSusChem, 2012, 5, 1039–1046 CrossRef CAS PubMed.
- C. N. Reddy, R. Kakarla and B. Min, in Microbial Electrochemical Technology, Elsevier, 2019, pp. 525–547 Search PubMed.
- J. M. Moradian, Z. A. Xu, Y. T. Shi, Z. Fang and Y. C. Yong, Int. J. Energy Res., 2020, 44, 325–333 CrossRef CAS.
- B. E. Logan and J. M. Regan, Trends Microbiol., 2006, 14, 512–518 CrossRef CAS PubMed.
- F. Hernández-Fernández, A. P. De Los Ríos, M. Salar-García, V. Ortiz-Martínez, L. Lozano-Blanco, C. Godínez, F. Tomás-Alonso and J. Quesada-Medina, Fuel Process. Technol., 2015, 138, 284–297 CrossRef.
- V. S. Sarathi and K. S. Nahm, Biosens. Bioelectron., 2013, 43, 461–475 CrossRef PubMed.
- S. B. Velasquez-Orta, T. P. Curtis and B. E. Logan, Biotechnol. Bioeng., 2009, 103, 1068–1076 CrossRef CAS PubMed.
- B. E. Logan, Microbial fuel cells, John Wiley & Sons, 2008 Search PubMed.
- J. Greenman, I. Gajda and I. Ieropoulos, Sustainable Energy Fuels, 2019, 3, 2546–2560 RSC.
- L. Gouveia, C. Neves, D. Sebastião, B. P. Nobre and C. T. Matos, Bioresour. Technol., 2014, 154, 171–177 CrossRef CAS PubMed.
- L. Chenghong, Y. Zhimin, T. Hailong, Y. Jing and H. Yan, Chin. J. Environ. Eng., 2015, 9, 5109–5112 Search PubMed.
- H. He, M. Zhou, J. Yang, Y. Hu and Y. Zhao, Bioprocess Biosyst. Eng., 2014, 37, 873–880 CrossRef CAS PubMed.
- R. Muñoz and B. Guieysse, Water Res., 2006, 40, 2799–2815 CrossRef PubMed.
- S. Kanamarlapudi, V. K. Chintalpudi and S. Muddada, in Biosorption, ed. D. Jan and V. Branislav, IntechOpen, Rijeka, 2018, Ch. 4, DOI:10.5772/intechopen.77315.
- S. S. Chan, K. S. Khoo, K. W. Chew, T. C. Ling and P. L. Show, Bioresour. Technol., 2022, 344, 126159 CrossRef CAS PubMed.
- L. Rodrigues de Assis, M. L. Calijuri, P. P. Assemany, T. A. Silva and J. S. Teixeira, J. Environ. Manage., 2020, 274, 111183 CrossRef CAS PubMed.
- M. Han, C. Zhang and S.-H. Ho, Environ. Sci. Ecotechnology, 2023, 14, 100227 CrossRef CAS PubMed.
- M. Manzoor, R. Ma, H. A. Shakir, F. Tabssum and J. I. Qazi, Punjab Univ. J. Zool., 2016, 31, 307–320 Search PubMed.
- S. R. Chia, K. W. Chew, H. Y. Leong, S.-H. Ho, H. S. H. Munawaroh and P. L. Show, Chem. Eng. J., 2021, 425, 131436 CrossRef CAS.
- E. J. Olguín, Biotechnol. Adv., 2012, 30, 1031–1046 CrossRef PubMed.
- Y. Feng, C. Li and D. Zhang, Bioresour. Technol., 2011, 102, 101–105 CrossRef CAS PubMed.
- J. Ankita and S. M. Ganti, AIMS Energy, 2017, 5, 239–257 Search PubMed.
- C. Xin, M. M. Addy, J. Zhao, Y. Cheng, Y. Ma, S. Liu, D. Mu, Y. Liu, P. Chen and R. Ruan, Bioresour. Technol., 2018, 250, 523–531 CrossRef CAS PubMed.
- E. W. Becker, Microalgae: biotechnology and microbiology, Cambridge University Press, 1994 Search PubMed.
- M. A. Borowitzka, J. Biotechnol., 1999, 70, 313–321 CrossRef CAS.
- A. Richmond, Handbook of microalgal culture: biotechnology and applied phycology, John Wiley & Sons, 2008 Search PubMed.
- A. L. Haag, Nature, 2007, 447, 520–521 CrossRef CAS PubMed.
- F. Metting, J. Ind. Microbiol., 1996, 17, 477–489 CAS.
- T. A. Norton, M. Melkonian and R. A. Andersen, Phycologia, 1996, 35, 308–326 CrossRef.
- R. Radakovits, R. E. Jinkerson, A. Darzins and M. C. Posewitz, Eukaryotic Cell, 2010, 9, 486–501 CrossRef CAS PubMed.
- A. R. Grossman, Plant Physiol., 2005, 137, 410–427 CrossRef CAS PubMed.
- M. S. Parker, T. Mock and E. V. Armbrust, Annu. Rev. Genet., 2008, 42, 619–645 CrossRef CAS PubMed.
- L. Jiang, Y. Li and H. Pei, Renewable Sustainable Energy Rev., 2021, 149, 111395 CrossRef CAS.
- O. Pulz, Appl. Microbiol. Biotechnol., 2001, 57, 287–293 CrossRef CAS PubMed.
- C. Inostroza, A. Solimeno, J. García, J. M. Fernández-Sevilla and F. G. Acién, Algal Res., 2021, 54, 102207 CrossRef.
- D. M. San Agustin, M. T. O. Ledesma, I. M. Ramírez, I. Y. Noguez, V. M. L. Pabello and S. B. Velasquez-Orta, Renewable Energy, 2022, 181, 592–603 CrossRef.
- Y. Y. Peng, F. Gao, W. J. W. Hang, H. L. Yang, W. H. Jin and C. Li, J. Chem. Technol. Biotechnol., 2019, 94, 3578–3584 CrossRef CAS.
- V. S. Muthuraman and N. Kasianantham, Process Safety and Environmental Protection, 2023 Search PubMed.
- S. Salim, R. Bosma, M. H. Vermuë and R. H. Wijffels, J. Appl. Phycol., 2011, 23, 849–855 CrossRef PubMed.
- S. Viswanaathan, P. K. Perumal and S. Sundaram, Sustainability, 2022, 14, 1075 CrossRef CAS.
- Y. T. Cheah and D. J. C. Chan, Bioengineered, 2021, 12, 7577–7599 CrossRef CAS PubMed.
- J. Sheehan, T. Dunahay, J. Benemann and P. Roessler, National Renewable Energy Laboratory, 19983281–294.
- P. J. l. B. Williams and L. M. Laurens, Energy Environ. Sci., 2010, 3, 554–590 RSC.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- Y. Chisti, Trends Biotechnol., 2008, 26, 126–131 CrossRef CAS PubMed.
- J. R. Benemann, Energy Convers. Manage., 1997, 38, S475–S479 CrossRef CAS.
- O. Hammouda, A. Gaber and N. Abdelraouf, Ecotoxicol. Environ. Saf., 1995, 31, 205–210 CrossRef CAS PubMed.
- N. Mallick, BioMetals, 2002, 15, 377–390 CrossRef CAS PubMed.
- W. Y. Chia, D. Y. Y. Tang, K. S. Khoo, A. N. K. Lup and K. W. Chew, Environ. Sci. Ecotechnology, 2020, 4, 100065 CrossRef PubMed.
- E. Becker, Handbook of microalgal mass culture, 1986, pp. 339–420 Search PubMed.
- P. W. Behrens and D. J. Kyle, J. Food Lipids, 1996, 3, 259–272 CrossRef CAS.
- M. Plaza, S. Santoyo, L. Jaime, G. G.-B. Reina, M. Herrero, F. J. Señoráns and E. Ibáñez, J. Pharm. Biomed. Anal., 2010, 51, 450–455 CrossRef CAS PubMed.
Footnote |
| † Authors contribute equally to the work. |
| This journal is © The Royal Society of Chemistry 2023 |